- 1Centro Universitario de Ciencias de la Salud, Doctorado en Ciencias Biomédicas, Universidad de Guadalajara, Guadalajara, Mexico
- 2Centro Universitario de Ciencias de la Salud, Instituto de Investigación en Reumatología y del Sistema Músculo-Esquelético (IIRSME), Universidad de Guadalajara, Guadalajara, Mexico
- 3Centro Universitario de Ciencias de la Salud, Departamento de Disciplinas Filosófico, Metodológicas e Instrumentales, Universidad de Guadalajara, Guadalajara, Mexico
- 4Hospital Civil Dr. Juan I. Menchaca, División de Medicina Interna, Servicio de Reumatología 004086, PNPC CONACyT, Guadalajara, Mexico
- 5Centro Universitario de Ciencias de la Salud, UDG-CA 703 Inmunología y Reumatología, Universidad de Guadalajara, Guadalajara, Mexico
- 6Departamento de Reumatología Centro Médico Nacional 20 de Noviembre, Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE), Ciudad de México, Mexico
- 7Centro Universitario de Ciencias de la Salud, Departamento de Fisiología, Universidad de Guadalajara, Guadalajara, Mexico
- 8Centro Universitario de Ciencias de la Salud, Departamento de Biología Molecular y Genómica, Universidad de Guadalajara, Guadalajara, Mexico
The idiopathic inflammatory myopathies (IIM) are characterized by muscular weakness, cutaneous manifestations, muscle damage revealed by increase of muscular enzymes, muscle biopsy, electromyography and changes on magnetic resonance imaging. However, the hallmark of these IIM, is the development of myositis specific antibodies (MSA) or myositis associated antibodies (MAA). The theories about their presence in the serum of IIM is not known. Some studies have suggested that some of these MSA, such as anti-Mi-2 increases according to the intensity of UV radiation. There is scarce information about the environmental factors that might contribute in order to be considered as triggering factors as UV radiation might be. In this review, we analyzed the reported prevalence of MSAs and MAAs regarding to their geographical location and the possible relation with UV radiation. We collected the prevalence data of fifteen MSA and thirteen MAA from 22 countries around the world and we were able to observe a difference in prevalence between countries and continents. We found differences in anti-PL7, anti-Ro52, anti-La and anti-Ku prevalence according to UV radiation level. Otherwise, we observed that anti-Mi-2 prevalence increases near to the Equator meanwhile anti-MJ/NXP2 and anti-ARS prevalence had an opposite behavior increasing their prevalence in the geographical locations farther to the Equator. Our results highlighted the importance to include the UV radiation and other environmental factors in IIM studies, in order to clarify its association with MSA and MAA prevalence as well as its possible role in the immunopathogenesis of these diseases.
Introduction
The idiopathic inflammatory myopathies (IIM), also known as myositis, represent a heterogeneous group of autoimmune rheumatic diseases. The main features are muscle weakness, multiorgan involvement including skin, joints, lungs, heart and gastrointestinal tract, as well as malignancy development (1, 2).
One of the more recent classification criteria of myositis, is the Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies established by the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) in 2017. According to this, the myositis subgroups encompass dermatomyositis (DM), amyopathic dermatomyositis (ADM), juvenile dermatomyositis (JDM), polymyositis (PM), inclusion body myositis (IBM), immune-mediated necrotizing myopathy (IMNM) and juvenile myositis (JM) (3).
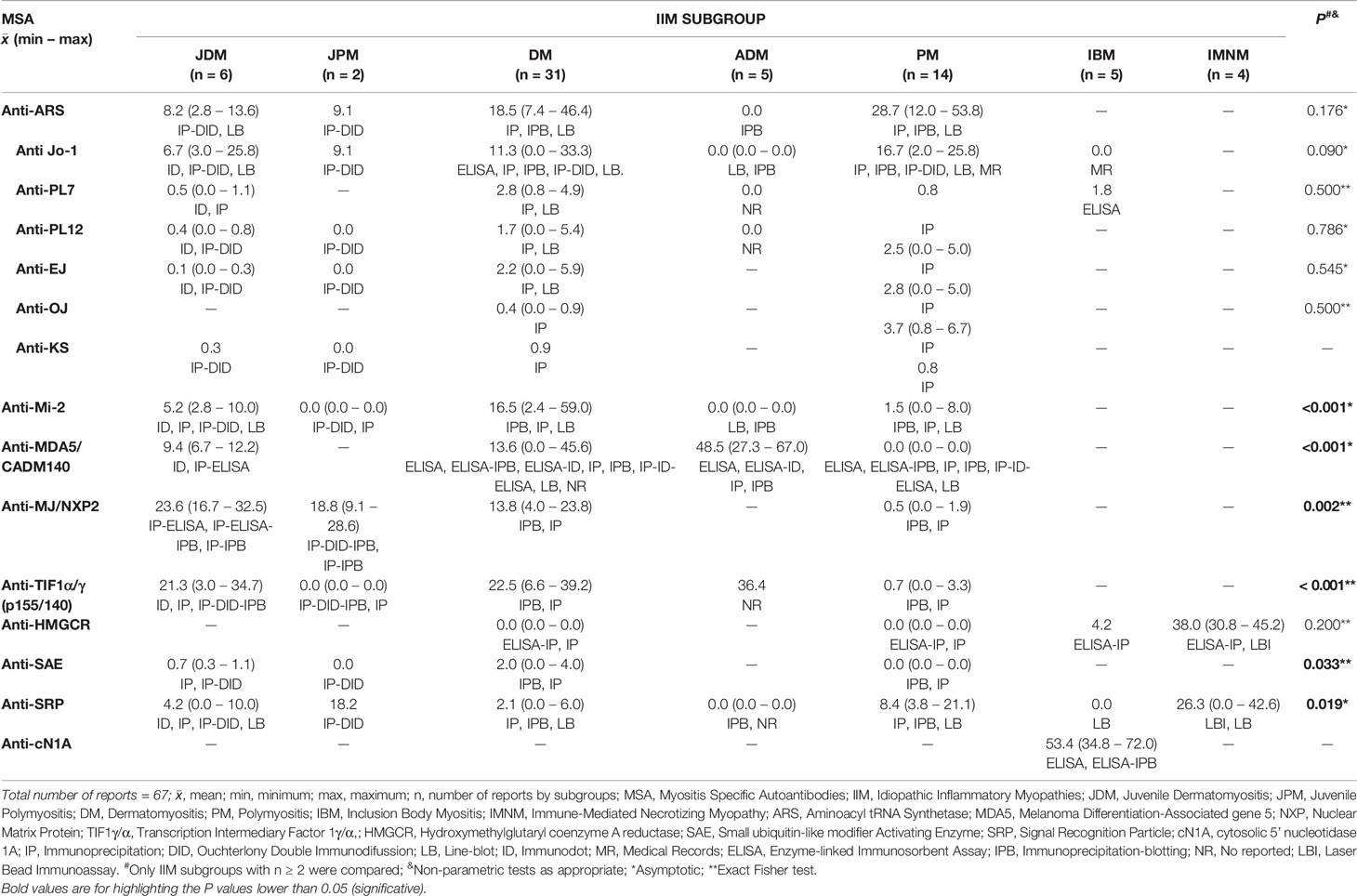
These subgroups differ in age, clinical manifestations and histopathological features. IIM are associated with the presence of myositis specific antibodies (MSA) and myositis associated antibodies (MAA) (Figure 1) (4).
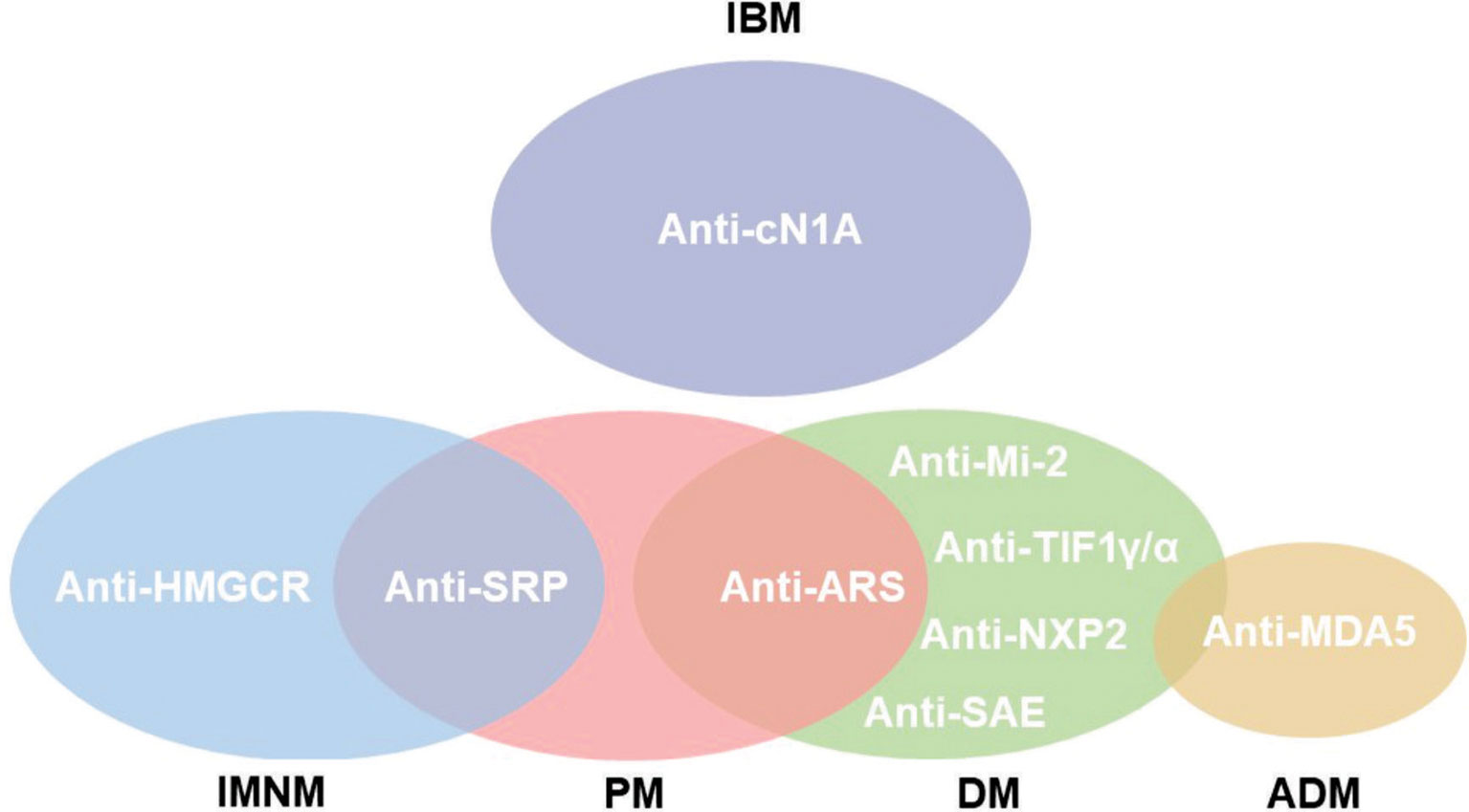
Figure 1 Subgroups of IIM according to autoantibody phenotype. Not all autoantibodies are exclusive for the myopathy subgroup, as is the case of anti-Signal Recognition Particle (SRP) that might be found in polymyositis (PM) and immune-mediated necrotizing myopathy (IMNM). Notwithstanding, anti-hydroxymethylglutaryl coenzyme A reductase (HMGCR) is classically observed in IMNM. The anti-aminoacyl tRNA synthetase (ARS) autoantibodies are related to anti-synthetase syndrome (ASSD). Inclusion body myositis (IBM) is associated but not exclusive for anti-cytosolic 5’nucleotidase 1A (cN1A). Dermatomyositis (DM) is associated to cancer development in positive patients for anti-Transcription Intermediary Factor 1γ/α (TIF1γ/α). Anti-Mi-2, Nuclear Matrix Protein 2 (NXP2) and anti-Small ubiquitin-like modifier Activating Enzyme (SAE) are also related to DM. The presence of anti-Melanoma Differentiation-Associate Gene 5 (MDA5) is associated with rapidly progressive interstitial lung disease (RPILD) in amyopathic dermatomyositis (ADM).
Environment and genetics might be involved in myositis pathogenesis. UV radiation has been identified as a risk factor for DM development (5). The geographic distribution of MSA for Mi-2 and its association with UV radiation and proximity to the equator area is observed (6–9).
UV as a Triggering Factor for Autoimmunity in Dermatomyositis
UV light is classified according to wavelength into UVA (315 – 400 nm), UVB (280 – 315 nm) and UVC (200 – 280 nm). In the case of UVB, it does not penetrate deeper than epidermis and is strongly absorbed by DNA and proteins, therefore it is an inducer of DNA damage of keratinocyte resulting in apoptosis and antigen re-localization (10, 11).
Other important aspect of UVB radiation, is that promotes the expression of adhesion molecules as well as keratinocyte stimulation to produce IL-1, IL-6, IL-8, IL-10, GM-CSF, TNF-α and IFN-I (12, 13). These cytokines might influence the type I IFN signature in IIM through the myocytes overexpression of MHC class I molecules as well as B cell activating factor belonging to the TNF family (BAFF) activation (14).
Prevalence of Anti-Mi-2, UV Radiation and Geographical Distribution
Anti-Mi-2 is an autoantibody mostly present in DM. Mi-2 antigen has two recognized isoforms named as alpha and beta; encoded by the chromodomain helicase DNA binding protein 3 (CHD3) and 4 (CHD4) genes, respectively. Mi-2 is part of the nucleosome and remodeling deacetylase (NuRD) complex, which is characterized by its different enzymatic activities: ATP-dependent nucleosome remodeling and histone deacetylase activity (15). Mi-2 as part of the complex NuRD, has been reported to show a check-point like activity during DNA replication as important factor for the stability of pericentric heterochromatin (16).
Using cell lines of male and female keratinocytes, it was demonstrated that UVB radiation increases the Mi-2 antigen expression (17). Notwithstanding, other studies have demonstrated that MSA autoantibodies are not dependent of the level of gene transcription for their particular autoantigen (18). In the last years, collaborative worldwide groups have been published studies dealing with clinical characteristics according to IIM classification.
Since it has been reported that Mexico has the highest prevalence of anti-Mi-2 autoantibodies (9, 19). In this review paper, we focused on literature regarding prevalence of MSA and MAA according to geographical location.
Methods
For the references revision, we used the PubMed and medical subject headings (MeSH) data base from the National Center for Biotechnology Information (NCBI). We considered the publication dates from 10/01/1999 to 10/07/2019 (8). With regard to search criteria, we included clinical studies, meta-analysis, observational studies and twin studies published only in English language.
We considered three key aspects: myositis, geographical location and antibodies in the MeSH database (Figure 2). We did the PubMed search on October 8th, at that moment, the final algorithm for our search in PubMed was:
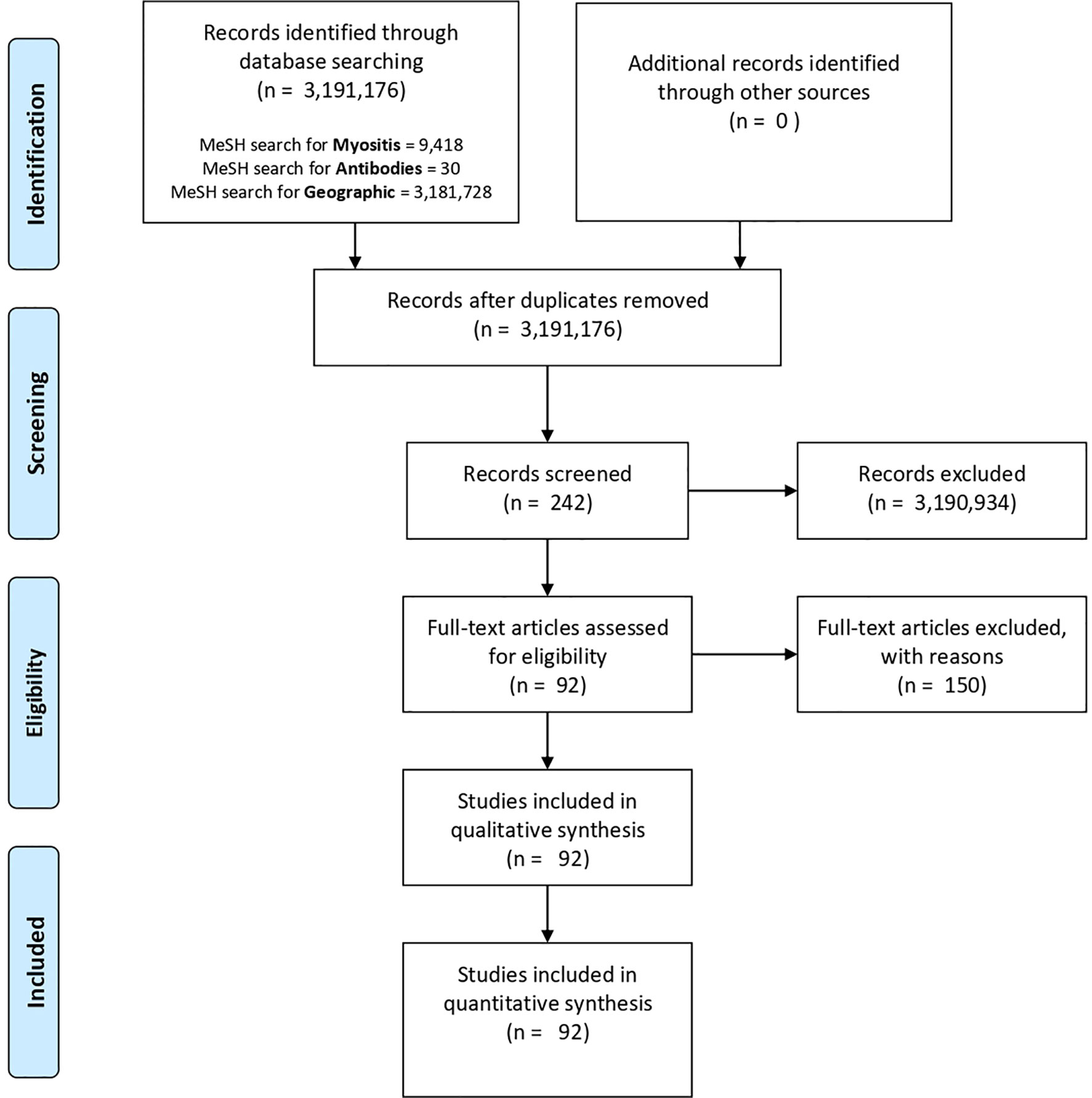
Figure 2 PubMed and MeSH database PRISMA flow diagram. Three key aspects were considered in our database search: myositis (myositis, polymyositis, inclusion body myositis or dermatomyositis), geographical location (epidemiology or geographic location) and antibodies (antibodies or anti-Mi-2). However, the only screened records were those papers related to the three important aspects in our search (n = 242). Finally, 150 of them were excluded and 92 were considered and reviewed in this study.
(((“Myositis”[Mesh] OR “Myositis, Inclusion Body”[Mesh] OR “Polymyositis”[Mesh] OR “Dermatomyositis”[Mesh]) AND (“1999/10/01”[PDAT]: “2019/10/07”[PDAT])) AND ((“Geographic Locations”[Mesh] OR “epidemiology”[Subheading]) AND (“1999/10/01”[PDAT]: “2019/10/07”[PDAT]))) AND ((“Mi-2 antibodies”[Supplementary Concept] OR “Antibodies”[Mesh]) AND (“1999/10/01”[PDAT]: “2019/10/07”[PDAT])) AND ((“1999/10/01”[PDAT]: “2019/10/07”[PDAT]) AND English[lang])
As a result of the final search, we obtained 242 articles. However, not all of them were included for this review (Supplementary Table 1). The exclusion criteria were: lack of relation with the central topic, absence or unclear prevalence of MSAs and MAAs, as well as overlapping with other diseases. In the case of multicentric studies they were considered only if the number of subjects per city was more than 10, including the autoantibody prevalence. Meta-analysis and review papers, were excluded because of data duplication. The final number of considered articles for this review was 92 (6, 9, 20–108).
We obtained the monthly city UV average from the website: https://www.worldweatheronline.com/. Initially, we tried to obtain the annual UV average of the year of the publication date or the year when the study was carried out; however, in some articles the period of collection data was very wide. The considered time for our reference search was established from 1999 to 2019, and we decided to consult the 2010 monthly UV average. From these data, we calculated the measures of central tendency including mean and median as well as the minimal and maximal UV radiation per year of every city.
Once we obtained the UV radiation level data, we classified them according to a collaboration of: World Health Organization, World Meteorological Organization, United Nations Environment Program and the International Commission on Non-Ionizing Radiation Protection. Then the Global Solar UV Index (UVI) that describes the level of solar UV radiation at Earth’s surface and its values were grouped into exposure categories in the next ranges: a) low: < 2; b) moderate: 3 to 5; c) high: 6 to 7; d) very high: 8 to 10 and; e) extreme: up to 11.
We also consider the latitude of every city from the website: (https://www.geodatos.net/en), afterwards, we classified the prevalence according to geographical areas: a) Antarctic polar circle to Tropic of Capricorn; b) Tropic of Capricorn to Equator; c) Equator to Tropic of Cancer, and; d) Tropic of Cancer to Artic polar circle.
All the autoantibody prevalence from the reviewed articles as well as the UV and latitude values, were analyzed by SPSS v.22 and graphed as appropriate by GraphPad Prism v.6 software. The obtained data was reported in tables as mean, minimum and maximum. The non-parametric (Kolmogorov-Smirnov, Mann-Whitney U, Kruskal-Wallis, Exact Fisher and Spearman’s Rho) statistics tests were carried out due to the type of variables and sample size. A P value lower than 0.05 was considered significant.
Results
The Prevalence of MSA but Not MAA Is Distinct According to IIM Phenotype
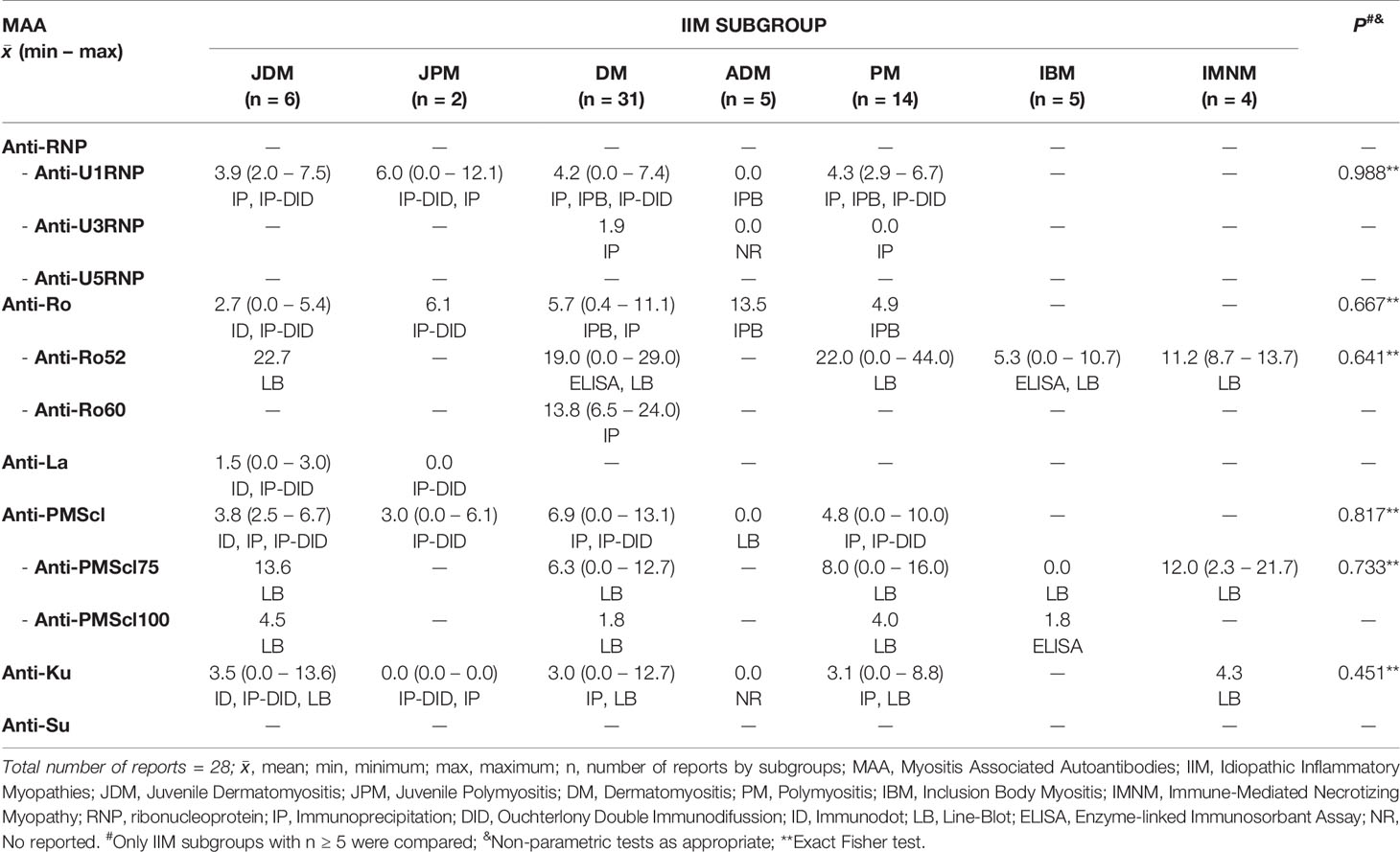
Within the 92 selected articles, we firstly identified the MSA and MAA prevalence, being statistically different for anti-Mi-2, Anti-MDA5/CADM140, anti-MJ/NXP2, Anti-TIF1α/γ, anti-SAE and anti-SRP. The IIM subgroups included JDM, JPM, DM, PM, IBM and IMNM (Tables 1 and 2).
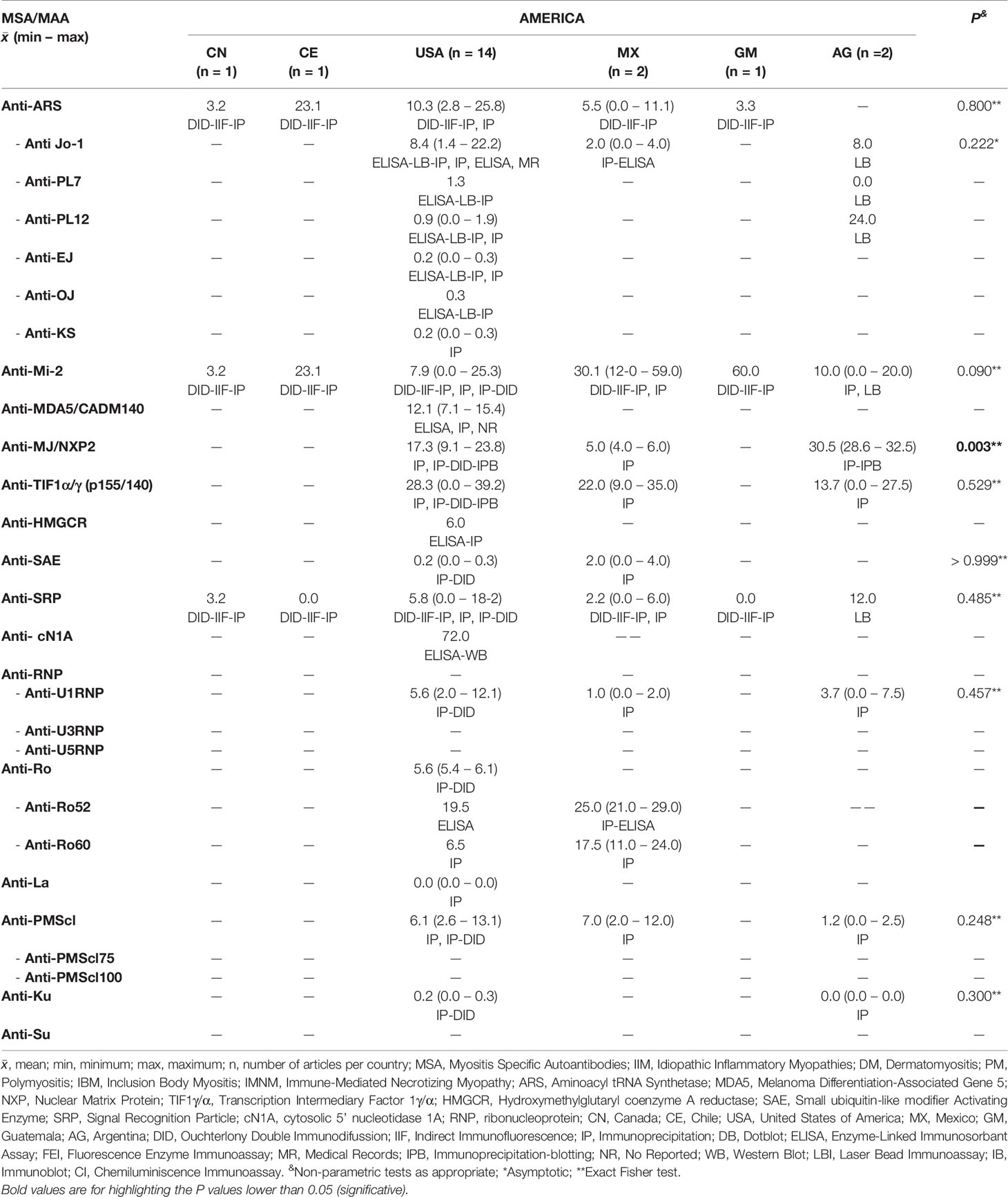
Not All the MSA Differ on Prevalence Between Countries, Continents or Geographical Areas
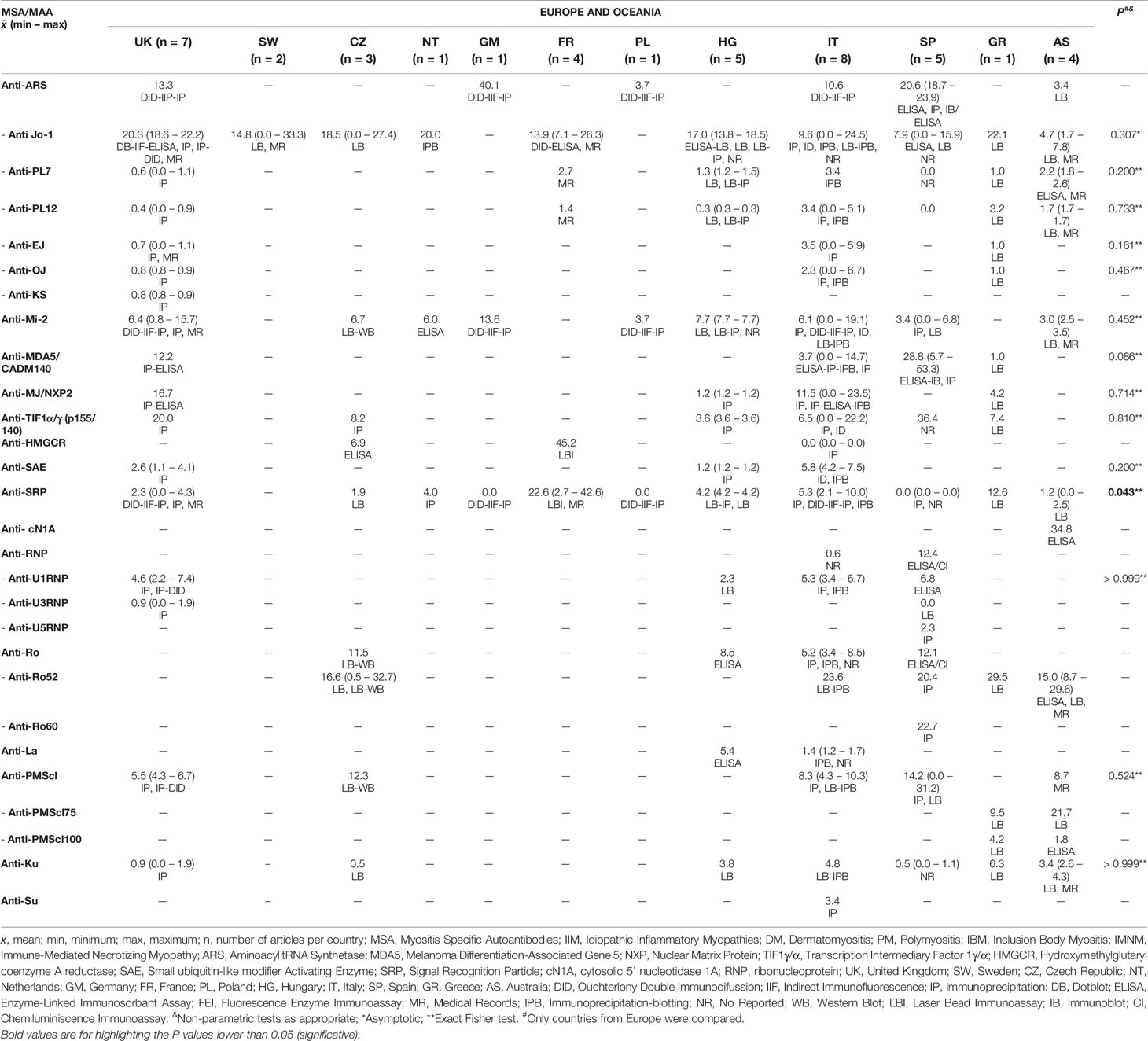
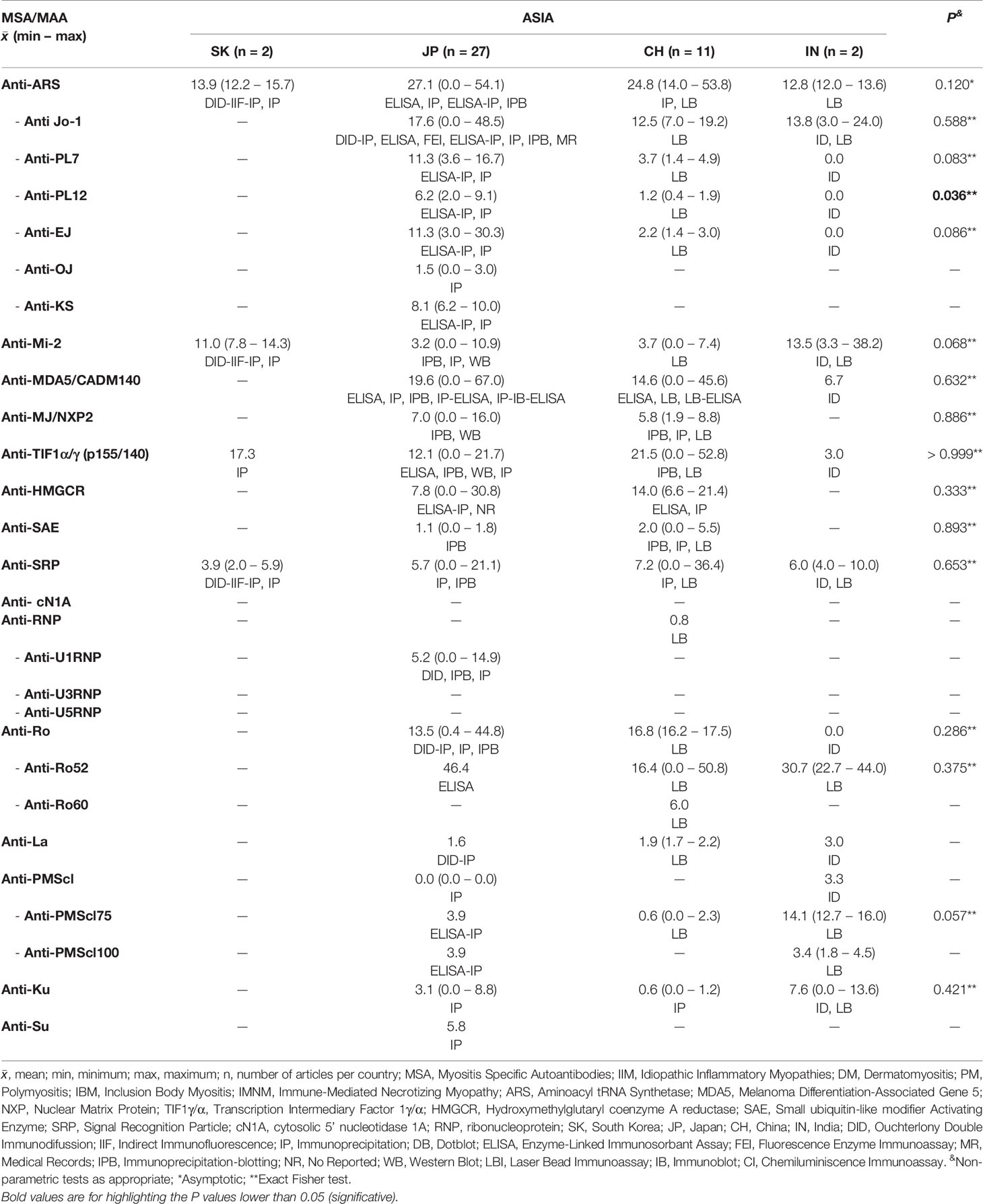
We obtained information of MSA and MAA prevalence from 22 countries around the world (Tables 3–5). Interestingly, when we compared the prevalence between countries, we only find differences in some autoantibodies: anti-SRP between European countries (P = 0.043) (Table 3), anti-PL12 (P = 0.036) in Asia (Table 4) and anti-MJ/NXP2 in American continent (P = 0.003) (Table 5). However, when we grouped and compared the prevalence between continents, we found differences in some MSA such as anti-aminoacyl tRNA synthetases (ARS) (P = 0.015), anti-Jo-1 (P = 0.049), anti-PL7 (P = 0.017) and anti-MJ/NXP2 (P = 0.023) (Supplementary Table 2).
Considering the annual mean of UV radiation per city, we categorized all the considered countries according to UVI scale (Supplementary Table 3). Within the 22 countries, we found that United Kingdom and Sweden had the lowest UV average radiation while India the highest.
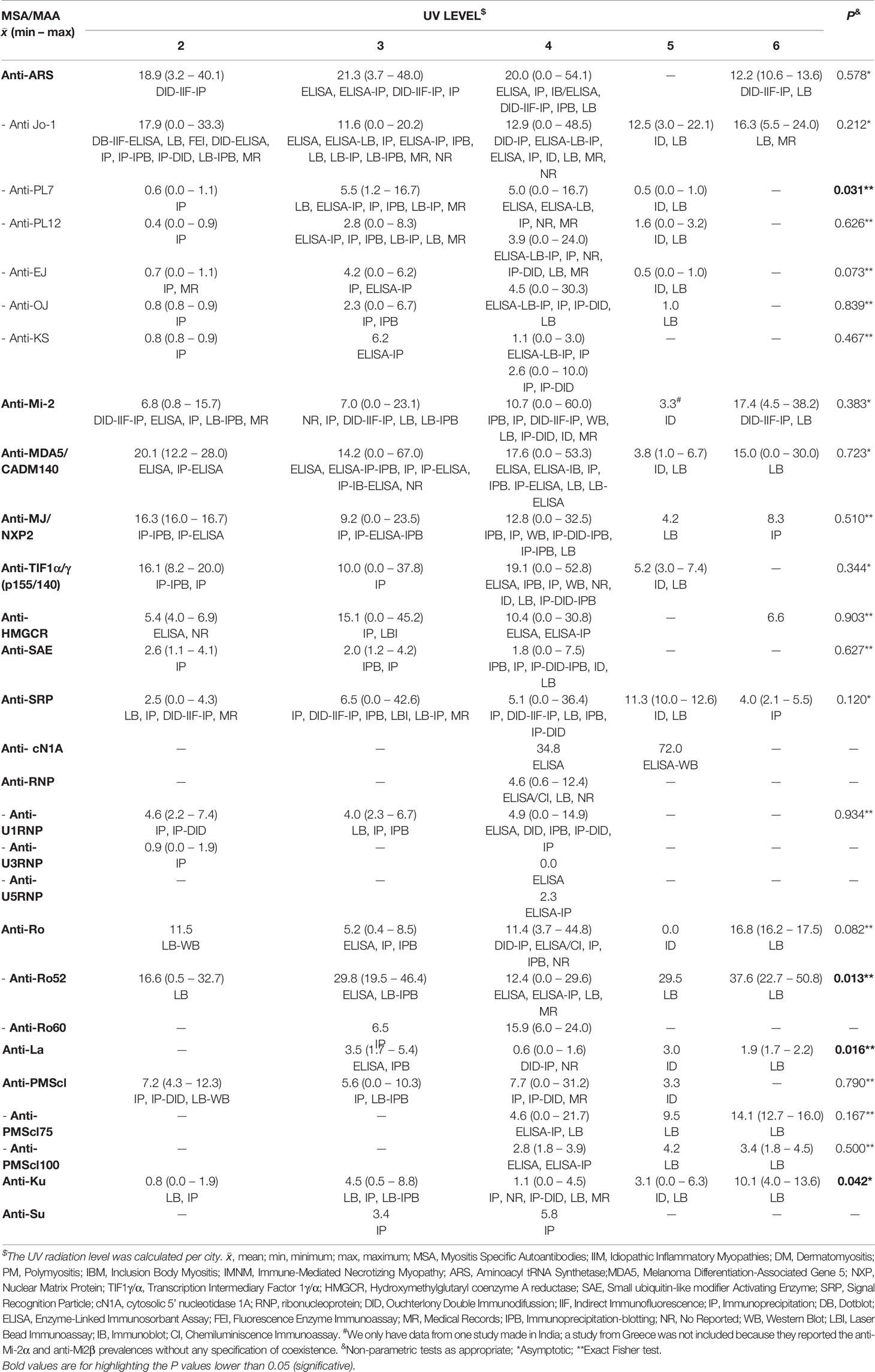
It is important to highlight that we obtained a mean of annual UV level per country. When we analyzed the autoantibody prevalence according to the UV, we only find differences in anti-PL7 (P = 0.031), anti-Ro52 (P = 0.013), anti-La (P = 0.016) and anti-Ku (P = 0.042) (Table 6). Anti-Mi-2 prevalence between UV radiation levels was not statistically significant, however, we could observe a trend to increase according to UV level (Table 6).
We also looked for a correlation between MSA, MAA and UV radiation in all IIM subgroups. Regarding anti-Mi-2, we found a correlation with annual minimum UV radiation (rs = 0.289, P = 0.028) (Figure 3).
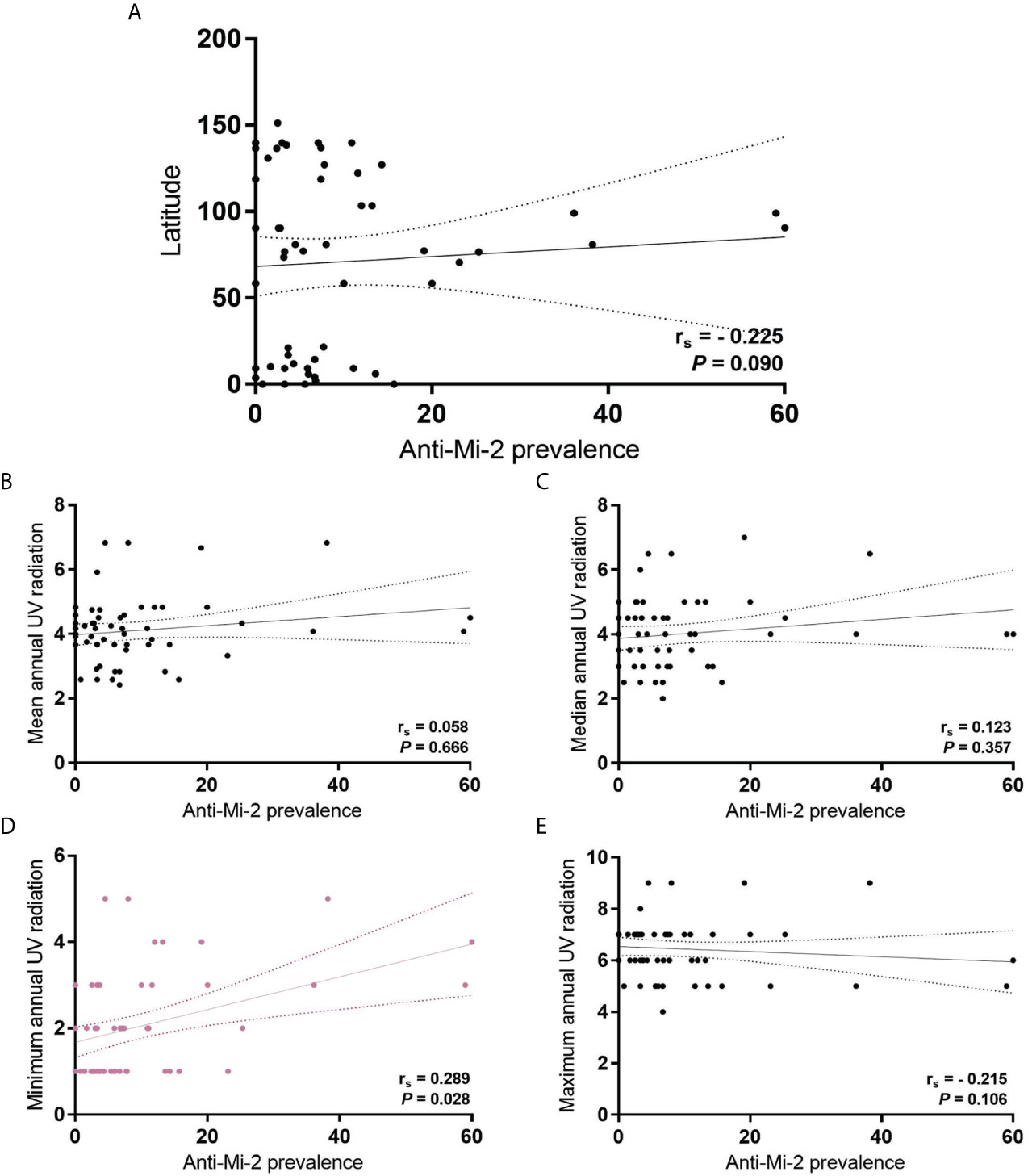
Figure 3 Correlation of anti-Mi-2 prevalence in all IIM subgroups with (A) latitude; (B) mean annual UV radiation; (C) median annual UV radiation; (D) minimum annual UV radiation, and; (E) maximum annual UV radiation.
Is It a Contradiction to State That Anti-Mi-2 Does Not Associate With UV Levels but Correlates With Annual Minimum UV?
One caveat of the studies analyzed for this review, is the lack of UV radiation real exposure data of the subjects enrolled. So, we think that in the field of environmental factors related to IIM study, it is important to consider the time and intensity to the UV exposure where the patients live. Then it is important to include this information on worldwide database. Because of the surprising behavior of our data, we decided to include the geographical latitude in this study, we re-classified the prevalence according to geographic location. We found differences between mean UV radiation of the considered countries according to the geographic location (P <0.001) (Figure 4). It is important to highlight that we also found a correlation between UV radiation and latitude, including mean (rs = -0.756, P <0.001), median (rs = -0.683, P <0.001), minimum (rs = -0.645, P <0.001) and maximum (rs = -0.645, P <0.001) (Supplementary Figure 1).
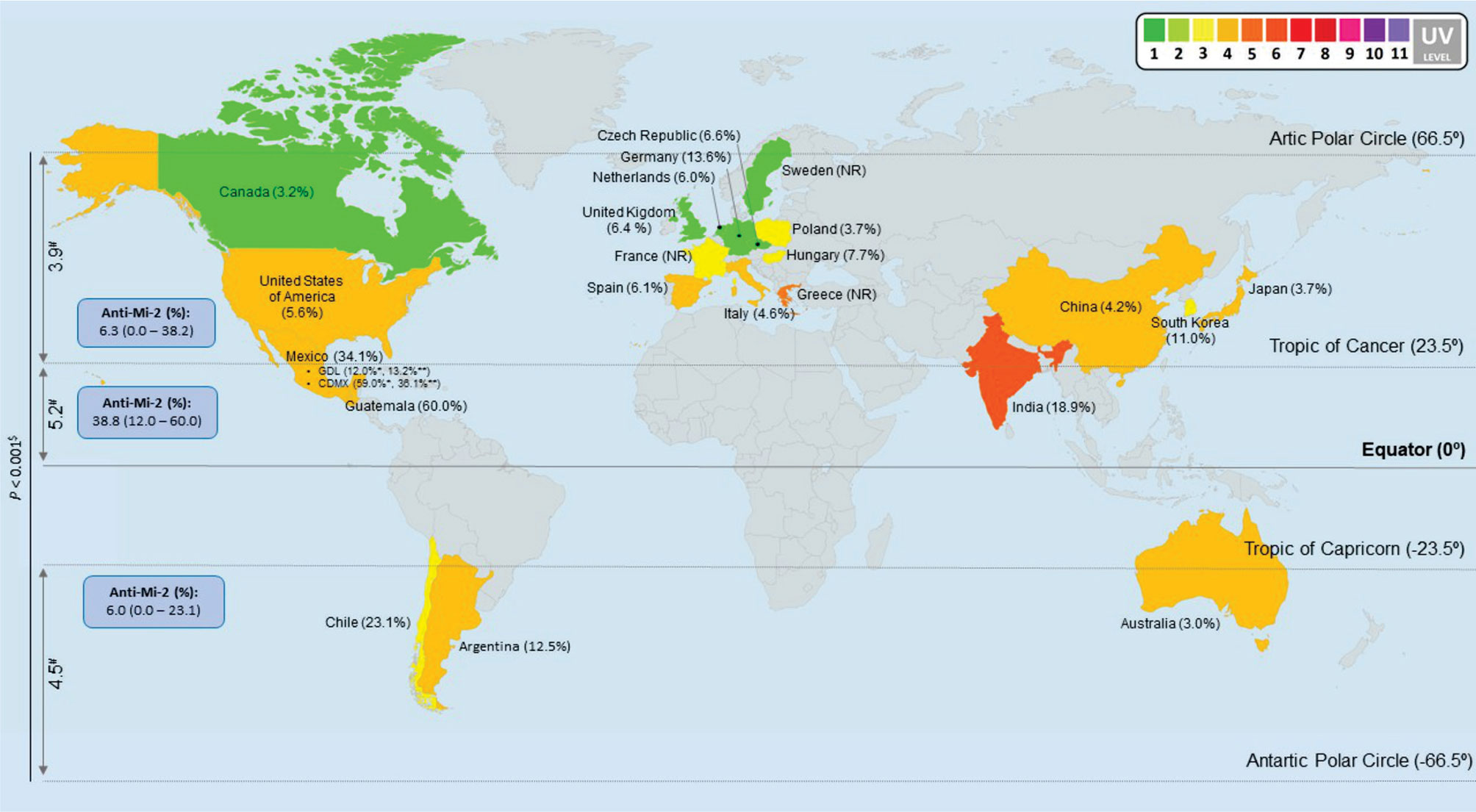
Figure 4 Anti-Mi-2 global prevalence in all IIM subgroups. We classified all our data per countries and according to geographic locations to obtain anti-Mi-2 prevalence. The map shows that the anti-Mi-2 prevalence increases in the geographic zone closer to the Equator and decreases in the locations farther to the Equator; likewise, UV radiation increases according to Equator proximity and it is different between geographic locations (P < 0.001). #UV annual average radiation, we only considered the colored countries in the map. $Non-parametric test, asymptotic significance. * (9). ** (6). NR: No Reported prevalence of anti-Mi-2 in these countries. Map image was made at: (https://mapchart.net/world.html).
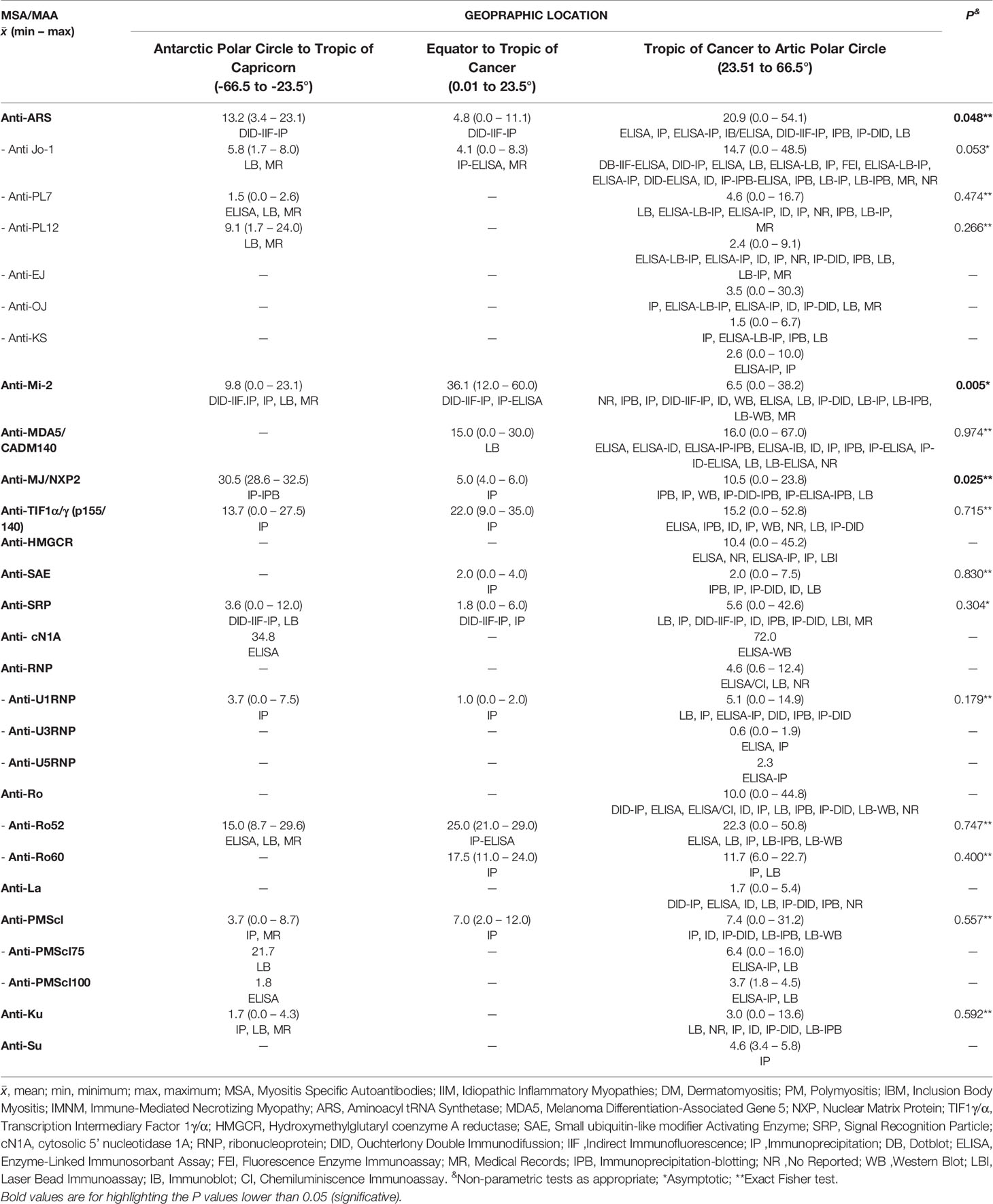
We decided to look forward for differences in the MSA and MAA prevalence according to geographic zones instead of UV radiation level. We found differences for anti-Mi-2 (P = 0.005), anti-MJ/NXP2 prevalence (P = 0.025) and anti-ARS (P = 0.048) (Table 7, Figure 5). Anti-Mi-2 shows a higher prevalence in the closest region to equator (Figure 4).
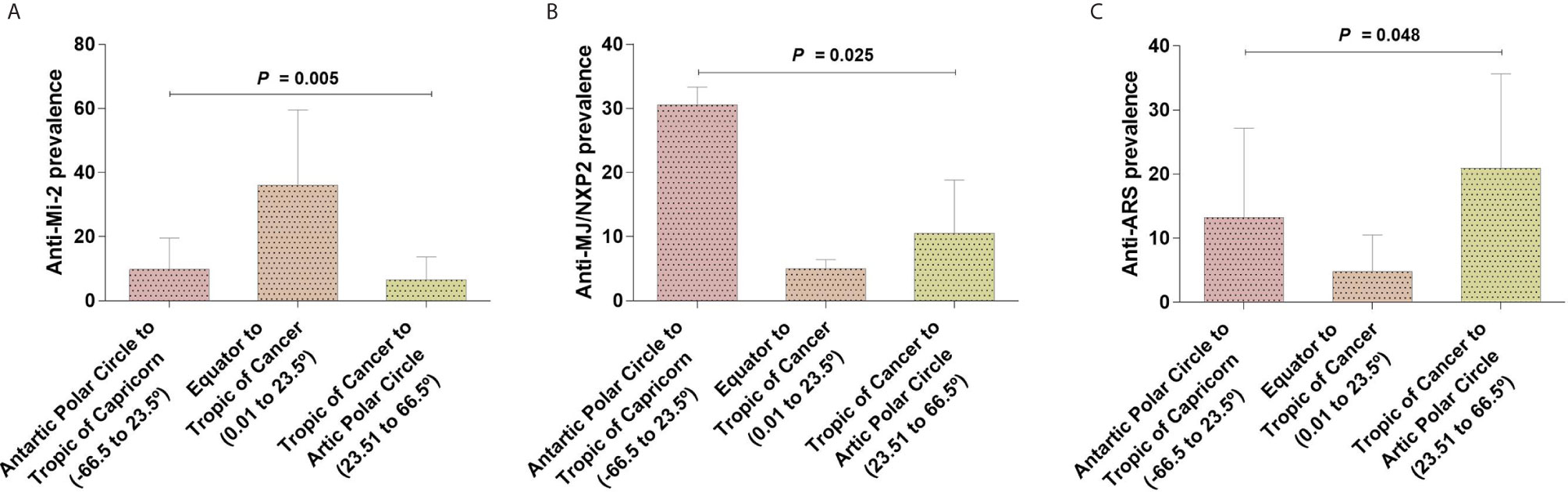
Figure 5 Differences of autoantibodies prevalence between geographical locations. (A) Anti-Mi-2 showed an increase in the geographical region closer to the Equator and a decrease in those farther to the Equator; (B) anti-MJ/NXP2 and (C) anti-ARS prevalence had an opposite behavior increasing in the geographical locations farther to the Equator.
Although we found differences, the data behavior was not the same; for example, in the case of anti-Mi-2, we observed that the prevalence increased in the region closer to the Equator and decreased in the other two geographic locations (Figures 4 and 5). In the case of anti-MJ/NXP2 we observed a major prevalence in the region from Antarctic Polar Circle to Tropic of Capricorn and this decreased while approaching to Equator (Figure 5) while anti-ARS prevalence increases in the zone from Tropic of Cancer to Artic Polar Circle (Figure 5).
Anti-Mi-2, Anti-PL12, Anti-Ro52 and Anti-PMScl-75 Autoantibodies Have a Negative Correlation With Geographical Latitude
We investigated the correlation between latitude and autoantibodies prevalence and we observed that anti-Mi-2 (Figure 4A), and anti-Ro-52 (Figure 6A) prevalence follows a trend to diminish while latitude increases. The prevalence of anti-PL12 and anti-PMScl-75 have a negative correlation with geographical latitude (Figures 6B, C). However, only anti-PMScl-75 autoantibody also showed a correlation with mean UV radiation (Figure 6C).
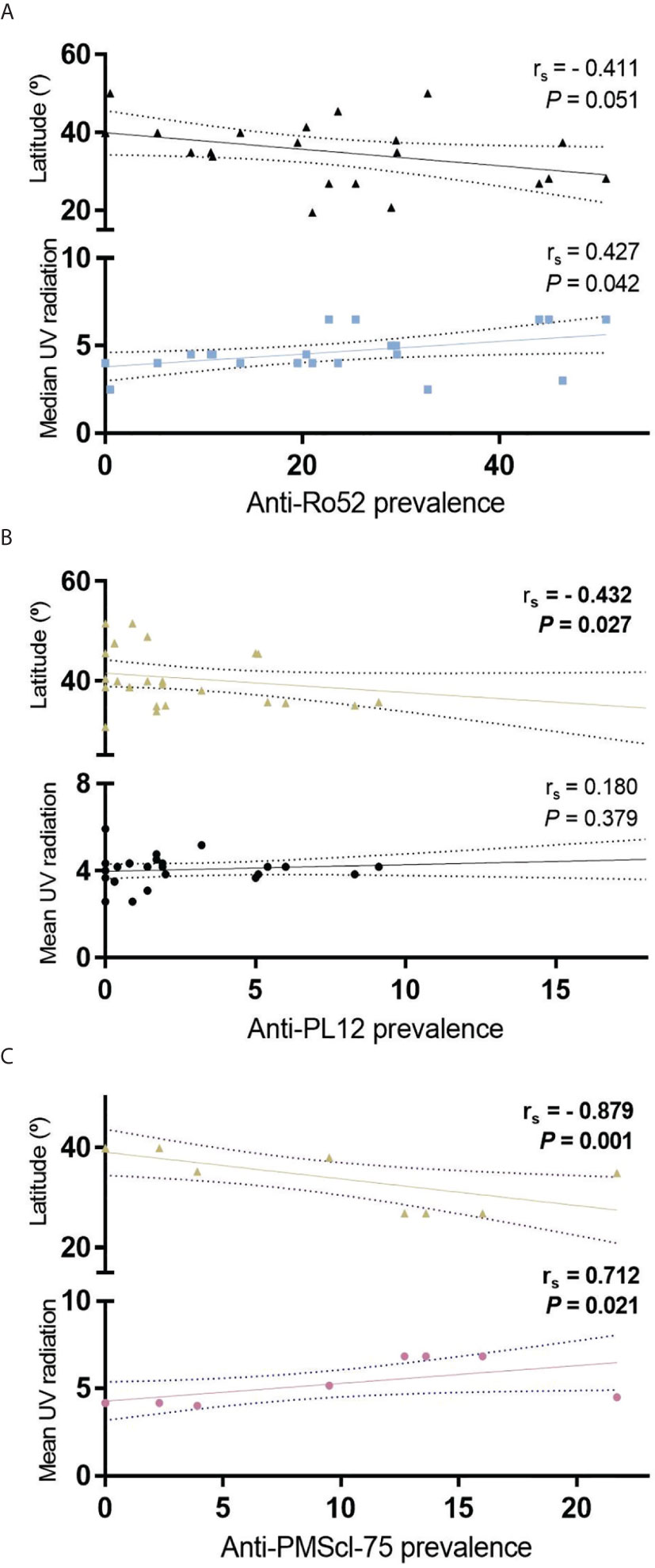
Figure 6 Correlation of geographical latitude and mean UV radiation with (A) anti-Ro52; (B) anti-PL12 and; (C) anti-PMScl-75 in all IIM subgroups.
Discussion
UV radiation intensity changes every day, for this reason its measure becomes complicated. In this systemic review, we tried to obtain an UV annual approximate of every city where autoantibodies prevalence was reported; however, the time period of patient recruitment was very wide (even up to 10 years). Since these difficulties, we could not observe a direct correlation between UV radiation and MSA. In order to get better results, we encourage to consider the UV radiation levels in future MSA and MAA prevalence reports.
Because of this situation, our alternative option was to consider geographic latitude; it gave us greater precision in our analysis. Additionally, we successfully achieved to show a correlation between latitude and UV radiation.
Our main results revealed that anti-PL7, anti-Ro52, anti-La and anti-Ku showed differences between UV radiation levels. Once we analyzed the prevalence according to latitude, we could observe a difference of anti-Mi-2 prevalence according to the geographical zone as well as a trend to a correlation between anti-Mi-2 prevalence and proximity to the Equator.
Interestingly, we noticed differences in the prevalence of anti-ARS and anti-MJ/NXP2 between the geographical locations, but they did not correlate with geographical latitude. In the case of anti-Mi-2, it was different between geographic zones and showed a trend to diminish in higher latitude.
Otherwise, we found that the prevalence of anti-PL12, and anti-PMScl-75 are correlated to geographical latitude. It is important to note that only anti-PMScl-75 correlated with both geographical latitude and UV radiation, might due to the lack of UV radiation accurate data.
The behavior of these data suggests that the prevalence of anti-PL12, anti-Ro52 and anti-PMScl-75 autoantibodies also increases according to the equator proximity. Anti-Ro52, had already been previously reported to follow this behavior (11); however, to our knowledge, this is the first study reporting the differential geographic distribution of anti-PL12 and anti-PMScl-75.
Although the mechanisms that regulate this behavior and geographical distribution remain unclear, they could be associated to UV radiation as a key factor in the pathogenesis of the disease. In addition, it has been documented that the source of autoantigens in other rheumatic autoimmune diseases such as Systemic Lupus Erythematosus (SLE), comes from debris released after apoptosis including Ro52, Ro60, La, U1, and even Mi-2 (109). It is important to highlight that we could observe an increment in autoantibodies against Mi-2 and Ro-52 according to their proximity to the Equator and higher UV radiation levels. This probably could be related with UV radiation.
Although we initially considered 242 articles, we only obtained relevant information of 92; however, there is still a poorly information and we had limitations such a lack of reports about prevalence of all the autoantibodies of interest in different countries and UV levels in order to get a better overview of the behavior of MSAs and MAAs according to geographical location and the role of UV radiation in the development of autoimmunity.
In this review we could obtained MSA and MAA prevalence data from 22 countries and we offer the evidence that there are differences in their prevalence between countries, as well as the fact that anti-Jo-1 is not the most prevalent autoantibody around the world, just in European countries. In Mexico, anti-Mi-2 is the most prevalent autoantibody reported maybe to UV radiation; however, other environment factors need to be considered. In summary, in the case of anti-Mi-2 we observed that the prevalence increased in the region closer to the Equator, meanwhile anti-MJ/NXP2 and anti-ARS demonstrated a major prevalence far from Equator zone.
Limitations
UV radiation intensity changes every day, for this reason its measure becomes complicated. In this systemic review, we tried to obtain an UV annual approximate of every city where autoantibodies prevalence was reported; however, the time period of patient recruitment was very wide (even up to 10 years). Since these difficulties, we could not observe a direct correlation between UV radiation and anti-MSA.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
AA-V. Contributed to the conception and design of the study, carried out the statistics and participated in analysis and interpretation of data. Drafted the paper and approved the final version of the manuscript. EC-A. Contributed to the conception and design of the study, carried out the statistics and participated in analysis and interpretation of data. Drafted the paper and approved the final version of the manuscript. OP-M. Contributed to the execution and participated in analysis and interpretation of data. Critically revised the draft of article and approved the final version of manuscript. LA-O. Contributed to the execution and participated in analysis and interpretation of data. Critically revised the draft of article and approved the final version of manuscript. AR-H. Contributed to the execution, carried out the statistics and participated in data interpretation. Critically revised the draft of article and approved the final version of manuscript. E-DR-A. Contributed to the execution, carried out the statistics and participated in data interpretation. Critically revised the draft of article and approved the final version of manuscript. MV-M. Contributed to the conception and the design of the study, execution, verified the analysis and interpretation of data. Critically revised the draft of article and approved of the final version of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Fondo de Desarrollo Cientifico (FODECIJAL) 2019 from Consejo Estatal de Ciencia y Tecnología de Jalisco (COECYTJAL), with approval number 1702512-8152.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We want to thanks to Alondra V. Espinosa-Herrera and Erick J. Diaz-Huerta for their collaboration in records collection. We are also thankful with Cynthia A. Gomez-Rios for her support in the administrative process of this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.672008/full#supplementary-material
Supplementary Figure 1 | Negative correlation of geographic latitude with (A) mean annual UV radiation; (B) median annual UV radiation; (C) minimum annual UV radiation, and; (D) maximum annual UV radiation.
References
1. Lundberg IE, de Visser M, Werth VP. Classification of Myositis. Nat Rev Rheumatol (2018) 14(5):269–78. doi: 10.1038/nrrheum.2018.41
2. Dalakas MC. Inflammatory Muscle Diseases. N Engl J Med (2015) 373(4):393–4. doi: 10.1056/NEJMc1506827
3. Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, Visser M, et al. European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Ann Rheum Dis (2017) 76(12):1955–64. doi: 10.1136/annrheumdis-2017-211468
4. Tieu J, Lundberg IE, Limaye V. Idiopathic Inflammatory Myositis. Best Pract Res Clin Rheumatol (2016) 30(1):149–68. doi: 10.1016/j.berh.2016.04.007
5. Miller FW, Lamb JA, Schmidt J, Nagaraju K. Risk Factors and Disease Mechanisms in Myositis. Nat Rev Rheumatol (2018) 14(5):255–68. doi: 10.1038/nrrheum.2018.48
6. Okada S, Weatherhead E, Targoff IN, Wesley R, Miller FW. Global Surface Ultraviolet Radiation Intensity may Modulate the Clinical and Immunologic Expression of Autoimmune Muscle Disease. Arthritis Rheum (2003) 48(8):2285–93. doi: 10.1002/art.11090
7. Love LA, Weinberg CR, McConnaughey DR, Oddis CV, Medsger TA Jr, Reveille JD, et al. Ultraviolet Radiation Intensity Predicts the Relative Distribution of Dermatomyositis and anti-Mi-2 Autoantibodies in Women. Arthritis Rheum (2009) 60(8):2499–504. doi: 10.1002/art.24702
8. Hengstman GJ, van Venrooij WJ, Vencovsky J, Moutsopoulos HM, van Engelen BG. The Relative Prevalence of Dermatomyositis and Polymyositis in Europe Exhibits a Latitudinal Gradient. Ann Rheum Dis (2000) 59(2):141–2. doi: 10.1136/ard.59.2.141
9. Petri MH, Satoh M, Martin-Marquez BT, Vargas-Ramirez R, Jara LJ, Saavedra MA, et al. Implications in the Difference of anti-Mi-2 and -p155/140 Autoantibody Prevalence in Two Dermatomyositis Cohorts From Mexico City and Guadalajara. Arthritis Res Ther (2013) 15(2):R48. doi: 10.1186/ar4207
10. Bijl M, Kallenberg CG. Ultraviolet Light and Cutaneous Lupus. Lupus (2006) 15(11):724–7. doi: 10.1177/0961203306071705
11. Casciola-Rosen LA, Anhalt GJ, Rosen A. DNA-Dependent Protein Kinase is One of a Subset of Autoantigens Specifically Cleaved Early During Apoptosis. J Exp Med (1995) 182(6):1625–34. doi: 10.1084/jem.182.6.1625
12. Takashima A, Bergstresser PR. Impact of UVB Radiation on the Epidermal Cytokine Network. Photochem Photobiol (1996) 63(4):397–400. doi: 10.1111/j.1751-1097.1996.tb03054.x
13. Skopelja-Gardner S, An J, Tai J, Tanaka L, Sun X, Hermanson P, et al. The Early Local and Systemic Type I Interferon Responses to Ultraviolet B Light Exposure are cGAS Dependent. Sci Rep (2020) 10(1):7908. doi: 10.1038/s41598-020-64865-w
14. Lundberg IE, Helmers SB. The Type I Interferon System in Idiopathic Inflammatory Myopathies. Autoimmunity (2010) 43(3):239–43. doi: 10.3109/08916930903510955
15. Basta J, Rauchman M. The Nucleosome Remodeling and Deacetylase Complex in Development and Disease. Transl Res (2015) 165(1):36–47. doi: 10.1016/j.trsl.2014.05.003
16. Sims JK, Wade PA. Mi-2/NuRD Complex Function is Required for Normal S Phase Progression and Assembly of Pericentric Heterochromatin. Mol Biol Cell (2011) 22(17):3094–102. doi: 10.1091/mbc.E11-03-0258
17. Burd CJ, Kinyamu HK, Miller FW, Archer TK. UV Radiation Regulates Mi-2 Through Protein Translation and Stability. J Biol Chem (2008) 283(50):34976–82. doi: 10.1074/jbc.M805383200
18. Pinal-Fernandez I, Amici DR, Parks CA, Derfoul A, Casal-Dominguez M, Pak K, et al. Myositis Autoantigen Expression Correlates With Muscle Regeneration but Not Autoantibody Specificity. Arthritis Rheumatol (2019) 71(8):1371–6. doi: 10.1002/art.40883
19. Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin Rev Allergy Immunol (2017) 52(1):1–19. doi: 10.1007/s12016-015-8510-y
20. Temmoku J, Sato S, Fujita Y, Asano T, Suzuki E, Kanno T, et al. Clinical Significance of Myositis-Specific Autoantibody Profiles in Japanese Patients With Polymyositis/Dermatomyositis. Medicine (Baltimore) (2019) 98(20):e15578. doi: 10.1097/md.0000000000015578
21. Huang L, Wang L, Yang Y, Chen H, Liu Y, Liu K, et al. Coexistence of anti-HMGCR and anti-MDA5 Identified by an Unlabeled Immunoprecipitation Assay in a Chinese Patient Cohort With Myositis. Medicine (Baltimore) (2018) 97(47):e13236. doi: 10.1097/md.0000000000013236
22. Cobo-Ibanez T, Lopez-Longo FJ, Joven B, Carreira PE, Munoz-Fernandez S, Maldonado-Romero V, et al. Long-Term Pulmonary Outcomes and Mortality in Idiopathic Inflammatory Myopathies Associated With Interstitial Lung Disease. Clin Rheumatol (2019) 38(3):803–15. doi: 10.1007/s10067-018-4353-2
23. Jiao Y, Cai S, Lin J, Zhu W, Xi J, Li J, et al. Statin-Naive anti-HMGCR Antibody-Mediated Necrotizing Myopathy in China. J Clin Neurosci (2018) 57:13–9. doi: 10.1016/j.jocn.2018.08.010
24. Zampeli E, Venetsanopoulou A, Argyropoulou OD, Mavragani CP, Tektonidou MG, Vlachoyiannopoulos PG, et al. Myositis Autoantibody Profiles and Their Clinical Associations in Greek Patients With Inflammatory Myopathies. Clin Rheumatol (2019) 38(1):125–32. doi: 10.1007/s10067-018-4267-z
25. Kubo S, Todoroki Y, Nakayamada S, Nakano K, Satoh M, Nawata A, et al. Significance of Nailfold Videocapillaroscopy in Patients With Idiopathic Inflammatory Myopathies. Rheumatology (Oxford) (2019) 58(1):120–30. doi: 10.1093/rheumatology/key257
26. Ueki M, Kobayashi I, Takezaki S, Tozawa Y, Okura Y, Yamada M, et al. Myositis-Specific Autoantibodies in Japanese Patients With Juvenile Idiopathic Inflammatory Myopathies. Mod Rheumatol (2019) 29(2):351–6. doi: 10.1080/14397595.2018.1452353
27. So H, Ip RW, Wong VT, Yip RM. Analysis of Anti-Melanoma Differentiation-Associated Gene 5 Antibody in Hong Kong Chinese Patients With Idiopathic Inflammatory Myopathies: Diagnostic Utility and Clinical Correlations. Int J Rheum Dis (2018) 21(5):1076–81. doi: 10.1111/1756-185x.13268
28. Chen F, Li S, Wang T, Shi J, Wang G. Clinical Heterogeneity of Interstitial Lung Disease in Polymyositis and Dermatomyositis Patients With or Without Specific Autoantibodies. Am J Med Sci (2018) 355(1):48–53. doi: 10.1016/j.amjms.2017.07.013
29. Yura H, Sakamoto N, Satoh M, Ishimoto H, Hanaka T, Ito C, et al. Clinical Characteristics of Patients With anti-aminoacyl-tRNA Synthetase Antibody Positive Idiopathic Interstitial Pneumonia. Respir Med (2017) 132:189–94. doi: 10.1016/j.rmed.2017.10.020
30. Yang H, Peng Q, Yin L, Li S, Shi J, Zhang Y, et al. Identification of Multiple Cancer-Associated Myositis-Specific Autoantibodies in Idiopathic Inflammatory Myopathies: A Large Longitudinal Cohort Study. Arthritis Res Ther (2017) 19: (1):259. doi: 10.1186/s13075-017-1469-8
31. Lin JM, Zhang YB, Peng QL, Yang HB, Shi JL, Gu ML, et al. Genetic Association of HLA-DRB1 Multiple Polymorphisms With Dermatomyositis in Chinese Population. Hla (2017) 90(6):354–9. doi: 10.1111/tan.13171
32. Ogawa-Momohara M, Muro Y, Satoh M, Akiyama M. Autoantibodies to Su/Argonaute 2 in Japanese Patients With Inflammatory Myopathy. Clin Chim Acta (2017) 471:304–7. doi: 10.1016/j.cca.2017.06.022
33. Shi J, Li S, Yang H, Zhang Y, Peng Q, Lu X, et al. Clinical Profiles and Prognosis of Patients With Distinct Antisynthetase Autoantibodies. J Rheumatol (2017) 44(7):1051–7. doi: 10.3899/jrheum.161480
34. Tanizawa K, Handa T, Nakashima R, Kubo T, Hosono Y, Watanabe K, et al. The Long-Term Outcome of Interstitial Lung Disease With anti-aminoacyl-tRNA Synthetase Antibodies. Respir Med (2017) 127:57–64. doi: 10.1016/j.rmed.2017.04.007
35. Pinal-Fernandez I, Casal-Dominguez M, Huapaya JA, Albayda J, Paik JJ, Johnson C, et al. A Longitudinal Cohort Study of the Anti-Synthetase Syndrome: Increased Severity of Interstitial Lung Disease in Black Patients and Patients With anti-PL7 and anti-PL12 Autoantibodies. Rheumatology (Oxford) (2017) 56(6):999–1007. doi: 10.1093/rheumatology/kex021
36. Hussain A, Rawat A, Jindal AK, Gupta A, Singh S. Autoantibodies in Children With Juvenile Dermatomyositis: A Single Centre Experience From North-West India. Rheumatol Int (2017) 37(5):807–12. doi: 10.1007/s00296-017-3707-4
37. Ge Y, Lu X, Shu X, Peng Q, Wang G. Clinical Characteristics of anti-SAE Antibodies in Chinese Patients With Dermatomyositis in Comparison With Different Patient Cohorts. Sci Rep (2017) 7(1):188. doi: 10.1038/s41598-017-00240-6
38. Limaye V, Smith C, Koszyca B, Blumbergs P, Otto S. Infections and Vaccinations as Possible Triggers of Inflammatory Myopathies. Muscle Nerve (2017) 56(5):987–9. doi: 10.1002/mus.25628
39. Rogers A, Chung L, Li S, Casciola-Rosen L, Fiorentino DF. Cutaneous and Systemic Findings Associated With Nuclear Matrix Protein 2 Antibodies in Adult Dermatomyositis Patients. Arthritis Care Res (Hoboken) (2017) 69(12):1909–14. doi: 10.1002/acr.23210
40. Albayda J, Pinal-Fernandez I, Huang W, Parks C, Paik J, Casciola-Rosen L, et al. Antinuclear Matrix Protein 2 Autoantibodies and Edema, Muscle Disease, and Malignancy Risk in Dermatomyositis Patients. Arthritis Care Res (Hoboken) (2017) 69(11):1771–6. doi: 10.1002/acr.23188
41. Svensson J, Holmqvist M, Tjärnlund A, Dastmalchi M, Hanna B, Magnusson Bucher S, et al. Use of Biologic Agents in Idiopathic Inflammatory Myopathies in Sweden: A Descriptive Study of Real Life Treatment. Clin Exp Rheumatol (2017) 35(3):512–5.
42. Tampoia M, Notarnicola A, Abbracciavento L, Fontana A, Giannini M, Louis Humbel R, et al. A New Immunodot Assay for Multiplex Detection of Autoantibodies in a Cohort of Italian Patients With Idiopathic Inflammatory Myopathies. J Clin Lab Anal (2016) 30(6):859–66. doi: 10.1002/jcla.21948
43. Tan TC, Wienholt L, Adelstein S. TEST Performance of a Myositis Panel in a Clinical Immunology Laboratory in New South Wales, Australia. Int J Rheum Dis (2016) 19(10):996–1001. doi: 10.1111/1756-185x.12792
44. Deakin CT, Yasin SA, Simou S, Arnold KA, Tansley SL, Betteridge ZE, et al. Muscle Biopsy Findings in Combination With Myositis-Specific Autoantibodies Aid Prediction of Outcomes in Juvenile Dermatomyositis. Arthritis Rheumatol (2016) 68(11):2806–16. doi: 10.1002/art.39753
45. Ceribelli A, Isailovic N, De Santis M, Generali E, Fredi M, Cavazzana I, et al. Myositis-Specific Autoantibodies and Their Association With Malignancy in Italian Patients With Polymyositis and Dermatomyositis. Clin Rheumatol (2017) 36(2):469–75. doi: 10.1007/s10067-016-3453-0
46. Gomez GN, Gargiulo Mde L, Perez N, Collado MV, Suarez LV, Khoury M, et al. Autoantibodies in Adult Patients With Idiopathic Inflammatory Myopathies in Buenos Aires. Medicina (B Aires) (2016) 76(3):129–34.
47. Srivastava P, Dwivedi S, Misra R. Myositis-Specific and Myositis-Associated Autoantibodies in Indian Patients With Inflammatory Myositis. Rheumatol Int (2016) 36(7):935–43. doi: 10.1007/s00296-016-3494-3
48. Pinal-Fernandez I, Parks C, Werner JL, Albayda J, Paik J, Danoff SK, et al. Longitudinal Course of Disease in a Large Cohort of Myositis Patients With Autoantibodies Recognizing the Signal Recognition Particle. Arthritis Care Res (Hoboken) (2017) 69(2):263–70. doi: 10.1002/acr.22920
49. Allenbach Y, Keraen J, Bouvier AM, Jooste V, Champtiaux N, Hervier B, et al. High Risk of Cancer in Autoimmune Necrotizing Myopathies: Usefulness of Myositis Specific Antibody. Brain (2016) 139(Pt 8):2131–5. doi: 10.1093/brain/aww054
50. Kawasumi H, Gono T, Kawaguchi Y, Kuwana M, Kaneko H, Katsumata Y, et al. Clinical Manifestations and Myositis-Specific Autoantibodies Associated With Physical Dysfunction After Treatment in Polymyositis and Dermatomyositis: An Observational Study of Physical Dysfunction With Myositis in Japan. BioMed Res Int (2016) 2016:9163201. doi: 10.1155/2016/9163201
51. Bodoki L, Nagy-Vincze M, Griger Z, Betteridge Z, Szollosi L, Jobanputra R, et al. Rare Myositis-Specific Autoantibody Associations Among Hungarian Patients With Idiopathic Inflammatory Myopathy. Acta Reumatol Port (2015) 40(4):337–47.
52. Limaye VS, Lester S, Blumbergs P, Greenberg SA. Anti- C N1A Antibodies in South Australian Patients With Inclusion Body Myositis. Muscle Nerve (2016) 53(4):654–5. doi: 10.1002/mus.24989
53. Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-Melanoma Differentiation-Associated Gene 5 Is Associated With Rapidly Progressive Lung Disease and Poor Survival in US Patients With Amyopathic and Myopathic Dermatomyositis. Arthritis Care Res (Hoboken) (2016) 68(5):689–94. doi: 10.1002/acr.22728
54. Klein M, Mann H, Plestilova L, Zamecnik J, Betteridge Z, McHugh N, et al. Increasing Incidence of Immune-Mediated Necrotizing Myopathy: Single-Centre Experience. Rheumatology (Oxford) (2015) 54(11):2010–4. doi: 10.1093/rheumatology/kev229
55. Chen Z, Hu W, Wang Y, Guo Z, Sun L, Kuwana M. Distinct Profiles of Myositis-Specific Autoantibodies in Chinese and Japanese Patients With Polymyositis/Dermatomyositis. Clin Rheumatol (2015) 34(9):1627–31. doi: 10.1007/s10067-015-2935-9
56. Muro Y, Hosono Y, Sugiura K, Ogawa Y, Mimori T, Akiyama M. Anti-PM/Scl Antibodies are Found in Japanese Patients With Various Systemic Autoimmune Conditions Besides Myositis and Scleroderma. Arthritis Res Ther (2015) 17:57. doi: 10.1186/s13075-015-0573-x
57. Goyal NA, Cash TM, Alam U, Enam S, Tierney P, Araujo N, et al. Seropositivity for NT5c1A Antibody in Sporadic Inclusion Body Myositis Predicts More Severe Motor, Bulbar and Respiratory Involvement. J Neurol Neurosurg Psychiatry (2016) 87(4):373–8. doi: 10.1136/jnnp-2014-310008
58. Hozumi H, Enomoto N, Kono M, Fujisawa T, Inui N, Nakamura Y, et al. Prognostic Significance of anti-aminoacyl-tRNA Synthetase Antibodies in Polymyositis/Dermatomyositis-Associated Interstitial Lung Disease: A Retrospective Case Control Study. PloS One (2015) 10(3):e0120313. doi: 10.1371/journal.pone.0120313
59. Watanabe Y, Suzuki S, Nishimura H, Murata KY, Kurashige T, Ikawa M, et al. Statins and Myotoxic Effects Associated With Anti-3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Autoantibodies: An Observational Study in Japan. Medicine (Baltimore) (2015) 94(4):e416. doi: 10.1097/md.0000000000000416
60. Narang NS, Casciola-Rosen L, Li S, Chung L, Fiorentino DF. Cutaneous Ulceration in Dermatomyositis: Association With Anti-Melanoma Differentiation-Associated Gene 5 Antibodies and Interstitial Lung Disease. Arthritis Care Res (Hoboken) (2015) 67(5):667–72. doi: 10.1002/acr.22498
61. Bodoki L, Nagy-Vincze M, Griger Z, Betteridge Z, Szollosi L, Danko K. Four Dermatomyositis-Specific autoantibodies-anti-TIF1gamma, anti-NXP2, anti-SAE and anti-MDA5-in Adult and Juvenile Patients With Idiopathic Inflammatory Myopathies in a Hungarian Cohort. Autoimmun Rev (2014) 13(12):1211–9. doi: 10.1016/j.autrev.2014.08.011
62. Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Tincani A, Selmi C, et al. Prevalence and Clinical Significance of anti-MDA5 Antibodies in European Patients With Polymyositis/Dermatomyositis. Clin Exp Rheumatol (2014) 32(6):891–7.
63. Cuesta-Mateos C, Colom-Fernández B, Portero-Sainz I, Tejedor R, García-García C, Concha-Garzón MJ, et al. Autoantibodies Against TIF-1-γ and CADM-140 in Spanish Patients With Clinically Amyopathic Dermatomyositis (CADM): Clinical Significance and Diagnostic Utility. J Eur Acad Dermatol Venereol (2015) 29(3):482–9. doi: 10.1111/jdv.12591
64. Klein M, Mann H, Plestilova L, Betteridge Z, McHugh N, Remakova M, et al. Arthritis in Idiopathic Inflammatory Myopathy: Clinical Features and Autoantibody Associations. J Rheumatol (2014) 41(6):1133–9. doi: 10.3899/jrheum.131223
65. Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A. Anti-MDA5 Antibodies in a Large Mediterranean Population of Adults With Dermatomyositis. J Immunol Res (2014) 2014:290797. doi: 10.1155/2014/290797
66. Wang L, Liu L, Hao H, Gao F, Liu X, Wang Z, et al. Myopathy With Anti-Signal Recognition Particle Antibodies: Clinical and Histopathological Features in Chinese Patients. Neuromuscul Disord (2014) 24(4):335–41. doi: 10.1016/j.nmd.2014.01.002
67. Taborda AL, Azevedo P, Isenberg DA. Retrospective Analysis of the Outcome of Patients With Idiopathic Inflammatory Myopathy: A Long-Term Follow-Up Study. Clin Exp Rheumatol (2014) 32(2):188–93.
68. Neri R, Barsotti S, Iacopetti V, Iacopetti G, Pepe P, d’Ascanio A, et al. Cancer-Associated Myositis: A 35-Year Retrospective Study of a Monocentric Cohort. Rheumatol Int (2014) 34(4):565–9. doi: 10.1007/s00296-013-2910-1
69. Fiorentino DF, Chung LS, Christopher-Stine L, Zaba L, Li S, Mammen AL, et al. Most Patients With Cancer-Associated Dermatomyositis Have Antibodies to Nuclear Matrix Protein NXP-2 or Transcription Intermediary Factor 1gamma. Arthritis Rheum (2013) 65(11):2954–62. doi: 10.1002/art.38093
70. Ishigaki K, Maruyama J, Hagino N, Murota A, Takizawa Y, Nakashima R, et al. Skin Ulcer is a Predictive and Prognostic Factor of Acute or Subacute Interstitial Lung Disease in Dermatomyositis. Rheumatol Int (2013) 33(9):2381–9. doi: 10.1007/s00296-013-2735-y
71. Shah M, Mamyrova G, Targoff IN, Huber AM, Malley JD, Rice MM, et al. The Clinical Phenotypes of the Juvenile Idiopathic Inflammatory Myopathies. Medicine (Baltimore) (2013) 92(1):25–41. doi: 10.1097/MD.0b013e31827f264d
72. Muro Y, Sugiura K, Akiyama M. Limitations of a Single-Point Evaluation of anti-MDA5 Antibody, Ferritin, and IL-18 in Predicting the Prognosis of Interstitial Lung Disease With anti-MDA5 Antibody-Positive Dermatomyositis. Clin Rheumatol (2013) 32(3):395–8. doi: 10.1007/s10067-012-2142-x
73. Marie I, Josse S, Hatron PY, Dominique S, Hachulla E, Janvresse A, et al. Interstitial Lung Disease in anti-Jo-1 Patients With Antisynthetase Syndrome. Arthritis Care Res (Hoboken) (2013) 65(5):800–8. doi: 10.1002/acr.21895
74. Gono T, Kawaguchi Y, Kuwana M, Sugiura T, Furuya T, Takagi K, et al. Brief Report: Association of HLA-DRB1*0101/*0405 With Susceptibility to Anti-Melanoma Differentiation-Associated Gene 5 Antibody-Positive Dermatomyositis in the Japanese Population. Arthritis Rheum (2012) 64(11):3736–40. doi: 10.1002/art.34657
75. Tarricone E, Ghirardello A, Rampudda M, Bassi N, Punzi L, Doria A. Anti-SAE Antibodies in Autoimmune Myositis: Identification by Unlabelled Protein Immunoprecipitation in an Italian Patient Cohort. J Immunol Methods (2012) 384(1-2):128–34. doi: 10.1016/j.jim.2012.07.019
76. Fujimoto M, Matsushita T, Hamaguchi Y, Kaji K, Asano Y, Ogawa F, et al. Autoantibodies to Small Ubiquitin-Like Modifier Activating Enzymes in Japanese Patients With Dermatomyositis: Comparison With a UK Caucasian Cohort. Ann Rheum Dis (2013) 72(1):151–3. doi: 10.1136/annrheumdis-2012-201736
77. Chua F, Higton AM, Colebatch AN, O’Reilly K, Grubnic S, Vlahos I, et al. Idiopathic Inflammatory Myositis-Associated Interstitial Lung Disease: Ethnicity Differences and Lung Function Trends in a British Cohort. Rheumatology (Oxford) (2012) 51(10):1870–6. doi: 10.1093/rheumatology/kes167
78. Gono T, Sato S, Kawaguchi Y, Kuwana M, Hanaoka M, Katsumata Y, et al. Anti-MDA5 Antibody, Ferritin and IL-18 are Useful for the Evaluation of Response to Treatment in Interstitial Lung Disease With anti-MDA5 Antibody-Positive Dermatomyositis. Rheumatology (Oxford) (2012) 51(9):1563–70. doi: 10.1093/rheumatology/kes102
79. Ceribelli A, Fredi M, Taraborelli M, Cavazzana I, Franceschini F, Quinzanini M, et al. Anti-MJ/NXP-2 Autoantibody Specificity in a Cohort of Adult Italian Patients With Polymyositis/Dermatomyositis. Arthritis Res Ther (2012) 14(2):R97. doi: 10.1186/ar3822
80. Koga T, Fujikawa K, Horai Y, Okada A, Kawashiri SY, Iwamoto N, et al. The Diagnostic Utility of Anti-Melanoma Differentiation-Associated Gene 5 Antibody Testing for Predicting the Prognosis of Japanese Patients With DM. Rheumatology (Oxford) (2012) 51(7):1278–84. doi: 10.1093/rheumatology/ker518
81. Ellis E, Ann Tan J, Lester S, Tucker G, Blumbergs P, Roberts-Thomson P, et al. Necrotizing Myopathy: Clinicoserologic Associations. Muscle Nerve (2012) 45(2):189–94. doi: 10.1002/mus.22279
82. Muro Y, Sugiura K, Hoshino K, Akiyama M, Tamakoshi K. Epidemiologic Study of Clinically Amyopathic Dermatomyositis and Anti-Melanoma Differentiation-Associated Gene 5 Antibodies in Central Japan. Arthritis Res Ther (2011) 13(6):R214. doi: 10.1186/ar3547
83. Chinoy H, Adimulam S, Marriage F, New P, Vincze M, Zilahi E, et al. Interaction of HLA-DRB1*03 and Smoking for the Development of anti-Jo-1 Antibodies in Adult Idiopathic Inflammatory Myopathies: A European-wide Case Study. Ann Rheum Dis (2012) 71(6):961–5. doi: 10.1136/annrheumdis-2011-200182
84. Ikeda N, Takahashi K, Yamaguchi Y, Inasaka M, Kuwana M, Ikezawa Z. Analysis of Dermatomyositis-Specific Autoantibodies and Clinical Characteristics in Japanese Patients. J Dermatol (2011) 38(10):973–9. doi: 10.1111/j.1346-8138.2011.01262.x
85. Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The Mucocutaneous and Systemic Phenotype of Dermatomyositis Patients With Antibodies to MDA5 (CADM-140): A Retrospective Study. J Am Acad Dermatol (2011) 65(1):25–34. doi: 10.1016/j.jaad.2010.09.016
86. Hamaguchi Y, Kuwana M, Hoshino K, Hasegawa M, Kaji K, Matsushita T, et al. Clinical Correlations With Dermatomyositis-Specific Autoantibodies in Adult Japanese Patients With Dermatomyositis: A Multicenter Cross-Sectional Study. Arch Dermatol (2011) 147(4):391–8. doi: 10.1001/archdermatol.2011.52
87. Nakajima A, Yoshino K, Soejima M, Kawaguchi Y, Satoh T, Kuwana M, et al. High Frequencies and Co-Existing of Myositis-Specific Autoantibodies in Patients With Idiopathic Inflammatory Myopathies Overlapped to Rheumatoid Arthritis. Rheumatol Int (2012) 32(7):2057–61. doi: 10.1007/s00296-011-1931-x
88. Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies Against 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase in Patients With Statin-Associated Autoimmune Myopathy. Arthritis Rheum (2011) 63(3):713–21. doi: 10.1002/art.30156
89. Kang EH, Nakashima R, Mimori T, Kim J, Lee YJ, Lee EB, et al. Myositis Autoantibodies in Korean Patients With Inflammatory Myositis: Anti-140-kDa Polypeptide Antibody is Primarily Associated With Rapidly Progressive Interstitial Lung Disease Independent of Clinically Amyopathic Dermatomyositis. BMC Musculoskelet Disord (2010) 11:223. doi: 10.1186/1471-2474-11-223
90. Ghirardello A, Rampudda M, Ekholm L, Bassi N, Tarricone E, Zampieri S, et al. Diagnostic Performance and Validation of Autoantibody Testing in Myositis by a Commercial Line Blot Assay. Rheumatology (Oxford) (2010) 49(12):2370–4. doi: 10.1093/rheumatology/keq281
91. Marie I, Menard JF, Hatron PY, Hachulla E, Mouthon L, Tiev K, et al. Intravenous Immunoglobulins for Steroid-Refractory Esophageal Involvement Related to Polymyositis and Dermatomyositis: A Series of 73 Patients. Arthritis Care Res (Hoboken) (2010) 62(12):1748–55. doi: 10.1002/acr.20325
92. Vancsa A, Gergely L, Ponyi A, Lakos G, Nemeth J, Szodoray P, et al. Myositis-Specific and Myositis-Associated Antibodies in Overlap Myositis in Comparison to Primary Dermatopolymyositis: Relevance for Clinical Classification: Retrospective Study of 169 Patients. Joint Bone Spine (2010) 77(2):125–30. doi: 10.1016/j.jbspin.2009.08.008
93. Espada G, Maldonado Cocco JA, Fertig N, Oddis CV. Clinical and Serologic Characterization of an Argentine Pediatric Myositis Cohort: Identification of a Novel Autoantibody (anti-MJ) to a 142-kDa Protein. J Rheumatol (2009) 36(11):2547–51. doi: 10.3899/jrheum.090461
94. Fujikawa K, Kawakami A, Kaji K, Fujimoto M, Kawashiri S, Iwamoto N, et al. Association of Distinct Clinical Subsets With Myositis-Specific Autoantibodies Towards Anti-155/140-kDa Polypeptides, Anti-140-kDa Polypeptides, and Anti-Aminoacyl tRNA Synthetases in Japanese Patients With Dermatomyositis: A Single-Centre, Cross-Sectional Study. Scand J Rheumatol (2009) 38(4):263–7. doi: 10.1080/03009740802687455
95. Suzuki Y, Hayakawa H, Miwa S, Shirai M, Fujii M, Gemma H, et al. Intravenous Immunoglobulin Therapy for Refractory Interstitial Lung Disease Associated With Polymyositis/Dermatomyositis. Lung (2009) 187(3):201–6. doi: 10.1007/s00408-009-9146-6
96. Selva-O’Callaghan A, Sampol G, Romero O, Lloberes P, Trallero-Araguas E, Vilardell-Tarres M. Obstructive Sleep Apnea in Patients With Inflammatory Myopathies. Muscle Nerve (2009) 39(2):144–9. doi: 10.1002/mus.21204
97. Takada T, Hirakata M, Suwa A, Kaneko Y, Kuwana M, Ishihara T, et al. Clinical and Histopathological Features of Myopathies in Japanese Patients With anti-SRP Autoantibodies. Mod Rheumatol (2009) 19(2):156–64. doi: 10.1007/s10165-008-0139-8
98. Betteridge ZE, Gunawardena H, Chinoy H, North J, Ollier WE, Cooper RG, et al. Clinical and Human Leucocyte Antigen Class II Haplotype Associations of Autoantibodies to Small Ubiquitin-Like Modifier Enzyme, a Dermatomyositis-Specific Autoantigen Target, in UK Caucasian Adult-Onset Myositis. Ann Rheum Dis (2009) 68(10):1621–5. doi: 10.1136/ard.2008.097162
99. Selva-O’Callaghan A, Labrador-Horrillo M, Solans-Laque R, Simeon-Aznar CP, Martinez-Gomez X, Vilardell-Tarres M. Myositis-Specific and Myositis-Associated Antibodies in a Series of Eighty-Eight Mediterranean Patients With Idiopathic Inflammatory Myopathy. Arthritis Rheum (2006) 55(5):791–8. doi: 10.1002/art.22237
100. Yamasaki Y, Yamada H, Nozaki T, Akaogi J, Nichols C, Lyons R, et al. Unusually High Frequency of Autoantibodies to PL-7 Associated With Milder Muscle Disease in Japanese Patients With Polymyositis/Dermatomyositis. Arthritis Rheum (2006) 54(6):2004–9. doi: 10.1002/art.21883
101. Chinoy H, Salway F, Fertig N, Shephard N, Tait BD, Thomson W, et al. In Adult Onset Myositis, the Presence of Interstitial Lung Disease and Myositis Specific/Associated Antibodies are Governed by HLA Class II Haplotype, Rather Than by Myositis Subtype. Arthritis Res Ther (2006) 8(1):R13. doi: 10.1186/ar1862
102. Rios G. Retrospective Review of the Clinical Manifestations and Outcomes in Puerto Ricans With Idiopathic Inflammatory Myopathies. J Clin Rheumatol (2005) 11(3):153–6. doi: 10.1097/01.rhu.0000164820.46979.52
103. Gergely P Jr, Blazsek A, Danko K, Ponyi A, Poor G. Detection of TT Virus in Patients With Idiopathic Inflammatory Myopathies. Ann N Y Acad Sci (2005) 1050:304–13. doi: 10.1196/annals.1313.032
104. Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-Kd Polypeptide, CADM-140, in Japanese Patients With Clinically Amyopathic Dermatomyositis. Arthritis Rheum (2005) 52(5):1571–6. doi: 10.1002/art.21023
105. Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV. Anti-Signal Recognition Particle Autoantibody in Patients With and Patients Without Idiopathic Inflammatory Myopathy. Arthritis Rheum (2004) 50(1):209–15. doi: 10.1002/art.11484
106. Marie I, Hachulla E, Cherin P, Dominique S, Hatron PY, Hellot MF, et al. Interstitial Lung Disease in Polymyositis and Dermatomyositis. Arthritis Rheum (2002) 47(6):614–22. doi: 10.1002/art.10794
107. Kubo M, Ihn H, Asano Y, Yamane K, Yazawa N, Tamaki K. Prevalence of 52-Kd and 60-Kd Ro/SS-A Autoantibodies in Japanese Patients With Polymyositis/Dermatomyositis. J Am Acad Dermatol (2002) 47(1):148–51. doi: 10.1067/mjd.2002.121037
108. Hengstman GJ, Brouwer R, Egberts WT, Seelig HP, Jongen PJ, van Venrooij WJ, et al. Clinical and Serological Characteristics of 125 Dutch Myositis Patients. Myositis Specific Autoantibodies Aid in the Differential Diagnosis of the Idiopathic Inflammatory Myopathies. J Neurol (2002) 249(1):69–75. doi: 10.1007/pl00007850
Keywords: idiopathic inflammatory myopathies (IIM), autoantibodies, prevalence, latitude, UV radiation
Citation: Aguilar-Vazquez A, Chavarria-Avila E, Pizano-Martinez O, Ramos-Hernandez A, Andrade-Ortega L, Rubio-Arellano ED and Vazquez-Del Mercado M (2021) Geographical Latitude Remains as an Important Factor for the Prevalence of Some Myositis Autoantibodies: A Systematic Review. Front. Immunol. 12:672008. doi: 10.3389/fimmu.2021.672008
Received: 25 February 2021; Accepted: 06 April 2021;
Published: 22 April 2021.
Edited by:
Lazaros Ignatios Sakkas, University of Thessaly, GreeceReviewed by:
Franco Franceschini, University of Brescia, ItalyAnna Ghirardello, University of Padua, Italy
Copyright © 2021 Aguilar-Vazquez, Chavarria-Avila, Pizano-Martinez, Ramos-Hernandez, Andrade-Ortega, Rubio-Arellano and Vazquez-Del Mercado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica Vazquez-Del Mercado, ZHJhdm1lQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Andrea Aguilar-Vazquez
Andrea Aguilar-Vazquez Efrain Chavarria-Avila
Efrain Chavarria-Avila Oscar Pizano-Martinez
Oscar Pizano-Martinez Alejandra Ramos-Hernandez4
Alejandra Ramos-Hernandez4 Edy-David Rubio-Arellano
Edy-David Rubio-Arellano Monica Vazquez-Del Mercado
Monica Vazquez-Del Mercado