- 1Laboratorio di Patologia Clinica, IRCCS S.D.N., Napoli, Italy
- 2Laboratorio di Patologia Clinica, Ospedale San Antonio, Tolmezzo—Azienda Sanitaria Universitaria Integrata di Udine, Udine, Italy
- 3 Laboratorio Diagnostico di Autoimmunologia, IRCCS Ospedale Policlinico San Martino, Genova, Italy
- 4Dipartimento di Medicina Interna e Specialità Mediche (DIMI), Università Degli Studi di Genova, Genova, Italy
The recent availability of automated computer-assisted diagnosis (CAD) systems for the reading and interpretation of the anti-nuclear antibody (ANA) test performed with the indirect immunofluorescence (IIF) method on HEp-2 cells, has improved the reproducibility of the results and initiated a process of harmonization of this test. Furthermore, CAD systems provide quantitative expression of fluorescence intensity, allowing the introduction of objective quality control procedures to the monitoring of the entire process. The calibration of the reading systems and the automated image interpretation are essential prerequisites for obtaining reproducible and harmonized IIF test results and form the basis for standardization, regardless of the computer algorithms used in the different systems. The use of automated CAD systems, facilitating control procedures, represents a step forward for the quality certification of the laboratory.
Introduction
The indirect immunofluorescence (IIF) assay on HEp-2 cells is considered the reference method for the screening of anti-nuclear antibodies (ANA) and plays a central role in the diagnosis of autoimmune rheumatic diseases. Its high diagnostic sensitivity allows the detection of over 30 different fluorescence patterns, corresponding to as many autoantibody specificities (1–4). However, the HEp-2 IIF method is currently limited by a low level of harmonization. Major drawbacks are high intra and inter-laboratory variability, semiquantitative expression of results and lack of specificity. The method is also time consuming and has a long turn-around-time (5–8). It was also pointed out that the high variability of the method jeopardizes the selection of patients to be included in clinical trials for the evaluation of therapeutic protocols (9). The main critical issues related to the search of ANA by HEp-2 IIF are shown in Table 1.
Probably the most important cause of variability in the detection of HEp-2 IIF ANA is represented by the subjectivity in titer and pattern interpretation, even when the reading is performed by expert personnel (10, 11). In this regard, external quality assessment (EQA) schemes have highlighted a significant discrepancy of the results, especially for samples with a cytoplasmic pattern and in the assessment of the antibody titer which, in some cases, may differ by more than two dilutions (12–14).
Other causes of variability are inherent in the reagents used. Differences in the HEp-2 substrate supplied by the various manufacturers mainly related to the growth time of cell cultures and the methods of cell fixation, are an important source of discrepancy (15, 16). The different substrates of HEp-2 cells available on the market significantly determine the non-uniform accuracy of the various diagnostic kits, not only in terms of overall sensitivity but also as regards the ability to detect autoantibodies directed against some antigenic specificities (17).
Another critical issue is the choice of the initial dilution of the screening test, which is directly linked to the diagnostic specificity of the method. There is now sufficient agreement that the threshold cutoff for ANA should no longer be fixed at 1:40. Accumulated evidence has made clear that the best compromise between sensitivity and specificity of the ANA test be at least 1:80. Furthermore, the choice of 1:80 as the best screening dilution is consistent with the results obtained by Tan et al. (18) on more than 22,000 healthy individuals, showing that this titer corresponds to the 95%ile of healthy controls, as recommended by the EASI group (4) and various national guidelines (19–21). The new classification criteria for systemic lupus erythematosus also recommend a screening dilution of 1:80 (22).
ANA HEp-2 IIF Detected by Automated Computer-Assisted Systems
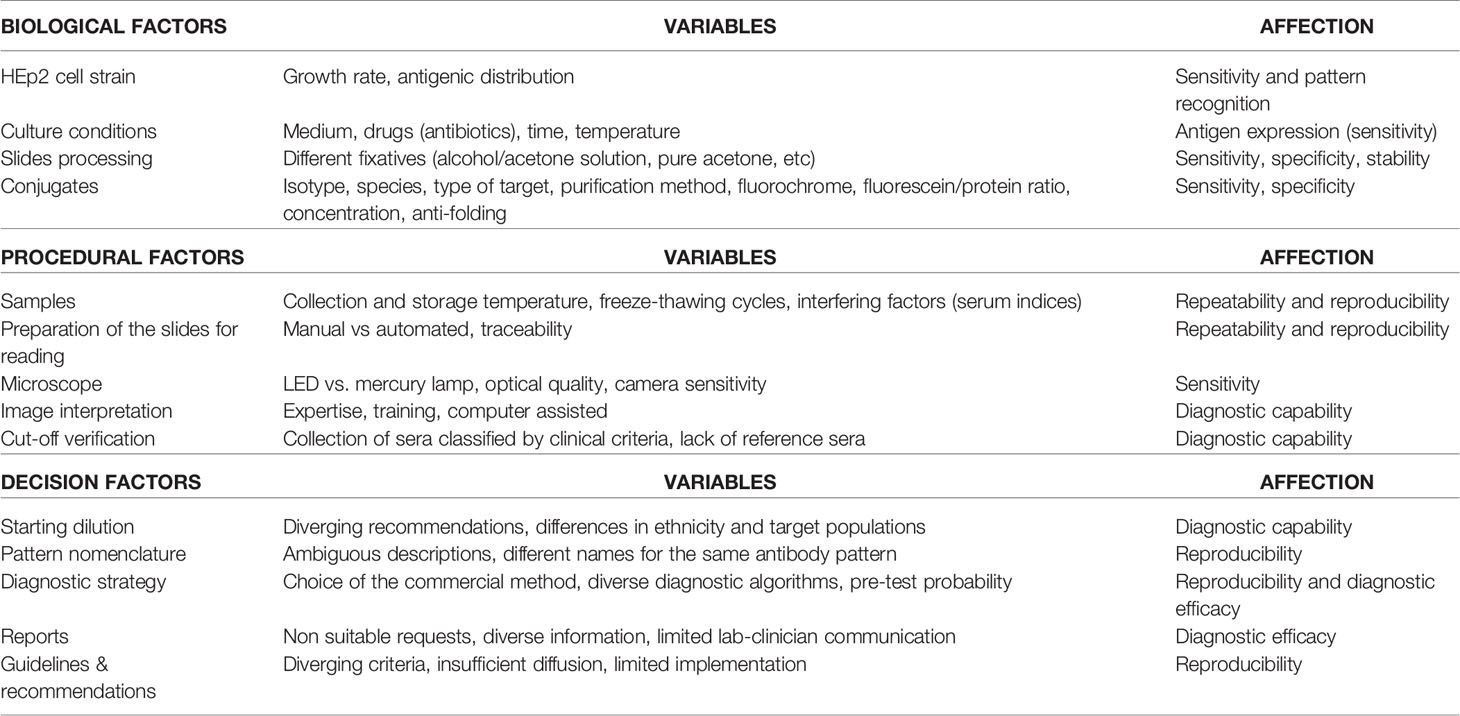
In an attempt to overcome some of the disadvantages of manual HEp-2 IIF tests, the biomedical industry, in addition to the development of fully automatic slide processors to standardize the pre-analytical phase, has developed computer-aided diagnosis (CAD) technologies to digitalize ANA HEp-2 IIF analysis (23–28). These systems arise from the combination of various hardware modules which, using software based on complex mathematical schemes and algorithms, are able to acquire, analyze and store the images in a fully automated way (29, 30) (Figure 1).
Figure 1 Complete processing cycle of automated HEp-2 cells assay reading by Aklides system (reproduced from Hiemann R, et al. Challenges of automated screening and differentiation of non-organ specific autoantibodies on HEp-2 cells. Autoimmunity Rev 2009; 9:17-22) (29).
One of the most important advantages of CAD systems is that they offer a more standardized, automated quantitative reading of the fluorescence signal, translated into system specific fluorescence intensity (FI) measures. In a meta-analysis that compared the diagnostic accuracy of CAD systems with that of manual methods for HEp-2 IIF, CAD systems showed overall greater agreement in the estimation of results and less variability in the definition of antibody levels compared to manual methods. Furthermore, in the screening of systemic autoimmune diseases, automated methods have proved more sensitive than manual ones (31).
Through the digitization of the images, CAD systems aim not only to determine the reduction of the variability of the HEp-2 IIF tests, minimizing the subjectivity of the interpretation of the fluorescence patterns (31–34), but also to increase the productivity of the laboratory, eliminate the use of the darkroom, allow the archiving of images for future check, ensure sample traceability through the barcode, and electronic data transmission (35).
However, despite the obvious improvements in the harmonization of results, given that these new computerized systems use HEp-2 cells, they still suffer from some of the inherent problems of the manual HEp-2 IIF method. Furthermore, like all analytical systems produced by various manufacturers, CAD systems differ in DNA counterstaining (DAPI, propidium iodide, none), substrate composition, run time, number of microscopic fields processed, type of recognized HEp-2 IIF patterns and the interpretative software of the acquired images (24, 27).
The nature of the light sources and the specifications of the microscope optics may also be a cause of inconsistency (36–38). Differences in the technical specifications of the light emitting devices, filters and lenses, can lead to a high variability in the intensity of the excitation light used in CAD systems. In these automated systems the drop in intensity of the LED lamp, the degradation of the camera sensor, the whitening of the fluorescent filter, the misalignment of the light path, may have an impact on the intensity of the emitted fluorescence (39, 40).
In a study involving 31 Belgian laboratories using different automated CAD systems, reproducibility of results and sufficient accuracy in estimating dilution was observed in a limited number of laboratories, while the overall results indicated that significant variability persisted in the detection of ANA. It should be noted that not only variability was found between the results of automated HEp-2 IIF assays from different manufacturers but also between those obtained from instruments of the same manufacturer (41).
Finally, as regards the interpretation of the pattern, it cannot be overlooked that automated CAD systems are currently able to recognize only some fluorescence patterns, mainly the homogeneous, speckled, nucleolar, centromeric, nuclear dots and cytoplasmic. Hence, visual reading by the operator at the monitor is still considered essential in order to assign the pattern and for subsequent reporting. To perform the diagnosis by looking at digital images on a workstation monitor allows the specialists to better concentrate on sample examination, e.g. to observe carefully fine details without take care of photobleaching effects. The observers were initially not accustomed to diagnose the sample using the workstation monitor, while they were well skilled in carrying out the diagnosis at the microscope. Therefore, the results on digital image classification could potentially remarkably improve as the expertise with this kind of diagnostic procedure increases and even the less frequent patterns, not recognized today by CAD systems, can be identified more accurately by the specialist.
Standardization/Harmonization of Automated ANA HEp-2 IIF Assays
The standardization of autoantibody tests is generally considered to be among the most challenging in the context of in vitro diagnostics (42). The main reason is that measurands, i.e. antibodies, are made up of a highly variable mixture of different molecules in terms of epitope recognition, degree and type of glycosylation, isotypes and subclass distribution, and degree of avidity (43, 44).
Standardization can be defined as the process of implementing a standard preparation capable of maximizing the compatibility, even quantitative, of test results and possibly achieving their uniformity. Harmonization, on the other hand, can be defined as mediation between different measurements obtained with different methods and procedures to make them mutually compatible. Harmonization is generally reached by agreement between the parties concerned and is formalized in recommendations and/or guidelines (45, 46).
Therefore, if standardization in autoimmunology is a very difficult goal to achieve and will likely take a long time, the use of automated CAD systems is expected to improve right away the harmonization of the reading of HEp-2 IIF. In particular, two important benefits are expected: greater agreement in discriminating between positive and negative ANA samples, and lower imprecision in the definition of antibody titer/concentration. Currently available data show that the concordance between conventional HEp-2 IIF interpretation and automated systems in correctly expressing positive and negative results varies between 92% and 99% (24, 25, 30, 47, 48). In samples with ANA tests that are clearly negative or highly positive, CAD systems achieve a degree of accuracy close to 100% (49). The greater reproducibility of the results provided by the new automatic methods was demonstrated in a study that compared the analytical imprecision of six CAD systems vs. the manual HEp-2 IIF method. The mean coefficient of variation (CV) was 12% for the CAD vs. 39% for manual IIF (24).
A further contribution to the harmonization of the process concerns the choice of the cutoff titer, which is fundamental for a correct classification of the samples as positive or negative. While it would be recommended for each laboratory to determine its own screening dilution for the local population to distinguish healthy and diseased states, in practice, this procedure is not followed by the vast majority of laboratories because there is a high consensus in the literature that the titer of 1:80 can be considered the best compromise between diagnostic sensitivity and specificity (21, 36, 50–52). Furthermore, since the titer 1:80 is the screening dilution adopted by all manufacturers of CAD systems for automated reading and interpretation of ANA (24), this methodological approach represents a first and concrete step to achieve the harmonization of ANA HEp-2 IIF results. Indeed, if different laboratories should adopt different cutoffs, this would diminish comparability of results and therefore decrease harmonization.
However, given that the fluorescence signal is strongly dependent on the antibody pattern because of the variable concentration and cell distribution of the self-antigens, different staining patterns are characterized by a different FI mean for the same end-point titer. This issue has been faced by manufacturers of CAD systems developing built-in calibration curves for each one of the most common ANA patterns. To prove this relationship, Carbone et al. calculated R2 on a single fitted lines plot obtained by plotting FI as a function of dilution factor for whole serum series and for 10 different antibody patterns. Regression analysis showed a close relationship between FI and titer dilution for each pattern (53).
Since an accurate extrapolation of antibody titer based on fluorescence intensity is not possible with only a single screening dilution and this method cannot be applied to mixed ANA patterns, Won (54) proposed to use the line slope titration (LST) method using at least two distant point dilutions (i.e., 1:80 and 1:320) which would enable a better prediction of end-point titers based on the measured FI and evaluate possible prozone effects avoiding serial dilutions. To this end, an interfacing middleware to calculate the endpoint titer using LST should be implemented between automated CAD software and the laboratory information system (54).
While the advent of CAD systems has already contributed to improving ANA HEp-2 IIF assay, for a wider harmonization of the test, other aspects must be considered. Uniform terminology is also needed in the description of the HEp-2 IIF patterns. In a context characterized by the absence of a universally accepted nomenclature and by a substantial subjectivity in the interpretation of fluorescence patterns, the International Consensus on ANA Patterns (ICAP) had the merit of laying the foundations for the harmonization of the terminology, of providing guidelines for the interpretation of test results and to indicate the reporting format (55–57). ICAP has also defined the clinical relevance of the distinct HEp-2 IIF patterns, also indicating the appropriate use of in-depth tests, and has promoted the translation of the information content into multiple languages, to facilitate the unambiguous diffusion of the classification system in different countries of the world (58).
Reporting the ANA test result as positive or negative in the presence of cytoplasmic and mitotic patterns (CMP) is still a controversial topic (22, 59). However, although there is still no general consensus, given that CMP are observable in the HEp-2 IIF assay along with the nuclear patterns, some guidelines have recommended that CMP should be included in the ANA positive definition (4, 60–62).
Quality Assessement
In addition to automated procedures for the validation of the analytical process, the HEp-2 IIF CAD systems, due to their ability to report FI quantitative results, allow the introduction of quality control (QC) procedures using objective acceptance criteria for each analytical session (7). Quality assurance can be based on daily monitoring of the measured FI values for positive and negative QC samples, evaluated with the traditional Westgard rules, 12CV as the alarm limit and 13CV as the limit to reject the series (63, 64). In this regard, however, it has been pointed out that the use of only internal quality control (iQC) materials provided by the manufacturers of the diagnostic kits cannot highlight all possible analytical errors (65) because iQC samples in the diagnostic kit are usually ready-to-use and do not require pre-dilution like routine patient samples. In addition, according to van der Bremt et al, the effect of some apparently trivial variables (i.e., the efficiency of the conjugate) is not evident using iQC samples associated with the highest FI values but only with those with FI values around the positivity limit (33). For a more adequate quality assurance, the introduction of additional quality indicators has been proposed, such as the evaluation of the median of the results of the FI of iQC samples obtained from pooled patient sera, and the monitoring of the percentage of ANA IIF positive results in the analytic session (65, 66).
Subsequently, a wider participation in EQA programs will be required to monitor the performance of each CAD system in order to comprehensively address the harmonization of the HEp-2 IIF test (33). In this context, it is important that EQA programs are dedicated to CAD assays or at least evaluated separately from manual methods (Figure 2).
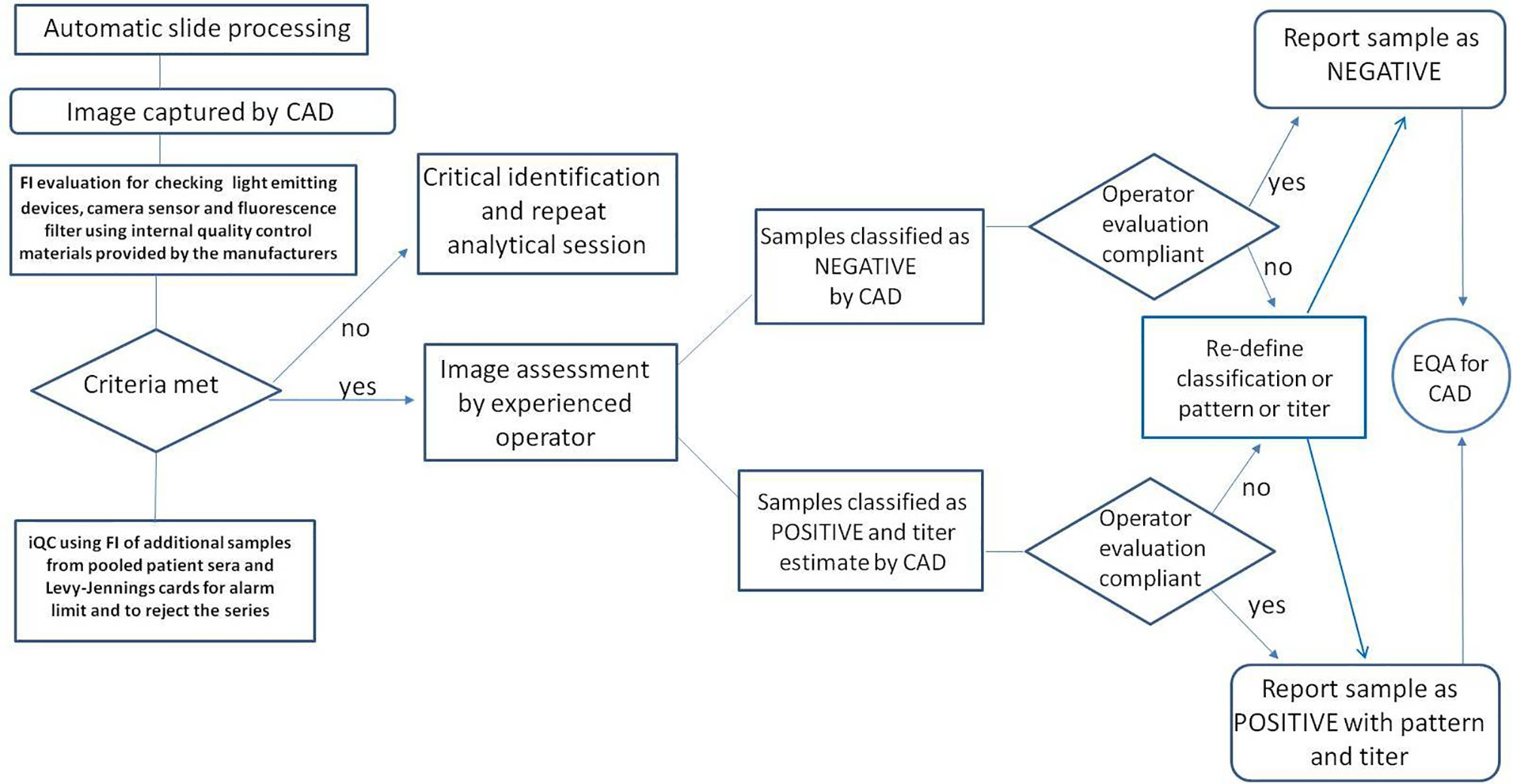
Figure 2 Steps related to quality control and interpretation of the results using the automated CAD procedure for the determination of ANA in indirect immunofluorescence.
Furthermore, integrating FI based iQC charts into the routine ANA IIF workflow offers a solution to current shortcomings of autoimmune laboratory testing in achieving ISO 15189 accreditation and could bring this branch of autoimmunity closer to other immunometric assays and their well-established rules (64, 65, 67–69). To this end, it is the responsibility of the laboratory autoimmunologist to evaluate and control all the variables that have a potential impact on the total processing of the HEp-2 IIF test (70, 71). In this context, neither pre-analytical variables such as the type and degree of suspected pathology underlying test request, nor analytical (errors in the washing or dispensing of reagents), or post-analytical ones (expression of results and introduction of interpretative notes in the report through the laboratory information system) should be neglected.
Discussion and Future Perspectives
In recent years, technological evolution has allowed the development of solid phase assays (SPA) for the research of ANA, which have proved to be slightly less sensitive but more specific than the HEp-2 IIF method (either manual or automated). In turn, this has led many researchers to propose the association of a SPA method with HEp-2 IIF as the best strategy to increase the diagnostic efficiency of ANA research (72–77). Whatever the choice, whether performed alone or in combination with SPA methods, the HEp-2 IIF method will continue to play a central role in the diagnosis of autoimmune rheumatic diseases. For this reason, efforts to further improve the performance of the HEp-2 IIF method and the test standardization and harmonization process should not be abandoned or slowed down.
The development of more characterized standards and reference materials is the first step towards the standardization of autoantibody tests. Such reference materials should ideally be homogeneous, stable, traceable, switchable, safe, ethically obtained, available and, ideally, certified. A promising and concrete initiative underway by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Committee on Harmonisation of Autoantibody Testing aims at the preparation of serum pools with monospecific samples obtained from an adequate number of donors (78). Numerous variants of the same antibody will be included in the pool to minimize batch-to-batch differences. However, the complexity and variability of antigens, antibodies and analytical methods makes it unlikely that the introduction of antibody standards alone will completely solve all standardization problems. It is more likely that it will represent the beginning of the standardization process of the entire supply chain including not only the antibody but also the antigenic substrate and the analytical method.
It is necessary that the biomedical industry produces a further effort aimed both at expanding the spectrum of patterns that can be identified (for example the dense fine speckled) consistently with those classified by ICAP, and at the recognition of mixed patterns (35, 79). The implementation of the ICAP nomenclature, despite being already widespread, is believed to be only a first step towards the common goal of harmonizing the interpretation of HEp-2 IIF tests. According to a recent survey by Lisa Peterson et al. for US respondents, there is a need for further guidelines, consent documents, control/reference materials to promote the formation of the skills necessary to uniquely report the rarest and complex fluorescence patterns (80).
The electronic setting of each CAD system should be optimized in each operational reality, providing for the possibility of modifying the IF threshold value established by the manufacturer to classify the test as positive or negative, based on the state of efficiency of the individual components of the analytical instrumentation, so that the IF threshold value always corresponds to the titer of 1:80 chosen as the discriminant cutoff.
Finally, assigning the likelihood ratio (LR) value or post-test probability of disease to the HEp-2 IIF test result represents a new reporting approach in the field of ANA testing that can facilitate the clinical interpretation of test results and, by improving the comparability of the results from different analytical methods, contribute to harmonizing autoimmune laboratory reporting (81). The CAD systems, expressing the ANA test results quantitatively as FI values make the calculation of the LR easier, especially if the relationship between pre and post-test probability is represented graphically as a function of LR (62, 82).
Conclusions
The standardization/harmonization of ANA tests is far from complete. A closer collaboration is necessary between autoimmunologists and the biomedical industry for the adjustment of diagnostic kits. The standardization process will be greatly accelerated when international standards and independent and certified calibrators are available and disseminated. The objectives are therefore to produce commutable materials that could be used as interim calibration material for autoantibody assays; to evaluate the impact of new reference material on the variability of autoantibody tests; and to identify areas where further harmonization would improve diagnostic accuracy. In this scenario, the international harmonization of diagnostic kits for HEp-2 IIF tests and the correct management of automated CAD systems for reading fluorescence preparations are the key points for the standardization of ANA research in immunofluorescence using HEp-2 cells.
Author Contributions
LC, NB, and GP drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Tozzoli R, Bizzaro N, Tonutti E, Villalta D, Bassetti D, Manoni F, et al. Guidelines for the laboratory use of autoantibody tests in the diagnosis and monitoring of autoimmune rheumatic diseases. Am J Clin Pathol (2002) 117:316–24. doi: 10.1309/Y5VF-C3DM-L8XV-U053
2. Solomon DH, Kavanaugh AJ, Schur PH, American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum (2002) 47:434–44. doi: 10.1002/art.10561
3. American College of Rheumatology. Current practice issues: ACR tracking concerns about ANA testing results. Atlanta, GA: American College of Rheumatology (2009).
4. Agmon-Levin N, Damoiseaux J, Kallenberg C, Sack U, Witte T, Herold M, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as antinuclear antibodies. Ann Rheum Dis (2014) 73:17–23. doi: 10.1136/annrheumdis-2014-205324
5. Copple SS, Giles SR, Jaskowski TD, Gardiner AE, Wilson AM, Hill HR. Screening for IgG antinuclear autoantibodies by HEp-2 indirect fluorescent antibody assays and the need for standardization. Am J Clin Pathol (2012) 137:825–30. doi: 10.1309/AJCPICNFG7UCES1S
6. Choi M, Fritzler MJ. Challenges and advances in SLE autoantibody detection and interpretation. Curr Treat Options Rheum (2019) 5:147–67. doi: 10.1007/s40674-019-00122-0
7. Jacobs JFM, Bossuyt X. Standardization and harmonization of autoimmune diagnostics. Clin Chem Lab Med (2018) 56:1563–7. doi: 10.1515/cclm-2018-0807
8. van Hoovels L, Bossuyt X. Harmonisation of laboratory tests for rheumatic diseases: still a long way to go. Ann Rheum Dis (2020) 79:e5. doi: 10.1136/annrheumdis-2018-214696
9. Pisetsky DS, Spencer DM, Lipsky PE, Rovin BH. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann Rheum Dis (2018) 77:911–3. annrheumdis-2017-212599. doi: 10.1136/annrheumdis-2017-212599
10. Mahler M, Meroni PL, Bossuyt X, Fritzler MJ. Current concepts and future directions for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. J Immunol Res (2014) 2014:1–18. doi: 10.1155/2014/315179
11. Rigon A, Infantino M, Merone M, Iannello G, Tincani A, Cavazzana I, et al. The inter-observer reading variability in antinuclear antibodies indirect (ANA) immunofluorescence test: a multicenter evaluation and a review of the literature. Autoimmun Rev (2017) 16:1224–9. doi: 10.1016/j.autrev.2017.10.006
12. Bizzaro N, Tozzoli R, Tonutti E, Piazza A, Manoni F, Ghirardello A, et al. Variability between methods to determine ANA, anti-dsDNA and anti-ENA autoantibodies: a collaborative study with the biomedical industry. J Immunol Methods (1998) 219:99–107. doi: 10.1016/S0022-1759(98)00140-9
13. Pham BN, Albarede S, Guyard A, Burg E, Maisonneuve P. Impact of external quality assessment on antinuclear antibody detection performance. Lupus (2005) 14:113–9. doi: 10.1191/0961203305lu2069oa
14. van Blerk M, Van Campenhout C, Bossuyt X, Duchateau J, Humbel R, Servais G, et al. Current practices in antinuclear antibody testing: results from the Belgian External Quality Assessment Scheme. Clin Chem Lab Med (2009) 47:102–8. doi: 10.1515/CCLM.2009.021
15. Monce’ NM Jr, Cappel V, Saqueton CB. A comparison of two fixatives on IFA HEp-2 slides for the detection of antinuclear antibodies. J Immunoassay (2006) 15:55–68. doi: 10.1080/15321819408009571
16. Hahm D, Anderer U. Establishment of HEp-2 cell preparation for automated analysis of ANA fluorescence pattern. Cytometry A (2006) 69:178–81. doi: 10.1002/cyto.a.20223
17. Mahler M, Ngo JT, Schulte-Pelkum J, Luettich T, Fritzler MJ. Limited reliability of the indirect immunofluorescence technique for the detection of anti-Rib-P antibodies. Arthritis Res Ther (2008) 10:R131. doi: 10.1186/ar2548
18. Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum (1997) 40:1601–11. doi: 10.1002/art.1780400909
19. Dellavance A, Junior AG, Cintra AFU, Ximenes AC, Nuccitelli B, Taliberti BH, et al. Brazilian consensus on antinuclear antibodies in HEp-2 cells. Definitions for standardization of autoantibody testing against the nucleus (ANA HEP-2), nucleolus, cytoplasm and mitotic apparatus, as well as its clinical associations. Rev Bras Reumatol (2003) 43:129–40. doi: 10.1590/S0482-50042003000300002
20. Cinquanta L, Bizzaro N, Villalta D, Morozzi G, Tonutti E, Bagnasco M, et al. Guidelines for the laboratory use of autoantibody tests in the diagnosis and monitoring of autoimmune rheumatic diseases. Revision 2015. Riv Ital Med Lab (2015) 11:205–24. [Italian]. doi: 10.1007/s13631-015-0099-x
21. Cruvinel WM, Andrade LEC, von Mühlen CA, Dellavance A, Ximenes AC, Bichara CD, et al. V Brazilian consensus guidelines for detection of anti-cell autoantibodies on HEp-2 cells. Adv Rheumatol (2019) 59:28. doi: 10.1186/s42358-019-0069-5
22. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis (2019) 78:1151–9. doi: 10.1136/annrheumdis-2018-214819
23. Tozzoli R, Antico A, Porcelli B, Bassetti D. Automation in indirect immunofluorescence testing: a new step in the evolution of the autoimmunology laboratory. Autoimmun Highlights (2012) 3:59–65. doi: 10.1007/s13317-012-0035-2
24. Bizzaro N, Antico A, Platzgummer S, Tonutti E, Bassetti D, Pesente F, et al. Automated antinuclear immunofluorescence antibody screening: a comparative study of six computer-aided diagnostic systems. Autoimmun Rev (2014) 13:292–8. doi: 10.1016/j.autrev.2013.10.015
25. van Beers JJBC, Hahn M, Fraune J, Mallet K, Krause C, Hormann W, et al. Performance analysis of automated evaluation of antinuclear antibody indirect immunofluorescent tests in a routine setting. Auto Immun Highlights (2018) 9:8. doi: 10.1007/s13317-018-0108-y
26. Copple SS, Jaskowski TD, Giles R, Hill HR. Interpretation of ANA indirect immunofluorescence test outside the darkroom using NOVA view compared to manual microscopy. J Immunol Res (2014) 2014:149316. doi: 10.1155/2014/149316
27. Krause C, Ens K, Fechner K, Voigt J, Fraune J, Rohwäder E, et al. EUROPattern Suite technology for computer-aided immunofluorescence microscopy in autoantibody diagnostics. Lupus (2015) 24:516–29. doi: 10.1177/0961203314559635
28. van Hoovels L, Schouwers S, van den Bremt S, Bogaert L, Vandeputte N, Vercammen M, et al. Analytical performance of the single well titer function of NOVA View: good enough to omit ANA IIF titer analysis? Clin Chem Lab Med (2018) 56:258–61. doi: 10.1515/cclm-2018-0338
29. Hiemann R, Büttner T, Krieger T, Roggenbuck D, Sack U, Conrad K. Challenges of automated screening and differentiation of non-organ specific autoantibodies on HEp-2 cells. Autoimmun Rev (2009) 9:17–22. doi: 10.1016/j.autrev.2009.02.033
30. Meroni PL, Bizzaro N, Cavazzana I, Borghi MO, Tincani A. Automated tests of ANA immunofluorescence as throughput autoantibody detection technology: strengths and limitations. BMC Med (2014) 12:38. doi: 10.1186/1741-7015-12-38
31. Kim J, Lee W, Kim GT, Kim HS, Ock S, Kim IS, et al. Diagnostic utility of automated indirect immunofluorescence compared to manual indirect immunofluorescence for anti-nuclear antibodies in patients with systemic rheumatic diseases: a systematic review and meta-analysis. Semin Arthritis Rheum (2019) 48:728–35. doi: 10.1016/j.semarthrit.2018.03.015
32. Rigon A, Soda P, Zennaro D, Iannello G, Afeltra A. Indirect immunofluorescence in autoimmune diseases: assessment of digital images for diagnostic purpose. Cytometry B Clin Cytom (2007) 72:472–7. doi: 10.1002/cyto.b.20356
33. van den Bremt S, Schouwers S, van Blerk M, van Hoovels L. ANA IIF Automation: moving towards harmonization? Results of a multicenter study. J Immunol Res (2017) 2017:6038137. doi: 10.1155/2017/6038137
34. Infantino M, Manfredi M, Soda P, Merone M, Afeltra A, Rigon A. ANA testing in ‘real life’. Ann Rheum Dis (2020) 79:e3. doi: 10.1136/annrheumdis-2018-214615
35. Hobson P, Lovell BC, Percannella G, Saggese A, Vento M, Wiliem A. Computer aided diagnosis for anti-nuclear antibodies HEp-2 images: Progress and challenges. Pattern Recognit Lett (2016) 82:3–11. doi: 10.1016/j.patrec.2016.06.013
36. Sack U, Conrad K, Csernok E, Frank I, Hiepe F, Krieger T, et al. Autoantibody detection using indirect immunofluorescence on HEp-2 cells, German EASI (European Autoimmunity Standardization Initiative). Ann N Y Acad Sci (2009) 1173:166–73. doi: 10.1111/j.1749-6632.2009.04735.x
37. Bradwell AR, Hughes RG, Karim AR. Immunofluorescent antinuclear antibody tests. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of Clinical Laboratory Immunology, 7th ed. Washington: ASM press (2006). p. 995–1006. doi: 10.1128/9781555815905.ch112
38. Infantino M, Manfredi M, Grossi V, Merone M, Soda P. The new era of LED microscopes in immunofluorescence anti-nuclear antibody (ANA) testing. Clin Chem Lab Med (2020) 58:e183–4. doi: 10.1515/cclm-2019-1103
39. Roggenbuck D, Hiemann R, Bogdanos D, Reinhold D, Conrad K. Standardization of automated interpretation of immunofluorescence tests. Clin Chim Acta (2013) 421:168–9. doi: 10.1016/j.cca.2013.03.019
40. Willitzki A, Hiemann R, Peters V, Sack U, Schierack P, Rödiger S, et al. New platform technology for comprehensive serological diagnostics of autoimmune disease. Clin Dev Immunol (2012) 2012:284740. doi: 10.1155/2012/284740
41. van Hoovels L, Schouwers S, van den Bremt S, Bossuyt X. Variation in antinuclear antibody detection by automated indirect immunofluorescence analysis. Ann Rheum Dis (2019) 78:e48. doi: 10.1136/annrheumdis-2018-213543
42. Paxton A. New momentum in harmonizing lab results. CAP Today (2018) 32:1–34. doi: 10.1111/1467-8322.12442
43. Tozzoli R, Villalta D, Bizzaro N. Challenges in the standardization of autoantibody testing: a comprehensive review. Clinic Rev Allergy Immunol (2017) 53:68–77. doi: 10.1007/s12016-016-8579-y
44. Damoiseaux J. Perspective on standardisation and harmonisation: the viewpoint of the EASI president. Autoimmun Highlights (2020) 11:4. doi: 10.1186/s13317-020-0127-3
45. Tate JR, Johnson R, Sikaris K. Harmonization in laboratory testing. Clin Biochem Rev (2012) 33:121–2.
46. Plebani M. Harmonization in laboratory medicine: the complete picture. Clin Chem Lab Med (2013) 51:741–51. doi: 10.1515/cclm-2013-0075
47. Kivity S, Gilburd B, Agmon-Levin N, Carrasco MG, Tzafrir Y, Sofer Y, et al. A novel automated indirect immunofluorescence autoantibody evaluation. Clin Rheumatol (2012) 31:503–9. doi: 10.1007/s10067-011-1884-1
48. Infantino M, Meacci F, Grossi V, Manfredi M, Benucci M, Merone M, et al. The burden of the variability introduced by the HEp-2 assay kit and the CAD system in ANA indirect immunofluorescence test. Immunol Res (2017) 65:345–54. doi: 10.1007/s12026-016-8845-3
49. Loock CD, Egerer K, Feist E, Burmester GR. Automated evaluation of ANA under real-life conditions. RMD Open (2017) 3:e000409. doi: 10.1136/rmdopen-2016-000409
50. Ghosh P, Dwivedi S, Naik S, Agarwal V, Verma A, Aggarwal A, et al. Antinuclear antibodies by indirect immunofluorescence : optimum screening dilution for diagnosis of systemic lupus erythematosus. Indian J Med Res (2007) 126:34–8.
51. Francescantonio PLC, Cruvinel WM, Dellavance A, Andrade LEC, Taliberti BH, Mühler CA, et al. IV Brazilian guidelines for autoantibodies on HEp-2 cells. Rev Bras Reumatol (2014) 54:44–50. doi: 10.1016/j.rbre.2014.02.006
52. Depincé-Berger AE, Moreau A, Bossy V, Genin C, Rinaudo M, Paul S. Comparison of screening dilution and automated reading for antinuclear antibody detection on HEp2 cells in the monitoring of connective tissue diseases. J Clin Lab Anal (2016) 30:471–8. doi: 10.1002/jcla.21881
53. Carbone T, Gilio M, Padula MC, Tramontano G, D’Angelo S, Pafundi V. A step towards standardization: A method for end-point titer determination by fluorescence index of an automated microscope. End-point titer determination by fluorescence index. J Immunol Methods (2018) 456:67–71. doi: 10.1016/j.jim.2018.02.014
54. Won DIL. Measurements of endpoint titers based on the fluorescence intensity trend in anti-nuclear antibody testing. Lab Med (2020) 51:469–77. doi: 10.1093/labmed/lmz087
55. Chan EK, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PL, et al. Report of the first international consensus on standardized nomenclature of antinuclear antibody HEp-2 cell patterns 2014-2015. Front Immunol (2015) 6:412. doi: 10.3389/fimmu.2015.00412
56. Chan EK, Damoiseaux J, de Melo Cruvinel W, Carballo OG, Conrad K, Francescantonio PL, et al. Report on the second International Consensus on ANA Pattern (ICAP) workshop in Dresden 2015. Lupus (2016) 25:797–804. doi: 10.1177/0961203316640920
57. Andrade LE, Klotz W, Herold M, Conrad K, Rönnelid J, Fritzler MJ, et al. International consensus on antinuclear antibody patterns: definition of the AC-29 pattern associated with antibodies to DNA topoisomerase I. Clin Chem Lab Med (2018) 56:1783–8. doi: 10.1515/cclm-2018-0188
58. Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann. Rheum Dis (2019) 78:879–8. doi: 10.1136/annrheumdis-2018-214436
59. Mahler M. Lack of standardisation of ANA and implications for drug development and precision medicine. Ann Rheum Dis (2019) 78:e33. doi: 10.1136/annrheumdis-2018-213374
60. Damoiseaux J, von Muhlen CA, Garcia-De La Torre I, Carballo OG, de Melo Cruvinel W, Francescantonio PL, et al. International Consensus on ANA Patterns (ICAP): the bumpy road towards a consensus on reporting ANA results. Autoimmun Highlights (2016) 7:1. doi: 10.1007/s13317-016-0075-0
61. Cinquanta L, Infantino M, Manfredi M, Daves M, Radice A, Villalta D, et al. Updating the Italian Society of Clinical Pathology and Laboratory Medicine guidelines on the use of autoantibody tests in the diagnosis and monitoring of systemic rheumatic autoimmune diseases: analytical methods for the screening of antibodies to cellular antigens (ANA). Riv Ital Med Lab (2019) 15:294–9. [Italian]. doi: 10.23736/S1825-859X.19.00043-4
62. Clinical and Laboratory Standards Institute (CLSI). Quality Assurance of Laboratory Tests for Autoantibodies to Nuclear Antigens: (1) Indirect Fluorescent Assay for Microscopy and (2) Microtiter Enzyme Immunoassay Methods. Approved Guideline, CLSI Document I/LA2-A2. 2nd edition. Wayne, Pa, USA: CLSI (2006).
63. Maenhout TM, Bonroy C, Verfaillie C, Stove V, Devreese K. Automated indirect immunofluorescence microscopy enables the implementation of a quantitative internal quality control system for anti-nuclear antibody (ANA) analysis. Clin Chem Lab Med (2014) 52:989–98. doi: 10.1515/cclm-2013-0912
64. van Hoovels L, Bossuyt X, Manfredi M, Grossi V, Benucci M, van den Bremt S, et al. Integrating quality assurance in autoimmunity: the changing face of the automated ANA IIF test. Clin Chem Lab Med (2021). (accepted). doi: 10.1515/cclm-2020-1669
65. Bogaert L, van den Bremt S, Schouwers S, Bossuyt X, van Hoovels L. Harmonizing by reducing inter-run variability: performance evaluation of a quality assurance program for antinuclear antibody detection by indirect immunofluorescence. Clin Chem Lab Med (2019) 57:990–8. doi: 10.1515/cclm-2018-0933
66. Bonroy C, Verfaillie C, Smith V, Persijn L, De Witte E, De Keyser F, et al. Automated indirect immunofluorescence antinuclear antibody analysis is a standardized alternative for visual microscope interpretation. Clin Chem Lab Med (2013) 51:1771–9. doi: 10.1515/cclm-2013-0016
67. Bizzaro N, Bossuyt X, Haapala AM, Shoenfeld Y, Sack U. Accreditation in autoimmune diagnostic laboratories. A position paper of the European Autoimmunity Standardisation Initiative (EASI). Autoimmun Rev (2017) 16:81–6. doi: 10.1016/j.autrev.2016.09.021
68. Sack U, Bossuyt X, Andreeva H, Antal-Szalmás P, Bizzaro N, Bogdanos D, et al. European Autoimmunity Standardisation Initiative. Quality and best practice in medical laboratories: specific requests for autoimmunity testing. Auto Immun Highlights (2020) 11:12. doi: 10.1186/s13317-020-00134-0
69. Tozzoli R, Bizzaro N. The clinical autoimmunologist and the laboratory autoimmunologist: The two sides of the coin. Autoimmun Rev (2012) 11:766–70. doi: 10.1016/j.autrev.2012.02.011
70. Tozzoli R, Bizzaro N. The clinical and the laboratory autoimmunologist: Where do we stand? Auto Immun Highlights (2020) 11:10. doi: 10.1186/s13317-020-00133-1
71. Bossuyt X, Fieuws S. Detection of antinuclear antibodies: added value of solid phase assay? Ann Rheum Dis (2014) 73:e10. doi: 10.1136/annrheumdis-2013-204793
72. Bizzaro N, Brusca I, Previtali G, Alessio MG, Daves M, Platzgummer S, et al. The association of solid-phase assays to immunofluorescence increases the diagnostic accuracy for ANA screening in patients with autoimmune rheumatic diseases. Autoimmun Rev (2018) 17:541–7. doi: 10.1016/j.autrev.2017.12.007
73. Bizzaro N. Can solid-phase assays replace immunofluorescence for ANA screening? Ann Rheum Dis (2020) 79:e32. doi: 10.1136/annrheumdis-2018-214805
74. Claessens J, Belmondo T, De Langhe E, Westhovens R, Poesen K, Hue S, et al. Solid phase assays versus automated indirect immunofluorescence for detection of antinuclear antibodies. Autoimmun Rev (2018) 17:533–40. doi: 10.1016/j.autrev.2018.03.002
75. Willems P, De Langhe E, Claessens J, Westhovens R, Van Hoeyveld E, Poesen K, et al. Screening for connective tissue disease-associated antibodies by automated immunoassay. Clin Chem Lab Med (2018) 56:909–18. doi: 10.1515/cclm-2017-0905
76. Pérez D, Gilburd B, Azoulay D, Shovman O, Bizzaro N, Shoenfeld Y. Antinuclear antibodies: Is the indirect immunofluorescence still the gold standard or should be replaced by solid phase assays? Autoimmun Rev (2018) 17:548–52. doi: 10.1016/j.autrev.2017.12.008
77. Sheldon J, Dellavance A. Strategies for building reference standards for autoantibodies. Front Immunol (2015) 6:194. doi: 10.3389/fimmu.2015.00194
78. Oyaert M, Bossuyt X, Ravelingien I, Van Hoovels L. Added value of indirect immunofluorescence intensity of automated antinuclear antibody testing in a secondary hospital setting. Clin Chem Lab Med (2016) 54:e63–6. doi: 10.1515/cclm-2015-0887
79. Peterson LK, Tebo AE, Wener MH, Copple SS, Fritzler MJ. Assessment of antinuclear antibodies by indirect immunofluorescence assay: report from a survey by the American Association of Medical Laboratory Immunologists. Clin Chem Lab Med (2020) 58:1489–97. doi: 10.1515/cclm-2019-1262
80. Bossuyt X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmun Rev (2009) 8:543–8. doi: 10.1016/j.autrev.2009.01.013
81. Bossuyt X, Claessens J, Belmondo T, De Langhe E, Westhovens R, Poesen K. Harmonization of clinical interpretation of antinuclear antibody test results by solid phase assay and by indirect immunofluorescence through likelihood ratios. Autoimmun Rev (2019) 18:102386. doi: 10.1016/j.autrev.2019.102386
Keywords: harmonization, standardization, anti-nuclear antibodies, computer-assisted systems, immunofluorescence, automation
Citation: Cinquanta L, Bizzaro N and Pesce G (2021) Standardization and Quality Assessment Under the Perspective of Automated Computer-Assisted HEp-2 Immunofluorescence Assay Systems. Front. Immunol. 12:638863. doi: 10.3389/fimmu.2021.638863
Received: 07 December 2020; Accepted: 18 January 2021;
Published: 25 February 2021.
Edited by:
Luis Eduardo Coelho Andrade, Federal University of São Paulo, BrazilReviewed by:
Carlos Alberto von Muhlen, Independent Researcher, San Diego, CA, United StatesJan Damoiseaux, Maastricht University Medical Centre, Netherlands
Copyright © 2021 Cinquanta, Bizzaro and Pesce. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Bizzaro, bmljLmJpenphcm9AZ21haWwuY29t
 Luigi Cinquanta
Luigi Cinquanta Nicola Bizzaro
Nicola Bizzaro Giampaola Pesce
Giampaola Pesce