
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 21 July 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.699848
This article is part of the Research Topic Emerging Insights in Controlling Autoimmunity View all 24 articles
 Efterpi Zafiriou1
Efterpi Zafiriou1 Athina I. Daponte1,2
Athina I. Daponte1,2 Vasileios Siokas2
Vasileios Siokas2 Christina Tsigalou3
Christina Tsigalou3 Efthymios Dardiotis2
Efthymios Dardiotis2 Dimitrios P. Bogdanos2*
Dimitrios P. Bogdanos2*Patients with psoriasis are frequently obese and experience anxiety or suffer from depressive disorders. The immunopathogenesis of psoriasis and indeed psoriatic arthritis is largely based on the pivotal role of IL-17/IL-23 axis, to an extent that currently monoclonal antibodies selectively inhibiting IL-17 or IL-23 are routinely used for the treatment of psoriatic diseases. Emerging data, demonstrating a decisive role for IL-17 and IL-17 producing cell subsets, such as Th17 in the induction and progression of obesity and depression has led authors to suggest that psoriatic disease, obesity and anxiety/depression may indeed be interconnected manifestation of a state of immunedysregulation, the linked being IL-17 and its related cells. We discuss this hypothetical link in depth taking into account the beneficial effects anti-IL17 and anti-IL-17 receptor inhibitors in treating psoriatic disease and the on-going debate as to whether these biologics may exert a direct or indirect effect in ameliorating concomitant obesity and depressive disorders, which are frequently noted in the same patient.
Depression is a frequent comorbidity of various autoimmune rheumatic and immune-mediated diseases, including psoriasis (Ps) and psoriatic arthritis (PsA) (1). More then a third of patients with these diseases experience anxiety, depression, and even suicidal ideation and behaviors (SIB), which is not always immediately relevant with the activity of the underlying disease (2–4). In fact, the rate of depression can be higher in patients with PsA than in those with Ps (5). The cause–effect bond between depression and Ps (as well as PsA) is puzzling (6). Remission of the disease, following successful treatment, especially with biologics has a positive effect on depression and anxiety, but not always. Chronic inflammation has been considered a favorable trigger of depression and chronic medical illness (7). The immunobiological basis of depression’s development has started to emerge following studies demonstrating a fine imbalance between pro-inflammatory and anti-inflammatory cytokines (8–10). IL-17 has emerged as a master cytokine for the immunopathogenesis of psoriatic disease, leading to the development of the plethora of agents specifically inhibiting its deleterious effect (11–15).
The direct or indirect effect of such biologics in fighting depression in patients with immune-mediated or autoimmune diseases such as Ps and PsA remains elusive (16). The lack of information resulting from inconsequential clinical data will become less elusive in the coming years as monoclonal antibodies (mAbs), such as those specifically inhibiting IL-17 and IL-17 receptors, are currently approved treatments for psoriatic disease patients and data are continuously analyzed and published (17). The impact of mAbs specifically inhibiting that; cytokine in depression, anxiety and even suicidal ideation and behaviors (SIB) will also become more evident, as animal models of depression become more sophisticated resembling more and more the human disease. The question arising from the clinical use of the approved drugs is whether anti-IL17 treatment is efficacious in combating depression, irrespectively of the disease patients are suffering from (Ps, PsA or other spondylarthropathies) and whether the magnitude of IL-17 inhibition can predict the performance as per depression scale.
So far, it has become apparent that patients suffering from depression have elevated levels of circulating IL-17 in their serum and that the percentage of Th17 cells, the T cell predominantly producing this cytokine, are also increased in people with depressive disorders (18). What is not clear and remains a matter of debate is whether this increase is an epiphenomenon resulting from the disease or whether the increase per se is playing a decisive role in the development and progression of neuroimmune depressive disorders in isolation or in combination with other non-immunologically relevant mechanisms (19). Research clinical trials using IL-17 or IL-17R blockers for the treatment of depressive disorders have not been initiated so far. It is worthy to mention data from two clinical trials, based on biologic therapy, and in particular that using infliximab which is a chimeric IgG1 mAb that blocks TNF-α for the treatment of refractory depression (20, 21). None of the two achieved its primary outcome, and infliximab did not appear to reduce symptoms of depression compared to placebo, though a favourable outcome has been reported in those patients with inflammatory indices (21).
It has also been well documented that people with depression are more frequently obese compared to those without and that obese people, because of their internal and external stigma, are experiencing more frequent depression. Again, it is not clear what is the pathophysiologal impact of IL-17 in the direct induction of obesity. A provocative hypothesis is taking into consideration that an impairment of immunoregulatory mechanisms, characterized by the functional inability of the immune system to promote the expression and production of IL-17 and other suppressory cytokines, is directly linked to the overexpression of IL-17 for cellular subsets such as—but not limited to—Th17.
If IL-17 is critical for the immunedysregulation noted in patients with depression, it should be expected that patients with that disorder at early stages of the disease will have well documented increased expression of IL-17, and that over time, serum IL-17 levels as well as the levels of Th17 and other IL-17-producing cells will become significantly amplified, especially in those patients who become more depressed and more obese because of the underlying disease. Prospective studies reporting on that are currently missing, but experimental data in animal models are rather informative and provide a wealth of information, which can lead to a better understanding of the complex nature and close interplay between immunedysregulation in psoriatic disease and its impact on depression and obesity.
We and others have shown that is IL-17 per se, as well as IL-17 axis, is pivotal for the development and progression of Ps and PsA (12, 13, 22–27). More recently, the imperative role of Il-17 in the induction and maintenance of ankylosing spondylitis and other spondylarthropathies has been revealed (28).
Four biologic therapies targeting either IL-17 or IL-17R have been approved for the treatment of Ps. Secukinumab, a fully human IgG1κ mAb (29) and ixekizumab, a humanized IgG4 mAb (30, 31) selectively bind and neutralize IL-17A. Brodalumab is a fully human IgG2 mAb that binds and inactivates the IL-17A receptor leading to the inhibition of either IL-17A, IL-17C, IL-17E and IL-17F (32). Finally bimekizumab, a humanized IgG1 mAb, which selectively neutralizes IL-17A and IL-17F, is approved for the treatment of Ps but is also efficacious in PsA (33).
The neurological and psychiatric disease related implication of Th17 and Il-17 mediated cell damage is the focus of intense research and has just been started emerging. Th17 cells have been considered likely inducer of brain damage (34). Th17 induce neuronal cell death and promote neuronal toxicity in experimental autoimmune encephalomyelitis, the animal model of multiple sclerosis (35) and IL-17 mRNA is overexpressed in active MS brain lesions (36), while IL-17 production from central nervous system resident T lymphocytes and glial cells are associated with disease-activity (37). CD8+ T cells producing IL-17 are elevated during disease-relapses compared to disease-remission (38). Work in mice has clear demonstrated that though not directly, IL-17 plays an important role in MS, as mice deficient for IL-17A/F escape from disease’s appearance. Immunome data demonstrate that paediatric patients with drug refractory epilepsy are characterized by an IL-17 inducing CD4 and CD8 cell subset profile, which likely contributes to epileptogenesis (39) while autism spectrum disorders are characterized by an imbalance between proinflammatory Th17 and suppressory T regulatory cells (Tregs) (40). Th17 cells are increased in patients with stable schizophrenia (41).
Depression is associated with the elevation of proinflammatory cytokines among which IL-17 appears to be one of those found elevated in patients with major depressive disorders (42, 43). Accumulated evidence suggest that the cytokine milieu noted in patients with depression, as well as that well-characterized in animal models of depressive disorders, underlined the important role of IL-1β, TNF-α and IL-6 (44–46). A recent meta-analysis investigating children and adolescents with depression has found that IL-6 predicts the future development of depression and conversely that the establishment of depression is a significant predictor of IL-6 increase (47). That effect appears to be influenced by other factors like gender or stressful life events. A longitudinal cross-lagged twin study has provided compelling evidence supporting the imperative role of IL-6 increase as an independent risk factor of depression rather than an epiphenomenal consequence of disease’s establishment (48). In animal models of depression, IL-6 (and/or IL-1β appears to be instrumental for the development of chronic stress and depression-like behaviors (49). Also, numerous studies have shown that patients with major depression have elevated levels of TNF-α and that fluctuation of this pro-inflammatory cytokine (as well as that of IL-6) influences the mood behavior of the affected individuals (for review see (50). Furthermore, some data suggest that antipsychotic drugs exert an anti-inflammatory effect and decrease TNF-α and IL-6 levels in murine models of the disease (51). Intriguingly, however, a recent systematic review, meta-analysis and meta-regression including 38 eligible studies representing 58,256 failed to identify a prospective association of depression with TNF-α and a small association with IL-6 (45). Also, as we mentioned previously, data from a limited number of clinical trials have failed to identify a beneficial effect of anti-TNF-α biologics in patients with mood disorders (20, 21), while such drugs are successfully used for the treatment of psoriatic disease, other autoimmune rheumatic diseases and several immune-mediated inflammatory diseases.
Experimental work has been redirected to other newly identified pro-inflammatory cytokines, including IL-17. IL-17A mediated disruption of the blood-brain barrier by Th17 cells is well documented, and treatment of mice with IL-17A neutralizing antibodies prevents such a disruption (52). Though limited, data from studies in patients with depressive disorders are also noteworthy (Table 1). Interestingly, a serological study has found higher IL-17 levels in blood samples in 41 patients with major depressive disorder compared to those noted in 40 healthy age-matched controls with no history of malignancies or autoimmune diseases (53). However, lack of an association between blood levels of IL-17A and depression has also been reported, as anti-depressants appear not to exert an effect on IL-17 levels (57). The levels of IL-17 gene expression among 190 patients with depression were higher compared to 100 healthy individuals, while the mean mRNA expression of the immunoregulatory Foxp3 was considerably reduced in patients suffering from depressive disorders compared to the control group (54). A study in 40 patients with major depressive disorders and 30 healthy controls has found an imbalance of Th17/Treg ratio compared to healthy controls documented by a significant increase in peripheral blood mononuclear cell Th17 cell numbers, and a decrease in T regulatory cells (55). The same study also reported higher mRNA expression levels of retinoic acid-related orphan receptor-γt (RORγt), which is the specific transcription factor of Th17 cell and increased IL-17 serum levels in depressed patients compared to healthy controls (55). Another study found that women with major depressive disorder have increased Th17 and increased serum IL-17 compared to controls (56).
Considerable evidence in mice supports the notion that Th17 cells endorse susceptibility to depression-like behaviors (58). The percentage of Th17 cells is significantly increased (up to a 3-fold) in the brain of mice demonstrating depressive-like behavior compared to mice that do not have such behavior (59). Of interest, at the experimental level oral administration of BALB/C to mice sensitized by ovalbumin with the tricyclic-antidepressant desipramine diminished symptoms of allergic rhinitis symptoms in mice, up regulated CD4+CD25+Foxp3+ Treg cells and reduced CD4+IL-17+ Th17 cells, which were significantly increased in mice not receiving this antidepressant. Finally, in vitro addition of serotonin and treatment of MDD patients with selective serotonin reuptake inhibitors (SSRIs) reduced the production of Th17/Tc17-related cytokines by CD4+= and CD8+ T cells (60). Treatment of depressed mice with anti-IL-17 mAb inhibits glial differentiation and ameliorates anxiety and depression behaviors (61). In the human setting, CD4+CD25+ Tregs are found decreased in patients with major depression (62) (Figure 1).
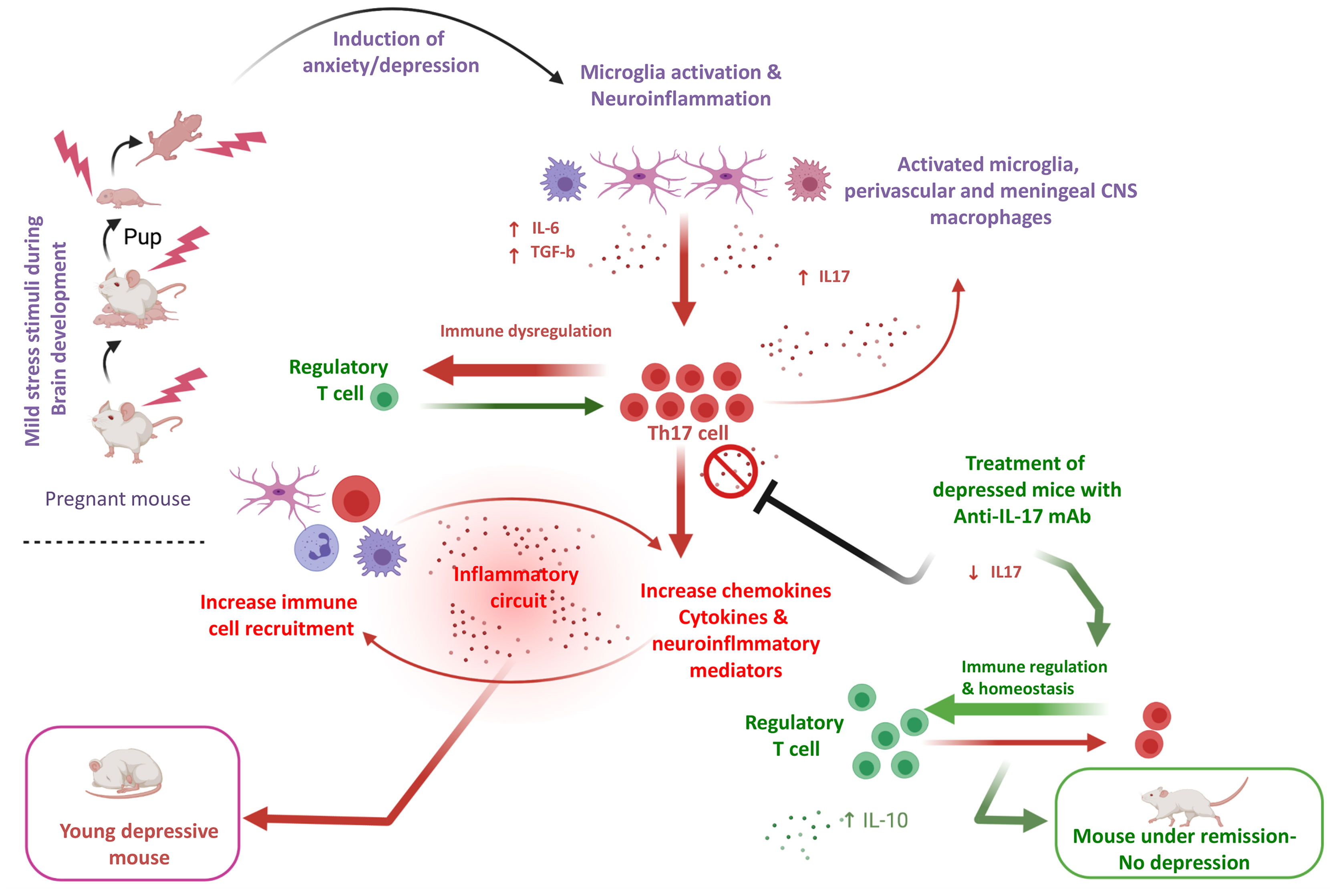
Figure 1 An-IL-17-mediated hypothesis of depressive disorder in an experimental model. In a young adult depression mouse model exposed to cumulative mild stress (CPMS) characterized by microglial activation, IL-17 in brain and blood, as well Th-17 cells are elevated. A hypothesis based on the assumption that microglia activation is pivotal for the increase of pro-inflammatory cytokines such as IL-16 and TGF-b, which polarize CD4+ T cells towards Th-17 is formulated. Experimental data suggest that anti-IL-17 mAb treatment, diminishes IL-17 induction and Th-17 differentiation and ameliorates anxiety and depression-like behaviors (61) (prepared with BioRender).
A population-based cohort study found that patients with Ps who have elevated levels of IL-17A also have increased risk for depression and anxiety disorders. In a murine imiquimod model of psoriatic disease, administration of IL-17A was associated with acceleration of depression-like symptoms, while treatment with an anti-IL17A antibody diminished depression-like symptomatology (63). A recent case-based study reported three patients with moderate-to-severe Ps and comorbid depression, who were successfully treated with brodalumab (PASI 100 after treatment with brodalumab); in two of those, depressive symptoms either improved or resolved suggesting that the use of brodalumab is able to improve both skin lesions and depression (64). These results rather contradict those suggesting a potential link between SIB and brodalumab use, as there are data from the clinical Phase 3 Studies (AMAGINE-2) which report three events of suicide attempt that occurred in only one individual out of 486 participants (Patient-yr = 379.7) who received a constant dose of brodalumab 210 mg Q2W (65), which makes dermatologists rather hesitant to prescribe that mAb (65, 66), despite the lack of concrete causality (67). The exact mechanism by which brodalumab may indeed exert or participates in suicidal behavior has not been studied in the affected individual or in any other setting. The distinctive mechanism of action of brodalumab involves the broader blockade of IL-17 isoforms binding IL-17RA. If this action can provide a mechanistical explanation of the assumed suicidal ideation induction remains elusive. Studies of the effect of anti-IL17 RA or other anti-IL17 neutralizing antibodies in animal models of suicidal behavior (68) may shed a light on this provocative topic.
A recent two-year US pharmacovigilance report on brodalumab usage collecting data from 2,677 patients with an estimated exposure of 1,656 patient-years reported just 25 reports of depression but no suicide attempts (69). Data on the safety of ixekizumab in adult patients with plaque Ps, PsA and axial spondylarthritis from 21 clinical trials of 8,228 patients with an ixekizumab exposure of 20,895.9 patient-years have reported depression during ixekizumab treatment occurred in 203 patients with Ps, 37 patients with PsA and 13 patients with axial spondylarthritis. The cumulative data over the total exposure period have shown incidence rates of reported depression to be low (≤2.2 per 100 PY across indications) and decreased across the treatment periods. Of importance, the same study found that suicidal behavior/self-injury was present in 17 patients with Ps, one patient with PsA and two patients with axial spondylarthritis (70). A very recent post hoc Analysis of the Italian SUPREME multi-centre (50 sites) study has found that anxiety was resolved in 67 and 71% of Ps patients at weeks 16 and 48, respectively and that depression symptoms were improved in 81.3 and 70.6% of patients at weeks 16 and 48, respectively (71).
Currently, it is projected that by 2030, 20% of the world’s population will be obese and 38% will be overweight (72). New piece of evidence suggests that the spatial prevalence of comorbid obesity/depression is not a random phenomenon and indeed common denominators at the cellular and immunophysiological level may account for mutual interactions between obesity and depression (73). Obesity is related to a higher grade of inflammation and this may hold true for depression too. Metabolic manifestations seen in obese people such as cardiovascular diseases and diabetes have been attributed at least in part in obesity-related chronic inflammation stemming from adipose tissue (74). Adipose tissue induces IL-6, which is an important mediator for CD4+ T cell polarization to Th17 cells (75). Blood concentrations of IL-17 (as well as IL-23) appear to be elevated in obese (BMI: 30–48 kg/m2) compared to slim women (BMI: 18–25 kg/m2) (76) (Table 2).
Of relevance, Ps and obesity are interconnected on the basis of various common denominators (82). For example, significant weight loss can improve psoriatic lesions and attributed to disease-remission. In addition, obesity is associated with higher incidence and prevalence of Ps. Finally, work in animals has shown that BMI increase participates in the development of Ps, while clinical studies have shown that increase in body weight participate in the relapse of psoriatic lesions and/or the de novo appearance of the disease (82). On the basis of that it becomes apparent that Ps, obesity and depression are interlinked and that IL-17, which significantly contributes to disease development may account for the induction and continuation of all three denominators in the same patient (Figure 2).
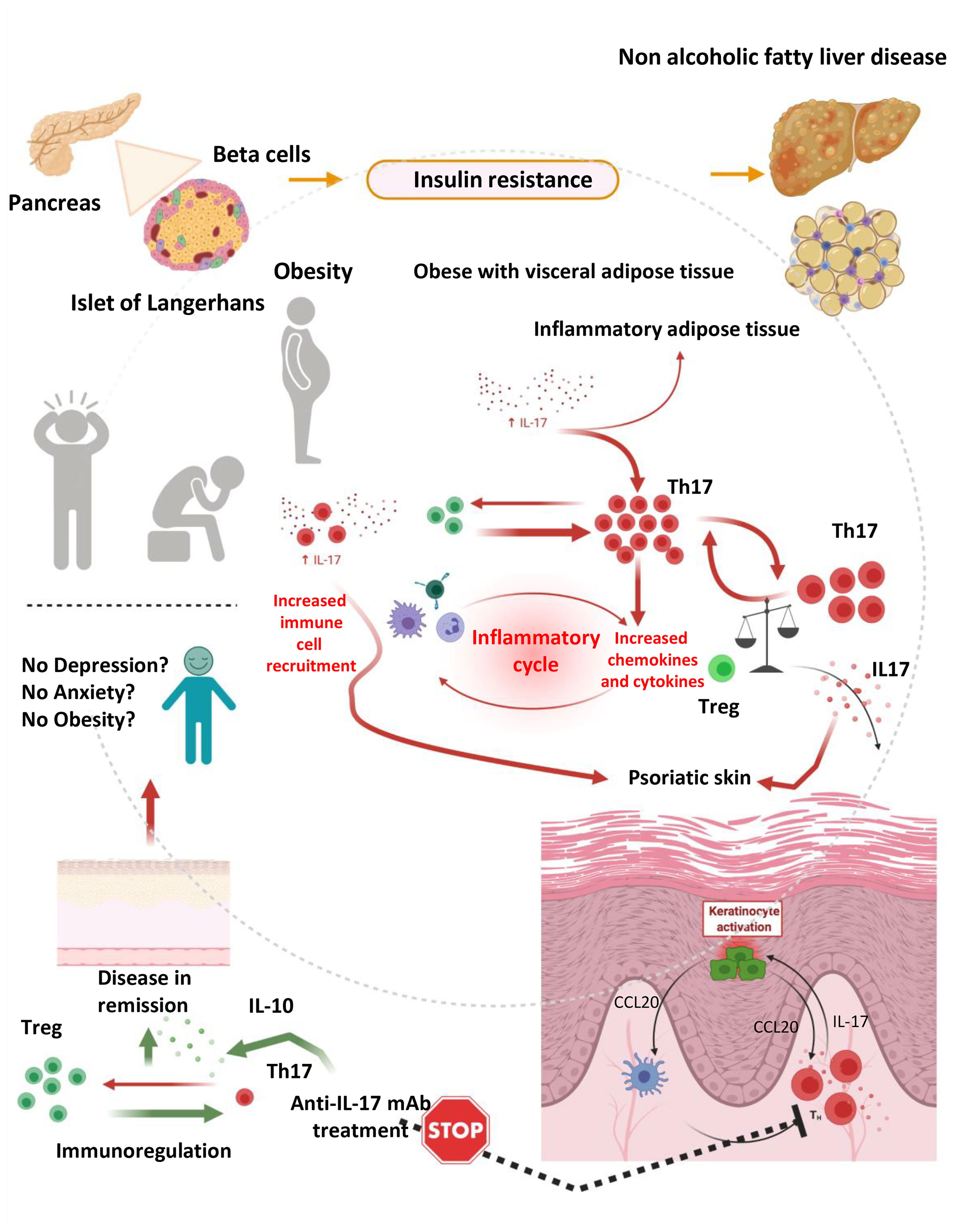
Figure 2 The hypothesis of immunedysregulation in obese depressed patients with psoriatic disease. An inflammatory response mediated by IL-17 producing cells such as Th17 is playing a pivotal role in the development of obesity, depression and psoriasis, in isolation or in combination. Immundysregulation manifested as increased expression of IL-17 and IL-17 producing cell subsets and diminished expression of regulatory cells (Tregs) and related cytokines (such as IL-10) is driving complex reactions leading to the development and progression of psoriatic disease and concomitant anxiety, depression and obesity. Those manifestations can partially be attributed to the inflammatory milieu, which fosters consistent inflammation, keratinocyte activation, cellular damage and skin destruction, as well as interconnected depressive disorders and weight gain. This vicious circle is stopped by IL-17 selective inhibitors, which can lead to the remission of skin lesions (and in the remission of arthritogenic features in the case of psoriatic arthritis or axial spondylarthritis) (prepared with BioRender).
Intriguingly, recent data has shown that ixekizumab was efficacious in the treatment of moderate-to-severe Ps irrespectively of body weight (83). In most research clinical trials, anti-IL-17 inhibitors did not exert any effect on body weight increase. In AMAGINE 1, a phase 3 trial of brodalumab, treatment with this mAb showed higher rates of disease skin improvement (PASI 75 and PASI 90) at weeks 12 and 52 in normal weight patients compared to that noted in obese psoriatic patients (66, 84). Notably, IL-17 inhibitors are very effective independently from body weight; however, they tend to present better clearance rates in normal weight patients.
However, a recent study failed no correlation between body mass index and IL-17 expression in 95 patients with depression (85) and secukinumab-induced skin remission in patients with Ps does not reduce body weight after 12 or 24 weeks of treatment (86). A phase 4 randomized, multi-centre, open label, parallel group, active comparator-controlled study with a duration of 28 weeks and a 28 week extension phase (MEDABOLYX, NCT03440736) recruits patients in an attempt to assess whether secukinumab with lifestyle intervention can improve both skin symptomatology and cardiometabolic status compared to secukinumab alone. Though, the design of the study is on immediately addressing the emerging issue as to whether the IL-17 blocker can improve body weight/BMI, the results of the study could be informative and are highly anticipated.
Several studies, including those conducted by our group, have revealed a negative correlation between IL-17-producing cells and IL-10-producing or other regulatory cell subsets in patients with Ps and PsA, but it is not clear whether these negative correlations are largely depicted in patients stratified in accordance to the presence of obesity (or depression) (22, 87, 88). Of relevance, secukinumab appears to exert an intriguing obliterating effect on disease-related autoantibodies in patients with Ps and PsA (89).
In the last decades, extensive piece of information underline that cutaneous Ps and PsA patients are at higher risk of developing obesity and cardiovascular disease. A major player for that increase, particularly in the industrialized countries, has been attributed to the adaptation of a western lifestyle with less physical activity and diet with high fat and carbohydrate, as well as excessive sodium consumption favor the development, all of which contribute to overweight and obesity (90). Depression is also a comorbidity, which patients with psoriatic disease frequently need to deal with. Whether these comorbidities contribute via an immunobiologically sound mechanism in disease development, relapses or progression or they are just epiphenomena, is a matter of heated debate. Needless to mention, that a significant impact on the resolution or improvement of anxiety and depression related symptoms, as well as on controlling weight, it is likely due to the positive effect the disease remission may exert in patients with Ps and PsA.
In conclusion, data from experimental models, and to a lesser extent from clinical studies in depression, obesity and psoriatic disease, provide the impetus for the understanding for the complex nature of the immunopathogenesis of these manifestations, and suggest that IL-17 could be a common denominator cross-linking some of involved features at the cellular and molecular level in susceptible individuals. However, these data must be treated with caution, as no clear cut evidence for a beneficial role of anti-IL-17 treatment (other than that related to skin lesions and arthritis) in managing anxiety, depression is currently provided, and data by no means are conclusive. Also, a direct effect of anti-IL17 inhibitors in obesity is not clearly documented and this is rather troubling for those promoting the hypothesis suggesting that anti-IL17 neutralizing antibodies may indeed have a clinical impact in fighting obesity. In the clinical setting, body weight does not appear to influence the efficacious effect of anti-IL17 inhibitors in patients with psoriatic disease and this cannot be underestimated. Circumstantial evidence supporting the opposite effect is also weak, raising the expectation of more well controlled, prospective studies investigating these issues in the near future. Limited data on male patients with PsA, have shown that secukinumab treatment is associated with a decrease in resestin and chemerin (but not adiponectin) at 6 months post-treatment compared to baseline. Such a decrease on adipokines was not noted in women with PsA. It must also be emphasized that, as per anti-TNF-α biologics it is not clear to what extent and under which circumstances anti-IL-17 biologics can cross the blood brain barrier, suggesting that their presumed ability to exert any anti-depressant effect would derive mainly from peripheral inhibition of IL-17 rather than a direct effect of those biologics on the brain (50). Nevertheless, the role played in by the IL-17 axis in the development of depression (and obesity) which are frequently regarded as comorbidities of psoriatic disease needs urgent attention and further exploration, mainly because it will assist efforts to better understand whether immunodysregulation involving IL-17 is indeed involved in the induction of either obesity or depression (or both).
EZ and DB had the original idea and scripted considerable part of original draft. AD and VS had performed extensive literature search and scripted part of the manuscript. ED scripted part of the original draft and edited the manuscript. DB prepared the artwork DB and EZ had edited the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded in part by the Special Account for Research Grants University of Thessaly, grant numbers 6357, 5158 & 5847.
EZ: Lilly, Genesis Pharma, Janssen, LEO Pharma, Novartis, UCB, Pfizer, Sanofi Genzyme, AbbVie—speaker honoraria/or paid investigator. ED: Allergan, Novartis, Genesis, ELPEN, Bayer, Teva, Merck-Serono, Genzyme-Sanofi, Roche, UCB: speaker or chairman horonaria, advisory or travel grants, and clinical research-educational support grants. DB: AbbVie, Novartis, Genesis, ELPEN, Pfizer, Aenorasis, Menarini, Kopper, ITF Hellas, Roche, MSD, GSK, Hospital Line-speaker or chairman horonaria or paid investigator or clinical research and educational support grants.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We appreciate the critical comments made by Dr. EI Rigopoulou.
1. Haugeberg G, Lund Nilsen TI, Kavanaugh A, Thomsen RS, Gulati AM, Hoff M. Physical and Psychosocial Burden of Psoriatic Arthritis: Longitudinal Data From a Population-Based Study in Norway. Arthritis Care Res (Hoboken) (2021) 73:138–45. doi: 10.1002/acr.24412
2. Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The Risk of Depression, Anxiety, and Suicidality in Patients With Psoriasis: A Population-Based Cohort Study. Arch Dermatol (2010) 146:891–5. doi: 10.1001/archdermatol.2010.186
3. Parisi R, Webb RT, Kleyn CE, Carr MJ, Kapur N, Griffiths CEM, et al. Psychiatric Morbidity and Suicidal Behaviour in Psoriasis: A Primary Care Cohort Study. Br J Dermatol (2019) 180:108–15. doi: 10.1111/bjd.17004
4. Wu JJ, Penfold RB, Primatesta P, Fox TK, Stewart C, Reddy SP, et al. The Risk of Depression, Suicidal Ideation and Suicide Attempt in Patients With Psoriasis, Psoriatic Arthritis or Ankylosing Spondylitis. J Eur Acad Dermatol Venereol (2017) 31:1168–75. doi: 10.1111/jdv.14175
5. McDonough E, Ayearst R, Eder L, Chandran V, Rosen CF, Thavaneswaran A, et al. Depression and Anxiety in Psoriatic Disease: Prevalence and Associated Factors. J Rheum (2014) 41:887–96. doi: 10.3899/jrheum.130797
6. Mathew AJ, Chandran V. Depression in Psoriatic Arthritis: Dimensional Aspects and Link With Systemic Inflammation. Rheum Ther (2020) 7:287–300. doi: 10.1007/s40744-020-00207-6
7. Kramer NE, Cosgrove VE, Dunlap K, Subramaniapillai M, McIntyre RS, Suppes T. A Clinical Model for Identifying an Inflammatory Phenotype in Mood Disorders. J Psychiatr Res (2019) 113:148–58. doi: 10.1016/j.jpsychires.2019.02.005
8. Koo J, Marangell LB, Nakamura M, Armstrong A, Jeon C, Bhutani T, et al. Depression and Suicidality in Psoriasis: Review of the Literature Including the Cytokine Theory of Depression. J Eur Acad Dermatol Venereol (2017) 31:1999–2009. doi: 10.1111/jdv.14460
9. Liu CS, Adibfar A, Herrmann N, Gallagher D, Lanctot KL. Evidence for Inflammation-Associated Depression. Curr Top Behav Neurosci (2017) 31:3–30. doi: 10.1007/7854_2016_2
10. Raison CL, Capuron L, Miller AH. Cytokines Sing the Blues: Inflammation and the Pathogenesis of Depression. Trends Immunol (2006) 27:24–31. doi: 10.1016/j.it.2005.11.006
11. Patel DD, Lee DM, Kolbinger F, Antoni C. Effect of IL-17A Blockade With Secukinumab in Autoimmune Diseases. Ann Rheum Dis (2013) 72(Suppl 2):ii116–23. doi: 10.1136/annrheumdis-2012-202371
12. Sakkas LI, Bogdanos DP. Are Psoriasis and Psoriatic Arthritis the Same Disease? The Il-23/IL-17 Axis Data. Autoimmun Rev (2017) 16:10–5. doi: 10.1016/j.autrev.2016.09.015
13. Sakkas LI, Zafiriou E, Bogdanos DP. Mini Review: New Treatments in Psoriatic Arthritis. Focus on the IL-23/17 Axis. Front Pharmacol (2019) 10:872. doi: 10.3389/fphar.2019.00872
14. Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: Il-17A and Beyond. Front Immunol (2018) 9:1682. doi: 10.3389/fimmu.2018.01682
15. Fasching P, Stradner M, Graninger W, Dejaco C, Fessler J. Therapeutic Potential of Targeting the Th17/Treg Axis in Autoimmune Disorders. Molecules (2017) 22(1):134. doi: 10.3390/molecules22010134
16. Kaushik SB, Lebwohl MG. Psoriasis: Which Therapy for Which Patient: Psoriasis Comorbidities and Preferred Systemic Agents. J Am Acad Dermatol (2019) 80:27–40. doi: 10.1016/j.jaad.2018.06.057
17. Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR Recommendations for the Management of Psoriatic Arthritis With Pharmacological Therapies: 2019 Update. Ann Rheum Dis (2020) 79:700–12. doi: 10.1136/annrheumdis-2020-217163
18. Osborne LM, Brar A, Klein SL. The Role of Th17 Cells in the Pathophysiology of Pregnancy and Perinatal Mood and Anxiety Disorders. Brain Behav Immun (2019) 76:7–16. doi: 10.1016/j.bbi.2018.11.015
19. Kowalczyk M, Szemraj J, Blizniewska K, Maes M, Berk M, Su KP, et al. An Immune Gate of Depression - Early Neuroimmune Development in the Formation of the Underlying Depressive Disorder. Pharmacol Rep (2019) 71:1299–307. doi: 10.1016/j.pharep.2019.05.022
20. Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. JAMA Psychiatry (2013) 70:31–41. doi: 10.1001/2013.jamapsychiatry.4
21. McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, et al. Efficacy of Adjunctive Infliximab vs Placebo in the Treatment of Adults With Bipolar I/Ii Depression: A Randomized Clinical Trial. JAMA Psychiatry (2019) 76:783–90. doi: 10.1001/jamapsychiatry.2019.0779
22. Mavropoulos A, Varna A, Zafiriou E, Liaskos C, Alexiou I, Roussaki-Schulze A, et al. Il-10 Producing Bregs Are Impaired in Psoriatic Arthritis and Psoriasis and Inversely Correlate With IL-17- and Ifngamma-Producing T Cells. Clin Immunol (2017) 184:33–41. doi: 10.1016/j.clim.2017.04.010
23. Kelepouri D, Mavropoulos A, Bogdanos DP, Sakkas LI. The Role of Flavonoids in Inhibiting Th17 Responses in Inflammatory Arthritis. J Immunol Res (2018) 2018:9324357. doi: 10.1155/2018/9324357
24. Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, et al. Psoriasis Is Characterized by Accumulation of Immunostimulatory and Th1/Th17 Cell-Polarizing Myeloid Dendritic Cells. J Invest Dermatol (2009) 129:79–88. doi: 10.1038/jid.2008.194
25. Ghoreschi K, Laurence A, Yang XP, Hirahara K, O’Shea JJ. T Helper 17 Cell Heterogeneity and Pathogenicity in Autoimmune Disease. Trends Immunol (2011) 32:395–401. doi: 10.1016/j.it.2011.06.007
26. Krueger JG, Brunner PM. Interleukin-17 Alters the Biology of Many Cell Types Involved in the Genesis of Psoriasis, Systemic Inflammation and Associated Comorbidities. Exp Dermatol (2018) 27:115–23. doi: 10.1111/exd.13467
27. Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal Role of Dermal IL-17-producing Gammadelta T Cells in Skin Inflammation. Immunity (2011) 35:596–610. doi: 10.1016/j.immuni.2011.08.001
28. Yin Y, Wang M, Liu M, Zhou E, Ren T, Chang X, et al. Efficacy and Safety of IL-17 Inhibitors for the Treatment of Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Arthritis Res Ther (2020) 22:111. doi: 10.1186/s13075-020-02208-w
29. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in Plaque Psoriasis–Results of Two Phase 3 Trials. N Engl J Med (2014) 371:326–38. doi: 10.1056/NEJMoa1314258
30. Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Uncover and U.-. Investigators, Comparison of Ixekizumab With Etanercept or Placebo in Moderate-to-Severe Psoriasis (UNCOVER-2 and UNCOVER-3): Results From Two Phase 3 Randomised Trials. Lancet (2015) 386:541–51. doi: 10.1016/S0140-6736(15)60125-8
31. Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an Interleukin-17A Specific Monoclonal Antibody, for the Treatment of Biologic-Naive Patients With Active Psoriatic Arthritis: Results From the 24-Week Randomised, Double-Blind, Placebo-Controlled and Active (Adalimumab)-Controlled Period of the Phase III Trial SPIRIT-P1. Ann Rheum Dis (2017) 76:79–87. doi: 10.1136/annrheumdis-2016-209709
32. Roman M, Chiu MW. Spotlight on Brodalumab in the Treatment of Moderate-to-Severe Plaque Psoriasis: Design, Development, and Potential Place in Therapy. Drug Des Devel Ther (2017) 11:2065–75. doi: 10.2147/DDDT.S113683
33. Papp KA, Merola JF, Gottlieb AB, Griffiths CEM, Cross N, Peterson L, et al. Dual Neutralization of Both Interleukin 17A and Interleukin 17F With Bimekizumab in Patients With Psoriasis: Results From BE ABLE 1, a 12-Week Randomized, Double-Blinded, Placebo-Controlled Phase 2b Trial. J Am Acad Dermatol (2018) 79:277–286 e10. doi: 10.1016/j.jaad.2018.03.037
34. Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential Regulation of Central Nervous System Autoimmunity by T(H)1 and T(H)17 Cells. Nat Med (2008) 14:337–42. doi: 10.1038/nm1715
35. Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, et al. In Vivo Imaging of Partially Reversible th17 Cell-Induced Neuronal Dysfunction in the Course of Encephalomyelitis. Immunity (2010) 33:424–36. doi: 10.1016/j.immuni.2010.08.018
36. Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-Microarray Analysis of Multiple Sclerosis Lesions Yields New Targets Validated in Autoimmune Encephalomyelitis. Nat Med (2002) 8:500–8. doi: 10.1038/nm0502-500
37. Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 Production in Central Nervous System-Infiltrating T Cells and Glial Cells is Associated With Active Disease in Multiple Sclerosis. Am J Pathol (2008) 172:146–55. doi: 10.2353/ajpath.2008.070690
38. Wang HH, Dai YQ, Qiu W, Lu ZQ, Peng FH, Wang YG, et al. Interleukin-17-Secreting T Cells in Neuromyelitis Optica and Multiple Sclerosis During Relapse. J Clin Neurosci (2011) 18:1313–7. doi: 10.1016/j.jocn.2011.01.031
39. Kumar P, Shih DCW, Lim A, Paleja B, Ling S, Li Yun L, et al. Pro-Inflammatory, IL-17 Pathways Dominate the Architecture of the Immunome in Pediatric Refractory Epilepsy. JCI Insight (2019) 4(8):e126337 (pages 1–12). doi: 10.1172/jci.insight.126337
40. Moaaz M, Youssry S, Elfatatry A, El Rahman MA. Th17/Treg Cells Imbalance and Their Related Cytokines (IL-17, IL-10 and TGF-β) in Children With Autism Spectrum Disorder. J Neuroimmunol (2019) 337:577071. doi: 10.1016/j.jneuroim.2019.577071
41. Borovcanin MM, Minic Janicijevic S, Jovanovic IP, Gajovic NM, Jurisevic MM, Arsenijevic NN. Type 17 Immune Response Facilitates Progression of Inflammation and Correlates With Cognition in Stable Schizophrenia. Diagn (Basel) (2020) 10(11):926 (pages 1–12). doi: 10.3390/diagnostics10110926
42. Beurel E, Lowell JA. Th17 Cells in Depression. Brain Behav Immun (2018) 69:28–34. doi: 10.1016/j.bbi.2017.08.001
43. Tsuboi H, Sakakibara H, Minamida Y, Tsujiguchi H, Matsunaga M, Hara A, et al. Elevated Levels of Serum Il-17A in Community-Dwelling Women With Higher Depressive Symptoms. Behav Sci (Basel) (2018) 8(11):102 (page 1–7). doi: 10.3390/bs8110102
44. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
45. Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The Longitudinal Associations of Inflammatory Biomarkers and Depression Revisited: Systematic Review, Meta-Analysis, and Meta-Regression. Mol Psychiatry (2020). doi: 10.1038/s41380-020-00867-4
46. Miller AH, Raison CL. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat Rev Immunol (2016) 16:22–34. doi: 10.1038/nri.2015.5
47. Colasanto M, Madigan S, Korczak DJ. Depression and Inflammation Among Children and Adolescents: A Meta-Analysis. J Affect Disord (2020) 277:940–8. doi: 10.1016/j.jad.2020.09.025
48. Huang M, Su S, Goldberg J, Miller AH, Levantsevych OM, Shallenberger L, et al. Longitudinal Association of Inflammation With Depressive Symptoms: A 7-Year Cross-Lagged Twin Difference Study. Brain Behav Immun (2019) 75:200–7. doi: 10.1016/j.bbi.2018.10.007
49. Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, et al. Evidence for Sustained Elevation of IL-6 in the CNS as a Key Contributor of Depressive-Like Phenotypes. Transl Psychiatry (2012) 2:e199. doi: 10.1038/tp.2012.120
50. Uzzan S, Azab AN. Anti-TNF-Alpha Compounds as a Treatment for Depression. Molecules (2021) 26(8):2368 (pages 2–9). doi: 10.3390/molecules26082368
51. Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y. Atypical Antipsychotics Suppress Production of Proinflammatory Cytokines and Up-Regulate Interleukin-10 in Lipopolysaccharide-Treated Mice. Prog Neuropsychopharmacol Biol Psychiatry (2009) 33:303–7. doi: 10.1016/j.pnpbp.2008.12.006
52. Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, et al. Cellular Mechanisms of IL-17-Induced Blood-Brain Barrier Disruption. FASEB J (2010) 24:1023–34. doi: 10.1096/fj.09-141978
53. Davami MH, Baharlou R, Ahmadi Vasmehjani A, Ghanizadeh A, Keshtkar M, Dezhkam I, et al. Elevated IL-17 and TGF-beta Serum Levels: A Positive Correlation Between T-Helper 17 Cell-Related Pro-Inflammatory Responses With Major Depressive Disorder. Basic Clin Neurosci (2016) 7:137–42. doi: 10.15412/J.BCN.03070207
54. Galecka M, Blizniewska-Kowalska K, Orzechowska A, Szemraj J, Maes M, Berk M, et al. Inflammatory Versus Anti-inflammatory Profiles in Major Depressive Disorders-The Role of IL-17, Il-21, IL-23, Il-35 and Foxp3. J Pers Med (2021) 11(2):66 (pages 1–14). doi: 10.3390/jpm11020066
55. Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, et al. Emerging Tendency Towards Autoimmune Process in Major Depressive Patients: A Novel Insight From Th17 Cells. Psychiatry Res (2011) 188:224–30. doi: 10.1016/j.psychres.2010.10.029
56. Alvarez-Mon MA, Gomez-Lahoz AM, Orozco A, Lahera G, Diaz D, Ortega MA, et al. Expansion of CD4 T Lymphocytes Expressing Interleukin 17 and Tumor Necrosis Factor in Patients With Major Depressive Disorder. J Pers Med (2021) 11(3):220 (pages 1–16). doi: 10.3390/jpm11030220
57. Kim JW, Kim YK, Hwang JA, Yoon HK, Ko YH, Han C, et al. Plasma Levels of IL-23 and IL-17 Before and After Antidepressant Treatment in Patients With Major Depressive Disorder. Psychiatry Investig (2013) 10:294–9. doi: 10.4306/pi.2013.10.3.294
58. Beurel E, Lowell JA, Jope RS. Distinct Characteristics of Hippocampal Pathogenic TH17 Cells in a Mouse Model of Depression. Brain Behav Immun (2018) 73:180–91. doi: 10.1016/j.bbi.2018.04.012
59. Beurel E, Harrington LE, Jope RS. Inflammatory T Helper 17 Cells Promote Depression-Like Behavior in Mice. Biol Psychiatry (2013) 73:622–30. doi: 10.1016/j.biopsych.2012.09.021
60. Sales MC, Kasahara TM, Sacramento PM, Rossi AD, Cafasso M, Oyamada HAA, et al. Selective Serotonin Reuptake Inhibitor Attenuates the Hyperresponsiveness of TLR2(+) and TLR4(+) Th17/Tc17-like Cells in Multiple Sclerosis Patients With Major Depression. Immunology (2021) 162:290–305. doi: 10.1111/imm.13281
61. Kim J, Suh YH, Chang KA. Interleukin-17 Induced by Cumulative Mild Stress Promoted Depression-Like Behaviors in Young Adult Mice. Mol Brain (2021) 14:11. doi: 10.1186/s13041-020-00726-x
62. Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, et al. Altered Expression of CD4(+)CD25(+) Regulatory T Cells and its 5-HT(1a) Receptor in Patients With Major Depression Disorder. J Affect Disord (2010) 124:68–75. doi: 10.1016/j.jad.2009.10.018
63. Nadeem A, Ahmad SF, Al-Harbi NO, Fardan AS, El-Sherbeeny AM, Ibrahim KE, et al. Il-17A Causes Depression-Like Symptoms Via NFkappaB and p38MAPK Signaling Pathways in Mice: Implications for Psoriasis Associated Depression. Cytokine (2017) 97:14–24. doi: 10.1016/j.cyto.2017.05.018
64. Rivera-Oyola R, Stanger R, Litchman GH, Thibodeaux Q, Koo J, Fried R, et al. The Use of Brodalumab in Three Patients With Psoriasis and Psychiatric Comorbidities. J Clin Aesthet Dermatol (2020) 13:44–8.
65. Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 Studies Comparing Brodalumab With Ustekinumab in Psoriasis. N Engl J Med (2015) 373:1318–28. doi: 10.1056/NEJMoa1503824
66. Papp KA, Reich K, Paul C, Blauvelt A, Baran W, Bolduc C, et al. A Prospective Phase III, Randomized, Double-Blind, Placebo-Controlled Study of Brodalumab in Patients With Moderate-to-Severe Plaque Psoriasis. Br J Dermatol (2016) 175:273–86. doi: 10.1111/bjd.14493
67. Lebwohl MG, Papp KA, Marangell LB, Koo J, Blauvelt A, Gooderham M, et al. Psychiatric Adverse Events During Treatment With Brodalumab: Analysis of Psoriasis Clinical Trials. J Am Acad Dermatol (2018) 78:81–89 e5. doi: 10.1016/j.jaad.2017.08.024
68. Gould TD, Georgiou P, Brenner LA, Brundin L, Can A, Courtet P, et al. Postolache, Animal Models to Improve Our Understanding and Treatment of Suicidal Behavior. Transl Psychiatry (2017) 7:e1092. doi: 10.1038/tp.2017.50
69. Lebwohl M, Leonardi C, Wu JJ, Armstrong A, Rawnsley N, Merchant M, et al. Two-Year US Pharmacovigilance Report on Brodalumab. Dermatol Ther (Heidelb) (2021) 11:173–80. doi: 10.1007/s13555-020-00472-x
70. Genovese MC, Mysler E, Tomita T, Papp KA, Salvarani C, Schwartzman S, et al. Safety of Ixekizumab in Adult Patients With Plaque Psoriasis, Psoriatic Arthritis and Axial Spondyloarthritis: Data From 21 Clinical Trials. Rheumatol (Oxford) (2020) 59:3834–44. doi: 10.1093/rheumatology/keaa189
71. Talamonti M, Malara G, Natalini Y, Bardazzi F, Conti A, Chiricozzi A, et al. Secukinumab Improves Patient Perception of Anxiety and Depression in Patients With Moderate to Severe Psoriasis: A Post Hoc Analysis of the SUPREME Study. Acta Derm Venereol (2021) 101:adv00422. doi: 10.2340/00015555-3712
72. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global Burden of Obesity in 2005 and Projections to 2030. Int J Obes (Lond) (2008) 32:1431–7. doi: 10.1038/ijo.2008.102
73. Chauvet-Gelinier JC, Roussot A, Cottenet J, Brindisi MC, Petit JM, Bonin B, et al. Depression and Obesity, Data From a National Administrative Database Study: Geographic Evidence for an Epidemiological Overlap. PLoS One (2019) 14:e0210507. doi: 10.1371/journal.pone.0210507
74. Nishimura S, Manabe I, Nagai R. Adipose Tissue Inflammation in Obesity and Metabolic Syndrome. Discov Med (2009) 8:55–60.
75. Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, et al. Functional Cooperation Between Interleukin-17 and Tumor Necrosis Factor-Alpha Is Mediated by CCAAT/enhancer-binding Protein Family Members. J Biol Chem (2004) 279:2559–67. doi: 10.1074/jbc.M308809200
76. Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, et al. Increased Activity of Interleukin-23/interleukin-17 Proinflammatory Axis in Obese Women. Int J Obes (Lond) (2009) 33:151–6. doi: 10.1038/ijo.2008.216
77. Elisia I, Lam V, Cho B, Hay M, Li MY, Kapeluto J, et al. Exploratory Examination of Inflammation State, Immune Response and Blood Cell Composition in a Human Obese Cohort to Identify Potential Markers Predicting Cancer Risk. PLoS One (2020) 15:e0228633. doi: 10.1371/journal.pone.0228633
78. Pirowska M, Obtulowicz A, Lipko-Godlewska S, Gozdzialska A, Podolec K, Wojas-Pelc A. The Level of Proinflammatory Cytokines: Interleukins 12, 23, 17 and Tumor Necrosis Factor Alpha in Patients With Metabolic Syndrome Accompanying Severe Psoriasis and Psoriatic Arthritis. Postepy Dermatol Alergol (2018) 35:360–6. doi: 10.5114/ada.2018.77665
79. Nikseresht M. Comparison of Serum Cytokine Levels in Men Who are Obese or Men Who are Lean: Effects of Nonlinear Periodized Resistance Training and Obesity. J Strength Cond Res (2018) 32:1787–95. doi: 10.1519/JSC.0000000000002039
80. Villarreal-Calderon JR, Castillo EC, Cuellar-Tamez RX, Garcia-Garza M, Elizondo-Montemayor L, Garcia-Rivas G. Reduced Th1 Response is Associated With Lower Glycolytic Activity in Activated Peripheral Blood Mononuclear Cells After Metabolic and Bariatric Surgery. J Endocrinol Invest (2021). doi: 10.1007/s40618-021-01587-4
81. Farhangi MA, Saboor-Yaraghi AA, Keshavarz SA. Vitamin A Supplementation Reduces the Th17-Treg - Related Cytokines in Obese and non-Obese Women. Arch Endocrinol Metab (2016) 60:29–35. doi: 10.1590/2359-3997000000125
82. Paroutoglou K, Papadavid E, Christodoulatos GS, Dalamaga M. Deciphering the Association Between Psoriasis and Obesity: Current Evidence and Treatment Considerations. Curr Obes Rep (2020) 9:165–78. doi: 10.1007/s13679-020-00380-3
83. Kaushik SB, Lebwohl MG. Psoriasis: Which Therapy for Which Patient: Focus on Special Populations and Chronic Infections. J Am Acad Dermatol (2019) 80:43–53. doi: 10.1016/j.jaad.2018.06.056
84. Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, et al. Systemic Pharmacological Treatments for Chronic Plaque Psoriasis: A Network Meta-Analysis. Cochrane Database Syst Rev (2017) 12:CD011535. doi: 10.1002/14651858.CD011535.pub2
85. Blizniewska-Kowalska K, Szewczyk B, Galecka M, Su KP, Maes M, Szemraj J, et al. Is Interleukin 17 (Il-17) Expression A Common Point in the Pathogenesis of Depression and Obesity? J Clin Med (2020) 9(12):4018 (pages 1–15). doi: 10.3390/jcm9124018
86. Wang HN, Huang YH. Changes in Metabolic Parameters in Psoriatic Patients Treated With Secukinumab. Ther Adv Chronic Dis (2020) 11:2040622320944777. doi: 10.1177/2040622320944777
87. Liu Y, Zhang C, Li B, Yu C, Bai X, Xiao C, et al. A Novel Role of IL-17A in Contributing to the Impaired Suppressive Function of Tregs in Psoriasis. J Dermatol Sci (2021) 101:84–92. doi: 10.1016/j.jdermsci.2020.09.002
88. Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ Regulatory T Cells of Psoriasis Patients Easily Differentiate Into IL-17A-producing Cells and Are Found in Lesional Skin. J Invest Dermatol (2011) 131:1853–60. doi: 10.1038/jid.2011.139
89. Patrikiou E, Liaskos C, Mavropoulos A, Ntavari N, Gkoutzourelas A, Simopoulou T, et al. Autoantibodies Against Specific Nuclear Antigens are Present in Psoriatic Disease and Are Diminished by Secukinumab. Clin Chim Acta (2020) 510:400–7. doi: 10.1016/j.cca.2020.07.037
Keywords: depression, IL-17, IL-23, immunity, obesity, psoriasis, psoriatic disease
Citation: Zafiriou E, Daponte AI, Siokas V, Tsigalou C, Dardiotis E and Bogdanos DP (2021) Depression and Obesity in Patients With Psoriasis and Psoriatic Arthritis: Is IL-17-Mediated Immune Dysregulation the Connecting Link? Front. Immunol. 12:699848. doi: 10.3389/fimmu.2021.699848
Received: 24 April 2021; Accepted: 08 June 2021;
Published: 21 July 2021.
Edited by:
Trine N. Jorgensen, Case Western Reserve University, United StatesReviewed by:
Eric Toussirot, CIC1431 Centre d’Investigation Clinique Besançon (INSERM), FranceCopyright © 2021 Zafiriou, Daponte, Siokas, Tsigalou, Dardiotis and Bogdanos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimitrios P. Bogdanos, Ym9nZGFub3NAbWVkLnV0aC5ncg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.