- 1CHU de Poitiers, Service de Médecine Interne et Maladies Infectieuses, Poitiers, France
- 2CHU de Poitiers, CIC-1402 Poitiers, France
- 3Haematology and Transplant Unit, The Christie NHS Foundation Trust, Manchester, United Kingdom
- 4Haemato-Oncology Department, Clatterbridge Cancer Centre, University of Liverpool, Liverpool, United Kingdom
- 5Department of Hematology, Oncology and Rheumatology, Internal Medicine V, University Hospital of Heidelberg, Heidelberg, Germany
- 6EBMT ADWP Office, Paris, France
- 7U.O. Ematologia Centro Trapianti Midollo - Ospedale Policlinico San Martino, Genova, Italy
- 8Department of Haematology and BM Transplantation, St Vincent’s Hospital Sydney and St Vincent’s Clinical School, Faculty of Medicine, University of New South Wales (UNSW) Sydney, Sydney, NSW, Australia
- 9Institute for Maternal and Child Health – IRCCS Burlo Garofolo, Trieste, Italy
- 10Haematology and Bone Marrow Transplant Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 11Vita-Salute San Raffaele University, Milan, Italy
- 12Department of Rheumatology and Clinical Immunology, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Charité - Universitätsmedizin Berlin, Berlin, Germany
- 13Deutsches Rheuma-Forschungszentrum (DRFZ), an Institute of the Leibniz Association, Berlin, Germany
- 14Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
Background: Behçet’s Disease (BD) is an autoimmune disease mostly presenting with recurrent oral and genital aphthosis, and uveitis. Patients are rarely refractory to immunosuppressive treatments. Autologous hematopoietic stem cell transplantation (aHSCT) is a standard of care in other autoimmune diseases. Some patients with BD have been treated with aHSCT based on compassionate use.
Objectives: Evaluate the outcome of aHSCT in adult patients with BD treated in member centers of the European Society for Blood and Marrow Transplantation (EBMT).
Methods: Adults who received aHSCT primarily for BD were identified retrospectively in the EBMT registry and/or in published literature. Data were extracted from either medical records of the patient or from publications.
Results: Eight out of 9 cases reported to the registry and extracted data of 2 further patients from literature were analyzed. Four were female, median age at onset of BD was 24y (range 9-50). Median age at aHSCT was 32y (27-51). Patients had received median 4 (2-11) previous lines of therapy (89% corticosteroids, 50% methotrexate, anti-TNFα therapy or cyclophosphamide). All patients had active disease before mobilization. Conditioning regimen was heterogeneous. Median follow-up was 48 months (range 6-240). No treatment-related mortality was reported. This procedure induced complete remission (CR) in 80%, partial remission in 10% and lack of response in 10% of the patients. Relapse rate was 30% (2 relapses in patients in CR and 1 relapse in the patient in PR) with panuveitis (n=1), aphthosis (n=2) and arthralgia (n=1). Six patients were in CR. No late complications were reported.
Conclusion: aHSCT has an acceptable safety profile and represents a feasible and relatively effective procedure in severe and conventional treatment-resistant cases of BD and has the potential to stabilize BD in patients with life-threatening involvements.
Key points
1. aHSCT has an acceptable safety profile and represents a feasible and relatively effective procedure in severe and conventional treatment-resistant cases of BD
2. aHSCT has the potential to stabilize BD in patients with life-threatening involvements.
3. The rate of failure and relapse seems to be low.
Introduction
Behçet´s disease (BD) is a rare autoimmune disease mostly presenting with recurrent oral and genital aphthosis, and uveitis. Other common symptoms include gastrointestinal, vascular, neurological and articular manifestations (1). The first internationally agreed criteria for BD were the International Study Group (ISG) criteria (2). They had very high specificity, but lacked satisfactory sensitivity, missing out on a sizable subset of patients. The International Criteria for Behçet’s Disease (ICBD) were created in 2006 to overcome this lack of sensitivity. This classification was revised in 2006. A score ≥ 3 is associated with sensitivity of 95% and specificity of 90.5% (3). Treatment is based on chronic immunosuppression with conventional disease-modifying or targeted biologic drugs such as corticosteroids, intravenous cyclophosphamide, cyclosporine or anti TNFα therapy (4). The most recent European League Against Rheumatism (EULAR) recommendations were updated in 2018 (5). They did not mention autologous hematopoietic stem cell transplantation (aHSCT), possibly due to limited specific data in BD.
Over the past 20 years, aHSCT has become a standard of care for early rapidly progressive Systemic Sclerosis (6–11) and has emerged as a promising treatment modality for severe Multiple Sclerosis (12–14) and Crohn’s Disease (12, 15). But patients with rarer diseases, such as BD, have undergone aHSCT based on compassionate use.
Led by the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT), a previously published retrospective analysis of HSCT covered all vasculitis (16), in which partial and complete responses were reported for two patients with BD. Other publications on autologous stem cell transplantation for BD [without a concomitant hematologic malignancy, as it has also been reported (17, 18)] only consist of case reports (19–23). Only one failure with aHSCT in BD has been reported (22). Further data and composite analysis are required to inform the balance of benefits and risks of aHSCT in patients with severe, refractory BD. The aim of this retrospective study was therefore to bring together a larger experience in order to describe the efficacy and toxicity of aHSCT in adults with BD treated in EBMT centers.
Methods
Study Design
The European Society for Blood and Marrow Transplantation (EBMT) is a medical and scientific organization that represents HSCT centers in Europe and worldwide. The EBMT registry contains details on transplants performed for auto-immune diseases since 1986, including over 3000 patients undergoing HSCT for autoimmune diseases. Patients provided written consent before transplant for the collection and analysis of anonymized data, and data were maintained in the central EBMT registry in line with legal and regulatory requirements. This is a multicenter retrospective study approved by the Autoimmune Diseases Working Party (ADWP) of the EBMT. Once eligible patients had been identified from the database, data already held within EBMT records were sourced and referring centers were asked to complete a questionnaire to provide further detailed information regarding patients’ pre- and post-transplant disease status. All relevant data were collated and held in a specially designed database within the EBMT. Data from published cases treated in Europe were extracted from the literature in PubMed and Science Direct. The algorithm was (“Behcet Syndrome”[Mesh]) AND “Hematopoietic Stem Cell Transplantation”[Mesh] in Pubmed and “Behcet” AND “Stem Cell transplantation” in Science Direct.
Patient Eligibility
Inclusion criteria were:
1. Behçet’s disease (BD) diagnosed according to International Criteria for Behçet’s disease (ICBD) classification criteria
2. Aged 18 years or older at the time of treatment with autologous stem cell transplantation treated in an EBMT member center
Exclusion criteria were:
1. aHSCT for a concomitant disease in patients with a diagnosis of Behcet’s disease
2. Patients with follow-up <6 months
Definitions of Disease Activity
The following definitions of disease activity and response were used:
● Active disease (persisting or recurring disease activity post-transplantation, insufficiently controlled by glucocorticoids and/or disease-modifying drugs).
● Drug-dependent partial response (DDPR) (any documented clinical and/or laboratory response in patients receiving glucocorticoids at a dose equivalent or greater than 7.5mg of prednisolone/day and/or disease-modifying drugs).
● Drug-dependent complete response (DDCR) (no evidence for disease activity in patients receiving glucocorticoids at a dose equivalent or greater than 7.5mg of prednisolone/day and/or disease-modifying drugs)
● Complete remission (CR) (no evidence for disease activity in patients with glucocorticoids at a dose below 7.5mg of prednisolone and absence of disease-modifying drugs).
A major relapse was considered in case of involvement of a life-threatening (pulmonary or vascular event for example) or functional-threatening organ (which was mainly eye). Other instances were considered as a minor relapse.
Study Endpoints
Primary endpoint was response to treatment at the last follow-up visit. Progression was defined as absence of improvement but also as relapse of the disease requiring reintroduction or re-escalation of immunosuppression.
Secondary endpoints were:
● overall survival,
● relapse rate, defined as recurrence or new development of ulcerative disease, ocular lesions or vascular event,
● number of patients in CR, DDPR, DDCR, Active disease at last follow-up visit,
● toxicities related to aHSCT,
● time to reintroduction or re-escalation of immunosuppressive treatment,
● Non-Relapse Mortality (NRM) at 100 days and 1 year, defined as any death following transplant that cannot be attributed to active disease.
Statistical Method
Due to the low number of patients and the heterogeneity of the population that do not allow any adjustment, statistics were only descriptive and no comparison was performed. Categorical variables were described as numbers and percentage and continuous variables using median and range.
Data Collection
Cases for inclusion in the study were identified by database queries in the EBMT Registry according to the abovementioned inclusion criteria. A letter of invitation to participate in the study was sent to the EBMT centers. Data were extracted locally retrospectively: demographic and general data (age at BD onset, sex, age at aHSCT, ICBD criteria, number of previous therapies before aHSCT l304 and l306, daily Prednisolone dosage at baseline, therapies prior to HSCT), mobilization regimen, transplant data (graft manipulation, stem cell yield and number of collections, conditioning regimen, Time to engraftment, Adverse event before 100 days), late complications (after day 100), BD status at mobilization, 6 months, 12 months, and yearly post-aHSCT, date of most recent follow-up, date of reintroduced immunosuppression with time-point of reintroduction, relapse or progression, survival status, cause of death if happened. In case of extraction of the case from the literature, the same data were collected.
Results
Selection
Nine patients were registered in the EBMT database who all signed informed consent. Data were available for 8 patients [two cases were already published but with shorter follow-up (23)]. Two patients were treated in an EBMT center but not reported in the registry. They were identified from the published literature (20). All in all, 10 patients were included in our study (Figure 1).
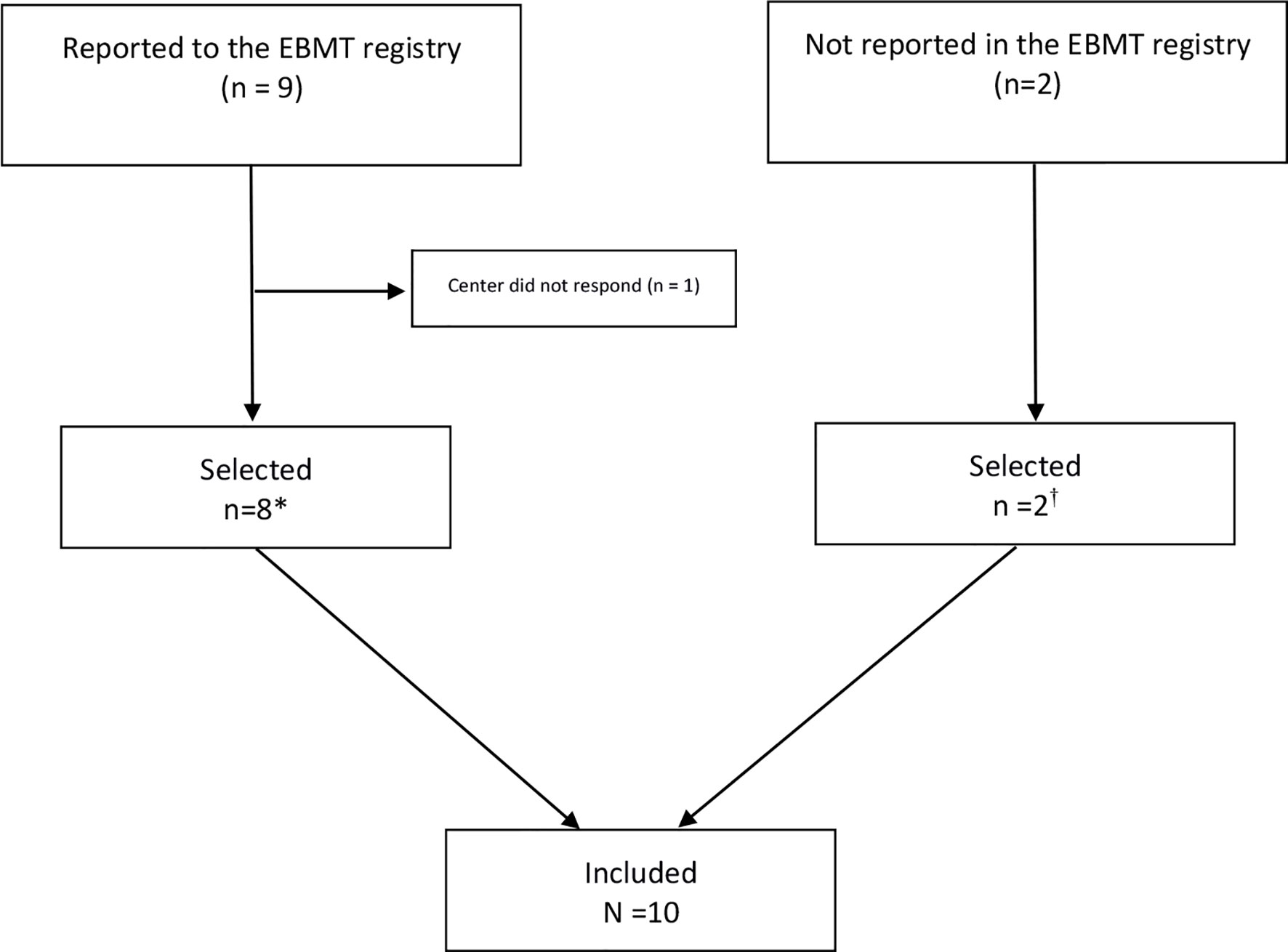
Figure 1 Flow Chart on identification of patients treated with autologous hematopoietic stem cell transplantation for Behçet’s Disease in European Society of Blood and Marrow Transplantation (EBMT) centers (N=10). *Preliminary results of two patients with shorter follow-up were previously published (23). Long-term follow-up data are included in this manuscript. †Two cases were already published: De Cata A, Intiso D, Bernal M, et al: Prolonged Remission of Neuro-Behcet Disease following Autologous Transplantation. Int J Immunopathol Pharmacol 20:91–96, 2007 (center of Foggia).
Patient Characteristics
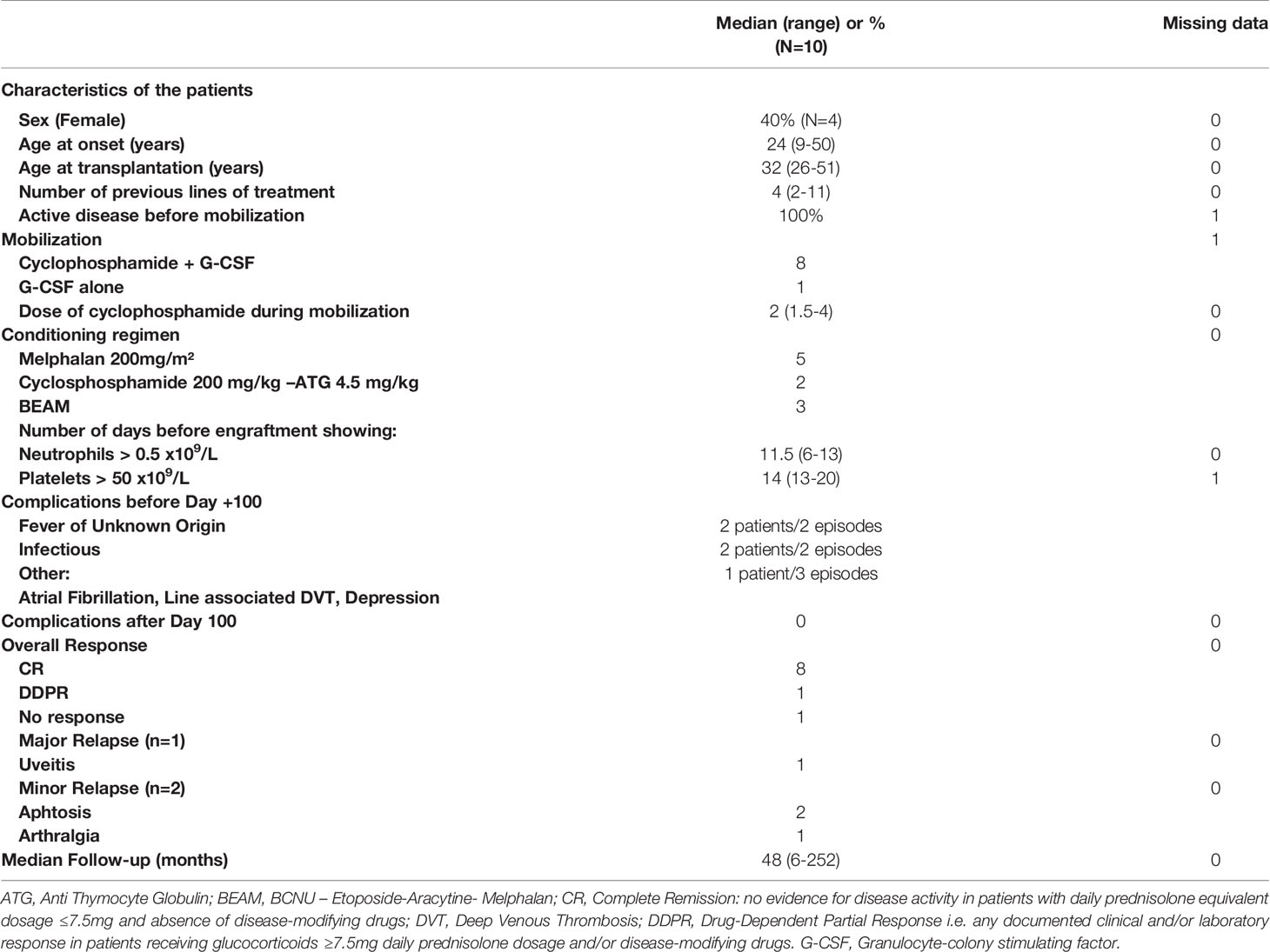
Demographic and clinical characteristics of patients are provided in Table 1. All patients from EBMT registry had an ICBD score greater than 4 points. For the 2 patients extracted from the literature, diagnosis was made according to ISG criteria. Four patients were female. Median age at the onset of the disease was 24 years (range 9-50y), and median age at the transplantation was 32 years (range 26-51y). Patients had received median 4 (2-11) previous lines of treatment: 89 % of the patients were treated with corticosteroids, and 50 % received either methotrexate, anti-TNFα therapy or cyclophosphamide. All patients had active disease before aHSCT (one missing data). Main indications were severe uncontrolled and usually multiple involvements: ocular (4 patients) gastro-intestinal (4 patients), neurological (3 patients), cardiac (2 patients), lung and kidney (1 patient). Aphtosis was not controlled in 4 patients. For one patient, indication of transplantation is unknown. Detailed observations are presented in Table 2.
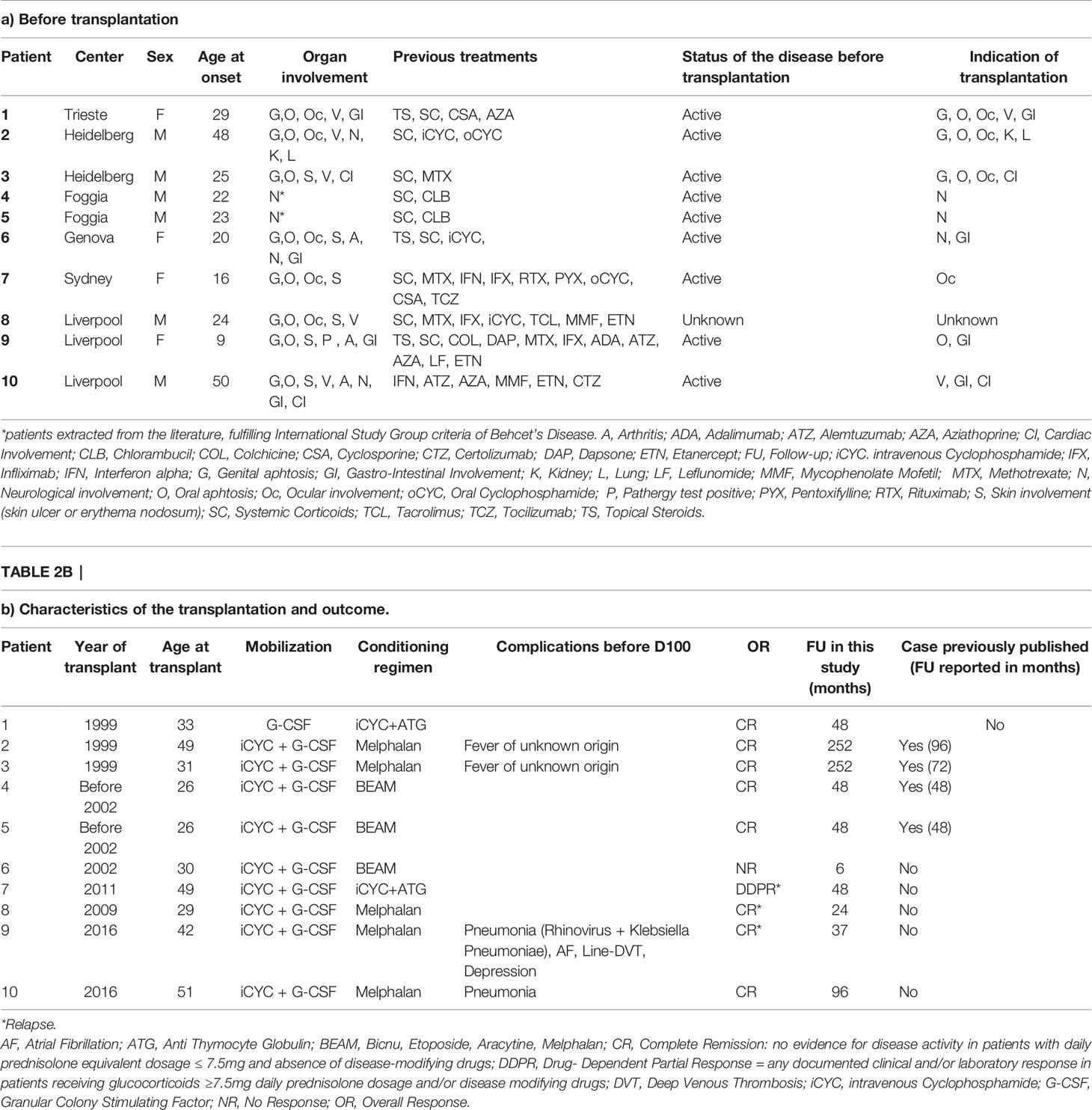
Table 2 Individual characteristics and outcome for patients with Behçet’s disease treated with autologous stem cell transplantation in European Society of Blood and Marrow Transplantation centers (N=10).
aHSCT
Cyclophosphamide and Granulocyte-Colony Stimulating Factor (G-CSF) were administrated during mobilization in 8 patients and G-CSF alone in 1 patient. Data was missing in 1 patient. Conditioning regimen was Melphalan 200mg/m² in 5 patients, BEAM (BCNU-Etoposide-Aracytine-Melphalan) in 3 patients, Cyclophosphamide 200 mg/kg plus Anti-Thymocyte-Globulin (ATG) in 2 patients. Median follow-up was 48 months (range 6-252 months). Detailed data are presented in Table 1.
Main Outcome: Response to Treatment
This procedure induced complete remission (CR) in 80%, partial remission in 10% and lack of response in 10% of the patients. Relapse rate was 30% (2 relapses in patients in CR and 1 relapse in the patient in PR) with panuveitis (n=1), aphthosis (n=2) and arthralgia (n=1) Only one patient did not respond with aHSCT at 6 months and was treated with allogeneic hematopoietic stem cell transplantation (HSCT). At the most recent follow-up, 60% of the patients were in CR without any treatment (including corticosteroids), three patients relapsed at 24, 37 and 48 months (Table 2).
Secondary Outcomes
During aHSCT (before day 100), two patients had a fever of unknown origin, two patients had infectious complications (pneumonia) and in addition to pneumonia a single patient had paroxysmal atrial fibrillation, line-associated deep venous thrombosis and depression. No complications were reported after Day 100. The patient who did not respond to aHSCT was treated with allogeneic HSCT. He died three years after allogeneic transplantation of chronic Graft versus Host Disease complicated by cytomegalovirus infection. No other late death was reported. At one year, 8 patients were in CR and one patient in DDPR. The patient with DDPR had a mild active uveitis. All other involvements improved (skin, eye, gastro-intestinal, cardiac, lung, kidney) but sometimes with irreversible organ damage (decreased visual acuity in patients 1,6,9 and moderate chronic kidney disease in patient 2). In case of neurological involvement (3 patients), 2 patients improved but with residual disability and one patient remained stable. The relapse rate was 3 out 9 (≈ 33%) with only one major relapse. The major involvement was an ocular panuveitis (patient 7) that occurred 48 months after aHSCT leading immediately to introduction of a new treatment. At last follow-up visit, the patient was in DDCR with steroids. Two minor relapses with aphthosis (n=2) and arthralgia (n=1) were reported at 24 and 37 months. Patients experiencing relapse were treated immediately with steroids and azathioprine (AZA) or AZA alone. At last follow-up visit, the patients who relapsed were only treated with AZA and were in DDCR. One of these patients was resistant to AZA before the aHSCT and the patient who relapsed with panuveitis was refractory to steroids before the aHSCT (Table 2).
Discussion
Our study is the first retrospective cohort study conducted in patients with BD who underwent aHSCT. The results showed a high rate of efficacy in heavily pretreated patients or in patients with life-threatening complications with acceptable toxicity profile and no aHSCT-related mortality. Two patients were in CR (ie without any treatment) up to 21 years after aHSCT, raising the question of the efficacy of aHSCT as an effective cure for patients with BD refractory to multiple conventional treatments. However, one patient did not respond, one patient had a major relapse and two patients had a minor relapse reflecting some limitations of the procedure. Relapses consisted of the same clinical presentation observed at the onset of the disease. But there was no life-threatening involvement and all relapses were controlled by conventional treatments, whereas patients were refractory to those treatments before aHSCT. This is consistent with observations following aHSCT in refractory Crohn’s Disease and use of anti-TNFα therapy after aHSCT (24) and in rheumatoid arthritis and use of disease-modifying agents after aHSCT (25), where there is renewed sensitivity to drugs associated with the “immune reset” after aHSCT.
Such “immune reset” after aHSCT may explain the efficacy in BD. Although no data are available in BD, this concept is well described in other auto-immune diseases treated with aHSCT, and the mechanisms seem to be similar whatever the disease (26). The destruction of the aberrant immune system leads to restoration of a new naïve and self-tolerant immune system (27–30). Renewal of T-cell repertoire is well documented in rheumatic diseases like systemic sclerosis or systemic lupus erythematosus with the disappearance of T-cell autoimmune clones (31) and of the pre-existing serum auto-antibodies (32). A functional renewal of regulatory T cells (33) and diversification of the T-cell receptor (TCR) repertoire (30) are also associated with favorable outcomes in patients with severe resistant autoimmune and inflammatory diseases treated with aHSCT (34).
Significantly, our results are congruent with published cases showing a high rate of efficacy (16, 19–22). By selecting the patients from EBMT registry with the inclusion of consecutive patients, this study avoided publication bias, i.e. reporting only success and not failure. Furthermore, this study is the first focusing on aHSCT in BD. Other studies had no more than 2 cases as they were focused on primary vasculitis, including BD among others (16, 22), with shorter follow-up (16, 19–22, 35).
tThis study has limitations including retrospective data collection (but missing data were rare, and they pertained to secondary data) and possible under-reporting of aHSCT for BD in the EBMT registry (the 2 cases extracted from the literature illustrate this issue (20), despite the obligation of full EBMT member centers to report all cases). This bias is likely to be independent from aHSCT outcome. The literature search improved sensitivity but may have been affected by publication bias. Another bias was the heterogeneity of patients characteristics at baseline (especially in terms of organ involvements), reflecting the absence of consensus or guidelines on the indication of aHSCT in BD. The long term risk/benefit balance of aHSCT in BD should be evaluated in prospective studies, ideally randomized controlled trials of anti-TNFα therapy refractory patients against other standard of care. But it would be unlikely to be feasible due to the rare incidence of patients with this severity of BD. More pragmatically, ongoing registry-based analyses may help to define the role of aHSCT in BD and further inform transplant technique and patient selection to optimize patient outcomes.
In conclusion, based on our data, aHSCT has an acceptable safety profile and represents a feasible and relatively effective procedure in severe and conventional treatment-resistant cases of BD. It should be considered as a treatment option for patients refractory to anti-TNFα with high disease burden. Further data ideally from randomized controlled trials are warranted.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: ALL. Drafting the work or revising it critically for important intellectual content: ALL. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: ALL. All authors contributed to the article and approved the submitted version.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Conflict of Interest
JS declares previous honoraria for speaking at educational events from Sanofi, Jazz, Janssen, Actelion, Mallinckrodt and Gilead, and is a member of a trial IDMC for Kiadis Pharma (none directly related to the subject of this analysis).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
To Myriam Labopin for her help in the design of the study.
To Jeffrey Arshaw for his help in editing the manuscript.
This work was presented as a e-poster during the virtual EBMT congress 2020.
References
1. Davatchi F, Chams-Davatchi C, Shams H, Shahram F, Nadji A, Akhlaghi M, et al. Behcet’s disease: epidemiology, clinical manifestations, and diagnosis. Expert Rev Clin Immunol (2017) 13(1):57–65. doi: 10.1080/1744666X.2016.1205486
2. O’Neill TW, Rigby AS, Silman AJ, Barnes C. Validation of the International Study Group criteria for Behçet’s disease. Br J Rheumatol (1994) 33(2):115–7. doi: 10.1093/rheumatology/33.2.115
3. Davatchi F. Diagnosis/Classification Criteria for Behcet’s Disease. Pathol Res Int (2012) 2012:1–5. doi: 10.1155/2012/607921
4. Vallet H, Riviere S, Sanna A, Deroux A, Moulis G, Addimanda O, et al. Efficacy of anti-TNF alpha in severe and/or refractory Behçet’s disease: Multicenter study of 124 patients. J Autoimmunity (2015) 62:67–74. doi: 10.1016/j.jaut.2015.06.005
5. Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F, et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis (2018) 77(6):808–18. doi: 10.1136/annrheumdis-2018-213225
6. van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA (2014) 311(24):2490–8. doi: 10.1001/jama.2014.6368
7. Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet (2011) 378(9790):498–506. doi: 10.1016/S0140-6736(11)60982-3
8. Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N Engl J Med (2018) 378(1):35–47.
9. Alexander T, Bondanza A, Muraro PA, Greco R, Saccardi R, Daikeler T, et al. SCT for severe autoimmune diseases: consensus guidelines of the European Society for Blood and Marrow Transplantation for immune monitoring and biobanking. Bone Marrow Transplant (2015) 50(2):173–80. doi: 10.1038/bmt.2014.251
10. Pugnet G, Castilla-Llorente C, Puyade M, Terriou L, Badoglio M, Deligny C, et al. Indications et suivi des autogreffes de cellules souches hématopoïétiques dans les maladies auto-immunes et auto-inflammatoires : recommandations de la Société francophone de greffe de moelle et de thérapie cellulaire (SFGM-TC). Bull du Cancer (2017) 104(12):S169–80. doi: 10.1016/j.bulcan.2017.06.019
11. Puyade M, Maltez N, Lansiaux P, Pugnet G, Roblot P, Colmegna I, et al. Health-related quality of life in systemic sclerosis before and after autologous haematopoietic stem cell transplant-a systematic review. Rheumatol (Oxford) (2019) 59(4):779–89. doi: 10.1093/rheumatology/kez300
12. Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, et al. Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv (2017) 1(27):2742–55. doi: 10.1182/bloodadvances.2017010041
13. for the European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT) and EBMT (JACIE), Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, et al. Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant (2019) 55(2):283–306. doi: 10.1038/s41409-019-0684-0
14. Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of Nonmyeloablative Hematopoietic Stem Cell Transplantation vs Continued Disease-Modifying Therapy on Disease Progression in Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial. JAMA (2019) 321(2):165. doi: 10.1001/jama.2018.18743
15. Hawkey CJ, Allez M, Clark MM, Labopin M, Lindsay JO, Ricart E, et al. Autologous Hematopoetic Stem Cell Transplantation for Refractory Crohn Disease: A Randomized Clinical Trial. JAMA (2015) 314(23):2524–34. doi: 10.1001/jama.2015.16700
16. Daikeler T, Kotter I, Bocelli Tyndall C, Apperley J, Attarbaschi A, Guardiola P, et al. Haematopoietic stem cell transplantation for vasculitis including Behcet’s disease and polychondritis: a retrospective analysis of patients recorded in the European Bone Marrow Transplantation and European League Against Rheumatism databases and a review of the literature. Ann Rheumatic Dis (2006) 66(2):202–7. doi: 10.1136/ard.2006.056630
17. Chauhan S, Olujohungbe A, Moots RJ. Treatment of Behçet’s syndrome and plasmacytoma in a patient with peripheral blood stem cell transplantation. Leuk Lymphoma (2008) 49(12):2377–9. doi: 10.1080/10428190802474447
18. Soysal T, Salihoğlu A, Esatoğlu SN, Gültürk E, Eşkazan AE, Hatemi G, et al. Bone marrow transplantation for Behcet’s disease: a case report and systematic review of the literature. Rheumatology (2014) 53(6):1136–41. doi: 10.1093/rheumatology/ket479
19. Hensel M, Breitbart A, Ho AD. Autologous Hematopoietic Stem-Cell Transplantation for Behçet’s Disease with Pulmonary Involvement. N Engl J Med (2001) 344(1):69–9. doi: 10.1056/NEJM200101043440121
20. De Cata A, Intiso D, Bernal M, Molinaro F, Mazzoccoli G, D’Alessandro V, et al. Prolonged Remission of Neuro-Behcet Disease following Autologous Transplantation. Int J Immunopathol Pharmacol (2007) 20(1):91–6. doi: 10.1177/039463200702000110
21. Rossi G, Moretta A, Locatelli F. Autologous hematopoietic stem cell transplantation for severe/refractory intestinal Behcet disease. Blood (2004) 103(2):748–50. doi: 10.1182/blood-2003-10-3617
22. Statkute L, Oyama Y, Barr WG, Sufit R, Ho S, Verda L, et al. Autologous non-myeloablative haematopoietic stem cell transplantation for refractory systemic vasculitis. Ann Rheumatic Dis (2008) 67(7):991–7. doi: 10.1136/ard.2007.070227
23. Fiehn C, Ho AD, Lorenz H-M. Hematopoietic stem cell transplantation (HSCT) for primary systemic vasculitis and related diseases. Autoimmunity (2008) 41(8):648–53. doi: 10.1080/08916930802197495
24. López-García A, Rovira M, Jauregui-Amezaga A, Marín P, Barastegui R, Salas A, et al. Autologous Haematopoietic Stem Cell Transplantation for Refractory Crohn’s Disease: Efficacy in a Single-Centre Cohort. J Crohns Colitis (2017) 11(10):1161–8. doi: 10.1093/ecco-jcc/jjx054
25. Snowden JA, Passweg J, Moore JJ, Milliken S, Cannell P, Van Laar J, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol (2004) 31(3):482–8.
26. Alexander T, Farge D, Badoglio M, Lindsay JO, Muraro PA, Snowden JA. Hematopoietic stem cell therapy for autoimmune diseases – Clinical experience and mechanisms. J Autoimmunity (2018) 92:35–46. doi: 10.1016/j.jaut.2018.06.002
27. Collins F, Kazmi M, Muraro PA. Progress and prospects for the use and the understanding of the mode of action of autologous hematopoietic stem cell transplantation in the treatment of multiple sclerosis. Expert Rev Clin Immunol (2017) 13(6):611–22. doi: 10.1080/1744666X.2017.1297232
28. Arruda LCM, Clave E, Moins-Teisserenc H, Douay C, Farge D, Toubert A. Resetting the immune response after autologous hematopoietic stem cell transplantation for autoimmune diseases. Curr Res Trans Med (2016) 64(2):107–13. doi: 10.1016/j.retram.2016.03.004
29. Arruda LCM, Lima-Júnior JR, Clave E, Moraes DA, Douay C, Fournier I, et al. Homeostatic proliferation leads to telomere attrition and increased PD-1 expression after autologous hematopoietic SCT for systemic sclerosis. Bone Marrow Transplant (2018) 53(10):1319–27. doi: 10.1038/s41409-018-0162-0
30. Farge D, Arruda LCM, Brigant F, Clave E, Douay C, Marjanovic Z, et al. Long-term immune reconstitution and T cell repertoire analysis after autologous hematopoietic stem cell transplantation in systemic sclerosis patients. J Hematol Oncol (2017) 10(1):21. doi: 10.1186/s13045-016-0388-5
31. Farge D, Henegar C, Carmagnat M, Daneshpouy M, Marjanovic Z, Rabian C, et al. Analysis of immune reconstitution after autologous bone marrow transplantation in systemic sclerosis. Arthritis Rheumatol (2005) 52(5):1555–63. doi: 10.1002/art.21036
32. Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood (2009) 113(1):214–23. doi: 10.1182/blood-2008-07-168286
33. Arruda LCM, Malmegrim KCR, Lima-Júnior JR, Clave E, Dias JBE, Moraes DA, et al. Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients. Blood Adv (2018) 2(2):126–41. doi: 10.1182/bloodadvances.2017011072
34. Snowden JA, Sharrack B, Akil M, Kiely DG, Lobo A, Kazmi M, et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases - a guide for the generalist. Clin Med (Lond) (2018) 18(4):329–34. doi: 10.7861/clinmedicine.18-4-329
Keywords: Behçet’s disease, autologous stem cell transplantation, efficacy, toxicity, immune reset
Citation: Puyade M, Patel A, Lim YJ, Blank N, Badoglio M, Gualandi F, Ma DD, Maximova N, Greco R, Alexander T and Snowden JA (2021) Autologous Hematopoietic Stem Cell Transplantation for Behçet’s Disease: A Retrospective Survey of Patients Treated in Europe, on Behalf of the Autoimmune Diseases Working Party of the European Society for Blood and Marrow Transplantation. Front. Immunol. 12:638709. doi: 10.3389/fimmu.2021.638709
Received: 07 December 2020; Accepted: 26 March 2021;
Published: 06 May 2021.
Edited by:
Ali Akbar Pourfathollah, Tarbiat Modares University, IranReviewed by:
Marco Infante, University of Miami, United StatesDonghai Wang, Duke University, United States
Copyright © 2021 Puyade, Patel, Lim, Blank, Badoglio, Gualandi, Ma, Maximova, Greco, Alexander and Snowden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mathieu Puyade, bWF0aGlldS5wdXlhZGVAY2h1LXBvaXRpZXJzLmZy; orcid.org/0000-0002-5639-0138
 Mathieu Puyade
Mathieu Puyade Amit Patel3
Amit Patel3 Norbert Blank
Norbert Blank Natalia Maximova
Natalia Maximova Raffaella Greco
Raffaella Greco Tobias Alexander
Tobias Alexander John A. Snowden
John A. Snowden