
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ERRATUM article
Front. Immunol. , 23 June 2021
Sec. Immunological Tolerance and Regulation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.722805
This article is an erratum on:
Systemic Characterization of Novel Immune Cell Phenotypes in Recurrent Pregnancy Loss
An Erratum on
Systemic Characterization of Novel Immune Cell Phenotypes in Recurrent Pregnancy Loss
By Liu H, Lin X-X, Huang X-B, Huang D-H, Song S, Chen Y-J, Tang J, Tao D, Yin Z-N, Mor G and Liao A-H (2021). Front. Immunol. 12:657552. doi: 10.3389/fimmu.2021.657552
Due to a production error, additional proof corrections submitted by the author were not implemented in the final version. Corrections have therefore been made throughout the article.
There was an error in Table 1, Table 2, Figure 1, and Figure 3. Instead of “Characteristics of 12 immune parameters with significant differences in NPW, NP and RPL groups”, Table 1 should say “Characteristics of 11 immune parameters with significant differences in NPW, NP and RPL groups”. Therefore, references to “V2+PD-1+” were removed in these tables and figures, as well as throughout the text, including the Abstract, Introduction and Results.
The corrected tables and figures appear below.
Additionally, Supplementary Figure 1, Supplementary Figure 3, and Supplementary Table 2 were not the latest versions. The correct versions appear below.
The publisher apologizes for these mistakes. The original version of this article has been updated.
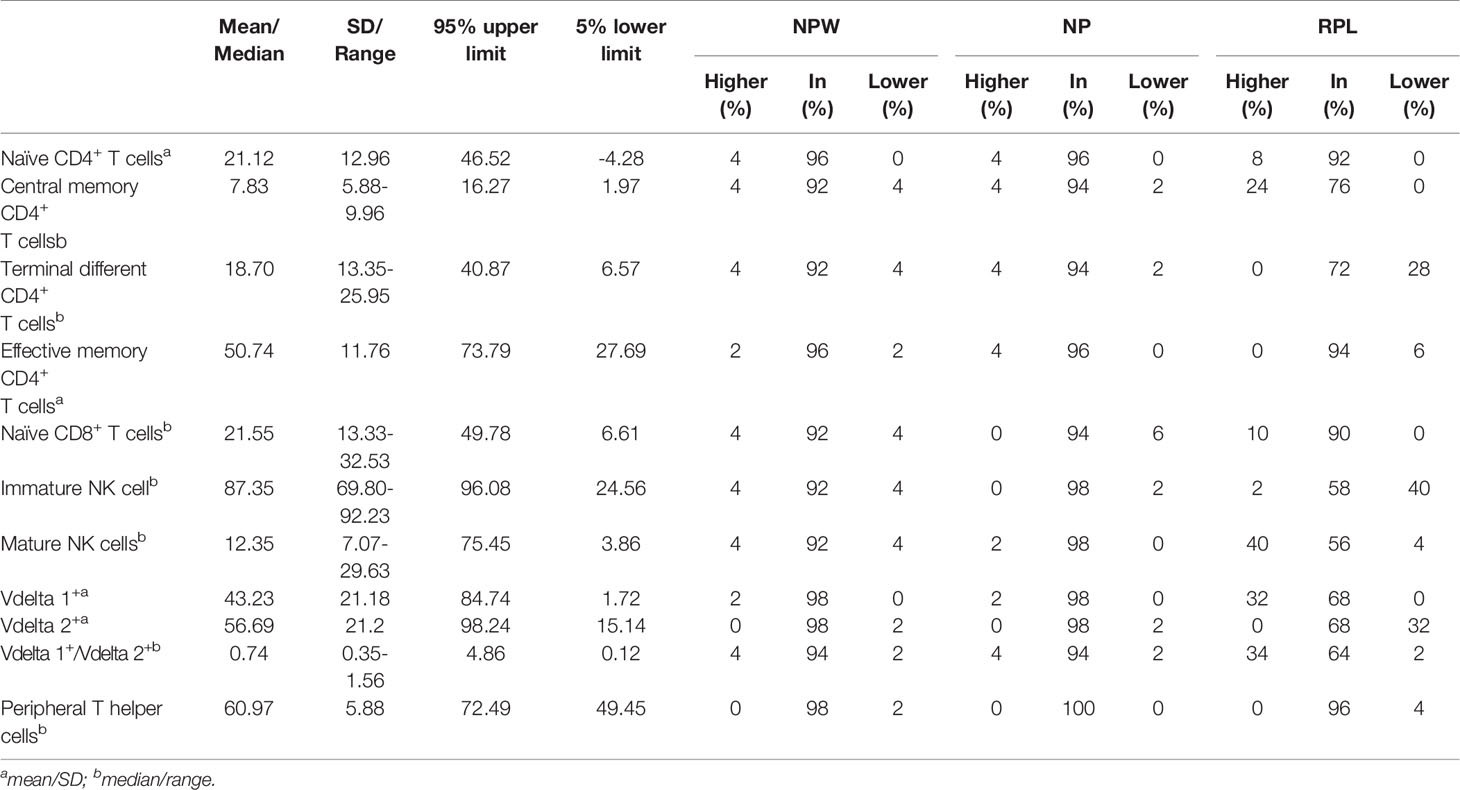
Table 1 Characteristics of 11 immune parameters with significant differences in NPW, NP and RPL groups.
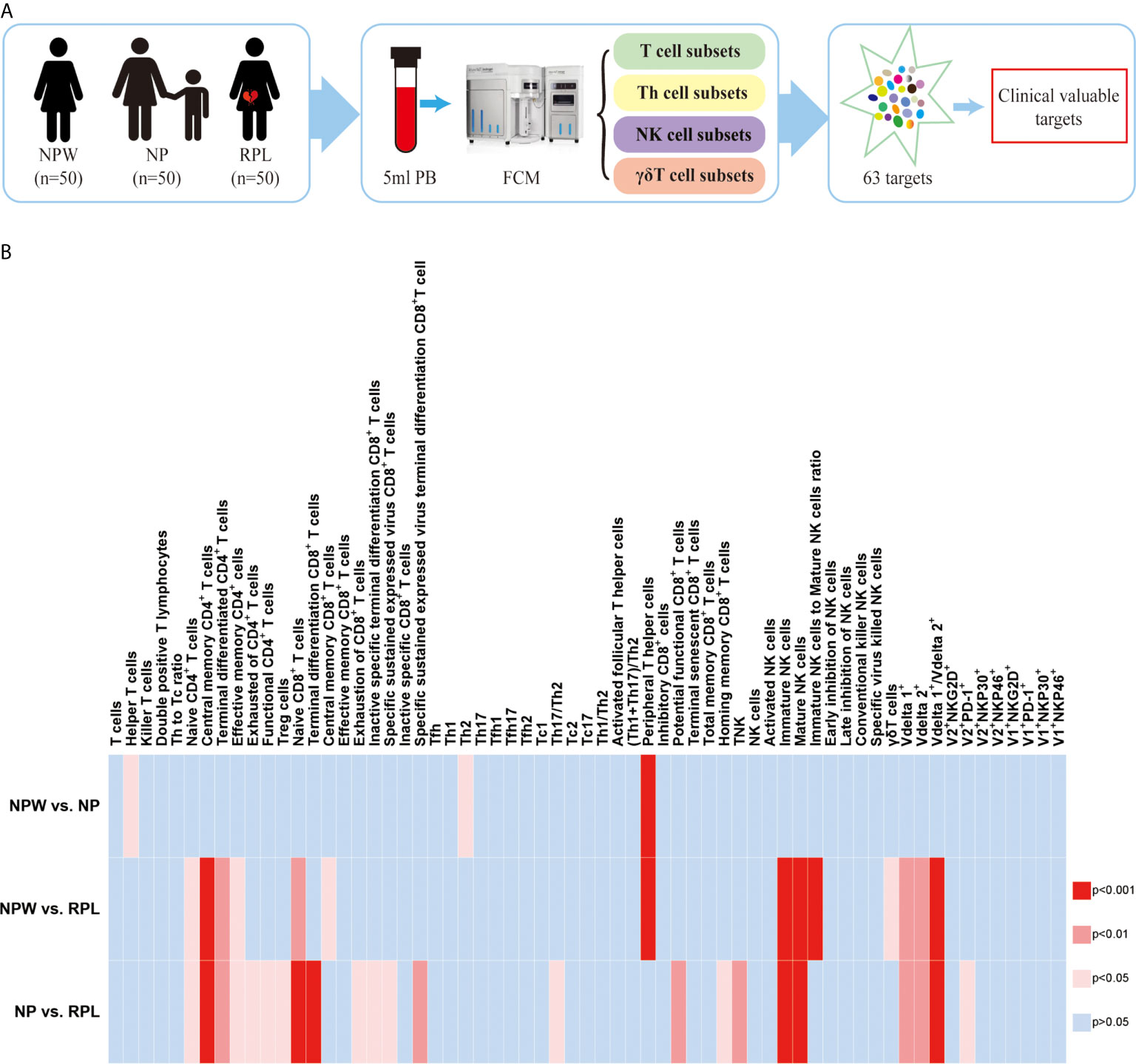
Figure 1 Study design and the overview of the different immunological parameters among the three groups. (A) Schedule of the study. Three groups were included in our study: NPW group (women who were never pregnant, n=50), NP group (women with the history of normal pregnancy, n=50) and RPL group (women with the history of RPL, n=50). Peripheral blood mononuclear cells (PBMCs) were isolated from 5 ml peripheral blood. Total 63 immune cell subsets were simultaneously detected by flow cytometry, including T cell, NK cell and gd T cell subsets. By analyzing the data, clinical-relevant immune parameters were finally identified. (B) Heat map of the significantly changed immune parameters. The 63 immunological parameters were compared among the three groups and presented as the heat map, which can directly show the differences. The colors represent the different significance among the comparisons. The deeper the color is red, the larger the differences are. The blue represents no significance. NPW, women never pregnant; NP, women with a history of normal pregnancy; RPL, women with ahistory of RPL.
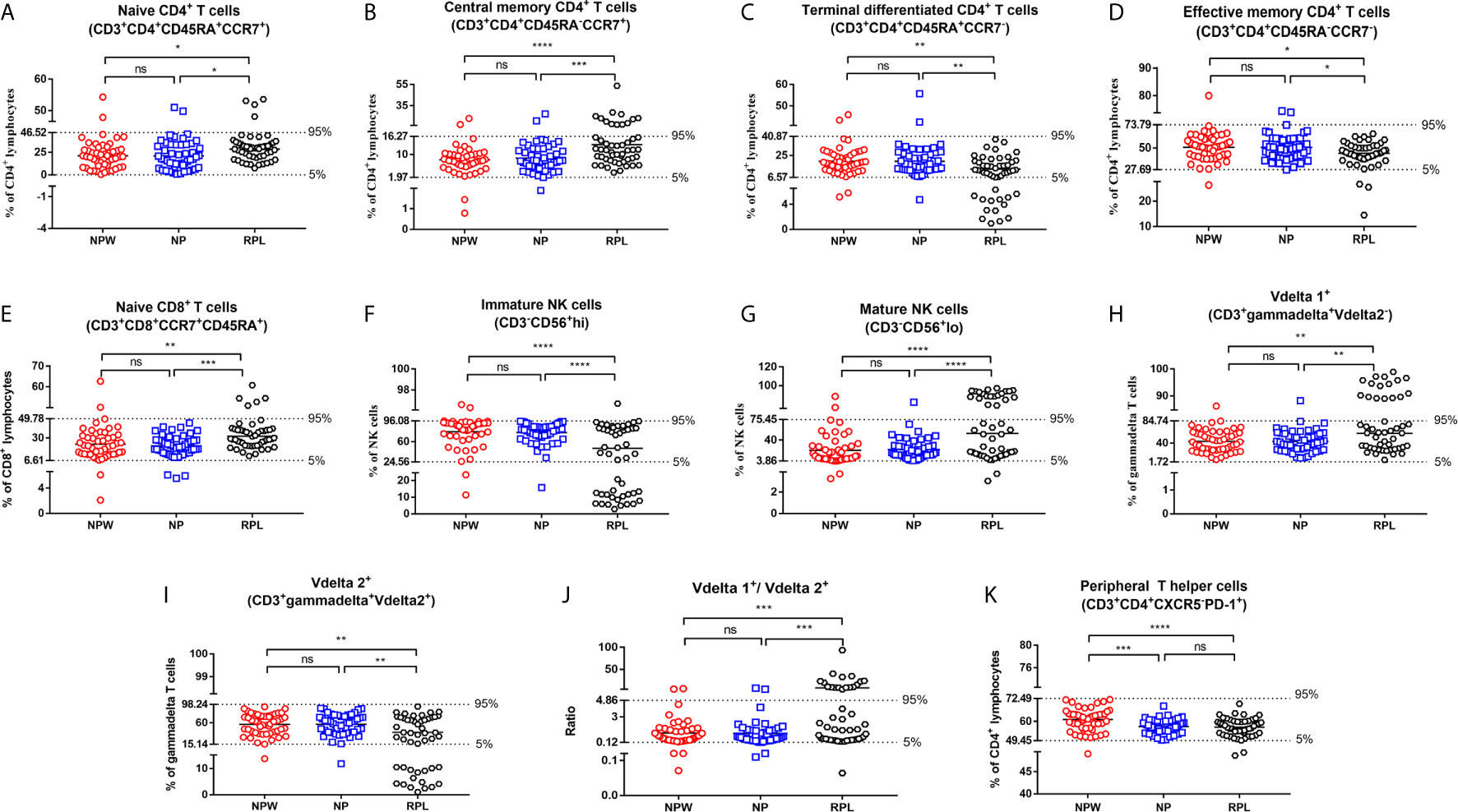
Figure 3 Reference ranges for the significantly changed immune parameters. (A–J) Reference ranges for significantly changed immune parameters related to RPL. The percentages of naïve CD4+ T cells (A), central memory CD4+ T cells (B), naïve CD8+ T cells (E), mature NK cells (G), Vδ1+ T cells (H) and the ratio of Vδ1+ T cells/Vδ2+ T cells (J) were significantly higher in the RPL group than those in the NPW and NP groups. The percentages of terminal differentiated CD4+ T cells (D), effective memory CD4+ T cells (D), immature NK cells (F) and Vδ2+ T cells (I) were significantly lower in the RPL group than those in the NPW and NP groups. The mean + 1.96 SD was used to measure the reference ranges for the normally distributed data. Median and 5th/95th percentiles represented the lower/upper limit to set up the reference ranges for skewed distribution data. In 14%- 40% of women with RPL, the percentages of central memory CD4+ T cells (B), mature NK cells (G), the ratio of Vδ1+ T cells/Vδ2+ T cells (J), and Vδ1+ T cells (H) were above the 95th percentile limit. In 28% - 40% of women with RPL group, the percentages of terminally differentiated CD4+ T cells (C), immature NK cells (F) and Vδ2+ T cells (I) were below the 5th percentile limit. The percentages of these different immunological parameters in the NP group were similar to those in the NPW group, and most were within the 5th percentile limit and 95th percentile limit. (K) Reference ranges for significantly changed immune parameters related to pregnancies. The percentage of TPH was significantly low in the NP and RPL groups compared with the NPW group. Vdelta1: Vd1; Vdelta2: Vd2. Significance levels were set to *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, and ns means not significant.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.722805/full#supplementary-material
Supplementary Figure 1 | Reference ranges for nonsignificant immune parameters in CD4+ and CD8+ T cells. (A) Th1 and Th17 cells were not significantly different in the RPL vs. NP and RPL vs. NPW groups. Th2 cells were significantly different in the NP vs. NPW groups but not in the RPL vs. NP and RPL vs. NPW groups. Treg cells were significantly different in the RPL vs. NP groups but not in the RPL vs. NPW groups. (B) T cells (a), killer T cells (b), effective memory CD8+ T cells (e), inactive specific CD8+ T cells (i), inhibitory CD8+ T cells (k), terminally senescent CD8+ T cells (m) and total memory CD8+ T cells (n) were not significantly different among the three groups. Some of the parameters were lower or higher than the 5th/95th percentile limits. Terminal differentiation CD8+ T cells (c), exhaustion of CD8+ T cells (f), inactive specific terminal differentiation CD8+ T cells (g), specific sustained expressed virus CD8+ T cells (h), specific sustained expressed virus terminal differentiation CD8+ T cells (j), potential functional CD8+ T cells (l), and homing memory CD8+ T cells (o) were significantly different in the RPL vs. NP groups but not in the RPL vs. NPW groups. Central memory CD8+ T cells (d) were significantly different in the NPW vs. RPL groups but not in RPL vs. NP groups. (C) CD4+ T cell DP T lymphocytes and activated Tfh cells were not significantly different among the three groups. Helper T cells were significantly different in the NP vs. NPW groups. The exhaustion of CD4+ T cells and functional CD4+ T cells was significant in the RPL vs. NP groups but not in the RPL vs. NPW groups. (D) Tfh cells (a) and cytotoxic T (Tc) cells (Tfh, Tfh1, Tfh2, Tfh17, Tc1, Tc2 and Tc17) (b–g) were not significantly different among the three groups. (E) The Th to Tc ratios (a), Th1/Th2 ratios (c) and (Th1+Th17)/Th2 ratios (d) were nonsense parameters among the three groups. Th17/Th2 (b) was significantly different in the RPL vs. NP groups. The ratio of immature NK cells to mature NK cells (e) was significantly different in the RPL vs. NPW groups. The mean ± 1.96 SD was used to measure the reference range for the normally distributed data. The median and 5th/95th percentiles represent the lower/upper limits of each reference range for skewed distribution data. Significance levels were set to *P < 0.05, **P < 0.01, and ***P < 0.001 and ns means not significant.
Supplementary Figure 3 | Reference ranges for nonsignificant immune parameters in gd T cells. (A) γδ T cells were significantly different in the RPL vs. NPW groups. (B–I) The percentages of V1+NKG2D+, V1+NKP30+, V1+NKP46+, V1+PD-1+, V2+NKG2D+, V2+NKP30+ and V2+NKP46+ γδ T cells were not significantly different among the three groups. The percentage of Vdelta2+PD-1+ γδ T cells was significantly different between the RPL and NP groups (P < 0.05). (I) The mean ± 1.96 SD was used to measure the reference range for the normally distributed data. The median and 5th/95th percentiles represent the lower/upper limits of the reference range for skewed distribution data. Significance levels were set to P < 0.05 (*), and ns means not significant.
32. Sprent J, Surh CDNormal T Cell Homeostasis: The Conversion of Naive Cells Into Memory-Phenotype Cells. Nat Immunol (2011) 12:478–84 doi: 10.1038/ni.2018
33. Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM, et alDengue Virus Infection Elicits Highly Polarized CX3CR1+ Cytotoxic CD4+ T Cells Associated with Protective Immunity. Proc Natl Acad Sci USA (2015) 112:E4256–63 doi: 10.1073/pnas.1505956112
34. Crotty SFollicular Helper CD4 T Cells (TFH). Annu Rev Immunol (2011) 29:621–63 doi: 10.1146/annurev-immunol-031210-101400
35. Cai D, Tang Y, Yao XChanges of GammadeltaT Cell Subtypes During Pregnancy and Their Influences in Spontaneous Abortion. J Reprod Immunol (2019) 131:57–62 doi: 10.1016/j.jri.2019.01.003
36. Uchida Y, Gherardini J, Schulte-Mecklenbeck A, Alam M, Cheret J, Rossi A Pro-Inflammatory Vdelta1(+)T-Cells infiltrates Are Present in and Around the Hair Bulbs of Non-Lesional and Lesional Alopecia Areata Hair Follicles. J Dermatol Sci (2020) 100:129–38 doi: 10.1016/j.jdermsci.2020.09.001
37. Brummelman J, Pilipow K, Lugli EThe Single-Cell Phenotypic Identity of Human CD8(+) and CD4(+) T Cells. Int Rev Cell Mol Biol (2018) 341:63–124 doi: 10.1016/bs.ircmb.2018.05.007
38. Gordon CL, Miron M, Thome JJ, Matsuoka N, Weiner J, Rak MA Tissue Reservoirs of Antiviral T Cell Immunity in Persistent Human CMV Infection. J Exp Med (2017) 214:651–67 doi: 10.1084/jem.20160758
39. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF A Human Memory T Cell Subset With Stem Cell-Like Properties. Nat Med (2011) 17:1290–7 doi: 10.1038/nm.2446
40. Peters C, Kabelitz D, Wesch DRegulatory Functions of Gammadelta T Cells. Cell Mol Life Sci (2018) 75:2125–35 doi: 10.1007/s00018-018-2788-x
41. Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T Crystal Structure of A Gammadelta T-Cell Receptor Specific for the Human MHC Class I Homolog MICA. Proc Natl Acad Sci USA (2011) 108:2414–9 doi: 10.1073/pnas.1015433108
42. Simoes AE, Di Lorenzo B, Silva–Santos BMolecular Determinants of Target Cell Recognition by Human Gammadelta T Cells. Front Immunol (2018) 9:929 doi: 10.3389/fimmu.2018.00929
43. Ribeiro ST, Ribot JC, Silva–Santos BFive Layers of Receptor Signaling in gammadelta T-Cell Differentiation and Activation. Front Immunol (2015) 6:15 doi: 10.3389/fimmu.2015.00015
44. Chen Y, Mo J, Jia X, He YThe B7 Family Member B7-H6: A New Bane of Tumor. Pathol Oncol Res (2018) 24:717–21 doi: 10.1007/s12253-017-0357-5
Keywords: recurrent pregnancy loss, flow cytometry, peripheral T helper cell, immune cell, NK cell, γδT cell
Citation: Frontiers Production Office (2021) Erratum: Systemic Characterization of Novel Immune Cell Phenotypes in Recurrent Pregnancy Loss. Front. Immunol. 12:722805. doi: 10.3389/fimmu.2021.722805
Received: 09 June 2021; Accepted: 09 June 2021;
Published: 23 June 2021.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2021 Frontiers Production Office. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frontiers Production Office, cHJvZHVjdGlvbi5vZmZpY2VAZnJvbnRpZXJzaW4ub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.