- 1College of Public Health and Social Justice, Saint Louis University, St. Louis, MO, United States
- 2Catholic Induced Pluripotent Stem Cell Research Center, College of Medicine, The Catholic University of Korea, Seoul, South Korea
- 3Division of Rheumatology, Department of Internal Medicine, Seoul St. Mary's Hospital, Institute of Medical Science, College of Medicine, The Catholic University of Korea, Seoul, South Korea
Mesenchymal stem cell (MSC) therapies have been used as cell-based treatments for decades, owing to their anti-inflammatory, immunomodulatory, and regenerative properties. With high expectations, many ongoing clinical trials are investigating the safety and efficacy of MSC therapies to treat arthritic diseases. Studies on osteoarthritis (OA) have shown positive clinical outcomes, with improved joint function, pain level, and quality of life. In addition, few clinical MSC trials conducted on rheumatoid arthritis (RA) patients have also displayed some optimistic outlook. The largely positive outcomes in clinical trials without severe side effects establish MSCs as promising tools for arthritis treatment. However, further research is required to investigate its applicability in clinical settings. This review discusses the most recent advances in clinical studies on MSC therapies for OA and RA.
Introduction
The first study on mesenchymal stem cells (MSCs) was published in 1966 by Fridenshtein et al., who cultured bone-forming cells from guinea-pig bone marrow and spleen cells (1, 2). Subsequent studies have characterized MSCs as clonogenic progenitor cells capable of differentiating into mesoderm-derived cells such as osteoblasts, chondrocytes, and adipocytes (1, 3–5). The term “mesenchymal stem cell” was first used in 1991 to represent cells originating from embryonic mesodermal tissues (5, 6). While, MSCs imply mesenchymal “stem” or “stromal” cells at the same time, it is suggested only to refer progenitor cells with self-renewal and differentiation ability as “mesenchymal stem cells.” Mesenchymal stromal cells, on the other hand, refer to a bulk population of cells with immunomodulatory and homing properties. Some researchers, however, have recently argued that MSCs should be renamed “medicinal signaling cells” because these cells secrete therapeutic regenerative bioactive factors to stimulate the site- and tissue-specific resident stem cells of patients rather than differentiating into tissue-producing cells (7). Nevertheless, the wide clinical potential of MSCs, which ranges from repairing simple tissue tears to regulating immunological diseases, remains to be fully elucidated (2). Hence, researchers worldwide continue to explore the applications of MSCs. Notably, there were 1,043 trials involving 47,548 patients conducted between 2011 and 2018 (2).
Although the criteria for defining human MSCs are not concrete and subject to changes, most researchers agree on the three defining characteristics of human MSCs established by the International Society of Cellular Therapy (ISCT) (8, 9). The first general characteristic of MSCs is plastic adherence where cells with clonal expansion ability can be maintained for several passages in plastic culture dishes while excluding the subpopulation of cells with hematopoietic functions (9, 10). This characteristic is generally believed to encompass all types of MSCs without any exceptions. The second feature of human MSCs is the unique set of positive and negative surface markers expressed on these cells; the ICST has proposed CD105 (endoglin), CD73 (ecto-5′-nucleotidase), and CD90 (Thy-1) as the surface markers of MSCs. In contrast, MSCs lack the expression of hematopoietic and endothelial markers, such as CD45, CD34, CD14, CD11b, CD79α, CD19, and human leukocyte antigen (HLA)-DR (8, 9). Although the list proposed by the ICST is generally agreed upon by researchers, this criterion is the most disputed; some researchers regard CD34, CD45, and CD14 as negative markers, while STRO-1, CD29, CD73, CD90, CD105, CD106, CD166, CD146, and CD44 are considered positive markers (11). Multiple studies have also discovered variations from this criterion. For instance, fractions of adipose tissue derived-MSCs (AT-MSCs) were observed to express CD34 when insulin-like growth factor 1 (IGF-1) was added to the culture media (12). Moreover, the expression of the negative marker HLA-DR was upregulated in murine and human MSCs after exposure to interferon-γ (IFN-γ) (13, 14). Hence, positive and negative surface markers are not widely used to classify in vitro-expanded MSCs, and further research is needed to clarify this criterion. The final and most defining characteristic of MSCs is the ability to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro (8). As mentioned earlier, the differentiation potential of MSCs into various cell lineages was a factor in their early classification as a type of stem cell and remains one of their key traits (6).
The first clinical studies involving MSCs assessed their therapeutic potential in hematopoiesis, osteogenesis imperfecta, Hurler syndrome, and metachromatic leukodystrophy (15–19). These studies provided initial safety assessments for MSCs and encouraged further research to thoroughly examine their clinical efficacy (15). Recent decades have seen clinical trials conducted on these cells, especially for autoimmune, neurodegenerative, cardiovascular, and bone and cartilage diseases (20). However, the number of approved MSC treatments worldwide remains limited. Asian countries have approved more MSC treatments than other countries; South Korea has approved four MSC therapies, whereas Japan and India have each approved one (Table 1).
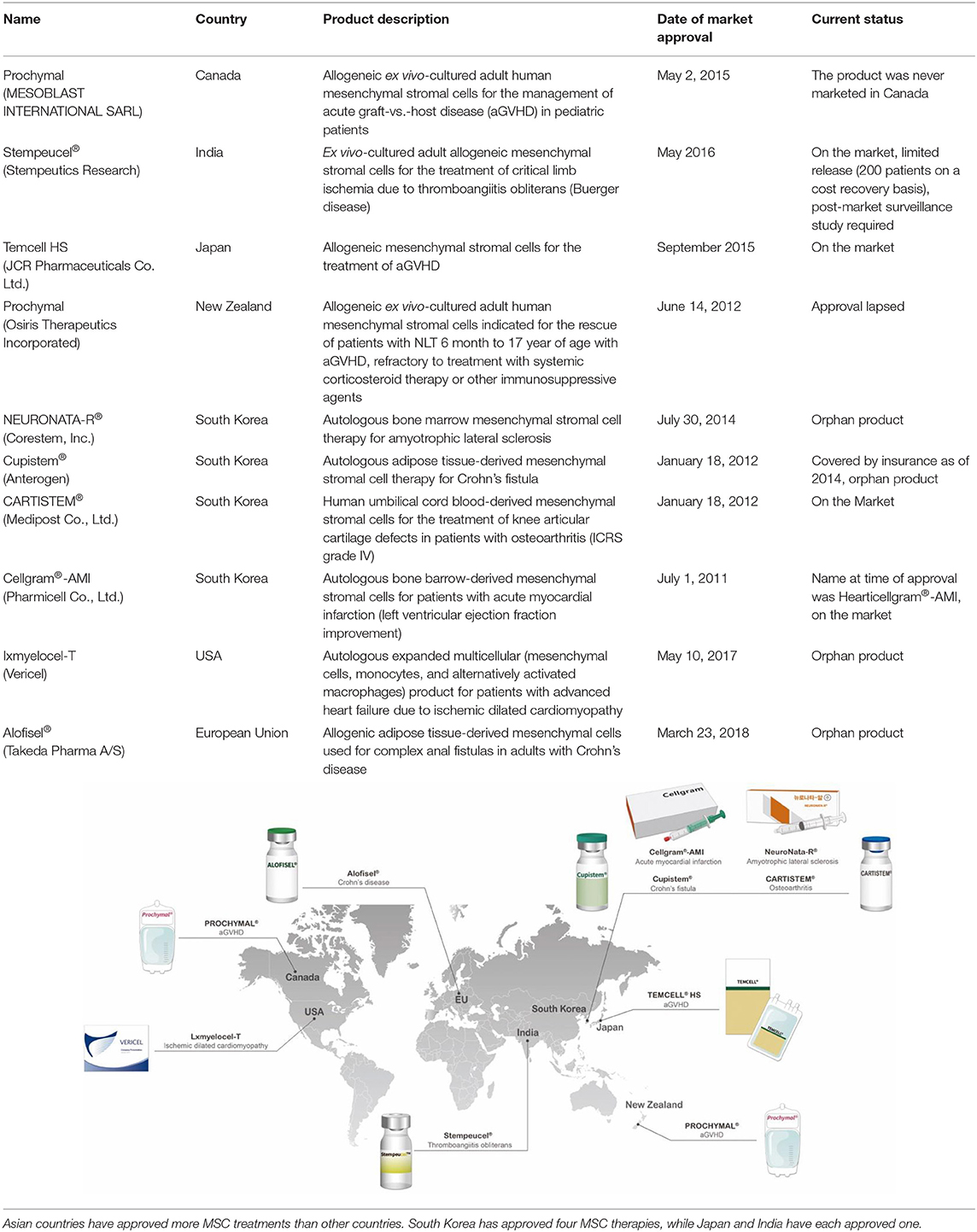
Table 1. A list of approved cell therapy products around the world and a graphical image of countries where MSC therapies are approved and clinically used.
Human Tissues Containing MSCs and the Various Potentials of These Cells
The ISCT MSC committee recommends not to use the term mesenchymal “stem” cells unless there is rigorous in vitro and in vivo functional evidence that can provide the self-renewal and differentiation ability (9). While MSCs are found in various parts of the human body, MSCs isolated from the bone marrow (BM), umbilical cord blood, periosteum, dental pulp, adipose tissue, and growth plate were confirmed to have stem cell-like properties [Figure 1; (21–23)]. In this section, we will discuss four sites where MSCs are frequently found and used for treatment of arthritic diseases: the bone marrow, umbilical cord, adipose tissue, and synovial membrane (24). To select a suitable MSC source for treatment, both advantages and disadvantages of MSC acquisition including the potential side effects and limitations (e.g., cell quality, number, and the difficulty and invasiveness of the isolation process) must be considered (25).
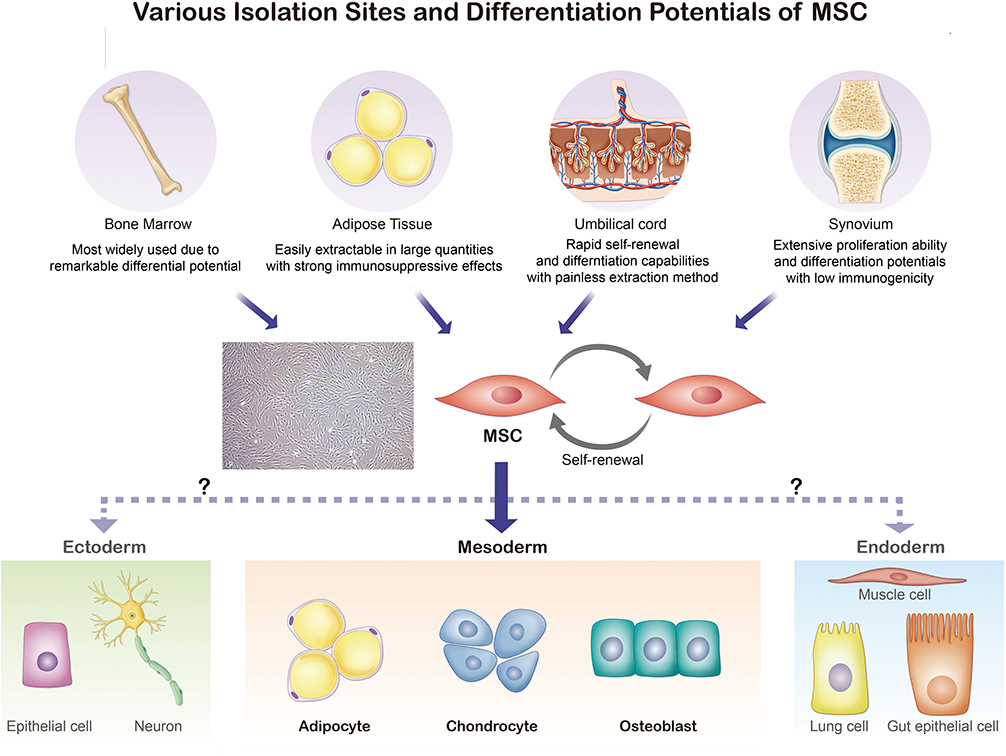
Figure 1. Various isolation sites and differentiation potentials of MSCs. Bone marrow, adipose tissue, umbilical cord, and synovium are common sites for MSC extraction. The isolated MSCs can be differentiated into adipocytes, chondrocytes, myocytes, and osteoblasts.
Bone marrow was the initial extraction site used by Fridenshtein et al. (1). After years of animal studies, the isolation and expansion of human bone marrow-derived MSCs (BM-MSCs) in culture were first conducted in 1992 (2, 26). With the safety and effectiveness of BM-MSCs confirmed through multiple clinical trials, they have become the most widely-used source of MSCs characterized by remarkable differentiation potential (27). However, there are several limitations of BM-MSCs. Most importantly, the yields, along with the differentiation and repair potential, are heavily dependent on the donor characteristics, such as disease condition and age (25). Furthermore, BM-MSC harvesting is challenging and inefficient, as only 0.001–0.01% of bone marrow cells are MSCs (27, 28). The risk of infection during the isolation of cells from the bone marrow also cannot be ignored. Consequently, a more effective and less invasive procedure is required, and scientists have attempted to identify new extraction sites (27, 28).
In 2000, human umbilical cord blood was recognized as an alternative source of MSCs (29). Umbilical cord blood-derived MSCs (UCB-MSCs) show rapid self-renewal and differentiation capabilities, thereby promoting tissue repair and modulation of immune responses; moreover, these cells are easy to access with painless extraction procedures (30). UCB-MSCs have rapid proliferation rates that are approximately three- to four-fold greater than that of adipose tissue (AT)-MSCs (31, 32). Furthermore, UCB-MSCs are known to secrete multiple growth factors associated with skin rejuvenation, such as epithelial growth factor (EGF), collagen type 1, hepatocyte growth factor (HGF), and growth differentiation factor-11 (GDF-11) (33). Indeed, UCB-MSCs have been reported to possess anti-wrinkling effects and the ability to increase dermal density. Because of these benefits, researchers claim that the clinical applications of UCB-MSCs extend beyond the limits of those of BM-MSCs (34). However, previous studies have also reported undesirable characteristics of UCB-MSCs, such as earlier morphological changes and faster loss of amplification ability, along with lower attachment efficiency (31, 35, 36).
Human AT-MSCs were identified as another promising source of MSCs in 2001, because of its accessibility and abundancy as well as its stronger immunosuppressive effects. Unlike BM-MSCs, AT-MSCs can be extracted in large, concentrated quantities (about 500 times more than BM-MSCs) using relatively simple procedures and local anesthesia (37). Another benefit of AT-MSCs is that they can be extracted from various human body sites; however, AT-MSCs extracted from different sites have shown varied traits (38). For instance, Nepali et al. concluded that orbital AT-MSCs have higher expressions of CD73, CD90, CD105, and CD146, but lower expressions of CD31, CD45, and HLA-DR, than abdominal AT-MSCs (38). Moreover, Kim et al. reported increased expression of HLA-ABC and HLA-DR in AT-MSCs after IFN-γ treatment, raising concerns about the application of allogenic AT-MSCs (32). Hence, more research investigating donor-matched AT-MSCs from different isolation sites and their respective traits is required to fully understand the defining phenotypes and increase the clinical efficiency of these MSCs (28, 38).
While the previously mentioned sites represent the most common tissues for MSC extraction, the synovial membrane also contains MSCs. Synovial membrane-derived MSCs (SM-MSCs) were first isolated in 2001 by De Bari et al. (39). Like AT-MSCs, SM-MSCs can be extracted from various sites, including the cotyloid fossa or paralabral synovium, with site-specific traits (40). Interestingly, SM-MSCs have extensive proliferative ability, multilineage differentiation potential, and low immunogenicity relative to other MSCs (39, 41). Due to higher expression of type II collagen, aggrecan, and SRY-box transcription factor 9 in SM-MSCs, they have demonstrated higher chondrogenic potential than MSCs from other sources and are expected to be more widely used for cartilage repair and joint homeostasis treatments (42–44). Moreover, a study by Sakaguchi et al. concluded that SM-MSCs and BM-MSCs have greater osteogenic and adipogenic potentials than other MSCs; however, SM-MSCs foster relatively low-density expansions in vitro compared to BM-MSCs (41, 45).
Applications of MSC Therapies for Cartilage Injuries
The safety and efficacy of MSCs in the treatment of joint-related diseases and cartilage injuries have been continuously examined over the recent decades. Concurrently, the prevalence of cartilage lesions have also significantly increased during this period, as the early incidence rates of this condition have roughly tripled from 1996 to 2011 (46, 47). Despite the high prevalence rate, a universally efficient method for articular cartilage repair is yet to be developed (48). Current surgical options include arthroplasty, microfracture, and autologous chondrocyte implantation (49). The promising qualities of MSC-based therapies could potentially provide effective, less invasive procedures to repair articular cartilage defects. In an experimental trial, BM-MSCs were transplanted into the patellae of two patients with full-thickness articular cartilage defects (50). Two years after transplantation, the arthroscopic results showed significant improvements in the walking abilities of both patients (50). Similarly, another case study involving a judo athlete diagnosed with a full-thickness cartilage defect in the medial femoral condyle exhibited recovery within months after the implantation of MSC-embedded collagen gel with reduced pain (51). Furthermore, a 2010 study compared the clinical outcomes of cartilage lesion repair between implantations of first-generation autologous chondrocytes and BM-MSCs in groups of 36 patients each; all patients showed improvements in quality of life with no significant differences between the groups (52). Therefore, it was concluded that BM-MSC treatment is a cost-efficient option for cartilage lesion repair with minimal donor-site morbidity and fewer surgical procedures than autologous chondrocyte implantation (52). Thus, multiple clinical trials have revealed the promising potential of MSC therapy in cartilage repair.
Variations in the general characteristics of rheumatoid arthritis (RA) and osteoarthritis (OA) largely depend on their etiologies and initial symptoms. RA is a chronic systemic autoimmune disease characterized by joint inflammation and bone erosion (53, 54), whereas OA is a degenerative joint disease that triggers the gradual loss of articular cartilage (55, 56). While increased bone spur growth is observed in osteoarthritic joints during the early stages, RA joints initially experience synovial inflammation (Figure 2). Ultimately, patients diagnosed with either joint condition suffer from severe cartilage inflammation and loss of mobility (56, 57). In this review, we will further discuss the use of MSCs in these two respective joint diseases.
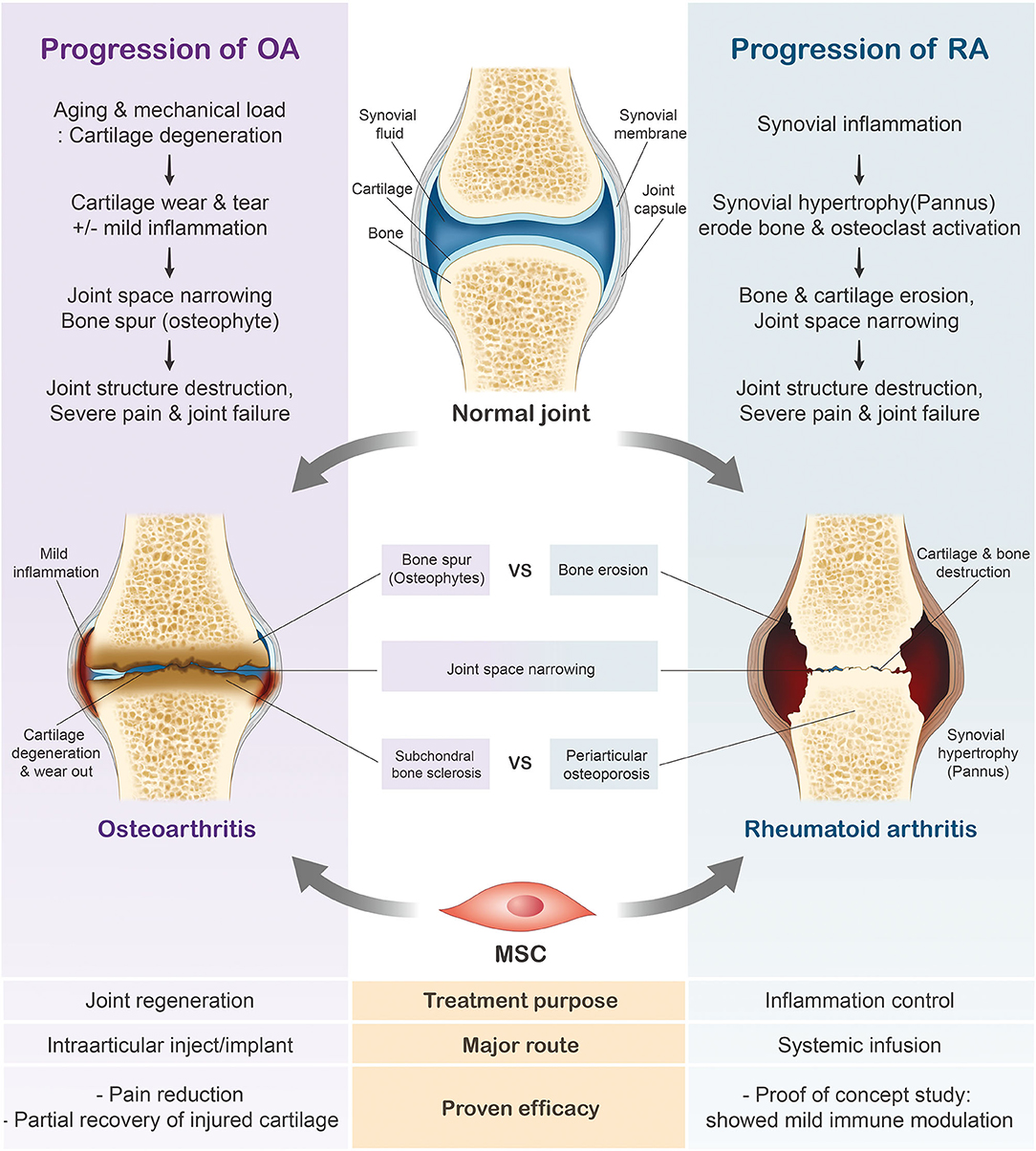
Figure 2. Progression of rheumatoid arthritis (RA) and osteoarthritis (OA). In contrast to the healthy joint, the osteoarthritic joint has thinned cartilage and the bone ends rub together. Joints diagnosed with RA have swollen, inflamed synovial membranes and undergo bone erosion. The cartilage erosion seen in the later stages of arthritis ultimately leads to the loss of mobility.
Applications of MSC Therapies in OA
General Characteristics of OA
OA is the most common degenerative joint disease (55). Its initial symptoms include loss of articular cartilage followed by progressive to joint stiffness, swelling, pain, and loss of mobility (56). The prevalence rate of OA is extremely high, affecting more than 250 million people worldwide (58–60). With increases in the aging and obese populations, the prevalence of OA is predicted to increase to 67 million by 2030 (61). Hence, an effective and safe OA treatment is urgently required.
Like RA, OA is also divided into two groups based on its etiology: primary and secondary (59). Primary OA is idiopathic and gene-dependent, whereas secondary OA mainly originates from traumatic events (58, 62, 63). Despite different etiologies, the two types of OA progress in similar directions, ultimately resulting in the loss and destruction of articular cartilage (55).
Although aging is one of the biggest risk factors for OA, the underlying mechanisms and related factors are yet to be definitively established (55, 56, 64). An imbalance in the production and activities of catabolic mediators in aging cells is a cause of the destruction and loss of articular cartilage (65). A disturbed ratio of the transforming growth factor β (TGF-β) receptors activin receptor-like kinase 1 (ALK1) and ALK5 triggers the downregulation of the TGF-β pathway and induces matrix metalloproteinase (MMP) expression, which degrades structural proteins in the cartilage (55, 66). The exact reason for this imbalance in signaling is assumed to be the senescent phenotype of OA chondrocytes, but a clear explanation is still required (65). Age-related mitochondrial dysfunction has also been suggested to promote the development of OA (66, 67). Aged articular chondrocytes and other cells display increased secretion of reactive oxygen species (ROS), and thereby elevated oxidative stress (67–69). The production of ROS ultimately alters mitochondrial function, leaving them unable to synthesize proteoglycans, the primary building blocks of the cartilage extracellular matrix (ECM) (70).
Genetic predisposition is another major risk factor of primary OA, with almost 30–65% of OA risk being genetically determined (56, 64, 71, 72). Recent genome-wide association studies (GWAS) have drastically expanded our understanding of the genetic risk factors of OA (71, 73). Currently, 90 loci are known to pose significant OA genetic risks, and 80 possible gene mutations or single nucleotide polymorphisms (SNPs) have been identified to be involved in OA pathogenesis (74). These include genes encoding other structural factors (Col2a1, Col9a1, and Col11a1) and bone morphogenetic proteins (Gdf5) (75–81). There are various ongoing studies focused on fully uncovering the genetic risk factors of OA. Most notably, a 2019 study analyzed ~77,000 patients with OA and 378,000 undiagnosed individuals from the UK Biobank cohort to identify 52 novel OA-associated signals (74). While most studies have specifically investigated OA susceptibility loci in Europeans or those of European descent, there have also been studies targeting other populations (82–85). A 2020 study by Zhao et al. showed a significant correlation between the SNP rs10896015 in the LTBP3 gene and hip OA among the Chinese population (86). Furthermore, another study revealed that the SNP rs4238326 in the ALDH1A2 gene, which was previously reported to trigger hand OA in European populations, is also linked to knee OA risk (86, 87).
Obesity has become a highly prevalent disease in contemporary society and it is estimated to spread to almost 20% of the global population by 2030 (88). Obese patients with unhealthy diets are exposed to multiple risk factors of OA, as one study reports a 24% increase in the likelihood of developing OA in obese individuals compared to those of healthy weight (89). First and foremost, mechanical overload in joints promotes ROS production by OA chondrocytes, which further aggravates cartilage degradation (90). A recent study concluded that there is a 2.5-times higher likelihood of patients with diabetic knee OA experiencing knee pain compared to patients with non-diabetic knee OA due to accelerated cartilage damage (91). Furthermore, obesity has also been associated with the secretion of adipokines, thus contributing to low-grade systemic inflammation (92–95). The expression levels of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α), are increased in obese individuals (96–101). Inflammatory factors activate the nuclear factor-κB (NF-κB) signaling pathway and ultimately result in the upregulation of MMPs, subsequently targeting the ECM (102–104). Lastly, meta-analysis studies have shown a significant relationship between obesity and dose-response. Patients with higher body mass indices are less likely to be dose-responsive and show continual clinical consequences (89, 105, 106).
Although moderate physical activity is encouraged to improve one's general health, repetition or incorrect execution of movements is the prevailing cause of OA in both young and older adults (56, 64, 107). From a comprehensive review of recent studies, common occupational activities such as sitting (hip and knee OA), lifting (knee OA), driving (knee OA), and squat (lower limb OA) have been associated with increased risk of OA (108, 109). These activities, if repeated, could be detrimental to the joint, as a study calculated the risk of developing localized OA to be twice as likely in individuals with occupations demanding repetitive physical activities compared to the average population (110). Moreover, although recreational sports activities are known to lower the occurrence of OA, elite athletes participating in competitive sports are extremely prone to OA, as incorrect execution of movements may disturb joint stabilization (111–114). In a systematic review including over 8,400 subjects, it was concluded that soccer, weightlifting, and wrestling were the sports with the highest prevalence of knee OA (112). Furthermore, it has been reported that OA prevalence rates in contact sports, such as rugby, are twice as high as those in non-contact sports (113).
Clinical Trials Using MSCs in OA
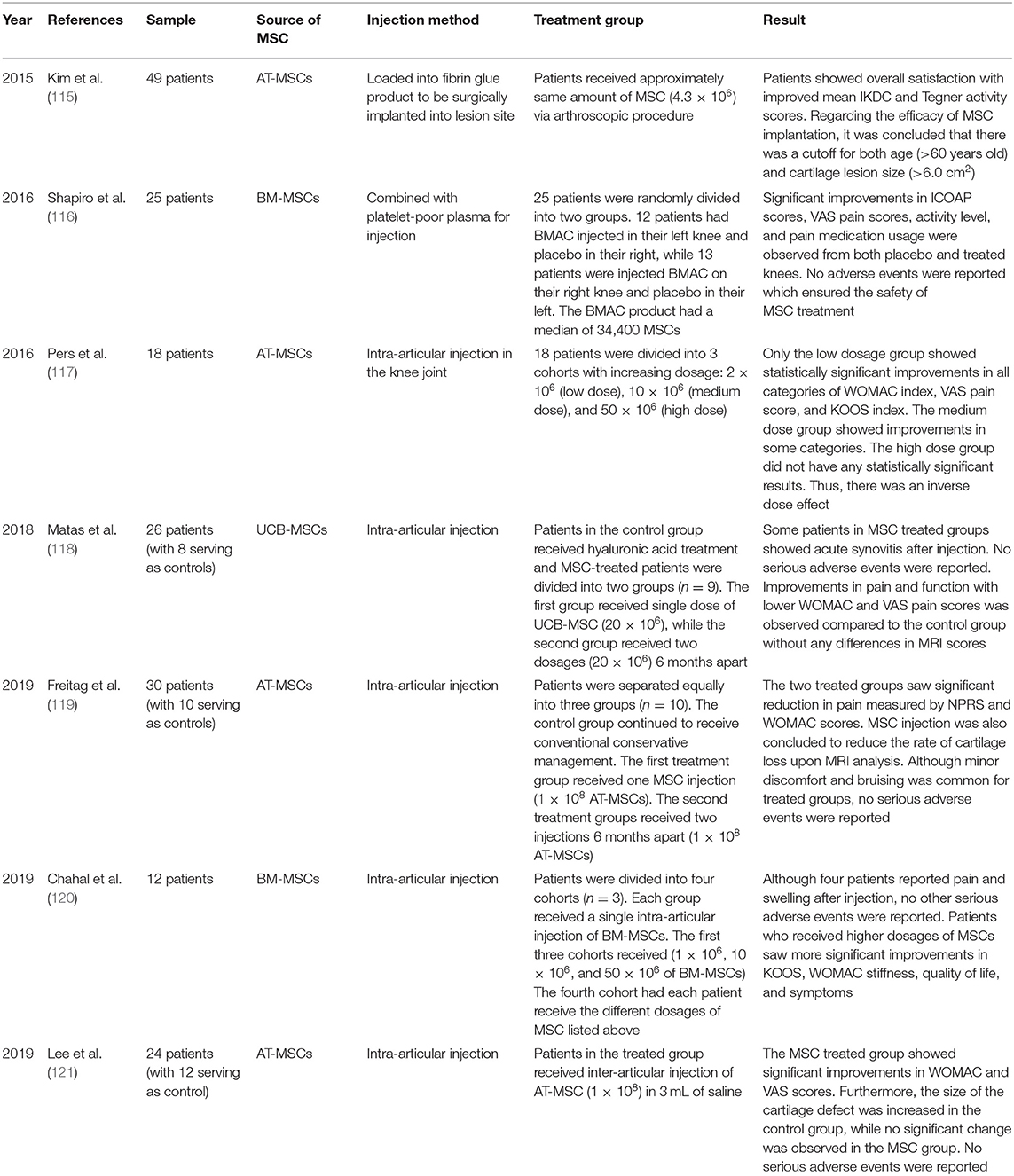
We also reviewed recent clinical trials that used MSC-based treatments in patients with OA (Table 2). A clinical trial by Kim et al. involved 49 patients (55 knees) with isolated full-thickness cartilage lesions and Kellgren-Lawrence (K-L) grade 1 or 2 OA with symptoms of knee joint pain and/or functional limitations despite non-surgical treatments for a minimum of 3 months (115). AT-MSCs were harvested from each patient's buttocks via tumescent liposuction. Upon isolation, AT-MSCs were loaded into a fibrin glue product and surgically implanted into the lesion site. Implanted knees were immobilized with a knee brace for 2 weeks post-surgery, followed by passive joint exercises. During the follow-ups, it was reported that the mean International Knee Documentation Committee (IKDC) score increased from 37.7 to 67.3, and the Tegner Activity Scale from 2.2 to 3.8. Both scores showed significant improvements in patients, with 74.5% of them expressing better satisfaction. In addition, age and lesion size were identified as independent factors affecting clinical outcomes. Patients over 60 years of age with lesion sizes >6.0 cm2 showed less favorable results. Although there were some variations in the results due to these factors, the overall clinical outcomes of MSC implantation in OA patients were encouraging, with successful results.
In 2016, Shapiro et al. conducted a randomized, single-blinded, placebo-controlled trial in 25 patients with mild to moderate bilateral knee OA who had previously received conventional treatments, such as activity modification or physical therapy (116). Each patient had ~52 mL of bone marrow harvested from their respective superior iliac crests. The marrow cells were then analyzed for the positive and negative co-expression of surface markers to fulfill the minimal criteria for defining MSCs. Upon confirmation, 5 mL of cells were mixed with 10 mL of previously separated platelet-poor bone marrow plasma to be injected into a randomly assigned knee of each patient (13 patients received MSCs in their right knee, and 12 patients in their left knee). The contralateral knees subsequently underwent an intra-articular injection of 15 mL of sterile saline, and served as controls. After 1 week, 3 months, and 6 months, the Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) questionnaire and visual analog scale (VAS) pain scores of the two groups were recorded. The VAS pain scores and answers to the ICOAP questionnaires indicated significant improvements throughout the follow-up periods in both the bone marrow aspirate concentration group and the placebo group. Furthermore, both groups showed increased activity levels and decreases in self-reported pain medication usage, with no difference in the degree of improvement from baseline. The efficacy of MSC injection was questioned, as there was no difference in pain-mediating capabilities between the knees treated with the BM-MSC injection vs. saline.
In 2016, Pers et al. published a phase I, prospective, bicentric, single-arm, open-label, dose-escalating clinical trial report of AT-MSC injection in patients with knee OA (117). The 18 patients selected for the trial were 50–75 years of age with K-L grade 3–4 knee OA. The subjects were first divided into three consecutive cohorts with increasing dosages: 2 × 106 (low dose), 10 × 106 (medium dose), and 50 × 106 (high dose) cells. The primary outcome assessed the safety of the trial, while the secondary outcome measured clinical efficacy. The AT-MSCs were extracted from the respective patients through liposuction and the prepared AT-MSC dosages were administered via intra-articular injections to the knee joints. In the primary outcome assessment, no adverse events from either liposuction or intra-articular injection were observed. However, one patient who had hypertension and hyperlipidemia suffered from unstable angina pectoris without increased levels of cardiac markers 3 months after treatment. In addition, four other patients reported minor knee pain/joint effusion that resolved spontaneously or after treatment with non-steroidal anti-inflammatory drugs. Thus, the safety of AT-MSC treatment was further demonstrated. The secondary outcome was initially assessed using magnetic resonance imaging (MRI), which showed no correlation between MRI and clinical results, in addition to histologic analysis that showed no indication of tumor proliferation. In contrast to other studies, only the low dosage group presented statistically significant results in all categories of the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index, VAS pain score, and Knee Injury and Osteoarthritis Outcome Score (KOOS). This inverse dose effect could possibly be due to increased inflammation in the low-dose group. Despite limited resources and unclear explanations, Pers et al. further demonstrated the safety and promising potential of MSC treatment.
Matas et al. led a randomized, double-blinded, controlled clinical trial including 29 patients aged 40–65 years with K-L grade 1–3 knee OA (118). Patients were divided into three groups and received two injections 6-months apart. The hyaluronic acid (HA) group (control) received two HA injections (3 mL of Durolane). The MSC-1 group received UCB-MSC (2 × 107 UCB-MSCs and 5% AB plasma in 3 mL of saline) at baseline and was later injection with placebo (5% of AB plasma in 3 mL of saline), while the MSC-2 group received two UCB-MSC injections (2 × 107 UCB-MSCs and 5% AB plasma in 3 mL of saline). Although acute synovitis was common after UCB-MSC injections, no serious adverse events were observed during the trial. Clinical assessment revealed that the MSC-2 group had significantly lower total WOMAC indices than the HA control group at 12 months (4.2 vs. 15.2). In parallel with this result, the VAS score of the MSC-2 group was 2.4, while the VAS score of the HA group was 22.1. In contrast to the MSC-2 group, the MSC-1 group did show improvements through the first 9 months, but later became ineffective after receiving an HA injection. Overall, no patients in any group showed evidence of chondral damage or intra-articular calcifications upon MRI follow-up. The clinically significant results indicated that repeated administration of UC-MSCs dosage is a favorable and safe means of improving the clinical outcomes of patients with knee OA.
Freitag et al. conducted a randomized trial involving 30 patients aged 18 years or older with K-L grade 2–3 knee OA who had previously undergone primary conservative management of OA, such as weight management programs and bracing (119). The participants were first randomly divided into three groups: two treatment groups and one control group. The first group was injected once with 1 × 108 MSCs, while the second group received two injections of 1 × 108 MSCs 6 months apart. The third group served as the control with continued conservative treatments. MSCs were harvested from the adipose tissues of the patients and cultured until passage 2 prior to injection. AT-MSCs were then injected under ultrasound guidance into the intra-articular knee space. The primary outcomes measured the pain and functional changes after the procedure; the secondary outcome involved an MRI analysis after 12 months. Relative to the control, the two treated groups showed significant improvements in pain according to the numeric pain rating scale (NPRS) (6.7 and 6.5 to 2.6 and 2.3) and WOMAC scores (59.6 and 54.4 to 84 and 87.3). MRI analysis showed that 37% of the participants in the first treatment group exhibited further cartilage loss compared to the control. However, ~89% of the patients in the second treatment group showed marked improvement or no progression in cartilage loss. Furthermore, no serious adverse events were observed in the two treated groups during follow-up. Thus, it was concluded that AT-MSC therapy is a safe and effective treatment for knee OA and that frequent injections are preferable.
In 2019, Chahal et al. published a non-randomized, open-label, dose-escalation clinical study (120). This study included patients aged 40–65 years, who were diagnosed with K-L grade 3–4 knee OA and had failed to derive benefits from non-operative treatment regimens for at least 6 months. A total of 12 patients were divided into four cohorts. The first three cohorts were injected with 1 × 106, 2 × 107, and 5 × 107 BM-MSCs (extracted from the posterior superior spine, respectively). In the fourth cohort, three patients each received different BM-MSCs dosages consistent with the increasing dosage levels injected into the three previous cohorts. The primary outcome ensured the safety of the trial, and the secondary outcomes involved clinical, radiological, and biomarker assessments. Without any adverse events, patients saw significant improvements in KOOS and WOMAC stiffness scores, quality of life, and symptoms 12 months post-BM-MSC treatment. Moreover, patients treated with higher dosages demonstrated better chances of significant improvements compared to those treated with lower dosages. Hence, it was concluded from this study that BM-MSC treatment is safe with positive clinical outcomes, specifically at higher dosages.
In 2019, Lee et al. conducted a randomized, double-blinded, placebo-controlled study in 24 patients with knee OA aged 18–75 years, who had a mean pain intensity (VAS score) of 4 or higher for a minimum of 12 weeks with at least one grade 3–4 lesion (121). Patients were randomly divided into MSC and control groups. MSCs were then isolated from the abdominal subcutaneous adipose tissue via lipoaspiration. After the isolated AT-MSCs were cultured to passage 3, the MSC group was treated with an intra-articular injection of 1 × 106 AT-MSCs in 3 mL of saline, while the control group was injected with 3 mL of saline alone. The primary clinical outcome was evaluated 6 months after injection using the WOMAC index. In addition, the secondary outcomes comprised clinical scores, physical examination, radiological evaluation, and safety assessment. In the follow-up, the control group showed no drastic changes in any of the outcomes. On the contrary, the MSC group showed significant reductions in WOMAC and VAS scores for knee pain, from 60.0 to 26.7 and 6.8 to 3.4, respectively. Moreover, the physical and radiological examination data showed that the MSC-injected patients demonstrated a wider range of knee motion (127.9°-134.6°) and unchanged cartilage defects, in contrast to the enlargement seen in the control group. Finally, with all adverse events below grade 3 of the National Cancer Institute-Common Terminology Criteria for Adverse Events scale, the use of intermittent acetaminophen could remediate all treatment-related adverse events without any treatment discontinuations. Although some evaluations (K-L grade, HKA angle, quadriceps power, and the presence of joint effusion) did not show any difference or improvement between the MSC and control groups, it was concluded that the intra-articular injection of AT-MSCs resulted in satisfactory clinical and functional outcomes without serious short-term safety concerns.
Applications of MSC Therapy in RA
General Characteristics of RA
RA is a chronic systemic autoimmune disease that causes progressive disability and premature death (53). This disease initially affects the synovial joints and later progresses to the skin, eyes, heart, kidneys, and lungs (53, 57). Ultimately, the patient suffers from joint failure characterized by cartilage damage and severely weakened tendons and ligaments (57, 122). The prevalence rate of RA was reported to be ~0.5–1.0% across the global population in 2002, with females being twice as more likely to be affected due to unknown factors (123, 124).
Based on the presence or absence of anti-citrullinated protein antibodies, RA is divided into two major subtypes (53), which show significant differences in their respective genomes and have completely different pathophysiologies (125). The primary genetic risk factors of RA include alleles encoding the HLA-DR region (53, 126–130). Other critical components are environmental risk factors, such as exposure to tobacco smoke, and lifestyle factors, such as dietary habits (53, 128). As the mechanisms of RA development and its specific targeting of the joints remain unclear, further research is required to fully understand this process (53). The fulminant stage of RA involves hyperplastic synovium, cartilage damage, and bone erosion (53). Along with bone loss, both inflammation and autoimmune responses are potential causes of RA progression (53). This cascade of reactions is activated when fibroblast-like synoviocytes (FLSs) interact with immune cells of the innate and adaptive immune systems (53). Some of the immune cells responsible for inflammation are monocytes, macrophages, T lymphocytes, and B cells (53, 131, 132). The synovial membrane and cartilage undergo significant inflammation, causing hyperplastic synovium and cartilage destruction that eventually lead to bone erosion (54). Hyperplastic synovium is a critical characteristic of RA, and there are two hypotheses regarding its cause. The first is that the abnormal proliferation of FLSs ultimately leads to the production of inflammatory cytokines and mediators that continue joint destruction (53, 133). The second is that the resistance to apoptosis due to defects in tumor protein p53 triggers the hyperplastic synovium (53, 134). Here, the shortage of chondrocytes caused by apoptosis would result in cartilage degeneration and joint-space narrowing via directed adhesion and invasion (53, 135, 136).
Clinical Trials Using MSCs in RA
Compared to OA, relatively few trials have been performed in RA with MSCs. In some cases, it is said that MSC therapy is not suitable for RA given the growing armamentarium of other efficient therapeutic agents available in contrast to OA. Systemic administration of autologous MSCs seemed to cause an exacerbation of RA in a collagen-induced arthritis (CIA) RA animal model, whereas the results of administration of allogeneic MSCs were more successful. These results suggest that allogeneic MSCs are more effective in treatment for autoimmune disorders (137, 138). Although there have been a limited number of clinical trials of MSC treatment in RA patients, the safety and efficacy of therapy have been confirmed in several studies (139). Here, we have briefly reviewed recent clinical trials that used MSC-based treatments in patients with RA (Table 3).
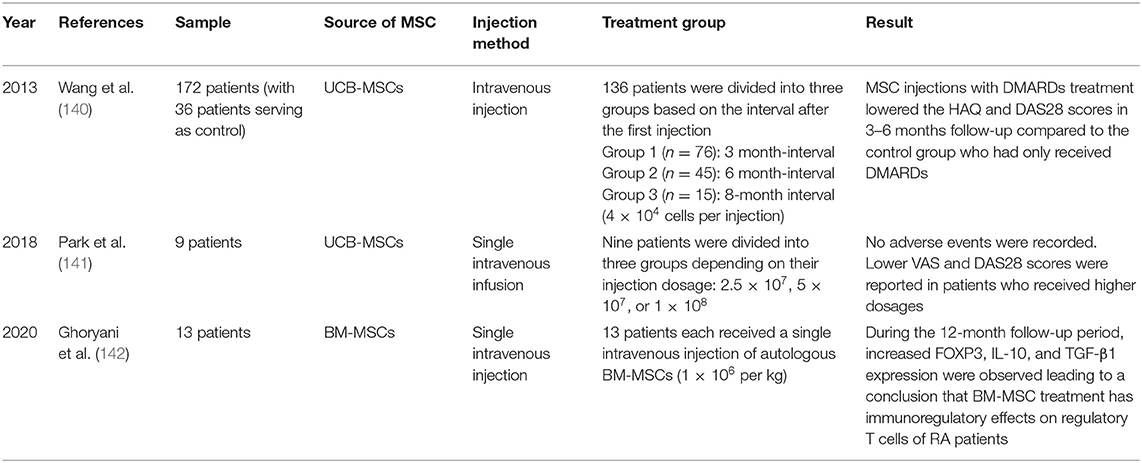
Wang et al. conducted a randomized controlled clinical trial with 172 patients with RA who previously underwent unsuccessful chemotherapy treatments and were currently prescribed disease-modifying anti-rheumatic drugs (DMARDs) (140). The MSC-treated group (n = 136) received 4.0 × 107 UC-MSCs in 40 mL of stem cell solvent, while the control group (n = 36) received only 40 mL of stem cell solvent via intravenous infusion. The MSC-treated group was then further divided into three groups based on the intervals after the first treatment: Group 1 had a 3-month interval, Group 2 had a 6-month interval, and Group 3 had an 8-month interval between injections. The safety of the trial was assessed through radiographic and physical examinations, while disease activity was monitored via disease activity score 28 (DAS28) and the Health Assessment Questionnaire (HAQ). This study did not find serious side effects other than minor fevers and chills. Two weeks after the intravenous injection, the MSC-treated groups showed higher quality of life with reduced joint pain/swelling compared to that of the control group. Moreover, decreased DAS28 and HAQ scores were recorded in the MSC-treated group with repeated treatments, indicating a steady reduction in disease activity. Thus, treatment with a combination of DMARDs and UCB-MSC via injection was concluded to be safe and effective in reducing the long-term disease activity of refractory RA compared to that of conventional DMARDs treatment alone.
Park et al. conducted a clinical trial to test the safety of short-term application of UCB-MSCs in patients with RA (141). The nine participating patients, all aged 18 years or older, had baseline DAS28 assessments and were on a stable dose of methotrexate for a minimum of 12 weeks. Patients received different concentrations of UCB-MSCs via intravenous infusion. Follow-ups for the assessment of clinical and safety parameters were conducted 24 h, 3 days, 1 week, and 4 weeks after infusion. At 4 weeks post-infusion, no abnormalities were detected in the hematologic and chemical profiles; only minor elevations in serum uric acid levels were observed among patients. Hence, it was reported that there were no serious adverse events or dose-limiting toxicities due to the application of UCB-MSCs. UCB-MSC treatment reduced the disease activity of RA and dose-dependently reduced the DAS28 score and VAS pain scale.
Ghoryani et al. conducted a clinical trial to test the immunoregulatory effects of MSCs on 13 female patients with refractory RA, who had previously received maximum dosages of DMARDs (142). The patients had their respective BM-MSCs transplanted via a single dose (1 × 106 per kg of body weight) and were followed up at 1, 6, and 12 months post-transplantation. Patients showed a significant reduction in DAS28 score (from 5.56 to 4.72) after 12 months of MSC treatment. Increased forkhead box P3 (FOXP3), IL-10, and TGF-β1 gene expressions were observed in patients treated with MSCs. Based on the increases in IL-10 and TGF-β, MSC therapy was concluded to have significant immunomodulatory effects in patients with refractory RA. Nevertheless, further research is required to investigate the possible effects of increasing/replicating the MSC dosages in patients for improved results.
Strategies for Future Use of MSCs
The terminology debate over “stem” versus “stromal” has been argued in the past and is still ongoing (9). In 2005, the ISCT committee issued a paper that clarifies that the term MSC is not equivalent (or interchangeable) with mesenchymal stromal cell (143). While the MSCs previously discussed in our study refers to cells with self-renewal and differentiation, mesenchymal stromal cells refers to a bulk population of cells with secretory, immunomodulatory effects with additional homing ability (144–146). This is a critical point in studies using MSCs, as the ISCT MSC committee recommends to clarify whether MSC stands for “mesenchymal stromal cells” or “mesenchymal stem cells.” However, currently, there is no surface marker that can be used to distinguish these two cell types. The ISCT MSC committee endorses the functional distinction between stromal and stem cells and suggests further analysis focused on their functionalities along with their secretomes. With advanced analysis at the single cells levels and mass cytometry using next-generation sequencing tools, it is important to distinguish the epigenomic, transcriptomic, and proteomic differences between the mesenchymal stromal cells and stem cells. Future studies that target treatment and regeneration of the defected joint tissue should consider thoroughly characterizing the attributes along with the stemness of the MSCs that are used in each study. Such detailed characterization of the investigated MSCs may suggest a unique subtype of MSCs for more direct targeting for the treatment of arthritic diseases.
Conclusion
In this review, we have summarized the current status of MSC therapies for OA and RA. While, OA had more promising studies and results compared to that of RA, MSC therapy has shown potential in both OA and RA treatments with reduced pain, improved joint function, and enhanced overall life satisfaction in patients. Clinical trials on OA and RA discussed in this review demonstrate that MSCs are a safe treatment option without serious adverse events. However, more studies are required to examine the long-term safety of MSC injections and their respective clinical applications. Future research studies employing the latest in technology can be the key to increasing scientific evidence concerning their efficacy and safety of MSC therapies. In addition, thorough examination and characterization of the studies already using MSCs are critical for the better understanding of MSCs and will allow them to become a leading candidate for the treatment of various diseases, including arthritic diseases.
Author Contributions
JH and JJ concepted the topic, collected data, and wrote the manuscript. YR and YN reviewed and revised the work. JJ supervised this project. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the Korea Healthcare Technology R&D project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (HI16C2177). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2019R1I1A1A01060753 and 2019R1I1A1A01062060) and a National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT, MSIT)(NRF-2020R1A1C3004123).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACPA, anti-citrullinated protein antibodies; AI, arthritis index; AIA, antigen-induced arthritis; ALK, activin receptor-like kinase; AT-MSC, adipose tissue-derived mesenchymal stem cell; BMAC, bone marrow aspirate concentration; BM-MSC, bone marrow-derived mesenchymal stem cell; CD, cluster of differentiation; DAS28, disease activity score; DMARD, disease-modifying anti-rheumatic drug; ECM, extracellular matrix; FLS, fibroblast-like synoviocytes; GFP-MSC, green fluorescent protein-positive mesenchymal stem cells; HA, hyaluronic acid; HAQ, health assessment questionnaire; HKA angle, hip-knee-ankle angle; HLA, human leukocyte antigen; ICOAP pain questionnaire, measure of Intermittent and Constant Osteoarthritis Pain questionnaire; ICST, International Society of Cellular Therapy; IFN- γ, interferon gamma; IGF-1, insulin-like growth factor 1; IKDC, International Knee Documentation Committee; IL, interleukin; K-L grade, Kellgren-Lawrence grade; KOOS, Knee Injury and Osteoarthritis Outcome Score; MMP, matrix metalloproteinases; MRI, magnetic resonance imaging; MSC, mesenchymal stem cell; NPRS, Numeric Pain Rating Scale; NF-κB, nuclear factor-kappa B; OA, osteoarthritis; OP-1, osteogenic protein 1; PGA, patient global assessment; RA, rheumatoid arthritis; ROS, reactive oxygen species; GWAS, genome-wide association studies; SF-36 Questionnaire, 36-Item Short Form Survey; SM-MSC, synovium-derived mesenchymal stem cell; SNP, single nucleotide polymorphism; TNF, tumor necrosis factor; UCB-MSC, umbilical cord blood-derived mesenchymal stem cell; VAS, visual analog scale; WOMAC index, Western Ontario and McMaster Universities Osteoarthritis index; WORMS, whole-organ MRI scoring.
References
1. Fridenshtein A, Piatetskii S II, Petrakova KV. [Osteogenesis in transplants of bone marrow cells]. Arkh Anat Gistol Embriol. (1969) 56:3–11.
2. Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. (2019) 4:22. doi: 10.1038/s41536-019-0083-6
3. Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. (1987) 20:263–72. doi: 10.1111/j.1365-2184.1987.tb01309.x
4. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. (1970) 3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x
5. Gomez-Salazar M, Gonzalez-Galofre ZN, Casamitjana J, Crisan M, James W, Peault B. Five decades later, are mesenchymal stem cells still relevant? Front Bioeng Biotechnol. (2020) 8:148. doi: 10.3389/fbioe.2020.00148
7. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. (2017). 6:1445–51. doi: 10.1002/sctm.17-0051
8. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
9. Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy [ISCT(R)] mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. (2019) 21:1019–24. doi: 10.1016/j.jcyt.2019.08.002
10. Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J. (2012) 2:154–62.
11. Saeed H, Ahsan M, Saleem Z, Iqtedar M, Islam M, Danish Z, et al. Mesenchymal stem cells (MSCs) as skeletal therapeutics - an update. J Biomed Sci. (2016) 23:41. doi: 10.1186/s12929-016-0254-3
12. Bellagamba BC, Grudzinski PB, Ely PB, Nader PJH, Nardi NB, da Silva Meirelles L. Induction of expression of CD271 and CD34 in mesenchymal stromal cells cultured as spheroids. Stem Cells Int. (2018) 2018:7357213. doi: 10.1155/2018/7357213
13. Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. (2006) 107:2570–7. doi: 10.1182/blood-2005-07-2793
14. Romieu-Mourez R, Francois M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. (2007) 179:1549–8. doi: 10.4049/jimmunol.179.3.1549
15. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. (2010) 12:87–117. doi: 10.1146/annurev-bioeng-070909-105309
16. Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. (1995) 16:557–64.
17. Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. (2000) 18:307–16. doi: 10.1200/JCO.2000.18.2.307
18. Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. (2002) 99:8932–7. doi: 10.1073/pnas.132252399
19. Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. (2002) 30:215–22. doi: 10.1038/sj.bmt.1703650
20. Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and modification strategies. Stem Cell Investig. (2019) 6:34. doi: 10.21037/sci.2019.08.11
21. Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. (2016) 6:897–913. doi: 10.1016/j.stemcr.2016.05.011
22. Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. (2002) 81:531–5. doi: 10.1177/154405910208100806
23. Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. (2008) 214:413–21. doi: 10.1002/jcp.21210
24. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. (2019) 8:886. doi: 10.3390/cells8080886
25. Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. (2020) 77:2771–94. doi: 10.1007/s00018-020-03454-6
26. Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. (1992) 13:81–8. doi: 10.1016/8756-3282(92)90364-3
27. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. (1999) 284:143–7. doi: 10.1126/science.284.5411.143
28. Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, et al. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. (2018) 9:168. doi: 10.1186/s13287-018-0914-1
29. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. (2000) 109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x
30. Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. (2014) 6:195–202. doi: 10.4252/wjsc.v6.i2.195
31. Chen JY, Mou XZ, Du XC, Xiang C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac J Trop Med. (2015) 8:739–46. doi: 10.1016/j.apjtm.2015.07.022
32. Kim JH, Jo CH, Kim HR, Hwang YI. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem Cells Int. (2018) 2018:8429042. doi: 10.1155/2018/8429042
33. Kim YJ, Seo DH, Lee SH, Lee SH, An GH, Ahn HJ, et al. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem Biophys Rep. (2018) 16:96–102. doi: 10.1016/j.bbrep.2018.10.007
34. Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. (2006) 91:1017–26.
35. Stanko P, Kaiserova K, Altanerova V, Altaner C. Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2014) 158:373–7. doi: 10.5507/bp.2013.078
36. Schmelzer E, McKeel DT, Gerlach JC. Characterization of human mesenchymal stem cells from different tissues and their membrane encasement for prospective transplantation therapies. Biomed Res Int. (2019) 2019:6376271. doi: 10.1155/2019/6376271
37. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. (2001) 7:211–28. doi: 10.1089/107632701300062859
38. Nepali S, Park M, Lew H, Kim O. Comparative analysis of human adipose-derived mesenchymal stem cells from orbital and abdominal fat. Stem Cells Int. (2018) 2018:3932615. doi: 10.1155/2018/3932615
39. De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. (2001) 44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P
40. Murata Y, Uchida S, Utsunomiya H, Hatakeyama A, Nakashima H, Chang A, et al. Synovial mesenchymal stem cells derived from the cotyloid fossa synovium have higher self-renewal and differentiation potential than those from the paralabral synovium in the hip joint. Am J Sports Med. (2018) 46:2942–53. doi: 10.1177/0363546518794664
41. Li N, Gao J, Mi L, Zhang G, Zhang L, Zhang N, et al. Synovial membrane mesenchymal stem cells: past life, current situation, and application in bone and joint diseases. Stem Cell Res Ther. (2020) 11:381. doi: 10.1186/s13287-020-01885-3
42. Kubosch EJ, Lang G, Furst D, Kubosch D, Izadpanah K, Rolauffs B, et al. The potential for synovium-derived stem cells in cartilage repair. Curr Stem Cell Res Ther. (2018) 13:174–84. doi: 10.2174/1574888X12666171002111026
43. Mochizuki T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, Koga H, et al. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. (2006) 54:843–53. doi: 10.1002/art.21651
44. Ogata Y, Mabuchi Y, Yoshida M, Suto EG, Suzuki N, Muneta T, et al. Purified human synovium mesenchymal stem cells as a good resource for cartilage regeneration. PLoS ONE. (2015) 10:e0129096. doi: 10.1371/journal.pone.0129096
45. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. (2005) 52:2521–29. doi: 10.1002/art.21212
46. Mor A, Grijota M, Norgaard M, Munthe J, Lind M, Deruaz A, et al. Trends in arthroscopy-documented cartilage injuries of the knee and repair procedures among 15-60-year-old patients. Scand J Med Sci Sports. (2015) 25:e400–7. doi: 10.1111/sms.12330
47. Martin AR, Patel JM, Zlotnick HM, Carey JL, Mauck RL. Emerging therapies for cartilage regeneration in currently excluded 'red knee' populations. NPJ Regen Med. (2019) 4:12. doi: 10.1038/s41536-019-0074-7
48. Zylinska B, Silmanowicz P, Sobczynska-Rak A, Jarosz L, Szponder T. Treatment of articular cartilage defects: focus on tissue engineering. In Vivo. (2018) 32:1289–300. doi: 10.21873/invivo.11379
49. Du D, Hsu P, Zhu Z, Zhang C. Current surgical options and innovation for repairing articular cartilage defects in the femoral head. J Orthop Translat. (2020) 21:122–8. doi: 10.1016/j.jot.2019.06.002
50. Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S, et al. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. (2004) 13:595–600. doi: 10.3727/000000004783983747
51. Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. (2007) 15:226–31. doi: 10.1016/j.joca.2006.08.008
52. Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. (2010) 38:1110–6. doi: 10.1177/0363546509359067
53. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J, et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. (2018) 6:15. doi: 10.1038/s41413-018-0016-9
54. Ouboussad L, Burska AN, Melville A, Buch MH. Synovial tissue heterogeneity in rheumatoid arthritis and changes with biologic and targeted synthetic therapies to inform stratified therapy. Front Med. (2019) 6:45. doi: 10.3389/fmed.2019.00045
55. Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. (2017) 5:16044. doi: 10.1038/boneres.2016.44
56. Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A, et al. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. (2015) 16:6093–112. doi: 10.3390/ijms16036093
57. Bullock J, Rizvi SAA, Saleh AM, Ahmed SS, Do DP, Ansari RA, et al. Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract. (2018) 27:501–7. doi: 10.1159/000493390
58. Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res. (2018) 11:2189–96. doi: 10.2147/JPR.S154002
59. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. (2015) 162:46–54. doi: 10.7326/M14-1231
60. Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. (2014) 43:701–12. doi: 10.1016/j.semarthrit.2013.11.012
61. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. (2006) 54:226–9. doi: 10.1002/art.21562
62. Ryder JJ, Garrison K, Song F, Hooper L, Skinner J, Loke Y, et al. Genetic associations in peripheral joint osteoarthritis and spinal degenerative disease: a systematic review. Ann Rheum Dis. (2008) 67:584–91. doi: 10.1136/ard.2007.073874
63. Nordenvall R, Bahmanyar S, Adami J, Stenros C, Wredmark T, Fellander-Tsai, et al. A population-based nationwide study of cruciate ligament injury in Sweden, 2001-2009: incidence, treatment, and sex differences. Am J Sports Med. (2012) 40:1808–13. doi: 10.1177/0363546512449306
64. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. (2018) 30:160–7. doi: 10.1097/BOR.0000000000000479
65. Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. (2013) 25:108–13. doi: 10.1097/BOR.0b013e32835a9428
66. Loeser RF. The role of aging in the development of osteoarthritis. Trans Am Clin Climatol Assoc. (2017) 128:44–54.
67. Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. (2011) 23:492–6. doi: 10.1097/BOR.0b013e3283494005
68. Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. (2011) 208:417–20. doi: 10.1084/jem.20110367
69. Goodwin W, McCabe D, Sauter E, Reese E, Walter M, Buckwalter JA, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. (2010) 28:1057–63. doi: 10.1002/jor.21091
70. Zahan OM, Serban O, Gherman C, Fodor D. The evaluation of oxidative stress in osteoarthritis. Med Pharm Rep. (2020) 93:12–22. doi: 10.15386/mpr-1422
71. Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. (2014) 28:5–15. doi: 10.1016/j.berh.2014.01.004
72. Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of persons with symptomatic knee osteoarthritis in the us: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. (2016) 68:1743–50. doi: 10.1002/acr.22897
73. Reynard LN, Barter MJ. Osteoarthritis year in review 2019: genetics, genomics and epigenetics. Osteoarthritis Cartilage. (2020) 28:275–84. doi: 10.1016/j.joca.2019.11.010
74. Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. (2019) 51:230–6. doi: 10.1038/s41588-018-0327-1
75. Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci USA. (2004) 101:9757–62. doi: 10.1073/pnas.0403456101
76. Bijsterbosch J, Kloppenburg M, Reijnierse M, Rosendaal FR, Huizinga TW, Slagboom PE, et al. Association study of candidate genes for the progression of hand osteoarthritis. Osteoarthritis Cartilage. (2013) 21:565–9. doi: 10.1016/j.joca.2013.01.011
77. Valdes AM, Spector TD, Tamm A, Kisand K, Doherty SA, Dennison EM, et al. Genetic variation in the SMAD3 gene is associated with hip and knee osteoarthritis. Arthritis Rheum. (2010) 62:2347–52. doi: 10.1002/art.27530
78. Rodriguez RR, Seegmiller RE, Stark MR, Bridgewater LC. A type XI collagen mutation leads to increased degradation of type II collagen in articular cartilage. Osteoarthritis Cartilage. (2004) 12:314–20. doi: 10.1016/j.joca.2003.12.002
79. Jeong C, Lee J.Y, Kim J, Chae H, Park HI, Kim M, et al. Novel COL9A3 mutation in a family diagnosed with multiple epiphyseal dysplasia: a case report. BMC Musculoskelet Disord. (2014) 15:371. doi: 10.1186/1471-2474-15-371
80. Carlson KM, Yamaga KM, Reinker KA, Hsia YE, Carpenter C, Abe LM, et al. Precocious osteoarthritis in a family with recurrent COL2A1 mutation. J Rheumatol. (2006) 33:1133–6.
81. Zhang R, Yao J, Xu P, Ji B, Luck JV, Chin B, et al. A comprehensive meta-analysis of association between genetic variants of GDF5 and osteoarthritis of the knee, hip and hand. Inflamm Res. (2015) 64:405–14. doi: 10.1007/s00011-015-0818-9
82. Styrkarsdottir U, Helgason H, Sigurdsson A, Norddahl GL, Agustsdottir AB, Reynard LN, et al. Whole-genome sequencing identifies rare genotypes in COMP and CHADL associated with high risk of hip osteoarthritis. Nat Genet. (2017) 49:801–5. doi: 10.1038/ng.3816
83. Zengini E, Hatzikotoulas K, Tachmazidou I, Steinberg J, Hartwig FP, Southam L, et al. Genome-wide analyses using UK Biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet. (2018) 50:549–58. doi: 10.1038/s41588-018-0079-y
84. Styrkarsdottir U, Lund SH, Thorleifsson G, Zink F, Stefansson OA, Sigurdsson JK, et al. Meta-analysis of Icelandic and UK data sets identifies missense variants in SMO, IL11, COL11A1 and 13 more new loci associated with osteoarthritis. Nat Genet. (2018) 50:1681–7. doi: 10.1038/s41588-018-0247-0
85. Styrkarsdottir U, Stefansson OA, Gunnarsdottir K, Thorleifsson G, Lund SH, Stefansdottir L, et al. Publisher Correction: GWAS of bone size yields twelve loci that also affect height, BMD, osteoarthritis or fractures. Nat Commun. (2019) 10:2358. doi: 10.1038/s41467-019-10425-4
86. Zhao T, Zhao J, Ma C, Wei J, Wei B, Liu J. Common variants in LTBP3 gene contributed to the risk of hip osteoarthritis in Han Chinese population. Biosci Rep. (2020) 40:BSR20192999. doi: 10.1042/BSR20192999
87. Chu M, Zhu X, Wang C, Rong J, Wang Y, Wang S, et al. The rs4238326 polymorphism in ALDH1A2 gene potentially associated with non-post traumatic knee osteoarthritis susceptibility: a two-stage population-based study. Osteoarthritis Cartilage. (2017) 25:1062–7. doi: 10.1016/j.joca.2017.01.003
88. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. (2008) 32:1431–7. doi: 10.1038/ijo.2008.102
89. Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. (2015) 17:86. doi: 10.1186/s13075-015-0601-x
90. Eymard F, Parsons C, Edwards MH, Petit-Dop F, Reginster JY, Bruyere O, et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis Cartilage. (2015) 23:851–9. doi: 10.1016/j.joca.2015.01.013
91. Alenazi AM, Alshehri MM, Alothman S, Alqahtani BA, Rucker J, Sharma N, et al. The association of diabetes with knee pain severity and distribution in people with knee osteoarthritis using data from the osteoarthritis initiative. Sci Rep. (2020) 10:3985. doi: 10.1038/s41598-020-60989-1
92. Xie C, Chen Q. Adipokines: new therapeutic target for osteoarthritis? Curr Rheumatol Rep. (2019) 21:71. doi: 10.1007/s11926-019-0868-z
93. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. (2013) 21:16–21. doi: 10.1016/j.joca.2012.11.012
94. Conde J, Scotece M, Gomez R, Lopez V, Gomez-Reino JJ, Gualillo O. Adipokines and osteoarthritis: novel molecules involved in the pathogenesis and progression of disease. Arthritis. (2011) 2011:203901. doi: 10.1155/2011/203901
95. Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. (2010) 2010:513948. doi: 10.1155/2010/513948
96. Visser M. Higher levels of inflammation in obese children. Nutrition. (2001) 17:480–1. doi: 10.1016/s0899-9007(01)00509-3
97. Aygun AD, Gungor S, Ustundag B, Gurgoze MK, Sen Y. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediators Inflamm. (2005) 2005:180–3. doi: 10.1155/MI.2005.180
98. Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Meigs JB, Lipinska I, Kathiresan S, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. (2007) 116:1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509
99. Straczkowski M, Dzienis-Straczkowska S, Stepien A, Kowalska I, Szelachowska M, Kinalska I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab. (2002) 87:4602–6. doi: 10.1210/jc.2002-020135
100. Shi C, Zhu L, Chen X, Gu N, Chen L, Zhu L, et al. IL-6 and TNF-alpha induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J Interferon Cytokine Res. (2014) 34:342–8. doi: 10.1089/jir.2013.0078
101. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. (2013) 2013:139239. doi: 10.1155/2013/139239
102. Xu L, Sun C, Zhang S, Xu X, Zhai L, Wang Y, et al. Sam68 promotes NF-kappaB activation and apoptosis signaling in articular chondrocytes during osteoarthritis. Inflamm Res. (2015) 64:895–902. doi: 10.1007/s00011-015-0872-3
103. Chow YY, Chin KY. The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm. (2020) 2020:8293921. doi: 10.1155/2020/8293921
104. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. (2011) 7:33–42. doi: 10.1038/nrrheum.2010.196
105. Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, et al. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. (2012) 79:291–7. doi: 10.1016/j.jbspin.2011.05.015
106. Raud B, Gay C, Guiguet-Auclair C, Bonnin A, Gerbaud L, Pereira B, et al. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci Rep. (2020) 10:3601. doi: 10.1038/s41598-020-60587-1
107. Chang AH, Lee JJ, Chmiel JS, Almagor O, Song J, Sharma L. Association of long-term strenuous physical activity and extensive sitting with incident radiographic knee osteoarthritis. JAMA Netw Open. (2020) 3:e204049. doi: 10.1001/jamanetworkopen.2020.4049
108. Schram B, Orr R, Pope R, Canetti E, Knapik J. Risk factors for development of lower limb osteoarthritis in physically demanding occupations: a narrative umbrella review. J Occup Health. (2020) 62:e12103. doi: 10.1002/1348-9585.12103
109. Gignac MAM, Irvin E, Cullen K, Van Eerd D, Beaton DE, Mahood Q, et al. Men and women's occupational activities and the risk of developing osteoarthritis of the knee, hip, or hands: a systematic review and recommendations for future research. Arthritis Care Res. (2020) 72:378–96. doi: 10.1002/acr.23855
110. Messier SP, Legault C, Mihalko S, Miller GD, Loeser RF, DeVita P, et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: design and rationale. BMC Musculoskelet Disord. (2009). 10:93. doi: 10.1186/1471-2474-10-93
111. Alentorn-Geli E, Samuelsson K, Musahl V, Green CL, Bhandari M, Karlsson J. The association of recreational and competitive running with hip and knee osteoarthritis: a systematic review and meta-analysis. J Orthop Sports Phys Ther. (2017) 47:373–90. doi: 10.2519/jospt.2017.7137
112. Driban JB, Hootman JM, Sitler MR, Harris KP, Cattano NM. Is participation in certain sports associated with knee osteoarthritis? A systematic review. J Athl Train. (2017) 52:497–506. doi: 10.4085/1062-6050-50.2.08
113. Hind K, Konerth N, Entwistle I, Theadom A, Lewis G, King D, et al. Cumulative sport-related injuries and longer term impact in retired male elite- and amateur-level rugby code athletes and non-contact athletes: a retrospective study. Sports Med. (2020) 50:2051–61. doi: 10.1007/s40279-020-01310-y
114. Bullock GS, Collins GS, Peirce N, Arden NK, Filbay SR. Playing sport injured is associated with osteoarthritis, joint pain and worse health-related quality of life: a cross-sectional study. BMC Musculoskelet Disord. (2020) 21:111. doi: 10.1186/s12891-020-3136-5
115. Kim YS, Choi YJ, Koh YG. Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes. Am J Sports Med. (2015) 43:2293–2301. doi: 10.1177/0363546515588317
116. Shapiro SA, Kazmerchak SE, Heckman MG, Zubair AC, O'Connor MI. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med. (2017) 45:82–90. doi: 10.1177/0363546516662455
117. Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl Med. (2016) 5:847–56. doi: 10.5966/sctm.2015-0245
118. Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, et al. Umbilical cord-derived Mesenchymal Stromal Cells (MSCs) for knee osteoarthritis: repeated msc dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. (2019) 8:215–24. doi: 10.1002/sctm.18-0053
119. Freitag J, Bates D, Wickham J, Shah K, Huguenin L, Tenen A, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. (2019) 14:213–30. doi: 10.2217/rme-2018-0161
120. Chahal J, Gomez-Aristizabal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med. (2019) 8:746–57. doi: 10.1002/sctm.18-0183
121. Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. (2019) 8:504–11. doi: 10.1002/sctm.18-0122
122. Lee JE, Kim IJ, Cho MS, Lee J. A case of rheumatoid vasculitis involving hepatic artery in early rheumatoid arthritis. J Korean Med Sci. (2017) 32:1207–10. doi: 10.3346/jkms.2017.32.7.1207
123. Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. (2002) 4(Suppl. 3):S265–72. doi: 10.1186/ar578
124. Myasoedova E, Crowson CS, Turesson C, Gabriel SE, Matteson EL. Incidence of extraarticular rheumatoid arthritis in Olmsted County, Minnesota, in 1995-2007 versus 1985-1994: a population-based study. J Rheumatol. (2011) 38:983–9. doi: 10.3899/jrheum.101133
125. Daha NA, Toes RE. Rheumatoid arthritis: are ACPA-positive and ACPA-negative RA the same disease? Nat Rev Rheumatol. (2011) 7:202–3. doi: 10.1038/nrrheum.2011.28
126. Okada Y, Kim K, Han B, Pillai NE, Ong RT, Saw WY, et al. Risk for ACPA-positive rheumatoid arthritis is driven by shared HLA amino acid polymorphisms in Asian and European populations. Hum Mol Genet. (2014) 23:6916–26. doi: 10.1093/hmg/ddu387
127. Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, Jia X, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. (2012) 44:291–6. doi: 10.1038/ng.1076
128. Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2017) 31:3–18. doi: 10.1016/j.berh.2017.08.003
129. Michou L, Croiseau P, Petit-Teixeira E, du Montcel ST, Michou L, Petit-Teixeira E, du Montcel ST, Lemaire I, Pierlot C, et al. Validation of the reshaped shared epitope HLA-DRB1 classification in rheumatoid arthritis. Arthritis Res Ther. (2006) 8:R79. doi: 10.1186/4ar1949
130. du Mellado ST, Michou L, Petit-Teixeira E, Osorio J, Lemaire I, Lasbleiz S, et al. New classification of HLA-DRB1 alleles supports the shared epitope hypothesis of rheumatoid arthritis susceptibility. Arthritis Rheum. (2005) 52:1063–8. doi: 10.1002/art.20989
131. Mellado M, Martinez-Munoz L, Cascio G, Lucas P, Pablos JL, Rodriguez-Frade JM. T cell migration in rheumatoid arthritis. Front Immunol. (2015) 6:384. doi: 10.3389/fimmu.2015.00384
132. Volkov M, van Schie KA, van der Woude D. Autoantibodies and B Cells: the ABC of rheumatoid arthritis pathophysiology. Immunol Rev. (2020) 294:148–63. doi: 10.1111/imr.12829
133. Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. (2013) 9:24–33. doi: 10.1038/nrrheum.2012.190
134. Lee YH, Ji JD, Kim A, Kim CH, Song GG. Expression of p53 protein in rheumatoid arthritis synovium. An immunohistochemical analysis. Korean J Intern Med. (1999) 14:59–65. doi: 10.3904/kjim.1999.14.1.59
135. Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis–two unequal siblings. Nat Rev Rheumatol. (2015) 11:606–15. doi: 10.1038/nrrheum.2015.95
136. Kato K, Yasojima N, Tamura K, Ichikawa S, Sutherland K, Kato M, et al. Detection of fine radiographic progression in finger joint space narrowing beyond human eyes: phantom experiment and clinical study with rheumatoid arthritis patients. Sci Rep. (2019) 9:8526. doi: 10.1038/s41598-019-44747-6
137. El-Jawhari JJ, El-Sherbiny YM, Jones EA, McGonagle D. Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM. (2014) 107:505–14. doi: 10.1093/qjmed/hcu033
138. Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. (2007) 56:1175–86. doi: 10.1002/art.22511
139. Zhang Q, Li Q, Zhu J, Guo H, Zhai Q, Li B, et al. Comparison of therapeutic effects of different mesenchymal stem cells on rheumatoid arthritis in mice. PeerJ. (2019) 7:e7023. doi: 10.7717/peerj.7023
140. Wang L, Wang L, Cong X, Liu G, Zhou J, Bai B, et al. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells Dev. (2013) 22:3192–202. doi: 10.1089/scd.2013.0023
141. Park EH, Lim HS, Lee S, Roh K, Seo KW, Kang KS, et al. Intravenous infusion of umbilical cord blood-derived mesenchymal stem cells in rheumatoid arthritis: a phase ia clinical trial. Stem Cells Transl Med. (2018) 7:636–42. doi: 10.1002/sctm.18-0031
142. Ghoryani M, Shariati-Sarabi Z, Tavakkol-Afshari J, Mohammadi M. The sufficient immunoregulatory effect of autologous bone marrow-derived mesenchymal stem cell transplantation on regulatory T cells in patients with refractory rheumatoid arthritis. J Immunol Res. (2020) 2020:3562753. doi: 10.1155/2020/3562753
143. Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. (2005) 7:393–5. doi: 10.1080/14653240500319234
144. Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. (2007) 131:324–36. doi: 10.1016/j.cell.2007.08.025
145. Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, et al. Identification of the human skeletal stem cell. Cell. (2018) 175:43–56.e21. doi: 10.1016/j.cell.2018.07.029
Keywords: mesenchymal stem cell, rheumatoid arthritis, osteoarthritis, cartilage, cell therapy
Citation: Hwang JJ, Rim YA, Nam Y and Ju JH (2021) Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front. Immunol. 12:631291. doi: 10.3389/fimmu.2021.631291
Received: 19 November 2020; Accepted: 04 February 2021;
Published: 08 March 2021.
Edited by:
Eric Toussirot, INSERM CIC1431 Centre d'Investigation Clinique Besançon, FranceReviewed by:
Guenter Steiner, Medical University of Vienna, AustriaPatricia Luz Crawford, University of the Andes, Chile
Copyright © 2021 Hwang, Rim, Nam and Ju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Hyeon Ju, juji@catholic.ac.kr
†These authors have contributed equally to this work
 Joel Jihwan Hwang1†
Joel Jihwan Hwang1†