
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 17 August 2021
Sec. Viral Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.711847
This article is part of the Research Topic Contemporary Challenges in Immunologic Testing in Clinical and Research Laboratories View all 28 articles
 Jeong Rae Yoo1‡
Jeong Rae Yoo1‡ Tae-Jin Kim2†‡
Tae-Jin Kim2†‡ Sang Taek Heo1‡
Sang Taek Heo1‡ Kyung-Ah Hwang3†
Kyung-Ah Hwang3† Hyunjoo Oh1
Hyunjoo Oh1 TaeHong Ha4
TaeHong Ha4 Hye Kyung Ko1
Hye Kyung Ko1 Seungjae Baek1
Seungjae Baek1 Ju Eun Kim1
Ju Eun Kim1 Jun Hyeong Kim1
Jun Hyeong Kim1 Jiin Lee1
Jiin Lee1 Min Ji Kang1
Min Ji Kang1 Mi Soo Yoo1
Mi Soo Yoo1 Jung Mogg Kim4
Jung Mogg Kim4 Kyung-Mi Lee2*
Kyung-Mi Lee2* Keun Hwa Lee4*
Keun Hwa Lee4*Severe fever with thrombocytopenia syndrome (SFTS) is a new tick-borne viral disease, and most SFTS virus (SFTSV) infections occur via bites from the tick Haemaphysalis longicornis; however, SFTSV transmission can also occur through close contact with an infected patient. SFTS is characterized by acute high fever, thrombocytopenia, leukopenia, elevated serum hepatic enzyme levels, gastrointestinal symptoms, and multiorgan failure and has a 16.2 to 30% mortality rate. In this study, we found that age, dyspnea rates, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase, multiorgan dysfunction score (MODS), viral load, IL-6 levels, and IL-10 levels were higher in patients with fatal disease than in patients with nonfatal disease during the initial clinical course of SFTS. In addition, we found that IL-6 and IL-10 levels, rather than viral load and neutralizing antibody titers, in patients with an SFTSV infection strongly correlated with outcomes (for severe disease with an ultimate outcome of recovery or death).
Severe fever with thrombocytopenia syndrome (SFTS), a new tick-borne viral disease with a high mortality rate, was first reported in China in 2009, South Korea in 2010, Japan in 2013, Vietnam in 2017, Myanmar in 2018, Taiwan in 2019, and Thailand and Pakistan in 2020 (1–8).
Most SFTSV infections occur via bites from the tick Haemaphysalis longicornis; however, SFTSV transmission can also occur through close contact with an infected patient (9).
SFTS is characterized by acute high fever, thrombocytopenia, leukopenia, elevated serum hepatic enzyme levels, gastrointestinal symptoms, and multiorgan failure and has a 16.2 to 30% mortality rate (1, 3, 6, 10).
In this study, we report the clinical and laboratory variables and clinical outcomes of confirmed SFTS patients with nonfatal and fatal disease from 2013 to 2019 in South Korea and show that age, dyspnea rates, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase, multiorgan dysfunction score (MODS), viral load, IL-6 levels, and IL-10 levels were higher in patients with fatal disease than in patients with nonfatal disease during the initial clinical course of SFTS.
In addition, we found that systemic IL-6 and IL-10 levels in patients with an SFTSV infection more strongly correlated with outcomes (for severe disease with an ultimate outcome of recovery or death) than did viral load and neutralizing antibodies.
We confirmed 62 SFTS patients treated at a single tertiary hospital on Jeju Island from April 2013 to December 2019 (case fatality rate (CFR = 11.2%), and 54 SFTS patients were enrolled in the study (Table 1).
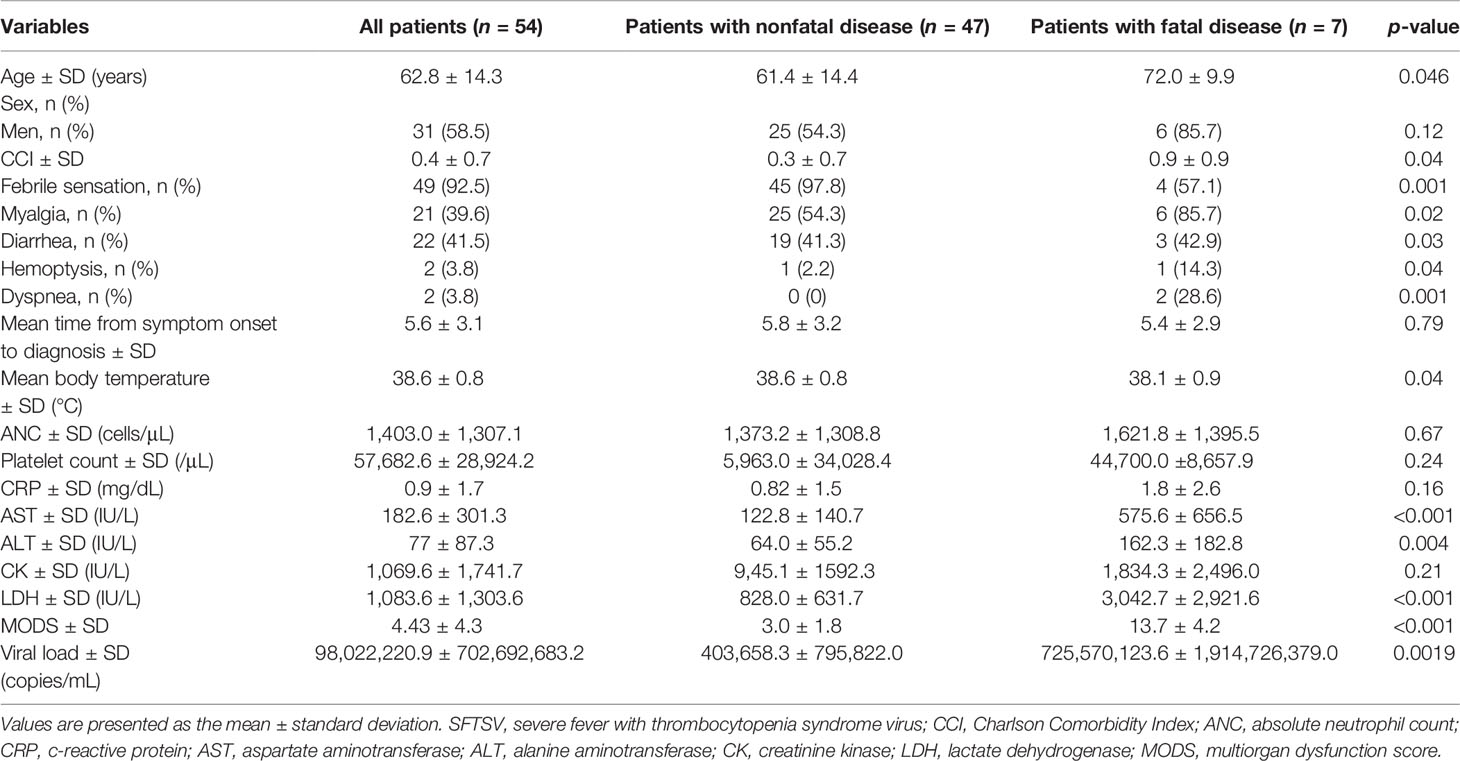
Table 1 Demographics and baseline characteristics of patients infected with SFTSV in Jeju, South Korea, from 2013 to 2019 (n=54).
To investigate demographic, clinical, and laboratory variables, including SFTS viral loads (Ct value) and the levels of cytokines (obtained during the first visit to the hospital), we collected 155 serum samples from 54 patients (patients with nonfatal disease: n = 47, mean age: 61.4 ± 14.4; patients with fatal disease: n = 7, mean age: 72.0 ± 9.9, CFR = 12.96%). Laboratory variables were confirmed in the Laboratory Department of Jeju National University Hospital (Table 1). This study was approved by the Institutional Review Board (IRB) of Jeju National University Hospital (IRB file no. 2018-11-002).
For molecular diagnosis of SFTSV and measurements of viral load, RNA was extracted from stored patient serum (155 serum samples from 54 patients) using a QIAamp Viral RNA Mini kit (QIAGEN, Hilden, Germany). Real-time one-step RT-PCR was performed using an Ezplex® SFTS virus Real-time PCR Kit (SMLGENETREE, South Korea) according to the manufacturer’s instructions. The patients were confirmed within one day in the hospital.
To characterize the effect of SFTSV infection on the production of serum cytokines in SFTS patients, interleukin-2 (IL-2), IL-4, IL-6, IL-10, IL-17A, tumor necrosis factor (TNF-α), and interferon-γ (IFN-γ) were measured using human Th1/Th2/Th17 CBA kits (BD Bioscience, San Diego, CA) according to the manufacturer’s instructions, with minor modifications. Sample acquisitions were performed with a FACS Canto II flow cytometer and analyzed by FCAP Array software version 3.0 (BD Bioscience, San Diego, CA).
All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). P values < 0.05 indicated statistical significance. To compare the mean difference between patients with fatal and nonfatal disease, we usually used a two-samples t-test. When using this method, we checked some assumptions, such as normality, equal variance, and independence. In this case, the two groups had quite different sample sizes (n = 47 and n = 7), and the normality assumption for each group did not hold. We used a nonparametric two-sample t-test called the Wilcoxon-Mann-Whitney test (Tables 1 and 2).
We confirmed 62 SFTS patients treated at a single tertiary hospital on Jeju Island from April 2013 to December 2019 (case fatality rate (CFR) = 11.2%, and 54 SFTS patients (patients with nonfatal disease: n = 47, mean age: 61.4 ± 14.4; patients with fatal disease: n = 7, mean age: 72.0 ± 9.9, CFR = 12.96%) enrolled in the study (Table 1).
Among the 54 SFTS patients, age, dyspnea rates, body temperature, AST, ALT, LDH, MODS, viral load, IL-6 levels, and IL-10 levels were significantly associated with the outcomes of patients with SFTSV (Tables 1, 2). Compared with patients with nonfatal disease, patients with fatal disease had higher age (p-value 0.046), dyspnea rates (0.001), AST (<0.001), ALT (0.004), LDH (<.0001), MODS (<.0001), viral load (0.0019), serum IL-6 levels (<.0001), and serum IL-10 levels (0.0003) and lower body temperature (0.04) during the initial clinical course of hospitalization (Tables 1, 2). However, there were no statistically significant differences in plasma levels of IL-2, IL-4, IL-17A, TNF-α, and IFN-γ between patients with nonfatal and fatal disease (Table 2).
We also studied the kinetics of the viral load and cytokine levels and compared them with the titer of neutralizing antibodies, which was previously shown to differ between patients with fatal severe disease and patients with nonfatal severe disease (11).
In patients with nonfatal severe disease, the levels of IL-6 and IL-10 were lower, and the viral load was higher than those of patients with fatal severe disease and decreased over time. In our previous paper, we showed that the titer of neutralizing antibodies in patients with nonfatal severe disease increased over time, although one patient with nonfatal severe disease did not produce neutralizing antibodies, similar to patients with fatal severe disease (Tables 3-1, 3-2).
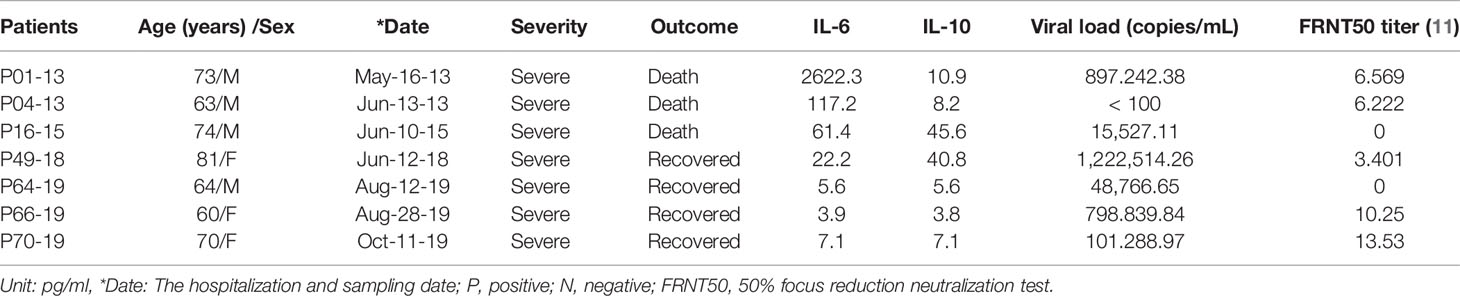
Table 3-1 Comparison of IL-6 and IL-10 concentrations, viral load, and neutralizing antibody titers between patients with fatal severe disease and patients with nonfatal severe disease.
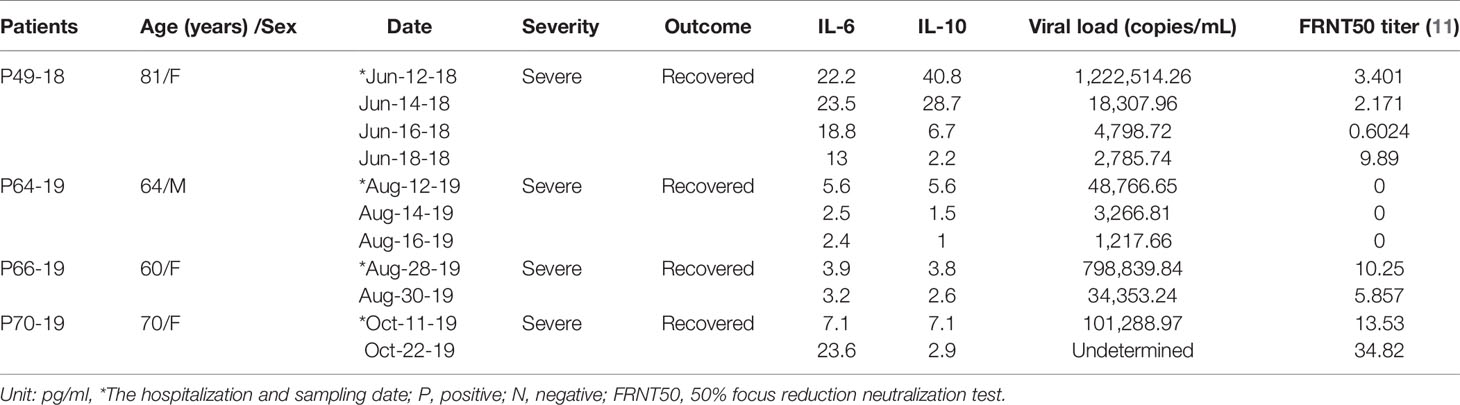
Table 3-2 Kinetics of IL-6 and IL-10 concentrations, viral load, and neutralizing antibody titers in patients with nonfatal severe disease.
IL-6, a proinflammatory cytokine, is essential for escalating the cell response to control persistent viral infection, and expression of IL-10, an important anti-inflammatory cytokine, is significantly elevated in SFTS patients, especially in patients with fatal disease.
The overproduction of IL-6 and IL-10 can create a cytokine storm, which is considered to contribute to the pathology of SFTS (12, 13).
In this study, high levels of IL-6 and IL-10 and high viral loads were found to coexist in patients with fatal and nonfatal disease.
In addition, IL-6 and IL-10 levels were higher in patients with fatal severe disease than in patients with nonfatal severe disease, and the levels of these cytokines were both decreased in patients with nonfatal severe disease.
The viral load was higher in patients with nonfatal severe disease than in patients with fatal severe disease at the first visit to the hospital and decreased over time. The titers of neutralizing antibodies for some patients with nonfatal severe disease was lower than that of patients with fatal severe disease at the first visit to the hospital but increased over time. However, one patient did not produce neutralizing antibodies such as a patient with fatal severe disease.
Therefore, we suggest that IL-6 and IL-10 determine the fate of patients (for severe disease with an ultimate outcome of recovery or death) more than viral load and the titer of neutralizing antibodies.
The limitations of our study include the relatively small number of patients studied (n = 54). However, this is a rigorous prospective study that took 7 years (from 2013 to 2019) in a representative hospital for the treatment of SFTS on Jeju Island, South Korea.
In summary, we reported that the levels of serum IL-6 and IL-10 were elevated in patients with fatal severe disease, while the levels these cytokines decreased in patients with nonfatal severe disease. This suggests that IL-6 and IL-10, rather than viral load and the titer of neutralizing antibodies, play an important role in determining the fate of patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
This study was approved by the Institutional Review Board (IRB) of Jeju National University Hospital (IRB file no. 2018-11-002). The patients/participants provided their written informed consent to participate in this study.
Conceptualization: KL. Methodology: KL, K-ML, JY, SH, TK, K-AH, HO, HK, SB, JEK, JHK, JL, MK, and TH. Supervision and validation: KL and K-ML. Formal analysis: KL, K-ML, JY, SH, TK, and K-AH. Funding acquisition: KL and K-ML. Data curation: KL and K-ML. Writing-original draft: KL, K-ML, JY, and TK. Writing-review and editing: KL and K-ML. All authors contributed to the article and approved the submitted version.
This work was supported by the National Research Foundation of Korea (NRF), the Ministry of Science, ICT, and Future Planning of South Korea (grant number: NRF-2020R1A2C2103061), and research funding from Hanyang University (HY-2020), and we thank S.Y. Bae for reviewing this paper.
K-AH was employed by SML Genetree.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever With Thrombocytopenia Associated With a Novel Bunyavirus in China. N Engl J Med (2011) 364:1523–32. doi: 10.1056/NEJMoa1010095
2. Kim YR, Yun Y, Bae SG, Park D, Kim SH, Lee JM, et al. Severe Fever With Thrombocytopenia Syndrome Virus Infection, South Korea, 2010. Emerg Infect Dis (2018) 24(11):2103–05. doi: 10.3201/eid2411.170756
3. Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, et al. The First Identification and Retrospective Study of Severe Fever With Thrombocytopenia Syndrome in Japan. J Infect Dis (2014) 209:816–27. doi: 10.1093/infdis/jit603
4. Tran XC, Yun Y, An LV, Kim SH, Thao NTP, Man PKC, et al. Endemic Severe Fever With Thrombocytopenia Syndrome, Vietnam. Emerg Infect Dis (2019) 25(5):1029–31. doi: 10.3201/eid2505.181463
5. Win AM, Nguyen YTH, Kim Y, Ha NY, Kang JG, Kim H, et al. Genotypic Heterogeneity of Orientia Tsutsugamushi in Scrub Typhus Patients and Thrombocytopenia Syndrome Co-Infection, Myanmar. Emerg Infect Dis (2020) 26(8):1878–81. doi: 10.3201/eid2608.200135
6. Peng SH, Yang SL, Tang SE, Wang TC, Hsu TC, Su CL, et al. Human Case of Severe Fever With Thrombocytopenia Syndrome Virus Infection, Taiwan, 2019. Emerg Infect Dis (2020) 26(7):1612–4. doi: 10.3201/eid2607.200104
7. Ongkittikul S. Severe Fever With Thrombocytopenia Syndrome Virus: The First Case Report in Thailand. Bangkok Med J (2020) 16(2):204–06. doi: 10.31524/bkkmedj.2020.22.001
8. Zohaib A, Zhang J, Saqib M, Athar MA, Hussain MH, Chen J, et al. Serologic Evidence of Severe Fever With Thrombocytopenia Syndrome Virus and Related Viruses in Pakistan. Emerg Infect Dis (2020) 26(7):1513–16. doi: 10.3201/eid2607.190611
9. Yoo JR, Heo ST, Park D, Kim H, Fukuma A, Fukushi S, et al. Family Cluster Analysis of Severe Fever With Thrombocytopenia Syndrome Virus Infection in Korea. Am J Trop Med Hyg (2017) 95(6):1351–57. doi: 10.4269/ajtmh.16-0527
10. Li H, Lu QB, Xing B, Zhang SF, Liu K, Du J, et al. Epidemiological and Clinical Features of Laboratory-Diagnosed Severe Fever With Thrombocytopenia Syndrome in China, 2011-17: A Prospective Observational Study. Lancet Infect Dis (2018) 18:30293–97. doi: 10.1016/S1473-3099(18)30293-7
11. Yoo JR, Kim JY, Heo ST, Kim J, Park HJ, Lee JY, et al. Neutralizing Antibodies to Severe Fever With Thrombocytopenia Syndrome Virus Among Survivors, Non-Survivors and Healthy Residents in South Korea. Front Cell Infect Microbiol (2021) 11:649570. doi: 10.3389/fcimb.2021.649570
12. Sun Y, Jin C, Zhan F, Wang X, Liang M, Zhang Q, et al. Host Cytokine Storm Is Associated With Disease Severity of Severe Fever With Thrombocytopenia Syndrome. J Infect Dis (2021) 206:1085–94. doi: 10.1093/infdis/jis452
Keywords: severe fever with thrombocytopenia syndrome, tick-borne viral diseases, IL-6, IL-10, South Korea
Citation: Yoo JR, Kim T-J, Heo ST, Hwang K-A, Oh H, Ha T, Ko HK, Baek S, Kim JE, Kim JH, Lee J, Kang MJ, Yoo MS, Kim JM, Lee K-M and Lee KH (2021) IL-6 and IL-10 Levels, Rather Than Viral Load and Neutralizing Antibody Titers, Determine the Fate of Patients With Severe Fever With Thrombocytopenia Syndrome Virus Infection in South Korea. Front. Immunol. 12:711847. doi: 10.3389/fimmu.2021.711847
Received: 19 May 2021; Accepted: 28 July 2021;
Published: 17 August 2021.
Edited by:
Stefan Vieths, Paul-Ehrlich-Institut (PEI), GermanyReviewed by:
Egidia Miftode, Grigore T. Popa University of Medicine and Pharmacy, RomaniaCopyright © 2021 Yoo, Kim, Heo, Hwang, Oh, Ha, Ko, Baek, Kim, Kim, Lee, Kang, Yoo, Kim, Lee and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keun Hwa Lee, eW9tdXN0N0BnbWFpbC5jb20=; Kyung-Mi Lee, a3l1bmdsZWVAa29yZWEuYWMua3I=
†Present address: Tae-Jin Kim, Division of Radiation Biomedical Research, Korea Institute of Radiological and Medical Science, Seoul, South Korea
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.