- Department of Nephro-urology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
Background: The global prevalence and recurrence rate of kidney stones is very high. Recent studies of Randall plaques and urinary components in vivo, and in vitro including gene manipulation, have attempted to reveal the pathogenesis of kidney stones. However, the evidence remains insufficient to facilitate the development of novel curative therapies. The involvement of renal and peripheral macrophages in inflammatory processes offers promise that might lead to the development of therapeutic targets. The present systematic literature review aimed to determine current consensus about the functions of macrophages in renal crystal development and suppression, and to synthesize evidence to provide a basis for future immunotherapy.
Methods: We systematically reviewed the literature during February 2021 according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Articles investigating the relationship between macrophages and urolithiasis, particularly calcium oxalate (CaOx) stones, were extracted from PubMed, MEDLINE, Embase, and Scopus. Study subjects, languages, and publication dates were unrestricted. Two authors searched and screened the publications.
Results: Although several studies have applied mixed modalities, we selected 10, 12, and seven (total, n = 29) of 380 articles that respectively described cultured cells, animal models, and human samples.
The investigative trend has shifted to macrophage phenotypes and signaling pathways, including micro (m)-RNAs since the discovery of macrophage involvement in kidney stones in 1999. Earlier studies of mice-associated macrophages with the acceleration and suppression of renal crystal formation. Later studies found that pro-inflammatory M1- and anti-inflammatory M2-macrophages are involved. Studies of human-derived and other macrophages in vitro and ex vivo showed that M2-macrophages (stimulated by CSF-1, IL-4, and IL-13) can phagocytose CaOx crystals, which suppresses stone development. The signaling mechanisms that promote M2-like macrophage polarization toward CaOx nephrocalcinosis, include the NLRP3, PPARγ-miR-23-Irf1/Pknox1, miR-93-TLR4/IRF1, and miR-185-5p/CSF1 pathways.
Proteomic findings have indicated that patients who form kidney stones mainly express M1-like macrophage-related proteins, which might be due to CaOx stimulation of the macrophage exosomal pathway.
Conclusions: This systematic review provides an update regarding the current status of macrophage involvement in CaOx nephrolithiasis. Targeting M2-like macrophage function might offer a therapeutic strategy with which to prevent stones via crystal phagocytosis.
Introduction
The prevalence of kidney stones has increased worldwide, and its high recurrence rate is also a factor that affects medical and economic resources (1–4). Considerable research effort has been directed toward finding a cure, but the pathology of kidney stone formation is complex and awaits elucidation despite recent technological innovations. Kidney stones are recognized as a multifactorial disease similar to metabolic syndrome (MetS) (5, 6). Renal function, mineral and lipid metabolism, inflammatory processes, oxidative stress, and insulin resistance can cause calcium oxalate (CaOx) crystals to develop (7).
Most kidney stones consist of calcium oxalate (CaOx) (8, 9). The hypotheses presented to account for the pathogenesis of CaOx stones are free- and fixed-particle mechanisms (10); the latter is also known as Randall plaques (RP) that comprise apatite formed by calcium phosphate that grow in the interstitial space around the loop of Henle (11). In contrast to stones that develop within the tubular lumen or renal collecting system, crystal precursors of RP are surrounded by other molecular and cellular structures, which might be influenced by impaired homeostasis (12, 13). Among such lithogenic environments, chemical and mineral component overload or other sources of inflammatory stimulation might act as first triggers that are followed by reactive oxygen species (ROS), which subsequently induce renal epithelial cell damage resulting in CaOx crystal deposition (14). The primary defense mechanism against such cellular impairment is autophagy involving endocytosis (15), and a secondary defense mechanism extends to peri-tubular cells in the interstitial space and immune cells from the circulation (16). The innate defense system that clears crystal deposits from renal tissues is key to finding a fundamental solution for developing novel treatments for kidney stones.
Understanding the role of macrophages (Mφ) in renal crystal formation can help to identify a solution. Renal or peripheral Mφ involvement in kidney stone disease was first reported by de Water et al. in 1999 (17). They discovered that Mφs migrate to crystal deposition sites and engulf the crystals. Given M1 pro- and M2 anti-inflammatory (18) polarization, the involvement of Mφ in renal crystal formation is probably diversified in different ways. Because the ability to phagocytose crystals is greater for M2- than M1-like Mφs (19), regulating their polarization might have therapeutic value (20). Much about the clinical application of Mφ to preventing kidney stone development has been reported over the last two decades (21).
This systematic review aimed to provide collective evidence of Mφ function in renal crystal development and suppression, and to provide ideas for the future direction of Mφ research into the clinical application of Mφ immunotherapy.
Materials and Methods
This systematic literature review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22).
Search Strategy
We searched PubMed, MEDLINE, Embase, and Scopus databases using the MeSH key terms, “macrophages” OR “macrophage”, OR “monocyte”, AND “urolithiasis”, OR “calcium oxalate”, OR “kidney stone”. Article type, publication date, language, or species were not restricted during the initial search.
Eligibility Criteria
Articles that focused on the relationship between Mφ and monocyte function and the pathogenesis of urolithiasis were included. Articles describing findings of inflammatory cytokines/chemokines secreted by Mφs and monocytes were excluded because direct connections with Mφs and/or monocytes were limited. Since we focused on experimental and translational findings, original articles were favored over reviews or commentary articles, which referred to and/or summarized findings published by others.
Data Collection and Description
Two authors (KT and RU) independently reviewed the titles and abstracts of the articles identified in the initial search during February 8th, 2021. Data were extracted from articles that met the eligibility criteria and reassessed in full-text articles during primary and secondary screening, respectively. The most recent of duplicate published articles was included. Disagreements and discrepancies between the two screens were resolved through discussion and consensus with the other authors. The following data were extracted from all eligible full-text articles: first author, journal name, year of publication, type of study, methodology, type of experimental sources, and main findings concerning Mφ/monocyte function.
The Mφ phenotypes were reported in accordance with the latest nomenclature, experimental guidelines (18), and reviews regarding organ-specific findings (20, 23, 24).
Results
We identified 380 articles that met the search strategy and criteria across all databases in the initial literature search. After the first screen of titles and abstracts, the second screen of full texts filtered them into 29 articles that were eligible for review. Figure 1 shows the PRISMA flowchart.
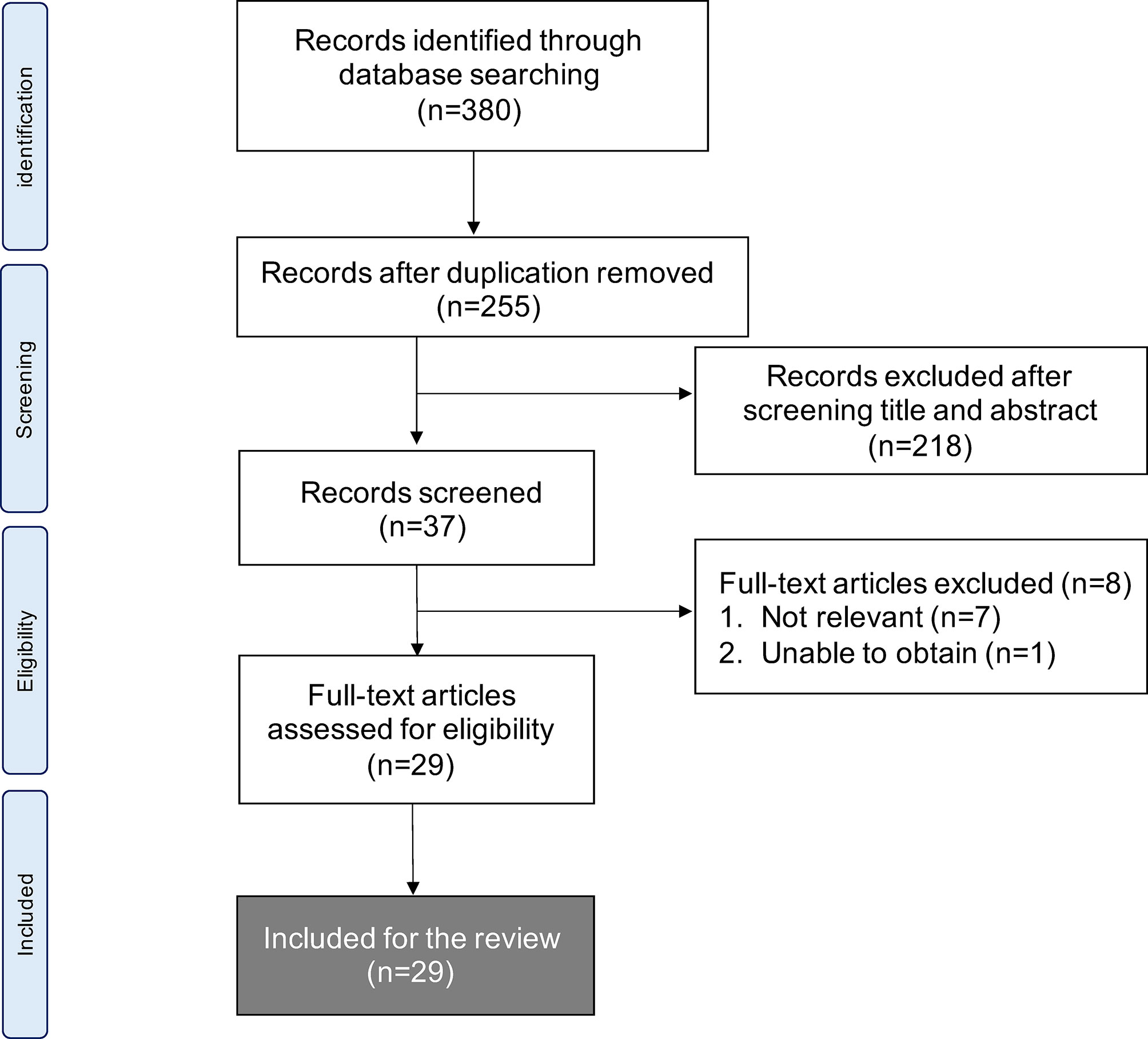
Figure 1 Flow chart of methods used to conduct systematic literature review in accordance with PRISMA guidelines.
Table 1 summarizes the findings of 29 articles describing various studies that were published in China, Germany, Japan, the Netherlands, Thailand, and the USA between 1999 and 2020. These articles described mostly translational research studies including rat and mouse models of nephrocalcinosis caused by hyperoxaluria in vivo, murine, canine, and human renal tubular epithelial cell lines in vitro, and human renal papillar, peripheral blood, and urine samples ex vivo.
In Vitro and Ex Vivo Human Monocyte Function Against Calcium Oxalate Crystals in Kidney Stone Formers
Thongboonkerd et al. were the first to report the proteomic interactions between human monocytes and CaOx monohydrate (COM) crystals. The researchers found that COM could drive oxidative stress, resulting in increased cellular apoptosis and expression of prohibitin, plasminogen activator inhibitor-2, Alix, lamin A/C, and moesin, as well as reduced cellular survival, protein synthesis and stability, mRNA stability, and lipid metabolism. In addition, levels of La protein, heterogeneous nuclear ribonucleoprotein H1, elongation factor-2, otubain-1, heat shock protein (HSP) 105, and acyl-CoA thioester hydrolase were reduced (25). The proteomics associated with the monocyte functions evoked by COM in renal tubular epithelial cells (RTECs) have also been investigated. The researchers identified six secreted proteins with significantly altered expression levels in Mardin-Darby canine kidney (MDCK) cells incubated with COM crystals. Furthermore, they reported that enolase-1, a COM crystal-binding protein, activated monocytic cell migration by binding to the surface of monocytic cells (26).
Williams et al. further investigated monocyte mitochondrial function in human serum specimens and reported significantly reduced monocyte mitochondrial maximal respiration, reserve capacities, and bioenergetic health indices in patients with CaOx stones, as compared to healthy controls (27).
In Vitro M (–) (Non-Polarized Macrophage) Phagocytosis and Calcium Oxalate Crystal Reaction
Confocal laser scanning microscopy revealed that I9.1 Mφs (derived from mouse spleen cells) that were co-incubated with macrophage colony-stimulating factor (M-CSF) dissolved internalized CaOx crystals (28). Okada et al. further investigated crystal dissolution by Mφs by using Lysotracker, transmission electron microscopy (TEM), and confocal light microscopy to study the murine J774.1 Mφ cell line. The authors confirmed the diachronic elimination of engulfed COM crystals in Mφs (Figure 2). Internalized COM crystals become surrounded by phagosomes that fuse with lysosomes to promote dissolution and spontaneous elimination (29). Mφ functions have also been investigated in RTEC. M-1 (murine collecting duct) and RAW264.7 cells incubated with murine Mφs developed into a pro-inflammatory state with increased COM crystal adhesion, especially when these cells were co-cultured with adipocytes. This paracrine mechanism is associated with increased mRNA and protein levels of OPN, MCP-1, and tumor necrosis factor (TNF)-α (30).
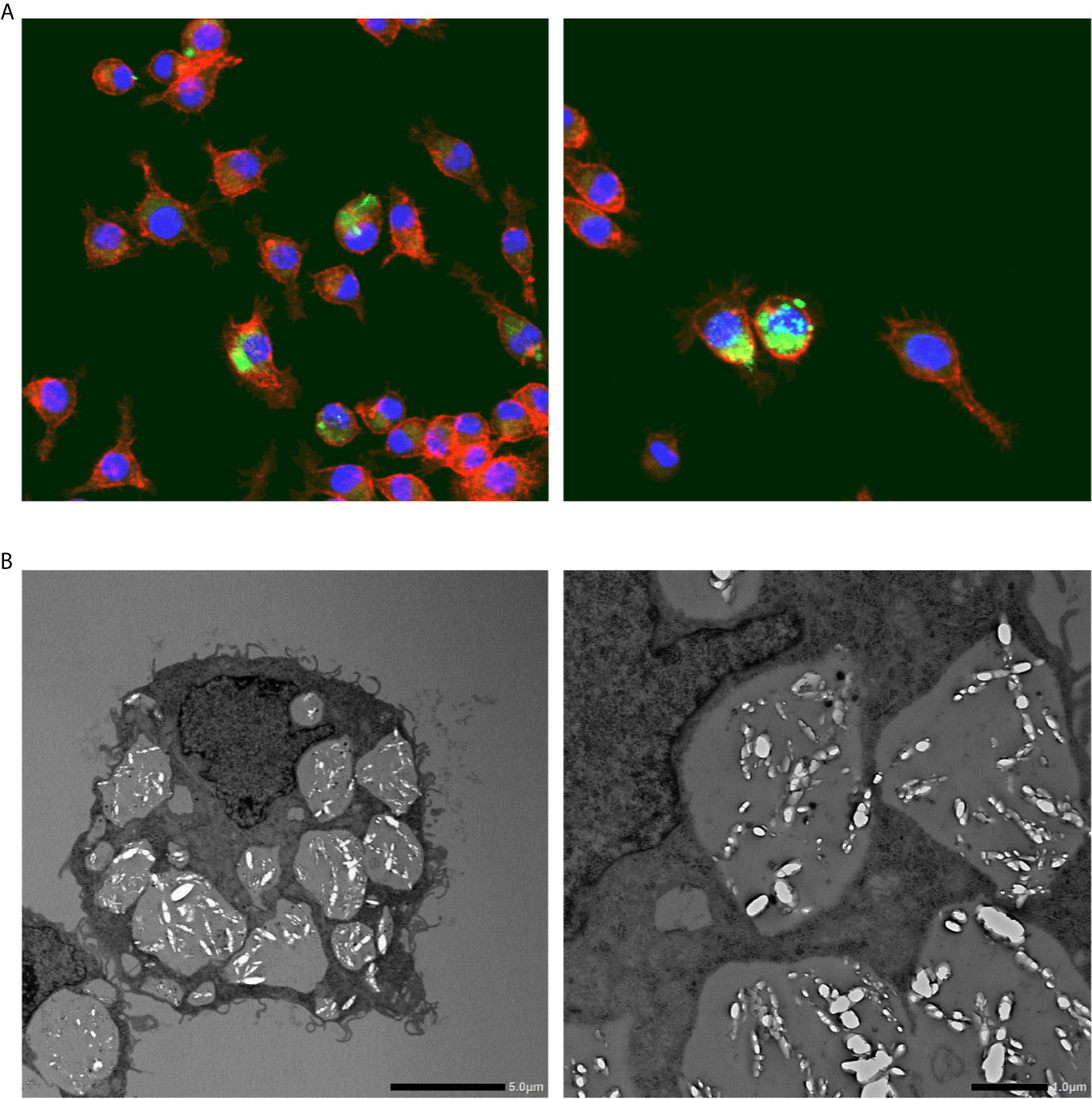
Figure 2 Calcium oxalate crystal phagocytosis by macrophages. (A) Representative photographs show fluorescent immunohistochemical staining of macrophages (RAW264.8) cultured with calcium oxalate monohydrate crystals. Red, phalloidin; Green: calcium oxalate monohydrate crystals; Blue, nucleus. Magnification: ×400. (B) Transmission electron microscopy images of macrophages (RAW264.8) engulfing calcium oxalate monohydrate crystals. Scale bars, left and right panels, 5 and 10 μm, respectively.
A previous study on Mφs derived from human monocytes incubated with COM crystals in vitro identified an association between HSP90 and F-actin on the phagosome membranes engulfing COM crystals. Blocking HSP90 with an siRNA reduced phagocytic activity and Mφ migration, suggesting that HSP90 and F-actin are involved in Mφ function during the CaOx interaction (31). Exosomes secreted by Mφs are also affected by COM crystals. In particular, COM-treated Mφ exosomes have increased membrane fragility and are able to enter the renal interstitium and trigger RTEC to release IL-8, which worsens tissue inflammation (32). Thus, COM-treated Mφ exosomes enhance monocyte and T-cell migration, monocyte activation, and Mφ phagocytosis. However, the suppression of vimentin with an siRNA abolishes these effects, suggesting that Mφ exosomal vimentin plays an important role in the immune response to COM crystals (33).
In Vivo Macrophage Involvement in Calcium Oxalate Nephrocalcinosis
While there are limitations in adapting the findings to human patients owing to differences between human kidney stone formation and nephrocalcinosis in rodents, CaOx nephrocalcinosis models in rats and mice by hyperoxaluria are widely utilized for research, on the basis of similarities in intratubular crystal formation and retention (34). Mφs and multinucleated giant cells encapsulating interstitial CaOx crystals in both kidneys in a hyperoxaluric rat model and in patients with oxalosis were described in 1999 (17). The authors further confirmed that ectodermal dysplasia 1 (ED1)-positive Mφs were predominantly increased around renal crystal deposition sites compared with CD45 and major histocompatibility complex (MHC) class II positive and other mononuclear cells during the time course of hyperoxaluria in rats (35). These early studies showed that Mφ dissolve CaOx crystals, which might be related to renal defense against stone development. Okada et al. found spontaneously eliminated renal CaOx crystal deposition in a mouse model of nephrocalcinosis induced by glyoxylate (36), and that subsequent transcriptome studies associated the activation of monocytes/macrophages with this phenomenon involved chemokine (C-C motif) ligand 6, vimentin, Cd14, cytochrome P450 family 1 subfamily b polypeptide 1, moesin, apolipoprotein E, histocompatibility 2 class II antigen A α, histocompatibility 2 class II antigen A β 1, and lipopolysaccharide (LPS)-induced tumor necrosis factor. Immunohistochemical findings also confirmed that F4/80-positive Mφs could be visualized as crystal deposition peaks (37). The expression of monocyte chemoattractant protein (MCP)-1, osteopontin (OPN), fibronectin, cluster of differentiation (CD)44, and major histocompatibility complex (MHC) class II is associated with amounts of renal crystal deposition and the expression of F4/80-positive Mφs. TEM also revealed the phagocytosis of crystals by renal Mφ (38).
In Vitro and Ex Vivo Polarized Macrophage Function Toward Calcium Oxalate Crystals
Examination of the roles of M1 and M2Mφs in CaOx crystals using murine bone marrow-derived Mφs revealed that M(IL-4) had a greater ability to phagocytose COM crystals than that does M(LPS) (39). In addition, M(IL-4+IL-13) suppresses COM crystal adherence to M-1 murine collecting duct cells and has higher COM-phagocytized cell rates than that does M(LPS+IFNγ) (19).
Yu et al. examined the role of Mφ in RP formation in vitro using human RTEC, HK-2 cells, with M(LPS) differentiated from the human monocytic cell line U937. They found that co-cultured M(LPS) and HK-2 cells incubated with hydroxyapatite increase oxidative stress, MCP-1 and OPN expression, and decrease fetuin-A (40). The same group assessed the role of M2Mφ in oxidative stress injury and apoptosis induced by CaOx crystals in HK-2 cells and found that M(IL-4+IL-13) differentiated from the human monocytic THP-1 cell line. Similar to apocynin, M(IL-4+IL-13) reduce the expression of NADPH oxidase p47phox protein, increase mitochondrial membrane potential, and inhibit the protein expression of cleaved caspase-3, cytochrome c, and phosphor-p38 MAPK, as well as ROS release in HK-2 cells incubated with CaOx crystals (41). These results indicated opposing roles of M1 and M2Mφs in the oxidative stress damage and apoptosis of RTEC during the development CaOx stones.
M(CSF-1) derived from human peripheral blood mononuclear cells demonstrate greater ability to phagocytose both CaOx crystals and natural kidney stones than that does M(GM-CSF). Inhibitor assays have demonstrated that kidney stone clearance is mediated through clathrin-dependent phagocytosis and endocytosis (42). Dominguez-Gutierrez et al. reported that CaOx, but not potassium or ZnOx, induced the M1-like Mφ differentiation of human monocyte cell lines and primary human monocytes expressing CD86 and CD68, and secrete cytokines and chemokines. Furthermore, supernatants of CaOx-treated monocytes can enhance M2Mφ CaOx crystal phagocytosis (43).
In Vivo Polarized Macrophage Function in Renal CaOx Crystal Development
Several studies have assessed Mφ polarization in renal stone formation. In accordance with the mouse model of nephrocalcinosis with hyperoxaluria, more renal and urinary crystal deposition develops in CSF-1-deficient mice with fewer M2-like Mφs (identified as CD11b+F4/80+CD163+CD206hi cells), than wild-type mice (39). In contrast, a surge in M1-like Mφs (identified as CD11b+F4/80+CD11cintLy6Chi cells) has been identified in MetS model mice with a leptin deficiency and CaOx crystal deposition in the kidney under treatment with ethylene glycol (EG), and fed with a high-fat diet (44). Further studies of the roles of M1 and M2Mφ roles in stone development have revealed that the induction/transfusion of M1- and M2-like Mφs respectively accelerated and attenuated renal crystal development in C57BL/6J wild-type mice administered daily with intra-abdominal glyoxylate (19). Anders et al. examined the role of the nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing 3 (NLRP3), a central molecular mediator of inflammation in crystallopathies, in CaOx nephrocalcinosis formation in Nlrp3-deficient mice on a high-oxalate diet. They found that NLRP3 inhibition induces a shift in infiltrating renal Mφs from the M1-like (CD45+F4/80+CD11b+CX3CR1+ CD206-) to the M2-like (CD45+F4/80+CD11b+CX3CR1+CD206+TGFβ -) phenotype and attenuation of renal fibrosis. Therefore NLRP3 appears to promote nephrocalcinosis-related fibrotic kidney disease by promoting a shift from anti-inflammatory M1, to pro-inflammatory and profibrotic M2-like Mφs (45).
Roles and Polarization of Macrophage in Human Tissue
Microarray analysis of human kidneys revealed decreased expression of the M2-like Mφ-related genes, peroxisome proliferator- activated receptor gamma (PPARγ), CD163, and CD206, and increased expression of M1-like Mφ-related genes, including nitric oxide synthase 2, CSF2, IL10, and C-C chemokine receptor type 2, in renal papillary tissues from patients with CaOx stones compared with those who do not form stones (19). The findings of causal network analyses associated the differentially expressed genes in RP papillary tissues with significantly higher immune cell activity, including Mφs and plasma cells, which are linked to IL11, PG-endoperoxide synthase 1, glutathione peroxidase 3, and monocyte-to-Mφ differentiation in RP, than in normal papillary tissues (46). Urinary Mφ-related cytokines in stone-forming adolescents have also been investigated. Levels of urinary IL-13/creatinine and Mφ inflammatory protein-1β (MIP-1β)/creatinine could serve as useful biomarkers for stones based on sensitivity with 50% and 58%, respectively, and 93% specificity for both in a study of a small sample (47). Urinary multiplex comparisons among individuals who did not form stones, and those who formed CaOx stones for the first time and those who formed recurrent CaOx stones, identified IL-1a, IL-1b, IL-4, IL-10, and GM-CSF as potential biomarkers affecting Mφ and neutrophil function in stone development (48). Furthermore, M1-like Mφ polarization increases pro-inflammatory cytokines such as TNFα, IL-1β, and IL-1, as well as M1/M2-like monocyte ratios in blood samples from patients with CaOx stones compared with those who do not form stones (49).
Therapeutic Target Altering Macrophage Phenotype Against Kidney Stone Disease
Sirtuin 3 (SIRT3), an NAD+-dependent deacetylase in the mitochondrial matrix, suppresses renal CaOx crystal deposition in vitro and in vivo by promoting M2-like Mφs through deacetylating forkhead box O1 (FOXO1) (49). The prevalence of kidney stones is influenced by sex hormones (47). Suppressing androgen receptor (AR) expression in RTEC in vitro increases Mφ recruitment and causes an M2 polarization shift, which increases the phagocytosis of intrarenal CaOx crystals (47). Administering renal tubule-specific AR knockout mice and hydroxy-L-proline treated rats with an AR degradation enhancer in vivo has revealed that AR signaling suppresses CSF-1 expression via the upregulation of miR-185-5p, which results in decreased M2-like Mφs and accelerated CaOx crystal development (50). The potential of pioglitazone, a PPARγ agonist, to treat kidney stones has been recognized (51, 52). Pioglitazone increases M2-like Mφ polarization and decreases renal CaOx crystal deposition and inflammatory damage and in murine bone marrow-derived Mφ in vitro and in CaOx nephrocalcinosis mouse models in vivo. These results indicated that PPARγ upregulates miR-23 expression and subsequently attenuates the expression of interferon regulatory factor 1 (Irf1) and Pknox1, which shifts the Mφ phenotype from M1 to M2 (53). The effects of nuclear factor erythroid 2-related factor 2 (Nrf2) on PPARγ and the anti-inflammatory process in individuals with CaOx stone have been investigated. The findings showed that Nrf2 attenuates the M1-like Mφ polarization shift by suppressing toll-like receptor 4 (TLR4) and IRF in vitro. Moreover, sulforaphane, an activator of Nrf2, plays a protective role against CaOx crystal formation and renal injury via the Nrf2-miR-93-TLR4/IRF1 axis, which promotes M2-like Mφ polarization and inhibits RTEC inflammation in vivo in mouse models of CaOx nephrocalcinosis (54). The involvement of the aryl hydrocarbon receptor (AhR) as a regulator of the phenotypic balance between M1- and M2-like Mφs in renal CaOx stone development has been investigated (55). Transcriptomic and proteomic analyses of bone marrow-derived Mφs and mouse models of CaOx nephrocalcinosis have revealed that stimulating the AhR-miR-142a-IRF1/hypoxia-inducible factor (HIF)-1α axis diminishes M1-like Mφs and promotes M2-like Mφs, leading to the suppression of renal CaOx crystal deposition and stone-related renal damage (55).
Limitations
This systematic review has several limitations. Firstly, most articles we found utilized the M1/M2Mφ definition to be consistent with the prior literature in the nephrology and urology fields; therefore, in some cases it was difficult to summarize them according to the latest nomenclature. Secondly, findings from nephrocalcinosis mouse models might not be applicable to kidney stone patients owing to pathological differences between intratubular crystal deposition and CaOx stone formation. Lastly, only a few studies have assessed the direct relationship between CaOx stones/crystals and Mφs in human tissues; thus, additional live tissue studies are required.
Conclusion
The roles of Mφs in the development of CaOx kidney stone have been investigated in vitro, in vivo, and in human specimens ex vivo. In vitro and ex vivo studies have demonstrated that monocytes and M(-) (non-polarized Mφs) have the ability to eliminate CaOx crystals via phagocytosis. The treatment of RTECs with COM crystals stimulates Mφ migration via cytokines, chemokines, and exosomes. In vivo studies have shown M1-like Mφs facilitate renal CaOx crystal development with renal inflammation, fibrosis, and cellular damage, whereas M2-like Mφs suppress CaOx crystal development. M2-like Mφs, such as M(CSF-1), M(IL-4), and M(IL-4+IL-13), have greater capability for phagocytosing CaOx crystals, which eventually dissolves crystal fragments, and autocrine and paracrine mechanisms along with RTEC enhance Mφ phagocytosis (Figure 3). Furthermore, while only a few studies have investigated Mφ polarization in human tissues, there may be a predominant M1-like Mφ cytokine/chemokine phenotype in the urine, serum, and renal tissues of kidney stone formers.
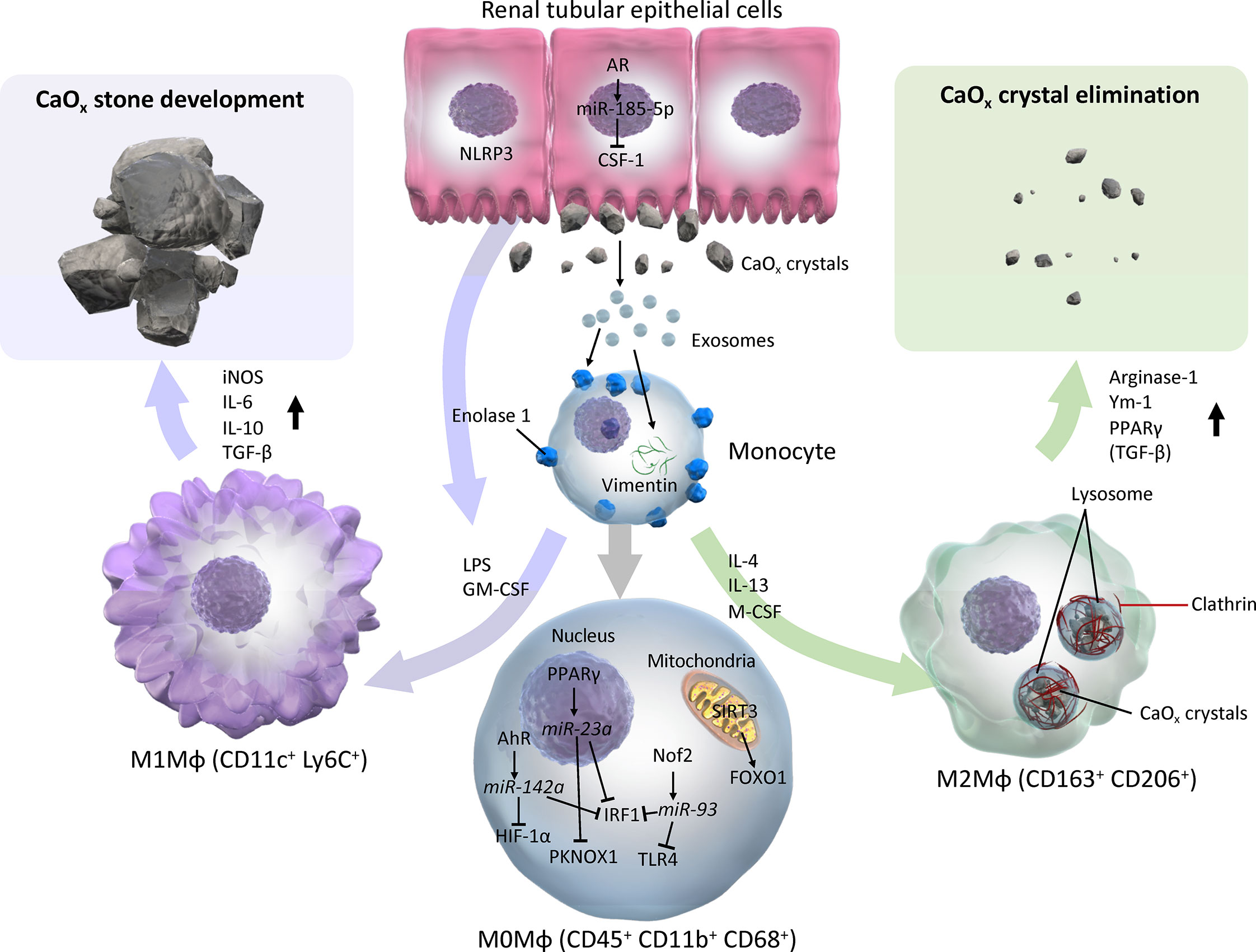
Figure 3 Schema of evidence synthesized from current literature regarding roles of M1 and M2 macrophages in CaOx stone development. M1 or M2Mφs are usually differentiated from monocytes and M0 (with neutral polarization) Mφs via various cytokines/chemokines by direct and indirect influences from CaOx crystals. M0 Mφs autocrine mechanism shifts phenotypes toward CaOx crystal development via AhR-miR-142a-IRF1/HIF-1α, PPARγ-miR-23a-IRF1/PKNOX1, Nrf2-miR-93-IRF/TLR4, and SIRT3-FOXO1 axes. Monocytes that reflect exosomes secreted by monocytes/Mφs and renal tubular epithelial cells via enolase-1 and vimentin, then become activated and change into either M1 or M2Mφs. In contrast, paracrine involvement of renal tubular epithelial cells via NLRP3 and AR-miR-185-5p-CSF-1 is also an important factor in regulation of Mφ polarization. While M1Mφs facilitate CaOx stone development by promoting pro-inflammatory and oxidative stress molecules such as iNOS, IL-6, IL-10, and TGF-β, M2Mφs attenuate the development of CaOx crystals, and eliminate them by phagocytosis via lysosomes and clathrin mediation, and induces anti-inflammatory molecules including Arginase-1, Ym-1, and PPARγ. AhR, aryl hydrocarbon receptor; AR, androgen receptor; CSF-1, colony-stimulating factor-1; FOXO1, forkhead box O1; GM-CSF, granulocyte-macrophage colony-stimulating factor; HIF-1α, hypoxia-inducible factor-1 alpha; iNOS, inducible nitric oxide synthase; IRF1, interferon regulatory factor 1; LPS, lipopolysaccharide; Mφ, macrophage; M-CSF, macrophage colony-stimulating factor; NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain containing 3; PPARγ, peroxisome proliferator-activated receptor-gamma; SIRT3, Sirtuin 3; TLR4, toll-like receptor 4.
Research focus has shifted from identifying Mφ expression to analyzing Mφ function and finding triggers that alter the Mφ phenotypes to create novel therapeutic targets. However, evidence regarding Mφs in urinary sediments is scant, and the direct manipulation of Mφ phenotypes for clinical use needs to be determined (56). Future investigations should strive to establish a urinary biomarker for liquid biopsies (57), and an Mφ-specific target therapy using antibodies, vectors, and nanoparticles (58, 59).
Author Contributions
KT, SH, and TY: Study conception and design. KT and RU: Data collection. KT, RU, and AO Data analysis. KT, SH, and AO: Data interpretation. KT and RU: Drafted the manuscript. SH, AO, and TY: Revised the manuscript. TY supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (nos. 19H03791, 19K09735, and 20K21658), as well as grants from the NOVARTIS Foundation (Japan), the Naito Foundation, and the Hori Sciences and Arts Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Editage (http://www.editage.com) for English language editing and review of the manuscript. We also thank Science Graphics Co., Ltd. (Kyoto, Japan) for helping with the schema.
Abbreviations
AhR, aryl hydrocarbon receptor; AR, androgen receptor; CaOx, calcium oxalate; COM, calcium oxalate monohydrate; CSF-1, colony stimulating factor-1; EG, ethylene glycol; FOXO1, forkhead box O1; GM-CSF, granulate macrophage colony stimulating factor-1; HIF-1α, hypoxia-inducible factor-1 alpha; HLP, hydroxy-L-proline; IFNγ, interferon gamma; Irf1, interferon regulatory factor 1; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein-1; MetS, metabolic syndrome; MHC, major histocompatibility complex; MIP1β, macrophage inflammatory protein-1β; Mφ, macrophage; NLRP3, nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing 3; Nrf2, nuclear factor erythroid 2-related factor 2; OPN, osteopontin; PPARγ, peroxisome proliferator-activated receptor-gamma; HSP, heat shock protein; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; ROS, reactive oxygen species; RP, Randall plaque; RTEC, renal tubular epithelial cell; SIRT3, sirtuin 3; TLR4, toll-like receptor 4; TNFα, tumor necrosis factor alpha.
References
1. Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of Stone Disease Across the World. World J Urol (2017) 35(9):1301–20. doi: 10.1007/s00345-017-2008-6
2. Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of Urolithiasis: Trends in Prevalence, Treatments, and Costs. Eur Urol Focus (2017) 3(1):18–26. doi: 10.1016/j.euf.2017.04.001
3. Roberson D, Sperling C, Shah A, Ziemba J. Economic Considerations in the Management of Nephrolithiasis. Curr Urol Rep (2020) 21(5):18. doi: 10.1007/s11934-020-00971-6
4. Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the National Health and Nutrition Examination Survey to Calculate the Impact of Obesity and Diabetes on Cost and Prevalence of Urolithiasis in 2030. Eur Urol (2014) 66(4):724–9. doi: 10.1016/j.eururo.2014.06.036
5. Wong Y, Cook P, Roderick P, Somani BK. Metabolic Syndrome and Kidney Stone Disease: A Systematic Review of Literature. J Endourol (2016) 30(3):246–53. doi: 10.1089/end.2015.0567
6. Kohjimoto Y, Sasaki Y, Iguchi M, Matsumura N, Inagaki T, Hara I. Association of Metabolic Syndrome Traits and Severity of Kidney Stones: Results From a Nationwide Survey on Urolithiasis in Japan. Am J Kidney Dis (2013) 61(6):923–9. doi: 10.1053/j.ajkd.2012.12.028
7. Yasui T, Okada A, Hamamoto S, Ando R, Taguchi K, Tozawa K, et al. Pathophysiology-Based Treatment of Urolithiasis. Int J Urol (2017) 24(1):32–8. doi: 10.1111/iju.13187
8. Xu LHR, Adams-Huet B, Poindexter JR, Maalouf NM, Moe OW, Sakhaee K. Temporal Changes in Kidney Stone Composition and in Risk Factors Predisposing to Stone Formation. J Urol (2017) 197(6):1465–71. doi: 10.1016/j.juro.2017.01.057
9. Siener R, Buchholz N, Daudon M, Hess B, Knoll T, Osther PJ, et al. Quality Assessment of Urinary Stone Analysis: Results of a Multicenter Study of Laboratories in Europe. PloS One (2016) 11(6):e0156606. doi: 10.1371/journal.pone.0156606
10. Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. Kidney Stones. Nat Rev Dis Prim (2016) 2:16008. doi: 10.1038/nrdp.2016.8
11. Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, et al. Randall’s Plaque of Patients With Nephrolithiasis Begins in Basement Membranes of Thin Loops of Henle. J Clin Invest (2003) 111(5):607–16. doi: 10.1172/JCI17038
12. Zhu Z, Huang F, Xia W, Zeng H, Gao M, Li Y, et al. Osteogenic Differentiation of Renal Interstitial Fibroblasts Promoted by Lncrna MALAT1 may Partially Contribute to Randall’s Plaque Formation. Front Cell Dev Biol (2021) 8:1747. doi: 10.3389/fcell.2020.596363
13. Taguchi K, Chen L, Usawachintachit M, Hamamoto S, Kang M, Sugino T, et al. Fatty Acid–Binding Protein 4 Downregulation Drives Calcification in the Development of Kidney Stone Disease. Kidney Int (2020) 97(5):1042–56. doi: 10.1016/j.kint.2020.01.042
14. Khan SR, Canales BK, Dominguez-Gutierrez PR. Randall’s Plaque and Calcium Oxalate Stone Formation: Role for Immunity and Inflammation. Nat Rev Nephrol (2021). doi: 10.1038/s41581-020-00392-1
15. Unno R, Kawabata T, Taguchi K, Sugino T, Hamamoto S, Ando R, et al. (Mechanistic Target of Rapamycin Kinase) is Responsible for Autophagy Defects Exacerbating Kidney Stone Development. Autophagy (2020) 16(4):709–23. doi: 10.1080/15548627.2019.1635382
16. Weisheit CK, Engel DR, Kurts C. Dendritic Cells and Macrophages: Sentinels in the Kidney. Clin J Am Soc Nephrol (2015) 10(10):1841–51. doi: 10.2215/CJN.07100714
17. de Water R, Noordermeer C, van der Kwast TH, Nizze H, Boevé ER, Kok DJ, et al. Calcium Oxalate Nephrolithiasis: Effect of Renal Crystal Deposition on the Cellular Composition of the Renal Interstitium. Am J Kidney Dis (1999) 33(4):761–71. doi: 10.1016/S0272-6386(99)70231-3
18. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity (2014) 41(1):14–20. doi: 10.1016/j.immuni.2014.06.008
19. Taguchi K, Okada A, Hamamoto S, Unno R, Moritoki Y, Ando R, et al. M1/M2-Macrophage Phenotypes Regulate Renal Calcium Oxalate Crystal Development. Sci Rep (2016) 6:35167. doi: 10.1038/srep35167
20. Chen T, Cao Q, Wang Y, Harris DCH. M2 Macrophages in Kidney Disease: Biology, Therapies, and Perspectives. Kidney Int (2019) 95(4):760–73. doi: 10.1016/j.kint.2018.10.041
21. Dominguez-Gutierrez PR, Kwenda EP, Khan SR, Canales BK. Immunotherapy for Stone Disease. Curr Opin Urol (2020) 30(2):183–9. doi: 10.1097/MOU.0000000000000729
22. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst Rev (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
23. Lee H, Fessler MB, Qu P, Heymann J, Kopp JB. Macrophage Polarization in Innate Immune Responses Contributing to Pathogenesis of Chronic Kidney Disease. BMC Nephrol (2020) 21(1):270. doi: 10.1186/s12882-020-01921-7
24. Engel JE, Chade AR. Macrophage Polarization in Chronic Kidney Disease: A Balancing Act Between Renal Recovery and Decline? Am J Physiol Renal Physiol (2019) 317(6):F1409–13. doi: 10.1152/ajprenal.00380.2019
25. Singhto N, Sintiprungrat K, Sinchaikul S, Chen ST, Thongboonkerd V. Proteome Changes in Human Monocytes Upon Interaction With Calcium Oxalate Monohydrate Crystals. J Proteome Res (2010) 9(8):3980–8. doi: 10.1021/pr100174a
26. Chiangjong W, Thongboonkerd V. Calcium Oxalate Crystals Increased Enolase-1 Secretion From Renal Tubular Cells That Subsequently Enhanced Crystal and Monocyte Invasion Through Renal Interstitium. Sci Rep (2016) 6(1):1–11. doi: 10.1038/srep24064
27. Williams J, Holmes RP, Assimos DG, Mitchell T. Monocyte Mitochondrial Function in Calcium Oxalate Stone Formers. Urology (2016) 93:e1–6:224. doi: 10.1016/j.urology.2016.03.004
28. de Water R, Leenen P, Noordermeer C, Nigg A, Houtsmuller A, Kok D, et al. Cytokine Production Induced by Binding and Processing of Calcium Oxalate Crystals in Cultured Macrophages. Am J Kidney Dis (2001) 38(2):331–8. doi: 10.1053/ajkd.2001.26098
29. Okada A, Aoki H, Onozato D, Kato T, Hashita T, Takase H, et al. Active Phagocytosis and Diachronic Processing of Calcium Oxalate Monohydrate Crystals in an In Vitro Macrophage Model. Kidney Blood Press Res (2019) 44(5):1014–25. doi: 10.1159/000501965
30. Zuo L, Tozawa K, Okada A, Yasui T, Taguchi K, Ito Y, et al. A Paracrine Mechanism Involving Renal Tubular Cells, Adipocytes and Macrophages Promotes Kidney Stone Formation in a Simulated Metabolic Syndrome Environment. J Urol (2014) 191(6):1906–12. doi: 10.1016/j.juro.2014.01.013
31. Singhto N, Sintiprungrat K, Thongboonkerd V. Alterations in Macrophage Cellular Proteome Induced by Calcium Oxalate Crystals: The Association of HSP90 and F-actin is Important for Phagosome Formation. J Proteome Res (2013) 12(8):3561–72. doi: 10.1021/pr4004097
32. Singhto N, Thongboonkerd V. Exosomes Derived From Calcium Oxalate-Exposed Macrophages Enhance IL-8 Production From Renal Cells, Neutrophil Migration and Crystal Invasion Through Extracellular Matrix. J Proteomics (2018) 185:64–76. doi: 10.1016/j.jprot.2018.06.015
33. Singhto N, Kanlaya R, Nilnumkhum A, Thongboonkerd V. Roles of Macrophage Exosomes in Immune Response to Calcium Oxalate Monohydrate Crystals. Front Immunol (2018) 9:316. doi: 10.3389/fimmu.2018.00316
34. Tzou DT, Taguchi K, Chi T, Stoller ML. Animal Models of Urinary Stone Disease. Int J Surg (2016) 36:596–606. doi: 10.1016/j.ijsu.2016.11.018
35. de Water R, Noordermeer C, Houtsmuller A, Nigg A, Stijnen T, Schröder F, et al. Role of Macrophages in Nephrolithiasis in Rats: An Analysis of the Renal Interstitium. Am J Kidney Dis (2000) 36(3):615–25. doi: 10.1053/ajkd.2000.16203
36. Okada A, Nomura S, Higashibata Y, Hirose M, Gao B, Yoshimura M, et al. Successful Formation of Calcium Oxalate Crystal Deposition in Mouse Kidney by Intraabdominal Glyoxylate Injection. Urol Res (2007) 35(2):89–99. doi: 10.1007/s00240-007-0082-8
37. Okada A, Yasui T, Hamamoto S, Hirose M, Kubota Y, Itoh Y, et al. Genome-Wide Analysis of Genes Related to Kidney Stone Formation and Elimination in the Calcium Oxalate Nephrolithiasis Model Mouse: Detection of Stone-Preventive Factors and Involvement of Macrophage Activity. J Bone Miner Res (2009) 24(5):908–24. doi: 10.1359/jbmr.081245
38. Okada A, Yasui T, Fujii Y, Niimi K, Hamamoto S, Hirose M, et al. Renal Macrophage Migration and Crystal Phagocytosis Via Inflammatory-Related Gene Expression During Kidney Stone Formation and Elimination in Mice: Detection by Association Analysis of Stone-Related Gene Expression and Microstructural Observation. J Bone Miner Res (2010) 25(12):2701–11. doi: 10.1002/jbmr.158
39. Taguchi K, Okada A, Kitamura H, Yasui T, Naiki T, Hamamoto S, et al. Colony-Stimulating Factor-1 Signaling Suppresses Renal Crystal Formation. J Am Soc Nephrol (2014) 25(8):1680–97. doi: 10.1681/ASN.2013060675
40. Yu J, Deng Y, Tao Z, Liang W, Guan X, Wu J, et al. The Effects of HAP and Macrophage Cells to the Expression of Inflammatory Factors and Apoptosis in HK-2 Cells of Vitro Co-Cultured System. Urolithiasis (2018) 46(5):429–43. doi: 10.1007/s00240-017-1032-8
41. Liu Q, Liu Y, Guan X, Wu J, He Z, Kang J, et al. Effect of M2 Macrophages on Injury and Apoptosis of Renal Tubular Epithelial Cells Induced by Calcium Oxalate Crystals. Kidney Blood Press Res (2019) 44(4):777–91. doi: 10.1159/000501558
42. Kusmartsev S, Dominguez-Gutierrez PR, Canales BK, Bird VG, Vieweg J, Khan SR. Calcium Oxalate Stone Fragment and Crystal Phagocytosis by Human Macrophages. J Urol (2016) 195(4P1):1143–51. doi: 10.1016/j.juro.2015.11.048
43. Dominguez-Gutierrez PR, Kusmartsev S, Canales BK, Khan SR. Calcium Oxalate Differentiates Human Monocytes Into Inflammatory M1 Macrophages. Front Immunol (2018) 9:1863. doi: 10.3389/fimmu.2018.01863
44. Taguchi K, Okada A, Hamamoto S, Iwatsuki S, Naiki T, Ando R, et al. Proinflammatory and Metabolic Changes Facilitate Renal Crystal Deposition in an Obese Mouse Model of Metabolic Syndrome. J Urol (2015) 194(6):1787–96. doi: 10.1016/j.juro.2015.07.083
45. Anders HJ, Suarez-Alvarez B, Grigorescu M, Foresto-Neto O, Steiger S, Desai J, et al. The Macrophage Phenotype and Inflammasome Component NLRP3 Contributes to Nephrocalcinosis-Related Chronic Kidney Disease Independent From IL-1–mediated Tissue Injury. Kidney Int (2018) 93(3):656–69. doi: 10.1016/j.kint.2017.09.022
46. Taguchi K, Hamamoto S, Okada A, Unno R, Kamisawa H, Naiki T, et al. Genome-Wide Gene Expression Profiling of Randall’s Plaques in Calcium Oxalate Stone Formers. J Am Soc Nephrol (2017) 28(1):333–47. doi: 10.1681/ASN.2015111271
47. Kusumi K, Ketz J, Saxena V, Spencer JD, Safadi F, Schwaderer A. Adolescents With Urinary Stones Have Elevated Urine Levels of Inflammatory Mediators. Urolithiasis (2019) 47(5):461–6. doi: 10.1007/s00240-019-01133-1
48. Okada A, Ando R, Taguchi K, Hamamoto S, Unno R, Sugino T, et al. Identification of New Urinary Risk Markers for Urinary Stones Using a Logistic Model and Multinomial Logit Model. Clin Exp Nephrol (2019) 23(5):710–6. doi: 10.1007/s10157-019-01693-x
49. Xi J, Chen Y, Jing J, Zhang Y, Liang C, Hao Z, et al. Sirtuin 3 Suppresses the Formation of Renal Calcium Oxalate Crystals Through Promoting M2 Polarization of Macrophages. J Cell Physiol (2019) 234(7):11463–73. doi: 10.1002/jcp.27803
50. Zhu W, Zhao Z, Chou F, Zuo L, Liu T, Yeh S, et al. Loss of the Androgen Receptor Suppresses Intrarenal Calcium Oxalate Crystals Deposition Via Altering Macrophage Recruitment/M2 Polarization With Change of the miR-185-5p/CSF-1 Signals. Cell Death Dis (2019) 10(4):1–19. doi: 10.1038/s41419-019-1358-y
51. Taguchi K, Okada A, Yasui T, Kobayashi T, Ando R, Tozawa K, et al. Pioglitazone, a Peroxisome Proliferator Activated Receptor γ Agonist, Decreases Renal Crystal Deposition, Oxidative Stress and Inflammation in Hyperoxaluric Rats. J Urol (2012) 188(3):1002–11. doi: 10.1016/j.juro.2012.04.103
52. Iba A, Kohjimoto Y, Mori T, Kuramoto T, Nishizawa S, Fujii R, et al. Insulin Resistance Increases the Risk of Urinary Stone Formation in a Rat Model of Metabolic Syndrome. BJU Int (2010) 106(10):1550–4. doi: 10.1111/j.1464-410X.2010.09216.x
53. Chen Z, Yuan P, Sun X, Tang K, Liu H, Han S, et al. Pioglitazone Decreased Renal Calcium Oxalate Crystal Formation by Suppressing M1 Macrophage Polarization Via the PPAR-γ-miR-23 Axis. Am J Physiol Physiol (2019) 317(1):F137–51. doi: 10.1152/ajprenal.00047.2019
54. Liu H, Yang X, Tang K, Ye T, Duan C, Lv P, et al. Sulforaphane Elicts Dual Therapeutic Effects on Renal Inflammatory Injury and Crystal Deposition in Calcium Oxalate Nephrocalcinosis. Theranostics (2020) 10(16):7319–34. doi: 10.7150/thno.44054
55. Yang X, Liu H, Ye T, Duan C, Lv P, Wu X, et al. Ahr Activation Attenuates Calcium Oxalate Nephrocalcinosis by Diminishing M1 Macrophage Polarization and Promoting M2 Macrophage Polarization. Theranostics (2020) 10(26):12011–25. doi: 10.7150/thno.51144
56. Sun P-P, Zhou X-J, Su J-Q, Wang C, Yu X-J, Su T, et al. Urine Macrophages Reflect Kidney Macrophage Content During Acute Tubular Interstitial and Glomerular Injury. Clin Immunol (2019) 205:65–74. doi: 10.1016/j.clim.2019.06.005
57. Yang JYC, Sarwal RD, Ky K, Dong V, Stoller M, Sarwal MM, et al. Non-Radiological Assessment of Kidney Stones Using the Kidney Injury Test (KIT), a Spot Urine Assay. BJU Int (2020) 125(5):732–8. doi: 10.1111/bju.14978
58. Bansal R, Ginhoux F, Bartneck M, Lanao JM, Colino CI, Gutierrez-Millan C. Targeting of Hepatic Macrophages by Therapeutic Nanoparticles. Front Immunol (2020) 11:218. doi: 10.3389/fimmu.2020.00218
Keywords: urolithiasis, nephrocalcinosis, calcium oxalate (CaOx), Randall plaque, macrophage, M1-macrophage, M2-macrophage, monocyte
Citation: Taguchi K, Okada A, Unno R, Hamamoto S and Yasui T (2021) Macrophage Function in Calcium Oxalate Kidney Stone Formation: A Systematic Review of Literature. Front. Immunol. 12:673690. doi: 10.3389/fimmu.2021.673690
Received: 28 February 2021; Accepted: 06 May 2021;
Published: 24 May 2021.
Edited by:
Uday Kishore, Brunel University London, United KingdomReviewed by:
Zhangqun Ye, Huazhong University of Science and Technology, ChinaPanagiotis F. Christopoulos, Oslo University Hospital, Norway
Copyright © 2021 Taguchi, Okada, Unno, Hamamoto and Yasui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazumi Taguchi, a3RhZ3VjaGlAbWVkLm5hZ295YS1jdS5hYy5qcA==
 Kazumi Taguchi
Kazumi Taguchi Atsushi Okada
Atsushi Okada Rei Unno
Rei Unno Shuzo Hamamoto
Shuzo Hamamoto Takahiro Yasui
Takahiro Yasui