
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 02 April 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.631869
 Chien-Hsien Lo1,2†
Chien-Hsien Lo1,2† James Cheng-Chung Wei3,4,5,6†
James Cheng-Chung Wei3,4,5,6† Yu-Hsun Wang7
Yu-Hsun Wang7 Chin-Feng Tsai1,2
Chin-Feng Tsai1,2 Kuei-Chuan Chan1,2
Kuei-Chuan Chan1,2 Li-Ching Li8
Li-Ching Li8 Tse-Hsien Lo9
Tse-Hsien Lo9 Chun-Hung Su1,2*
Chun-Hung Su1,2*Objectives: Hydroxychloroquine (HCQ) is widely used to treat rheumatic diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and Sjögren’s syndrome (SS). Cardiac arrhythmia has been concerned as important safety issue for HCQ. The aim of this study was to investigate whether hydroxychloroquine increases new-onset arrhythmia among patients with RA, SLE or SS.
Methods: This was a retrospective cohort study that conducted from the longitudinal health insurance database of Taiwan. Patients with newly diagnosed RA, SLE or SS with age ≥20 years old were selected from 2000 to 2012. Patients who received HCQ and without HCQ treatment groups were matched by propensity score to minimize the effect of selection bias and confounders. The Cox proportional hazard model was used to analyze the risk of arrhythmia between the two groups after controlling for related variables.
Results: A total of 15892 patients were selected to participate and finally 3575 patients were enrolled in each group after matching. There was no different risk of all arrhythmia in patients using HCQ than without HCQ (adjusted hazards ratio 0.81, 95% CI 0.61–1.07) and ventricular arrhythmia as well. The incidence of arrhythmia did not increase when HCQ co-administrated with macrolides. The arrhythmia risk was also not different regardless of daily HCQ dose <400mg or ≥400mg or follow-up duration of ≦4 months or >4 months.
Conclusion: The administration of HCQ did not increase the risk of all cardiac arrhythmia and ventricular arrhythmia regardless of different duration of treatment (≦4 months or >4 months) or cumulative dose (<400mg or ≥400mg) in patients with common autoimmune diseases such as RA, SLE and SS.
Hydroxychloroquine (HCQ) is an antimalarial drug that also has been extensively used in certain rheumatic diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and Sjögren’s syndrome (SS) to control disease activity and improve survival for several decades (1–5). HCQ can modulate prothrombotic signaling pathways and protect against systemic inflammation by inhibiting endosomal NADPH oxidase (6). Coronavirus disease 2019 (Covid-19) become pandemic since 2019 December, currently still spreads rapidly, rising number of cases and deaths worldwide (7, 8). Because of Covid-19 pandemic, the efficacy and safety of HCQ has been more concerned and explored recently. Cardiac arrhythmia is one of the safety issues in patients receiving HCQ. Previous studies had been debating on the effect of HCQ and the risk of cardiac arrhythmia (9–11). A recent meta-analysis including 45 articles concluded that HCQ use was not associated with mortality, benefit or harm in Covid-19 patient (12). A prospective trial reported that prolongation of QTc interval was found more frequently in the HCQ group, but there was no increase in arrhythmia (13). Rosenberg et al. reported more patients in the HCQ experienced arrhythmias compared with non-HCQ group (14). McGhie et al. pointed that cumulative antimalarial dose did not significant associate with ECG structural abnormalities, while was protective for ECG conduction abnormalities (15). Recent study for hospitalized patients showed that the risk of supraventricular tachycardia, ventricular arrhythmia and AV block were not increased in HCQ group (16). The data about the treatment of HCQ and clinical outcome are mixing and still inconsistent. Therefore, we designed a retrospective cohort study from large population-based dataset to investigate whether HCQ increase the risk of arrhythmia or not in patients with rheumatic diseases including RA, SLE and SS.
We conducted a retrospective cohort study using data from the Taiwan National Health Insurance Research Database (NHIRD), which contains information on outpatient visits, emergency care, hospitalization, medical procedures, and medications. It contains one million people randomly sampled from the NHIRD with International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes (17) and data were collected from 1999 to 2013. This study was approved by the Research Ethics Committee of Chung Shan Medical University and Hospital (CS-17114).
This data was collected from 1999 to 2013 from the Longitudinal Health Insurance Database. We enrolled all people with age ≧20 years-old, who were newly diagnosed with RA (ICD-9-CM=714.0) (18, 19), SLE (ICD-9-CM=710.0) (6, 20) and SS (ICD-9-CM=710.2) with at least three outpatient clinic visits or one admission from 2000 to 2012. Patients with a diagnosis of arrhythmia (ICD-9-CM=426–427), before the diagnosis of disease RA, SLE or SS were excluded. The first date of HCQ after a disease diagnosis was set as the index date. The comparison group was non-use of HCQ after disease diagnosis. The comparison group included patients who had not been diagnosed with arrhythmia after the disease diagnosis. The primary outcome was defined as a new diagnosis of all arrhythmias including conduction disorders such as atrioventricular block and bundle branch block (ICD-9-CM=426), supraventricular tachyarrhythmias, ventricular tachyarrhythmias and sinus node dysfunction (ICD-9-CM=427). Ventricular tachyarrhythmias alone (ICD-9-CM=427.1, 427.4–427.5) was also analyzed as secondary outcome. The study period was from the index date to the first onset of arrhythmia, withdraw from the national health insurance system, or 2013/12/31, which came first.
To minimize the effect of confounding factors, we used propensity score (PS) matching to obtain a 1:1 matched by the age, gender, comorbidities such as hypertension (HTN: ICD-9-CM=401–405), hyperlipidemia (ICD-9-CM=272.0–272.4), chronic liver disease (ICD-9-CM=571), chronic kidney disease (CKD: ICD-9-CM=585), diabetes mellitus (DM: ICD-9-CM=250), chronic obstructive pulmonary disease (COPD: ICD-9-CM=491,492,496), ischemic heart disease (ICD-9-CM=410–414), heart failure (ICD-9-CM=428), stroke (ICD-9-CM=430–438), history of beta (β)-blocker usage, antibiotic macrolides treatment and index year. The pre-existing comorbidity was diagnosed one year before index date. The medications were used during the study period. PS matching is a statistical matching technique that can reduce potential confounding caused by unbalanced covariates in non-experimental settings. PS matching is the probability calculated via a logistic regression model. The score is a unit with certain characteristics, which can be used to reduce or eliminate selection bias.
To compare the characteristics of HCQ and non-HCQ groups, Chi-square test for categorical variables and independent t test for continuous variables were used. The incidence of events was determined by the number of events divided by the observed person-years. The Kaplan-Meier method was applied to obtain the cumulative incidences of newly diagnosed arrhythmia and log-rank test to perform the significance. We used a Cox proportional hazard model to estimate the crude hazard ratios (HR), adjusted HR, and 95% confidence intervals (CIs) among the two groups. The per-day HCQ dosage was also calculated to perform the risk of arrhythmia. All statistical analyses were conducted using software SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). A p value less than 0.05 between the two groups was considered to be statistically significant.
The flow chart for patient selection is demonstrated in Figure 1. Among the one million patients, a total of 15892 patients with newly diagnosed RA, SLE and SS were selected to participate in the study. 2988 patients were excluded due to previous arrhythmia before their RA diagnosis, those using antiarrhythmic agents such as amiodarone, dronedarone, or propafenone. After PS matching with 1:1 ratio, a total of 3575 patients were enrolled in the both groups respectively.
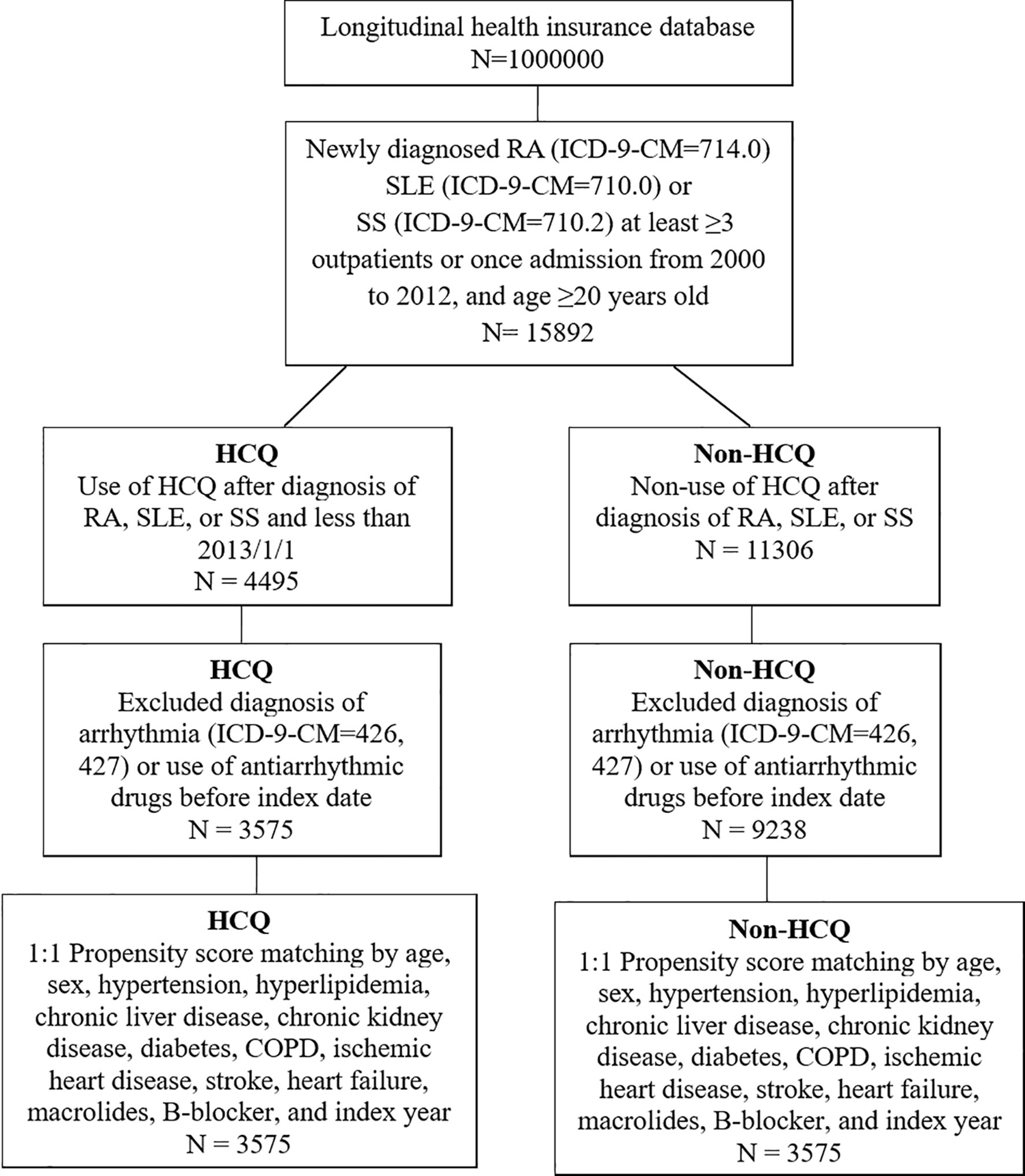
Figure 1 Flow chart of patient selection for those with rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) or Sjögren’s syndrome (SS), who were using hydroxychloroquine (HCQ) (study group) and or not using HCQ (non-HCQ control group) from the National Health Insurance Research Database.
Baseline demographic and clinical characteristics of the participants are summarized in Table 1. The mean (SD) age of the patients was 51.1 (13) years in the both groups. About 80% of the study population was female. Most underlying comorbidities were higher in non-HCQ group which were balanced after PS matching. 438 (12.3%) used a macrolide antibiotic combined with HCQ, whereas 420 (11.7%) patients used macrolide antibiotics in the non-HCQ group (p=0.512).
The main arrhythmias outcome was illustrated in Figure 2. The cumulative risk of both all arrhythmia (Figure 2A) and ventricular tachyarrhythmia (Figure 2B) were not different in the HCQ group compared with non-HCQ group (log-rank test, p=0.165 and p=0.548 respectively).
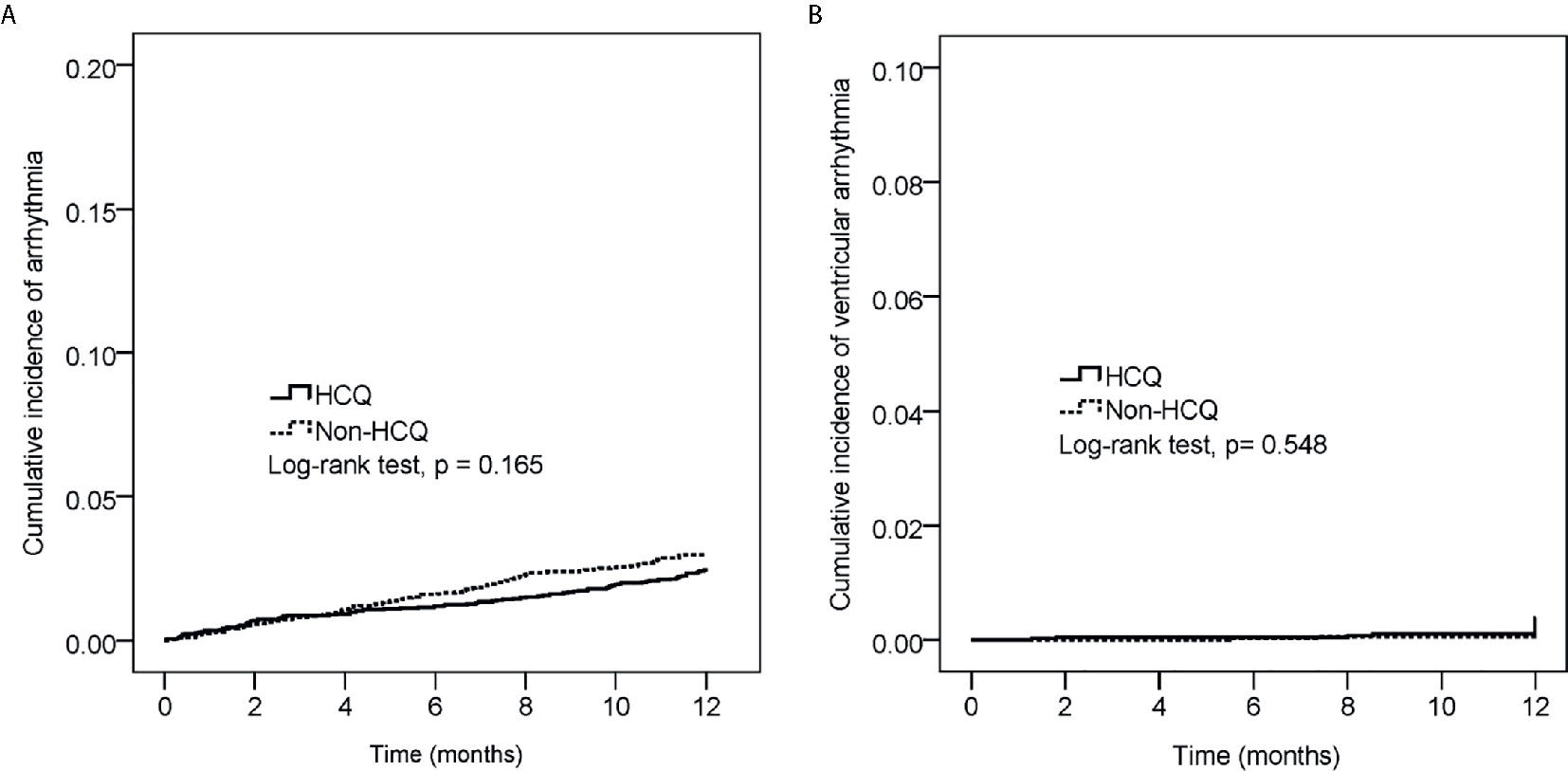
Figure 2 The cumulative incidence of all cardiac arrhythmia (A) and ventricular tachyarrhythmia (B) between the hydroxychloroquine (HCQ) group and the non-HCQ group (p-value=0.165 and p-value=0.548 respectively, Log-rank test).
The result of Cox regressions to determine the hazard ratios for arrhythmia is listed in Table 2. The incidence of arrhythmia did not increase in HCQ group with an adjusted HR of 0.81, 95% CI 0.61–1.07. The incidence of arrhythmia was 106 per 41916 person-months in the non-HCQ group and 87 per 42057 person-months in the HCQ group (Table S1). There was no different in ventricular arrhythmia in HCQ usage than in the non-HCQ group with an adjusted HR of 1.35, 95% CI 0.61–2.99 (Table S2). Age ≥50 years had higher risk of arrhythmia (adjusted HR 1.64, 95% CI 1.18–2.28). People with underlying hypertension, chronic kidney disease, ischemic heart disease, stroke and using β-blocker had significantly higher numbers of arrhythmias. Other comorbidities were not significantly different for the risk of arrhythmia.
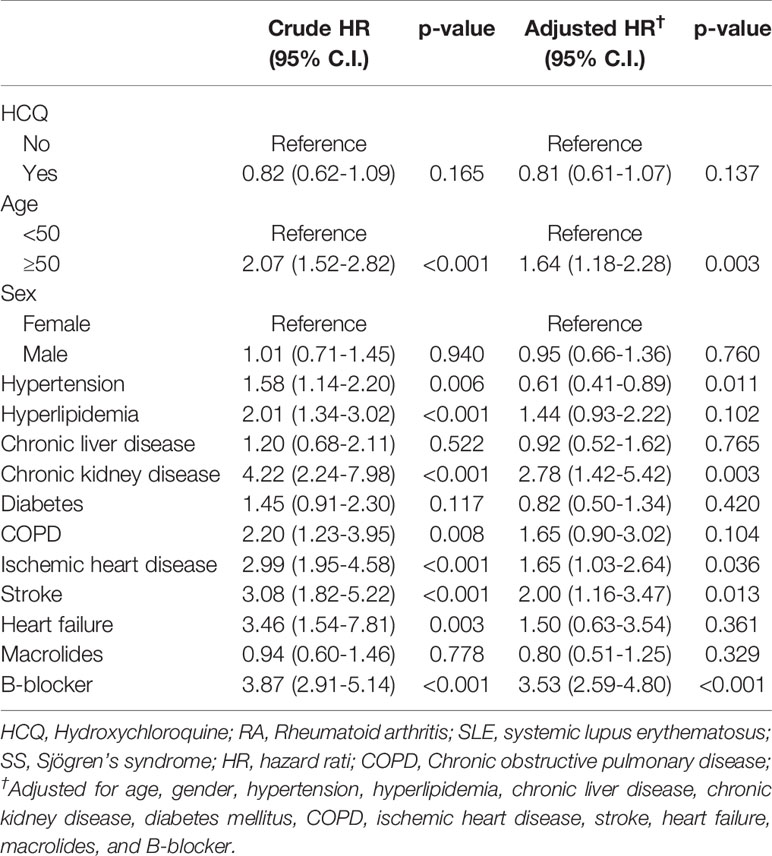
Table 2 Association of arrhythmia in RA, SLE or SS patients with multivariable analysis and Cox proportional hazard analysis.
The subgroup analysis for association of arrhythmia between HCQ and non-HCQ group is shown in Figure 3 and Table S3. There was no statistically significant difference between the combination of HCQ and macrolide antibiotics and incidence of arrhythmia (only 13 events over 438 patients in HCQ group compared with 9 events among 420 patients in the non HCQ group) (adjusted HR of 1.38, 95% CI 0.59–3.23). Age above or below 50 years, gender and β-blocker usage also did not increase the risk of arrhythmia either patients using HCQ or not. Figure 4, Tables S4, S5 provide the Cox regression hazard ratios for the relationships between arrhythmia and HCQ cumulative dose. All three diseases as well as individual RA, SLE and SS were also analyzed. We found that the risk of arrhythmia for HCQ did not significantly different regardless of the daily dose of <400 mg (adjusted HR 0.84, 95% CI 0.61–1.17) or ≥400 mg (adjusted HR 0.76, 95% CI 0.51–1.12), and follow-up duration of ≦4 months (adjusted HR 0.85, 95% CI 0.53–1.36) or >4 months (adjusted HR 0.78, 95% CI 0.55–1.12) compared with non HCQ usage. The different dose result was also consistent in all sub-analyzed individual disease.
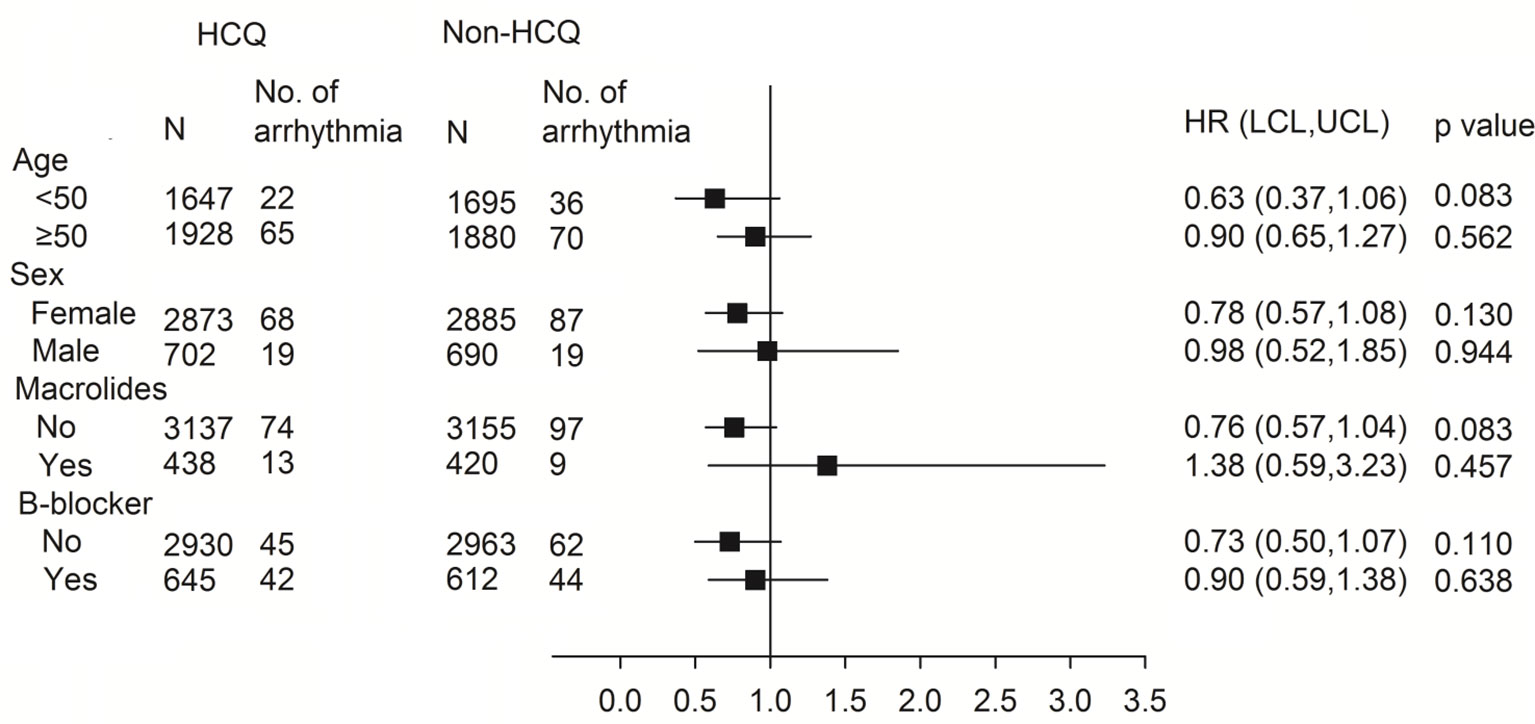
Figure 3 Subgroup analysis using the Cox proportional hazard model for the association between arrhythmia and HCQ. HR, hazard ratio; HCQ, hydroxychloroquine.
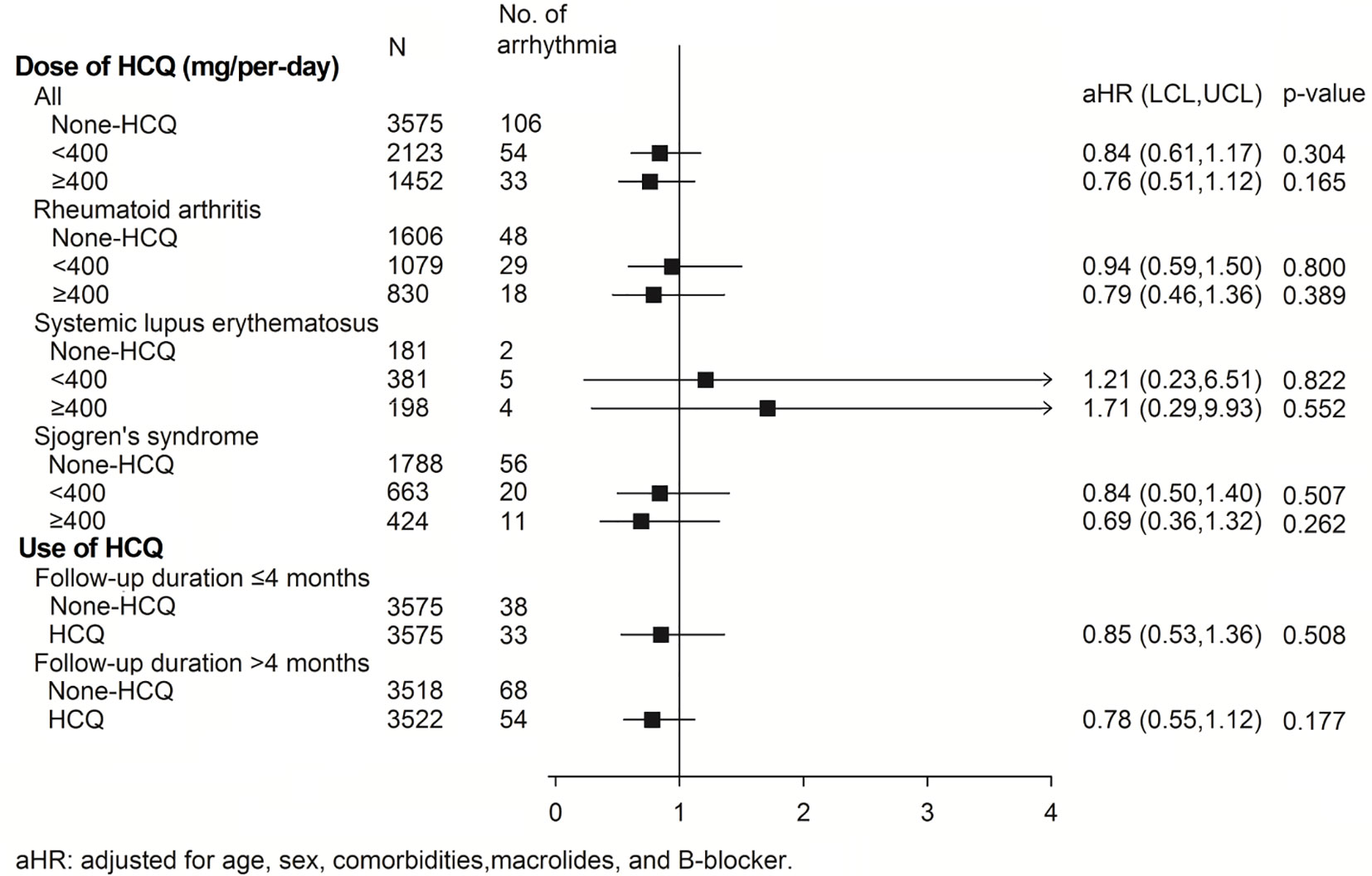
Figure 4 The risk of arrhythmias in the subgroup analysis of individual diseases and those with different daily HCQ doses and follow-up durations. aHR, adjusted hazard ratio; HCQ, hydroxychloroquine.
The aim of this study was to determine the safety concern of HCQ treatment and the risk of arrhythmia. The results indicated that common rheumatic disease patients using HCQ did not have higher risks of all kinds of cardiac arrhythmias including ventricular tachyarrhythmia. Furthermore, the risk of arrhythmia was not dependent on a longer duration of HCQ treatment >4 months or higher daily dose of ≥400mg.
HCQ has been used in many thousands of patients all over the world for the treatment of Covid-19 in early outbreak. However, recently observational and prospective studies have reported on the efficacy of HCQ treatment for Covid-19. Although some retrospective data disclosed use of HCQ alone and in combination with azithromycin decreased the Covid-19 associated mortality (21), most reports showed that HCQ treatment did not improve their clinical status or reduce the risk of intubation or death (13, 14, 16, 22). A recent systemic review and meta-analysis conducted by Putman et al. who collected 45 articles including 4 randomized controlled trials, 29 cohort studies and 12 case series, concluded that HCQ use was not associated with benefit or harm with regard to Covid-19 mortality (12). In addition, the safety of HCQ treatment in cardiac arrhythmia also had been discussed and results were inconsistent. Cavalcanti et al. conducted a prospective trial with 667 patients (13), and reported that prolongation of QTc interval was found more frequently in the HCQ group, but there was no increase in arrhythmia. Rosenberg et al. investigated 1438 hospitalized patients (14), reported 16% of patients in the HCQ experienced arrhythmias compared with 10% in the non-HCQ group. McGhie et al. analyzed 453 patients treated with antimalarial including HCQ and chloroquine which showed that cumulative antimalarial dose did not significant associate with ECG structural abnormalities, while was protective for ECG conduction abnormalities (15). Recent study from the RECOVERY collaborative group with 4716 hospitalized patients (16) showed that the risk of supraventricular tachycardia, ventricular arrhythmia and AV block were not increased in HCQ group. The study design, relatively small sample size and low event rate may have affected the outcomes and results. In the present study, we found no significant different between cardiac arrhythmia and the use of HCQ in most rheumatic patients.
An open-label trial discloses azithromycin added to HCQ was significantly more efficient for virus elimination (23). HCQ with or without azithromycin were used as a treatment option for Covid-19 in several countries during early outbreak (23, 24). However, co-administration of HCQ with other drugs such as azithromycin might amplify the arrhythmia risk. Some antibiotics, including macrolides, show pharmacodynamic evidence of iKr inhibition, which results in QT prolongation and dispersion of recovery across the ventricular wall. This phenomenon also has the chance to induce TdP and increase the risk of cardiovascular death (25, 26). In animal studies, there are no synergistic arrhythmic effects of azithromycin with or without chloroquine (27). Nevertheless, one retrospective cohort study reported that patients taking azithromycin had increased risk of mortality, including cardiovascular death, compared with those without antibiotic use during the 5 days of therapy (26). Several studies report the combination of HCQ and azithromycin in Covid-19 treatment prolonged the QT interval, however inconsistent outcomes about the development of life-threatening arrhythmia and mortality were found (13, 28–30). Hence, we also analyzed whether arrhythmia increased or not in the specific subgroups using HCQ with added macrolide treatment for other reasons, which showed that combination therapy of HCQ with macrolides also did not increase any arrhythmia (Figure 3 and Table S3). Nonetheless, the event numbers (arrhythmias) are low in both the HCQ and non-HCQ groups. Even so, it may support the safety of HCQ combined with macrolide therapy.
Regarding comorbidities (Table 2), patients with CKD, ischemic heart disease and stroke had a significantly increased risk of arrhythmia. The anticipated result of CKD come from CKD related various arrhythmogenic alternation including autonomic nervous system, metabolic hemostasis, pharmacokinetics and pharmacodynamics of drugs that may facilitate cardiac arrhythmias (31, 32). β-blockers provide an antiarrhythmic effect because they decrease sympathetic activity by inhibition of the β1 adrenergic receptor and reduce atrial also ventricular tachyarrhythmia (33, 34). But they are also widely used in other cardiovascular diseases rather than arrhythmia such as post myocardial infarction, heart failure, and hypertension (35, 36). In our study, β-blockers were matched by PS matching to reduce possible confounding bias. We found that patients taking β-blocker developed more arrhythmia with an adjusted hazard ratio of 3.53. It may be due to patients taking β-blocker had more underlying comorbidities. In the subgroup analysis, β-blocker did not further increase or decrease risk of arrhythmia either patients using HCQ or not.
Recently we have reported that patients with RA using HCQ did not have a higher risk of cardiac arrhythmia regardless of the daily HCQ dose or follow-up duration (37), which result is consistent with the current study. In addition to RA population, our study includes SLE and SS which increase the sample size, better matching between the 2 groups and further subgroup analysis. We also determined the sensitivity analysis for development of only ventricular tachyarrhythmia as secondary outcome, which also disclosed consistent outcome. The sub-group analysis of the individual disease of RA, SLE and SS, all revealed no significant increase risk of arrhythmia. Our data showed that the administration of HCQ had a neutral effect on the development of all arrhythmias and ventricular tachyarrhythmias. The previous study reports cardiotoxicity of HCQ was possibly associated with cumulative dose (38). The chronic use of HCQ has also been reported to provoke cardiac arrhythmia (10). According to the sub-analysis (Figure 4, Tables S4 and S5), we did not find the incidence of cardiac arrhythmia increase with larger HCQ dose or longer follow-up duration. These data suggest the safety of HCQ regardless of different dose (<400mg or ≥400mg) or treatment duration (≦4 months or >4 months).
There are some strengths and limitations in our study. The strength of this study stands its large NHIRD system, covers 99% of the Taiwanese population, which minimize bias from selection, poor recall or participation (39). Limitations were also notified. First, electrocardiography (ECG) is recommended to measure the QTc interval in individuals receiving HCQ administration (40). In the database, we could not determine the QTc interval by ECG. Nevertheless, our result is safe for risk of arrhythmia in HCQ therapy even we did not provide a baseline ECG indicated that routine ECG data is not always required. Second, only macrolide antibiotics were analyzed as combination therapy, and we did not further investigate other possible drugs that could have prolonged QT. Third, duration and dose of macrolides are not reported as the event numbers is less. Thus claims of association between HCQ and macrolides should be limited. Fourth, diseases were defined according to ICD-9 codified data without any procedure codes, which may lead to over diagnosis. Thence, we added disease codes with at least three outpatient clinic visits or one admission to reduce this bias. Fifth, our study population limited for the patients with RA, SLE and SS hence the results should not be totally applied on patients using HCQ for other diseases. Finally, this is a retrospective cohort design so patient follow-up is not by protocol. There may remain as the possibility of having mild cases of arrhythmias in either group which may have gone undetected. Further larger prospective or randomized control trials are necessary to verify the outcomes of this study.
The administration of HCQ in patients with RA, SLE and SS did not increase the risk of cardiac arrhythmia as well as ventricular tachyarrhythmia regardless of the treatment duration (≦4 months or >4 months) or cumulative dose (<400mg or ≥400mg). The outcome is also consistent with others for combination HCQ with macrolide antibiotics.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Research Ethics Committee of Chung Shan Medical University and Hospital (approval no. CS-17114). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
C-HL and JC-CW designed the study, generated the figures and wrote the manuscript. Y-HW analyzed the data and generated the figures. C-FT, K-CC, L-CL and T-HL performed the bioinformatics analysis and wrote the manuscript. C-HS made substantial contributions to the design of the study, conducted the data analysis and figure generation, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.631869/full#supplementary-material
1. Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol (2012) 42:145–53. doi: 10.1007/s12016-010-8243-x
2. Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis (2007) 66:1168–72. doi: 10.1136/ard.2006.068676
3. Group CHS. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med (1991) 324:150–4. doi: 10.1056/NEJM199101173240303
4. Rempenault C, Combe B, Barnetche T, Gaujoux-Viala C, Lukas C, Morel J, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis (2018) 77:98–103. doi: 10.1136/annrheumdis-2017-211836
5. Wang SQ, Zhang LW, Wei P, Hua H. Is hydroxychloroquine effective in treating primary Sjogren’s syndrome: a systematic review and meta-analysis. BMC Musculoskelet Disord (2017) 18:186. doi: 10.1186/s12891-017-1543-z
6. Yang DH, Leong PY, Sia SK, Wang YH, Wei JC. Long-Term Hydroxychloroquine Therapy and Risk of Coronary Artery Disease in Patients with Systemic Lupus Erythematosus. J Clin Med (2019) 8:796. doi: 10.3390/jcm8060796
7. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
8. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
9. Yanturali S, Aksay E, Demir O. Massive hydroxychloroquine overdose RA. Acta Anaesthesiol Scand (2004) 48:379–81. doi: 10.1111/j.0001-5172.2004.0302.x
10. Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila) (2006) 44:173–5. doi: 10.1080/15563650500514558
11. Costedoat-Chalumeau N, Hulot JS, Amoura Z, Leroux G, Lechat P, Funck-Brentano C, et al. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatol (Oxford) (2007) 46:808–10. doi: 10.1093/rheumatology/kel402
12. Putman M, Chock YPE, Tam H, Kim AHJ, Sattui SE, Berenbaum F, et al. Antirheumatic Disease Therapies for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Arthritis Rheumatol (2021) 73:36–47. doi: 10.1002/art.41469
13. Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med (2020) 383:2041–52. doi: 10.1056/NEJMoa2019014
14. Rosenberg ES, Elizabeth M Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA (2020) 23:2493–502. doi: 10.1001/jama.2020.8630
15. McGhie TK, Harvey P, Su J, Anderson N, Tomlinson G, Touma Z. Electrocardiogram abnormalities related to anti-malarials in systemic lupus erythematosus. Clin Exp Rheumatol (2018) 36:545–51.
16. Group RC, Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med (2020) 383:2030–40. doi: 10.1056/NEJMoa2022926
17. Cheng TM. Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (2003) 22:61–76. doi: 10.1377/hlthaff.22.3.61
18. Chang YJ, Lee YH, Leong PY, Wang YH, Wei JC. Impact of Rheumatoid Arthritis on Alopecia: A Nationwide Population-Based Cohort Study in Taiwan. Front Med (Lausanne) (2020) 7:150. doi: 10.3389/fmed.2020.00150
19. Liu Y-T, Tsou H-K, Chiou JY, Wang YH, Chou MC, Wei JC. Association of Human Papillomavirus Infection With Risk for Rheumatoid Arthritis: A Population-Based Cohort Study. Ann Rheum Dis (2019) 78:1734–6. doi: 10.1136/annrheumdis-2019-215931
20. Wu CY, Tan M, Huang JY, Chiou JY, Wei JC. Hydroxychloroquine is neutral in risk of chronic kidney disease in patients with systemic lupus erythematosus. Ann Rheum Dis (2020) 217728. doi: 10.1136/annrheumdis-2020-217728
21. Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis (2020) 97:396–403. doi: 10.1016/j.ijid.2020.06.099
22. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med (2020) 382:2411–8. doi: 10.1056/NEJMoa2012410
23. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents (2020) 56:105949. doi: 10.1016/j.ijantimicag.2020.105949
24. Bhimraj A, Morgan R, Shumake A, Lavergne V, Baden L, Vincent CC, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis (2020), ciaa478. doi: 10.1093/cid
25. Yap Y, Camm J. Risk of torsades de pointes with non-cardiac drugs. BMJ (2000) 320:1158–9. doi: 10.1136/bmj.320.7243.1158
26. Ray W, Murray K, Hall K, Arbogast P, Stein M. Azithromycin and the Risk of Cardiovascular Death. N Engl J Med (2012) 366:1881–90. doi: 10.1056/NEJMoa1003833
27. Fossa A, Wisialowski T, Duncan J, Deng S, Dunne M. Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. Am J Trop Med Hyg (2007) 77:929–38. doi: 10.4269/ajtmh.2007.77.929
28. Cipriani A, Zorzi A, Ceccato D, Capone F, Parolin M, Donato F, et al. Arrhythmic profile and 24-hour QT interval variability in COVID-19 patients treated with hydroxychloroquine and azithromycin. Int J Cardiol (2020) 316:280–4. doi: 10.1016/j.ijcard.2020.05.036
29. Duska F, Waldauf P, Halacova M, Zvonicek V, Bala J, Balik M, et al. Azithromycin added to hydroxychloroquine for patients admitted to intensive care due to coronavirus disease 2019 (COVID-19)-protocol of randomised controlled trial AZIQUINE-ICU. Trials (2020) 21:631. doi: 10.1186/s13063-020-04566-x
30. Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect (2020) 50:384. doi: 10.1016/j.medmal.2020.03.006
31. Potpara TS, Jokic V, Dagres N, Marin F, Prostran MS, Blomstrom-Lundqvist C, et al. Cardiac Arrhythmias in Patients With Chronic Kidney Disease: Implications of Renal Failure for Antiarrhythmic Drug Therapy. Curr Med Chem (2016) 23:2070–83. doi: 10.2174/0929867323666160309114246
32. Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J (2018) 39:2314–25. doi: 10.1093/eurheartj/ehy060
33. Coumel P, Leclercq J-F, Escoubet B. Beta-blockers: Use for arrhythmias. Eur Heart J (1987) 8(supplment A):41–52. doi: 10.1093/eurheartj/8.suppl_A.41
34. Zicha S, Tsuji Y, Shiroshita-Takeshita A, Nattel S. Beta-blockers as antiarrhythmic agents. Handb Exp Pharmacol (2006) 171:235–66.
35. Borrello F, Beahan M, Klein L, Gheorghiade M. Reappraisal of beta-blocker therapy in the acute and chronic post-myocardial infarction period. Rev Cardiovasc Med (2003) 4(Suppl 3):S13–24. doi: 10.1016/j.amjcard.2004.01.021
36. Domanski M, Krause-Steinrauf H, Massie B, Deedwania P, Follmann D, Kovar D, et al. A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail (2003) 9:354–63. doi: 10.1054/S1071-9164(03)00133-7
37. Lo CH, Wang YH, Tsai CF, Chan KC, Li LC, Lo TH, et al. Correspondence on ‘Festina lente: hydroxychloroquine, COVID-19 and the role of the rheumatologist’ by Graef et al. Ann Rheum Dis (2020) 218589. doi: 10.1136/annrheumdis-2020-218589
38. Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac Complications Attributed to Chloroquine and Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf (2018) 41:919–31. doi: 10.1007/s40264-018-0689-4
39. Hsing A, Ioannidis J. Nationwide Population Science: Lessons From the Taiwan National Health Insurance Research Database. JAMA Intern Med (2015) 175:1527–9. doi: 10.1001/jamainternmed.2015.3540
40. Kapoor A, Pandurangi U, Arora V, Gupta A, Jaswal A, Nabar A, et al. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19 patients: A scientific statement from the Indian Heart Rhythm Society. Indian Pacing Electrophysiol J (2020) 20:117–20. doi: 10.1016/j.ipej.2020.04.003
Keywords: Hydroxychloroquine, arrhythmia, rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome
Citation: Lo C-H, Wei JC-C, Wang Y-H, Tsai C-F, Chan K-C, Li L-C, Lo T-H and Su C-H (2021) Hydroxychloroquine Does Not Increase the Risk of Cardiac Arrhythmia in Common Rheumatic Diseases: A Nationwide Population-Based Cohort Study. Front. Immunol. 12:631869. doi: 10.3389/fimmu.2021.631869
Received: 21 November 2020; Accepted: 11 March 2021;
Published: 02 April 2021.
Edited by:
Amr Sawalha, University of Pittsburgh, United StatesReviewed by:
Graciela Alarcon, University of Alabama at Birmingham, United StatesCopyright © 2021 Lo, Wei, Wang, Tsai, Chan, Li, Lo and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Hung Su, c3VjaDE5NzQwOEBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.