
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 14 April 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.654376
The Janus kinases (JAKs) are intracellular tyrosine kinases involved in a broad variety of inflammatory cascades participating in the pathogenesis of systemic lupus erythematosus (SLE). Diffuse non-scarring alopecia is one of the most frequent cutaneous manifestations in SLE, resulting in devastating psychosocial consequences. Although recent studies have shown promising outcomes of the JAK inhibitors in SLE treatment, the efficacy of tofacitinib in diffuse non-scarring alopecia due to SLE has never been reported. Here we present a 29-year-old SLE patient with a 10-year history of refractory severe diffuse non-scarring alopecia who experienced dramatic hair regrowth with tofacitinib. Furthermore, we have made a systematic review regarding the potential effectiveness of tofacitinib in systemic and cutaneous lupus erythematosus. To the best of our knowledge, this is the first case study depicting an SLE patient with refractory alopecia who experienced impressive hair regrowth with the JAK1/3 inhibitor tofacitinib therapy, which contributes to expanding the field of possible uses of tofacitinib in SLE patients with difficult-to-treat cutaneous involvement, including severe alopecia.
Alopecia is one of the most frequent cutaneous manifestations in systemic lupus erythematosus (SLE) (1), potentially reflecting SLE disease activity (2). It often manifests as a diffuse non-scarring form of hair loss, sometimes even involving more than 50% of the scalp surface area. Although alopecia is not life-threatening, it can be associated with devastating psychosocial consequences, resulting in depression, anxiety, and poor quality of life (3).
The Janus kinases (JAKs) are intracellular tyrosine kinases involved in a broad variety of inflammatory cascades participating in the pathogenesis of SLE (4). Tofacitinib is a JAK1/3 inhibitor licensed for the treatment of moderate to severe active rheumatoid arthritis, psoriatic arthritis and ulcerative colitis (5). It has been shown that tofacitinib ameliorates both clinical and histological features of lupus-associated skin inflammation in murine lupus (6). A recent study including 10 patients with SLE demonstrated that tofacitinib could rapidly improve the signs and symptoms of arthritis and partially ameliorate skin rash (7). However, the efficacy of JAK1/3 inhibitors in diffuse non-scarring alopecia due to SLE has never been reported. Here, we describe an SLE patient with long-standing severe diffuse non-scarring alopecia who experienced dramatic hair regrowth with tofacitinib.
A 29-year-old Chinese woman presented with a 10-year history of diffuse non-scarring alopecia and recurrent rash. At the age of 12, she was diagnosed with SLE due to severe generalized rash with pruritus, alopecia, hypocomplementaemia with complement 3 to be 0.53 g/L (reference range 0.80 - 1.81 g/L) and complement 4 to be 0.11 g/L (reference range 0.15 - 0.57 g/L), an elevated titre of anti-nuclear antibodies (ANA, 1:1000, speckled immunofluorescent pattern; reference range < 1:100) and positive anti-double-stranded (ds) DNA antibodies determined by indirect immunofluorescence. Her anti-Sjögren’s syndrome antigen A (SSA) antibodies were also positive by immunoblotting and the level of serum immunoglobulin G was 18.66 g/L (reference range 8.0 - 18.0 g/L) with normal complete blood cell count and 24-hour urine protein. SLE disease activity was stable after remission for 7 years with low-dose methylprednisolone (4 mg/day) and hydroxychloroquine (200 mg/day) for maintenance therapy.
At the ages of 19, 23, and 24, she had three SLE flares with rash and hair loss progressing over several days to nearly her entire scalp. Methylprednisolone (40 mg/day) combined with cyclosporine (75 mg twice a day) following high dose pulsed methylprednisolone at 250 mg/day for 3 days significantly relieved her symptoms, with methylprednisolone (4 mg/day) and hydroxychloroquine (200 mg/day) for maintenance therapy. However, at the age of 25, her diffuse non-scarring alopecia relapsed again. The severe alopecia was persistent for the next 4 years without any therapeutic benefit from the previous regimen mentioned above (methylprednisolone at a dose of 40 mg/day combined with cyclosporine after steroid pulse therapy), methotrexate, mycophenolate mofetil, hydroxychloroquine, or oral or topical tacrolimus. Nevertheless, she refused to undergo scalp skin biopsy. Moreover, her rash had also recurred intermittently during this period. Two weeks before admission, methylprednisolone (24 mg/day) was administered in a local hospital due to the recurrence of a generalized rash with pruritus, but it had a negligible effect.
Physical examination on admission revealed generalized rash with skin scratches and diffuse non-scarring alopecia involving nearly the entire scalp (Figures 1A, B). Laboratory findings revealed positive ANA (1:320, speckled immunofluorescent pattern; reference range < 1:100) with negative anti-dsDNA antibodies by indirect immunofluorescence and normal levels of complement components, as well as normal complete blood cell count and 24-hour urine protein. Owing to the reported promising outcomes of the JAK1/3 inhibitor tofacitinib for cutaneous involvement in SLE (7) and alopecia areata (8), tofacitinib (5 mg twice a day) was initiated along with hydroxychloroquine (200 mg twice a day), and methylprednisolone was gradually tapered. Surprisingly, prominent hair regrowth on the scalp was observed after 4 weeks, without any rash. At 8 weeks, the methylprednisolone dose was reduced to 8 mg/day with no relapse of alopecia or rash (Figures 1C, D).
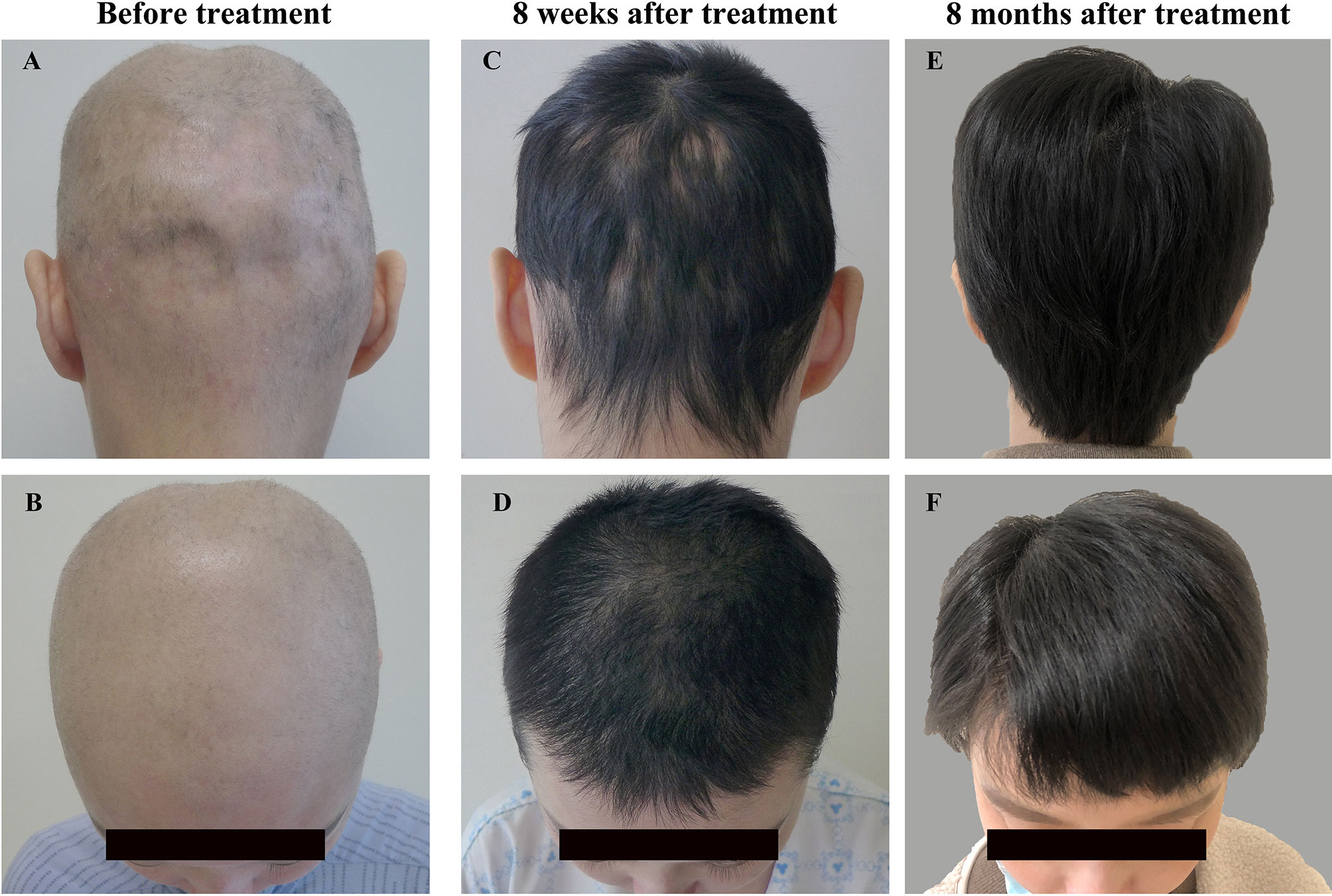
Figure 1 Changes in scalp hair on admission (A, B), 8 weeks (C, D) and 8 months after tofacitinib therapy (E, F).
At 7 months, she had her hair cut short because of her wedding. The tofacitinib dose was also tapered to 5 mg/day due to her plan to quit tofacitinib therapy and become pregnant. At the time of writing this report (8 months after tofacitinib therapy was initiated), the patient was being treated with 5 mg/day tofacitinib combined with 4 mg/day methylprednisolone and 200 mg twice a day hydroxychloroquine, with stable hair regrowth (Figures 1E, F). The timeline of the disease and treatment is shown in Table 1. Tofacitinib has been well tolerated, and no adverse events, including infection, have been observed in the patient up until the time of this report.
In this case study, although there was no other systemic involvement and current lupus immunologic evidence was almost absent except for positive ANA, a definitive diagnosis of SLE can be made in the patient due to her recurrent generalized rash, alopecia and hypocomplementaemia, as well as a high titre of ANA and positive anti-dsDNA antibodies. To the best of our knowledge, we describe for the first time, an SLE patient with long-standing refractory diffuse non-scarring alopecia who experienced dramatic hair regrowth with the JAK1/3 inhibitor tofacitinib, which contributes to expanding the field of possible uses of tofacitinib in SLE patients with difficult-to-treat cutaneous involvement, including severe alopecia.
The JAK-signal transducer and activator of transcription (STAT) pathway is regarded as a central communication node for the immune system that promotes many SLE associated pathogenic proinflammatory cytokines and chemokines, including interferons (IFNs), interleukin-6 (IL-6), IL-12 and IL-23 (9). The dysregulation of type I and II IFNs is a key signature associated with SLE, which provides evidence for using JAK inhibitors in patients with SLE (10). The pathogenesis of alopecia in SLE is still not clear. Previous studies have revealed that alopecia areata is driven by cytotoxic T lymphocytes and can be reversed by JAK inhibition through eliminating the IFN signature and subsequently inhibiting the downstream signaling pathway (11). Notably, according to the evidence mentioned above, a definitive diagnosis of SLE was made rather than idiopathic alopecia areata in this patient. Intriguingly, the severe diffuse non-scarring alopecia of our patient in whom standard treatments including pulse steroids and other immunosuppressive therapy (methotrexate, mycophenolate mofetil, hydroxychloroquine, or oral or topical tacrolimus) were not effective was successfully reversed by the JAK1/3 inhibitor tofacitinib. Therefore, it is reasonable to speculate that the IFN-JAK-STAT pathway may be involved in the pathogenesis of alopecia in SLE.
Tofacitinib is the first JAK inhibitor approved for RA, and recent studies have shown promising outcomes of tofacitinib in SLE treatment. In vitro and in vivo evidence regarding the potential effectiveness of tofacitinib in SLE and cutaneous lupus erythematosus is summarized in Table 2. Zhou et al. found that targeting JAK/STAT signaling by tofacitinib effectively controlled the fate of CD8+CD103+ tissue-resident memory T cells in lupus nephritis, contributing to reduced renal inflammation and damage in MRL/lpr mice (12). Tofacitinib challenge has been revealed to be effective in reducing the levels of anti-dsDNA antibodies and proteinuria, as well as in ameliorating nephritis in mouse models (13, 14). Moreover, lupus-associated skin inflammation could be significantly ameliorated by tofacitinib in murine lupus (6). In addition, Yamamoto et al. reported that tofacitinib decreased anti-dsDNA levels in one patient with inactive SLE complicated by rheumatoid arthritis (16). Intriguingly, a recent small cohort study demonstrated that tofacitinib could rapidly improve the signs and symptoms of arthritis and partially ameliorate skin rash in patients with SLE (7). Notably, our SLE patient presented with long-standing refractory alopecia and recurrent rash in whom there was negligible therapeutic benefit from standard treatments. Therefore, tofacitinib was initiated in the patient due to its reported promising outcomes for skin involvement in SLE (6, 7) and alopecia areata (8). Our case study further suggests that the JAK1/3 inhibitor tofacitinib may be a therapeutic option for patients with SLE, including individuals with difficult-to-treat cutaneous involvement in whom standard treatments are ineffective. Importantly, tofacitinib is now being investigated in SLE patients in a phase II clinical trial (NCT03288324).
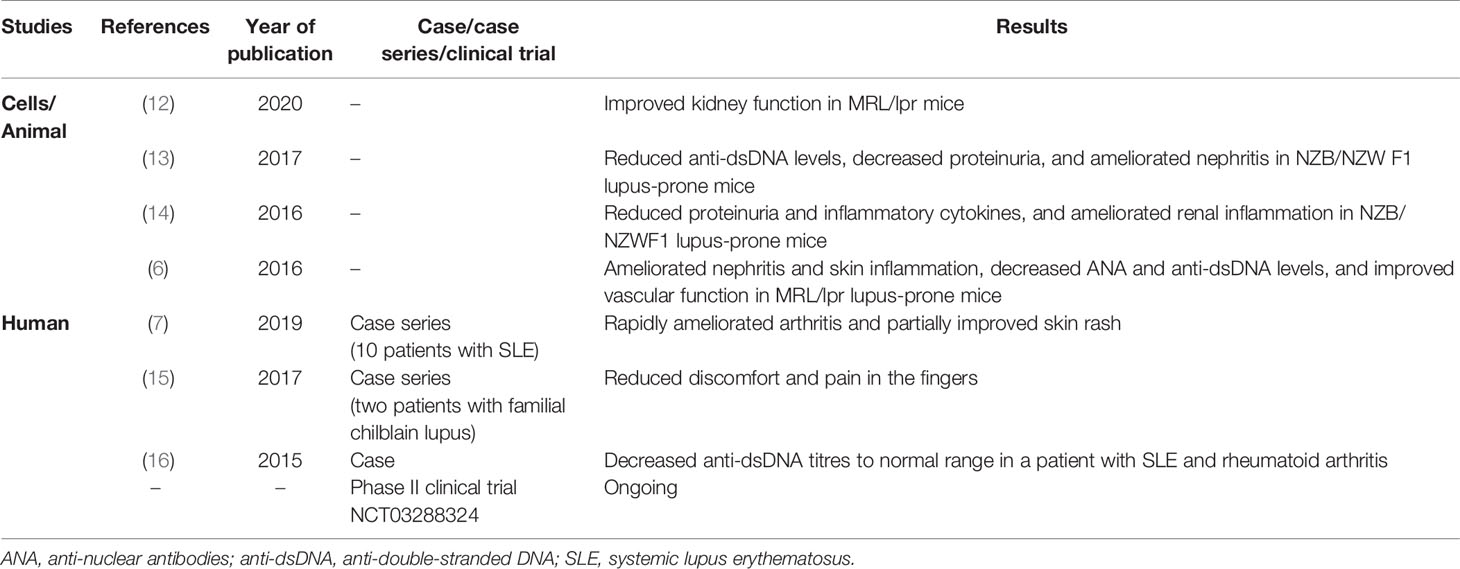
Table 2 The effect of tofacitinib in systemic and cutaneous lupus erythematosus previously reported.
Other JAK inhibitors have shown the potential effectiveness in SLE and cutaneous lupus erythematosus. Evidence from a phase II clinical trial showed positive results with the JAK 1/2 inhibitor baricitinib in SLE, particularly at a dose of 4 mg/day (17). Fornaro et al. described one SLE patient with refractory papulosquamous subacute lesions who was successfully treated with baricitinib (18). Of note, one recent case report showed that a patient with SLE experienced substantial improvement in diffuse non-scarring alopecia following JAK1/2 inhibitor baricitinib therapy (19). Additionally, anti-extractable nuclear antigen (ENA) and anti-dsDNA antibody production in SLE patients could be abrogated in the presence of another JAK1/2 inhibitor, ruxolitinib (20). Moreover, Wenzel et al. reported a patient with chilblain lupus erythematosus that was successfully controlled by ruxolitinib therapy (21).
There is a paucity of evidence regarding the effect of JAK inhibitors on alopecia due to SLE. One recent case of diffuse non-scarring alopecia due to SLE successfully treated with the JAK1/2 inhibitor baricitinib therapy has been reported (19). The main limitation of the current study is that this is a case report. However, we have performed a systematic review, and to the best of our knowledge, this is the first case study depicting an SLE patient with refractory alopecia who experienced impressive hair regrowth with the JAK1/3 inhibitor tofacitinib, which exerted a profoundly positive effect on her quality of life. Therefore, our case adds to the small body of existing evidence on the promising outcomes of JAK inhibitors in alopecia caused by SLE. The efficacy and safety of tofacitinib in patients with SLE should be further defined by randomized controlled trials.
We describe the first case of an SLE patient with difficult-to-treat diffuse non-scarring alopecia who experienced remarkable hair regrowth with tofacitinib therapy, which may constitute a promising therapeutic option in SLE patients, especially in those in whom standard treatments are ineffective.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of Shenzhen People’s Hospital (approval number: LL-KT-2018358). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors contributed to the final manuscript. Y-LC analyzed documents and drafted the manuscript. Corresponding authors D-ZL and X-PH read and revised the manuscript. L-XL participated in drafting the manuscript. QH and X-YL collected the clinical data. All authors contributed to the article and approved the submitted version.
Shenzhen Science and Technology Innovation Program (grant number JCYJ20190807144418845); Sanming Project of Medicine in Shenzhen (grant number SYJY201901); National Natural Science Foundation of China (grant number 81971464); and National Key Research and Development Program of China (grant number 2019YFC0840603).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All authors thank the patient in this study.
1. Udompanich S, Chanprapaph K, Suchonwanit P. Hair and scalp changes in cutaneous and systemic lupus erythematosus. Am J Clin Dermatol (2018) 19(5):679–94. doi: 10.1007/s40257-018-0363-8
2. Chanprapaph K, Udompanich S, Visessiri Y, Ngamjanyaporn P, Suchonwanit P. Nonscarring alopecia in systemic lupus erythematosus: a cross-sectional study with trichoscopic, histopathologic, and immunopathologic analyses. J Am Acad Dermatol (2019) 81(6):1319–29. doi: 10.1016/j.jaad.2019.05.053
3. Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med (2012) 366(16):1515–25. doi: 10.1056/NEJMra1103442
4. Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discovery (2017) 16(12):843–62. doi: 10.1038/nrd.2017.201
5. Roskoski RJ. Properties of FDA-approved small molecule protein kinase inhibitors: a 2020 update. Pharmacol Res (2020) 152:104609. doi: 10.1016/j.phrs.2019.104609
6. Furumoto Y, Smith CK, Blanco L, Zhao W, Brooks SR, Thacker SG, et al. Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol (2016) 69(1):148–60. doi: 10.1002/art.39818
7. You H, Zhang G, Wang Q, Zhang S, Zhao J, Tian X, et al. Successful treatment of arthritis and rash with tofacitinib in systemic lupus erythematosus: the experience from a single centre. Ann Rheum Dis (2019) 78(10):1441–3. doi: 10.1136/annrheumdis-2019-215455
8. Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol (2017) 76(1):22–8. doi: 10.1016/j.jaad.2016.09.007
9. Mok CC. The Jakinibs in systemic lupus erythematosus: progress and prospects. Expert Opin Investig Drugs (2019) 28(1):85–92. doi: 10.1080/13543784.2019.1551358
10. Kawasaki M, Fujishiro M, Yamaguchi A, Nozawa K, Kaneko H, Takasaki Y, et al. Possible role of the JAK/STAT pathways in the regulation of T cell-interferon related genes in systemic lupus erythematosus. Lupus (2011) 20(12):1231–9. doi: 10.1177/0961203311409963
11. Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med (2014) 20(9):1043–9. doi: 10.1038/nm.3645
12. Zhou M, Guo C, Li X, Huang Y, Li M, Zhang T, et al. JAK/STAT signaling controls the fate of CD8+CD103+ tissue-resident memory T cell in lupus nephritis. J Autoimmunity (2020) 109:102424. doi: 10.1016/j.jaut.2020.102424
13. Ikeda K, Hayakawa K, Fujishiro M, Kawasaki M, Hirai T, Tsushima H, et al. JAK inhibitor has the amelioration effect in lupus-prone mice: the involvement of IFN signature gene downregulation. BMC Immunol (2017) 18(1):41. doi: 10.1186/s12865-017-0225-9
14. Ripoll E, de Ramon L, Draibe BJ, Merino A, Bolanos N, Goma M, et al. JAK3-STAT pathway blocking benefits in experimental lupus nephritis. Arthritis Res Ther (2016) 18(1):134. doi: 10.1186/s13075-016-1034-x
15. Konig N, Fiehn C, Wolf C, Schuster M, Cura CE, Tungler V, et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis (2017) 76(2):468–72. doi: 10.1136/annrheumdis-2016-209841
16. Yamamoto M, Yokoyama Y, Shimizu Y, Yajima H, Sakurai N, Suzuki C, et al. Tofacitinib can decrease anti-DNA antibody titers in inactive systemic lupus erythematosus complicated by rheumatoid arthritis. Modern Rheumatol (2015) 26(4):1–2. doi: 10.3109/14397595.2015.1069473
17. Wallace DJ, Furie RA, Tanaka Y, Kalunian KC, Mosca M, Petri MA, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet (2018) 392(10143):222–31. doi: 10.1016/S0140-6736(18)31363-1
18. Fornaro M, Coladonato L, Venerito V, Cacciapaglia F, Lopalco G, Iannone F. Efficacy of baricitinib on refractory skin papulosquamous rash in a patient with systemic lupus erythematosus. Rheumatol (Oxford) (2019) 10:kez442. doi: 10.1093/rheumatology/kez442
19. Maeshima K, Shibata H. Efficacy of JAK 1/2 inhibition in the treatment of diffuse non-scarring alopecia due to systemic lupus erythematosus. Ann Rheum Dis (2020) 79(5):674–5. doi: 10.1136/annrheumdis-2019-216571
20. de la Varga Martínez R, Rodríguez-Bayona B, Añez GA, Medina Varo F, Pérez Venegas JJ, Brieva JA, et al. Clinical relevance of circulating anti-ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur J Immunol (2017) 47(7):1211–9. doi: 10.1002/eji.201646872
Keywords: Janus kinase inhibitor, tofacitinib, diffuse non-scarring alopecia, hair regrowth, systemic lupus erythematosus
Citation: Chen Y-L, Liu L-X, Huang Q, Li X-Y, Hong X-P and Liu D-Z (2021) Case Report: Reversal of Long-Standing Refractory Diffuse Non-Scarring Alopecia Due to Systemic Lupus Erythematosus Following Treatment With Tofacitinib. Front. Immunol. 12:654376. doi: 10.3389/fimmu.2021.654376
Received: 16 January 2021; Accepted: 22 March 2021;
Published: 14 April 2021.
Edited by:
Federico Alberici, University of Brescia, ItalyReviewed by:
Angela Tincani, University of Brescia, ItalyCopyright © 2021 Chen, Liu, Huang, Li, Hong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong-Zhou Liu, bGl1X2R6MjAwMUBzaW5hLmNvbQ==; Xiao-Ping Hong, aG9uZ194aWFvcGluZ0Bob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.