
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 21 July 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.713225
 Miki Nakano1†
Miki Nakano1† Masahiro Ayano1,2*
Masahiro Ayano1,2* Kazuo Kushimoto1
Kazuo Kushimoto1 Shotaro Kawano1
Shotaro Kawano1 Kazuhiko Higashioka1†
Kazuhiko Higashioka1† Shoichiro Inokuchi1
Shoichiro Inokuchi1 Hiroki Mitoma1
Hiroki Mitoma1 Yasutaka Kimoto3
Yasutaka Kimoto3 Mitsuteru Akahoshi1†
Mitsuteru Akahoshi1† Nobuyuki Ono1
Nobuyuki Ono1 Yojiro Arinobu1
Yojiro Arinobu1 Koichi Akashi1
Koichi Akashi1 Takahiko Horiuchi3
Takahiko Horiuchi3 Hiroaki Niiro4
Hiroaki Niiro4Background: CD226, an activating receptor expressed on the surface of natural killer (NK) cells and T cells, is also seen on B cells and CD226 polymorphism is associated with systemic lupus erythematosus (SLE). Because the specific roles of CD226+ B cells in SLE are still unknown, we investigated the association of CD226+ B cells with SLE.
Methods: We measured CD226 expression on B cells and its subsets using flow cytometry in 48 SLE patients and 24 healthy controls (HCs). We assessed the relationships between CD226+ B cells and SLE Disease Activity Index 2000 (SLEDAI-2K), clinical manifestations, laboratory data, and prognosis after 12 months.
Results: The proportions of CD226+ cells in whole B cells and all its subsets were significantly higher in SLE patients than HCs. In SLE patients, the proportions of CD226+ B cells and CD226+ switched-memory (SM) B cells were significantly correlated with SLEDAI-2K scores and anti-dsDNA antibody titers, and negatively correlated with serum complement levels. Moreover, basal percentages of CD226+ B cells and CD226+ SM B cells were low in patients who were in Lupus Low Disease Activity State after 12 months. In patients with renal involvement, the proportion of CD226+ B cells increased. Additionally, the proportion of CD226+ B cells was higher in patients who were not in complete renal remission after 12 months.
Conclusions: Increased proportion of CD226+ B cells was associated with disease activity and prognosis of SLE. CD226+ B cells may be a useful biomarker for the management of SLE.
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with various clinical manifestations and its management is often difficult (1–3). Recently, the concept of treat-to-target (T2T) in SLE has been established, and it has been more important to monitor disease activity and predict the prognosis of SLE (3–5). Because of this, many studies have tried to find potential novel biomarkers from the molecules involved in the pathogenesis of SLE (6, 7).
Although the pathogenesis of SLE is not yet fully understood, it is known that genetic factors contribute to the development of SLE (2, 8–10) and that various immune cells are involved in the immune responses in SLE (11). In particular, B cells play an important role in the pathogenesis of SLE by autoantibody and cytokine production and antigen presentation (8, 11–13). Some B cell phenotypes have been reported as useful biomarkers for disease activity of SLE (7, 13); however, these have not yet been established.
Several genome-wide association studies have reported the importance of the CD226 gene in the susceptibility to SLE in multiple ancestries (14–17). CD226 is expressed on the cell surface of T cells, natural killer (NK) cells, B cells, monocytes, and platelets (18). It also acts as an activating receptor and mediates cytotoxicity and has been reported to modulate various immune functions as well (18–21). Moreover, several studies have shown the involvement of CD226 in autoimmune diseases such as SLE (22), rheumatoid arthritis (23), and systemic sclerosis (24). On the other hand, the immune functions of CD226 on B cells remain unclear, although a recent study suggested CD226 was involved in adaptive immune responses of B cells (25).
This study aimed to reveal the involvement of CD226 on B cells in the pathogenesis of SLE by measuring CD226 expression on B cells using flow cytometry in SLE patients and by assessing the relationship between CD226-expressing B cells and the disease activity, clinical manifestations, and prognosis of SLE.
We investigated 48 Japanese patients who were treated for SLE at the Kyushu University hospital between 2018 and 2020. We included patients who fulfilled at least four of the American College of Rheumatology revised criteria for SLE (26) and had no other autoimmune diseases, infections, or cancers. Some patients were treated with corticosteroids, hydroxychloroquine, and immunosuppressive drugs, alone or in combination. Of these 48 SLE patients, we were able to assess 5 patients both before and after treatment. We also investigated 24 healthy controls (HCs).
This study was approved by the ethics committee of Kyushu University Hospital (approval number 2019-481) in accordance with the Helsinki Declaration. All participants gave written informed consent.
We obtained the following information from the medical records of the patients: demographic data, clinical manifestations, medications, and laboratory findings including C3, C4, and anti-dsDNA antibody titers at baseline and after 12 months. The serum anti-dsDNA antibody titers were measured using chemiluminescence enzyme immunoassay, and the positive cut-off value used was >10 IU/ml. Disease activity was assessed by the SLE Disease Activity Index 2000 (SLEDAI-2K) (27) and the clinical SLEDAI-2K (28), with active SLE being defined as having a SLEDAI-2K score of ≥11 (29). The clinical SLEDAI-2K (28) is obtained by excluding the serological descriptors such as increased anti-dsDNA antibodies and low complement from the SLEDAI-2K. Clinical manifestations were classified based on the SLEDAI-2K descriptors (27), and renal manifestations were evaluated using the renal SLEDAI-2K scores (30), which were the renal descriptors of the SLEDAI-2K including urinary casts, hematuria, proteinuria, and pyuria. Active nephritis was defined as having a renal SLEDAI-2K score of ≥4. Previous lupus nephritis (LN) and neuropsychiatric systemic lupus erythematosus (NPSLE) were defined as having once before a renal SLEDAI-2K score of ≥4 and one or more neuropsychiatric descriptors of the SLEDAI-2K, respectively. Low disease activity (LDA) was assessed by the definitions of Lupus Low Disease Activity State (LLDAS): a SLEDAI-2K score of ≤4, with no activity in major organ systems (renal, central nervous system, cardiopulmonary, vasculitis, fever) and no hemolytic anemia or gastrointestinal activity; no new lupus disease activity compared with the previous assessment; physician global assessment (scale 0–3) of ≤1; a current prednisolone (or equivalent) dose of ≤7.5 mg/day; and well-tolerated standard maintenance doses of immunosuppressive drugs and approved biological agents (31). Complete renal remission (CR) was defined as proteinuria <500 mg/24 hours and serum creatinine levels within 10% from baseline (5).
Peripheral blood mononuclear cells (PBMCs) from SLE patients and HCs were isolated from the heparinized fresh blood by Lymphoprep (Stemcell Technologies, Vancouver, Canada). PBMCs obtained were pre-treated with FcR blocking reagent (Miltenyi Biotec, Bergisch Gladbach, Germany) and stained with the following fluorescence-conjugated anti-human antibodies: anti-CD3-PE-Cyanine (Cy) 7 (SK7), anti-CD4-Brilliant Violet (BV) 421 (RPA-T4), anti-CD8a-BV510 (RPA-T8), anti-CD19-APC (HIB19), anti-CD56-FITC (HCD56), anti-CD3-PerCP-Cy5.5 (UCHT1), anti-CD19-BV421 (HIB19), anti-CD20-PE-Cy7 (2H7), anti-CD27-BV510 (O323), anti-IgD-FITC (IA6-2), anti-CD38-APC-Cy7 (HIT2; all from BioLegend, San Diego, CA, USA), and anti-CD226-PE (DX11; Miltenyi Biotec). Isotype control antibodies (BD Biosciences, San Jose, CA, USA) were used to determine the level of background staining. Samples were analyzed with the FACS Aria II flow cytometer (BD Biosciences). Data analysis was performed using the FlowJo Software (Tree Star, Ashland, OR, USA). CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), NK cells (CD3−CD56+), B cells (CD3−CD19+), naive B cells (CD3−CD19+IgD+CD27−), IgD+-memory B cells (CD3−CD19+IgD+CD27+), switched-memory (SM) B cells (CD3−CD19+IgD−CD27+), and plasmablasts (CD3−CD19+CD20−CD38++) were all assessed by flow cytometry.
Data are presented as the median and interquartile range unless otherwise stated. Differences between two groups were analyzed using the Student’s t-test for normally distributed continuous variables or using the Mann–Whitney U test for non-normally distributed variables. The relations between two continuous variables were analyzed using Spearman’s rank correlation. All tests were two-tailed and P-values < 0.05 were considered significant. All analyses were performed using the JMP software, version 15 (SAS Institute, Cary, NC, USA).
To investigate the involvement of CD226 in the pathogenesis of SLE, we first measured CD226 expression on PBMCs using flow cytometry in 48 SLE patients (mean age, 41.4 years; 43 females) and 24 HCs (mean age, 38.0 years; 23 females). No significant differences were found between SLE patients and HCs in terms of age and gender. The baseline characteristics of the SLE patients are described in Table 1. The proportions of CD226+ B cells and CD226+ CD8+ T cells were significantly higher in SLE patients than in HCs (Figure 1A and Supplementary Figure 1A), whereas those of CD226+ CD4+ T cells and CD226+ NK cells were almost the same between the two groups (Supplementary Figures 1B, C).
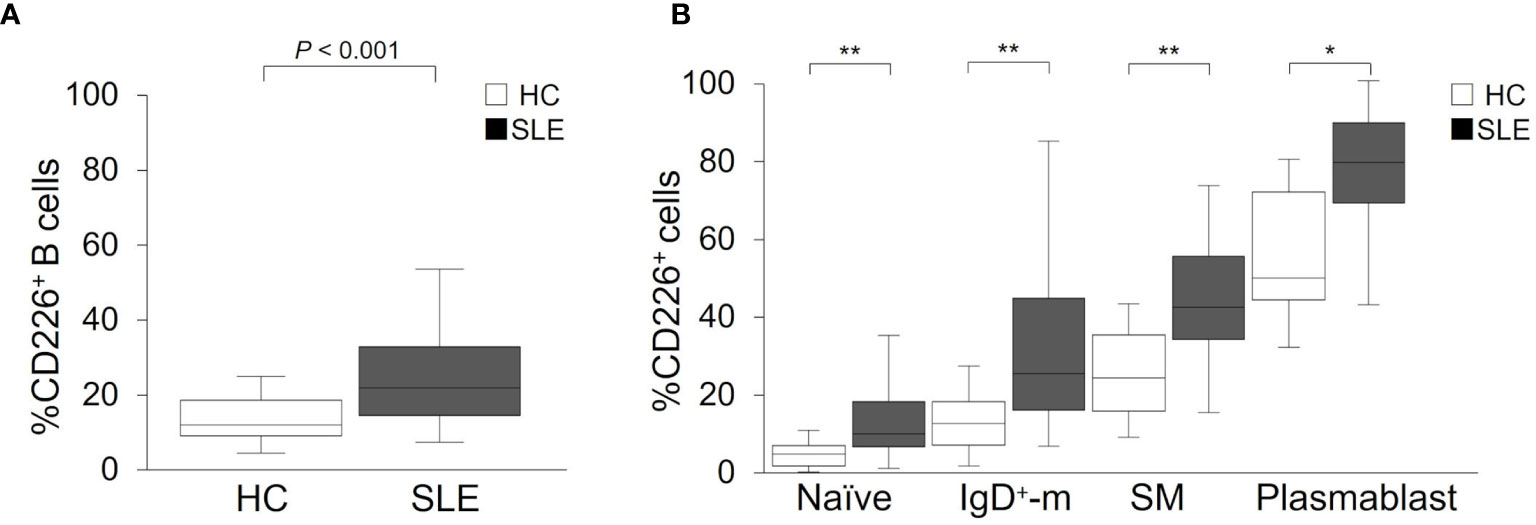
Figure 1 CD226 expression on B cell subsets in SLE patients and healthy controls (HCs). Proportions of CD226+ cells in B cells (A), naive B cells, IgD+-memory (IgD+-m) B cells, switched-memory (SM) B cells, and plasmablasts (B) were compared between SLE patients and HCs. The median (interquartile range) proportions of CD226+ cells from SLE patients and HCs were 21.9% (14.4–32.9) and 11.9% (9.0–18.5) in B cells; 10.6% (7.4–18.7) and 5.4% (2.4–7.5) in naive B cells; 25.9% (16.6–44.9) and 13.2% (7.6–18.8) in IgD+-m B cells; 42.6% (34.5–55.5) and 24.8% (16.4–35.6) in SM B cells; 79.3% (69.0–89.3) and 50.0% (44.6–71.8) in plasmablasts. Box plots represent the median values, interquartile ranges, and the range of values. Statistical differences among groups were evaluated using the Mann–Whitney U test (*P < 0.005, **P < 0.001).
To reveal whether increased proportions of CD226+ cells occurred in a particular B cell subset, we assessed CD226 expression on each subset: naive B cells, IgD+-memory B cells, SM B cells, and plasmablasts. In both SLE patients and HCs, the proportions of CD226+ cells increased in differentiated B cells, such as SM B cells and plasmablasts (Figure 1B). Interestingly, the proportions of CD226+ cells in all B cell subsets were higher to same extent in SLE patients compared with HCs (Figure 1B). Thus, the proportions of CD226+ B cells increased in SLE patients and such increases were seen in all B cell subsets, with no specificity to a particular subset.
We studied the relation between CD226+ B cells and disease activity of SLE. We first confirmed that the proportion of CD226+ B cells had no obvious correlation with prednisolone equivalent dose (ρ = 0.09; P = 0.54) and were almost the same between SLE patients with immunosuppressive agents and those without immunosuppressive agents [23.0% (15.5–31.7) vs 21.4% (12.1–37.0); P = 0.76]. The percentage of CD226+ B cells had a significant correlation with SLEDAI-2K scores (ρ = 0.40; P = 0.005) and clinical SLEDAI-2K scores (ρ = 0.37; P = 0.009) (Figures 2A, B). This percentage also had a significant positive correlation with anti-dsDNA antibody titers (ρ = 0.41; P = 0.004) (Figure 2C) and an inverse correlation with serum levels of C3 (ρ = −0.28; P = 0.055) and C4 (ρ = −0.32; P = 0.027) (Figures 2D, E). Across all B cell subsets, the percentage of CD226+ SM B cells was also significantly correlated positively with SLEDAI-2K scores (ρ = 0.36; P = 0.012), clinical SLEDAI-2K scores (ρ = 0.30; P = 0.037), and anti-dsDNA antibody titers (ρ = 0.47; P = 0.001) and inversely correlated with C3 (ρ = −0.36; P = 0.011) and C4 levels (ρ = −0.39; P = 0.007). Similarly, the percentage of CD226+ plasmablasts was correlated positively but non-significantly with SLEDAI-2K scores (ρ = 0.27; P = 0.066), clinical SLEDAI-2K scores (ρ = 0.27; P = 0.059), and anti-dsDNA antibody titers (ρ = 0.27; P = 0.066), whereas CD226 expression on the other B cell subsets had no obvious relation to such parameters (Supplementary Table 1).
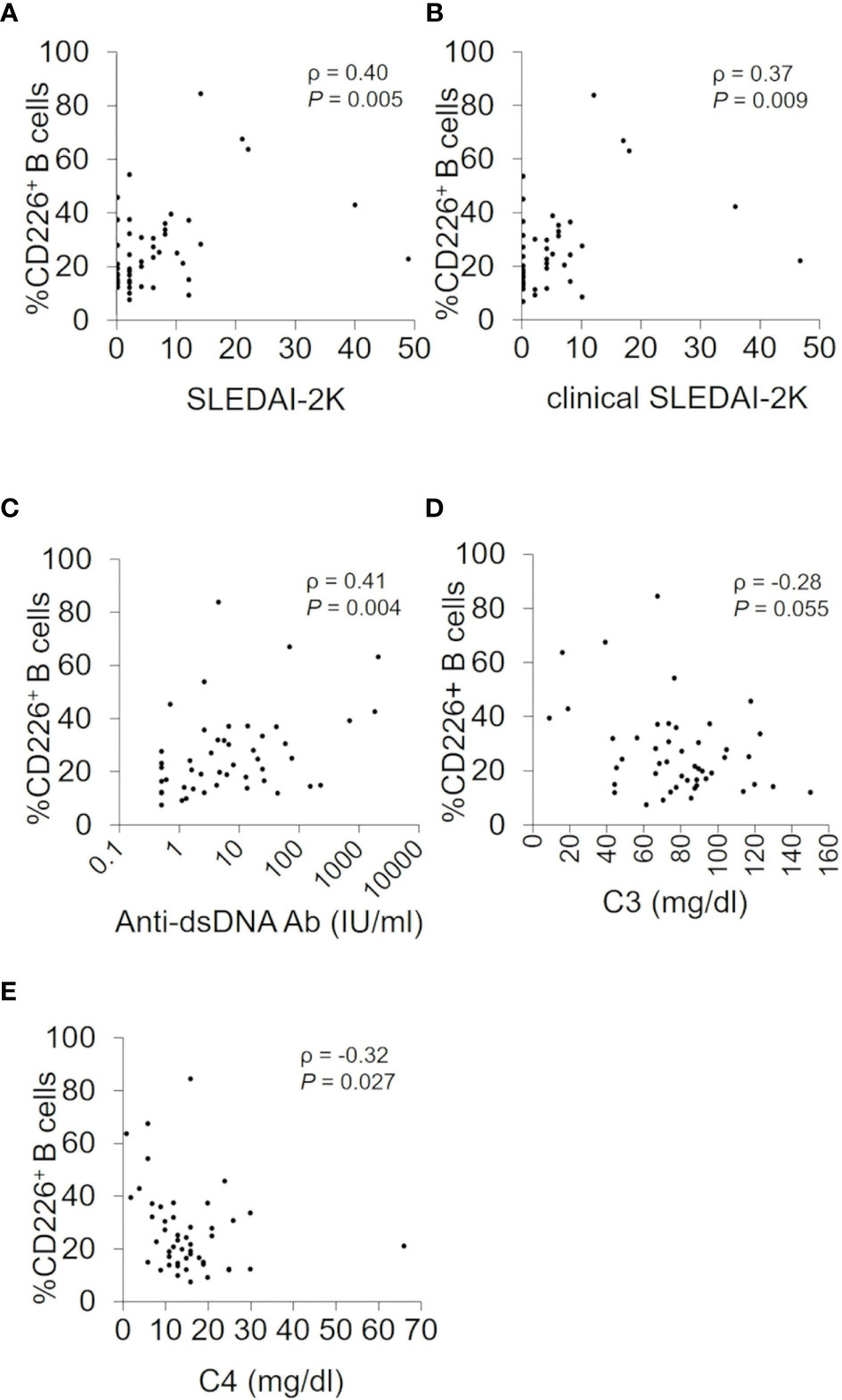
Figure 2 Associations between CD226+ B cells and SLEDAI-2K, clinical SLEDAI-2K, anti-dsDNA antibody titers, serum C3, and C4 levels. Correlations between the percentage of CD226+ B cells and SLEDAI-2K scores (A), clinical SLEDAI-2K (B), anti-dsDNA antibody (Ab) titers (C), the serum C3 (D), and C4 levels (E) in SLE patients. Each data point represents a single subject. Correlation analyses were evaluated using Spearman’s rank correlation. SLEDAI-2K, SLE Disease Activity Index 2000.
We also assessed the association between CD226+ B cells and clinical manifestations of SLE and found that the proportion of CD226+ B cells was significantly higher in patients with renal, musculoskeletal, and/or hematological manifestations (Supplementary Table 2). Thus, CD226+ B cells are associated with disease activity and clinical manifestations.
To further investigate the relation of CD226+ B cells and disease activity of SLE, we examined CD226+ B cells in 5 patients with active SLE before and after treatment (median follow-up duration, 1.0 month). In 4 SLE patients who had improved SLEDAI-2K scores and renal SLEDAI-2K scores after treatment, the frequency of CD226+ B cells decreased after medication (Figures 3A–C, shown in solid lines). In contrast, the frequency of CD226+ B cells increased in one SLE patient who had impaired disease activity (Figures 3A–C, shown in dashed lines). When we measured CD226 expression on each B cell subset in the same patients, the frequency of each CD226+ B cell subset showed no significant differences before and after treatment (data not shown). These findings suggest that CD226+ B cells reflect disease activity.
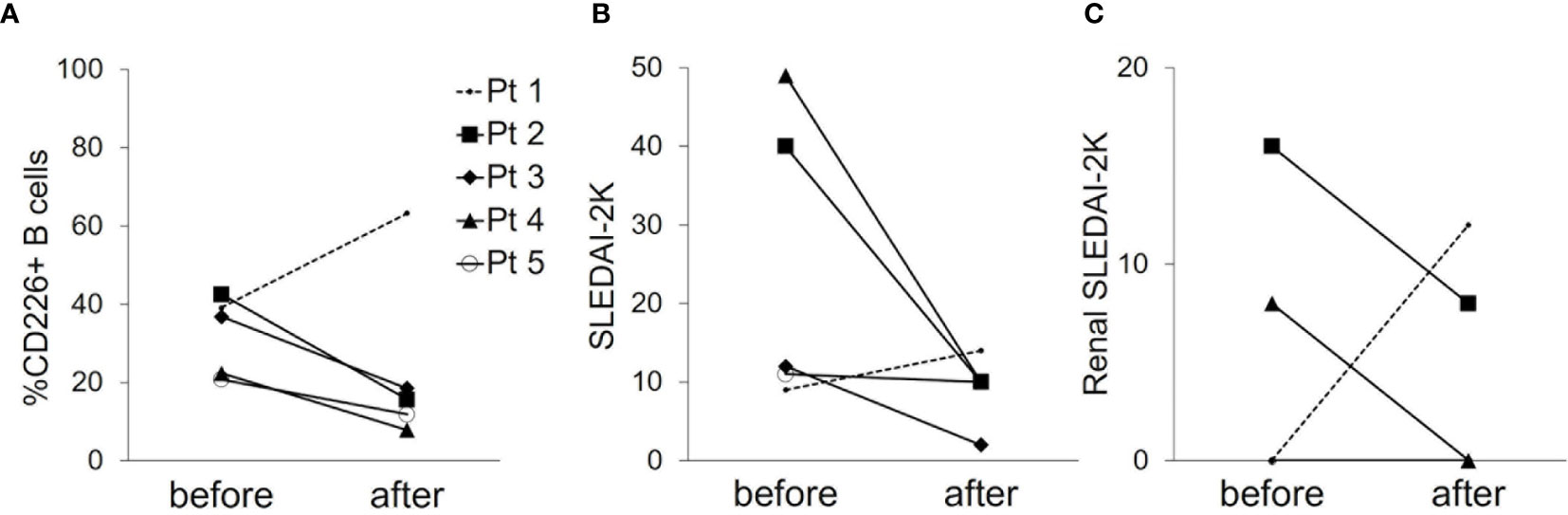
Figure 3 Changes in the frequency of CD226+ B cells and disease activity before and after treatment. The frequency of CD226+ B cells (A), SLEDAI-2K scores (B), and renal SLEDAI-2K scores (C) before and after treatment in 5 SLE patients are shown. Each data point represents a single subject. SLEDAI-2K, SLE Disease Activity Index 2000.
Because SLE treatment should aim at remission or low disease activity and prevention of flares in all organs in 2019 EULAR recommendation (5), we assessed the total disease activity and prognosis first. Of the 48 SLE patients examined, 10 were in an active state and 16 were in LLDAS at baseline, and the percentage of CD226+ B cells was higher in an active state than in LLDAS [32.4% (19.3–64.2) vs 18.8% (13.4–30.7); P = 0.078]. To determine if CD226+ B cells at baseline can predict disease prognosis after 12 months, we compared the proportion of CD226+ B cells at baseline between patients who were in LLDAS after 12 months and those who were not (Figure 4A). In LLDAS patients at baseline, the proportion of CD226+ B cells and CD226+ SM B cells was significantly lower in patients who remained in LLDAS after 12 months than in patients who were not (Figures 4B, D). In active state patients at baseline, the percentage of CD226+ cells in B cells, especially SM B cells was low in patients who achieved LLDAS after 12 months (Figures 4C, E). These results suggest that CD226+ B cells, especially CD226+ SM B cells, are associated with SLE outcomes.
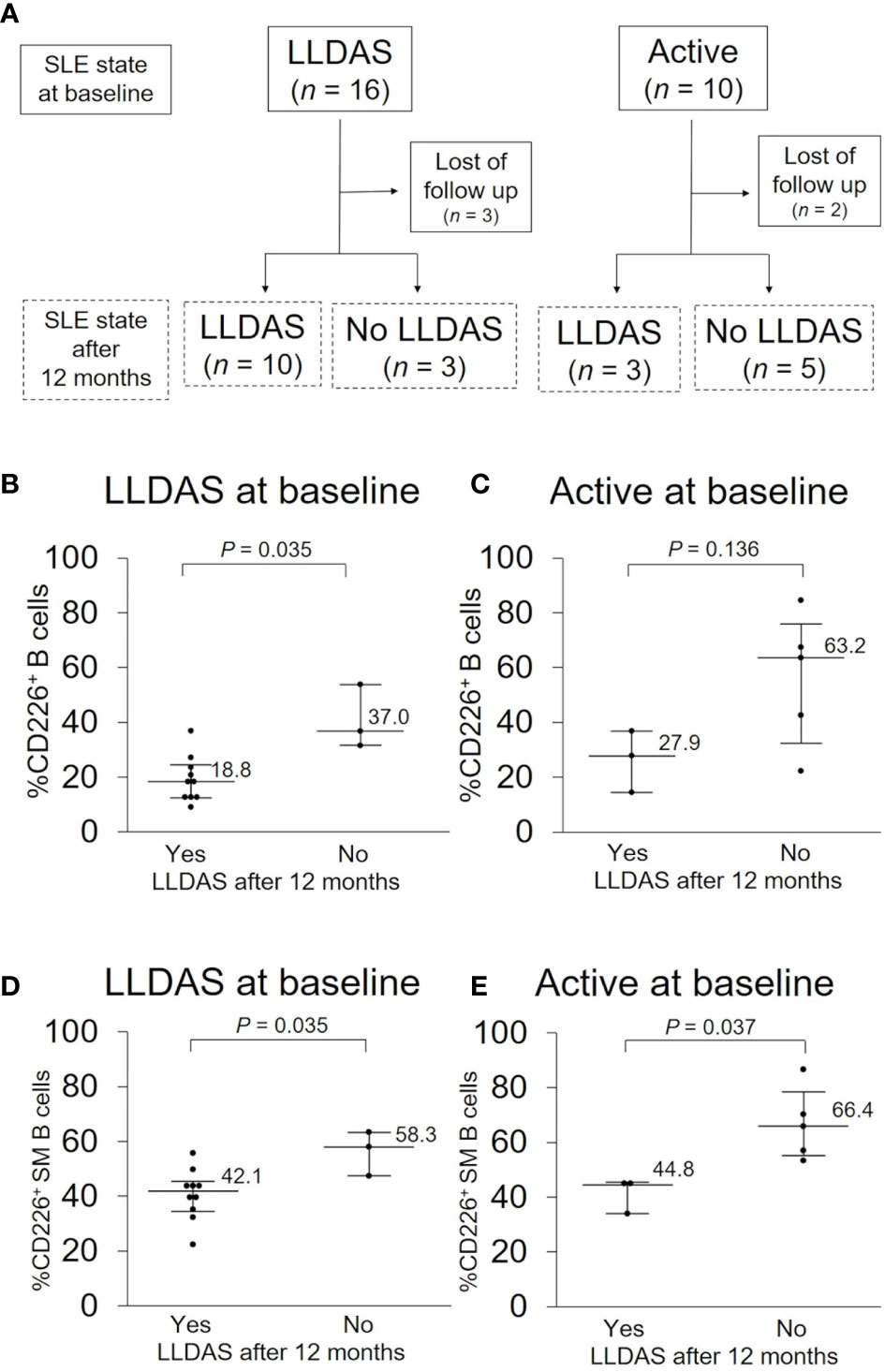
Figure 4 CD226+ B cells at baseline and disease prognosis after 12 months. (A) SLE activity state at baseline and after 12 months. In each activity state at baseline, basal proportions of CD226+ cells in B cells (B, C) and switched-memory (SM) B cells (D, E) were compared between patients who were in Lupus Low Disease Activity State (LLDAS) after 12 months and patients who were not. Each data point represents a single subject. Horizontal lines show the median and error bars represent interquartile ranges. Statistical differences among groups were evaluated using the Mann–Whitney U test.
Because renal involvement is one of the major causes of morbidity and mortality in SLE patients (32) and our study included many patients with renal involvement, we wanted to further investigate the relationship between CD226+ B cells and renal manifestation. There were 16 patients with active nephritis at baseline, and they had a significantly elevated percentage of CD226+ B cells (Figure 5A and Supplementary Table 2) which had a significant correlation with renal SLEDAI-2K scores (ρ = 0.37; P = 0.009) (Figure 5B). To investigate the association of CD226+ B cells and renal prognosis, we then compared the percentage of CD226+ B cells between patients who achieved CR after 12 months and patients who did not. In patients who achieved CR after 12 months, the frequency of CD226+ B cells at baseline was lower than in patients who did not (Figure 5C). The percentages of CD226+ cells in each B cell subset were almost the same between the two groups (data not shown). These findings indicate that CD226+ B cells may be associated with renal involvement and prognosis.
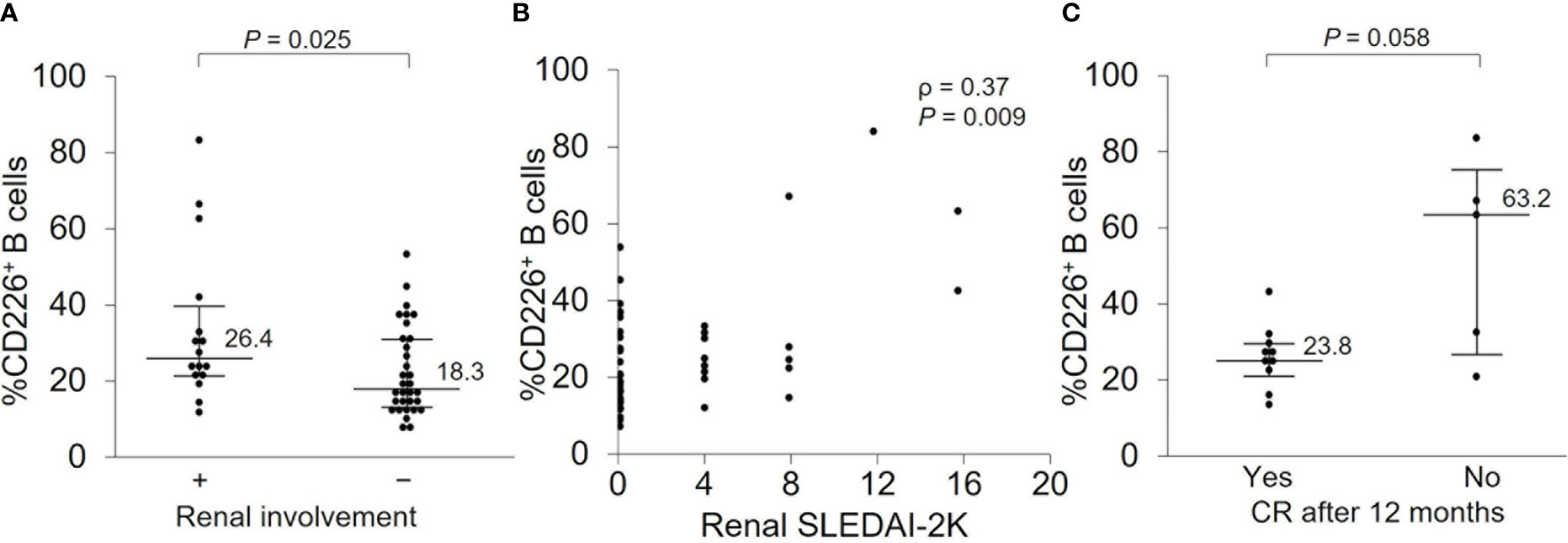
Figure 5 CD226+ B cells at baseline and renal prognosis after 12 months. (A) The frequency of CD226+ B cells was compared between SLE patients with renal involvement and those without. (B) Correlation between the percentage of CD226+ B cells and renal SLEDAI-2K scores. (C) In patients with renal involvement at baseline, the percentage of CD226+ B cells was compared between patients who achieved complete renal remission (CR) after 12 months and those who did not. Each data point represents a single subject. Horizontal lines show the median and error bars represent interquartile ranges. Statistical differences among groups were evaluated using the Mann–Whitney U test. Correlation analyses were evaluated using Spearman’s rank correlation. SLEDAI-2K, SLE Disease Activity Index 2000.
In this study, we showed that the proportion of CD226+ B cells was significantly higher in SLE patients and was associated with disease activity and renal involvement. We also demonstrated that the proportion of CD226+ B cells predicted disease outcome and renal prognosis after 12 months.
CD226 is a costimulatory adhesion molecule expressed on NK cells and T cells which mediates cytotoxic signals (18–21). T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT), a paired receptor of CD226, is a coinhibitory receptor that inhibits the activation of T cells and NK cells (33, 34). Both CD226 and TIGIT bind to CD112 and CD155, and TIGIT inhibits the interaction between CD155 and CD226 (33). Some studies have shown the involvement of CD226 and TIGIT in SLE (14–17, 22, 35). In mouse SLE models, treatment with the TIGIT-Ig fusion protein was effective for the reduction of autoantibody production and increase in survival rate (35). Regarding CD226, several studies have reported that the nonsynonymous rs763361 polymorphism in CD226 was associated with SLE in multiple ancestries (14–17). Furthermore, another study has shown that the proportion of CD226+ NK cells was decreased in active SLE patients, and this increased in some patients during therapy (22). In this previous study, CD226 expression was assessed in SLE patients with severe disease activity for a short period of time. CD226 is also expressed on the cell surface of B cells (25) which play a pivotal role in SLE (8, 11–13); however, little is known regarding the associations of CD226 on B cells with SLE. Therefore, our study focused on CD226 expression on B cells in SLE patients with various disease activity for a longer period of time.
In our study, the proportion of CD226+ B cells was significantly higher in SLE patients compared to HCs. Additionally, the percentage of CD226+ B cells was associated with disease activity and positively correlated with anti-dsDNA antibody titers. Across all B cell subsets, CD226+ SM B cells and CD226+ plasmablasts were associated with disease activity of SLE, consistent with the reports that showed SM B cells and plasmablasts increased in SLE patients and were associated with disease activity (36–39). Although we did not investigate the immune functions of CD226 on B cells in SLE, a recent study with healthy subjects reported that CD226+ B cells were upregulated by stimulation via toll-like receptor (TLR) 9 and were also involved in IL-10 and antibody productions (25). Furthermore, many studies have shown that both TLR9 and IL-10 play pivotal roles in B cell immunity in SLE (40–46). These findings suggest that CD226+ B cells, especially CD226+ SM B cells and CD226+ plasmablasts are involved in the pathogenesis of SLE.
Our analysis showed that the proportions of CD226+ cells increased in differentiated B cell subsets such as SM B cells and plasmablasts in SLE patients, consistent with a recent report involving HCs (25). B cells have been classified into several phenotypes based on differentiation stage (47), and their functions in immune responses are different (48–50). Other reports claim that the proportions of some B cell subsets such as SM B cells and plasmablasts are altered in SLE patients (13, 36–39, 51). In our study, however, the proportions of CD226+ cells in all B cell subsets were higher in SLE patients than in HCs, indicating that CD226 upregulation may not reflect alterations in the proportions of B cell subsets in SLE. A recent study reported that CD226+ B cells were upregulated by stimulation via TLR 9 (25). Given that TLR 9 expression has been known to increase in differentiated B cell subsets such as SM B cells and plasmablasts (43, 44), TLR 9 may affect the CD226 upregulation according to differentiation stage of B cells. Further analyses of the mechanism of CD226 upregulation on B cells are required to clarify this.
In this study, increased proportion of CD226+ B cells was associated with disease activity. Moreover, CD226+ B cells at baseline were low in patients who were in LLDAS after 12 months. According to the 2019 EULAR recommendation, SLE treatment should aim for remission or LDA monitoring disease activity (5). Among various definitions of LDA, LLDAS has been used in many studies (31, 52, 53). While there are many biomarkers for monitoring disease activity (6, 7), there are no useful markers for predicting LLDAS. In our study, conventional biomarkers such as anti-dsDNA titers and complement levels were associated with disease activity; however, these biomarkers could not predict the prognosis after 12 months (data not shown). Our study showed that CD226+ B cells were associated with disease activity and the prognosis of SLE. Although some studies have tried to find B cell phenotypes associated with disease activity of SLE, these studies need to measure the expression of various surface molecules, but these were not established (13, 38, 39). Although our results are needed to be validated in a prospective study with a larger sample size, CD226+ B cells may be a candidate of a useful biomarker for disease activity and prognosis of SLE.
In this study, we further investigated the relationship between CD226+ B cells and renal manifestation. The results showed that the proportion of CD226+ B cells was higher in patients with renal involvement; this reflected renal disease activity. Among various clinical manifestations of SLE, LN is a major cause of morbidity and mortality (32). Therefore, it is recommended that treatment for LN should aim for CR (5, 54, 55). However, no useful biomarkers for predicting CR have yet been developed. In our study, we showed that patients with a higher percentage of CD226+ B cells at baseline did not achieve CR after 12 months. Although it is necessary to investigate the infiltration of CD226+ cells into the kidney and urinary analysis, these findings indicate the utility of CD226+ B cells as a biomarker for predicting renal outcome.
Our study had some limitations. First, this study was a single-center study with small sample size. Because our institution is a tertiary referral hospital, a relatively large number of patients with refractory manifestations, such as LN and NPSLE, were included in this study. Moreover, although we targeted patients with various disease activity and clinical manifestations, there were few active patients without renal manifestations in our study. There is a need to replicate this study with a larger sample size in a multicenter setting and assess the prognosis in active patients with other than renal manifestations. Second, the functions of CD226 on B cells in SLE are still unknown; further studies are required to reveal the mechanisms of CD226 on B cells in SLE. Lastly, this study was a retrospective study, and anti-dsDNA antibody titers using Farr assay, which is defined in SLEDAI-2K (27), were not measured. To confirm the association of CD226 on B cells with the prognosis of SLE, a prospective study needs to be performed as well.
In conclusion, we demonstrated that the proportion of CD226+ B cells increased in SLE patients and could be associated with disease activity and prognosis. These findings enable more precise control for the T2T strategy in SLE.
The original contributions presented in the study are included in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the ethics committee of Kyushu University Hospital (approval number 2019-481). The patients/participants provided their written informed consent to participate in this study.
MN and MAy participated in study conception and design. MN, MAy, KK, SK, KH, and SI participated in data acquisition and analysis. MN, MAy, KK, HM, YK, MAk, NO, YA, KA, TH, and HN contributed to the interpretation of results. MN was a major contributor in writing the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Japan Society for the Promotion of Science [grant number JSPS KAKENHI 19K17887].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Enago for the English language review.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.713225/full#supplementary-material
1. Tsokos GC. Systemic Lupus Erythematosus. N Engl J Med (2011) 365:2110–21. doi: 10.1056/NEJMra1100359
2. Tsokos GC. Autoimmunity and Organ Damage in Systemic Lupus Erythematosus. Nat Immunol (2020) 21:605–14. doi: 10.1038/s41590-020-0677-6
3. Gatto M, Zen M, Iaccarino L, Doria A. New Therapeutic Strategies in Systemic Lupus Erythematosus Management. Nat Rev Rheumatol (2019) 15:30–48. doi: 10.1038/s41584-018-0133-2
4. Van Vollenhoven RF, Mosca M, Bertsias G, Isenberg D, Kuhn A, Lerstrøm K, et al. Treat-to-Target in Systemic Lupus Erythematosus: Recommendations From an International Task Force. Ann Rheum Dis (2014) 73:958–67. doi: 10.1136/annrheumdis-2013-205139
5. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 Update of the EULAR Recommendations for the Management of Systemic Lupus Erythematosus. Ann Rheum Dis (2019) 78:736–45. doi: 10.1136/annrheumdis-2019-215089
6. Arriens C, Wren JD, Munroe ME, Mohan C. Systemic Lupus Erythematosus Biomarkers: The Challenging Quest. Rheumatol (Oxford) (2017) 56:i32–45. doi: 10.1093/rheumatology/kew407
7. Capecchi R, Puxeddu I, Pratesi F, Migliorini P. New Biomarkers in SLE: From Bench to Bedside. Rheumatol (Oxford) (2020) 59:v12–18. doi: 10.1093/rheumatology/keaa484
8. Tsokos GC, Lo MS, Reis PC, Sullivan KE. New Insights Into the Immunopathogenesis of Systemic Lupus Erythematosus. Nat Rev Rheumatol (2016) 12:716–30. doi: 10.1038/nrrheum.2016.186
9. Harley ITW, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic Susceptibility to SLE: New Insights From Fine Mapping and Genome-Wide Association Studies. Nat Rev Genet (2009) 10:285–90. doi: 10.1038/nrg2571
10. Bentham J, Morris DL, Cunninghame Graham DS, Pinder CL, Tombleson P, Behrens TW, et al. Genetic Association Analyses Implicate Aberrant Regulation of Innate and Adaptive Immunity Genes in the Pathogenesis of Systemic Lupus Erythematosus. Nat Genet (2015) 47:1457–64. doi: 10.1038/ng.3434
11. Sanz I, Lee FEH. B Cells as Therapeutic Targets in SLE. Nat Rev Rheumatol (2010) 6:326–37. doi: 10.1038/nrrheum.2010.68
12. Dörner T, Lipsky PE. Beyond pan-B-cell-directed Therapy-New Avenues and Insights Into the Pathogenesis of SLE. Nat Rev Rheumatol (2016) 12:645–57. doi: 10.1038/nrrheum.2016.158
13. Carvajal Alegriaw G, Gazeau P, Hillion S, Daïen CI, Cornec DYK. Could Lymphocyte Profiling be Useful to Diagnose Systemic Autoimmune Diseases? Clin Rev Allergy Immunol (2017) 53:219–36. doi: 10.1007/s12016-017-8608-5
14. Sun C, Molineros JE, Looger LL, Zhou XJ, Kim K, Okada Y, et al. High-Density Genotyping of Immune-Related Loci Identifies New SLE Risk Variants in Individuals With Asian Ancestry. Nat Genet (2016) 48:323–30. doi: 10.1038/ng.3496
15. Nie D, Li H, Yan G, Wang Z, He Z, Zhou W. Gene–Gene Interaction Between CD40 and CD226 Gene on Systemic Lupus Erythematosus in the Chinese Han Population. Rheumatol Int (2016) 36:1657–62. doi: 10.1007/s00296-016-3570-8
16. Wang YF, Zhang Y, Zhu Z, Wang TY, Morris DL, Shen JJ, et al. Identification of ST3AGL4, Mfhas1, CSNK2A2 and CD226 as Loci Associated With Systemic Lupus Erythematosus (SLE) and Evaluation of SLE Genetics in Drug Repositioning. Ann Rheum Dis (2018) 77:1078–84. doi: 10.1136/annrheumdis-2018-213093
17. Maiti AK, Kim-Howard X, Viswanathan P, Guillén L, Qian X, Rojas-Villarraga A, et al. Non-Synonymous Variant (Gly307Ser) in CD226 Is Associated With Susceptibility to Multiple Autoimmune Diseases. Rheumatol (Oxford) (2010) 49:1239–44. doi: 10.1093/rheumatology/kep470
18. Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanashan T, et al. Dnam-1, A Novel Adhesion Molecule Involved in the Cytolytic Function of T Lymphocytes. Immunity (1996) 4:573–81. doi: 10.1016/S1074-7613(00)70060-4
19. Xu Z, Jin B. A Novel Interface Consisting of Homologous Immunoglobulin Superfamily Members With Multiple Functions. Cell Mol Immunol (2010) 7:11–9. doi: 10.1038/cmi.2009.108
20. Martinet L, Smyth MJ. Balancing Natural Killer Cell Activation Through Paired Receptors. Nat Rev Immunol (2015) 15:243–54. doi: 10.1038/nri3799
21. Huang Z, Qi G, Miller JS, Zheng SG. Cd226: An Emerging Role in Immunologic Diseases. Front Cell Dev Biol (2020) 8:1–9. doi: 10.3389/fcell.2020.00564
22. Huang Z, Fu B, Zheng SG, Li X, Sun R, Tian Z, et al. Involvement of CD226+ Nk Cells in Immunopathogenesis of Systemic Lupus Erythematosus. J Immunol (2011) 186:3421–31. doi: 10.4049/jimmunol.1000569
23. Fasth AER, Björkström NK, Anthoni M, Malmberg KJ, Malmström V. Activating NK-Cell Receptors Co-Stimulate CD4+CD28– T Cells in Patients With Rheumatoid Arthritis. Eur J Immunol (2010) 40:378–87. doi: 10.1002/eji.200939399
24. Ayano M, Tsukamoto H, Kohno K, Ueda N, Tanaka A, Mitoma H, et al. Increased CD226 Expression on CD8+ T Cells Is Associated With Upregulated Cytokine Production and Endothelial Cell Injury in Patients With Systemic Sclerosis. J Immunol (2015) 195:892–900. doi: 10.4049/jimmunol.1403046
25. Nagayama-Hasegawa Y, Honda S, Shibuya A, Shibuya K. Expression and Function of DNAM-1 on Human B-Lineage Cells. Cytometry B Clin Cytom (2020) 98:368–74. doi: 10.1002/cyto.b.21859
26. Hochberg MC. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum (1997) 40:1725. doi: 10.1002/art.1780400928
27. Gladman DD, Ibañez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol (2002) 29:288–91.
28. Uribe AG, Vila LM, McGwin G, Sanchez ML, Reveille JD, Alarcon GS. The Systemic Lupus Activity Measure-Revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a Modified SLEDAI-2K Are Adequate Instruments to Measure Disease Activity in Systemic Lupus Erythematosus. J Rheumatol (2004) 31:1934–40.
29. Cook RJ, Gladman DD, Pericak D, Urowitz MB. Prediction of Short Term Mortality in Systemic Lupus Erythematosus With Time Dependent Measures of Disease Activity. J Rheumatol (2000) 27:1892–5.
30. Hanaoka M, Gono T, Kawaguchi Y, Uchida K, Koseki Y, Katsumata Y, et al. Urinary Free Light Chain Is a Potential Biomarker for ISN/RPS Class III/IV Lupus Nephritis. Rheumatol (Oxford) (2013) 52:2149–57. doi: 10.1093/rheumatology/ket108
31. Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, et al. Definition and Initial Validation of a Lupus Low Disease Activity State (Lldas). Ann Rheum Dis (2016) 75:1615–21. doi: 10.1136/annrheumdis-2015-207726
32. Hanly JG, O’Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, et al. The Frequency and Outcome of Lupus Nephritis: Results From an International Inception Cohort Study. Rheumatol (Oxford) (2015) 55:252–62. doi: 10.1093/rheumatology/kev311
33. Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, et al. The Surface Protein TIGIT Suppresses T Cell Activation by Promoting the Generation of Mature Immunoregulatory Dendritic Cells. Nat Immunol (2009) 10:48–57. doi: 10.1038/ni.1674
34. Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, et al. The Interaction of TIGIT With PVR and PVRL2 Inhibits Human NK Cell Cytotoxicity. Proc Natl Acad Sci USA (2009) 106:17858–63. doi: 10.1073/pnas.0903474106
35. Liu S, Sun L, Wang C, Cui Y, Ling Y, Li T, et al. Treatment of Murine Lupus With TIGIT-Ig. Clin Immunol (2019) 203:72–80. doi: 10.1016/j.clim.2019.04.007
36. Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, et al. Disturbed Peripheral B Lymphocyte Homeostasis in Systemic Lupus Erythematosus. J Immunol (2000) 165:5970–9. doi: 10.4049/jimmunol.165.10.5970
37. Kosalka J, Jakiela B, Musial J. Changes of Memory B-and T-Cell Subsets in Lupus Nephritis Patients. Folia Histochem Cytobiol (2016) 54:32–41. doi: 10.5603/FHC.a2016.0005
38. Jin W, Luo Z, Yang H. Peripheral B Cell Subsets in Autoimmune Diseases: Clinical Implications and Effects of B Cell-Targeted Therapies. J Immunol Res (2020) 2020:9518137. doi: 10.1155/2020/9518137
39. Iwata S, Tanaka Y. B-Cell Subsets, Signaling and Their Roles in Secretion of Autoantibodies. Lupus (2016) 25:850–6. doi: 10.1177/0961203316643172
40. Marshak-Rothstein A. Toll-Like Receptors in Systemic Autoimmune Disease. Nat Rev Immunol (2006) 6:823–35. doi: 10.1038/nri1957
41. Nakano S, Morimoto S, Suzuki J, Nozawa K, Amano H, Tokano Y, et al. Role of Pathogenic Auto-Antibody Production by Toll-like Receptor 9 of B Cells in Active Systemic Lupus Erythematosus. Rheumatol (Oxford) (2008) 47:145–9. doi: 10.1093/rheumatology/kem327
42. Yuan Y, Zhao L, Ye Z, Ma H, Wang X, Jiang Z. Association of Toll-Like Receptor 9 Expression With Prognosis of Systemic Lupus Erythematosus. Exp Ther Med (2019) 17:3247–54. doi: 10.3892/etm.2019.7290
43. Bernasconi NL, Onai N, Lanzavecchia A. A Role for Toll-like Receptors in Acquired Immunity: Up-Regulation of TLR9 by BCR Triggering in Naive B Cells and Constitutive Expression in Memory B Cells. Blood (2003) 101:4500–4. doi: 10.1182/blood-2002-11-3569
44. Papadimitraki ED, Choulaki C, Koutala E, Bertsias G, Tsatsanis C, Gergianaki I, et al. Expansion of Toll-Like Receptor 9-Expressing B Cells in Active Systemic Lupus Erythematosus: Implications for the Induction and Maintenance of the Autoimmune Process. Arthritis Rheum (2006) 54:3601–11. doi: 10.1002/art.22197
45. Peng H, Wang W, Zhou M, Li R, HF P, Ye DQ. Role of interleukin-10 and Interleukin-10 Receptor in Systemic Lupus Erythematosus. Clin Rheumatol (2013) 32:1255–66. doi: 10.1007/s10067-013-2294-3
46. Llorente L, Richaud-Patin Y, García-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, et al. Clinical and Biologic Effects of Anti-Interleukin-10 Monoclonal Antibody Administration in Systemic Lupus Erythematosus. Arthritis Rheum (2000) 43:1790–800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2
47. Maecker HT, McCoy JP, Nussenblatt R. Standardizing Immunophenotyping for the Human Immunology Project. Nat Rev Immunol (2012) 12:191–200. doi: 10.1038/nri3158
48. Cyster JG, Allen CDC. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell (2019) 177:524–40. doi: 10.1016/j.cell.2019.03.016
49. Kurosaki T, Kometani K, Ise W. Memory B Cells. Nat Rev Immunol (2015) 15:149–59. doi: 10.1038/nri3802
50. Hiepe F, Radbruch A. Plasma Cells as an Innovative Target in Autoimmune Disease With Renal Manifestations. Nat Rev Nephrol (2016) 12:232–40. doi: 10.1038/nrneph.2016.20
51. Tanaka Y, Kubo S, Iwata S, Yoshikawa M, Nakayamada S. B Cell Phenotypes, Signaling and Their Roles in Secretion of Antibodies in Systemic Lupus Erythematosus. Clin Immunol (2018) 186:21–5. doi: 10.1016/j.clim.2017.07.010
52. Zen M, Iaccarino L, Gatto M, Saccon F, Larosa M, Ghirardello A, et al. Lupus Low Disease Activity State Is Associated With a Decrease in Damage Progression in Caucasian Patients With SLE, But Overlaps With Remission. Ann Rheum Dis (2018) 77:104–10. doi: 10.1136/annrheumdis-2017-211613
53. Petri M, Magder LS. Comparison of Remission and Lupus Low Disease Activity State in Damage Prevention in a United States Systemic Lupus Erythematosus Cohort. Arthritis Rheumatol (2018) 70:1790–5. doi: 10.1002/art.40571
54. Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (Eular/Era-EDTA) Recommendations for the Management of Lupus Nephritis. Ann Rheum Dis (2020) 79:713–23. doi: 10.1136/annrheumdis-2020-216924
Keywords: systemic lupus erythematosus, CD226, B cells, lupus low disease activity, lupus nephritis
Citation: Nakano M, Ayano M, Kushimoto K, Kawano S, Higashioka K, Inokuchi S, Mitoma H, Kimoto Y, Akahoshi M, Ono N, Arinobu Y, Akashi K, Horiuchi T and Niiro H (2021) Increased Proportion of CD226+ B Cells Is Associated With the Disease Activity and Prognosis of Systemic Lupus Erythematosus. Front. Immunol. 12:713225. doi: 10.3389/fimmu.2021.713225
Received: 22 May 2021; Accepted: 05 July 2021;
Published: 21 July 2021.
Edited by:
Linda L. Kusner, George Washington University, United StatesReviewed by:
Johannes Nossent, University of Western Australia, AustraliaCopyright © 2021 Nakano, Ayano, Kushimoto, Kawano, Higashioka, Inokuchi, Mitoma, Kimoto, Akahoshi, Ono, Arinobu, Akashi, Horiuchi and Niiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masahiro Ayano, YXlhbm8ubWFzYWhpcm8uODExQG0ua3l1c2h1LXUuYWMuanA=
†Present address: Miki Nakano, Department of Rheumatology, Fukuoka National Hospital, Fukuoka, Japan
Kazuhiko Higashioka, Center for Rheumatology, Iizuka Hospital, Fukuoka, Japan
Mitsuteru Akahoshi, Department of Rheumatology, Saga University Hospital, Saga, Japan
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.