
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 26 August 2021
Sec. Inflammation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.718895
 Tobias Moser1*
Tobias Moser1* Michael Seiberl1
Michael Seiberl1 Julia Feige1
Julia Feige1 Lara Bieler1
Lara Bieler1 Richard F. Radlberger1
Richard F. Radlberger1 Ciara O’Sullivan1
Ciara O’Sullivan1 Georg Pilz1
Georg Pilz1 Andrea Harrer1
Andrea Harrer1 Kerstin Schwenker1
Kerstin Schwenker1 Elisabeth Haschke-Becher2
Elisabeth Haschke-Becher2 Lukas Machegger3
Lukas Machegger3 Jochen Grimm3
Jochen Grimm3 Monika Redlberger-Fritz4
Monika Redlberger-Fritz4 Arabella Buchmann5
Arabella Buchmann5 Michael Khalil5
Michael Khalil5 Erich Kvas6
Erich Kvas6 Eugen Trinka1,7
Eugen Trinka1,7 Peter Wipfler1
Peter Wipfler1Background: Efficacy of vaccines and disease activity linked to immunization are major concerns among people with multiple sclerosis (pwMS).
Objective: To assess antibody responses to seasonal influenza antigens and vaccine-associated neuroaxonal damage utilizing serum neurofilament light chain (sNfL) in pwMS receiving dimethyl fumarate (DMF).
Methods: In this prospective study, the 2020/2021 seasonal tetravalent influenza vaccine was administered to 20 pwMS treated with DMF and 15 healthy controls (HCs). The primary endpoints were responder rate of strain-specific antibody production (seroconversion or significant (4-fold) increase in influenza-antibody titers for ≥2/4 strains) at 30 days post-vaccination and changes in sNfL levels.
Results: All patients treated with DMF fulfilled the responder criteria for immunization compared with 53% of the controls. However, higher proportions of HCs already had influenza-antibody titers ≥1:40 at baseline (53% vs. 41%, p = 0.174). sNfL levels were comparable among both groups at baseline and did not increase 34 days after vaccination. In addition, no clinical or radiological disease reactivation was found.
Conclusion: DMF-treated patients mount an adequate humoral immune response to influenza vaccines. Within the limits of the small cohort investigated, our data suggest that influenza immunization is not associated with clinical or subclinical disease reactivation.
Vaccines are a major achievement of science, and many of them protect from life-threatening infectious disease or have eradicated global health threats such as smallpox (1). However, false studies or misguided reports, including lay advice on social media, impact public attitude and contribute to the growing vaccine hesitancy (2, 3). Anti-vaccine sentiments constitute a major obstacle in times when immunization coverage is crucial to combatting a global pandemic. People suffering from chronic immune-mediated disorders including multiple sclerosis (MS) are confronted with particular vaccine-related concerns. First, people with MS (pwMS) are more susceptible to infections (4) mainly due to suppressive properties of disease-modifying therapies (DMTs) on normal immune functions (5). Infections, on the other hand, frequently trigger neurological deteriorations, which have been reported to be more severe than spontaneous relapses (6, 7). Immunizations hence not only are essential to prevent infections but may even be considered neuroprotective in MS. In fact, the seasonal influenza vaccine is highly recommended for MS patients (8). Another concern is that the immunomodulating/immunosuppressive effects of MS drugs may reduce vaccine efficacy. Trials in this regard have primarily assessed humoral responses to seasonal influenza vaccinations. Findings indicate that, apart from beta-interferons, many DMTs diminish immune responses to vaccinations (9). For dimethyl fumarate (DMF), one of the most frequently used MS therapeutics (10), humoral response against bacterial antigens was shown to be preserved (11), but data on vaccine efficacy to viral pathogens is completely lacking.
Irrespective of infectious concerns, patients also fear neurological sequelae following immunization, again fostering vaccine reluctance in MS. Several reports have found no link between seasonal flu vaccination and MS exacerbation assessed by the current standard of care with magnetic resonance imaging (MRI) scans and/or clinical examination (12–16), while two small studies could not refute an association (17, 18).
More recently, serum neurofilament light chain (sNfL) has been proposed as a biomarker for neuronal injury (19). NfL represents a major constituent of the axonal cytoskeleton in neurons, and it is released into the cerebrospinal fluid and the peripheral circulation upon neuro-axonal injury. In fact, a growing body of evidence supports its role as surrogate for disease activity and potentially for subclinical neuro-axonal damage (19–22). sNfL therefore appears to be a sensitive marker to unveil contrasting results from the literature regarding the impact of vaccines on disease activity.
The aim of this study was to elucidate whether vaccine-induced immunological consequences are linked to increases in sNfL in pwMS. Moreover, we investigated whether the immunomodulating properties of DMF blunt the efficacy of viral vaccines.
We conducted a prospective study to assess efficacy and safety of seasonal flu immunization in MS patients treated with DMF. We recruited patients aged 18 to 65 with relapsing MS according to the McDonald criteria 2017 (23) from an outpatient MS clinic in a large university center and 15 healthy controls (HCs). Eligible patients were required to receive DMF in the approved dose (240 mg twice daily) for at least 3 months and have not been treated with steroids within 4 weeks from vaccination. Patients with prior immunosuppressive drugs or concomitant, clinically significant systemic diseases at baseline (BL) were excluded.
Serum samples for each individual were drawn before and 4 weeks after vaccination and stored at −80°C. Participants were immunized with injectable seasonal influenza vaccines 2020/2021 (VaxigripTetra®, Sanofi Pasteur Europe; or Influvac Tetra®, Mylan Healthcare GmbH) comprising antigens of A(H1N1)pdm09 A/Guangdong-Maonan/SWL1536/2019, A(H3N2) A/Hong Kong/2671/2019, B/Victoria lineage B/Washington/02/2019 (B/Vic), and B/Yamagata lineage B/Phuket/3073/2013 (B/Yam) strains (Table 1). Both vaccines contained 15 µg of each strain. Amounts of strain-specific antibodies were quantitatively obtained by hemagglutination inhibition assay (HAI). HAIs were performed blinded and in duplicates. Disease activity following influenza vaccination was gauged by clinical, radiological, and laboratory parameters. Clinical and sNfL evaluations were determined just prior to vaccination (BL) and 30 days thereafter. sNfL concentrations were assessed by a commercially available single-molecule array (SIMOA) assay NF-light® kit on the SR-X Analyzer (Quanterix, Lexington, MA). Cerebral MRI (cMRI) analyses were performed on 3-tesla MRIs 4 weeks post-vaccination and compared with the most recent pre-immunization image carried out on the same scanner. All cMRIs included T1-weighted images before and after administration of contrast agent [gadolinium (Gd)] and T2/fluid-attenuated inversion recovery (FLAIR) sequences. Images were analyzed by two independent neuroradiologists (JG and LM).
The primary influenza-vaccine efficacy outcome was responder rate at 30 days from immunization in accordance with European Guidelines (24). The responder rate was determined as the proportion of individuals to fulfill either the criteria for seroconversion or a significant antibody increase for at least two of the four influenza strains. Participants who had BL titers of ≤1:10 and after immunization reached the cut-off for seroprotection were defined as seroconverters. An antibody increase by 4-fold was considered significant based on regulatory guidelines for vaccination trials. Seroprotection was defined as a HAI of ≥1:40 according to literature recommendations (25). The primary safety outcome was determined by sNfL. Also, relapses and Expanded Disability Status Scale (EDSS) changes during the study period were assessed by MS specialists. Post-vaccine cMRI scans were primarily evaluated for Gd enhancement but also for new/enlarging lesions compared with pre-vaccine MRI. In addition, we assessed routine laboratory parameters within the MS cohort including inflammatory proteins (IL-6 and C-reactive protein (CRP)) and main immune cell subsets.
This project was an exploratory study, and therefore, all analyses have to be seen on a descriptive level. After testing for normality using the Kolmogorov–Smirnov test, results were presented as median [interquartile range (IQR)] and/or mean ± standard deviation (SD), as appropriate. Qualitative variables are shown as absolute counts and/or percentages.
Quantification of sNfL levels pre- and post-vaccination as well as the comparison between MS patients and HCs was investigated using the Wilcoxon signed-rank test for changes over time and the Mann–Whitney U or Fisher test (as appropriate) to compare groups at each time point. All other quantitative variables were analyzed according to the primary objective.
Statistical testing and 95% confidence intervals were used to detect possible signals and not to confirm planned hypotheses. Significance levels were set at nominal p-values of p ≤ 0.05, and no corrections for multiple testing were performed. Statistical analyses were done using IBM SPSS Statistics Version 24 (IBM Corp., Armonk, NY, USA). Graphs were designed by GraphPad PRISM8 (GraphPad Software, San Diego, CA, USA).
The study was approved by the local ethics committee (415-E/1612/11-2018) and conducted according to the Good Clinical Practice and the ethical principles of the Declaration of Helsinki. All participants provided written informed consent.
We enrolled 20 pwMS treated with DMF and 15 age-matched controls. The mean age of the MS cohort was 37.6 years ( ± 9), with a median EDSS at BL of 1.0 (IQR 0–1.5). Patients had received DMF on average for 19.2 months ( ± 12.1). The demographics and vaccine distribution of the two cohorts and the EDSS score of the MS cohort are displayed in Table 1. The time from vaccination to the follow-up visit was longer for HCs (means 40.7 vs. 29.1 days, p < 0.05).
At BL, seroprotection (antibody titers ≥1:40) was more frequent among HCs in three of the four influenza strains (Figure 1A). Across all strains, the proportions of patients vs. controls who met the criteria for seroprotection was 41% vs. 53% (p = 0.174), respectively. The cut-off was evident in patients vs. controls in 55% (11/20) and 66% (10/15) for A(H1N1)pdm09, in 20% (4/20) and 47% (7/15) for A(H3N2), in 80% (16/20) and 60% (9/15) for B/Vic, and in 10% (2/20) and 40% (6/15) for B/Yam.
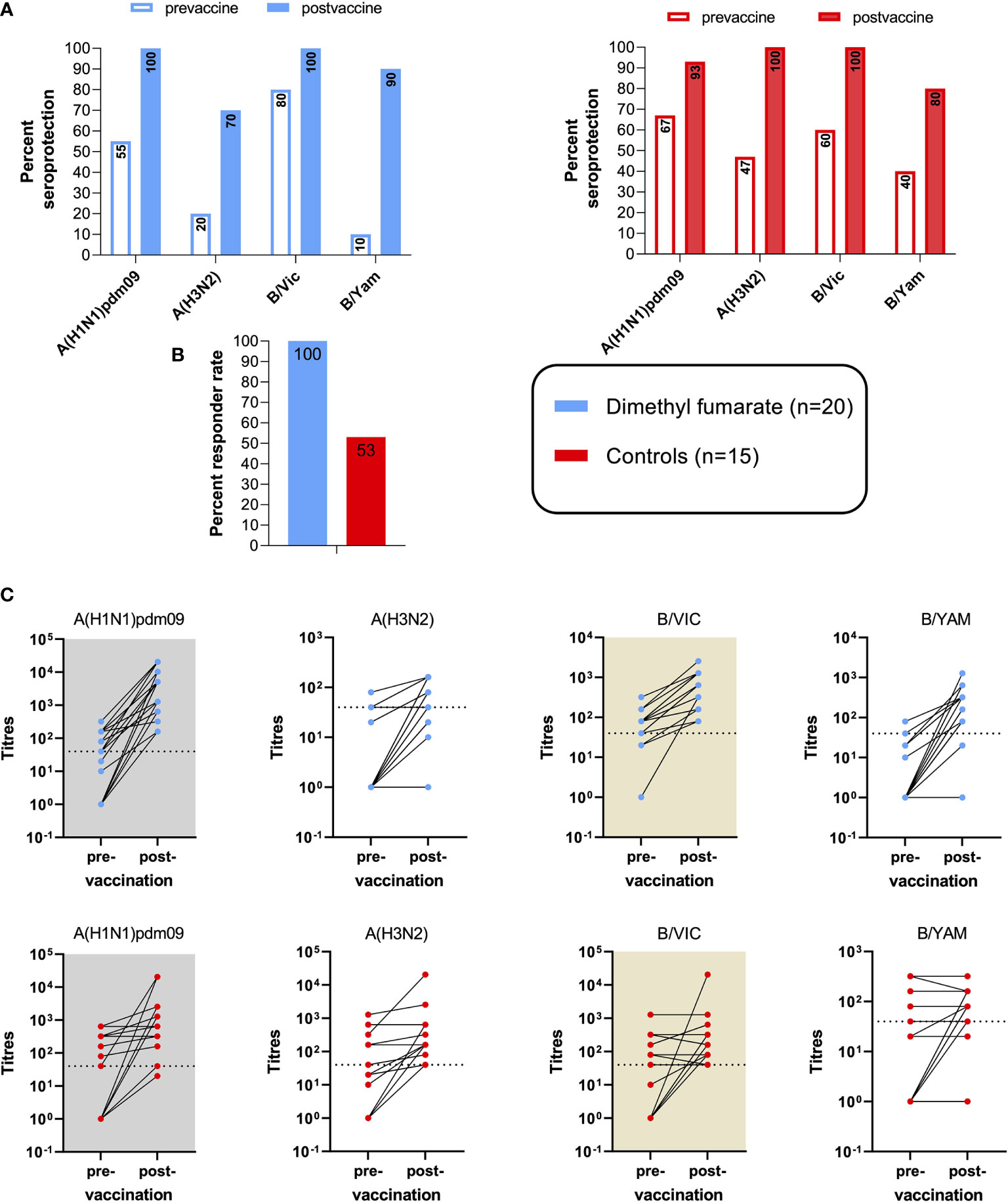
Figure 1 Vaccine efficacy to influenza immunization in multiple sclerosis (MS) patients on dimethyl fumarate (DMF) and healthy controls. (A) Pre- and post-vaccine seroprotection rates, defined by a strain-specific anti-influenza titer of ≥1:40. (B) Vaccine responder rates among the two cohorts. Vaccine response was defined by seroconversion and/or significant (≥4-fold) specific titer increases in ≥2/4 influenza strains. (C) Increases in strain-specific antibody titers among the two cohorts at 34.1 days ( ± 9.4) post-vaccination compared with baseline. Dotted lines indicate the cut-off titer for seroprotection.
At BL, average A(H3N2) titers were higher in the control group (p = 0.007), while no significant differences were found among the other strains.
The primary efficacy endpoint was responder rate at 30 days from vaccination. This outcome was reached in 100% of DMF-treated patients, compared with 53% of controls (Figure 1B).
A seroconversion and/or 4-fold increase in antibody titers for DMF-treated MS patients vs. controls was achieved in 95% vs. 47% for A(H1N1)pdm09, in 60% vs. 40% for A(H3N2), in 85% vs. 53% for B/Vic, and in 90% vs. 40% for B/Yam. The increases in strain-specific antibody titers are shown in Figure 1C. Over all strains, a significant (4-fold) increase in antibody titers for DMF-treated MS patients and controls was found in 49% and 15%, respectively; and the criteria for seroconversion were met by 36% and 30%, respectively.
Thirty days post-vaccination, seroprotection was evident in 100% (20/20) and 93% (14/15) for A(H1N1)pdm09, in 70% (14/20) and 100% (15/15) for A(H3N2), in 100% (20/20) and 100% (15/15) for B/Vic, and in 90% (18/20) and 80% (12/15) for B/Yam in DMF patients and controls, respectively (Figure 1A). Across all four strains, seroprotection was reached by 90% of MS patients and by 93% of the controls at follow-up.
At follow-up, the increase of average antibody levels was statistically significant for MS and HCs against A(H1N1)pdm09 (p < 0.001 and p = 0.003, respectively), against A(H3N2) (p < 0.001 and p = 0.001, respectively), and against B/Vic (p < 0.001 and p = 0.050, respectively). For the B/Yam strain, a statistically significant increase was found only among the MS cohort (p < 0.001). Regarding inter-group differences of humoral vaccine responses, average titer increases against A(H1N1)pdm09, B/Vic, and B/Yam were more pronounced among the MS group (p = 0.014, p = 0.003, and p < 0.001, respectively).
No relapses or neurological deteriorations were reported within the observational period of 4 weeks after vaccination. Also, post-vaccination cMRI scans did not show any Gd-enhancing lesions. Compared with the most recent pre-immunization MRI scan (28.4 ± 18.8 weeks apart from BL), no new or enlarging lesions were found in 16 patients (80%). Four patients (20%) showed one or two new FLAIR hyperintense lesions as compared with pre-vaccine MRI (41 ± 12.6 weeks apart).
The primary safety variable was vaccine-associated increases in sNfL (Figure 2). Mean pre-vaccine NfL levels from MS patients were 7.64 pg/ml ( ± 2.67 pg/ml) and did not increase 4 weeks after immunization (7.5 ± 2.7 pg/ml). There were no significant differences in sNfL levels between pwMS and HCs (BL: 9.6 ± 4.9 pg/ml; follow-up: 10.7 ± 8.3 pg/ml) at either measuring point.
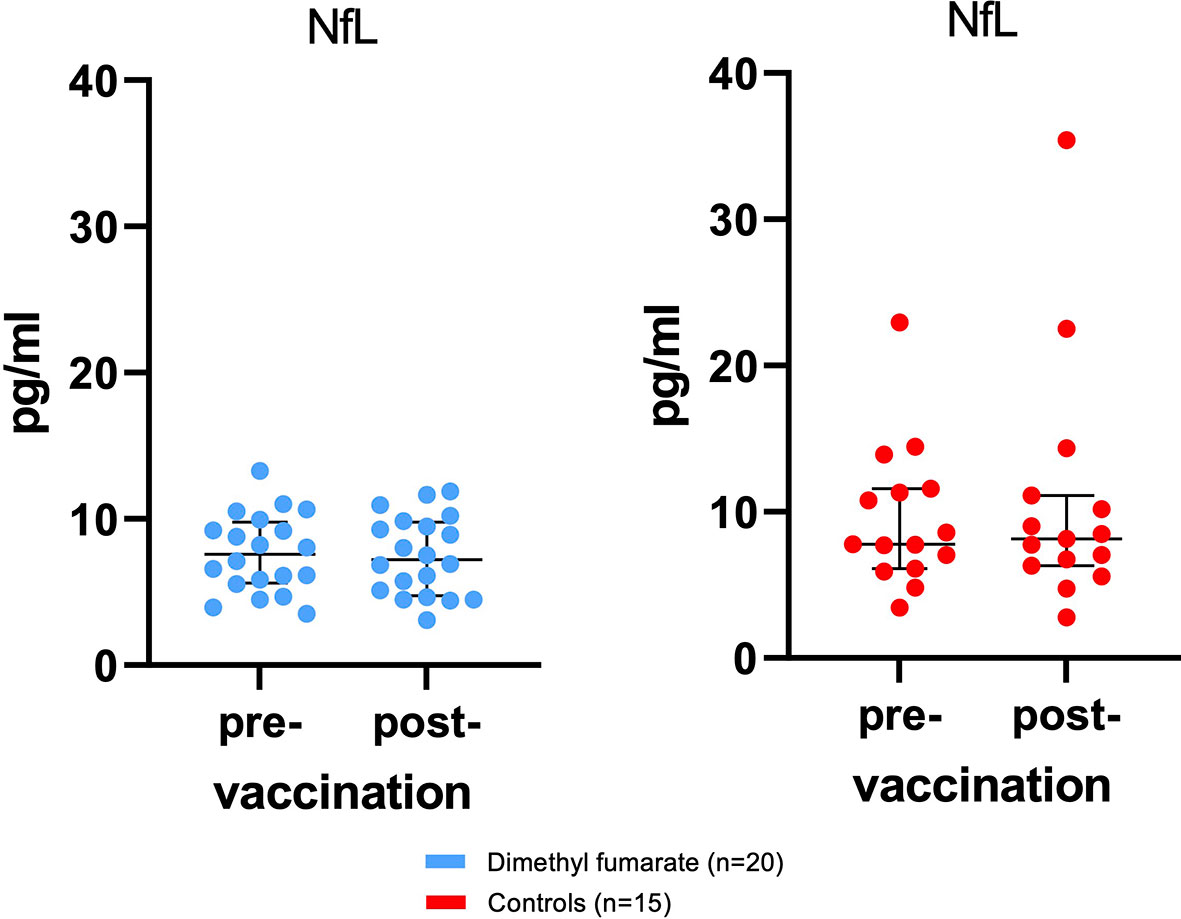
Figure 2 No subclinical disease activity as measured by serum neurofilament light chain (sNfL) associated with influenza vaccination was found. Displayed are sNfL values for patients and controls at baseline and 34.1 days ( ± 9.4) after immunization with influenza vaccine. Bars indicate median and interquartile range (IQR).
During the investigational period, two controls and one MS patient suffered from a COVID-19 infection (two SARS-CoV-2 PCR confirmed cases (one in each cohort) and one probable case without laboratory test among the HCs). NfL levels of the two controls increased above average by >10 pg/ml (from 11.3 to 22.5 pg/ml and from 22.9 to 35.4 pg/ml), while sNfL from the MS patient on DMF remained stable (from 6.1 to 5.1 pg/ml).
Additional laboratory parameters were assessed at BL and 4 weeks after immunization for the pwMS. No patient exhibited either Grade 3 or 4 lymphopenia, while Grade 2 lymphopenia was found in four patients (20%). CD19+ B cells were below the limit of normal in 5% (1/20). The courses of the main immune subsets and inflammatory parameters (interleukin 6 and CRP) are displayed in Figure 3.
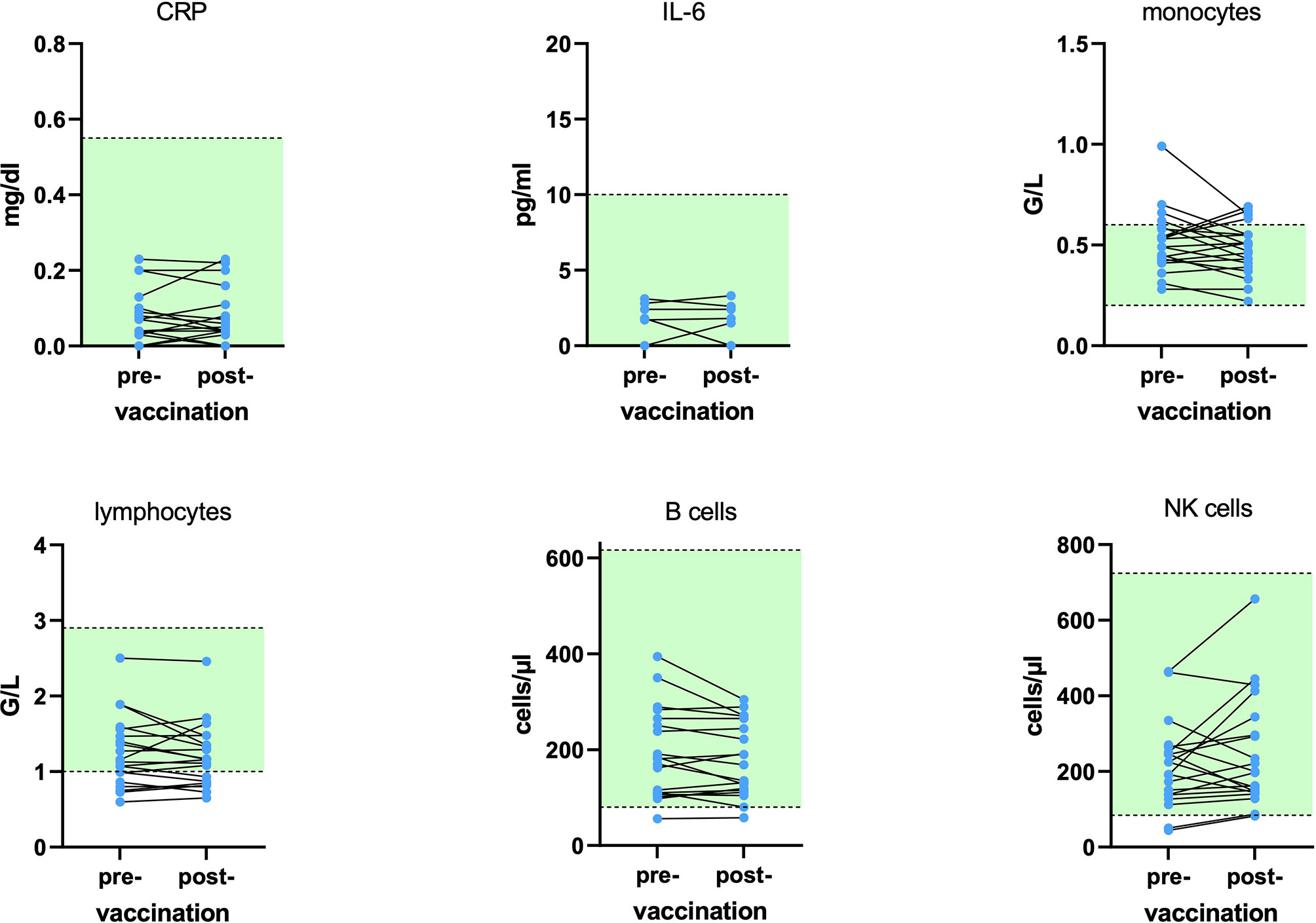
Figure 3 Course of inflammatory proteins and main immune cells at baseline and 29.1 ( ± 2.9) days after influenza vaccination for 20 patients on dimethyl fumarate (DMF). No statistically significant alterations were found. Green areas display the reference range. CRP, C-reactive protein; IL-6, interleukin 6; NK, natural killer.
There were no safety concerns for either cohort in this study. The seasonal influenza vaccine was well tolerated by all participants.
This pilot study assessing efficacy and safety of influenza vaccination in 20 MS patients treated with DMF reports on two key findings. First, despite its multiple immunomodulatory effects (26), DMF preserves humoral immune responses to specific viral antigens. In fact, we observed a 100% responder rate to tetravalent 2020/2021 influenza immunizations in DMF-treated patients, which was even higher than that of controls. This is likely influenced by the responder rate definition, since controls have had higher pre-vaccine seroprotection rates (53% vs. 41%). Across all strains, seroprotection increased to 93% and 90% for controls versus pwMS after immunization. Moreover, the period from vaccination to titer assessment was longer for controls. Less likely, the usage of two vaccines from different companies (both containing the same strains and doses) impacted on the outcome. In line with our results, vaccine efficacy to bacterial antigens was also shown to be adequate in DMF-treated pwMS (11). Together, the mode of action of DMF appears not to interfere with specific antibody production, assuming that immune responses to other vaccines, including COVID-19 mRNA and adenovirus vectors, to deliver immunization would be preserved. Unimpaired immune functions to control pathogens are also supported by the fact that DMF treatment, despite induction of lymphopenia, is not linked to an increased risk of infections (27, 28). In contrast to adequate humoral immune functions under DMF, diminished vaccine responses have been reported for several DMTs including fingolimod, glatiramer acetate, natalizumab, and CD20-depleting agents (29–31).
In accordance with prior studies, we confirm that seasonal influenza vaccination is not linked to clinical or radiological deteriorations in MS (12–16). In addition, our study is, to the best of our knowledge, the first to show that immunization is not associated with an increase in sNfL in DMF-treated pwMS or in HCs. This is crucial, as NfL represents a specific biomarker for neuro-axonal cell damage, able to detect even discreet, subclinical neuroinflammation leading to neuro-axonal injury. Moreover, NfL increase not only is restricted to axonal damage of the brain but also reflects pathology within the spinal cord (19). In contrast to the limited ability of MRI scans to detect ongoing (sub-)clinical inflammation of the gray matter and neuro-axonal degeneration, NfL can serve as a holistic biomarker for disease activity (19).
Our data support the rationale that vaccine-induced processes are restricted to the peripheral immune system without comprising the functionality of the blood–brain barrier and therefore not precipitating inflammation in the CNS of MS patients. This is crucial, as immune reactions associated with systemic infections appear to impact the integrity of the CNS, eventually resulting in MS exacerbations (6, 7). In fact, recent studies revealed elevated NfL levels during COVID-19, irrespective of the clinical course (32, 33). Moreover, increased NfL concentrations at the time of admission of COVID-19 patients were linked to a higher mortality risk (34). This is of interest as SARS-CoV-2 primarily affects the respiratory system, and a direct impact on neurons has not ultimately been clarified (35–37). In line with these reports, we found relevant increases in sNfL among two controls infected by SARS-CoV-2. Intriguingly, NfL from the DMF patient who also suffered from COVID-19 during the study period remained stable. To date, sNfL levels during systemic infections other than COVID-19 have not been extensively studied. However, the reports on NfL increases during COVID-19 together with our findings on safety and efficacy of vaccines argue that, particularly in light of the current pandemic, preventing infections by immunization should therefore be strongly considered in vulnerable populations like MS patients.
The small number of patients enrolled mainly limits our study, and the results must be interpreted in this context. Another limitation is that no specific BL MRI and no spinal cord images were available, with the most recent cMRI prior to vaccination being used for comparison. Also, the observational period of 4 weeks post-immunization appears short for clinical evaluations, but as sNfL assessment was the primary outcome parameter, the interval was considered appropriate. However, we cannot ultimately rule out disease reactivation after the follow-up period. Considering data from the literature, we strongly believe, that a) disease activity associated with vaccination, if any, would occur within 4 weeks and b) sNfL would increase within this period similar to early increases found in small vessel disease (38) and traumatic brain injuries (39–41). sNfL is currently considered the most appropriate serum biomarker for neuroaxonal damage, yet stable values cannot exclude neuroaxonal pathology with absolute certainty. Even though we found no clinical and serological evidence for neurological damages associated with immunization, safe administration of vaccines without any signs of induction of disease activity cannot ultimately be proven by our study design. Lastly, no patients with severe lymphopenia were included, and we can make no statement on the vaccine response in such patients.
In spite of the small number of participants and the limitations mentioned above, we conclude that the seasonal influenza immunization is effective and safe among MS patients treated with DMF.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethikkommission für das Bundesland Salzburg. The patients/participants provided their written informed consent to participate in this study.
TM and PW: design and concept of study, data collection and analysis, and drafting and revision of manuscript. MS, JF, LB, RR, CO’S, GP, AH, KS, EH-B, LM, JG, MR-F, AB, MK, EK, and ET: data collection, and drafting and revision of manuscript. All authors contributed to the article and approved the submitted version.
This study was financially supported by Biogen. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Author EK was employed by company Hermesoft. TM received travel support and honoraria for presentations or participation on advisory boards from Biogen Idec, Celgene, Novartis, Roche, Sanofi, Merck, and Teva. ET has received consultation fees and/or speakers honoraria from Arvelle, Argenix, Angellini, Bial, Biogen Idec, Boehringer Ingelheim, Eisai, Epilog, GL Pharma, GW Pharmaceuticals, Ever Pharma, Hikma, LivaNova, Marinus, Medtronics, Newbridge, Novartis, Sanofi, Genzyme, and UCB Pharma. MK has received speaker honoraria from Bayer, Novartis, Merck, Biogen Idec, and Teva Pharmaceutical Industries Ltd. and serves on scientific advisory boards for Biogen Idec, Merck Serono, Roche, Novartis, Bristol-Myers Squibb, and Gilead. He received research grants from Teva Pharmaceutical Industries Ltd., Biogen, and Novartis. AB was trained within the frame of the PhD Program Molecular Medicine of the Medical University of Graz. JF received travel support and honoraria for presentations from Biogen, Merck, Roche, and Sanofi. MS received travel support from Biogen, Merck, Bristol-Myers Squibb, Sanofi, Roche, Teva, and Novartis. PW has received consultation fees and/or speakers honoraria from Bayer, Biogen Idec, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi Genzyme, and Teva Pharmaceutical Industries Ltd. He received research grants from Biogen Idec and Merck. EK has received consultation fees from Astra Zeneca, Biogen, Bristol-Myers Squibb, Chiesi, Genzyme-Sanofi, Gilead, Glock, Merck, Novartis Pharma, Pfizer, Roche, Servier, and Vertex.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Greenwood B. The Contribution of Vaccination to Global Health: Past, Present and Future. Philos Trans R Soc Lond B Biol Sci (2014) 369(1645):20130433. doi: 10.1098/rstb.2013.0433
2. World Health Organization (WHO). Report of the SAGE Working Group on Vaccine Hesitancy. Vaccine Hesitancy (2014) 33:4161–64. doi: 10.1016/j.vaccine.2015.04.036
3. Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding Vaccine Hesitancy Around Vaccines and Vaccination From a Global Perspective: A Systematic Review of Published Literature, 2007-2012. Vaccine (2014) 32(19):2150–9. doi: 10.1016/j.vaccine.2014.01.081
4. Williamson EM, Berger JR. Infection Risk in Patients on Multiple Sclerosis Therapeutics. CNS Drugs (2015) 29(3):229–44. doi: 10.1007/s40263-015-0226-2
5. Wingerchuk DM, Carter JL. Multiple Sclerosis: Current and Emerging Disease-Modifying Therapies and Treatment Strategies. Mayo Clin Proc (2014) 89(2):225–40. doi: 10.1016/j.mayocp.2013.11.002
6. Marrodan M, Alessandro L, Farez MF, Correale J. The Role of Infections in Multiple Sclerosis. Mult Scler (2019) 25(7):891–901. doi: 10.1177/1352458518823940
7. Correale J, Fiol M, Gilmore W. The Risk of Relapses in Multiple Sclerosis During Systemic Infections. Neurology (2006) 67(4):652–9. doi: 10.1212/01.wnl.0000233834.09743.3b
8. Farez MF, Correale J, Armstrong MJ, Rae-Grant A, Gloss D, Donley D, et al. Practice Guideline Update Summary: Vaccine-Preventable Infections and Immunization in Multiple Sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology (2019) 93(13):584–94. doi: 10.1212/WNL.0000000000008157
9. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS Disease-Modifying Therapies on Responses to Vaccinations: A Review. Mult Scler Relat Disord (2020) 45:102439. doi: 10.1016/j.msard.2020.102439
10. Kern DM, Cepeda MS. Treatment Patterns and Comorbid Burden of Patients Newly Diagnosed With Multiple Sclerosis in the United States. BMC Neurol (2020) 20(1):296. doi: 10.1186/s12883-020-01882-2
11. von Hehn C, Howard J, Liu S, Meka V, Pultz J, Mehta D, et al. Immune Response to Vaccines Is Maintained in Patients Treated With Dimethyl Fumarate. Neurol Neuroimmunol Neuroinflamm (2018) 5(1):e409. doi: 10.1212/NXI.0000000000000409
12. Bamford CR, Sibley WA, Laguna JF. Swine Influenza Vaccination in Patients With Multiple Sclerosis. Arch Neurol (1978) 35(4):242–3. doi: 10.1001/archneur.1978.00500280060012
13. Myers LW, Ellison GW, Lucia M, Novom S, Holevoet M, Madden D, et al. Swine Influenza Virus Vaccination in Patients With Multiple Sclerosis. J Infect Dis (1977) 136 Suppl:S546–54. doi: 10.1093/infdis/136.Supplement_3.S546
14. Farez MF, Ysrraelit MC, Fiol M, Correale J. H1N1 Vaccination Does Not Increase Risk of Relapse in Multiple Sclerosis: A Self-Controlled Case-Series Study. Mult Scler (2012) 18(2):254–6. doi: 10.1177/1352458511417253
15. De Keyser J, Zwanikken C, Boon M. Effects of Influenza Vaccination and Influenza Illness on Exacerbations in Multiple Sclerosis. J Neurol Sci (1998) 159(1):51–3. doi: 10.1016/S0022-510X(98)00139-7
16. Miller AE, Morgante LA, Buchwald LY, Nutile SM, Coyle PK, Krupp LB, et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Influenza Immunization in Multiple Sclerosis. Neurology (1997) 48(2):312–4. doi: 10.1212/WNL.48.2.312
17. Salvetti M, Pisani A, Bastianello S, Millefiorini E, Buttinelli C, Pozzilli C. Clinical and MRI Assessment of Disease Activity in Patients With Multiple Sclerosis After Influenza Vaccination. J Neurol (1995) 242(3):143–6. doi: 10.1007/BF00936886
18. McNicholas N, Chataway J. Relapse Risk in Patients With Multiple Sclerosis After H1N1 Vaccination, With or Without Seasonal Influenza Vaccination. J Neurol (2011) 258(8):1545–7. doi: 10.1007/s00415-011-5944-x
19. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as Biomarkers in Neurological Disorders. Nat Rev Neurol (2018) 14(10):577–89. doi: 10.1038/s41582-018-0058-z
20. Siller N, Kuhle J, Muthuraman M, Barro C, Uphaus T, Groppa S, et al. Serum Neurofilament Light Chain Is a Biomarker of Acute and Chronic Neuronal Damage in Early Multiple Sclerosis. Mult Scler (2019) 25(5):678–86. doi: 10.1177/1352458518765666
21. Varhaug KN, Barro C, Bjornevik K, Myhr KM, Torkildsen O, Wergeland S, et al. Neurofilament Light Chain Predicts Disease Activity in Relapsing-Remitting MS. Neurol Neuroimmunol Neuroinflamm (2018) 5(1):e422. doi: 10.1212/NXI.0000000000000422
22. Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, et al. Serum Neurofilament Light: A Biomarker of Neuronal Damage in Multiple Sclerosis. Ann Neurol (2017) 81(6):857–70. doi: 10.1002/ana.24954
23. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the Mcdonald Criteria. Lancet Neurol (2018) 17(2):162–73. doi: 10.1016/S1474-4422(17)30470-2
24. European Agency for the Evaluation of Medicinal Products. Committee for Proprietary Medicinal Products: Note for Guidance on Harmonization of Requirements for Influenza Vaccines. London: European Agency for the Evaluation of Medicinal Products (1997).
25. Nauta JJ, Beyer WE, Osterhaus AD. On the Relationship Between Mean Antibody Level, Seroprotection and Clinical Protection From Influenza. Biologicals (2009) 37(4):216–21. doi: 10.1016/j.biologicals.2009.02.002
26. Moser T, Akgun K, Proschmann U, Sellner J, Ziemssen T. The Role of TH17 Cells in Multiple Sclerosis: Therapeutic Implications. Autoimmun Rev (2020) 19(10):102647. doi: 10.1016/j.autrev.2020.102647
27. Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. N Engl J Med (2012) 367(12):1098–107. doi: 10.1056/NEJMoa1114287
28. Gold R, Arnold DL, Bar-Or A, Fox RJ, Kappos L, Chen C, et al. Safety and Efficacy of Delayed-Release Dimethyl Fumarate in Patients With Relapsing-Remitting Multiple Sclerosis: 9 Years’ Follow-Up of DEFINE, CONFIRM, and ENDORSE. Ther Adv Neurol Disord (2020) 13:1756286420915005. doi: 10.1177/1756286420915005
29. Kappos L, Mehling M, Arroyo R, Izquierdo G, Selmaj K, Curovic-Perisic V, et al. Randomized Trial of Vaccination in Fingolimod-Treated Patients With Multiple Sclerosis. Neurology (2015) 84(9):872–9. doi: 10.1212/WNL.0000000000001302
30. Olberg HK, Cox RJ, Nostbakken JK, Aarseth JH, Vedeler CA, Myhr KM. Immunotherapies Influence the Influenza Vaccination Response in Multiple Sclerosis Patients: An Explorative Study. Mult Scler (2014) 20(8):1074–80. doi: 10.1177/1352458513513970
31. Bar-Or A, Calkwood JC, Chognot C, Evershed J, Fox EJ, Herman A, et al. Effect of Ocrelizumab on Vaccine Responses in Patients With Multiple Sclerosis: The VELOCE Study. Neurology (2020) 95(14):e1999–2008. doi: 10.1212/WNL.0000000000010380
32. Ameres M, Brandstetter S, Toncheva AA, Kabesch M, Leppert D, Kuhle J, et al. Association of Neuronal Injury Blood Marker Neurofilament Light Chain With Mild-to-Moderate COVID-19. J Neurol (2020) 267(12):3476–8. doi: 10.1007/s00415-020-10050-y
33. Sutter R, Hert L, De Marchis GM, Twerenbold R, Kappos L, Naegelin Y, et al. Serum Neurofilament Light Chain Levels in the Intensive Care Unit: Comparison Between Severely Ill Patients With and Without Coronavirus Disease 2019. Ann Neurol (2020) 89(3):610–16. doi: 10.1002/ana.26004
34. Aamodt AH, Hogestol EA, Popperud TH, Holter JC, Dyrhol-Riise AM, Tonby K, et al. Blood Neurofilament Light Concentration at Admittance: A Potential Prognostic Marker in COVID-19. J Neurol (2021) 20:1–10. doi: 10.1007/s00415-021-10517-6
35. Lapostolle F, Schneider E, Vianu I, Dollet G, Roche B, Berdah J, et al. Clinical Features of 1487 COVID-19 Patients With Outpatient Management in the Greater Paris: The COVID-Call Study. Intern Emerg Med (2020) 15(5):813–7. doi: 10.1007/s11739-020-02379-z
36. Kremer S, Lersy F, de Seze J, Ferre JC, Maamar A, Carsin-Nicol B, et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology (2020) 297(2):E242–51. doi: 10.1148/radiol.2020202222
37. Pajo AT, Espiritu AI, Apor A, Jamora RDG. Neuropathologic Findings of Patients With COVID-19: A Systematic Review. Neurol Sci (2021) 42(4):1255–66. doi: 10.1007/s10072-021-05068-7
38. Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, et al. Serum Neurofilament Light Is Sensitive to Active Cerebral Small Vessel Disease. Neurology (2017) 89(20):2108–14. doi: 10.1212/WNL.0000000000004645
39. Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum Neurofilament Light as a Biomarker for Mild Traumatic Brain Injury in Contact Sports. Neurology (2017) 88(19):1788–94. doi: 10.1212/WNL.0000000000003912
40. Wirsching A, Chen Z, Bevilacqua ZW, Huibregtse ME, Kawata K. Association of Acute Increase in Plasma Neurofilament Light With Repetitive Subconcussive Head Impacts: A Pilot Randomized Control Trial. J Neurotrauma (2019) 36(4):548–53. doi: 10.1089/neu.2018.5836
Keywords: vaccination, immunization, NfL, titers, influenza, COVID-19, antibody response
Citation: Moser T, Seiberl M, Feige J, Bieler L, Radlberger RF, O’Sullivan C, Pilz G, Harrer A, Schwenker K, Haschke-Becher E, Machegger L, Grimm J, Redlberger-Fritz M, Buchmann A, Khalil M, Kvas E, Trinka E and Wipfler P (2021) Tetravalent Influenza Vaccine Is Not Associated With Neuroaxonal Damage in Multiple Sclerosis Patients. Front. Immunol. 12:718895. doi: 10.3389/fimmu.2021.718895
Received: 01 June 2021; Accepted: 04 August 2021;
Published: 26 August 2021.
Edited by:
Anne L. Astier, INSERM UMR1291 – CNRS UMR5051 - Université Toulouse III, FranceReviewed by:
Marina Herwerth, University of Zurich, SwitzerlandCopyright © 2021 Moser, Seiberl, Feige, Bieler, Radlberger, O’Sullivan, Pilz, Harrer, Schwenker, Haschke-Becher, Machegger, Grimm, Redlberger-Fritz, Buchmann, Khalil, Kvas, Trinka and Wipfler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tobias Moser, dC5tb3NlckBzYWxrLmF0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.