- 1Department of Rheumatology, Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- 2Department of Radiology, Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
Objective: Evaluate the MRI evidence of active inflammatory and chronic structural damages in radiographic axial spondyloarthritis (r-axSpA) and non-radiographic axial spondyloarthritis (nr-axSpA).
Methods: A retrospective review of 253 patients who underwent sacroiliac joint (SIJ) MRI between June 2014 and December 2019 was performed. MRI images including short tau inversion recovery scan and T1-weighted spin echo scans were assessed using the Spondyloarthritis Research Consortium of Canada (SPARCC) score and SPARCC MRI SIJ structural score by two independent readers.
Results: Higher mean score of inflammatory (SPARCC) was seen in r-axSpA patients when compared with nr-axSpA patients (8.08 vs 4.37, P<0.05). Frequencies of MRI structural lesions in r-axSpA patients and nr-axSpA patients were as follows: erosion (65.84 vs 88.23%, P=0.002), backfill (33.17 vs 13.73%, P<0.001), fat metaplasia (79.21 vs 60.78%, P=0.01), and ankylosis (37.13 vs 1.96%, P<0.001). Patients with r-axSpA had a higher mean score for fat metaplasia (8.93 vs 4.06, P=0.0003) and ankylosis (4.49 vs 0.04, P<0.001).
Conclusion: More active inflammatory and chronic structural damages except for erosion were seen in r-axSpA patients than nr-axSpA patients, while higher percentage of nr-axSpA patients presented with erosion in MRI.
Introduction
Spondyloarthritis (SpA) is a group of inflammatory diseases mainly affecting the sacroiliac (SI) joint and spine. Axial spondyloarthritis (axSpA) includes non-radiographic axial spondyloarthritis (nr-axSpA) and radiographic axial spondyloarthritis (r-axSpA); the latter one is also termed ankylosing spondylitis (AS). Nr-axSpA and r-axSpA share some similarities such as disease activity (defined by Bath Ankylosing Spondylitis Disease Activity Index—BASDAI), physical function, prevalence of HLA-B27 positivity, and comorbidity burden (1–3), while there are several differences including disease course, gender predilection, and levels of inflammatory markers (4).
MRI is an objective measurement detecting acute inflammatory and chronic structural changes recommended by the Assessment of SpondyloArthritis International Society (5). Bone marrow edema (BME), capsulitis, and subligamentous enthesitis are defined as active inflammatory changes in SpA according to the update of the ASAS MRI working group (6). Chronic structural changes including subchondral sclerosis, erosion, backfill, fat metaplasia, and ankylosis are present better and earlier in CT (7) or MRI (8) than radiographs.
Inflammatory and chronic structural changes are objective signs of axSpA. Previous studies indicated that sacroiliitis on radiographs is usually bilateral and symmetric in AS (9). However, the differences of structural damages between r-axSpA and nr-axSpA patients on MRI are limited. The aim of this study was to investigate whether there are differences between Chinese r-axSpA and nr-axSpA patients in inflammatory and chronic structural lesions on MRI, and clinical factors related to MRI changes.
Methods
Patients
A retrospective study was performed, and patients with MRI and radiographic imaging of the sacroiliac joint and diagnosed with axSpA between June 2014 and December 2019 were included. The local ethics committee waived approval because of the retrospective nature of this study. The patients provided their written informed consent to anonymize the use of relative data in further study according to daily clinical practices in this department. Demographic characteristics and inflammatory indicators such as C-reactive protein (CRP) and Erythrocyte Sedimentation Rate (ESR) are collected.
MRI Protocol and Assessment
All MR images were acquired on a 1.5 Tesla or 3 Tesla MR scanner in a coronal plane titled parallel to the long axis of the SI joint with 3–4 mm slice thickness and 12–15 slices acquired. Short-tau inversion recovery (STIR) sequences were used for the assessment of inflammatory lesion. The T1-weighted MRI sequences were used for the assessment of chronic/structural changes. The Spondyloarthritis Research Consortium of Canada SPARCC MRI scoring system was used to assess inflammation and structural damages in SI joint. SPARCC SIJ scores were based on the measurement of six consecutive slices, with the largest score of 12 for edema, intensity, and depth per slice, and a total score of 0–72 was calculated. SPARCC SI structural lesion score (SSS) (10) was used to assess the structural lesions, and four kinds of lesions were assessed based on five consecutive slices through the SIJ. Fat metaplasia is defined as an increased signal in bone marrow on T1WSE, and the lesion has to demonstrate homogeneous signal with more than 1 cm in depth from the joint surface. Erosion is defined as the full-thickness loss of the dark appearance of either iliac or sacral cortical bone at its anticipated location and loss of the normal bright appearance of adjacent bone marrow. Backfill is defined as complete loss of iliac or sacral cortical bone at its anticipated location and increased signal that is clearly demarcated from adjacent normal marrow by irregular dark signal reflecting sclerosis at the border of the eroded bone. Ankylosis is defined as bone marrow signal on T1WSE sequences extending between the sacral and iliac bone marrow with a full-thickness loss of the dark appearance of the iliac and sacral cortical bone. The presence/absence of lesions is scored using an online data entry system in SIJ quadrants (fat, erosion) or halves (backfill, ankylosis) with a scoring range of 0–40 for quadrants lesions and 0–20 for halves lesions. Examples of these inflammatory and structural damages are shown in Figure 1. Images were assessed by two radiologists independently, who were blinded to all patient data. The mean score of two radiologists was used for analysis. For SPARCC SIJ score, two readers had to discuss and reach consensus if a difference of >3 points existed (11). A SPARCC SSS >0 was required from both readers for identification of a structural lesion of erosion, backfill, fat metaplasia, or ankylosis (12). The before image evaluation, a training session including 20 test images, was carried out by an expert rheumatologist. A score ≥2 for SI joint bone marrow edema (BME) was considered positive MRI evidence of inflammation, and this cutoff has been validated in the literature (3, 13).
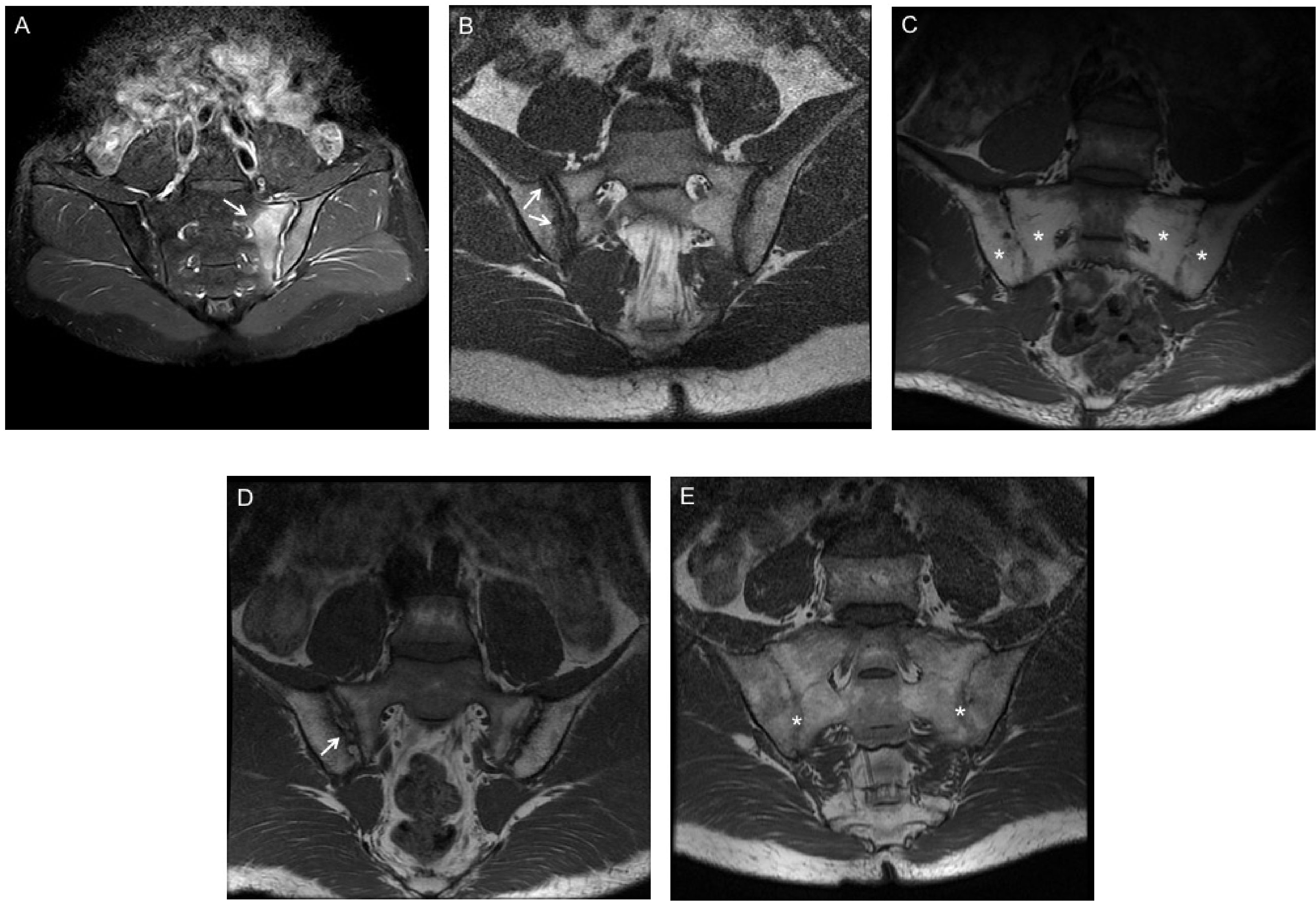
Figure 1 MRI findings of inflammatory and structural damage of SpA. (A) Bone marrow edema (BME) at the left side of sacroiliac joints (white arrows) in Coronal oblique STIR sequence. (B) Erosion of the right iliac bone on T1 weighted spin echo MRI (white arrows). (C) Fat metaplasia at the bilateral sacroiliac joints on T1 weighted spin echo MRI (white stars). (D) Backfill of the right iliac bone on T1 weighted spin echo MRI (white arrows). (E) Ankylosis of the bilateral sacroiliac joints on T1 weighted spin echo MRI (white stars).
Statistical Analysis
Statistical analyses were performed using Stata Version 15.0. Summary statistics such as frequency, percentage, mean, and standard deviation were used to analyze demographic characteristics and inflammatory and structural changes of axSpA patients. T-test was used to evaluate the difference of demographic characteristics and MRI scores between AS and nr-axSpA patients. Spearman correlations were used to determine the correlation between SPARCC scores and clinical factors. Then we conducted multivariate stepwise regression analyses to determine the best significant predictors for inflammatory and structural lesions. All tests were two-sided, and a significance level of P < 0.05 was assumed. Interobserver reliability for imaging scores was assessed using the intraclass correlation coefficient (ICC). The ICC value of <0.4 was designed as fair, ≥0.4 but <0.6 as moderate, ≥0.6 but <0.8 as good, ≥0.8 but <0.9 as very good, and ≥0.9 as excellent.
Results
Demographic
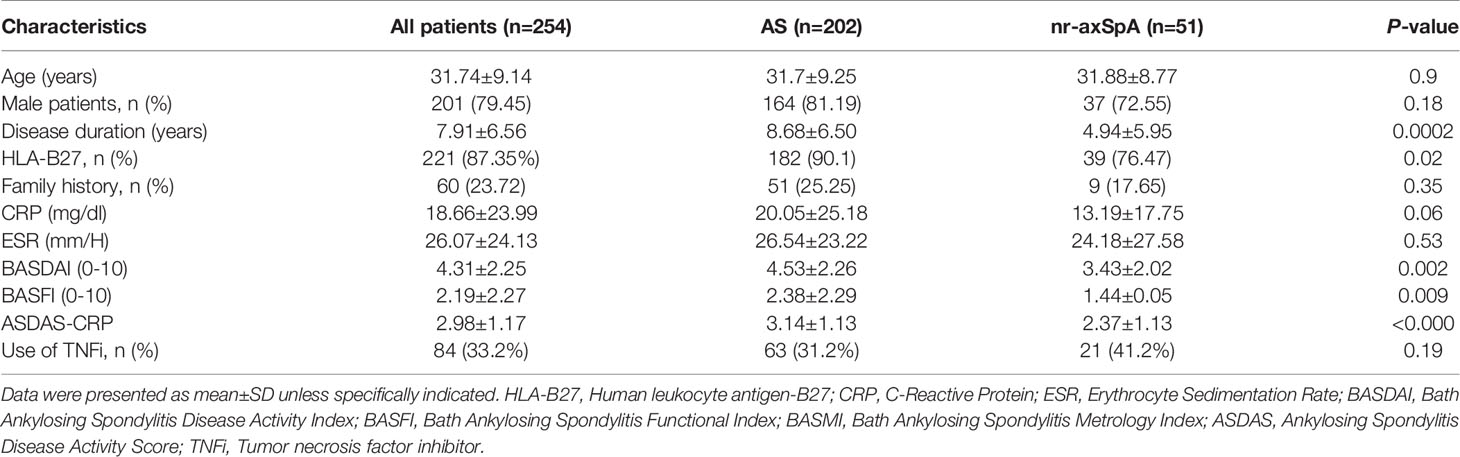
A total of 253 patients with a clinical diagnosis of axSpA were included for analysis during June 2014 and December 2019 in the third affiliated hospital of SUN YAT-SEN University. Fifty-one patients were classified as nr-axSpA, while others fulfilled the diagnosis of r-axSpA. The average age of patients was 31.74 years with a mean disease duration of 7.91 years. A total of 87.35% (n=221) of patients were HLA-B27 positive, and 79.45% of them were male. The mean BASDAI and BASFI scores were 4.31 ± 2.25 and 2.19 ± 2.27 respectively, and 33.2% (n=84) of patients had used tumor necrosis factor inhibitor (TNFi) in the recent 3 months. Longer disease duration, higher positive rate of HLA-B27, and worse function, as measured by BASFI, were seen in r-axSpA group. The disease activity markers of BASDAI, ASDAS, and SPARCC MRI SIJ inflammation were significantly higher in r-axSpA group. No significant differences in other demographic features were noted between the groups (Table 1).
Inflammatory and Structural Changes on MRI
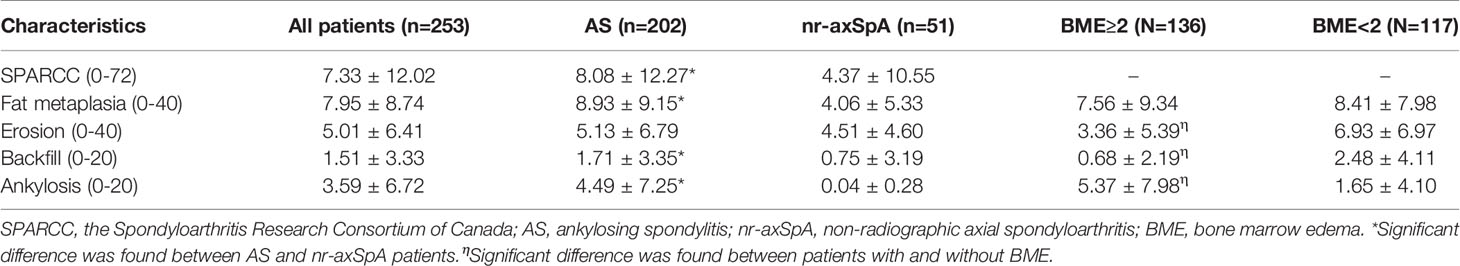
Higher SPARCC score was seen in r-axSpA patients than in nr-axSpA patients. Of the 253 patients evaluated, 117 (46.25%) had no BME seen on MRI. There was no significant difference in percentage of patients with BME between r-axSpA and nr-axSpA groups. Clinical characteristics such as age, sex, BASDAI, and CRP did not differ between patients with and without BME. There was significant difference in the mean score of erosion, backfill, and ankylosis between patients with and without SIJ BME (Table 2). Relative frequencies of MRI structural lesions in patients with versus without SIJ BME were as follows: erosion (78/136, 57.35% vs 100/117, 85.47%; P<0.001), backfill (23/136, 16.91% vs 51/117, 43.59%; P<0.001), fat metaplasia (93/136, 68.38% vs 98/117, 83.76%; P=0.005), and ankylosis (50/136, 36.76% vs 26/117, 22.22%; P=0.01).
The majority of patients had a score of >0 for structural damages for at least one of fat metaplasia, erosion, backfill, and ankylosis in the SI joint. A total of 242 (95.65%) patients had ≥1 structural lesion on MRI comprising erosion 71.54%, backfill 29.24%, fat metaplasia 75.49%, and ankylosis 30.04%. In SSS structural scores, significant difference was found between r-axSpA and nr-axSpA groups in fat metaplasia, backfill, and ankylosis (Table 2). Frequencies of MRI structural lesions in r-axSpA patients and nr-axSpA patients were as follows: erosion (65.84 vs 88.23%, P=0.002), backfill (33.17 vs 13.73%, P<0.001), fat metaplasia (79.21 vs 60.78%, P=0.01), and ankylosis (37.13 vs 1.96%, P<0.001).
Correlations Between MRI Inflammatory and Structural Lesions
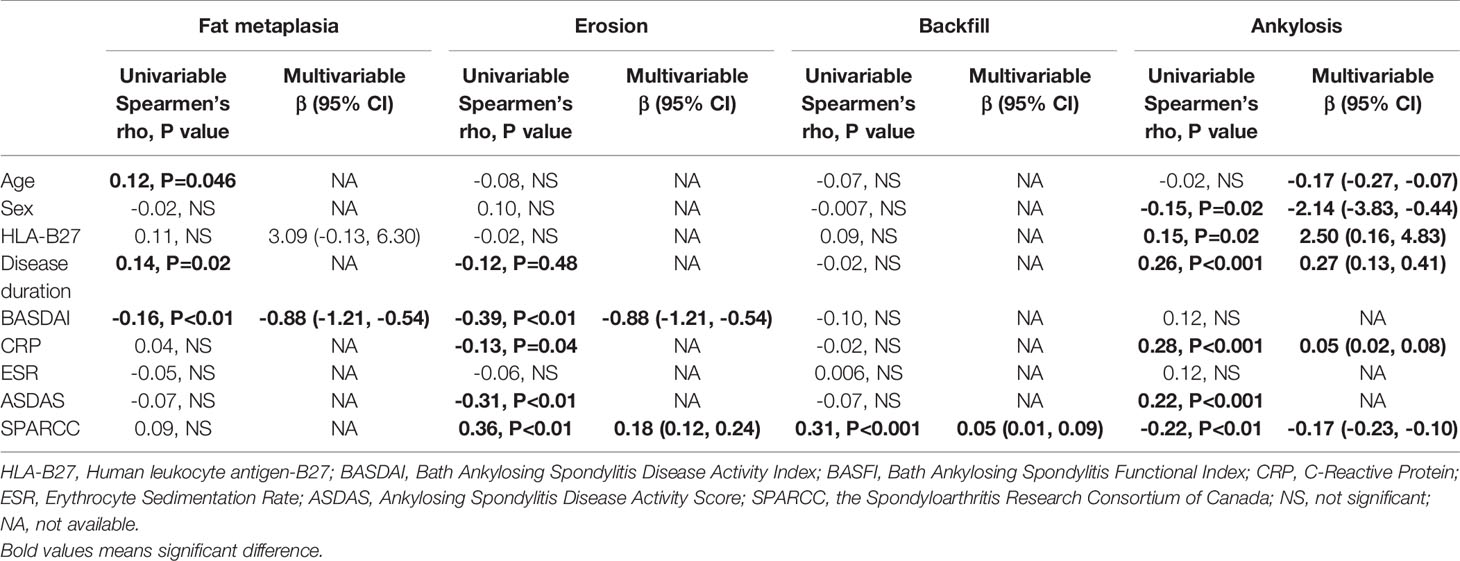
Significant correlation was found between SPARCC score and age (−0.21, P <0.01), sex (−0.15, P =0.02). In multivariable stepwise regression analysis, age (−0.28, P =0.001) and HLA-B27 (4.53, P =0.046) were found significantly associated with SPARCC score.
Table 3 shows factors associated with SSS scores. Fat metaplasia was associated with age, sex, and BASDAI, and only BASDAI (β: −0.56, P =0.02) remained significant in multivariable analysis. For erosion, significant association was found with BASDAI (β: −0.88, P <0.01) and SPARCC (β: 0.18, P <0.01) in multivariable analysis. Backfill was associated with SPARCC (β: 0.05, P <0.01) in multivariable analysis. For ankylosis, age, sex, HLA-B27, disease duration, CRP, and SPARCC were found significantly associated.
Interobserver Reliability
ICC was computed to test interobserver reliability. Excellent correlation was achieved for rating of SPARCC score and ankylosis (ICC scores of 0.98 [95% CI 0.93–0.99] and 0.95 [95% CI 0.91–0.98], respectively). Good correlation was achieved for identification of erosion, fat metaplasia, and backfill with ICC scores of 0.85 (95% CI 0.71–0.93) for fat metaplasia, 0.87 (95% CI 0.75–0.94) for erosion, and 0.74 (95% CI 0.52–0.87) for backfill.
Discussion
The results of this study demonstrate that structural lesions on MRI may occur in early stage of SpA (nr-axSpA) and in the absence of SIJ BME on MRI. R-axSpA patients have more SIJ inflammation on MRI, and fat metaplasia is seen more in r-axSpA than nr-axSpA patients. Our data also support that structural damages in SIJ are associated with active inflammation.
Conventional radiograph is still preferred for the analysis of SIJ structural damage and for the diagnosis of axSpA. It has been verified that T1-weighted MRI sequences were superior to conventional radiographs in the detection of erosion of SIJ in axSpA patients (14). Besides, additional lesions such as fat metaplasia, backfill, and ankylosis can be observed clearly on MRI. The distribution pattern of inflammation and structural lesions may differ between AS and nr-axSpA patients; unilateral fat metaplasia and erosion were more seen in nr-axSpA patients (15) with a small simple size. This study compared the inflammation and structural lesions in r-axSpA and nr-axSpA patients using SPARCC scoring system and analyzed factor related to MRI changes.
Fat metaplasia, one of the chronic lesions and usually observed in the bone marrow of SI joint and spine, is assumed to be an intermediate stage between active inflammation and formation of new bone (16). Prospective studies indicated that fat metaplasia and backfill are associated with the development of new ankylosis (8, 14, 16). However, the proportion of fat marrow increases over time, and a previous study reported that a very high prevalence of fat metaplasia (50.6% in the age groups less than 45years, 94.4% in patients ≥75 years) was found in the SIJ of asymptomatic patients, while erosions were extremely uncommon (17). Fat metaplasia with certain features such as a distinct border, proximity to subchondral bone, and homogeneity of T1-weighted signal, combined with other abnormalities (bone marrow edema and erosion), is assumed to be highly specific for SpA but does no help for diagnosis (18).
The relationship between inflammation, fat metaplasia, and ankylosis has been studied before. It is reported that baseline SPARCC SIJ inflammation and SIJ backfill scores independently predicted progression in SPARCC SIJ ankylosis (19). TNFi can retard not only inflammation but also structural progression rate on MRI in both short-term (11, 20) and long-term follow-up (19). It is reported that etanercept has more effect than usual care on SIJ erosion development; attaining sustained ASDAS inactive disease is relevant to the amelioration of erosion (21). Reduction of inflammation, erosion, and increase of fat metaplasia were seen in SpA patients treated with adalimumab compared with placebo group (20). Other clinical factors such as disease activity, demographics, and HLA-27 were not associated with development of MRI structural features according to previous study (22).
There are several limitations of this study. First is the retrospective nature, which is subject to inherent observation bias. Second is the interpretation of the MRI T1 sequence. The structural damages such as erosion and backfill are heterogeneous lesions, which are difficult to discern and need caution when interpreting the results. Besides, these damages could be presented in other diseases such as scoliosis and degenerative disease. Further validation of structural damages using both computed tomography and MRI is needed for better understanding.
Conclusions
This study indicated that higher inflammatory and structural damages except for erosion were seen in r-axSpA patients when compared with nr-axSpA patients. In patients without MRI SIJ BME, structural lesions are also present. Prospective study is necessary to understand the relationship between structural damages and treatments.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by third affiliated hospital of SUN YAT-SEN University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JG and LT. Acquisition, analysis, or interpretation of data: all authors. Drafting the manuscript: LT and JG. Critical revision of the manuscript for important intellectual content: all authors. All authors contributed to the article and approved the submitted version.
Funding
This study is funded by Guangdong Science & Technology Infrastructure Construction Project (Number: 2019B030316004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We specially thank the participants who made this study possible.
References
1. Rudwaleit M, Haibel H, Baraliakos X, Listing J, Marker-Hermann E, Zeidler H, et al. The Early Disease Stage in Axial Spondylarthritis: Results From the German Spondyloarthritis Inception Cohort. Arthritis Rheumatism (2009) 60(3):717–27. doi: 10.1002/art.24483
2. Kiltz U, Baraliakos X, Karakostas P, Igelmann M, Kalthoff L, Klink C, et al. Do Patients With non-Radiographic Axial Spondylarthritis Differ From Patients With Ankylosing Spondylitis? Arthritis Care Res (Hoboken) (2012) 64(9):1415–22. doi: 10.1002/acr.21688
3. Zhao SS, Ermann J, Xu C, Lyu H, Tedeschi SK, Liao KP, et al. Comparison of Comorbidities and Treatment Between Ankylosing Spondylitis and non-Radiographic Axial Spondyloarthritis in the United States. Rheumatology (2019) 58(11):2025–30. doi: 10.1093/rheumatology/kez171
4. Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. New Engl J Med (2016) 374(26):2563–74. doi: 10.1056/NEJMra1406182
5. Lambert RG, Bakker PA, van der Heijde D, Weber U, Rudwaleit M, Hermann KG, et al. Defining Active Sacroiliitis on MRI for Classification of Axial Spondyloarthritis: Update by the ASAS MRI Working Group. Ann Rheumatic Dis (2016) 75(11):1958–63. doi: 10.1136/annrheumdis-2015-208642
6. Maksymowych WP, Lambert RG, Ostergaard M, Pedersen SJ, Machado PM, Weber U, et al. MRI Lesions in the Sacroiliac Joints of Patients With Spondyloarthritis: An Update of Definitions and Validation by the ASAS MRI Working Group. Ann Rheumatic Dis (2019) 78(11):1550–8. doi: 10.1136/annrheumdis-2019-215589
7. Baraliakos X, Braun J. Imaging Scoring Methods in Axial Spondyloarthritis. Rheumatic Dis Clinics North Am (2016) 42(4):663–78. doi: 10.1016/j.rdc.2016.07.006
8. Weber U, Pedersen SJ, Ostergaard M, Rufibach K, Lambert RG, Maksymowych WP. Can Erosions on MRI of the Sacroiliac Joints be Reliably Detected in Patients With Ankylosing Spondylitis? - A cross-sectional study. Arthritis Res Ther (2012) 14(3):R124. doi: 10.1186/ar3854
9. Jang JH, Ward MM, Rucker AN, Reveille JD, Davis JC Jr, Weisman MH, et al. Ankylosing Spondylitis: Patterns of Radiographic Involvement–a Re-Examination of Accepted Principles in a Cohort of 769 Patients. Radiology (2011) 258(1):192–8. doi: 10.1148/radiol.10100426
10. Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Development and Preliminary Validation of the Spondyloarthritis Research Consortium of Canada Magnetic Resonance Imaging Sacroiliac Joint Structural Score. J Rheumatol (2015) 42(1):79–86. doi: 10.3899/jrheum.140519
11. Maksymowych WP, Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, et al. Clinical and MRI Responses to Etanercept in Early Non-Radiographic Axial Spondyloarthritis: 48-Week Results From the EMBARK Study. Ann Rheumatic Dis (2016) 75(7):1328–35. doi: 10.1136/annrheumdis-2015-207596
12. Maksymowych WP, Wichuk S, Dougados M, Jones H, Szumski A, Bukowski JF, et al. MRI Evidence of Structural Changes in the Sacroiliac Joints of Patients With non-Radiographic Axial Spondyloarthritis Even in the Absence of MRI Inflammation. Arthritis Res Ther (2017) 19(1):126. doi: 10.1186/s13075-017-1342-9
13. van der Heijde D, Sieper J, Maksymowych WP, Brown MA, Lambert RG, Rathmann SS, et al. Spinal Inflammation in the Absence of Sacroiliac Joint Inflammation on Magnetic Resonance Imaging in Patients With Active Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol (2014) 66(3):667–73. doi: 10.1002/art.38283
14. Diekhoff T, Hermann KG, Greese J, Schwenke C, Poddubnyy D, Hamm B, et al. Comparison of MRI With Radiography for Detecting Structural Lesions of the Sacroiliac Joint Using CT as Standard of Reference: Results From the SIMACT Study. Ann Rheumatic Dis (2017) 76(9):1502–8. doi: 10.1136/annrheumdis-2016-210640
15. Motamedi D, Patel R, Devulapalli KK, Gensler LS, Steinbach L. MR Distribution of Active Inflammatory and Chronic Structural Sacroiliac Joint Changes in Axial Spondyloarthropathy: Challenging Conventional Wisdom. Clin Imaging (2019) 58:70–3. doi: 10.1016/j.clinimag.2019.06.003
16. Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Fat Metaplasia and Backfill Are Key Intermediaries in the Development of Sacroiliac Joint Ankylosis in Patients With Ankylosing Spondylitis. Arthritis Rheumatol (2014) 66(11):2958–67. doi: 10.1002/art.38792
17. Ziegeler K, Eshkal H, Schorr C, Sieper J, Diekhoff T, Makowski MR, et al. Age- and Sex-Dependent Frequency of Fat Metaplasia and Other Structural Changes of the Sacroiliac Joints in Patients Without Axial Spondyloarthritis: A Retrospective, Cross-Sectional MRI Study. J Rheumatol (2018) 45(7):915–21. doi: 10.3899/jrheum.170904
18. Weber U, Pedersen SJ, Zubler V, Rufibach K, Chan SM, Lambert RG, et al. Fat Infiltration on Magnetic Resonance Imaging of the Sacroiliac Joints has Limited Diagnostic Utility in Nonradiographic Axial Spondyloarthritis. J Rheumatol (2014) 41(1):75–83. doi: 10.3899/jrheum.130568
19. Pedersen SJ, Weber U, Said-Nahal R, Sorensen IJ, Loft AG, Kollerup G, et al. Structural Progression Rate Decreases Over Time on Serial Radiography and Magnetic Resonance Imaging of Sacroiliac Joints and Spine in a Five-Year Follow-Up Study of Patients With Ankylosing Spondylitis Treated With Tumour Necrosis Factor Inhibitor. Scand J Rheumatol (2019) 48(3):185–97. doi: 10.1080/03009742.2018.1506822
20. Pedersen SJ, Poddubnyy D, Sorensen IJ, Loft AG, Hindrup JS, Thamsborg G, et al. Course of Magnetic Resonance Imaging-Detected Inflammation and Structural Lesions in the Sacroiliac Joints of Patients in the Randomized, Double-Blind, Placebo-Controlled Danish Multicenter Study of Adalimumab in Spondyloarthritis, as Assessed by the Berlin and Spondyloarthritis Research Consortium of Canada Methods. Arthritis Rheumatol (2016) 68(2):418–29. doi: 10.1002/art.39434
21. Maksymowych WP, Claudepierre P, de Hooge M, Lambert RG, Landewe R, Molto A, et al. Structural Changes in the Sacroiliac Joint on MRI and Relationship to ASDAS Inactive Disease in Axial Spondyloarthritis: A 2-Year Study Comparing Treatment With Etanercept in EMBARK to a Contemporary Control Cohort in DESIR. Arthritis Res Ther (2021) 23(1):43. doi: 10.1186/s13075-021-02428-8
22. Pedersen SJ, Wichuk S, Chiowchanwisawakit P, Lambert RG, Maksymowych WP. Tumor Necrosis Factor Inhibitor Therapy But Not Standard Therapy is Associated With Resolution of Erosion in the Sacroiliac Joints of Patients With Axial Spondyloarthritis. Arthritis Res Ther (2014) 16(2):R100. doi: 10.1186/ar4548
Keywords: ankylosing spondylitis, axial spondyloarthritis, inflammation, structural damage, MRI
Citation: Tu L, Lin C, Xie Y, Wang X, Wei Q, Zhang Y and Gu J (2021) Active Inflammatory and Chronic Structural Damages of Sacroiliac Joint in Patients With Radiographic Axial Spondyloarthritis and Non-Radiographic Axial Spondyloarthritis. Front. Immunol. 12:700260. doi: 10.3389/fimmu.2021.700260
Received: 25 April 2021; Accepted: 12 July 2021;
Published: 27 July 2021.
Edited by:
Giuseppe Lopalco, University of Bari Aldo Moro, ItalyReviewed by:
Augusta Ortolan, University of Padua, ItalyMarco Di Carlo, Marche Polytechnic University, Italy
Copyright © 2021 Tu, Lin, Xie, Wang, Wei, Zhang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jieruo Gu, Z3VqaWVydW9AMTYzLmNvbQ==
 Liudan Tu
Liudan Tu Churong Lin
Churong Lin Ya Xie
Ya Xie Xiaohong Wang2
Xiaohong Wang2 Qiujing Wei
Qiujing Wei Yanli Zhang
Yanli Zhang Jieruo Gu
Jieruo Gu