
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 20 September 2021
Sec. Immunological Tolerance and Regulation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.686439
This article is part of the Research TopicCharacterization, Functions, and Roles of Antigen-Specific Regulatory T Cells in Health and DiseaseView all 18 articles
 Yannick D. Muller1,2†
Yannick D. Muller1,2† Leonardo M. R. Ferreira1,2,3†
Leonardo M. R. Ferreira1,2,3† Emilie Ronin1,2
Emilie Ronin1,2 Patrick Ho1,2,3
Patrick Ho1,2,3 Vinh Nguyen1,2
Vinh Nguyen1,2 Gaetano Faleo1,2
Gaetano Faleo1,2 Yu Zhou4
Yu Zhou4 Karim Lee1
Karim Lee1 Kevin K. Leung5
Kevin K. Leung5 Nikolaos Skartsis1,6
Nikolaos Skartsis1,6 Anupurna M. Kaul1
Anupurna M. Kaul1 Arend Mulder7
Arend Mulder7 Frans H. J. Claas7
Frans H. J. Claas7 James A. Wells5
James A. Wells5 Jeffrey A. Bluestone2,3
Jeffrey A. Bluestone2,3 Qizhi Tang1,2*
Qizhi Tang1,2*Infusion of regulatory T cells (Tregs) engineered with a chimeric antigen receptor (CAR) targeting donor-derived human leukocyte antigen (HLA) is a promising strategy to promote transplant tolerance. Here, we describe an anti-HLA-A2 CAR (A2-CAR) generated by grafting the complementarity-determining regions (CDRs) of a human monoclonal anti-HLA-A2 antibody into the framework regions of the Herceptin 4D5 single-chain variable fragment and fusing it with a CD28-ζ signaling domain. The CDR-grafted A2-CAR maintained the specificity of the original antibody. We then generated HLA-A2 mono-specific human CAR Tregs either by deleting the endogenous T-cell receptor (TCR) via CRISPR/Cas9 and introducing the A2-CAR using lentiviral transduction or by directly integrating the CAR construct into the TCR alpha constant locus using homology-directed repair. These A2-CAR+TCRdeficient human Tregs maintained both Treg phenotype and function in vitro. Moreover, they selectively accumulated in HLA-A2-expressing islets transplanted from either HLA-A2 transgenic mice or deceased human donors. A2-CAR+TCRdeficient Tregs did not impair the function of these HLA-A2+ islets, whereas similarly engineered A2-CAR+TCRdeficientCD4+ conventional T cells rejected the islets in less than 2 weeks. A2-CAR+TCRdeficient Tregs delayed graft-versus-host disease only in the presence of HLA-A2, expressed either by co-transferred peripheral blood mononuclear cells or by the recipient mice. Altogether, we demonstrate that genome-engineered mono-antigen-specific A2-CAR Tregs localize to HLA-A2-expressing grafts and exhibit antigen-dependent in vivo suppression, independent of TCR expression. These approaches may be applied towards developing precision Treg cell therapies for transplant tolerance.
Regulatory T cells (Tregs) are a small subset of CD4+ T cells that are key for maintaining self-tolerance and preventing autoimmune disease (1). A plethora of preclinical models have shown that the infusion of Tregs can suppress graft rejection and promote transplant tolerance (2). Several phase I/II clinical studies using Tregs have been reported (3, 4). For instance, the ONE Study is the largest coordinated international study of regulatory cell therapies in kidney transplantation. The study includes 28 patients who received Treg therapy in 4 non-randomized single-arm phase I/IIa trials. The results demonstrated feasibility, safety, and potential benefit of Treg-based therapies to reduce the burden of immunosuppression (5). While a significant fraction of Tregs in the polyclonal pool can react to allogeneic donor antigens, data from preclinical models show that donor-reactive Tregs are more effective than polyclonal Tregs in promoting transplant tolerance (6). Unfortunately, donor alloantigen-reactive Tregs may be functionally altered or induced to migrate out of the peripheral blood following transplantation, thus limiting the frequency of alloantigen-reactive clones within polyclonal Treg products and thereby posing challenges for consistent expansion of donor-reactive Tregs (2).
Redirecting Tregs with transgenic T cell receptors or chimeric antigen receptors (CAR) have been reported by multiple labs since 2005 (7–14). In transplantation, engineering alloantigen reactivity using an alloantigen-specific CAR has also been reported (15–17). Previous studies have shown that a CAR, consisting of a mouse anti-HLA-A2 (A2) antibody-derived single chain variable fragment (scFv) coupled to a CD28-ζ signaling domain, could be introduced in human Tregs using lentivirus. These A2-CAR Tregs demonstrated superior efficacy in preventing xenogeneic graft-versus-host disease (GvHD) in NSG mice when compared to polyclonal Tregs or Tregs transduced with an irrelevant CAR (18). The therapeutic potential of A2-CAR Tregs for organ transplantation was subsequently demonstrated by two separate groups which independently applied A2-CAR Tregs to prevent the rejection of A2+ human skin grafts in humanized mouse models, further bolstering the enthusiasm for evaluating this technology in humans (19, 20).
In all these studies, the CAR constructs were introduced into the Tregs via lentivirus and the engineered Tregs also expressed their endogenous TCR. Lentiviral transduction results in random integration of the CAR construct in the genome that can lead to variable levels of CAR expression, transcriptional silencing, or accidental disruption of important genes. A previous study has shown that site-specific integration of a CD19-CAR into the TCR alpha constant region (TRAC) of T cells results in a more uniform distribution and TCR-like regulation of CAR surface expression, thereby mitigating T-cell exhaustion and enhancing anti-tumor activity (21). In addition, we recently observed that CARhi human T effector cells exhibited a surprisingly robust proliferative response to anti-CD28 stimulation alone, independent of CAR or TCR engagement, whereas CARlo T effector cells did not (22). Thus, lentivirally engineered Tregs may result in heterogeneous CAR expression and unexpected properties of the engineered cells. Knocking a CAR into the TRAC locus and deleting the endogenous TCR may more precisely control CAR Treg activity. Furthermore, this strategy avoids confounding effects from xenoreactivity of the endogenous human TCR against mouse antigens when testing the in vivo function of CAR Tregs in humanized mouse models. However, it is unclear whether CAR Tregs can function without the endogenous TCR. We thus conducted the current study by generating TCRdeficient A2-CAR human Tregs and assessed their trafficking, survival, and function in humanized NSG mouse hosts.
Human peripheral blood from de-identified healthy donors was purchased from STEMCELL Technologies (Vancouver, Canada), which collects and distributes de-identified human blood products with consent forms, according to protocols approved by the Institutional Review Board (IRB). Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll (GE Healthcare, Chicago, IL) density gradient centrifugation. T cells were further enriched using the EasySep Human T Cell Isolation Kit (STEMCELL Technologies), as per the manufacturer’s instructions. Enriched CD3+ T cells, or CD4+CD127+CD25low conventional T cells (Tconv) or CD4+CD127lowCD25high regulatory T cells (Tregs) purified by fluorescence-assisted cell sorting (FACS) using a BD FACS Aria II Cell Sorter (Beckton Dickinson, Franklin Lakes, NJ) were used for experiments. Tregs were expanded as previously described (23). Antibodies utilized for flow cytometry are summarized in Supplementary Table 1.
A human B-cell derived hybridoma (clone SN607D8) was used as source material to produce an anti-HLA-A2 scFv. This hybridoma produces an IgG1κ monoclonal antibody that recognizes HLA serotypes A2 and A28 (24). RNA from the SN607D8 hybridoma was used as template for RT-PCR amplification of the VL and VH chains of the IgG. The scFv gene was then constructed in a VH-(GGGS)3linker-VL format and incorporated into the pHEN1 phage display vector (25). The binding activity of phage-displayed scFv was assessed using two tumor cell lines, THP-1 [HLA-A*02:01/02:01, HLA-B*15:11/15:11 (26)] and RPMI 8226 [HLA-A*30:01/68:02, HLA-B*15:03/15:10 (27)]. Binding to these cell lines was measured using sequential staining with a biotinylated anti-phage antibody and fluorochrome-conjugated streptavidin followed by flow cytometric analysis.
The CDR regions of the anti-HLA-A2 scFv from hybridoma SN607D8 were grafted onto the 4D5 human antibody scaffold used in herceptin (trastuzumab) by pairwise alignment of amino acid residues using the software Jalview (28). The specific CDR3 regions of the anti-HLA-A2 scFv were predicted using the software Paratome (29). The grafted scFv was constructed in the VH-(GGGGS)3linker-VL format.
The A2-specific CAR was created by generating a chimeric DNA sequence encoding a MYC-tag upstream of the grafted anti-HLA-A2 scFv, an IgG4 hinge, CD28 transmembrane domain, and a CD28-CD3zeta tandem signaling domain (purchased as gblocks from Integrated DNA Technologies, IDT, Coralville, IA). The resulting DNA fragment was subcloned into a pCDH lentiviral vector containing an EF1α promoter [addgene-plasmid-64874 (30)]. The CAR construct was linked to a truncated EGFR (EGFRt) or a luciferase gene via a 2A self-cleaving peptide sequence. All constructs used in subsequent experiments were confirmed by Sanger sequencing. Lentivirus was produced as previously described (31). Briefly, HEK293T cells were seeded at 3 × 106 cells on 10 cm cell culture dishes 24 hours prior to transfection with 4 µg of plasmid DNA, 2 µg of the packaging vector pCMV-dR8.9, 2 µg of VSV envelope vector pMD2.G and 15 nmol linear 25 kDa polyethylenimine (Millipore Sigma, Burlington, MA). Media was replaced 24 hours later and ViralBoost Reagent (Alstem, Richmond, CA) was added. The supernatant was collected 24 and 48 hours later. Virus was concentrated using LentiX concentrator (Takara, Shiga, Japan).
A pAAV-MCS plasmid containing inverted terminal repeats (ITRs) from AAV serotype 2 (Agilent Technologies, Santa Clara, CA) was utilized as backbone for AAV6 plasmid construction [naturally occurring AAV6 has an AAV2 ITR (32)]. Cloning was performed with in-fusion cloning tools and protocols provided by Takara. Large scale DNA preparation was performed using a Zymopure plasmid maxiprep kit (Zymo Research, Irvine, CA). All constructs used in subsequent experiments were confirmed by Sanger sequencing. For AAV production, 30 µg of pDGM6 helper plasmid (a gift from Dr. YY Chen, University of California, Los Angeles), 40 µg of pAAV helper containing the VA, E2A and E4 regions (33), a gift from Dr. YY Chen, University of California, Los Angeles), and 15 nmol linear polyethylenimine were used. AAV6 vector production was carried out by iodixanol gradient purification as previously described (34, 35). After ultracentrifugation, AAVs were extracted by puncture and further concentrated using a 50 ml Amicon column (Millipore Sigma) and titrated directly on primary human T cells.
HLA allele cross-reactivity of the A2-CAR-expressing Tregs was determined based on a previously reported method (36). In brief, 2.5 × 104 FACS-purified A2-CAR Tregs, as well as 2.5 × 104 control untransduced polyclonal Tregs, were incubated with 0.5 µl to 5 µl PE-labeled FlowPRA Single HLA Antigen bead panels (FL1HD01 and FL1HD02, OneLambda, Los Angeles, CA), a fixable viability dye (Ghost Dye BV510, Tonbo Biosciences, San Diego, CA), and anti-CD45 e450 (clone HI30, eBioscience, San Diego, CA) for 30 minutes at 37°C. After incubation, the suspensions were washed with DPBS, fixed with 0.5% neutral buffered formalin (VWR International, West Chester, PA), washed again with DPBS, and run in a BD LSRII flow cytometer. Single antigen beads decorated with different HLAs fluoresce in the PE channel with distinct intensity, allowing one to discern the individual HLA alleles. The abundance of unbound beads was quantified in the presence of either A2-CAR Tregs or untransduced Tregs for each single HLA antigen group. Percentage relative binding of A2-CAR Tregs to each HLA allele was then calculated using the following formula
i.e. by dividing the normalized (norm.) number of beads in the untransduced (UT) Treg condition for a specific HLA minus the normalized number of beads in the A2-CAR Treg condition for that same HLA by the normalized number of beads in the untransduced Treg condition, multiplied by 100. HLA antigen bead numbers were normalized using the following formula
i.e. by multiplying the number of beads of interest in each HLA peak by 200, divided by the number of negative control beads in the sample, to correct for variations in the absolute number of negative control beads acquired in each sample.
CRISPR/Cas9 genome editing in Tregs and bulk T cells was carried out using ribonucleoprotein (RNP) electroporation as previously described (37). Briefly, RNPs were produced by complexing a two-component guide RNA (gRNA) to Cas9. crRNAs and tracrRNAs were chemically synthesized (Dharmacon, IDT) and Cas9-NLS (nuclear localization signal) was recombinantly produced and purified (QB3 Macrolab). Lyophilized RNA was resuspended at a concentration of 160 µM, and stored in single use aliquots at −80°C. crRNA and tracrRNA aliquots were thawed, mixed 1:1 by volume, and annealed at 37 °C for 30 min. 40 µM recombinant Cas9 was mixed 1:1 by volume with the 80 µM gRNA (2:1 gRNA to Cas9 molar ratio) at 37 °C for 15 min to form an RNP complex at 20 µM. RNPs were electroporated immediately after complexing into Tregs and T cells resuspended in supplemented P3 buffer (Lonza).
Guide RNA sequences used for gene editing were:
TRAC: CAGGGTTCTGGATATCTGT
TRBC: CCCACCAGCTCAGCTCCACG
HLA-A2: CCTCGTCCTGCTACTCTCGG
In Figure 1, A2-CAR lentivirus alone was used to engineer Tregs. In Figures 2, 3 (and Supplementary Figures 4–6), the TCR was removed using a constant region of the TCR beta chain (TRBC)-specific CRISPR/Cas9 RNP and an A2-CAR lentivirus to engineer the CAR Tregs. Lentivirus was transduced at a multiplicity of infection (MOI) of 1 by spinoculation for 30 min at 1200 G. The next day, cells were washed to remove residual virus from the medium and further expanded with recombinant human IL-2 (300IU/ml). In Figure 2, A2-CAR+ cells were FACS-purified on Day 9 based on MYC-tag expression and the TCR was deleted by electroporating a CRISPR/Cas9 RNP complex targeting TRBC thereafter. In Figure 3, the TCR was deleted after Treg/Teff cell purification and prior to Treg/Teff cell activation with anti-CD3/CD28 beads.
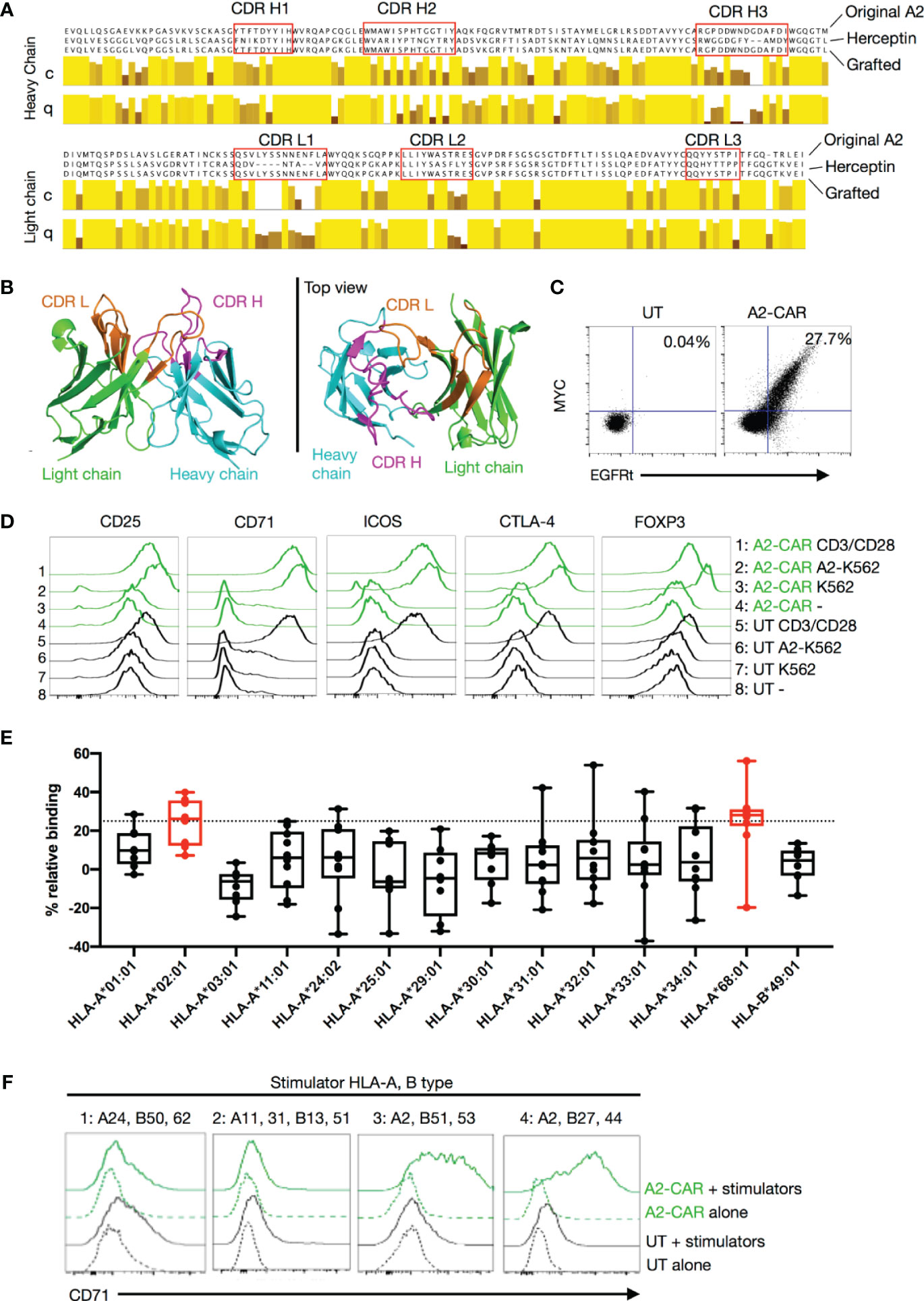
Figure 1 Generation of a grafted A2-CAR. (A) Grafting strategy comparing the original VH and VL chain sequences of the SN607D8 hybridoma and of the Herceptin (trastuzumab) 4D5 scaffold. The grafted amino acid sequences are shown. The sequences of SN607D8 and 4D5 HER2 were aligned using the software Jalview and the level of conservation (C) and quality (Q) of each amino acid between SN607D8 and 4D5 sequences were compared. Conservation reflects similarity of the physicochemical properties of amino acid residues. Identical residues are shown as light-yellow columns and residues with more dissimilar physicochemical properties are marked with darker column colors. Quality measures the likelihood of observing a mutation in any particular amino acid residue position (38). CDRs were predicted using Paratome (29). (B) The conformation of the grafted antibody was predicted with ABodyBuilder (39) and displayed using PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, CA). (C) EGFRt and MYC-tag expression on Day 6 of culture of human Tregs transduced with the grafted A2-CAR-2A-EGFRt lentivirus. (D) On Day 9, A2-CAR Tregs were cultured for another 48 hours alone, with anti-CD3/CD28 beads, or with irradiated (4000 rad) parental A2– K562 or A2-expressing K562 cells. CD25, CD71, ICOS, CTLA-4, and FOXP3 expression were analyzed thereafter using flow cytometry. (E) OneLambda FlowPRA Single HLA Antigen bead panels FL1HD01 and FL1HD02. Percentage relative binding of A2-CAR Tregs to each HLA allele was calculated as described in the Materials and Methods section. Plotted averages of at least 5 independent experiments. Red coloring indicates HLA allele beads surpassing the 25% binding threshold to be considered binders. (F) On Day 9, A2-CAR or UT T cells were cultured for another 48 hours alone or with dissociated islet cells from 4 allogeneic donors. Expression of CD71 was analyzed thereafter using flow cytometry. HLA-A and -B alleles expressed by the 4 allogeneic donors are indicated above the histograms. For donors 1, 3, and 4, A2-CAR and UT Tregs were used in the assay and for donor 2, A2-CAR and UT CD4+ Tconv cells are used. Results are a summary of 4 independent experiments using T cells from unrelated healthy donors. scFv, single-chain variable fragment; CDR, complementarity-defining region; A2, HLA-A2; UT, untransduced; Tconv, conventional T cells.
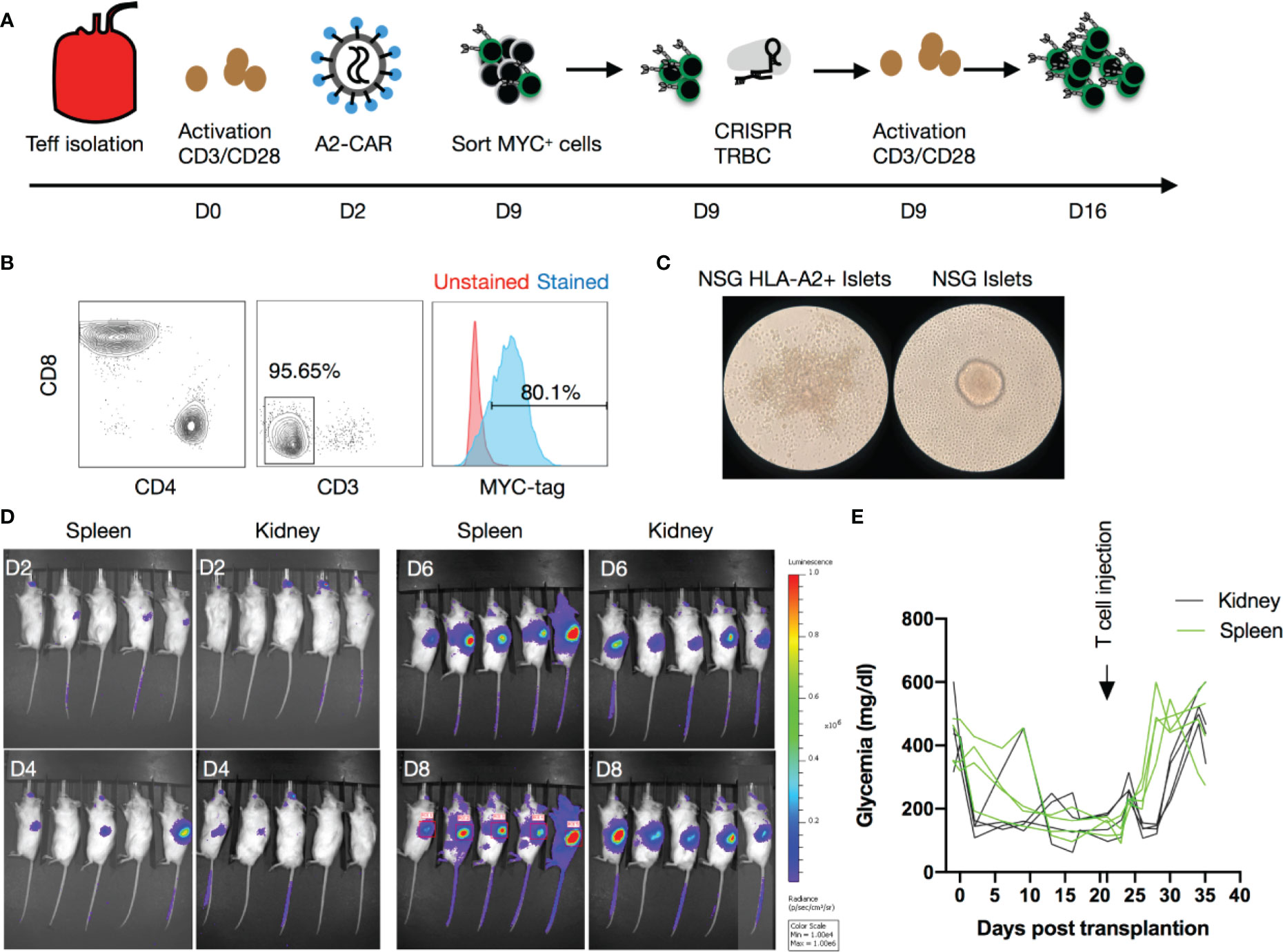
Figure 2 Human A2-CAR T cell trafficking and function in an islet transplantation model. (A) Experimental design of human T-cell engineering and expansion. (B) Editing efficiency as measured by CD3 and MYC-tag expression on T cells prior to infusion from an HLA-A2 negative donor after CRISPR editing of the TCR (TRBC) and transduced with a lentivirus consisting of a A2-CAR linked to a luciferase reporter gene by a 2A self-cleaving peptide. (C) WT NSG or HLA-A2 transgenic NSG mouse islets pictured 48h after co-culture with A2-CAR T cells (100 IEQ with 1 x 105 T cells). Result shown is representative of two experiments using Tregs from 3 unrelated donors. (D) Luciferase activity over time after infusion of 2 x 106 A2-CAR T cells in mice that received an A2 transgenic murine islet transplant either under the left kidney capsule or into the spleen. (E) Glycemia monitoring after streptozotocin (STZ) injection, islet transplantation, and T-cell infusion (2 x 106/mouse from a HLA-A2 negative donor). An insulin pellet was placed subcutaneously in mice with partial graft function (blood glucose >200mg/dl) on Day 10 after transplantation.
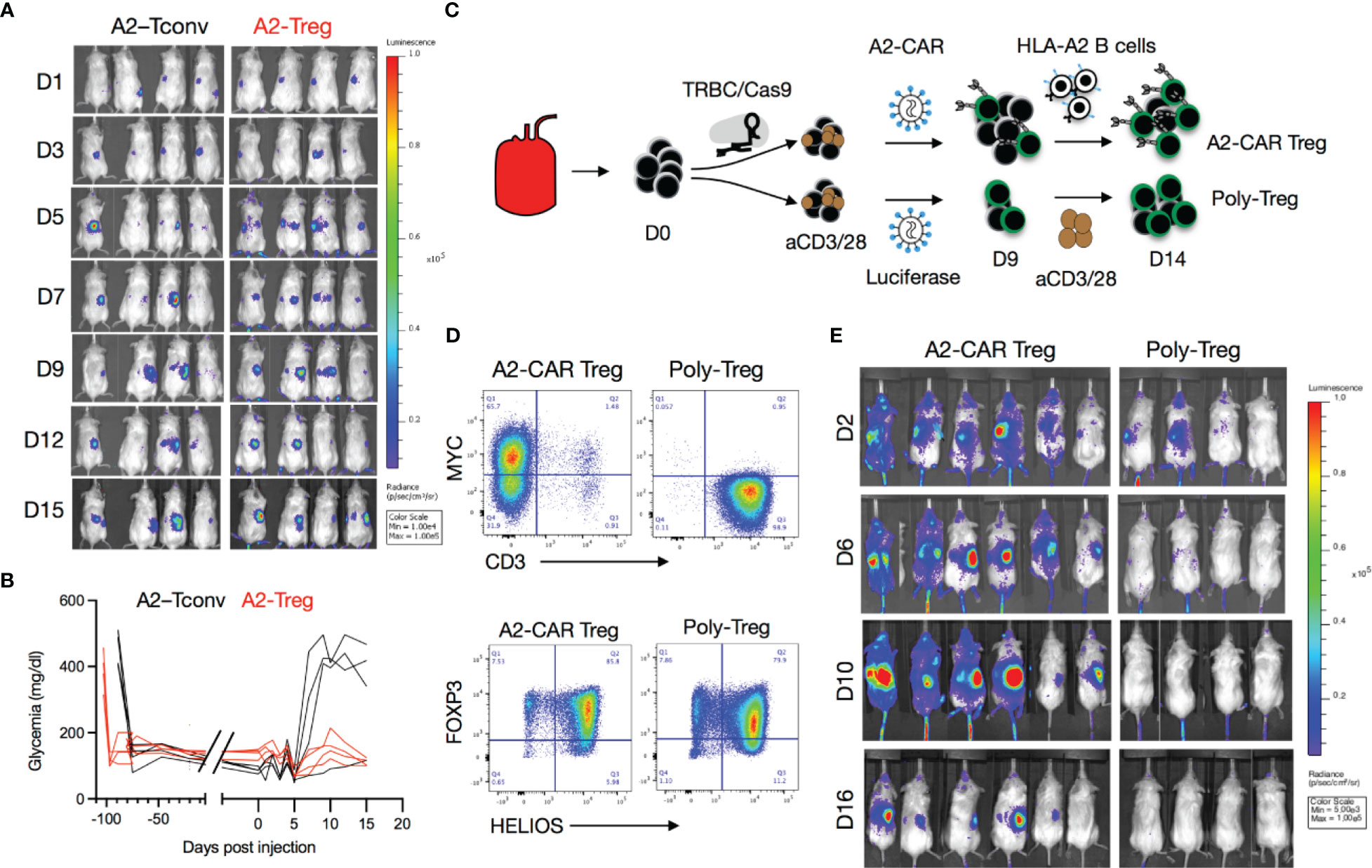
Figure 3 Trafficking of A2-CAR Tregs to islet grafts. (A) TCR deficient (TRBC-CRISPR edited), lentiviral transduced, 2 x 106 A2-CAR CD4+ Tconv cells or A2-CAR Tregs, (harvested on Day 10 after sorting from peripheral blood of an HLA-A2 negative donor) were infused into mice that had been stably transplanted with HLA-A2 transgenic mouse islets. Luciferase activity of A2-CAR CD4+ Tconv cells or A2-CAR Tregs over time is shown. (B) Glycemia was monitored over time after cell infusion. (C) Tregs were edited with CRISPR/Cas9 ribonucleoprotein (RNP) complexes targeting the TRBC locus after cell sorting, activated with anti-CD3/28 beads, and transduced with a lentivirus consisting of an A2-CAR linked to a luciferase reporter gene by a 2A self-cleaving peptide two days later. On Day 9, Tregs were re-stimulated with HLA-A2+ stimulated B cells (sBCs) for another 5 days and were thereafter injected in diabetic mice (2 x 106/animal) transplanted with HLA-A2+ human islets. In parallel, polyclonal Tregs were activated with anti-CD3/28 beads, transduced with a lentivirus expressing a luciferase reporter gene alone, and restimulated with anti-CD3/28 beads on Day 9 and injected on Day 14 in diabetic mice (2 x 106/animal) transplanted with HLA-A2+ human islets. (D) Editing efficiency was measured by MYC-tag and CD3 surface expression in two independent donors. Treg purity was assessed by FOXP3 and HELIOS expression in the same donors. (E) Luciferase activity of A2-CAR Tregs and polyclonal Tregs (transduced with a lentivirus expressing a luciferase reporter alone) over time.
In Figures 4–6, two days after anti-CD3/CD28 bead-mediated activation, cells were electroporated with a TRAC-specific and an HLA-A2-specific CRISPR/Cas9 RNP (1:1 ratio, final volume 5 µl). Tregs and T cells were replated for expansion together with an AAV6 containing the A2-CAR homology-directed repair (HDR) template. The next day, the cells were washed to remove residual virus from the medium and further expanded with recombinant human IL-2 (300IU/ml). In this case, the HLA-A2 gene was removed, as A2-CAR Tregs cannot be engineered from HLA-A2 positive donors (data not shown).
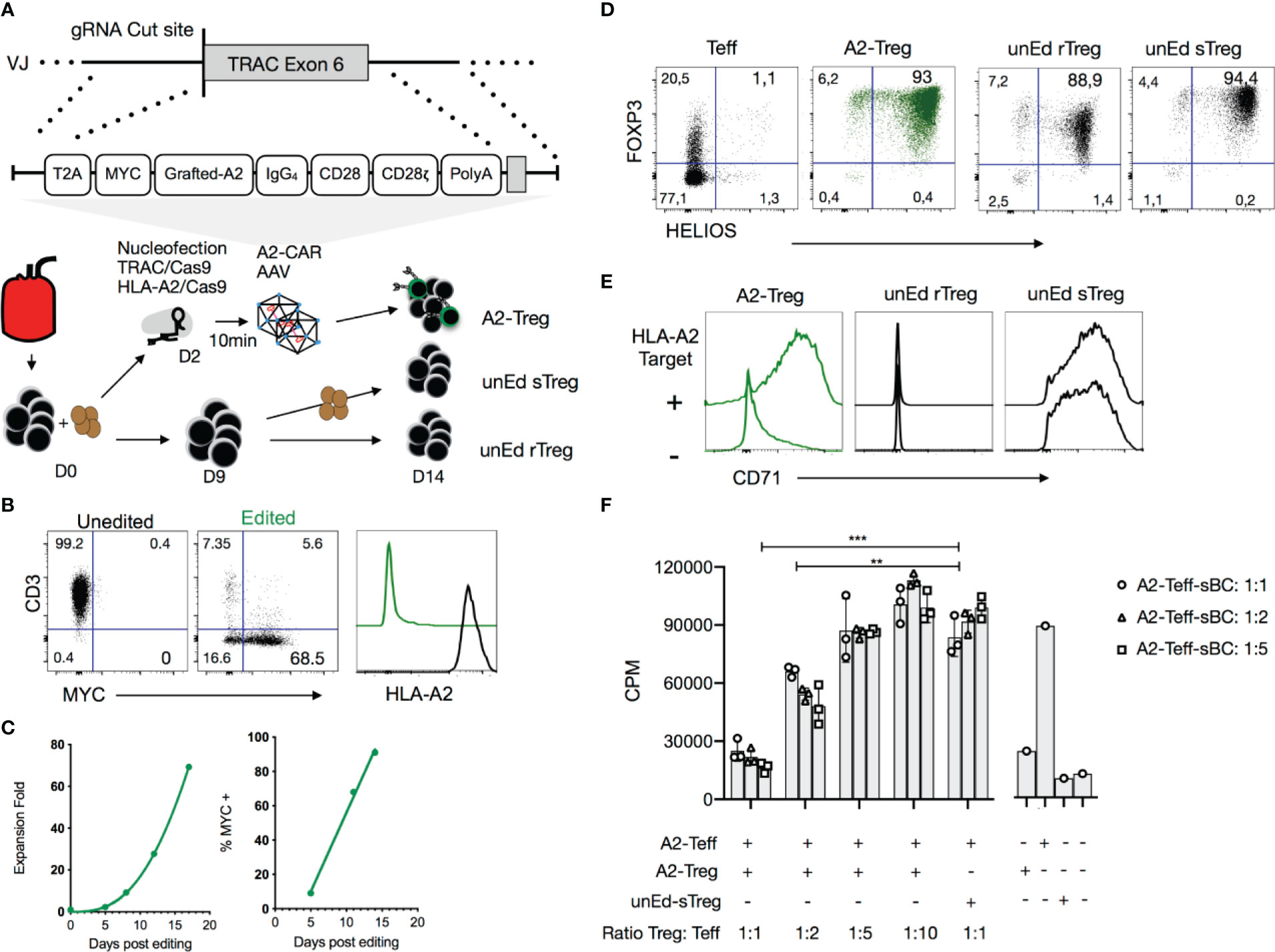
Figure 4 Precision engineering of an A2-CAR into the TRAC locus of human Tregs. (A) Expansion strategy of A2-CAR Tregs, unedited resting Tregs (unEd rTreg) and unedited stimulated Tregs after sorting from peripheral blood of an HLA-A2 positive donor. For homology-directed repair-mediated integration into the TRAC locus, the A2-CAR template was inserted using AAV6 transduction after electroporation of two CRISPR/Cas9 ribonucleoprotein (RNP) complexes targeting the TRAC and HLA-A loci. (B) Representative flow cytometry of the editing efficiency measured 10 days later in 3 independent experiments. CD3, MYC-tag, and HLA-A2 surface expression is shown. (C) Treg fold-expansion and percentage of MYC-tag+ Tregs over time. Fitted line plots are shown. (D) Fourteen days after activation, FOXP3 and HELIOS expression were assessed on edited Tregs and compared to that of unedited T cells and unedited Day 9 stimulated Tregs (unEd sTregs). (E) The same cells were co-cocultured with or without irradiated (4000 rad) HLA-A2+ NALM6 cells. CD71 expression was assessed 48 hours later. (F) In vitro suppression assays were performed using HLA-A2+ stimulated B cells (sBCs) as stimulator cells, A2-CAR+TCRdeficient CD4+ T cells as responder cells (0.05 x 106 cell/96well) and A2-CAR+TCRdeficient Tregs at different ratios. After 3 days, 0.5μCi/well of 3[H] thymidine (Perkin Elmer, Waltham, MA) was added for the final 16 h of culture. Proliferation was assessed by 3[H] thymidine incorporation (counts per minute - cpm). Two-way ANOVA was used to determine the statistical significance of the difference. Data corresponds to the cells infused in Figure 5. Similar results were obtained with 3 independent donors. **p < 0.01; ***p < 0.001. Teff, CD4+ T effector cells; A2-Treg, A2-CAR+TCRdeficient Treg; unEd sTreg, unedited Day 9 stimulated Tregs; A2-Teff, A2-CAR+TCRdeficient Teff; sBC, stimulated B cells; CPM, counts per minute.
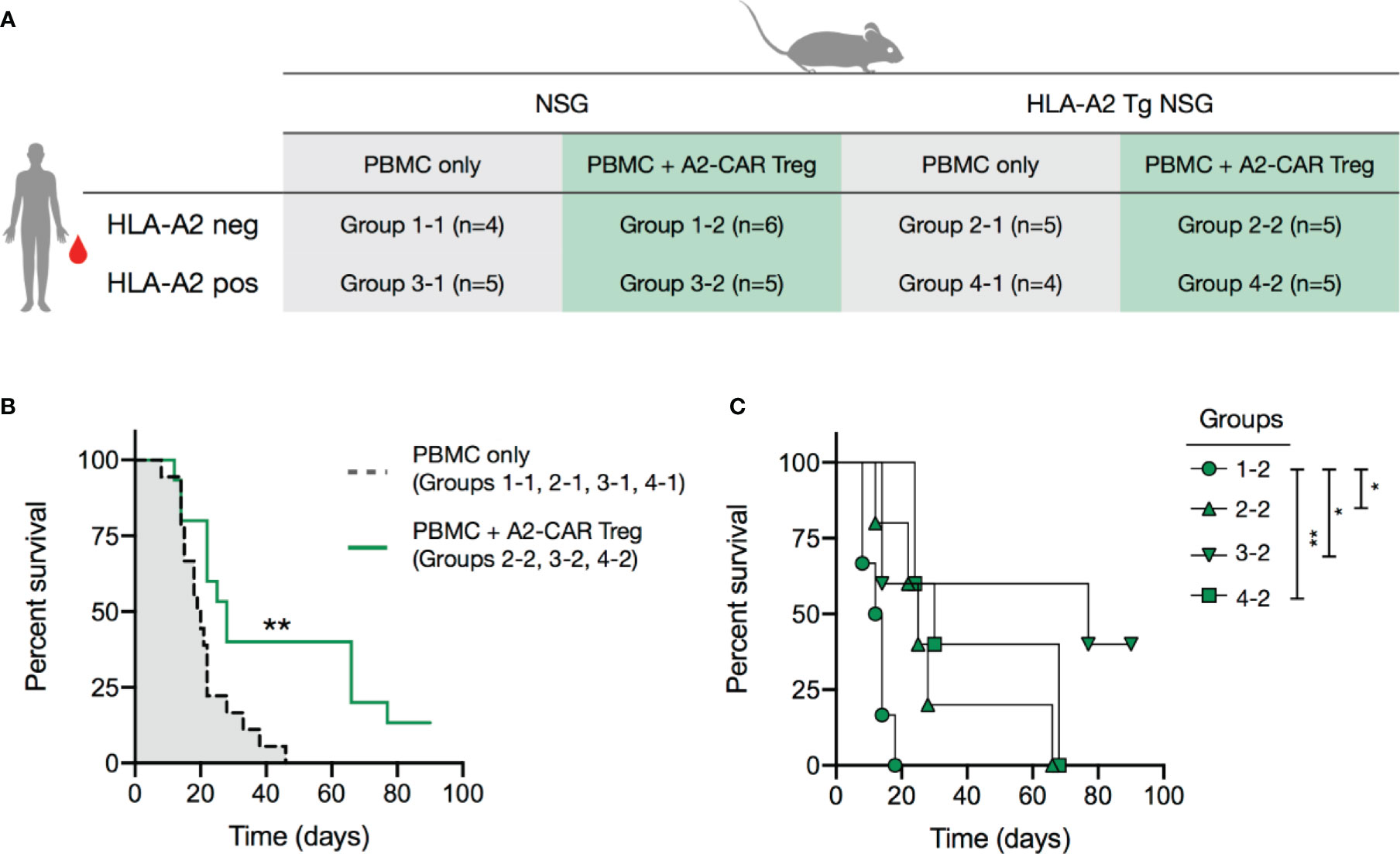
Figure 5 A2-CAR Tregs confer protection against graft-versus-host disease. (A) 2.5 x 106 AAV CRISPR-edited A2-CAR+TCRdeficient Tregs (generated as described in Figure 4) were co-injected with 5 x 106 A2 negative or positive PBMC in wild type or A2 transgenic immunodeficient NSG mice. PBMCs and Tregs were injected into contralateral retro-orbital plexus. (B) Overall survival of mice that received PBMCs (A2 positive or negative, NSG A2 positive or negative) or PBMCs and A2-CAR+TCRdeficient Tregs, excluding the condition where Tregs remained unstimulated (group 1-2). (C) Overall survival of mice treated with A2-CAR+TCRdeficient Tregs comparing NSG PMBC (group 1.2, round), A2-NSG PBMC (group 2-2, triangle), NSG A2+ PBMC (group 3-2, inverted triangle, n=6), and A2-NSG A2+ PBMC (group 4-2, square). Log-rank (Mantel-Cox) test was used for statistical analysis *p < 0.05, **p < 0.01.
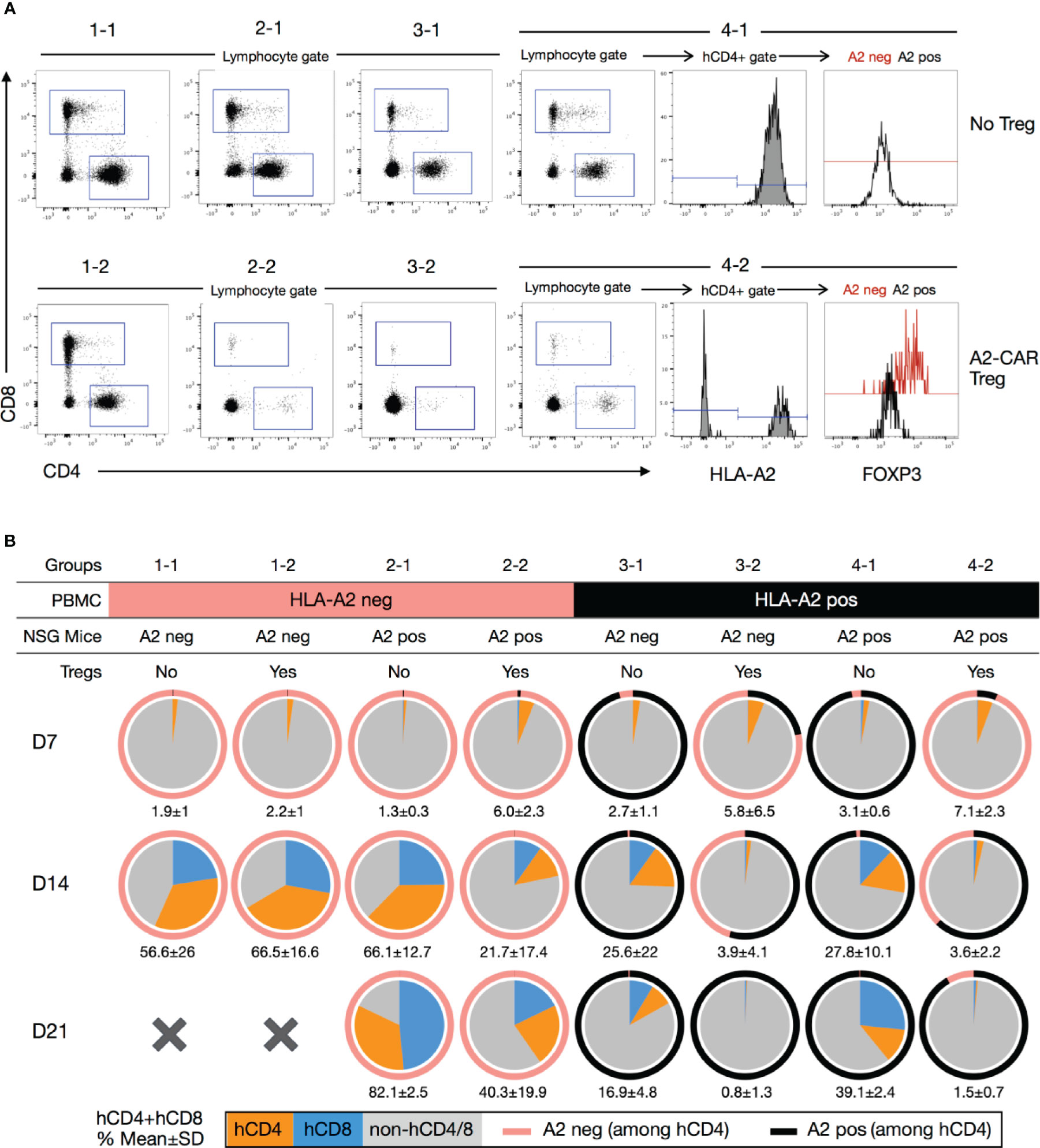
Figure 6 Blood monitoring of immune reconstitution in xenogeneic graft-versus-host disease. (A) Representative staining of human CD8+, human CD4+ and hCD4+A2negFOXP3+ subpopulations after dead cell (Ghost fixable viability dye positive) exclusion. Representative example of mice from each group (1-1, 1-2, 2-1, 2-2, 3-1, 3-2, 4-1, 4-2). (B) Pie charts showing the mean percentage of human CD4+ (orange) and CD8+ (blue) cells in the peripheral blood of mice 7 (n=4-6), 14 (n=2-5) and 21 days (n=2-4) after injection. The numbers below each pie chart represent the mean ± standard deviation of the combined percentage of human T cells (hCD4+ and hCD8+). A2, HLA-A2.
Female or male NSG mice were rendered diabetic by a single intraperitoneal (i.p.) injection of streptozotocin (STZ) at 220mg/kg and islets were transplanted 72-96 hours later. Blood glucose levels were monitored 2-3 times per week using a glucometer (Nova Max Plus Blood glucose and Ketone Monitor, Nova Diabetes care, Billerica, MA). Only mice with blood glucose levels above 300mg/dl were used for transplantation. Pancreatic islets from NSG.HLA-A2 transgenic mice (A2-NSG, NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(HLA-A/H2-D/B2M)1Dvs/SzJ, Jackson Laboratories, Bar Harbor, ME, Stock number 014570) were isolated as previously described (40). Human pancreata were procured from deceased multi-organ donors with research use consents and approval from UCSF institutional review board. Human research islets were isolated by the UCSF Diabetes Center Islet Core following standard protocols (41). A total of either 500 mouse islets or 3000 human islet equivalents (IEQs) were transplanted under the kidney capsule or into the spleen. Blood glucose levels of < 200mg/dl on two consecutive days were defined as successful islet engraftment. Mice that only attained partial graft function (blood glucose range 200-500mg/dl) by 10 to 14 days after transplant were given subcutaneous insulin pellets (Linbit, LinShin Canada) to support graft function. Luciferase-expressing A2-CAR Tregs or A2-CAR T cells were infused intravenously in STZ-induced diabetic mice transplanted with mouse HLA-A2+ islets. Luciferase activity was monitored 2-3 times per week. These animals were anesthetized in an isofluorane chamber, injected i.p. with 100 μl of 15 mg/ml D-Luciferin (Biosynth, Staad, Switzerland) and, 7 min later, imaged in a Xenogen IVIS Spectrum Imaging System (PerkinElmer, Richmond, California). Luciferase data analysis was performed using Living Image software (PerkinElmer).
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) and NOD.Cg Prkdcscid Il2rgtm1Wjl/Tg(HLA-DRB1)31Dmz/SzJ/H2-Ab1tm1Gru x NOD.Cg-Tg(HLA-A/H2-D/B2M)1Dvs/SzJ (A2-NSG) were obtained from Jackson Laboratories. For GvHD induction, animals were irradiated (2.5Gy) 24 hours prior to retroorbital intravenous (i.v.) infusion of 5 x 106 freshly isolated PBMCs from either an HLA-A2-positive or an HLA-A2-negative donor with or without 2.5 x 106 ex vivo expanded third-party A2-CAR Tregs. All mouse experiments were performed according to a UCSF Institutional Animal Care and Use Committee (IACUC) approved protocol.
To engineer an anti-A2 CAR, we first cloned the variable regions of the heavy (VH) and light (VL) chains of an A2-specific IgG1κ antibody from a hybridoma (SN607D8) produced using B cells isolated from a previously described sensitized donor (24). This antibody was reported to bind to HLA serotypes A2 and A28 (broad antigen which includes HLA-A68 and A69 alleles as split antigen). After cloning the SN607D8 scFv from the hybridoma, we evaluated phage-displayed SN607D8 scFv binding to two human tumor cell lines. The THP-1 monocytic cell line expresses HLA-A2, but not A28, whereas the RPMI 8226 myeloma cell line is HLA-A2– but has a genotype of HLA-A*6802 and is thus HLA-A28+. The results showed that the SN607D8 scFv indeed binds to both cell lines (Supplementary Figure 1), demonstrating the retention of the original specificity of the antibody.
We then cloned the SN607D8 scFv into a construct that contained an IgG4 hinge, the CD28 transmembrane domain, and a signaling domain composed of the CD28 and CD3ζ intracellular domains. Unexpectedly, the CAR failed to express on the surface of human T cells (data not shown). To rescue the expression, we grafted the complementarity-determining regions (CDRs) of the heavy and light chains of the SN607D8 scFv into the framework regions of an scFv derived from the anti-HER2 antibody Herceptin (trastuzumab), which is known to be compatible with CAR surface expression (42). The resulting grafted heavy and light chains (Figure 1A) were connected via a 15 amino acid linker (GGGGS)3 to form a new grafted scFv, termed QT007YL. Automated computer modeling with an antibody structure prediction tool, ABodyBuilder (39), showed that the grafted scFv folds as expected (Figure 1B). We then generated a new A2-CAR for expression in human T cells by fusing the QT007YL scFv to an IgG4 hinge, the CD28 transmembrane domain, and a CD28-ζ signaling domain. The resulting A2-CAR was cloned with an N-terminal MYC-tag into a pCDH lentiviral vector behind an EF1α promoter (30). A truncated EGFR (EGFRt) was cloned in-frame behind the CAR separated by a 2A self-cleaving peptide to enable facile evaluation of lentiviral transduction using expression of EGFRt. To enable in vivo tracking of CAR-expressing cells, we also generated a version of the lentiviral construct with a luciferase gene behind the CAR separated by a 2A peptide (Supplementary Figure 2A). We first transduced HLA-A2-negative Jurkat T cells to assess the expression and function of the grafted A2-CAR. Detection of the MYC-tag via flow cytometry verified efficient surface expression of the A2-CAR (Supplementary Figure 2B). Lentiviral transduction of primary human Tregs with the grafted A2-CAR also resulted in co-detection of the MYC-tag and EGFRt on the cell surface (Figure 1C). Moreover, A2-CAR Jurkat T cells upregulated CD69 and CD25 expression specifically when co-cultured with irradiated HLA-A2+ K562 tumor cells (Supplementary Figure 2C), suggesting that the grafted A2-CAR is able to activate T-cell signaling in response to HLA-A2. Finally, we evaluated A2-CAR-mediated in vitro activation of human Tregs following co-culture with either HLA-A2+ or HLA-A2- K562 cells (Figure 1D). A2-CAR Tregs upregulated CD25, CD71, ICOS, FOXP3, and CTLA4 48 hours after stimulation with HLA-A2+, but not with HLA-A2– cells.
The HLA-A2 molecule contains 41 polymorphic epitopes (called eplets) that are shared with other HLA class I molecules (Supplementary Table 2). The parental monoclonal antibody SN607D8 used to generate the grafted A2-CAR has a specificity for the eplet 144TKH (142T, 144K, and 145H residues), which is shared among HLA-A2, -A68, and -A69, but not with HLA-A3, -A11, or -A24 (43). To verify that the grafted A2-CAR QT007YL retained the specificity for 144TKH, we tested binding of grafted A2-CAR transduced Tregs to a FlowPRA Single Antigen bead panel. In this assay, CAR specificity is defined as an increase in binding to HLA-bearing beads over control beads of at least 25%, a threshold used to define the binding specificity of a previously reported A2-CAR (36). The grafted A2-CAR reacted with HLA-A2 and HLA-A68, but not HLA-A3, -A11, -A24, -A25, -A30-A34 (Figure 1E). This pattern of reactivity ruled out all other eplets but the two overlapping eplets of 144TKH and 144KHA, consistent with the specificity of the parental antibody. To further demonstrate the specificity of the QT007YL A2-CAR for HLA naturally expressed on human cells, we incubated A2-CAR T cells or Tregs with dissociated HLA-typed primary human islet cells and measured their activation 48 hrs later by CD71 upregulation. A2-CAR+ cells were activated by stimulator cells expressing HLA-A2, but not those expressing HLA-A11, -A24, or -A31 (Figure 1F). These results, together with the FlowPRA data, demonstrate that the QT007YL A2-CAR retained the specificity of the parental clone SN607D8.
Next, we injected QT007YL A2-CAR-expressing T cells in NSG mice to determine whether CAR expression could redirect T cells to HLA-A2-expressing tissues in vivo. A significant fraction of human T cells can recognize mismatched HLA and trigger rejection of transplanted allogeneic human tissue in NSG mice (44). Additionally, human T cells have conspicuous reactivity against xenogeneic antigens expressed in the mouse host, with the potential to divert T cells away from human grafts and also eventually cause GvHD (45). To avoid these confounding issues, we first lentivirally transduced primary human T cells to express the A2-CAR. We subsequently generated A2-CAR+TCRdeficient T cells by CRISPR/Cas9-mediated knockout of endogenous TCR expression from Day 9 FACS-purified A2-CAR+ cells (Figure 2A). After editing, we restimulated the cells with anti-CD3/CD28 beads for another 7 days, resulting in 95.65% CD3– and 80.1% MYC-tag+ cells (Figure 2B). Co-culturing these TCRdeficient A2-CAR T cells with islets from HLA-A2 transgenic NSG (A2-NSG) or WT NSG mice for 48 hours resulted in the selective destruction of A2-NSG transgenic mouse islets (Figure 2C), demonstrating that the grafted A2-CAR can be specifically activated by HLA-A2 molecules expressed on islet tissue in vitro.
To determine if TCRdeficient A2-CAR T cells can recognize A2-expressing islets in vivo, we first transplanted HLA-A2 transgenic mouse islets into STZ-induced diabetic NSG mice, and then infused the mice with 2 x 106 TCRdeficient A2-CAR T cells after the islet grafts had been established. The kidney capsule is a standard site for islet transplantation. However, human CD4+ T cells efficiently trafficked to the lungs, livers and spleens, but not kidneys of NSG recipients (Supplementary Figure 3). It has been previously reported that infusion of 2 x 107 allogeneic human PBMCs at the time of islet transplant can lead to rejection of human islets transplanted under the kidney capsule in NSG mice within 3 weeks. However, after the graft has been stably engrafted, 2 x 107 allogeneic human PBMCs cannot consistently reject the islets before the mice succumb to GvHD due to the xenogeneic response of the human PBMCs against the mouse hosts (46). We reasoned that, at the time of islet transplant, ischemic injury to the islets led to the release of chemokines and other inflammatory mediators to attract the PBMCs. Once islet grafts are established and the inflammation subsides, PBMCs may not efficiently traffic to the kidney to mediate the rejection. Primary human islets from deceased donors become available for research with very short (< 2 days) advanced notice and need to be transplanted right away to ensure optimal function. This, unfortunately, does not allow time to produce CAR T cells, so we had to rely on the use of a pre-engrafted islet model. To improve the chance of A2-CAR T cell encounter with the islets, we transplanted the islets into the spleen of the NSG mice and included the standard left kidney capsule site for comparison. Three weeks after transplantation, we infused the mice with luciferase-expressing A2-CAR T cells and monitored their migration using bioluminescence imaging. We observed progressive increase in luciferase signal in the recipient mice, although it was difficult to discern the accumulation of A2-CAR T cells in the spleen versus the left kidney (Figure 2D). Moreover, we observed a sharp synchronous rise in blood glucose first among mice with islet grafts in the spleen, followed by mice with islets grafted in the kidney capsule, indicative of rejection of the A2-NSG islets. The median survival was 6 days in the spleen and 11 days under the kidney capsule (Figure 2E), indicating that A2-CAR T cells trafficked to the transplanted kidney to mediate the rejection. To further substantiate graft-specific trafficking of A2-CAR T cells, we transplanted A2 transgenic murine islets either in the liver, the spleen, under the left or left kidney capsule. Luminescence signals were detected only in the locations corresponding to sites of the islet grafts (Supplementary Figure 4). Altogether, these data demonstrated that the A2-CAR was able to direct the trafficking and accumulation of human T cells to sites of antigen deposition outside the default route of T cell migration.
We then used the kidney capsule islet transplantation model to evaluate the in vivo trafficking of A2-CAR Tregs. Luciferase-labeled human TCRdeficient A2-CAR Tregs were generated as described in Figure 2A and the resulting cells expressed Treg lineage markers FOXP3 and CD25 (Supplementary Figure 5). We then infused these cells into STZ-induced diabetic animals transplanted with A2-NSG transgenic islets. In this experiment, the islets were transplanted under the right kidney capsule to enable greater spatial separation from the spleen during bioluminescence imaging. To support Treg persistence in the absence of human IL-2-producing cells, mice infused with 2 x 106 A2-CAR Tregs received daily i.p. injections of recombinant human IL-2 (50,000 IU/day/mouse). For comparison, we separately infused a cohort of mice with 2 x 106 TCRdeficient A2-CAR conventional CD4+ T cells (Tconv), without IL-2 injection. In both groups, luciferase activity was observed first in the spleen and 3-7 days later in the right kidney (Figure 3A) demonstrating that both A2-CAR Treg and Tconv cells can traffic to the A2+ grafts. Importantly, islet rejection was observed in 3 out of 4 mice that received A2-CAR CD4+ Tconv cells, but none of the 4 mice that received A2-CAR Tregs (Figure 3B). Despite accumulation within the grafts, the lack of mouse islet destruction by the human A2-CAR Tregs suggests that CAR Tregs do not have overt toxicity against islets.
To validate that A2-CAR Tregs can traffic to human A2+ islets, we repeated this experiment with human HLA-A2+ islets transplanted under the right kidney capsule. In this experiment, human Tregs were first treated with CRISPR/Cas9 ribonucleoprotein (RNP) complexes targeting the TCR beta constant (TRBC) locus to eliminate endogenous TCR expression prior to lentiviral transduction with an A2-CAR-2A-luciferase construct. To expand sufficient numbers of TCRdeficient A2-CAR Tregs, cells were re-stimulated with HLA-A2+ stimulated B cells (sBCs) on Day 9 of culture for an additional 5 days (Figure 3C). As a control, TCR-unedited polyclonal Tregs were transduced with a luciferase-only construct and restimulated with anti-CD3/28 beads on Day 9. Tregs maintained FOXP3 and HELIOS expression prior to infusion (Figure 3D). Mice received 2 × 106 Tregs intravenously (i.v.) with subsequent daily i.p. IL-2 injections. A2-CAR-expressing, but not polyclonal Tregs, trafficked from the spleen to the right kidney (Figure 3E). Together, these results demonstrate that TCRdeficientA2-CAR Tregs efficiently traffic to and accumulate in human HLA-A2+ islets in vivo.
To investigate A2-CAR function independently of the endogenous TCR, we employed homology-directed repair (HDR) to site-specifically integrate the A2-CAR into the TCR alpha constant (TRAC) locus, replacing expression of the endogenous TCR with expression of the A2-CAR (Figure 4A). CD4+CD25highCD127low Tregs were FACS-purified and activated with anti-CD3/CD28 beads in the presence of IL-2 (300 IU/ml). Two days later, anti-CD3/CD28 beads were magnetically removed, and the cells were electroporated with Cas9-gRNA ribonucleoprotein (RNP) complexes targeting the TRAC locus and transduced with AAV6 encoding the QT007YL A2-CAR HDR template. Because this blood donor happened to be HLA-A2+, we also included Cas9-gRNA RNP designed to target the HLA-A2 gene locus. TRAC and HLA-A2 gene knockout efficiencies were approximately 85% and 95%, respectively. A minor (5%) population of MYC-tag+TCR+ cells was observed, likely resulting from incomplete TRAC inactivation and either monoallelic A2-CAR genomic integration into the other TRAC locus or off-target integration (Figure 4B). Importantly, while the percentage of A2-CAR-expressing MYC-tag+ (edited) Tregs was initially low (9%), A2-CAR+TCRdeficient Tregs preferentially expanded in vitro, presumably due to activation by residual HLA-A2 surface expression shortly after CRISPR/Cas9-mediated HLA-A2 gene knockout. In the absence of further exogenous stimulation, 91% of cells were MYC-tag+ after 14 days (Figure 4C).
On Day 14 of culture, we evaluated FOXP3 and HELIOS expression among edited A2-CAR+TCRdeficient Tregs, unedited CD4+ Tconv, and unedited Tregs [unEd sTreg, stimulated by anti-CD3/CD28 beads on Days 0 and 9, as per our standard protocol for polyclonal Treg expansion (47)]. Over 99% of A2-CAR+TCRdeficient Tregs were FOXP3 positive and 93% were HELIOS and FOXP3 double positive (Figure 4D). Co-culture of the A2-CAR+TCRdeficient Tregs (A2-CAR Treg) with NALM6, an HLA-A2-positive B cell-derived leukemia cell line, led to a marked and specific upregulation of surface CD71 expression, demonstrating the antigen-driven activation of the edited cells. Unedited resting Tregs (unEd rTreg) remained CD71 negative, whereas anti-CD3/28 bead-stimulated unedited Tregs (unEd sTreg) were CD71 positive regardless of the presence of NALM6, as expected (Figure 4E). Finally, we evaluated the suppressive function of the A2-CAR+TCRdeficient Tregs in vitro by co-culturing them with A2-CAR+TCRdeficient T cells and HLA-A2+ irradiated sBCs and assessing T cell proliferation. A2-CAR+TCRdeficient Tregs suppressed the proliferation of A2-CAR+TCRdeficient T cells in the presence of HLA-A2+ sBCs, whereas unedited polyclonal Tregs did not (Figure 4F). The lack of suppression observed with polyclonal Treg may be explained by the low frequency of allogenic Tregs which could interact with A2-expressing B cells and by the strong activation mediated by the CAR in responder T cells, as previously reported (48, 49).
We next tested the in vivo functionality of A2-CAR+TCRdeficient Tregs in vivo within models of xenogeneic GvHD, induced by human PBMCs in sub-lethally irradiated NSG mice. In these experiments, we induced GvHD by infusing PBMCs from an HLA-A2+ or an HLA-A2- donor into HLA-A2-transgenic or wild-type (i.e. lacking HLA-A2 expression) NSG mice (Figure 5A and Supplementary Figure 6A). This created 4 experimental groups with regards to the expression of HLA-A2: 1 absent; 2 expressed by the NSG recipients, 3 expressed by the infused PBMCs; and 4 expressed by both NSG recipients and PBMCs. A subset of mice in each of the 4 groups also received A2-CAR+TCRdeficient Tregs at the time of PBMC infusion. We first confirmed in a mixed lymphocyte reaction (MLR) that A2-CAR+TCRdeficient Tregs upregulated CD71 only in the presence of the PBMCs from the HLA-A2+ donor used for the immune reconstitution (Supplementary Figure 6B). To avoid direct contact between the PMBCs and Tregs during infusion, we injected the cells separately into contralateral retro-orbital plexus. A2-CAR+TCRdeficient Tregs delayed GvHD in mice that had HLA-A2 expressed by the PBMCs, the NSG recipients, or both (Figure 5B) and failed to confer any protection against GvHD in wild-type NSG animals reconstituted with HLA-A2– PBMC, with a median survival of 13 days (Figure 5C). This demonstrates that A2-CAR Tregs can function in the absence of the endogenous TCR and that A2-CAR Treg-mediated protection from GvHD depends on the presence of the HLA-A2 antigen.
To further investigate the mechanism of A2-CAR+TCRdeficient Treg-mediated protection, we determined the percentage of circulating human CD4+ and CD8+ T cells in the peripheral blood of treated animals at Days 7, 14, and 21 following cell injection (Figure 6A). As previously reported (18), we observed that HLA-A2+ T cells barely engrafted in mice that also received A2-CAR+TCRdeficient Tregs, irrespective of HLA-A2 expression by the host mice (Figures 6A, B). Meanwhile, the frequency of engrafted HLA-A2– T cells was vastly reduced, but not completely eliminated, when co-injected with A2-CAR+TCRdeficient Tregs in HLA-A2+ transgenic host mice. In HLA-A2 transgenic mice reconstituted with HLA-A2+ PBMCs (groups 4-1 and 4-2), we found that the circulating HLA-A2– cells, i.e. the engineered A2-CAR+TCRdeficient Tregs, remained FOXP3+ (Figure 6A), albeit with limitations in the number of acquired events due to the marked decrease in the number of CD4+ and CD4+HLA-A2- cells over time (Figure 6B).
Here, we report the successful development of a novel human anti-HLA-A2 CAR. Two other human and humanized A2-CARs have been previously described: one by the group of Megan Levings, where a mouse anti–A2 BB7.2 hybridoma was humanized (36), and a second by the groups of Giovanna Lombardi and Elmar Jaeckel, generated from a previously published anti-HLA-A2 antibody sequence (50) (clone 3PB2 VH and DPK1 VL) derived from a sensitized blood transfusion patient (19, 20). Our original anti-HLA-A2 hybridoma (SN607D8), first described in 2003, was isolated from a woman sensitized during her pregnancies (24). Its HLA specificity was determined by complement-dependent cytotoxicity on a large panel (n>230) of HLA-typed peripheral blood lymphocytes (51), where it was found to cross-react with HLA-A28, a split antigen that encompasses HLA-A68 and HLA-A69 alleles. The epitope responsible for this cross-reactivity has also been pinpointed, being defined by the amino acids 142T/145H (52). Such extensive characterization was instrumental for us to confirm the preservation of the specificity of the original and the grafted A2-CAR scFv.
Our initial failure to express an A2-CAR constructed with an scFv derived from the original SN607D8 hybridoma on the surface of human T cells suggests possible conformational instability. We then grafted the scFv CDR regions into an scFv framework (trastuzumab) known to be compatible with CAR surface expression (42). Thus, the trastuzumab framework may confer greater stability to scFvs for CAR protein folding and expression. However, it should be noted that we have not tested this grafting strategy with other scFvs, and thus cannot be certain of the broader applicability of this CDR-grafting approach. Nevertheless, our success in grafting the specificity of the SN607D8 antibody shows that this approach may be useful when designing CARs for new targets.
It is currently unknown whether the endogenous TCR impacts the function of CAR Tregs. Thymically derived Tregs have a highly diverse TCR repertoire that is skewed towards recognizing autoantigens (53), and work in mice has demonstrated that Tregs require continuous TCR signaling to maintain normal immune homeostasis (54). Thus, retaining the TCR in CAR Tregs might support their homeostasis in vivo. However, in the context of solid organ transplantation, A2-CAR Tregs traffic to the HLA-A2-expressing graft, as shown in previous work (19, 20) and in this study, thus receiving continuous signaling via the CAR, ultimately inducing bystander suppression and supporting homeostasis independently from their endogenous TCR. Our islet transplantation experiments show that A2-CAR Tregs with or without endogenous TCR efficiently traffic to the site of antigen expression. Moreover, the finding that TCR-deficient A2-CAR+ Tregs suppress GvHD, in an HLA-A2-dependent manner, shows that CAR Tregs can function without their endogenous TCR.
Our results are consistent with previous work that has shown efficient GvHD prevention by A2-CAR Tregs in NSG mice infused with HLA-A2+ PBMCs (18). By analyzing PBMC engraftment, it was apparent that the protection from GvHD was a result of preventing T cell engraftment, possibly due to direct recognition of HLA-A2+ PBMCs by A2-CAR Tregs. Yet, low T cell engraftment is not a desirable outcome of Treg therapy for GvHD. On the contrary, immune reconstitution, and subsequent recovery of protective immunity, is needed to safeguard cancer patients who receive a bone marrow transplant against infectious agents and residual cancer cells (55, 56). Our results show that A2-CAR Tregs can delay GvHD not only when the PBMCs themselves express HLA-A2, but also when HLA-A2 is expressed by the recipients and not by the PBMCs.
In the conditions of HLA-A2+ PBMC and A2-CAR Treg co-transfer (group 3-2 and 4-2), we were able to track the infused A2-CAR Tregs by their hCD4+HLA-A2- phenotype. We observed that A2-CAR Tregs dominated in the first week, but their percentages among human CD4+ T cells (pink portion of the outer rings of the pie charts) were reduced by the 2nd week and barely detectable by the 3rd week. The cause of poor A2-CAR Treg persistence is not clear, but likely secondary to low human T cell engraftment, thus limited IL-2 availability, needed for Treg survival. The lack of A2-CAR Treg persistence might allow the residual human T cells unopposed by Tregs to recover, eventually resulting in lethal GvHD in these mice.
One potential benefit of eliminating endogenous TCR expression is to more precisely control Treg specificity, especially in the context of universal CAR Tregs for off-the-shelf use. Creating and banking such universal CAR Tregs may circumvent the challenge of expanding Tregs from immunosuppressed transplant recipients and the long production time that precludes their use in acute conditions. In this vein, we show in this study that we can simultaneously ablate HLA-A2 and TCR expression at high efficiency in human Tregs, while maintaining stable FOXP3 and HELIOS expression, as well as antigen-specific suppressive function in vitro and in vivo. Recently, progress has been made towards the generation of universal human pluripotent stem cells, which portend potential inexhaustible sources of universally compatible cells, tissues, and organs for therapy (57, 58). Our data support the feasibility of developing universal engineered Tregs with precisely controlled specificity while evading host immune destruction to induce immune tolerance. Future experiments aimed at further characterizing the suppressive potency and longevity of CAR Tregs in vivo will shed additional light on the efficiency, safety, and feasibility of this strategy for Treg-based cell therapy.
In conclusion, we demonstrated that it is feasible to engineer a grafted CAR directly into the TRAC locus of human Tregs. This strategy is highly efficient, does not cause Treg destabilization, and allowed for the generation of Tregs with CAR-restricted specificity that delayed GvHD in a target antigen-dependent manner. This strategy can be applied for precision engineering of therapeutic Tregs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by IACUC, University of California, San Francisco.
Designed the project: QT and JB. Supervised the project: QT, JB, and JW. Designed experiments: YM, LF, KL, YZ, and QT. Performed experiments: YM, LF, ER, PH, GF, VN, KL, YZ, NS, and AK. Analyzed data: YM, LF, and QT. Provided reagents and advice: AM and FC. Wrote the manuscript: YM, LF, and QT. All authors contributed to the article and approved the submitted version.
This project was funded in part by grants from the NIDDK (UC4 DK116264 and P30 DK063720), Juno Therapeutics (to QT and JB), and JDRF (SRA-2019-776-S-B). JB acknowledges the support of the Sean N. Parker Autoimmune Laboratory. YM was supported by the Swiss National Science Foundation (Advanced Postdoctoral Mobility Grant no. P300PB_174500) and a fellowship grant from the University Hospital of Geneva. LF was supported by a Jeffrey G. Klein Family Diabetes fellowship and a HIRN Emerging Leader in Type 1 Diabetes grant.
A provisional patent on A2-CAR Tregs has been submitted. QT is a co-founder and scientific advisor of Sonoma Biotherapeutics. JB is a co-founder and the Chief Executive Officer and President of Sonoma Biotherapeutics. JW is co-Founder of Soteria Biotherapeutics developing small molecule switchable biologics, on the SAB of Spotlight, and recipient of sponsored research from Bristol Myers Squibb.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Alexander Marson for sharing the Lonza 4D 96-well electroporation system, and Juan Du, Roxxana Beltran-Valencia, and Hashim Shaikh for technical assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.686439/full#supplementary-material
1. Esensten JH, Muller YD, Bluestone JA, Tang Q. Regulatory T Cell Therapy for Autoimmune and Autoinflammatory Diseases: The Next Frontier. J Allergy Clin Immunol (2018) 142:1710–18. doi: 10.1016/j.jaci.2018.10.015
2. Tang Q, Vincenti F. Transplant Trials With Tregs: Perils and Promises. J Clin Invest (2017) 127:2505–12. doi: 10.1172/JCI90598
3. Chandran S, Tang Q, Sarwal M, Laszik ZG, Putnam AL, Lee K, et al. Polyclonal Regulatory T Cell Therapy for Control of Inflammation in Kidney Transplants. Am J Transplant (2017) 17:2945–54. doi: 10.1111/ajt.14415
4. Mathew JM, H-Voss J, LeFever A, Konieczna I, Stratton C, He J, et al. A Phase I Clinical Trial With Ex Vivo Expanded Recipient Regulatory T Cells in Living Donor Kidney Transplants. Sci Rep (2018) 8:7428. doi: 10.1038/s41598-018-25574-7
5. Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, et al. Regulatory Cell Therapy in Kidney Transplantation (The ONE Study): A Harmonised Design and Analysis of Seven non-Randomised, Single-Arm, Phase 1/2A Trials. Lancet (2020) 395:1627–39. doi: 10.1016/S0140-6736(20)30167-7
6. Lee K, Nguyen V, Lee KM, Kang SM, Tang Q. Attenuation of Donor-Reactive T Cells Allows Effective Control of Allograft Rejection Using Regulatory T Cell Therapy. Am J Transplant (2014) 14:27–38. doi: 10.1111/ajt.12509
7. Mekala DJ, Alli RS, Geiger TL. IL-10-Dependent Suppression of Experimental Allergic Encephalomyelitis by Th2-Differentiated, Anti-TCR Redirected T Lymphocytes. Blood (2005) 174(6):3789–97. doi: 10.4049/jimmunol.174.6.3789
8. Elinav E, Waks T, Eshhar Z. Redirection of Regulatory T Cells With Predetermined Specificity for the Treatment of Experimental Colitis in Mice. Gastroenterology (2008) 134(7):2014–24. doi: 10.1053/j.gastro.2008.02.060
9. Hombach AA, Kofler D, Rappl G, Abken H. Redirecting Human CD4+CD25+ Regulatory T Cells From the Peripheral Blood With Pre-Defined Target Specificity. Gene Ther (2009) 16(9):1088–96. doi: 10.1038/gt.2009.75
10. Lee JC, Hayman E, Pegram HJ, Santos E, Heller G, Sadelain M, et al. In Vivo Inhibition of Human CD19-Targeted Effector T Cells by Natural T Regulatory Cells in a Xenotransplant Murine Model of B Cell Malignancy. Cancer Res (2011) 71(8):2871–81. doi: 10.1158/0008-5472.CAN-10-0552
11. Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z. Suppression of Murine Colitis and its Associated Cancer by Carcinoembryonic Antigen-Specific Regulatory T Cells. Mol Ther (2014) 22(5):1018–28. doi: 10.1038/mt.2014.41
12. Zhang AH, Yoon J, Kim YC, Scott DW. Targeting Antigen-Specific B Cells Using Antigen-Expressing Transduced Regulatory T Cells. J Immunol (2018) 201(5):1434–41. doi: 10.4049/jimmunol.1701800
13. Kim YC, Zhang AH, Yoon J, Culp WE, Lees JR, Wucherpfennig KW, et al. Engineered MBP-Specific Human Tregs Ameliorate MOG-Induced EAE Through IL-2-Triggered Inhibition of Effector T Cells. J Autoimmun (2018) 92:77–86. doi: 10.1016/j.jaut.2018.05.003
14. Yoon J, Schmidt A, Zhang AH, Königs C, Kim YC, Scott DW. FVIII-Specific Human Chimeric Antigen Receptor T-Regulatory Cells Suppress T- and B-Cell Responses to FVIII. Blood (2017) 129(2):238–45. doi: 10.1182/blood-2016-07-727834
15. Wagner JC, Tang Q. CAR-Tregs as a Strategy for Inducing Graft Tolerance. Curr Transplant Rep (2020) 7:205–14. doi: 10.1007/s40472-020-00285-z
16. Sicard A, Levings MK, Scott DW. Engineering Therapeutic T Cells to Suppress Alloimmune Responses Using TCRs, CARs, or BARs. Am J Transplant (2018) 18:1305–11. doi: 10.1111/ajt.14747
17. Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-Generation Regulatory T Cell Therapy. Nat Rev Drug Discov (2019) 18:749–69. doi: 10.1038/s41573-019-0041-4
18. MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, et al. Alloantigen-Specific Regulatory T Cells Generated With a Chimeric Antigen Receptor. J Clin Invest (2016) 126:1413–24. doi: 10.1172/JCI82771
19. Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MA, Hannen RF, Cooper D, et al. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am J Transplant (2017) 17:931–43. doi: 10.1111/ajt.14185
20. Noyan F, Zimmermann K, Hardtke-Wolenski M, Knoefel A, Schulde E, Geffers R, et al. Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am J Transplant (2017) 17:917–30. doi: 10.1111/ajt.14175
21. Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC Locus With CRISPR/Cas9 Enhances Tumour Rejection. Nature (2017) 543:113–17. doi: 10.1038/nature21405
22. Muller YD, Nguyen DP, Ferreira LMR, Ho P, Raffin C, Valencia RVB, et al. The CD28-Transmembrane Domain Mediates Chimeric Antigen Receptor Heterodimerization With CD28. Front Immunol (2021) 12:639818. doi: 10.3389/fimmu.2021.639818
23. Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, et al. Expansion of Human Regulatory T-Cells From Patients With Type 1 Diabetes. Diabetes (2009) 58:652–62. doi: 10.2337/db08-1168
24. Mulder A, Eijsink C, Kardol MJ, Franke-van Dijk ME, van der Burg SH, Kester M, et al. Identification, Isolation, and Culture of HLA-A2-Specific B Lymphocytes Using MHC Class I Tetramers. J Immunol (2003) 171:6599–603. doi: 10.4049/jimmunol.171.12.6599
25. Sheets MD, Amersdorfer P, Finnern R, Sargent P, Lindquist E, Schier R, et al. Efficient Construction of a Large Nonimmune Phage Antibody Library: The Production of High-Affinity Human Single-Chain Antibodies to Protein Antigens. Proc Natl Acad Sci USA (1998) 95:6157–62. doi: 10.1073/pnas.95.11.6157
26. Battle R, Poole K, Haywood-Small S, Clark B, Woodroofe MN. Molecular Characterisation of the Monocytic Cell Line THP-1 Demonstrates a Discrepancy With the Documented HLA Type. Int J Cancer (2013) 132:246–7. doi: 10.1002/ijc.27661
27. Adams S, Robbins FM, Chen D, Wagage D, Holbeck SL, Morse HC, et al. HLA Class I and II Genotype of the NCI-60 Cell Lines. J Transl Med (2005) 3:11. doi: 10.1186/1479-5876-3-11
28. Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2–a Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics (2009) 25:1189–91. doi: 10.1093/bioinformatics/btp033
29. Kunik V, Ashkenazi S, Ofran Y. Paratome: An Online Tool for Systematic Identification of Antigen-Binding Regions in Antibodies Based on Sequence or Structure. Nucleic Acids Res (2012) 40:W521–4. doi: 10.1093/nar/gks480
30. Yousefzadeh MJ, Wyatt DW, Takata K, Mu Y, Hensley SC, Tomida J, et al. Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ. PloS Genet (2014) 10:e1004654. doi: 10.1371/journal.pgen.1004654
31. Ho P, Ede C, Chen YY. Modularly Constructed Synthetic Granzyme B Molecule Enables Interrogation of Intracellular Proteases for Targeted Cytotoxicity. ACS Synth Biol (2017) 6:1484–95. doi: 10.1021/acssynbio.6b00392
32. Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene Therapy Vectors Based on Adeno-Associated Virus Type 1. J Virol (1999) 73:3994–4003. doi: 10.1128/JVI.73.5.3994-4003.1999
33. Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, et al. Adeno-Associated Virus Vectors Can Be Efficiently Produced Without Helper Virus. Gene Ther (1998) 5(7):938–45. doi: 10.1038/sj.gt.3300680
34. Grieger JC, Choi VW, Jude Samulski R. Production and Characterization of Adeno-Associated Viral Vectors. Nat Protoc (2006) 1:1412–28. doi: 10.1038/nprot.2006.207
35. Zah E, Nam E, Bhuvan V, Tran U, Ji BY, Gosliner SB, et al. Systematically Optimized BCMA/CS1 Bispecific CAR-T Cells Robustly Control Heterogeneous Multiple Myeloma. Nat Commun (2020) 11(1):2283. doi: 10.1038/s41467-020-16160-5
36. Dawson NA, Lamarche C, Hoeppli RE, Bergqvist P, Fung VC, McIver E, et al. Systematic Testing and Specificity Mapping of Alloantigen-Specific Chimeric Antigen Receptors in Regulatory T Cells. JCI Insight (2019) 4:e123672. doi: 10.1172/jci.insight.123672
37. Roth TL, Puig-Saus C, Yu R, Shifrut E, Carnevale J, Li PJ, et al. Reprogramming Human T Cell Function and Specificity With Non-Viral Genome Targeting. Nature (2018) 559:405–9. doi: 10.1038/s41586-018-0326-5
38. Vlachakis D, Koumandou VL, Kossida S. A Holistic Evolutionary and Structural Study of Flaviviridae Provides Insights Into the Function and Inhibition of HCV Helicase. Peer J (2013) 1:e74. doi: 10.7717/peerj.74
39. Leem J, Dunbar J, Georges G, Shi J, Deane CM. ABodyBuilder: Automated Antibody Structure Prediction With Data-Driven Accuracy Estimation. MAbs (2016) 8:1259–68. doi: 10.1080/19420862.2016.1205773
40. Szot GL, Koudria P, Bluestone JA. Transplantation of Pancreatic Islets Into the Kidney Capsule of Diabetic Mice. J Vis Exp (2007) 9):404. doi: 10.3791/404
41. Szot GL, Lee MR, Tavakol MM, Lang J, Dekovic F, Kerlan RK, et al. Successful Clinical Islet Isolation Using a GMP-Manufactured Collagenase and Neutral Protease. Transplantation (2009) 88:753–6. doi: 10.1097/TP.0b013e3181b443ae
42. Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A Herceptin-Based Chimeric Antigen Receptor With Modified Signaling Domains Leads to Enhanced Survival of Transduced T Lymphocytes and Antitumor Activity. J Immunol (2009) 183:5563–74. doi: 10.4049/jimmunol.0900447
43. Mulder A, Kardol M, Regan J, Buelow R, Claas F. Reactivity of Twenty-Two Cytotoxic Human Monoclonal HLA Antibodies Towards Soluble HLA Class I in an Enzyme-Linked Immunosorbent Assay (PRA-STAT). Hum Immunol (1997) 56:106–13. doi: 10.1016/s0198-8859(97)00146-8
44. DeWolf S, Grinshpun B, Savage T, Lau SP, Obradovic A, Shonts B, et al. Quantifying Size and Diversity of the Human T Cell Alloresponse. JCI Insight (2018) 3(15):e121256. doi: 10.1172/jci.insight.121256
45. Gress RE, Nathenson SG, Lucas PJ. Fine Specificity of Xenogeneic Antigen Recognition by Human T Cells. Transplantation (1989) 48:93–8. doi: 10.1097/00007890-198907000-00022
46. King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, et al. A New Hu-PBL Model for the Study of Human Islet Alloreactivity Based on NOD-Scid Mice Bearing a Targeted Mutation in the IL-2 Receptor Gamma Chain Gene. Clin Immunol (2008) 126(3):303–14. doi: 10.1016/j.clim.2007.11.001
47. Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 Diabetes Immunotherapy Using Polyclonal Regulatory T Cells. Sci Transl Med (2015) 7:315ra189. doi: 10.1126/scitranslmed.aad4134
48. Boroughs AC, Larson RC, Choi BD, Bouffard AA, Riley LS, Schiferle E, et al. Chimeric Antigen Receptor Costimulation Domains Modulate Human Regulatory T Cell Function. JCI Insight (2019) 5(8):e126194. doi: 10.1172/jci.insight.126194
49. Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 Costimulation: From Mechanism to Therapy. Immunity (2016) 44(5):973–88. doi: 10.1016/j.immuni.2016.04.020
50. Watkins NA, Brown C, Hurd C, Navarrete C, Ouwehand WH. The Isolation and Characterisation of Human Monoclonal HLA-A2 Antibodies From an Immune V Gene Phage Display Library. Tissue Antigens (2000) 55:219–28. doi: 10.1034/j.1399-0039.2000.550305.x
51. Rijkers M, Schmidt D, Lu N, Kramer CSM, Heidt S, Mulder A, et al. Anti-HLA Antibodies With Complementary and Synergistic Interaction Geometries Promote Classical Complement Activation on Platelets. Haematologica (2019) 104:403–16. doi: 10.3324/haematol.2018.201665
52. El-Awar N, Jucaud V, Nguyen A. HLA Epitopes: The Targets of Monoclonal and Alloantibodies Defined. J Immunol Res (2017) 2017:3406230. doi: 10.1155/2017/3406230
53. Wyss L, Stadinski BD, King CG, Schallenberg S, McCarthy NI, Lee JY, et al. Affinity for Self Antigen Selects Treg Cells With Distinct Functional Properties. Nat Immunol (2016) 17:1093–101. doi: 10.1038/ni.3522
54. Levine AG, Arvey A, Jin W, Rudensky AY. Continuous Requirement for the TCR in Regulatory T Cell Function. Nat Immunol (2014) 15:1070–8. doi: 10.1038/ni.3004
55. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ Regulatory T Cells Preserve Graft-Versus-Tumor Activity While Inhibiting Graft-Versus-Host Disease After Bone Marrow Transplantation. Nat Med (2003) 9:1144–50. doi: 10.1038/nm915
57. Han X, Wang M, Duan S, Franco PJ, Kenty JH, Hedrick P, et al. Generation of Hypoimmunogenic Human Pluripotent Stem Cells. Proc Natl Acad Sci USA (2019) 116:10441–6. doi: 10.1073/pnas.1902566116
Keywords: chimeric antigen receptor, regulatory T cells, genome editing, transplantation, humanized mouse model, immune tolerance, HLA, Treg
Citation: Muller YD, Ferreira LMR, Ronin E, Ho P, Nguyen V, Faleo G, Zhou Y, Lee K, Leung KK, Skartsis N, Kaul AM, Mulder A, Claas FHJ, Wells JA, Bluestone JA and Tang Q (2021) Precision Engineering of an Anti-HLA-A2 Chimeric Antigen Receptor in Regulatory T Cells for Transplant Immune Tolerance. Front. Immunol. 12:686439. doi: 10.3389/fimmu.2021.686439
Received: 26 March 2021; Accepted: 26 August 2021;
Published: 20 September 2021.
Edited by:
Nirupama Darshan Verma, University of New South Wales, AustraliaReviewed by:
Jean-Christophe Bories, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2021 Muller, Ferreira, Ronin, Ho, Nguyen, Faleo, Zhou, Lee, Leung, Skartsis, Kaul, Mulder, Claas, Wells, Bluestone and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qizhi Tang, cWl6aGkudGFuZ0B1Y3NmLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.