- 1Department of Pediatrics, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait
- 2Allergy and Clinical Immunology Unit, Pediatric Department, Al-Sabah Hospital, Kuwait City, Kuwait
- 3Department of Quantitative Methods and Information Systems, College of Business Administration, Kuwait University, Kuwait City, Kuwait
- 4As’ad Al-Hamad Dermatology Center, Kuwait City, Kuwait
Background and Objectives: Reports on skin manifestations in inborn errors of immunity (IEI) are based on retrospective analysis, small series, or isolated case reports. The present prospective study aimed to determine the spectrum of skin manifestations in children with IEI and their relevance to specific molecular defects.
Materials and Methods: The data were obtained from the Kuwait National Primary Immunodeficiency Disorders Registry during the period of 2004–2020.
Results: A total of 313 pediatric cases of IEI, 71% diagnosed at molecular level, were registered with a cumulative follow-up period of 29,734 months. Skin manifestations were seen in 40.3% of the patients, and they were among the presenting manifestations in 33%. Patients with skin manifestations were older at both onset and diagnosis ages of IEI symptoms, but this was statistically significant for the latter only. The diagnosis delay was significantly longer in patients with skin manifestations. There was a statistically significant association between having skin manifestations and IEI category, being more common in patients with complement deficiencies, combined immunodeficiencies, and diseases of immune dysregulation. There was no statistically significant association between having skin manifestations and both gender and survival. Skin infections were the most frequent manifestations followed by eczema and autoimmune associations. Among IEI with more than 10 cases, skin lesions were a consistent finding in dedicator of cytokinesis 8 (DOCK8) deficiency, hyper IgE syndrome, ataxia-telangiectasia, and recombination activation gene (RAG)1 deficiency.
Conclusions: Skin manifestations are common in IEI patients, and they had significant diagnosis delay and referral to specialists. Improvement of awareness about IEI is needed among pediatricians and dermatologists.
Introduction
Around 6 million people worldwide are suggested to be suffering from inborn errors of immunity (IEI) (1). According to a recent review, a total of 104,614 have been reported from different registries, with molecular defects identified only in 13.2% (2). Hence, a great majority of IEI patients remain undiagnosed.
Skin manifestations have been reported in 40%–70% of the patients with IEI, and they are among the presenting features in majority of them (3–8). There are only a few studies that have focused on the whole spectrum of cutaneous manifestations seen among IEI patients (4–8). Most data are restricted to cutaneous manifestations of one disorder, case series, or isolated case reports (9). We had earlier published a prospective report on skin manifestations of primary immunodeficient children registered in the Kuwait National Primary Immunodeficiency Registry (KNPIDR) over a span of 6 years (6, 10). Now, 10 years later, we have 313 children diagnosed with IEI. With improved diagnosis facilities and genetic testing, molecular diagnosis has been settled in most of them.
The present study aimed to determine the frequencies and characteristics of skin manifestations in children with IEI and to determine their relevance to specific molecular defects. This will hopefully highlight the importance of such manifestations to help in early diagnosis and timely management of IEI patients.
Materials and Methods
Patients’ Data
The data were obtained from the KNPIDR as part of a study approved by The Research and Ethics Committee of the Ministry of Health in Kuwait and by the Kuwait University Health Sciences Center Ethical Committee in accordance with the Declaration of Helsinki (10). An informed written consent was obtained from patients and/or families for whom testing was done for research purposes. A written informed consent was obtained from the patients and/or families for publishing the images. The subjects were diagnosed between January 2004 and December 2020 and followed prospectively. The patients were followed prospectively by WA-H and AN whenever they showed skin manifestations. Patients were categorized according to the International Union of Immunological Societies (IUIS) Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity (2019) (11). Secondary immunodeficiencies were ruled out by obtaining a complete history and by performing proper testing when needed. The diagnosis of skin manifestations was made clinically and was supported by a skin biopsy and other relevant investigations whenever indicated.
Statistics
Minitab 19.2020.1 (Minitab LLC, PA, USA) software was used to carry out all statistical tests. Pearson’s chi-square test was used to assess whether skin manifestations have any relation with death count, gender, or IEI categories. A two-sample t-test was used to assess whether there is a difference in onset age, diagnosis age, and diagnosis delay between the group of patients with and without skin manifestations. The Kaplan–Meier survival analysis was used to estimate the survival function. Survivals were calculated from the date of diagnosis until the date of death (uncensored) or until the end of the study period (censored). The p-value ≤0.05 was used as the cutoff level for statistical significance.
Results
Patients
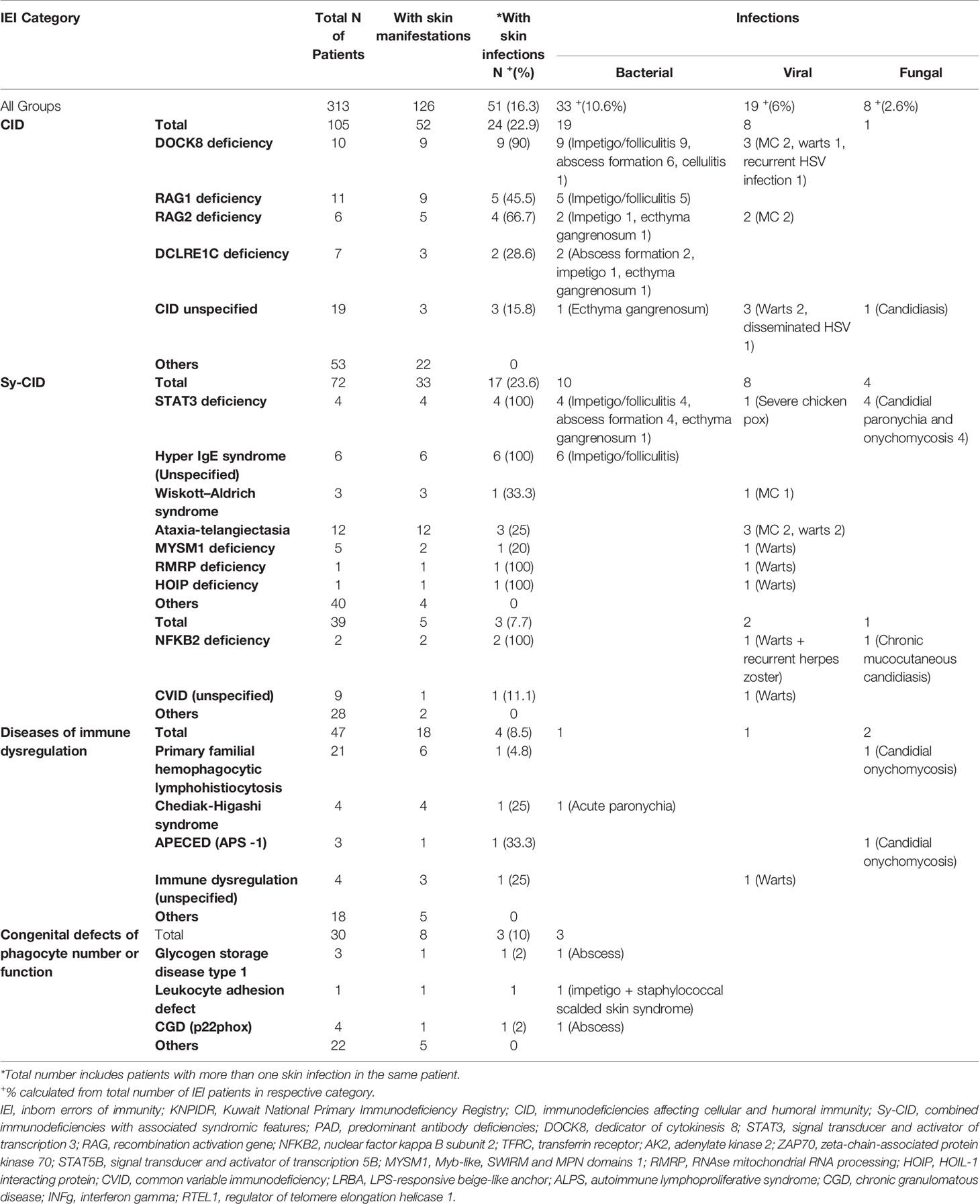
A total of 313 children with IEI (162 males and 151 females) were diagnosed during the study period and followed prospectively. Two hundred twenty-two patients (71%) were diagnosed at the molecular level. The cumulative follow-up period for all patients was 29,734 months (2,477 years) [mean 95 months, standard deviation (SD) 65.59, 0–204 months]. The distribution of these patients according to IEI categories (11) showed predominance of immunodeficiencies affecting cellular and humoral immunity (CIDs) (33.54%), followed by combined immunodeficiencies with associated syndromic features (Sy-CIDs) (23%), diseases of immune dysregulation (15.01%), predominant antibody deficiencies (PADs) (12.46%), congenital defects of phagocyte number and functions (9.58%), complement deficiencies (5.11%), defects of intrinsic and innate immunity (0.63%), and autoinflammatory disorders and bone marrow failure (0.31% each). Table 1 shows the frequencies of skin manifestations among the registered patients based on IEI categories.
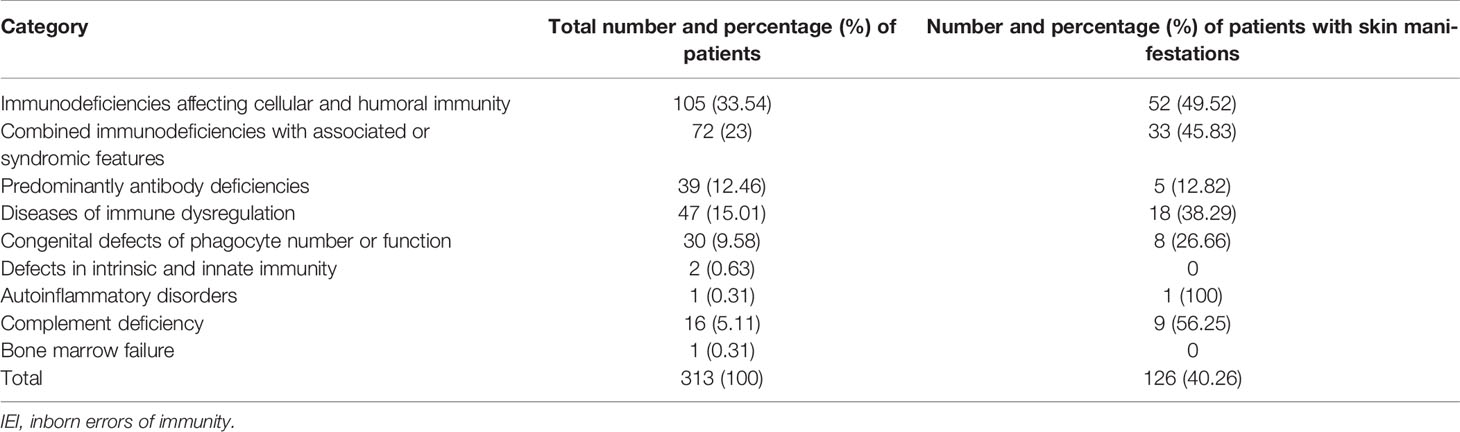
Table 1 Frequency of skin manifestations in children with IEI registered in the Kuwait National Primary Immunodeficiency Registry.
Characteristics of Skin Manifestations
One hundred twenty-six (40.3%) patients were observed to have skin manifestations, and in 103 (33% of all patients and 82% of those with skin lesions), they were among the presenting signs. Patients with skin manifestations were older at both onset and diagnosis ages of IEI symptoms compared to those with no such manifestations, but this was statistically significant for the latter only (Table 2). The diagnosis delay was longer in patients with skin manifestations, and it reached the level of significance (Table 2). There was no statistically significant association between having skin manifestations and gender (66 males and 60 females, p = 0.856). Table 3 shows the distribution of skin manifestations among the registered patients. Skin manifestations affected >50% of patients with complement deficiencies and almost half of patients with CID and Sy-CID. There was a statistically significant association between having skin manifestations and IEI category after merging together patients who belong to defects in intrinsic and innate immunity, autoinflammatory disorders, complement deficiencies, and bone marrow failure categories due to low numbers (p = 0.001) (Table 1).
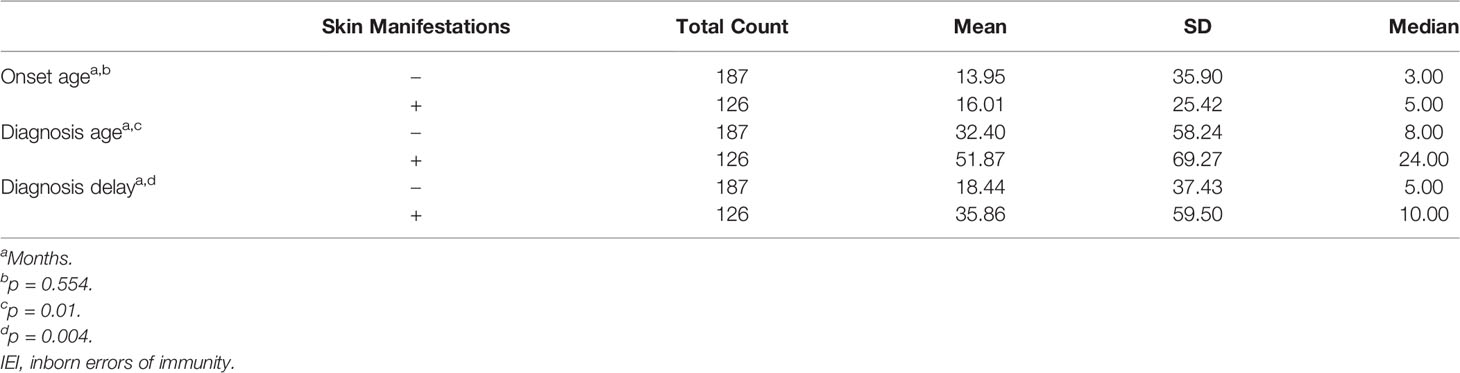
Table 2 Onset age, diagnosis age, and diagnosis delay of patients with IEI with respect to having skin manifestations.
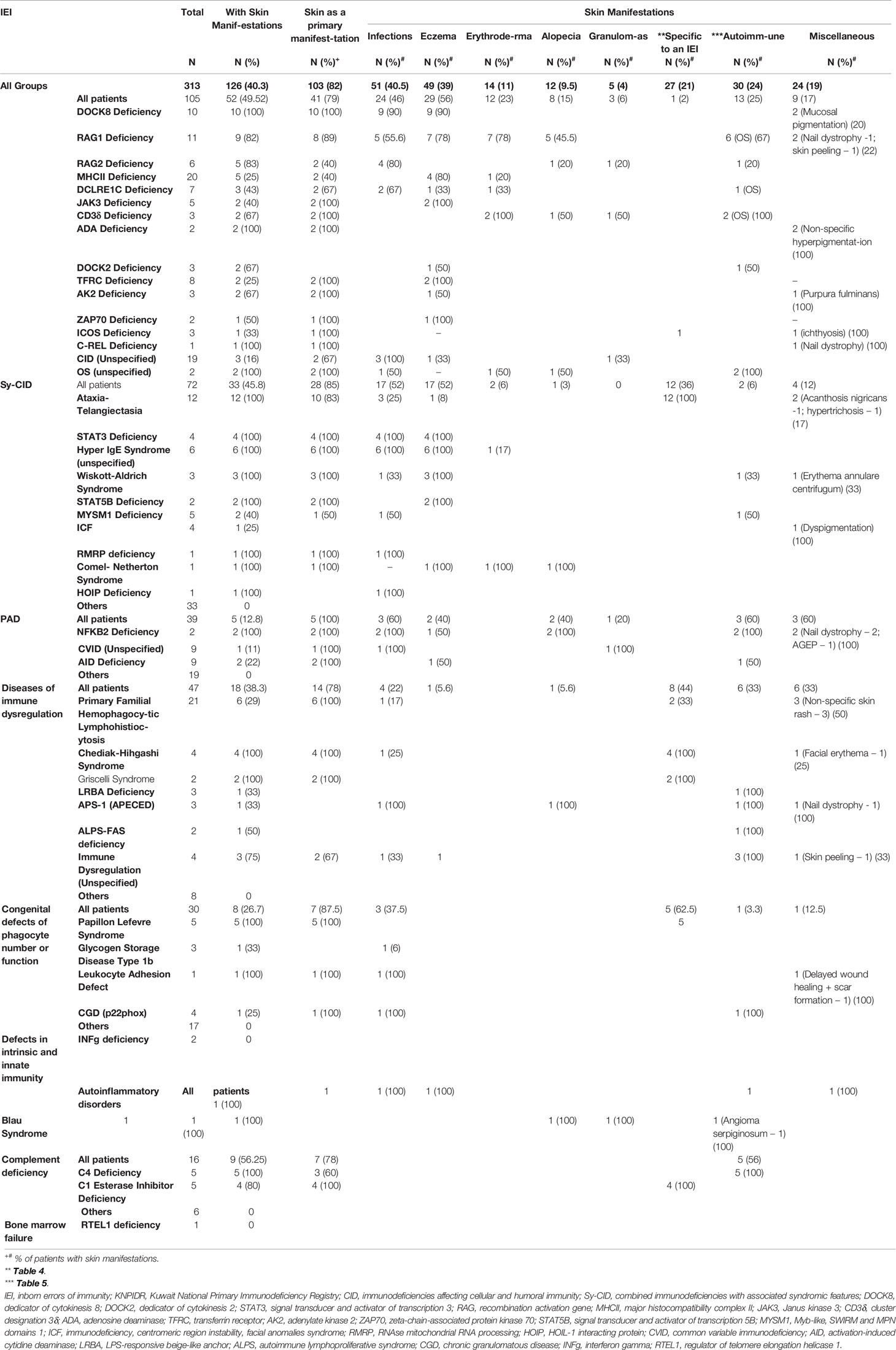
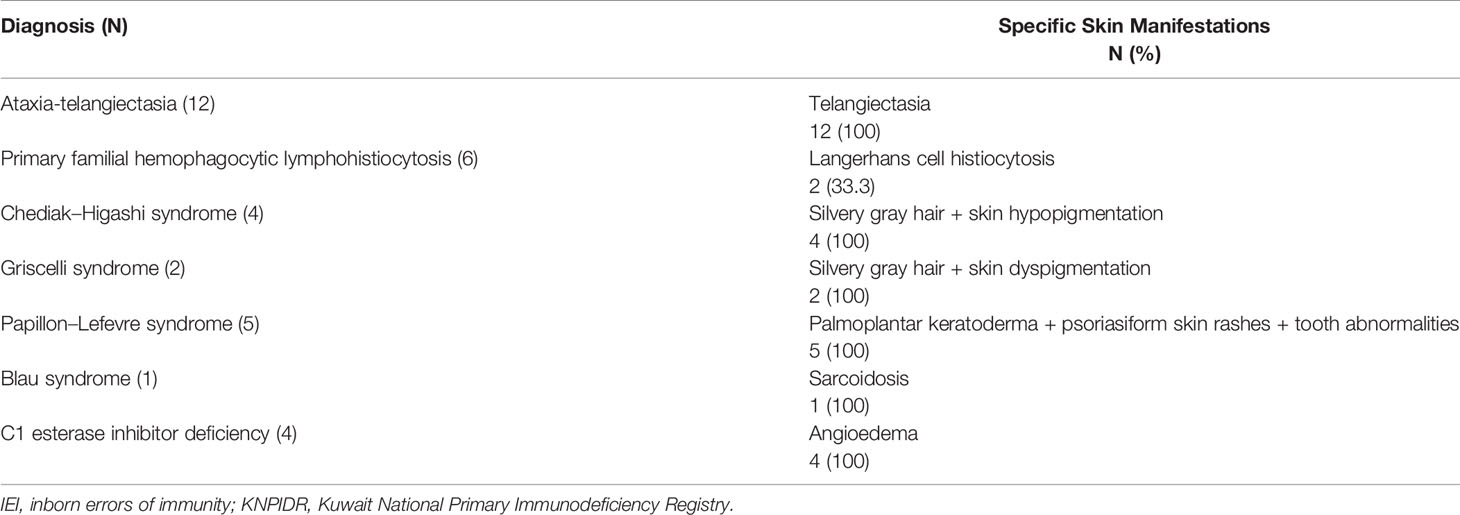
Table 3 Characterization of skin manifestations according to specific diagnosis among children with IEI registered in the KNPIDR.
Among 126 patients with skin manifestation, 60 (47.6%) had one skin manifestation, 28 (22.2%) had two, and 38 (30.2%) had three or more during the course of the disease. Skin infections were the most prevalent, followed by eczema/eczematoid rashes and autoimmune skin associations (Table 3). Miscellaneous skin manifestations not peculiar to a specific IEI were seen in 19% of the patients with skin manifestations. Among specific IEI with more than 10 patients registered in the KNPIDR, skin manifestations were present in 100% of the patients with dedicator of cytokinesis 8 (DOCK8) deficiency, hyper IgE syndrome (HIGE) [both signal transducer and activator of transcription 3 (STAT3) deficiency and unspecified], and ataxia-telangiectasia (AT) and in 82% of recombination activation gene 1 (RAG1) deficiency, and they were among the presenting signs in 79%, 100%, 83%, and 89% of them, respectively (Table 3).
Among infectious manifestations seen in 51 patients, bacterial infections were more prevalent and seen followed by viral and fungal infections (Table 4). Skin infections were prevalent among patients with Sy-CID followed by CID (Tables 3, 4). Skin infections with widespread eczematous/eczematoid rashes were a consistent finding in all patients with HIGE. Abscess formation was seen in all patients with STAT3 deficiency and DNA Cross-Link Repair 1C (DCLREIC) deficiency and in the majority of patients with DOCK8 deficiency. Four patients (8%) were confirmed to have ecthyma gangrenosum (EG) due to Pseudomonas infection that was caused by either primary skin invasion or as part of secondary invasion following Pseudomonas septicemia (Figure 1A). Various viral infections including warts (Figures 1B, C) and molluscum contagiosum (MC) (Figure 1D) were observed to be more widespread and recurrent.
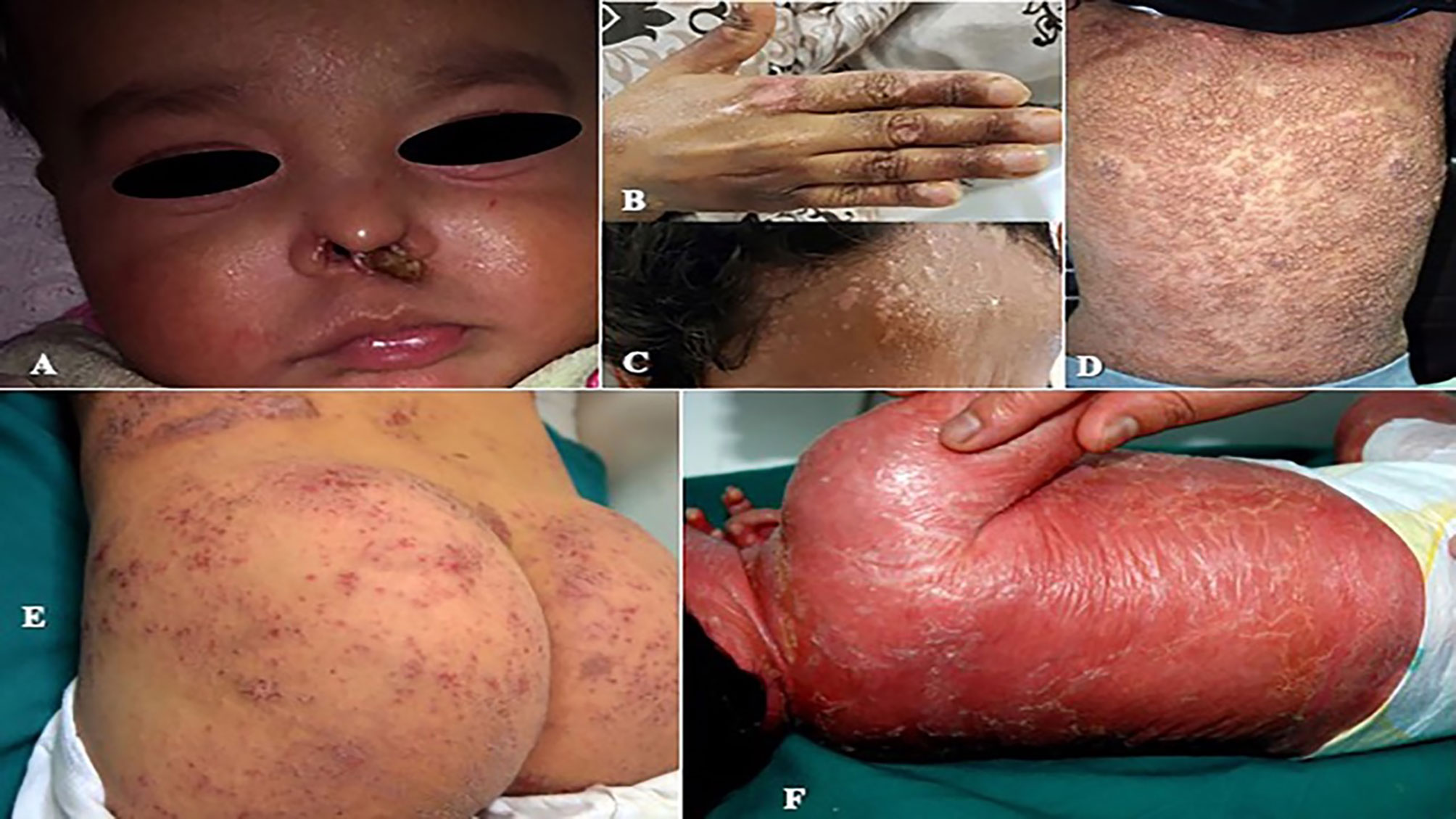
Figure 1 (A) Perinasal ecthyma gangrenosum in a patient with DCLRE1C deficiency. (B, C) Multiple recalcitrant warts in a patient with common variable immunodeficiency (CVID). (D) Generalized molluscum contagiosum in a patient with dedicator of cytokinesis 8 (DOCK8) deficiency. (E) Severe eczematous rashes in a patient with signal transducer and activator of transcription 3 (STAT3) deficiency. (F) Erythroderma in a patient with Omenn syndrome due to recombination activation gene 1 (RAG1) deficiency.
Eczema and eczematoid rashes were more often seen in patients with CID and Sy-CID and were consistent signs in patients with HIGE and Wiskott–Aldrich syndrome (WAS) and DOCK8 deficiency (Figure 1E). Among 14 cases with erythroderma, 12 (86%) were diagnosed with CID and was a consistent sign in patients with Omenn syndrome (OS) (Figure 1F). The other two cases with erythroderma included a case of HIGE and Comel–Netherton syndrome each. Among 12 cases of alopecia, eight were the patients with CID that included seven patients with OS. Three cases among other four were of alopecia areata (Figure 2A) associated with NFKB deficiency in two and autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) syndrome in one. Among five patients with cutaneous granulomas, three were associated with CID and one each with common variable immunodeficiency (CVID) and Blau syndrome.

Figure 2 (A) Alopecia areata in a patient with nuclear factor kappa B subunit 2 (NFKB2) deficiency. (B–D) Erythematous scaly plaques and palmoplantar keratoderma in a patient with Papillon–Lefèvre syndrome. (E–G) Tumid lupus erythematosus lesions on the face, skin histopathology showing features of lupus erythematosus, and direct immunofluorescence with granular deposition of IgM at the basement membrane zone in a patient with activation-induced cytidine deaminase (AID) deficiency.
Table 5 shows the specific skin manifestations seen in IEI patients. Mucocutaneous telangiectasia was observed to be a consistent feature in all patients with AT. Silvery gray hair and hypopigmentation of skin were consistent findings in patients with Chediak–Higashi syndrome and Griscelli syndrome; scaly rashes on the extremities, palmoplantar keratoderma (Figures 2B–D), tooth abnormalities, and gingivitis were consistent findings of Papillon–Lefevre syndrome.
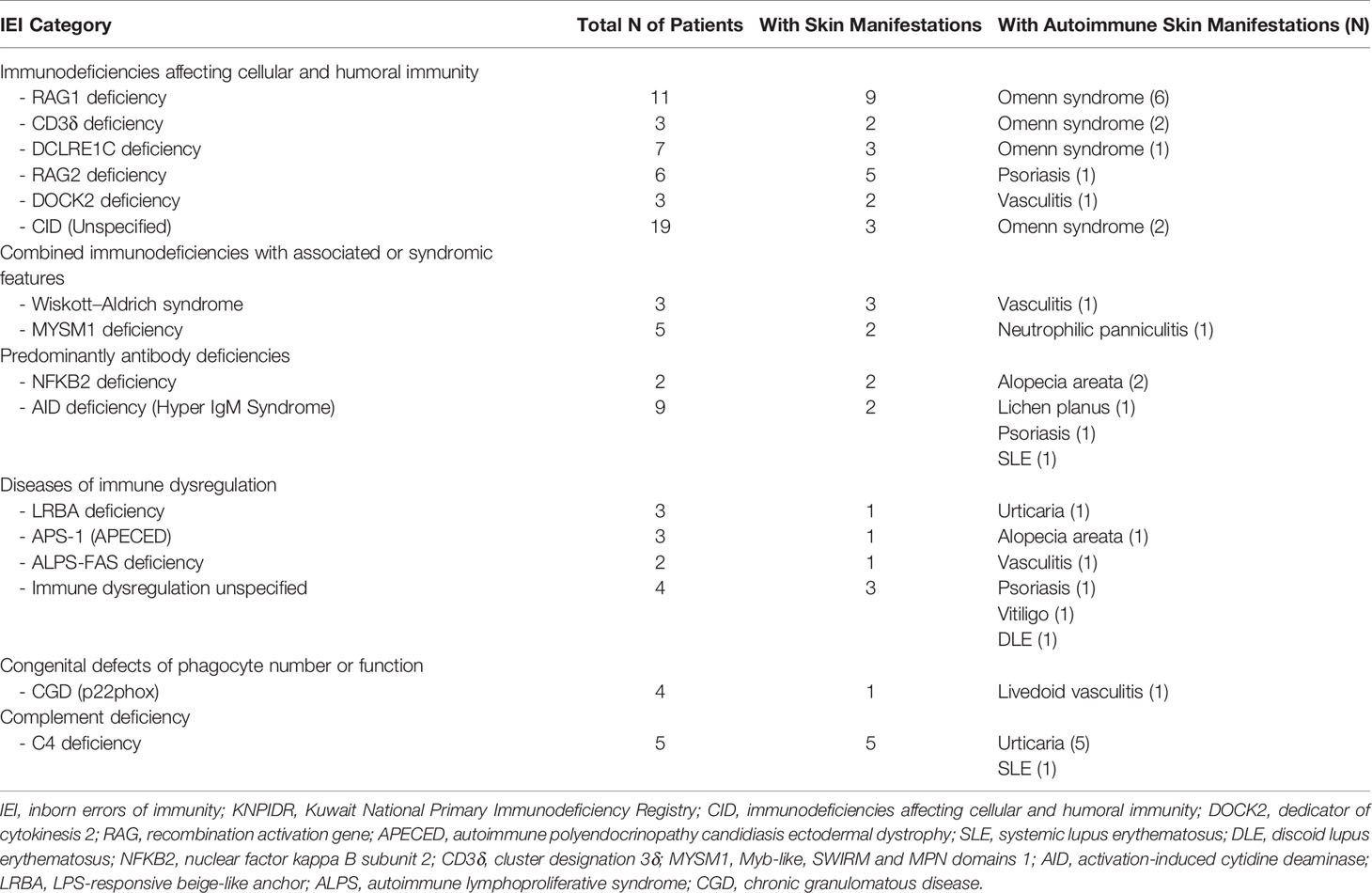
Autoimmune skin diseases were seen among 9.5% of total IEI cases and were more often encountered in patients with complement deficiencies followed by diseases of immune dysregulation (Table 6, Figures 2E–G).
Survival Analysis
There were 85 deaths during the study period. There was no significant statistical association between having skin manifestations and death (p = 0.644). Figure 3 shows the Kaplan–Meier survival curves of children with IEI with respect to having skin manifestations.
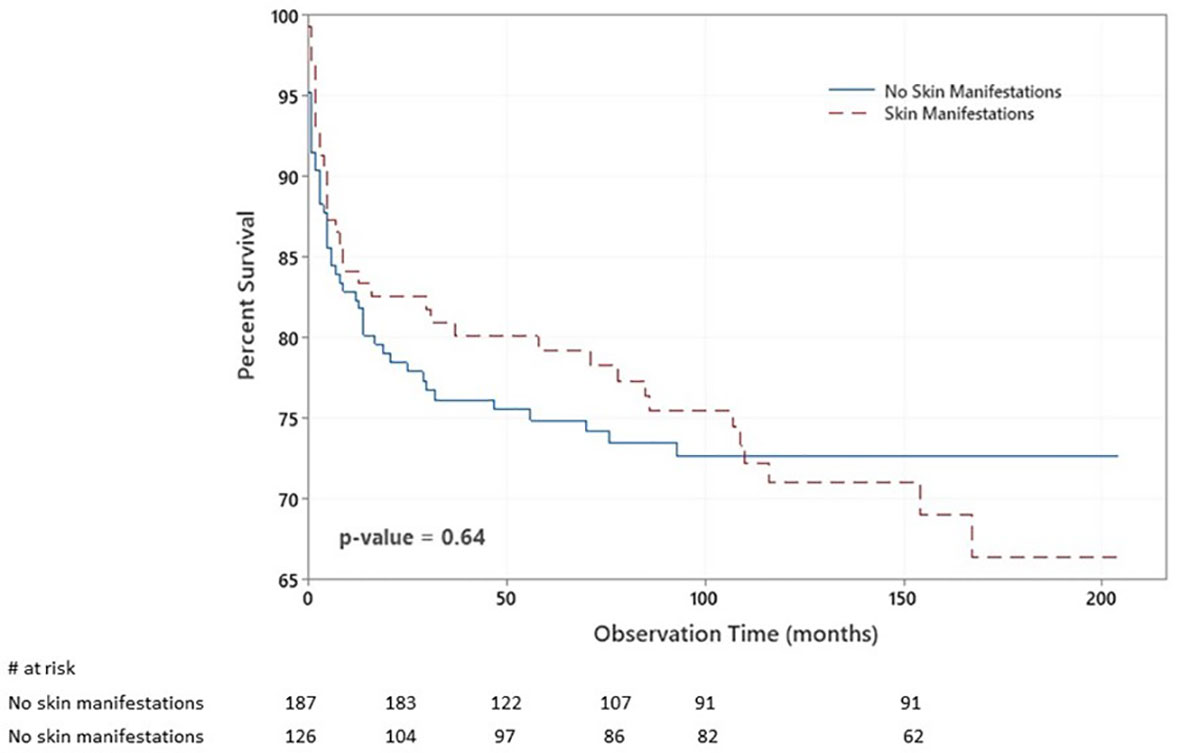
Figure 3 Kaplan–Meier survival plot of the chance of survival among children with inborn errors of immunity (IEI) with respect to having skin manifestations. The probabilities that patients with no skin manifestations survived 2, 6, and 14 years after diagnosis were 77%, 74%, and 72%. At the same time, they were 82%, 74%, and 42% among patients with skin manifestations.
Discussion
The present study aimed to determine the frequencies and characteristics of skin manifestations in children with IEI who were followed prospectively over 17 years and registered in a national registry and to determine their relevance to specific molecular defects. Skin is an important organ affected in IEI patients, and skin manifestations are the presenting features in a vast number of patients. As the list of IEI continues to widen, so is expected the spectrum of cutaneous manifestations.
Compared to our previous study (6), the number of patients in the current report is doubled and they are more characterized at the molecular level. Hence, the frequency and characteristics of skin manifestations in IEI are better defined. An important finding of our report is that the IEI patients with skin manifestation had longer delay in diagnosis compared to patients with no such manifestations. This is probably due to un-awareness of primary physicians, general pediatricians and dermatologist that such manifestations could be caused or related to IEI and the presumption that they could be due to common skin diseases of childhood like atopic dermatitis and/or skin infections. This signifies delay in clinical suspicion of IEI and referral to the specialists and warrants to develop more awareness among the primary physicians, general dermatologists and the pediatricians.
We observed skin manifestations in 40% of our patients, and they were the presenting signs in 33%, representing 82% of all with skin manifestations. This high number compared to previous reports (4, 5, 7, 8) is partly due to the prospective type of our study and due to the relatively high number of patients with combined immunodeficiencies registered in the KNPIDR. Skin manifestations were observed to be a consistent finding in diseases with more than 10 patients registered like DOCK8 deficiency, HIGE, RAG1 deficiency, and AT. However, no definite inference can be drawn in diseases with small number of patients.
The prevalence of skin infections in the present report (16.3%) is much lower than that reported from Colombia, Tunisia, Iran, and Mexico (56.63%, 36.55%, 47.1%, and 61.5%, respectively) (4, 5, 7, 8) as well as from that reported by us earlier from Kuwait (30%) (6). This represents improved awareness of infection control care of our IEI patients over the years. Skin infections were significantly more often seen in patients with Sy-CID and CID as compared to other groups of IEI. This can be explained by the more severe type of immune defects affecting patients in these categories. Similar to our earlier observations and those reported from elsewhere (4–9, 12), Staphylococcus aureus abscess formation was a consistent feature in HIGE syndrome and DOCK8 deficiency patients. Besides the common staphylococcal skin infections, patients are also prone to get rare infections due to Gram-negative bacteria that may result in EG, which has been reported earlier in patients with X-linked agammaglobulinemia, chronic granulomatous disease, and hemophagocytic lymphohistiocytosis (13–15).
Eczematous rashes have been reported in 13%–57% of the patients with IEI in previous reports (4–8). We feel that the low frequency of eczematous/eczematoid rashes in this report (15.7%) as compared to our earlier report (19%) (6) is due to widening of our IEI registry including several patients with IEI that are not associated with eczematous rashes. However, comparable to our earlier observations and those reported from elsewhere (4–9, 12), eczema is a consistent feature of HIGE syndrome and WAS and a predominant feature in patients with CID including DOCK8, RAG1, and MHC II deficiency.
Non-infectious cutaneous granulomas are a rare manifestation of IEI. Although they have been reported with several diseases, they are more often seen in patients with AT, CID, and CVID (16, 17). They were reported to constitute 0.77% of total IEI children in a report from France (17). Five patients with cutaneous granulomas in the present report were observed to constitute 1.6% of all patients with IEI. Four of these patients have been earlier reported (18).
Manifestations specific to various IEI were seen in 21% of the patients. These are the manifestations that can be seen in patients other than IEI, but when present in patients who are suspected to have an IEI, they raise the suspicion of a specific disease that needs to be ruled out.
Autoimmune manifestations related to skin, hair, and nail were second common after cytopenias were previously reported and constituted 21% of the patients with all autoimmune manifestations (19). Autoimmune diseases have been reported to be more frequent by a factor of 10 in patients with IEI than the general population, they tend to occur throughout the lifetime, and they have a prognostic significance (20). We observed autoimmune skin manifestations among 9.5% of patients with IEI, and they represented 24% of total patients with skin manifestations. They were more often seen in patients with complement deficiencies, followed by diseases of immune dysregulation, CID and PAD. The high prevalence of autoimmune diseases in IEI demonstrates the intricate relationships between the mechanisms involved in these two conditions and is a result of defects in central and peripheral tolerance with the influence of chronic and recurrent infections (21).
To conclude, skin is a common organ affected in the IEI, and being apparent can be more important than any other organ in terms of providing a first clue to diagnosis and thus in aiding in early detection and management of these patients. The strength of the current study is the inclusion of a relatively large number of molecularly defined patients who were followed prospectively over a long period of time by the same investigators. Limitations of the present study include the small number of cases of various individual IEI among most groups and restriction to one ethnic group that may not be representative of the true spectrum of IEI and their skin manifestations across the globe. Hence, more studies, both global and from different regions that include patients with different IEI or disease specific, are needed to have a better understanding of the effects of IEI on the skin.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Research and Ethics Committee of the Ministry of Health in Kuwait, and by the Kuwait University Health Sciences Center Ethical Committee, in accordance with the Declaration of Helsinki. An informed written consent was obtained from patients and/or families for whom testing was done for research purposes. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individuals, and minors’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
WA-H contributed to the establishment of the KNPIDR, patients’ diagnosis, data collection and analysis, writing the article, approval of the submitted article, and agreement to be accountable for the content of the work. MZ contributed to the data analysis and statistics, approval of the submitted article, and agreement to be accountable for the content of the work. AN participated in patients’ diagnosis, data collection and analysis, writing the article, approval of the submitted article, and agreement to be accountable for the content of the work. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We want to thank our patients and their families to whom our work is dedicated.
Abbreviations
IEI, inborn errors of immunity; KNPIDR, Kuwait National Primary Immunodeficiency Registry; IUIS, International Union of Immunological Societies; SD, standard deviation; CID, immunodeficiencies affecting cellular and humoral immunity; Sy-CID, combined immunodeficiencies with associated syndromic features; PAD, predominant antibody deficiencies; DOCK8, dedicator of cytokinesis 8; DOCK2, dedicator of cytokinesis 2; STAT3, signal transducer and activator of transcription 3; RAG, recombination activation gene; EG, ecthyma gangrenosum; MC, molluscum contagiosum; WAS, Wiskott–Aldrich syndrome; OS, Omenn syndrome; HIGE, hyper IgE syndrome; APECED, autoimmune polyendocrinopathy candidiasis ectodermal dystrophy; AT, ataxia telangiectasia; SLE, systemic lupus erythematosus; DLE, discoid lupus erythematosus; NFKB2, nuclear factor kappa B subunit 2; MHCII, major histocompatibility complex II; JAK3, Janus kinase 3; CD3δ, cluster designation 3δ; ADA, adenosine deaminase; TFRC, transferrin receptor; AK2, adenylate kinase 2; ZAP70, zeta-chain-associated protein kinase 70; STAT5B, signal transducer and activator of transcription 5B; MYSM1, Myb-like, SWIRM and MPN domains 1; ICF, immunodeficiency, centromeric region instability, facial anomalies syndrome; RMRP, RNAse mitochondrial RNA processing; HOIP, HOIL-1 interacting protein; CVID, common variable immunodeficiency; AID, activation-induced cytidine deaminase; LRBA, LPS-responsive beige-like anchor; ALPS, autoimmune lymphoproliferative syndrome; CGD, chronic granulomatous disease; INFg, interferon gamma; RTEL1, regulator of telomere elongation helicase 1.
References
1. Bousfiha AA, Jeddane L, Ailal F, Benhsaien I, Mahlaoui N, Casanova JL, et al. Primary Immunodeficiency Diseases Worldwide More Common Than Generally Thought. J Clin Immunol (2013) 38:1–7. doi: 10.1007/s10875-012-9751-7
2. Abolhassani H, Aziz G, Sharifi L, Yazdani R, Mohsenzadegan M, Delavari S, et al. Global Systematic Review of Primary Immunodeficiency Registries. Exp Rev Clin Immunol (2020) 16(7):717–32. doi: 10.1080/1744666X.2020.1801422
3. Lewis DJ, Wu JH, Boyd M, Duvic M, Feldman SR. Cutaneous Manifestations of Genodermatoses and Primary Immunodeficiency. Dermatol Online J (2019) 25(6):13030/qt1gj1n07j. doi: 10.5070/D3256044442
4. Berron-Ruiz A, Berron-Perez R, Ruiz-Maldonado R. Cutaneous Markers of Primary Immunodeficiency Diseases in Children. Pediatr Dermatol (2000) 17(3):91–6. doi: 10.1046/j.1525-1470.2000.01721.x
5. Moin A, Harhoudi A, Moin M, PourPpak Z, Bazargan N. Cutaneous Manifestations of Primary Immunodeficiency Diseases in Children. Iran J Allergy Asthma Immunol (2006) 5(3):121–6.
6. Al-Herz W, Nanda A. Skin Manifestations in Primary Immunodeficient Children. Pediatr Dermatol (2011) 28(5):494–501. doi: 10.1111/j.1525-1470.2011.01409.x
7. Guirat Dhouib N, Ben Khaled M, Ouederni M, Ben-Mustapha I, Kouki R, Besbes H. Cutaneous Manifestations of Primary Immunodeficiency Diseases in Tunisian Children. Mediterr J Hematol Infect Dis (2018) 10(1):e2018065. doi: 10.4084/MJHID.2018.065
8. López-Qintero W, Cleves D, Gomez-Vasco J, Pérez P, Patiño J, Medina valencia D, et al. Skin Manifestations in Pediatric Patients With Primary Immunodeficiency Diseases (PIDs) in a Tertiary Care Hospital in Colombia. World Allergy Organ J (2021) 14(3):e100527. doi: 10.1016/j.waojou.2021.100527
9. de Wit J, Brada JK, van Veldhuizen J, Dalm VASH, Pasmans SGMA. Skin Disorders are Prominent Features in Primary Immunodeficiency Diseases: A Systematic Overview of Current Data. Allergy (2019) 74:464–82. doi: 10.1111/all.13681
10. Al-Herz W, Al-Ahmad M, Al-Khabaz A, Husain A, Sadek A, Othman Y. The Kuwait National Primary Immunodeficiency Registry 2004-2018. Front Immunol (2019) 10:1754. doi: 10.3389/fimmu.2019.01754
11. Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunnigham Rundles C, Etzioni A, et al. Human Inborn Errors of Immunity 2019 Update on the Classification From the International Union of Immunological Societies Expert Committee. J Clin Immunol (2020) 40(1):24–64. doi: 10.1007/s10875-019-00737-x
12. Al-Herz W, Ragupathy R, Massaad MJ, Al-Attiyah R, Nanda A, Engelhardt KR, et al. Clinical, Immunologic and Genetic Profiles of DOCK8-Deficient Patients in Kuwait. Clin Immunol (2012) 143(3):266–72. doi: 10.1016/j.clim.2012.03.002
13. Huang H, Bai K, Fu Y, Yan J, Li J. Ecthyma Gangrenosum Due to Pseudomonas Aeruginosa Sepsis as Initial Manifestation of X-Linked Agammaglobulinemia: A Case Report. BMC Pediatr (2020) 20(1):540. doi: 10.1186/s12887-020-02436-8
14. Martínez-Longoria J, Ocampo-Candiani J, Rosales-Solis GM, Guerrero-González GA. Ecthyma Gangrenosum: A Report of Eight Cases. Bras Dermatol (2017) 92(5):698–700. doi: 10.1590/abd1806-4841.20175580
15. Nanda A, Al-Abboh H, Zahra A, Al-Sabah H, Gupta A, Adekile AD. Neutrophilic Panniculitis in a Child With MYSM1 Deficiency. Pediatr Dermatol (2019) 36(2):258–9. doi: 10.1111/pde.13757
16. Harp J, Coggshall K, Ruben BS, Ramírez-Valle F, He SY, Berger TG. Cutaneous Granulomas in the Setting of Primary Immunodeficiency: A Report of Four Cases and Review of the Literature. Int J Dermatol (2015) 54(6):617–25. doi: 10.1111/ijd.12765
17. Leclerc-Mercier S, Moshous D, Neven B, Mahlaoui N, Martin L, Pellier I, et al. Cutaneous Granulomas With Primary Immunodeficiency: A Report of 17 New Patients and a Review of the Literature. J Eur Acad Dermatol Venereol (2019) 33(7):1412–20. doi: 10.1111/jdv.15568
18. Nanda A, Al-Herz W, Al-Sabah H, Al-Ajmi H. Noninfectious Cutaneous Granulomas in Primary Immunodeficiency Disorders: Report of a National Registry. Am J Dermatopathol (2014) 36(10):832–7. doi: 10.1097/DAD.0000000000000112
19. Massaad MJ, Zainal M, Al-Herz W. Frequency and Manifestations of Autoimmunity Among Children Registered in Kuwait National Primary Immunodeficiency Registry. Front Immunol (2020) 11:1119. doi: 10.3389/fimmu.2020.01119
20. Fischer A, Provot J, Jais JP, Alcais A, Mahlaoui N, Members of the CEREDIH French PID Study Group, et al. Autoimmune and Inflammatory Manifestations Occur Frequently in Primary Immunodeficiencies. J Allergy Clin Immunol (2017) 140(5):1388–93. doi: 10.1016/j.jaci.2016.12.978
Keywords: skin manifestations, inborn errors of immunity, eczematoid rashes, cutaneous infections, autoimmunity, non-infectious cutaneous granulomas, immunodeficiency
Citation: Al-Herz W, Zainal M and Nanda A (2021) A Prospective Survey of Skin Manifestations in Children With Inborn Errors of Immunity From a National Registry Over 17 Years. Front. Immunol. 12:751469. doi: 10.3389/fimmu.2021.751469
Received: 01 August 2021; Accepted: 10 September 2021;
Published: 30 September 2021.
Edited by:
Guzide Aksu, Ege University, TurkeyReviewed by:
Ricardo U. Sorensen, Louisiana State University, United StatesAnete S. Grumach, Faculdade de Medicina do ABC, Brazil
Copyright © 2021 Al-Herz, Zainal and Nanda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waleed Al-Herz, d2VtaEBob3RtYWlsLmNvbQ==; d2FsZWVkLmFsaGVyekBrdS5lZHUua3c=
 Waleed Al-Herz
Waleed Al-Herz Mohammad Zainal
Mohammad Zainal Arti Nanda
Arti Nanda