
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 29 March 2021
Sec. Viral Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.666983
The emergence of viruses with pandemic potential such as the SARS-CoV-2 coronavirus causing COVID-19 poses a global health challenge. There is remarkable progress in vaccine technology in response to this threat, but their design often overlooks the innate arm of immunity. Gamma Delta (γδ) T cells are a subset of T cells with unique features that gives them a key role in the innate immune response to a variety of homeostatic alterations, from cancer to microbial infections. In the context of viral infection, a growing body of evidence shows that γδ T cells are particularly equipped for early virus detection, which triggers their subsequent activation, expansion and the fast deployment of antiviral functions such as direct cytotoxic pathways, secretion of cytokines, recruitment and activation of other immune cells and mobilization of a trained immunity memory program. As such, γδ T cells represent an attractive target to stimulate for a rapid and effective resolution of viral infections. Here, we review the known aspects of γδ T cells that make them crucial component of the immune response to viruses, and the ways that their antiviral potential can be harnessed to prevent or treat viral infection.
It’s estimated that on average, a human being will be infected with about 10 different viral species over a lifetime (1), including influenza viruses, coronaviruses, noroviruses and rhinoviruses. Most of these viral infections result in either no disease or mild symptoms, and viral clearance in a matter of days or weeks. However, the increasing emergence of new viruses, to which human populations have no existing immunity, raises the potential for pandemics posing a threat to global human health that needs to be addressed.
During a viral infection, the successive and functional cooperation of the innate and adaptive immune systems is crucial in order to control the viral load and lead to a successful resolution of disease. The early detection and reaction by the immune system to viral infection is fundamental for the subsequent course of infection. This early response includes the production of cytokines and cytotoxic factors by first-line innate effector cells including macrophages, neutrophils, natural killer cells and Gamma Delta (γδ) T cells. This early ‘innate’ arm of the immune system also begins to recruit the adaptive arm to tailor the response and lead to immune memory. γδ T cells in particular are of the utmost importance as their large numbers in tissues, their pre-activated phenotype and rapidity of response make them a central player in the fight against viruses (2). They represent 1-5% of blood lymphocytes and constitute between 10–100% of T cells in “barrier” sites such as lung, gut and skin (3). γδ T cells migrate to these organs during early development and persist there as resident cells (4) with non-redundant features of surveillance compared to the other tissue-resident lymphocytes (5, 6). In addition, γδ T cells acquire a pre-activated phenotype early in their development that allows the rapid induction of effector functions upon detecting cellular stress and infection. Indeed, γδ T cells have been shown to be one of the first immune cells to react to viral entry (7). The importance of γδ T cells for an efficient antiviral response is illustrated by γδ T cell-deficient mice which show severely impaired responses to both primary and secondary infection (8, 9). These mice also demonstrate substantial increases in viral titers immediately post-infection as well as increased mortality compared with control mice. The precise mechanisms deployed by human γδ T cells against viruses are still incompletely understood, but their ability in early sensing of infection, quick activation and cytotoxicity against a wide array of viruses, including cytomegalovirus (CMV), influenza A virus, hepatitis B (HBV) and C (HCV) virus, human immunodeficiency virus (HIV) and severe acute respiratory syndrome-related coronavirus (SARS-CoV), has triggered interest in a better definition of these under-studied lymphocytes and in ways of harnessing their potential for therapies (2). This review aims to provide an insight into γδ T cells’ protective functions in human pathologies and to illustrate the necessity of including innate immunity in the design of antiviral strategies.
Despite their active roles in many human infectious diseases, the pathways used by γδ T cells to sense pathogens and initiate rapid responses remain largely unknown. In this section, we will explore some of the principal signals that are critical for γδ-T cell-mediated antiviral activity.
In addition to their strategic position, γδ T cells express a diversity of receptors for sensing both viral particles directly and infected cells. Firstly, the presence on γδ T cells of both membrane expressed and intracellular pattern recognition receptors (PRRs), which bind conserved pathogen-associated molecular patterns (PAMPs), is a major tool for virus detection. Of particular importance are Toll-like receptors (TLRs) that respond independently of any other receptors to stimulation by virus-derived molecules.
TLRs are expressed on the cell membrane, where they can directly recognize PAMPs like viral glycoproteins and glycolipids (TLR2 & 4) (10–12). They are also present on endosomes and lysosomes where they detect viral single-stranded (TLR7) and double-stranded (TLR3) RNA (13), as well as CpG nucleotides (TLR9) present in the extracellular environment or produced during intracellular replication of many viruses. All TLRs (but TLR8) are expressed on γδ T cells in peripheral blood of human donors (14), and they are quickly upregulated during activation (e.g. by TCR stimulation) (15).
The binding of viral ligands to TLRs leads to the activation of several transcription factors such as interferon regulatory factor 3, 5, and 7 (IRFs) and nuclear factor-κB (NF-κB) (16). This activation induces an antiviral program, including production of interferons, pro-inflammatory cytokines (IL-1, TNF-α) and other associated molecules. Through positive feedback processes, interferons are able to enhance many TLRs (17).
In addition to PRRs, γδ T cells also express several other receptors that mediate their optimal activation during viral infection, by directly triggering their own signaling effect, and/or modulating TCR signaling. Among these are NK type receptors (NKRs) including natural killer group 2-member D (NKG2D), DNAX Accessory Molecule-1 (DNAM1) and the Natural Cytotoxicity receptors (NCRs) NKp30, NKp44 and NKp46.
The activating NKG2D molecule is an important stimulatory receptor expressed on γδ T cells which provides a critical role in stress antigen recognition (18). In humans, the ligands of NKG2D have been identified as stress‐inducible MHC class I related molecules A/B (MICA/MICB) and members of the UL16-binding protein family (ULBPs) (19). These molecules have been shown to be upregulated in response to stress, including viral infection. For example, during CMV infection of fibroblasts, MICA and ULBP1-3 have been shown to be upregulated (20). MICB is induced in macrophages infected by influenza A or Sendai virus (21). CD4+ lymphocytes infected by HIV also display an upregulation in ULBP1-3 (22). Furthermore, MICA, MICB and ULBP4 have been shown to be upregulated in response to Epstein-Barr virus (EBV) infection allowing activation of γδ T cells (23, 24). Recognition of these ligands induces signaling through NKG2D and rapid Ca2+ responses, triggering protein kinase C (PKC)-dependent co-stimulation of the TCR (25), but can also signal independently of TCR signaling (18). Blockade of NKG2D but not TCR resulted in decreased killing suggesting that recognition is principally mediated by NKG2D, and activation achieved through TCR (26). Ligand recognition might actually involve the two receptors, as ULBPs have been suggested to engage both NKG2D and Vγ9Vδ2 TCR (24). Alternatively, the binding of TCR and NKG2D to MICA has been reported to be mutually exclusive, with a dynamic influenced by the higher affinity for the latter (27).
DNAM1 or CD226 is another NKR involved in γδ T cell activation. It is expressed at a low level constitutively and is upregulated following stimulation of the cell (28). The ligands of this receptor include poliovirus receptor PVR (CD155) and nectin-2 (CD112), key receptors that play a role in viral entry and have been shown to be upregulated in response to cellular stress such as infection by viruses including CMV, HIV, EBV (29–31). Interaction of DNAM1 with its ligands triggers γδ T cell effector functions, notably cytolytic granule exocytosis and interferon-gamma (IFN-γ) production against tumors (28), but more studies are needed to establish if it has similar effects during a viral infection.
Finally, γδ T cells have been shown to express members of the NCR family, including NKp30, NKp44 and NKp46. These receptors were originally documented on NK cells and were shown to coordinate cytotoxic responses against tumor and infected cells. They play a key role in infection by CMV, as infected cells express NKp30 ligand B7-H6 (32). NKp44 and NKp46 bind hemagglutinin (HA) present on influenza (33, 34) and vaccinia viruses (35) as well as hemagglutinin-neuraminidase (HN) on Newcastle disease virus (NDV) (36). Numerous other pathogens such as West Nile and dengue viruses have also been shown to bind these receptors via unidentified proteins (37). While not expressed constitutively on γδ T cells, studies have shown that the expression of NCRs can be induced following activation (38). NCRs are instrumental for γδ T cells antiviral function, as shown for example in the case of HIV suppression via NKp30-dependent activation of γδ T cells (39), or cytotoxicity inhibition by specific blockade of NKp44 (40). These receptors have been shown to mediate granzyme B production and cytotoxicity in a TCR-independent manner (38).
Gamma delta T cells are also capable of responding to infected cells via their T-Cell Receptor (TCR). The TCR recognition of γδ T cells is independent of MHC restrictions (41) and has been shown to bind to a variety of non-processed antigens (42) including MHC-like molecules (43), HSPs (44) and HSP-regulated proteins (45), several glycoproteins, lipoproteins and phosphoantigens (pAg) (46). Many of these antigens are upregulated in an infectious context, as shown earlier for MICA and MICB, and γδ T cells rely on them for optimal activation and antiviral function, as exemplified by the correlation between pAg synthesis of EBV- or influenza A-infected cells and γδ T cells cytotoxicity against them (47, 48). The role of the γδ TCR is illustrated by blocking studies, resulting in the loss of recognition, for example in CMV-infected cells (49). Conversely, transferring TCR from a CMV-reactive clone to a TCR-deficient cell line is sufficient to confer reactivity against CMV-infected targets (50).
In humans, γδ T cells can be classified into two main populations according to their TCR expression: Vδ1 and Vδ2 γδ T cells (51). Vδ1 γδ T cells are generally resident lymphocytes, abundant in mucosal surfaces and epithelia of the digestive, respiratory and urogenital tracts; in contrast, Vδ2 γδ T cells are circulating lymphocytes and constitute the majority of peripheral blood γδ T cells (52). There is some evidence to suggests that the tissue specificity of γδ T cells is shaped by the selective activation resulting from the interaction between the TCR and a family of presenting molecules called butyrophilins (BTN) and butyrophilins-like proteins (BTNL) (53, 54).
Vδ1 γδ T cells proliferate during some chronic viral infections, including HCV and HIV (55, 56). They display antiviral potential with the production of T-helper cell type 1 cytokines (57) and direct cytotoxicity toward infected cells (58). Similarly, activation and proliferation of Vδ2 γδ T cells have also been shown to be increased early during the acute phase of many viral infections. These cells can display potent antiviral responses and mainly recognize pAg synthesized by infected cells via the interaction between their TCR and the BTN3A1 (CD277) presenting molecule (59, 60). This activating signal is capable of stimulating Vδ2 γδ T cells independently of the virus type (48).
Activation of γδ T cells by the integrated signals from the PRRs, NKRs and TCRs induce an antiviral state characterized by proliferation and phenotypic specialization. Indeed, as seen for example in hepatitis C virus (HCV) patients (2), during infection by herpes simplex virus (HSV) (61), or following an encounter with EBV (62, 63), there is a rapid proliferation of γδ T cells seen in the blood where they can expand from approximately 1% of circulating T cells in steady-state to over 50% following viral infection. These expanded γδ T cells express activation markers like CD69, CD38 and HLA-DR absent in healthy individuals (64, 65), but also effector molecules such as perforin, granzymes, granulysin contained in cytolytic granules and FasL or TRAIL.
The strategic position of γδ T cells for immune surveillance, and their capacity to recognize a unique and wide array of danger signals allows them to rapidly detect viral infection. This activation generates a high number of functionally active cells, ready to deploy their full antiviral potential via multiple routes, either direct killing of infected cells or indirect inhibition through production of noncytolytic factors and interactions with other components of the immune system.
γδ T cell-mediated direct cytotoxicity is executed by diverse pathways, including secretion of cytotoxic mediators stored in granules such as perforin (66), granzymes (67, 68) and granulysin (69) and expression of members of the death-inducing TNF family of ligands and receptors, including tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL) (70) and FasL.
γδ T cells uniformly express abundant perforin, granzymes and granulysin in their cytoplasmic granules (71–74) and are able to degranulate after specific recognition of virus-infected cells (75). Interestingly, the granules’ content varies with cell type and immunological context, influencing the outcome. For example, Granzyme M, which is highly expressed by γδ T cells, is regulated differently than Granzyme B and initiates a unique cell death pathway independent of caspase activation (76, 77). In addition to the induced apoptosis of infected cells, Granzyme M also directly inhibits viral replication by cleavage of essential virus proteins (78). Similarly, γδ T cell granules contain Granzyme H and K which have various antiviral activity against adenoviruses, Influenza virus, HBV and HCV (68, 79–82).
Despite the central role of the cytolytic granules in immune-induced apoptosis, several observations of target cell death in the absence of Ca2+, perforin, or granule exocytosis suggests the existence of alternative pathways of cytotoxicity. The FasL-Fas pathway is such an alternative mechanism of direct killing used by γδ T cells (83). Fas is induced in the membrane of virally infected cells (84) and binds to FasL expressed on γδ T cells. This leads to caspases activation and apoptosis in a manner not dissimilar to the one triggered by Granzyme B (85). γδ T cells upregulate FasL as early as 1 hour after stimulation (via NF-kB), and are capable of keeping a high and sustained expression during an immune response (86).
Mounting evidence indicates that γδ T cells also exert their protective function in the elimination of pathogens by producing cytokines, chemokines, and interacting with other components of the immune system.
During a viral infection, targeted cells can produce cytokines like TNF-α, IL-1, IL-6, IL-18 (87) which participate in the activation of γδ T cells both in situ and in the peripheral blood. During activation, these γδ T cells upregulate the chemokine receptors CXCR3/5, and CCR1/5, allowing additional recruitment to the site of inflammation, rich in CCL3/4/5 and CXCL9/10/11 [86–88].
Within a few hours of activation, γδ T cells release high amounts of cytokines, among which is IFN-γ, a key antiviral molecule capable of suppressing viral replication as well as recruiting and activating complementary immune cells like NK, macrophage or killer T cells. In vitro, the non-cytolytic antiviral activity of IFN-γ has been demonstrated in infections with hepatitis viruses (HBV & HCV), herpesviruses, orthopoxviruses, picornaviruses, retroviruses, influenza and others (88). IFN-γ induces the transcription of several genes called Interferon-Stimulated Genes (ISGs), which exhibit numerous functions such as targeting viral entry, RNA expression, protein synthesis, assembly or release through multiple mechanisms (89–91). For example, members of the IFN-inducible transmembrane (IFITM) family have the capacity of limiting viral entry and replication (92, 93). Another noticeable effect of IFN-γ is the induction of the OAS (oligoadenylate synthetase)-RNase L (latent ribonuclease L) pathway which functions to detect foreign RNA and to cleave both host and viral RNA (94). At the other end of the viral life cycle, Viperin (virus inhibitory protein, endoplasmic reticulum-associated, IFN-inducible) inhibits the virus release by blocking budding at the plasma membrane (95). Interestingly, Viperin acts in a similar manner as bisphosphonates, a class of drugs known to activate γδ T cells. Indeed, it inhibits farnesyl diphosphate synthase (FPPS), altering membrane fluidity by disrupting lipid rafts and interfering with virus budding as a consequence (96). Thus, one can hypothesize that administration of bisphosphonates for in vivo γδ T cells activation, as routinely done clinically (Cf. Part 4), will have a beneficial synergistic antiviral action.
γδ T cells produce a high amount of IFN-γ upon stimulation (97–100), commencing as early as 4 hours post-activation (101). Several studies show the central role of γδ T cell-secreted IFN-γ in the antiviral response (102–104). As an additional immunostimulatory mechanism, the high concentration of IFNs produced by infected cells and immune cells including γδ T cells themselves in inflamed areas (105) will reinforce activation of the immune cell pool, therefore augmenting the antiviral response (106).
Due to the evolutionary pressure of the anti-viral effects of IFN-γ, numerous strategies have arisen in viruses to subvert this protective mechanism. Other complementary and non-redundant mechanisms, such as TNF-α, which is also produced by the γδ T cell, are required. TCR triggering induces massive production of TNF-α by γδ T cells, as early as 20 minutes after stimulation (107, 108). The protective effect of TNF-α for antiviral immunity has been shown in a number of cases, such as infection by CMV (109), HSV (110) and vaccinia virus (111). In addition to its effect on infected cells, TNF-α is necessary for inducing resistance in uninfected cells, and for optimal activation of γδ T cells and their cytokine production. In this regard, TNF-α can act as a co-stimulatory signal for a sustained response to TCR triggering (112) which implies a positive feedback loop not dissimilar to the one observed with IFN-γ.
After activation via the TCR, even if the majority of γδ T cells were expressing only IFN-γ, the appearance of cells producing both IFN-γ and TNF-α has been noted (113), suggesting that different subsets with diverging antiviral functions might appear during activation, depending on the context (114). It is known that TNF-α and IFN-γ have a synergistic effect, providing a heightened antiviral function to the γδ T cells with the capacity to produce both (115). A diverse range of other cytokines including GM-CSF, IL-4, IL-5 and IL-8 are produced by γδ T cells following viral infection (116, 117), participating in the systemic immune response. Similar to other sentinel cells, γδ T cells also secrete chemokines such as CCL2, CCL3, CCL4, CCL5, and CCL22 to recruit pro-inflammatory effectors, accelerating the elimination of pathogens and the repair of damaged tissues (116, 118).
In addition to their direct anti-infection activities and their recruitment of other immune cells, γδ T cells help to establish the adaptive response by contributing to dendritic cell maturation (119–121) but also by acting as professional Antigen Presenting Cells (APC) themselves (122). Indeed, they can efficiently internalize, process and present pathogen-related antigens from both free viral particles (123) and infected cells (124) to other effector immune cells (125). These γδ-T APCs express approximately similar levels of the MHC-II antigen-presenting molecule HLA-DR and of the costimulatory molecules CD80/CD86 to conventional APCs such as dendritic cells, allowing an efficient induction of CD4+ αβ-T-cell responses (126). Moreover, γδ-T APCs’ ability for cross-presentation (a process describing the internalization of exogenous antigens and their degradation for peptide loading on MHC-I antigen-presenting molecules) allow them to equal or even exceed dendritic cells’ capacity to induce CD8+ αβ-T-cell proliferation and effector functions (126, 127). In addition to their capacity for antigen presentation, γδ-T APCs change their migratory properties during activation, including the expression of the chemokine receptor CCR7, allowing their homing to the draining lymph nodes where they can activate virus-specific αβ-T-cells (128).
Another role for γδ T cells in the initiation of adaptive immunity is their helper function for the B cell-mediated humoral immunity (129). Besides their role in antibody production, γδ T cells are also key players in antibody-dependent cell-mediated cytotoxicity (ADCC) via their expression of FcγRIII (CD16) (130, 131). Moreover, in the case of CMV infection, CD16 has been shown to be upregulated in γδ T cells (132) and implicated in viral inhibition via direct recognition of IgG-opsonized virions and stimulation of IFN-γ production (133). Interestingly, CD56 expression, upregulated upon stimulation (134) and associated with cytolytic effector functions in γδ T cells (135) might be only a marker of co-expression with CD16. Thus, the better observed antiviral activity of CD56+ γδ T cells would be essentially due to the CD16-mediated degranulation pathway (136).
The antiviral capacity of γδ T cells has been illustrated by different studies using a variety of in vitro infected cells. They highlight the relative importance of each pathway and their modulation depending on the infectious context. For example, in a model of influenza virus-infected A549 lung alveolar epithelial cell line, Li et al. have proven by targeted inhibition the reliance of γδ T cells on the perforin and Granzyme B pathway, as well as NKG2D, FasL, TRAIL and IFN-γ (116, 137). This cytotoxic profile was confirmed in different in vitro models, including EBV-infected B cell lines (23) and HIV-infected lymphocytes (58, 138).
In vivo, activated γδ T cells have also proven to efficiently clear human influenza virus in humanized mice models (139). In humans, a study in 205 renal allograft recipients showed that CMV infection directly precedes γδ T cell expansion, and is the only clinical parameter associated with this expansion (140). Importantly, CMV-infected patients who develop delayed γδ T cell expansion have a higher viral load, more symptoms and longer disease than patients with early expansion, showing another link between γδ T cells and viral infection (141). This resolution is likely to be dependent on TCR stimulation triggering the perforin-granzyme B pathway as well as the production of IFN-γ (142, 143). Both αβ and γδ T cells respond to viral infection, as in the case of EBV-induced mononucleosis, but only the latter keeps a high frequency during the convalescent phase, consistent with their immune surveillance role (65). In acute hepatitis B, peripheral γδ T cells are activated and exhibit increased cytotoxicity and capacity for viral clearance (144). There is a negative correlation between activated γδ T cells and clinical markers of hepatitis progression (145), and in chronically-infected patients there is a marked reduction in the proportion and cytotoxicity of circulating γδ T cells compared to healthy donors, this decreased antiviral function correlating with the persistence of HBV (146, 147). Early HIV infection is also associated with reduced number and function of γδ T cells in the blood and endocervix (148, 149). This loss is proportional to viremia (150, 151) and might be a contributing factor in the establishment of viral persistence in AIDS, notably by reducing the level of IFN-γ (152). Interestingly, this appears to precede the loss of CD4+ αβ T cells, the major target of HIV, suggesting that γδ T cell impairment is one of the very first immune failings during HIV infection (153). Moreover, HIV‐infected elite controllers have elevated levels of circulating γδ T cells compared with HIV‐negative controls or HIV‐infected individuals on antiretroviral therapy (154), highlighting again a link between γδ T cells and disease outcome. In this latter category of antiretroviral treated patients, a slow but steady reconstitution of the γδ T cell pool to near-normal levels is observed (155, 156). Combined treatment with zoledronate (a γδ T cell-stimulating drug) and Interleukin-2 (IL2) in HIV patients induced activation and expansion of their circulating γδ T cells, and a subsequent heightened immune response characterized by dendritic cell maturation and CD8+ T cells responses (157) showing the efficiency of such intervention.
To illustrate the points discussed above, the next part of this review will focus on the case of the SARS-CoV-2 virus, responsible for the 2020 pandemic, which has generated a worldwide effort and an unprecedented amount of data for a better understanding of viral infection and the immune response to it.
SARS-CoV-2 belongs to the betacoronavirus genus and causes a highly infectious respiratory disease called COVID-19. Its closest relative among human coronaviruses is SARS-CoV, with 79% genetic similarity (158). The pathophysiology of SARS-CoV-2 infection resembles that of SARS-CoV infection, with progression in some individuals to acute respiratory distress syndrome (ARDS) characterized by aggressive inflammatory responses in the lower airways and responsible for 28% of fatal COVID-19 cases. As such, severe COVID-19 is not only due to direct effects of the virus but also in part to a dysregulated immune response inflicting multi-organ damage, especially in the cardiac, hepatic and renal systems (159).
This immunopathology is defined by a suppression of the early pro-inflammatory response. Indeed, SARS-CoV-2 is able to inhibit several transcription factors pivotal for the antiviral response such as NF-kB and IRF3/7, resulting in limited IFN production and signaling, reduced recruitment of immune cells and viral evasion. This precipitates pathogenesis and mortality in susceptible individuals (160). Reports on severe COVID-19 patients also showed altered immune composition, with increased total neutrophils and reduced lymphocyte count in the peripheral blood (161), and a correlation between lymphocytopenia, serum IL-6 concentration (a hallmark of cytokine storm), and disease severity (162, 163). Moreover, as patients progress toward symptomatic stages, an increasing proportion of exhausted PD1+ and TIM3+ lymphocytes are seen, highlighting the failure of the adaptive system to control infection in these cases (164). COVID-19 is also characterized by its demographics, with a high susceptibility among older males (14.8% case fatality ratio after age 80 Vs 2.3% total; men roughly 1.5x more likely to die than women) (165, 166). Indeed, most children with COVID-19 are asymptomatic and have a normal lymphocyte count (167). One of the striking differences between young and elderly immunity is the strong innate responses observed in the former (168), leading to early control of infection at the site of entry. Multiple innate immunity aberrations have been reported in the elderly: desensitization of dendritic cells, reduced TLR responses, dysregulated IFN response, decreased macrophage and neutrophil function, reduced NK activity, and relevant to this discussion, decreased γδ T cell proliferation and number (169–171). It has also been observed that there is altered function and phenotype among circulating γδ T cell in the elderly, notably a lower response and a lack of memory cells (172–174). In women, this phenotypic change is not observed, and the γδ T cell reduction occurs later in life and is less pronounced than in men (175).
So innate immunity status and particularly γδ T cell function can shape the viral response and be a determinant of disease progression. Currently, only a few studies are available on the host innate immune response of COVID‐19 infected patients. It’s been shown that as the first line of defense, innate immunity must block the virus in the upper airways in the first 10-12 days from infection (5-7 from the disease onset) for an efficient resolution of the infection (176) and that it indeed performs with great efficiency in the majority of individuals (177). But in the case of the deleterious inflammation associated with severe COVID-19, a body of evidence suggest that it is due to a failure to activate the immune system during a critical early time window, and to a subsequent primary cytokine release syndrome triggered as a delayed emergency response to uncontrolled SARS-CoV-2 replication (178, 179). The priority therefore would be to promote an early and robust immune response for effective viral clearance and the prevention of symptomatic infection as well as viral transmission.
During the 2003 coronavirus outbreak, health care workers that survived SARS-CoV infection had a selective expansion of the blood Vδ2 γδ T cells, observed 3 months after the disease onset (180). No expansion of non-innate αβ T cells was detected at this timepoint. Interestingly, these γδ T cells were able to directly kill SARS-CoV infected target cells in an IFN-γ-dependent way, and their increase was proportional with anti-SARS-CoV IgG titers, suggesting their protective role during coronavirus infections.
There is currently a paucity of studies including the γδ T cells in their immune characterization of COVID-19, but the few studies that investigated this population gives us an interesting perspective on their role during the fight against SARS-CoV-2:
In accordance with the general lymphocytopenia, the percentage of γδ T cells in the blood of patients hospitalized for COVID-19 (on average 10 days after the onset of clinical symptoms) is lower than that of healthy controls (181, 182). Interestingly, there is a shift in γδ T cell phenotype during the 2 weeks of hospital admission, with a transition toward effector (memory) cells more capable of tissue infiltration, as confirmed by Odak et al. (183). The blood γδ T cell reduction is indeed associated with their recruitment in the airway tissues (184, 185). Moreover, γδ T cells’ level of stimulation (CD69 positivity) is increased in the blood compared to healthy controls and is even higher in the infected tissues than in the blood, showing their activation at the injury epicenter (186). Lei et al. (187) confirmed the γδ T cell activation in blood, with increasing expression of CD4 and CD25, and showed no sign of exhaustion as assessed by PD1 expression. The expansion of a CD16+ γδ T cell population in COVID-19 has been observed in single-cell transcriptional profiling of 13 patients. In the study, the presence of this CD16+ γδ T cells subset is strongly associated with moderate disease and almost absent in the severe condition (188). Another team comparing immune signatures between 63 COVID-19 patients and 55 Healthy Controls also confirmed the depletion of γδ T cells in the blood and showed that while the number of Vδ1 is not different from controls or between severity groups, the Vδ2 depletion is proportional to the disease severity (189). The authors then suggest that it could be used as a diagnostic or prognostic marker, a suggestion supported by Carissimo et al. who showed that a Neutrophil/Vδ2 ratio is a better prognostic marker of COVID-19 severity than the Neutrophil/CD8+ Lymphocytes ratio (190). They also showed that γδ T cells are generally activated, as seen by their upregulation of the activation marker CD38 and differentiate into central memory cells after recovery. Expansion of the γδ T cell pool has also been noted concomitantly of the remission phase in a single-cell analysis of 2 severe COVID-19 patients (191).
All the advantages highlighted above, including rapid activation, MHC independency, ability to traffic to infected tissues and potent antiviral function makes γδ T cells attractive candidates as therapeutic tools (192) (Figure 1). In the next section, we will focus on this therapeutic potential.
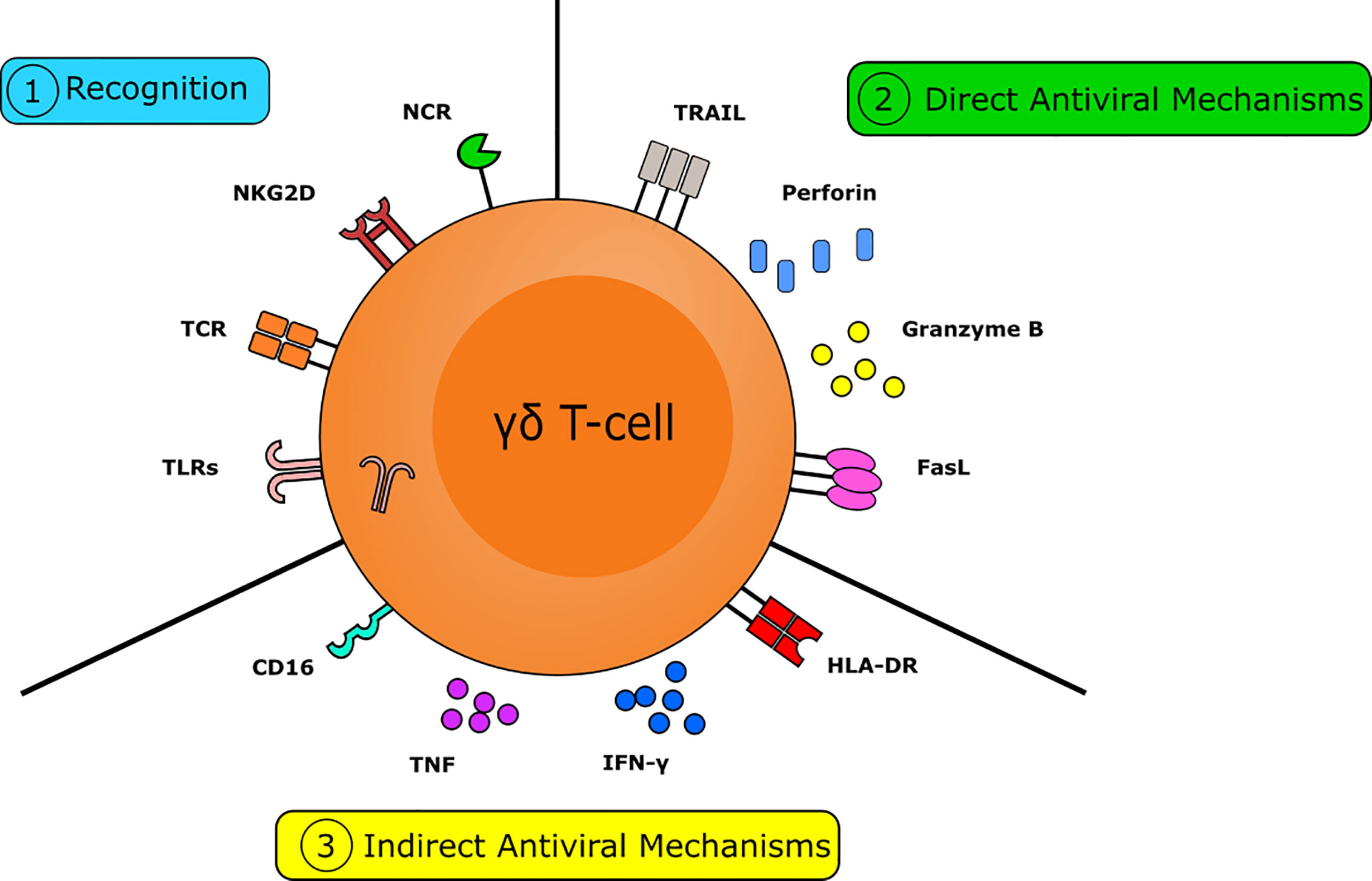
Figure 1 The multifactorial capacity for the γδ T-cell to interact with viruses and virally infected targets. Numerous pathways are crucial in the γδ T-cell mediated antiviral response. γδ T-cells are capable of rapidly recognizing virally infected cells. This can occur via the detection of isopentenyl pyrophosphate (IPP) by the T-cell receptor (TCR), via recognition of stress-induced molecules by NKG2D, or via the recognition of viral molecules and PAMPs by NK-type receptors and TLR, respectively. γδ T-cells have numerous mechanisms to directly combat viral infection. Direct antiviral mechanisms are mediated by cytolytic molecules, such as perforin and granzyme B, to induce cytolysis and by the expression of death receptors, including FasL and TRAIL, to induce apoptosis. γδ T-cells also have several indirect mechanisms capable of combatting viral infection. Indirect antiviral mechanisms are mediated by cytokines, such as IFNγ and TNF, by the expression of MHC-II allowing them to act as APC to direct the adaptive immune response and via expression of CD16 to trigger antibody-dependent cellular cytotoxicity. Together these actions make the γδ T-cell a crucial component in the immune response to viruses.
There are 2 major modalities for taking advantage of γδ T cell capabilities in a clinical context: ex vivo activation with a subsequent adoptive transfer, or direct in vivo activation.
The ex vivo approach relies on γδ T cell isolation from Peripheral Blood Mononuclear Cells (PBMCs), in vitro stimulation with products such as bisphosphonates, pAg or monoclonal antibodies (193), and injection of the activated cells into patients (194). The safety and efficacy of this approach have long been proven in the treatment of cancers, with dozens of clinical trials involving isolation, expansion and adoptive transfer of up to 1x1010 γδ T cells (195).
This strategy is also implemented as antiviral therapy against various infections and has shown promising results. The first necessity for an optimal cell product is to stimulate γδ T cells in a way that maximizes their antiviral response. This has been achieved for example in a model of H1N1-infected macrophage, where γδ T cells expanded with isopentenyl pyrophosphate (IPP), a phosphoantigen, are able to effectively kill target cells and to inhibit viral replication, notably due to their high production of IFN-γ (116, 196). Similarly, when expanded with Pamidronate (PAM), a bisphosphonate, γδ T cells can also effectively kill influenza-infected lung alveolar epithelial cells in vitro thus inhibiting viral replication (137). These results have been confirmed in models of HCV as well as CMV infection (104, 197). Furthermore, Zoledronic Acid (ZA), another bisphosphonate, has been used ex vivo in PBMCs from HIV+ individuals and resulted in expansion of γδ T cells displaying cytotoxic capabilities and potent ADCC function, demonstrating that this protocol is able to reactivate effector functions in patient’s cells (198). PAM expanded cells from HIV-infected patients showed similar cytotoxicity against HIV-infected cells (199), illustrating that various avenues can be chosen to harness γδ T cells’ antiviral functions in a clinical setting.
The second step of this strategy involves the adoptive transfer of activated γδ T cells, which have been shown to be safe and effective in pre-clinical models of infectious disease. In mice infected with enterovirus or CMV, the adoptive transfer of γδ T cells was able to provoke a Th1-type response associated with viral control and better survival (200–202). In humanized mice infected by the influenza virus, injection of PAM-activated γδ T cells resulted in controlled viral replication and reduced disease severity and mortality (203).
Thus, γδ T cell-based adoptive cell therapies have the potential to be used as an allogeneic “off-the-shelf” antiviral product, akin to the strategies used for example with NK cells (https://clinicaltrials.gov/ct2/show/NCT04365101). Despite this potential, clinical efficacy has yet to be proven, and the logistical challenges that come with an ex vivo cell product may hinder the development of this specific strategy. Hence, directly stimulating a patient’s γδ T cells in vivo could appear more desirable.
The in vivo approach involves systemic stimulation and expansion of γδ T cells, usually by administration of bisphosphonates or pAg. It’s also used routinely for cancer treatment, with no severe adverse effects and an efficient in vivo expansion of IFN-γ+ Perforin+ effector γδ T cells (204, 205) associated with stable disease or partial remission (206).
The use of humanized mouse models has generated interesting data in influenza infection. In vivo activation with PAM resulted in accumulation of γδ T cells in lungs and fewer symptoms, associated with reduced lung inflammation, fewer cell infiltrates and decreased levels of mediators such as IL-6, TNF-α or IP-10 (203). This finding has been supported by others, who also describe a 3-fold increase of γδ T cells 2 days after treatment, and lower viral replication and mortality (139). Non-human primate models provide an alternative to humanized mice in the interrogation of in vivo γδ T cells responses. The pAg HMBPP ((E)-4-Hydroxy-3-Methyl-But-2-enyl Pyrophosphate), in combination with IL2, has been shown to cause expansion of circulating IFN-γ+ Perforin+ γδ T cells in vivo, and accumulation in the lungs lasting at least 3-4 months, long after circulating levels had returned to normal (207). In a similar study, γδ T cells accumulated in the lungs were able to protect from pulmonary lesions caused by Yersinia pestis infection (208). Finally, in a model of tuberculosis, IFN-γ+ Perforin+ γδ T cells accumulating in the lungs attenuated the lesions and stimulated a CD8+ T cell adaptive immune response (209). These findings are consistent with the paradigm that circulating γδ T cells can traffic to the lungs for homeostatic protection against tissue damage during infection, suggesting their potential as immunotherapeutics against a variety of pulmonary pathogens. In humans, administration of ZA with IL2 has been carried out in HIV‐infected, antiretroviral naïve patients and was associated with γδ T cell expansion, dendritic cell activation and increased HIV‐specific CD8+ T‐cell responses (210), suggesting that this strategy can be used to restore impaired immune response observed in AIDS (211).
The advantage of bisphosphonates such as ZA and PAM is that they are already clinically approved, inexpensive and relatively safe drugs (212). Moreover, in the context of viral infection, they might have an additive clinical benefit, as they’ve been shown not only to stimulate γδ T cells but also inhibit the protein prenylation pathway and the cholesterol synthesis, both required for virus assembly (113, 213). Taken together, these effects strengthen the argument for their use as antiviral agents.
Another known mechanism of in vivo γδ T cell activation is by microbial products like listeria, mycobacteria or salmonella-derived vaccines (214–216). Indeed, there is accumulating evidence that innate immunity, including γδ T cells, is boosted by specific vaccination in addition to targeted adaptive immunity (217). For example, the influenza vaccine is able to induce virus-specific γδ T cell expansion along with CD4+ and CD8+ T cells stimulation (218), and the differentiation of these γδ T cells into an effector/memory phenotype, with increased perforin expression (219). Vaccination in a model of Simian Immunodeficiency Virus (SIV) in macaques has been shown to block infection early at mucosal sites, and this protection was associated with expansion of γδ T cells and maturation of dendritic cells (220). In addition to their designed effects, vaccines have long been shown to protect beyond their target antigen through induction of innate immune mechanisms termed non-specific heterologous effects and trained immunity (221). Thus, certain adjuvants such as TLR agonists (222), as well as live vaccines like polio (223) or measles (224, 225) induce long-term cross-protection against various infections through epigenetic, transcriptional, and functional reprogramming of innate immune cells such as macrophages, NK cells or γδ T cells (226). This reprogramming results in enhanced activation, and ultimately protection against secondary infection, resembling immune memory (227, 228). The most well-studied inducer of trained immunity is the Bacillus Calmette–Guérin (BCG) vaccine (229). It is composed of a live attenuated strain of Mycobacterium bovis originally given to young children to protect against tuberculosis, but recent studies demonstrated that its administration more broadly reduced mortalities from infectious diseases over the neonatal period (230, 231). It has then been postulated that the relative protection from COVID-19 reported in children might be attributed to their frequent vaccinations, and indeed some correlations between BCG vaccination policies and reduced infection and mortality rates due to SARS-CoV-2 have been reported (232–235). Indeed, even after correcting for many socioeconomic and pandemic-related confounders, data shows that for every 10% increase in the BCG index (degree of national universal vaccination), there is a 10.4% reduction in COVID-19 mortality (236). These results are still under debate (237) but have initiated numerous studies and clinical trials investigating the effect of BCG on nonspecific protection against SARS-CoV-2 infection or its severity (238–240) (https://clinicaltrials.gov/ct2/show/NCT04369794, NCT04362124, NCT04379336, NCT04350931, NCT04327206, NCT04373291, NCT04328441, NCT04348370). This non-specific protection could be harnessed independently of age, as a randomized controlled trial in elderly (60–75 years old) who received BCG vaccinations, showed a reduction of the incidence of acute upper respiratory tract infection (241). It has also been proven to protect against a variety of viruses like yellow fever, influenza, papillomavirus (HPV), Respiratory syncytial virus (RSV) or HSV (242, 243).
As a key cell type in the innate immune response, it is clear γδ T cells also play a role in contributing to trained immunity. Many studies have documented expansion of the γδ T cell population following vaccination with BCG, with these cells being one of the key producers of IFN-y in immunized children (244–246). Mycobacteria stimulation also induces γδ T cell cytotoxicity toward virus-infected cells (HSV and vaccinia), typical of the heterologous effect observed in trained immunity (247). Moreover, γδ T cells expanded after viral infection or BCG stimulation, differentiate into effector memory cells capable of a faster and more efficient response to a second infection (248–251). So BCG can be used to expand cytotoxic γδ T cells capable of eventually differentiating in long-lived memory cells allowing enhanced protection against subsequent infections.
The contribution of γδ T cells to the regression of BCG-treated melanoma patients has already been proven (252), and highlights the clinical potential suggested above for a similar setting in treatments of viral infections. Thus, BCG or its derivatives (253, 254) are attractive candidates for establishing trained immunity and stimulating early clearance of subsequent viral infection (255). Integrating innate immunity stimulation in the design of vaccines would also be a way of harnessing this under-considered potential (256). Indeed, by the choice of delivery route (257, 258) or adjuvant (259), one could balance the immune response to allow for complementary protection in instances where the adaptive immunity is failing. BCG itself could be used as an adjuvant or in a prime-boost strategy, as it has been shown to orient toward an antiviral Th1-type response and to enhance vaccine efficiency (260).
As highlighted here, the varied characteristics of γδ T cells support their role in controlling viral diseases in general and COVID-19 in particular. Considering the accumulating evidence on their multiple antiviral functions and their capacity to react early and to quickly prevent viral spread, we’re advocating for better inclusion of γδ T cells in the therapeutic armamentarium against viral infections. For example, a cheap and effective way of harnessing anti-viral innate immunity such as that mediated by γδ T cells would be to vaccinate the population with BCG in cases where there is no access to a specific vaccine, or as a supplementary boost to it, and the ongoing clinical trials using these strategies will be of tremendous importance for the optimization of γδ T cell-based therapies against viruses.
JC designed, wrote, and revised the manuscript. LR wrote and revised the manuscript. MB-S revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Institute for Cancer Vaccines and Immunotherapy (Registered Charity Number 1080343).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Xu GJ, Kula T, Xu Q, Li MZ, Vernon SD, Ndung’u T, et al. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science (2015) 348:aaa0698. doi: 10.1126/science.aaa0698
2. Poccia F, Agrati C, Martini F, Capobianchi MR, Wallace M, Malkovsky M. Antiviral reactivities of gammadelta T cells. Microbes Infect (2005) 7:518–28. doi: 10.1016/j.micinf.2004.12.009
3. Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol (2017) 17:733–45. doi: 10.1038/nri.2017.101
4. Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol (2010) 10:467–78. doi: 10.1038/nri2781
5. Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol (2001) 2:997–1003. doi: 10.1038/ni1101-997
6. Cruz MS, Diamond A, Russell A, Jameson JM. Human αβ and γδ T Cells in Skin Immunity and Disease. Front Immunol (2018) 9:1304. doi: 10.3389/fimmu.2018.01304
7. Chien Y, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol (2014) 32:121–55. doi: 10.1146/annurev-immunol-032713-120216
8. Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells. J Immunol (2001) 166:6784–94. doi: 10.4049/jimmunol.166.11.6784
9. Wang T, Gao Y, Scully E, Davis CT, Anderson JF, Welte T, et al. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J Immunol (2006) 177:1825–32. doi: 10.4049/jimmunol.177.3.1825
10. Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Gamma interferon secretion by human Vgamma2Vdelta2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-Like receptor 2 agonist Pam3Cys. Infect Immun (2006) 74:4505–11. doi: 10.1128/IAI.00088-06
11. Cui Y, Kang L, Cui L, He W. Human gammadelta T cell recognition of lipid A is predominately presented by CD1b or CD1c on dendritic cells. Biol Direct (2009) 4:47. doi: 10.1186/1745-6150-4-47
12. Lester SN, Li K. Toll-like receptors in antiviral innate immunity. J Mol Biol (2014) 426:1246–64. doi: 10.1016/j.jmb.2013.11.024
13. Wesch D, Beetz S, Oberg H-H, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J Immunol (2006) 176:1348–54. doi: 10.4049/jimmunol.176.3.1348
14. Pietschmann K, Beetz S, Welte S, Martens I, Gruen J, Oberg H-H, et al. Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scand J Immunol (2009) 70:245–55. doi: 10.1111/j.1365-3083.2009.02290.x
15. Wesch D, Peters C, Oberg H-H, Pietschmann K, Kabelitz D. Modulation of γδ T cell responses by TLR ligands. Cell Mol Life Sci (2011) 68:2357–70. doi: 10.1007/s00018-011-0699-1
16. Kawai T, Akira S. TLR signaling. Cell Death Differ (2006) 13:816–25. doi: 10.1038/sj.cdd.4401850
17. Sirén J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol (2005) 174:1932–7. doi: 10.4049/jimmunol.174.4.1932
18. Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol (2005) 175:2144–51. doi: 10.4049/jimmunol.175.4.2144
19. González S, López-Soto A, Suarez-Alvarez B, López-Vázquez A, López-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol (2008) 29:397–403. doi: 10.1016/j.it.2008.04.007
20. Rölle A, Mousavi-Jazi M, Eriksson M, Odeberg J, Söderberg-Nauclér C, Cosman D, et al. Effects of human cytomegalovirus infection on ligands for the activating NKG2D receptor of NK cells: up-regulation of UL16-binding protein (ULBP)1 and ULBP2 is counteracted by the viral UL16 protein. J Immunol (2003) 171:902–8. doi: 10.4049/jimmunol.171.2.902
21. Sirén J, Sareneva T, Pirhonen J, Strengell M, Veckman V, Julkunen I, et al. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J Gen Virol (2004) 85:2357–64. doi: 10.1099/vir.0.80105-0
22. Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood (2007) 110:1207–14. doi: 10.1182/blood-2006-06-028175
23. Xiang Z, Liu Y, Zheng J, Liu M, Lv A, Gao Y, et al. Targeted activation of human Vγ9Vδ2-T cells controls epstein-barr virus-induced B cell lymphoproliferative disease. Cancer Cell (2014) 26:565–76. doi: 10.1016/j.ccr.2014.07.026
24. Kong Y, Cao W, Xi X, Ma C, Cui L, He W. The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood (2009) 114:310–7. doi: 10.1182/blood-2008-12-196287
25. Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D Costimulates Human Vγ9Vδ2 T Cell Antitumor Cytotoxicity through Protein Kinase Cθ-Dependent Modulation of Early TCR-Induced Calcium and Transduction Signals. J Immunol (2010) 185:55–63. doi: 10.4049/jimmunol.1000373
26. Lança T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood (2010) 115:2407–11. doi: 10.1182/blood-2009-08-237123
27. Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, et al. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci USA (2011) 108:2414–9. doi: 10.1073/pnas.1015433108
28. Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. Eur J Immunol (2009) 39:1361–8. doi: 10.1002/eji.200838409
29. Pignoloni B, Fionda C, Dell’Oste V, Luganini A, Cippitelli M, Zingoni A, et al. Distinct Roles for Human Cytomegalovirus Immediate Early Proteins IE1 and IE2 in the Transcriptional Regulation of MICA and PVR/CD155 Expression. J Immunol (2016) 197:4066–78. doi: 10.4049/jimmunol.1502527
30. Vassena L, Giuliani E, Matusali G, Cohen ÉA, Doria M. The human immunodeficiency virus type 1 Vpr protein upregulates PVR via activation of the ATR-mediated DNA damage response pathway. J Gen Virol (2013) 94:2664–9. doi: 10.1099/vir.0.055541-0
31. Pappworth IY, Wang EC, Rowe M. The switch from latent to productive infection in epstein-barr virus-infected B cells is associated with sensitization to NK cell killing. J Virol (2007) 81:474–82. doi: 10.1128/JVI.01777-06
32. Charpak-Amikam Y, Kubsch T, Seidel E, Oiknine-Djian E, Cavaletto N, Yamin R, et al. Human cytomegalovirus escapes immune recognition by NK cells through the downregulation of B7-H6 by the viral genes US18 and US20. Sci Rep (2017) 7:8661. doi: 10.1038/s41598-017-08866-2
33. Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature (2001) 409:1055–60. doi: 10.1038/35059110
34. Ho JW, Hershkovitz O, Peiris M, Zilka A, Bar-Ilan A, Nal B, et al. H5-type influenza virus hemagglutinin is functionally recognized by the natural killer-activating receptor NKp44. J Virol (2008) 82:2028–32. doi: 10.1128/JVI.02065-07
35. Jarahian M, Fiedler M, Cohnen A, Djandji D, Hämmerling GJ, Gati C, et al. Modulation of NKp30- and NKp46-mediated natural killer cell responses by poxviral hemagglutinin. PloS Pathog (2011) 7:e1002195. doi: 10.1371/journal.ppat.1002195
36. Jarahian M, Watzl C, Fournier P, Arnold A, Djandji D, Zahedi S, et al. Activation of natural killer cells by newcastle disease virus hemagglutinin-neuraminidase. J Virol (2009) 83:8108–21. doi: 10.1128/JVI.00211-09
37. Hershkovitz O, Rosental B, Rosenberg LA, Navarro-Sanchez ME, Jivov S, Zilka A, et al. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol (2009) 183:2610–21. doi: 10.4049/jimmunol.0802806
38. Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood (2011) 118:992–1001. doi: 10.1182/blood-2011-02-339135
39. Hudspeth K, Fogli M, Correia DV, Mikulak J, Roberto A, Della Bella S, et al. Engagement of NKp30 on Vδ1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood (2012) 119:4013–6. doi: 10.1182/blood-2011-11-390153
40. von Lilienfeld-Toal M, Nattermann J, Feldmann G, Sievers E, Frank S, Strehl J, et al. Activated gammadelta T cells express the natural cytotoxicity receptor natural killer p 44 and show cytotoxic activity against myeloma cells. Clin Exp Immunol (2006) 144:528–33. doi: 10.1111/j.1365-2249.2006.03078.x
41. Urban EM, Chapoval AI, Pauza CD. Repertoire development and the control of cytotoxic/effector function in human gammadelta T cells. Clin Dev Immunol (2010) 2010:732893. doi: 10.1155/2010/732893
42. Born WK, Kemal Aydintug M, O’Brien RL. Diversity of γδ T-cell antigens. Cell Mol Immunol (2013) 10:13–20. doi: 10.1038/cmi.2012.45
43. Spada FM, Grant EP, Peters PJ, Sugita M, Melián A, Leslie DS, et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med (2000) 191:937–48. doi: 10.1084/jem.191.6.937
44. Hirsh MI, Junger WG. Roles of heat shock proteins and gamma delta T cells in inflammation. Am J Respir Cell Mol Biol (2008) 39:509–13. doi: 10.1165/rcmb.2008-0090TR
45. Das H, Groh V, Kuijl C, Sugita M, Morita CT, Spies T, et al. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity (2001) 15:83–93. doi: 10.1016/s1074-7613(01)00168-6
46. Fournié JJ, Bonneville M. Stimulation of gamma delta T cells by phosphoantigens. Res Immunol (1996) 147:338–47. doi: 10.1016/0923-2494(96)89648-9
47. Djaoud Z, Guethlein LA, Horowitz A, Azzi T, Nemat-Gorgani N, Olive D, et al. Two alternate strategies for innate immunity to Epstein-Barr virus: One using NK cells and the other NK cells and γδ T cells. J Exp Med (2017) 214:1827–41. doi: 10.1084/jem.20161017
48. Jameson JM, Cruz J, Costanzo A, Terajima M, Ennis FA. A role for the mevalonate pathway in the induction of subtype cross-reactive immunity to influenza A virus by human gammadelta T lymphocytes. Cell Immunol (2010) 264:71–7. doi: 10.1016/j.cellimm.2010.04.013
49. Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X, et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. J Clin Invest (1999) 103:1437–49. doi: 10.1172/JCI5409
50. Kaminski H, Marsères G, Cosentino A, Guerville F, Pitard V, Fournié J-J, et al. Understanding human γδ T cell biology toward a better management of cytomegalovirus infection. Immunol Rev (2020) 298:264–88. doi: 10.1111/imr.12922
51. Jin Y, Xia M, Saylor CM, Narayan K, Kang J, Wiest DL, et al. Cutting edge: Intrinsic programming of thymic γδT cells for specific peripheral tissue localization. J Immunol (2010) 185:7156–60. doi: 10.4049/jimmunol.1002781
52. Chennupati V, Worbs T, Liu X, Malinarich FH, Schmitz S, Haas JD, et al. Intra- and intercompartmental movement of gammadelta T cells: intestinal intraepithelial and peripheral gammadelta T cells represent exclusive nonoverlapping populations with distinct migration characteristics. J Immunol (2010) 185:5160–8. doi: 10.4049/jimmunol.1001652
53. Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific γδ T Cell Compartments. Cell (2016) 167:203–18.e17. doi: 10.1016/j.cell.2016.08.030
54. Jandke A, Melandri D, Monin L, Ushakov DS, Laing AG, Vantourout P, et al. Butyrophilin-like proteins display combinatorial diversity in selecting and maintaining signature intraepithelial γδ T cell compartments. Nat Commun (2020) 11:3769. doi: 10.1038/s41467-020-17557-y
55. Agrati C, D’Offizi G, Narciso P, Abrignani S, Ippolito G, Colizzi V, et al. Vdelta1 T lymphocytes expressing a Th1 phenotype are the major gammadelta T cell subset infiltrating the liver of HCV-infected persons. Mol Med (2001) 7:11–9. doi: 10.1016/j.arr.2018.11.003
56. De Paoli P, Gennari D, Martelli P, Basaglia G, Crovatto M, Battistin S, et al. A subset of gamma delta lymphocytes is increased during HIV-1 infection. Clin Exp Immunol (1991) 83:187–91. doi: 10.1111/j.1365-2249.1991.tb05612.x
57. Agrati C, D’Offizi G, Narciso P, Selva C, Pucillo LP, Ippolito G, et al. Gammadelta T cell activation by chronic HIV infection may contribute to intrahepatic vdelta1 compartmentalization and hepatitis C virus disease progression independent of highly active antiretroviral therapy. AIDS Res Hum Retroviruses (2001) 17:1357–63. doi: 10.1089/08892220152596614
58. Fausther-Bovendo H, Wauquier N, Cherfils-Vicini J, Cremer I, Debré P, Vieillard V. NKG2C is a major triggering receptor involved in the V[delta]1 T cell-mediated cytotoxicity against HIV-infected CD4 T cells. AIDS (2008) 22:217–26. doi: 10.1097/QAD.0b013e3282f46e7c
59. Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol (2013) 14:908–16. doi: 10.1038/ni.2665
60. Boutin L, Scotet E. Towards Deciphering the Hidden Mechanisms That Contribute to the Antigenic Activation Process of Human Vγ9Vδ2 T Cells. Front Immunol (2018) 9:828. doi: 10.3389/fimmu.2018.00828
61. Verjans GMGM, Roest RW, van der Kooi A, van Dijk G, van der Meijden WI, Osterhaus A ‘D ME. Isopentenyl pyrophosphate-reactive Vgamma9Vdelta 2 T helper 1-like cells are the major gammadelta T cell subset recovered from lesions of patients with genital herpes. J Infect Dis (2004) 190:489–93. doi: 10.1086/422393
62. Zhong H, Hu X, Janowski AB, Storch GA, Su L, Cao L, et al. Whole transcriptome profiling reveals major cell types in the cellular immune response against acute and chronic active Epstein-Barr virus infection. Sci Rep (2017) 7:17775. doi: 10.1038/s41598-017-18195-z
63. Hassan J, Feighery C, Bresnihan B, Whelan A. Elevated T cell receptor gamma delta + T cells in patients with infectious mononucleosis. Br J Haematol (1991) 77:255–6. doi: 10.1111/j.1365-2141.1991.tb07990.x
64. Yin W, Tong S, Zhang Q, Shao J, Liu Q, Peng H, et al. Functional dichotomy of Vδ2 γδ T cells in chronic hepatitis C virus infections: role in cytotoxicity but not for IFN-γ production. Sci Rep (2016) 6:26296. doi: 10.1038/srep26296
65. De Paoli P, Gennari D, Martelli P, Cavarzerani V, Comoretto R, Santini G. Gamma delta T cell receptor-bearing lymphocytes during Epstein-Barr virus infection. J Infect Dis (1990) 161:1013–6. doi: 10.1093/infdis/161.5.1013
66. Koizumi H, Liu CC, Zheng LM, Joag SV, Bayne NK, Holoshitz J, et al. Expression of perforin and serine esterases by human gamma/delta T cells. J Exp Med (1991) 173:499–502. doi: 10.1084/jem.173.2.499
67. Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol (2003) 3:361–70. doi: 10.1038/nri1083
68. Bade B, Boettcher HE, Lohrmann J, Hink-Schauer C, Bratke K, Jenne DE, et al. Differential expression of the granzymes A, K and M and perforin in human peripheral blood lymphocytes. Int Immunol (2005) 17:1419–28. doi: 10.1093/intimm/dxh320
69. Sparrow E, Bodman-Smith MD. Granulysin: The attractive side of a natural born killer. Immunol Lett (2020) 217:126–32. doi: 10.1016/j.imlet.2019.11.005
70. Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity (1997) 7:831–6. doi: 10.1016/s1074-7613(00)80401-x
71. Nakata M, Smyth MJ, Norihisa Y, Kawasaki A, Shinkai Y, Okumura K, et al. Constitutive expression of pore-forming protein in peripheral blood gamma/delta T cells: implication for their cytotoxic role in vivo. J Exp Med (1990) 172:1877–80. doi: 10.1084/jem.172.6.1877
72. De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Herzenberg LA, et al. Ontogeny of gamma delta T cells in humans. J Immunol (2004) 172:1637–45. doi: 10.4049/jimmunol.172.3.1637
73. Nakata M, Kawasaki A, Azuma M, Tsuji K, Matsuda H, Shinkai Y, et al. Expression of perforin and cytolytic potential of human peripheral blood lymphocyte subpopulations. Int Immunol (1992) 4:1049–54. doi: 10.1093/intimm/4.9.1049
74. Dieli F, Troye-Blomberg M, Ivanyi J, Fournié JJ, Krensky AM, Bonneville M, et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis (2001) 184:1082–5. doi: 10.1086/323600
75. Farnault L, Gertner-Dardenne J, Gondois-Rey F, Michel G, Chambost H, Hirsch I, et al. Clinical evidence implicating gamma-delta T cells in EBV control following cord blood transplantation. Bone Marrow Transplant (2013) 48:1478–9. doi: 10.1038/bmt.2013.75
76. de Koning PJA, Tesselaar K, Bovenschen N, Colak S, Quadir R, Volman TJH, et al. The cytotoxic protease granzyme M is expressed by lymphocytes of both the innate and adaptive immune system. Mol Immunol (2010) 47:903–11. doi: 10.1016/j.molimm.2009.10.001
77. de Koning PJA, Kummer JA, Bovenschen N. Biology of granzyme M: a serine protease with unique features. Crit Rev Immunol (2009) 29:307–15. doi: 10.1615/critrevimmunol.v29.i4.20
78. van Domselaar R, Philippen LE, Quadir R, Wiertz EJHJ, Kummer JA, Bovenschen N. Noncytotoxic inhibition of cytomegalovirus replication through NK cell protease granzyme M-mediated cleavage of viral phosphoprotein 71. J Immunol (2010) 185:7605–13. doi: 10.4049/jimmunol.1001503
79. Andrade F, Fellows E, Jenne DE, Rosen A, Young CSH. Granzyme H destroys the function of critical adenoviral proteins required for viral DNA replication and granzyme B inhibition. EMBO J (2007) 26:2148–57. doi: 10.1038/sj.emboj.7601650
80. Zhong C, Li C, Wang X, Toyoda T, Gao G, Fan Z. Granzyme K inhibits replication of influenza virus through cleaving the nuclear transport complex importin α1/β dimer of infected host cells. Cell Death Differ (2012) 19:882–90. doi: 10.1038/cdd.2011.178
81. Tang H, Li C, Wang L, Zhang H, Fan Z. Granzyme H of cytotoxic lymphocytes is required for clearance of the hepatitis B virus through cleavage of the hepatitis B virus X protein. J Immunol (2012) 188:824–31. doi: 10.4049/jimmunol.1102205
82. Romero V, Fellows E, Jenne DE, Andrade F. Cleavage of La protein by granzyme H induces cytoplasmic translocation and interferes with La-mediated HCV-IRES translational activity. Cell Death Differ (2009) 16:340–8. doi: 10.1038/cdd.2008.165
83. Rouvier E, Luciani MF, Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med (1993) 177:195–200. doi: 10.1084/jem.177.1.195
84. Huber SA. T cells expressing the gamma delta T cell receptor induce apoptosis in cardiac myocytes. Cardiovasc Res (2000) 45:579–87. doi: 10.1016/s0008-6363(99)00267-9
85. Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science (1994) 265:528–30. doi: 10.1126/science.7518614
86. Yamashita S, Tanaka Y, Tsutsumi S, Aburatani H, Minato N, Ihara S. Analysis of mechanism for human gammadelta T cell recognition of nonpeptide antigens. Biochem Biophys Res Commun (2005) 334:349–60. doi: 10.1016/j.bbrc.2005.06.100
87. Guerville F, Daburon S, Marlin R, Lartigue L, Loizon S, Pitard V, et al. TCR-dependent sensitization of human γδ T cells to non-myeloid IL-18 in cytomegalovirus and tumor stress surveillance. Oncoimmunology (2015) 4:e1003011. doi: 10.1080/2162402X.2014.1003011
88. DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol (2004) 2:401–13. doi: 10.1038/nrmicro878
89. Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol (2014) 32:513–45. doi: 10.1146/annurev-immunol-032713-120231
90. Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev (2001) 14:778–809. doi: 10.1128/CMR.14.4.778-809.2001
91. Crosse KM, Monson EA, Beard MR, Helbig KJ. Interferon-Stimulated Genes as Enhancers of Antiviral Innate Immune Signaling. J Innate Immun (2018) 10:85–93. doi: 10.1159/000484258
92. Brass AL, Huang I-C, Benita Y, John SP, Krishnan MN, Feeley EM, et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell (2009) 139:1243–54. doi: 10.1016/j.cell.2009.12.017
93. Huang I-C, Bailey CC, Weyer JL, Radoshitzky SR, Becker MM, Chiang JJ, et al. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PloS Pathog (2011) 7:e1001258. doi: 10.1371/journal.ppat.1001258
94. Chakrabarti A, Jha BK, Silverman RH. New insights into the role of RNase L in innate immunity. J Interferon Cytokine Res (2011) 31:49–57. doi: 10.1089/jir.2010.0120
95. Szretter KJ, Brien JD, Thackray LB, Virgin HW, Cresswell P, Diamond MS. The interferon-inducible gene viperin restricts West Nile virus pathogenesis. J Virol (2011) 85:11557–66. doi: 10.1128/JVI.05519-11
96. Wang X, Hinson ER, Cresswell P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe (2007) 2:96–105. doi: 10.1016/j.chom.2007.06.009
97. Dunne MR, Madrigal-Estebas L, Tobin LM, Doherty DG. (E)-4-hydroxy-3-methyl-but-2 enyl pyrophosphate-stimulated Vgamma9Vdelta2 T cells possess T helper type 1-promoting adjuvant activity for human monocyte-derived dendritic cells. Cancer Immunol Immunother (2010) 59:1109–20. doi: 10.1007/s00262-010-0839-8
98. Sant S, Jenkins MR, Dash P, Watson KA, Wang Z, Pizzolla A, et al. Human γδ T-cell receptor repertoire is shaped by influenza viruses, age and tissue compartmentalisation. Clin Transl Immunol (2019) 8:e1079. doi: 10.1002/cti2.1079
99. Yin Z, Zhang DH, Welte T, Bahtiyar G, Jung S, Liu L, et al. Dominance of IL-12 over IL-4 in gamma delta T cell differentiation leads to default production of IFN-gamma: failure to down-regulate IL-12 receptor beta 2-chain expression. J Immunol (2000) 164:3056–64. doi: 10.4049/jimmunol.164.6.3056
100. García VE, Sieling PA, Gong J, Barnes PF, Uyemura K, Tanaka Y, et al. Single-cell cytokine analysis of gamma delta T cell responses to nonpeptide mycobacterial antigens. J Immunol (1997) 159:1328–35.
101. Battistini L, Borsellino G, Sawicki G, Poccia F, Salvetti M, Ristori G, et al. Phenotypic and cytokine analysis of human peripheral blood gamma delta T cells expressing NK cell receptors. J Immunol (1997) 159:3723–30.
102. Barcy S, De Rosa SC, Vieira J, Diem K, Ikoma M, Casper C, et al. Gamma delta+ T cells involvement in viral immune control of chronic human herpesvirus 8 infection. J Immunol (2008) 180:3417–25. doi: 10.4049/jimmunol.180.5.3417
103. Conroy MJ, Mac Nicholas R, Taylor M, O’Dea S, Mulcahy F, Norris S, et al. Increased Frequencies of Circulating IFN-γ-Producing Vδ1(+) and Vδ2(+) γδ T Cells in Patients with Asymptomatic Persistent Hepatitis B Virus Infection. Viral Immunol (2015) 28:201–8. doi: 10.1089/vim.2014.0133
104. Agrati C, Alonzi T, De Santis R, Castilletti C, Abbate I, Capobianchi MR, et al. Activation of Vgamma9Vdelta2 T cells by non-peptidic antigens induces the inhibition of subgenomic HCV replication. Int Immunol (2006) 18:11–8. doi: 10.1093/intimm/dxh337
105. Lundqvist C, Baranov V, Teglund S, Hammarström S, Hammarström ML. Cytokine profile and ultrastructure of intraepithelial gamma delta T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol (1994) 153:2302–12.
106. Cimini E, Bonnafous C, Bordoni V, Lalle E, Sicard H, Sacchi A, et al. Interferon-α improves phosphoantigen-induced Vγ9Vδ2 T-cells interferon-γ production during chronic HCV infection. PloS One (2012) 7:e37014. doi: 10.1371/journal.pone.0037014
107. Lang F, Peyrat MA, Constant P, Davodeau F, David-Ameline J, Poquet Y, et al. Early activation of human V gamma 9V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol (1995) 154:5986–94.
108. Lafont V, Liautard J, Sable-Teychene M, Sainte-Marie Y, Favero J. Isopentenyl pyrophosphate, a mycobacterial non-peptidic antigen, triggers delayed and highly sustained signaling in human gamma delta T lymphocytes without inducing eown-modulation of T cell antigen receptor. J Biol Chem (2001) 276:15961–7. doi: 10.1074/jbc.M008684200
109. Pavić I, Polić B, Crnković I, Lucin P, Jonjić S, Koszinowski UH. Participation of endogenous tumour necrosis factor alpha in host resistance to cytomegalovirus infection. J Gen Virol (1993) 74(Pt 10):2215–23. doi: 10.1099/0022-1317-74-10-2215
110. Rossol-Voth R, Rossol S, Schütt KH, Corridori S, de Cian W, Falke D. In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J Gen Virol (1991) 72(Pt 1):143–7. doi: 10.1099/0022-1317-72-1-143
111. Sambhi SK, Kohonen-Corish MR, Ramshaw IA. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc Natl Acad Sci USA (1991) 88:4025–9. doi: 10.1073/pnas.88.9.4025
112. Li H, Luo K, Pauza CD. TNF-alpha is a positive regulatory factor for human Vgamma2 Vdelta2 T cells. J Immunol (2008) 181:7131–7. doi: 10.4049/jimmunol.181.10.7131
113. Poccia F, Agrati C, Martini F, Mejia G, Wallace M, Malkovsky M. Vgamma9Vdelta2 T cell-mediated non-cytolytic antiviral mechanisms and their potential for cell-based therapy. Immunol Lett (2005) 100:14–20. doi: 10.1016/j.imlet.2005.06.025
114. Lafont V, Sanchez F, Laprevotte E, Michaud H-A, Gros L, Eliaou J-F, et al. Plasticity of γδ T Cells: Impact on the Anti-Tumor Response. Front Immunol (2014) 5:622. doi: 10.3389/fimmu.2014.00622
115. Bartee E, McFadden G. Cytokine synergy: an underappreciated contributor to innate anti-viral immunity. Cytokine (2013) 63:237–40. doi: 10.1016/j.cyto.2013.04.036
116. Qin G, Liu Y, Zheng J, Ng IHY, Xiang Z, Lam K-T, et al. Type 1 responses of human Vγ9Vδ2 T cells to influenza A viruses. J Virol (2011) 85:10109–16. doi: 10.1128/JVI.05341-11
117. Dong P, Ju X, Yan Y, Zhang S, Cai M, Wang H, et al. γδ T Cells Provide Protective Function in Highly Pathogenic Avian H5N1 Influenza A Virus Infection. Front Immunol (2018) 9:2812. doi: 10.3389/fimmu.2018.02812
118. Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. Chemokine expression by intraepithelial gamma delta T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol (1996) 157:985–92.
119. Sparrow EL, Fowler DW, Fenn J, Caron J, Copier J, Dalgleish AG, et al. The cytotoxic molecule granulysin is capable of inducing either chemotaxis or fugetaxis in dendritic cells depending on maturation: a role for Vδ2+ γδ T cells in the modulation of immune response to tumour? Immunology (2020) 161:245–58. doi: 10.1111/imm.13248
120. Münz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med (2005) 202:203–7. doi: 10.1084/jem.20050810
121. Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol (2005) 174:252–60. doi: 10.4049/jimmunol.174.1.252
122. Himoudi N, Morgenstern DA, Yan M, Vernay B, Saraiva L, Wu Y, et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol (2012) 188:1708–16. doi: 10.4049/jimmunol.1102654
123. Meuter S, Eberl M, Moser B. Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells. Proc Natl Acad Sci USA (2010) 107:8730–5. doi: 10.1073/pnas.1002769107
124. Lamichhane PP, Samarasinghe AE. The Role of Innate Leukocytes during Influenza Virus Infection. J Immunol Res (2019) 2019:8028725. doi: 10.1155/2019/8028725
125. Moser B, Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci (2011) 68:2443–52. doi: 10.1007/s00018-011-0706-6
126. Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science (2005) 309:264–8. doi: 10.1126/science.1110267
127. Brandes M, Willimann K, Bioley G, Lévy N, Eberl M, Luo M, et al. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci U.S.A. (2009) 106:2307–12. doi: 10.1073/pnas.0810059106
128. Brandes M, Willimann K, Lang AB, Nam K-H, Jin C, Brenner MB, et al. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood (2003) 102:3693–701. doi: 10.1182/blood-2003-04-1016
129. Caccamo N, Battistini L, Bonneville M, Poccia F, Fournié JJ, Meraviglia S, et al. CXCR5 identifies a subset of Vgamma9Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol (2006) 177:5290–5. doi: 10.4049/jimmunol.177.8.5290
130. He X, Liang H, Hong K, Li H, Peng H, Zhao Y, et al. The potential role of CD16+ Vγ2Vδ2 T cell-mediated antibody-dependent cell-mediated cytotoxicity in control of HIV type 1 disease. AIDS Res Hum Retroviruses (2013) 29:1562–70. doi: 10.1089/AID.2013.0111
131. Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto A-H, Scaglione V, Ingoure S, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood (2009) 113:4875–84. doi: 10.1182/blood-2008-08-172296
132. Couzi L, Pitard V, Moreau J-F, Merville P, Déchanet-Merville J. Direct and Indirect Effects of Cytomegalovirus-Induced γδ T Cells after Kidney Transplantation. Front Immunol (2015) 6:3. doi: 10.3389/fimmu.2015.00003
133. Couzi L, Pitard V, Sicard X, Garrigue I, Hawchar O, Merville P, et al. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood (2012) 119:1418–27. doi: 10.1182/blood-2011-06-363655
134. Alexander AAZ, Maniar A, Cummings J-S, Hebbeler AM, Schulze DH, Gastman BR, et al. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res (2008) 14:4232–40. doi: 10.1158/1078-0432.CCR-07-4912
135. Urban EM, Li H, Armstrong C, Focaccetti C, Cairo C, Pauza CD. Control of CD56 expression and tumor cell cytotoxicity in human Vgamma2Vdelta2 T cells. BMC Immunol (2009) 10:50. doi: 10.1186/1471-2172-10-50
136. Qin G, Liu Y, Zheng J, Xiang Z, Ng IHY, Malik Peiris JS, et al. Phenotypic and functional characterization of human γδ T-cell subsets in response to influenza A viruses. J Infect Dis (2012) 205:1646–53. doi: 10.1093/infdis/jis253
137. Li H, Xiang Z, Feng T, Li J, Liu Y, Fan Y, et al. Human Vγ9Vδ2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells. Cell Mol Immunol (2013) 10:159–64. doi: 10.1038/cmi.2012.70
138. Wallace M, Bartz SR, Chang WL, Mackenzie DA, Pauza CD, Malkovsky M. Gamma delta T lymphocyte responses to HIV. Clin Exp Immunol (1996) 103:177–84. doi: 10.1046/j.1365-2249.1996.d01-625.x
139. Tu WW, Lau YL, Peiris JSM. Use of humanised mice to study antiviral activity of human γδ-T cells against influenza A viruses. Hong Kong Med J (2014) 20 Suppl 6:4–6.
140. Déchanet J, Merville P, Bergé F, Bone-Mane G, Taupin JL, Michel P, et al. Major expansion of gammadelta T lymphocytes following cytomegalovirus infection in kidney allograft recipients. J Infect Dis (1999) 179:1–8. doi: 10.1086/314568
141. Lafarge X, Merville P, Cazin MC, Bergé F, Potaux L, Moreau JF, et al. Cytomegalovirus infection in transplant recipients resolves when circulating gammadelta T lymphocytes expand, suggesting a protective antiviral role. J Infect Dis (2001) 184:533–41. doi: 10.1086/322843
142. Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med (2005) 201:1567–78. doi: 10.1084/jem.20041851
143. Kaminski H, Ménard C, El Hayani B, Adjibabi A-N, Marsères G, Courant M, et al. Characterization of a unique γδ T cell subset as a specific marker of CMV infection severity. J Infect Dis (2020) 223(4):655–66. doi: 10.1093/infdis/jiaa400
144. Jia Z-H, Li Y-Y, Wang J-Y, Zhang J-Y, Huang A, Guo X-D, et al. Activated γδ T cells exhibit cytotoxicity and the capacity for viral clearance in patients with acute hepatitis B. Clin Immunol (2019) 202:40–8. doi: 10.1016/j.clim.2019.03.005
145. Chen M, Hu P, Peng H, Zeng W, Shi X, Lei Y, et al. Enhanced peripheral γδT cells cytotoxicity potential in patients with HBV-associated acute-on-chronic liver failure might contribute to the disease progression. J Clin Immunol (2012) 32:877–85. doi: 10.1007/s10875-012-9678-z
146. Chen M, Zhang D, Zhen W, Shi Q, Liu Y, Ling N, et al. Characteristics of circulating T cell receptor gamma-delta T cells from individuals chronically infected with hepatitis B virus (HBV): an association between V(delta)2 subtype and chronic HBV infection. J Infect Dis (2008) 198:1643–50. doi: 10.1086/593065
147. Rajoriya N, Fergusson JR, Leithead JA, Klenerman P. Gamma Delta T-lymphocytes in Hepatitis C and Chronic Liver Disease. Front Immunol (2014) 5:400. doi: 10.3389/fimmu.2014.00400
148. Olusola BA, Kabelitz D, Olaleye DO, Odaibo GN. Early HIV infection is associated with reduced proportions of gamma delta T subsets as well as high creatinine and urea levels. Scand J Immunol (2020) 91:e12868. doi: 10.1111/sji.12868
149. Strbo N, Alcaide ML, Romero L, Bolivar H, Jones D, Podack ER, et al. Loss of Intra-Epithelial Endocervical Gamma Delta (GD) 1 T Cells in HIV-Infected Women. Am J Reprod Immunol (2016) 75:134–45. doi: 10.1111/aji.12458
150. Hermier F, Comby E, Delaunay A, Petitjean J, Favennec L, Bazin C, et al. Decreased blood TcR gamma delta+ lymphocytes in AIDS and p24-antigenemic HIV-1-infected patients. Clin Immunol Immunopathol (1993) 69:248–50. doi: 10.1006/clin.1993.1176
151. Li H, Peng H, Ma P, Ruan Y, Su B, Ding X, et al. Association between Vgamma2Vdelta2 T cells and disease progression after infection with closely related strains of HIV in China. Clin Infect Dis (2008) 46:1466–72. doi: 10.1086/587107
152. Enders PJ, Yin C, Martini F, Evans PS, Propp N, Poccia F, et al. HIV-mediated gammadelta T cell depletion is specific for Vgamma2+ cells expressing the Jgamma1.2 segment. AIDS Res Hum Retroviruses (2003) 19:21–9. doi: 10.1089/08892220360473934
153. Wallace M, Scharko AM, Pauza CD, Fisch P, Imaoka K, Kawabata S, et al. Functional gamma delta T-lymphocyte defect associated with human immunodeficiency virus infections. Mol Med (1997) 3:60–71. doi: 10.1007/bf03401668
154. Riedel DJ, Sajadi MM, Armstrong CL, Cummings J-S, Cairo C, Redfield RR, et al. Natural viral suppressors of HIV-1 have a unique capacity to maintain gammadelta T cells. AIDS (2009) 23:1955–64. doi: 10.1097/QAD.0b013e32832ff1ff
155. Bordon J, Evans PS, Propp N, Davis CE, Redfield RR, Pauza CD. Association between longer duration of HIV-suppressive therapy and partial recovery of the V gamma 2 T cell receptor repertoire. J Infect Dis (2004) 189:1482–6. doi: 10.1086/382961
156. Chaudhry S, Cairo C, Venturi V, Pauza CD. The γδ T-cell receptor repertoire is reconstituted in HIV patients after prolonged antiretroviral therapy. AIDS (2013) 27:1557–62. doi: 10.1097/QAD.0b013e3283611888
157. Poccia F, Gioia C, Martini F, Sacchi A, Piacentini P, Tempestilli M, et al. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vgamma9Vdelta2 T cells. AIDS (2009) 23:555–65. doi: 10.1097/QAD.0b013e3283244619
158. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8
159. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe (2020) 27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009
160. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: Immunology and treatment options. Clin Immunol (2020) 215:108448. doi: 10.1016/j.clim.2020.108448
161. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest (2020) 130:2620–9. doi: 10.1172/JCI137244
162. Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang Y-Q, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther (2020) 5:33. doi: 10.1038/s41392-020-0148-4
163. Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J Gen Intern Med (2020) 35:1545–9. doi: 10.1007/s11606-020-05762-w
164. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol (2020) 11:827. doi: 10.3389/fimmu.2020.00827
165. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
166. Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis (2020) 20:669–77. doi: 10.1016/S1473-3099(20)30243-7
167. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 Infection in Children. N Engl J Med (2020) 382:1663–5. doi: 10.1056/NEJMc2005073
168. Teran R, Mitre E, Vaca M, Erazo S, Oviedo G, Hübner MP, et al. Immune system development during early childhood in tropical Latin America: evidence for the age-dependent down regulation of the innate immune response. Clin Immunol (2011) 138:299–310. doi: 10.1016/j.clim.2010.12.011
169. Molony RD, Malawista A, Montgomery RR. Reduced dynamic range of antiviral innate immune responses in aging. Exp Gerontol (2018) 107:130–5. doi: 10.1016/j.exger.2017.08.019
170. Meyer KC. The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly. Semin Respir Crit Care Med (2010) 31:561–74. doi: 10.1055/s-0030-1265897
171. Roux A, Mourin G, Larsen M, Fastenackels S, Urrutia A, Gorochov G, et al. Differential impact of age and cytomegalovirus infection on the γδ T cell compartment. J Immunol (2013) 191:1300–6. doi: 10.4049/jimmunol.1202940
172. Re F, Poccia F, Donnini A, Bartozzi B, Bernardini G, Provinciali M. Skewed representation of functionally distinct populations of Vgamma9Vdelta2 T lymphocytes in aging. Exp Gerontol (2005) 40:59–66. doi: 10.1016/j.exger.2004.09.008
173. Argentati K, Re F, Donnini A, Tucci MG, Franceschi C, Bartozzi B, et al. Numerical and functional alterations of circulating gammadelta T lymphocytes in aged people and centenarians. J Leukoc Biol (2002) 72:65–71. doi: 10.1189/jlb.72.1.65
174. Xu W, Lau ZWX, Fulop T, Larbi A. The Aging of γδ T Cells. Cells (2020) 9(5):1181. doi: 10.3390/cells9051181
175. Caccamo N, Dieli F, Wesch D, Jomaa H, Eberl M. Sex-specific phenotypical and functional differences in peripheral human Vgamma9/Vdelta2 T cells. J Leukoc Biol (2006) 79:663–6. doi: 10.1189/jlb.1105640
176. Matricardi PM, Dal Negro RW, Nisini R. The first, holistic immunological model of COVID-19: Implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol (2020) 31:454–70. doi: 10.1111/pai.13271
177. Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: Comparison with SARS and MERS. Rev Med Virol (2020) 30:e2107. doi: 10.1002/rmv.2107
178. Hirano T, Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity (2020) 52:731–3. doi: 10.1016/j.immuni.2020.04.003
179. Remy KE, Mazer M, Striker DA, Ellebedy AH, Walton AH, Unsinger J, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight (2020) 5(17):e140329. doi: 10.1172/jci.insight.140329
180. Poccia F, Agrati C, Castilletti C, Bordi L, Gioia C, Horejsh D, et al. Anti-severe acute respiratory syndrome coronavirus immune responses: the role played by V gamma 9V delta 2 T cells. J Infect Dis (2006) 193:1244–9. doi: 10.1086/502975
181. Rijkers G, Vervenne T, van der Pol P. More bricks in the wall against SARS-CoV-2 infection: involvement of γ9δ2 T cells. Cell Mol Immunol (2020) 17:771–2. doi: 10.1038/s41423-020-0473-0
182. Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med (2020) 26:1070–6. doi: 10.1038/s41591-020-0944-y
183. Odak I, Barros-Martins J, Bošnjak B, Stahl K, David S, Wiesner O, et al. Reappearance of effector T cells is associated with recovery from COVID-19. EBioMedicine (2020) 57:102885. doi: 10.1016/j.ebiom.2020.102885
184. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med (2020) 26:842–4. doi: 10.1038/s41591-020-0901-9
185. Chen X-J, Li K, Xu L, Yu Y-J, Wu B, He Y-L, et al. Novel insight from the first lung transplant of a COVID-19 patient. Eur J Clin Invest (2021) 51:e13443. doi: 10.1111/eci.13443
187. Lei L, Qian H, Yang X, Zhang X, Zhang D, Dai T, et al. The phenotypic changes of γδ T cells in COVID-19 patients. J Cell Mol Med (2020) 24:11603–6. doi: 10.1111/jcmm.15620
188. Zhang J-Y, Wang X-M, Xing X, Xu Z, Zhang C, Song J-W, et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat Immunol (2020) 21:1107–18. doi: 10.1038/s41590-020-0762-x
189. Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med (2020) 26:1623–35. doi: 10.1038/s41591-020-1038-6
190. Carissimo G, Xu W, Kwok I, Abdad MY, Chan Y-H, Fong S-W, et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat Commun (2020) 11:5243. doi: 10.1038/s41467-020-19080-6
191. Guo C, Li B, Ma H, Wang X, Cai P, Yu Q, et al. Single-cell analysis of two severe COVID-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat Commun (2020) 11:3924. doi: 10.1038/s41467-020-17834-w
192. Yazdanifar M, Mashkour N, Bertaina A. Making a case for using γδ T cells against SARS-CoV-2. Crit Rev Microbiol (2020) 46:689–702. doi: 10.1080/1040841X.2020.1822279
193. Harly C, Guillaume Y, Nedellec S, Peigné C-M, Mönkkönen H, Mönkkönen J, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood (2012) 120:2269–79. doi: 10.1182/blood-2012-05-430470
194. Deniger DC, Moyes JS, Cooper LJN. Clinical applications of gamma delta T cells with multivalent immunity. Front Immunol (2014) 5:636. doi: 10.3389/fimmu.2014.00636
195. Buccheri S, Guggino G, Caccamo N, Li Donni P, Dieli F. Efficacy and safety of γδT cell-based tumor immunotherapy: a meta-analysis. J Biol Regul Homeost Agents (2014) 28:81–90.
196. Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan P-L, et al. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis (2009) 200:858–65. doi: 10.1086/605413
197. Daguzan C, Moulin M, Kulyk-Barbier H, Davrinche C, Peyrottes S, Champagne E. Aminobisphosphonates Synergize with Human Cytomegalovirus To Activate the Antiviral Activity of Vγ9Vδ2 Cells. J Immunol (2016) 196:2219–29. doi: 10.4049/jimmunol.1501661
198. Poonia B, Pauza CD. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy (2012) 14:173–81. doi: 10.3109/14653249.2011.623693
199. Garrido C, Clohosey ML, Whitworth CP, Hudgens M, Margolis DM, Soriano-Sarabia N. γδ T cells: an immunotherapeutic approach for HIV cure strategies. JCI Insight (2018) 3(12):e120121. doi: 10.1172/jci.insight.120121
200. Huber S, Shi C, Budd RC. Gammadelta T cells promote a Th1 response during coxsackievirus B3 infection in vivo: role of Fas and Fas ligand. J Virol (2002) 76:6487–94. doi: 10.1128/jvi.76.13.6487-6494.2002
201. Khairallah C, Netzer S, Villacreces A, Juzan M, Rousseau B, Dulanto S, et al. γδ T cells confer protection against murine cytomegalovirus (MCMV). PloS Pathog (2015) 11:e1004702. doi: 10.1371/journal.ppat.1004702
202. Sell S, Dietz M, Schneider A, Holtappels R, Mach M, Winkler TH. Control of murine cytomegalovirus infection by γδ T cells. PloS Pathog (2015) 11:e1004481. doi: 10.1371/journal.ppat.1004481
203. Tu W, Zheng J, Liu Y, Sia SF, Liu M, Qin G, et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a gammadelta T cell population in humanized mice. J Exp Med (2011) 208:1511–22. doi: 10.1084/jem.20110226
204. Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res (2007) 67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199
205. Pressey JG, Adams J, Harkins L, Kelly D, You Z, Lamb LS. In vivo expansion and activation of γδ T cells as immunotherapy for refractory neuroblastoma: A phase 1 study. Med (Baltimore) (2016) 95:e4909. doi: 10.1097/MD.0000000000004909
206. Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood (2003) 102:200–6. doi: 10.1182/blood-2002-12-3665
207. Ali Z, Shao L, Halliday L, Reichenberg A, Hintz M, Jomaa H, et al. Prolonged (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-driven antimicrobial and cytotoxic responses of pulmonary and systemic Vgamma2Vdelta2 T cells in macaques. J Immunol (2007) 179:8287–96. doi: 10.4049/jimmunol.179.12.8287
208. Huang D, Chen CY, Ali Z, Shao L, Shen L, Lockman HA, et al. Antigen-specific Vgamma2Vdelta2 T effector cells confer homeostatic protection against pneumonic plaque lesions. Proc Natl Acad Sci USA (2009) 106:7553–8. doi: 10.1073/pnas.0811250106
209. Chen CY, Yao S, Huang D, Wei H, Sicard H, Zeng G, et al. Phosphoantigen/IL2 expansion and differentiation of Vγ2Vδ2 T cells increase resistance to tuberculosis in nonhuman primates. PloS Pathog (2013) 9:e1003501. doi: 10.1371/journal.ppat.1003501
210. Poccia F, Gioia C, Martini F, Sacchi A, Piacentini P, Tempestilli M, et al. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vgamma9Vdelta2 T cells. AIDS (2009) 23:555–65. doi: 10.1097/QAD.0b013e3283244619
211. Juno JA, Kent SJ. What Can Gamma Delta T Cells Contribute to an HIV Cure? Front Cell Infect Microbiol (2020) 10:233. doi: 10.3389/fcimb.2020.00233
212. Billington EO, Reid IR. Benefits of Bisphosphonate Therapy: Beyond the Skeleton. Curr Osteoporos Rep (2020) 18:587–96. doi: 10.1007/s11914-020-00612-4
213. Brufsky A, Marti JLG, Nasrazadani A, Lotze MT. Boning up: amino-bisphophonates as immunostimulants and endosomal disruptors of dendritic cell in SARS-CoV-2 infection. J Transl Med (2020) 18:261. doi: 10.1186/s12967-020-02433-6
214. Frencher JT, Shen H, Yan L, Wilson JO, Freitag NE, Rizzo AN, et al. HMBPP-deficient Listeria mutant immunization alters pulmonary/systemic responses, effector functions, and memory polarization of Vγ2Vδ2 T cells. J Leukoc Biol (2014) 96:957–67. doi: 10.1189/jlb.6HI1213-632R
215. Fowler DW, Copier J, Dalgleish AG, Bodman-Smith MD. Tripartite immune cell co-operation in the Bacillus Calmette Guérin-induced activation of γδ T cells. Immunol Cell Biol (2013) 91:461–8. doi: 10.1038/icb.2013.30
216. Workalemahu G, Wang H, Puan K-J, Nada MH, Kuzuyama T, Jones BD, et al. Metabolic engineering of Salmonella vaccine bacteria to boost human Vγ2Vδ2 T cell immunity. J Immunol (2014) 193:708–21. doi: 10.4049/jimmunol.1302746
217. Dantzler KW, de la Parte L, Jagannathan P. Emerging role of γδ T cells in vaccine-mediated protection from infectious diseases. Clin Transl Immunol (2019) 8:e1072. doi: 10.1002/cti2.1072
218. Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis (2011) 204:845–53. doi: 10.1093/infdis/jir436
219. Re F, Donnini A, Provinciali M. Induction of alphadelta- and alphabeta-mediated T cell responses in healthy elderly subjects after influenza vaccination. Biogerontology (2006) 7:249–59. doi: 10.1007/s10522-006-9024-z
220. Tenner-Racz K, Stahl Hennig C, Uberla K, Stoiber H, Ignatius R, Heeney J, et al. Early protection against pathogenic virus infection at a mucosal challenge site after vaccination with attenuated simian immunodeficiency virus. Proc Natl Acad Sci USA (2004) 101:3017–22. doi: 10.1073/pnas.0308677101
221. Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science (2016) 352:aaf1098. doi: 10.1126/science.aaf1098
222. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity (2010) 33:492–503. doi: 10.1016/j.immuni.2010.10.002
223. Sørup S, Stensballe LG, Krause TG, Aaby P, Benn CS, Ravn H. Oral Polio Vaccination and Hospital Admissions With Non-Polio Infections in Denmark: Nationwide Retrospective Cohort Study. Open Forum Infect Dis (2016) 3:ofv204. doi: 10.1093/ofid/ofv204
224. Aaby P, Samb B, Simondon F, Seck AM, Knudsen K, Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ (1995) 311:481–5. doi: 10.1136/bmj.311.7003.481
225. Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol (2013) 34:431–9. doi: 10.1016/j.it.2013.04.004
226. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol (2020) 20:375–88. doi: 10.1038/s41577-020-0285-6
227. Netea MG, Quintin J, van der Meer JWM. Trained immunity: a memory for innate host defense. Cell Host Microbe (2011) 9:355–61. doi: 10.1016/j.chom.2011.04.006
228. Lau CM, Adams NM, Geary CD, Weizman O-E, Rapp M, Pritykin Y, et al. Epigenetic control of innate and adaptive immune memory. Nat Immunol (2018) 19:963–72. doi: 10.1038/s41590-018-0176-1
229. Uthayakumar D, Paris S, Chapat L, Freyburger L, Poulet H, De Luca K. Non-specific Effects of Vaccines Illustrated Through the BCG Example: From Observations to Demonstrations. Front Immunol (2018) 9:2869. doi: 10.3389/fimmu.2018.02869
230. Biering-Sørensen S, Aaby P, Lund N, Monteiro I, Jensen KJ, Eriksen HB, et al. Early BCG-Denmark and Neonatal Mortality Among Infants Weighing <2500 g: A Randomized Controlled Trial. Clin Infect Dis (2017) 65:1183–90. doi: 10.1093/cid/cix525
231. de Castro MJ, Pardo-Seco J, Martinón-Torres F. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin Infect Dis (2015) 60:1611–9. doi: 10.1093/cid/civ144
232. Miller A, Reandelar MJ. Correlation between universal BCG vaccination policy and reduced mortality for COVID-19. medRxiv (2020). doi: 10.1101/2020.03.24.20042937
233. Shet A, Ray D. Differential COVID-19-attributable mortality and BCG vaccine use in countries. medRxiv (2020). doi: 10.1101/2020.04.01.20049478
234. Dayal D, Gupta S. Connecting BCG Vaccination and COVID-19: Additional Data. medRxiv (2020). doi: 10.1101/2020.04.07.20053272
235. Covián C, Retamal-Díaz A, Bueno SM, Kalergis AM. Could BCG Vaccination Induce Protective Trained Immunity for SARS-CoV-2? Front Immunol (2020) 11:970 doi: 10.3389/fimmu.2020.00970
236. Rivas MN, Ebinger JE, Wu M, Sun N, Braun J, Sobhani K, et al. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J Clin Invest (2021) 131(2):e145157. doi: 10.1172/JCI145157
237. Hensel J, McAndrews KM, McGrail DJ, Dowlatshahi DP, LeBleu VS, Kalluri R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci Rep (2020) 10:18377. doi: 10.1038/s41598-020-75491-x
238. Kleen T-O, Galdon AA, MacDonald AS, Dalgleish AG. Mitigating Coronavirus Induced Dysfunctional Immunity for At-Risk Populations in COVID-19: Trained Immunity, BCG and “New Old Friends.” Front Immunol (2020) 11:2059. doi: 10.3389/fimmu.2020.02059
239. Netea MG, Giamarellos-Bourboulis EJ, Domínguez-Andrés J, Curtis N, van Crevel R, van de Veerdonk FL, et al. Trained Immunity: a Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell (2020) 181:969–77. doi: 10.1016/j.cell.2020.04.042
240. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19. Lancet (2020) 395:1545–6. doi: 10.1016/S0140-6736(20)31025-4
241. Wardhana, Datau EA, Sultana A, Mandang VVV, Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones (2011) 43:185–90.
242. Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang S-Y, Oosting M, et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe (2018) 23:89–100.e5. doi: 10.1016/j.chom.2017.12.010
243. Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect (2019) 25:1473–8. doi: 10.1016/j.cmi.2019.04.020
244. Mazzola TN, Da Silva MTN, Moreno YMF, Lima SCBS, Carniel EF, Morcillo AM, et al. Robust gammadelta+ T cell expansion in infants immunized at birth with BCG vaccine. Vaccine (2007) 25:6313–20. doi: 10.1016/j.vaccine.2007.06.039
245. Taştan Y, Arvas A, Demir G, Alikaşifoğlu M, Gür E, Kiray E. Influence of Bacillus Calmette-Guèrin vaccination at birth and 2 months old age on the peripheral blood T-cell subpopulations [gamma/delta and alpha-beta T cell]. Pediatr Allergy Immunol (2005) 16:624–9. doi: 10.1111/j.1399-3038.2005.00329.x
246. Zufferey C, Germano S, Dutta B, Ritz N, Curtis N. The contribution of non-conventional T cells and NK cells in the mycobacterial-specific IFNγ response in Bacille Calmette-Guérin (BCG)-immunized infants. PloS One (2013) 8:e77334. doi: 10.1371/journal.pone.0077334
247. Bukowski JF, Morita CT, Brenner MB. Recognition and destruction of virus-infected cells by human gamma delta CTL. J Immunol (1994) 153:5133–40.
248. Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon M-E, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood (2008) 112:1317–24. doi: 10.1182/blood-2008-01-136713
249. Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guérin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol (1998) 161:1045–54.
250. Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science (2002) 295:2255–8. doi: 10.1126/science.1068819
251. Chen ZW, Letvin NL. Adaptive immune response of Vgamma2Vdelta2 T cells: a new paradigm. Trends Immunol (2003) 24:213–9. doi: 10.1016/s1471-4906(03)00032-2
252. Yang J, Jones MS, Ramos RI, Chan AA, Lee AF, Foshag LJ, et al. Insights into Local Tumor Microenvironment Immune Factors Associated with Regression of Cutaneous Melanoma Metastases by Mycobacterium bovis Bacille Calmette-Guérin. Front Oncol (2017) 7:61. doi: 10.3389/fonc.2017.00061
253. Gupta PK. New disease old vaccine: Is recombinant BCG vaccine an answer for COVID-19? Cell Immunol (2020) 356:104187. doi: 10.1016/j.cellimm.2020.104187
254. Dalgleish AG, Mudan S, Fusi A. Enhanced effect of checkpoint inhibitors when given after or together with IMM-101: significant responses in four advanced melanoma patients with no additional major toxicity. J Transl Med (2018) 16:227. doi: 10.1186/s12967-018-1602-8
255. O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol (2020) 20:335–7. doi: 10.1038/s41577-020-0337-y
256. Sánchez-Ramón S, Conejero L, Netea MG, Sancho D, Palomares Ó, Subiza JL. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations. Front Immunol (2018) 9:2936. doi: 10.3389/fimmu.2018.02936
257. Tuvim MJ, Evans SE, Clement CG, Dickey BF, Gilbert BE. Augmented lung inflammation protects against influenza A pneumonia. PloS One (2009) 4:e4176. doi: 10.1371/journal.pone.0004176
258. Shen L, Frencher J, Huang D, Wang W, Yang E, Chen CY, et al. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc Natl Acad Sci U.S.A. (2019) 116:6371–8. doi: 10.1073/pnas.1811380116
259. Fenoglio D, Zocchi MR, Parodi A, Durando P, Gabutitp G, Gasparini R, et al. MF-59 adjuvant influence on the functions of gammadelta T cells in HIV-1+ adults immunized with influenza seasonal vaccine. J Prev Med Hyg (2011) 52:137–41.
Keywords: gamma delta T cell, innate immunity, trained immunity, antiviral, virus, COVID-19, BCG, vaccine
Citation: Caron J, Ridgley LA and Bodman-Smith M (2021) How to Train Your Dragon: Harnessing Gamma Delta T Cells Antiviral Functions and Trained Immunity in a Pandemic Era. Front. Immunol. 12:666983. doi: 10.3389/fimmu.2021.666983
Received: 11 February 2021; Accepted: 12 March 2021;
Published: 29 March 2021.
Edited by:
Xu Yu, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Jodi L. McGill, Iowa State University, United StatesCopyright © 2021 Caron, Ridgley and Bodman-Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Bodman-Smith, bWJvZG1hbnNAc2d1bC5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.