- 1Research Service, Department of Veterans Affairs Medical Center, Memphis, TN, United States
- 2Department of Medicine, University of Tennessee Health Science Center, Memphis, TN, United States
- 3School of Molecular Sciences, The University of Western Australia, Perth, WA, Australia
- 4Department of Pathology, University of Alabama at Birmingham, Birmingham, AL, United States
- 5Research Service, Department of Veterans Affairs Medical Center, Birmingham, AL, United States
The ability to use large doses of vitamin D3 (D3) to chronically treat autoimmune diseases such as rheumatoid arthritis (RA) is prohibitive due to its calcemic effect which can damage vital organs. Cytochrome P450scc (CYP11A1) is able to convert D3 into the noncalcemic analog 20S-hydroxyvitamin D3 [20S(OH)D3]. We demonstrate that 20S(OH)D3 markedly suppresses clinical signs of arthritis and joint damage in a mouse model of RA. Furthermore, treatment with 20S(OH)D3 reduces lymphocyte subsets such as CD4+ T cells and CD19+ B cells leading to a significant reduction in inflammatory cytokines. The ratio of T reg cells (CD4+CD25+Foxp3+ T cells) to CD3+CD4+ T cells is increased while there is a decrease in critical complement-fixing anti-CII antibodies. Since pro-inflammatory cytokines and antibodies against type II collagen ordinarily lead to destruction of cartilage and bone, their decline explains why arthritis is attenuated by 20(OH) D3. These results provide a basis for further consideration of 20S(OH)D3 as a potential treatment for RA and other autoimmune disorders.
Introduction
Rheumatoid arthritis (RA) is one of the more common autoimmune diseases (affecting approximately 0.5-1% of the world’s population (1, 2). Although, multiple diathroidial joints are the main targets of autoimmune attack, other organ systems are also involved, reducing life expectancy by about 10 years in patients with RA (3, 4). Standard disease modifying anti-rheumatic drugs, (DMARDs), such as methotrexate (MTX) and “biologics” that target specific cytokines (e.g., TNFα) or surface molecules on immune cells (e.g., CTLA-4 on T cells), do reduce both joint damage and certain systemic complications of RA. However, their use occasionally triggers adverse events such as increased infections and development of certain neoplasms, other autoimmune diseases, or interstitial lung disease (5–8). Clearly, safer and effective therapeutic agents for RA are needed.
In humans, vitamin D3 (D3 aka cholecalciferol) is synthesized in the skin via ultraviolet B (UVB) induced photoconversion of 7-dehydrocholestrol (7-DHC), a precursor to cholesterol (9, 10). D3, which is inert as a pro-hormone, undergoes two hydroxylation steps: first in the liver (or skin itself) by a 25-hydroxylase (CYP2R1 or CYP27A1) to form 25(OH)D3, and then in the kidney and other tissues by 25-hydroxyvitamin D-1 alpha hydroxylase (CYP27B1) to form 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) (11–15). 1,25(OH)2D3 exerts positive or negative influences on D3-dependent gene expression by binding to the ligand binding domain of the vitamin D receptor (VDR) (16). A heterodimer is then formed within the retinoic acid X receptor (RXR), which, (with the contributions of basal transcriptional machinery plus coactivators and corepressors) forms transcription complexes at vitamin D response elements (VDRE) of target genes (17).
1,25(OH)2D3 is the most extensively characterized active naturally occurring D3 metabolite that, not only systematically regulates calcium homeostasis and bone metabolism, but also possesses immunomodulatory properties. Clinically, normal D3 level is associated with better outcomes in patients with a variety of autoimmune diseases (18–21). In RA, disease activity, C reactive protein and disability scores are inversely related to serum levels of 25(OH)D, and anticyclic citrullinated peptide antibody positivity in RA patients is correlated with D3 insufficiency [25(OH)D, 21-29 ng/ml] and deficiency [25(OH)D <20 ng/ml] (18, 22–25). Furthermore, the VDR Fok1 polymorphism may confer susceptibility to RA in Europeans and Native Americans (26, 27). These observations suggest D3 may have salutary effects in RA. Earlier studies demonstrated 1,25(OH)2D3 inhibited arthritis in the type II collagen (CII)-induced arthritis (CIA) model of RA in mice fed a low calcium diet to protect against development of hypercalcemia (28). Unfortunately, 1,25(OH)2D3 or its precursors, 25(OH)D3 or D3 (cholecalciferol), induce hypercalcemic toxicity when given chronically at the pharmacological doses needed to maximally suppress arthritis and autoimmunity, limiting the amounts that can be given chronically to patients to treat autoimmune diseases such as RA.
We have discovered a novel pathway of D3 metabolism operative in humans, mediated by cytochrome P450scc (CYP11A1), which is modified by CYP27B1, that generates additional biologically active products (29–31). These are at least as potent as classical 1,25(OH)2D3 when tested in vitro and in vivo in several model systems and, like 1,25(OH)2D3, bind to the VDR (32–37). The main and first product of the pathway, 20S(OH)D3, in contrast to 25(OH)D3 or 1,25(OH)2D3, is nontoxic (i.e., noncalcemic and nontoxic to the hematopoietic system, liver, kidney, and heart) at doses as high as 60 µg/kg, while 25(OH)D3 or 1,25(OH)2D3 induce hypercalcemia at doses ≤2 µg/kg (38–41). Finally, 20S(OH)D3 is produced in vivo, being present in human serum at 1/20th the concentration of classical 25(OH)D3 (31) and has also been detected in honey (42). The cytochrome SCC enzymes are unusual in that they hydroxylate vitamin D3 to produce 20S-hydroxyvitamin D3 [20(OH)D3] rather than cleaving the side chains of D3 (43). These properties define 20S(OH)D3 as a hither to unrecognized novel endogenous regulator/natural product.
In the present study, CIA in DBA/1 mice (the most widely studied animal model of RA) was used to collect preclinical data on 20S(OH)D3 as a potential treatment for human RA. When immunized with bovine CII, DBA/1 mice rapidly develop an anti-CII Th1 and Th17 T cell (IFNγ, IL-2, GM-CSF, TNFα, and IL-17) and later a B cell response characterized by production of IgG1 and complement fixing IgG2a antibodies to CII that triggers inflammation (44–49). At about 3 weeks post immunization, arthritis (which histologically resembles RA) begins to develop in peripheral joints (44, 46, 50–52). As time passes arthritis develops in more joints and with greater degrees of inflammation and damage. We demonstrated that treatment of mice with CIA with 20S(OH)D3 reduces the severity of clinical arthritis accompanied by reduction in joint destruction, in serum anti-CII antibodies, in lymphoid organ CD4+ T and CD19+ B cells, and production by cultured draining lymph node cells of TH1, TH17, and inflammatory cytokines and chemokines.
Materials and Methods
Production and Purification of 20(OH)D3
20S(OH)D3 was generated by enzymatic hydroxylation of D3 catalyzed by CYP11A1 as previously described by our group (29, 53). Briefly, 10 mM D3 in 45% 2-hydroxpropyl-β-cyclodextrin was prepared. Buffer comprising 20 mM HEPES (pH 7.4), 100 mM NaCl, 0.1mM dithiothreitol, 2 µM CYP11A1, 0.1 mM EDTA, 0.3 µM adrenodoxin reductase, 10 µM adrendoxin, 2 mM glucose 6-phosphate, 2U/ml glucose 6-phosphate dehydrogenase, and 50 µM NADPH was mixed with the D3 stock solution rendering a final D3 concentration of 200 µM and 0.9% concentration of 2-hydroxypropyl-β-cyclodexrtin. Following 8 min pre-incubation, the reaction was initiated by adding NADPH, after which the samples were incubated for 3 h at 37°C with gentle shaking. Ice cold dichloromethane (20 ml) was then added to stop the reactions after which the reaction products were extracted with dichloromethane as previously described (53–55). The 20(OH)D3 product was purified by preparative thin-layer chromatography, followed by reverse phase high performance liquid chromatography, as previously described (55). Routinely 0.3 mg of purified 20S(OH)D3 was recovered from 12.5 ml of the starting incubation mixture. Aliquots of the purified 20S(OH)D3 were dried under nitrogen and stored at -80°C until used.
Mice
Female DBA/1 Lac J mice, age 6 weeks old (Jackson Laboratories, Bar Harbour, ME) were housed in a pathogen-free AAALAC-approved animal care facility at the University of Tennessee Health Science Center (UTHSC) and Department of Veterans Affairs Medical Center (VAMC), Memphis, TN. Mice were fed regular laboratory chow and water ad libitam, and housed under a 12 h light and 12 h darkness cycle. Animal protocols for the study were approved by the Institutional Animal Care and Use Committees at UTHSC and VAMC Memphis.
Induction and Assessment of CIA
Mice were immunized with native bovine CII prepared (as previously described from fetal calf articular cartilage) (47). Groups of 12 mice (for assessment of arthritis development) or groups of 5 or 6 mice (for other studies) were administered different amounts of 20S(OH)D3 dissolved in sterile sesame oil (S.O) or propylene glycol [(PG) (Sigma-Aldrich, St. Louis, MO)]. The S.O. or PG, with-or-without 20S(OH)D3, was administered daily intraperitoneally (i.p.) or via daily oral gavage in volumes of 50 µl and 100 µl, respectively. For studies using PG to solubilize 20S(OH)D3, the PG, with-or-without 20S(OH)D3, was diluted 1:5 by volume with sterile normal saline, and 100 µl was administered daily by oral gavage. Rheumatrex tablets (DAVA Pharmaceuticals, Inc., Fort Lee, NJ) were used as a source of methotrexate sodium. Methotrexate 2.5 mg/kg was administered in 100 µl normal saline weekly by oral gavage. The tablets were crushed and solubilized in sterile normal saline.
Arthritis severity was assessed in each paw every other day by two observers (one of whom was blinded to treatment) using the following scale: 0=no swelling or redness, 1=slight swelling and redness, 2=moderate swelling or redness, 3=marked swelling and redness, and 4=marked swelling and redness with some deformity (47). For histological assessment of joint tissue, mice were euthanized, all paws were removed, decalcified, processed and scored histologically, as previously described (56). Evaluation of each joint was done in a blinded manner using 4 parameters (0–3 scale for each parameter): synovial inflammation and thickness, synovial leukocyte invasion into the joint, cartilage unevenness caused by inflammation related cartilage damage, and subchondral bone erosion. The total histologic score represented the sum of the 4 parameters. The maximal histologic score per mouse paw was 12 and 48 per mouse.
Quantitation of Anti-CII Antibodies in Sera
Specific murine IgG1, IgG2a, and Ig2b anti-CII antibodies were quantitated in sera using a commercial ELISA, according to the manufacturer’s instructions (Chondrex, Redwood, WA).
Flow Cytometric Assessment of Lymphoid Cells
Isolated cells from spleen or draining para aortic, popliteal or inguinal lymph nodes were labeled as follows: Alexa-700 labeled rat anti-mouse CD3, PE-Cy7-labeled rat anti-mouse CD25, Per CP-Cy5.5-labeled rat anti-mouse CD4, FoxP3 Tregs were detected by FoxP3 staining kit, and FITC-labeled rat anti-mouse CD-19 (BD Bioscience, San Diego, CA). Flow cytometry was performed on a SORP BD LSRII instrument and results analyzed by FlowJo.
Cytokine Quantitation
Draining lymph node cells were isolated and cultured at 2x106 cells/ml in RPMI 1640 medium containing 9% fetal calf serum, penicillin 100 u/ml, streptomycin 100 µg/ml, 1% glutamax, 1% pyruvate, and 1% 2-mercaptoethanol. Supernatants were collected after 48 h or 120 h of culture and analyzed by multiplex sandwich immunoassay (Bio-plex mouse cytokine/chemokine kits, Bio Rad) for levels of different cytokines using a Luminex instrument according to the manufacturer’s protocol.
Quantitation of Total Serum Calcium
Levels of calcium in mice serum were quantitated by atomic absorption spectroscopy as previously described (57).
Statistical Analyses
Differences between groups were analyzed using 2 way RM ANOVA when multiple comparisons were made, Student’s 2-sample t-test was used for single comparisons between groups with normally distributed data, or by Mann-Whitney rank sum test for data not normally distributed. The level of significance was set at P < 0.05.
Results
20S(OH)D3 Treatment Suppresses Development of CIA and Associated Joint Damage
To determine whether 20S(OH)D3 treatment started day 14 post CII immunization, after T cell priming to CII is firmly established, would suppress the development of CIA, two groups of mice (12 per group) were immunized with CII and from days 14-to-40 post immunization treated daily with i.p. injections of sterile 50 µl S.O. or 50 µl S.O. containing 20S(OH)D3 at a dose of 2.4 µg/kg/day. The 20S(OH)D3 treatment markedly reduced the mean arthritis severity score (Figure 1A) and arthritis incidence (Figure 1B). The curves comparing drug to vehicle are roughly parallel, suggesting that the greatest effect is on the magnitude of the immune response rather than elicitation of the response. The histologic scores reflecting inflammation, cartilage damage and subchondral bone erosion in joints harvested at sacrifice on Day 40 post CII immunization were significantly reduced in 20S(OH)D3 treated versus S.O. treated CIA mice (Figure 1E), reflecting the ability of 20(OH)D3 to protect joints from damage in this model.
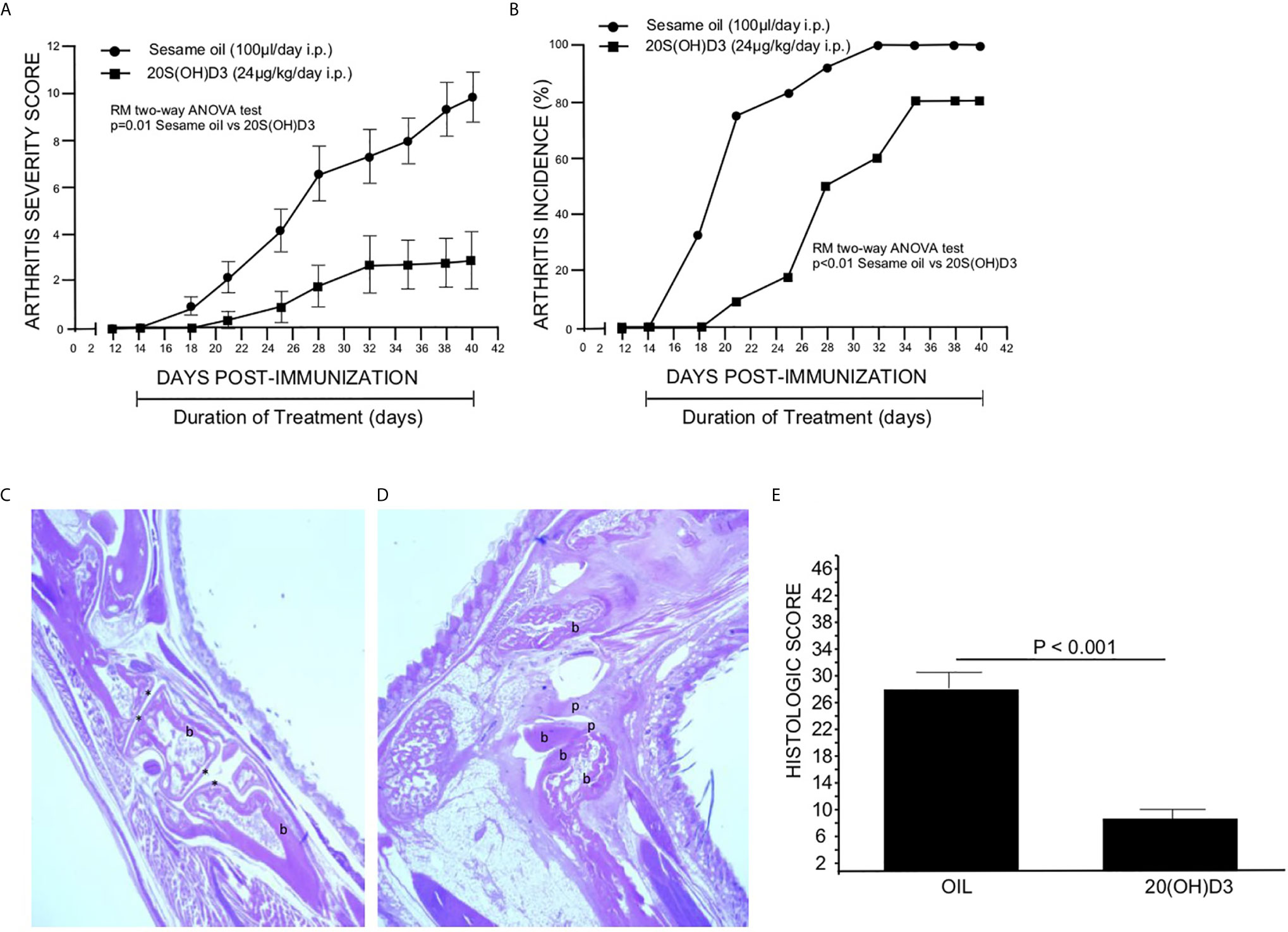
Figure 1 20S(OH)D3 suppresses CIA: 24 DBA/1 Lac J female mice at 6 wks of age were immunized with bovine CII and 14 days later 12 mice were treated daily with intraperitoneal (i.p.) 50 µl S.O. and 12 mice with 50 µl S.O. containing 2.4 µg/kg 20S(OH)D3. Arthritis severity (A) and incidence (B) were assessed by a blinded observer and each paw was scored on a scale of 0 to 4 with 16 being the maximal score per mouse. All mice survived to day 40. On Day 40, mice were euthanized, sera obtained, and paws processed for histology and scored histologically as described in Methods. An H&E stained section of the decalcified hind paw of a mouse treated with 20S(OH)D3 shows preservation of joint structures with minimal inflammation (10X magnification, b, bone, * identifies joint space) (C) while S.O.-treated mouse hind paws showed marked joint destruction with extensive loss of cartilage and bone with invasion of pannus (20X magnification, b, bone; p, pannus) (D). 20S(OH)D3- treated CIA mice had a lower histologic score than S.O. treated CIA mice (E). Photographs of typical paws for the scoring system we used are available online at the Hooke Laboratories Web Site (https://hookelabs.com/services/cro/cia/MouseCIAscoring.html).
Representative images of hematoxylin and eosin-stained sections of hind paws harvested on Day 40 post CII immunization show marked destruction of joint structures in S.O. treated CIA mice (Figure 1D), while joint structure was maintained in mice with CIA treated with 20S(OH)D3 (Figure 1C). Aliquots of sera were subjected to analysis of calcium content by atomic absorption spectroscopy. There was no difference in levels of serum calcium between 20S(OH)D3- and S.O.-treated mice with CIA (S.O. = 9.50 ± 0.50 mg/dL, 20S(OH)D3 = 9.57± 0.50 mg/dL, p = NS). This is compatible with our earlier report that 20S(OH)D3 does not induce hypercalcemia at doses up to 60 µg/kg when administered to C57BL/6 daily by i.p. injections for 21 days (39).
20S(OH)D3 Treatment Reduces Levels of Serum Antibodies to CII
Earlier studies demonstrated that the generation of complement fixing anti-CII antibodies is essential for development of CIA (58, 59). The major complement fixing anti-CII antibodies generated in DBA/1 mice immunized with CII are of the IgG2a subclass (59). However, the less potent complement fixing anti CII IgG1 and anti CII Ig G2b antibodies are also generated (59). Therefore, it was essential to assess whether 20S(OH)D3 versus S.O. vehicle treatment of mice immunized with CII developed less IgG2a and IgG1 anti-CII antibodies. On Day 40, sera from mice in Figure 1A were analyzed for anti-CII specific antibodies by a commercial ELISA kit from Chondrex specific for IgG1, IgG2a and Ig2b anti-CII antibodies. Significant reductions in serum levels of anti-CII antibodies of the IgG1 (Figure 2A) and IgG2a (Figure 2B) subclasses (P=0.017 and P=0.03), respectively occurred in 20S(OH)D3 treated mice compared to S.O. treated mice with CIA. However, IgG2b anti-CII antibody levels were not significantly different between 20S(OH)D3- and S.O.- treated cohort with CIA (data not shown). These reduced complement fixing anti-CII antibodies likely resulted in reduced arthritis severity observed in mice treated with CIA treated with 20S(OH)D3.
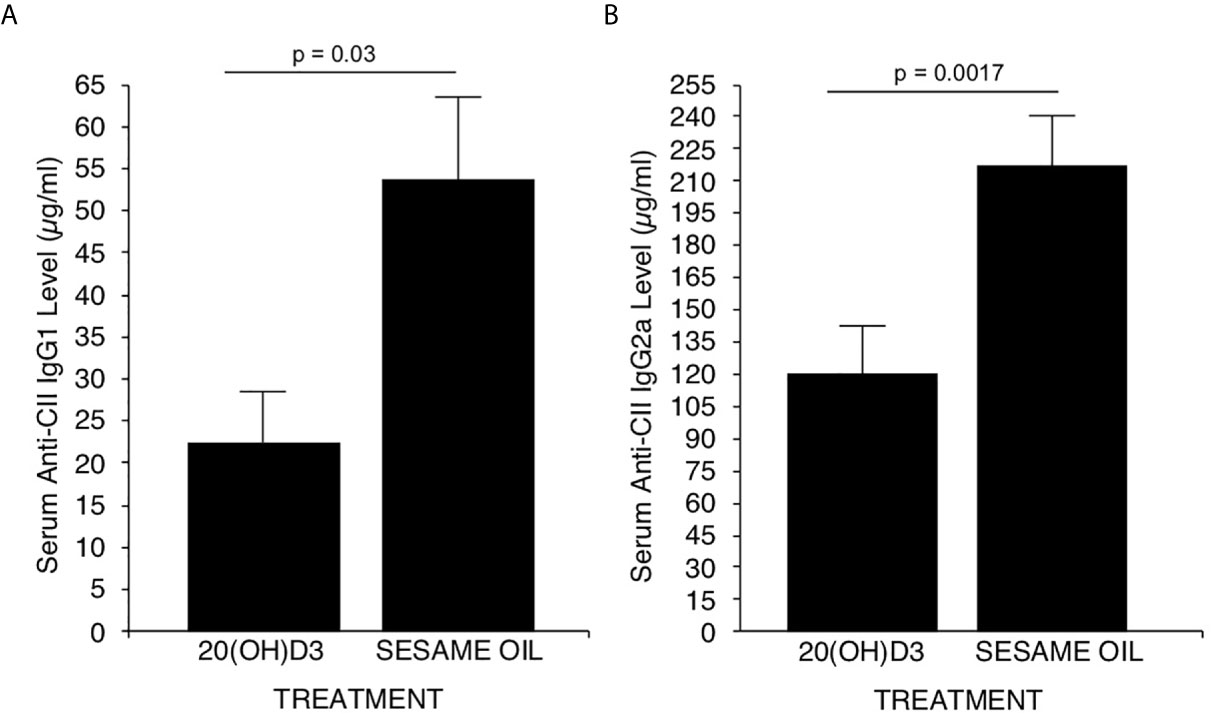
Figure 2 20(OH)D3 reduces serum levels of anti-CII antibodies in CIA mice: Aliquots of sera harvested at day 40 post-immunization with CII from mice described in Figure 1A were analyzed for levels of CII specific murine IgG1, IgG2a and IgG2b antibodies as described in Methods. 20S(OH)D3 treatment significantly reduced levels of anti CII IgG1 and IgG2a (A, B) but not of anti-CII IgG2b antibodies (data not shown).
20S(OH)D3 Treatment Modulates Production of Cytokines and Chemokines by Cultured Draining LN Cells
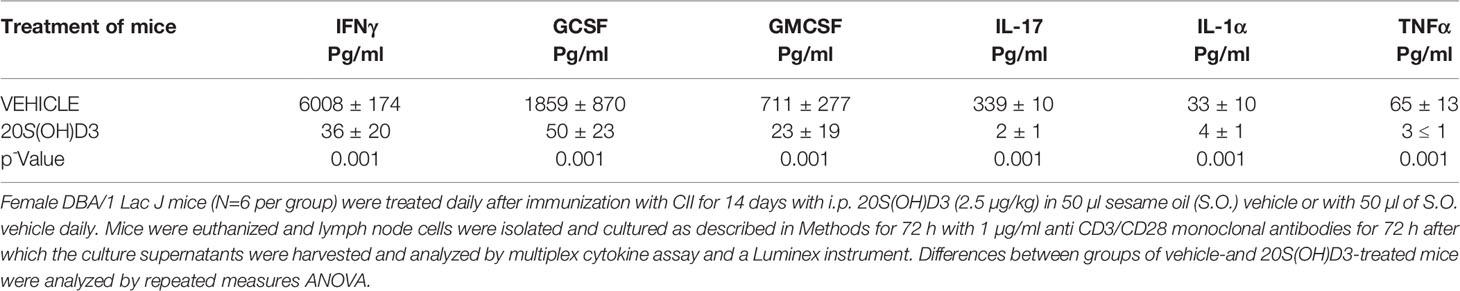
Popliteal and inguinal lymph nodes contain immune cells that traffic to and from the joints in the hind limbs of mice with CIA (50, 60). Cytokine and chemokine production by draining LN cells from mice with CIA, in part, reflect production of cytokines and chemokines by immune cells in arthritic joints (61). To assess whether cytokine and/or chemokine production are changed by treatment of mice with CIA with 20S(OH)D3, groups of 9 mice immunized with CII were treated beginning on the day of CII immunization with i.p. administration of 2.4 µg/kg 20S(OH)D3 or S.O. daily for 14 days. The mice were then euthanized and isolated popliteal and inguinal LN cells were cultured for 48 h to allow cytokines and chemokines to be released into the culture medium. Levels of Th1, Th2, Th17, and inflammatory cytokines were significantly reduced in the supernatants of the draining LN from mice treated with 20S(OH)D3 vs S.O. vehicle (Table 1). Similar reductions in production of these types of cytokines were observed when we cultured spleen cells from a similarly treated different group of CIA mice (data not shown).
Treatment of CIA Mice With 20S(OH)D3 Reduces Percentages of CD4+ T Cells and CD19+ B Cells From Draining LN Cells and Increases T Regulatory (Treg) CD4+T Cell Ratio
Since arthritis in mice with CIA results from a contribution by CD4+ T cells and B cells which may be suppressed by CD4+CD25+Foxp3+ Tregs (62), it was important to determine whether percentages of these cell populations were changed by 20S(OH)D3 treatment. Groups of DBA/1 mice were immunized with CII and treated with 20S(OH)D3 (N=12) or S.O. (N=12) following the same protocol as in the experiment depicted in Figure 1A, except mice were euthanized on Day 14 post CII immunization. Draining LN cells were isolated and subjected to analysis by flow cytometry as described under Materials and Methods. The mice treated with 20S(OH)D3 had reduced percentages of CD3+ CD4+ T cells and CD19+ B cells (P=0.004 and P=0.004, respectively) (Table 2). We also assessed percentages of CD4+CD25+ Foxp3+ Tregs in this experiment and found no absolute change in the percentage of these Tregs in the draining LN population (data not shown). However, the ratio of CD4+CD25+ Foxp3+ Tregs to CD3+ CD4+ T cells in mice with CIA treated with 20S(OH)D3 increased, indicating the equilibrium was shifted in favor of less CD3+ CD4+ T cells compared to this type of Treg (Table 2).
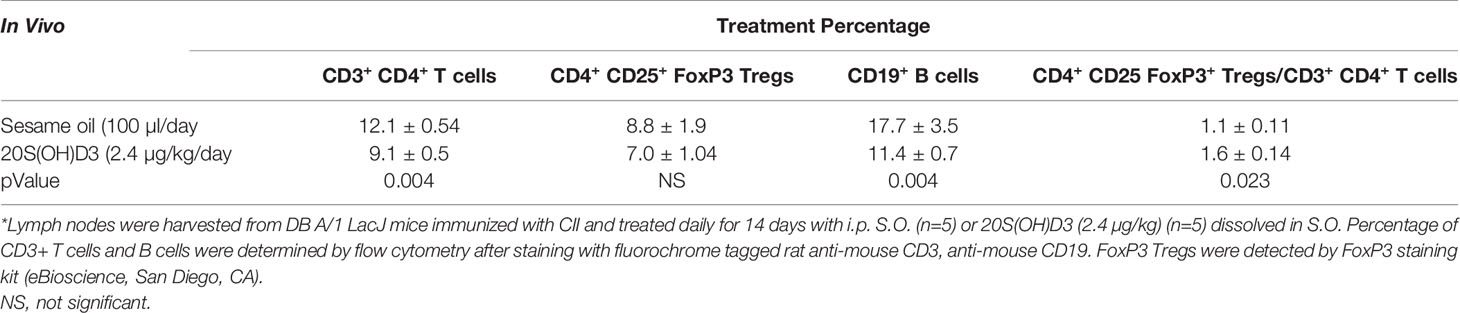
Table 2 Treatment of CIA mice with 20S(OH)D3 reduces percentages of CD 4+ T cells and B cells in draining lymph nodes while elevating FoxP3 Treg/CD3+ CD4+ ratio*.
20S(OH)D3 Administered by Oral Gavage Suppresses Development of CIA
Since 20S(OH)D3 would be given via the oral route to humans with RA, if eventually approved to treat this disease, we evaluated whether CIA would be suppressed if 20S(OH)D3 were administered via the oral route using gavage and how it compared to methotrexate in its ability to suppress CIA. Groups of DBA/1 mice (N=10-12 per group) were immunized with CII and were assigned to different treatments as follows: daily oral gavage 100 µl 1:5 diluted PG containing 15 µg/kg 20S(OH)D3 and 100 µl normal saline by oral gavage every 7 days daily oral gavage 100 µl 1:5 diluted PG and 100 µl normal saline by oral gavage every 7 days; and daily oral gavage 100 µl 1:5 diluted PG and every 7 days by oral gavage methotrexate (MTX) 2.5 mg/kg dissolved in 100 µl normal saline. Treatments began at Day 13 Post CII immunization of the mice and continued through 48 days post CII immunization. Arthritis Severity Scores were significantly lower in 20S(OH)D3- and MTX-treated mice than in PG saline vehicle–treated mice (Figure 3A). The incidence of arthritis (percentage of mice with one or more arthritic joints) was also significantly lower in 20S(OH)D3–treated mice, but not in MTX-treated mice (Figure 3B). This experiment demonstrates that, like MTX (a commonly used medication to treat RA), 20S(OH)D3 can suppress CIA when given via the oral route.
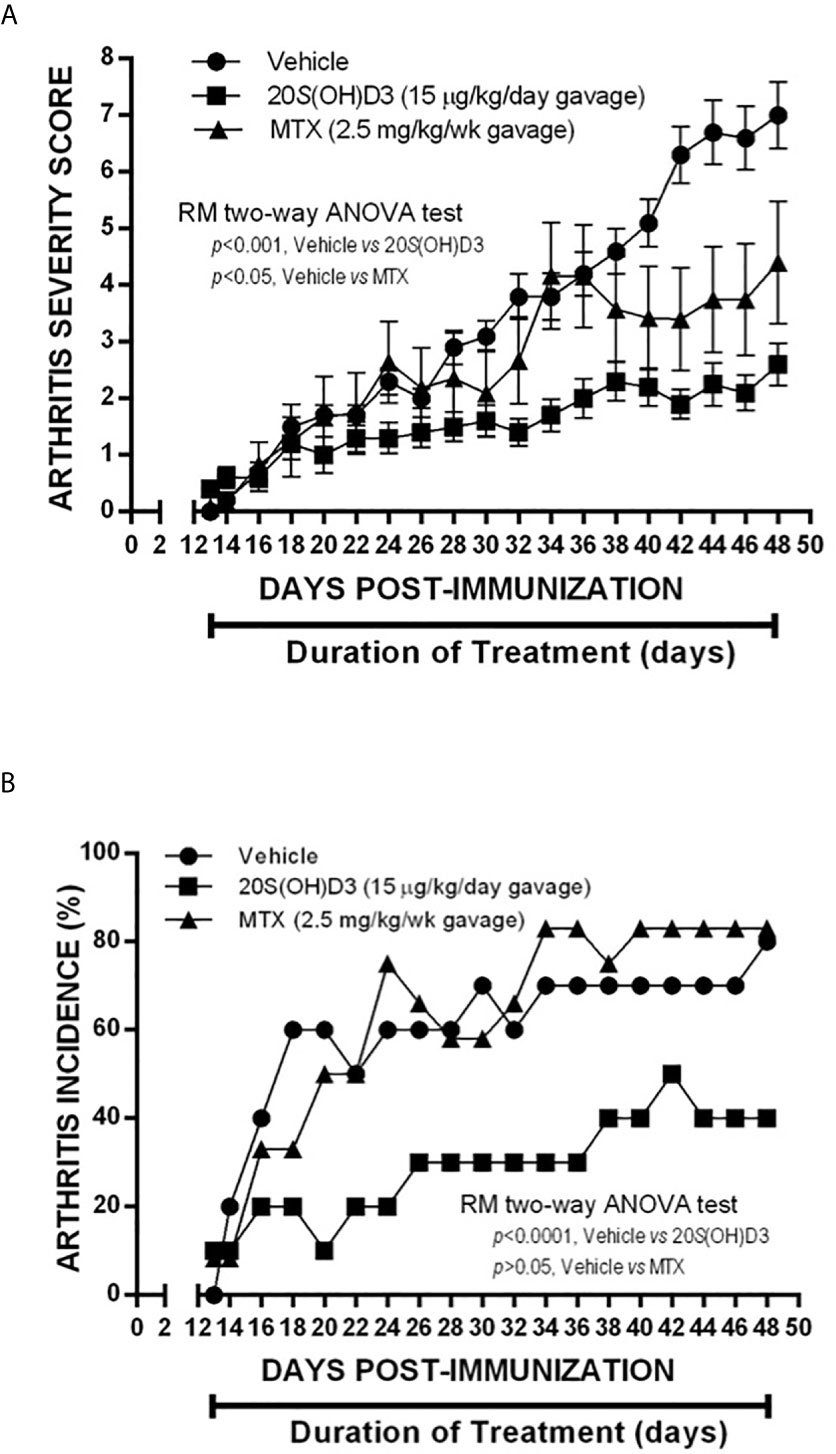
Figure 3 Suppression of CIA by 20S(OH)D3 given by gavage: 36 DBA/Lac J female mice 6 weeks of age were immunized with bovine CII (Day 0) and groups of 12 were gavaged orally beginning on day 13 post CII immunization with daily 15 µg/kg 20S(OH)D3 in 50 µl 1:5 diluted PG and 50 µl normal saline by oral gavage every 7 days; oral gavage daily with 50 µl 1:5 diluted PG and every 7 days by oral gavage with MTX 2.5 mg/kg dissolved in 50 µl normal saline; or oral gavage daily with 50 µl 1:5 diluted PG and 50 µl normal saline by oral gavage every 7 days. Treatment began at Day 13 after CII immunization and continued through 48 days post CII immunization. Arthritis severity (A) and arthritis incidence (B) were assessed every 2 days. All mice survived until termination of the experiment.
20S(OH)D3 Administered by Gavage Suppresses Established Arthritis in CIA Mice
To determine whether 20S(OH)D3 would suppress arthritis severity when started later after CII immunization when arthritis is firmly established, we immunized 24 mice with CII and waited until arthritis was present to begin treatment with 20S(OH)D3 and S.O. Beginning Day 21, after CII immunization when the mean arthritis severity score was 2.4, we treated 12 of the mice with S.O. administered by daily gavage and 12 with S.O. containing 20S(OH)D3 administered by daily gavage at a dose of 30 µg/kg/day. After 46 days treatment, mice were euthanized. As shown in Figure 4, mice treated with 20S(OH)D3 had less severe arthritis, demonstrating 20S(OH)D3 can suppress CIA during the inflammatory phase when anti-CII antibodies play a major role in mediating inflammation in the joints (59, 63). On Day 21 under the conditions we employ, arthritis is highly inflammatory and driven by complement fixing anti-CII antibodies. This suggests 20S(OH)D3 also has anti-inflammatory effects.
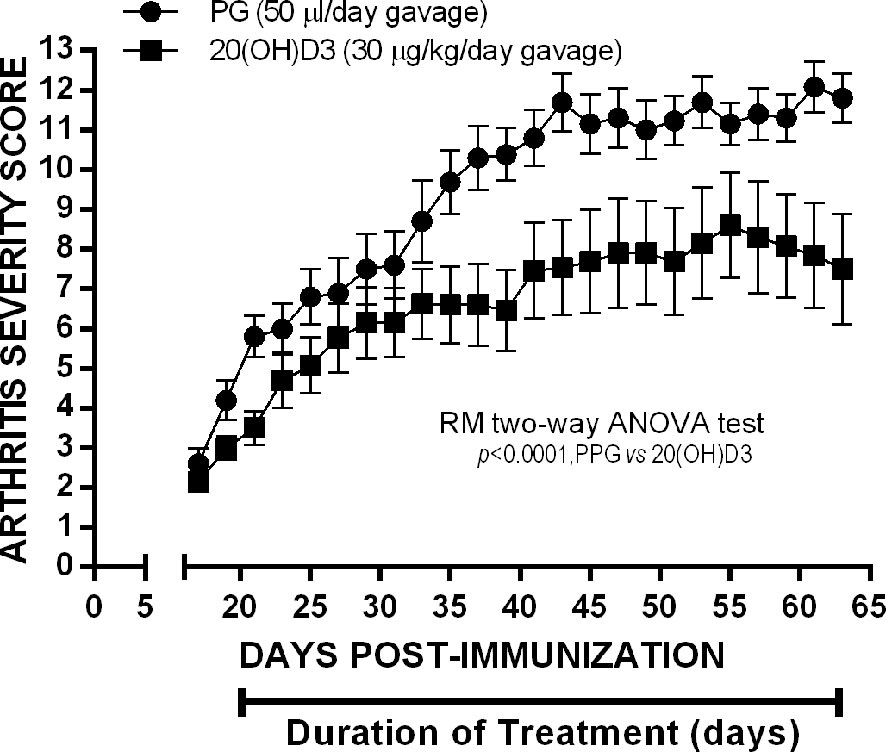
Figure 4 20S(OH)D3 given by oral gavage Suppresses established arthritis: 24 6 wk old DBA/1 Lac J female mice were immunized on day 0 with CII and scored for arthritis by a blinded observer every other day for 64 days post CII immunization. Treatment was started day 20 post CII immunization by daily oral gavage with (N=12) 100 µl/day PPG 1:5 dilution in sterile saline (N=12) or 100µl of the PPG 1:5 dilution containing 30 µg/kg 20S(OH)D3.
Discussion
This is the first demonstration that a natural noncalcemic D3 analog, 20S(OH)D3, which is normally produced in humans, suppresses the CIA model of human RA, both clinical arthritis and joint destruction, providing a rationale for further consideration of 20S(OH)D3 as a potential mono or adjunctive therapy for RA and other autoimmune diseases. It must be noted that CYP11A1 is also expressed outside the classical steroidogenic organs including skin (64, 65), brain, gastrointestinal tract (66, 67), and immune system (68–71). This suggests that 20S(OH)D3 may also be produced by the immune cells. Furthermore, 20S(OH)D3 has been detected in honey identifying it as a natural product (42). The mechanisms by which 20S(OH)D3 downregulates arthritis severity in the CIA model is likely related to reduction in CD4+ T cells, CD19+ B cells, anti-CII antibodies, and maintenance of CD4+CD24+FoxP3+Tregs. Treatment with 20(OH) D3 leads to a significant reduction in inflammatory cytokines, likely caused by reduction in the numbers of CD4+ T cells together with an increase in the ratio of T reg cells (CD4+CD25+Foxp3+ T cells) to CD3+CD4+ T cells. The decrease in arthritis was also accentuated by a decrease in critical complement-fixing anti-CII antibodies together with a reduction in the number of CD19+ B cells. Since pro-inflammatory mediators interact to produce an inflammatory cascade and antibodies against type II collagen lead to destruction of cartilage and bone, these data explain why arthritis is attenuated by 20(OH) D3. These results have some similarities to results obtained using a natural plant product (72). The currently used and/or FDA approved therapeutics to treat RA have the potential to cause mild-to-severe life-threatening adverse events such as bacterial, fungal, or mycobacterium tuberculosis infections, neoplasms such as skin cancers and lymphoma, vasculitis, SLE, MS, and interstitial lung disease, etc (73–75).
20S(OH)D3 is produced in vivo by the hydroxylation of D3 by CYP11A1 and is non-calcemic in rats and mice (38–41). The serum levels in normal humans of 20S(OH)D3 is approximately 5% of 25(OH)D3. In preclinical studies on C57BL6 mice treated with 20S(OH)D3 up to 60 µg/kg given i.p. daily for 3 weeks, there was no evidence of hematologic, renal, or liver toxicity (39). In addition, 20S(OH)D3 in vitro exhibited anti-inflammatory and pro-differentiatory effects on epidermal cells (32, 34, 76, 77). In contrast, C57B/L6 mice given either 2 µ/kg 1,25(OH)2D3 or 25(OH)D3 i.p. daily for 3 weeks displayed hypercalcemia (41). This hypercalcemic property of 1,25(OH)2D3 and 25(OH)D3 markedly limits the dosages that can be safely administered to humans on a chronic basis that would be required to treat autoimmune diseases such as RA (78). 20S(OH)D3 in addition to inhibiting CIA, shares some other biological properties with 1,25(OH)2D3 (32). 20S(OH)D3 like 1,25(OH)2D3 inhibits collagen synthesis by dermal fibroblasts in vitro, and at a dose of 3 µ/kg in vivo inhibits fibrosis induced by repeated subcutaneous injection of bleomycin into mice (36). 20S(OH)D3 also like 1,25(OH)2D3 inhibits growth of melanoma cells in vitro (37, 40, 79), and it inhibits growth of melanoma at a dose of 30 µg/kg applied daily in vivo (37). This is in addition to the aforementioned anti-cancer, pro-differentiation and photoprotective activities of 20S(OH)D3 in cells of different origins (80–82). Of significance are anti-inflammatory and immunomodulatory (downregulation of T-cell responses) properties of 20S(OH)D3 in conjunction with its ability to decrease NF-kB activity by increasing IkBα levels and inhibiting translocation of NF-kB to the nucleus (76, 81) and inhibit production of IL-17, interferon-γ, TNF-α, and IL-2 (32, 83) and inverse agonism on RORγ (77). These immunomodulatory properties are consistent with the beneficial effect of 20S(OH)D3 in the CII-induced arthritis model of RA reported in this paper.
In addition, 20S(OH)D3, in contrast to the classical 1,25(OH)2D3, is non-calcemic and acts as a biased agonist on the VDR having different interactions with the ligand binding domain in comparison to 1,25(OH)2D3 or 1,20(OH)2D3 (83, 84). Furthermore, 20S(OH)D3 acts as an inverse agonist on RORα and γ (77, 84) and acts as an agonist on the aryl hydrocarbon receptor (AhR) (85). Defining the relative contribution of these nuclear receptors (80, 86) to the reported attenuation of the RA will be addressed in the future studies using transgenic mice with silenced VDR, RORs and AhR receptors.
In summary, we provided for the first time preclinical evidence that 20S(OH)D3 can significantly attenuate the progression of arthritis in a murine model of RA in vivo through suppression of immune responses by T and B-cells. Thus, we synthesized a novel non-calcemic and nontoxic vitamin D3 hydroxyderivative and demonstrated it to be an excellent candidate for clinical trials in RA and other autoimmune diseases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by University of Tennessee IACUC Committee.
Author Contributions
AP and AS conceived and designed the study. RT, TK, WL, SB, LM, and DB contributed data. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by NIH grants 1RO1 AR052190 and 1R21 AR066505 to AS and AP, 1R01AR073004-01A1, R01AR071189-01A1 and VA merit grant 1I01BX004293-01A1 to AS, and VA Program Project Grant IP1BX001607 to AP and R21 AI149267 to CR and AS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the excellent technical assistance of Patricia Wheller, David Cullins and Jiwen Luo. The assistance of Angela Cody in preparing the draft of this manuscript is gratefully acknowledged.
In Memoriam
This article is dedicated to our distinguished colleague, the late Professor Arnold E. Postlethwaite, in loving memory of his humanity, humility,integrity and seminal contributions to the broad field of Rheumatology, spanning over five decades.
References
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid Arthritis. Lancet (2016) 388:2023–38. doi: 10.1016/S0140-6736(16)30173-8
2. Minichiello E, Semerano L, Boissier MC. Time Trends in the Incidence, Prevalence, and Severity of Rheumatoid Arthritis: A Systematic Literature Review. Joint Bone Spine (2016) 83:625–30. doi: 10.1016/j.jbspin.2016.07.007
3. Myllykangas M, Pekkanen J, Rasi V, Haukkala A, Vahtera E, Salomaa V. Haemostatic and Other Cardiovascular Risk Factors, and Socioeconomic Status Among Middle-Aged Finnish Men and Women. Int J Epidemiol (1995) 24:1110–6. doi: 10.1093/ije/24.6.1110
4. Kvien TK. Epidemiology and Burden of Illness of Rheumatoid Arthritis. Pharmacoeconomics (2004) 22:1–12. doi: 10.2165/00019053-200422001-00002
5. Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial Lung Disease Induced by Drugs and Radiation. Respiration (2004) 71:301–26. doi: 10.1159/000079633
6. Nalbant S, Ozyurt M, Yildirim M, Kuskucu M. Pulmonary Tuberculosis and Tuberculous Arthritis of Knee Joint Associated With Rheumatoid Arthritis Treated With Anti-Tumor Necrosis Factor (TNF)-Alpha Medication: A Case Report. Rheumatol Int (2012) 32:2863–6. doi: 10.1007/s00296-011-2119-0
7. Fromont A, De Seze J, Fleury MC, Maillefert JF, Moreau T. Inflammatory Demyelinating Events Following Treatment With Anti-Tumor Necrosis Factor. Cytokine (2009) 45:55–7. doi: 10.1016/j.cyto.2008.11.002
8. Ramos-Casals M, Brito-Zeron P, Munoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune Diseases Induced by TNF-Targeted Therapies: Analysis of 233 Cases. Med (Baltimore) (2007) 86:242–51. doi: 10.1097/MD.0b013e3181441a68
10. Bikle DD. Vitamin D and the Skin. J Bone Miner Metab (2010) 28:117–30. doi: 10.1007/s00774-009-0153-8
11. Jenkinson C. The Vitamin D Metabolome: An Update on Analysis and Function. Cell Biochem Funct (2019) 37:408–23. doi: 10.1002/cbf.3421
12. Tuckey RC, Cheng CYS, Slominski AT. The Serum Vitamin D Metabolome: What We Know and What Is Still to Discover. J Steroid Biochem Mol Biol (2019) 186:4–21. doi: 10.1016/j.jsbmb.2018.09.003
13. Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal Expression of 25-Hydroxyvitamin D(3)-1 Alpha-Hydroxylase. J Clin Endocrinol Metab (2001) 86:888–94. doi: 10.1210/jcem.86.2.7220
14. Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-Orphanization of Cytochrome P450 2R1: A Microsomal Vitamin D 25-Hydroxilase. J Biol Chem (2003) 278:38084–93. doi: 10.1074/jbc.M307028200
15. DeLuca HF. Overview of General Physiologic Features and Functions of Vitamin D. Am J Clin Nutr (2004) 80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S
16. Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, et al. The Vitamin D Hormone and Its Nuclear Receptor: Molecular Actions and Disease States. J Endocrinol (1997) 154 Suppl:S57–73.
17. Chun RF. New Perspectives on the Vitamin D Binding Protein. Cell Biochem Funct (2012) 30:445–56. doi: 10.1002/cbf.2835
18. Turhanoglu AD, Guler H, Yonden Z, Aslan F, Mansuroglu A, Ozer C. The Relationship Between Vitamin D and Disease Activity and Functional Health Status in Rheumatoid Arthritis. Rheumatol Int (2011) 31:911–4. doi: 10.1007/s00296-010-1393-6
19. Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of Vitamin D and Risk of Type 1 Diabetes: A Birth-Cohort Study. Lancet (2001) 358:1500–3. doi: 10.1016/S0140-6736(01)06580-1
20. Wright TB, Shults J, Leonard MB, Zemel BS, Burnham JM. Hypovitaminosis D Is Associated With Greater Body Mass Index and Disease Activity in Pediatric Systemic Lupus Erythematosus. J Pediatr (2009) 155:260–5. doi: 10.1016/j.jpeds.2009.02.033
21. Amital H, Szekanecz Z, Szucs G, Danko K, Nagy E, Csepany T, et al. Serum Concentrations of 25-OH Vitamin D in Patients With Systemic Lupus Erythematosus (SLE) are Inversely Related to Disease Activity: Is it Time to Routinely Supplement Patients With SLE With Vitamin D? Ann Rheum Dis (2010) 69:1155–7. doi: 10.1136/ard.2009.120329
22. Haque UJ, Bartlett SJ. Relationships Among Vitamin D, Disease Activity, Pain and Disability in Rheumatoid Arthritis. Clin Exp Rheumatol (2010) 28:745–7.
23. Rossini M, Maddali Bongi S, La Montagna G, Minisola G, Malavolta N, Bernini L, et al. Vitamin D Deficiency in Rheumatoid Arthritis: Prevalence, Determinants and Associations With Disease Activity and Disability. Arthritis Res Ther (2010) 12:R216. doi: 10.1186/ar3195
24. Kerr GS, Sabahi I, Richards JS, Caplan L, Cannon GW, Reimold A, et al. Prevalence of Vitamin D Insufficiency/Deficiency in Rheumatoid Arthritis and Associations With Disease Severity and Activity. J Rheumatol (2011) 38:53–9. doi: 10.3899/jrheum.100516
25. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
26. Lee YH, Bae SC, Choi SJ, Ji JD, Song GG. Associations Between Vitamin D Receptor Polymorphisms and Susceptibility to Rheumatoid Arthritis and Systemic Lupus Erythematosus: A Meta-Analysis. Mol Biol Rep (2011) 38:3643–51. doi: 10.1007/s11033-010-0477-4
27. Hitchon CA, Sun Y, Robinson DB, Peschken CA, Bernstein CN, Siminovitch KA, et al. Vitamin D Receptor Polymorphism Rs2228570 (Fok1) Is Associated With Rheumatoid Arthritis in North American Natives. J Rheumatol (2012) 39:1792–7. doi: 10.3899/jrheum.120387
28. Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol Inhibits the Progression of Arthritis in Murine Models of Human Arthritis. J Nutr (1998) 128:68–72. doi: 10.1093/jn/128.1.68
29. Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, et al. The Cytochrome P450scc System Opens an Alternate Pathway of Vitamin D3 Metabolism. FEBS J (2005) 272:4080–90. doi: 10.1111/j.1742-4658.2005.04819.x
30. Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, et al. In Vivo Evidence for a Novel Pathway of Vitamin D(3) Metabolism Initiated by P450scc and Modified by CYP27B1. FASEB J (2012) 26:3901–15. doi: 10.1096/fj.12-208975
31. Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EKY, et al. Detection of Novel CYP11A1-Derived Secosteroids in the Human Epidermis and Serum and Pig Adrenal Gland. Sci Rep (2015) 5:14875. doi: 10.1038/srep14875
32. Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The Role of CYP11A1 in the Production of Vitamin D Metabolites and Their Role in the Regulation of Epidermal Functions. J Steroid Biochem Mol Biol (2014) 144 Pt A:28–39. doi: 10.1016/j.jsbmb.2013.10.012
33. Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, et al. Correlation Between Secosteroid-Induced Vitamin D Receptor Activity in Melanoma Cells and Computer-Modeled Receptor Binding Strength. Mol Cell Endocrinol (2012) 361:143–52. doi: 10.1016/j.mce.2012.04.001
34. Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, et al. 20-Hydroxyvitamin D3, A Product of Vitamin D3 Hydroxylation by Cytochrome P450scc, Stimulates Keratinocyte Differentiation. J Invest Dermatol (2008) 128:2271–80. doi: 10.1038/jid.2008.62
35. Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM Jr., Slominski AT. 20,23-Dihydroxyvitamin D3, Novel P450scc Product, Stimulates Differentiation and Inhibits Proliferation and NF-kappaB Activity in Human Keratinocytes. J Cell Physiol (2010) 223:36–48. doi: 10.1002/jcp.21992
36. Slominski A, Janjetovic Z, Tuckey RC, Nguyen MN, Bhattacharya KG, Wang J, et al. 20S-Hydroxyvitamin D3, Noncalcemic Product of CYP11A1 Action on Vitamin D3, Exhibits Potent Antifibrogenic Activity In Vivo. J Clin Endocrinol Metab (2013) 98:E298–303. doi: 10.1210/jc.2012-3074
37. Skobowiat C, Oak AS, Kim TK, Yang CH, Pfeffer LM, Tuckey RC, et al. Noncalcemic 20-Hydroxyvitamin D3 Inhibits Human Melanoma Growth in In Vitro and In Vivo Models. Oncotarget (2017) 8:9823–34. doi: 10.18632/oncotarget.14193
38. Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, et al. Products of Vitamin D3 or 7-Dehydrocholesterol Metabolism by Cytochrome P450scc Show Anti-Leukemia Effects, Having Low or Absent Calcemic Activity. PloS One (2010) 5:e9907. doi: 10.1371/journal.pone.0009907
39. Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, et al. Novel Vitamin D Analogs as Potential Therapeutics: Metabolism, Toxicity Profiling, and Antiproliferative Activity. Anticancer Res (2014) 34:2153–63.
40. Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, et al. 20-Hydroxyvitamin D2 Is a Noncalcemic Analog of Vitamin D With Potent Antiproliferative and Prodifferentiation Activities in Normal and Malignant Cells. Am J Physiol Cell Physiol (2011) 300:C526–41. doi: 10.1152/ajpcell.00203.2010
41. Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, et al. 20-Hydroxyvitamin D(3) Inhibits Proliferation of Cancer Cells With High Efficacy While Being Non-Toxic. Anticancer Res (2012) 32:739–46.
42. Kim TK, Atigadda V, Brzeminski P, Fabisiak A, Tang EKY, Tuckey RC, et al. Detection of 7-Dehydrocholesterol and Vitamin D3 Derivatives in Honey. Molecules (2020) 25(11):1–14. doi: 10.3390/molecules25112583
43. Arbiser JL, Arbiser ZK, Majzoub JA. Differential Regulation of Choriocarcinoma Gene Expression by DNA Synthesis Inhibitors. Endocr J (1993) 40:263–8. doi: 10.1507/endocrj.40.263
44. Thornton S, Duwel LE, Boivin GP, Ma Y, Hirsch R. Association of the Course of Collagen-Induced Arthritis With Distinct Patterns of Cytokine and Chemokine Messenger RNA Expression. Arthritis Rheum (1999) 42:1109–18. doi: 10.1002/1529-0131(199906)42:6<1109::AID-ANR7>3.0.CO;2-7
45. Postigo J, Genre F, Iglesias M, Fernandez-Rey M, Buelta L, Carlos Rodriguez-Rey J, et al. Exacerbation of Type II Collagen-Induced Arthritis in Apolipoprotein E-Deficient Mice in Association With the Expansion of Th1 and Th17 Cells. Arthritis Rheum (2011) 63:971–80. doi: 10.1002/art.30220
46. Doncarli A, Stasiuk LM, Fournier C, Abehsira-Amar O. Conversion In Vivo From an Early Dominant Th0/Th1 Response to a Th2 Phenotype During the Development of Collagen-Induced Arthritis. Eur J Immunol (1997) 27:1451–8. doi: 10.1002/eji.1830270623
47. Rosloniec EF, Cremer M, Kang AH, Myers LK, Brand DD. Collagen-Induced Arthritis. Curr Protoc Immunol (2010) 15:Unit 15.5.1–25. doi: 10.1002/0471142735.im1505s89
48. Stasiuk LM, Abehsira-Amar O, Fournier C. Collagen-Induced Arthritis in DBA/1 Mice: Cytokine Gene Activation Following Immunization With Type II Collagen. Cell Immunol (1996) 173:269–75. doi: 10.1006/cimm.1996.0277
49. Cook AD, Turner AL, Braine EL, Pobjoy J, Lenzo JC, Hamilton JA. Regulation of Systemic and Local Myeloid Cell Subpopulations by Bone Marrow Cell-Derived Granulocyte-Macrophage Colony-Stimulating Factor in Experimental Inflammatory Arthritis. Arthritis Rheum (2011) 63:2340–51. doi: 10.1002/art.30354
50. Cho YG, Cho ML, Min SY, Kim HY. Type II Collagen Autoimmunity in a Mouse Model of Human Rheumatoid Arthritis. Autoimmun Rev (2007) 7:65–70. doi: 10.1016/j.autrev.2007.08.001
51. Postigo J, Iglesias M, Cerezo-Wallis D, Rosal-Vela A, Garcia-Rodriguez S, Zubiaur M, et al. Mice Deficient in CD38 Develop an Attenuated Form of Collagen Type II-Induced Arthritis. PloS One (2012) 7:e33534. doi: 10.1371/journal.pone.0033534
52. Brand DD, Latham KA, Rosloniec EF. Collagen-Induced Arthritis. Nat Protoc (2007) 2:1269–75. doi: 10.1038/nprot.2007.173
53. Tuckey RC, Nguyen MN, Slominski A. Kinetics of Vitamin D3 Metabolism by Cytochrome P450scc (CYP11A1) in Phospholipid Vesicles and Cyclodextrin. Int J Biochem Cell Biol (2008) 40:2619–26. doi: 10.1016/j.biocel.2008.05.006
54. Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, et al. Production of 22-Hydroxy Metabolites of Vitamin D3 by Cytochrome P450scc (CYP11A1) and Analysis of Their Biological Activities on Skin Cells. Drug Metab Dispos (2011) 39:1577–88. doi: 10.1124/dmd.111.040071
55. Tuckey RC, McKinley AJ, Headlam MJ. Oxidized Adrenodoxin Acts as a Competitive Inhibitor of Cytochrome P450scc in Mitochondria From the Human Placenta. Eur J Biochem (2001) 268:2338–43. doi: 10.1046/j.1432-1327.2001.02113.x
56. Lin H, Ah Kioon MD, Lalou C, Larghero J, Launay JM, Khatib AM, et al. Protective Role of Systemic Furin in Immune Response-Induced Arthritis. Arthritis Rheum (2012) 64:2878–86. doi: 10.1002/art.34523
57. Bhattacharya SK. Determination of Calcium and Magnesium in Human Blood Serum by Atomic Absorption Spectrophotometer. Anal Lett (1977) 10:817–30. doi: 10.1080/00032717708067830
58. Hietala MA, Jonsson IM, Tarkowski A, Kleinau S, Pekna M. Complement Deficiency Ameliorates Collagen-Induced Arthritis in Mice. J Immunol (2002) 169:454–9. doi: 10.4049/jimmunol.169.1.454
59. Watson WC, Townes AS. Genetic Susceptibility to Murine Collagen II Autoimmune Arthritis. Proposed Relationship to the IgG2 Autoantibody Subclass Response, Complement C5, Major Histocompatibility Complex (MHC) and Non-MHC Loci. J Exp Med (1985) 162:1878–91. doi: 10.1084/jem.162.6.1878
60. Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship Between Th1/Th2 Cytokine Patterns and the Arthritogenic Response in Collagen-Induced Arthritis. Eur J Immunol (1996) 26:1511–8. doi: 10.1002/eji.1830260716
61. Nacher-Juan J, Terencio MC, Alcaraz MJ, Ferrandiz ML. Osteostatin Inhibits Collagen-Induced Arthritis by Regulation of Immune Activation, Pro-Inflammatory Cytokines, and Osteoclastogenesis. Int J Mol Sci (2019) 20(16):1–14. doi: 10.3390/ijms20163845
62. Chen W, Wang J, Xu Z, Huang F, Qian W, Ma J, et al. Apremilast Ameliorates Experimental Arthritis via Suppression of Th1 and Th17 Cells and Enhancement of CD4(+)Foxp3(+) Regulatory T Cells Differentiation. Front Immunol (2018) 9:1662. doi: 10.3389/fimmu.2018.01662
63. Banda NK, Levitt B, Wood AK, Takahashi K, Stahl GL, Holers VM, et al. Complement Activation Pathways in Murine Immune Complex-Induced Arthritis and in C3a and C5a Generation In Vitro. Clin Exp Immunol (2010) 159:100–8. doi: 10.1111/j.1365-2249.2009.04035.x
64. Slominski RM, Raman C, Elmets C, Jetten AM, Slominski AT, Tuckey RC. The Significance of CYP11A1 Expression in Skin Physiology and Pathology. Mol Cell Endocrinol (2021) 111238:111238. doi: 10.1016/j.mce.2021.111238
65. Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, et al. A Novel Pathway for Sequential Transformation of 7-Dehydrocholesterol and Expression of the P450scc System in Mammalian Skin. Eur J Biochem/FEBS (2004) 271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x
66. Huang SC, Lee CT, Chung BC. Tumor Necrosis Factor Suppresses NR5A2 Activity and Intestinal Glucocorticoid Synthesis to Sustain Chronic Colitis. Sci Signal (2014) 7:ra20. doi: 10.1126/scisignal.2004786
67. Phan TS, Merk VM, Brunner T. Extra-Adrenal Glucocorticoid Synthesis at Epithelial Barriers. Genes Immun (2019) 20:627–40. doi: 10.1038/s41435-019-0058-z
68. Slominski RM, Tuckey RC, Manna PR, Jetten AM, Postlethwaite A, Raman C, et al. Extra-Adrenal Glucocorticoid Biosynthesis: Implications for Autoimmune and Inflammatory Disorders. Genes Immun (2020) 21:150–68. doi: 10.1038/s41435-020-0096-6
69. Mahata B, Pramanik J, van der Weyden L, Polanski K, Kar G, Riedel A, et al. Tumors Induce De Novo Steroid Biosynthesis in T Cells to Evade Immunity. Nat Commun (2020) 11:3588. doi: 10.1038/s41467-020-17339-6
70. Costa B, Pini S, Gabelloni P, Da Pozzo E, Abelli M, Lari L, et al. The Spontaneous Ala147Thr Amino Acid Substitution Within the Translocator Protein Influences Pregnenolone Production in Lymphomonocytes of Healthy Individuals. Endocrinology (2009) 150:5438–45. doi: 10.1210/en.2009-0752
71. Mahata B, Zhang X, Kolodziejczyk AA, Proserpio V, Haim-Vilmovsky L, Taylor AE, et al. Single-Cell RNA Sequencing Reveals T Helper Cells Synthesizing Steroids De Novo to Contribute to Immune Homeostasis. Cell Rep (2014) 7:1130–42. doi: 10.1016/j.celrep.2014.04.011
72. Munroe ME, Arbiser JL, Bishop GA. Honokiol, a Natural Plant Product, Inhibits Inflammatory Signals and Alleviates Inflammatory Arthritis. J Immunol (2007) 179:753–63. doi: 10.4049/jimmunol.179.2.753
73. Dixon WG, Hyrich KL, Watson KD, Lunt M, Consortium BCC, Symmons DP, et al. Influence of Anti-TNF Therapy on Mortality in Patients With Rheumatoid Arthritis-Associated Interstitial Lung Disease: Results From the British Society for Rheumatology Biologics Register. Ann Rheum Dis (2010) 69:1086–91. doi: 10.1136/ard.2009.120626
74. Wu CY, Chen DY, Shen JL, Ho HJ, Chen CC, Kuo KN, et al. The Risk of Cancer in Patients With Rheumatoid Arthritis Taking Tumor Necrosis Factor Antagonists: A Nationwide Cohort Study. Arthritis Res Ther (2014) 16:449. doi: 10.1186/s13075-014-0449-5
75. Wadstrom H, Frisell T, Askling J, G. Anti-Rheumatic Therapy in Sweden Study. Malignant Neoplasms in Patients With Rheumatoid Arthritis Treated With Tumor Necrosis Factor Inhibitors, Tocilizumab, Abatacept, or Rituximab in Clinical Practice: A Nationwide Cohort Study From Sweden. JAMA Intern Med (2017) 177:1605–12. doi: 10.1001/jamainternmed.2017.4332
76. Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20-Hydroxycholecalciferol, Product of Vitamin D3 Hydroxylation by P450scc, Decreases NF-kappaB Activity by Increasing IkappaB Alpha Levels in Human Keratinocytes. PloS One (2009) 4:e5988. doi: 10.1371/journal.pone.0005988
77. Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, et al. RORalpha and ROR Gamma Are Expressed in Human Skin and Serve as Receptors for Endogenously Produced Noncalcemic 20-Hydroxy- and 20,23-Dihydroxyvitamin D. FASEB J (2014) 28:2775–89. doi: 10.1096/fj.13-242040
78. Alshahrani F, Aljohani N, Vitamin D. Deficiency, Sufficiency and Toxicity. Nutrients (2013) 5:3605–16. doi: 10.3390/nu5093605
79. Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, et al. High Basal NF-kappaB Activity in Nonpigmented Melanoma Cells Is Associated With an Enhanced Sensitivity to Vitamin D3 Derivatives. Br J Cancer (2011) 105:1874–84. doi: 10.1038/bjc.2011.458
80. Slominski AT, Chaiprasongsuk A, Janjetovic Z, Kim TK, Stefan J, Slominski RM, et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem Biophys (2020) 78:165–80. doi: 10.1007/s12013-020-00913-6
81. Chaiprasongsuk A, Janjetovic Z, Kim TK, Tuckey RC, Li W, Raman C, et al. CYP11A1-Derived Vitamin D3 Products Protect Against UVB-Induced Inflammation and Promote Keratinocytes Differentiation. Free Radic Biol Med (2020) 155:87–98. doi: 10.1016/j.freeradbiomed.2020.05.016
82. Chaiprasongsuk A, Janjetovic Z, Kim TK, Jarrett SG, D’Orazio JA, Holick MF, et al. Protective Effects of Novel Derivatives of Vitamin D3 and Lumisterol Against UVB-Induced Damage in Human Keratinocytes Involve Activation of Nrf2 and P53 Defense Mechanisms. Redox Biol (2019) 24:101206. doi: 10.1016/j.redox.2019.101206
83. Lin Z, Marepally SR, Goh ESY, Cheng CYS, Janjetovic Z, Kim TK, et al. Investigation of 20S-Hydroxyvitamin D3 Analogs and Their 1alpha-OH Derivatives as Potent Vitamin D Receptor Agonists With Anti-Inflammatory Activities. Sci Rep (2018) 8:1478. doi: 10.1038/s41598-018-19183-7
84. Slominski AT, Kim TK, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, et al. Endogenously Produced Nonclassical Vitamin D Hydroxy-Metabolites Act as “Biased” Agonists on VDR and Inverse Agonists on RORalpha and RORgamma. J Steroid Biochem Mol Biol (2017) 173:42–56. doi: 10.1016/j.jsbmb.2016.09.024
85. Slominski AT, Kim TK, Janjetovic Z, Brozyna AA, Zmijewski MA, Xu H, et al. Differential and Overlapping Effects of 20,23(OH)(2)D3 and 1,25(OH)(2)D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)(2)D3. Int J Mol Sci (2018) 19(10):1–35. doi: 10.3390/ijms19103072
Keywords: vitamin D, arthritis, RA, 20S(OH)D3, mouse, collagen
Citation: Postlethwaite AE, Tuckey RC, Kim T-K, Li W, Bhattacharya SK, Myers LK, Brand DD and Slominski AT (2021) 20S-Hydroxyvitamin D3, a Secosteroid Produced in Humans, Is Anti-Inflammatory and Inhibits Murine Autoimmune Arthritis. Front. Immunol. 12:678487. doi: 10.3389/fimmu.2021.678487
Received: 30 March 2021; Accepted: 15 June 2021;
Published: 30 June 2021.
Edited by:
Silvano Sozzani, Sapienza University of Rome, ItalyReviewed by:
Martin Hewison, University of Birmingham, United KingdomArup Kumar Indra, Oregon State University, United States
Copyright © 2021 Postlethwaite, Tuckey, Kim, Li, Bhattacharya, Myers, Brand and Slominski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda K. Myers, bG15ZXJzQHV0aHNjLmVkdQ==
†Deceased
 Arnold E. Postlethwaite
Arnold E. Postlethwaite Robert C. Tuckey
Robert C. Tuckey Tae-Kang Kim4
Tae-Kang Kim4 Wei Li
Wei Li Syamal K. Bhattacharya
Syamal K. Bhattacharya Linda K. Myers
Linda K. Myers David D. Brand
David D. Brand Andrzej T. Slominski
Andrzej T. Slominski