
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 23 September 2021
Sec. Vaccines and Molecular Therapeutics
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.742732
 Verena Fuhrmann1
Verena Fuhrmann1 Huey-Jy Huang1
Huey-Jy Huang1 Aysegul Akarsu2
Aysegul Akarsu2 Igor Shilovskiy3
Igor Shilovskiy3 Olga Elisyutina3
Olga Elisyutina3 Musa Khaitov3,4
Musa Khaitov3,4 Marianne van Hage5
Marianne van Hage5 Birgit Linhart1
Birgit Linhart1 Margarete Focke-Tejkl1,6
Margarete Focke-Tejkl1,6 Rudolf Valenta1,3,6,7*
Rudolf Valenta1,3,6,7* Bulent Enis Sekerel2
Bulent Enis Sekerel2Peanuts and tree nuts are two of the most common elicitors of immunoglobulin E (IgE)-mediated food allergy. Nut allergy is frequently associated with systemic reactions and can lead to potentially life-threatening respiratory and circulatory symptoms. Furthermore, nut allergy usually persists throughout life. Whether sensitized patients exhibit severe and life-threatening reactions (e.g., anaphylaxis), mild and/or local reactions (e.g., pollen-food allergy syndrome) or no relevant symptoms depends much on IgE recognition of digestion-resistant class I food allergens, IgE cross-reactivity of class II food allergens with respiratory allergens and clinically not relevant plant-derived carbohydrate epitopes, respectively. Accordingly, molecular allergy diagnosis based on the measurement of allergen-specific IgE levels to allergen molecules provides important information in addition to provocation testing in the diagnosis of food allergy. Molecular allergy diagnosis helps identifying the genuinely sensitizing nuts, it determines IgE sensitization to class I and II food allergen molecules and hence provides a basis for personalized forms of treatment such as precise prescription of diet and allergen-specific immunotherapy (AIT). Currently available forms of nut-specific AIT are based only on allergen extracts, have been mainly developed for peanut but not for other nuts and, unlike AIT for respiratory allergies which utilize often subcutaneous administration, are given preferentially by the oral route. Here we review prevalence of allergy to peanut and tree nuts in different populations of the world, summarize knowledge regarding the involved nut allergen molecules and current AIT approaches for nut allergy. We argue that nut-specific AIT may benefit from molecular subcutaneous AIT (SCIT) approaches but identify also possible hurdles for such an approach and explain why molecular SCIT may be a hard nut to crack.
Nuts are nutrient-dense foods that receive increasing attention due to reports regarding their possible health-promoting properties and their pleasant taste (1, 2). At the same time, tree nuts and peanuts are among the most common elicitors of anaphylaxis, a severe, potentially life-threatening hypersensitivity reaction mediated by allergen-specific IgE antibody-induced mast cell and basophil activation (3–6). The possibility of accidental nut ingestion and the associated fear of experiencing severe allergic reactions is particularly challenging for nut-allergic children and their parents and results in a considerable reduction in quality of life (7–10).
In allergology, a distinction is made between tree nuts and peanuts by defining a nut according to what is considered a nut in the culinary sense and less according to the botanical definition. Generally, “real” botanical nuts like the hazelnut, but also several seeds and drupes that grow on trees are considered tree nuts. Peanuts, which grow underground, are classified as legumes (11). Walnut, pistachio, pecan, hazelnut, almond, cashew, Brazil nut and macadamia are responsible for most allergic reactions to tree nuts and therefore included in this review under the umbrella of “tree nuts” (11) and the term “nut” used herein generally refers to peanuts and tree nuts unless otherwise specified.
True food allergy (class I) is characterized by the primary sensitization to the allergy-causing food via the gastrointestinal tract (12, 13) (Figure 1). Therefore, class I food allergens have usually higher stability to gastric digestion than other allergens (14). Immediate allergic reactions to nuts in sensitized patients occur within minutes after nut ingestion. It has been also speculated that IgE sensitization to class I food allergens may occur by epicutaneous sensitization (15) but on the other hand it was found that epicutaneous allergen application does not induce or boost allergen-specific IgE responses (16).
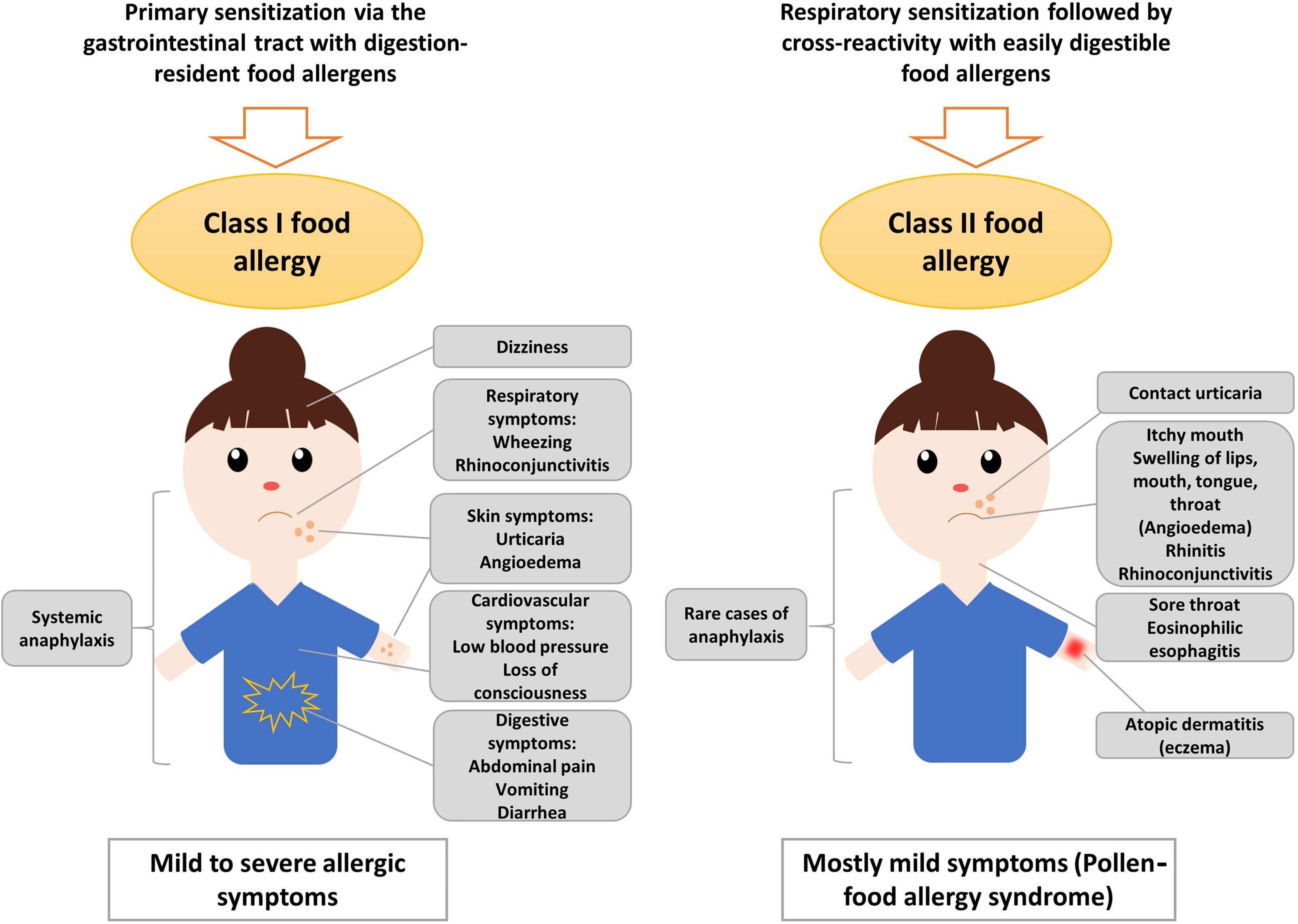
Figure 1 Sensitization to class I and class II nut allergens is associated with different clinical symptoms. Sensitization to class II nut allergens usually occurs by respiratory sensitization to cross-reactive respiratory allergens (e.g., pollen allergens) and is associated with mild symptoms such as oral allergy syndrome, local reactions in the oropharynx, esophagus and may trigger atopic dermatitis and/or urticaria. Sensitization to class I digestion-resistant nut allergens usually occurs via the gastrointestinal tract and eventually via the skin and is associated with systemic and severe manifestations such as anaphylaxis but also mild symptoms are possible.
The severity of the allergic reaction depends on the amount of allergen to which the patient is exposed and on other factors such as barrier function and allergen-specific sensitivity which often is associated with specific IgE levels. Class I food allergens often contain sequential IgE epitopes in addition to conformational IgE epitopes which indicates that sensitization occurs also to allergen fragments emerging through digestion in the gastrointestinal tract (17–19). Allergic reactions to nuts are typically IgE-mediated (type I reactions) and might cause symptoms affecting the gastrointestinal tract (abdominal pain, vomiting), the skin (urticaria, angioedema), the respiratory tract (rhino-conjunctivitis, wheezing) and, in severe cases, the cardiovascular system (loss of consciousness, low blood pressure) (Figure 1). Anaphylactic shock characterized by drop in blood pressure and cardiovascular failure involves several organ systems and requires immediate treatment with epinephrine (20). Several factors such as mast cell activation and/or load, existing co-allergies or asthma might enhance the risk of anaphylactic reactions to tree nuts (21).
Class II food allergy is associated with sensitization to pollen allergens. Patients are usually first sensitized to a pollen allergen and produce IgE antibodies which cross-react with allergens present in food. Examples include the major birch pollen allergen, Bet v 1 and the panallergen, profilin which were discovered first in birch pollen (22–26). IgE sensitization to class II food allergens is usually associated with mild allergic reactions known as pollen-food allergy syndrome (PFAS) or oral allergy syndrome (OAS) (20, 27). Clinical characteristics of PFAS include mainly oropharyngeal symptoms (27). Interestingly, it has been indicated that ingestion of birch pollen-related allergens from food sources such as Cor a 1 from hazelnut, could activate allergen-specific T cells independent of IgE, leading to late-phase and chronic allergic inflammation and this might further cause disorders such as atopic dermatitis in sensitized patients (28, 29). Moreover, pollen-related nut allergens causing PFAS might be associated with eosinophilic esophagitis, although they seem to be of less relevance than homologues from fruits and vegetables (30). However, eosinophilic esophagitis can be caused also by class I food allergens from milk, egg and wheat, while peanut and tree nuts seem to be of minor relevance (31). Major features of class II food allergens are that they contain mainly conformational but not sequential IgE epitopes which are sensitive to digestion and heating (32–34). The sensitization to class II food allergens is initially caused by pollen allergens and results in IgE and T cell cross-reactivity with the related food allergens (35, 36). IgE sensitization to class II food allergens is highly prevalent in countries with high exposure to the cross-reactive pollen allergens (37, 38). Accordingly, diagnostics including the measurement of IgE against the originally sensitizing pollen allergens (39) and allergen-specific immunotherapy to the cross-reactive pollen allergens can improve not only pollen allergy but also the associated food allergy to some extent (40, 41).
Diagnosis of nut allergy usually starts with a thorough evaluation of the patient’s history. Allergic sensitization can be detected by skin prick tests (SPT) and in vitro diagnostics with allergen extracts. However, sensitization determined by measurement of specific IgE antibodies and SPT does not always indicate clinical food allergy, which can only be confirmed by the occurrence of specific allergic symptoms after food exposure. Double-blind placebo-controlled food challenges (DBPCFC) are still considered the “gold standard” of food allergy testing, although patients are at risk of anaphylaxis during the procedure (42, 43). Lip dose challenge (LDC) is another possibility of testing which has a good predictive value for nut allergy (44). However, in recent years, molecular diagnosis with defined and mainly recombinant allergens by IgE serology has turned out to be very helpful in diagnosing nut allergy, in particular when it is combined with a thorough medical history (45). Another key problem in therapy of nut allergy is the lack of modern and effective allergen-specific treatment options. At present, avoidance of the disease-causing allergens is a possible option but there is also evidence that early introduction of for example of peanuts in the diet of sensitized but not yet symptomatic children may have beneficial effects (46). Accordingly, there are different opinions whether avoidance or rather intake should be recommended for sensitized children. Another major problem is that there is currently little progress regarding the development of modern molecular immunotherapy forms for nut allergy. Hypoallergenic allergen derivatives have been described (47) but no clinical studies have been performed so far. Sensitization to different nut allergens varies in different parts of the world due to dietary habits in diverse populations and varying allergen exposure in different areas but this is undergoing changes due to globalization and migration.
The prevalence of nut allergies among children and adults has been investigated in several studies (11, 48–51). However, there are large variations regarding methodology and study design which make it difficult to compare the studies and to understand the true nut allergy rates. It seems that reports on nut allergy prevalence do not provide accurate information regarding actual prevalences in the different populations due to several reasons. First of all, most studies that include a representative study population are limited to self-reports and do not contain a detailed clinical evaluation of patients. Moreover, several studies do not distinguish between sensitization to class I and class II food allergens. In this context, it must be considered that allergic reactions to nuts might be due to cross-reactivity with pollen allergens and are not caused by primary nut sensitization (11). Especially in studies from Europe, hazelnut allergy prevalence might thus be overestimated and sensitization should therefore be evaluated by molecular diagnosis to clearly distinguish between birch pollen allergic patients and those with true hazelnut allergy. This applies also to several other nuts that contain cross-reactive panallergens and cross-reactive carbohydrate determinants (CCDs). For example, many subjects who were tested positive by IgE serology using peanut allergen extracts in Zimbabwe were found to be sensitized to CCDs which usually do not cause allergic reactions (52).
Table 1 provides an overview of nut allergy prevalence studies, in particular from Europe, Northern America, Asia, Australia and Africa (38, 48–50, 52–84).

Table 1 Importance of peanut and different tree nuts as allergen sources in different parts of the world.
Importantly, the worldwide prevalence of nuts causing allergy correlates strongly with the nuts that are consumed in this region. However, for improved nut allergy management it is more relevant to consider the sensitization profile of nut allergic patients on a molecular level. As an example, sensitization to allergens of the family of pathogenesis-related class 10 (PR-10) proteins is widespread in northern countries, while IgE reactivity to non-specific lipid transfer proteins (nsLTPs) is predominant in the Mediterranean region. Molecular diagnostics significantly helps to distinguish between cross-reactive allergens and those that are a true indicator of sensitization to a particular nut.
In Europe, regional as well as ethnical differences in the sensitization profile of nut allergic patients have been observed (48, 50, 56). Generally, self-reported prevalence is significantly higher than food challenge-confirmed nut allergy (58). Several studies that investigated peanut allergy prevalence in Europe revealed varying prevalence rates (53–55, 59). In Russia, peanut allergy does not seem to play a major role in food allergy (38). Peanuts and cashew nuts are among the most common elicitors of anaphylaxis (85). Co-sensitization to different nuts correlates strongest between nuts of the same botanical family such as cashew and pistachio or pecan and walnut (60).
In the US, peanut is one of the most common foods causing allergy (76–78). Among tree nuts, walnut and cashew cause most of the allergic reactions, followed by almond, pistachio, Brazil nut, hazelnut and macadamia (76). Similar results were seen in a Canadian study with peanut allergy being most prevalent, predominantly in children (81).
In Central and South America, few studies reported sensitization of allergic patients to peanut and almond, although in this region, allergy to nuts seems to be low in general (79, 80, 86, 87). In most Latin American countries, frequent foods that cause allergy include fish, seafood, milk, egg, vegetables and fruits (87, 88).
In Asia, peanut allergy prevalence seems to be low compared to US and certain western countries (76, 89–92). Cashew nut is one of the most common reported tree nuts causing allergy in Asia (67, 70, 71, 74). However, tree nut allergy prevalence varies significantly across Asia especially between East and Southeast Asia and the Middle East (62, 63, 66, 70, 74). It can be assumed that the availability of nuts in certain regions contributes to the prevalence of allergies to these nuts, as can be seen by the increased frequency of pistachio allergy in pistachio cultivation regions (64).
In Australia, peanut allergy is one of the most frequent elicitors of IgE-mediated food allergy (49, 93). Tree nut allergy in Australia is less common than peanut allergy and prevalence rates of individual tree nut allergies vary significantly between studies (82, 83, 93).
Peanut allergens are the most frequently recognized nut allergens in South Africa (84) as determined in allergic children whereas IgE recognition of peanut allergens seems to be often asymptomatic as reported for Zimbabwe (52) but data regarding the prevalence of nut allergies in Africa are rare.
Figure 2 provides an overview of the role of different nuts as allergen sources for different regions of the world. Peanut allergy seems to be most frequent in most parts of the world whereas in Europe hazel nut allergy seems to be more important. Interestingly, different molecular IgE sensitization patterns can be observed in different geographic regions depending on birch pollen exposure involving IgE reactivity to Ara h 8, sensitization to lipid transfer proteins in southern Europe with sensitization to Ara h 9, and the classical peanut sensitization involving storage proteins such as Ara h 1, Ara h 2, Ara h 3 and Ara h 6 (94–96). In South America, nut allergy seems to be less common than in other parts of the world. Only few data are available for Africa indicating a need for further studies. It seems that early introduction of peanut in the diet as it occurs in Zimbabwe results in a low rate of symptomatic peanut allergy (52).
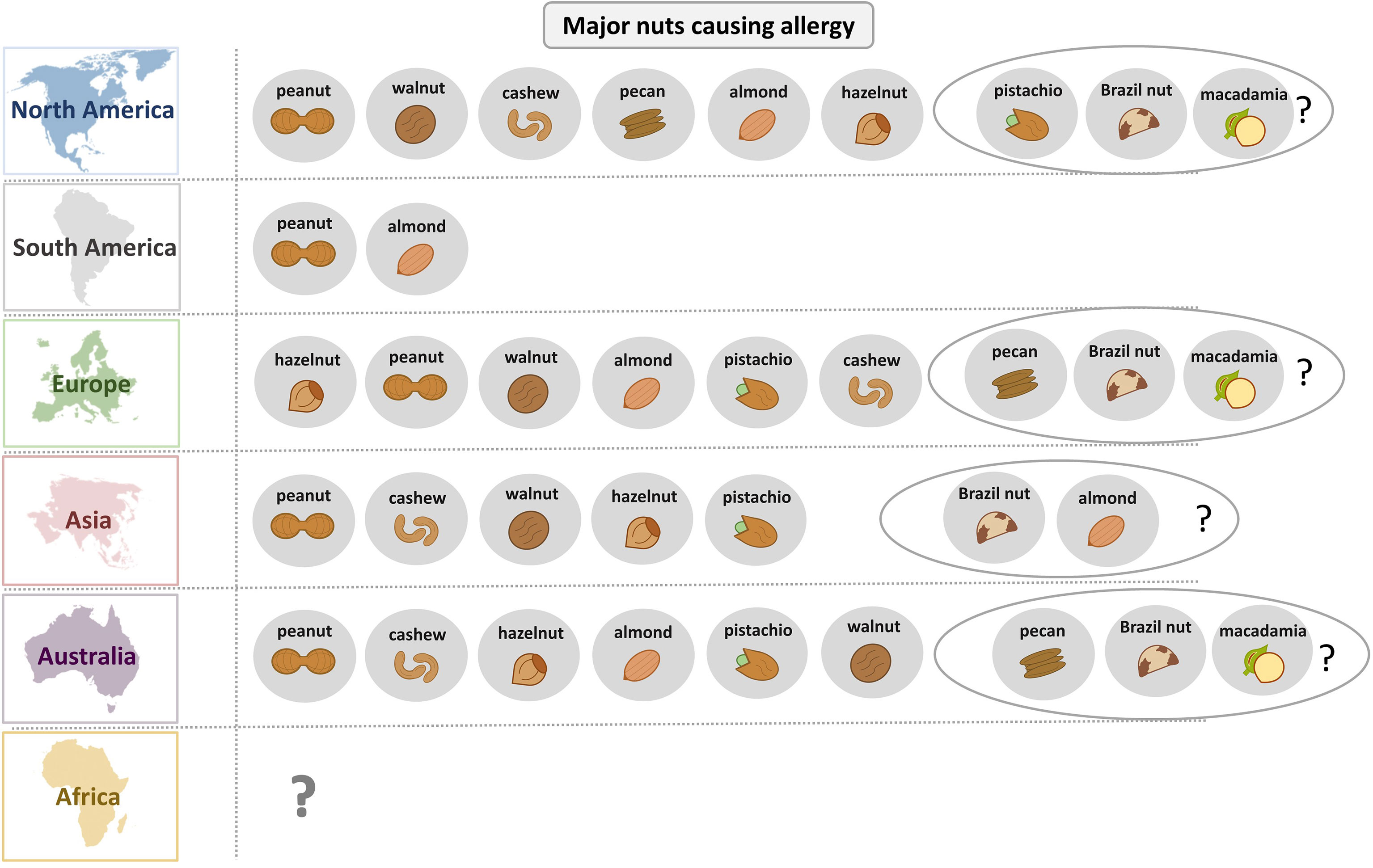
Figure 2 Overview of the relevance of different nuts as allergen sources in different parts of the world.
Notably, reports on the prevalence of nut allergies among adults are rare and most studies have been conducted in children. More studies taking into account the molecular IgE sensitization profiles and symptoms verified by highly indicative case history and/or provocation testing in children and adults are needed to obtain a more complete picture of the dominating nut allergies in different parts of the world.
Peanut allergy is a good example for the importance of molecular diagnosis for identifying the culprit sensitizing allergen source. Patients may be allergic to peanut due to primary sensitization to birch pollen and cross-reactivity of PR-10 allergen (i.e., cross-reactivity between Bet v 1 and Ara h 8), some are sensitized to lipid transfer proteins from fruits and eventually certain pollen (e.g., cross-reactivity between Pru p 3 and Ara h 9), others may be genuinely sensitized to peanut and the corresponding peanut-specific marker allergens (Ara h 1, 2, 3 and 6) and there can be mixed sensitizations (94–96). The deconvolution of the molecular IgE sensitization profiles is therefore of high importance for identifying the genuinely sensitizing allergen source, predicting clinical manifestations (mild or severe forms of allergy), prevention and treatment based on avoidance/diet and AIT (13). New approaches for the diagnosis and therapy of nut allergies will be increasingly based on individual nut allergen molecules. The clinical relevance of different allergens significantly varies by region and age. In the overview of nut allergen molecules in Table 2 (94, 97–161) a clear distinction has been made between cross-reactive class I food allergens, such as lipid transfer proteins, and confirmed and putative class II food allergens. Key references are given for each of the allergen molecules and reference is made to the WHO/IUIS allergen nomenclature data base (94, 97–161).
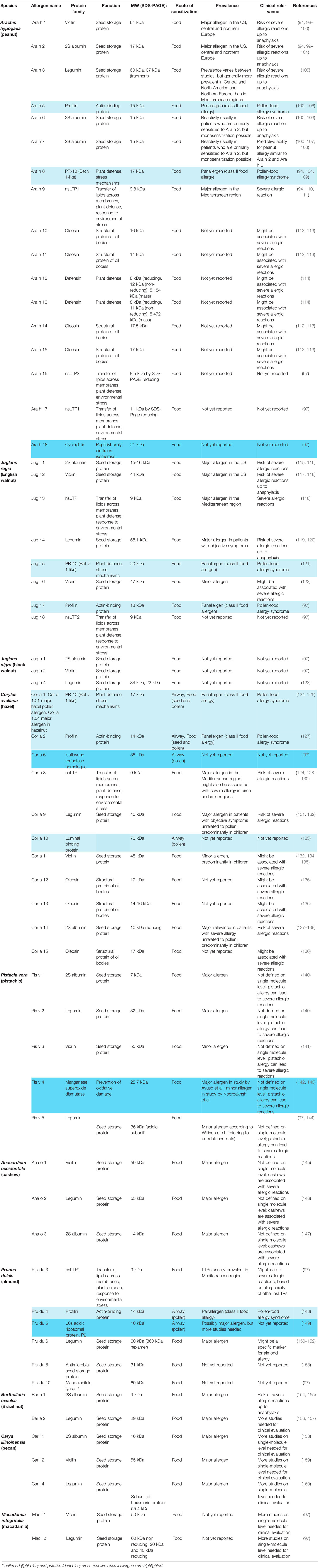
Table 2 Nut allergen molecules according to the WHO/IUIS allergen nomenclature (97) including information regarding biochemical, immunological and clinical features with key references.
At present, 17 peanut (Arachis hypogaea) allergens – Ara h 1 to Ara h 18 – have been identified, with exception of Ara h 4 which was identified as isoform of Ara h 3 (97) (Table 2). Peanut allergens belong either to the prolamin superfamily (Ara h 2, Ara h 6, Ara h 7, Ara h 9, Ara h 16, Ara h 17), the cupin superfamily (Ara h 1, Ara h 3) or different other proteins such as profilin (Ara h 5), Bet v 1-like (Ara h 8), oleosins (Ara h 10, Ara h 11, Ara h 14, Ara h 15) or defensins (Ara h 12, Ara h 13) (97). Recently, the cyclophilin-peptidyl-prolyl cis-trans isomerase Ara h 18 was officially recognized as peanut allergen by the WHO/IUIS Allergen Nomenclature Sub-committee (97).
In America, Central and Northern Europe, Ara h 1 and Ara h 2 are major peanut allergens (94, 99). Valcour et al. showed that in the US, patients with reported peanut allergy most frequently recognized Ara h 2 but IgE reactivity to Ara h 1 and Ara h 3 was also highly prevalent in the tested patients (104). Kleber-Janke et al. reported IgE reactivity to Ara h 1 in 65% and to Ara h 2 in 85% of sera from patients (n = 40) with reported peanut allergy (100). Koppelman et al. compared the IgE reactivity of 32 peanut-allergic patients to Ara h 1, Ara h 2 and Ara h 3 and showed that of these three allergens, Ara h 2 was most frequently recognized (26/32) (102). Importantly, sensitization to Ara h 2 is associated with severe allergic reactions (103). Ara h 2 further has the potential to cross-react with other 2S albumins such as Ara h 6 and Ara h 7, with Ara h 2 possibly representing the primary sensitizing agent (108, 162). However, in rare cases, monosensitization to Ara h 6 and Ara h 7 might be observed and thus must be considered for accurate diagnosis (108, 163). It has been shown that detection of IgE reactivity to peanut extract together with reactivity to rAra h 2 and rAra h 6 allows reliable peanut allergy diagnosis and Ara h 2 could significantly increase diagnostic specificity (164). In comparison to Ara h 1 and Ara h 2, sensitization to Ara h 3 is less frequently observed (94, 102, 105).
In the Mediterranean region, sensitization to the nsLTP Ara h 9 is common and has high cross-reactive potential with homologous allergens of the Rosaceae family, in particular the peach nsLTP Pru p 3 (94, 110, 111, 165).
Schwager et al. reported sensitization to peanut oleosins in patients with a history of severe allergic reactions (113). According to the authors, roasting of peanuts seemed to increase the IgE-binding capacity of oleosins. Previously, several studies have reported that roasting might enhance the allergenic activity of peanut allergens (166–169).
So far, little is known regarding the clinical relevance of peanut defensins and the nsLTPs Ara h 16 and Ara h 17 as well as the currently approved cyclophilin-peptidyl-prolyl cis-trans isomerase Ara h 18 which may be cross-reactive with corresponding pollen and respiratory allergens.
For the English walnut (Juglans regia), which belongs to the Juglandaceae family, 8 allergens have been officially approved by the allergen nomenclature (Jug r 1 to 8), making it the clinically most relevant walnut species (97, 116) (Table 2). For the black walnut (Juglans nigra) 3 allergens have been identified (Jug n 1, 2, 4) (97). However, their clinical relevance is not yet well described in the literature.
Teuber et al. reported that 12 out of 16 walnut-allergic patients showed IgE reactivity to a 2S albumin from English walnut, designated Jug r 1, thus identifying it as a major walnut allergen (115).
IgE reactivity to another major walnut allergen, the vicilin Jug r 2, was detected in 9 out of 15 patients from the US (117). In a study by Pastorello et al., IgE reactivity to vicilin-like protein precursors and vicilin precursors of 9 kD was observed in 10 out of 46 sera from Italian patients, suggesting a minor role of vicilins in allergic patients in the Mediterranean region (118).
Pastorello et al. further reported that 37 out of 46 sera showed IgE binding to the walnut nsLTP Jug r 3, leading to the conclusion that in southern Europe, Jug r 3 represents a major allergen of walnut (118). Notably, peach LTP (Pru p 3) completely inhibited IgE binding to Jug r 3, indicating strong cross-reactivity between walnut and peach.
In 2003, Teuber et al. observed IgE sensitization of patients who experienced life-threatening systemic reactions after walnut consumption to a walnut protein of the legumin group, designated Jug r 4 (119). IgE binding to a recombinant Jug r 4 fusion protein was observed in 15 out of 23 tested sera, suggesting major importance of Jug r 4 in patients with confirmed symptoms. Another study showed IgE reactivity to recombinant Jug r 4 in 21 out of 37 sera from walnut-allergic patients (120).
Jug r 6, like Jug r 2 and Jug r 4, is a member of the cupin superfamily. Although Jug r 2 and Jug r 6 belong to the same protein family, they share only 44% identity (122). In comparison to Jug r 2, which was identified as a major walnut allergen by Teuber et al., Jug r 6 showed IgE reactivity in 20 of 77 walnut-allergic patients, indicating it is of minor clinical relevance (117, 122). Interestingly, cross-reactivity has been shown between Jug r 6 and homologues from pistachio, sesame and hazelnut, which, however, did not apply for Jug r 2 (122).
So far, 11 allergens from common hazel (Corylus avellana) are registered in the WHO/IUIS database (97) (Table 2).
Sensitization to the nsLTP, Cor a 8 predominantly occurs in patients from the Mediterranean region and has been associated with severe allergic reactions (128, 130). However, also in birch-endemic regions, sensitization to Cor a 8 was found in children who had objective reactions during DBPCFC (129). Pastorello et al. reported IgE reactivity to Cor a 8 in patients with a history of anaphylactic reactions to hazelnuts and demonstrated inhibition of IgE binding to Cor a 8 by the purified Pru p 3 (124).
Severe allergic reactions unrelated to pollen allergy have also been reported from patients with sensitization to the 11S globulin Cor a 9 and the 7S globulin Cor a 11 (132). IgE reactivity to Cor a 9 was detected in 12 of 14 patients with a history of systemic reactions to hazelnuts (131). In hazelnut-allergic patients from birch-endemic regions, age-related differences regarding the sensitization to Cor a 9 were observed (126). In total, 65% of pre-school children and 50% of schoolchildren, but only 17% of adults with systemic reactions were sensitized to Cor a 9. In a study by Lauer et al., IgE sensitization to Cor a 11 was observed in less than 50% of 65 hazelnut-allergic patients and the allergen demonstrated significantly lower biological activity in comparison to Cor a 1, suggesting that Cor a 11 is a less relevant hazelnut allergen (134). Similar to Cor a 9, in birch-endemic regions, sensitization to Cor a 11 is age-dependent and is recognized predominantly by children with objective symptoms (135).
The 2S albumin Cor a 14 was first identified in 2010 (137). In a study by Faber et al., IgE reactivity of hazelnut-allergic patients to Cor a 14 was analyzed in different age groups, revealing that Cor a 14 was predominantly recognized in pre-school (18/20) and school-aged children (8/10) (139). In Dutch patients with hazelnut allergy, sensitization to Cor a 14 and Cor a 9 was shown to be highly specific for predicting more severe hazelnut allergy (138). Similar results were obtained in another study that examined the role of component resolved diagnostics for the prediction of clinical allergy in hazelnut-allergic children (170). Specific IgE to Cor a 14 was found to be reliable for the discrimination between patients with clinical reactivity and those that were nonreactive.
The hazelnut oleosins Cor a 12, Cor a 13 and Cor a 15 might be associated with severe allergic reactions (136, 171). However, more studies are needed to establish their clinical relevance. In Europe, sensitization to Cor a 12 in patients with reported reactions to hazelnuts ranged from 10 to 25% and appeared to be more frequent in children than adults (172). The clinical relevance of Cor a 6, a isoflavone reductase-related protein, and Cor a 10 a luminal binding protein with possible pollen cross-reactivity remains to be determined.
Five allergens from Pistacia vera (Pis v 1, Pis v 2, Pis v 3, Pis v 4 and Pis v 5) have been officially approved (97) (Table 2). The sensitization profile of patients with pistachio allergy varies significantly across Europe, indicating age-related, demographic and ethnic differences among the population (56, 60, 63). The clinical relevance of individual pistachio allergens has not been investigated in detail, but it has been shown that pistachio allergy can lead to severe allergic reactions (173).
Ahn et al. reported IgE reactivity in the serum of 19 out of 28 pistachio-allergic patients to a 7 kDa 2S albumin, which was designated Pis v 1. Moreover, 14 out of 28 patients showed IgE binding to the legumin-like protein Pis v 2 (140). These allergens were further identified as homologous of the cashew allergens Ana o 3 and Ana o 2, respectively. The cashew tree belongs just like pistachio to the Anacardiaceae family, which explains the high structural similarity of the proteins and indicates cross-reactivity.
IgE sensitization to the 7S globulin Pis v 3 was shown in 7 of 19 patients who had a history of allergic reactions to pistachio and/or cashew (141). The patients with IgE reactivity to rPis v 3 also reacted to rAna o 1 from cashew nut.
In 16 out of 27 sera from pistachio-allergic patients, IgE reactivity to a manganese superoxide dismutase (MnSOD)-like protein, designated Pis v 4, from pistachio was detected (142). MnSOD-like proteins are known as cross-reactive respiratory allergens (174) and hence Pis v 4 may be considered as a class II food allergen. In 2010, Noorbakhsh et al. reported the expression and purification of recombinant Pis v 4, which exhibited IgE reactivity in 10 of 25 patients (143). Moreover, cross-reactivity with other MnSODs was suggested by the authors.
Pis v 5 is another legumin of pistachio nut, but little is known about the clinical relevance of this protein (97). However, it was described as minor pistachio allergen by Willison et al., referring to unpublished data that reported IgE reactivity in 10 out of 28 patients (144).
Currently, three cashew (Anacardium occidentale) allergens are registered in the database of the WHO/IUIS (97) (Table 2). The vicilin Ana o 1, the legumin Ana o 2 and the 2S albumin Ana o 3 are listed as the major allergens of cashew nut.
Wang et al. reported IgE reactivity to rAna o 1 in 10 out of 20 patients with a history of severe reactions to cashew (145). IgE reactivity to rAna o 2 was shown in 13 out of 21 cashew-allergic patients (146). Robotham et al. detected IgE reactivity to rAna o 3 in 21 of 26 patients with cashew nut allergy (147). Cross-reactivity between the botanically related cashew and pistachio nuts, both members of the Anacardiaceae family, has been observed in several studies (64, 141, 175).
So far 6 allergens from Prunus dulcis (Pru du 3, Pru du 4, Pru du 5, Pru du 6, Pru du 8 and Pru du 10) have been officially recognized by the WHO/IUIS (97) (Table 2).
Pru du 3 belongs to the nsLTP family, which is usually associated with high allergenic activity and cross-reactivity between members of the Rosaceae family, mainly in the Mediterranean region (176, 177). However, large clinical studies evaluating the prevalence of IgE sensitization to Pru du 3 in almond-allergic patients from different regions are needed.
The 60s acidic ribosomal protein P2 has been identified as Pru du 5, and IgE reactivity to a recombinant variant of the protein was shown in 4 of 8 almond-sensitized patients (149). Acid ribosomal proteins have been identified in molds as allergens and it may therefore be considered that this allergen may represent a class II food allergen (178).
Reactivity to recombinant variants of the amandin Pru du 6, Pru du 6.01 and Pru du 6.02, was seen in 9 of 18 and 5 of 18 almond-allergic patients, respectively, while only 4 of the tested patients showed IgE reactivity to both isoforms (151). Kabasser et al. suggested that Pru du 6 might be a specific marker for almond allergy since 16 of 18 almond-allergic patients showed IgE reactivity to the allergen (152). Moreover, positive sIgE to Pru du 6 provided a specificity of 78% and a sensitivity of 83% for almond allergy, while at the same threshold level, the detection of sIgE to almond extract significantly lacked specificity (33%). In comparison, Pru du 8 and Pru du 10 had specificities of 100% and 61% but were less sensitive (41% and 67%) (152). The antigenicity of almond amandin does not seem to be influenced by roasting, blanching or autoclaving, indicating high protein stability (179, 180).
In 2019, Che et al. reported that Pru du 8 might be a member of a novel food allergen family with antimicrobial properties and demonstrated IgE reactivity against rPru du 8 in 6 of 18 patients (153).
To this date, the 2S albumin Ber e 1 and the 11S globulin Ber e 2 from Brazil nut (Bertholletia excelsa) have been registered in the allergen data base (97) (Table 2).
Pastorello et al. reported that each of 11 patients with a history of anaphylaxis after the consumption of Brazil nut, showed IgE reactivity to a 2S albumin, implying that it represents a major allergen from Brazil nut (154). Rayes et al. suggested improvement of allergy diagnosis by measurement of IgE to recombinant Ber e 1, which provides higher sensitivity without loss of specificity compared to whole nut extract (181). Beyer et al. reported the identification of a 11S globulin, designated Ber e 2, as another major allergen from Brazil nut, showing IgE reactivity to the native protein in 56% and the recombinant variant in 44% of sera from Brazil nut-sensitized patients (n = 27) (157).
Three proteins from Carya illinoinensis, the 2S albumin Car i 1, the vicilin Car i 2 and the legumin Car i 4 have been officially approved as allergens (97) (Table 2).
In 2011, the 2S albumin Car i 1 was characterized and IgE binding to recombinant Car i 1 was detected in 22 of 28 patients with pecan allergy (158). The same study showed that pecan and walnut extracts inhibited IgE binding to recombinant Car i 1, indicating strong cross-reactivity with homologous proteins from these nuts. In 2016, Zhang et al. reported that 6 out of 25 patients with DBPCFC-confirmed pecan allergy, showed IgE reactivity to pecan vicilin Car i 2 (159). In a study by Sharma et al., an 11S globulin from pecan, designated Cari i 4, was recognized by IgE from 16 out of 28 patients with pecan allergy (160). Furthermore, extracts from pecan as well as walnut inhibited IgE binding to rCar i 4, suggesting cross-reactivity with legumins from other tree nuts.
To date, 2 allergens from macadamia nut (Macadamia integrifolia), the vicilin Mac i 1 and the legumin Mac i 2, are included in the allergen list of the WHO/IUIS Allergen Nomenclature Sub-committee (97) (Table 2).
In a study by Sutherland et al., IgE reactivity to a 17.4 kDa protein from macadamia was shown in the serum of a patient that had experienced anaphylaxis after consumption of a cake made with macadamia meal (182). Herbst et al. reported IgE reactivity to a macadamia protein of 45 kDa and, under non-reducing conditions, to another protein of 12 kDa (183). Recently, Ehlers et al. reported IgE recognition of vicilin-like antimicrobial peptides in 24 of 82 nut-allergic patients, including 3 patients with a history of systemic reactions to macadamia nut (184). According to available data, measurement of specific IgE to macadamia nut does not always predict clinical allergy and might lead to false-negative results (185, 186). However, single allergen molecules of macadamia nut for component resolved diagnosis are lacking and it must be considered that macadamia extracts might not contain all relevant allergens and thus provide low diagnostic sensitivity (186). Therefore, the identification and characterization of macadamia proteins with established allergenic potential is urgently needed. Possible cross-reactivity between macadamia and hazelnut has been suggested (182, 183).
In peanuts, one of the most relevant panallergens is the Bet v 1-like homologue Ara h 8, which is of major importance in patients from birch-endemic regions where allergic reactions to peanuts can be strongly associated with sensitization to birch pollen (94, 104, 109). Similarly, IgE reactivity to the profilin Ara h 5 is associated with previous sensitization to pollen (106). In walnut, the pathogenesis-related protein (PR-10) Jug r 5 is associated with IgE cross-reactivity between homologous allergens from different plant sources and of minor relevance for patients with primary walnut allergy (121). The Bet v 1-like Cor a 1 and the profilin Cor a 2 are cross-reactive allergens of hazelnut and sensitization to these allergens is typically seen in birch-endemic regions (50, 125, 127). Both allergens are expressed in hazelnut as well as in hazel pollen. The profilin Pru du 4 is a minor allergen of almond and cross-reactivity with profilins from grass pollen was reported (148). It is quite likely that additional “food allergens” (Table 2, light blue) will be identified for which sensitization occurs by respiratory allergen sources and symptoms of food allergy will be low because the allergens are not heat stable and/or become easily digested and then lose their allergenic activity. Ara h 18, Cor a 6, Pis v 4 and Pru du 5 are possible candidates and there may be more discovered in the future (Table 2, dark blue). IgE reactivity to the class II nut allergens is not due to genuine nut sensitization and symptoms caused by these allergens may be treated by AIT directed to the originally sensitizing respiratory allergens.
Diagnosis of nut allergies usually starts with the evaluation of the medical history of the patient. While in the past, diagnosis was mainly achieved by allergen extract-based tests (SPT, OFC), these are increasingly being replaced by modern molecular techniques using specific allergen molecules (Figure 3) (187). Figure 3 compares traditional allergen extract-based diagnosis for nut allergy with modern molecular allergy diagnosis. Traditional extract-based diagnosis uses allergen extracts prepared from the allergen sources for serology and provocation testing in conjunction with the clinical history to determine food which can elicit allergic reactions. Molecular allergy diagnosis is based on IgE serology to a broad panel of defined allergen molecules in combination with the clinical history. In this pathway provocation testing is reduced and usually only performed if necessary to confirm clinically relevant allergy if this cannot be determined by molecular testing and medical history. Molecular testing offers high precision regarding the identification of the culprit allergen molecules is fast and helps to reduce provocation testing which can give rise to severe reactions (187).
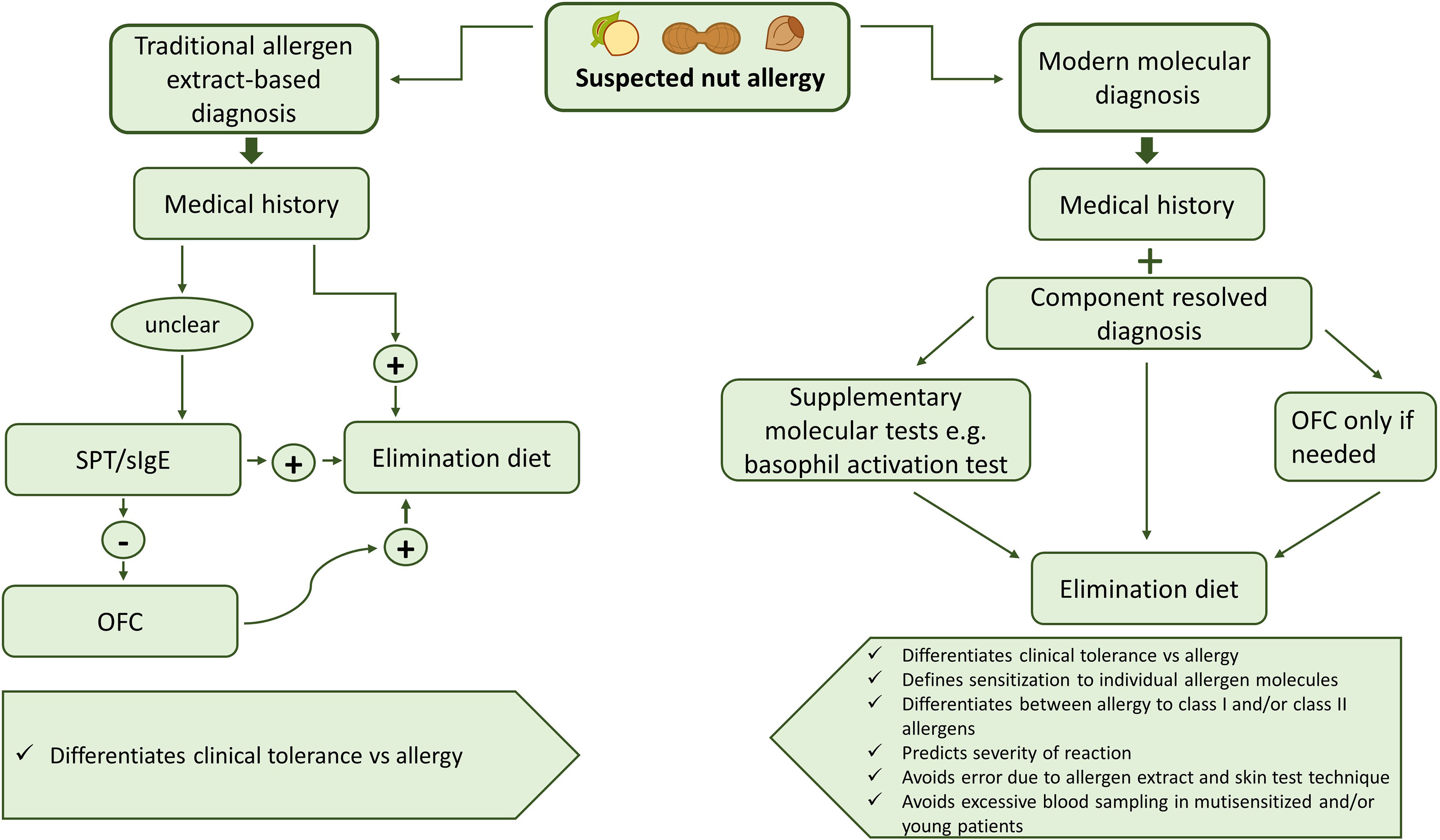
Figure 3 Overview of traditional allergen extract-based nut allergy diagnosis in comparison with modern molecular diagnosis.
Double-blind, placebo-controlled oral food challenge is still a common procedure for food allergy diagnosis, although in the case of strong clinical suspicion, this is usually avoided. Generally, it is recommended that DBPCFC is performed in a standardized procedure under consideration of several patient-related and procedure-related parameters (188, 189). Nevertheless, it must be taken into account that oral food challenges (OFC) bear the risk of potentially fatal anaphylaxis during the procedure (43). This applies particularly to nuts, which are among the most common foods causing anaphylaxis (5). In recent studies, lip dose challenges (LDC), using fresh nuts or nut paste, were suggested as a supplement for oral challenges for nut allergy diagnosis (44, 190). LDC might be performed as a preliminary test to an OFC but currently cannot replace the latter. However, LDC, in combination with modern molecular diagnostic, might reduce the need for OFC in the future.
In principle, two types of skin tests can be performed for diagnostics purposes. Skin prick testing measures the induction of mast cell degranulation caused by cross-linking of IgE bound to the high affinity IgE receptor (FcϵRI) (191) whereas atopy patch testing (APT) detects allergen-specific T cell activation even in the absence of IgE-mediated effects (191, 192). Accordingly, SPT may be considered as surrogate test for IgE-mediated immediate allergic inflammation and APT as surrogate test for chronic, T cell-mediated allergic inflammation. SPT and the detection of food-specific serum IgE with allergen extracts have been traditionally used for allergy diagnosis but have major weaknesses. First of all, these tests are performed with poorly defined allergen extracts and hence do not identify the sensitizing allergen molecules (193). Second, both methods cannot be used to predict clinical sensitivity with certainty because the extent to which digestion affects allergenic activity cannot be measured with these methods. Several authors suggested that the use of fresh food might increase test sensitivity (194, 195). Therefore, food challenge tests are still recommended despite the associated risk factors.
Molecular allergy diagnosis is based on the use of purified allergen molecules, mainly recombinant allergens, to determine the IgE sensitization profile of allergic patients (45). There are also attempts to improve the diagnosis of nut allergy by combining different forms of allergen extracts-based diagnosis. For example, it has been shown that prediction of clinical reactivity to pistachio and cashew was improved by SPT in combination with measurement of sIgE (196). However, nowadays native purified or recombinant single allergen molecules are increasingly replacing conventional extracts in in vitro diagnostics. Molecular tests that allow the detection of specific IgE antibodies to individual allergen molecules are also known under the term component-resolved diagnostics (CRD) (197). For peanut allergy, it was demonstrated that by measuring Ara h 2-specific IgE, the diagnostic accuracy could be considerably improved (198–201). When measured together, sIgE reactivity to Ara h 6 and Ara h 2 was shown to be predictive for severe peanut allergy (103). For the prediction of positive outcomes of food challenges in children, it was demonstrated that Ara h 2-specific IgE levels of 14.4 kUA/L and Cor a 14-specific IgE levels of 47.8 kUA/L had an estimated probability of 90% for predicting a positive peanut or hazelnut challenge (202). In another study, Cor a 14-specific IgE levels of 0.5 and 1.0 kUA/L had a probability of 50% and 95% to predict clinical reactivity to hazelnut in sensitized patients, respectively (170). Moreover, it was shown that measurement of sIgE levels for Cor a 9 in hazelnut-sensitized patients might improve the diagnostic accuracy for the prediction of hazelnut allergy in Japanese children (203). For cashew it was found that sIgE to individual allergen molecules from cashew nut had a predictive value for the diagnosis of clinical allergy (204–206). Measurement of Jug r 1-specific IgE was suggested for the prediction of walnut allergy in children due to improved clinical specificity in comparison with IgE to walnut extracts (207).
Several assays have been developed for the detection of serum IgE to either a single allergen analyte (singleplex assay) or various allergens at a time (multiplex assay) (187, 208, 209). The availabilities of single allergens and advanced microarray technology have made it possible to obtain a quick insight into the sensitization profile of a patient (210). In order to enable quantitative conversion between different multiplex IgE test-platforms for nut allergens, statistical models have been established recently (211). For the European MeDALL research project, an allergen chip with 170 allergen molecules, including natural purified and recombinant allergens from almond, cashew, pistachio and peanut, was developed which could be used even for dried blood samples (212). Recently, a study showed moderate agreement of microarray-based analysis in comparison with clinical diagnosis but high sensitivity of the microarray was seen for tree nuts (213). Moreover, the microarray results for tree nuts correlated with SPT results, promising a superior role of component resolved diagnostic for nut allergies in the future.
Another interesting approach for in vitro allergy diagnosis of nut allergy is the basophil activation test (BAT). Since the early description of allergen-induced histamine release from basophils (214) and the demonstration of the applicability of basophil activation testing for recombinant allergens (215), basophil activation testing has continuously developed (216). Importantly, basophil activation can discriminate between IgE-reactive antigens with no or poor ability to induce IgE-mediated receptor aggregation from potent allergens which induce basophil activation already at low doses (32, 217). Thus basophil activation testing is useful to address a major problem of in vitro allergy diagnostics, i.e., the possibility of false-positive results due to the presence of cross-reactive carbohydrate determinants (218). In plants, these IgE-binding carbohydrate structures are usually N-glycans with a core α-1,3-linked fucose residue. It is well established that CCDs are responsible for IgE cross-reactivity between a wide range of plant allergens and other unrelated allergen sources (219). Furthermore, the presence of N-glycans in cellulose-based ImmunoCap assays could lead to false-positive results in patients with high levels of CCD-reactive IgE antibodies (220). Possibilities to overcome IgE reactivity to CCDs are the production of non-glycosylated recombinant allergen molecules or the use of specific CCD inhibitors (221). CCD-directed IgE antibodies seem to have poor biological activity and are not associated with clinical symptoms (222–224). In basophil activation tests, flow cytometry can be used to analyze basophil activation, which, for example can be defined by the upregulation of the lineage-specific basophil marker CD203c together with the degranulation marker CD63 (225) as has been shown for hazelnut allergy (226). Alternatively, rat basophil cell lines transfected with human FcϵRI can be loaded with serum IgE and then stimulated with allergens (227). Basophil activation was found useful for predicting clinical reactions in peanut allergic patients. Glaumann et al. reported that negative basophil allergen threshold sensitivity correlated with negative DBPCFC in children with peanut allergy (228). Moreover, 92% with positive DBPCFC had positive threshold sensitivity results and increased levels of IgE antibodies to the major peanut allergens Ara h 1, Ara h 2 and Ara h 3. More recently, basophil activation testing was reported to have high accuracy for the diagnosis of peanut and tree nut allergy but it has not been studied if it can be used to differentiate between sensitization to class I and class II food allergens, causing mild and severe systemic anaphylactic reactions, respectively (229).
Basophil activation testing is also a useful tool to investigate the efficacy of AIT for nut allergy by demonstrating the ability of allergen-specific immunoglobulin G (IgG) antibodies to block IgE-mediated immediate allergic reactions (230, 231).
Most of the strategies for treatment and prevention of food allergy and in particular of nut allergy (e.g., allergen avoidance, diet, use of hypoallergenic food products, AIT) are tightly connected with the accurate identification of the culprit allergens. However, some measures like the management of severe acute and chronic inflammation may be achieved by drugs such as epinephrine injection for treatment of acute anaphylactic reactions, immunosuppressive drugs and anti-IgE treatment (232). Besides diet, AIT is the most important form of allergen-specific treatment. The immunological mechanisms underlying AIT include a modified allergen-specific antibody, cellular and cytokine response (233). Besides complex alterations of the cellular and cytokine responses it has become clear that the induction of allergen-specific IgG and perhaps of allergen-specific IgA antibodies which block IgE binding to the allergen and accordingly the IgE antibody-mediate pathology is a key mechanism of AIT (234–236). This has been evidenced in clinical studies using molecular approaches for AIT (237, 238) and by the demonstration that passive immunization with allergen-specific blocking IgG antibodies is clinically effective (239–241).
Regarding the treatment of respiratory allergy by AIT subcutaneous injection immunotherapy remains to be the most frequently used and effective form of AIT as documented by a large number of clinical studies although a huge effort has been done to promote sublingual immunotherapy (SLIT) in multiple studies (235, 242). However, SCIT is more effective than SLIT and patients adherence to SCIT is much better than to SLIT (235, 243). Regarding AIT of food allergy it is of note, that there are only few early studies regarding SCIT (244, 245) and it seems that due to unfavorable side effect profiles SCIT has not been further pursued for food allergy. Instead, oral immunotherapy (OIT) has been developed for class I food allergens which are resistant to digestion whereas OIT studies for respiratory allergens and class II food allergens which are sensitive to digestion have not been successful (246–248). Another important aspect is that only few attempts were made to introduce molecular forms of AIT for food allergy whereas different forms of molecular AIT have been evaluated for respiratory allergy (235). One possible reason for this could be that many more patients suffer from respiratory allergy than from food allergy and usually new forms of treatment are mainly evaluated for frequently occurring forms of allergy because the costs for the preclinical and clinical development of novel vaccines are high. Accordingly, the majority of AIT trials for food allergy have been performed with allergen extracts and by using the OIT approach.
OIT is based on the controlled ingestion of the allergen-causing food, intending to achieve sustained desensitization in the patients. It has been shown that similar as for SCIT, the success of treatment is associated with the development of allergen-specific IgG blocking antibodies which have actually been measured in many of the OIT studies. Table 3 provides and overview of OIT studies (249–279) informing about the number of participants, the study design, clinical and immunological outcomes, side effects and references and/or trial registration numbers which allow to track the studies in the Clinical Trials data base (https://clinicaltrials.gov/). Most of the studies were conducted for peanut allergy whereas OIT studies for tree nut allergies are scarce (Table 3). A study by Andorf et al. (280) is one of the few studies providing evidence for effects of OIT to several different nuts when OIT was combined with anti-IgE treatment.

Table 3 Overview of clinical studies performed for peanut and tree nut allergy grouped according to the route of administration (OIT, SLIT, EPIT, rectal application).
There are methods available for determining major peanut allergens in natural allergen extracts (281) but the precise concentrations of the individual peanut allergens in the natural extracts is not known. Currently, there is no standardized procedure for OIT neither regarding the study design nor are there defined vaccines with known composition. Usually, OIT starts with a dose-escalation day, followed by a buildup phase during which increasing amounts of the allergen are ingested until the maintenance dose is reached. DBPCFC might be performed after a defined food avoidance period to confirm sustained desensitization in the treated subjects. Already in 2009, Jones et al. reported a clinical trial of peanut OIT (249). Since then, the efficacy and safety of peanut OIT have been extensively studied. OIT studies demonstrated successful desensitization and the production of protective IgG4 antibodies but reports of adverse reactions raised safety concerns (267, 269). Adverse reactions affecting the gastrointestinal and respiratory tract during peanut OIT are common (282). To reduce the risk of side effects and to accelerate the desensitization process, the supplementation of OIT with omalizumab, an anti-IgE monoclonal antibody, has been suggested (283–285). The optimal time point to start OIT, treatment duration and length of the maintenance phase are still a matter of debate. With exception of few studies (261, 265, 267–270), most studies involved less than 100 patients and the achieved clinical benefits were relatively modest when put into context with side effects. Accordingly there are different opinions about OIT. One metanalysis (286) concluded: “In patients with peanut allergy, high-certainty evidence shows that available peanut oral immunotherapy regimens considerably increase allergic and anaphylactic reactions over avoidance or placebo, despite effectively inducing desensitization. Safer peanut allergy treatment approaches and rigorous randomized controlled trials that evaluate patient-important outcomes are needed.” whereas another opinion was more optimistic (287). Nevertheless, Aimmune’s peanut OIT has been approved by FDA in the USA and is now marketed as “Palforzia” (https://www.fda.gov/vaccines-blood-biologics/allergenics/palforzia).
Another possible form of immunotherapy for nut allergy is sublingual immunotherapy, which is given in the form of allergen-containing tablets or drops that must be kept under the tongue. One intention for the development of SLIT was the reduction of side effects and its simplified application for self-administration by the patients. However, clinical effects of SLIT are less pronounced than for SCIT for respiratory allergens (235) and there are only few studies, most of them performed in few patients for nut allergy (Table 3) (255, 271–275). Although few studies showed desensitization in some of the participants by the end of the therapy, the results regarding sustained unresponsiveness and long-term compliance are not encouraging (255, 274).
Epicutaneous immunotherapy (EPIT) is a more recent approach which has been developed originally for AIT of respiratory allergy (288) but has now been evaluated also for AIT of peanut allergy (235, 289). EPIT is based on the direct application of an allergen-containing patch on the patient´s skin, similar as it is performed in APT. In theory, EPIT promises a reduced risk of systemic reactions and an uncomplicated application, also for children, due to its non-invasive nature. Table 3 provides an overview of current EPIT studies for nut allergy (276–278), which, however, is currently limited exclusively to peanut. Moderate success for the treatment of peanut allergy was reported, with one study showing some efficacy in children between 6 and 11 years (277). A review of available data states that “EPIT might induce desensitization in peanut allergy and an increased risk of local adverse events (AEs). These findings should be interpreted with caution owing to the limited study and heterogeneity. More data in the older (children ≥ 12 years and adults) and other allergic diseases are needed” (289). The analysis of systemic peanut allergen-specific IgG responses has shown that epicutaneous allergen administration induces only a very modest production of allergen-specific IgG and mainly specific T cell activation (16).
As already mentioned above, SCIT has not been developed for AIT of allergy to class I food allergens, most likely because of the risk of inducing anaphylactic side effects when natural allergen extracts are used (244, 245).
Regarding molecular AIT we found only one published study in which peanut allergic subjects had been treated by a molecular form of AIT using recombinant modified Ara h 1, 2 and 3 encapsulated in inactivated Escherichia coli (279) (Table 3) but half of the subjects (5/10) in this trial experienced adverse reactions, and two of them had anaphylactic reactions.
For AIT of respiratory allergy, several molecular AIT approaches have been evaluated already in clinical trials (Figure 4), yielding encouraging results in terms of inducing protective IgG responses, alterations of cellular immune responses and evidence for clinical efficacy (235). These approaches include SCIT with recombinant or purified major allergen molecules (290), SCIT based on recombinant hypoallergenic allergen derivatives with (291) and without allergen-specific T cell epitopes (237, 292). For the latter approaches the induction of allergen-specific blocking IgG antibodies has been demonstrated and evidence for clinical efficacy has been obtained. SCIT with allergen-derived T cell epitope-containing peptides has not been successful and an induction of allergen-specific IgG has only been demonstrated when relatively long peptides had been used [reviewed in (235)].
Regarding the development of molecular AIT approaches for treatment of allergy to class I food allergens, important and promising results have been collected for the major fish allergen parvalbumin which such as the major nut allergens represents a digestion-resistant and highly allergenic molecule (293). Within the European Union-funded research program FAST, a hypoallergenic recombinant mutant protein of the major carp allergen Cyp c 1 (294) has been produced, characterized and shown to be hypoallergenic in vivo (295–297). Furthermore, safety and ability to induce protective specific IgG responses has been demonstrated in first clinical trials for this molecular vaccine (https://clinicaltrials.gov/: NCT02017626; NCT02382718). Thus it has been proven that it is possible to develop recombinant hypoallergens for SCIT of class I allergens. First recombinant hypoallergenic derivatives of peanut allergens have been characterized in preclinical studies. In fact, several studies reported the production of modified allergen variants of the peanut allergens Ara h 1, Ara h 2 and Ara h 3 and demonstrated reduced IgE reactivity by immunoblotting using patient’s sera (298–300). More recently, the generation of hypoallergenic variants of Ara h 2 and Ara h 6 with decreased allergenic activity but preserved T-cell proliferation capacity has been described (301). Similarly, Tscheppe et al. reported the production of a novel Ara h 2 hypoallergen lacking linear and conformational IgE epitopes (47). IgE reactivity to the unfolded mutant was tested using sera from Ara h 2-sensitized patients and showed reduced IgE-binding capacity compared to natural Ara h 2. The Ara h 2 mutant exhibited low basophil activation ability but still induced T-cell proliferation.
It is known that for allergy to class II food allergens beneficial effects can be obtained by SCIT with the genuinely sensitizing cross-reactive respiratory allergens (40, 302) but the effects on food allergy seem to be lower due to limited cross-reactivity of the induced IgG antibodies (303).
Likewise, molecular AIT with recombinant hypoallergenic birch pollen allergen derivatives was found to induce also cross-protective IgG antibodies to cross-reactive food allergens (41, 291) but similar as for natural allergen extracts, there seems to be limited cross-reactivity of therapy-induced IgG with the cross-reactive food allergens. This has been observed in the clinical trials but also in preclinical studies investigating the cross-protective potential of antibodies induced with molecular vaccines made for the treatment of respiratory allergy (304, 305). Accordingly, it has been suggested to develop recombinant hypoallergens which incorporate also epitopes of the cross-reactive food allergen molecules (306).
Originally, recombinant hypoallergenic allergen derivatives have been made to incorporate allergen-specific T cell epitopes but it has been realized that also non-IgE reactive T cell epitopes can cause side effects by activating allergen-specific T cells leading to late phase side effects (192, 307, 308). The more recently developed technology of replacing allergen-specific T cell epitopes by unrelated carrier proteins (309) seems to reduce T cell-mediated side effects and has been shown to yield promising clinical data with approximately 25% improvement of symptoms over placebo when tested for SCIT of grass pollen allergy (237). One may therefore consider the development of carrier-based B cell epitope-containing vaccines by combining peptides derived from the IgE binding sites of the respiratory allergens and the corresponding cross-reactive class II food allergens to obtain combination vaccines for treatment of pollen allergy and the associated oral allergy syndrome (Figure 4, lower, right).
The technology of producing fusion proteins consisting of hypoallergenic peptides derived from IgE binding sites of allergens and allergen-unrelated carrier proteins may be applicable also for class I food allergens. However, it needs to be born in mind that it may be more difficult to identify hypoallergenic peptides in class I food allergens because they may harbor not only conformational IgE epitopes which can be easily disrupted but also sequential IgE epitopes of which some may be cryptic (i.e., hidden in the intact allergen structure and exposed only after digestion). It may therefore be difficult to identify non-allergenic peptides derived from the IgE binding sites of class I food allergens which are needed for the construction of the carrier-bound B cell epitope-containing vaccines. SCIT with recombinant purified class I food allergens is in principle possible but vaccines based on purified wild-type allergens may cause severe side effects. SCIT with recombinant T cell epitope-containing hypoallergens derived from class I food allergens seems possible and effective if the vaccines induce allergen-specific protective IgG antibodies but late phase, T cell-mediated side effects may occur. Treatment with T cell epitope-containing peptides from class I food allergens will likely not be successful because short peptides fail to induce protective IgG antibodies but tolerogenic peptides may be considered for preventive approaches (Figure 4).
If one performs an analysis of strengths and weaknesses of current allergen extract-based AIT approaches for nut allergy and future molecular AIT vaccines several aspects need to be considered (Figure 5). Without doubt, advances have been made regarding the development of allergen extract-based AIT for nut allergy and experience has been collected in several clinical trials (Figure 5 and Table 3). However, the major limitation for allergen extract-based forms of treatment resides in the fact that allergen extracts represent natural products which have major limitations regarding quality, allergen composition, purity and allergenic activity which only can be overcome by introducing molecular approaches for treatment (Figure 5) (193). It seems to be due to side effects that SCIT approaches with natural allergen extracts for treating allergy to class I food allergens were not pursued. Instead, mainly OIT approaches have been investigated in larger trials whereas SLIT and EPIT are still in an experimental stage. Side effects are still a concern in OIT with allergen extracts and may be overcome with molecular AIT technologies using hypoallergenic allergen derivatives (Figure 5).
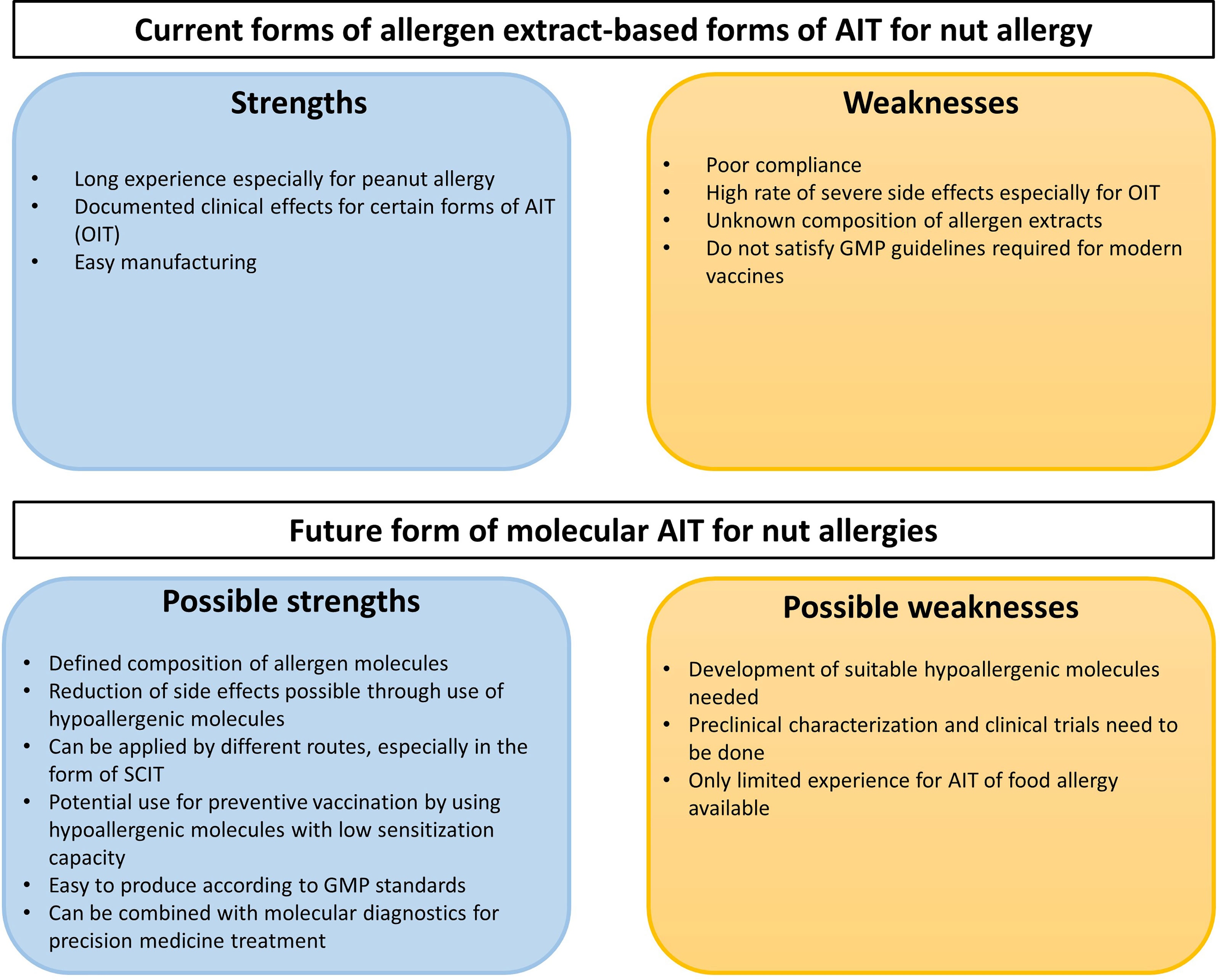
Figure 5 SWOT analysis of existing allergen extract-based forms of AIT for nut allergy and future molecular AIT approaches.
Studies performed with molecular AIT approaches indicate high potential but more efforts are needed to advance this treatment into clinical trials and into clinical use. Accordingly, hypoallergenic derivatives need to be developed for the most important allergens, and thus a thorough preclinical and clinical characterization needs to be performed which will require large efforts and investment into the development (Figure 5). Most of the experiences have been collected for AIT of respiratory allergy but experience from preclinical and clinical trials in food allergy suggest a common mode of action indicating that SCIT with recombinant nut hypoallergens should be safe, induce protective IgG responses and exhibit clinical efficacy but clinical studies are lacking. Clear advantages of molecular AIT forms are the defined mode of production which satisfies Good Manufacturing Practice requirements needed for clinical studies. A major possible advantage is that molecular design will allow to develop safe and effective forms of AIT for allergy to class I food allergens. Furthermore, molecular AIT can be ideally combined with the already established forms of molecular diagnosis allowing the adequate selection of patients for treatment and also the monitoring of the treatment using molecular biomarkers (209, 236, 310).
Figure 5 provides a summary of the SWOT analysis of existing allergen extract-based forms of AIT for nut allergy and future forms of molecular AIT but much more needs to be done regarding the preclinical and clinical development of molecular AIT forms for food allergy.
Nut allergies might lead to severe allergic reactions or even death, and yet the only current treatment option is avoidance of the allergen source. For AIT as well as in nut allergy diagnosis, extract-based methods are still used. Molecular diagnosis is an alternative to traditional allergen-extract based diagnosis and molecular AIT is a promising future perspective. Molecular AIT approaches require knowledge of molecular sensitization profiles in the population intended to treat. It is evident that currently available studies regarding prevalence of sensitization and allergy to nuts are highly heterogeneous regarding design and only few contain information about molecular sensitization profiles. Therefore, there is a need for molecular studies to obtain comparable data regarding the prevalence of allergy to certain nuts. Molecular IgE-based diagnosis for nut allergy diagnosis may reduce the risk of side effects by reducing the need for provocation tests and promises more comprehensive results. At the moment mainly oral forms of allergen-specific immunotherapy are studied which suffer from poor patients compliance and severe side effects. Molecular AIT is not yet well investigated for treatment of nut allergy although it promises a reduction of side effects through the use of recombinant hypoallergens.
RV, VF, and BES wrote the manuscript. RV, VF, and BES designed the figures and tables. VF, MvH, BL, MF-T, IS, OE, AA and MK contributed materials. VF, RV, BES, H-JH, MH, BL, MF-T, IS, OE, MK, MF-T, and AA critically read and revised the manuscript. All authors contributed to the article and approved the submitted version.
Supported by the Danube Allergy Research Cluster funded by the Country of Lower Austria, by the MCCA PhD program of the Austrian Science Fund (FWF), by the Russian Academic Excellence Project 5-100, by a Megagrant of the Government of the Russian Federation, grant No 14.W03.31.0024, by a research grant from Worg Pharmaceuticals, Hangzhou, China and by grant from HVD Life Science, Vienna, Austria.
RV has received research grants from HVD Life Science, Vienna Austria, Viravaxx, Vienna, Austria and Worg Pharmaceuticals, Hangzhou, China and serves as a consultant for Viravaxx and Worg. MvH has received personal fees from Thermo Fisher Scientific, Sweden, and Hycor Biomedical LLC, CA, US., outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge the support of the Medical University of Vienna, Austria for the research infrastructure.
Ig, Immunoglobulin; AIT, Allergen-specific immunotherapy; SCIT, Subcutaneous immunotherapy; PFAS, Pollen-food allergy syndrome; SPT, Skin prick test; DBPCFC, Double-blind placebo-controlled food challenges; LDC, Lip dose challenge; BAT, Basophil activation test; CCD, Cross-reactive carbohydrate determinant; PR-10, Pathogenesis-related class 10; nsLTP, Non-specific lipid transfer protein; OFC, Oral food challenge; APT, Atopy patch test; CRD, Component resolved diagnostics; SLIT, Sublingual immunotherapy; OIT, Oral immunotherapy; EPIT, Epicutaneous immunotherapy.
1. Blanco Mejia S, Kendall CW, Viguiliouk E, Augustin LS, Ha V, Cozma AI, et al. Effect of Tree Nuts on Metabolic Syndrome Criteria: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMJ Open (2014) 4:e004660. doi: 10.1136/bmjopen-2013-004660
2. Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D. Effects of Tree Nuts on Blood Lipids, Apolipoproteins, and Blood Pressure: Systematic Review, Meta-Analysis, and Dose-Response of 61 Controlled Intervention Trials. Am J Clin Nutr (2015) 102:1347–56. doi: 10.3945/ajcn.115.110965
3. Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities Due to Anaphylactic Reactions to Foods. J Allergy Clin Immunol (2001) 107:191–3. doi: 10.1067/mai.2001.112031
4. Bock SA, Muñoz-Furlong A, Sampson HA. Further Fatalities Caused by Anaphylactic Reactions to Food, 2001-2006. J Allergy Clin Immunol (2007) 119:1016–8. doi: 10.1016/j.jaci.2006.12.622
5. Gonzalez-Estrada A, Silvers SK, Klein A, Zell K, Wang XF, Lang DM. Epidemiology of Anaphylaxis at a Tertiary Care Center: A Report of 730 Cases. Ann Allergy Asthma Immunol (2017) 118:80–5. doi: 10.1016/j.anai.2016.10.025
6. Kahveci M, Akarsu A, Koken G, Sahiner UM, Soyer O, Sekerel BE. Food-Induced Anaphylaxis in Infants, as Compared to Toddlers and Preschool Children in Turkey. Pediatr Allergy Immunol (2020) 31:954–61. doi: 10.1111/pai.13320
7. Sicherer SH, Burks AW, Sampson HA. Clinical Features of Acute Allergic Reactions to Peanut and Tree Nuts in Children. Pediatrics (1998) 102:e6. doi: 10.1542/peds.102.1.e6
8. Sicherer SH, Furlong TJ, DeSimone J, Sampson HA. The US Peanut and Tree Nut Allergy Registry: Characteristics of Reactions in Schools and Day Care. J Pediatr (2001) 138:560–5. doi: 10.1067/mpd.2001.111821
9. Yu JW, Kagan R, Verreault N, Nicolas N, Joseph L, St Pierre Y, et al. Accidental Ingestions in Children With Peanut Allergy. J Allergy Clin Immunol (2006) 118:466–72. doi: 10.1016/j.jaci.2006.04.024
10. King RM, Knibb RC, Hourihane JO. Impact of Peanut Allergy on Quality of Life, Stress and Anxiety in the Family. Allergy (2009) 64:461–8. doi: 10.1111/j.1398-9995.2008.01843.x
11. McWilliam V, Koplin J, Lodge C, Tang M, Dharmage S, Allen K. The Prevalence of Tree Nut Allergy: A Systematic Review. Curr Allergy Asthma Rep (2015) 15:54. doi: 10.1007/s11882-015-0555-8
12. Han Y, Kim J, Ahn K. Food Allergy. Korean J Pediatr (2012) 55:153–8. doi: 10.3345/kjp.2012.55.5.153
13. Valenta R, Hochwallner H, Linhart B, Pahr S. Food Allergies: The Basics. Gastroenterology (2015) 148:1120–31.e4. doi: 10.1053/j.gastro.2015.02.006
14. Astwood JD, Leach JN, Fuchs RL. Stability of Food Allergens to Digestion In Vitro. Nat Biotechnol (1996) 14:1269–73. doi: 10.1038/nbt1096-1269
15. Brough HA, Nadeau KC, Sindher SB, Alkotob SS, Chan S, Bahnson HT, et al. Epicutaneous Sensitization in the Development of Food Allergy: What is the Evidence and How can This be Prevented? Allergy (2020) 75:2185–205. doi: 10.1111/all.14304
16. Campana R, Moritz K, Neubauer A, Huber H, Henning R, Brodie TM, et al. Epicutaneous Allergen Application Preferentially Boosts Specific T Cell Responses in Sensitized Patients. Sci Rep (2017) 7:11657. doi: 10.1038/s41598-017-10278-1
17. Lin J, Sampson HA. The Role of Immunoglobulin E-Binding Epitopes in the Characterization of Food Allergy. Curr Opin Allergy Clin Immunol (2009) 9:357–63. doi: 10.1097/ACI.0b013e32832d05ba
18. Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA. Mapping and Mutational Analysis of the IgE-Binding Epitopes on Ara H 1, a Legume Vicilin Protein and a Major Allergen in Peanut Hypersensitivity. Eur J Biochem (1997) 245:334–9. doi: 10.1111/j.1432-1033.1997.t01-1-00334.x
19. Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, et al. Identification and Mutational Analysis of the Immunodominant IgE Binding Epitopes of the Major Peanut Allergen Ara H 2. Arch Biochem Biophys (1997) 342:244–53. doi: 10.1006/abbi.1997.9998
20. Stiefel G, Anagnostou K, Boyle RJ, Brathwaite N, Ewan P, Fox AT, et al. BSACI Guideline for the Diagnosis and Management of Peanut and Tree Nut Allergy. Clin Exp Allergy (2017) 47:719–39. doi: 10.1111/cea.12957
21. Cetinkaya PG, Buyuktiryaki B, Soyer O, Sahiner UM, Sekerel BE. Factors Predicting Anaphylaxis in Children With Tree Nut Allergies. Allergy Asthma Proc (2019) 40:180–6. doi: 10.2500/aap.2019.40.4211
22. Breiteneder H, Pettenburger K, Bito A, Valenta R, Kraft D, Rumpold H, et al. The Gene Coding for the Major Birch Pollen Allergen Betv1, is Highly Homologous to a Pea Disease Resistance Response Gene. EMBO J (1989) 8:1935–8. doi: 10.1002/j.1460-2075.1989.tb03597.x
23. Valenta R, Duchêne M, Pettenburger K, Sillaber C, Valent P, Bettelheim P, et al. Identification of Profilin as a Novel Pollen Allergen; IgE Autoreactivity in Sensitized Individuals. Science (1991) 253:557–60. doi: 10.1126/science.1857985
24. Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, et al. Profilins Constitute a Novel Family of Functional Plant Pan-Allergens. J Exp Med (1992) 175:377–85. doi: 10.1084/jem.175.2.377
25. Valenta R, Ferreira F, Grote M, Swoboda I, Vrtala S, Duchêne M, et al. Identification of Profilin as an Actin-Binding Protein in Higher Plants. J Biol Chem (1993) 268:22777–81. doi: 10.1016/S0021-9258(18)41594-3
26. Valenta R, Kraft D. Type I Allergic Reactions to Plant-Derived Food: A Consequence of Primary Sensitization to Pollen Allergens. J Allergy Clin Immunol (1996) 97:893–5. doi: 10.1016/s0091-6749(96)80062-5
27. Kim M, Ahn Y, Yoo Y, Kim DK, Yang HJ, Park HS, et al. Clinical Manifestations and Risk Factors of Anaphylaxis in Pollen-Food Allergy Syndrome. Yonsei Med J (2019) 60:960–8. doi: 10.3349/ymj.2019.60.10.960
28. Reekers R, Busche M, Wittmann M, Kapp A, Werfel T. Birch Pollen–Related Foods Trigger Atopic Dermatitis in Patients With Specific Cutaneous T-Cell Responses to Birch Pollen Antigens. J Allergy Clin Immunol (1999) 104:466–72. doi: 10.1016/S0091-6749(99)70395-7
29. Wassmann-Otto A, Heratizadeh A, Wichmann K, Werfel T. Birch Pollen-Related Foods can Cause Late Eczematous Reactions in Patients With Atopic Dermatitis. Allergy (2018) 73:2046–54. doi: 10.1111/all.13454
30. Letner D, Farris A, Khalili H, Garber J. Pollen-Food Allergy Syndrome is a Common Allergic Comorbidity in Adults With Eosinophilic Esophagitis. Dis Esophagus (2018) 31. doi: 10.1093/dote/dox122
31. Spergel J, Aceves SS. Allergic Components of Eosinophilic Esophagitis. J Allergy Clin Immunol (2018) 142:1–8. doi: 10.1016/j.jaci.2018.05.001
32. Valenta R, Karaulov A, Niederberger V, Gattinger P, van Hage M, Flicker S, et al. Molecular Aspects of Allergens and Allergy. Adv Immunol (2018) 138:195–256. doi: 10.1016/bs.ai.2018.03.002
33. Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, et al. Conversion of the Major Birch Pollen Allergen, Bet V 1, Into Two Nonanaphylactic T Cell Epitope-Containing Fragments: Candidates for a Novel Form of Specific Immunotherapy. J Clin Invest (1997) 99:1673–81. doi: 10.1172/JCI119330
34. Bohle B, Zwölfer B, Heratizadeh A, Jahn-Schmid B, Antonia YD, Alter M, et al. Cooking Birch Pollen-Related Food: Divergent Consequences for IgE- and T Cell-Mediated Reactivity In Vitro and In Vivo. J Allergy Clin Immunol (2006) 118:242–9. doi: 10.1016/j.jaci.2006.03.011
35. Kazemi-Shirazi L, Pauli G, Purohit A, Spitzauer S, Fröschlc R, Hoffmann-Sommergruber K, et al. Quantitative IgE Inhibition Experiments With Purified Recombinant Allergens Indicate Pollen-Derived Allergens as the Sensitizing Agents Responsible for Many Forms of Plant Food Allergy. J Allergy Clin Immunol (2000) 105:116–25. doi: 10.1016/S0091-6749(00)90186-6
36. Fritsch R, Bohle B, Vollmann U, Wiedermann U, Jahn-Schmid B, Krebitz M, et al. Bet V 1, the Major Birch Pollen Allergen, and Mal D 1, the Major Apple Allergen, Cross-React at the Level of Allergen-Specific T Helper Cells. J Allergy Clin Immunol (1998) 102:679–86. doi: 10.1016/s0091-6749(98)70287-8
37. Westman M, Lupinek C, Bousquet J, Andersson N, Pahr S, Baar A, et al. Early Childhood IgE Reactivity to Pathogenesis-Related Class 10 Proteins Predicts Allergic Rhinitis in Adolescence. J Allergy Clin Immunol (2015) 135:1199–206.e1-11. doi: 10.1016/j.jaci.2014.10.042
38. Elisyutina O, Lupinek C, Fedenko E, Litovkina A, Smolnikov E, Ilina N, et al. IgE-Reactivity Profiles to Allergen Molecules in Russian Children With and Without Symptoms of Allergy Revealed by Micro-Array Analysis. Pediatr Allergy Immunol (2021) 32:251–63. doi: 10.1111/pai.13354
39. Elisyutina O, Fedenko E, Campana R, Litovkina A, Ilina N, Kudlay D, et al. Bet V 1-Specific IgE Levels and PR-10 Reactivity Discriminate Silent Sensitization From Phenotypes of Birch Allergy. Allergy (2019) 74:2525–8. doi: 10.1111/all.13931
40. Bucher X, Pichler WJ, Dahinden CA, Helbling A. Effect of Tree Pollen Specific, Subcutaneous Immunotherapy on the Oral Allergy Syndrome to Apple and Hazelnut. Allergy (2004) 59:1272–6. doi: 10.1111/j.1398-9995.2004.00626.x
41. Niederberger V, Reisinger J, Valent P, Krauth MT, Pauli G, van Hage M, et al. Vaccination With Genetically Modified Birch Pollen Allergens: Immune and Clinical Effects on Oral Allergy Syndrome. J Allergy Clin Immunol (2007) 119:1013–6. doi: 10.1016/j.jaci.2006.12.661
42. Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Report of the NIAID-Sponsored Expert Panel. J Allergy Clin Immunol (2010) 126:S1–58. doi: 10.1016/j.jaci.2010.10.007
43. Upton J, Alvaro M, Nadeau K. A Perspective on the Pediatric Death From Oral Food Challenge Reported From the Allergy Vigilance Network. Allergy (2019) 74:1035–6. doi: 10.1111/all.13791
44. Akarsu A, Soyer O, Sahiner UM, Valenta R, Sekerel BE. Improving the Diagnostic Utility of Lip Dose Challenges to Diagnose Tree Nut Allergy. J Allergy Clin Immunol Pract (2021) 9:534–6.e2. doi: 10.1016/j.jaip.2020.08.061
45. Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI Molecular Allergology User’s Guide. Pediatr Allergy Immunol (2016) 27:1–250. doi: 10.1111/pai.12563
46. Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N Engl J Med (2015) 372:803–13. doi: 10.1056/NEJMoa1414850
47. Tscheppe A, Palmberger D, van Rijt L, Kalic T, Mayr V, Palladino C, et al. Development of a Novel Ara H 2 Hypoallergen With No IgE Binding or Anaphylactogenic Activity. J Allergy Clin Immunol (2020) 145:229–38. doi: 10.1016/j.jaci.2019.08.036
48. Burney P, Summers C, Chinn S, Hooper R, van Ree R, Lidholm J. Prevalence and Distribution of Sensitization to Foods in the European Community Respiratory Health Survey: A EuroPrevall Analysis. Allergy (2010) 65:1182–8. doi: 10.1111/j.1398-9995.2010.02346.x
49. Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of Challenge-Proven IgE-Mediated Food Allergy Using Population-Based Sampling and Predetermined Challenge Criteria in Infants. J Allergy Clin Immunol (2011) 127:668–76. doi: 10.1016/j.jaci.2011.01.039
50. Burney PG, Potts J, Kummeling I, Mills EN, Clausen M, Dubakiene R, et al. The Prevalence and Distribution of Food Sensitization in European Adults. Allergy (2014) 69:365–71. doi: 10.1111/all.12341
51. Nwaru BI, Hickstein L, Panesar S, Roberts G, Muraro A, Sheikh A. Prevalence of Common Food Allergies in Europe: A Systematic Review and Meta-Analysis. Allergy (2014) 69:992–1007. doi: 10.1111/all.12423
52. Wollmann E, Hamsten C, Sibanda E, Ochome M, Focke-Tejkl M, Asarnoj A, et al. Natural Clinical Tolerance to Peanut in African Patients is Caused by Poor Allergenic Activity of Peanut IgE. Allergy (2015) 70:638–52. doi: 10.1111/all.12592
53. Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, et al. Time Trends in the Prevalence of Peanut Allergy: Three Cohorts of Children From the Same Geographical Location in the UK. Allergy (2010) 65:103–8. doi: 10.1111/j.1398-9995.2009.02176.x
54. Hourihane JO, Aiken R, Briggs R, Gudgeon LA, Grimshaw KE, DunnGalvin A, et al. The Impact of Government Advice to Pregnant Mothers Regarding Peanut Avoidance on the Prevalence of Peanut Allergy in United Kingdom Children at School Entry. J Allergy Clin Immunol (2007) 119:1197–202. doi: 10.1016/j.jaci.2006.12.670
55. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or Tolerance in Children Sensitized to Peanut: Prevalence and Differentiation Using Component-Resolved Diagnostics. J Allergy Clin Immunol (2010) 125:191–7.e1-13. doi: 10.1016/j.jaci.2009.10.008
56. Luyt DK, Vaughan D, Oyewole E, Stiefel G. Ethnic Differences in Prevalence of Cashew Nut, Pistachio Nut and Almond Allergy. Pediatr Allergy Immunol (2016) 27:651–4. doi: 10.1111/pai.12582
57. Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early Consumption of Peanuts in Infancy is Associated With a Low Prevalence of Peanut Allergy. J Allergy Clin Immunol (2008) 122:984–91. doi: 10.1016/j.jaci.2008.08.039
58. Osterballe M, Mortz CG, Hansen TK, Andersen KE, Bindslev-Jensen C. The Prevalence of Food Hypersensitivity in Young Adults. Pediatr Allergy Immunol (2009) 20:686–92. doi: 10.1111/j.1399-3038.2008.00842.x
59. Pénard-Morand C, Raherison C, Kopferschmitt C, Caillaud D, Lavaud F, Charpin D, et al. Prevalence of Food Allergy and its Relationship to Asthma and Allergic Rhinitis in Schoolchildren. Allergy (2005) 60:1165–71. doi: 10.1111/j.1398-9995.2005.00860.x
60. Uotila R, Kukkonen AK, Pelkonen AS, Mäkelä MJ. Cross-Sensitization Profiles of Edible Nuts in a Birch-Endemic Area. Allergy (2016) 71:514–21. doi: 10.1111/all.12826
61. Mustafayev R, Civelek E, Orhan F, Yüksel H, Boz AB, Sekerel BE. Similar Prevalence, Different Spectrum: IgE-Mediated Food Allergy Among Turkish Adolescents. Allergol Immunopathol (Madr) (2013) 41:387–96. doi: 10.1016/j.aller.2012.05.005
62. Orhan F, Karakas T, Cakir M, Aksoy A, Baki A, Gedik Y. Prevalence of Immunoglobulin E-Mediated Food Allergy in 6-9-Year-Old Urban Schoolchildren in the Eastern Black Sea Region of Turkey. Clin Exp Allergy (2009) 39:1027–35. doi: 10.1111/j.1365-2222.2009.03263.x
63. Kaya A, Erkoçoğlu M, Civelek E, Çakır B, Kocabaş CN. Prevalence of Confirmed IgE-Mediated Food Allergy Among Adolescents in Turkey. Pediatr Allergy Immunol (2013) 24:456–62. doi: 10.1111/pai.12097
64. Noorbakhsh R, Mortazavi SA, Sankian M, Shahidi F, Tehrani M, Azad FJ, et al. Pistachio Allergy-Prevalence and In Vitro Cross-Reactivity With Other Nuts. Allergol Int (2011) 60:425–32. doi: 10.2332/allergolint.10-OA-0222
65. Khazaei HA, Hashemi SR, Aghamohammadi A, Farhoudi F, Rezaei N. The Study of Type 1 Allergy Prevalence Among People of South-East of Iran by Skin Prick Test Using Common Allergens. Iran J Allergy Asthma Immunol (2003) 2:165–8.
66. Jeong K, Lee SY, Ahn K, Kim J, Lee HR, Suh DI, et al. A Multicenter Study on Anaphylaxis Caused by Peanut, Tree Nuts, and Seeds in Children and Adolescents. Allergy (2017) 72:507–10. doi: 10.1111/all.13096
67. Sun X, Zhao J, Wang Q, Shi G, Yang J, Ming L. Prevalence of Allergen Sensitization Among 15,534 Patients With Suspected Allergic Diseases in Henan Province, China. Asian Pac J Allergy Immunol (2019) 37:57–64. doi: 10.12932/AP-160817-0137
68. Chen J, Hu Y, Allen KJ, Ho MH, Li H. The Prevalence of Food Allergy in Infants in Chongqing, China. Pediatr Allergy Immunol (2011) 22:356–60. doi: 10.1111/j.1399-3038.2011.01139.x
69. Hu Y, Chen J, Li H. Comparison of Food Allergy Prevalence Among Chinese Infants in Chongqing, 2009 Versus 1999. Pediatr Int (2010) 52:820–4. doi: 10.1111/j.1442-200X.2010.03166.x
70. Lee MP, Saffari SE, Loh W, Goh SH, Goh A, Chiang WC, et al. A 5-Year Retrospective Review of Children With Peanut Allergy in the Largest Paediatric Hospital in Singapore. Asia Pac Allergy (2020) 10:e6. doi: 10.5415/apallergy.2020.10.e6
71. Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, et al. A Population-Based Questionnaire Survey on the Prevalence of Peanut, Tree Nut, and Shellfish Allergy in 2 Asian Populations. J Allergy Clin Immunol (2010) 126:324–31. doi: 10.1016/j.jaci.2010.06.003
72. Chiang WC, Pons L, Kidon MI, Liew WK, Goh A, Burks AW. Serological and Clinical Characteristics of Children With Peanut Sensitization in an Asian Community. Pediatr Allergy Immunol (2010) 21:e429–38. doi: 10.1111/j.1399-3038.2009.00930.x
73. Liew WK, Chiang WC, Goh AE, Lim HH, Chay OM, Chang S, et al. Paediatric Anaphylaxis in a Singaporean Children Cohort: Changing Food Allergy Triggers Over Time. Asia Pac Allergy (2013) 3:29–34. doi: 10.5415/apallergy.2013.3.1.29
74. Cheng CW, Lin YC, Nong BR, Liu PY, Huang YF, Lu LY, et al. Nut Sensitization Profile in Southern Taiwan. J Microbiol Immunol Infect (2020) 53:791–6. doi: 10.1016/j.jmii.2018.12.005
75. Imamura T, Kanagawa Y. Ebisawa M. A Survey of Patients With Self-Reported Severe Food Allergies in Japan. Pediatr Allergy Immunol (2008) 19:270–4. doi: 10.1111/j.1399-3038.2007.00621.x
76. Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US Prevalence of Self-Reported Peanut, Tree Nut, and Sesame Allergy: 11-Year Follow-Up. J Allergy Clin Immunol (2010) 125:1322–6. doi: 10.1016/j.jaci.2010.03.029
77. Sicherer SH, Muñoz-Furlong A, Burks AW, Sampson HA. Prevalence of Peanut and Tree Nut Allergy in the US Determined by a Random Digit Dial Telephone Survey. J Allergy Clin Immunol (1999) 103:559–62. doi: 10.1016/s0091-6749(99)70224-1
78. Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of Peanut and Tree Nut Allergy in the United States Determined by Means of a Random Digit Dial Telephone Survey. J Allergy Clin Immunol (2003) 112:1203–7. doi: 10.1016/S0091-6749(03)02026-8
79. Ruiz Segura LT, Figueroa Pérez E, Nowak-Wegrzyn A, Siepmann T, Larenas-Linnemann D. Food Allergen Sensitization Patterns in a Large Allergic Population in Mexico. Allergol Immunopathol (Madr) (2020) 48:553–9. doi: 10.1016/j.aller.2020.02.004
80. Bedolla-Barajas M, Bedolla-Pulido TR, Macriz-Romero N, Morales-Romero J, Robles-Figueroa M. Prevalence of Peanut, Tree Nut, Sesame, and Seafood Allergy in Mexican Adults. Rev Invest Clin (2015) 67:379–86.
81. Ben-Shoshan M, Harrington DW, Soller L, Fragapane J, Joseph L, St Pierre Y, et al. A Population-Based Study on Peanut, Tree Nut, Fish, Shellfish, and Sesame Allergy Prevalence in Canada. J Allergy Clin Immunol (2010) 125:1327–35. doi: 10.1016/j.jaci.2010.03.015
82. McWilliam V, Peters R, Tang ML, Dharmage S, Ponsonby AL, Gurrin L, et al. Patterns of Tree Nut Sensitization and Allergy in the First 6 Years of Life in a Population-Based Cohort. J Allergy Clin Immunol (2019) 143:644–650.e5. doi: 10.1016/j.jaci.2018.07.038
83. Sasaki M, Koplin JJ, Dharmage SC, Field MJ, Sawyer SM, McWilliam V, et al. Prevalence of Clinic-Defined Food Allergy in Early Adolescence: The SchoolNuts Study. J Allergy Clin Immunol (2018) 141:391–398.e4. doi: 10.1016/j.jaci.2017.05.041
84. Mittermann I, Dzoro S, Gattinger P, Botha M, Basera W, Facey-Thomas HE, et al. Molecular IgE Sensitization Profiles of Urban and Rural Children in South Africa. Pediatr Allergy Immunol (2021) 32:234–41. doi: 10.1111/pai.13377
85. Vetander M, Helander D, Flodström C, Ostblom E, Alfvén T, Ly DH, et al. Anaphylaxis and Reactions to Foods in Children–a Population-Based Case Study of Emergency Department Visits. Clin Exp Allergy (2012) 42:568–77. doi: 10.1111/j.1365-2222.2011.03954.x
86. Soto-Quiros M, Gutierrez I, Calvo N, Araya C, Karlberg J, Hanson LA, et al. Allergen Sensitization of Asthmatic and Nonasthmatic Schoolchildren in Costa Rica. Allergy (1998) 53:1141–7. doi: 10.1111/j.1398-9995.1998.tb03833.x
87. Martínez J, Méndez C, Talesnik E, Campos E, Viviani P, Sánchez I. Pruebas Cutáneas De Hipersensibilidad Inmediata En Una Población Pediátrica Seleccionada. Rev Med Chil (2005) 133:195–201. doi: 10.4067/s0034-98872005000200007
88. Rodríguez-Ortiz PG, Muñoz-Mendoza D, Arias-Cruz A, González-Díaz SN, Herrera-Castro D, Vidaurri-Ojeda AC. Características Epidemiológicas De Pacientes Con Alergia a Alimentos Atendidos En El Centro Regional De Alergias E Inmunologia Clínica De Monterrey. Rev Alerg Mex (2009) 56:185–91.
89. Chen J, Liao Y, Zhang H, Zhao H, Chen J, Li H. Prevalence of Food Allergy in Children Under 2 Years of Age in Three Cities in China. Zhonghua Er Ke Za Zhi (2012) 50:5–9.
90. Lee AJ, Thalayasingam M, Lee BW. Food Allergy in Asia: How Does it Compare? Asia Pac Allergy (2013) 3:3–14. doi: 10.5415/apallergy.2013.3.1.3
91. Venter C, Maslin K, Patil V, Kurukulaaratchy R, Grundy J, Glasbey G, et al. The Prevalence, Natural History and Time Trends of Peanut Allergy Over the First 10 Years of Life in Two Cohorts Born in the Same Geographical Location 12 Years Apart. Pediatr Allergy Immunol (2016) 27:804–11. doi: 10.1111/pai.12616
92. Kim M, Lee JY, Jeon HY, Yang HK, Lee KJ, Han Y, et al. Prevalence of Immediate-Type Food Allergy in Korean Schoolchildren in 2015: A Nationwide, Population-Based Study. Allergy Asthma Immunol Res (2017) 9:410–6. doi: 10.4168/aair.2017.9.5.410
93. Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The Prevalence of Food Allergy and Other Allergic Diseases in Early Childhood in a Population-Based Study: HealthNuts Age 4-Year Follow-Up. J Allergy Clin Immunol (2017) 140:145–153.e8. doi: 10.1016/j.jaci.2017.02.019
94. Vereda A, van Hage M, Ahlstedt S, Ibañez MD, Cuesta-Herranz J, van Odijk J, et al. Peanut Allergy: Clinical and Immunologic Differences Among Patients From 3 Different Geographic Regions. J Allergy Clin Immunol (2011) 127:603–7. doi: 10.1016/j.jaci.2010.09.010
95. Asarnoj A, Movérare R, Ostblom E, Poorafshar M, Lilja G, Hedlin G, et al. IgE to Peanut Allergen Components: Relation to Peanut Symptoms and Pollen Sensitization in 8-Year-Olds. Allergy (2010) 65:1189–95. doi: 10.1111/j.1398-9995.2010.02334.x
96. Asarnoj A, Hamsten C, Lupinek C, Melén E, Andersson N, Anto JM, et al. Prediction of Peanut Allergy in Adolescence by Early Childhood Storage Protein-Specific IgE Signatures: The BAMSE Population-Based Birth Cohort. J Allergy Clin Immunol (2017) 140:587–590.e7. doi: 10.1016/j.jaci.2016.12.973
97. Allergen Nomenclature, the Official Site for the Systematic Allergen Nomenclature (2021). Available at: http://www.allergen.org.
98. Burks AW, Cockrell G, Stanley JS, Helm RM, Bannon GA. Recombinant Peanut Allergen Ara H I Expression and IgE Binding in Patients With Peanut Hypersensitivity. J Clin Invest (1995) 96:1715–21. doi: 10.1172/JCI118216
99. Clarke MC, Kilburn SA, Hourihane JO, Dean KR, Warner JO, Dean TP. Serological Characteristics of Peanut Allergy. Clin Exp Allergy (1998) 28:1251–7. doi: 10.1046/j.1365-2222.1998.00386.x
100. Kleber-Janke T, Crameri R, Appenzeller U, Schlaak M, Becker WM. Selective Cloning of Peanut Allergens, Including Profilin and 2S Albumins, by Phage Display Technology. Int Arch Allergy Immunol (1999) 119:265–74. doi: 10.1159/000024203
101. Burks AW, Williams LW, Connaughton C, Cockrell G, O’Brien TJ, Helm RM, et al. Identification and Characterization of a Second Major Peanut Allergen, Ara h II, With Use of the Sera of Patients With Atopic Dermatitis and Positive Peanut Challenge. J Allergy Clin Immunol (1992) 90:962–9. doi: 10.1016/0091-6749(92)90469-i
102. Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara H1, Ara H2 and Ara H3 in Peanut-Allergic Patients, as Determined by Immunoglobulin E Western Blotting, Basophil-Histamine Release and Intracutaneous Testing: Ara H2 is the Most Important Peanut Allergen. Clin Exp Allergy (2004) 34:583–90. doi: 10.1111/j.1365-2222.2004.1923.x
103. Kukkonen AK, Pelkonen AS, Mäkinen-Kiljunen S, Voutilainen H, Mäkelä MJ. Ara H 2 and Ara 6 Are the Best Predictors of Severe Peanut Allergy: A Double-Blind Placebo-Controlled Study. Allergy (2015) 70:1239–45. doi: 10.1111/all.12671
104. Valcour A, Jones JE, Lidholm J, Borres MP, Hamilton RG. Sensitization Profiles to Peanut Allergens Across the United States. Ann Allergy Asthma Immunol (2017) 119:262–266.e1. doi: 10.1016/j.anai.2017.06.021
105. Rabjohn P, Helm EM, Stanley JS, West CM, Sampson HA, Burks AW, et al. Molecular Cloning and Epitope Analysis of the Peanut Allergen Ara H 3. J Clin Invest (1999) 103:535–42. doi: 10.1172/JCI5349
106. Cabanos C, Tandang-Silvas, Odijk V, Brostedt P, Tanaka A, Utsumi S, et al. Expression, Purification, Cross-Reactivity and Homology Modeling of Peanut Profilin. Protein Expr Purif (2010) 73:36–45. doi: 10.1016/j.pep.2010.03.005
107. Schmidt H, Krause S, Gelhaus C, Petersen A, Janssen O, Becker WM. Detection and Structural Characterization of Natural Ara H 7, the Third Peanut Allergen of the 2S Albumin Family. J Proteome Res (2010) 9:3701–9. doi: 10.1021/pr1002406
108. Blankestijn MA, Otten HG, Suer W, Weimann A, Knol EF, Knulst AC. Specific IgE to Peanut 2S Albumin Ara H 7 has a Discriminative Ability Comparable to Ara H 2 and 6. Clin Exp Allergy (2018) 48:60–5. doi: 10.1111/cea.13030
109. Mittag D, Akkerdaas J, Ballmer-Weber BK, Vogel L, Wensing M, WM B, et al. Ara H 8, a Bet V 1-Homologous Allergen From Peanut, Is a Major Allergen in Patients With Combined Birch Pollen and Peanut Allergy. J Allergy Clin Immunol (2004) 114:1410–7. doi: 10.1016/j.jaci.2004.09.014
110. Krause S, Reese G, Randow S, Zennaro D, Quaratino D, Palazzo P, et al. Lipid Transfer Protein (Ara H 9) as a New Peanut Allergen Relevant for a Mediterranean Allergic Population. J Allergy Clin Immunol (2009) 124:771–778.e5. doi: 10.1016/j.jaci.2009.06.008
111. Lauer I, Dueringer N, Pokoj S, Rehm S, Zoccatelli G, Reese G, et al. The non-Specific Lipid Transfer Protein, Ara H 9, is an Important Allergen in Peanut. Clin Exp Allergy (2009) 39:1427–37. doi: 10.1111/j.1365-2222.2009.03312.x
112. Schwager C, Kull S, Krause S, Schocker F, Petersen A, Becker WM, et al. Development of a Novel Strategy to Isolate Lipophilic Allergens (Oleosins) From Peanuts. PloS One (2015) 10:e0123419. doi: 10.1371/journal.pone.0123419
113. Schwager C, Kull S, Behrends J, Röckendorf N, Schocker F, Frey A, et al. Peanut Oleosins Associated With Severe Peanut Allergy-Importance of Lipophilic Allergens for Comprehensive Allergy Diagnostics. J Allergy Clin Immunol (2017) 140:1331–1338.e8. doi: 10.1016/j.jaci.2017.02.020
114. Petersen A, Kull S, Rennert S, Becker WM, Krause S, Ernst M, et al. Peanut Defensins: Novel Allergens Isolated From Lipophilic Peanut Extract. J Allergy Clin Immunol (2015) 136:1295–301. doi: 10.1016/j.jaci.2015.04.010
115. Teuber SS, Dandekar AM, Peterson WR, Sellers CL. Cloning and Sequencing of a Gene Encoding a 2S Albumin Seed Storage Protein Precursor From English Walnut (Juglans Regia), a Major Food Allergen. J Allergy Clin Immunol (1998) 101:807–14. doi: 10.1016/S0091-6749(98)70308-2
116. Costa J, Carrapatoso I, Oliveira MB, Mafra I. Walnut Allergens: Molecular Characterization, Detection and Clinical Relevance. Clin Exp Allergy (2014) 44:319–41. doi: 10.1111/cea.12267
117. Teuber SS, Jarvis KC, Dandekar AM, Peterson WR, Ansari AA. Identification and Cloning of a Complementary DNA Encoding a Vicilin-Like Proprotein, Jug R 2, From English Walnut Kernel (Juglans Regia), a Major Food Allergen. J Allergy Clin Immunol (1999) 104:1311–20. doi: 10.1016/s0091-6749(99)70029-1
118. Pastorello EA, Farioli L, Pravettoni V, Robino AM, Scibilia J, Fortunato D, et al. Lipid Transfer Protein and Vicilin are Important Walnut Allergens in Patients Not Allergic to Pollen. J Allergy Clin Immunol (2004) 114:908–14. doi: 10.1016/j.jaci.2004.06.020
119. Teuber SS, Peterson WR, Uratsu S, Dandekar A, Roux KH, Sathe SK. Identification and Cloning of Jug R 4, a Major Food Allergen From English Walnut Belonging to the Legumin Group. J Allergy Clin Immunol (2003) 111:S248. doi: 10.1016/S0091-6749(03)80879-5
120. Wallowitz M, Peterson WR, Uratsu S, Comstock SS, Dandekar AM, Teuber SS. Jug R 4, a Legumin Group Food Allergen From Walnut (Juglans Regia Cv. Chandler). J Agric Food Chem (2006) 54:8369–75. doi: 10.1021/jf061329s
121. Wangorsch A, Jamin A, Lidholm J, Gräni N, Lang C, Ballmer-Weber B, et al. Identification and Implication of an Allergenic PR-10 Protein From Walnut in Birch Pollen Associated Walnut Allergy. Mol Nutr Food Res (2017) 61. doi: 10.1002/mnfr.201600902
122. Dubiela P, Kabasser S, Smargiasso N, Geiselhart S, Bublin M, Hafner C, et al. Jug R 6 is the Allergenic Vicilin Present in Walnut Responsible for IgE Cross-Reactivities to Other Tree Nuts and Seeds. Sci Rep (2018) 8:11366. doi: 10.1038/s41598-018-29656-4
123. Zhang YZ, Du WX, Fan Y, Yi J, Lyu SC, Nadeau KC, et al. Purification and Characterization of a Black Walnut (Juglans Nigra) Allergen, Jug N 4. J Agric Food Chem (2017) 65:454–62. doi: 10.1021/acs.jafc.6b04387
124. Pastorello EA, Vieths S, Pravettoni V, Farioli L, Trambaioli C, Fortunato D, et al. Identification of Hazelnut Major Allergens in Sensitive Patients With Positive Double-Blind, Placebo-Controlled Food Challenge Results. J Allergy Clin Immunol (2002) 109:563–70. doi: 10.1067/mai.2002.121946
125. Hofmann C, Scheurer S, Rost K, Graulich E, Jamin A, Foetisch K, et al. Cor a 1-Reactive T Cells and IgE are Predominantly Cross-Reactive to Bet V 1 in Patients With Birch Pollen-Associated Food Allergy to Hazelnut. J Allergy Clin Immunol (2013) 131:1384–92.e6. doi: 10.1016/j.jaci.2012.10.037
126. De Knop KJ, Verweij MM, Grimmelikhuijsen M, Philipse E, Hagendorens MM, Bridts CH, et al. Age-Related Sensitization Profiles for Hazelnut (Corylus Avellana) in a Birch-Endemic Region. Pediatr Allergy Immunol (2011) 22:e139–49. doi: 10.1111/j.1399-3038.2011.01112.x
127. Hirschwehr R, Valenta R, Ebner C, Ferreira F, Sperr WR, Valent P, et al. Identification of Common Allergenic Structures in Hazel Pollen and Hazelnuts: A Possible Explanation for Sensitivity to Hazelnuts in Patients Allergic to Tree Pollen. J Allergy Clin Immunol (1992) 90:927–36. doi: 10.1016/0091-6749(92)90465-e
128. Schocker F, Lüttkopf D, Scheurer S, Petersen A, Cisteró-Bahima A, Enrique E, et al. Recombinant Lipid Transfer Protein Cor a 8 From Hazelnut: A New Tool for In Vitro Diagnosis of Potentially Severe Hazelnut Allergy. J Allergy Clin Immunol (2004) 113:141–7. doi: 10.1016/j.jaci.2003.09.013
129. Flinterman AE, Akkerdaas JH, den Hartog Jager CF, Rigby NM, Fernandez-Rivas M, Hoekstra MO, et al. Lipid Transfer Protein-Linked Hazelnut Allergy in Children From a non-Mediterranean Birch-Endemic Area. J Allergy Clin Immunol (2008) 121:423–428.e2. doi: 10.1016/j.jaci.2007.10.009
130. Blanc F, Bernard H, Ah-Leung S, Przybylski-Nicaise L, Skov PS, Purohit A, et al. Further Studies on the Biological Activity of Hazelnut Allergens. Clin Transl Allergy (2015) 5:26. doi: 10.1186/s13601-015-0066-7
131. Beyer K, Grishina G, Bardina L, Grishin A, Sampson HA. Identification of an 11S Globulin as a Major Hazelnut Food Allergen in Hazelnut-Induced Systemic Reactions. J Allergy Clin Immunol (2002) 110:517–23. doi: 10.1067/mai.2002.127434
132. Ebo DG, Verweij MM, Sabato V, Hagendorens MM, Bridts CH, De Clerck LS. Hazelnut Allergy: A Multi-Faced Condition With Demographic and Geographic Characteristics. Acta Clin Belg (2012) 67:317–21. doi: 10.2143/ACB.67.5.2062683
133. Gruehn S, Suphioglu C, O’Hehir RE, Volkmann D. Molecular Cloning and Characterization of Hazel Pollen Protein (70 Kd) as a Luminal Binding Protein (BiP): A Novel Cross-Reactive Plant Allergen. Int Arch Allergy Immunol (2003) 131:91–100. doi: 10.1159/000070924
134. Lauer I, Foetisch K, Kolarich D, Ballmer-Weber BK, Conti A, Altmann F, et al. Hazelnut (Corylus Avellana) Vicilin Cor a 11: Molecular Characterization of a Glycoprotein and its Allergenic Activity. Biochem J (2004) 383:327–34. doi: 10.1042/BJ20041062
135. Verweij MM, Hagendorens MM, Trashin S, Cucu T, De Meulenaer B, Devreese B, et al. Age-Dependent Sensitization to the 7S-Vicilin-Like Protein Cor a 11 From Hazelnut (Corylus Avellana) in a Birch-Endemic Region. J Investig Allergol Clin Immunol (2012) 22:245–51.
136. Zuidmeer-Jongejan L, Fernández-Rivas M, Winter MG, Akkerdaas JH, Summers C, Lebens A, et al. Oil Body-Associated Hazelnut Allergens Including Oleosins are Underrepresented in Diagnostic Extracts But Associated With Severe Symptoms. Clin Transl Allergy (2014) 4:4. doi: 10.1186/2045-7022-4-4
137. Garino C, Zuidmeer L, Marsh J, Lovegrove A, Morati M, Versteeg S, et al. Isolation, Cloning, and Characterization of the 2S Albumin: A New Allergen From Hazelnut. Mol Nutr Food Res (2010) 54:1257–65. doi: 10.1002/mnfr.200900456
138. Masthoff LJ, Mattsson L, Zuidmeer-Jongejan L, Lidholm J, Andersson K, Akkerdaas JH, et al. Sensitization to Cor a 9 and Cor a 14 is Highly Specific for a Hazelnut Allergy With Objective Symptoms in Dutch Children and Adults. J Allergy Clin Immunol (2013) 132:393–9. doi: 10.1016/j.jaci.2013.02.024
139. Faber MA, De Graag M, van der Heijden C, Sabato V, Hagendorens MM, Bridts CH, et al. Cor a 14: Missing Link in the Molecular Diagnosis of Hazelnut Allergy? Int Arch Allergy (2014) 164:200–6. doi: 10.1159/000365050
140. Ahn K, Bardina L, Grishina G, Beyer K, Sampson HA. Identification of Two Pistachio Allergens, Pis V 1 and Pis V 2, Belonging to the 2S Albumin and 11S Globulin Family. Clin Exp Allergy (2009) 39:926–34. doi: 10.1111/j.1365-2222.2009.03259.x
141. Willison LN, Tawde P, Robotham JM, Penney RM, Teuber SS, Sathe SK, et al. Pistachio Vicilin, Pis V 3, is Immunoglobulin E-Reactive and Cross-Reacts With the Homologous Cashew Allergen, Ana O 1. Clin Exp Allergy (2008) 38:1229–38. doi: 10.1111/j.1365-2222.2008.02998.x
142. Ayuso R, Grishina G, Ahn K, Bardina L, Beyer K, Sampson H. Identification of a MnSOD-Like Protein as a New Major Pistachio Allergen. J Allergy Clin Immunol (2007) 119:S115. doi: 10.1016/j.jaci.2006.11.433
143. Noorbakhsh R, Mortazavi SA, Sankian M, Shahidi F, Assarehzadegan MA, Varasteh A. Cloning, Expression, Characterization, and Computational Approach for Cross-Reactivity Prediction of Manganese Superoxide Dismutase Allergen From Pistachio Nut. Allergol Int (2010) 59:295–304. doi: 10.2332/allergolint.10-OA-0174
144. Willison LN, Sathe SK, Roux KH. Production and Analysis of Recombinant Tree Nut Allergens. Methods (2014) 66:34–43. doi: 10.1016/j.ymeth.2013.07.033
145. Wang F, Robotham JM, Teuber SS, Tawde P, Sathe SK, Roux KH. Ana O 1, a Cashew (Anacardium Occidental) Allergen of the Vicilin Seed Storage Protein Family. J Allergy Clin Immunol (2002) 110:160–6. doi: 10.1067/mai.2002.125208
146. Wang F, Robotham JM, Teuber SS, Sathe SK, Roux KH. Ana O 2, a Major Cashew (Anacardium Occidentale L.) Nut Allergen of the Legumin Family. Int Arch Allergy Immunol (2003) 132:27–39. doi: 10.1159/000073262
147. Robotham JM, Wang F, Seamon V, Teuber SS, Sathe SK, Sampson HA, et al. Ana O 3, an Important Cashew Nut (Anacardium Occidentale L.) Allergen of the 2S Albumin Family. J Allergy Clin Immunol (2005) 115:1284–90. doi: 10.1016/j.jaci.2005.02.028
148. Tawde P, Venkatesh YP, Wang F, Teuber SS, Sathe SK, Roux KH. Cloning and Characterization of Profilin (Pru Du 4), a Cross-Reactive Almond (Prunus Dulcis) Allergen. J Allergy Clin Immunol (2006) 118:915–22. doi: 10.1016/j.jaci.2006.05.028
149. Abolhassani M, Roux KH. cDNA Cloning, Expression and Characterization of an Allergenic 60s Ribosomal Protein of Almond (Prunus Dulcis). Iran J Allergy Asthma Immunol (2009) 8:77–84.
150. Sathe SK, Wolf WJ, Roux KH, Teuber SS, Venkatachalam M, Sze-Tao KW. Biochemical Characterization of Amandin, the Major Storage Protein in Almond (Prunus Dulcis L.). J Agric Food Chem (2002) 50:4333–41. doi: 10.1021/jf020007v
151. Willison LN, Tripathi P, Sharma G, Teuber SS, Sathe SK, Roux KH. Cloning, Expression and Patient IgE Reactivity of Recombinant Pru Du 6, an 11S Globulin From Almond. Int Arch Allergy Immunol (2011) 156:267–81. doi: 10.1159/000323887
152. Kabasser S, Hafner C, Chinthrajah S, Sindher SB, Kumar D, Kost LE, et al. Identification of Pru Du 6 as a Potential Marker Allergen for Almond Allergy. Allergy (2020) 76:1463–72. doi: 10.1111/all.14613
153. Che H, Zhang Y, Jiang S, Jin T, Lyu SC, Nadeau KC, et al. Almond (Prunus Dulcis) Allergen Pru Du 8, the First Member of a New Family of Food Allergens. J Agric Food Chem (2019) 67:8626–31. doi: 10.1021/acs.jafc.9b02781
154. Pastorello EA, Farioli L, Pravettoni V, Ispano M, Conti A, Ansaloni R, et al. Sensitization to the Major Allergen of Brazil Nut is Correlated With the Clinical Expression of Allergy. J Allergy Clin Immunol (1998) 102:1021–7. doi: 10.1016/S0091-6749(98)70341-0
155. Alcocer MJ, Murtagh GJ, Bailey K, Dumoulin M, Meseguer AS, Parker MJ, et al. The Disulphide Mapping, Folding and Characterisation of Recombinant Ber E 1, an Allergenic Protein, and SFA8, Two Sulphur-Rich 2S Plant Albumins. J Mol Biol (2002) 324:165–75. doi: 10.1016/S0022-2836(02)01061-6
156. Guo F, Jin T, Howard A, Zhang YZ. Purification, Crystallization and Initial Crystallographic Characterization of Brazil-Nut Allergen Ber E 2. Acta Cryst F (2007) 63:976–9. doi: 10.1107/S1744309107051445
157. Beyer K, Bardina L, Grishina G, Ashraf A, Teuber S, Niggemann B, et al. Identification of a New Brazil Nut Allergen - Ber E 2. J Allergy Clin Immunol (2008) 121:247. doi: 10.1016/j.jaci.2007.12.980
158. Sharma GM, Irsigler A, Dhanarajan P, Ayuso R, Bardina L, Sampson HA, et al. Cloning and Characterization of 2S Albumin, Car I 1, a Major Allergen in Pecan. J Agric Food Chem (2011) 59:4130–9. doi: 10.1021/jf104319d
159. Zhang Y, Lee B, Du WX, Lyu SC, Nadeau KC, Grauke LJ, et al. Identification and Characterization of a New Pecan [Carya Illinoinensis (Wangenh.) K. Koch] Allergen, Car I 2. J. Agric Food Chem (2016) 64:4146–51. doi: 10.1021/acs.jafc.6b00884
160. Sharma GM, Irsigler A, Dhanarajan P, Ayuso R, Bardina L, Sampson HA, et al. Cloning and Characterization of an 11S Legumin, Car I 4, a Major Allergen in Pecan. J Agric Food Chem (2011) 59:9542–52. doi: 10.1021/jf2017447
161. Morales M, López-Matas MÁ, Moya R, Carnés J. Cross-Reactivity Among non-Specific Lipid-Transfer Proteins From Food and Pollen Allergenic Sources. Food Chem (2014) 165:397–402. doi: 10.1016/j.foodchem.2014.05.101
162. Hemmings O, Du Toit G, Radulovic S, Lack G, F. Santos A. Ara H 2 is the Dominant Peanut Allergen Despite Similarities With Ara H 6. J Allergy Clin Immunol (2020) 146:621–630.e5. doi: 10.1016/j.jaci.2020.03.026
163. Asarnoj A, Glaumann S, Elfström L, Lilja G, Lidholm J, Nilsson C, et al. Anaphylaxis to Peanut in a Patient Predominantly Sensitized to Ara H 6. Int Arch Allergy Immunol (2012) 159:209–12. doi: 10.1159/000336027
164. Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain A-C, et al. A Novel Immunoassay Using Recombinant Allergens Simplifies Peanut Allergy Diagnosis. Int Arch Allergy Immunol (2011) 154:216–26. doi: 10.1159/000321108
165. Ballmer-Weber BK, Lidholm J, Fernández-Rivas M, Seneviratne S, Hanschmann KM, Vogel L, et al. IgE Recognition Patterns in Peanut Allergy are Age Dependent: Perspectives of the EuroPrevall Study. Allergy (2015) 70:391–407. doi: 10.1111/all.12574
166. Maleki SJ, Chung SY, Champagne ET, Raufman JP. The Effects of Roasting on the Allergenic Properties of Peanut Proteins. J Allergy Clin Immunol (2000) 106:763–8. doi: 10.1067/mai.2000.109620
167. Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks A, et al. Effects of Cooking Methods on Peanut Allergenicity. J Allergy Clin Immunol (2001) 107:1077–81. doi: 10.1067/mai.2001.115480
168. Mondoulet L, Paty E, Drumare MF, Ah-Leung S, Scheinmann P, Willemot RM, et al. Influence of Thermal Processing on the Allergenicity of Peanut Proteins. J Agric Food Chem (2005) 53:4547–53. doi: 10.1021/jf050091p
169. Vissers YM, Blanc F, Skov PS, Johnson PE, Rigby NM, Przybylski-Nicaise L, et al. Effect of Heating and Glycation on the Allergenicity of 2S Albumins (Ara H 2/6) From Peanut. PloS One (2011) 6:e23998. doi: 10.1371/journal.pone.0023998
170. Buyuktiryaki B, Cavkaytar O, Sahiner UM, Yilmaz EA, Yavuz ST, Soyer O, et al. Cor a 14, Hazelnut-Specific IgE, and SPT as a Reliable Tool in Hazelnut Allergy Diagnosis in Eastern Mediterranean Children. J Allergy Clin Immunol Pract (2016) 4:265–72.e3. doi: 10.1016/j.jaip.2015.12.012
171. Akkerdaas JH, Schocker F, Vieths S, Versteeg S, Zuidmeer L, Hefle SL, et al. Cloning of Oleosin, a Putative New Hazelnut Allergen, Using a Hazelnut cDNA Library. Mol Nutr Food Res (2006) 50:18–23. doi: 10.1002/mnfr.200500147
172. Datema MR, Zuidmeer-Jongejan L, Asero R, Barreales L, Belohlavkova S, de Blay F, et al. Hazelnut Allergy Across Europe Dissected Molecularly: A EuroPrevall Outpatient Clinic Survey. J Allergy Clin Immunol (2015) 136:382–91. doi: 10.1016/j.jaci.2014.12.1949
173. Costa J, Silva I, Vicente AA, Oliveira M, Mafra I. Pistachio Nut Allergy: An Updated Overview. Crit Rev Food Sci Nutr (2019) 59:546–62. doi: 10.1080/10408398.2017.1379947
174. Flückiger S, Scapozza L, Mayer C, Blaser K, Folkers G, Crameri R. Immunological and Structural Analysis of IgE-Mediated Cross-Reactivity Between Manganese Superoxide Dismutases. Int Arch Allergy Immunol (2002) 128:292–303. doi: 10.1159/000063862
175. Hasegawa M, Inomata N, Yamazaki H, Morita A, Kirino M, Ikezawa Z. Clinical Features of Four Cases With Cashew Nut Allergy and Cross-Reactivity Between Cashew Nut and Pistachio. Allergol Int (2009) 58:209–15. doi: 10.2332/allergolint.08-OA-0010
176. Salcedo G, Sánchez-Monge R, Barber D, Díaz-Perales A. Plant non-Specific Lipid Transfer Proteins: An Interface Between Plant Defence and Human Allergy. Biochim Biophys Acta (2007) 1771:781–91. doi: 10.1016/j.bbalip.2007.01.001
177. Zuidmeer L, van Ree R. Lipid Transfer Protein Allergy: Primary Food Allergy or Pollen/Food Syndrome in Some Cases. Curr Opin Allergy Clin Immunol (2007) 7:269–73. doi: 10.1097/ACI.0b013e32814a5401
178. Mayer C, Appenzeller U, Seelbach H, Achatz G, Oberkofler H, Breitenbach M, et al. Humoral and Cell-Mediated Autoimmune Reactions to Human Acidic Ribosomal P2 Protein in Individuals Sensitized to Aspergillus Fumigatus P2 Protein. J Exp Med (1999) 189:1507–12. doi: 10.1084/jem.189.9.1507
179. Roux KH, Teuber SS, Robotham JM, Sathe SK. Detection and Stability of the Major Almond Allergen in Foods. J Agric Food Chem (2001) 49:2131–6. doi: 10.1021/jf001307k
180. Venkatachalam M, Teuber SS, Roux KH, Sathe SK. Effects of Roasting, Blanching, Autoclaving, and Microwave Heating on Antigenicity of Almond (Prunus Dulcis L.) Proteins. J Agric Food Chem (2002) 50:3544–8. doi: 10.1021/jf020012z
181. Rayes H, Raza AA, Williams A, Matthews S, Arshad SH. Specific IgE to Recombinant Protein (Ber E 1) for the Diagnosis of Brazil Nut Allergy. Clin Exp Allergy (2016) 46:654–6. doi: 10.1111/cea.12693
182. Sutherland MF, O’Hehir RE, Czarny D, Suphioglu C. Macadamia Nut Anaphylaxis: Demonstration of Specific IgE Reactivity and Partial Cross-Reactivity With Hazelnut. J Allergy Clin Immunol (1999) 104:889–90. doi: 10.1016/S0091-6749(99)70304-0
183. Herbst RA, Wahl R, Frosch PJ. Specific IgE Reactivity and Identification of Potential Allergens in Macadamia Allergy. J Eur Acad Dermatol Venereol (2010) 24:1361–3. doi: 10.1111/j.1468-3083.2010.03642.x
184. Ehlers AM, Rohwer S, Otten HG, Brix B, Le TM, Suer W, et al. IgE-Binding to Vicilin-Like Antimicrobial Peptides is Associated With Systemic Reactions to Macadamia Nut. Clin Transl Allergy (2020) 10:55. doi: 10.1186/s13601-020-00364-5
185. Knott E, Gürer CK, Ellwanger J, Ring J, Darsow U. Macadamia Nut Allergy. J Eur Acad Dermatol Venereol (2008) 22:1394–5. doi: 10.1111/j.1468-3083.2008.02657.x
186. Ekbote A, Hayman G, Bansal A. Macadamia Nut Allergy: Potentially Misleading Specific IgE Results. Allergy (2010) 65:1345. doi: 10.1111/j.1398-9995.2010.02354.x
187. van Hage M, Hamsten C, Valenta R. ImmunoCAP Assays: Pros and Cons in Allergology. J Allergy Clin Immunol (2017) 140:974–7. doi: 10.1016/j.jaci.2017.05.008
188. Bindslev-Jensen C, Ballmer-Weber BK, Bengtsson U, Blanco C, Ebner C, Hourihane J, et al. Standardization of Food Challenges in Patients With Immediate Reactions to Foods–Position Paper From the European Academy of Allergology and Clinical Immunology. Allergy (2004) 59:690–7. doi: 10.1111/j.1398-9995.2004.00466.x
189. Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, Sicherer S, Teuber SS, Burks AW, et al. Standardizing Double-Blind, Placebo-Controlled Oral Food Challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL Consensus Report. J Allergy Clin Immunol (2012) 130:1260–74. doi: 10.1016/j.jaci.2012.10.017
190. Vazquez-Ortiz M, Ludman S, Aston A, Noimark L, Turner PJ. Lip Dose Challenges in Food Allergy: Current Practice and Diagnostic Utility in the United Kingdom. J Allergy Clin Immunol Pract (2019) 7:2770–2774.e3. doi: 10.1016/j.jaip.2019.04.037
191. Ansotegui IJ, Melioli G, Canonica GW, Caraballo L, Villa E, Ebisawa M, et al. IgE Allergy Diagnostics and Other Relevant Tests in Allergy, a World Allergy Organization Position Paper. World Allergy Organ J (2020) 13:100080. doi: 10.1016/j.waojou.2019.100080
192. Campana R, Moritz K, Marth K, Neubauer A, Huber H, Henning R, et al. Frequent Occurrence of T Cell-Mediated Late Reactions Revealed by Atopy Patch Testing With Hypoallergenic Rbet V 1 Fragments. J Allergy Clin Immunol (2016) 137:601–609.e8. doi: 10.1016/j.jaci.2015.08.042
193. Valenta R, Karaulov A, Niederberger V, Zhernov Y, Elisyutina O, Campana R, et al. Allergen Extracts for In Vivo Diagnosis and Treatment of Allergy: Is There a Future? J Allergy Clin Immunol Pract (2018) 6:1845–1855.e2. doi: 10.1016/j.jaip.2018.08.032
194. Zivanovic M, Atanasković-Marković M, Medjo B, Gavrović-Jankulović M, Smiljanić K, Tmušić V, et al. Evaluation of Food Allergy in Children by Skin Prick Tests With Commercial Extracts and Fresh Foods, Specific IgE and, Open Oral Food Challenge-Our Five Years Experience in Food Allergy Work-Up. Iran J Allergy Asthma Immunol (2017) 16:127–32.
195. Rancé F, Juchet A, Brémont F, Dutau G. Correlations Between Skin Prick Tests Using Commercial Extracts and Fresh Foods, Specific IgE, and Food Challenges. Allergy (1997) 52:1031–5. doi: 10.1111/j.1398-9995.1997.tb02427.x
196. Cetinkaya PG, Karaguzel D, Esenboğa S, Sahiner UM, Soyer O, Buyuktiryaki B, et al. Pistachio and Cashew Nut Allergy in Childhood: Predictive Factors Towards Development of a Decision Tree. Asian Pac J Allergy Immunol (2021) 39:53–61. doi: 10.12932/AP-281018-0429
197. Valenta R, Lidholm J, Niederberger V, Hayek B, Kraft D, Grönlund H. The Recombinant Allergen-Based Concept of Component-Resolved Diagnostics and Immunotherapy (CRD and CRIT). Clin Exp Allergy (1999) 29:896–904. doi: 10.1046/j.1365-2222.1999.00653.x
198. Dang TD, Tang M, Choo S, Licciardi PV, Koplin JJ, Martin PE, et al. Increasing the Accuracy of Peanut Allergy Diagnosis by Using Ara H 2. J Allergy Clin Immunol (2012) 129:1056–63. doi: 10.1016/j.jaci.2012.01.056
199. Klemans RJ, Broekman HC, Knol EF, Bruijnzeel-Koomen CA, Otten HG, Pasmans SG, et al. Ara H 2 is the Best Predictor for Peanut Allergy in Adults. J Allergy Clin Immunol Pract (2013) 1:632–8.e1. doi: 10.1016/j.jaip.2013.07.014
200. Klemans RJ, Otte D, Knol M, Knol EF, Meijer Y, Gmelig-Meyling FH, et al. The Diagnostic Value of Specific IgE to Ara H 2 to Predict Peanut Allergy in Children is Comparable to a Validated and Updated Diagnostic Prediction Model. J Allergy Clin Immunol (2013) 131:157–63. doi: 10.1016/j.jaci.2012.08.010
201. Lieberman JA, Glaumann S, Batelson S, Borres MP, Sampson HA, Nilsson C. The Utility of Peanut Components in the Diagnosis of IgE-Mediated Peanut Allergy Among Distinct Populations. J Allergy Clin Immunol Pract (2013) 1:75–82. doi: 10.1016/j.jaip.2012.11.002
202. Beyer K, Grabenhenrich L, Härtl M, Beder A, Kalb B, Ziegert M, et al. Predictive Values of Component-Specific IgE for the Outcome of Peanut and Hazelnut Food Challenges in Children. Allergy (2015) 70:90–8. doi: 10.1111/all.12530
203. Inoue Y, Sato S, Takahashi K, Yanagida N, Yamamoto H, Shimizu N, et al. Component-Resolved Diagnostics can be Useful for Identifying Hazelnut Allergy in Japanese Children. Allergol Int (2020) 69:239–45. doi: 10.1016/j.alit.2019.10.001
204. Lange L, Lasota L, Finger A, Vlajnic D, Büsing S, Meister J, et al. Ana O 3-Specific IgE is a Good Predictor for Clinically Relevant Cashew Allergy in Children. Allergy (2017) 72:598–603. doi: 10.1111/all.13050
205. van der Valk JP, Gerth van Wijk R, Vergouwe Y, Steyerberg EW, Reitsma M, Wichers HJ, et al. Sige Ana O 1, 2 and 3 Accurately Distinguish Tolerant From Allergic Children Sensitized to Cashew Nuts. Clin Exp Allergy (2017) 47:113–20. doi: 10.1111/cea.12794
206. Sato S, Movérare R, Ohya Y, Ito K, Nagao M, Borres MP, et al. Ana O 3-Specific IgE is a Predictive Marker for Cashew Oral Food Challenge Failure. J Allergy Clin Immunol Pract (2019) 7:2909–2911.e4. doi: 10.1016/j.jaip.2019.04.049
207. Sato S, Yamamoto M, Yanagida N, Ito K, Ohya Y, Imai T, et al. Jug R 1 Sensitization is Important in Walnut-Allergic Children and Youth. J Allergy Clin Immunol Pract (2017) 5:1784–1786.e1. doi: 10.1016/j.jaip.2017.04.025
208. Karsonova A, Riabova K, Villazala-Merino S, Campana R, Niederberger V, Eckl-Dorna J, et al. Highly Sensitive ELISA-Based Assay for Quantification of Allergen-Specific IgE Antibody Levels. Allergy (2020) 75:2668–70. doi: 10.1111/all.14325
209. Huang HJ, Campana R, Akinfenwa O, Curin M, Sarzsinszky E, Karsonova A, et al. Microarray-Based Allergy Diagnosis: Quo Vadis? Front Immunol (2020) 11:594978. doi: 10.3389/fimmu.2020.594978
210. Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, et al. Microarrayed Allergen Molecules: Diagnostic Gatekeepers for Allergy Treatment. FASEB J (2002) 16:414–6. doi: 10.1096/fj.01-0711fje
211. Hoang JA, Celik A, Lupinek C, Valenta R, Duan L, Dai R, et al. Modeling the Conversion Between Specific IgE Test Platforms for Nut Allergens in Children and Adolescents. Allergy (2020) 76:831–41. doi: 10.1111/all.14529
212. Garib V, Rigler E, Gastager F, Campana R, Dorofeeva Y, Gattinger P, et al. Determination of IgE and IgG Reactivity to More Than 170 Allergen Molecules in Paper-Dried Blood Spots. J Allergy Clin Immunol (2019) 143:437–40. doi: 10.1016/j.jaci.2018.08.047
213. Dubiela P, Dölle-Bierke S, Aurich S, Worm M, Hoffmann-Sommergruber K. Component-Resolved Diagnosis in Adult Patients With Food-Dependent Anaphylaxis. World Allergy Organ J (2021) 14:100530. doi: 10.1016/j.waojou.2021.100530
214. Lichtenstein LM, Osler AG. Studies on the Mechanisms of Hypersensitivity Phenomena. IX. Histamine Release From Human Leukocytes by Ragweed Pollen Antigen. J Exp Med (1964) 120:507–30. doi: 10.1084/jem.120.4.507
215. Valenta R, Sperr W, Ferreira F, Valent P, Sillaber C, Tejkl M, et al. Induction of Specific Histamine Release From Basophils With Purified Natural and Recombinant Birch Pollen Allergens. J Allergy Clin Immunol (1993) 91:88–97. doi: 10.1016/0091-6749(93)90300-5
216. Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, et al. The Clinical Utility of Basophil Activation Testing in Diagnosis and Monitoring of Allergic Disease. Allergy (2015) 70:1393–405. doi: 10.1111/all.12698
217. Caraballo L, Valenta R, Acevedo N, Zakzuk J. Are the Terms Major and Minor Allergens Useful for Precision Allergology? Front Immunol (2021) 12:651500. doi: 10.3389/fimmu.2021.651500
218. Mari A, Iacovacci P, Afferni C, Barletta B, Tinghino R, Di Felice G, et al. Specific IgE to Cross-Reactive Carbohydrate Determinants Strongly Affect the In Vitro Diagnosis of Allergic Diseases. J Allergy Clin Immunol (1999) 103:1005–11. doi: 10.1016/S0091-6749(99)70171-5
219. Gattinger P, Mittermann I, Lupinek C, Hofer G, Keller W, Bidovec Stojkovic U, et al. Recombinant Glycoproteins Resembling Carbohydrate-Specific IgE Epitopes From Plants, Venoms and Mites. EBioMedicine (2019) 39:33–43. doi: 10.1016/j.ebiom.2018.12.002
220. Hemmer W, Altmann F, Holzweber F, Gruber C, Wantke F, Wöhrl S. ImmunoCAP Cellulose Displays Cross-Reactive Carbohydrate Determinant (CCD) Epitopes and can Cause False-Positive Test Results in Patients With High Anti-CCD IgE Antibody Levels. J Allergy Clin Immunol (2018) 141:372–381.e3. doi: 10.1016/j.jaci.2017.04.028
221. Altmann F. Coping With Cross-Reactive Carbohydrate Determinants in Allergy Diagnosis. Allergol J Int (2016) 25:98–105. doi: 10.1007/s40629-016-0115-3
222. Cabauatan CR, Lupinek C, Scheiblhofer S, Weiss R, Focke-Tejkl M, Bhalla PL, et al. Allergen Microarray Detects High Prevalence of Asymptomatic IgE Sensitizations to Tropical Pollen-Derived Carbohydrates. J Allergy Clin Immunol (2014) 133:910–4.e5. doi: 10.1016/j.jaci.2013.10.004
223. Mari A. IgE to Cross-Reactive Carbohydrate Determinants: Analysis of the Distribution and Appraisal of the In Vivo and In Vitro Reactivity. Int Arch Allergy Immunol (2002) 129:286–95. doi: 10.1159/000067591
224. van der Veen MJ, van Ree R, Aalberse RC, Akkerdaas J, Koppelman SJ, Jansen HM, et al. Poor Biologic Activity of Cross-Reactive IgE Directed to Carbohydrate Determinants of Glycoproteins. J Allergy Clin Immunol (1997) 100:327–34. doi: 10.1016/S0091-6749(97)70245-8
225. Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, et al. Recombinant Allergens Promote Expression of CD203c on Basophils in Sensitized Individuals. J Allergy Clin Immunol (2002) 110:102–9. doi: 10.1067/mai.2002.125257
226. Lötzsch B, Dölle S, Vieths S, Worm M. Exploratory Analysis of CD63 and CD203c Expression in Basophils From Hazelnut Sensitized and Allergic Individuals. Clin Transl Allergy (2016) 6:45. doi: 10.1186/s13601-016-0134-7
227. Kaul S, Lüttkopf D, Kastner B, Vogel L, Höltz G, Vieths S, et al. Mediator Release Assays Based on Human or Murine Immunoglobulin E in Allergen Standardization. Clin Exp Allergy (2007) 37:141–50. doi: 10.1111/j.1365-2222.2006.02618.x
228. Glaumann S, Nopp A, Johansson SG, Rudengren M, Borres MP, Nilsson C. Basophil Allergen Threshold Sensitivity, CD-Sens, IgE-Sensitization and DBPCFC in Peanut-Sensitized Children. Allergy (2012) 67:242–7. doi: 10.1111/j.1398-9995.2011.02754.x
229. Duan L, Celik A, Hoang JA, Schmidthaler K, So D, Yin X, et al. Basophil Activation Test Shows High Accuracy in the Diagnosis of Peanut and Tree Nut Allergy: The Markers of Nut Allergy Study. Allergy (2021) 76:1800–12. doi: 10.1111/all.14695
230. Patil SU, Steinbrecher J, Calatroni A, Smith N, Ma A, Ruiter B, et al. Early Decrease in Basophil Sensitivity to Ara H 2 Precedes Sustained Unresponsiveness After Peanut Oral Immunotherapy. J Allergy Clin Immunol (2019) 144:1310–1319.e4. doi: 10.1016/j.jaci.2019.07.028
231. Orgel K, Burk C, Smeekens J, Suber J, Hardy L, Guo R, et al. Blocking Antibodies Induced by Peanut Oral and Sublingual Immunotherapy Suppress Basophil Activation and are Associated With Sustained Unresponsiveness. Clin Exp Allergy (2019) 49:461–70. doi: 10.1111/cea.13305
232. de Silva D, Geromi M, Panesar SS, Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. Acute and Long-Term Management of Food Allergy: Systematic Review. Allergy (2014) 69:159–67. doi: 10.1111/all.12314
233. Larché M, Akdis CA, Valenta R. Immunological Mechanisms of Allergen-Specific Immunotherapy. Nat Rev Immunol (2006) 6:761–71. doi: 10.1038/nri1934
234. Shamji MH, Layhadi JA, Sharif H, Penagos M, Durham SR. Immunological Responses and Biomarkers for Allergen-Specific Immunotherapy Against Inhaled Allergens. J Allergy Clin Immunol Pract (2021) 9:1769–78. doi: 10.1016/j.jaip.2021.03.029
235. Dorofeeva Y, Shilovskiy I, Tulaeva I, Focke-Tejkl M, Flicker S, Kudlay D, et al. Past, Present, and Future of Allergen Immunotherapy Vaccines. Allergy (2020) 76:131–49. doi: 10.1111/all.14300
236. Shamji MH, Valenta R, Jardetzky T, Verhasselt V, Durham SR, Würtzen PA, et al. The Role of Allergen-Specific IgE, IgG and IgA in Allergic Disease. Allergy (2021). doi: 10.1111/all.14908
237. Niederberger V, Neubauer A, Gevaert P, Zidarn M, Worm M, Aberer W, et al. Safety and Efficacy of Immunotherapy With the Recombinant B-Cell Epitope-Based Grass Pollen Vaccine BM32. J Allergy Clin Immunol (2018) 142:497–509.e9. doi: 10.1016/j.jaci.2017.09.052
238. Eckl-Dorna J, Weber M, Stanek V, Linhart B, Ristl R, Waltl EE, et al. Two Years of Treatment With the Recombinant Grass Pollen Allergy Vaccine BM32 Induces a Continuously Increasing Allergen-Specific IgG4 Response. EBioMedicine (2019) 50:421–32. doi: 10.1016/j.ebiom.2019.11.006
239. Orengo JM, Radin AR, Kamat V, Badithe A, Ben LH, Bennett BL, et al. Treating Cat Allergy With Monoclonal IgG Antibodies That Bind Allergen and Prevent IgE Engagement. Nat Commun (2018) 9:1421. doi: 10.1038/s41467-018-03636-8
240. Gevaert P, De Craemer J, De Ruyck N, Rottey S, de Hoon J, Hellings PW, et al. Novel Antibody Cocktail Targeting Bet V 1 Rapidly and Sustainably Treats Birch Allergy Symptoms in a Phase 1 Study. J Allergy Clin Immunol (2021) 11:S0091–6749(21)00904–0. doi: 10.1016/j.jaci.2021.05.039
241. Shamji MH, Singh I, Layhadi JA, Ito C, Karamani A, Kouser L, et al. Passive Prophylactic Administration With a Single Dose of Anti-Fel D 1 Monoclonal Antibodies REGN1908-1909 in Cat Allergen-Induced Allergic Rhinitis: A Randomized, Double-Blind, Placebo Controlled Trial. Am J Respir Crit Care Med (2021) 204:23–33. doi: 10.1164/rccm.202011-4107OC
242. Passalacqua G, Bagnasco D, Canonica GW. 30 Years of Sublingual Immunotherapy. Allergy (2020) 75:1107–20. doi: 10.1111/all.14113
243. Kiel MA, Röder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten-van Mölken MP. Real-Life Compliance and Persistence Among Users of Subcutaneous and Sublingual Allergen Immunotherapy. J Allergy Clin Immunol (2013) 132:353–60.e2. doi: 10.1016/j.jaci.2013.03.013
244. Oppenheimer J, Nelson H, Bock S, Christensen F, Leung D. Treatment of Peanut Allergy With Rush Immunotherapy. J Allergy Clin Immunol (1992) 90:256–62. doi: 10.1016/0091-6749(92)90080-l
245. Nelson H, Lahr J, Rule R, Bock A, Leung D. Treatment of Anaphylactic Sensitivity to Peanuts by Immunotherapy With Injections of Aqueous Peanut Extract1. J Allergy Clin Immunol (1997) 99:744–51. doi: 10.1016/S0091-6749(97)80006-1
246. Cooper PJ, Darbyshire J, Nunn AJ, Warner JO. A Controlled Trial of Oral Hyposensitization in Pollen Asthma and Rhinitis in Children. Clin Allergy (1984) 14:541–50. doi: 10.1111/j.1365-2222.1984.tb02242.x
247. Taudorf E, Laursen LC, Djurup R, Kappelgaard E, Pedersen CT, Søborg M, et al. Oral Administration of Grass Pollen to Hay Fever Patients. An Efficacy Study in Oral Hyposensitization. Allergy (1985) 40:321–35. doi: 10.1111/j.1398-9995.1985.tb00243.x
248. Möller C, Dreborg S, Lanner A, Björkstén B. Oral Immunotherapy of Children With Rhinoconjunctivitis Due to Birch Pollen Allergy. A Double Blind Study. Allergy (1986) 41:271–9. doi: 10.1111/j.1398-9995.1986.tb02028.x
249. Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical Efficacy and Immune Regulation With Peanut Oral Immunotherapy. J Allergy Clin Immunol (2009) 124:292–30197. doi: 10.1016/j.jaci.2009.05.022
250. Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral Peanut Immunotherapy in Children With Peanut Anaphylaxis. J Allergy Clin Immunol (2010) 126:83–91.e1. doi: 10.1016/j.jaci.2010.04.030
251. Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A Randomized Controlled Study of Peanut Oral Immunotherapy: Clinical Desensitization and Modulation of the Allergic Response. J Allergy Clin Immunol (2011) 127:654–60. doi: 10.1016/j.jaci.2010.12.1111
252. Anagnostou K, Clark A, King Y, Islam S, Deighton J, Ewan P. Efficacy and Safety of High-Dose Peanut Oral Immunotherapy With Factors Predicting Outcome. Clin Exp Allergy (2011) 41:1273–81. doi: 10.1111/j.1365-2222.2011.03699.x
253. Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, et al. Assessing the Efficacy of Oral Immunotherapy for the Desensitisation of Peanut Allergy in Children (STOP II): A Phase 2 Randomised Controlled Trial. Lancet (2014) 383:1297–304. doi: 10.1016/S0140-6736(13)62301-6
254. Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained Unresponsiveness to Peanut in Subjects Who Have Completed Peanut Oral Immunotherapy. J Allergy Clin Immunol (2014) 133:468–475.e6. doi: 10.1016/j.jaci.2013.11.007
255. Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, et al. A Randomized, Double-Blind, Placebo-Controlled Pilot Study of Sublingual Versus Oral Immunotherapy for the Treatment of Peanut Allergy. J Allergy Clin Immunol (2015) 135:1275–82. doi: 10.1016/j.jaci.2014.11.005
256. Bird JA, Feldman M, Arneson A, Dougherty I, Brown LS, Burk CM, et al. Modified Peanut Oral Immunotherapy Protocol Safely and Effectively Induces Desensitization. J Allergy Clin Immunol Pract (2015) 3:433–5. doi: 10.1016/j.jaip.2014.11.020
257. Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a Probiotic With Peanut Oral Immunotherapy: A Randomized Trial. J Allergy Clin Immunol (2015) 135:737–44.e8. doi: 10.1016/j.jaci.2014.11.034
258. Kukkonen AK, Uotila R, Malmberg LP, Pelkonen AS, Mäkelä MJ. Double-Blind Placebo-Controlled Challenge Showed That Peanut Oral Immunotherapy was Effective for Severe Allergy Without Negative Effects on Airway Inflammation. Acta Paediatr (2017) 106:274–81. doi: 10.1111/apa.13668
259. Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early Oral Immunotherapy in Peanut-Allergic Preschool Children is Safe and Highly Effective. J Allergy Clin Immunol (2017) 139:173–181.e8. doi: 10.1016/j.jaci.2016.05.027
260. Bird JA, Spergel JM, Jones SM, Rachid R, Assa’ad AH, Wang J, et al. Efficacy and Safety of AR101 in Oral Immunotherapy for Peanut Allergy: Results of ARC001, a Randomized, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. J Allergy Clin Immunol Pract (2018) 6:476–485.e3. doi: 10.1016/j.jaip.2017.09.016
261. PALISADE Group, Vickery BP, Vereda A, Casale TB, Beyer K, Du Toit G, et al. AR101 Oral Immunotherapy for Peanut Allergy. N Engl J Med (2018) 379:1991–2001. doi: 10.1056/NEJMoa1812856
262. Nagakura KI, Yanagida N, Sato S, Nishino M, Asaumi T, Ogura K, et al. Low-Dose Oral Immunotherapy for Children With Anaphylactic Peanut Allergy in Japan. Pediatr Allergy Immunol (2018) 29:512–8. doi: 10.1111/pai.12898
263. Fauquert JL, Michaud E, Pereira B, Bernard L, Gourdon-Dubois N, Rouzaire PO, et al. Peanut Gastrointestinal Delivery Oral Immunotherapy in Adolescents: Results of the Build-Up Phase of a Randomized, Double-Blind, Placebo-Controlled Trial (PITA Study). Clin Exp Allergy (2018) 48:862–74. doi: 10.1111/cea.13148
264. Blumchen K, Trendelenburg V, Ahrens F, Gruebl A, Hamelmann E, Hansen G, et al. Efficacy, Safety, and Quality of Life in a Multicenter, Randomized, Placebo-Controlled Trial of Low-Dose Peanut Oral Immunotherapy in Children With Peanut Allergy. J Allergy Clin Immunol Pract (2019) 7:479–491.e10. doi: 10.1016/j.jaip.2018.10.048
265. Wasserman RL, Hague AR, Pence DM, Sugerman RW, Silvers SK, Rolen JG, et al. Real-World Experience With Peanut Oral Immunotherapy: Lessons Learned From 270 Patients. J Allergy Clin Immunol Pract (2019) 7:418–426.e4. doi: 10.1016/j.jaip.2018.05.023
266. Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Pontoppidan B, et al. Walnut Oral Immunotherapy for Desensitisation of Walnut and Additional Tree Nut Allergies (Nut CRACKER): A Single-Centre, Prospective Cohort Study. Lancet Child Adolesc Health (2019) 3:312–21. doi: 10.1016/S2352-4642(19)30029-X
267. Chinthrajah RS, Purington N, Andorf S, Long A, O’Laughlin KL, Lyu SC, et al. Sustained Outcomes in a Large Double-Blind, Placebo-Controlled, Randomized Phase 2 Study of Peanut Immunotherapy. Lancet (2019) 394:1437–49. doi: 10.1016/S0140-6736(19)31793-3
268. Moraly T, Pelletier de Chambure D, Verdun S, Preda C, Seynave M, Vilain AC, et al. Oral Immunotherapy for Hazelnut Allergy: A Single-Center Retrospective Study on 100 Patients. J Allergy Clin Immunol Pract (2020) 8:704–709.e4. doi: 10.1016/j.jaip.2019.10.045
269. Hourihane JO, Beyer K, Abbas A, Fernández-Rivas M, Turner PJ, Blumchen K, et al. Efficacy and Safety of Oral Immunotherapy With AR101 in European Children With a Peanut Allergy (ARTEMIS): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 3 Trial. Lancet Child Adolesc Health (2020) 4:728–39. doi: 10.1016/S2352-4642(20)30234-0
270. Vickery BP, Vereda A, Nilsson C, Du Toit G, Shreffler WG, Burks AW, et al. Continuous and Daily Oral Immunotherapy for Peanut Allergy: Results From a 2-Year Open-Label Follow-on Study. J Allergy Clin Immunol Pract (2021) 9:1879–1889.e14. doi: 10.1016/j.jaip.2020.12.029
271. Enrique E, Pineda F, Malek T, Bartra J, Basagaña M, Tella R, et al. Sublingual Immunotherapy for Hazelnut Food Allergy: A Randomized, Double-Blind, Placebo-Controlled Study With a Standardized Hazelnut Extract. J Allergy Clin Immunol (2005) 116:1073–9. doi: 10.1016/j.jaci.2005.08.027
272. Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual Immunotherapy for Peanut Allergy: Clinical and Immunologic Evidence of Desensitization. J Allergy Clin Immunol (2011) 127:640–646.e1. doi: 10.1016/j.jaci.2010.12.1083
273. Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual Immunotherapy for Peanut Allergy: A Randomized, Double-Blind, Placebo-Controlled Multicenter Trial. J Allergy Clin Immunol (2013) 131:119–127.e7. doi: 10.1016/j.jaci.2012.11.011
274. Burks AW, Wood RA, Jones SM, Sicherer SH, Fleischer DM, Scurlock AM, et al. Sublingual Immunotherapy for Peanut Allergy: Long-Term Follow-Up of a Randomized Multicenter Trial. J Allergy Clin Immunol (2015) 135:1240–8. doi: 10.1016/j.jaci.2014.12.1917
275. Kim EH, Yang L, Ye P, Guo R, Li Q, Kulis MD, et al. Long-Term Sublingual Immunotherapy for Peanut Allergy in Children: Clinical and Immunologic Evidence of Desensitization. J Allergy Clin Immunol (2019) 144:1320–1326.e1. doi: 10.1016/j.jaci.2019.07.030
276. Jones SM, Sicherer SH, Burks AW, Leung DY, Lindblad RW, Dawson P, et al. Epicutaneous Immunotherapy for the Treatment of Peanut Allergy in Children and Young Adults. J Allergy Clin Immunol (2017) 139:1242–1252.e9. doi: 10.1016/j.jaci.2016.08.017
277. Sampson HA, Shreffler WG, Yang WH, Sussman GL, Brown-Whitehorn TF, Nadeau KC, et al. Effect of Varying Doses of Epicutaneous Immunotherapy vs. Placebo on Reaction to Peanut Protein Exposure Among Patients With Peanut Sensitivity: A Randomized Clinical Trial. JAMA (2017) 318:1798–809. doi: 10.1001/jama.2017.16591
278. Fleischer DM, Greenhawt M, Sussman G, Bégin P, Nowak-Wegrzyn A, Petroni D, et al. Effect of Epicutaneous Immunotherapy vs. Placebo on Reaction to Peanut Protein Ingestion Among Children With Peanut Allergy: The PEPITES Randomized Clinical Trial. JAMA (2019) 321:946–55. doi: 10.1001/jama.2019.1113
279. Wood RA, Sicherer SH, Burks AW, Grishin A, Henning AK, Lindblad R, et al. A Phase 1 Study of Heat/Phenol-Killed, E. Coli-Encapsulated, Recombinant Modified Peanut Proteins Ara H 1, Ara H 2, and Ara H 3 (EMP-123) for the Treatment of Peanut Allergy. Allergy (2013) 68:803–8. doi: 10.1111/all.12158
280. Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, et al. Anti-IgE Treatment With Oral Immunotherapy in Multifood Allergic Participants: A Double-Blind, Randomised, Controlled Trial. Lancet Gastroenterol Hepatol (2018) 3:85–94. doi: 10.1016/S2468-1253(17)30392-8
281. Berglund JP, Szczepanski N, Penumarti A, Beavers A, Kesselring J, Orgel K, et al. Preparation and Analysis of Peanut Flour Used in Oral Immunotherapy Clinical Trials. J Allergy Clin Immunol Pract (2017) 5:1098–104. doi: 10.1016/j.jaip.2016.11.034
282. Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a Peanut Oral Immunotherapy Protocol in Children With Peanut Allergy. J Allergy Clin Immunol (2009) 124:286–91. doi: 10.1016/j.jaci.2009.03.045
283. Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A Pilot Study of Omalizumab to Facilitate Rapid Oral Desensitization in High-Risk Peanut-Allergic Patients. J Allergy Clin Immunol (2013) 132:1368–74. doi: 10.1016/j.jaci.2013.09.046
284. MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab Facilitates Rapid Oral Desensitization for Peanut Allergy. J Allergy Clin Immunol (2017) 139:873–881.e8. doi: 10.1016/j.jaci.2016.08.010
285. Yee CS, Albuhairi S, Noh E, El-Khoury K, Rezaei S, Abdel-Gadir A, et al. Long-Term Outcome of Peanut Oral Immunotherapy Facilitated Initially by Omalizumab. J Allergy Clin Immunol Pract (2019) 7:451–461.e7. doi: 10.1016/j.jaip.2018.09.015
286. Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral Immunotherapy for Peanut Allergy (PACE): A Systematic Review and Meta-Analysis of Efficacy and Safety. Lancet (2019) 393:2222–32. doi: 10.1016/S0140-6736(19)30420-9
287. Eiwegger T, Anagnostou K, Arasi S, Bégin P, Ben-Shoshan M, Beyer K, et al. Conflicting Verdicts on Peanut Oral Immunotherapy From the Institute for Clinical and Economic Review and US Food and Drug Administration Advisory Committee: Where do We Go From Here? J Allergy Clin Immunol (2020) 145:1153–6. doi: 10.1016/j.jaci.2019.10.021
288. Senti G, Graf N, Haug S, Rüedi N, von Moos S, Sonderegger T, et al. Epicutaneous Allergen Administration as a Novel Method of Allergen-Specific Immunotherapy. J Allergy Clin Immunol (2009) 124:997–1002. doi: 10.1016/j.jaci.2009.07.019
289. Xiong L, Lin J, Luo Y, Chen W, Dai J. The Efficacy and Safety of Epicutaneous Immunotherapy for Allergic Diseases: A Systematic Review and Meta-Analysis. Int Arch Allergy Immunol (2020) 181:170–82. doi: 10.1159/000504366
290. Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, et al. Efficacy of Recombinant Birch Pollen Vaccine for the Treatment of Birch-Allergic Rhinoconjunctivitis. J Allergy Clin Immunol (2008) 122:951–60. doi: 10.1016/j.jaci.2008.09.017
291. Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination With Genetically Engineered Allergens Prevents Progression of Allergic Disease. Proc Natl Acad Sci U.S.A. (2004) 101 Suppl 2:14677–82. doi: 10.1073/pnas.0404735101
292. Zieglmayer P, Focke-Tejkl M, Schmutz R, Lemell P, Zieglmayer R, Weber M, et al. Mechanisms, Safety and Efficacy of a B Cell Epitope-Based Vaccine for Immunotherapy of Grass Pollen Allergy. EBioMedicine (2016) 11:43–57. doi: 10.1016/j.ebiom.2016.08.022
293. Swoboda I, Bugajska-Schretter A, Verdino P, Keller W, Sperr WR, Valent P, et al. Recombinant Carp Parvalbumin, the Major Cross-Reactive Fish Allergen: A Tool for Diagnosis and Therapy of Fish Allergy. J Immunol (2002) 168:4576–84. doi: 10.4049/jimmunol.168.9.4576
294. Swoboda I, Bugajska-Schretter A, Linhart B, Verdino P, Keller W, Schulmeister U, et al. A Recombinant Hypoallergenic Parvalbumin Mutant for Immunotherapy of IgE-Mediated Fish Allergy. J Immunol (2007) 178:6290–6. doi: 10.4049/jimmunol.178.10.6290
295. Douladiris N, Linhart B, Swoboda I, Gstöttner A, Vassilopoulou E, Stolz F, et al. In Vivo Allergenic Activity of a Hypoallergenic Mutant of the Major Fish Allergen Cyp C 1 Evaluated by Means of Skin Testing. J Allergy Clin Immunol (2015) 136:493–5.e8. doi: 10.1016/j.jaci.2015.01.015
296. Zuidmeer-Jongejan L, Huber H, Swoboda I, Rigby N, Versteeg SA, Jensen BM, et al. Development of a Hypoallergenic Recombinant Parvalbumin for First-in-Man Subcutaneous Immunotherapy of Fish Allergy. Int Arch Allergy Immunol (2015) 166:41–51. doi: 10.1159/000371657
297. Zuidmeer-Jongejan L, Fernandez-Rivas M, Poulsen LK, Neubauer A, Asturias J, Blom L, et al. FAST: Towards Safe and Effective Subcutaneous Immunotherapy of Persistent Life-Threatening Food Allergies. Clin Transl Allergy (2012) 2:5. doi: 10.1186/2045-7022-2-5
298. King N, Helm R, Stanley JS, Vieths S, Lüttkopf D, Hatahet L, et al. Allergenic Characteristics of a Modified Peanut Allergen. Mol Nutr Food Res (2005) 49:963–71. doi: 10.1002/mnfr.200500073
299. Rabjohn P, West CM, Connaughton C, Sampson HA, Helm RM, Burks AW, et al. Modification of Peanut Allergen Ara H 3: Effects on IgE Binding and T Cell Stimulation. Int Arch Allergy Immunol (2002) 128:15–23. doi: 10.1159/000057999
300. Bannon GA, Cockrell G, Connaughton C, West CM, Helm R, Stanley JS, et al. Engineering, Characterization and In Vitro Efficacy of the Major Peanut Allergens for Use in Immunotherapy. Int Arch Allergy Immunol (2001) 124:70–2. doi: 10.1159/000053672
301. Bublin M, Kostadinova M, Radauer C, Varga EM, Hafner C, Schmidthaler K, et al. Engineering of Structural Variants of the Major Peanut Allergens Ara H 2 and Ara H 6 for Allergen-Specific Immunotherapy. J Allergy Clin Immunol (2019) 143:1226–1229.e10. doi: 10.1016/j.jaci.2018.10.039
302. Mauro M, Russello M, Incorvaia C, Gazzola G, Frati F, Moingeon P, et al. Birch-Apple Syndrome Treated With Birch Pollen Immunotherapy. Int Arch Allergy Immunol (2011) 156:416–22. doi: 10.1159/000323909
303. Gadermaier E, Flicker S, Aberer W, Egger C, Reider N, Focke M, et al. Analysis of the Antibody Responses Induced by Subcutaneous Injection Immunotherapy With Birch and Fagales Pollen Extracts Adsorbed Onto Aluminum Hydroxide. Int Arch Allergy Immunol (2010) 151:17–27. doi: 10.1159/000232567
304. Marth K, Breyer I, Focke-Tejkl M, Blatt K, Shamji MH, Layhadi J, et al. A Nonallergenic Birch Pollen Allergy Vaccine Consisting of Hepatitis PreS–fused Bet V 1 Peptides Focuses Blocking IgG Toward IgE Epitopes and Shifts Immune Responses to a Tolerogenic and Th1 Phenotype. J Immunol (2013) 190:3068–78. doi: 10.4049/jimmunol.1202441
305. Campana R, Vrtala S, Maderegger B, Jertschin P, Stegfellner G, Swoboda I, et al. Hypoallergenic Derivatives of the Major Birch Pollen Allergen Bet V 1 Obtained by Rational Sequence Reassembly. J Allergy Clin Immunol (2010) 126:1024–31. doi: 10.1016/j.jaci.2010.05.023
306. Hofer H, Asam C, Hauser M, Nagl B, Laimer J, Himly M, et al. Tackling Bet V 1 and Associated Food Allergies With a Single Hybrid Protein. J Allergy Clin Immunol (2017) 140:525–533.e10. doi: 10.1016/j.jaci.2016.09.055
307. Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, et al. Clinical Effects of Immunotherapy With Genetically Modified Recombinant Birch Pollen Bet V 1 Derivatives. Clin Exp Allergy (2008) 38:1514–25. doi: 10.1111/j.1365-2222.2008.03042.x
308. Haselden BM, Kay AB, Larché M. Immunoglobulin E-Independent Major Histocompatibility Complex-Restricted T Cell Peptide Epitope-Induced Late Asthmatic Reactions. J Exp Med (1999) 189:1885–94. doi: 10.1084/jem.189.12.1885
309. Valenta R, Campana R, Niederberger V. Recombinant Allergy Vaccines Based on Allergen-Derived B Cell Epitopes. Immunol Lett (2017) 189:19–26. doi: 10.1016/j.imlet.2017.04.015
Keywords: allergen molecules, component, food allergy, immunotherapy, molecular allergy diagnosis, peanut, tree nut
Citation: Fuhrmann V, Huang H-J, Akarsu A, Shilovskiy I, Elisyutina O, Khaitov M, van Hage M, Linhart B, Focke-Tejkl M, Valenta R and Sekerel BE (2021) From Allergen Molecules to Molecular Immunotherapy of Nut Allergy: A Hard Nut to Crack. Front. Immunol. 12:742732. doi: 10.3389/fimmu.2021.742732
Received: 16 July 2021; Accepted: 23 August 2021;
Published: 23 September 2021.
Edited by:
Sandip D. Kamath, James Cook University, AustraliaReviewed by:
Michael D. Kulis, University of North Carolina at Chapel Hill, United StatesCopyright © 2021 Fuhrmann, Huang, Akarsu, Shilovskiy, Elisyutina, Khaitov, van Hage, Linhart, Focke-Tejkl, Valenta and Sekerel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rudolf Valenta, UnVkb2xmLnZhbGVudGFAbWVkdW5pd2llbi5hYy5hdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.