- Department of Stomatology, China-Japan Union Hospital of Jilin University, Changchun, China
Aim: Periodontitis is an inflammatory disease that destroys both soft and hard periodontal tissues. However, a complex periodontal cytokine network remains unclear. This systematic review explored multiple cytokine gene polymorphisms in the pathogenesis of periodontitis.
Material and Methods: A systematic search was performed using the databases from previous publications, which indicated the association between cytokine polymorphisms and periodontitis pathogenesis. Meta-analysis was conducted using fixed or randomized models to calculate the significance of multiple cytokine polymorphisms. A total of 147 articles were analyzed with polymorphisms in 12 interleukins [Th1 (IL-2, IFN-γ, and TNF-α), Th2 (IL-4 and IL-13), Th17 (IL-1α, IL-1β, IL-6, and IL-17), and Treg cytokines (IL-10 and TGF-β)]. Doi plot was used to probe the occurrence of publication bias.
Results: The polymorphisms of IL-2 and TNF-α of Th1 cytokine family may be associated with the pathogenesis or the prevention of periodontitis risk, while the polymorphism of IFN-γ is not related to periodontitis risk. The polymorphisms for IL-4 and IL-13 of Th2 cytokine family are not found to be associated with the pathogenesis of periodontitis. For the polymorphisms of the members of Th17 cytokine family, different IL-1α polymorphisms may have inverse actions in the pathogenesis of periodontitis. IL-1β is a noteworthy cytokine biomarker in periodontitis development and progression. IL-6 may have a protective function in the inflammatory responses of periodontitis, and IL-17 has a weak relationship the inflammatory responses. The polymorphisms for the members of Treg cell cytokines may have a protective function against periodontitis risk. LFK indexes show the major asymmetry due to publication bias.
Conclusion: IL-1β is a notable cytokine biomarker in periodontitis risk. Treg cytokines favor an anti-inflammatory and protective environment. Further data are needed to confirm the present conclusion due to publication bias.
Introduction
Periodontitis is a chronic destructive inflammatory disease that destroys the tooth-supporting structures and results in tooth loss (1). The cytokine network plays an important role in periodontitis development and immune responses (2), and its association with periodontitis remains widely unclear. Cytokine expression profiles are closely related to Th1, Th2, Th17, and Treg cells, which play an immunoregulatory role (3). Interferon‐γ (IFN-γ), tumor necrosis factor α (TNF-α), and IL-2 belong to Th1 cytokines (4); and T follicular helper cells have been shown to be a significant source of IL-4 and IL-13 in the lymph nodes during the Th2 activity (5). Th17 cells are the major source of interleukin-17 (6), TGF-β (7), IL-1 and IL-6 (8), and IL-23 (9). Anti-inflammatory IL-10 (10) and TGF-β (11) are secreted by B cell-endowed Tregs, involved in the suppressive function of regulatory T cells, and play critical roles in maintaining immune homeostasis.
Many studies have proven gene polymorphisms in cytokines such as IL‐1α (12–28), IL-1β (12, 16, 18, 24, 29–55), IL-2 (56–60), IL-4 (61–73), IL‐6 (74–86), IL-23 (87), IL-10 (16, 42, 84–86, 88–110), IL-13 (66, 111, 112), IL-17 (60, 73, 87, 113–117), TNF-α (16, 35, 38, 44, 48, 85, 118–132), IFN (70, 85, 102, 133–136), and TGF (42, 137–143), which often play various vital roles in periodontitis immune pathogenesis or in the prevention of periodontitis immune responses.
However, the genetic polymorphisms of these cytokines were often analyzed separately and seldom compared together. The significant differences remain unclear. Therefore, in the current meta‐analysis, a total of 147 studies were included to further identify the contributions of these genetic polymorphisms using a broader collection of patients and races.
Materials and Methods
Search Strategy and Inclusion Criteria
The criteria for inclusion in our meta‐analysis were as follows: 1) case–control studies; 2) the case groups consisted of patients diagnosed with periodontitis, and the control groups consisted of periodontally healthy individuals; 3) the genetic polymorphisms were detected, and sufficient data supporting the genotype distribution were provided for the calculation of odds ratios (ORs) and corresponding 95% confidence intervals (CIs); and 4) studies with no repeated data. Studies that did not fit any of the criteria were excluded.
A systematic literature search was performed using the databases PubMed, the Cochrane Library, Medical Abstracts, TOXLINE, OVID, EMBASE, Web of Science, EBSCO, VIP Full Text, CNKI, and Wanfang, including studies published up to January 31, 2021. In addition, the reference lists of the selected manuscripts and related reviews were also manually searched and screened to ensure that a comprehensive search was performed. Furthermore, no language restriction was applied. Two authors independently searched all the databases. The following key terms were used for searching; “interleukin‐1”, “interleukin 1”, “IL‐1”, “IL1”, “interleukin‐2”, “interleukin 2”, “IL‐2”, “IL2”, “interleukin‐4”, “interleukin 4”, “IL‐4”, “IL4”, “interleukin‐6”, “interleukin 6”, “IL‐6”, “IL6”, “interleukin-10”, “interleukin 10”, “IL‐10”, “IL10”, “interleukin‐13”, “interleukin 13”, “IL‐13”, “IL13”, “interleukin‐17”, “interleukin 17”, “IL‐17”, “IL17”, “interferon‐gamma”, “interferon gamma”, “IFN-gamma”, “IFN gamma”, “interferon‐γ”, “interferon γ”, “IFN-γ”, “IFN γ”, “tumor necrosis factor‐alpha”, “tumor necrosis factor alpha”, “TNF‐alpha”, “TNF alpha”, “tumor necrosis factor‐α”, “tumor necrosis factor α”, “TNF‐α”, “TNF α”, “transforming growth factor-beta”, “transforming growth factor beta”, “TGF‐beta”, “TGF beta”, “transforming growth factor-β”, “transforming growth factor β”, “TGF‐β”, “TGF β”, “polymorphism”, “periodontal diseases”, “periodontitis”, and their combined phrase.
Study Selection and Data Extraction
During the data selection, duplicate researches were removed using Endnote. The abstracts of selected papers were further screened, and the full text was checked based on the selection criteria (Figure 1). The results were examined by two authors, and an expert was referred if inconsistencies appeared. Two authors obtained the following characteristics, and inconsistencies were determined via discussion: 1) the first author name and publication years; 2) nationality and/or ethnicity; 3) population size; 4) genetic polymorphism; and 5) Hardy–Weinberg equilibrium (HWE) in these populations.
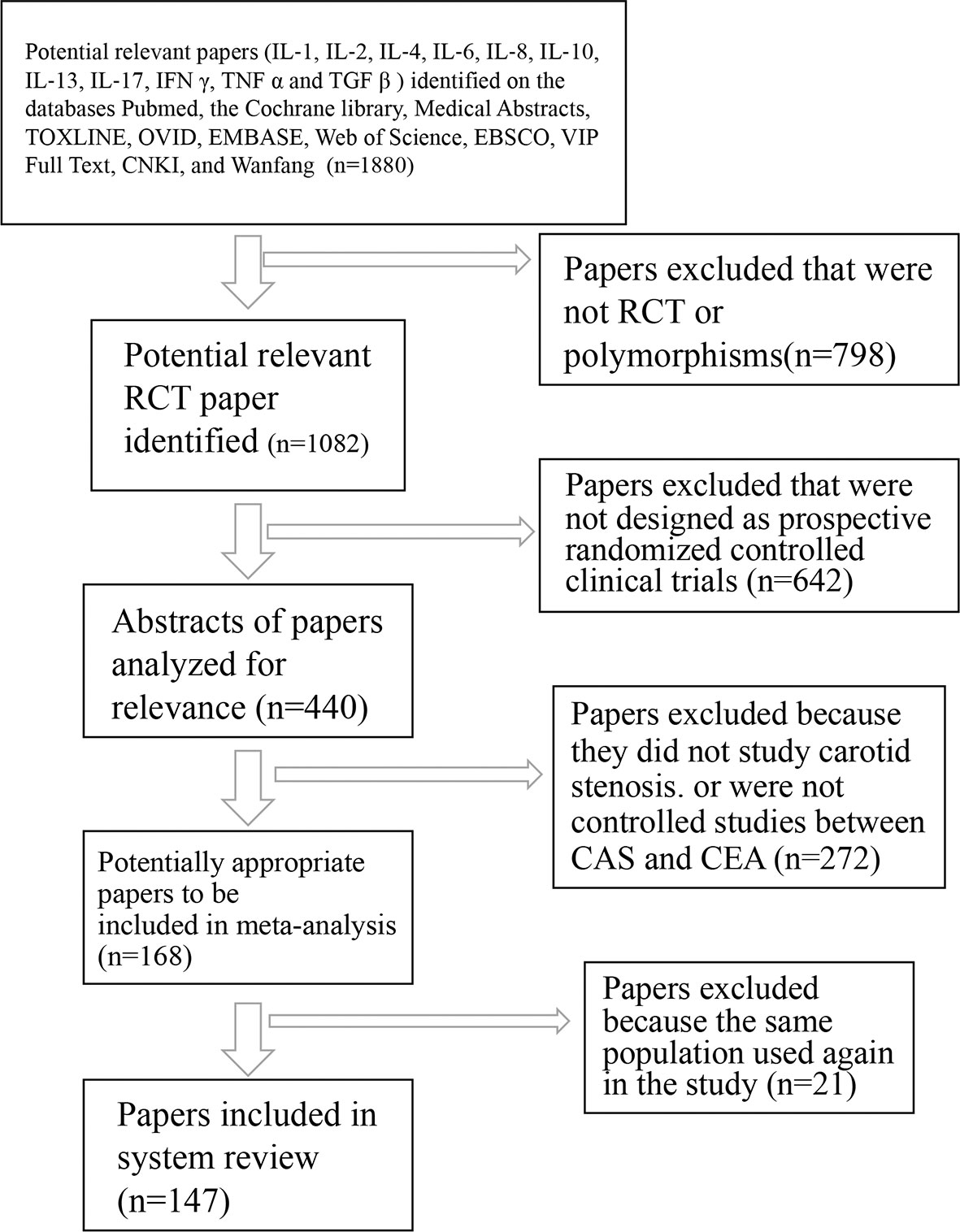
Figure 1 Flowchart of trial search and selection for meta-analysis. RCT, randomized and controlled trial; IL‐1, interleukin‐1; IL‐2, interleukin-2; IL‐4, interleukin‐4; IL‐6, interleukin‐6; IL‐8, interleukin‐8; IL‐10, interleukin-10; IL‐13, interleukin‐13; IL‐17, interleukin‐17; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor beta.
Data Analysis
ORs and 95% CIs were analyzed to evaluate the relationship between genetic polymorphisms in 12 interleukins [Th1 (IL-2, IFN-γ, and TNF-α), Th2 (IL-4 and IL-13), Th17 (IL-1 α, IL-1β, IL-6, TGF-β, and IL-17), and Treg cytokines (IL-10 and TGF-β)] and periodontitis. The interstudy heterogeneity was estimated using I2, where I2 > 50% and p < 0.05 were regarded as significant heterogeneity. Subsequently, the random-effects model (Mantel–Haenszel method) was performed in this study. I2 < 50% and p > 0.05, and the fixed‐effects model (Mantel–Haenszel method) was performed to confirm collective effectiveness. One or all the following genetic polymorphisms were analyzed: 1) genetic specific allele; 2) dominant model; and/or 3) recessive model. Statistical analyses were carried out using MetaXL (https://www.epigear.com/index_files/metaxl.html).
Publication Bias
Publication bias is a major problem in meta-analysis, which affects the strength of the final conclusion and remains suboptimal, and impedes the cogency and explanation of meta-analysis discoveries. When bias occurs, it typically differentially affects studies manifesting as an association between precision and effect size, and photographic asymmetry of common funnel plots. However, the asymmetry is quantified using Egger’s regression, and the sensitivity of Egger’s regression is difficult to detect such asymmetry when the amounts of studies are limited. A new graphical method, the Doi plot, to visualize asymmetry using the LFK index can detect the asymmetry of study effects well. The LFK index also has a higher sensitivity than Egger’s regression (144). Therefore, publication bias was assessed using LFK index test proposed by Furuya-Kanamori et al. LFK index zero represents complete symmetry, and the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry.
Results
Study Selection
According to the study flowchart (Figure 1), a total of 147 articles were included in the final meta‐analysis on genetic polymorphism in the 12 cytokine genes (Th1 (IL-2, IFN-γ, and TNF-α), Th2 (IL-4 and IL-13), Th17 (IL-1α, IL-1β, IL-6, TGF-β, and IL-17), and Treg cytokines (IL-10 and TGF-β)), including IL‐1α (12–28), IL-1β (12, 16, 18, 24, 29–55), IL-2 (56–60), IL-4 (61–73), IL‐6 (74–85, 145), IL-10 (16, 42, 84–86, 88–107, 109, 110, 118, 146, 147), IL-13 (66, 111, 112), IL-17 (60, 73, 87, 113–117), TNF‐α (16, 35, 38, 44, 48, 85, 118–132), IFN (70, 85, 102, 133–136), and TGF (42, 137–143). These studies encompassed various periodontitis cases and healthy controls, which were involved in the analysis of the gene polymorphism and play important roles in promoting or preventing either chronic or aggressive periodontitis. We explored the relationship between the genetic polymorphism and the occurrence of periodontitis risk or prevention of periodontitis development using these studies (Tables S1–S12), in which the data were used to test the association of alleles and genotype with periodontitis risk.
Th1 Cell Cytokines
Th1 cytokines are structurally related to IL-2, IFN-γ, and TNF-α, whose polymorphisms are possibly associated with periodontitis risk. IL-2 −330G allele had a weak relationship with the periodontitis development with OR (95% CI), 0.96 (0.72–1.20) (Figure 2A). LFK index showed that there was major asymmetry and significant publication bias for the result of IL-2 −330G allele (Figure 2B). In contrast, IL-2 −330T allele had a strong relationship with the periodontitis risk with OR (95% CI), 0.80 (0.35–1.24) (Figure 2C). However, a higher level of LFK index also indicated that there was major asymmetry and publication bias for the result of IL-2 −330T allele (Figure 2D).
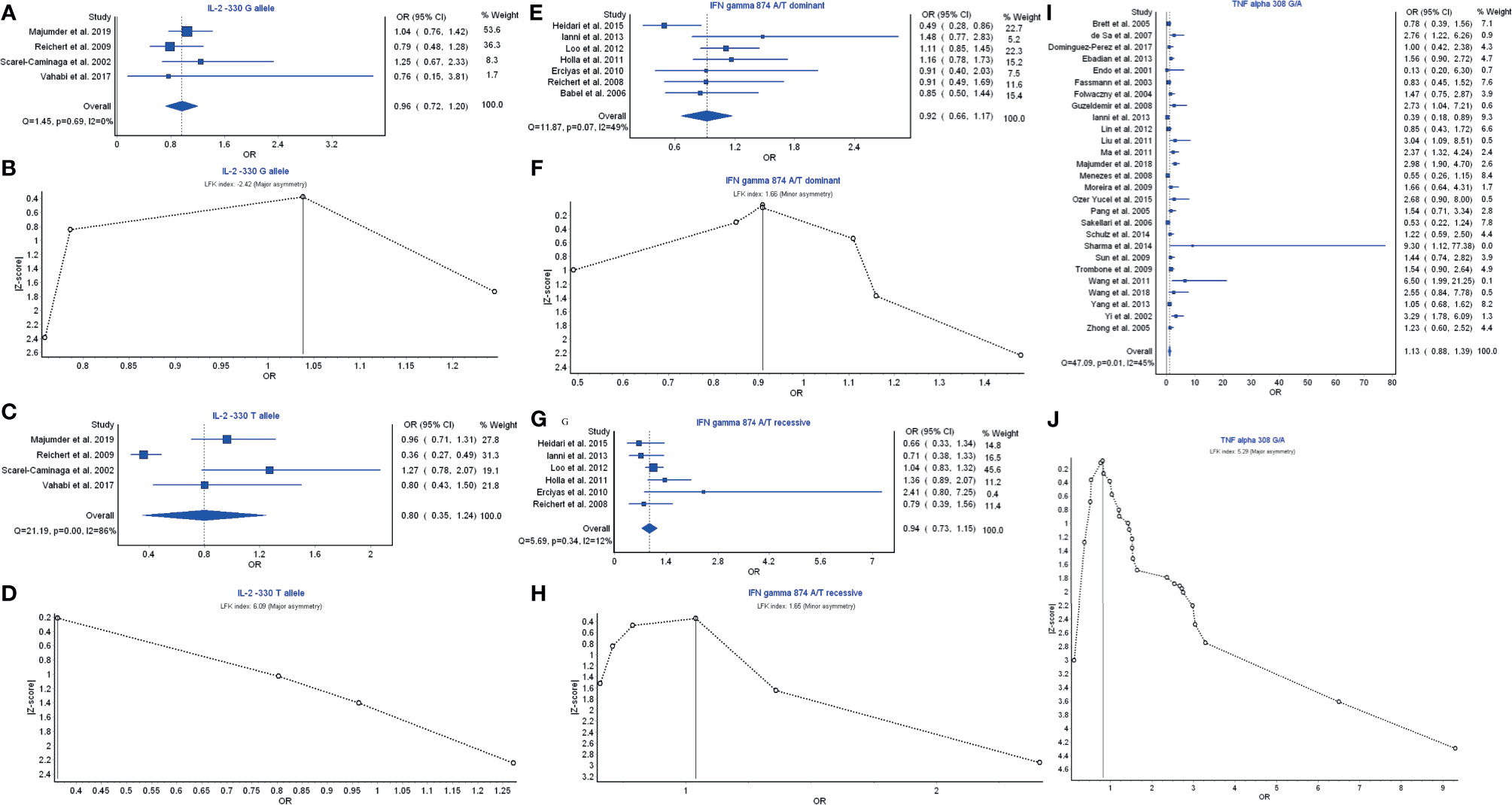
Figure 2 The association of Th1-related cytokine polymorphism with chronic periodontitis. (A) Forest plot of the association of IL‐2 −330 G allele polymorphism with chronic periodontitis. (B) The Doi plot is used to visualize the asymmetry of the association of IL‐2 −330 G allele polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry. (C) Forest plot of the association of IL‐2 −330 T allele polymorphism with chronic periodontitis. (D) The Doi plot is used to visualize the asymmetry of the association of IL‐2 −330 T allele polymorphism with chronic periodontitis using the LFK index. (E) Forest plot of the association of IFN-γ 874 A/T dominant polymorphism with chronic periodontitis. (F) The Doi plot is used to visualize the asymmetry of the association of IFN-γ 874 A/T dominant polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry. (G) Forest plot of the association of IFN-γ 874 A/T recessive polymorphism with chronic periodontitis. (H) The Doi plot is used to visualize the asymmetry of the association of IFN-γ 874 A/T recessive polymorphism with chronic periodontitis using the LFK index. (I) Forest plot of the association of TNF-α 308 G/A polymorphism with chronic periodontitis. (J) The Doi plot is used to visualize the asymmetry of the association of TNF-α 308 G/A polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry.
IFN-γ 874 A/T dominant model had a weak relationship with the periodontitis risk with OR (95% CI), 0.92 (0.66–1.17) (Figure 2E). LFK index showed that there was minor asymmetry and publication bias for the result of IFN-γ 874 A/T dominant model (Figure 2F). Similarly, IFN-γ 874 A/T recessive model also had a weak relationship with the periodontitis development with OR (95% CI), 0.94 (0.73–1.15) (Figure 2G). LFK index also indicated that there was minor asymmetry and publication bias for the result of IFN-γ 874 A/T recessive model (Figure 2H).
TNF-α 308 G/A polymorphism had a significant relationship in the prevention of periodontitis risk with OR (95% CI), 1.13 (0.88–1.39) (Figure 2I). LFK index showed that there was major asymmetry and significant publication bias for the result of TNF-α 308 G/A polymorphism (Figure 2J). Taken together, these results suggest that the members IL-2 and TNF-α of Th1 cytokine family may be associated with the pathogenesis of periodontitis or prevention of periodontitis risk, and their polymorphism can affect periodontitis risk while the polymorphism of IFN-γ is not associated with periodontitis risk.
Th2 Cell Cytokines
IL-4 and IL-13 are the members of Th2 cell cytokines. IL-4 −590 C/T T allele had a weak relationship with the periodontitis risk with OR (95% CI), 0.92 (0.72–1.11) (Figure 3A). LFK index showed that there was major asymmetry and significant publication bias for the result of IL-4 −590 C/T T allele (Figure 3B). IL-13 −1112C/T C allele had a weak relationship with the periodontitis risk with OR (95% CI), 0.91 (0.62–1.21) (Figure 3C). LFK index showed that there was major asymmetry and publication bias for the result of IL-13 −1112C/T C allele model (Figure 3D). Similarly, IL-13 −1112C/T T allele also had a weak relationship with the periodontitis development with OR (95% CI), 0.92 (0.45–1.39) (Figure 3E). LFK index indicated that there was no asymmetry or publication bias for the result of IL-13 −1112C/T T allele model (Figure 3F). Taken together, these results suggest that the members IL-4 and IL-13 of Th2 cytokine family may not be associated with the pathogenesis of periodontitis.
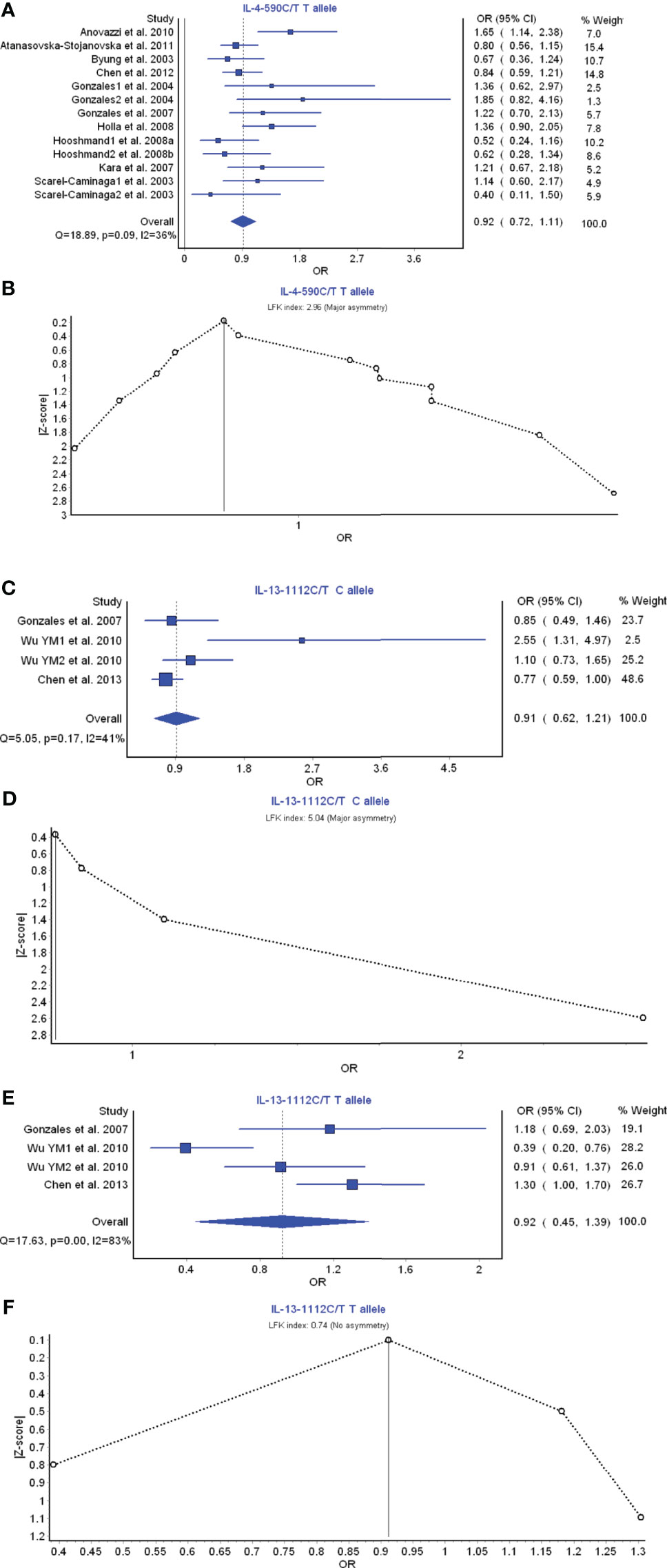
Figure 3 The association of Th2-related cytokine polymorphism with chronic periodontitis. (A) Forest plot of the association of IL-4 −590 C/T T allele polymorphism with chronic periodontitis. (B) The Doi plot is used to visualize the asymmetry of the association of IL-4 −590 C/T T allele polymorphism with chronic periodontitis using the LFK index. (C) Forest plot of the association of IL-13 −1112C/T C allele polymorphism with chronic periodontitis. (D) The Doi plot is used to visualize the asymmetry of the association of IL-13 −1112C/T C allele polymorphism with chronic periodontitis using the LFK index. (E) Forest plot of the association of IL-13 −1112C/T T allele polymorphism with chronic periodontitis. (F) The Doi plot is used to visualize the asymmetry of the association of IL-13 −1112C/T T allele polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry.
Th17 Cell Cytokines
IL-1 α, IL-1β, IL-6, and IL-17 belong to the members of Th17 cell cytokine family. IL-17A, secreted by Th17 cells, was found upregulated in human inflammatory and autoimmune disease (148). IL-1α −889C/T T allele had a favorable relationship in the prevention of periodontitis risk with OR (95% CI), 1.12 (0.99–1.25) (Figure 4A). LFK index showed that there was major asymmetry and publication bias for the result of IL-1α −889C/T T allele model (Figure 4B). In contrast, IL-1α −889C/T C allele also had a strong relationship with the periodontitis development with OR (95% CI), 0.75 (0.66–0.85) (Figure 4C). LFK index indicated that there was major asymmetry and publication bias for the result of IL-1α −889C/T C allele model (Figure 4D).
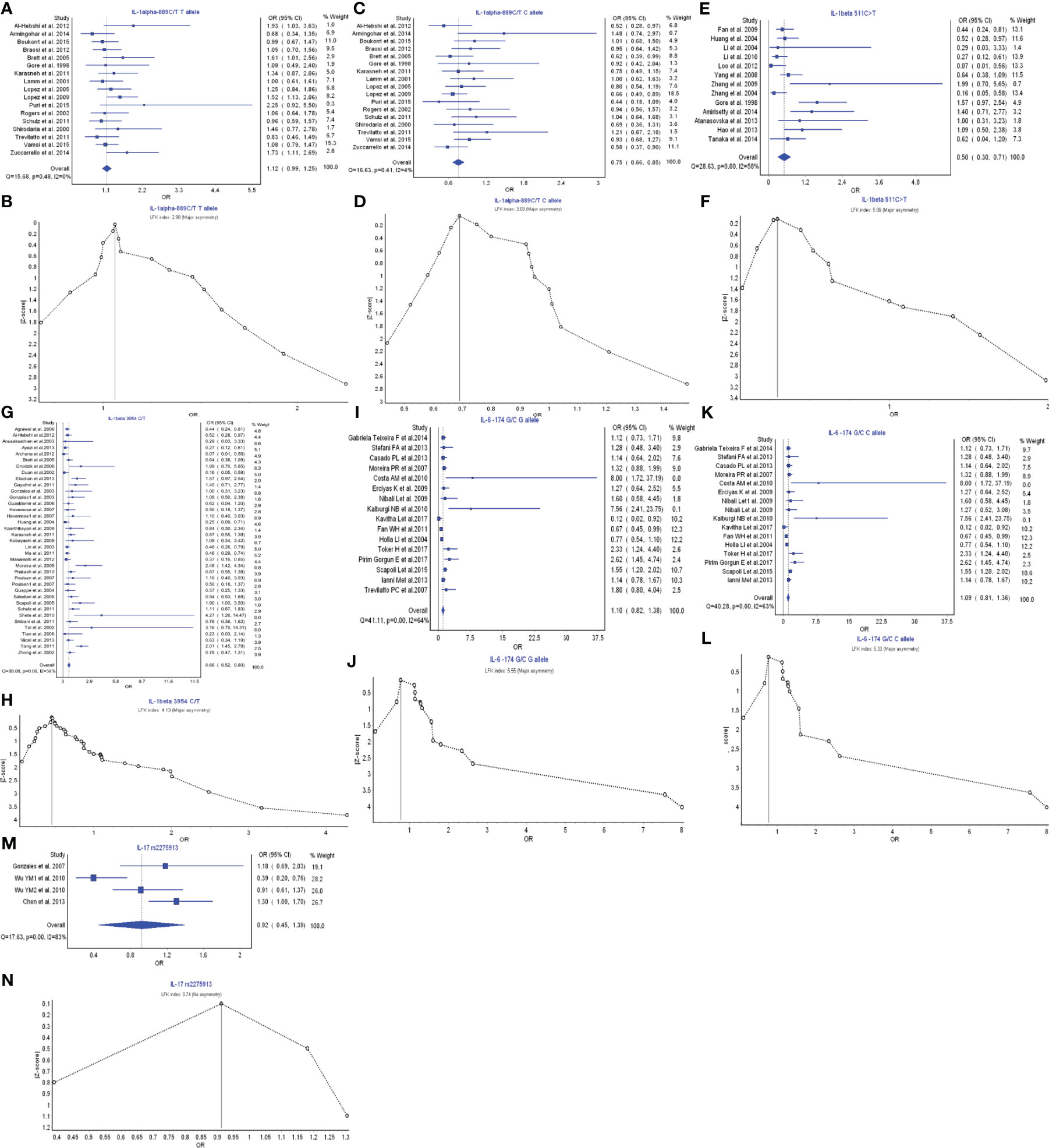
Figure 4 The association of Th17-related cytokine polymorphism with chronic periodontitis. (A) Forest plot of the association of IL-1α −889C/T T allele polymorphism with chronic periodontitis. (B) The Doi plot is used to visualize the asymmetry of the association of IL-1α −889C/T T allele polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry. (C) Forest plot of the association of IL-1α −889C/T C allele polymorphism with chronic periodontitis. (D) The Doi plot is used to visualize the asymmetry of the association of IL-1α −889C/T C allele polymorphism with chronic periodontitis using the LFK index. (E) Forest plot of the association of IL-1β −511C>T polymorphism with chronic periodontitis. (F) The Doi plot is used to visualize the asymmetry of the association of IL-1β −511C>T polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry. (G) Forest plot of the association of IL-1β 3954C>T polymorphism with chronic periodontitis. (H) The Doi plot is used to visualize the asymmetry of the association of IL-1β 3954C>T polymorphism with chronic periodontitis using the LFK index. (I) Forest plot of the association of IL-6 −174G/C G allele polymorphism with chronic periodontitis. (J) The Doi plot is used to visualize the asymmetry of the association of IL-6 −174G/C G allele polymorphism with chronic periodontitis using the LFK index. (K) Forest plot of the association of IL-6 −174G/C C allele polymorphism with chronic periodontitis. (L) The Doi plot is used to visualize the asymmetry of the association of IL-6 −174G/C C allele polymorphism with chronic periodontitis using the LFK index. (M) Forest plot of the association of IL-17 rs2275913 polymorphism with chronic periodontitis. (N) The Doi plot is used to visualize the asymmetry of the association of IL-17 rs2275913 polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry.
IL-1β −511C>T polymorphism had a very strong relationship in the periodontitis risk with OR (95% CI), 0.50 (0.30–0.71) (Figure 4E). LFK index showed that there was major asymmetry and publication bias for the result of IL-1β −511C>T polymorphism (Figure 4F). Similarly, IL-1β 3954C>T polymorphism also had a very strong relationship with the periodontitis development with OR (95% CI), 0.66 (0.52–0.80) (Figure 4G). LFK index indicated that there was major asymmetry and publication bias for the result of IL-1β 3954C>T polymorphism (Figure 4H).
IL-6 −174G/C G allele model had a weak relationship in the prevention of periodontitis risk with OR (95% CI), 1.10 (0.82–1.38) (Figure 4I). LFK index showed that there was major asymmetry and publication bias for the result of IL-6 −174G/C G allele model (Figure 4J). Similarly, IL-6 −174G/C C allele model also had a weak relationship in the prevention of periodontitis development with OR (95% CI), 1.09 (0.81–1.36) (Figure 4K). LFK index indicated that there was major asymmetry and publication bias for the result of IL-6 −174G/C C allele model (Figure 4L).
IL-17 rs2275913 polymorphism had a weak relationship in the periodontitis risk with OR (95% CI), 0.92 (0.45–1.39) (Figure 4M). LFK index showed that there was no asymmetry or publication bias for the result of IL-17 rs2275913 polymorphism (Figure 4N). Taken together, in the members of Th17 cytokine family, different IL-1 α polymorphisms may have inverse functions in the pathogenesis of periodontitis. IL-1β is a significant cytokine biomarker in the periodontitis development. IL-6 may have protective function in the inflammatory responses of periodontitis, and IL-17 has a weak relationship with the inflammatory responses.
Treg Cell Cytokines
Treg cell cytokines include TGF-β; and the IL-10 family, and TGF-β and IL-10 are the most relevant members for immune regulation. IL-10 592C>A A allele model had a strong relationship in the prevention of periodontitis risk with OR (95% CI), 1.21 (1.03–1.39) (Figure 5A). LFK index showed that there was major asymmetry and publication bias for the result of IL-10 592C>A A allele model (Figure 5B). IL-10 1082A>G A allele model also had a weak relationship with the periodontitis development with OR (95% CI), 1.04 (0.81–1.28) (Figure 5C). LFK index indicated that there was major asymmetry and publication bias for the result of L-10 1082A>G A allele model (Figure 5D). TGF-β rs1800469 polymorphism had a relationship in the prevention of periodontitis risk with OR (95% CI), 1.13 (0.93–1.34) (Figure 5E). LFK index showed that there was major asymmetry and publication bias for the result of TGF-β rs1800469 polymorphism (Figure 5F). Taken together, the members of Treg cell cytokines may have a protective function in the periodontitis risk by reducing the inflammatory responses.
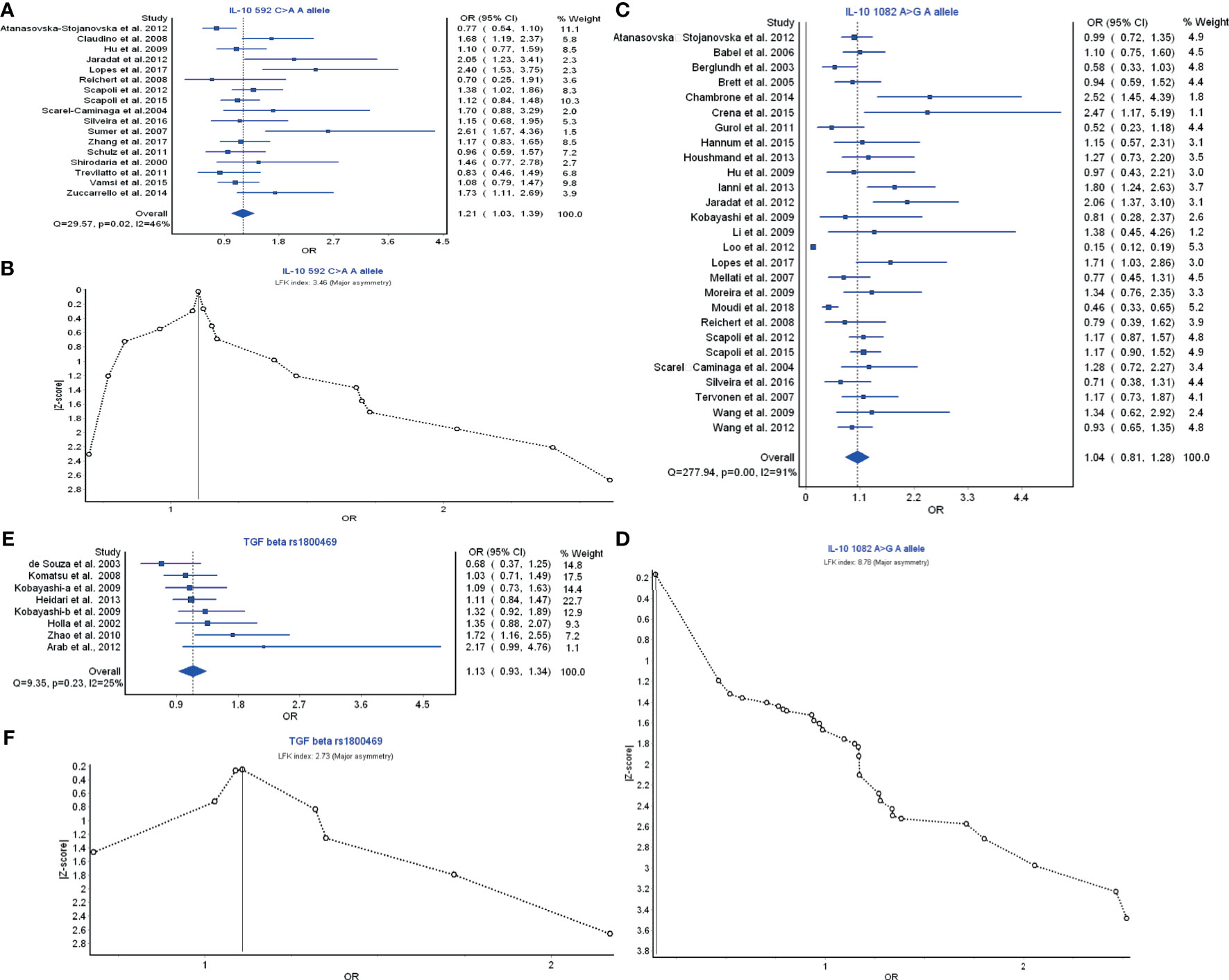
Figure 5 The association of Treg-related cytokine polymorphism with chronic periodontitis. (A) Forest plot of the association of IL-10 592C>A A allele polymorphism with chronic periodontitis. (B) The Doi plot is used to visualize the asymmetry of the association of IL-10 592C>A A allele polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry. (C) Forest plot of the association of IL-10 1082A>G A allele polymorphism with chronic periodontitis. (D) The Doi plot is used to visualize the asymmetry of the association of IL-10 1082A>G A allele polymorphism with chronic periodontitis using the LFK index. (E) Forest plot of the association of TGF-β rs1800469 polymorphism with chronic periodontitis. (F) The Doi plot is used to visualize the asymmetry of the association of TGF-β rs1800469 polymorphism with chronic periodontitis using the LFK index. LFK index zero represents complete symmetry, the limits of symmetry were set at ±1, and values beyond ±1 were inconsistent with symmetry.
Discussion
Pro-inflammatory CD4+ T helper cells mainly belong to the Th1, Th2, Th17, and Treg cells, which secrete various cytokines and are closely associated with periodontal diseases (Figure 6) (149). The present meta-analysis showed that the polymorphisms of the members IL-2 and TNF-α of Th1 cytokine family may be associated with the pathogenesis of periodontitis or the prevention of periodontitis risk, while the polymorphism of IFN-γ is not related to periodontitis risk. The result is consistent with previous meta-analysis that IFN-γ may not contribute to periodontitis susceptibility (150). Cytokine receptors also can induce inflammatory signals by binding specifically to certain types of cytokines. The activity of IL-2 receptor in the periodontitis patients was also found to be higher than that in the control subjects (151). Previous report also could not find any evidence to support that polymorphisms in an IFN-γ receptor‐1 contributes to periodontitis risk either (152).
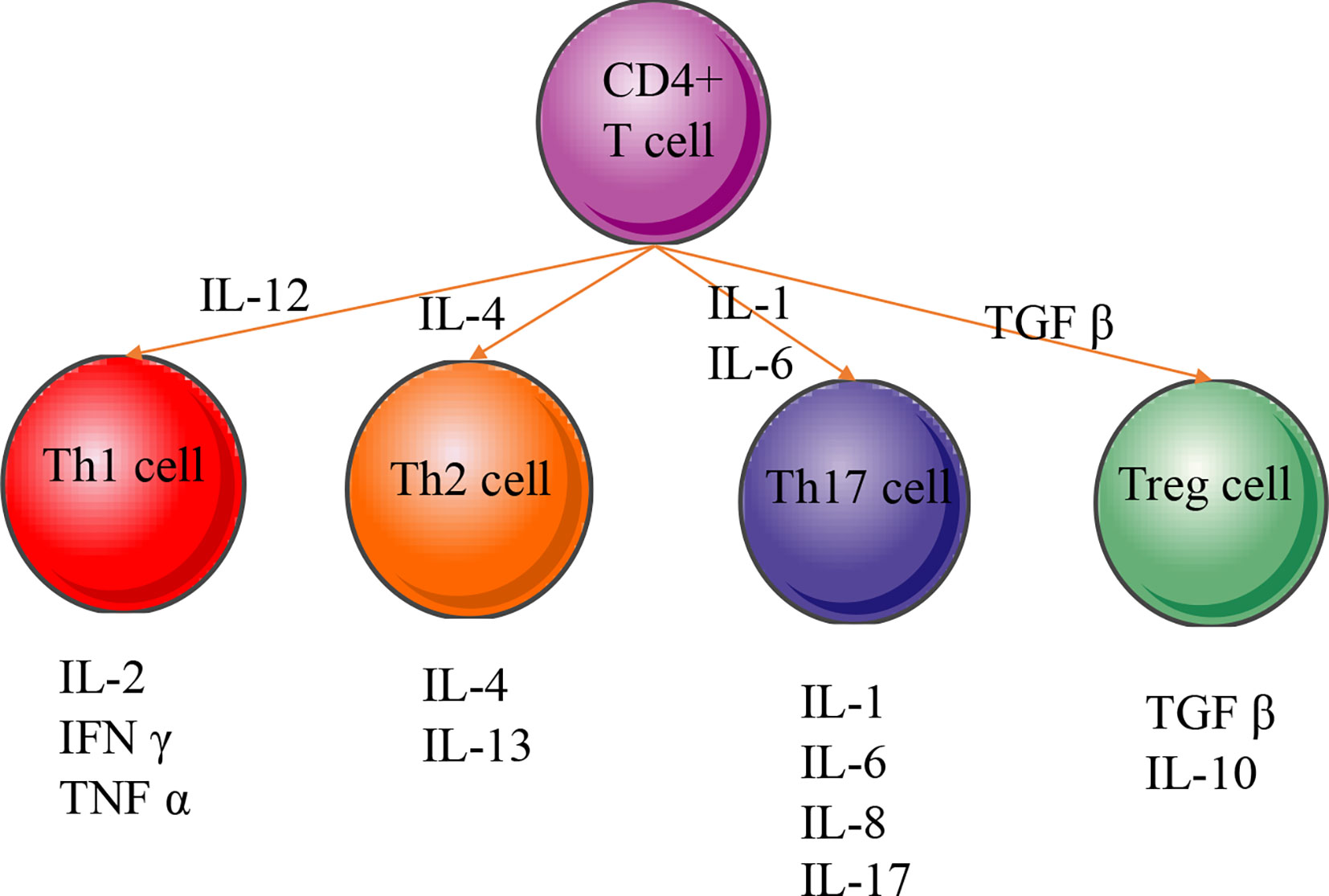
Figure 6 CD4+ T-cell differentiation and cellular phenotype. Naive CD4+ T cells differentiate into many different types of T helper (Th) or regulatory T (Treg) cells. The regulating transcription factors of subsets and their characteristic effector cytokines are indicated in the figure. IL‐1, interleukin‐1; IL‐2, interleukin-2; IL‐4, interleukin‐4; IL‐6, interleukin‐6; IL‐8, interleukin‐8; IL‐10, interleukin-10; IL‐13, interleukin‐13; IL‐17, interleukin‐17; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor beta.
TNF-α, one of the essential pro-inflammatory cytokines in periodontitis patients, has been regarded as a potential biomarker for diagnosis of a periodontal disease (153). Anti-TNF-α will be a potential way to prevent periodontitis risk (154). Apical periodontitis patients were treated with anti-TNF-α biologic medications and showed faster healing than the controls (155).
The polymorphisms for the members IL-4 and IL-13 of Th2 cytokine family are not found to be associated with the pathogenesis of periodontitis. The statistical differences for IL-4 receptor polymorphism were insignificant among mild, moderate, and severe chronic periodontitis subjects (156). Further work also did not show that the polymorphisms of IL-4 receptor were associated with the risk of severe chronic periodontitis (157).
For the polymorphisms of the members of Th17 cytokine family, different IL-1α polymorphisms may have inverse actions in the pathogenesis of periodontitis. IL-1β is a noteworthy cytokine biomarker in periodontitis development and progression. IL-6 may have a protective function in the inflammatory responses of periodontitis, and IL-17 has a weak relationship the inflammatory responses. The relationship between interleukin-1 receptor and periodontitis risk was seldom reported. The polymorphism (rs2234663) of interleukin-1 receptor antagonist (IL-1ra), an agent that binds to interleukin-1 receptor, was found to contribute to serious periodontitis susceptibility (158). Salivary level of IL-1Ra was also reported to be statistically higher in the periodontitis patients than the controls (159). Gingival crevicular fluid-soluble IL-6 receptor was also higher in the inflammatory sites than in the healthy controls in periodontitis patients (160).
Clinical evidence shows that IL-1β, a pro-inflammatory cytokine, is raised and contributes to periodontitis susceptibility. Evaluated levels of IL-1β initiate a chain of inflammatory responses and increase bone resorption (161). Therefore, IL-1β is an important therapeutic target for periodontitis. The application of IL-1ra (a natural inhibitor of IL-1β) suggests the possible function of IL-1ra in controlling periodontal inflammation in an experimental periodontitis model (162).
The present study showed that IL-2 −330T allele had a strong relationship with the periodontitis risk (Figure 2C), which was consistent with the previous report that the expression of IL-2 was increased in the tenacity stage of periodontitis and that IL-2 exerts an inhibitory function in the development of periodontitis (163). IL-2 −330T allele may affect its normal protective function. Preclinical experiments indicated that IL-2/anti-IL-2-antibody complexes activated the percent of Treg cells and reduced the inflammatory responses in various diseases (164, 165).
The present findings showed that the members IL-4 and IL-13 of Th2 cytokine family may not be associated with the pathogenesis of periodontitis. The results are inconsistent with the previous report that IL-4 has been found to be decreased in chronic periodontitis subjects and increased after therapy, which implies that IL-4 in Th2 cells may have protective effects in the prevention of periodontitis (166). Furthermore, it has been also reported that IL-4 can control pro-inflammatory cytokine levels (167) and restrain the occurrence of osteoclastogenesis (168).
The present findings showed that the members of Treg cell cytokines may have protective function in the periodontitis risk by reducing the inflammatory responses, which are consistent with popular reports. TGF-β and IL-10 are the main effector cytokines in Treg cells and may have synergetic functions (169, 170). Treg cells might gather at infecting sites of dental infection and might reduce immune actions and bone resorption in the periodontal area (171). The upregulation of TGF-β results in collagen production and reduces collagen-destroying MMP-1 production, which will be beneficial to protect against periodontitis (172). IL-10 polymorphism is of high clinical relevance and a valuable marker to recognize patients who are at higher risk for periodontitis (173). On the other hand, regulatory T cells play an important role in modulating the immunity in periodontitis patients by defending against inflammation and autoimmunity (174). Modulating the Th17/Treg imbalance during periodontitis may be a promising approach to cure periodontitis (175, 176) by affecting the levels of TGF-β and IL-10 (177).
Strengths and Limitations
This meta‐analysis has several strengths, including an unrestricted search process and duplicate review procedures for the search and strict assessments of the risk of bias and the quality of literature.
There are some limitations in the present paper. First, although a broad search in different databases of various cytokines was carried out, it is difficult to approve that whether all the studies for exploring the relationship between these genetic polymorphisms of various cytokines and periodontitis risk were included in the present study. Second, there were obvious heterogeneities and potential publication biases in these meta-analyses. To reduce some potential heterogeneities, the related literatures were removed; however, some heterogeneities still existed. Third, we used Doi plot to probe the occurrence of publication bias in the studies used for our meta‐analysis, and the results showed that all LFK indexes showed the major asymmetry due to publication bias. Therefore, to understand whether the different Th cell cytokines were associated with periodontitis, more unbiased data are required. IL-23 plays a critical role in the development of periodontitis (9, 178–181). However, the present research focuses on the relationship between cytokine polymorphisms and periodontitis and not between the cytokine level and periodontitis. We have tried to investigate the relationship between IL-23 polymorphisms and periodontitis. Unfortunately, the related contents were seldom reported, and the meta-analysis was not performed.
Conclusions
Within the limitations of this study, the present meta‐analysis indicated that genetic polymorphism of Th17 cytokines might be a risk factor for the development of periodontitis, while Treg cells may show protective function in the prevention of periodontitis inflammation. Further studies on larger population will be needed to verify these findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
XL and HL contributed to conception, design of the study, collect all literatures, analyze all data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The present project was supported by the Department of Science and Technology of Jilin Province (Grant No. 20200201519JC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.713198/full#supplementary-material
References
1. Pacheco CMF, Maltos KLM, Shehabeldin MS, Thomas LL, Zhuang Z, Yoshizawa S, et al. Local Sustained Delivery of Anti-IL-17a Antibodies Limits Inflammatory Bone Loss in Murine Experimental Periodontitis. J Immunol (2021) 206:2386–92. doi: 10.4049/jimmunol.2001432
2. Plemmenos G, Evangeliou E, Polizogopoulos N, Chalazias A, Deligianni M, Piperi C. Central Regulatory Role of Cytokines in Periodontitis and Targeting Options. Curr Med Chem (2021) 28(15):3032–58. doi: 10.2174/0929867327666200824112732
3. Azevedo M, Marques H, Binelli LS, Malange MSV, Devides AC, Silva EA, et al. Garlet GP: Simultaneous Analysis of Multiple T Helper Subsets in Leprosy Reveals Distinct Patterns of Th1, Th2, Th17 and Tregs Markers Expression in Clinical Forms and Reactional Events. Med Microbiol Immunol (2017) 206:429–39. doi: 10.1007/s00430-017-0519-9
4. Kisuya J, Chemtai A, Raballah E, Keter A, Ouma C. The Diagnostic Accuracy of Th1 (IFN-γ, TNF-α, and IL-2) and Th2 (IL-4, IL-6 and IL-10) Cytokines Response in AFB Microscopy Smear Negative PTB-HIV Co-Infected Patients. Sci Rep (2019) 9:1–12. doi: 10.1038/s41598-019-39048-x
5. Prout MS, Kyle RL, Ronchese F, Le Gros G. IL-4 Is a Key Requirement for IL-4-and IL-4/IL-13-Expressing CD4 Th2 Subsets in Lung and Skin. Front Immunol (2018) 9:1211. doi: 10.3389/fimmu.2018.01211
6. Ghazavi A, Ganji A, Keshavarzian N, Rabiemajd S, Mosayebi G. Cytokine Profile and Disease Severity in Patients With COVID-19. Cytokine (2021) 137:155323. doi: 10.1016/j.cyto.2020.155323
7. dos Santos Schiavinato JL, Haddad R, Saldanha-Araujo F, Baiochi J, Araujo AG, Scheucher PS, et al. TGF-Beta/atRA-Induced Tregs Express a Selected Set of microRNAs Involved in the Repression of Transcripts Related to Th17 Differentiation. Sci Rep (2017) 7:1–17. doi: 10.1038/s41598-017-03456-8
8. Lucherini OM, Lopalco G, Cantarini L, Emmi G, Lopalco A, Venerito V, et al. Critical Regulation of Th17 Cell Differentiation by Serum Amyloid-A Signalling in Behcet’s Disease. Immunol Lett (2018) 201:38–44. doi: 10.1016/j.imlet.2018.10.013
9. Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int J Mol Sci (2019) 20:3394. doi: 10.3390/ijms20143394
10. Tarique M, Naz H, Kurra SV, Saini C, Naqvi RA, Rai R, et al. Interleukin-10 Producing Regulatory B Cells Transformed CD4+ CD25– Into Tregs and Enhanced Regulatory T Cells Function in Human Leprosy. Front Immunol (2018) 9:1636. doi: 10.3389/fimmu.2018.01636
11. Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, et al. Transforming Growth Factor-β Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity (2017) 46:660–74. doi: 10.1016/j.immuni.2017.03.015
12. Al-Hebshi NN, A-aA S. Al-Ak’hali MS: Interleukin-1 Two-Locus Haplotype is Strongly Associated With Severe Chronic Periodontitis Among Yemenis. Mol Biol Int (2012) 2012. doi: 10.1155/2012/231309
13. Armingohar Z, Jorgensen JJ, Kristoffersen AK, Schenck K, Dembic Z. Polymorphisms in the Interleukin-1 Gene Locus and Chronic Periodontitis in Patients With Atherosclerotic and Aortic Aneurysmal Vascular Diseases. Scand J Immunol (2014) 79:338–45. doi: 10.1111/sji.12166
14. Boukortt KN, Saidi-Ouahrani N, Boukerzaza B, Ouhaibi-Djellouli H, Hachmaoui K, Benaissa FZ, et al. Association Analysis of the IL-1 Gene Cluster Polymorphisms With Aggressive and Chronic Periodontitis in the Algerian Population. Arch Oral Biol (2015) 60:1463–70. doi: 10.1016/j.archoralbio.2015.06.018
15. Braosi AP, de Souza CM, Luczyszyn SM, Dirschnabel AJ, Claudino M, Olandoski M, et al. Analysis of IL1 Gene Polymorphisms and Transcript Levels in Periodontal and Chronic Kidney Disease. Cytokine (2012) 60:76–82. doi: 10.1016/j.cyto.2012.06.006
16. Brett PM, Zygogianni P, Griffiths GS, Tomaz M, Parkar M, D’Aiuto F, et al. Functional Gene Polymorphisms in Aggressive and Chronic Periodontitis. J Dent Res (2005) 84:1149–53. doi: 10.1177/154405910508401211
17. Gore EA, Sanders JJ, Pandey JP, Palesch Y, Galbraith GM. Interleukin-1beta+3953 Allele 2: Association With Disease Status in Adult Periodontitis. J Clin Periodontol (1998) 25:781–5. doi: 10.1111/j.1600-051X.1998.tb02370.x
18. Karasneh JA, Ababneh KT, Taha AH, Al-Abbadi MS, Ollier WE. Investigation of the Interleukin-1 Gene Cluster Polymorphisms in Jordanian Patients With Chronic and Aggressive Periodontitis. Arch Oral Biol (2011) 56:269–76. doi: 10.1016/j.archoralbio.2010.10.001
19. Laine ML, Farre MA, Gonzalez G, van Dijk LJ, Ham AJ, Winkel EG, et al. Polymorphisms of the Interleukin-1 Gene Family, Oral Microbial Pathogens, and Smoking in Adult Periodontitis. J Dent Res (2001) 80:1695–9. doi: 10.1177/00220345010800080301
20. Lopez NJ, Jara L, Valenzuela CY. Association of Interleukin-1 Polymorphisms With Periodontal Disease. J Periodontol (2005) 76:234–43. doi: 10.1902/jop.2005.76.2.234
21. Lopez NJ, Valenzuela CY, Jara L. Interleukin-1 Gene Cluster Polymorphisms Associated With Periodontal Disease in Type 2 Diabetes. J Periodontol (2009) 80:1590–8. doi: 10.1902/jop.2009.090134
22. Puri K, Chhokra M, Dodwad V, Puri N. Association of Interleukin-1 Alpha (-889) Gene Polymorphism in Patients With Generalized Aggressive and Chronic Periodontitis. Dent Res J (Isfahan) (2015) 12:76–82. doi: 10.4103/1735-3327.150338
23. Rogers MA, Figliomeni L, Baluchova K, Tan AE, Davies G, Henry PJ, et al. Do Interleukin-1 Polymorphisms Predict the Development of Periodontitis or the Success of Dental Implants? J Periodontal Res (2002) 37:37–41. doi: 10.1034/j.1600-0765.2002.00651.x
24. Schulz S, Stein JM, Altermann W, Klapproth J, Zimmermann U, Reichert Y, et al. Single Nucleotide Polymorphisms in Interleukin-1gene Cluster and Subgingival Colonization With Aggregatibacter Actinomycetemcomitans in Patients With Aggressive Periodontitis. Hum Immunol (2011) 72:940–6. doi: 10.1016/j.humimm.2011.05.009
25. Shirodaria S, Smith J, McKay IJ, Kennett CN, Hughes FJ. Polymorphisms in the IL-1A Gene Are Correlated With Levels of Interleukin-1alpha Protein in Gingival Crevicular Fluid of Teeth With Severe Periodontal Disease. J Dent Res (2000) 79:1864–9. doi: 10.1177/00220345000790110801
26. Trevilatto PC, de Souza Pardo AP, Scarel-Caminaga RM, de Brito RB Jr., Alvim-Pereira F, Alvim-Pereira CC, et al. Association of IL1 Gene Polymorphisms With Chronic Periodontitis in Brazilians. Arch Oral Biol (2011) 56:54–62. doi: 10.1016/j.archoralbio.2010.09.004
27. Lavu V, Venkatesan V, Venkata Kameswara Subrahmanya Lakkakula B, Venugopal P, Paul SF, Rao SR. Polymorphic Regions in the Interleukin-1 Gene and Susceptibility to Chronic Periodontitis: A Genetic Association Study. Genet Test Mol Biomarkers (2015) 19:175–81. doi: 10.1089/gtmb.2014.0275
28. Zuccarello D, Bazzato MF, Ferlin A, Pengo M, Frigo AC, Favero G, et al. Role of Familiarity Versus, Interleukin-1 Genes Cluster Polymorphisms in Chronic Periodontitis. Gene (2014) 535:286–9. doi: 10.1016/j.gene.2013.11.016
29. Agrawal AA, Kapley A, Yeltiwar RK, Purohit HJ. Assessment of Single Nucleotide Polymorphism at IL-1A+ 4845 and IL-1B+ 3954 as Genetic Susceptibility Test for Chronic Periodontitis in Maharashtrian Ethnicity. J Periodontol (2006) 77:1515–21. doi: 10.1902/jop.2006.050427
30. Anusaksathien O, Sukboon A, Sitthiphong P, Teanpaisan R. Distribution of Interleukin-1beta(+3954) and IL-1alpha(-889) Genetic Variations in a Thai Population Group. J Periodontol (2003) 74:1796–802. doi: 10.1902/jop.2003.74.12.1796
31. Ayazi G, Pirayesh M, Yari K. Analysis of Interleukin-1beta Gene Polymorphism and Its Association With Generalized Aggressive Periodontitis Disease. DNA Cell Biol (2013) 32:409–13. doi: 10.1089/dna.2012.1905
32. Archana P, Salman AA, Kumar TS, Saraswathi P, Panishankar K, Kumarasamy P. Association Between Interleukin-1 Gene Polymorphism and Severity of Chronic Periodontitis in a South Indian Population Group. J Indian Soc Periodontol (2012) 16:174. doi: 10.4103/0972-124X.99258
33. Droździk A, Kurzawski M, Safronow K. Banach J: Polymorphism in Interleukin-1beta Gene and the Risk of Periodontitis in a Polish Population. Adv Med Sci (2006) 51:13–7.
34. Duan H, Zhang J, Zhang Y. The Association Between IL-1 Gene Polymorphisms and Susceptibility to Severe Periodontitis. Hua xi kou qiang yi xue za zhi= Huaxi kouqiang yixue zazhi= West China J Stomatol (2002) 20:48–51.
35. Ebadian AR, Radvar M, Afshari JT, Sargolzaee N, Brook A, Ganjali R, et al. Gene Polymorphisms of TNF-α and IL-1β are Not Associated With Generalized Aggressive Periodontitis in an Iranian Subpopulation. Iran J Allergy Asthma Immunol (2013) 12(4):345–51.
36. Gayathri R, Saadi AV, Bhat KM, Bhat SG, Satyamoorthy K. Allele, Genotype, and Composite Genotype Effects of IL-1A +4845 and IL-1B +3954 Polymorphisms for Chronic Periodontitis in an Indian Population. Indian J Dent Res (2011) 22:612. doi: 10.4103/0970-9290.90323
37. Gonzales JR, Michel J, Rodriguez EL, Herrmann JM, Bodeker RH, Meyle J. Comparison of Interleukin-1 Genotypes in Two Populations With Aggressive Periodontitis. Eur J Oral Sci (2003) 111:395–9. doi: 10.1034/j.1600-0722.2003.00071.x
38. Guzeldemir E, Gunhan M, Ozcelik O, Tastan H. Interleukin-1 and Tumor Necrosis Factor-Alpha Gene Polymorphisms in Turkish Patients With Localized Aggressive Periodontitis. J Oral Sci (2008) 50:151–9. doi: 10.2334/josnusd.50.151
39. Havemose-Poulsen A, Sørensen LK, Bendtzen K, Holmstrup P. Polymorphisms Within the IL-1 Gene Cluster: Effects on Cytokine Profiles in Peripheral Blood and Whole Blood Cell Cultures of Patients With Aggressive Periodontitis, Juvenile Idiopathic Arthritis, and Rheumatoid Arthritis. J Periodontol (2007) 78:475–92. doi: 10.1902/jop.2007.060135
40. Huang H, Zhang J. Investigation on the Association of Interleukin-1 Genotype Polymorphism With Chronic Periodontitis. Hua xi kou qiang yi xue za zhi= Huaxi kouqiang yixue zazhi= West China J Stomatol (2004) 22:415–9.
41. Kaarthikeyan G, Jayakumar N, Padmalatha O, Sheeja V, Sankari M, Anandan B. Analysis of the Association Between Interleukin-1beta (+3954) Gene Polymorphism and Chronic Periodontitis in a Sample of the South Indian Population. Indian J Dent Res (2009) 20:37–40. doi: 10.4103/0970-9290.49061
42. Kobayashi T, Murasawa A, Ito S, Yamamoto K, Komatsu Y, Abe A, et al. Cytokine Gene Polymorphisms Associated With Rheumatoid Arthritis and Periodontitis in Japanese Adults. J Periodontol (2009) 80:792–9. doi: 10.1902/jop.2009.080573
43. Lin L, Pan Y, Yin L-Y. Study on the Correlation of Cytokine Gene Polymorphism With Chronic Periodontitis. Shanghai kou qiang yi xue= Shanghai J Stomatol (2003) 12:456–9.
44. Ma M, Li G, Han C, Huang Y. Correlation Study on Polymorphisms of the Interleukin-1 and Tumor Necrosis Factoralpha Gene in Hui Patients With Chronic Periodontitis in Ningxia. J Modern Stomatol (2011) 25:94–7.
45. Masamatti SS, Kumar A, Baron TK, Mehta DS, Bhat K. Evaluation of Interleukin -1B (+3954) Gene Polymorphism in Patients With Chronic and Aggressive Periodontitis: A Genetic Association Study. Contemp Clin Dent (2012) 3:144–9. doi: 10.4103/0976-237X.96815
46. Moreira PR, De Sá AR, Xavier GM, Costa JE, Gomez RS, Gollob KJ, et al. A Functional Interleukin-1β Gene Polymorphism Is Associated With Chronic Periodontitis in a Sample of Brazilian Individuals. J Periodontal Res (2005) 40:306–11. doi: 10.1111/j.1600-0765.2005.00801.x
47. Quappe L, Jara L, Lopez NJ. Association of Interleukin-1 Polymorphisms With Aggressive Periodontitis. J Periodontol (2004) 75:1509–15. doi: 10.1902/jop.2004.75.11.1509
48. Sakellari D, Katsares V, Georgiadou M, Kouvatsi A, Arsenakis M, Konstantinidis A. No Correlation of Five Gene Polymorphisms With Periodontal Conditions in a Greek Population. J Clin Periodontol (2006) 33:765–70. doi: 10.1111/j.1600-051X.2006.00983.x
49. Scapoli C, Trombelli L, Mamolini E, Collins A. Linkage Disequilibrium Analysis of Case-Control Data: An Application to Generalized Aggressive Periodontitis. Genes Immun (2005) 6:44–52. doi: 10.1038/sj.gene.6364152
50. Shete AR, Joseph R, Vijayan NN, Srinivas L, Banerjee M. Association of Single Nucleotide Gene Polymorphism at Interleukin-1β+ 3954,– 511, and– 31 in Chronic Periodontitis and Aggressive Periodontitis in Dravidian Ethnicity. J Periodontol (2010) 81:62–9. doi: 10.1902/jop.2009.090256
51. Shibani K, Shhab R, Khattab R. Analysis of IL-1alpha(-889) and IL-1b(+3953) Gene Polymorphism in Syrian Patients With Aggressive Periodontitis: A Pilot Study. ISRN Dent (2011) 2011:682564.
52. Tai H, Endo M, Shimada Y, Gou E, Orima K, Kobayashi T, et al. Association of Interleukin-1 Receptor Antagonist Gene Polymorphisms With Early Onset Periodontitis in Japanese. J Clin Periodontol (2002) 29:882–8. doi: 10.1034/j.1600-051X.2002.291002.x
53. Tian Y-G, Pan Y-P, Lin L, Zhang D-M, Zhao J. The Relationship of IL-1beta Expressed in Buccal Cells and the Polymorphisms of IL-1beta (+ 3953) With Chronic Periodontitis. Shanghai kou qiang yi xue= Shanghai J Stomatol (2006) 15:456–60.
54. Yang L, Xie X, Ma L, Liu Z, Pan Y, Liu Y. A Study of Association Between the Interleukin-1 Single Nucleotide Polymorphism and Risk of Chronic Periodontitis Among the Hui and Dongxiang Minorities in Gansu Province. West China J Stomatol (2011) 29:365–8.
55. Zhong L, Zhang Y, Zhang J, Yang A, Huang H. [The Association of Interleukin-1 Gene Polymorphisms With the Susceptibility to Chronic Periodontitis in Uighur]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi (2002) 19:405–8.
56. Majumder P, Panda SK, Ghosh S, Dey SK. Interleukin Gene Polymorphisms in Chronic Periodontitis: A Case-Control Study in the Indian Population. Arch Oral Biol (2019) 101:156–64. doi: 10.1016/j.archoralbio.2019.03.015
57. Li G, Yue Y, Tian Y, Wang M, Liang H, Liao P, et al. Association of Matrix Metalloproteinase (MMP)-1, 3, 9, Interleukin (IL)-2, 8 and Cyclooxygenase (COX)-2 Gene Polymorphisms With Chronic Periodontitis in a Chinese Population. Cytokine (2012) 60:552–60. doi: 10.1016/j.cyto.2012.06.239
58. Reichert S, Machulla HK, Klapproth J, Zimmermann U, Reichert Y, Glaser C, et al. Interleukin-2 -330 and 166 Gene Polymorphisms in Relation to Aggressive or Chronic Periodontitis and the Presence of Periodontopathic Bacteria. J Periodontal Res (2009) 44:628–35. doi: 10.1111/j.1600-0765.2008.01173.x
59. Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Line SR. Investigation of an IL-2 Polymorphism in Patients With Different Levels of Chronic Periodontitis. J Clin Periodontol (2002) 29:587–91. doi: 10.1034/j.1600-051X.2002.290701.x
60. Vahabi S, Nazemisalman B, Hosseinpour S, Salavitabar S, Aziz A. Interleukin-2, -16, and -17 Gene Polymorphisms in Iranian Patients With Chronic Periodontitis. J Investig Clin Dent (2018) 9:e12319. doi: 10.1111/jicd.12319
61. Anovazzi G, Kim YJ, Viana AC, Curtis KM, Orrico SR, Cirelli JA, et al. Polymorphisms and Haplotypes in the Interleukin-4 Gene Are Associated With Chronic Periodontitis in a Brazilian Population. J Periodontol (2010) 81:392–402. doi: 10.1902/jop.2009.090392
62. Atanasovska-Stojanovska A, Trajkov D, Nares S, Angelov N, Spiroski M. IL4 Gene Polymorphisms and Their Relation to Periodontal Disease in a Macedonian Population. Hum Immunol (2011) 72:446–50. doi: 10.1016/j.humimm.2011.02.005
63. Kang BY, Choi YK, Choi WH, Kim KT, Choi SS, Kim K, et al. Two Polymorphisms of Interleukin-4 Gene in Korean Adult Periodontitis. Arch Pharm Res (2003) 26:482–6. doi: 10.1007/BF02976867
64. Chen D, Wei N, Bao X, Wang L-M, Zhou C-F, Zhang Y-L, et al. Analysis of Correlation Between IL-6, IL-6R and IL-4 Single Nucleotide Polymorphism and the Susceptibility of Chronic Periodontitis Among Shanghai Patients of Han Nationality. Stomatology (2012) 32:518–20.
65. Gonzales JR, Kobayashi T, Michel J, Mann M, Yoshie H, Meyle J. Interleukin-4 Gene Polymorphisms in Japanese and Caucasian Patients With Aggressive Periodontitis. J Clin Periodontol (2004) 31:384–9. doi: 10.1111/j.1600-051X.2004.00492.x
66. Gonzales JR, Mann M, Stelzig J, Bodeker RH, Meyle J. Single-Nucleotide Polymorphisms in the IL-4 and IL-13 Promoter Region in Aggressive Periodontitis. J Clin Periodontol (2007) 34:473–9. doi: 10.1111/j.1600-051X.2007.01086.x
67. Holla LI, Fassmann A, Augustin P, Halabala T, Znojil V, Vanek J. The Association of Interleukin-4 Haplotypes With Chronic Periodontitis in a Czech Population. J Periodontol (2008) 79:1927–33. doi: 10.1902/jop.2008.080035
68. Hooshmand B, Hajilooi M, Rafiei A, Mani-Kashani KH, Ghasemi R. Interleukin-4 (C-590T) and Interferon-Gamma (G5644A) Gene Polymorphisms in Patients With Periodontitis. J Periodontal Res (2008) 43:111–5. doi: 10.1111/j.1600-0765.2007.01006.x
69. Kara N, Keles GC, Sumer P, Gunes SO, Bagci H, Koprulu H, et al. Association of the Polymorphisms in Promoter and Intron Regions of the Interleukin-4 Gene With Chronic Periodontitis in a Turkish Population. Acta Odontol Scand (2007) 65:292–7. doi: 10.1080/00016350701644040
70. Loo WT, Fan CB, Bai LJ, Yue Y, Dou YD, Wang M, et al. Et Al: Gene Polymorphism and Protein of Human Pro- and Anti-Inflammatory Cytokines in Chinese Healthy Subjects and Chronic Periodontitis Patients. J Transl Med (2012) 10 Suppl 1:S8. doi: 10.1186/1479-5876-10-S1-S8
71. Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB Jr., Line SR. Investigation of IL4 Gene Polymorphism in Individuals With Different Levels of Chronic Periodontitis in a Brazilian Population. J Clin Periodontol (2003) 30:341–5. doi: 10.1034/j.1600-051X.2003.00317.x
72. Chen D, T-l Z. Wang X: Association Between Polymorphisms in Interleukins 4 and 13 Genes and Chronic Periodontitis in a Han Chinese Population. BioMed Res Int (2016) 2016. doi: 10.1155/2016/8389020
73. Jain N, Joseph R, Balan S, Arun R, Banerjee M. Association of Interleukin-4 and Interleukin-17F Polymorphisms in Periodontitis in Dravidian Ethnicity. Indian J Hum Genet (2013) 19:58–64. doi: 10.4103/0971-6866.112891
74. Gabriela Teixeira F, Mendonça SA, Menezes Oliveira K, Barbosa dos Santos D, Miranda Marques L, Mendonça Amorim M, et al. Interleukin-6 C.-174G> C Polymorphism and Periodontitis in a Brazilian Population. Mol Biol Int (2014) 2014. doi: 10.1155/2014/490308
75. Stefani FA, Viana MB, Dupim AC, Brito JA, Gomez RS, da Costa JE, et al. Expression, Polymorphism and Methylation Pattern of Interleukin-6 in Periodontal Tissues. Immunobiology (2013) 218:1012–7. doi: 10.1016/j.imbio.2012.12.001
76. Ladeira Casado P, Villas-Boas R, de Mello W, Leite Duarte ME, Mauro Granjeiro J. Peri-Implant Disease and Chronic Periodontitis: Is Interleukin-6 Gene Promoter Polymorphism the Common Risk Factor in a Brazilian Population? Int J Oral Maxillofac Implants (2013) 28(1):35–43. doi: 10.11607/jomi.2867
77. Costa A, Guimarães M, De Souza ER, Nóbrega O, Bezerra A. Interleukin-6 (G-174C) and Tumour Necrosis Factor-Alpha (G-308A) Gene Polymorphisms in Geriatric Patients With Chronic Periodontitis. Gerodontology (2010) 27:70–5. doi: 10.1111/j.1741-2358.2009.00291.x
78. Karaoglan I, Pehlivan S, Namiduru M, Pehlivan M, Kilinçarslan C, Balkan Y, et al. TNF-Alpha, TGF-Beta, IL-10, IL-6 and IFN-Gamma Gene Polymorphisms as Risk Factors for Brucellosis. New Microbiol (2009) 32:173–8.
79. Kalburgi NB, Bhatia A, Bilichodmath S, Patil SR, Mangalekar SB, Bhat K. Interleukin-6 Promoter Polymorphism (-174 G/C) in Indian Patients With Chronic Periodontitis. J Oral Sci (2010) 52:431–7. doi: 10.2334/josnusd.52.431
80. Kavitha L, Vijayshree Priyadharshini J, Sivapathasundharam B. Association Among Interleukin-6 Gene Polymorphisms, Type 2 Diabetes Mellitus, and Chronic Periodontitis: A Pilot Study. J Invest Clin Dentistry (2017) 8:e12230. doi: 10.1111/jicd.12230
81. Fan W, Liu D, Xiao L, Xie C, Sun S, Zhang J. Coronary Heart Disease and Chronic Periodontitis: Is Polymorphism of Interleukin-6 Gene the Common Risk Factor in a Chinese Population? Oral Dis (2011) 17:270–6. doi: 10.1111/j.1601-0825.2010.01736.x
82. Holla LI, Fassmann A, Stejskalová A, Znojil V, Vaněk J, Vacha J. Analysis of the Interleukin-6 Gene Promoter Polymorphisms in Czech Patients With Chronic Periodontitis. J Periodontol (2004) 75:30–6. doi: 10.1902/jop.2004.75.1.30
83. Toker H, Görgün EP, Korkmaz EM. Analysis of IL-6, IL-10 and NF-κB Gene Polymorphisms in Aggressive and Chronic Periodontitis. Cent Eur J Public Health (2017) 25:157–62. doi: 10.21101/cejph.a4656
84. Scapoli L, Girardi A, Palmieri A, Martinelli M, Cura F, Lauritano D, et al. Interleukin-6 Gene Polymorphism Modulates the Risk of Periodontal Diseases. J Biol Regul Homeost Agents (2015) 29:111–6.
85. Ianni M, Bruzzesi G, Pugliese D, Porcellini E, Carbone I, Schiavone A, et al. Variations in Inflammatory Genes Are Associated With Periodontitis. Immun Ageing (2013) 10:1–8. doi: 10.1186/1742-4933-10-39
86. Pirim Gorgun E, Toker H, Korkmaz EM, Poyraz O. IL-6 and IL-10 Gene Polymorphisms in Patients With Aggressive Periodontitis: Effects on GCF, Serum and Clinic Parameters. Braz Oral Res (2017) 31:e12. doi: 10.1590/1807-3107BOR-2017.vol31.0012
87. Saraiva AM, Alves e Silva MR, Correia Silva Jde F, da Costa JE, Gollob KJ, Dutra WO, et al. Evaluation of IL17A Expression and of IL17A, IL17F and IL23R Gene Polymorphisms in Brazilian Individuals With Periodontitis. Hum Immunol (2013) 74:207–14. doi: 10.1016/j.humimm.2012.10.026
88. Atanasovska-Stojanovska A, Trajkov D, Popovska M, Spiroski M. IL10 -1082, IL10 -819 and IL10 -592 Polymorphisms are Associated With Chronic Periodontitis in a Macedonian Population. Hum Immunol (2012) 73:753–8. doi: 10.1016/j.humimm.2012.04.009
89. Claudino M, Trombone AP, Cardoso CR, Ferreira SB Jr., Martins W Jr, Assis GF, et al. The Broad Effects of the Functional IL-10 Promoter-592 Polymorphism: Modulation of IL-10, TIMP-3, and OPG Expression and Their Association With Periodontal Disease Outcome. J Leukoc Biol (2008) 84:1565–73. doi: 10.1189/jlb.0308184
90. Garlet GP, Trombone APF, Menezes R, Letra A, Repeke CE, Vieira AE, et al. Santos CFd: The Use of Chronic Gingivitis as Reference Status Increases the Power and Odds of Periodontitis Genetic Studies–A Proposal Based in the Exposure Concept and Clearer Resistance and Susceptibility Phenotypes Definition. J Clin Periodontol (2012) 39:323–32. doi: 10.1111/j.1600-051X.2012.01859.x
91. Hu KF, Huang KC, Ho YP, Lin YC, Ho KY, Wu YM, et al. Interleukin-10 (-592 C/A) and Interleukin-12B (+16974 A/C) Gene Polymorphisms and the Interleukin-10 ATA Haplotype are Associated With Periodontitis in a Taiwanese Population. J Periodontal Res (2009) 44:378–85. doi: 10.1111/j.1600-0765.2008.01116.x
92. Jaradat SM, Ababneh KT, Jaradat SA, Abbadi MS, Taha AH, Karasneh JA, et al. Association of Interleukin-10 Gene Promoter Polymorphisms With Chronic and Aggressive Periodontitis. Oral Dis (2012) 18:271–9. doi: 10.1111/j.1601-0825.2011.01872.x
93. Li Y, Zhao H, Zhang J. Interleukin-10 Gene Promoter Polymorphism in Chinese Patients With Generalized Aggressive Periodontitis. J Dental Prev Treat (2009) 17:472–5.
94. Lopes CB, Barroso RFF, Burbano RMR, Garcia PA, Pinto P, Santos N, et al. Effect of Ancestry on Interleukin-10 Haplotypes in Chronic Periodontitis. Front Biosci (Elite Ed) (2017) 9:276–85. doi: 10.2741/e802
95. Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H, Moudi M. Analysis of Interleukin-10 Gene Polymorphisms in Patients With Chronic Periodontitis and Healthy Controls. Dent Res J (Isfahan) (2018) 15:71–9. doi: 10.4103/1735-3327.223614
96. Reichert S, Machulla H, Klapproth J, Zimmermann U, Reichert Y, Gläser C, et al. The Interleukin-10 Promoter Haplotype ATA is a Putative Risk Factor for Aggressive Periodontitis. J Periodontal Res (2008) 43:40–7. doi: 10.1111/j.1600-0765.2007.00992.x
97. Scapoli L, Girardi A, Palmieri A, Carinci F, Testori T, Zuffetti F, et al. IL6 and IL10 Are Genetic Susceptibility Factors of Periodontal Disease. Dent Res J (Isfahan) (2012) 9:S197–201. doi: 10.4103/1735-3327.109754
98. Scarel-Caminaga RM, Trevilatto PC, Souza AP, Brito RB, Camargo LE, Line SR. Interleukin 10 Gene Promoter Polymorphisms Are Associated With Chronic Periodontitis. J Clin Periodontol (2004) 31:443–8. doi: 10.1111/j.1600-051X.2004.00500.x
99. Silveira VR, Pigossi SC, Scarel-Caminaga RM, Cirelli JA, Rego R, Nogueira NA. Analysis of Polymorphisms in Interleukin 10, NOS2A, and ESR2 Genes in Chronic and Aggressive Periodontitis. Braz Oral Res (2016) 30:e105. doi: 10.1590/1807-3107BOR-2016.vol30.0105
100. Sumer AP, Kara N, Keles GC, Gunes S, Koprulu H, Bagci H. Association of Interleukin-10 Gene Polymorphisms With Severe Generalized Chronic Periodontitis. J Periodontol (2007) 78:493–7. doi: 10.1902/jop.2007.060309
101. Yuhui Z, Ping H, Jing L, Jin Z. Correlation Between Interleukin-10 Polymorphisms and Susceptibility to Chronic Periodontitis Among Uygur Adults in the Moyu Area. West China J Stomatol (2017) 35:514–9.
102. Babel N, Cherepnev G, Babel D, Tropmann A, Hammer M, Volk HD, et al. Analysis of Tumor Necrosis Factor-Alpha, Transforming Growth Factor-Beta, Interleukin-10, IL-6, and Interferon-Gamma Gene Polymorphisms in Patients With Chronic Periodontitis. J Periodontol (2006) 77:1978–83. doi: 10.1902/jop.2006.050315
103. Berglundh T, Donati M, Hahn-Zoric M, Hanson LA, Padyukov L. Association of the -1087 IL 10 Gene Polymorphism With Severe Chronic Periodontitis in Swedish Caucasians. J Clin Periodontol (2003) 30:249–54. doi: 10.1034/j.1600-051X.2003.10274.x
104. Chambrone L, Ascarza A, Guerrero ME, Pannuti C, de la Rosa M, Salinas-Prieto E, et al. Association of -1082 Interleukin-10 Gene Polymorphism in Peruvian Adults With Chronic Periodontitis. Med Oral Patol Oral Cir Bucal (2014) 19:e569–573. doi: 10.4317/medoral.19823
105. Crena J, Sangeetha Subramanian DJV, Gnana PPS, Ramanathan A. Single Nucleotide Polymorphism at– 1087 Locus of Interleukin-10 Gene Promoter Is Associated With Severe Chronic Periodontitis in Nonsmoking Patients. Eur J Dentistry (2015) 9:387. doi: 10.4103/1305-7456.163237
106. Gurol C, Kazazoglu E, Dabakoglu B, Korachi M. A Comparative Study of the Role of Cytokine Polymorphisms Interleukin-10 and Tumor Necrosis Factor Alpha in Susceptibility to Implant Failure and Chronic Periodontitis. Int J Oral Maxillofac Implants (2011) 26:955–60.
107. Quan-Gao DU, Ting-ting WU, Gang FU. Association Between IL-10-1082G/A Gene Polymorphism and Susceptibility of Recurrent Oral Ulcer: Meta Analysis. Shanghai J Stomatol (2018) 27:554–60.
108. Loo WT, C-b F, L-j B, Yue Y, Dou Y-d, Wang M, et al. Gene Polymorphism and Protein of Human Pro-and Anti-Inflammatory Cytokines in Chinese Healthy Subjects and Chronic Periodontitis Patients. In: Journal of Translational Medicine. Hong Kong: BioMed Central (2012). p. 1–10.
109. Mellati E, Arab HR, Tavakkol-Afshari J, Ebadian AR, Radvar M. Analysis of -1082 IL-10 Gene Polymorphism in Iranian Patients With Generalized Aggressive Periodontitis. Med Sci Monit (2007) 13:CR510–4. doi: 10.12659/MSM.935123
110. Wang J, Yuan W, Fu F, Yang J, Tang F. A Case-Control Study on Interleukin-10 Genetic Polymorphisms and Susceptibility to Chronic Periodontitis. Int J Epidemiol Infect Dis (2012) 39:306–9.
111. Wu YM, Chuang HL, Ho YP, Ho KY, Tsai CC. Investigation of Interleukin-13 Gene Polymorphisms in Individuals With Chronic and Generalized Aggressive Periodontitis in a Taiwanese (Chinese) Population. J Periodontal Res (2010) 45:695–701. doi: 10.1111/j.1600-0765.2010.01287.x
112. Chen L, Shen Y, Liu L, Li X, Wang T, Wen F. Interleukin-13 -1112 C/T Promoter Polymorphism Confers Risk for COPD: A Meta-Analysis. PloS One (2013) 8:e68222. doi: 10.1371/journal.pone.0068222
113. Borilova Linhartova P, Kastovsky J, Lucanova S, Bartova J, Poskerova H, Vokurka J, et al. Interleukin-17a Gene Variability in Patients With Type 1 Diabetes Mellitus and Chronic Periodontitis: Its Correlation With IL-17 Levels and the Occurrence of Periodontopathic Bacteria. Mediators Inflamm (2016) 2016:2979846. doi: 10.1155/2016/2979846
114. Chaudhari HL, Warad S, Ashok N, Baroudi K, Tarakji B. Association of Interleukin-17 Polymorphism (-197G/A) in Chronic and Localized Aggressive Periodontitis. Braz Oral Res (2016) 30. doi: 10.1590/1807-3107BOR-2016.vol30.0026
115. Corrêa JD, Madeira MFM, Resende RG, Correia-Silva J, Gomez RS, Souza D, et al. Association Between Polymorphisms in Interleukin-17A and-17F Genes and Chronic Periodontal Disease. Mediators Inflamm (2012) 2012. doi: 10.1155/2012/846052
116. Zacarias JM, Sippert EA, Tsuneto PY, Visentainer JE, de Oliveira e Silva C, Sell AM. The Influence of Interleukin 17A and IL17F Polymorphisms on Chronic Periodontitis Disease in Brazilian Patients. Mediators Inflamm (2015) 2015:147056. doi: 10.1155/2015/147056
117. Erdemir EO, Hendek MK, Kocakap DBS, Ozkan SY. Interleukin (IL)-17F (H161R) and IL-23R (R381Q) Gene Polymorphisms in Turkish Population With Periodontitis. J Res Med Dental Sci (2015) 3:2–7. doi: 10.5455/jrmds.2015322
118. Moreira P, Costa J, Gomez R, Gollob K, Dutra W. TNFA and IL10 Gene Polymorphisms Are Not Associated With Periodontitis in Brazilians. Open Dentistry J (2009) 3:184. doi: 10.2174/1874210600903010184
119. Domínguez-Pérez RA, Loyola-Rodriguez JP, Abud-Mendoza C, Alpuche-Solis AG, Ayala-Herrera JL, Martínez-Martínez RE. Association of Cytokines Polymorphisms With Chronic Peridontitis and Rheumatoid Arthritis in a Mexican Population. Acta Odontol Scand (2017) 75:243–8. doi: 10.1080/00016357.2017.1280846
120. Endo M, Tai H, Tabeta K, Kobayashi T, Yamazaki K, Yoshie H. Analysis of Single Nucleotide Polymorphisms in the 5’-Flanking Region of Tumor Necrosis Factor-Alpha Gene in Japanese Patients With Early-Onset Periodontitis. J Periodontol (2001) 72:1554–9. doi: 10.1902/jop.2001.72.11.1554
121. Fassmann A, Holla LI, Buckova D, Vasku A, Znojil V, Vanek J. Polymorphisms in the +252(A/G) Lymphotoxin-Alpha and the -308(A/G) Tumor Necrosis Factor-Alpha Genes and Susceptibility to Chronic Periodontitis in a Czech Population. J Periodontal Res (2003) 38:394–9. doi: 10.1034/j.1600-0765.2003.00661.x
122. Folwaczny M, Glas J, Török HP, Mende M, Folwaczny C. Lack of Association Between the Tnfα G– 308 A Promoter Polymorphism and Periodontal Disease. J Clin Periodontol (2004) 31:449–53. doi: 10.1111/j.1600-051x.2004.00499.x
123. Lin XH, Chen L, Wu B, Wei B. Association of the Tumour Necrosis Factor-α 308 Gene Polymorphism and Susceptibility to Severe Chronic Periodontitis. Chin J Birth Health Hered (2012) 20:13–4.
124. Bo L, Ning Y, Li-si T, Jing-bo L, Yan G, YA-ping P. A Study of Frequency of TNF Alpha Gene With Type 2 Diabetes Mellitus With Chronic Periodontitis. Shanghai J Stomatol (2011) 20(2):169–73.
125. Majumder P, Thou K, Bhattacharya M, Nair V, Ghosh S, Dey SK. Association of Tumor Necrosis Factor-Alpha (TNF-Alpha) Gene Promoter Polymorphisms With Aggressive and Chronic Periodontitis in the Eastern Indian Population. Biosci Rep (2018) 38. doi: 10.1042/BSR20171212
126. Ozer Yucel O, Berker E, Mesci L, Eratalay K, Tepe E, Tezcan I. Analysis of TNF-Alpha (-308) Polymorphism and Gingival Crevicular Fluid TNF-Alpha Levels in Aggressive and Chronic Periodontitis: A Preliminary Report. Cytokine (2015) 72:173–7. doi: 10.1016/j.cyto.2015.01.001
127. Pang R, Chen K, Zhang J, Xu C, Zhang X. Association of TNFA-308 Gene Polymorphisms With Susceptibility to Chronic Periodontitis in Chinese Patients. Shanghai J Stomatol (2005) 14:586–9.
128. Schulz S, Reichert S, Streetz K, Trautwein C, Reichert Y, Glaser C, et al. Tumor Necrosis Factor-Alpha and Oral Inflammation in Patients With Crohn Disease. J Periodontol (2014) 85:1424–31. doi: 10.1902/jop.2014.130644
129. Sharma N, Joseph R, Arun R, Chandni R, Srinivas KL, Banerjee M. Cytokine Gene Polymorphism (Interleukin-1beta +3954, Interleukin-6 [-597/-174] and Tumor Necrosis Factor-Alpha -308) in Chronic Periodontitis With and Without Type 2 Diabetes Mellitus. Indian J Dent Res (2014) 25:375–80. doi: 10.4103/0970-9290.138343
130. Trombone A, Cardoso C, Repeke C, Ferreira S, Martins W, Campanelli A, et al. Tumor Necrosis Factor-Alpha– 308G/A Single Nucleotide Polymorphism and Red-Complex Periodontopathogens Are Independently Associated With Increased Levels of Tumor Necrosis Factor-α in Diseased Periodontal Tissues. J Periodontal Res (2009) 44:598–608. doi: 10.1111/j.1600-0765.2008.01166.x
131. Yang W, Jia Y, Wu H. Four Tumor Necrosis Factor Alpha Genes Polymorphisms and Periodontitis Risk in a Chinese Population. Hum Immunol (2013) 74:1684–7. doi: 10.1016/j.humimm.2013.08.009
132. Zhong L, Zhang Y, Y-s L, Nie J, Wang X. Association Between Tumour Necrosis Factor A-308 Genotype and Chronic Periodontitis of Uighur Patients. Chin J Conservative Dentistry (2005) 15:550–2.
133. Heidari Z, Mahmoudzadeh-Sagheb H, Hashemi M, Ansarimoghaddam S, Moudi B, Sheibak N. Association between IFN-γ +874A/T and IFN-γR1 (-611A/G, +189T/G, and +95C/T) Gene Polymorphisms and Chronic Periodontitis in a Sample of Iranian Population. Int J Dent (2015) 2015:375353. doi: 10.1155/2015/375359
134. Holla LI, Hrdlickova B, Linhartova P, Fassmann A. Interferon-γ+ 874a/T Polymorphism in Relation to Generalized Chronic Periodontitis and the Presence of Periodontopathic Bacteria. Arch Oral Biol (2011) 56:153–8. doi: 10.1016/j.archoralbio.2010.09.005
135. Erciyas K, Pehlivan S, Sever T, Igci M, Arslan A, Orbak R. Association Between TNF-Alpha, TGF-Beta1, IL-10, IL-6 and IFN-Gamma Gene Polymorphisms and Generalized Aggressive Periodontitis. Clin Invest Med (2010) 33:E85.
136. Reichert S, Machulla HK, Klapproth J, Zimmermann U, Reichert Y, Gläser C, et al. Interferon-Gamma and Interleukin-12 Gene Polymorphisms and Their Relation to Aggressive and Chronic Periodontitis and Key Periodontal Pathogens. J Periodontol (2008) 79:1434–43. doi: 10.1902/jop.2008.070637
137. de Souza AP, Trevilatto PC, Scarel-Caminaga RM, de Brito RB, Line SR. Analysis of the TGF-Beta1 Promoter Polymorphism (C-509T) in Patients With Chronic Periodontitis. J Clin Periodontol (2003) 30:519–23. doi: 10.1034/j.1600-051X.2003.00323.x
138. Komatsu Y, Galicia JC, Kobayashi T, Yamazaki K, Yoshie H. Association of Interleukin-1 Receptor Antagonist +2018 Gene Polymorphism With Japanese Chronic Periodontitis Patients Using a Novel Genotyping Method. Int J Immunogenet (2008) 35:165–70. doi: 10.1111/j.1744-313X.2008.00757.x
139. Heidari Z, Mahmoudzadeh-Sagheb H, Hashemi M, Rigi-Ladiz MA. Quantitative Analysis of Interdental Gingiva in Patients With Chronic Periodontitis and Transforming Growth Factor-β1 29c/T Gene Polymorphisms. J Periodontol (2014) 85:281–9. doi: 10.1902/jop.2013.130087
140. Kobayashi T, Ito S, Kuroda T, Yamamoto K, Sugita N, Narita I, et al. The Interleukin-1 and Fcgamma Receptor Gene Polymorphisms in Japanese Patients With Rheumatoid Arthritis and Periodontitis. J Periodontol (2007) 78:2311–8. doi: 10.1902/jop.2007.070136
141. Holla LI, Fassmann A, Benes P, Halabala T, Znojil V. 5 Polymorphisms in the Transforming Growth Factor-Beta 1 Gene (TGF-Beta 1) in Adult Periodontitis. J Clin Periodontol (2002) 29:336–41. doi: 10.1034/j.1600-051X.2002.290409.x
142. Zhao X, Guan Z, Zhang Y. Relationship Between Transforming Growth Factor Beta-1 Gene-509C/T Polymorphism and Severe Chronic Periodontitis. Chin J Stomatol (2010) 45:610–3.
143. Arab H, Afshari J, Radvar M, Taghavi A, Sargolzaee N, Mokhtari M, et al. Association Between TGF-β1-509 Gene Polymorphism With Aggressive Periodontitis. Int J Genet Eng (2012) 2:33–7.
144. Furuya-Kanamori L, Barendregt JJ, Doi SA. A New Improved Graphical and Quantitative Method for Detecting Bias in Meta-Analysis. Int J Evidence-Based Healthc (2018) 16:195–203. doi: 10.1097/XEB.0000000000000141
145. Pirim GE, Toker H, Korkmaz EM, Poyraz O. IL-6 and IL-10 Gene Polymorphisms in Patients With Aggressive Periodontitis: Effects on GCF, Serum and Clinic Parameters. Braz Oral Res (2017) 31.
146. Tervonen T, Raunio T, Knuuttila M, Karttunen R. Polymorphisms in the CD14 and IL-6 Genes Associated With Periodontal Disease. J Clin Periodontol (2007) 34:377–83. doi: 10.1111/j.1600-051X.2007.01067.x
147. Wang C, Zhang J, Zhao H, Fan W, Xiao L, Xie B. Correlation of Interleukin-10-1082 G/A Single Nucleotide Polymorphism to the Risk of Severe Chronic Periodontitis in Chinese: A Case-Control Study. J Dental Prev Treat (2009) 17:354–6.
148. Chen J, Yang S, Li W, Yu W, Fan Z, Wang M, et al. IL-17A Secreted by Th17 Cells Is Essential for the Host Against Streptococcus Agalactiae Infections. J Microbiol Biotechnol (2021) 31:667–75. doi: 10.4014/jmb.2103.03053
149. Gaffen S, Hajishengallis G. A New Inflammatory Cytokine on the Block: Re-Thinking Periodontal Disease and the Th1/Th2 Paradigm in the Context of Th17 Cells and IL-17. J Dental Res (2008) 87:817–28. doi: 10.1177/154405910808700908
150. Shi Q, Cai C, Xu J, Liu J, Liu H, Huo N. Is There an Association Between IFN-γ+ 874a/T Polymorphism and Periodontitis Susceptibility?: A Meta-Analysis. Medicine (2017) 96. doi: 10.1097/MD.0000000000007288
151. McFarlane CG, Meikle MC. Interleukin-2, Interleukin-2 Receptor and Interleukin-4 Levels Are Elevated in the Sera of Patients With Periodontal Disease. J Periodontal Res (1991) 26:402–8. doi: 10.1111/j.1600-0765.1991.tb01729.x
152. Fraser DA, Loos BG, Boman U, Jan van Winkelhoff A, van der Velden U, Schenck K, et al. Polymorphisms in an Interferon-γ Receptor-1 Gene Marker and Susceptibility to Periodontitis. Acta Odontol Scand (2003) 61:297–302. doi: 10.1080/00016350310006168
153. Madureira DF, Lima ILDA, Costa GC, Lages EMB, Martins CC, Da Silva TA. Tumor Necrosis Factor-Alpha in Gingival Crevicular Fluid as a Diagnostic Marker for Periodontal Diseases: A Systematic Review. J Evid Based Dental Pract (2018) 18:315–31. doi: 10.1016/j.jebdp.2018.04.001
154. Kim J-H, Kim AR, Choi YH, Jang S, Woo G-H, Cha J-H, et al. Tumor Necrosis Factor-α Antagonist Diminishes Osteocytic RANKL and Sclerostin Expression in Diabetes Rats With Periodontitis. PloS One (2017) 12:e0189702. doi: 10.1371/journal.pone.0189702
155. Cotti E, Mezzena S, Schirru E, Ottonello O, Mura M, Ideo F, et al. Healing of Apical Periodontitis in Patients With Inflammatory Bowel Diseases and Under Anti–tumor Necrosis Factor Alpha Therapy. J Endodontics (2018) 44:1777–82. doi: 10.1016/j.joen.2018.09.004
156. Khoshhal M, Moradi Haghgoo J, Torkzaban P, Arabi SR, Vafaee F, Hajiloie M, et al. Association of Interleukin-4 Receptor Gene Polymorphism With Chronic Periodontitis. Avicenna J Clin Med (2011) 18:63–9.
157. Donati M, Berglundh T, Hytönen AM, Hahn-Zoric M, Hanson LÅ, Padyukov L. Association of the– 159 CD14 Gene Polymorphism and Lack of Association of the– 308 TNFA and Q551R IL-4RA Polymorphisms With Severe Chronic Periodontitis in Swedish Caucasians. J Clin Periodontol (2005) 32:474–9. doi: 10.1111/j.1600-051X.2005.00697.x
158. Ding C, Zhao L, Sun Y, Li L, Xu Y. Interleukin-1 Receptor Antagonist Polymorphism (Rs2234663) and Periodontitis Susceptibility: A Meta-Analysis. Arch Oral Biol (2012) 57:585–93. doi: 10.1016/j.archoralbio.2012.01.016
159. Baidaa TA, Maha S. Assessment of Salivary Interleukin-1 Receptor Antagonist and Obesity Measures of Patients With Chronic Periodontitis in Comparison to Healthy Control. Int J Med Res Health Sci (2017) 6:173–8.
160. Kajiura Y, Lew J-H, Ikuta T, Nishikawa Y, J-i K, Nagata T. Naruishi K: Clinical Significance of GCF sIL-6R and Calprotectin to Evaluate the Periodontal Inflammation. Ann Clin Biochem (2017) 54:664–70. doi: 10.1177/0004563216680232
161. Cheng R, Wu Z, Li M, Shao M, Hu T. Interleukin-1β is a Potential Therapeutic Target for Periodontitis: A Narrative Review. Int J Oral Sci (2020) 12:1–9. doi: 10.1038/s41368-019-0068-8
162. Lu J, Ren B, Wang L, Li M, Liu Y. Preparation and Evaluation of IL-1ra-Loaded Dextran/PLGA Microspheres for Inhibiting Periodontal Inflammation In Vitro. Inflammation (2020) 43:168–78. doi: 10.1007/s10753-019-01107-w
163. Ebersole JL, Kirakodu S, Novak MJ, Stromberg AJ, Shen S, Orraca L, et al. Cytokine Gene Expression Profiles During Initiation, Progression and Resolution of Periodontitis. J Clin Periodontol (2014) 41:853–61. doi: 10.1111/jcpe.12286
164. Goldstein JD, Perol L, Zaragoza B, Baeyens A, Marodon G, Piaggio E. Role of Cytokines in Thymus- Versus Peripherally Derived-Regulatory T Cell Differentiation and Function. Front Immunol (2013) 4:155. doi: 10.3389/fimmu.2013.00155
165. Lee SY, Cho ML, Oh HJ, Ryu JG, Park MJ, Jhun JY, et al. Et Al: Interleukin-2/Anti-Interleukin-2 Monoclonal Antibody Immune Complex Suppresses Collagen-Induced Arthritis in Mice by Fortifying Interleukin-2/STAT5 Signalling Pathways. Immunology (2012) 137:305–16. doi: 10.1111/imm.12008
166. Stadler AF, Angst PD, Arce RM, Gomes SC, Oppermann RV, Susin C. Gingival Crevicular Fluid Levels of Cytokines/Chemokines in Chronic Periodontitis: A Meta-Analysis. J Clin Periodontol (2016) 43:727–45. doi: 10.1111/jcpe.12557
167. Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and Immune Pathways in the Pathogenesis of Periodontal Disease. Periodontol 2000 (2014) 64:57–80. doi: 10.1111/prd.12002
168. Hajishengallis G, Korostoff JM. Revisiting the Page & Schroeder Model: The Good, the Bad and the Unknowns in the Periodontal Host Response 40 Years Later. Periodontol 2000 (2017) 75:116–51. doi: 10.1111/prd.12181
169. Moaaz M, Youssry S, Elfatatry A, El Rahman MA. Th17/Treg Cells Imbalance and Their Related Cytokines (IL-17, IL-10 and TGF-Beta) in Children With Autism Spectrum Disorder. J Neuroimmunol (2019) 337:577071. doi: 10.1016/j.jneuroim.2019.577071
170. Rouas R, Merimi M, Najar M, El Zein N, Fayyad-Kazan M, Berehab M, et al. Et Al: Human CD8(+) CD25 (+) CD127 (Low) Regulatory T Cells: microRNA Signature and Impact on TGF-Beta and IL-10 Expression. J Cell Physiol (2019) 234:17459–72. doi: 10.1002/jcp.28367
171. Wei L, Xu M, Xiong H. An Update of Knowledge on the Regulatory Role of Treg Cells in Apical Periodontitis. Oral Dis (2020) 27:1356–65. doi: 10.1111/odi.13450
172. Beklen A. Effects of IL-13 on TGF-Beta and MMP-1 in Periodontitis. Biotech Histochem (2017) 92:374–80. doi: 10.1080/10520295.2017.1312526
173. Mashhadiabbas F, Dastgheib SA, Hashemzehi A, Bahrololoomi Z, Asadian F, Neamatzadeh H, et al. Association of IL-10 -1082a>G, -819c>T, and -592C>A Polymorphisms With Susceptibility to Chronic and Aggressive Periodontitis: A Systematic Review and Meta-Analysis. Inflamm Res (2021) 70:509–24. doi: 10.1007/s00011-021-01448-z
174. Ilango P, Kumar D, Mahalingam A, Thanigaimalai A, Reddy VK. Evidence Revealing the Role of T Cell Regulators (Tregs) in Periodontal Diseases: A Review. J Indian Soc Periodontol (2021) 25:278. doi: 10.4103/jisp.jisp_308_20
175. Rajendran M, Looney S, Singh N, Elashiry M, Meghil MM, El-Awady AR, et al. Systemic Antibiotic Therapy Reduces Circulating Inflammatory Dendritic Cells and Treg–Th17 Plasticity in Periodontitis. J Immunol (2019) 202:2690–9. doi: 10.4049/jimmunol.1900046
176. Cafferata EA, Terraza-Aguirre C, Barrera R, Faúndez N, González N, Rojas C, et al. Interleukin-35 Inhibits Alveolar Bone Resorption by Modulating the Th17/Treg Imbalance During Periodontitis. J Clin Periodontol (2020) 47:676–88. doi: 10.1111/jcpe.13282
177. Alvarez C, Suliman S, Almarhoumi R, Vega ME, Rojas C, Monasterio G, et al. Regulatory T Cell Phenotype and Anti-Osteoclastogenic Function in Experimental Periodontitis. Sci Rep (2020) 10:1–12. doi: 10.1038/s41598-020-76038-w
178. Allam JP, Duan Y, Heinemann F, Winter J, Gotz W, Deschner J, et al. IL-23-Producing CD68(+) Macrophage-Like Cells Predominate Within an IL-17-Polarized Infiltrate in Chronic Periodontitis Lesions. J Clin Periodontol (2011) 38:879–86. doi: 10.1111/j.1600-051X.2011.01752.x
179. Luo Z, Wang H, Wu Y, Sun Z, Wu Y. Clinical Significance of IL-23 Regulating IL-17A and/or IL-17F Positive Th17 Cells in Chronic Periodontitis. Mediators Inflamm (2014) 2014:627959. doi: 10.1155/2014/627959
180. Ohyama H, Kato-Kogoe N, Kuhara A, Nishimura F, Nakasho K, Yamanegi K, et al. The Involvement of IL-23 and the Th17 Pathway in Periodontitis. J Dent Res (2009) 88:633–8. doi: 10.1177/0022034509339889
Keywords: meta-analysis, periodontitis, T helper cell, interleukin-1, genetic polymorphism
Citation: Liu X and Li H (2022) A Systematic Review and Meta-Analysis on Multiple Cytokine Gene Polymorphisms in the Pathogenesis of Periodontitis. Front. Immunol. 12:713198. doi: 10.3389/fimmu.2021.713198
Received: 22 May 2021; Accepted: 10 August 2021;
Published: 03 January 2022.
Edited by:
Roba M. Talaat, University of Sadat City, EgyptReviewed by:
Saikat Majumder, University of Pittsburgh, United StatesYong Cheng, Minzu University of China, China
Copyright © 2022 Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, bGlodWk5OUBqbHUuZWR1LmNu
 Xin Liu
Xin Liu Hui Li
Hui Li