- 1Key Laboratory for Genetic Breeding of Aquatic Animals and Aquaculture Biology, Freshwater Fisheries Research Center (FFRC), Chinese Academy of Fishery Sciences (CAFS), Wuxi, China
- 2Wuxi Fisheries College, Nanjing Agricultural University, Wuxi, China
- 3Tongwei Co., Ltd., Chengdu, China
- 4Healthy Aquaculture Key Laboratory of Sichuan Province, Chengdu, China
The present study aimed to assess the role of tributyrin (TB) in regulating the growth and health status of juvenile blunt snout bream (Megalobrama amblycephala) through an 8-week feeding experiment. Six groups were fed experimental diets with added TB percentages of 0% (control group), 0.03%, 0.06%, 0.09%, 0.12% and 0.15%. The present results showed that TB supplementation in feed had some positive impacts on FW, WG, FCR and SGR, and the best results were found in the 0.06% TB group (P<0.05). However, TB supplementation in feed had no significant effects on SR, CF, VSI or whole-body composition (P>0.05). TB supplementation in feed increased antioxidant capacity and immunological capacity and attenuated the inflammatory response by increasing the activity of T-SOD, GPx, CAT and the levels of anti-inflammatory cytokines (IL-10 and TGF-β) and decreasing the levels of MDA and anti-inflammatory cytokines (TNF-α) (P<0.05). Furthermore, TB supplementation improved immunity by increasing the levels of immunoglobulins (IgM and IgG), C3 and IFN-γ (P<0.05). Surprisingly, 0.06%-0.12% TB supplementation significantly increased the content of IL-1β (P<0.05). However, TB supplementation in feed had no significant effects on the plasma content of GSH, HSP70, IL-8 and the activity of T-AOC (P>0.05). The possible mechanism was that TB activated PI3K/Akt/Nrf2 and inhibits the NF-κB signaling pathway, further regulating the mRNA levels of key genes with antioxidant capacity and the inflammatory response; for example, it increased the mRNA levels of Nrf2, Cu/Zn-SOD, HO-1, CAT, Akt, PI3K, GPx, IL-10, and TGF-β and decreased the mRNA levels of NF-κB and TNF-α (P<0.05). In addition, 0.06%-0.15% TB supplementation significantly increased the mRNA levels of IL-1β (P<0.05). TB supplementation in feed had no significant effects on the mRNA levels of HSP70, Mn-SOD and IL-8 (P>0.05). Evidence was presented that TB supplementation decreased the mortality rate caused by Aeromonas hydrophila challenge. In pathological examination, TB supplementation prevented hepatic and intestinal damage. Generally, TB supplementation improved the growth performance of juvenile blunt snout bream. Furthermore, TB supplementation activated PI3K/Akt/Nrf2 and inhibited the NF-κB signaling pathway, regulating health status and preventing hepatic and intestinal damage.
Introduction
Butyric acid is a short-chain fatty acid in the intestinal tract and an important energy source of the colon (1). Butyric acid plays important roles in regulating growth performance, gastrointestinal function, immunity, gastrointestinal microecological balance and intestinal pH, and it has bactericidal and bacteriostatic functions (2, 3). Despite its many functions, the direct use of butyric acid as a liquid is very limited because of its volatility, corrosivity, very unpleasant odor and excessive absorption rate before its arrival in the small intestine. These disadvantages have greatly limited the popularization and application of butyric acid in farmed animal feed. To solve this industry problem, butyric acid is mostly added in the form of sodium butyrate; however, this practice does not solve many problems, such as easy delirium and unpleasant odor of butyric acid (4, 5). Hence, applications remain limited in husbandry and aquaculture. In recent years, tributyrin (TB), the precursor of butyric acid and a colorless oily liquid, has been found to address the unfavorable characteristics of butyric acid very well and has better palatability in that it has almost no smell or a slightly fatty fragrance; thus, it has great application potential in aquaculture (6–8).
TB is composed of trimolecular butyric acid and a molecule of glycerol, which is a short-chain fatty acid ester. Under the action of intestinal lipase, it is decomposed into butyric acid, glycerol butyrate and oil. Hence, TB has a function similar to that of butyric acid. Some studies have shown that TB has a positive effect on growth performance in various terrestrial animals, such as broiler chickens (1, 9), weaned pigs (10, 11), and small tail sheep ewes (12). In addition, TB can promote the healthy development, digestion and collection of nutrients of the intestinal tract. TB supplementation can improve the development of the intestine, including by increasing villus height, villus width, muscular thickness and villus area in the duodenum, while significantly decreasing crypt depth in intrauterine growth‐retarded piglets (13). It has been found to have similar results in nursery pigs (14). The improvements in intestinal development may be closely related to the immune state of the intestine. Studies have shown that the breakdown of TB into butyric acid can promote hydrogen ion accumulation and reduce intestinal pH, which can destroy the membranes of some harmful bacteria and stimulate the breeding of beneficial acidophilic microorganisms and the development of the intestinal mucosa (15). Furthermore, TB can improve intestinal immunity by regulating the antioxidant system and the inflammatory factor system (16, 17). There are only a few reports that TB supplementation in feed also has positive effects on growth performance, intestinal development and immunity in aquatic animals, such as snakehead (Channa argus) (18), black sea bream (Acanthopagrus schlegelii) (19) and yellow drum (Nibea albiflora) (20, 21). However, research on the effects of TB mainly focuses on livestock and poultry, and aquatic animals are rarely reported, and the mechanisms of growth and immune regulation are still unclear in aquatic animals.
As an important economic fish in China, blunt snout bream (Megalobrama amblycephala) has many advantages such as delicious meat, high economic value, and fast growth; thus, it is widely distributed all over the world and loved by many consumers. However, with the expansion of farming scale, diseases have begun to break out, resulting in increased mortality and economic losses, especially in summer (22). Therefore, to improve growth and health status, studies on the anti-stress function of feed are urgently needed. To date, no studies regarding the effects of TB supplementation on blunt snout bream have been reported, and the mechanism by which TB regulates health status is also unclear. Hence, the present study investigated the effect of TB supplementation on growth performance, plasma antioxidant and immune capacity indexes, PI3K/Akt/Nrf2 signaling and inhibition of the NF-κB signaling pathway and performed pathological examinations to assess the role of TB in regulating health status and to elucidate the related mechanism in juvenile blunt snout bream.
Materials and Methods
Experimental Ethics Statement
The protocols used on the experimental fish followed the guidelines of the Institutional Animal Care and Ethics Committee of Nanjing Agricultural University, Nanjing, China. [Permit number: SYXK (Su) 2011-0036].
Diets
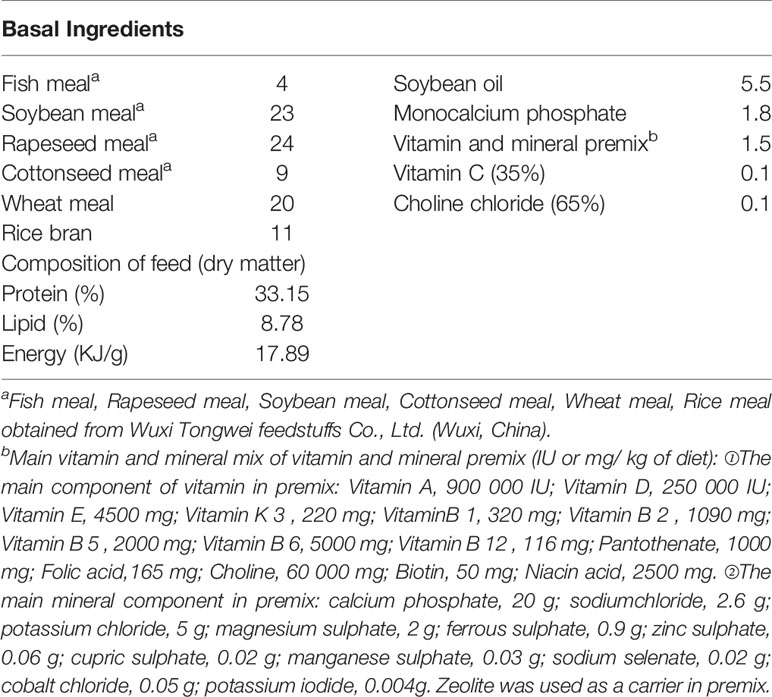
Commercial feed was used in the experiment. TB (50% active ingredient) was purchased from Qingdao Keneng Biotechnology Co., Ltd. (Qingdao, China). Six groups were fed experimental feed with different percentages of TB: 0% (the control group), 0.03%, 0.06%, 0.09%, 0.12% and 0.15%. The main protein sources and lipid sources were listed in Table 1. The ingredients were crushed, proportioned according to the feed formula, mixed and then granulated though a granulator (F-26 (II), South China University of Technology, Guangzhou, China). After granulation, the feed was dried in a drying oven at 65°C.
Experimental Procedure
Juvenile blunt snout bream based on health (lively, no scars, no signs of bleeding) and similar size were obtained from the breeding base of the Freshwater Fisheries Research Center (FFRC) and acclimatized to the experimental conditions in recirculatory tanks (φ820×700mm) for two weeks. The juvenile fish (6.51 ± 0.01 g) were randomly distributed into 18 recirculatory tanks (in triplicate). During the period of breeding, the fish were fed three times a day (at 08:00, 12:00 and 16:00) until apparent satiation by visual observation of the feeding behavior of the fish. The water quality was measured once a week with an YSI ProPlus multiparameter water quality analyzer (YSI China, Hong Kong, China). The water temperature was kept at 28.45 ± 0.41°C, the pH at 7.31 ± 0.16, the ammonia nitrogen concentration at 0.027 ± 0.003 mg/L, and the dissolved oxygen concentration at 6.13 ± 0.40 mg/L. The light exposure was controlled with a photoperiod of 12L:12 D (light: dark).
Sample Collection
After a 56-day feeding trial, the fish were fasted for 24 h to allow evacuation of the contents of the alimentary tract. Two fish (per recirculation tank) were obtained for whole-body analysis. Furthermore, three fish (per recirculation tank) were randomly selected and anesthetized using 100 mg/L MS-222. Immediately, blood samples were collected from the caudal vein using an injection syringe and centrifuged at 3,500 x g at 4°C for 10 minutes for collection of plasma. The intestine was also collected for gene expression analysis and pathological analysis, and the liver was collected for pathological analysis (the detailed collection methods are shown in section 2.7 on hematoxylin and eosin (HE) staining). The collected intestine samples were stored at -80°C until analysis.
Proximate Composition and Plasma Biochemical Analysis
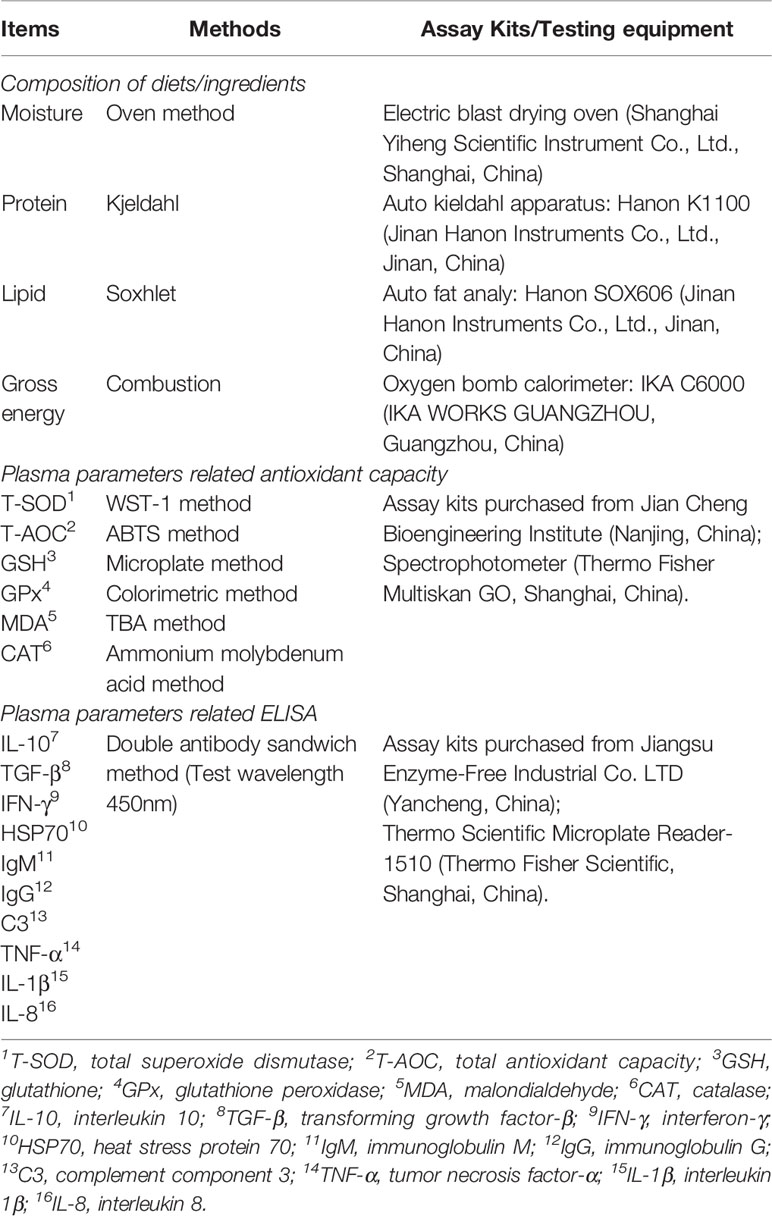
Based on the established methods of the AOAC (23), the chemical compositions of the dried whole fish, ingredients and experimental diets were assessed with regard to lipids (ID 991.36), crude protein (ID 984.13), ash (ID 923.03) and moisture (ID 920.36). Enzyme-linked immunosorbent assays (ELISAs) were used for evaluation of interferon-γ (IFN-γ), heat stress protein 70 (HSP70), immunoglobulin M (IgM), immunoglobulin G (IgG), complement component 3 (C3), interleukin 10 (IL-10), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β) and interleukin 8 (IL-8) in plasma. The methods, kits and equipment used in this study were presented in Table 2.
Tissue RNA Extraction and Real-Time PCR Analysis
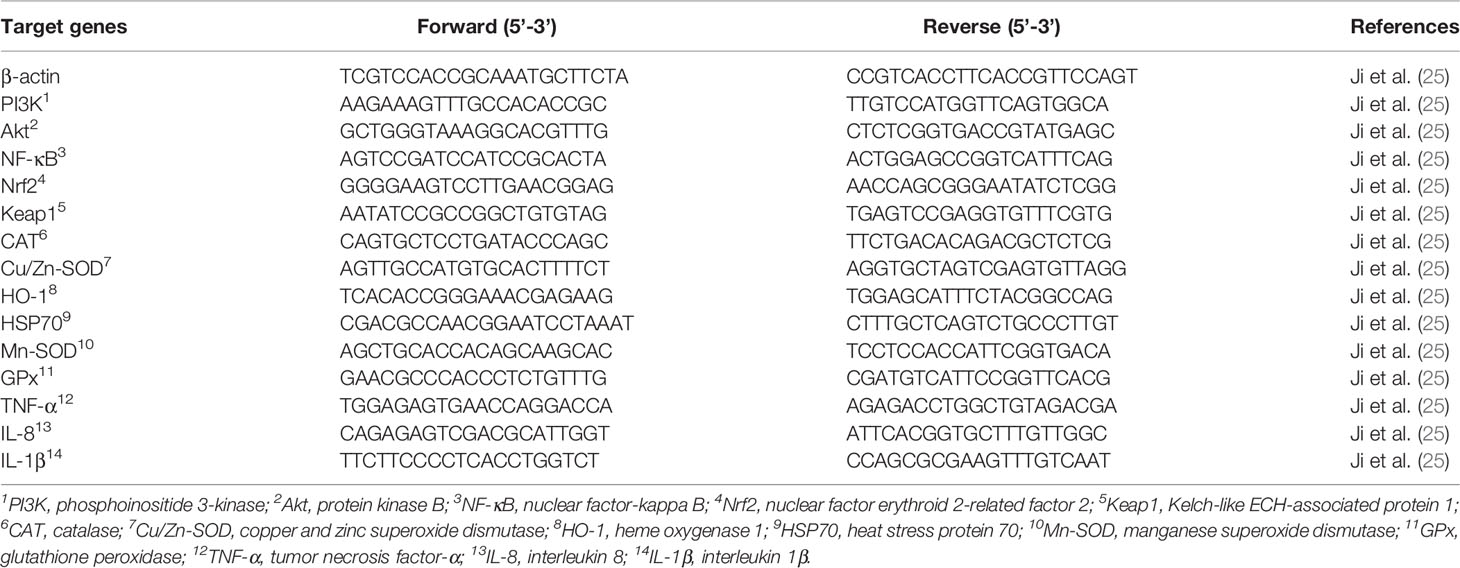
The method was described in our previous study (24). Tissue RNA was first extracted, and then the quality and quantity were determined. Later, the mRNA levels of all genes were tested with a 7500 real-time PCR machine (Applied Biosystems, USA). Initially, cDNA (2.0 μL) was reacted with 10.0 μL of SYBR® Premix Ex Taq II (2×), 0.8 μL of forward and reverse primers (10 μM each), 0.4 μL of ROX reference dye II (50×), and 6.0 μL of RNase-free distilled water in a 20 μL final reaction volume. The primer sequences used for qRT-PCR were described in our previous study (25), and the specific primers for the target genes used are shown in Table 3. β-actin was used as a nonregulated reference gene, and no obvious change was observed in its gene expression (24, 26, 27). Relative gene expression was calculated using Pfaffl’s mathematical model for CT calculation (28).
Hematoxylin and Eosin (HE) Staining
HE staining of liver and intestine samples was performed as described in our previous study (29). In brief, the method included (1) tissue fixation by 4% paraformaldehyde, (2) gradient alcohol dehydration and methyl salicylate clearing, (3) paraffin embedding, (4) slicing and patching, (5) HE staining, (6) dehydration sealing, and other procedures. Finally, pathological changes in the liver and intestine were observed and analyzed with a Zeiss microscope (Axioplan 2, Oberkochen, Germany).
Aeromonas hydrophila Challenge Test
The remaining 15 fish from each tank were challenged with Aeromonas hydrophila (A. hydrophila). The method was described in our previous study (30). The pre-experiment was carried out before the challenged experiment. The five concentration gradients were set as 1x105 CFU/mL, 1x106 CFU/mL, 1x107 CFU/mL, 1x108 CFU/mL, 1x109 CFU/mL, respectively. The half lethal concentration was determined to be 1x107 CFU/mL. Whereafter, the A. hydrophila was resuspended and amplified by inoculation in nutrient broth and incubation in a shaker at 160 rpm (37°C) for 24 h. The challenge concentration w as adjusted to 1x107 CFU/mL using a bacterial turbidimeter (SGZ-6AXJ, Yue Feng Instrument Co. Ltd., Shanghai, China). The fish were challenged by intraperitoneal injection with 1 mL/100 g (1% of body weight).
Calculations and Statistical Analysis
Parameters were calculated based on the following equations:
The data were subjected to normality and homogeneity tests where necessary. Statistical analysis was performed using IBM SPSS Statistics 23. Tukey’s test was used to determine whether significant differences existed between means. The data are expressed as the mean with S.E (M± S.E).
Results
Growth Performance, Whole-Body Composition and Physique Parameters
TB supplementation in feed had some positive impacts on final weight (FW), weight gain rate (WG), feed conversion ratio (FCR) and specific growth rate (SGR), and the best results were found in the 0.06% TB group (P<0.05). However, TB supplementation in feed had no significant effect on survival rate (SR), condition factor (CF), visceral somatic index (VSI) or the whole-body compositions of protein, lipids, moisture and ash (P>0.05) (Tables 4 and 5).
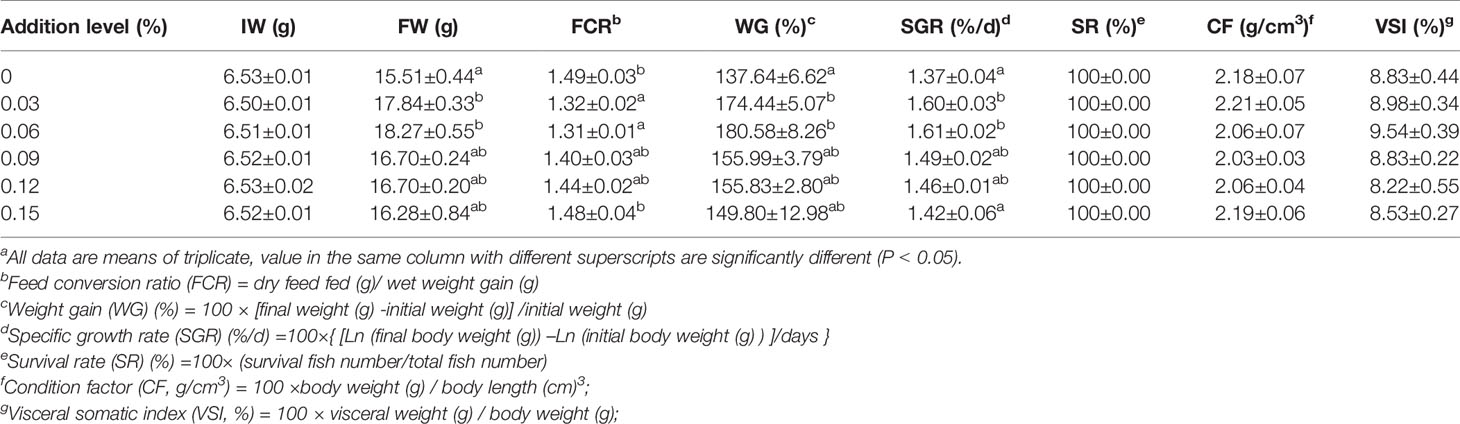
Table 4 Effect of TB supplementation on growth performance and physical indexes of juvenile blunt snout breama.
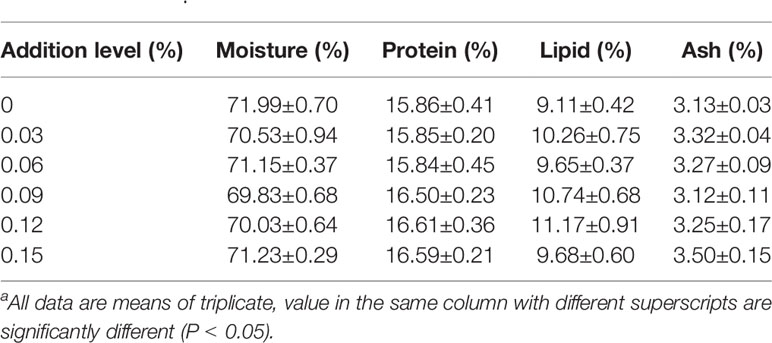
Table 5 Effect of TB supplementation on whole body composition of juvenile blunt snout breama.
Plasma Biochemical Composition
Compared with the control group (without TB supplementation, 0%), the group with 0.06% TB supplementation in feed exhibited a significantly lower plasma content of malondialdehyde (MDA) (P<0.05). The groups with 0.06% and 0.09% TB supplementation in feed exhibited significantly increased plasma activity levels of total superoxide dismutase (T-SOD) and glutathione peroxidase (GPx) (P<0.05). Furthermore, 0.03%-0.15% TB supplementation in feed significantly increased the catalase (CAT) activity in plasma (P<0.05). However, TB supplementation in feed had no significant effect on the plasma content of glutathione (GSH) or the activity of total antioxidant capacity (T-AOC) (P>0.05) (Figures 1A, B).
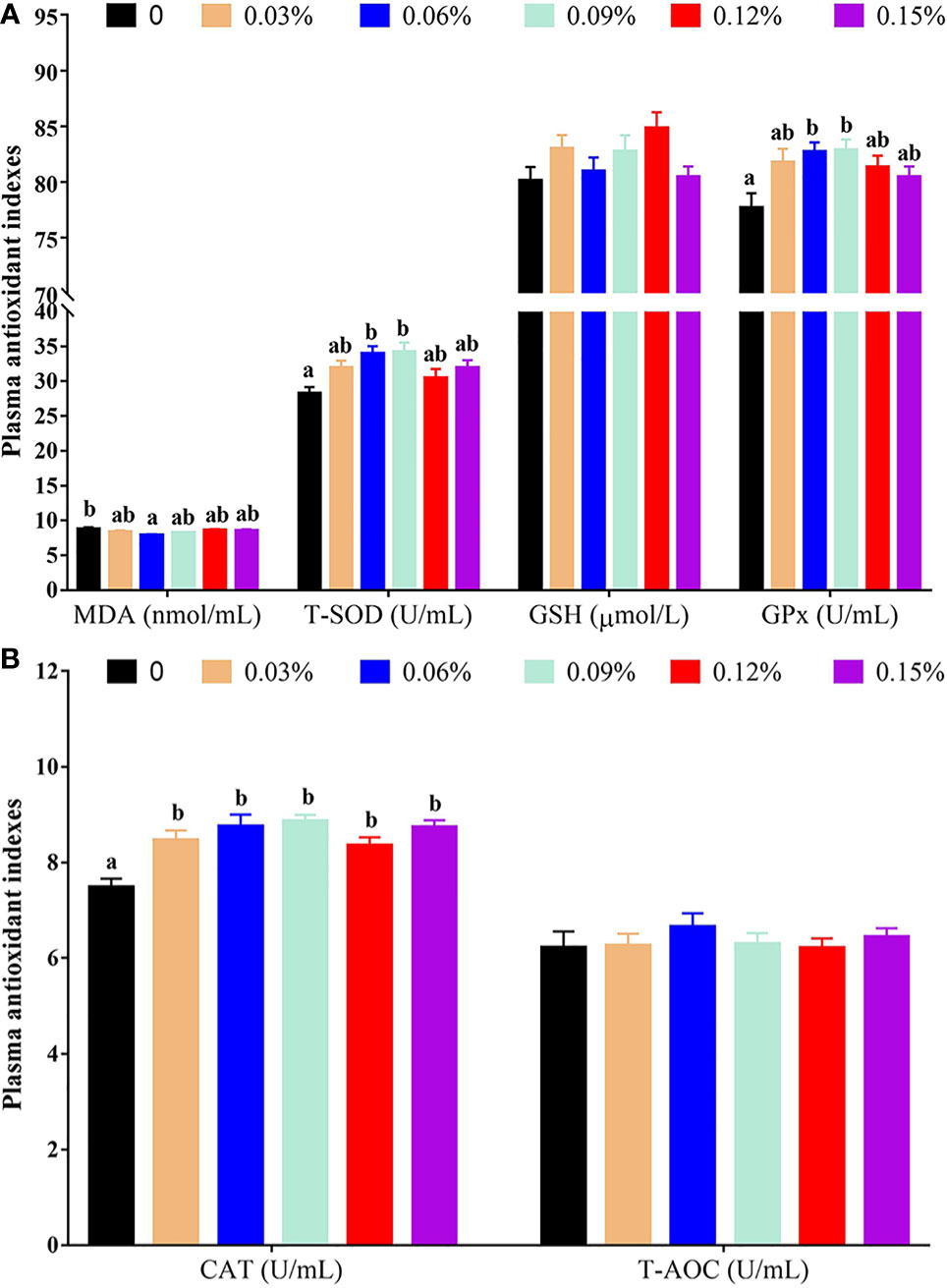
Figure 1 The results of plasma antioxidant indexes with different levels of TB supplementation. (A) MDA, malondialdehyde; T-SOD, total superoxide dismutase; GSH, glutathione; GPx, glutathione peroxidase; (B) CAT, catalase; T-AOC, total antioxidant capacity. Data are expressed as means with S.E. Value with different superscripts are significantly different (P < 0.05).
Plasma ELISA revealed that compared with the control group (without TB supplementation, 0%), the group with 0.06% TB supplementation in feed exhibited significantly higher levels of IFN-γ, IgM and IgG (P<0.05), and the groups with 0.06% and 0.09% TB supplementation in feed exhibited significantly higher content of C3 (P<0.05) (Figure 2). With regard to inflammatory cytokines, compared with the control group, the groups with 0.03%-0.09% and 0.06%-0.15% TB supplementation in feed exhibited significantly higher levels of IL-10 and TGF-β (anti-inflammatory cytokines), respectively (P<0.05). The groups with 0.06% and 0.09% TB supplementation in feed exhibited significantly reduced levels of TNF-α (pro-inflammatory cytokine) (P<0.05) (Figure 3). However, TB supplementation in feed had no significant effects on the levels of HSP70 and IL-8 (P>0.05) (Figures 2 and 3). Surprisingly, 0.06%-0.12% TB supplementation significantly increased the content of IL-1β (P<0.05) (Figure 3).
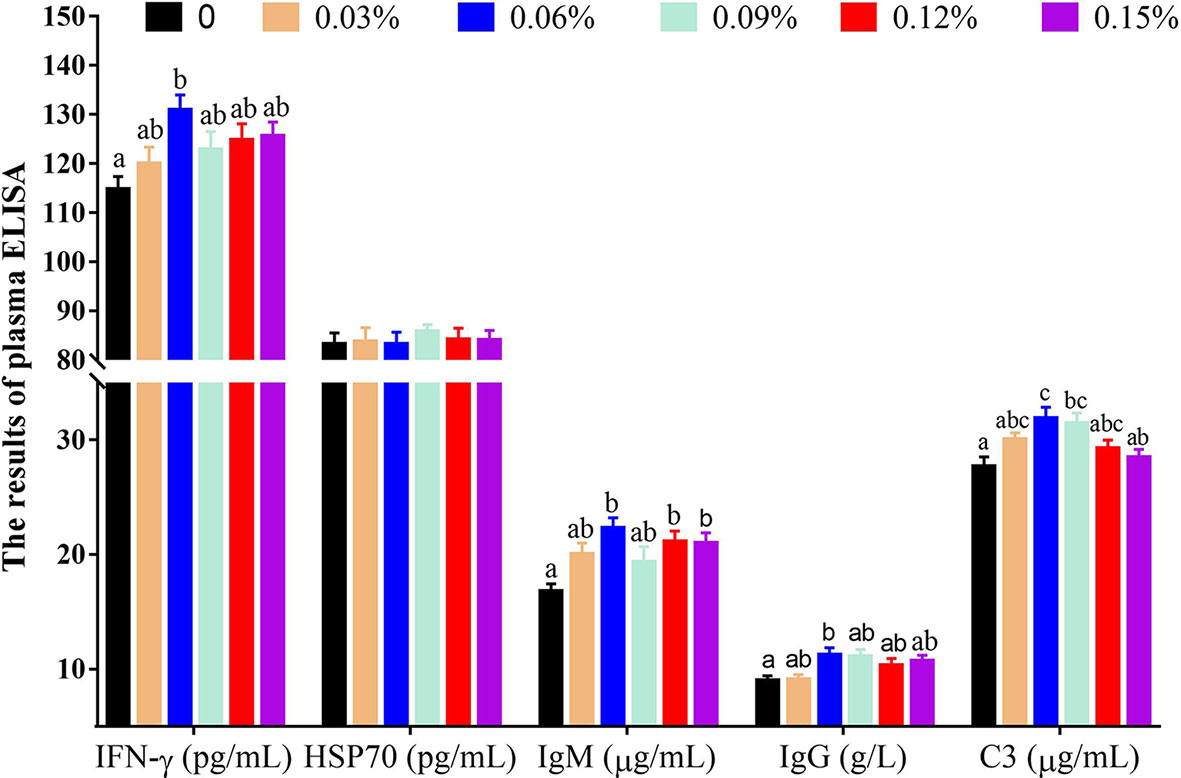
Figure 2 The contents of plasma immune factors with different levels of TB supplementation. IFN-γ, interferon-γ; HSP70, heat stress protein 70; IgM, immunoglobulin M; IgG, immunoglobulin G; C3, complement component 3. Data are expressed as means with S.E. Value with different superscripts are significantly different (P < 0.05).
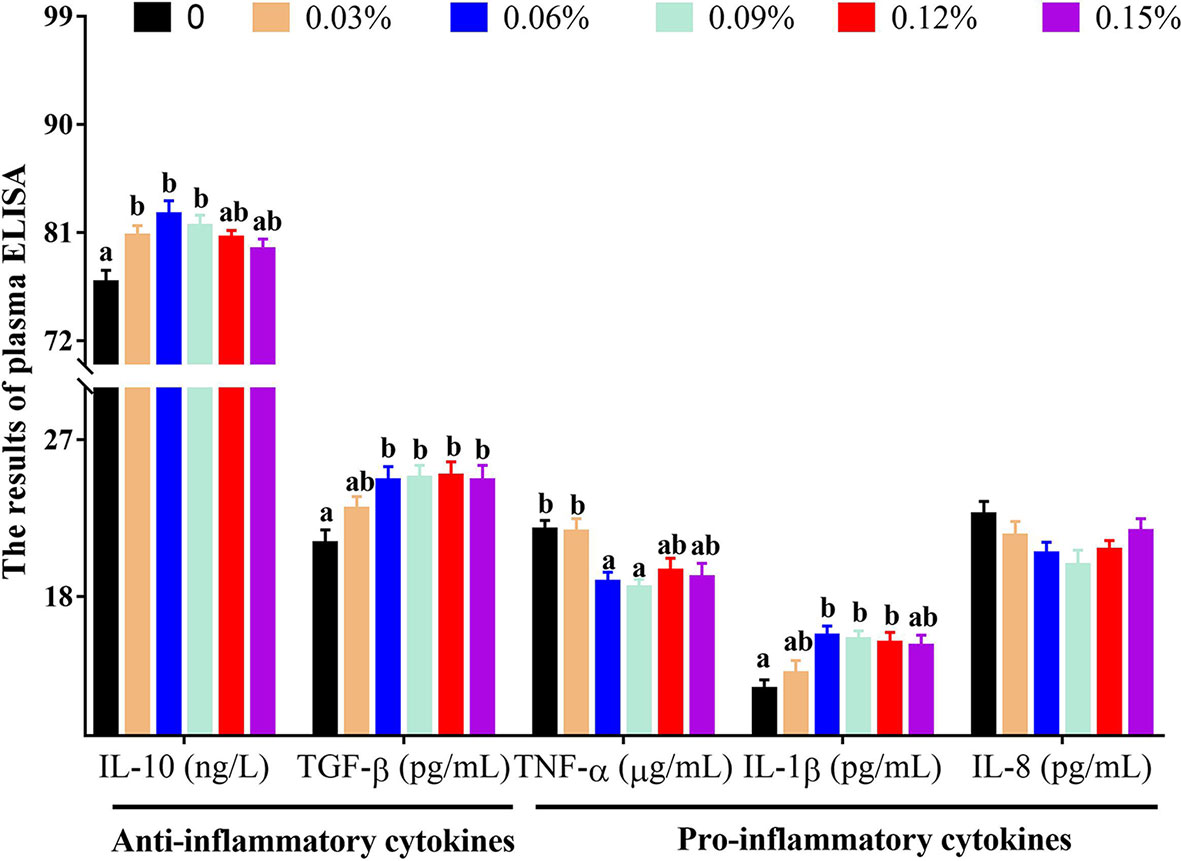
Figure 3 The contents of plasma inflammatory cytokines with different levels of TB supplementation. IL-10, interleukin 10; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; IL-1β, interleukin 1β; IL-8, interleukin 8. Data are expressed as means with S.E. Value with different superscripts are significantly different (P < 0.05).
Relative Expression of Genes in the Intestine
With regard to antioxidant genes, the relative expression of nuclear factor erythroid 2-related factor 2 (Nrf2) showed a trend of increasing first and then decreasing with the addition levels of TB in feed, and the highest levels was observed in 0.06% TB supplementation in feed (P<0.05), however, the relative expression of Kelch-like ECH-associated protein 1 (Keap1) showed an opposite trend with Nrf2. Furthermore, with the addition levels of TB in feed, the relative expression of CAT, protein kinase B (Akt), heme oxygenase 1 (HO-1), phosphoinositide 3-kinase (PI3K) and GPx were increased with increasing dietary TB supplementation level up to 0.06% (P<0.05), and thereafter showed a decreasing trend, while, the highest expression levels of copper and zinc superoxide dismutase (Cu/Zn-SOD) was observed in 0.09% TB supplementation in feed (P<0.05) (Figure 4). With regard to inflammatory cytokines, 0.03%-0.15% TB supplementation in feed significantly reduced the relative expression of nuclear factor-kappa B (NF-κB) and 0.03%-0.12% TB supplementation in feed significantly reduced the relative expression of TNF-α. Furthermore, 0.06% and 0.03%-0.06% TB supplementation in feed significantly increased the relative expression of IL-10 and TGF-β, respectively (P<0.05). Surprisingly, 0.06%-0.15% TB supplementation significantly increased the mRNA levels of IL-1β (P<0.05). However, TB supplementation in feed had no significant effect on the relative expression of HSP70, manganese superoxide dismutase (Mn-SOD) or IL-8 (P>0.05) (Figures 4 and 5).
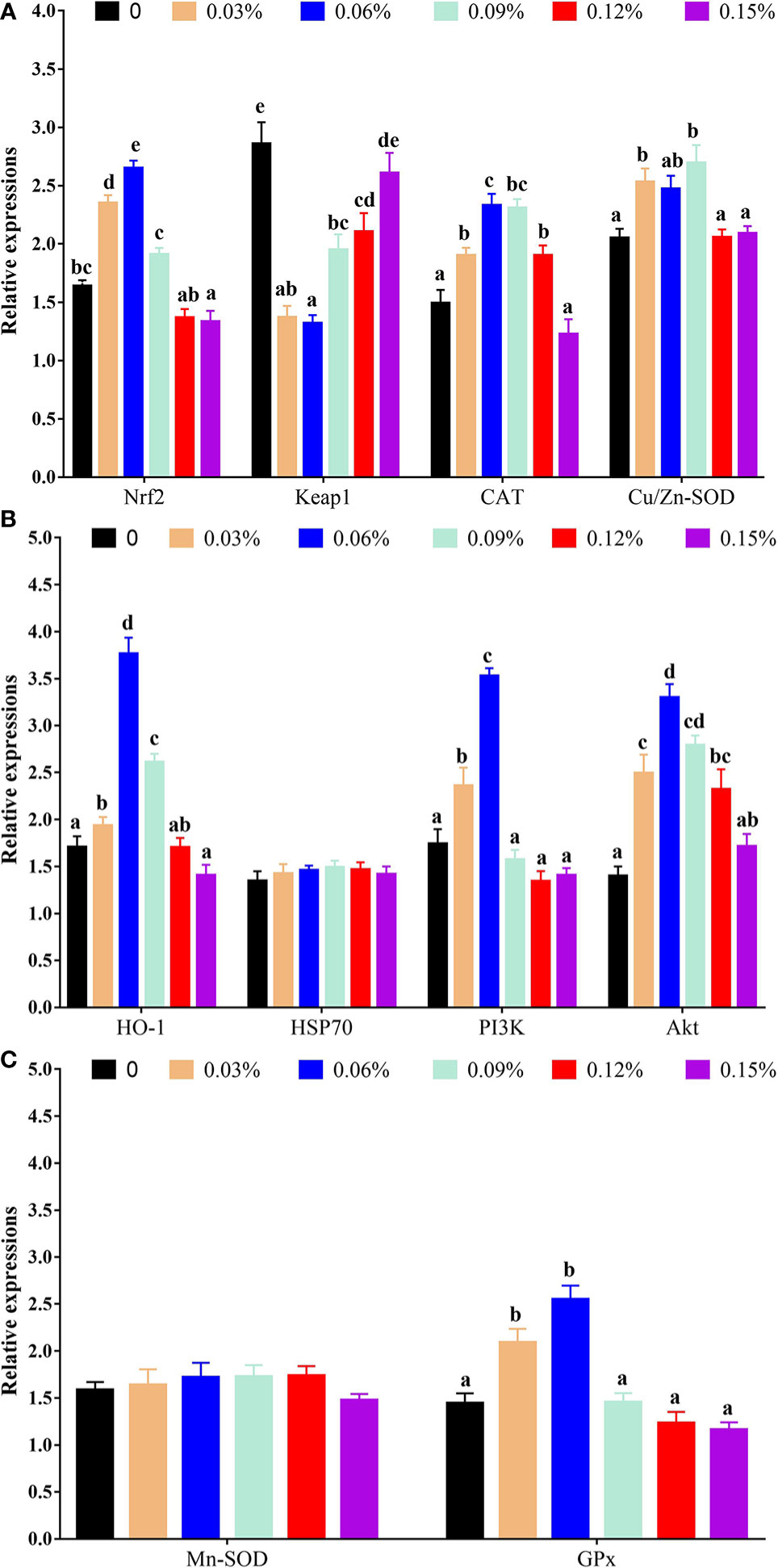
Figure 4 The relative expressions of vital gene in PI3K/Akt/Nrf2 signaling pathway with different levels of TB supplementation. (A) Nrf2, Nuclear factor erythroid 2-related factor 2; Keap1, Kelch-like ECH-associated protein 1; CAT, Catalase; Cu/Zn-SOD, Copper zinc superoxide dismutase; (B) HO-1, heme oxygenase 1; HSP70, heat stress protein 70; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; (C) Mn-SOD, manganese superoxide dismutase; GPx, glutathione peroxidase. Value with different superscripts are significantly different (P < 0.05).
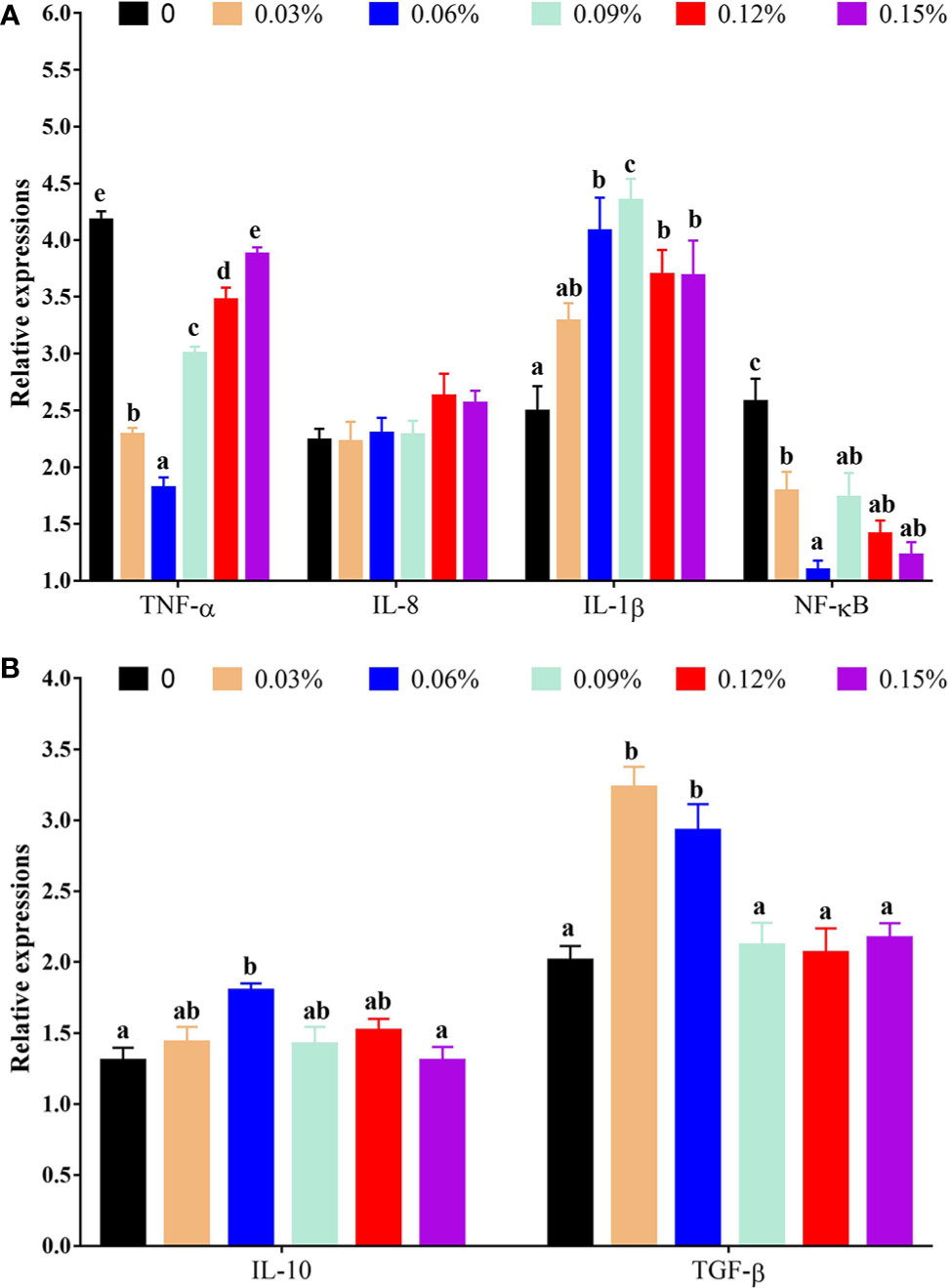
Figure 5 The relative expressions of vital gene in NF-κB signaling pathway with different levels of TB supplementation. (A) TNF-α, tumor necrosis factor-α; IL-8, interleukin 8; IL-1β, interleukin 1β; NF-κB, nuclear factor-kappa (B); (B) IL-10, interleukin 10; TGF-β, transforming growth factor-β. Data are expressed as means with S.E. Value with different superscripts are significantly different (P < 0.05).
Aeromonas hydrophila Challenge Test
After A. hydrophila challenge, the highest mortality rate of blunt snout bream was observed among fish given food with 0% TB supplementation at 144 h (P<0.05). The lowest mortality rate of blunt snout bream was observed among fish given food with 0.09% and 0.12% TB supplementation at 144 h (P<0.05) (Figure 6).

Figure 6 Mortality rate with Aeromonas hydrophila challenge after during 144h with different levels of TB supplementation. Data are expressed as means with S.E.
Hepatic and Intestinal Histopathology
HE staining of juvenile blunt snout bream intestinal tissue revealed lysis and necrosis at the tips of the intestinal villi and indicated that the cell structure disappeared in fish given food with 0% TB supplementation. Furthermore, in the groups of fish given diet with 0.03% and 0.15% TB supplementation, a small number of intestinal villi fused with each other, and the intestinal villi became wider; however, in other groups, the structure of each layer of the intestine was clear, the mucosal epithelial cells were not shed, the intestinal villi were abundant and arranged regularly, and goblet cells were visible (Figure 7). Hepatic HE staining of juvenile blunt snout bream tissues revealed that a small number of inflammatory cells were locally infiltrated in fish given food with 0% TB supplementation; however, tissues from fish in other groups, the hepatic cells were arranged neatly with clear outlines, and the hepatic sinusoids were normal (Figure 8).
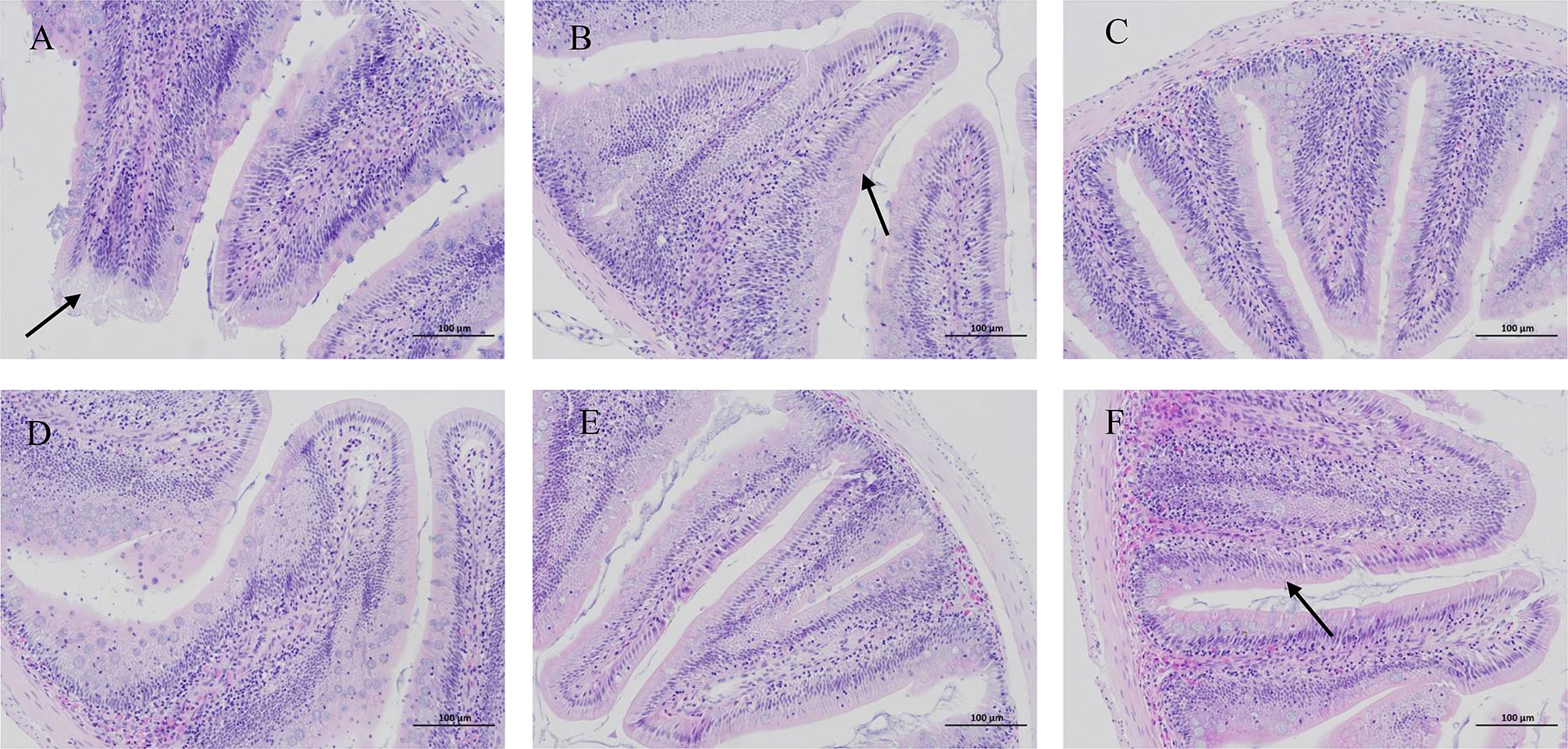
Figure 7 The intestinal HE staining of juvenile blunt snout bream (200X). (A–F) were corresponding to 0%, 0.03%, 0.06%, 0.09%, 0.12% and 0.15% TB supplementation. The black arrow indicated that there was lysis and necrosis at the top of the intestinal villi, and the cell structure disappeared (A); a small number of intestinal villi fused with each other and the intestinal villi become wider (B, F).
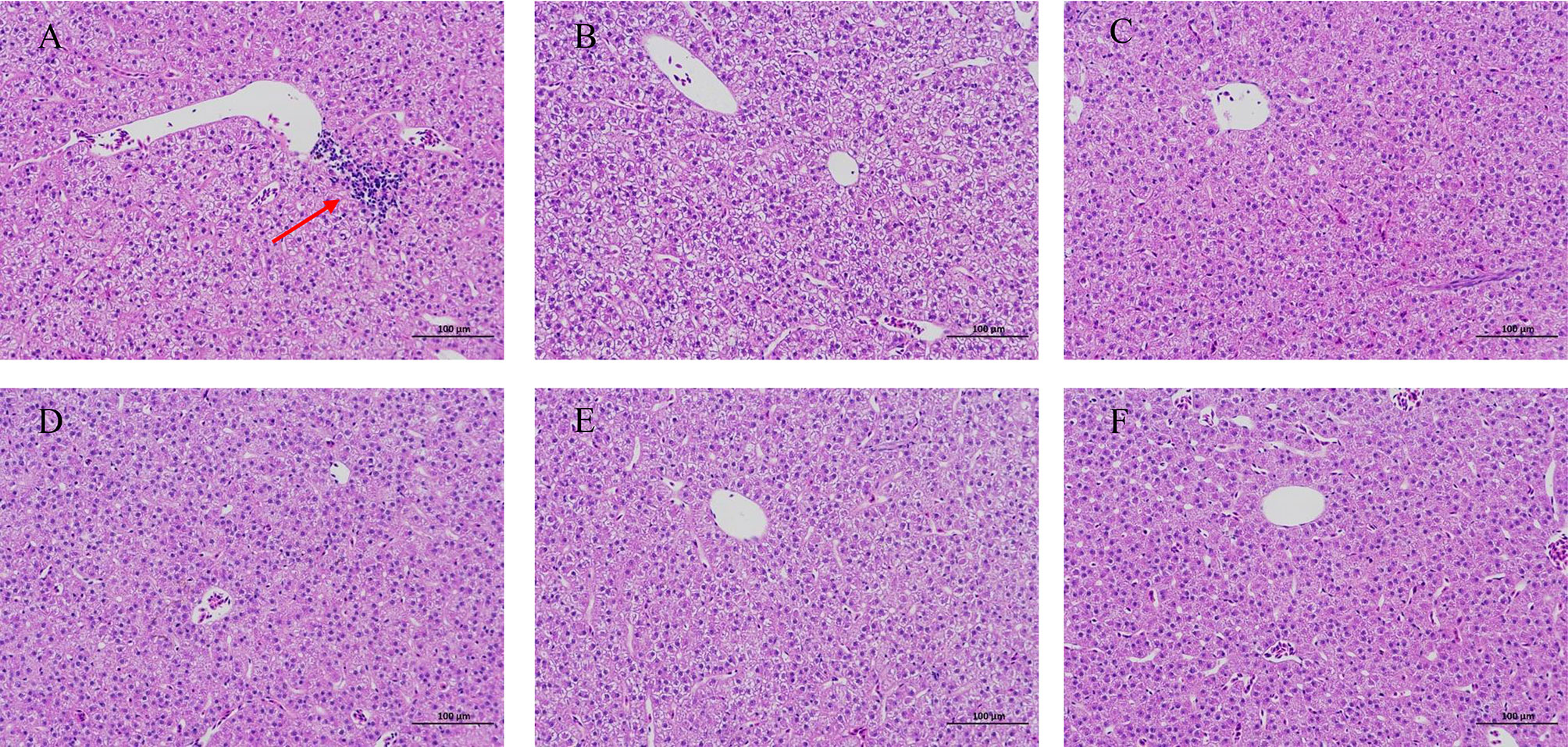
Figure 8 The hepatic HE staining of juvenile blunt snout bream (200X). (A–F) were corresponding to 0%, 0.03%, 0.06%, 0.09%, 0.12% and 0.15% TB supplementation, respectively. The red arrow indicated that a small number of inflammatory cells were locally infiltrated (A).
Discussion
Effect of TB Supplementation on Growth and Whole-Body Composition
TB has better palatability than butyric acid or sodium butyrate because it has almost no smell or only a slightly fatty fragrance, and it has great potential for use in aquatic feed. In this study, the growth performance results showed that TB supplementation in feed had some positive impacts on FW, WG, FCR and SGR, and the best results were found in the 0.06% TB group. These results indicated that TB supplementation in feed was effective in enhancing fish performance, at least in Megalobrama amblycephala. Researchers have demonstrated the availability and functionality of TB supplemented in the feed of terrestrial animals, such as broiler chickens (9, 10), weaned pigs (11), and small tail sheep ewes (12). Among aquatic animals, the effects of TB supplementation in feed have also been assessed in some fish species. TB supplementation in feed can also improve the growth performance of snakehead (18), black sea bream (19) and juvenile yellow drum (20, 21). The relevant mechanism may be that TB is decomposed into butyric acid under the action of intestinal lipase, promoting the proliferation and differentiation of mucosal cells after absorption by the intestine (31), which can expand the digestion and absorption area to improve intestinal physical barrier function, nutrient absorption, and nutrient utilization (20, 31). In addition, TB supplementation in feed had no significant effect on CF, VSI or whole-body composition of components including protein, lipids, moisture and ash, similar to the case in black sea bream (19), however, Tan et al. (20) reported that TB supplementation in a high soya bean meal diet significantly reduces body fat content in juvenile yellow drum. These differing results may have occurred because of the different species, different amounts of added butyrate, different durations of the experiments, and other differences.
Effect of TB Supplementation on Antioxidant Status
Antioxidant status plays a vital role in the growth of fish (27, 30). In the present study, compared with the control group (0% TB supplementation), the group with 0.06% TB supplementation in feed improved antioxidant capacity, as indicated by reduced plasma levels of MDA and increased plasma activity of T-SOD and GPx, suggesting that TB can alleviate or reduce intestinal oxidative stress. TB supplementation effectively decreases MDA content in piglets challenged with diquat (32). Jiang et al. (33) discovered that sodium butyrate reduces MDA levels in broiler chickens challenged with corticosterone. TB supplementation (0.05%) significantly improves intestinal antioxidative capacity in LPS-challenged broilers (34). Among aquatic animals, TB supplementation effectively improves oxidative capacity in snakehead (18) and black sea bream (19). The activity levels of enzymes affect the corresponding gene expression (25, 27). Studies have shown that the PI3K/Akt signaling pathway is crucial for cell survival, inhibition of the development of cancer cells and the antioxidative system (25, 35). In this study, 0.03% and 0.06% TB supplementation in feed significantly improved PI3K and Akt mRNA levels. Furthermore, various reports have demonstrated that the Nrf2-Keap1 signaling pathway plays an important role in regulating antioxidative capacity (36, 37). In this study, 0.03% -0.06% or 0.09% TB supplementation in feed significantly increased the relative expression of Nrf2, Cu/Zn-SOD, HO-1, CAT, and GPx and reduced the relative expression of Keap1, which was correlated with PI3K and Akt mRNA levels. There were evidences that Nrf2 signaling was activated via the PI3K/Akt pathway (38, 39). In our previous studies, we also found that Nrf2 signaling pathway has a Pearson correlation with the PI3K/Akt pathway and Nrf2 signaling was activated via the PI3K/Akt pathway in blunt snout bream (25, 40). The results of this study suggest that the optimum TB supplementation may induce Nrf2/Keap1 pathway signaling partly by activating the PI3K/Akt pathway, which further regulates antioxidant gene expression and the activity of related enzymes to improve the antioxidant capacity of blunt snout bream. The possible mechanism is that TB supplementation can increase the content of adenosine triphosphate (ATP) (34), which plays an important role in activating PI3K/Akt signaling (41), further activating the Nrf2 signaling pathway to regulate antioxidant ability.
Effect of TB Supplementation on Immunocompetence
Immunity is an important factor in the maintenance of healthy growth and disease resistance in animals (42). Immunoglobulins, the complement system and interferons play important roles in immune regulation in humans and animals (43–45). Hence, we also investigated the effect of TB on immunity. In the present study, 0.06% TB supplementation in feed improved immunocompetence by increasing the levels of IFN-γ, IgM, IgG and C3 in plasma. Similarly, various previous studies have reported that TB or butyric acid supplementation in feed can also increase the production of immunoglobulins (13) and IFN-γ (44). Furthermore, IL-10 and TGF-β are two important anti-inflammatory cytokines (16, 46), and TNF-α and IL-8 are two important pro-inflammatory cytokines (46). In the present study, 0.06% and 0.09% TB supplementation in feed significantly increased the levels of IL-10 and TGF-β and significantly decreased the levels of TNF-α. TB supplementation significantly decreases the levels of TNF-α in the plasma of rats after LPS administration (47). Studies have reported that TB supplementation can increase the levels of IL-10 in retroperitoneal adipose tissue (46) and colitis+TBT mice (16), and the content of TGF-β is also increased by TB supplementation in experimental colitis (16). These results are consistent with our results and indicate that TB supplementation can improve immunocompetence by regulating the production of inflammatory cytokines. Interestingly, in mice fed with tributyrin-supplemented diet, the levels of TNF-α have been found to be elevated principally in retroperitoneal and epididymal adipose tissue (46). These differing results may have occurred because of the different amounts of supplemented TB, the different durations of the experiments or the different study species.
TB supplementation attenuates the inflammatory response via inhibition of NF-kB activation (47, 48). In the present study, 0.03%-0.15% TB supplementation inhibited the relative expression of NF-κB, and the lowest expression was observed in fish fed the diet with 0.06% TB supplementation. Based on these results, TB supplementation might have a positive effect on the inflammatory response in this fish. Studies have demonstrated that NF-κB is a redox-responsive transcription factor that regulates the expression of downstream inflammatory cytokines, including pro-inflammatory cytokines and anti-inflammatory cytokines (49). In the present study, the 0.06% TB supplementation group showed the lowest expression of TNF-α and the highest expression of IL-10, while the 0.03% TB supplementation group showed the highest expression of TGF-β, which were positively correlated or negatively correlated with NF-κB expression. Various studies have reported that TB or butyrate supplementation can increase the mRNA levels of anti-inflammatory cytokines, including IL-10 in the distal intestines of juvenile yellow drum (21) and TGF-β in intestinal epithelial-like Caco-2 cells (50), and can reduce the mRNA levels of the pro-inflammatory cytokine TNF-α in piglets challenged with diquat (32), rats challenged with LPS (47) and juvenile yellow drum fed a SO-based diet with 0.20% TB (21). These results support the conclusion that TB supplementation attenuates the inflammatory response via inhibition of NF-kB activation to regulate pro-inflammatory cytokines and anti-inflammatory cytokines. The possible mechanism is that TB might activate PI3K/Akt, which further regulates the NF-κB signaling pathway (51–53). In the A. hydrophila challenge test, a lower mortality rate was observed in the groups of blunt snout bream fed diets with 0.06%-0.15% TB supplementation at 144 h than in the other groups, which also supports the results for the immune indexes and antioxidant indexes in this study. Interestingly, the mRNA and content of the pro-inflammatory cytokine IL-1β were increased by TB supplementation in this study, similar to the effects of TB supplementation in experimental colitis (16). This may be because cytokines play other roles in immune regulation. IL-1β might play major roles in the improvement of mucosal architecture, cell proliferation, and mucosal regeneration, rather than a role of pro-inflammatory cytokine (16). Furthermore, other studies have also reported that IL-1β might be involved in regulating several important physiological processes, including cell proliferation, differentiation and apoptosis (54, 55). In contrast to these results, Li et al. (34) reported that TB supplementation decreases the levels of pro-inflammatory cytokines such as IL-1β in the intestines of broilers challenged with LPS. Hence, the relevant mechanisms need to be further explored.
Protective Effects of TB Supplementation on the Liver and Intestine
In the present study, appropriate TB supplementation had positive protective effects on the liver and intestine, preventing lysis and necrosis of the intestine and inflammatory cell infiltration of the liver. TB supplementation or oral administration exerts important restorative effects in the liver or intestines, such as in growth‐retarded piglets (13), lipopolysaccharide-treated rats (47), snakehead (18), black sea bream (19) and yellow drum (20). These findings are in agreement with the results of the present study. The possible reason is that TB can activate the Nrf2 signaling pathway and inhibit the NF-κB signaling pathway, which further improves antioxidant capacity and attenuates the inflammatory response. Other possible reasons are that butyrate ions can regulate the intestinal mucosa and repair damage to the intestinal mucosa; TB can not only release sufficient butyrate ions at designated points in the intestine but also provide necessary energy to complete the repairs, and the liver is able to take up butyrate in patients with normal liver function (56).
Conclusion
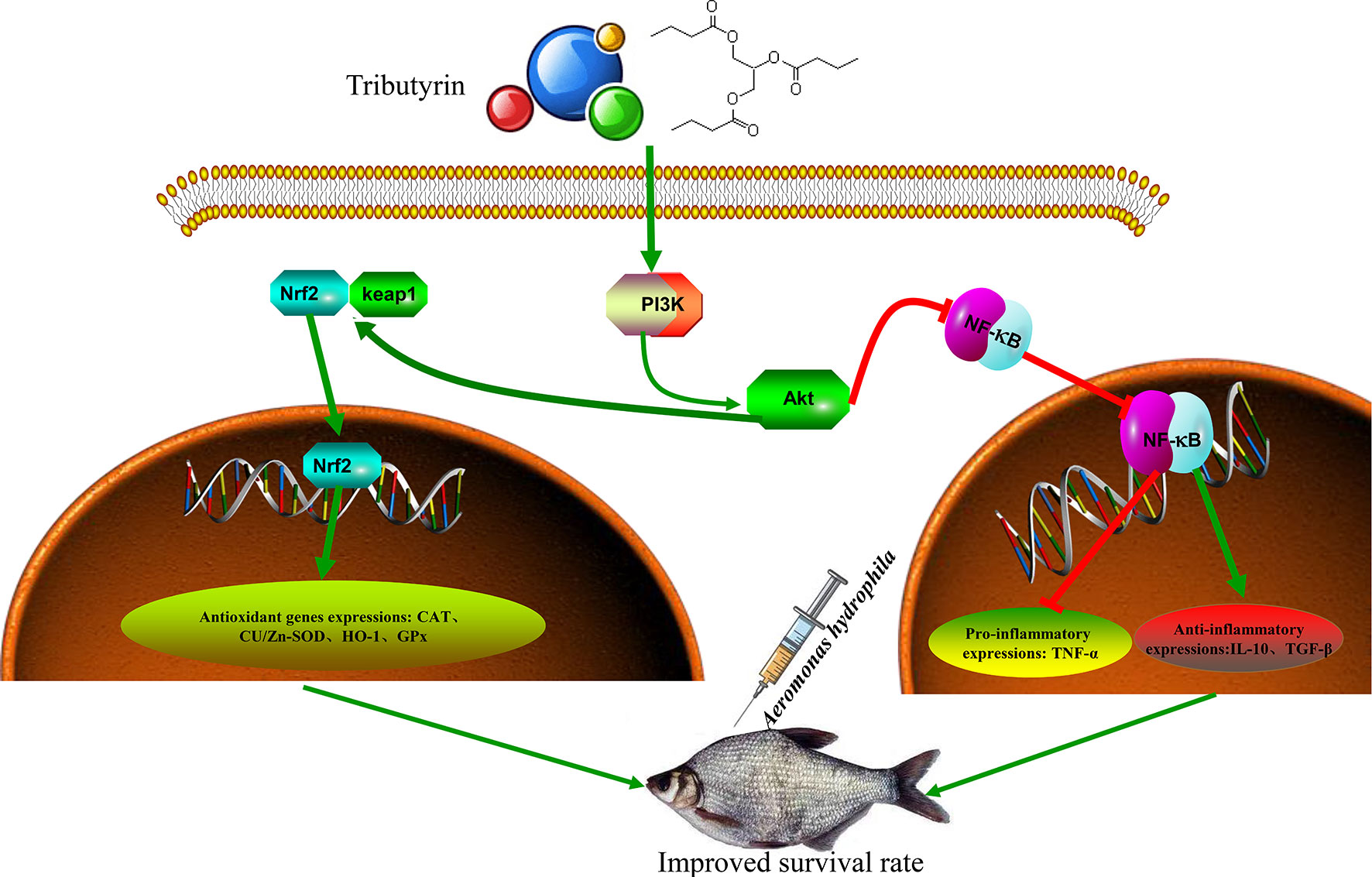
TB supplementation improves the growth performance of juvenile blunt snout bream. Furthermore, TB supplementation activates PI3K/Akt/Nrf2 and inhibits the NF-κB signaling pathway, regulating antioxidant capacity and the inflammatory response and preventing hepatic and intestinal damage (Figure 9). Based on the growth performance and immunocompetence, the recommended additive amount was 0.06% in the feed of juvenile blunt snout bream.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by The protocols used on the experimental fish followed the guidelines of the Institutional Animal Care and Ethics Committee of Nanjing Agricultural University, Nanjing, China. [Permit number: SYXK (Su) 2011-0036].
Author Contributions
MR and LZ designed the study. HL carried out the experiments and wrote the manuscript. KJ provided technical assistance. BX provided technical guidance. XC provided technical guidance. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by the National Key R&D Program of China (2019YFD0900200), the Natural Science Foundation of Jiangsu Province (BK20200169), National Natural Science Foundation of China, NSFC (31772820), the Modern Agriculture Industrial Technology System special project the National Technology System for Conventional Freshwater Fish Industries (CARS-45).
Conflict of Interest
LZ and XC were employed by company Tongwei Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Mahdavi R, Torki M. Study on usage period of dietary protected butyric acid on performance, carcass characteristics, serum metabolite levels and humoral immune response of broiler chickens. J Anim Vet Adv (2009) 8(9):1702–9. doi: 10.1016/j.fsi.2009.06.017
2. Guilloteau P, Zabielski R, David JC, Blum JW, Morisset JA, Biernat M, et al. Sodium-butyrate as a growth promoter in milk replacer formula for young calves. J Dairy Sci (2009) 92(3):1038–49. doi: 10.3168/jds.2008-1213
3. Malhi M, Gui HB, Yao L, Aschenbach JR, Gäbel G, Shen ZM. Increased papillae growth and enhanced short-chain fatty acid absorption in the rumen of goats are associated with transient increases in cyclin D1 expression after ruminal butyrate infusion. J Dairy Sci (2013) 96(12):7603–16. doi: 10.3168/jds.2013-6700
4. Smith DJ, Barri A, Herges G, Hahn J, Yersin AG, Jourdan A. In vitro dissolution and in vivo absorption of calcium [1-(14)C] butyrate in free or protected forms. J Agric Food Chem (2012) 60(12):3151–7. doi: 10.1021/jf3001058
5. Lacorn M, Goerke M, Claus R. Inulin-coated butyrate increases ileal MCT1 expression and affects mucosal morphology in the porcine ileum by reduced apoptosis. J Anim Physiol Anim Nutr (Berl) (2010) 94(5):670–6. doi: 10.1111/j.1439-0396.2009.00955.x
6. Clarke KO, Feinman R, Harrison LE. Tributyrin, an oral butyrate analogue, induces apoptosis through the activation of caspase-3. Cancer Lett (2001) 171(1):57–65. doi: 10.1016/s0304-3835(01)00574-2
7. Li Y, Maux SL, Xiao H, McClements DJ. Emulsion-based delivery systems for tributyrin, a potential colon cancer preventative agent. J Agric Food Chem (2009) 57(19):9243–9. doi: 10.1021/jf901836f
8. Lum J, Sygall R, Ros Felip JM. Comparison of tributyrin and coated sodium butyrate as sources of butyric acid for improvement of growth performance in Ross 308 broilers. Int J Poult Sci (2018) 17(6):290–4. doi: 10.3923/ijps.2018.290.294
9. Antongiovanni M, Buccioni A, Petacchi F, Leeson S, Minieri S, Martini A, et al. Butyric acid glycerides in the diet of broiler chickens: effects on gut histology and carcass composition. Ital J Anim Sci (2007) 6(1):19–25. doi: 10.4081/ijas.2007.19
10. Sotira S, Dell’Anno M, Caprarulo V, Hejna M, Pirrone F, Callegari ML, et al. Effects of tributyrin supplementation on growth performance, insulin, blood metabolites and gut microbiota in weaned piglets. Anim (Basel) (2020) 10(4):726. doi: 10.3390/ani10040726
11. Hou YQ, Liu YL, Hu J, Shen WH. Effects of lactitol and tributyrin on growth performance, small intestinal morphology and enzyme activity in weaned pigs. Asian-Australas J Anim Sci (2006) 19:1470–7. doi: 10.5713/ajas.2006.1470
12. Ren QC, Xuan JJ, Wang LK, Zhan QW, Yin DZ, Hu ZZ, et al. Effects of tributyrin supplementation on ruminal microbial protein yield, fermentation characteristics and nutrients degradability in adult Small Tail ewes. Anim Sci J (2018) 89:1271–9. doi: 10.1111/asj.13033
13. Li D, Xiang Z, He J, Zhang L, Bai K, Wen X, et al. Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin Nutr (2016) 35(2):399–407. doi: 10.1016/j.clnu.2015.03.002
14. Piva A, Prandini A, Fiorentini L, Morlacchini M, Galvano F, Luchansky JB. Tributyrin and lactitol synergistically enhanced the trophic status of the intestinal mucosa and reduced histamine levels in the gut of nursery pigs. J Anim Sci (2002) 80(3):670–80. doi: 10.2527/2002.803670x
15. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. The role of butyrate on colonic function. Aliment Pharmacol Ther (2010) 27(2):104–19. doi: 10.1111/j.1365-2036.2007.03562.x
16. Leonel AJ, Teixeira LG, Oliveira RP, Santiago AF, Batista NV, Ferreira TR, et al. Antioxidative and immunomodulatory effects of tributyrin supplementation on experimental colitis. Br J Nutr (2013) 109(8):1396–407. doi: 10.1017/S000711451200342X
17. Dwidar M, Park JY, Mitchell RJ, Sang B-I. The future of butyric acid in industry. Sci World J (2012) 2012:471417. doi: 10.1100/2012/471417
18. Hou YB, Hou Y, Yao L, Chen S, Fan JH, Qian LC. Effects of chromium yeast, tributyrin and bile acid on growth performance, digestion and metabolism of Channa argus. Aquac Res (2019) 50(3):836–46. doi: 10.1111/are.13954
19. Volatiana JA, Wang L, Gray N, Tong SG, Zhang GW, Shao QJ. Tributyrin-supplemented high-soya bean meal diets of juvenile black sea bream, Acanthopagrus schlegelii: Study on growth performance and intestinal morphology and structure. Aquac Res (2020) 51:135–46. doi: 10.1111/are.14355
20. Tan P, Wu X, Zhu WL, Lou B, Chen RL, Wang LG. Effect of tributyrin supplementation in high-soya bean meal diet on growth performance, body composition, intestine morphology and microbiota of juvenile yellow drum (Nibea albiflora). Aquac Res (2020) 51(5):2004–19. doi: 10.1111/are.14552
21. Zhu WL, Tan P, Lou B, Chen RY, Wang LG, Xu DD. Supplementation of a soybean oil-based diet with tributyrin influences growth, muscle composition, intestinal morphology, and expression of immune-related genes of juvenile yellow drum (Nibea albiflora richardson, 1846). Aquacult Int (2020) 28(5):2027–43. doi: 10.1007/s10499-020-00572-7
22. He LJ, Liao LK, Yuan JF, Tang HY, Wu Q, Zhang GW. Pathological observation of bacterial septicemia in Megalobrama amblycephala. J Southwest Agri Univ (2006) 28(3):483–90. doi: 10.3969/j.issn.1673-9868.2006.03.036
23. AOAC. Association of Official Analytical Chemists. Official methods of analysis of the association of official analytical chemists. 15th edn. USA: Association of Official Analytical Chemists Inc, Arlington (2003).
24. Liang HL, Ren MC, Habte-Tsion HM, Ge XP, Xie J, Mi HF, et al. Dietary arginine affects growth performance, plasma amino acids contents and gene expressions of TOR signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture (2016) 461:1–8. doi: 10.1016/j.aquaculture.2016.04.009
25. Ji K, Liang HL, Ren MC, Ge XP, Mi HF, Pan LK, et al. The immunoreaction and antioxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala) involves the PI3K/Akt/Nrf2 and NF-κB signal pathways in response to dietary methionine levels. Fish Shellfish Immunol (2020) 105:126–34. doi: 10.1016/j.fsi.2020.07.005
26. Habte-Tsion HM, Ge XP, Liu B, Xie J, Ren MC, Zhou QL, et al. A deficiency or an excess of dietary threonine level affects weight gain, enzyme activity, immune response and immune-related gene expression in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol (2015) 42:439–46. doi: 10.1016/j.fsi.2014.11.021
27. Liang HL, Mokrania A, Ji K, Ge XP, Ren MC, Pan LK, et al. Effects of dietary arginine on intestinal antioxidant status and immunity involved in Nrf2 and NF-κB signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Fish Shellfish Immunol (2018) 82:243–9. doi: 10.1016/j.fsi.2018.08.026
28. Pfaffl MW. A new mathematical model for relative quantification in real-time RTPCR. Nucleic Acids Res (2001) 29:2002–7. doi: 10.1093/nar/29.9.e45
29. Li MY, Liang HL, Xie J, Chao W, Zou FQ, Ge XP, et al. Diet supplemented with a novel Clostridium autoethanogenum protein have a positive effect on the growth performance, antioxidant status and immunity in juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Rep (2021) 19:100572. doi: 10.1016/j.aqrep.2020.100572
30. Liang HL, Ji K, Ge XP, Ren MC, Liu B, Xi BW, et al. Effects of dietary arginine on antioxidant status and immunity involved in AMPK-NO signaling pathway in juvenile blunt snout bream. Fish Shellfish Immunol (2018) 78:69–78. doi: 10.1016/j.fsi.2018.04.028
31. Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: Induction of cyclin D3 and p21 expression. Gut (2000) 46(4):507–14. doi: 10.1136/gut.46.4.507
32. Wang C, Cao S, Zhang Q, Shen Z, Feng J, Hong Q, et al. Dietary tributyrin attenuates intestinal inflammation, enhances mitochondrial function, and induces mitophagy in piglets challenged with diquat. J Agric Food Chem (2019) 67(5):1409–17. doi: 10.1021/acs.jafc.8b06208
33. Jiang Y, Zhang WH, Gao F, Zhou GH. Micro-encapsulated sodium butyrate attenuates oxidative stress induced by corticosterone exposure and modulates apoptosis in intestinal mucosa of broiler chickens. Anim Prod Sci (2015) 55:587–94. doi: 10.1071/AN13348
34. Li J, Hou Y, Yi D, Zhang J, Wang L, Qiu H, et al. Effects of tributyrin on intestinal energy status, antioxidative capacity and immune response to Lipopolysaccharide challenge in broilers. Asian-Australas J Anim Sci (2015) 28(12):1784–93. doi: 10.5713/ajas.15.0286
35. Sasaki T, Takasuga S, Sasaki J, Kofuji S, Eguchi S, Yamazaki M, et al. Mammalian phosphoinositide kinases and phosphatases. Prog Lipid Res (2009) 48(6):307–43. doi: 10.1016/j.plipres.2009.06.001
36. Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA (2002) 99(18):11908–13. doi: 10.1073/pnas.172398899
37. Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol (2013) 53(1):401–26. doi: 10.1146/annurev-pharmtox-011112-140320
38. Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol Cell Biol (2012) 32(17):3486–99. doi: 10.1128/MCB.00180-12
39. Vivarini ADC, Cristina CST, Mattos SA, Sampaio BV, Jaqueline FC, Ricardo K, et al. Systems approach reveals nuclear factor erythroid 2-related factor 2/protein kinase r crosstalk in human cutaneous leishmaniasis. Front Immunol (2017) 8:1127. doi: 10.3389/fimmu.2017.01127
40. Pan WJ, Miao LH, Lin Y, Huang X, Ge XP, Moosa SL, et al. Regulation mechanism of oxidative stress induced by high glucose through PI3K/Akt/Nrf2 pathway in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol (2017) 70:66–75. doi: 10.1016/j.fsi.2017.09.005
41. Chen J, Shao C, Lu W, Yan C, Yao Q, Zhu M, et al. Adenosine Triphosphate-Induced Rabbit Corneal Endothelial Cell Proliferation in vitro via the P2Y2-PI3K/Akt Signaling Axis. Cells Tissues Organs (2014) 199(2-3):131–9. doi: 10.1159/000365654
42. Liang HL, Mokrani A, Ji K, Ge XP, Ren MC, Xie J, et al. Dietary leucine modulates growth performance, Nrf2 antioxidant signaling pathway and immune response of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol (2018) 73:57–65. doi: 10.1016/j.fsi.2017.11.048
43. Thom V, Arumugam TV, Tim M, Gelderblom M. Therapeutic potential of intravenous immunoglobulin in acute brain injury. Front Immunol (2017) 8:875. doi: 10.3389/fimmu.2017.00875
44. Sekiguchi K, Ogawa E, Kurohane K, Konishi H, Mochizuki N, Manabe K, et al. Adjuvant effect of short chain triacylglycerol tributyrin on a mouse contact hypersensitivity model. Toxicol Lett (2018) 284:56–62. doi: 10.1016/j.toxlet.2017.11.036
45. Merle NS, Elizabeth CS, Veronique FB, Roumenina LT. Complement system part I-molecular mechanisms of activation and regulation. Front Immunol (2015) 6:262. doi: 10.3389/fimmu.2015.00262
46. Biondo LA, Teixeira AAS, Silveira LS, Souza CO, Costa RGF, Diniz TA, et al. Tributyrin in inflammation: does white adipose tissue affect colorectal cancer? Nutrients (2019) 11:110. doi: 10.3390/nu11010110
47. Miyoshi M, Sakaki H, Usami M, Iizuka N, Shuno K, Aoyama M, et al. Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin Nutr (2011) 30(2):252–8. doi: 10.1016/j.clnu.2010.09.012
48. Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, et al. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res (2008) 28:321–8. doi: 10.1016/j.nutres.2008.02.012.19083427
49. Zduńczyk Z, Jankowski J, Kubińska M, Ognik K, Czech A, Juśkiewicz J. The effect of different dietary levels of dl-methionine and dl-methionine hydroxy analogue on the antioxidant and immune status of young turkeys. Arch Anim Nutr (2017) 71(5):347–61. doi: 10.1080/1745039X.2017.1352328
50. Saegusa S, Totsuka M, Kaminogawa S, Hosoi T. Cytokine responses of intestinal epithelial-like CaCO-2 cells to non-pathogenic and opportunistic pathogeni yeasts in the presence of butyric acid. Biosci Biotechnol Biochem (2007) 71:2428–34. doi: 10.1271/bbb.70172
51. Wang S, Tian SD, Li MZ, Li ZC. Methionine attenuates the intensity of rheumatoid arthritis by downregulating NF-κB and iNOS expression in neonatal rats. 3 Biotech (2018) 8(7):303–9. doi: 10.1007/s13205-018-1311-2
52. Li XD, Chen F, Zhu QH, Ding BJ, Zhong QX, Huang KK, et al. Gli-1/PI3K/AKT/NF-κB pathway mediates resistance to radiation and is a target for reversion of responses in refractory acute myeloid leukemia cells. Oncotarget (2016) 7(22):33004–15. doi: 10.18632/oncotarget.8844
53. Sharif O, Brunner JS, Vogel A, Schabbauer G. Macrophage rewiring by nutrient associated PI3K dependent pathways. Front Immunol (2019) 10:2002. doi: 10.3389/fimmu.2019.02002
54. Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol (2007) 178:4641–9. doi: 10.4049/jimmunol.178.7.4641
55. Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease-enhanced production during active disease. Gut (1990) 31:686–9. doi: 10.1136/gut.31.6.686
Keywords: juvenile blunt snout bream, tributyrin, growth, antioxidant capacity, immune responses
Citation: Liang H, Ji K, Ge X, Xi B, Ren M, Zhang L and Chen X (2021) Tributyrin Plays an Important Role in Regulating the Growth and Health Status of Juvenile Blunt Snout Bream (Megalobrama amblycephala), as Evidenced by Pathological Examination. Front. Immunol. 12:652294. doi: 10.3389/fimmu.2021.652294
Received: 12 January 2021; Accepted: 22 March 2021;
Published: 12 April 2021.
Edited by:
Jun Xie, Chinese Academy of Fishery Sciences, ChinaCopyright © 2021 Liang, Ji, Ge, Xi, Ren, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingchun Ren, cmVubWNAZmZyYy5jbg==; Lu Zhang, emhhbmdsMjFAdG9uZ3dlaS5jb20=
 Hualiang Liang
Hualiang Liang Ke Ji2
Ke Ji2 Mingchun Ren
Mingchun Ren