- Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China
Ewing’s sarcoma (EWS) is a malignant and aggressive tumor type that predominantly occurs in children and adolescents. Traditional treatments such as surgery, radiotherapy and chemotherapy, while successful in the early disease stages, are ineffective in patients with metastases and relapses who often have poor prognosis. Therefore, new treatments for EWS are needed to improve patient’s outcomes. Chimeric antigen receptor (CAR)-T cells therapy, a novel adoptive immunotherapy, has been developing over the past few decades, and is increasingly popular in researches and treatments of various cancers. CAR-T cell therapy has been approved by the Food and Drug Administration (FDA) for the treatment of leukemia and lymphoma. Recently, this therapeutic approach has been employed for solid tumors including EWS. In this review, we summarize the safety, specificity and clinical transformation of the treatment targets of EWS, and point out the directions for further research.
Introduction
Ewing’s sarcoma (EWS), a malignant cancer of bones or soft tissues, occurs predominantly in children and young adults and is the second most frequent primary bone tumor after osteosarcoma. Traditional treatments, including aggressive neoadjuvant and adjuvant chemotherapy in combination with surgery and/or radiotherapy, have greatly improved the long-term survival of patients suffering from localized disease, with a 5-year survival rate of more than 70% (1–3). However, once the tumor cells have metastasized or recurred, patients often show poor outcomes (4), indicating the need for new treatments for EWS. To improve the efficacy and eliminate adverse side effects, such new therapies need to the following characteristics: 1) High specificity to the tumor lesions, often referred to as targeted therapy, which can reduce damage to normal tissues. 2) Efficacy for metastatic and recurrent tumor lesions. In the past decades, new immunotherapies have emerged, such as immune checkpoint blockers, therapeutic cancer vaccines, and so on (5, 6). Among them, immune checkpoint blockers and chimeric antigen receptor (CAR)-T cells meet the above requirements and have been widely used in researches and treatments of various cancers in recent years (7, 8). CAR-T therapy is a form of treatment that combines tumor specific antibody receptors with cytotolytic T cell activity (9). Excitingly, CAR-T therapy has been used to treat hematologic tumors with encouraging results (10, 11). Recently, CAR-T therapy has also been used for solid tumors (12, 13). Furthermore, research has focused on the application of CAR-T cells for primary bone tumors (14). Therefore, we attempted to summarize the recent knowledge accumulated on CAR-T cell therapy for EWS, so that researchers can have a comprehensive understanding of all aspects of this kind of therapy.
Overview of CAR-T Cell Therapy
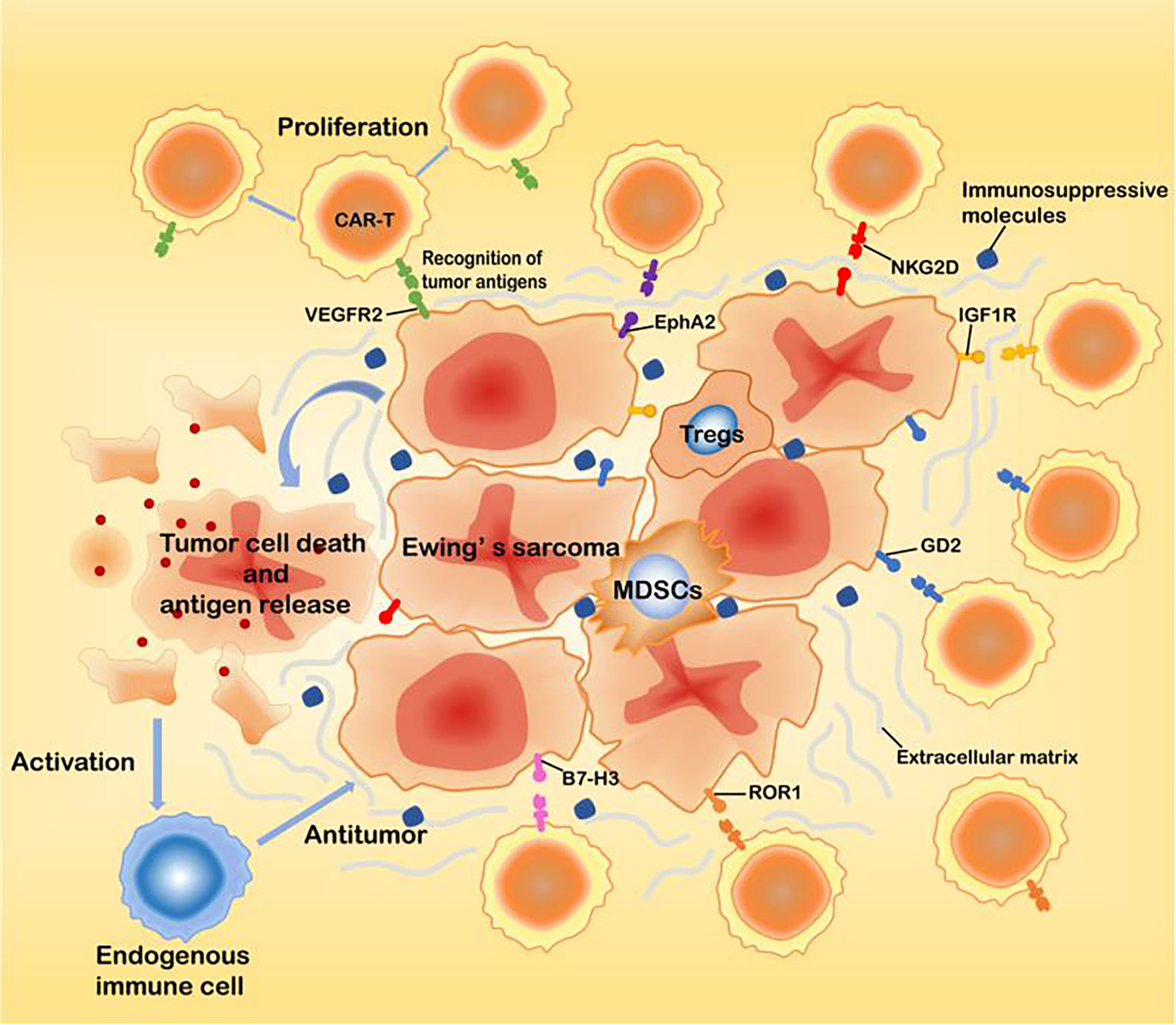
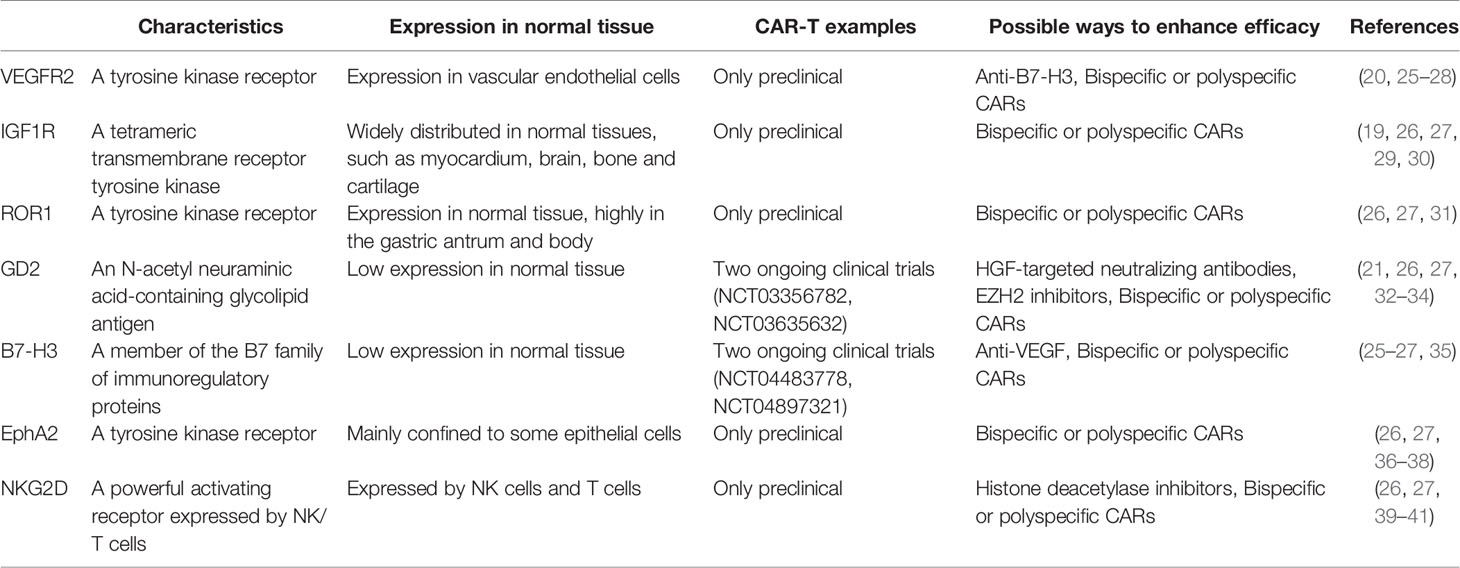
Adoptive cell therapy (ACT) is a treatment strategy where immune cells with antitumor activity are introduced into a cancer patient (15). CAR-T cell therapy, a novel type of adoptive immunotherapy, has developed rapidly in recent years. CARs are engineered receptors composed of an extracellular single-chain variable fragment (scFv) derived from a monoclonal antibody, a transmembrane domain and an intracellular domain. The intracellular domains of CAR-T cells are usually derived from the T cell receptor CD3-ζ chain, which can bind to costimulatory molecules such as CD28 or 41BB (16). The first-generation CARs usually contain only the CD3-ζ chain signal transduction domain. The addition of one costimulatory molecule to the first-generation CARs resulted in the so-called second-generation CARs, while the third-generation CARs include the addition of two costimulatory molecules to first-generation CARs (17). This approach not only makes the immune cells have the targeting property but can also overcome the immune tolerance dilemma, such that the modified immune cells will have strong antitumor activity. In addition, CAR-based T cells therapies show potent antitumor activity without the limitation of traditional major histocompatibility complexes(MHC). After transplantation, CAR-T cells can proliferate in large numbers and exhibit long-lasting antitumor activity (11). The effective application of CAR-T cells in the treatment of tumors requires that the engineered CAR-T cells be specific to the tumor cells and equally lethal to metastases (18). Therefore, the pursuit of finding tumor-specific antigens is a highly important task. Currently, targets identified in EWS include the vascular endothelial growth factor receptor 2 (VEGFR2), type I insulin-like growth factor receptor (IGF1R), receptor tyrosine kinase-like orphan receptor 1 (ROR1), ganglioside2 (GD2), B7-H3 (CD276), hepatocellular receptor tyrosine kinase class A2 (EphA2), and natural-killer group 2D ligands (NKG2D ligands) (19–24)(Figure 1, Table 1). These receptors may serve as effective therapeutic targets for CAR-T cells to treat EWS and inhibit metastases.
Research on CAR-T Cells Targeting EWS Antigens
VEGFR2 (Vascular Endothelial Growth Factor Receptor 2)
The vascular endothelial growth factor (VEGF) is an endothelial cell-specific mitogen that induces physiological and pathological angiogenesis. VEGF is a member of a larger family of growth factors that include VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factors. The most frequently studied member of this family is VEGF-A, commonly known as VEGF, which has several distinct variants (VEGF121, VEGF145, VEGF148, VEGF165, VEGF183, VEGF189 and VEGF206). VEGF receptors (VEGFRs) include the three types of VEGFR1, VEGFR2 and VEGFR3, among which VEGFR2 plays a major role in regulating VEGF signaling in endothelial cells. The VEGF-mediated signaling pathway has been demonstrated to occur in tumor cells, and it plays a key role in tumorigenesis, including cancer stem cell function and tumor initiation (42, 43). Recently, there has been growing interest in the role of VEGF in EWS (44, 45). Surita Dalal et al. demonstrated that EWS secretes VEGF, and that the expression of VEGF is independently related to microvascular density, suggesting that VEGF may be the most important regulator of neovascularization in ESW. Moreover, Flk-1/KDR receptor tyrosine kinase inhibitors and anti-VEGF agents significantly inhibited EWS growth in the mouse model (46). In one study, researchers generated CAR constructs against both human and murine VEGFR2 to enable preclinical studies of the xenograft model of EWS. This study showed that VEGFR2-specific CAR-T cells effectively lysed VEGFR2-positive cells of the respective species and responded with potent antigen-specific degranulation responses, cytokine secretion, and proliferation (20). Thus, VEGFR2 is likely to be a suitable target for CAR-T cell therapy in EWS. VEGFR2-targeted CAR-T cells have been used in early clinical trials of metastatic melanoma and epithelial carcinoma (NCT01218867), but the respective clinical response has been poor. Therefore, to enhance the efficiency of VEGFR2-targeted CAR-T cells and translate them into clinical applications, further research on targeting the VEGF signaling pathway is essential to better serve EWS therapy. Several studies have reported that VEGF165 plays a significant role in EWS angiogenesis and tumor growth, and targeting VEGF165 can inhibit EWS growth (47–50). In addition, VEGF165 could promote osteolytic bone destruction in EWS (51). Therefore, it seems worthwhile to investigate whether targeting VEGFR and VEGF simultaneously increases the antitumor effect of EWS.
IGF1R (Type I Insulin-Like Growth Factor Receptor)
IGF1R is a tetramer transmembrane receptor tyrosine kinase. The binding of ligand to the IGF1Rα subunit leads to the autophosphorylation of β subunit and the recruitment of adaptor proteins, ultimately resulting in the activation of signaling cascades that in turn contributes to proliferation, survival, transformation, metastasis, and angiogenesis (19, 29, 30). As IGF1R is expressed in EWS, many experiments have used it as an immune target for EWS treatment (52, 53). Various monoclonal antibodies have also been developed to treat EWS with a certain level of efficacy (54, 55). Thus, IGF1R-targeted CAR-T cell therapy for EWS appears to be a viable approach. According to a study by Xin Huang et al, IGF1R-targeted CAR-T cells showed specific cytotoxicity in vitro and mainly released IFN-γ, TNF-α, and IL-13 cytokines against sarcomas. These cells significantly inhibited sarcoma growth in both localized and disseminated pre-established sarcoma xenograft models. In addition, IGF1R-targeted CAR-T cells have also resulted in the benefit of prolonged survival in a localized sarcoma model (19). Although IGF1R-targeted CAR-T cells have a certain antitumor activity, it is not clear whether they have any toxic side effects on the body. Related studies have shown that IGF1R is also expressed in normal tissues (30). In a phase II study of EWS, researchers found that patients experienced adverse events such as neutropenia and leukopenia after treatment with ganitumab(a fully human anti-IGF1R antibody) (56). Xin Huang et al. reported that both lymphocytes and monocytes had low expression of cell-surface IGF1R, which made them not easily recognizable to IGF1R-targeted CAR-T cells (19). Off-target toxicity may be solved by the means of changing the affinity of CAR-T cells to the target or by adjusting the therapeutic dose of CAR-T cells. However, the systemic evaluation of off-target toxicity of IGF1R CAR-T cells should be performed before realizing their clinical application.
ROR 1 (Receptor Tyrosine Kinase-Like Orphan Receptor 1)
Receptor tyrosine kinase orphan receptors 1 (ROR1) is one of the twenty different RTK families and is highly conserved in evolution. It consists of three distinct extracellular domains, including the immunoglobulin-like domain, cysteine-rich (CRD) and Kringle (KNG) domains, and the intracellular TK domain. The cytoplasm contains the TK domain with protein kinase activity, which is rich in serine, threonine, and proline motifs further downstream. ROR1 is not expressed in normal adult tissues, but is overexpressed in several human malignancies and may act as a survival factor for tumor cells (57). Experiments by Jenny Potratz et al. have shown that ROR1 is expressed in EWS cell lines and ROR1 silencing impairs EWS cell survival and migration (58). Moreover, Xin Huang et al. further demonstrated that ROR1 is highly expressed in sarcoma cell lines including EWS, osteosarcoma, rhabdomyosarcoma, and fibrosarcoma. Furthermore, the in vitro and in vivo anti-sarcoma activity of ROR1-targeted CAR-T cells were indicated (19). The safety of ROR1-targeted CAR-T cells was demonstrated in primates (59). However, it was recently shown that ROR1 expression is not specific to tumor tissue. Cell surface ROR1 has been observed in several areas of the parathyroid gland, pancreatic islet, and intestinal tract in humans, and it was particularly abundant in the stomach antrum and gastric body, although experiments in the macaque model have shown no significant adverse effects (31). Shivani Srivastava et al. designed a Logic-Gated ROR1 CAR that can save healthy tissues and target tumor cells, addressing the issue of off-target toxicity (60). In the “AND” gate of the logic-gated receptor, CAR-T cell activity only eliminates tumors that express both antigens A and B. Because the synthetic Notch receptor is specific for antigen A induced expression of CAR-specific antigen B, the “AND” logic gate can integrate multiple signals to regulate T cell function, thus allowing a more precise distinction between tumor tissue and normal tissue (61). Although the above studies have proved that ROR1-targeted CAR-T cells have a certain efficacy in treating EWS, ROR1-targeted CAR-T cells have not yet been subject to clinical trials, and the latest progress comes from an ongoing recruitment study (NCT02706392), revealing that it has great development potential.
GD2 (Ganglioside2)
GD2, a cell surface molecule with a heavily restricted expression pattern, is highly expressed in EWS (62, 63). Due to the limited distribution of GD2 in normal tissues, it is safe for immunotargeting (32). S Kailayangiri et al. demonstrated that GD2 is expressed on the surface of EWS cell lines and primary EWS cells, and they proved that GD2-targeted CAR-T cells exert potent cytolytic responses against EWS cells (64). A different study also confirmed the antitumor activity of GD2-targeted CAR-T cells against EWS (65). However, other researches have shown that GD2-targeted CAR-T cells alone do not eliminate metastatic or orthotopically injected EWS cells. In fact, GD2-targeted CAR-T cells can prevent primary tumor growth and metastasis in EWS when combined with HGF-targeted neutralizing antibodies (21). In addition, Sareetha Kailayangiri et al. reported that the inhibition of enhancer of zeste homolog 2 (EZH2) enhanced the killing effect of GD2-targeted CAR-T cells against EWS (33). In a phase I study, researchers treated patients with neuroblastoma with GD2-targeted CAR-T cells and found no objective clinical response to treatment with GD2-targeted CAR-T cells alone (66). Therefore, the means to enhance the antitumor effect of GD2-targeted CAR-T cells is crucial to its successful clinical application. One strategy is to combine GD2 with other viable targets to construct T cells expressing multiple CARs. Another approach can combine GD2-targeted CAR-T cells with immune checkpoint inhibitors to improve efficacy.
B7-H3 (CD276)
B7-H3 (CD276), a member of the B7 family of immunoregulatory proteins, is frequently overexpressed at high levels by solid tumor cells. B7 proteins bind to members of the CD28/CTLA-4 family which act as costimulatory signals in T cell activation (67). Moreover, B7-H3 is overexpressed during pathological angiogenesis, which may make it an attractive target for the selective destruction of tumor vasculature (68). Another study showed that B7-H3 may be a receptor expressed by cytotoxic lymphocytes inhibiting the activation thereof, and its deficiency or lack of inhibitive effect results in increased cytotoxic lymphocyte function in tumor-bearing mice (69). Taken together, the B7-H3 checkpoint may serve as a novel target for immunotherapy against cancer. In recent years, the use of B7-H3-targeted CAR-T cells for the treatment of solid tumors have received significant attention. In in vitro orthotopic and metastatic xenografts in mouse models of pancreatic ductal adenocarcinoma, ovarian cancer, and neuroblastoma, B7-H3-targeted CAR-T cells showed promising efficacy with no significant adverse effects (70). One study used indirect immunofluorescence to detect 8H9 (a monoclonal antibody targeting tumor-associated B7-H3) immunoreactivity in Ewing/primitive neuroectodermal tumor cell lines, in which two-third of samples were strongly positive and the rest were weakly positive, strongly supporting the presence of B7-H3 expression in EWS (71). The use of B7-H3 targets to produce CAR-T cells or antibodies for EWS treatment has also been attempted (72). Robbie GM et al. tried to use B7-H3-targeted CAR-T cells against pediatric solid tumors. They found that greater than 90% of the tested pediatric sarcomas expressed B7-H3 with high expression of EWS. Further experiments showed that B7-H3-targeted CAR-T cells can eradicate EWS xenografts in vivo, leading to a significant survival advantage compared to treatment control in mice (22). Therefore, B7-H3-targeted CAR-T cell therapy may be a viable option to treat EWS. Similar studies have shown that the inhibition or suppression of B7-H3 expression can increase the response of tumor cells to alkylation agents, drugs targeting DNA replication, PI3K/Akt/mTOR, and Ras/Rraf/MEK signaling inhibitors (73–76). Accordingly, the combination of B7-H3-targeted CAR-T cells with immune checkpoint inhibitors or traditional chemotherapy agents seems to be a feasible alternative. Chao Xie et al. reported that VEGF expression in tumor cells may be mediated by soluble B7-H3 (25), suggesting that the combination of anti-VEGFR and anti-B7-H3 or the construction of bispecific CAR-T cells embedded with VEGFR and B7-H3 may constitute a sound approach. The knowledge of the specific mechanism of B7-H3 in tumors will enable us to better treat B7-H3-positive tumors.
EphA2(Hepatocellular Receptor Tyrosine Kinase Class A2)
Members of the Eph family are involved in cell transformation, metastasis, and angiogenesis. There are two classes of Eph receptor ligands: ephrin-A and ephrin-B. EphA2 is overexpressed in a variety of cancers, including breast cancer, melanoma, and prostate cancer (77). Associated studies have shown that EphA2 is upregulated in EWS cells, and participates in endothelial cell migration toward tumors to assist with tumor angiogenesis. Furthermore, EphA2 may be related to the aggressiveness of EWS (78–80). Therefore, blocking its function may be a promising method for EWS treatment. Kenneth Hsu et al. showed that EphA2-targeted CAR-T cells effectively killed EWS cells in mice, which was associated with prolonged survival. However, only a small percentage of EWS cells expressed EphA2 (23). It has been evidenced that EphA2 promotes EWS angiogenesis, tumor growth, and metastasis (79, 80), and that EphA2 CAR-T cells also exhibit antitumor activity in mice (23). Therefore, it is speculated that EphA2 CAR-T cells mainly influence EWS growth and metastasis by acting on tumor angiogenesis, which hypothesis clearly requires further experiments to test.
NKG2D (Natural-Killer Group 2D)
NKG2D is a powerful activating receptor expressed by natural killer (NK) cells and T cells (39). In recent years, the role of NKG2D and its ligands in EWS have been the focus of increased attention (40, 81, 82). Moreover, interference with NKG2D expression may affect the efficacy of NK cells against EWS; activated NK cells have been shown to kill EWS cells with high efficiency (81, 83). The restoration of NKG2D receptor expression on immune effector cells may contribute to therapeutic strategies for EWS. Manfred Lehner et al. constructed NKG2D-specific CAR-T cells by lentiviral transduction or mRNA transfection, and these CAR-T cells effectively eliminated EWS cells in vitro (24). On the one hand, to kill EWS cells more efficiently with NKG2D-specific CAR-T cells, we can modify T cells through the CARs editing technology. On the other hand, we can increase the efficiency by adding NKG2D ligands on tumor cells. Histone deacetylase inhibitors have been reported to upregulate the expression of NKD2G ligands in EWS (40, 41). Therefore, the combination of NKG2D-specific CAR-T cells and histone deacetylase inhibitors is considered as a recommended treatment for EWS.
Other Potential Targets
Several additional targets have been found to be expressed in EWS, such as EGFR, CD99, PAPP-A, STEAP1 and endosialin, but these have not yet been used in CAR-T cell therapy for EWS (84–88). Furthermore, EGFR has been employed in a phase I clinical trial of metastatic pancreatic carcinoma (89). The above targets can all serve as potential targets for EWS treatment by CAR-T therapy.
Discussion
Ewing’s sarcoma, an aggressive form of childhood cancer, is the second most common primary bone tumor. With the introduction of dose-intensive multiagent chemotherapy, the 5-year overall survival for the localized disease has improved to 70-75%, while the 5-year overall survival for metastatic or relapsed disease remains only at 20-30% (90, 91). Early hematogenous metastasis of EWS seriously affects the prognosis of patients, and the five-year survival rate of patients with metastasis is significantly lower than that of patients without metastasis (91, 92), which comprises the difficulty of EWS treatment. Traditional strategies including intensive chemotherapy fail to kill tumor cells completely in the blood, which are the key factors to EWS metastasis and recurrence (92). Targeted therapy and immunotherapy have shown some progress in EWS. Georgia J B McCaughan et al. reported an intensive pre-treated patient who had recurrent metastatic EWS achieved a clinical and radiological remission via PD-1 blockade (93), which is an encouraging result for the treatment of EWS. It is a pity that, there is little research in this field. For the treatment of EWS, there is still an urgent need for novel and effective treatments.
CAR-T cell therapy is a promising immunotherapeutical approach with encouraging results for tumors of the hematologic and lymphatic system (94). In comparison to traditional adoptive T cell therapy, editable CAR-T cells do not require MHC antigen presentation and can directly bind to target cell epitope for antitumor activity. Thus, CAR-T cells can overcome tumor escape and immune tolerance (95). Compared with checkpoint inhibitors, CAR-T cells can recognize lower levels of antigens, secrete cytokines that kill tumor cells, and self-proliferate to exert long-lasting antitumor effects (11, 96–99). Therefore, CAR-T cell therapy is clearly worthy of further studies. As EWS occurs more frequently in children and adolescents carrying more naive cells, and reports have suggested that adoptively transferred effector cells derived from naive T cells mediate superior antitumor effects, CAR-T therapy may exert superior antitumor effects in these EWS patients (100, 101). However, the adverse effects of CAR-T therapy on children and adolescents also should be focused. Currently, the main side effects of CAR-T therapy in children and adolescents remain the cytokine release syndrome and neurotoxicity, which can be prevented and treated by appropriate measures (102, 103). Furthermore, because the physiology of children and adolescents during growth is different from that of adults, it also should be discussed whether CAR-T cells therapy may have unique adverse effects. Therefore, more rigorous studies and clinical trials should be performed to explore the unique adverse effects of this therapy on children and adolescents. Excitingly, researches on CAR-T cells in the treatment of EWS have been pursued with certain encouraging developments. Currently, antigen epitopes used in CAR-T cells mainly include VEGFR2, IGF1R, ROR1, GD2, B7-H3, EphA2, and NKG2D. In most of the corresponding studies, researchers found that engineered targeted CAR-T cells exhibited antitumor activity in in vitro or in vivo preclinical models that were associated with a certain extent of extended survival. Nonetheless, these targets also have their limitations, such as off-target toxicity, insufficient effect and low expression, and they are still in their infancy, thus far from clinical applications. Therefore, more research is needed to address these issues before such targets can be translated into clinical practice. Regardless, CAR-T cell therapy for EWS is still worth undertaking. A considerable number of targets have been subject to clinical trials in solid tumor CAR-T cell therapy; even though their expression has not been established in EWS, targeted studies to address this gap can provide more options for EWS treatment by CAR-T, and are therefore worth conducting (104) (Table 2).
On the whole, for CAR-T cells to be translated into clinical applications, the following issues need to be effectively addressed: 1. Certain targets are not tumor-specific and are present in normal tissue. For example, Balakrishnan A et al. reported that ROR1 is not only expressed in EWS and cell surface, but has also been detected in several areas of the parathyroid gland, pancreatic islet, and intestinal tract in humans, and is particularly abundant in the gastric antrum and body (31). 2.Although CAR-T cell therapy has shown encouraging results in the treatment of hematological malignancies, formidable obstacles limit its success in treating the vast majority of solid tumors, which may include antigen selection, tumor trafficking and the tumor microenvironment (TME) (105, 106). 3. The extensive side effects of CAR-T cells also need to be addressed, such as Cytokine Release Syndrome, or Immune Effector Cell-Associated Neurotoxicity Syndrome (6, 107).
The following measures can be adopted to solve the above problems: 1. Firstly, targets can be identified that are more specific to tumors. For instance, EGFRvlll has been found to express almost exclusively on tumor cells, but not in normal tissues, indicating that EGFRvlll is tumor specific (108, 109). Secondly, when the target is highly expressed in tumor cells and lowly expressed in normal cells, the damage to normal tissue can be reduced by controlling the therapeutic threshold. Thirdly, the affinity between CARs and cognate antigens can be adjusted to achieve targeting (110–112). Furthermore, an alternative approach is to design CARs targeting tumor-associated abnormal glycosylated glycopeptide epitopes (113–116). 2. The obstacles of CAR-T cell therapy, such as tumor penetration, resistance to killing, antigen escape and immunosuppression, can be addressed from the following aspects. 1) CAR-T cells can be engineered to express chemokine receptors to recognize upregulated chemokines in TME, thus increasing the infiltration of CAR-T cells (117, 118). In addition, T cell infiltration can be enhanced by designing CAR-T cells capable of degrading the extracellular matrix proteins that constitute the physical barrier to TME (119). 2) A combination of CAR-T cells with conventional therapies or immune checkpoint inhibitors may be worth exploring. For example, Christian Spurny et al. showed that, when antibodies were used to block HLA-G upregulation, it could help T cells to infiltrate EWS, thereby enhancing the antitumor activity of T cells (120). 3) Constructing immune cells expressing multiple CARs or combining multiple CAR-T cells may provide a higher efficacy in tumor cell destruction (26). Bispecific CARs have been designed for the treatment of hematological tumors (27). However, this approach may also increase toxicity to normal tissues and therefore requires rigorous evaluation for practical applications. 4) More co-stimulatory expression receptors can be introduced into CAR-T cells, and CAR-T cells can be constructed that target tumor antigens and immunosuppressive cytokines or immunosuppressive cells in TME to resist the tumor immunosuppressive effects on T cells (121, 122). 5) Currently, certain drugs can upregulate the low expression of antigen epitopes in tumors via epigenetics and increase the killing effect of CAR-T cells on tumors (33, 41, 106). As a whole, the treatment of solid tumors is not confined to the treatment of tumor cells themselves, and the role of TME should not be ignored. Since the TME can affect the infiltration of T cells towards the tumor, the immune escape of tumor cells and T cell exhaustion may occur (123). Therefore, the interaction between CAR-T cells and TME may be the key to the transformation of CAR-T into clinical applications, which prompts the need for urgent consideration. 3. With regards to adverse effects such as cytokine storm, targeted toxicity by CAR-T cells can be limited appropriately by the introduction of operations such as co-expression of suicide genes or inhibition of receptors (124). However, further and rigorous scientific studies are needed on CAR-T cells to rule out adverse effects.
Conclusion
Therapies for EWS that involve CAR-T cells is a promising avenue, however, practicality and safety issues require further investigations.
Author Contributions
WL selected the topic and revised the manuscript. ZL searched the literature, wrote the manuscript and figures, and ZW searched the literature. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by special funds for the construction of innovative provinces in Hunan Province (2020RC3058).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Xiangya Hospital for their support of this writing.
References
1. Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, et al. Dose-Intensified Compared With Standard Chemotherapy for Nonmetastatic Ewing Sarcoma Family of Tumors: A Children's Oncology Group Study. J Clin Oncol (2009) 27:2536–41. doi: 10.1200/jco.2008.19.1478
2. Esiashvili N, Goodman M, Marcus RB Jr. Changes in Incidence and Survival of Ewing Sarcoma Patients Over the Past 3 Decades: Surveillance Epidemiology and End Results Data. J Pediatr Hematol/Oncol (2008) 30:425–30. doi: 10.1097/MPH.0b013e31816e22f3
3. Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of Ifosfamide and Etoposide to Standard Chemotherapy for Ewing's Sarcoma and Primitive Neuroectodermal Tumor of Bone. N Engl J Med (2003) 348:694–701. doi: 10.1056/NEJMoa020890
4. Burdach S, Jürgens H, Peters C, Nürnberger W, Mauz-Körholz C, Körholz D, et al. Myeloablative Radiochemotherapy and Hematopoietic Stem-Cell Rescue in Poor-Prognosis Ewing's Sarcoma. J Clin Oncol Off J Am Soc Clin Oncol (1993) 11:1482–8. doi: 10.1200/jco.1993.11.8.1482
5. Saxena M, van der Burg S, Melief C, Bhardwaj N. Therapeutic Cancer Vaccines. Nat Rev Cancer (2021) 21:360–78. doi: 10.1038/s41568-021-00346-0
6. Kennedy L, Salama A. A Review of Cancer Immunotherapy Toxicity. CA: Cancer J Clin (2020) 70:86–104. doi: 10.3322/caac.21596
7. Hong M, Clubb J, Chen Y. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell (2020) 38:473–88. doi: 10.1016/j.ccell.2020.07.005
8. Weiner G. Building Better Monoclonal Antibody-Based Therapeutics. Nat Rev Cancer (2015) 15:361–70. doi: 10.1038/nrc3930
9. Mackall C, Merchant M, Fry T. Immune-Based Therapies for Childhood Cancer. Nat Rev Clin Oncol (2014) 11:693–703. doi: 10.1038/nrclinonc.2014.177
10. June C, O'Connor R, Kawalekar O, Ghassemi S, Milone M. CAR T Cell Immunotherapy for Human Cancer. Sci (NY NY) (2018) 359:1361–5. doi: 10.1126/science.aar6711
11. June C, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med (2018) 379:64–73. doi: 10.1056/NEJMra1706169
12. Britten C, Shalabi A, Hoos A. Industrializing Engineered Autologous T Cells as Medicines for Solid Tumours. Nat Rev Drug Discov (2021) 20:476–88. doi: 10.1038/s41573-021-00175-8
13. Comoli P, Chabannon C, Koehl U, Lanza F, Urbano-Ispizua A, Hudecek M, et al. Development of Adaptive Immune Effector Therapies in Solid Tumors. Ann Oncol Off J Eur Soc Med Oncol (2019) 30:1740–50. doi: 10.1093/annonc/mdz285
14. Folkert I, Devalaraja S, Linette G, Weber K, Haldar M. Primary Bone Tumors: Challenges and Opportunities for CAR-T Therapies. J Bone Miner Res Off J Am Soc Bone Mineral Res (2019) 34:1780–8. doi: 10.1002/jbmr.3852
15. Rosenberg SA, Restifo NP. Adoptive Cell Transfer as Personalized Immunotherapy for Human Cancer. Science (2015) 348:62–8. doi: 10.1126/science.aaa4967
16. Sadelain M, Brentjens R, Rivière I. The Promise and Potential Pitfalls of Chimeric Antigen Receptors. Curr Opin Immunol (2009) 21:215–23. doi: 10.1016/j.coi.2009.02.009
17. Yu S, Li A, Liu Q, Li T, Yuan X, Han X, et al. Chimeric Antigen Receptor T Cells: A Novel Therapy for Solid Tumors. J Hematol Oncol (2017) 10:78. doi: 10.1186/s13045-017-0444-9
18. Köksal H, Müller E, Inderberg E, Bruland Ø., Wälchli S. Treating Osteosarcoma With CAR T Cells. Scand J Immunol (2019) 89:e12741. doi: 10.1111/sji.12741
19. Huang X, Park H, Greene J, Pao J, Mulvey E, Zhou S, et al. IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas. PloS One (2015) 10:e0133152. doi: 10.1371/journal.pone.0133152
20. Englisch A, Altvater B, Kailayangiri S, Hartmann W, Rossig C. VEGFR2 as a Target for CAR T Cell Therapy of Ewing Sarcoma. Pediatr Blood Cancer (2020) 67:e28313. doi: 10.1002/pbc.28313
21. Charan M, Dravid P, Cam M, Audino A, Gross A, Arnold M, et al. GD2-Directed CAR-T Cells in Combination With HGF-Targeted Neutralizing Antibody (AMG102) Prevent Primary Tumor Growth and Metastasis in Ewing Sarcoma. Int J Cancer (2020) 146:3184–95. doi: 10.1002/ijc.32743
22. Majzner R, Theruvath J, Nellan A, Heitzeneder S, Cui Y, Mount C, et al. CAR T Cells Targeting B7-H3, A Pan-Cancer Antigen, Demonstrate Potent Preclinical Activity Against Pediatric Solid Tumors and Brain Tumors. Clin Cancer Res Off J Am Assoc Cancer Res (2019) 25:2560–74. doi: 10.1158/1078-0432.ccr-18-0432
23. Hsu K, Middlemiss S, Saletta F, Gottschalk S, McCowage G, Kramer B. Chimeric Antigen Receptor-Modified T Cells Targeting EphA2 for the Immunotherapy of Paediatric Bone Tumours. Cancer Gene Ther (2021) 28:321–34. doi: 10.1038/s41417-020-00221-4
24. Lehner M, Götz G, Proff J, Schaft N, Dörrie J, Full F, et al. Redirecting T Cells to Ewing's Sarcoma Family of Tumors by a Chimeric NKG2D Receptor Expressed by Lentiviral Transduction or mRNA Transfection. PloS One (2012) 7:e31210. doi: 10.1371/journal.pone.0031210
25. Xie C, Liu D, Chen Q, Yang C, Wang B, Wu H. Soluble B7-H3 Promotes the Invasion and Metastasis of Pancreatic Carcinoma Cells Through the TLR4/NF-κb Pathway. Sci Rep (2016) 6:27528. doi: 10.1038/srep27528
26. Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, et al. Trivalent CAR T Cells Overcome Interpatient Antigenic Variability in Glioblastoma. Neuro Oncol (2018) 20:506–18. doi: 10.1093/neuonc/nox182
27. Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol Res (2016) 4:498–508. doi: 10.1158/2326-6066.cir-15-023
28. Holzer TR, Fulford AD, Nedderman DM, Umberger TS, Hozak RR, Joshi A, et al. Tumor Cell Expression of Vascular Endothelial Growth Factor Receptor 2 Is an Adverse Prognostic Factor in Patients With Squamous Cell Carcinoma of the Lung. PloS One (2013) 8:e80292–2. doi: 10.1371/journal.pone.0080292
29. Pollak M. The Insulin and Insulin-Like Growth Factor Receptor Family in Neoplasia: An Update. Nat Rev Cancer (2012) 12:159–69. doi: 10.1038/nrc3215
30. Chitnis M, Yuen J, Protheroe A, Pollak M, Macaulay V. The Type 1 Insulin-Like Growth Factor Receptor Pathway. Clin Cancer Res Off J Am Assoc Cancer Res (2008) 14:6364–70. doi: 10.1158/1078-0432.ccr-07-4879
31. Balakrishnan A, Goodpaster T, Randolph-Habecker J, Hoffstrom B, Jalikis F, Koch L, et al. Analysis of ROR1 Protein Expression in Human Cancer and Normal Tissues. Clin Cancer Res Off J Am Assoc Cancer Res (2017) 23:3061–71. doi: 10.1158/1078-0432.ccr-16-2083
32. Dobrenkov K, Cheung N. GD2-Targeted Immunotherapy and Radioimmunotherapy. Semin Oncol (2014) 41:589–612. doi: 10.1053/j.seminoncol.2014.07.003
33. Kailayangiri S, Altvater B, Lesch S, Balbach S, Göttlich C, Kühnemundt J, et al. EZH2 Inhibition in Ewing Sarcoma Upregulates G Expression for Targeting With Gene-Modified T Cells. Mol Ther J Am Soc Gene Ther (2019) 27:933–46. doi: 10.1016/j.ymthe.2019.02.014
34. Rashidijahanabad Z, Huang X. Recent Advances in Tumor Associated Carbohydrate Antigen Based Chimeric Antigen Receptor T Cells and Bispecific Antibodies for Anti-Cancer Immunotherapy. Semin Immunol (2020) 47:101390. doi: 10.1016/j.smim.2020.101390
35. Yang S, Wei W, Zhao Q. B7-H3, A Checkpoint Molecule, as a Target for Cancer Immunotherapy. Int J Biol Sci (2020) 16:1767–73. doi: 10.7150/ijbs.41105
36. Flanagan JG, Vanderhaeghen P. The Ephrins and Eph Receptors in Neural Development. Annu Rev Neurosci (1998) 21:309–45. doi: 10.1146/annurev.neuro.21.1.309
37. Lindberg RA, Hunter T. cDNA Cloning and Characterization of Eck, an Epithelial Cell Receptor Protein-Tyrosine Kinase in the Eph/Elk Family of Protein Kinases. Mol Cell Biol (1990) 10:6316–24. doi: 10.1128/mcb.10.12.6316
38. Kang BH, Jensen KJ, Hatch JA, Janes KA. Simultaneous Profiling of 194 Distinct Receptor Transcripts in Human Cells. Sci Signaling (2013) 6:rs13. doi: 10.1126/scisignal.2003624
39. Eagle RA, Trowsdale J. Promiscuity and the Single Receptor: NKG2D. Nat Rev Immunol (2007) 7:737–44. doi: 10.1038/nri2144
40. Berghuis D, Schilham MW, Vos HI, Santos SJ, Kloess S, Buddingh EP, et al. Histone Deacetylase Inhibitors Enhance Expression of NKG2D Ligands in Ewing Sarcoma and Sensitize for Natural Killer Cell-Mediated Cytolysis. Clin sarcoma Res (2012) 2:8. doi: 10.1186/2045-3329-2-8
41. Idso JM, Lao S, Schloemer NJ, Knipstein J, Burns R, Thakar MS, et al. Entinostat Augments NK Cell Functions via Epigenetic Upregulation of IFIT1-STING-STAT4 Pathway. Oncotarget (2020) 11:1799–815. doi: 10.18632/oncotarget.27546
42. Goel H, Mercurio A. VEGF Targets the Tumour Cell. Nat Rev Cancer (2013) 13:871–82. doi: 10.1038/nrc3627
43. Ferrara N. VEGF and the Quest for Tumour Angiogenesis Factors. Nat Rev Cancer (2002) 2:795–803. doi: 10.1038/nrc909
44. Zhou Z, Bolontrade M, Reddy K, Duan X, Guan H, Yu L, et al. Suppression of Ewing's Sarcoma Tumor Growth, Tumor Vessel Formation, and Vasculogenesis Following Anti Vascular Endothelial Growth Factor Receptor-2 Therapy. Clin Cancer Res Off J Am Assoc Cancer Res (2007) 13:4867–73. doi: 10.1158/1078-0432.ccr-07-0133
45. Kreuter M, Paulussen M, Boeckeler J, Gerss J, Buerger H, Liebscher C, et al. Clinical Significance of Vascular Endothelial Growth Factor-A Expression in Ewing's Sarcoma. Eur J Cancer (Oxf Engl) (2006) 1990) 42:1904–11. doi: 10.1016/j.ejca.2006.01.063
46. Dalal S, Berry A, Cullinane C, Mangham D, Grimer R, Lewis I, et al. Vascular Endothelial Growth Factor: A Therapeutic Target for Tumors of the Ewing's Sarcoma Family. Clin Cancer Res Off J Am Assoc Cancer Res (2005) 11:2364–78. doi: 10.1158/1078-0432.ccr-04-1201
47. Lee TH, Bolontrade MF, Worth LL, Guan H, Ellis LM, Kleinerman ES. Production of VEGF165 by Ewing's Sarcoma Cells Induces Vasculogenesis and the Incorporation of CD34+ Stem Cells Into the Expanding Tumor Vasculature. Int J Cancer (2006) 119:839–46. doi: 10.1002/ijc.21916
48. Reddy K, Cao Y, Zhou Z, Yu L, Jia SF, Kleinerman ES. VEGF165 Expression in the Tumor Microenvironment Influences the Differentiation of Bone Marrow-Derived Pericytes That Contribute to the Ewing's Sarcoma Vasculature. Angiogenesis (2008) 11:257–67. doi: 10.1007/s10456-008-9109-1
49. Zhou Z, Reddy K, Guan H, Kleinerman ES. VEGF(165), But Not VEGF(189), Stimulates Vasculogenesis and Bone Marrow Cell Migration Into Ewing's Sarcoma Tumors In Vivo. Mol Cancer Res (2007) 5:1125–32. doi: 10.1158/1541-7786.mcr-07-0174
50. Rennel E, Waine E, Guan H, Schüler Y, Leenders W, Woolard J, et al. The Endogenous Anti-Angiogenic VEGF Isoform, VEGF165b Inhibits Human Tumour Growth in Mice. Br J Cancer (2008) 98:1250–7. doi: 10.1038/sj.bjc.6604309
51. Guan H, Zhou Z, Cao Y, Duan X, Kleinerman ES. VEGF165 Promotes the Osteolytic Bone Destruction of Ewing's Sarcoma Tumors by Upregulating RANKL. Oncol Res (2009) 18:117–25. doi: 10.3727/096504009789954627
52. Toretsky J, Gorlick R. IGF-1R Targeted Treatment of Sarcoma. Lancet Oncol (2010) 11:105–6. doi: 10.1016/s1470-2045(09)70391-2
53. Ho A, Schwartz G. Targeting of Insulin-Like Growth Factor Type 1 Receptor in Ewing Sarcoma: Unfulfilled Promise or a Promising Beginning? J Clin Oncol Off J Am Soc Clin Oncol (2011) 29:4581–3. doi: 10.1200/jco.2011.38.2374
54. Juergens H, Daw N, Geoerger B, Ferrari S, Villarroel M, Aerts I, et al. Preliminary Efficacy of the Anti-Insulin-Like Growth Factor Type 1 Receptor Antibody Figitumumab in Patients With Refractory Ewing Sarcoma. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29:4534–40. doi: 10.1200/jco.2010.33.0670
55. Pappo A, Patel S, Crowley J, Reinke D, Kuenkele K, Chawla S, et al. R1507, a Monoclonal Antibody to the Insulin-Like Growth Factor 1 Receptor, in Patients With Recurrent or Refractory Ewing Sarcoma Family of Tumors: Results of a Phase II Sarcoma Alliance for Research Through Collaboration Study. J Clin Oncol Off J Am Soc Clin Oncol (2011) 29:4541–7. doi: 10.1200/jco.2010.34.0000
56. Tap WD, Demetri G, Barnette P, Desai J, Kavan P, Tozer R, et al. Phase II Study of Ganitumab, A Fully Human Anti-Type-1 Insulin-Like Growth Factor Receptor Antibody, in Patients With Metastatic Ewing Family Tumors or Desmoplastic Small Round Cell Tumors. J Clin Oncol (2012) 30:1849–56. doi: 10.1200/jco.2011.37.2359
57. Hojjat-Farsangi M, Moshfegh A, Daneshmanesh A, Khan A, Mikaelsson E, Osterborg A, et al. The Receptor Tyrosine Kinase ROR1–An Oncofetal Antigen for Targeted Cancer Therapy. Semin Cancer Biol (2014) 29:21–31. doi: 10.1016/j.semcancer.2014.07.005
58. Potratz J, Tillmanns A, Berning P, Korsching E, Schaefer C, Lechtape B, et al. Receptor Tyrosine Kinase Gene Expression Profiles of Ewing Sarcomas Reveal ROR1 as a Potential Therapeutic Target in Metastatic Disease. Mol Oncol (2016) 10:677–92. doi: 10.1016/j.molonc.2015.12.009
59. Berger C, Sommermeyer D, Hudecek M, Berger M, Balakrishnan A, Paszkiewicz P, et al. Safety of Targeting ROR1 in Primates With Chimeric Antigen Receptor-Modified T Cells. Cancer Immunol Res (2015) 3:206–16. doi: 10.1158/2326-6066.cir-14-0163
60. Srivastava S, Salter AI, Liggitt D, Yechan-Gunja S, Sarvothama M, Cooper K, et al. Logic-Gated ROR1 Chimeric Antigen Receptor Expression Rescues T Cell-Mediated Toxicity to Normal Tissues and Enables Selective Tumor Targeting. Cancer Cell (2019) 35:489–503.e8. doi: 10.1016/j.ccell.2019.02.003
61. Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell (2016) 164:770–9. doi: 10.1016/j.cell.2016.01.011
62. Lipinski M, Braham K, Philip I, Wiels J, Philip T, Dellagi K, et al. Phenotypic Characterization of Ewing Sarcoma Cell Lines With Monoclonal Antibodies. J Cell Biochem (1986) 31:289–96. doi: 10.1002/jcb.240310406
63. Lipinski M, Braham K, Philip I, Wiels J, Philip T, Goridis C, et al. Neuroectoderm-Associated Antigens on Ewing's Sarcoma Cell Lines. Cancer Res (1987) 47:183–7.
64. Kailayangiri S, Altvater B, Meltzer J, Pscherer S, Luecke A, Dierkes C, et al. The Ganglioside Antigen G(D2) Is Surface-Expressed in Ewing Sarcoma and Allows for MHC-Independent Immune Targeting. Br J Cancer (2012) 106:1123–33. doi: 10.1038/bjc.2012.57
65. Liebsch L, Kailayangiri S, Beck L, Altvater B, Koch R, Dierkes C, et al. Ewing Sarcoma Dissemination and Response to T-Cell Therapy in Mice Assessed by Whole-Body Magnetic Resonance Imaging. Br J Cancer (2013) 109:658–66. doi: 10.1038/bjc.2013.356
66. Straathof K, Flutter B, Wallace R, Jain N, Loka T, Depani S, et al. Antitumor Activity Without on-Target Off-Tumor Toxicity of GD2-Chimeric Antigen Receptor T Cells in Patients With Neuroblastoma. Sci Trans Med (2020) 12:eabd6169. doi: 10.1126/scitranslmed.abd6169
67. Flem-Karlsen K, Fodstad Ø., Tan M, Nunes-Xavier CE. B7-H3 in Cancer - Beyond Immune Regulation. Trends Cancer (2018) 4:401–4. doi: 10.1016/j.trecan.2018.03.010
68. Seaman S, Zhu Z, Saha S, Zhang X, Yang M, Hilton M, et al. Eradication of Tumors Through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell (2017) 31:501–15.e8. doi: 10.1016/j.ccell.2017.03.005
69. Lee Y, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, et al. Inhibition of the B7-H3 Immune Checkpoint Limits Tumor Growth by Enhancing Cytotoxic Lymphocyte Function. Cell Res (2017) 27:1034–45. doi: 10.1038/cr.2017.90
70. Du H, Hirabayashi K, Ahn S, Kren N, Montgomery S, Wang X, et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell (2019) 35:221–37.e8. doi: 10.1016/j.ccell.2019.01.002
71. Modak S, Kramer K, Gultekin S, Guo H, Cheung N. Monoclonal Antibody 8H9 Targets a Novel Cell Surface Antigen Expressed by a Wide Spectrum of Human Solid Tumors. Cancer Res (2001) 61:4048–54.
72. He L, Li Z. B7-H3 and Its Role in Bone Cancers. Pathol Res Pract (2019) 215:152420. doi: 10.1016/j.prp.2019.04.012
73. Flem-Karlsen K, Tekle C, Andersson Y, Flatmark K, Fodstad Ø., Nunes-Xavier CE. Immunoregulatory Protein B7-H3 Promotes Growth and Decreases Sensitivity to Therapy in Metastatic Melanoma Cells. Pigment Cell Melanoma Res (2017) 30:467–76. doi: 10.1111/pcmr.12599
74. Kasten BB, Arend RC, Katre AA, Kim H, Fan J, Ferrone S, et al. B7-H3-Targeted (212)Pb Radioimmunotherapy of Ovarian Cancer in Preclinical Models. Nucl Med Biol (2017) 47:23–30. doi: 10.1016/j.nucmedbio.2017.01.003
75. Liu H, Tekle C, Chen YW, Kristian A, Zhao Y, Zhou M, et al. B7-H3 Silencing Increases Paclitaxel Sensitivity by Abrogating Jak2/Stat3 Phosphorylation. Mol Cancer Ther (2011) 10:960–71. doi: 10.1158/1535-7163.mct-11-0072
76. Nunes-Xavier CE, Karlsen KF, Tekle C, Pedersen C, Øyjord T, Hongisto V, et al. Decreased Expression of B7-H3 Reduces the Glycolytic Capacity and Sensitizes Breast Cancer Cells to AKT/mTOR Inhibitors. Oncotarget (2016) 7:6891–901. doi: 10.18632/oncotarget.6902
77. Macrae M, Neve R, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, et al. A Conditional Feedback Loop Regulates Ras Activity Through Epha2. Cancer Cell (2005) 8:111–8. doi: 10.1016/j.ccr.2005.07.005
78. Nordberg J, Mpindi J, Iljin K, Pulliainen A, Kallajoki M, Kallioniemi O, et al. Systemic Analysis of Gene Expression Profiles Identifies ErbB3 as a Potential Drug Target in Pediatric Alveolar Rhabdomyosarcoma. PloS One (2012) 7:e50819. doi: 10.1371/journal.pone.0050819
79. Sáinz-Jaspeado M, Huertas-Martinez J, Lagares-Tena L, Martin Liberal J, Mateo-Lozano S, de Alava E, et al. EphA2-Induced Angiogenesis in Ewing Sarcoma Cells Works Through bFGF Production and Is Dependent on Caveolin-1. PloS One (2013) 8:e71449. doi: 10.1371/journal.pone.0071449
80. Garcia-Monclús S, López-Alemany R, Almacellas-Rabaiget O, Herrero-Martín D, Huertas-Martinez J, Lagares-Tena L, et al. EphA2 Receptor Is a Key Player in the Metastatic Onset of Ewing Sarcoma. Int J Cancer (2018) 143:1188–201. doi: 10.1002/ijc.31405
81. Verhoeven DH, de Hooge AS, Mooiman EC, Santos SJ, ten Dam MM, Gelderblom H, et al. NK Cells Recognize and Lyse Ewing Sarcoma Cells Through NKG2D and DNAM-1 Receptor Dependent Pathways. Mol Immunol (2008) 45:3917–25. doi: 10.1016/j.molimm.2008.06.016
82. Ahn YO, Weigel B, Verneris MR. Killing the Killer: Natural Killer Cells to Treat Ewing's Sarcoma. Clin Cancer Res (2010) 16:3819–21. doi: 10.1158/1078-0432.ccr-10-1368
83. Pahl JH, Ruslan SE, Kwappenberg KM, van Ostaijen-Ten Dam MM, van Tol MJ, Lankester AC, et al. Antibody-Dependent Cell Lysis by NK Cells Is Preserved After Sarcoma-Induced Inhibition of NK Cell Cytotoxicity. Cancer Immunol Immunother (2013) 62:1235–47. doi: 10.1007/s00262-013-1406-x
84. Guerzoni C, Fiori V, Terracciano M, Manara M, Moricoli D, Pasello M, et al. CD99 Triggering in Ewing Sarcoma Delivers a Lethal Signal Through P53 Pathway Reactivation and Cooperates With Doxorubicin. Clin Cancer Res Off J Am Assoc Cancer Res (2015) 21:146–56. doi: 10.1158/1078-0432.ccr-14-0492
85. Heitzeneder S, Sotillo E, Shern J, Sindiri S, Xu P, Jones R, et al. Pregnancy-Associated Plasma Protein-A (PAPP-A) in Ewing Sarcoma: Role in Tumor Growth and Immune Evasion. J Natl Cancer Inst (2019) 111:970–82. doi: 10.1093/jnci/djy209
86. Grunewald T, Ranft A, Esposito I, da Silva-Buttkus P, Aichler M, Baumhoer D, et al. High STEAP1 Expression Is Associated With Improved Outcome of Ewing's Sarcoma Patients. Ann Oncol Off J Eur Soc Med Oncol (2012) 23:2185–90. doi: 10.1093/annonc/mdr605
87. D'Onofrio A, Gano L, Melo R, Mendes F, Oliveira MC, Denoël T, et al. Biological Evaluation of New TEM1 Targeting Recombinant Antibodies for Radioimmunotherapy: In Vitro, In Vivo and in Silico Studies. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V (2021) 158:233–44. doi: 10.1016/j.ejpb.2020.11.015
88. Kersting N, Kunzler Souza B, Araujo Vieira I, Pereira Dos Santos R, Brufatto Olguins D, José Gregianin L, et al. Epidermal Growth Factor Receptor Regulation of Ewing Sarcoma Cell Function. Oncology (2018) 94:383–93. doi: 10.1159/000487143
89. Liu Y, Guo Y, Wu Z, Feng K, Tong C, Wang Y, et al. Anti-EGFR Chimeric Antigen Receptor-Modified T Cells in Metastatic Pancreatic Carcinoma: A Phase I Clinical Trial. Cytotherapy (2020) 22:573–80. doi: 10.1016/j.jcyt.2020.04.088
90. Balamuth N, Womer R. Ewing's Sarcoma. Lancet Oncol (2010) 11:184–92. doi: 10.1016/s1470-2045(09)70286-4
91. Grünewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, de Álava E, Kovar H, et al. Ewing Sarcoma. Nat Rev Dis Primers (2018) 4:5. doi: 10.1038/s41572-018-0003-x
92. Gaspar N, Hawkins D, Dirksen U, Lewis I, Ferrari S, Le Deley M, et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. J Clin Oncol Off J Am Soc Clin Oncol (2015) 33:3036–46. doi: 10.1200/jco.2014.59.5256
93. McCaughan GJ, Fulham MJ, Mahar A, Soper J, Hong AM, Stalley PD, et al. Programmed Cell Death-1 Blockade in Recurrent Disseminated Ewing Sarcoma. J Hematol Oncol (2016) 9:48. doi: 10.1186/s13045-016-0278-x
94. Elsallab M, Levine B, Wayne A, Abou-El-Enein M. CAR T-Cell Product Performance in Haematological Malignancies Before and After Marketing Authorisation. Lancet Oncol (2020) 21:e104–16. doi: 10.1016/s1470-2045(19)30729-6
95. Sha H, Wang D, Yan D, Hu Y, Yang S, Liu S, et al. Chimaeric Antigen Receptor T-Cell Therapy for Tumour Immunotherapy. Biosci Rep (2017) 37:BSR20160332. doi: 10.1042/bsr20160332
96. Feins S, Kong W, Williams E, Milone M, Fraietta J. An Introduction to Chimeric Antigen Receptor (CAR) T-Cell Immunotherapy for Human Cancer. Am J Hematol (2019) 94:S3–9. doi: 10.1002/ajh.25418
97. Ahmed N, Salsman V, Yvon E, Louis C, Perlaky L, Wels W, et al. Immunotherapy for Osteosarcoma: Genetic Modification of T Cells Overcomes Low Levels of Tumor Antigen Expression. Mol Ther J Am Soc Gene Ther (2009) 17:1779–87. doi: 10.1038/mt.2009.133
98. Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence That a Single Peptide-MHC Complex on a Target Cell Can Elicit a Cytolytic T Cell Response. Immunity (1996) 4:565–71. doi: 10.1016/s1074-7613(00)80483-5
99. Anders K, Blankenstein T. Molecular Pathways: Comparing the Effects of Drugs and T Cells to Effectively Target Oncogenes. Clin Cancer Res Off J Am Assoc Cancer Res (2013) 19:320–6. doi: 10.1158/1078-0432.ccr-12-3017
100. Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. Adoptively Transferred Effector Cells Derived From Naive Rather Than Central Memory CD8+ T Cells Mediate Superior Antitumor Immunity. Proc Natl Acad Sci USA (2009) 106:17469–74. doi: 10.1073/pnas.0907448106
101. Hong MS, Dan JM, Choi JY, Kang I. Age-Associated Changes in the Frequency of Naïve, Memory and Effector CD8+ T Cells. Mech Ageing Dev (2004) 125:615–8. doi: 10.1016/j.mad.2004.07.001
102. Jasinski S, De Los Reyes FA, Yametti GC, Pierro J, Raetz E, Carroll WL. Immunotherapy in Pediatric B-Cell Acute Lymphoblastic Leukemia: Advances and Ongoing Challenges. Pediatr Drugs (2020) 22:485–99. doi: 10.1007/s40272-020-00413-3
103. Shalabi H, Wolters P, Martin S, Toledo-Tamula M, Roderick M, Struemph K, et al. Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy. J Immunother (Hagerstown Md. 1997) (2018) 41:350–8. doi: 10.1097/cji.0000000000000241
104. Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, et al. CAR T Cells in Solid Tumors: Challenges and Opportunities. Stem Cell Res Ther (2021) 12:81. doi: 10.1186/s13287-020-02128-1
105. Klebanoff C, Rosenberg S, Restifo N. Prospects for Gene-Engineered T Cell Immunotherapy for Solid Cancers. Nat Med (2016) 22:26–36. doi: 10.1038/nm.4015
106. Steffin D, Heslop H. Epigenetic Inhibition Puts Target Antigen in the Crosshairs of CAR T Cells. Mol Ther J Am Soc Gene Ther (2019) 27:900–1. doi: 10.1016/j.ymthe.2019.04.007
107. Morgan R, Yang J, Kitano M, Dudley M, Laurencot C, Rosenberg S. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced With a Chimeric Antigen Receptor Recognizing ERBB2. Mol Ther J Am Soc Gene Ther (2010) 18:843–51. doi: 10.1038/mt.2010.24
108. Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The Somatic Genomic Landscape of Glioblastoma. Cell (2013) 155:462–77. doi: 10.1016/j.cell.2013.09.034
109. Johnson LA, Scholler J, Ohkuri T, Kosaka A, Patel PR, McGettigan SE, et al. Rational Development and Characterization of Humanized Anti-EGFR Variant III Chimeric Antigen Receptor T Cells for Glioblastoma. Sci Trans Med (2015) 7:275ra22. doi: 10.1126/scitranslmed.aaa4963
110. Chmielewski M, Hombach A, Heuser C, Adams GP, Abken H. T Cell Activation by Antibody-Like Immunoreceptors: Increase in Affinity of the Single-Chain Fragment Domain Above Threshold Does Not Increase T Cell Activation Against Antigen-Positive Target Cells But Decreases Selectivity. J Immunol (Baltimore Md. 1950) (2004) 173:7647–53. doi: 10.4049/jimmunol.173.12.7647
111. Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, et al. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index Against Tumors in Mice. Cancer Res (2015) 75:3596–607. doi: 10.1158/0008-5472.can-15-0159
112. Caruso HG, Hurton LV, Najjar A, Rushworth D, Ang S, Olivares S, et al. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer Res (2015) 75:3505–18. doi: 10.1158/0008-5472.can-15-0139
113. Stone JD, Aggen DH, Schietinger A, Schreiber H, Kranz DM. A Sensitivity Scale for Targeting T Cells With Chimeric Antigen Receptors (CARs) and Bispecific T-Cell Engagers (BiTEs). Oncoimmunology (2012) 1:863–73. doi: 10.4161/onci.20592
114. Posey AD Jr., Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity (2016) 44:1444–54. doi: 10.1016/j.immuni.2016.05.014
115. He Y, Schreiber K, Wolf SP, Wen F, Steentoft C, Zerweck J, et al. Multiple Cancer-Specific Antigens Are Targeted by a Chimeric Antigen Receptor on a Single Cancer Cell. JCI Insight (2019) 4:e130416. doi: 10.1172/jci.insight.130416
116. Sharma P, Marada V, Cai Q, Kizerwetter M, He Y, Wolf SP, et al. Structure-Guided Engineering of the Affinity and Specificity of CARs Against Tn-Glycopeptides. Proc Natl Acad Sci USA (2020) 117:15148–59. doi: 10.1073/pnas.1920662117
117. Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, et al. Enhanced Tumor Trafficking of GD2 Chimeric Antigen Receptor T Cells by Expression of the Chemokine Receptor CCR2b. J Immunother (Hagerstown Md. 1997) (2010) 33:780–8. doi: 10.1097/CJI.0b013e3181ee6675
118. Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, et al. Expression of a Functional CCR2 Receptor Enhances Tumor Localization and Tumor Eradication by Retargeted Human T Cells Expressing a Mesothelin-Specific Chimeric Antibody Receptor. Clin Cancer Res (2011) 17:4719–30. doi: 10.1158/1078-0432.ccr-11-0351
119. Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, et al. Heparanase Promotes Tumor Infiltration and Antitumor Activity of CAR-Redirected T Lymphocytes. Nat Med (2015) 21:524–9. doi: 10.1038/nm.3833
120. Spurny C, Kailayangiri S, Altvater B, Jamitzky S, Hartmann W, Wardelmann E, et al. T Cell Infiltration Into Ewing Sarcomas Is Associated With Local Expression of Immune-Inhibitory HLA-G. Oncotarget (2018) 9:6536–49. doi: 10.18632/oncotarget.23815
121. Sukumaran S, Watanabe N, Bajgain P, Raja K, Mohammed S, Fisher WE, et al. Enhancing the Potency and Specificity of Engineered T Cells for Cancer Treatment. Cancer Discov (2018) 8:972–87. doi: 10.1158/2159-8290.cd-17-1298
122. Finney HM, Akbar AN, Lawson AD. Activation of Resting Human Primary T Cells With Chimeric Receptors: Costimulation From CD28, Inducible Costimulator, CD134, and CD137 in Series With Signals From the TCR Zeta Chain. J Immunol (Baltimore Md. 1950) (2004) 172:104–13. doi: 10.4049/jimmunol.172.1.104
123. Hou A, Chen L, Chen Y. Navigating CAR-T Cells Through the Solid-Tumour Microenvironment. Nat Rev Drug Discov (2021) 20:531–50. doi: 10.1038/s41573-021-00189-2
Keywords: Ewing’s sarcoma, CAR-T therapy, solid tumors, immune targets, targeted therapy
Citation: Lin Z, Wu Z and Luo W (2021) A Novel Treatment for Ewing’s Sarcoma: Chimeric Antigen Receptor-T Cell Therapy. Front. Immunol. 12:707211. doi: 10.3389/fimmu.2021.707211
Received: 09 May 2021; Accepted: 27 August 2021;
Published: 10 September 2021.
Edited by:
Sutatip Pongcharoen, Naresuan University, ThailandReviewed by:
Hinrich Abken, Regensburg Center for Interventional Immunology (RCI), GermanyFrank James Ward, University of Aberdeen, United Kingdom
Copyright © 2021 Lin, Wu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Luo, bHVvd2VpMDkyOEAxMjYuY29t
 Zili Lin
Zili Lin Ziyi Wu
Ziyi Wu Wei Luo
Wei Luo