- 1Department of Neurology, Tongde Hospital of Zhejiang Province, Hangzhou, China
- 2Department of Neurology, Second Affiliated Hospital School of Medicine Zhejiang University, Hangzhou, China
- 3Department of Neurology, Zhejiang Hospital, Hangzhou, China
Anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) encephalitis, a rare subtype of autoimmune encephalitis, was first reported by Lai et al. The AMPAR antibodies target against extracellular epitopes of the GluA1 or GluA2 subunits of the receptor. AMPARs are expressed throughout the central nervous system, especially in the hippocampus and other limbic regions. Anti-AMPAR encephalitis was more common in middle-aged women and most patients had an acute or subacute onset. Limbic encephalitis, a classic syndrome of anti-AMPAR encephalitis, was clinically characterized by a subacute disturbance of short-term memory loss, confusion, abnormal behavior and seizure. Magnetic resonance imaging often showed T2/fluid-attenuated inversion-recovery hyperintensities in the bilateral medial temporal lobe. For suspected patients, paired serum and cerebrospinal fluid (CSF) testing with cell-based assay were recommended. CSF specimen was preferred given its higher sensitivity. Most patients with anti-AMPAR encephalitis were complicated with tumors, such as thymoma, small cell lung cancer, breast cancer, and ovarian cancer. First-line treatments included high-dose steroids, intravenous immunoglobulin and plasma exchange. Second-line treatments, including rituximab and cyclophosphamide, can be initiated in patients who were non-reactive to first-line treatment. Most patients with anti-AMPAR encephalitis showed a partial neurologic response to immunotherapy.
Introduction
Encephalitis is an infectious or inflammatory disorder of the brain parenchyma (1). There were 5-10 per 100,000 inhabitants suffering from encephalitis every year across all age groups (2, 3). Though classically attributed to infection, an autoimmune basis was reported with similar frequency for encephalitis (4). Autoimmune encephalitis (AE) is the umbrella term for autoimmune disorders in the central nervous system (CNS) characterized by the presence of autoantibodies against intracellular or membrane antigens (5). Over the past decade, the identification of an increasing number of antibodies have aided in the identification and characterization of AE (4). Anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) encephalitis, a rare subtype of AE mediated by AMPAR antibodies, was first reported by Lai et al. in 2009 (6). More than half of the patients were characterized by limbic encephalitis (LE), including short-term memory loss, confusion, abnormal behavior and seizures. In recent years, anti-AMPAR encephalitis has been increasingly reported with atypical clinical manifestations.
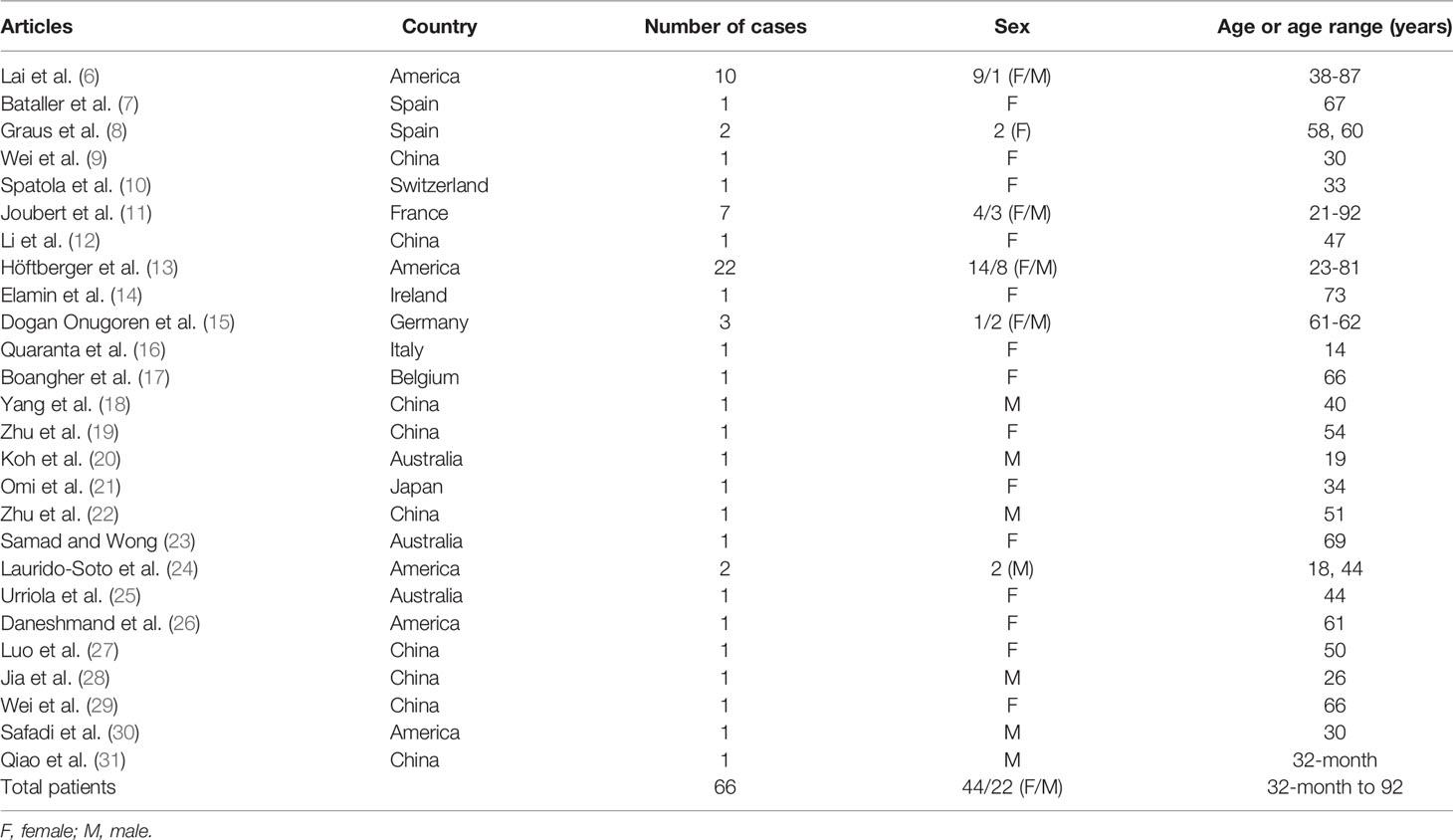
We searched PubMed, Web of Science and Embase for all articles published in English between April 2009 and November 2020, using the search terms [(AMPA OR AMPAR OR anti-AMPA OR anti-AMPAR OR AMPAR-antibody OR AMPA receptor OR anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) AND (encephalitis OR autoimmune encephalitis OR limbic encephalitis)]. We identified 66 cases from 6 case series and 20 individual case reports (6–31) (Table 1 and Supplementary Figure 1). We summarized the clinical presentations, diagnostic tests and treatments of anti-AMPAR encephalitis, in order to raise the awareness among neurologists (Supplementary Table 1).
Etiology and Pathogenesis
Unlike anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis, only a few patients reported prodromal viral infections (6/66). Forty patients (60.6%) had a history of tumor or detected tumors, with thymoma being the most common (16/40, most of them under 60 years old), followed by small cell lung cancer (SCLC) (8/40), breast cancer (6/40) and ovarian cancer (3/40). There were also cases reporting concomitant medullary thyroid carcinoma, bladder carcinoma, melanoma and Ewing’s sarcoma.
AMPARs are ionotropic receptors which belong to glutamate receptors. AMPARs are mainly heterotetramers and are composed of four subunits (GluA1-4) with several auxiliary subunits (32, 33). AMPARs, together with other ionotropic glutamate receptors, mediate the majority of excitatory synaptic transmission (32, 34). AMPARs, especially GluA1 and GluA2 subunits, are ubiquitously expressed throughout the central nervous system. In particular, there is a rich expression of GluA1/2 and GluA2/3 levels in the hippocampus and other limbic regions (35). The majority of synaptic AMPARs are GluA1/2 subunits and, to a less extent, GluA2/3 subunits in hippocampus (32, 33). GluA1/2 subunits are also widely expressed in the cerebellum, basal ganglia and cerebral cortex (35). The AMPAR antibodies target against the extracellular portion of cell surface proteins, i.e. the extracellular epitopes of the GluA1 or GluA2 subunits of the receptor (36). In vitro experimental studies indicated that AMPAR antibodies selectively decreased the surface synaptic AMPAR clusters and disrupted the balance between internalization and reinsertion of AMPARs, leading to the accumulation of internalized AMPARs (37). The AMPAR-mediated decrease in synaptic transmission led to a compensatory decrease of inhibitory synaptic transmission and an increase of intrinsic excitability (33, 37). Synaptic and neuronal changes induced by antibodies may contribute to the short-term memory loss and seizures in anti-AMPAR encephalitis patients.
Clinical Manifestations
Most patients (46/66) had an acute or subacute onset. The median age of onset was 57 years old with a wide range from 32-month-old to 92-year-old. The vast majority of patients developed the disease in adulthood (64/66) and patients aged 50 to 70 years old account for nearly 50% among adults. Anti-AMPAR encephalitis was more common in women, with a male to female ratio of about 1:2. The clinical manifestations of encephalitis were varied (Table 2).
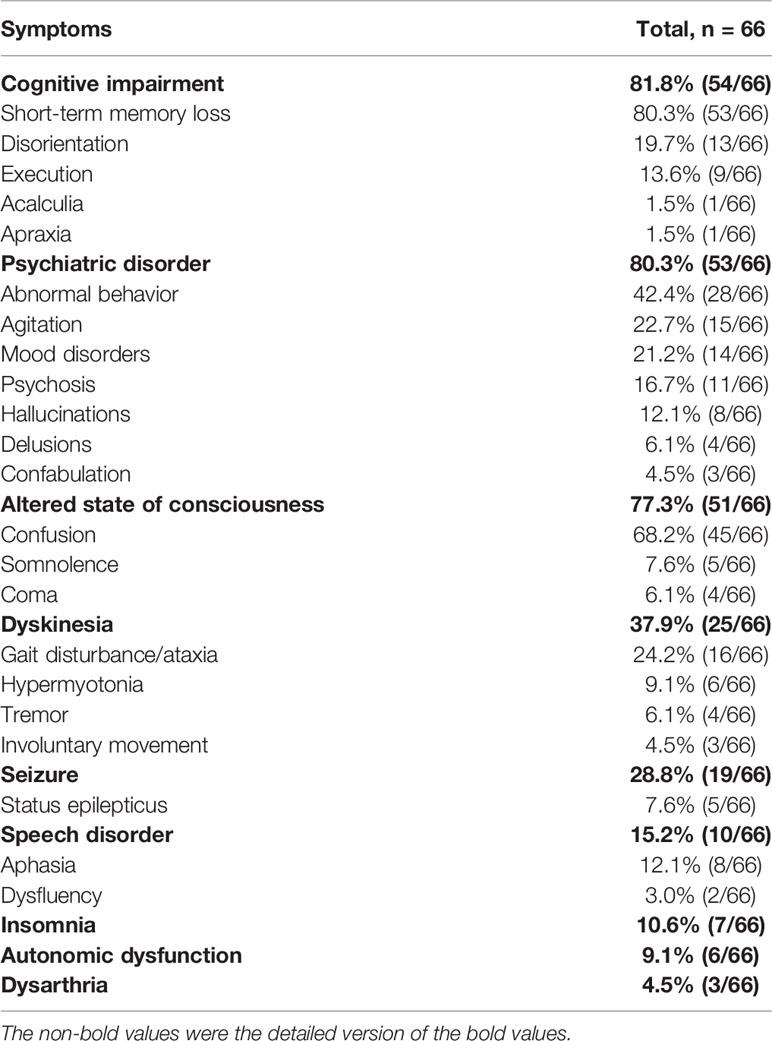
Cognitive dysfunction was the most common (54/66) clinical manifestation of anti-AMPAR encephalitis, including short-term memory loss (53/66), disorientation (13/66), executive dysfunction (9/66), etc. It could progress to dementia in severe cases. Psychiatric symptom was the second most common clinical manifestation (53/66), among which abnormal behaviors were most frequent (28/66). Less frequently, manifestations in order of decreasing frequency included agitation (15/66), mood disorders (14/66) and psychosis (11/66). There were 3 patients who developed mutism. An altered level of consciousness was not uncommon as well (51/66). Confusion could develop in most patients (45/66). Patients with dyskinesia (25/66) could manifest as gait disturbance or ataxia, parkinsonism and involuntary movement. Nineteen patients had seizures during the disease course, with only 5 patients having status epilepticus. There were various types of seizures, including myoclonic seizures, paroxysmal feeling of tightness, etc. Speech disorder occurred in 10 patients, mainly manifested as aphasia. In addition, some patients may have insomnia (7/66), autonomic dysfunction (6/66) and dysarthria (3/66).
Auxiliary Examination
Magnetic Resonance Imaging
There were 65 patients who underwent magnetic resonance imaging (MRI) scan, with 49 patients showing abnormalities (Figure 1). Among them, 39 patients showed increased T2/fluid-attenuated inversion-recovery (FLAIR) sequency signal in the temporal lobe, and the medial temporal lobe was involved in 21 patients (Figure 2). Bilateral temporal lobe involvement was more frequent (17 bilateral and 10 unilateral). There were also 11 patients with T2/FLAIR hyperintensity in the basal ganglia, which mainly involved caudate, followed by corpus striatum, putamen. In addition, insula, frontal lobe, cerebellum, parietal lobe, cingulum and occipital lobe were also affected. Leptomeningeal enhancement in the temporal-parietal regions (18) and mild transient contrast enhancement in the hippocampus (6) were reported. In the long term, hippocampal (10, 15) or cortical (9, 25) atrophy could also be seen.
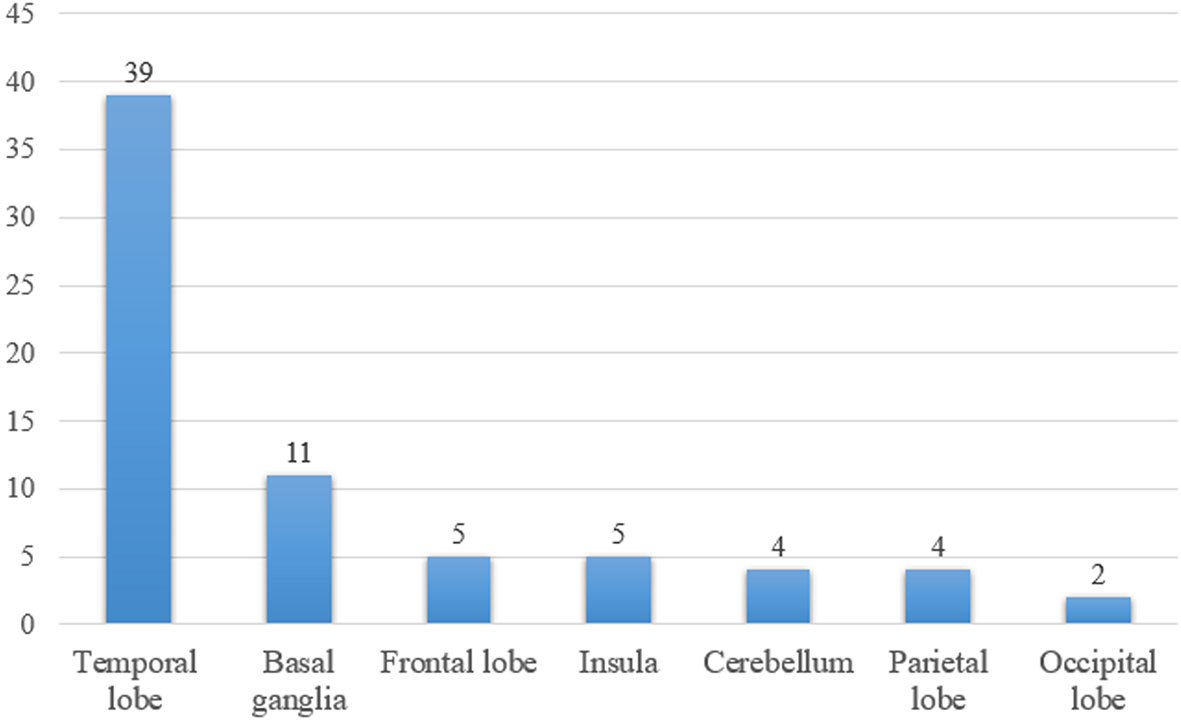
Figure 1 The number of patients with T2/fluid-attenuated inversion-recovery hyperintensity lesions in different brain areas.
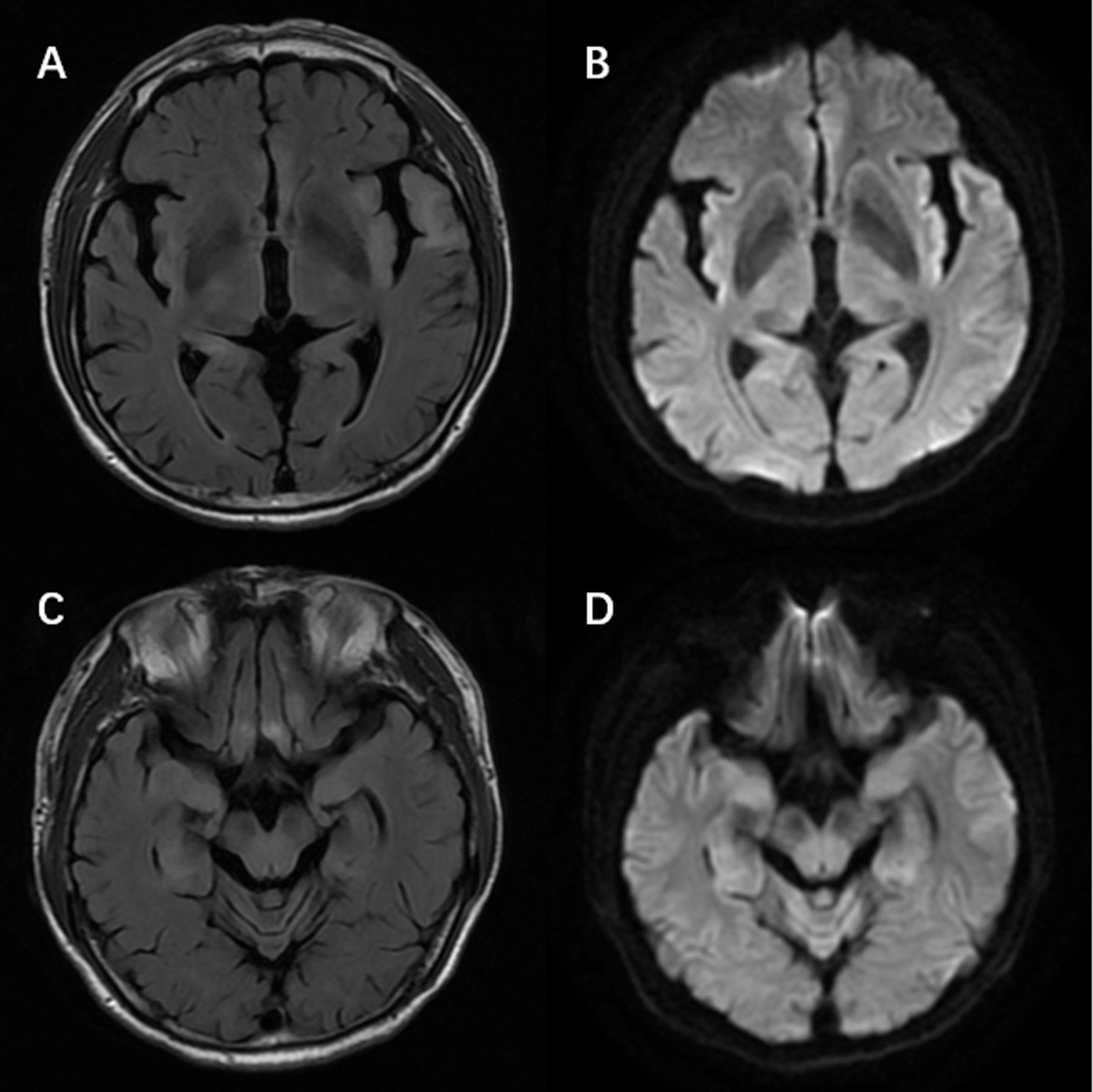
Figure 2 Magnetic resonance imaging of a patient with anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor encephalitis showed T2/fluid-attenuated inversion-recovery sequency hyperintensity in left temporal lobe, bilateral hippocampus, frontal lobes, insula (A, C) with restricted diffusion on diffusion weight imaging at the corresponding region (B, D).
18F-fluorodeoxyglucose Positron Emission Tomography
There were only 8 cases describing the manifestation of 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET). Four showed increased metabolism at the onset of disease (cerebellar, medial temporal lobe, hippocampus, striatum, parietal lobe and occipital lobe, basal ganglia) (10, 11, 24), 2 showed normal metabolism (though 1 patient had global hypometabolism on PET 3.5 weeks later) (8, 24), and 2 showed decreased metabolism (caudate, frontal, temporal, occipital and parietal areas) (9, 29). However, the regions with abnormal MRI signals did not match perfectly with those with abnormal 18FDG-PET metabolism, mainly because those 2 imaging modalities had a different emphasis on metabolism and structure. The inconsistency might be also due to the different time points when MRI and PET examinations were conducted along the disease course (38). Previous 18FDG-PET/MRI studies of AE patients (mainly anti-NMDAR encephalitis) indicated a higher sensitivity of PET than MRI in AE (39, 40). Given the limited sample size, we were unable to determine the sensitivity of PET and MRI for diagnosis of anti-AMPAR encephalitis.
Electroencephalogram
Among the 53 patients with reported electroencephalogram (EEG) at disease onset, 20 had normal EEG findings. There were 15 patients with epileptiform discharges, such as sharp waves and spike waves. And in 19 patients, EEG showed generalized or focal slowing. A few (4 cases) had epileptiform discharges and slow waves at the same time. However, there were 2 cases with epileptiform waves but no seizures.
Blood Tests
Routine blood tests showed no obvious abnormalities. Hyponatremia was present in 7 patients. Patients may also have other autoimmune antibodies, such as acetylcholine receptor antibody, thyroid peroxidase antibody and thyroglobulin antibody, autoimmune hepatitis antibodies, anti-nuclear antibody, cardiolipin antibodies, etc.
Cerebrospinal Fluid
Results of CSF analysis were available for 64 patients, including white blood cells (WBC) in 63 patients and protein levels in 59 patients. More than half of patients (38 cases) had an elevated WBC, with a maximum of 220 cells/μL, mainly composed of lymphocytes. CSF protein was abnormal in 27 patients, with a maximum of 425 mg/dL. Oligoclonal bands were detected in 5 patients.
Antibody Detection
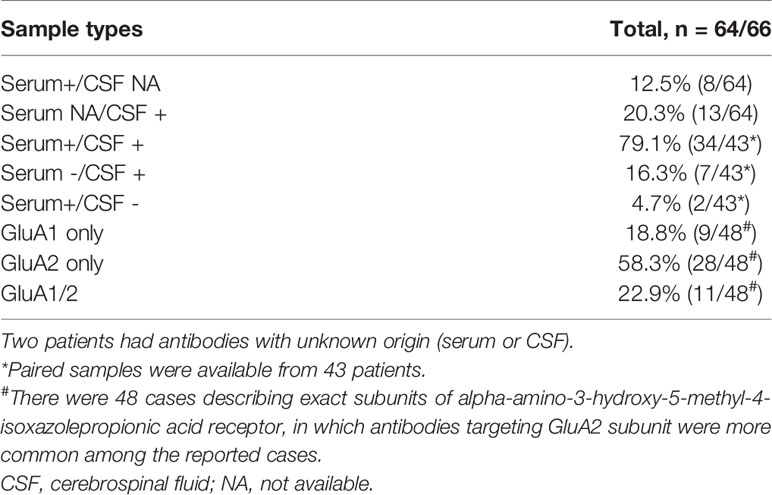
The positive serum and/or CSF antibody against AMPAR could be used as a reference for the diagnosis of this disease. Cell-based assay (CBA) was recommended due to its high sensitivity and specificity (2). The exact threshold of antibody level for anti-AMPAR encephalitis diagnosis remained inconclusive. Paired serum and CSF samples were available from 43 patients, where 36 had seropositivity and 41 had positive antibodies in the CSF (Table 3). In general, CSF AMPAR antibody examination had a higher sensitivity. Considering the distribution of AMPAR and intrathecal synthesis of antibodies (6), the specificity of CSF antibodies was relatively high. Therefore, paired testing of both the serum and CSF samples was recommended, of which the CSF was preferred (41). In cases with matched serum and CSF tests, the titers of AMPAR antibody in serum were higher than that in CSF (15, 27). Notably, patients with only a low-titer of serum antibody should be diagnosed with caution. It had been reported that the AMPAR antibody titer in the CSF gradually decreased after immunosuppressive treatment, as the clinical symptoms relieved (10). However, the association between antibody titer and disease severity remained unknown.
Antibodies against GluA1 and GluA2 subunits, the 2 main subunits of AMPAR, could be detected simultaneously or separately. GluA2-specific antibodies were more commonly reported (Table 3). There were no significant differences among clinical presentations between the two subtypes (13).
Nineteen patients had overlapping neural antibodies. Those patients usually had a worse prognosis. The most common concomitant antibody was collapsin response-mediator protein-5 (CRMP5) antibody (6 cases), followed by NMDAR antibody (4 cases), glutamic acid decarboxylase (GAD) antibody (3 cases), voltage-gated potassium channels (VGKC) antibody (3 cases), Sry-like high mobility group box (SOX1) antibody (3 cases), gamma-aminobutyric acid receptor (GABAR) antibody (1 case), antinuclear neuronal antibody type 1 (Hu) antibody (1 case), leucine-rich glioma-inactivated 1 (LGI1) antibody (1 case), amphiphysin antibody (1 case) and voltage-gated calcium channels (VGCC) antibody (1 case).
Pathology
Unfortunately, there was still no report on the pathology of brain tissue in anti-AMPAR encephalitis. A few cases reported pathological findings coming from concomitant neoplasms that GluA1/2 subunits present in patients’ tumor tissues, which correlated with the patients’ antibody specificity. This indicated that some types of tumors might play a role in triggering this autoimmune disorder (6).
Diagnosis and Differential Diagnosis
The diagnosis of anti-AMPAR encephalitis was based on the criteria published in the Lancet by Graus et al. (2). In particular, anti-AMPAR encephalitis should be considered in patients with the following characteristics: 1) acute or subacute onset, mostly manifested as short-term memory loss, confusion, abnormal behavior, dyskinesia and seizure; 2) MRI may present with unilateral or bilateral limbic lobe T2/FLAIR hyperintensity; 3) reactive to immunotherapy. In addition, for patients suspected with anti-AMPA encephalitis, a thorough evaluation for tumors, such as thymoma and SCLC, should be conducted.
Diseases mimicking anti-AMPAR encephalitis abound, including infectious, neoplastic, and other immunological diseases (Table 4) (2, 42–44). Caution is needed to identify anti-AMPAR-encephalitis from other types of encephalitis, like anti-NMDAR encephalitis and viral encephalitis (Table 5) (43, 45, 46).
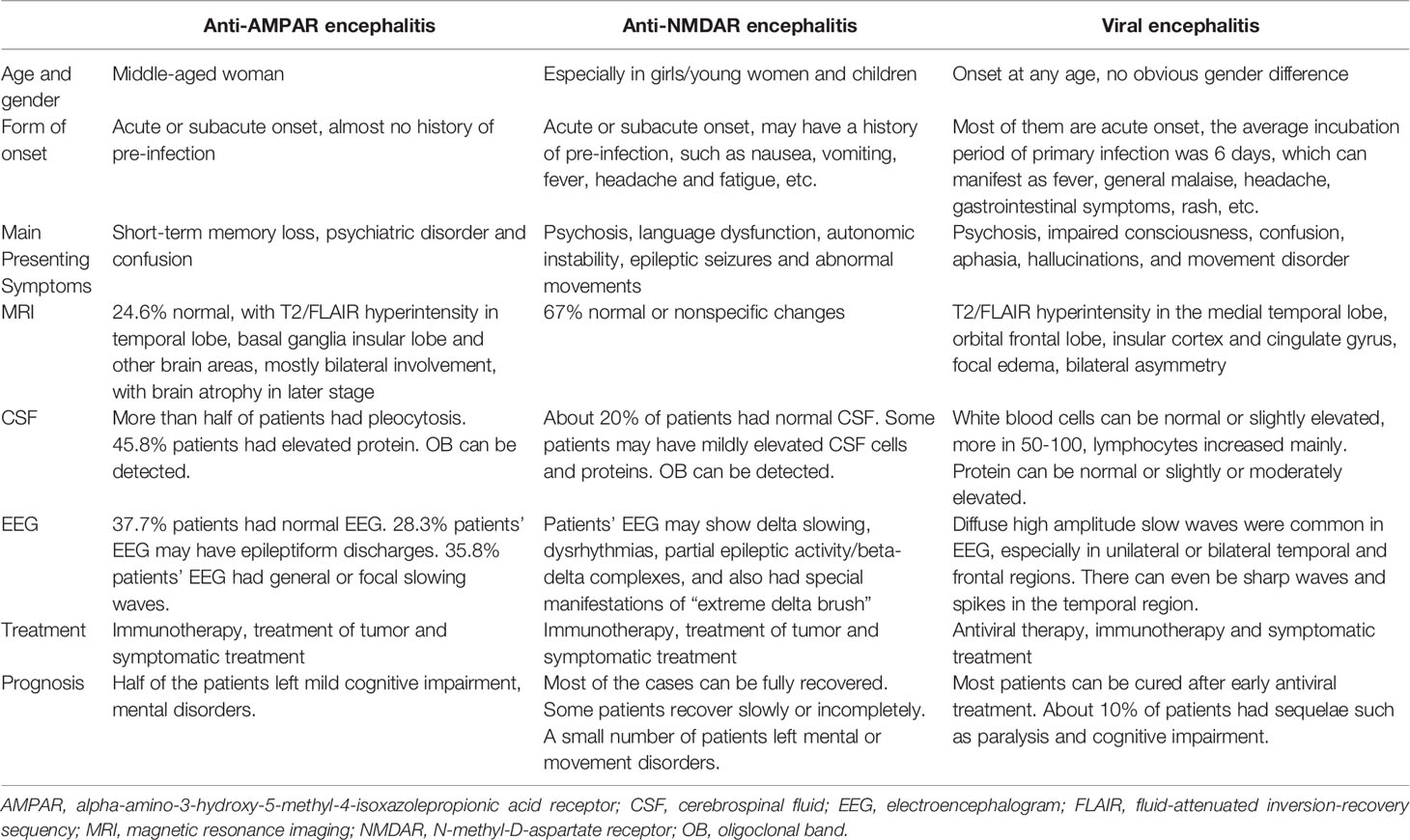
Table 5 General features distinguishing anti-AMPAR encephalitis from important differential diagnoses (43, 45, 46).
Treatment and Prognosis
There was no standard management for anti-AMPAR encephalitis. Therapies were usually chosen with reference to other autoimmune encephalitis (47), such as anti-NMDAR encephalitis. About 60-80% of autoimmune encephalitis with antibodies against neuronal surface antigens responded well to immunotherapy (48, 49). Similar to other neuronal surface autoantibody-mediated encephalitis, immunotherapy was recommended as early as possible after diagnosis (2, 50, 51).
First-line treatments included high-dose corticosteroids (methylprednisolone 1000 mg intravenously for 3 to 5 days), intravenous immunoglobulin (0.4 g/kg/day for 5 days) and plasma exchange. High-dose corticosteroids subsequently were followed by a tapering schedule. However, the optimal duration of steroid treatment remains inconclusive. There were 17 patients starting second-line treatment after first-line drugs. Timely initiation of second-line treatment was critical for patients non-reactive to first-line treatment. The exact timing of initiation of second-line therapy was unknown and may depend on the patients’ acceptance to side effects of first-line treatments, drug availability and neurologist’s preference. Major second-line drugs included rituximab and cyclophosphamide. Due to the small number of cases, the impact of second-line prevention on prognosis remained unclear. It was reported that patients who received second-line treatments during the first episode had a lower relapse rate and death rate than those who did not receive second-line immunotherapies (13, 48). Eight patients received long-term treatment in the remission period to prevent recurrence. Long-term treatment included azathioprine and mycophenolate mofetil. However, whether long-term treatment was needed for relapse prevention were still unclear.
In addition, symptomatic treatments were needed for mental disorders and seizures associated with anti-AMPAR-encephalitis. The effect of symptomatic treatment alone may be limited, and combined immunotherapy is usually required.
There were no significant differences in prognosis between patients with and without tumors in anti-AMPAR-encephalitis (13). Nevertheless, early treatment of tumors was important for a good prognosis (52). In 40 patients with concomitant tumors, 27 cases were treated for the neoplasms, such as tumor resection, chemotherapy and radiotherapy. Tumor screening should be conducted regularly for at least 2 years for anti-AMPAR encephalitis patients, even after resolution of neurological deficit (49, 53).
Most anti-AMPAR encephalitis patients were responsive to immunotherapy. However, 18.5% patients (12/65, prognosis of 1 patient was not available) had a poor response. There were 30.8% patients (20/65) who returned to baseline. 50.8% patients (33/65) with anti-AMPAR encephalitis left some sequelae such as cognitive impairment and mental disorder (Figure 3). Previous study reported that the relapse rate was about 23.8% (48), which was higher in those who did not receive aggressive therapy (chemotherapy or rituximab) (13). The relapses of encephalitis did not mean the recurrence of tumors (6). Death rate was 16.7% (11/66), which was mostly related to the progression of the primary tumor. In a few cases, the causes of death were status epilepticus, cardiorespiratory arrest, myocardial infarction and urinary sepsis (6, 11).
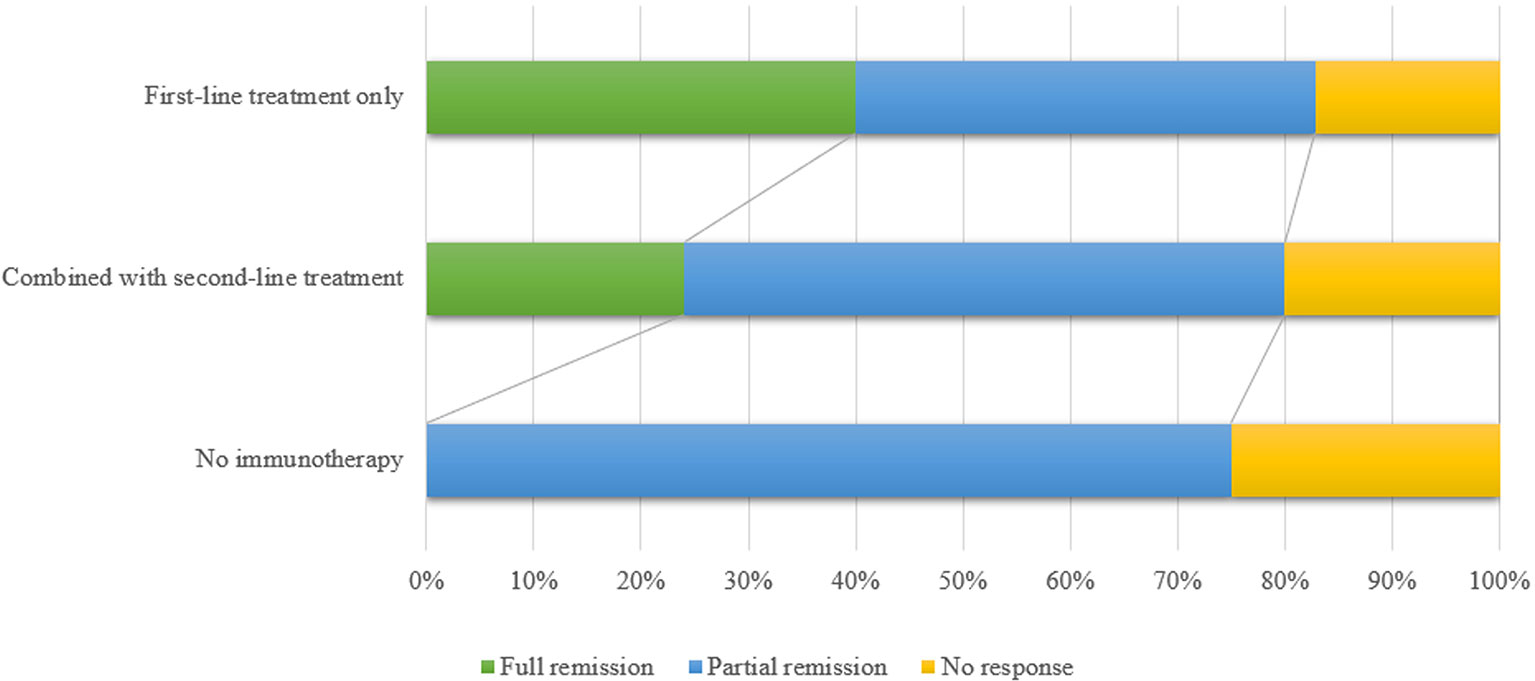
Figure 3 The figure shows the comparison of the efficacy between first-line immunotherapy only, first-line immunotherapy combined with second-line immunotherapy and no immunotherapy. Patients without immunotherapy had a relatively poor prognosis. First-line treatments included high-dose steroid pulse therapy, intravenous immunoglobulin and plasma exchange. Main second-line immunotherapy drugs included rituximab, cyclophosphamide and azathioprine. Full remission means the patients returned to baseline; partial remission means the patients showed partial recovery and partial sequelae; no response means patients did not respond to treatment medication.
Conclusion
Anti-AMPAR encephalitis is an autoimmune disease of the central nervous system. Given the small number of reported cases, our understanding of anti-AMPAR encephalitis is still limited. Consensus was not reached regarding its diagnosis and standard management strategies. For patients with cognitive-psychiatric disorders with an acute or subacute onset, anti-AMPAR encephalitis should be considered. Serum and CSF AMPAR antibody should be tested as soon as possible for further confirmation. Timely immunotherapy should also be initiated upon diagnosis. An extensive screening for tumors, especially thymoma and lung cancer, is warranted in such patients.
Author Contributions
T-YZ and M-TC drafted the initial manuscript, summarized available data and selected the references. YZ, Q-LL, and C-HS contributed to the manuscript. Y-XZ and SQ designed and revised the manuscript. All authors approved the final version of the manuscript.
Funding
This study was supported by the Science and Technology Program of Zhejiang Province (2018C37132).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.652820/full#supplementary-material
References
1. Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case Definitions, Diagnostic Algorithms, and Priorities in Encephalitis: Consensus Statement of the International Encephalitis Consortium. Clin Infect Dis (2013) 57(8):1114–28. doi: 10.1093/cid/cit458
2. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A Clinical Approach to Diagnosis of Autoimmune Encephalitis. Lancet Neurol (2016) 15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9
3. Hasbun R, Rosenthal N, Balada-Llasat JM, Chung J, Duff S, Bozzette S, et al. Epidemiology of Meningitis and Encephalitis in the United States, 2011-2014. Clin Infect Dis (2017) 65(3):359–63. doi: 10.1093/cid/cix319
4. Budhram A, Dubey D, Sechi E, Flanagan EP, Yang L, Bhayana V, et al. Neural Antibody Testing in Patients With Suspected Autoimmune Encephalitis. Clin Chem (2020). doi: 10.1093/clinchem/hvaa254
5. Sell J, Haselmann H, Hallermann S, Hust M, Geis C. Autoimmune Encephalitis: Novel Therapeutic Targets at the Preclinical Level. Expert Opin Ther Targets (2021) 25(1):37–47. doi: 10.1080/14728222.2021.1856370
6. Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, et al. AMPA Receptor Antibodies in Limbic Encephalitis Alter Synaptic Receptor Location. Ann Neurol (2009) 65(4):424–34. doi: 10.1002/ana.21589
7. Bataller L, Galiano R, Garcia-Escrig M, Martinez B, Sevilla T, Blasco R, et al. Reversible Paraneoplastic Limbic Encephalitis Associated With Antibodies to the AMPA Receptor. Neurology (2010) 74(3):265–7. doi: 10.1212/WNL.0b013e3181cb3e52
8. Graus F, Boronat A, Xifro X, Boix M, Svigelj V, Garcia A, et al. The Expanding Clinical Profile of anti-AMPA Receptor Encephalitis. Neurology (2010) 74(10):857–9. doi: 10.1212/WNL.0b013e3181d3e404
9. Wei YC, Liu CH, Lin JJ, Lin KJ, Huang KL, Lee TH, et al. Rapid Progression and Brain Atrophy in anti-AMPA Receptor Encephalitis. J Neuroimmunol (2013) 261(1-2):129–33. doi: 10.1016/j.jneuroim.2013.05.011
10. Spatola M, Stojanova V, Prior JO, Dalmau J, Rossetti AO. Serial Brain (1)(8)FDG-PET in anti-AMPA Receptor Limbic Encephalitis. J Neuroimmunol (2014) 271(1-2):53–5. doi: 10.1016/j.jneuroim.2014.04.002
11. Joubert B, Kerschen P, Zekeridou A, Desestret V, Rogemond V, Chaffois MO, et al. Clinical Spectrum of Encephalitis Associated With Antibodies Against the Alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptor: Case Series and Review of the Literature. JAMA Neurol (2015) 72(10):1163–9. doi: 10.1001/jamaneurol.2015.1715
12. Li X, Mao YT, Wu JJ, Li LX, Chen XJ. Anti-AMPA Receptor Encephalitis Associated With Thymomatous Myasthenia Gravis. J Neuroimmunol (2015) 281:35–7. doi: 10.1016/j.jneuroim.2015.02.011
13. Hoftberger R, van Sonderen A, Leypoldt F, Houghton D, Geschwind M, Gelfand J, et al. Encephalitis and AMPA Receptor Antibodies: Novel Findings in a Case Series of 22 Patients. Neurology (2015) 84(24):2403–12. doi: 10.1212/WNL.0000000000001682
14. Elamin M, Lonergan R, Killeen RP, O’Riordan S, Tubridy N, McGuigan C. Posterior Cortical and White Matter Changes on MRI in Anti-AMPA Receptor Antibody Encephalitis. Neurol Neuroimmunol Neuroinflamm (2015) 2(4):e118. doi: 10.1212/NXI.0000000000000118
15. Dogan Onugoren M, Deuretzbacher D, Haensch CA, Hagedorn HJ, Halve S, Isenmann S, et al. Limbic Encephalitis Due to GABAB and AMPA Receptor Antibodies: A Case Series. J Neurol Neurosurg Psychiatry (2015) 86(9):965–72. doi: 10.1136/jnnp-2014-308814
16. Quaranta G, Maremmani AG, Perugi G. Anti-AMPA-Receptor Encephalitis Presenting as a Rapid-Cycling Bipolar Disorder in a Young Woman With Turner Syndrome. Case Rep Psychiatry (2015) 2015:273192. doi: 10.1155/2015/273192
17. Boangher S, Mespouille P, Filip CM, Goffette S. Small-Cell Lung Cancer With Positive Anti-NMDAR and Anti-AMPAR Antibodies Paraneoplastic Limbic Encephalitis. Case Rep Neurol Med (2016) 2016:3263718. doi: 10.1155/2016/3263718
18. Yang S, Qin J, Li J, Gao Y, Zhao L, Wu J, et al. Rapidly Progressive Neurological Deterioration in anti-AMPA Receptor Encephalitis With Additional CRMP5 Antibodies. Neurol Sci (2016) 37(11):1853–5. doi: 10.1007/s10072-016-2680-0
19. Zhu M, Yu X, Liu C, Duan C, Li C, Zhu J, et al. Hashimoto’s Encephalitis Associated With AMPAR2 Antibodies: A Case Report. BMC Neurol (2017) 17(1):37. doi: 10.1186/s12883-017-0823-4
20. Koh D, Lau T, Teoh E, Lau KK. An Uncommon Presentation of a Primary Bone Tumor: Anti-AMPA (Anti-alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid) Receptor Limbic/Paraneoplastic Encephalitis as a Presenting Feature of Ewing Sarcoma. J Pediatr Hematol Oncol (2018) 40(7):555–7. doi: 10.1097/MPH.0000000000001304
21. Omi T, Kinoshita M, Nishikawa A, Tomioka T, Ohmori K, Fukada K, et al. Clinical Relapse of Anti-AMPAR Encephalitis Associated With Recurrence of Thymoma. Intern Med (2018) 57(7):1011–3. doi: 10.2169/internalmedicine.9682-17
22. Zhu H, Hou YB, Gao JG, Zhao T, Zhou CK, Liu JY. Limbic Encephalitis Associated With Antibodies Against the alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptor: A Case Report. Neuro Endocrinol Lett (2018) 39(2):85–7.
23. Samad N, Wong J. Anti-AMPA Receptor Encephalitis Associated With Medullary Thyroid Cancer. BMJ Case Rep (2018) 2018:bcr2018225745. doi: 10.1136/bcr-2018-225745
24. Laurido-Soto O, Brier MR, Simon LE, McCullough A, Bucelli RC, Day GS. Patient Characteristics and Outcome Associations in AMPA Receptor Encephalitis. J Neurol (2019) 266(2):450–60. doi: 10.1007/s00415-018-9153-8
25. Urriola N, Soosapilla K, Drummond J, Thieben M. Fulminant Thymomatous AMPAR-antibody Limbic Encephalitis With Hypertonic Coma, Bruxism, an Isoelectric Electroencephalogram and Temporal Cortical Atrophy, With Recovery. BMJ Case Rep (2019) 12(2):e227893. doi: 10.1136/bcr-2018-227893
26. Daneshmand A, Goyal G, Markovic S, Zekeridou A, Wijdicks EFM, Hocker SE. Autoimmune Encephalitis Secondary to Melanoma. Ann Intern Med (2019) 170(12):905–6. doi: 10.7326/L18-0593
27. Luo Q, Wu X, Huang W. Anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptor GluR2 Encephalitis in a Myasthenia Gravis Patient With Complete Thymectomy: A Case Report. BMC Neurol (2019) 19(1):126. doi: 10.1186/s12883-019-1358-7
28. Jia Y, Wang J, Xue L, Hou Y. Limbic Encephalitis Associated With AMPA Receptor and CRMP5 Antibodies: A Case Report and Literature Review. Brain Behav (2020) 10(3):e01528. doi: 10.1002/brb3.1528
29. Wei YC, Tseng JR, Wu CL, Su FC, Weng WC, Hsu CC, et al. Different FDG-PET Metabolic Patterns of anti-AMPAR and anti-NMDAR Encephalitis: Case Report and Literature Review. Brain Behav (2020) 10(3):e01540. doi: 10.1002/brb3.1540
30. Safadi AL, Wang T, Maria GD, Starr A, Delasobera BE, Mora CA, et al. Recurrent Thymoma-Associated Paraneoplastic Encephalitis Resulting From Multiple Antibodies: A Case Report. Neurohospitalist (2021) 10(2):139–42. doi: 10.1177/1941874419880423
31. Qiao S, Wu HK, Wang KM, Zang KJ, Liu XW. Long-Term Follow-Up of a Child With anti-AMPA Receptor Encephalitis. Neurol Sci (2021) 42(5):2107–9. doi: 10.1007/s10072-020-04900-w
32. Greger IH, Watson JF, Cull-Candy SG. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron (2017) 94(4):713–30. doi: 10.1016/j.neuron.2017.04.009
33. Haselmann H, Mannara F, Werner C, Planaguma J, Miguez-Cabello F, Schmidl L, et al. Human Autoantibodies Against the AMPA Receptor Subunit GluA2 Induce Receptor Reorganization and Memory Dysfunction. Neuron (2018) 100(1):91–105.e9. doi: 10.1016/j.neuron.2018.07.048
34. Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol Rev (2010) 62(3):405–96. doi: 10.1124/pr.109.002451
35. Sprengel R. Role of AMPA Receptors in Synaptic Plasticity. Cell Tissue Res (2006) 326(2):447–55. doi: 10.1007/s00441-006-0275-4
36. Dalmau J, Geis C, Graus F. Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol Rev (2017) 97(2):839–87. doi: 10.1152/physrev.00010.2016
37. Peng X, Hughes EG, Moscato EH, Parsons TD, Dalmau J, Balice-Gordon RJ. Cellular Plasticity Induced by anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptor Encephalitis Antibodies. Ann Neurol (2015) 77(3):381–98. doi: 10.1002/ana.24293
38. Bordonne M, Chawki MB, Doyen M, Kas A, Guedj E, Tyvaert L, et al. Brain (18)F-FDG PET for the Diagnosis of Autoimmune Encephalitis: A Systematic Review and a Meta-Analysis. Eur J Nucl Med Mol Imaging (2021). doi: 10.1007/s00259-021-05299-y
39. Solnes LB, Jones KM, Rowe SP, Pattanayak P, Nalluri A, Venkatesan A, et al. Diagnostic Value of (18)F-FDG PET/CT Versus MRI in the Setting of Antibody-Specific Autoimmune Encephalitis. J Nucl Med (2017) 58(8):1307–13. doi: 10.2967/jnumed.116.184333
40. Bacchi S, Franke K, Wewegama D, Needham E, Patel S, Menon D. Magnetic Resonance Imaging and Positron Emission Tomography in anti-NMDA Receptor Encephalitis: A Systematic Review. J Clin Neurosci (2018) 52:54–9. doi: 10.1016/j.jocn.2018.03.026
41. Rossling R, Pruss H. SOP: Antibody-Associated Autoimmune Encephalitis. Neurol Res Pract (2020) 2:1. doi: 10.1186/s42466-019-0048-7
42. Keshavan MS, Kaneko Y. Secondary Psychoses: An Update. World Psychiatry (2013) 12(1):4–15. doi: 10.1002/wps.20001
43. Endres D, Leypoldt F, Bechter K, Hasan A, Steiner J, Domschke K, et al. Autoimmune Encephalitis as a Differential Diagnosis of Schizophreniform Psychosis: Clinical Symptomatology, Pathophysiology, Diagnostic Approach, and Therapeutic Considerations. Eur Arch Psychiatry Clin Neurosci (2020) 270(7):803–18. doi: 10.1007/s00406-020-01113-2
44. Seo JH, Lee YJ, Lee KH, Gireesh E, Skinner H, Westerveld M. Autoimmune Encephalitis and Epilepsy: Evolving Definition and Clinical Spectrum. Clin Exp Pediatr (2020) 63(8):291–300. doi: 10.3345/kjp.2019.00598
45. Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, et al. Treatment and Prognostic Factors for Long-Term Outcome in Patients With anti-NMDA Receptor Encephalitis: An Observational Cohort Study. Lancet Neurol (2013) 12(2):157–65. doi: 10.1016/S1474-4422(12)70310-1
46. Tyler KL. Acute Viral Encephalitis. N Engl J Med (2018) 379(6):557–66. doi: 10.1056/NEJMra1708714
47. Abboud H, Probasco JC, Irani S, Ances B, Benavides DR, Bradshaw M, et al. Autoimmune Encephalitis: Proposed Best Practice Recommendations for Diagnosis and Acute Management. J Neurol Neurosurg Psychiatry (2021). doi: 10.1136/jnnp-2020-325300
48. Nosadini M, Mohammad SS, Ramanathan S, Brilot F, Dale RC. Immune Therapy in Autoimmune Encephalitis: A Systematic Review. Expert Rev Neurother (2015) 15(12):1391–419. doi: 10.1586/14737175.2015.1115720
49. Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and Antibodies to Synaptic and Neuronal Cell Surface Proteins. Neurology (2011) 77(2):179–89. doi: 10.1212/WNL.0b013e318224afde
50. Lancaster E, Dalmau J. Neuronal Autoantigens–Pathogenesis, Associated Disorders and Antibody Testing. Nat Rev Neurol (2012) 8(7):380–90. doi: 10.1038/nrneurol.2012.99
51. Broadley J, Seneviratne U, Beech P, Buzzard K, Butzkueven H, O’Brien T, et al. Prognosticating Autoimmune Encephalitis: A Systematic Review. J Autoimmun (2019) 96:24–34. doi: 10.1016/j.jaut.2018.10.014
52. Shin YW, Lee ST, Park KI, Jung KH, Jung KY, Lee SK, et al. Treatment Strategies for Autoimmune Encephalitis. Ther Adv Neurol Disord (2018) 11:1756285617722347. doi: 10.1177/1756285617722347
Keywords: alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, neuronal surface antibody, autoimmune encephalitis, limbic encephalitis, immunotherapy
Citation: Zhang T-Y, Cai M-T, Zheng Y, Lai Q-L, Shen C-H, Qiao S and Zhang Y-X (2021) Anti-Alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptor Encephalitis: A Review. Front. Immunol. 12:652820. doi: 10.3389/fimmu.2021.652820
Received: 13 January 2021; Accepted: 05 May 2021;
Published: 21 May 2021.
Edited by:
Hongzhi Guan, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Sudarshini Ramanathan, The University of Sydney, AustraliaTarun Singhal, Brigham and Women’s Hospital and Harvard Medical School, United States
Copyright © 2021 Zhang, Cai, Zheng, Lai, Shen, Qiao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin-Xi Zhang, enl4LW5ldXJvbG9neUB6anUuZWR1LmNu
Song Qiao, cWlhb3NvbmdpY3VAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Tian-Yi Zhang
Tian-Yi Zhang Meng-Ting Cai
Meng-Ting Cai Yang Zheng
Yang Zheng Qi-Lun Lai
Qi-Lun Lai Chun-Hong Shen
Chun-Hong Shen Song Qiao
Song Qiao Yin-Xi Zhang
Yin-Xi Zhang