
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 24 September 2021
Sec. Immunological Tolerance and Regulation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.718380
This article is part of the Research Topic The Placenta, Fetomaternal Tolerance and Beyond: A Tribute to Sir Peter Medawar on the 60th Anniversary of his Nobel Prize View all 24 articles
Estradiol (E2) and progesterone (P) have potent effects on immune function in the human uterine endometrium which is essential for creating an environment conducive for successful reproduction. Type III/lambda (λ) interferons (IFN) are implicated in immune defense of the placenta against viral pathogens, which occurs against the backdrop of high E2 and P levels. However, the effect of E2 and P in modulating the expression and function of IFNλ1 in the non-pregnant human uterine endometrium is unknown. We generated purified in vitro cultures of human uterine epithelial cells and stromal fibroblast cells recovered from hysterectomy specimens. Poly (I:C), a viral dsRNA mimic, potently increased secretion of IFNλ1 by both epithelial cells and fibroblasts. The secretion of IFNλ1 by epithelial cells significantly increased with increasing age following poly (I:C) stimulation. Stimulation of either cell type with E2 (5x10-8M) or P (1x10-7M) had no effect on expression or secretion of IFNλ1 either alone or in the presence of poly (I:C). E2 suppressed the IFNλ1-induced upregulation of the antiviral IFN-stimulated genes (ISGs) MxA, OAS2 and ISG15 in epithelial cells, but not fibroblasts. Estrogen receptor alpha (ERα) blockade using Raloxifene indicated that E2 mediated its inhibitory effects on ISG expression via ERα. In contrast to E2, P potentiated the upregulation of ISG15 in response to IFNλ1 but had no effect on MxA and OAS2 in epithelial cells. Our results demonstrate that the effects of E2 and P on IFNλ1-induced ISGs are cell-type specific. E2-mediated suppression, and selective P-mediated stimulation, of IFNλ1-induced ISG expression in uterine epithelial cells suggest that the effects of IFNλ1 varies with menstrual cycle stage, pregnancy, and menopausal status. The suppressive effect of E2 could be a potential mechanism by which ascending pathogens from the lower reproductive tract can infect the pregnant and non-pregnant endometrium.
Unique among mucosal sites, the immune system in the uterine endometrium has evolved to protect against incoming pathogens while creating an environment essential for successful reproduction. Key to this are the sex hormones estradiol (E2) and progesterone (P) whose concentrations change across the menstrual cycle, during pregnancy, and following menopause. The changing levels of hormones regulate multiple aspects of the innate and adaptive immune systems in the female reproductive tract (FRT), and particularly in the uterine endometrium, to allow for successful fertilization, implantation, and survival of a semi-allogeneic fetus (1). However, changes in immune function due to changing hormone levels are linked to altered susceptibility to sexually transmitted infections (STIs) (1), which can negatively impact reproduction.
Interferons (IFNs) encompass three different families of cytokines (I, II, and III) and are essential for preventing infections. The Type III IFNs are a family of antiviral cytokines consisting of IFNλ1, λ2, λ3 and λ4 (2–4) that are produced in response to pathogens. Previous studies have shown that stimulation of pattern recognition receptors (PRR) such as Toll-like receptor 3 (TLR3) and TLR9 increase the secretion of IFNλ1-3 in vitro (5–7). In turn, Type III IFNs exert their effects in an autocrine and paracrine fashion via a heterodimeric receptor complex consisting of IL28Rα (IFNLR1) and IL10Rβ (IL10R2) (2, 3). Similar to the Type I IFNs such as IFNβ, Type III IFN receptor activation initiates JAK/STAT signaling that upregulates expression of downstream IFN stimulated genes (ISG) such as Myxovirus A (MxA), Oligoadenylate Synthetase (OAS) 1-3, and ISG15. These ISGs can inhibit different stages of the viral lifecycle, thus creating an intracellular antiviral state hostile to pathogen survival. While previous studies have shown that sex hormones can modulate the secretion of, and sensitivity to, Type I IFNs in dendritic cells (8, 9), their effect on Type III IFNs is relatively unknown.
Type III IFNs function primarily at mucosal surfaces, in contrast to the Type I IFNs that induce antiviral responses throughout the body (10). For example, murine intestinal epithelial cells lacking functional Type III IFN receptors are more susceptible to rotavirus infection than wild-type mice (11). Type III IFNs increased the resistance of respiratory epithelial cells against influenza A virus and severe acute respiratory syndrome coronavirus, while IFNλ1 reduced infection of nasal epithelial cells by respiratory syncytial virus (12, 13). However, our understanding of Type III IFNs in the human FRT mucosa is sparse, with research focusing on the lower FRT (vagina and ectocervix). In the murine vagina, CD11c+ dendritic cells reduced Herpes Simplex Virus (HSV) 2 infection via the secretion of IFNλ1 (14), while induction of IFNλ1 in the End1/E6E7 human cervical epithelial cell line also inhibited HSV-2 (15). Differences in IFNλ1, IL28Rα, and IL10Rβ expression in human cervical epithelial cells is thought to be associated with low- versus high-risk Human Papilloma Virus (HPV) progression (16). Type III IFNs are also essential for the immune defense of the placenta against viral pathogens such as Zika Virus (ZIKV) (17, 18). Recognizing that the uterine endometrium is an anatomical compartment with a unique immunological environment that is distinct from the lower FRT (1, 19, 20), there exists a particular need to understand the contribution of Type III IFNs to innate immune protection at this unique site.
In this study we used IFNλ1 as model to study the effects of sex hormones and age on the secretion of, and sensitivity to, Type III IFNs by uterine epithelial cells and fibroblasts, the major non-hematopoietic cell types at the uterine mucosal surface. Both uterine cell types secrete IFNλ1 in response to the dsRNA viral agonist, poly (I:C), independent of E2 and P. However, E2 suppressed IFNλ1-induced upregulation of the antiviral genes OAS2 and ISG15 in epithelial cells via estrogen receptor alpha (ERα) signaling. P potentiated the upregulation of ISG15 by epithelial cells following treatment with IFNλ1 but had no effect on MxA and OAS2. In contrast, neither E2 nor P had any effect on IFNλ1-induced gene expression in stromal fibroblasts. These results demonstrate that the effects IFNλ1 vary by cell type and hormone exposure in the human uterine endometrium while also implicating E2 and P as regulators of IFNλ1 signaling.
Human uterine tissue was obtained from women (28-81 years old) undergoing hysterectomy surgery at Dartmouth-Hitchcock Medical Center (Lebanon, NH). Reasons for surgery were menorrhagia, adenomyosis, adnexal mass, fibroids and prolapse. All tissues used in this study were distal to the sites of pathology and were determined to be unaffected with disease upon inspection by a pathologist. Tissues were excluded under conditions of cancer. All investigations involving human subjects were conducted according to the principles expressed in the Declaration of Helsinki and carried out with the approval from the Committee for the Protection of Human Subjects (CPHS), Dartmouth Hitchcock Medical Center, and with written informed consent obtained from the patients before surgery.
Tissues were minced under sterile conditions into 1 to 2mm fragments and subjected to enzymatic digestion using an enzyme mixture containing 0.05% collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 0.01% DNAse (Worthington Biochemical, Lakewood, NJ) in 1x HBSS (Invitrogen). After enzymatic digestion for 1 hr at 37°C, cells were dispersed through a 250-µm mesh screen, washed, and resuspended in Hank’s Balanced Salt Solution (ThermoScientific, Logan, UT).
Epithelial cell sheets were separated from stromal fibroblasts by filtration through a 20-µm nylon mesh filter (Small Parts, Miami Lakes, FL). Epithelial sheets were retained on the 20-µm filter, while the stromal fraction containing the fibroblasts passed through and were collected as part of the filtrate. Epithelial sheets were recovered by rinsing and backwashing the filter with Complete medium, centrifuged (500 x g, 10 min), and analyzed for cell number and viability.
To establish an in vitro cell culture system of polarized human uterine epithelial cells, uterine epithelial cells were cultured in Falcon cell culture inserts coated with Human Extracellular Matrix (Becton Dickinson, Franklin Lakes, NJ) in 24-well culture plates (Fisher Scientific, Pittsburgh, PA). Apical and basolateral compartments had 300 and 500 µl of complete medium respectively. Complete medium consisted of DMEM/F12 supplemented with 20 mM HEPES (Invitrogen), 2 mM L-glutamine (Invitrogen), 50 mg/ml primocin (Invivogen) and 10% heat-inactivated defined Fetal Bovine Serum (FBS) (ThermoScientific). Incubation media was changed every 2 days.
To establish a purified population of uterine stromal fibroblasts, the stromal filtrate was centrifuged (500 x g, 10 min) and the pellet resuspended in complete media. Cells were placed in culture in a 75 cm2 cell culture flask (Fisher Scientific) in complete medium until they reached confluence. The medium was changed every 2 days. After reaching confluence, cells were trypsinized and 1x106 cells added to a fresh 75 cm2 flask. This was repeated at least twice, after which cells were recovered and plated (1x105 cells/well) in 24-well cell culture dishes (Fisher) in 500 μl of complete medium with charcoal dextran-stripped FBS for at least 48 hrs prior to treatment. Sequential passaging allows the fibroblasts to outcompete any other cell types present in the original stromal filtrate, leaving a population of fibroblasts that are positive for the stromal fibroblast markers vimentin and CD90, and negative for the hematopoietic cell marker CD45 as previously described (21, 22).
The ECC-1 cell line (originally established by Dr. Pondichery Satyaswaroop and kindly provided by George Olt, Penn State College of Medicine, Milton S Hershey Medical Center, PA) is a human uterine epithelial cell line, characteristic of the luminal uterine epithelium, that is responsive to sex hormones (23) and is used by us to study innate immune responses of FRT epithelial cells. To establish a culture system of polarized human ECC-1 cells with both apical and basolateral compartments, ECC-1 cells were cultured in uncoated Falcon cell culture inserts in 24-well culture dishes (Fisher). Apical and basolateral compartments had 300 and 500 μl of complete medium respectively as described above for primary cells.
As an indicator of tight junction formation and polarization of uterine epithelial cell monolayers, transepithelial resistance was assessed every 48 hrs using an EVOM electrode and Voltohmmeter (World Precision Instruments Inc., Sarasota, FL) as described previously (24). Matrigel-coated inserts without any cells were used as controls and had transepithelial resistance values of 200-240 ohms.
Cells were stimulated with HMW-poly (I:C) (Invitrogen) at 0.25-25 µg/ml in complete media with 10% defined FBS, except as noted below, for up to 24 hrs. Recombinant human IFNλ1 (PBL Assay Science, Piscataway, NJ) was used at 1-1000 ng/ml in complete media with 10% charcoal dextran-stripped FBS (Gemini, West Sacramento, CA) for up to 24 hrs. For polarized primary epithelial and ECC-1 cells, poly (I:C) was added to the apical compartment only, while IFNλ1 was added to both the apical and basolateral compartments.
Type I IFN receptor blockade experiments used a mouse monoclonal anti-human interferon receptor 2 (IFNAR2) blocking antibody (R&D) or a matched IgG2A isotype control at a final concentration of 10μg/ml for 1 hr and then stimulated with poly (I:C) for 24 hrs (25). Type III IFN receptor blockade experiments used a mouse monoclonal anti-human αIL10Rβ blocking antibody (R&D). In all experiments, blocking antibody was maintained in the culture media (complete media with 10% charcoal dextran-stripped FBS) throughout the experiment.
For hormone experiments, E2, P, or the estrogen receptor (ER) antagonist Raloxifene (Rx) (Calbiochem, Gibbstown, NJ) were dissolved in 100% ethanol for an initial concentration of 1x10-3M, evaporated to dryness and resuspended in complete media containing charcoal dextran-stripped FBS to a concentration of 1x10-5M, as previously described (21, 25, 26). Further dilutions were made to achieve final working concentrations of E2 ranging from 5x10-8M to 5x10-10M. P was used at 1x10-7M. As a control, an equivalent amount of 100% ethanol without dissolved hormone was initially evaporated. For polarized uterine epithelial cells and ECC-1 cells, E2 or P was added to both the apical and basolateral compartments. For ER blockade experiments, cells were pretreated with Raloxifene, a selective estrogen response modulator that antagonizes estrogen effects in uterine cells (27) and has been used by us in other studies (28), at 5x10-6M (100-fold excess) for at least 1 hr prior to the introduction of E2. Raloxifene was maintained in the cell culture media throughout the experiment.
For hormone experiments, and receptor-blockade experiments, cells were transferred from complete medium with 10% defined FBS to complete medium with 10% charcoal dextran-stripped FBS for at least 48 hrs prior to hormone treatment to remove the influence of any steroid hormones present in defined FBS that could otherwise confound the effects of exogenous E2 or P.
Total mRNA was isolated and purified using a RNeasy mini kit (Qiagen, Valencia, CA) with on-column DNase digestion using the RNase-Free DNase set (Qiagen) according to the manufacturer’s recommendations. 400ng of total RNA was reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s recommendations. Relative mRNA expression levels of IFNλ1 (Hs00601677_g1), MxA (Hs00895608_m1), OAS2 (Hs00942643_m1), ISG15 (Hs01921425_m1), IL28Rα (Hs00417120_m1), and IL10Rβ (Hs00175123_m1) were measured using the 5’ fluorogenic nuclease assay in real-time quantitative PCR using TaqMan chemistry on the ABI 7300 Prism real-time PCR instrument (Applied Biosystems, Carlsbad, CA). PCR was conducted using the following cycle parameters: 50°C, 2 mins, 1 cycle; 95°C, 10 mins, 1 cycle; 95°C, 15 s, 40 cycles; 60°C, 1 min, 1 cycle. Analysis was conducted using the sequence detection software supplied with the ABI 7300. In several patients, the expression of IFNλ1 was undetectable in untreated samples. In these cases, the expression of IFNλ1 was assigned a Ct value of 40, which is the lowest possible value in our system, for the purposes of calculating the fold-increase in IFNλ1 mRNA expression following treatment with poly (I:C).
Data are presented either as relative expression normalized to β-Actin, or as fold-change in mRNA expression. When presented as relative expression, the expression of the gene of interest is normalized to the expression of housekeeping gene β-Actin. Data are also presented as fold change in mRNA expression, where fold change refers to the change in expression of a gene of interest between the control and treatment group(s) which are both normalized to β-Actin expression and calculated using the formula t. When expressing our data as fold change, the control or untreated group is set to 1. For the purposes of clarity, in some cases the control group is absent from the fold change figures. However, this is noted in both y-axis legends and the figure legends.
Following treatment, conditioned media were recovered from epithelial cells (apical and basolateral) and fibroblasts and centrifuged at 10,000 x g in a microfuge. Supernatants were aliquoted and stored at -80°C. ELISA was used to determine the secretion of IFNλ1, CCL4, elafin, CCL20, RANTES (all R&D Systems, Minneapolis, MN), and HBD2 (Peprotech, Cranbury, NJ) by uterine epithelial cells and fibroblasts according to the manufacturer’s instructions.
Data analysis was performed using the GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). A two-sided P value <0.05 was considered statistically significant. Comparison of treatment groups vs. control group was performed applying Mann-Whitney U test for non-matched samples or Wilcoxon matched-pairs signed rank test for matched samples. Comparison of three or more groups was performed applying Kruskal-Wallis test for non-matched samples or Friedman test for matched samples, followed by Dunns-post test for multiple comparison correction. Correlations of age with protein secretion or mRNA expression were performed using the nonparametric Spearman correlation with a two-tailed p-value. Best-fit trend lines were generated using a simple linear regression.
We have previously shown that stimulation of FRT epithelial cells and stromal fibroblasts with the synthetic dsRNA viral ligand poly (I:C) induces a potent innate immune response (21, 22, 25, 26, 29, 30). However, whether IFNλ1 is part of the innate immune response of uterine epithelial cells and fibroblasts in response to viral pathogens is unknown. As part of our initial studies, we used uterine ECC-1 epithelial cells. This uterine epithelial cell line, which we have used extensively, displays some of the innate immune characteristics of primary uterine epithelial cells, including polarization and recognition of poly (I:C) (25, 30). We treated polarized uterine ECC-1 epithelial cells with multiple doses of poly (I:C) (0.25, 2.5 & 25 μg/ml) for 3-24 hrs. Poly (I:C) dose-dependently increased the expression of IFNλ1 mRNA in ECC-1 cells with maximum levels reached between 12-24 hrs after stimulation with 25μg/ml (Figure 1A). Based upon these results, we treated primary uterine epithelial cells with poly (I:C) for 24 hrs. Following 24 hrs of poly (I:C) exposure, IFNλ1 mRNA expression was significantly upregulated by an average of 790-fold (Figure 1B) and IFNλ1 secretion in the apical compartment by approximately 4500 pg/ml (Figure 1C). Overall, constitutive apical IFNλ1 protein secretion by untreated samples was low with an average of 20.5 pg/ml. However, in many patient samples there was no detectable IFNλ1 secretion by untreated samples. In contrast to the significantly increased secretion of IFNλ1 in the apical compartment, there was no change in the levels of IFNλ1 in the basolateral compartment following poly (I:C) treatment (Figure 1D). There was a wide range in the increased expression and secretion of IFNλ1 between different patient samples following poly (I:C) exposure. For example, the fold change in epithelial IFNλ1 mRNA expression ranged between 0.04- to 4800-fold after 24 hrs of poly (I:C) treatment (Figure 1B) while the increased secretion of IFNλ1 ranged between 120-12300 pg/ml (Figure 1C).
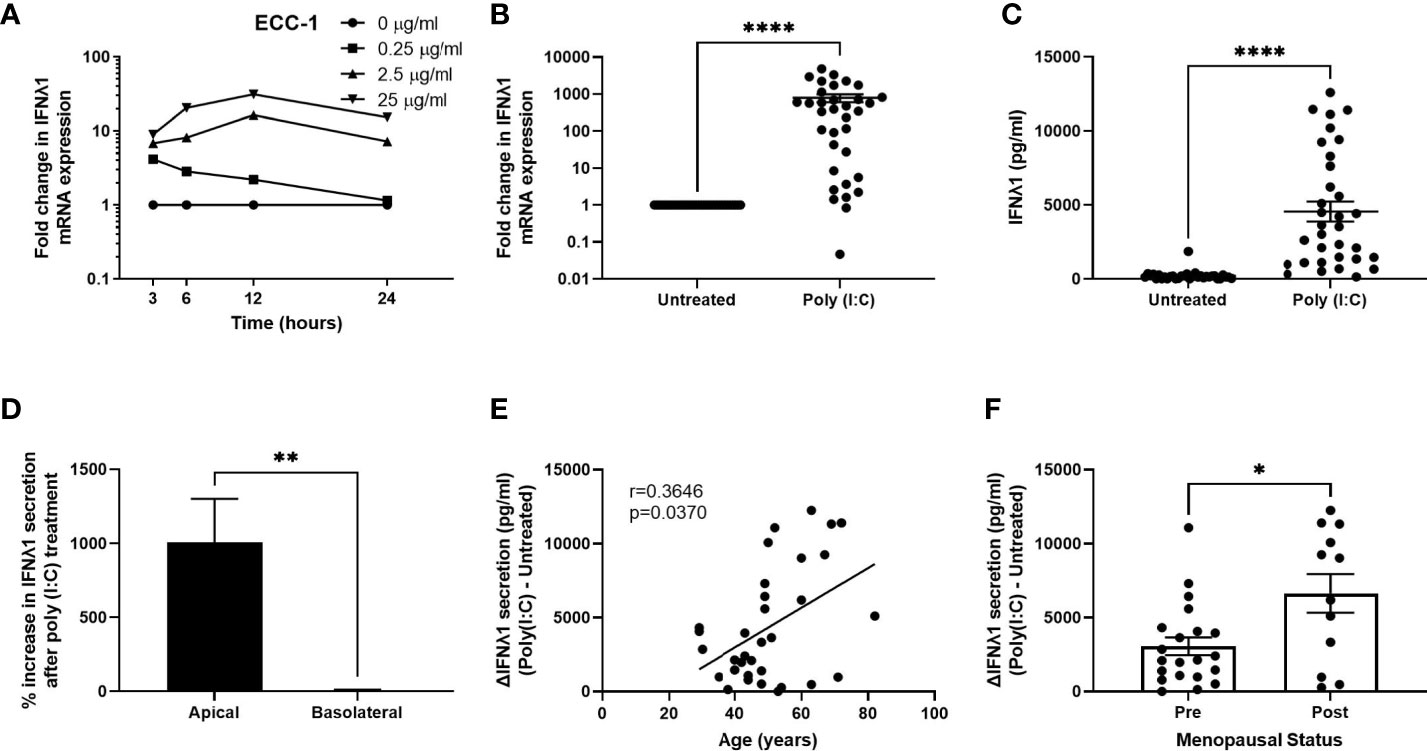
Figure 1 Poly (I:C) induces IFNλ1 expression and secretion by uterine epithelial cells. (A) ECC-1 uterine epithelial cells were stimulated with poly (I:C) for 3-24 hrs and IFNλ1 expression measured by RT-PCR. (B, C) Primary uterine epithelial cells were stimulated with poly (I:C) (25μg/ml) for 24 hrs prior to analysis of mRNA expression by real-time RT-PCR (B) and protein secretion (C) by ELISA. Each circle represents an individual patient (n = 34). (D) Percent increase in IFNλ1 secretion in the apical and basolateral compartments of transwell inserts following treatment of uterine epithelial cells with poly (I:C) (25μg/ml) for 24hrs versus untreated wells (n = 34). (E, F) Values for IFNλ1 secretion from panel E by poly (IC)-treated epithelial cells were subtracted from untreated wells to determine the difference in IFNλ1 secretion between matched poly (I:C)-treated and untreated wells (ΔIFNλ1 pg/ml) which was then plotted against patient age (E) (n = 34) or menopausal status (F) (n = 34). Each circle represents an individual patient. Data is shown as mean +/- SEM. Wilcoxon matched-pairs signed rank test (B–D). Non-parametric Spearman correlation analysis (E). Mann-Whitney non-parametric t-test (F). *p < 0.05; **p < 0.01; ****p < 0.0001.
We then calculated the difference in IFNλ1 secretion in the apical compartment between untreated cells and poly (I:C)-treated wells (ΔIFNλ1) and stratified the resulting values by patient age. As seen in Figure 1E, the poly (I:C)-induced secretion of IFNλ1 significantly increases with increasing age suggesting that the contribution of IFNλ1 to innate immune protection increases with age. We then stratified the results based on menopausal status. Consistent with our results in Figure 1E, we found that poly (I:C)-induced secretion of IFNλ1 was significantly higher by epithelial cells recovered from post-menopausal women compared to pre-menopausal women (Figure 1F).
We have previously shown that fibroblasts from throughout the FRT are capable of mounting a potent innate immune response following exposure to viral mimics such as poly (I:C) (21, 22, 26). To determine whether IFNλ1 was a part of this innate response, we stimulated primary uterine stromal fibroblasts with poly (I:C) for 24 hrs after which we measured IFNλ1 expression and secretion. There was a significant increase in IFNλ1 mRNA expression by an average of 5247-fold (Figure 2A) and secretion by 1390 pg/ml (Figure 2B) after 24 hrs. Similar to epithelial cells, constitutive IFNλ1 protein secretion by untreated samples was low, with no detectable IFNλ1 secretion in many samples. Furthermore, there was a wide range in both IFNλ1 mRNA expression (1014-13083-fold increase) and protein secretion (0-4832 pg/ml) following treatment with poly (I:C). However, in contrast to epithelial cells (Figure 1E), there was no effect of age on poly (I:C) induced secretion of IFNλ1 by fibroblasts (Figure 2C).
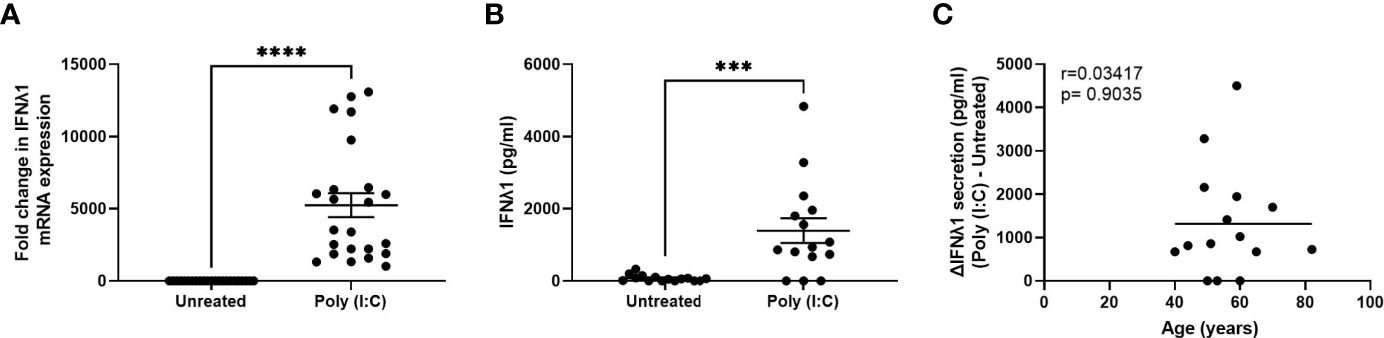
Figure 2 Poly (I:C) induces IFNλ1 expression and secretion by uterine stromal fibroblasts. Primary uterine fibroblasts were stimulated with poly (I:C) (25 μg/ml) for 24 hrs followed by analysis of (A) mRNA expression by real-time RT-PCR (n = 23) and (B) protein secretion by ELISA (n = 15). Each circle represents an individual patient. (C) Values for IFNλ1 secretion from panel B by poly (IC)-treated fibroblasts were subtracted from untreated wells to determine the difference in IFNλ1 secretion between matched poly (I:C)-treated and untreated wells (ΔIFNλ1 pg/ml) which was then plotted against patient age (n = 15). Data is shown as mean +/- SEM. Wilcoxon matched-pairs signed rank test (A, B). Non-parametric Spearman correlation analysis (C). ***p < 0.001; ****p < 0.0001.
Previous studies have reported that Type I IFNs, such as IFNβ, can regulate Type III IFN expression (31). To investigate the potential role of IFNβ in regulating IFNλ1 in our system, ECC-1 cells were treated with recombinant human IFNβ for 24 hrs. IFNβ dose-dependently induced expression of IFNλ1, which was inhibited in the presence of an IFNβ neutralizing antibody (Figure 3A). Primary uterine epithelial cells or fibroblasts were then pre-treated with αIFNAR2, a blocking antibody directed against the IFNAR2 subunit of the heterodimeric Type I IFN receptor complex. When stimulated with poly (I:C), αIFNAR2 partially inhibited the upregulation of IFNλ1 secretion in both epithelial cells (Figure 3B) and fibroblasts (Figure 3C). This suggests that IFNλ1 expression in response to incoming poly (I:C) and viral stimuli is mediated partly via the Type I IFNs.
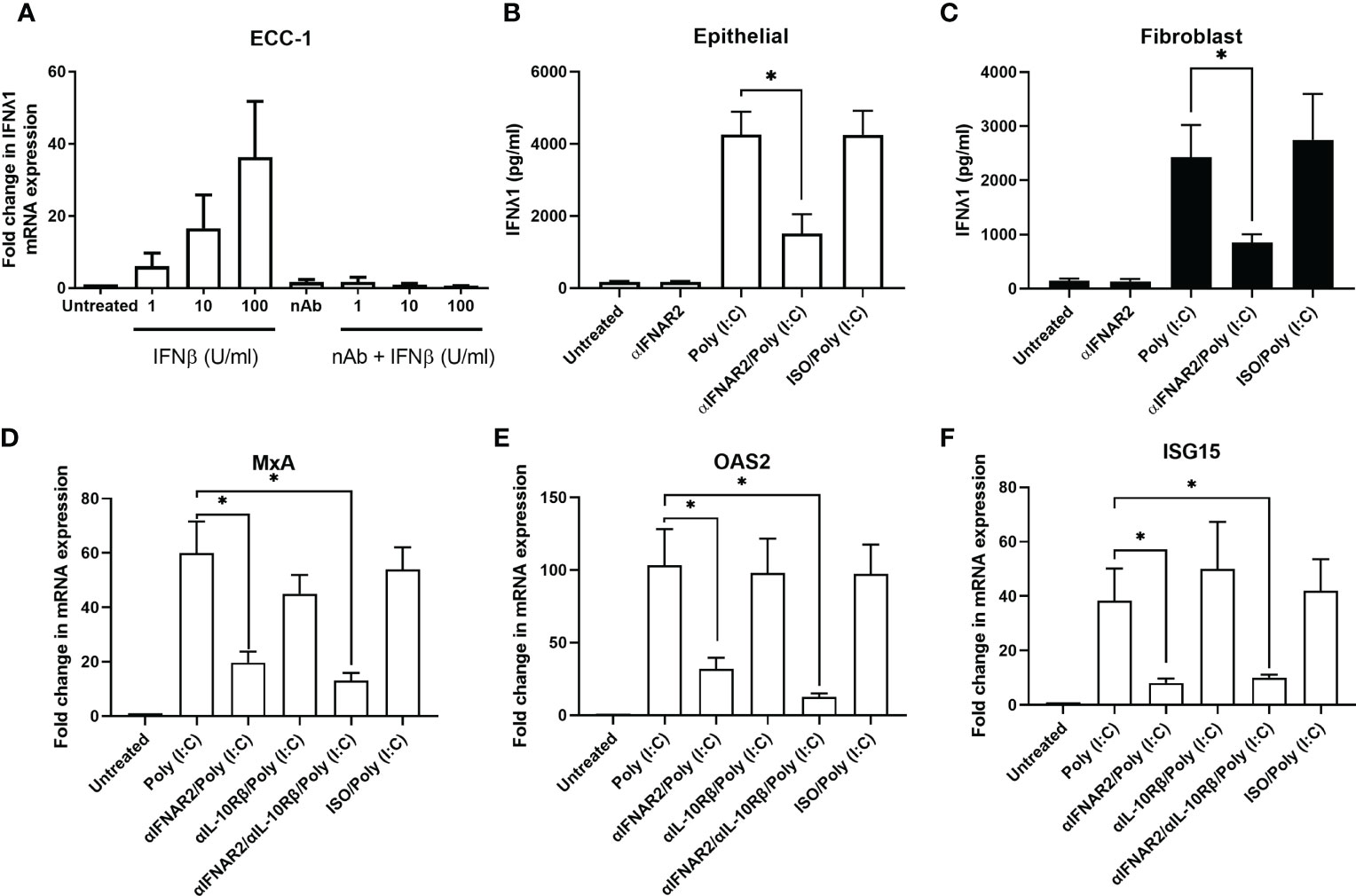
Figure 3 Blockade of Type I IFN signaling inhibits IFNλ1 secretions and signaling by uterine epithelial cells. (A) ECC-1 uterine epithelial cells were treated with recombinant human IFNβ for 24 hrs in the presence or absence of IFNβ neutralizing antibody after which IFNλ1 mRNA expression was determined (n = 3). (B) Uterine epithelial cells (n = 6) and (C) fibroblasts (n = 6) were pre-treated with a blocking antibody against IFNAR2 (αIFNAR2) or an isotype control (αISO) for 1 hr prior to the introduction of poly (I:C) (25 μg/ml) for 24 hrs, during which αIFNAR2 and αISO were maintained in the culture media. IFNλ1 secretion by epithelial cells (B) and fibroblasts (C) were determined by ELISA. Data is shown as mean IFNλ1 (pg/ml) +/- SEM. (D) Primary human uterine (n = 6) epithelial cells were pretreated with αIFNAR2, αIL-10Rβ, or a combination of both prior to treatment poly (I:C) for 24 hrs, after which mRNA expression of MxA (D), OAS2 (E), and ISG15 (F) was determined by RT-PCR. Data is shown as mean +/- SEM. Wilcoxon matched-pairs signed rank test (B–F). *p < 0.05.
Building upon our past studies demonstrating that uterine epithelial cells upregulate ISGs in response to poly (I:C) (25), we investigated the contribution of IFNλ1 to this antiviral response by using a blocking antibody against IL10Rβ, one half of the heterodimeric IFNλ1 receptor complex. Primary uterine epithelial cells were pre-treated with αIL10Rb, αIFNAR2, or a combination of both prior to treatment with poly (I:C) for 24hrs after which MxA, OAS2, and ISG15 expression levels were measured. As seen in Figures 3D–F, blockade of Type I IFN signaling significantly decreased the expression of MxA, OAS2, and ISG15. However, blockade of Type III IFN signaling had no effect on MxA and OAS2 expression levels. When both Type I and Type III IFN signaling was blocked, expression of MxA, OAS2, and ISG15 was no different than Type I IFN alone. Together this suggests that Type I IFNs are the primary mediators of ISG expression by epithelial cells following exposure to poly (I:C).
Previous studies have shown that E2 can upregulate the secretion of secretory leukocyte protease inhibitor (SLPI) and inhibit IL-1β-mediated mRNA expression and secretion of human β-defensin-2 and CXCL8 by primary uterine epithelial cells (19). To determine if E2 regulates IFNλ1 secretion, we treated uterine epithelial cells, fibroblasts, and ECC-1 cells with E2 (5x10-8M – 5x10-10M) for up to 72 hrs. E2 did not alter the expression or secretion of IFNλ1 compared to untreated cells (data not shown). Pretreatment of uterine epithelial cells or stromal fibroblasts with E2 (5x10-8M) for 48 hrs followed by poly (I:C) stimulation also had no effect on IFNλ1 secretion by either cell type (Figures 4A, B). Similarly, we found that P (1x10-7M) either alone or in combination with poly (I:C) had no effect on the expression or secretion of IFNλ1 by epithelial cells and fibroblasts (Figures 4C, D). Together these studies indicate that sex hormones do not modulate the secretion of IFNλ1 by epithelial cells and fibroblasts in the uterine endometrium following exposure to viral pathogens.
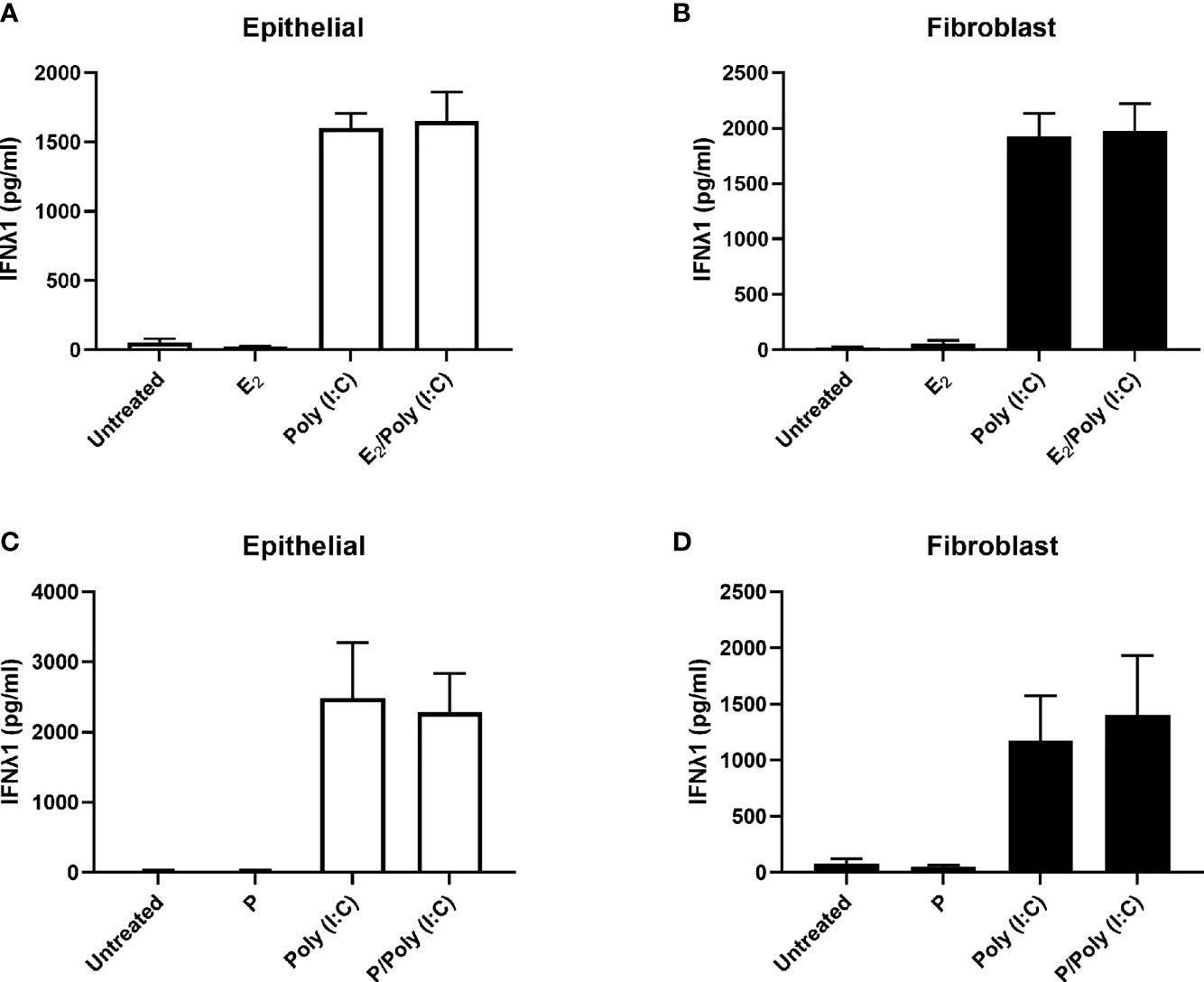
Figure 4 IFNλ1 secretion by uterine epithelial cells and uterine fibroblasts in response to poly (I:C) is independent of estradiol (E2) and progesterone (P). Primary human uterine epithelial cells (A, C) or uterine fibroblasts (B, D) were pretreated with E2 (5x10-8M) (A, B) or P (1x10-7M) (C, D) for 48 hrs after which media was replenished in the presence or absence of poly (I:C) (25 μg/ml) for a further 24 hrs. During this period E2 or P was maintained in the culture media. IFNλ1 secretion was determined by ELISA. Data is shown as mean +/- SEM. n = 3 (A, B). n = 6 (C, D).
Key to IFNλ1 sensitivity is the expression of its heterodimeric receptor complex composed of IL28Rα (IFNLR1) and IL10Rβ (IL10R2). While IL28Rα is exclusively used by the Type III IFNs, IL10Rβ is shared with other members of the IL10 family (3). Therefore, we hypothesized that IL28Rα expression would be lower than IL10Rβ. Analysis of mRNA obtained from uterine epithelial cells demonstrated that they express IL28Rα and IL10Rβ, with IL10Rβ being expressed at significantly higher levels than IL28Rα (Figure 5A), with no effect of age on the expression of either receptor (Figures 5B, C), suggesting that uterine epithelial cells can respond to IFNλ1 present in the extracellular environment. To determine if poly(I:C) could upregulate the expression of either subunit in epithelial cells as part of the antiviral response, we measured the expression of IL28Rα and IL10Rβ following 24 hrs of stimulation with poly (I:C). As seen in Figure 5D, poly (I:C) had no effect on expression of either subunit by epithelial cells.
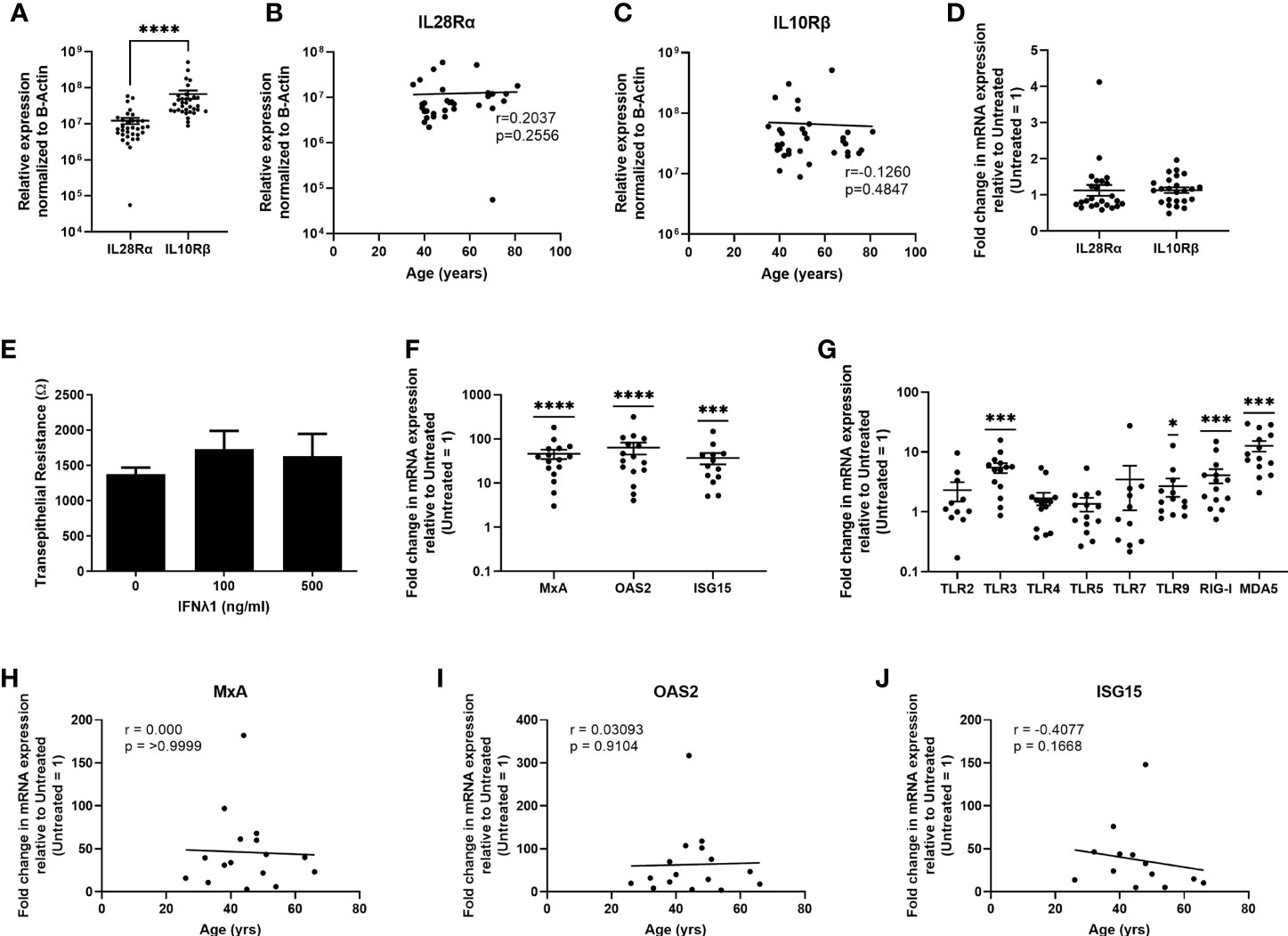
Figure 5 Uterine epithelial cells express Type III IFN receptors and respond to IFNλ1 by upregulating ISGs and pattern recognition receptors. (A) mRNA from uterine epithelial cells was recovered and analyzed for relative expression of IL28Rα and IL10Rβ normalized to β-Actin. Each circle represents an individual patient (n = 33). Values for IL28Rα (B) and IL10Rβ (C) from panel A were plotted against patient age. Each circle represents an individual patient (n = 33). (D) Fold change in mRNA expression of IL28Rα and IL10Rβ following stimulation of primary uterine epithelial cells with poly (I:C) (25ug/ml) for 24 hrs. Each circle represents an individual patient (n = 24). (E) Transepithelial resistance of primary uterine epithelial cells following dose-response of IFNλ1 (100, 500 ng/ml) stimulation for 24 hrs. (F) mRNA expression of MxA, OAS2, and ISG15 by primary uterine epithelial cells following treatment with IFNλ1 (500 ng/ml) for 24 hrs. Untreated control = 1. Each circle represents an individual patient (n = 13-16) (G) mRNA expression of TLR2, TLR3, TLR4, TLR5, TLR7, TLR9, RIG-I, and MDA5 by primary uterine epithelial cells following treatment with IFNλ1 (500 ng/ml) for 24 hrs. Untreated control = 1. Each circle represents an individual patient (n = 14). (H–J) Values for MxA, OAS2, and ISG15 mRNA expression from panel (F) were plotted against patient age. Each circle represents an individual patient (n = 13-16). mRNA expression data in panels (A–C) is normalized to the housekeeping gene β-Actin. mRNA expression data in panels (D–I) is normalized to the housekeeping gene β-Actin, and then further normalized to the untreated control whose value is then set to 1. Data is shown as mean +/- SEM. Each circle represents an individual patient. Wilcoxon matched-pairs signed rank test (A). Non-parametric Spearman correlation analysis (B, C, H–J). One-sample non-parametric Wilcoxon test (F, G) *p < 0.05; ***p < 0.001; ****p < 0.0001.
To determine if epithelial cells respond to IFNλ1, we stimulated ECC-1 cells for 24 hrs IFNλ1 (1-1000ng/ml) and measured ISG expression (Supplementary Figure 1A). Maximal upregulation of ISG expression was at 1000 ng/ml of IFNλ1 with MxA, OAS2 and ISG15 increasing by approximately 3-, 18-, and 8-fold respectively. There was also no effect of IFNλ1 on barrier function of ECC-1 cells as measured by transepithelial resistance (Supplementary Figure 1B). Building on our ECC-1 findings, we stimulated primary uterine epithelial cells with IFNλ1 (500ng/ml) for 24 hrs. Similar to ECC-1 cells, IFNλ1 had no effect on epithelial barrier function as measured by transepithelial resistance (Figure 5E). We then measured the expression of MxA, OAS2, and ISG15. As shown in Figure 5F, uterine epithelial cells significantly upregulated MxA, OAS2 and ISG15 by an average of approximately 46-, 63- and 37-fold respectively. We also measured the effect of IFNλ1 on the expression of pattern recognition receptors (PRRs) which are essential for the detection of incoming viral, bacterial, and fungal pathogens. IFNλ1 significantly upregulated expression of the viral PRRs TLR3, TLR9, RIG-I and MDA5 by approximately 5-, 3-, 4- and 13-fold respectively (Figure 5G), but had no effect on the expression of TLR2, TLR4, TLR5, and TLR7. As seen in Figures 5H–J, IFNλ1 had no effect on MxA, OAS2 or ISG15 expression with increasing age.
Similar to the epithelial cells, uterine stromal fibroblasts expressed IL28Rα and IL10Rβ, with significantly higher mRNA levels of IL10Rβ compared to IL28Rα (Figure 6A) and no effect of age on their expression (Figures 6B, C). When compared to epithelial cells, fibroblasts expressed significantly lower levels of both subunits (Figure 6D). Poly (I:C) had no effect on the expression of either subunit (Figure 6E). Following stimulation with IFNλ1 (500ng/ml) for 24 hrs, fibroblasts significantly upregulated expression of MxA, OAS2, and ISG15. However, in comparison to epithelial cells, fibroblasts were weakly sensitive to the presence of IFNλ1 (500ng/ml), with MxA, OAS2 and ISG15 upregulation by 6.3-, 4.7-, and 3.1-fold (Figure 6F). Similarly, fibroblasts only weakly upregulated TLR3, TLR9, RIG-I and MDA5 (Figure 6H). As shown in Figures 6H–J, IFNλ1 had no effect on MxA, OAS2 or ISG15 upregulation in stromal fibroblasts with increasing age.
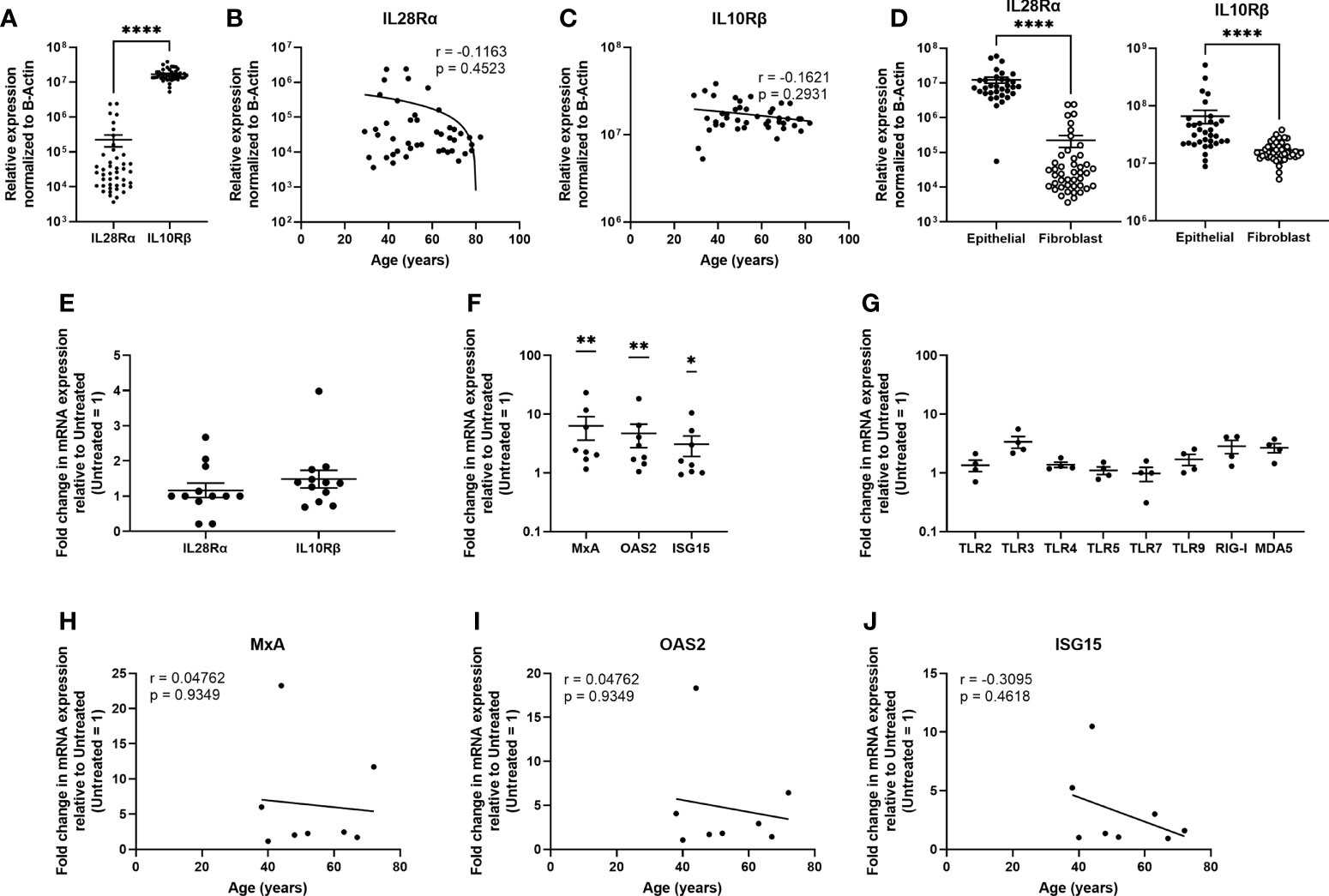
Figure 6 Uterine stromal fibroblasts express Type III IFN receptors and respond to IFNλ1 by upregulating ISGs and pattern recognition receptors. (A) mRNA from uterine stromal fibroblasts was recovered and analyzed for relative expression of IL28Rα and IL10Rβ normalized to β-Actin. Each circle represents an individual patient (n = 44). Values for IL28Rα (B) and IL10Rβ (C) from panel A were plotted against patient age. Each circle represents an individual patient (n = 44) (D) Comparison of IL28Rα and IL10Rβ mRNA expression between non-matched uterine epithelial cells (n = 33) and uterine stromal fibroblasts (n = 44). Each circle represents an individual patient. (E) Fold change in mRNA expression of IL28Rα and IL10Rβ following stimulation of uterine stromal fibroblasts with poly (I:C) (25ug/ml) for 24 hrs. Each circle represents an individual patient (n = 12). (F) mRNA expression of MxA, OAS2, and ISG15 by primary uterine stromal fibroblasts following treatment with IFNλ1 (500 ng/ml) for 24 hrs. Untreated control = 1. Each circle represents an individual patient (n = 8) (G) mRNA expression of TLR2, TLR3, TLR4, TLR5, TLR7, TLR9, RIG-I, and MDA5 by primary uterine stromal fibroblasts following treatment with IFNλ1 (500 ng/ml) for 24 hrs. Untreated control = 1. Each circle represents an individual patient (n = 4). (I–K) Values for MxA, OAS2, and ISG15 mRNA expression from panel (F) were plotted against patient age. Each circle represents an individual patient (n = 8). mRNA expression data in panels (A–D) is normalized to the housekeeping gene β-Actin. mRNA expression data in panels (E, F–J) is normalized to the housekeeping gene β-Actin, and then further normalized to the untreated control whose value is set to 1. Data is shown as mean +/- SEM. Each circle represents an individual patient. Wilcoxon matched-pairs signed rank test (A). Non-parametric Spearman correlation analysis (B, C, H–J). Mann-Whitney t-test (D). One-sample non-parametric Wilcoxon test (E, F, G). *p < 0.05; **p < 0.01; ****p < 0.0001.
Beyond IFNλ1, poly (I:C) stimulation of uterine epithelial cells and fibroblasts also leads to increased secretion of a broad range of inflammatory cytokines and antimicrobials such as human beta defensin 2 (HBD2), elafin, RANTES, CCL4 and CCL20 (29, 32–34). In addition to being cytokines, these proteins also inhibit survival of pathogens such as HIV (19, 21, 32, 33). To investigate whether poly (I:C)-induced IFNλ1 secretion could be responsible for the upregulation of these antimicrobials and cytokines, we measured the secretion of the HBD2, RANTES, CCL20, elafin and CCL4 in response to IFNλ1 (500ng/ml for 24 hrs). There was no effect of IFNλ1 stimulation on the secretion of HBD2, elafin, RANTES, CCL20 and CCL4 by either epithelial cells or fibroblasts (Supplementary Figure 2). This suggests that the primary effect of IFNλ1 is to upregulate the intracellular expression of antiviral ISGs.
Since neither E2 or P altered the upregulation of IFNλ1 in response to poly (I:C), we asked whether E2 could affect the stimulatory effect of IFNλ1 on ISG expression by epithelial cells or fibroblasts. We pretreated uterine epithelial cells, fibroblasts, and ECC-1 cells with E2 (5x10-8M) for 48 hrs prior to treatment with IFNλ1 (500ng/ml) for a subsequent 24 hrs in the presence or absence of E2. E2 significantly reduced the upregulation of OAS2 and ISG15 mRNA in ECC-1 cells after IFN treatment from approximately 6- and 4-fold to below 2-fold respectively (Figure 7A). A similar inhibitory effect on OAS2 and ISG15 was observed in primary uterine epithelial cells treated with under the same conditions (Figure 7B). In contrast, there was no E2 effect in uterine fibroblasts (Figure 7C).
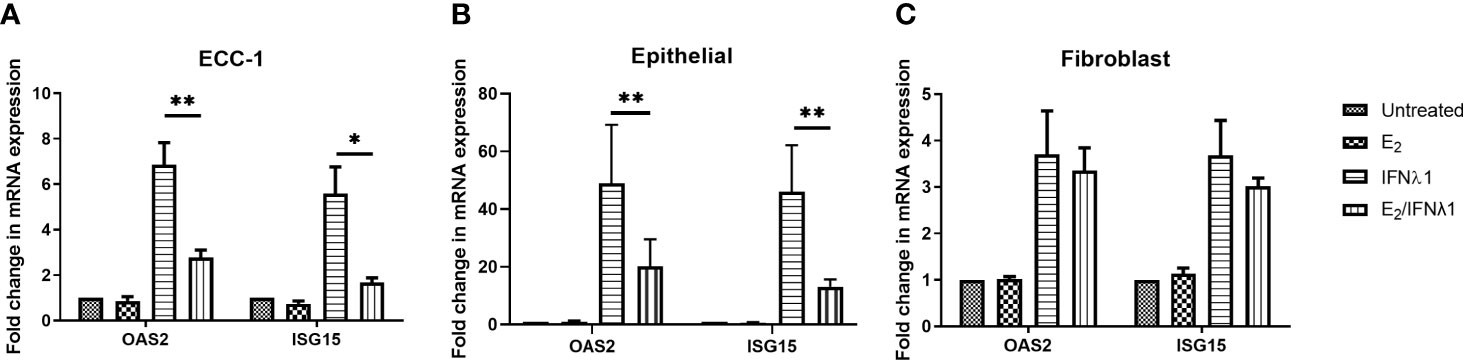
Figure 7 Estradiol (E2) inhibits IFNλ1 signaling in ECC-1 cells and uterine epithelial cells. (A) Polarized ECC-1 cells (n = 5), (B) uterine epithelial cells (n = 8) and (C) uterine fibroblasts (n = 3) were pretreated with E2 (5x10-8M) for 48 hrs after which, media was replenished in the presence or absence of IFNλ1 (500 ng/ml) for a further 24 hrs. During this period, E2 was maintained in the culture media. Subsequently, mRNA was analyzed for OAS2 and ISG15 expression by RT-PCR. mRNA expression data is presented as mean-fold +/- SEM change in gene expression over untreated control and is normalized to the housekeeping gene β-Actin, and then further normalized to the untreated control whose value is set to 1. Wilcoxon matched-pairs signed rank test. *p < 0.05; **p < 0.01.
E2 exerts its effects via estrogen receptor (ER) α and β. To determine the pathway of E2 action in ECC-1 cells, we used the selective estrogen response modulator, Raloxifene (Rx), which blocks ERα signaling. Pretreatment of ECC-1 cells with Rx (5x10-6M, 48 hrs) blocked the inhibitory effects of E2 on the upregulation of OAS2 and ISG15 after treatment with IFNλ1 (Figure 8A). Thus, direct E2-ERα interactions can block IFNλ1 signaling and potentially inhibit the antiviral response.
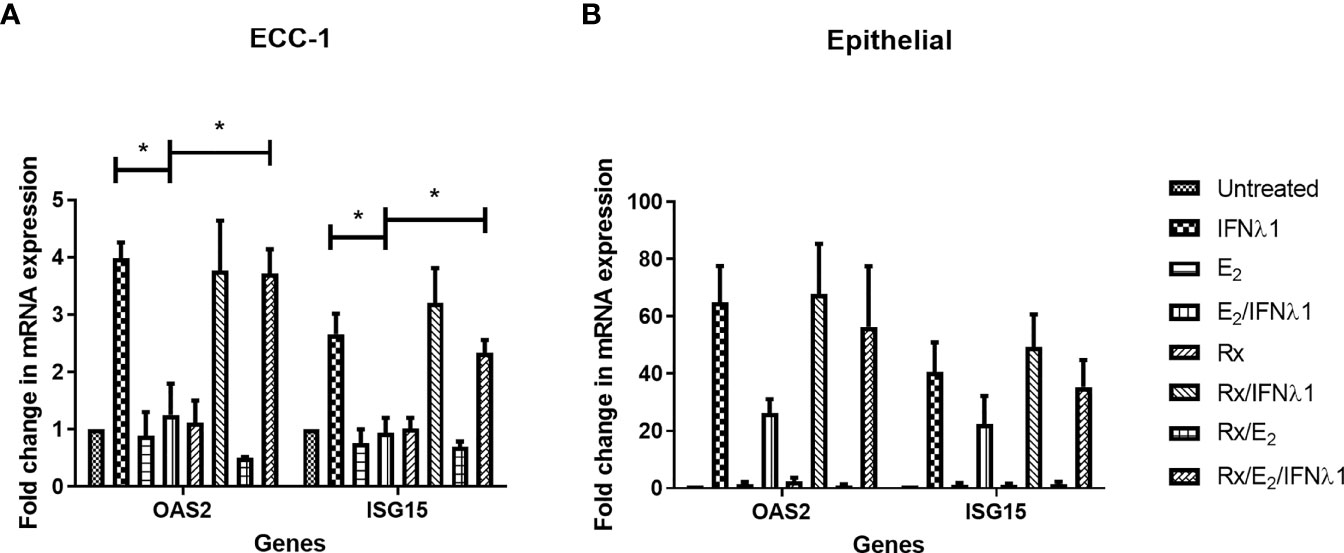
Figure 8 Estradiol (E2) inhibits IFNλ1-induced upregulation of OAS2 and ISG15 in ECC-1 cells and uterine epithelial cells via ERα. (A) Polarized ECC-1 cells (n = 3) or (B) uterine epithelial cells (n = 3) were pretreated with Raloxifene (Rx) (5x10-6M) for 1 hr prior to introduction of E2 (5x10-8M) for a subsequent 48 hrs. Following washout, Rx, E2 or IFNλ1 (500 ng/ml) were added to the cells in the combinations listed for a further 24 hrs prior to mRNA analysis of OAS2 and ISG15 expression by real-time RT-PCR. mRNA expression data is presented as mean-fold +/- SEM change in gene expression over untreated control and is normalized to the housekeeping gene β-Actin, and then further normalized to the untreated control whose value is set to 1. Wilcoxon matched-pairs signed rank test. *p < 0.05.
As a part of these studies, Rx was used to block E2 binding to ERα in primary uterine epithelial cells. As seen in Figure 8B, in primary epithelial cells Rx blocked the suppression of OAS2, and ISG15 by E2 following treatment with IFNλ1.
We then investigated whether P could modulate the IFNλ1-induced upregulation of MxA, OAS2, or ISG15. Epithelial cells and fibroblasts were treated with P (1x10-7M) for 48 hrs prior to treatment with IFNλ1 (500ng/ml) for a subsequent 24 hrs in the presence or absence of P. As seen in Figure 9, P had no effect on the IFNλ1-induced upregulation of MxA or OAS2 in either epithelial cells (Figures 9A, B) or fibroblasts (Figures 9D, E). However, P did significantly potentiate the upregulation of ISG15 by epithelial cells following IFNλ1 treatment (Figure 9C). In contrast, there was no effect of P on the IFNλ1-induced upregulation of ISG15 by fibroblasts (Figure 9F). Together this data shows that the effects of P are selective to specific ISGs and cell type.
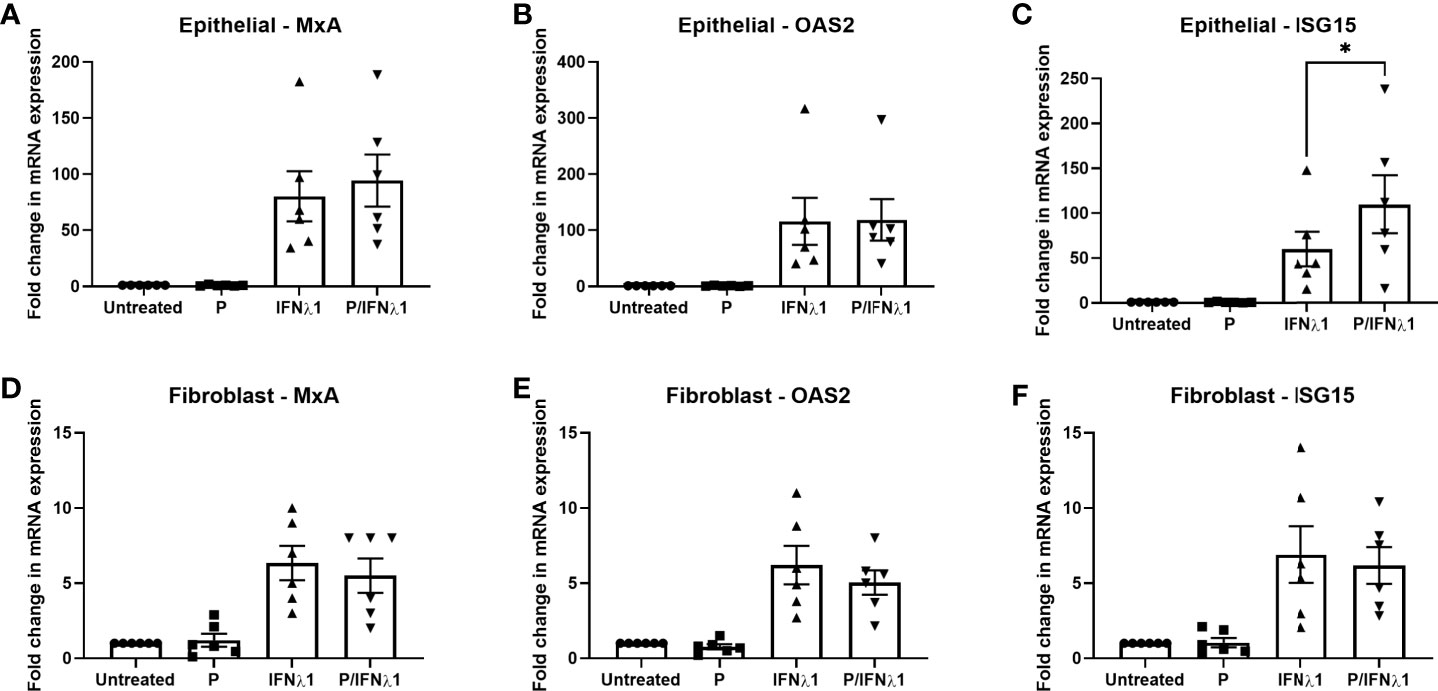
Figure 9 Progesterone (P) enhances IFNλ1-induced upregulation of ISG15 in uterine epithelial cells. Polarized uterine epithelial cells (A–C) (n = 6) or uterine fibroblasts (D–F) (n = 6) were pretreated with P (1x10-7M) for 48 hrs after which media was replenished in the presence or absence of IFNλ1 (500 ng/ml) for a further 24 hrs. During this period, P was maintained in the culture media. Subsequently, mRNA was analyzed for MxA (A, D), OAS2 (B, E), or ISG15 (C, F) expression by RT-PCR. Each symbol represents an individual patient. mRNA expression data is presented as mean-fold +/- SEM change in gene expression over untreated control and is normalized to the housekeeping gene β-Actin, and then further normalized to the untreated control whose value is set to 1. Wilcoxon matched-pairs signed rank test. *p < 0.05.
We found that primary human uterine epithelial cells, uterine fibroblasts, and ECC-1 uterine epithelial cells rapidly upregulate IFNλ1 expression and secretion in response to the viral mimic poly (I:C), independently of E2 or P. The quantity of IFNλ1 secreted following poly (I:C) treatment was significantly greater by epithelial cells obtained from older women compared to younger women. In contrast, there was no effect of age on the secretion of IFNλ1 by fibroblasts. IFNλ1 in turn induced the expression of antiviral ISGs and viral PRRs in epithelial cells, but only weakly in fibroblasts. E2 inhibited the upregulation of MxA, OAS2 and ISG15 by IFNλ1 in epithelial cells via ERα but had no effect in uterine fibroblasts. Lastly, P had no effect on MxA and OAS2 expression by either epithelial cells or fibroblasts following IFNλ1 treatment. However, P did significantly increase the upregulation of ISG15 by epithelial cells following treatment with IFNλ1 compared to IFNλ1-treated cells alone.
A fundamental role of the innate immune system is the recognition and response to pathogens present in the external environment. As part of this response, the secretion of IFNs is essential for the induction and propagation of an immune response to viral pathogens via the upregulation of ISGs. We have shown, for the first time, that in response to poly (I:C), IFNλ1 is upregulated in primary uterine epithelial cells and stromal fibroblasts. These findings extend our understanding of the role of IFNλ1 since other studies have shown that poly (I:C) upregulates the expression of IFNλ1 in blood-derived dendritic cells, macrophages, microglia and epithelial cells (nasal, intestinal and alveolar) (13–15, 35). The upregulation of IFNλ1 in response to poly (I:C) demonstrates that IFNλ1 is a part of the early innate immune response to viral pathogens in the uterine endometrium. This builds upon our earlier studies showing that IFNβ (25) and IL-27 (26), which are also stimulators of ISG expression, are upregulated by poly (I:C) in uterine epithelial cells and fibroblasts. Together, these studies demonstrate that viral exposure in the uterine endometrium leads to an immune response characterized by the upregulation antiviral cytokines such as IFNλ1, IFNβ, and IL-27 which in turn can induce the expression of antiviral ISGs.
While the role of Type III IFNs in protecting the mucosal surfaces, particularly the gastrointestinal and lung mucosa is well established, increasing evidence demonstrates that Type III IFNs protect the FRT from incoming pathogens. Studies have shown that IFNλ1 protects the placenta from bacterial (36) and viral infections including ZIKV (17, 18). Blockade of Type III IFN signaling in an endocervical epithelial cell line leads to increased infection by HSV-2 (15). In addition, IFNλ1 and IFNλ2 also reduce HIV infection of macrophages, CD4+ T cells and peripheral blood lymphocytes (37–40). Thus, the potential exists for IFNλs to play a central role in determining the outcome of HIV transmission, as well as other STIs, that enter the FRT. While we have not addressed whether IFNλ1 secretion and signaling prevent infection by pathogens of uterine epithelial cells and fibroblasts, it is likely that the increased expression of ISGs creates an antiviral state within the cells that reduces the possibility of successful infection.
Aging is linked to altered immune function in the FRT (41) but whether increased age leads to a decrease in immune protection in the FRT is unclear. Previous studies by our group have shown that antibacterial activity of apical uterine epithelial cell secretions against Staphylococcus aureus decrease after menopause (42). In the current study, we show for the first time that increased age leads to an increase in IFNλ1 secretion by uterine epithelial cells, but not stromal fibroblasts, following stimulation with the viral mimic poly (I:C). The mechanism behind this increased secretion is unknown, but our observation could suggest that the contribution of IFNλ1 to antiviral protection of the uterine epithelium increases with age. Whether aging has a similar effect with other Type III IFNs requires further study. As we observed no effect of sex hormones on the secretion of IFNλ1, it is unlikely that the reduced levels of sex hormones in post-menopausal women are responsible for the increased secretion following poly (I:C) stimulation.
Since IFNλ1 is secreted apically but not basolaterally by uterine epithelial cells, our findings suggest that IFNλ1 secretion by an epithelial cell is primarily targeted towards adjacent epithelial cells lining the FRT to enhance intracellular protection following pathogen exposure. Additionally, increased IFNλ1 secretion by uterine epithelial cells may compensate for decreased immune protection elsewhere in the FRT (34, 41, 43). Further studies are required to understand the mechanistic basis for differential effects of menopause and aging on IFNλ1 secretion in the different tissues and cell types in the FRT.
In contrast to the increased secretion of IFNλ1 by epithelial cells with age, we found no effect of age on the sensitivity of both uterine epithelial cells and fibroblasts to IFNλ1 stimulation. This suggests that while the production of IFNλ1 by epithelial cells changes with age, the ability of IFNλ1 to induce an antiviral response does not, particularly since the expression of the Type III IFN receptors, IL28Rα and IL10Rβ, also do not change with age. The lack of an aging effect on IFNλ1-induced ISG expression in uterine epithelial cells and fibroblasts does not preclude age-dependent effects on IFNλ1 signaling in other cell types in the FRT, at other mucosal sites, or by other Type III IFNs. However, there are several important caveats to consider in our study. First, is our small population size, particularly for the IFNλ1 stimulation experiments, where we used epithelial cells and fibroblasts from 16 and 8 different women respectively. Given this small population size, and the wide range in ISG expression in our results, any aging effects may not be detectable unless a larger population cohort is used. Second, our analyses were performed after 24 hrs of IFNλ1 stimulation and more extensive time course studies may be useful to determine whether aging affects the response to IFNλ1 beyond 24 hrs, particularly since some studies suggest that Type III IFNs are important in maintaining the antiviral response over longer time periods (44, 45). Third, we used a high dose of IFNλ1 which may have masked any aging effects. Whether aging could exert an effect on gene expression in the presence of a lower concentration of IFNλ1 is important to define. Lastly, we measured ISG mRNA, but not protein, expression in response to IFNλ1 stimulation. While unlikely, it is possible that the mRNA expression does not correlate with protein levels. These questions should be addressed in future studies.
The premenopausal and pregnant uterine endometrium is exposed to changing concentrations of sex hormones that regulate multiple aspects of innate and adaptive immunity within the FRT, including cytokine and antimicrobial secretion, barrier function and immune cell numbers (1). While the secretion of IFNλ1 was unaffected by E2 or P, both hormones altered the actions of IFNλ1 on ISG upregulation in uterine epithelial cells. In response to IFNλ1, E2 suppresses the upregulation of OAS2, and ISG15 by uterine epithelial cells, while P enhances the expression of ISG15. That this stimulatory effect of P was not observed with MxA or OAS2 emphasizes the unique regulatory control of intracellular innate protection in epithelial cells by sex hormones. Others have shown that ISG15 is upregulated at the time of implantation (46, 47) which suggests that it may function in a way that transcends its antiviral effects (48). Our finding builds on this study by demonstrating that P can further increase ISG15 expression following IFNλ1 stimulation through direct effects on epithelial cells in the uterine endometrium. To the best of our knowledge, these findings are the first demonstration that the actions of IFNλ1 in the human uterus are regulated by sex hormones. This suggests that the contribution of IFNλ1 to innate immune protection may vary across the menstrual cycle and at different stages of pregnancy.
We have previously hypothesized that immune protection against HIV and other sexually transmitted infections is downregulated in the uterus at mid-secretory phase of the menstrual cycle (49). Several studies have shown that transmission of pathogens in the FRT varies with sex hormone levels and menstrual cycle stage. For example, vaginal infection of pigtail macaques by SHIV is higher during secretory phase than proliferative phase (50–52). Similarly, HIV infection is greater in cervico-vaginal explants recovered from secretory phase (51). In ovariectomized mice and in vitro there is increased vaginal HSV-2 transmission following treatment with progesterone (53, 54) while in other studies using ovariectomized mice treated with IFNλ1, vaginal, cervical, and uterine ZIKV infection was greater in mice treated with P alone, compared to mice treated with both E2 and P (55). Our findings suggest that the modulatory effect of sex hormones on the action of IFNλ1 can potentially decrease protection against incoming pathogens, and thus increase the risk of successful transmission of pathogens that enter the FRT. However, whether the IFNλ1-mediated antiviral response by uterine epithelial cells is capable of protecting the uterine endometrium against incoming pathogens, and whether its potential contribution changes across the menstrual cycle, is unknown and not addressed in our study. Future studies will need to address whether exposure to IFNλ1 prevents the infection of uterine epithelial cells and fibroblasts by relevant pathogens.
Beyond the FRT, whether sex hormones modulate IFNλ1-mediated responses at mucosal sites outside the FRT such as the lung, skin, and gastrointestinal tracts and thus alter protection against pathogens that enter at these surfaces remains to be determined but is of considerable importance given the ubiquity of pathogens that can enter the body at these locations. Recognizing that the FRT is exposed to higher levels of sex hormones than other mucosal surfaces (56–61), future studies will need to use lower doses of sex hormones that are representative of levels seen at these sites.
Of particular interest is whether, in addition to being upregulated as part of the innate immune response, the secretion or effects of IFNλ1 are increased at the time of implantation when the semi-allogeneic fertilized egg contacts uterine epithelial cells, fibroblasts and decidual cells at the implantation site. Basic reproductive processes are often accompanied by a limited degree of inflammation and Type I IFNs, such as IFNτ, are thought to be important in mediating uterine receptivity in mammals (62). In animal models, Type I IFNs such as IFNτ are upregulated to as part of the implantation process (63). In turn, IFNs induce ISGs such as ISG15 which are essential for successful implantation (46, 47). Since implantation occurs when P levels are high, this could explain why P enhanced the expression of ISG15 following stimulation of epithelial cells with IFNλ1. Whether Type III IFNs have a similar function in regulating the expression of a larger panel of ISGs as part of the reproductive process is unclear.
As part of the reproductive process, the immune system in the FRT is precisely regulated to achieve a balance between immune tolerance which allows the survival of allogeneic sperm and potentially a semi-allogeneic fetus, while maintaining immune protection against external pathogens. High levels of E2 as seen during ovulation and mid-secretory phase in the non-pregnant endometrium could reduce the innate antiviral response increasing the chances for successful fertilization. The dose of P (1x10-7M) at which we observed a stimulatory effect on IFNλ1-induced ISG15 expression is representative of levels at mid-secretory phase in the endometrium when implantation occurs. Therefore, P may modulate IFNλ1-induced ISG15 expression as part of a successful implantation program.
Through its intracellular receptors, E2 is a potent modulator of immune function in the FRT (1). Our investigation of the effects of E2 on IFNλ1-induced gene expression demonstrate reduced the upregulation of OAS2 and ISG15 by IFNλ1 via ERα. While the precise regulation of IFNλ1 signaling downstream of ERα remains unclear, binding of IFNλ1 to IL28Rα and IL10Rβ leads to the phosphorylation of JAK2 as part of the signal transduction cascade (64, 65). In HuH7 and T-47D cells, E2 inhibits growth hormone-induced signaling by preventing phosphorylation of JAK2 via upregulation of Suppressor of Cytokine Signaling (SOCS) 2 (66). Therefore, disruption of JAK-mediated signaling may be a possible mechanism for E2-mediated inhibition of IFNλ1 in uterine epithelial cells.
Consistent with our results previous studies have shown that IFNλ1 expression is regulated by Type I IFNs, and is therefore an ISG itself (31). This suggests that a Type I IFN response is necessary for optimal antiviral protection in uterine epithelial cells. Other studies have shown that ISG expression induced by Type I IFNs peaks earlier and higher than ISG expression induced by Type III IFNs which maintains the antiviral response for longer (44, 45). This suggests that Type I IFNs are responsible for the upregulation of ISGs early in the antiviral response, while Type III IFNs are responsible for increased duration of the antiviral response. Since our studies were performed over an acute timepoint (24 hrs), the possibility remains that IFNλ1 is necessary for long term upregulation of ISGs but is not necessary for the immediate upregulation of ISGs following poly (I:C) stimulation of uterine epithelial cells and fibroblasts. Future studies will need to address the effects of long-term IFNλ1 stimulation on ISG expression in the FRT.
Together our results demonstrate that the FRT is a unique mucosal site in which the endocrine and immune systems intersect to create a unique and complex immune environment that balances protection with procreation. IFNλ1 is an important component of innate immune protection within the human FRT against potential viral pathogens. The selective effects of E2 and P on IFNλ1 function suggests that the antiviral efficacy of IFNλ may vary in women with stage of the menstrual cycle. Since IFNλs are increasingly used in a therapeutic setting, it is important to understand how endogenous (E2 and P) and exogenous (contraceptives and hormone therapy) hormone exposure may alter IFNλ-mediated protection both in the FRT, and at other sites in the body in women of all ages.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Committee for the Protection of Human Subjects Dartmouth-Hitchcock Medical Center. The patients/participants provided their written informed consent to participate in this study.
MVP, DCH, and FDB performed the experiments. MVP prepared the figures. MVP and CRW wrote the manuscript. All authors contributed to the article and approved the submitted version.
Supported by NIH AI102838, AI071761, AI117739 and AG064794 (CRW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge the patients from whom hysterectomy samples were obtained. We also acknowledge the surgeons, nurses, and other support staff from DHMC. We would like to thank Richard M. Rossoll for excellent technical assistance in carrying out these studies.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.718380/full#supplementary-material
Supplementary Figure 1 | IFNλ1 upregulates MxA, OAS2, and ISG15 expression by ECC-1 cells but does not affect TER. ECC-1 uterine epithelial cells were treated recombinant human IFNλ1 (10, 100, 1000 ng/ml) for 24 hrs prior to analysis of gene expression by real-time RT-PCR (A) or transepithelial resistance (B). Data is shown as mean +/- SEM.
Supplementary Figure 2 | IFNλ1 has no effect on the secretion of HBD2, elafin, CCL20, RANTES, and CCL4 by uterine epithelial cells. Primary human uterine epithelial cells (n = 6) and fibroblasts (n = 5) were treated with recombinant IFNλ1 (500 ng/ml) for 24 hrs after which secretions were recovered and analyzed by ELISA. Data is shown as mean +/- SEM.
1. Wira CR, Rodriguez-Garcia M, Patel MV. The Role of Sex Hormones in Immune Protection of the Female Reproductive Tract. Nat Rev Immunol (2015) 15(4):217–30. doi: 10.1038/nri3819
2. Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-Lambdas Mediate Antiviral Protection Through a Distinct Class II Cytokine Receptor Complex. Nat Immunol (2003) 4(1):69–77. doi: 10.1038/ni875
3. Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, et al. IL-28, IL-29 and Their Class II Cytokine Receptor IL-28r. Nat Immunol (2003) 4(1):63–8. doi: 10.1038/ni873
4. Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, et al. A Variant Upstream of IFNL3 (IL28B) Creating a New Interferon Gene IFNL4 Is Associated With Impaired Clearance of Hepatitis C Virus. Nat Genet (2013) 45(2):164–71. doi: 10.1038/ng.2521
5. Yang K, Puel A, Zhang S, Eidenschenk C, Ku C-L, Casrouge A, et al. Human TLR-7-, -8-, and -9-Mediated Induction of IFN-α/β and -λ Is IRAK-4 Dependent and Redundant for Protective Immunity to Viruses. Immunity (2005) 23(5):465–78. doi: 10.1016/j.immuni.2005.09.016
6. Zhou L, Wang X, Wang YJ, Zhou Y, Hu S, Ye L, et al. Activation of Toll-Like Receptor-3 Induces Interferon-λ Expression in Human Neuronal Cells. Neuroscience (2009) 159(2):629–37. doi: 10.1016/j.neuroscience.2008.12.036
7. Ioannidis I, Ye F, McNally B, Willette M, Flaño E. Toll-Like Receptor Expression and Induction of Type I and Type III Interferons in Primary Airway Epithelial Cells. J Virol (2013) 87(6):3261–70. doi: 10.1128/JVI.01956-12
8. Escribese MM, Kraus T, Rhee E, Fernandez-Sesma A, López CB, Moran TM. Estrogen Inhibits Dendritic Cell Maturation to RNA Viruses. Blood (2008) 112(12):4574–84. doi: 10.1182/blood-2008-04-148692
9. Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting Edge: Progesterone Regulates IFN-Alpha Production by Plasmacytoid Dendritic Cells. J Immunol (2008) 180(4):2029–33. doi: 10.4049/jimmunol.180.4.2029
10. Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-Lambda (IFN-λ) Is Expressed in a Tissue-Dependent Fashion and Primarily Acts on Epithelial Cells In Vivo. PloS Pathog (2008) 4(3):e1000017. doi: 10.1371/journal.ppat.1000017
11. Pott J, Mahlakõiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, et al. IFN-λ Determines the Intestinal Epithelial Antiviral Host Defense. Proc Natl Acad Sci (2011) 108(19):7944–9. doi: 10.1073/pnas.1100552108
12. Mordstein M, Neugebauer E, Ditt V, Jessen B, Rieger T, Falcone V, et al. Lambda Interferon Renders Epithelial Cells of the Respiratory and Gastrointestinal Tracts Resistant to Viral Infections. J Virol (2010) 84(11):5670–7. doi: 10.1128/JVI.00272-10
13. Okabayashi T, Kojima T, Masaki T, Yokota SI, Imaizumi T, Tsutsumi H, et al. Type-III Interferon, Not Type-I, Is the Predominant Interferon Induced by Respiratory Viruses in Nasal Epithelial Cells. Virus Res (2011) 160(1–2):360–6. doi: 10.1016/j.virusres.2011.07.011
14. Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda Interferon (IFN-λ), a Type III IFN, Is Induced by Viruses and IFNs and Displays Potent Antiviral Activity Against Select Virus Infections In Vivo. J Virol (2006) 80(9):4501–9. doi: 10.1128/JVI.80.9.4501-4509.2006
15. Zhou L, Li J-L, Zhou Y, Liu J-B, Zhuang K, Gao J-F, et al. Induction of Interferon-λ Contributes to TLR3 and RIG-I Activation-Mediated Inhibition of Herpes Simplex Virus Type 2 Replication in Human Cervical Epithelial Cells. Mol Hum Reprod (2015) 21(2):917–29. doi: 10.1093/molehr/gav058
16. Cannella F, Scagnolari C, Selvaggi C, Stentella P, Recine N, Antonelli G, et al. Interferon Lambda 1 Expression in Cervical Cells Differs Between Low-Risk and High-Risk Human Papillomavirus-Positive Women. Med Microbiol Immunol (2014) 203(3):177–84. doi: 10.1007/s00430-014-0330-9
17. Jagger BW, Miner JJ, Cao B, Arora N, Smith AM, Kovacs A, et al. Gestational Stage and IFN-λ Signaling Regulate ZIKV Infection In Utero. Cell Host Microbe (2017) 22(3):366–76.e3. doi: 10.1016/j.chom.2017.08.012
18. Casazza RL, Lazear HM, Miner JJ. Protective and Pathogenic Effects of Interferon Signaling During Pregnancy. Viral Immunol (2020) 33(1):3–11. doi: 10.1089/vim.2019.0076
19. Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, et al. Estradiol Selectively Regulates Innate Immune Function by Polarized Human Uterine Epithelial Cells in Culture. Mucosal Immunol (2008) 1(4):317–25. doi: 10.1038/mi.2008.20
20. Patel MV, Fahey JV, Rossoll RM, Wira CR. Innate Immunity in the Vagina (Part I): Estradiol Inhibits HBD2 and Elafin Secretion by Human Vaginal Epithelial Cells. Am J Reprod Immunol (2013) 69(5):463–74. doi: 10.1111/aji.12078
21. Patel MV, Shen Z, Rossoll RM, Wira CR. Estradiol-Regulated Innate Antiviral Responses of Human Endometrial Stromal Fibroblasts. Am J Reprod Immunol (2018) 80(5):e13042. doi: 10.1111/aji.13042
22. Patel MV, Shen Z, Wira CR. Poly (I:C) and LPS Induce Distinct Immune Responses by Ovarian Stromal Fibroblasts. J Reprod Immunol (2018) 127:36–42. doi: 10.1016/j.jri.2018.05.001
23. Mo B, Vendrov AE, Palomino WA, DuPont BR, Apparao KBC, Lessey BA. ECC-1 Cells: A Well-Differentiated Steroid-Responsive Endometrial Cell Line With Characteristics of Luminal Epithelium. Biol Reprod (2006) 75(3):387–94. doi: 10.1095/biolreprod.106.051870
24. Richardson JM, Kaushic C, Wira CR. Polymeric Immunoglobin (Ig) Receptor Production and IgA Transcytosis in Polarized Primary Cultures of Mature Rat Uterine Epithelial Cells. Biol Reprod (1995) 53(3):488–98. doi: 10.1095/biolreprod53.3.488
25. Patel MV, Ghosh M, Fahey JV, Wira CR. Uterine Epithelial Cells Specifically Induce Interferon-Stimulated Genes in Response to Polyinosinic-Polycytidylic Acid Independently of Estradiol. PloS One (2012) 7(4):e35654. doi: 10.1371/journal.pone.0035654
26. Patel MV, Shen Z, Rossoll RM, Wira CR. IL-27 Expression and Responsiveness in Human Uterine Epithelial Cells and Fibroblasts In Vitro and the Role of Estradiol. J Interferon Cytokine Res (2018) 38(3):101–10. doi: 10.1089/jir.2017.0038
27. Bläuer M, Heinonen PK, Rovio P, Ylikomi T. Effects of Tamoxifen and Raloxifene on Normal Human Endometrial Cells in an Organotypic In Vitro Model. Eur J Pharmacol (2008) 592(1–3):13–8. doi: 10.1016/j.ejphar.2008.06.091
28. Rodriguez-Garcia M, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C, et al. Estradiol Reduces Susceptibility of CD4+ T Cells and Macrophages to HIV-Infection. PloS One (2013) 8(4):e62069. doi: 10.1371/journal.pone.0062069
29. Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate Immunity in the Human Female Reproductive Tract: Antiviral Response of Uterine Epithelial Cells to the TLR3 Agonist Poly(I:C). J Immunol (2005) 174(2):992–1002. doi: 10.4049/jimmunol.174.2.992
30. Schaefer TM, Fahey JV, Wright JA, Wira CR. Migration Inhibitory Factor Secretion by Polarized Uterine Epithelial Cells Is Enhanced in Response to the TLR3 Agonist Poly (I:C). Am J Reprod Immunol (2005) 54(4):193–202. doi: 10.1111/j.1600-0897.2005.00298.x
31. Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, et al. An Important Role for Type III Interferon (IFN-λ/IL-28) in TLR-Induced Antiviral Activity. J Immunol (2008) 180(4):2474–85. doi: 10.4049/jimmunol.180.4.2474
32. Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: A Novel Innate Anti-Human Immunodeficiency Virus-1 Molecule of the Human Female Reproductive Tract. Immunology (2010) 129(2):207–19. doi: 10.1111/j.1365-2567.2009.03165.x
33. Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3α Is a Novel Anti-HIV-1 Molecule of the Human Female Reproductive Tract. Am J Reprod Immunol (2009) 62(1):60–71. doi: 10.1111/j.1600-0897.2009.00713.x
34. Rodriguez-Garcia M, Shen Z, Fortier JM, Wira CR. Differential Cytotoxic Function of Resident and Non-Resident CD8+ T Cells in the Human Female Reproductive Tract Before and After Menopause. Front Immunol (2020) 11:1096. doi: 10.3389/fimmu.2020.01096
35. Hillyer P, Mane VP, Schramm LM, Puig M, Verthelyi D, Chen A, et al. Expression Profiles of Human Interferon-Alpha and Interferon-Lambda Subtypes Are Ligand- and Cell-Dependent. Immunol Cell Biol (2012) 90(8):774–83. doi: 10.1038/icb.2011.109
36. Bierne H, Travier L, Mahlakõiv T, Tailleux L, Subtil A, Lebreton A, et al. Activation of Type III Interferon Genes by Pathogenic Bacteria in Infected Epithelial Cells and Mouse Placenta. PloS One (2012) 7(6):e39080. doi: 10.1371/journal.pone.0039080
37. Hou W, Wang X, Ye L, Zhou L, Yang Z-Q, Riedel E, et al. Lambda Interferon Inhibits Human Immunodeficiency Virus Type 1 Infection of Macrophages. J Virol (2009) 83(8):3834–42. doi: 10.1128/JVI.01773-08
38. Tian RR, Guo HX, Wei JF, Yang CK, He SH, Wang JH. IFN-Lambda Inhibits HIV-1 Integration and Post-Transcriptional Events In Vitro, But There Is Only Limited In Vivo Repression of Viral Production. Antiviral Res (2012) 95(1):57–65. doi: 10.1016/j.antiviral.2012.04.011
39. Wang Y, Li J, Wang X, Zhou Y, Zhang T, Ho W. Comparison of Antiviral Activity of Lambda-Interferons Against HIV Replication in Macrophages. J Interferon Cytokine Res (2015) 35(3):213–21. doi: 10.1089/jir.2014.0064
40. Serra C, Biolchini A, Mei A, Kotenko S, Dolei A. Type III and I Interferons Increase HIV Uptake and Replication in Human Cells That Overexpress CD4, CCR5, and CXCR4. AIDS Res Hum Retroviruses (2008) 24(2):173–80. doi: 10.1089/aid.2007.0198
41. Rodriguez-Garcia M, Patel MV, Shen Z, Wira CR. The Impact of Aging on Innate and Adaptive Immunity in the Human Female Genital Tract. Aging Cell (2021) 20(5):e13361. doi: 10.1111/acel.13361
42. Fahey JV, Wira CR. Effect of Menstrual Status on Antibacterial Activity and Secretory Leukocyte Protease Inhibitor Production by Human Uterine Epithelial Cells in Culture. J Infect Dis (2002) 185(11):1606–13. doi: 10.1086/340512
43. Rodriguez-Garcia M, Fortier JM, Barr FD, Wira CR. Aging Impacts CD103(+) CD8(+) T Cell Presence and Induction by Dendritic Cells in the Genital Tract. Aging Cell (2018) 17(3):e12733. doi: 10.1111/acel.12733
44. Klinkhammer J, Schnepf D, Ye L, Schwaderlapp M, Gad HH, Hartmann R, et al. IFN-λ Prevents Influenza Virus Spread From the Upper Airways to the Lungs and Limits Virus Transmission. Elife (2018) 7. doi: 10.7554/eLife.33354
45. Pervolaraki K, Rastgou Talemi S, Albrecht D, Bormann F, Bamford C, Mendoza JL, et al. Differential Induction of Interferon Stimulated Genes Between Type I and Type III Interferons Is Independent of Interferon Receptor Abundance. PloS Pathog (2018) 14(11):e1007420. doi: 10.1371/journal.ppat.1007420
46. Austin KJ, Bany BM, Belden EL, Rempel LA, Cross JC, Hansen TR. Interferon-Stimulated Gene-15 (Isg15) Expression Is Up-Regulated in the Mouse Uterus in Response to the Implanting Conceptus. Endocrinology (2003) 144(7):3107–13. doi: 10.1210/en.2002-0031
47. Joyce MM, White FJ, Burghardt RC, Muñiz JJ, Spencer TE, Bazer FW, et al. Interferon Stimulated Gene 15 Conjugates to Endometrial Cytosolic Proteins and Is Expressed at the Uterine-Placental Interface Throughout Pregnancy in Sheep. Endocrinology (2005) 146(2):675–84. doi: 10.1210/en.2004-1224
48. Hansen TR, Pru JK. ISGylation: A Conserved Pathway in Mammalian Pregnancy. Adv Exp Med Biol (2014) 759:13–31. doi: 10.1007/978-1-4939-0817-2_2
49. Wira CR, Fahey JV. A New Strategy to Understand How HIV Infects Women: Identification of a Window of Vulnerability During the Menstrual Cycle. AIDS (2008) 22(15):1909–17. doi: 10.1097/QAD.0b013e3283060ea4
50. Kersh EN, Henning T, Vishwanathan SA, Morris M, Butler K, Adams DR, et al. SHIV Susceptibility Changes During the Menstrual Cycle of Pigtail Macaques. J Med Primatol (2014) 43(5):310–6. doi: 10.1111/jmp.12124
51. Saba E, Origoni M, Taccagni G, Ferrari D, Doglioni C, Nava A, et al. Productive HIV-1 Infection of Human Cervical Tissue Ex Vivo Is Associated With the Secretory Phase of the Menstrual Cycle. Mucosal Immunol (2013) 6(6):1081–90. doi: 10.1038/mi.2013.2
52. Morris MR, Byrareddy SN, Villinger F, Henning TC, Butler K, Ansari AA, et al. Relationship of Menstrual Cycle and Vaginal Infection in Female Rhesus Macaques Challenged With Repeated, Low Doses of Sivmac251. J Med Primatol (2015) 44(5):301–5. doi: 10.1111/jmp.12177
53. Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. Estradiol Regulates Susceptibility Following Primary Exposure to Genital Herpes Simplex Virus Type 2, While Progesterone Induces Inflammation. J Virol (2005) 79(5):3107–16. doi: 10.1128/JVI.79.5.3107-3116.2005
54. Lee Y, Dizzell SE, Leung V, Nazli A, Zahoor MA, Fichorova RN, et al. Effects of Female Sex Hormones on Susceptibility to HSV-2 in Vaginal Cells Grown in Air-Liquid Interface. Viruses (2016) 8(9):241. doi: 10.3390/v8090241
55. Caine EA, Scheaffer SM, Arora N, Zaitsev K, Artyomov MN, Coyne CB, et al. Interferon Lambda Protects the Female Reproductive Tract Against Zika Virus Infection. Nat Commun (2019) 10(1):280–. doi: 10.1038/s41467-018-07993-2
56. Baird DT, Fraser IS. Concentration of Oestrone and Oestradiol in Follicular Fluid and Ovarian Venous Blood of Women. Clin Endocrinol (1975) 4(3):259–66. doi: 10.1111/j.1365-2265.1975.tb01533.x
57. McNatty KP, Baird DT, Bolton A, Chambers P, Corker CS, McLean H. Concentration of Oestrogens and Androgens in Human Ovarian Venous Plasma and Follicular Fluid Throughout the Menstrual Cycle. J Endocrinol (1976) 71(1):77–85. doi: 10.1677/joe.0.0710077
58. Baird DT, Fraser IS. Blood Production and Ovarian Secretion Rates of Estradiol-17β and Estrone in Women Throughout the Menstrual Cycle. J Clin Endocrinol Metab (1974) 38(6):1009–17. doi: 10.1210/jcem-38-6-1009
59. Lloyd CW, Lobotsky J, Baird DT, McCracken JA, Weisz J, Pupkin M, et al. Concentration of Unconjugated Estrogens, Androgens and Gestagens in Ovarian and Peripheral Venous Plasma of Women: The Normal Menstrual Cycle. J Clin Endocrinol Metab (1971) 32(2):155–66. doi: 10.1210/jcem-32-2-155
60. Huhtinen K, Desai R, Ståhle M, Salminen A, Handelsman DJ, Perheentupa A, et al. Endometrial and Endometriotic Concentrations of Estrone and Estradiol Are Determined by Local Metabolism Rather Than Circulating Levels. J Clin Endocrinol Metab (2012) 97(11):4228–35. doi: 10.1210/jc.2012-1154
61. Cicinelli E, Einer-Jensen N, Cignarelli M, Mangiacotti L, Luisi D, Schonauer S. Preferential Transfer of Endogenous Ovarian Steroid Hormones to the Uterus During Both the Follicular and Luteal Phases. Hum Reprod (2004) 19(9):2001–4. doi: 10.1093/humrep/deh404
62. Bazer FW, Burghardt RC, Johnson GA, Spencer TE, Wu G. Interferons and Progesterone for Establishment and Maintenance of Pregnancy: Interactions Among Novel Cell Signaling Pathways. Reprod Biol (2008) 8(3):179–211. doi: 10.1016/S1642-431X(12)60012-6
63. Bazer FW, Kim J, Song G, Ka H, Tekwe CD, Wu G. Select Nutrients, Progesterone, and Interferon Tau Affect Conceptus Metabolism and Development. Ann New York Acad Sci (2012) 1271(1):88–96. doi: 10.1111/j.1749-6632.2012.06741.x
64. Lee S-J, Kim W-J, Moon S-K. Role of the P38 MAPK Signaling Pathway in Mediating Interleukin-28A-Induced Migration of UMUC-3 Cells. Int J Mol Med (2012) 30(4):945–52. doi: 10.3892/ijmm.2012.1064
65. Odendall C, Dixit E, Stavru F, Bierne H, Franz KM, Durbin AF, et al. Diverse Intracellular Pathogens Activate Type III Interferon Expression From Peroxisomes. Nat Immunol (2014) 15(8):717–26. doi: 10.1038/ni.2915
Keywords: interferon lambda (IFN-λ), estradiol, progesterone, uterine epithelial cell, uterine stromal cell, interferon-stimulated gene (ISG)
Citation: Patel MV, Hopkins DC, Barr FD and Wira CR (2021) Sex Hormones and Aging Modulate Interferon Lambda 1 Production and Signaling by Human Uterine Epithelial Cells and Fibroblasts. Front. Immunol. 12:718380. doi: 10.3389/fimmu.2021.718380
Received: 31 May 2021; Accepted: 12 August 2021;
Published: 24 September 2021.
Edited by:
Surendra Sharma, Women & Infants Hospital of Rhode Island, United StatesReviewed by:
Ali A. Ashkar, McMaster University, CanadaCopyright © 2021 Patel, Hopkins, Barr and Wira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mickey V. Patel, TWlja2V5LlYuUGF0ZWxARGFydG1vdXRoLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.