
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 23 February 2021
Sec. B Cell Biology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.616650
This article is part of the Research Topic The B-Side of B Cells View all 10 articles
We have previously shown that obesity is associated with increased secretion of IgG antibodies with anti-self-reactivity. In this paper, we confirm and extend our previous findings. We show that the plasma of individuals with obesity is enriched in autoimmune antibodies whose levels are positively associated with blood frequencies of the subset of Double Negative (DN) B cells, which is the most pro-inflammatory B cell subset. We also show that DN B cells, significantly increased in the blood of obese versus lean individuals, are characterized by higher expression of immune activation markers and of the transcription factor T-bet, both associated with autoimmunity. The removal of DN B cells from the peripheral B cell pool significantly decreases in vitro secretion of anti-self IgG antibodies. These results altogether confirm the crucial role of DN B cells in the secretion of anti-self IgG antibodies in individuals with obesity.
Obesity, defined as body-mass index (BMI) ≥ 30 kg/m2 by the Centers for Disease Control and Prevention and the World Health Organization, is a condition associated with chronic low-grade systemic inflammation, known as inflammaging (1). Inflammaging has been shown to induce chronic immune activation (IA), which contributes to functional impairment of immune cells and decreased immunity. Obesity and associated inflammation lead to several debilitating chronic diseases such as type-2 diabetes, cancer, atherosclerosis, and inflammatory bowel disease (2–9).
We have previously shown that obesity is associated with decreased antibody responses to the influenza vaccine and decreased B cell function (10), measured by activation-induced cytidine deaminase (AID) after in vivo or in vitro stimulation with mitogens, antigens and vaccines. AID is the enzyme that regulates Ig class switch recombination (CSR) and somatic hypermutation (SHM) (11), two processes leading to the generation of high affinity protective antibodies (12–14). The reduced B cell resposes in individuals with obesity are likely due to the fact that B cells from obese individuals, as compared to those from lean individuals, are enriched in memory B cells, and in particular in the subset of Double Negative (DN) B cells, which is the most pro-inflammatory B cell subset (10, 15), reported to be increased in the blood of individuals with inflammatory conditions and diseases. These include aging (16–18), autoimmune diseases such as Rheumatoid Arthritis (19), Systemic Lupus Erythematosus (SLE) (20, 21), Multiple Sclerosis (22), Alzheimer’s disease (23), Sjogren’s disease (24) and pemphigus (25). DN B cells have also been reported to be increased in the blood of patients affected by chronic infectious diseases such as HIV (26), Hepatitis C (27) and Malaria (28). These results have suggested that these cells likely expand in vivo after chronic exposure to autoantigens or pathogen-derived antigens, leading to the production of autoimmune or protective antibodies, respectively. DN B cells are also expanded in the blood of COVID-19 patients and associated with anti-viral antibody responses and poor clinical outcomes, as recently shown (29).
In this paper, we show that the plasma of individuals with obesity is enriched in anti-self IgG antibodies and we tested three different antigenic specificities: double strand (ds)DNA, malondihyldehyde (MDA) and adipocyte-derived antigens. We chose these antigenic specificities because obesity is associated with increased DNA damage (measured by dsDNA) (30), increased oxidative stress and lipid peroxidation (measured by MDA) (31, 32), and increased fat mass (measured by adipocyte-associated antigens released by the adipose tissue) (33). Plasma levels of these anti-self IgG antibodies are positively associated with blood frequencies of DN B cells. We confirmed our previous findings that the frequencies of DN B cells are increased in the blood of obese versus lean individuals. Moreover, we found that DN B cells show higher expression of IA markers and of the transcription factor T-bet associated with autoimmunity. The removal of DN B cells from the total B cell pool significantly reduced in vitro secretion of anti-self IgG antibodies. These results reveal a critical role for DN B cells in the secretion of anti-self IgG antibodies in individuals with obesity.
Experiments were performed using blood isolated from lean (n=20, 30–54 years) and obese (n=20, 27–55 years) adult female individuals, with average body Mass Index (BMI, kg/m2) 21 ± 1 and 42 ± 3, respectively. The individuals participating in the study were screened for diseases known to alter the immune response or for consumption of medications that could alter the immune response. We excluded subjects with autoimmune diseases, congestive heart failure, cardiovascular disease, chronic renal failure, malignancies, renal or hepatic diseases, infectious disease, trauma or surgery, pregnancy, or documented current substance and/or alcohol abuse.
Study participants provided written informed consent. The study was reviewed and approved by our Institutional Review Board (IRB, protocols 20070481 and 20160542), which reviews all human research conducted under the auspices of the University of Miami.
PBMC were collected using Vacutainer CPT tubes (BD 362761) and cryopreserved. PBMC (1x106/ml) were thawed and cultured in complete medium (c-RPMI, RPMI 1640, supplemented with 10% FCS, 10 µg/ml Pen-Strep, 1 mM Sodium Pyruvate, and 2 x 10–5 M 2-ME and 2 mM L-glutamine).
After thawing, PBMC (2 x 106/ml) were stained for 20 min at room temperature with the following antibodies: anti-CD45 (BioLegend 368540), anti-CD19 (BD 555415), anti-CD27 (BD 555441), and anti-IgD (BD 555778) to measure naive (IgD+CD27-), IgM memory (IgD+CD27+), switched memory (IgD-CD27+), and DN (IgD-CD27-) B cells. To measure membrane expression of markers associated with IA, B cells were also stained with anti-CD95 (BioLegend 305635), anti-CD21 (BioLegend 354911), anti-CD11c (BioLegend 301625), anti-CD86 (BioLegend 374215), anti-HLADR (BioLegend 307617), anti-PD1 (BioLegend 329907) antibodies. Up to 104 events in the B cell gate were acquired on an LSR-Fortessa (BD) and analyzed using FlowJo 10.0.6 software. Single color controls were included in every experiment for compensation. Isotype controls were also used in every experiment to set up the gates.
After thawing, B cells were isolated from PBMC using magnetic CD19 Microbeads (Miltenyi), following manufacturer’s instructions. Cell preparations were typically >98% pure. B cells were stimulated in c-RPMI with CpG (InvivoGen ODN2006, 10 μg/ml) for 10 days. Supernatants were collected and IgG specificity was measured by ELISA.
To evaluate the effects of DN B cells on IgG autoantibody secretion, CD19+ B cells isolated with magnetic beads were stained with anti-CD27 and anti-IgD antibodies. DN B cells were sorted out in a Sony SH800 cell sorter. Total B cells and total B cells without DN B cells were stimulated for 10 days with CpG, and supernatants analyzed for IgG autoantibody specificity by ELISA.
Total RNA was extracted from unstimulated DN B cells, resuspended in TRIzol, according to the manufacturer’s protocol, then resuspended into 10 µl of preheated H2O, and stored at -80°C until use. Reverse Transcriptase (RT) reactions were performed in a Mastercycler Eppendorf Thermocycler to obtain cDNA. Briefly, 2 µl of RNA at the concentration of 0.5 µg/µl were used as template for cDNA synthesis in the RT reaction. Conditions were: 40 min at 42°C and 5 min at 65°C. Five µl of cDNA were used for qPCR. Reactions were conducted in MicroAmp 96-well plates and run in the ABI 7300 machine. Calculations were made with ABI software. Briefly, we determined the cycle number at which transcripts reached a significant threshold (Ct) for each target gene and for GAPDH as control. A value of the target gene, relative to GAPDH, was calculated and expressed as ΔCt. Reagents and primers (Taqman) were from ThermoFisher.
For dsDNA-specific and Malondihyldehyde (MDA)-specific IgG antibodies we used the Signosis EA-5002 and MyBioSource MBS390120 kits, respectively.
For adipocyte-specific IgG antibodies, we isolated the adipocytes from the subcutaneous adipose tissue of patients undergoing weight reduction surgeries (bilateral breast reduction), as previously described (33). After isolation, the adipocytes were centrifuged in a 5415C Eppendorf microfuge (2,000 rpm, 5 min). Total cell lysates were obtained using the M-PER (Mammalian Protein Extraction Reagent, ThermoFisher), according to the manufacturer’s instructions. Aliquots of the protein extracts were stored at -80°C. Protein content was determined by Bradford (34).
To examine differences between groups, unpaired Student’s t tests (two-tailed) were used. To examine relationships between variables, bivariate Pearson’s correlation analyses were performed, using GraphPad Prism version 8 software, which was used to construct all graphs. Principal Component Analyses (PCA) were generated using RStudio Version 1.1.463.
Plasma samples were isolated from individuals with obesity and from lean controls. Samples were tested for the presence of IgG antibodies specific for ds-DNA, MDA and adipocyte-derived antigens. Figure 1 shows significantly higher amounts of IgG for the 3 different antigenic specificities in obese versus lean individuals.
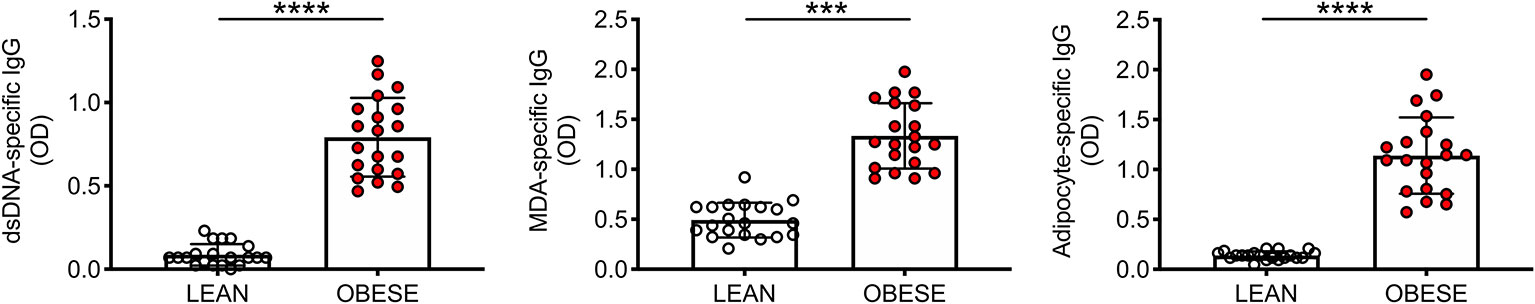
Figure 1 The plasma of individuals with obesity is enriched in IgG antibodies specific for dsDNA, MDA and adipocyte-derived antigens. Plasma samples were isolated from individuals with obesity and from lean controls. Mean comparisons between groups were performed by Student’s t test (two-tailed). ***p < 0.001, ****p < 0.0001.
We also measured IgM antibodies specific for the above autoantigens. Results show no significant differences in lean versus obese individuals for anti-ds-DNA IgM antibodies (0.84 ± 0.11 vs. 0.93 ± 0.13, p=0.60, n=6), for MDA IgM antibodies (1.39 ± 0.09 vs. 1.58 ± 0.08, p=0.15, n=6), and for IgM specific for adipocyte-derived antigens (1.26 ± 0.13 vs. 1.33 ± 0.09, p=0.06, n=18).
We have previously shown that DN B cells present in the blood and in the adipose tissue of individuals with obesity are responsible for the secretion of anti-adipocyte-specific IgG antibodies (15). Here, we tested the hypothesis that DN B cells were also associated with/responsible for the secretion of anti-dsDNA and anti-MDA IgG antibodies in the blood of obese individuals.
We therefore compared the frequencies of DN B cells in this cohort of obese and lean individuals. Figure 2 (top) shows the major B cell subsets, gated on leukocytes (CD45+): naive (IgD+CD27-), IgM memory (IgD+CD27+), switched memory (swIg, IgD-CD27+) and DN (IgD-CD27-). Results in Figure 2 (bottom) show the significant increase in the frequencies of DN B cells in obese versus lean individuals, confirming and extending to this cohort our previously published findings (10, 15). Results in Figure 2 (bottom) also show the frequencies of the other B cell subsets. We observed a significant increase in the frequencies of naïve and a significant decrease in the frequencies of IgM memory B cells in obese versus lean individuals, whereas the frequencies of swIg were found not significantly different between the two groups. These results are slightly different from those we have previously published (10), likely because in this study we have included individuals that are older (27–55 years) than those in our previous study (20–40 years).
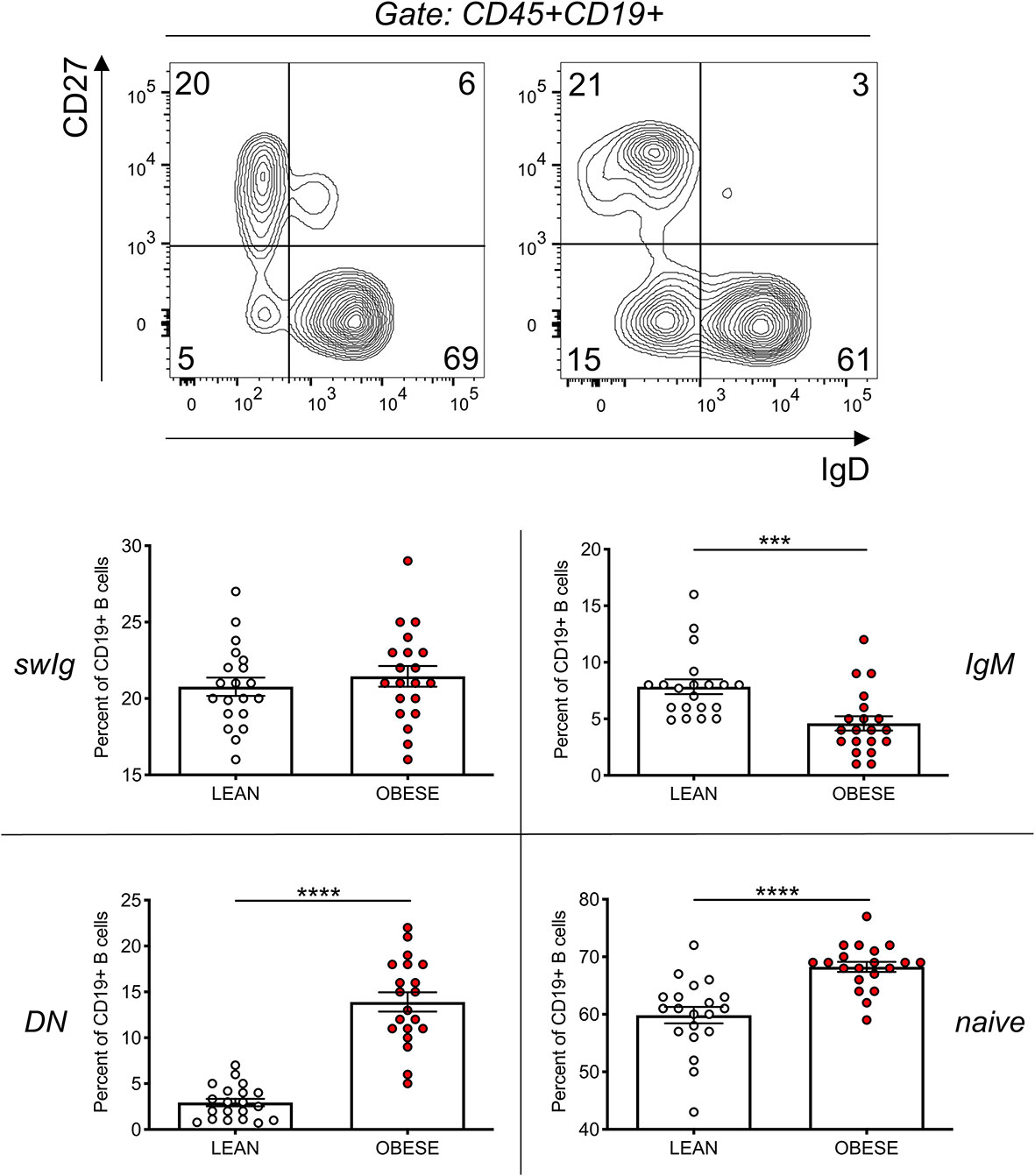
Figure 2 The frequencies of DN B cells significantly increase in the blood of obese versus lean individuals. Top. Gating strategies and a representative dot plot from one lean and one obese individual. Bottom. Results show frequencies of the four B cell subsets. Mean comparisons between groups were performed by Student’s t test (two-tailed). ***p < 0.001, ****p < 0.0001.
As expected, IgG antibodies specific for the self-antigens in Figure 1 were positively associated with blood frequencies of DN B cells in Figure 2 (Figure 3).
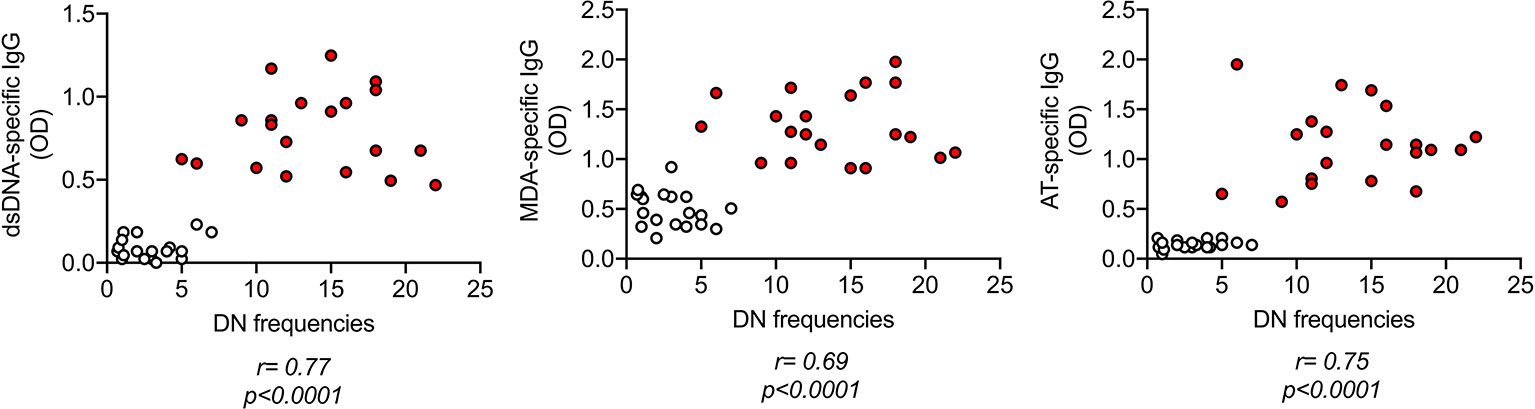
Figure 3 IgG antibodies specific for dsDNA, MDA, adipocyte-derived antigens are positively associated with blood frequencies of DN B cells. IgG antibodies were measured in plasma as indicated in Figure 1. DN B cell frequencies were measured by flow cytometry as indicated in Figure 2. Correlation coefficients and p values are shown for each antibody specificity.
In order to characterize the phenotype of DN B cells present in the blood of individuals with obesity and of lean controls, we examined membrane expression of markers of IA, previously shown to be present on DN B cells from patients with autoimmunity. Briefly, we measured the following: CD21, the complement receptor for C3d (35); CD95, Fas ligand (36); CD11c, the Itgax integrin involved in antigen presentation to T cells (37); CD86 and HLADR, also involved in antigen presentation to T cells (38, 39); PD1, a marker of IA and of cell exhaustion (40). Results in Figure 4A show that DN B cells from individuals with obesity are characterized by lower levels of expression of CD21, and higher levels of expression of CD95, CD11c, CD86, HLADR, PD1, as compared to those from lean controls. These results are in agreement with previously published observations showing the association of the membrane phenotype CD21lowCD95+CD11c+CD86+HLADR+PD1+ with autoimmune B cell subsets, and clearly demonstrate that obesity induces the expansion of DN B cells characterized by this autoimmune phenotype. In the PCA analysis in Figure 4B distinct clustering of DN B cells from the two groups of individuals are shown.
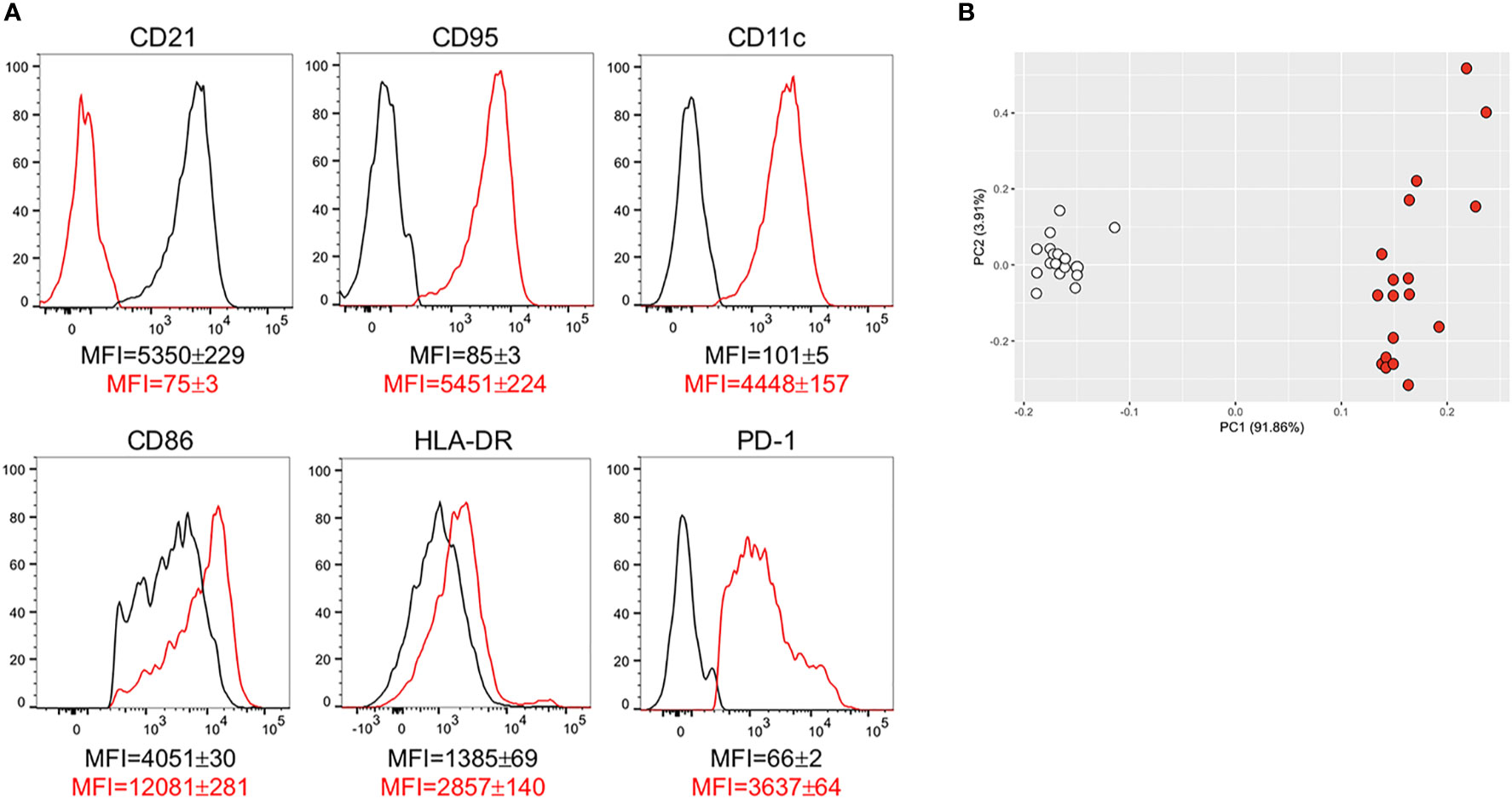
Figure 4 DN B cells are characterized by higher expression of IA markers associated with autoimmunity. (A) Cells were stained to evaluate the expression of several markers of IA on DN B cells from individuals with obesity and from lean controls. Results show mean fluorescence intensity (MFI)± SE for each marker in DN B cells from lean (black line) and obese (red line) individuals (18 individuals/group). (B) PCA analysis with the axes showing the percentage of variation explained by PC1 and PC2. Each symbol indicates an individual. White symbols: lean individuals. Red symbols: obese individuals.
Next, we evaluated if DN B cells with the membrane phenotype associated with autoimmunity were expressing not only the transcription factor T-bet, known to be involved in the secretion of anti–self-antibodies, but also the expression of transcription factors and enzymes crucial for CSR. Briefly, we measured RNA expression of T-bet (tbx21) and other transcription factors involved in CSR (E47, Pax-5), in germinal center reactions (bcl6), in plasma cell differentiation (prdm1, XBP1), as well as RNA expression of AID (aicda). Results in Figure 5 show that tbx21, bcl6, aicda, prdm1 and XBP1 are all significantly up-regulated in unstimulated DN B cells from individuals with obesity as compared to lean controls. No differences were observed for E47 and Pax-5. These results show that DN B cells isolated from the blood of individuals with obesity, as compared to those isolated from lean controls, are not only already pre-activated, as indicated by their higher expression of IA markers, but also show spontaneous expression of the transcription factors associated with antibody secretion, including T-bet, associated with the secretion of IgG antibodies with anti–self–specificity.
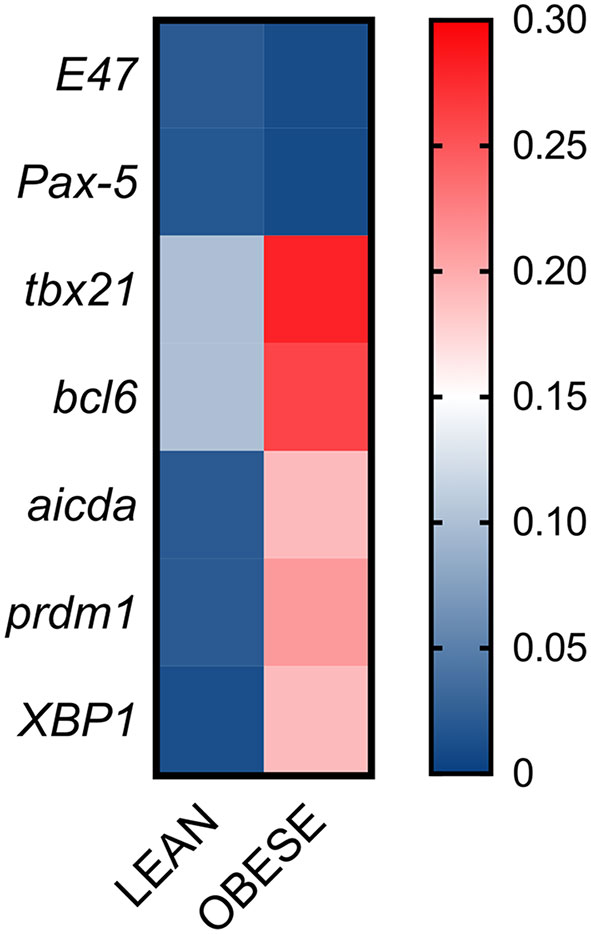
Figure 5 DN B cells are characterized by higher expression of transcription factors associated with autoimmunity. DN B cells were sorted from the peripheral blood of individuals with obesity and of lean controls and left unstimulated. Total RNA was extracted to evaluate by qPCR the expression of transcription factors. Heatmap shows qPCR values (2-ΔΔCt) of several transcription factors, normalized to GAPDH. Results show average qPCR values from 18 individuals/group.
We have previously shown that DN B cells sorted from the breast adipose tissue of obese female patients undergoing weight reduction surgeries secrete autoimmune IgG antibodies that are specific for adipocyte-derived antigens (15). These experiments have been possible because from surgery patients we get large pieces of discarded tissue and, also, because DN B cell frequencies in the adipose tissue reach up to 80% of the total B cell pool, a frequency never observed in the peripheral blood. To further confirm that DN B cells are responsible for the secretion of autoimmune IgG antibodies in the blood of individuals with obesity, we performed the following experiment. B cells, as well as B cells without DN B cells, isolated from the blood of individuals with obesity, were stimulated for 10 days with the B cell mitogen CpG. Stimulation is necessary to allow the stimulation/expansion of IgG secreting B cells. After stimulation, supernatants were collected and IgG autoimmune antibodies measured by ELISA. Results in Figure 6 show that the removal of DN B cells from the pool of total B cells of obese individuals significantly decreased in vitro secretion of anti-dsDNA, anti-MDA and anti-adipocyte IgG specific antibodies.
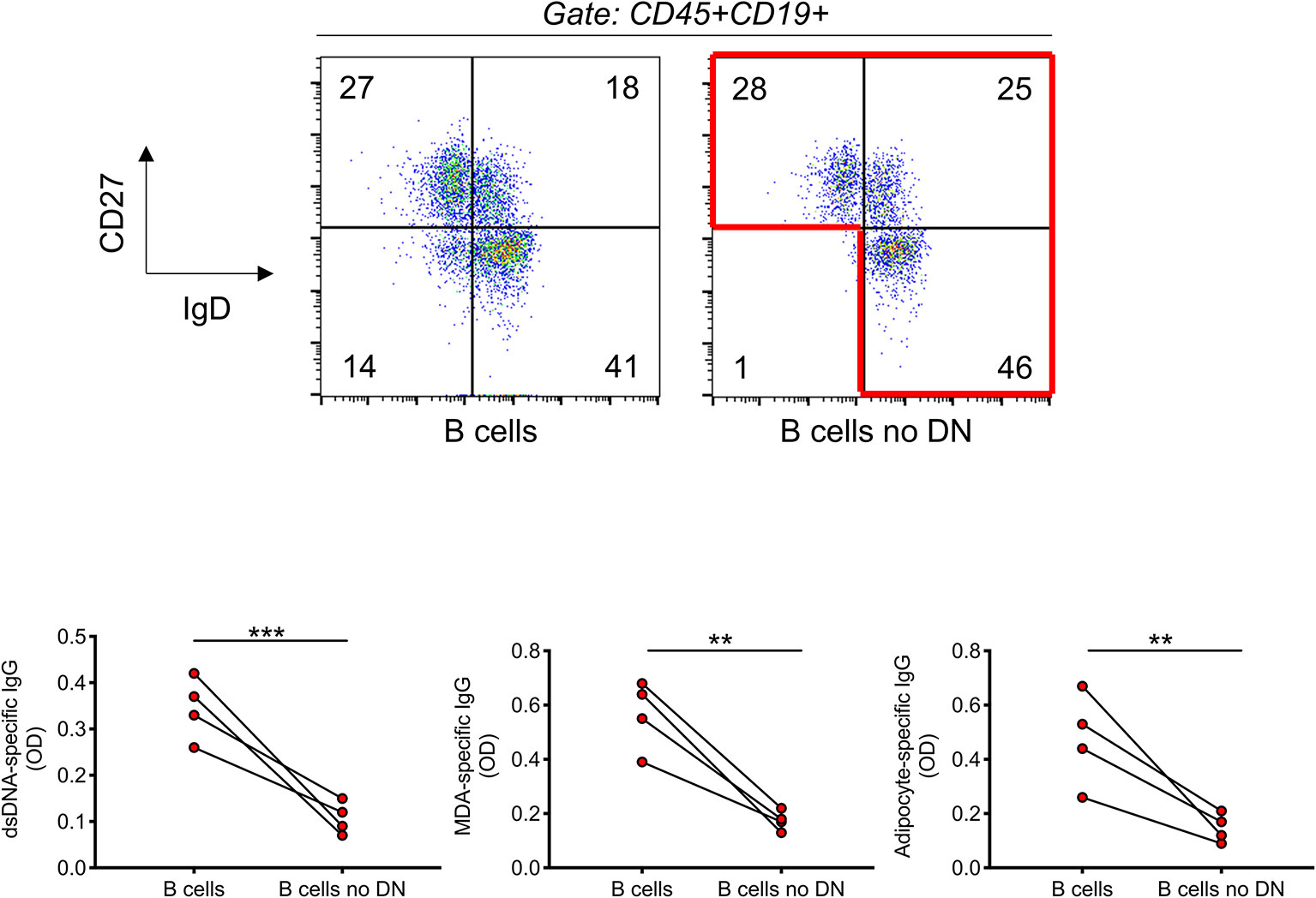
Figure 6 The removal of DN B cells significantly reduces the secretion of IgG autoimmune antibodies. B cells were isolated with magnetic beads from the blood of four individuals with obesity. Top. Gating strategies to remove DN B cells. B cells were stained with anti-CD27 and anti-IgD antibodies. DN B cells were sorted out using a Sony SH800 cell sorter. Bottom. After stimulation of total B cells and total B cells without DN B cells for 10 days with CpG, supernatants were collected and analyzed for the presence of anti-dsDNA, anti-MDA, and anti-adipocyte IgG by ELISA. **p < 0.01, ***p < 0.001.
The subset of DN B cells has been the focus of increasing interest in the last few years, as shown by a large number of dedicated publications. DN B cells expand in healthy aging, in autoimmune diseases, in chronic and acute infections. DN B cells also increase in the blood of individuals with obesity and reach significantly high frequencies in the obese subcutaneous adipose tissue, where they secrete large amounts of autoimmune antibodies with different specificities. As we have recently demonstrated, these specificities include adipocyte-derived products, mainly cell-associated proteins and nucleic acids, not known as autoantigens but released in large amounts in the obese adipose tissue under conditions associated with hypoxia and cell death (41). The finding that anti-dsDNA, anti-MDA and anti-adipocyte specific antibodies are increased in the plasma of healthy elderly individuals (15, 42) and obese individuals has suggested that obesity may accelerate age-associated B cell defects. Fat mass indeed increases with age in humans (43, 44) and this is associated with increased inflammaging (1), metabolic dysfunction (5, 45) and development of insulin resistance which also increases with age (46). Moreover, an age-associated increase in the ectopic deposit of triglycerides in several tissues (liver, muscle, heart, pancreas, kidney) (47–51) and in blood vessels (52) occurs, and this is associated with the development and/or progression of age-associated diseases.
Data herein clearly show that DN B cells from individuals with obesity express higher levels of membrane markers of IA associated with autoimmunity as compared to lean controls and are characterized by the phenotype CD21lowCD95+CD11c+CD86+HLADR+PD1+. They also spontaneously express higher RNA levels for transcription factors involved in the secretion of autoimmune antibodies (tbx21, prdm1, XBP1), suggesting that DN B cells from obese individuals are already pre-activated, a status leading to spontaneous secretion of autoimmune antibodies, as shown in autoimmune diseases (53), and in the obese adipose tissue at least for some specificities (33, 41). Because the IA phenotype of DN B cells from obese individuals is associated with increased energy demands, DN B cells engage in robust metabolic reprogramming to generate sufficient energy to fuel these demands and support autoantibody secretion (15).
Human DN B cells have many similarities with mouse splenic Age-associated B Cells (ABCs) (54, 55), identified as CD19+AA4.1-CD43-CD21-CD23- cells (54–56). DN B cells and ABCs originate from mature B cell subsets (naïve in humans, follicular B cells in mice) after in vivo or in vitro stimulation with the Toll-like receptors TLR7 or TLR9, alone or together with BCR cross-linking, demonstrating that BCR is also an active signaling system in these subsets. It has been shown that TLR agonists plus IL-21 and IFN-γ regulate T-bet expression, the transcription factor for the secretion of autoimmune antibodies (57), whereas TLR agonists plus IL-21 alone promote CD11c expression independently of T-bet (58). In agreement with the expression of T-bet, both human DN B cells and mouse ABCs secrete anti-ds-DNA (our results herein) or anti-chromatin (55) autoimmune IgG antibodies. Moreover, T-bet+ ABCs carry somatically mutated Ig, suggesting that they originate during T-dependent B cell responses (59). T-bet+ ABCs appear and persist indefinitely after influenza infection in mice (58, 59). These cells represent the spleen-resident population of memory B cells responsible for the secretion of HA stalk-specific IgG2c antibodies and of durable neutralizing antibodies (60). Previous results from Swain’s group have also demonstrated that mouse ABCs are specific for a live influenza virus (A/PR8/34) and these influenza-specific ABCs differentiate into antibody-secreting cells, some of which home to the bone marrow and to the lungs where they persist for months, suggesting their role in providing significant protection (61). Human T-bet+ B cells also have also recently been shown to mediate influenza-specific humoral memory (60). Similar to mouse T-bet+ ABCs, they have an activated phenotype, they are spleen-resident and secrete HA-specific IgG1 antibodies recognizing H1 or H3 viral strains. IgG1 antibodies represent the equivalent of mouse IgG2c.
DN B cells are heterogeneous with two major subsets, DN1 and DN2. DN1 B cells are exclusively involved in follicular T-dependent antibody responses. DN2 B cells, conversely, represent the DN B cell subset that participates in extra-follicular B cell responses. DN1 B cells represent the major DN B cell subset in healthy individuals, whereas DN2 B cells increase in the blood of SARS-CoV-2-infected patients as compared to uninfected controls, suggesting a pathogenic role of DN2 B cells in COVID-19 patients (29). DN2 B cells also increase in the blood of SLE patients, as shown by the same group (62). In both cases, DN2 B cells are characterized by decreased expression of the chemokine receptor CXCR5, associated with follicular homing predisposition, and by a concomitant increased expression of CXCR3, a marker of homing to inflamed tissues. We haven’t been able to identify DN1 and DN2 B cells in the blood and in the adipose tissue of individuals with obesity, likely because the individuals recruited in our studies are healthy, and either acute infection (COVID-19) or active disease (SLE) may be needed to allow the expansion of these extra-follicular B cells. We believe that the DN B cells in obese individuals are predominantly DN2, as they secrete autoimmune antibodies as observed in SLE patients.
In conclusion, our results confirm and extend our previous findings showing that frequencies of DN B cells increase in the blood of obese as compared to lean individuals and are positively correlated with the amounts of plasma autoimmune IgG antibodies. DN B cells are characterized by higher expression of IA markers and of the transcription factor T-bet, both associated with autoimmunity. When we removed DN B cells from the B cell pool we saw a significant decrease in the in vitro secretion of anti-self IgG antibodies. We believe that the results herein strongly support the role of DN B cells in the secretion of anti-self IgG antibodies in individuals with obesity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by institutional review board (IRB) protocol 20070481 and 20160542. The patients/participants provided their written informed consent to participate in this study.
DF wrote the paper. AD, MR, and DF performed the experiments and acquired and analyzed data. DF and BB were involved in funding acquisition. All authors contributed to the article and approved the submitted version.
This study was supported by NIH awards AG32576, AG059719, and AG023717.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci (2000) 908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x
2. Apovian CM, Gokce N. Obesity and cardiovascular disease. Circulation (2012) 125(9):1178–82. doi: 10.1161/CIRCULATIONAHA.111.022541
3. Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets (2014) 14(4):245–54. doi: 10.2174/1871530314666140922153350
4. Hass DJ, Brensinger CM, Lewis JD, Lichtenstein GR. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin Gastroenterol Hepatol (2006) 4(4):482–8. doi: 10.1016/j.cgh.2005.12.015
5. Hotamisligil GS. Inflammation and metabolic disorders. Nature (2006) 444(7121):860–7. doi: 10.1038/nature05485
6. Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell (2013) 152(4):673–84. doi: 10.1016/j.cell.2013.01.041
7. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (2008) 371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X
8. Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med (2007) 167(15):1670–5. doi: 10.1001/archinte.167.15.1670
9. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest (2006) 116(7):1793–801. doi: 10.1172/JCI29069
10. Frasca D, Ferracci F, Diaz A, Romero M, Lechner S, Blomberg BB. Obesity decreases B cell responses in young and elderly individuals. Obesity (Silver Spring) (2016) 24(3):615–25. doi: 10.1002/oby.21383
11. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell (2000) 102(5):553–63. doi: 10.1016/s0092-8674(00)00078-7
12. Frasca D, Diaz A, Romero M, Landin AM, Phillips M, Lechner SC, et al. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine (2010) 28(51):8077–84. doi: 10.1016/j.vaccine.2010.10.023 doi: S0264-410X(10)01492-1 [pii].
13. Frasca D, Diaz A, Romero M, Phillips M, Mendez NV, Landin AM, et al. Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine. Int Immunol (2012) 24:175–82. doi: 10.1093/intimm/dxr123 doi: dxr123 [pii].
14. Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in-vivo following pandemic 2009-H1N1 vaccination in humans. PloS Pathog (2012) 8(9):e1002920. doi: 10.1371/journal.ppat.1002920
15. Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Metabolic requirements of human pro-inflammatory B cells in aging and obesity. PloS One (2019) 14(7):e0219545. doi: 10.1371/journal.pone.0219545
16. Colonna-Romano G, Bulati M, Aquino A, Pellicano M, Vitello S, Lio D, et al. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev (2009) 130(10):681–90. doi: 10.1016/j.mad.2009.08.003
17. Frasca D, Diaz A, Romero M, Blomberg BB. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol (2017) 87(Pt A):113–20. doi: 10.1016/j.exger.2016.12.001
18. Nevalainen T, Autio A, Kummola L, Salomaa T, Junttila I, Jylha M, et al. CD27- IgD- B cell memory subset associates with inflammation and frailty in elderly individuals but only in males. Immun Ageing (2019) 16:19. doi: 10.1186/s12979-019-0159-6
19. Adlowitz DG, Barnard J, Biear JN, Cistrone C, Owen T, Wang W, et al. Expansion of Activated Peripheral Blood Memory B Cells in Rheumatoid Arthritis, Impact of B Cell Depletion Therapy, and Biomarkers of Response. PloS One (2015) 10(6):e0128269. doi: 10.1371/journal.pone.0128269
20. Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, et al. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun (2018) 9(1):1758. doi: 10.1038/s41467-018-03750-7
21. Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter HH, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol (2004) 113(2):161–71. doi: 10.1016/j.clim.2004.05.010
22. Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, et al. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. J Immunol (2016) 197(12):4576–83. doi: 10.4049/jimmunol.1502448
23. Martorana A, Balistreri CR, Bulati M, Buffa S, Azzarello DM, Camarda C, et al. Double negative (CD19+IgG+IgD-CD27-) B lymphocytes: a new insight from telomerase in healthy elderly, in centenarian offspring and in Alzheimer’s disease patients. Immunol Lett (2014) 162(1 Pt B):303–9. doi: 10.1016/j.imlet.2014.06.003
24. Saadoun D, Terrier B, Bannock J, Vazquez T, Massad C, Kang I, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjogren’s syndrome-associated lymphoproliferation. Arthritis Rheum (2013) 65(4):1085–96. doi: 10.1002/art.37828
25. Golinski ML, Demeules M, Derambure C, Riou G, Maho-Vaillant M, Boyer O, et al. CD11c(+) B Cells Are Mainly Memory Cells, Precursors of Antibody Secreting Cells in Healthy Donors. Front Immunol (2020) 11:32. doi: 10.3389/fimmu.2020.00032
26. Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med (2008) 205(8):1797–805. doi: 10.1084/jem.20072683
27. Chang LY, Li Y, Kaplan DE. Hepatitis C viraemia reversibly maintains subset of antigen-specific T-bet+ tissue-like memory B cells. J Viral Hepat (2017) 24(5):389–96. doi: 10.1111/jvh.12659
28. Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol (2013) 190(3):1038–47. doi: 10.4049/jimmunol.1202438
29. Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol (2020) 21(12):1506–16. doi: 10.1038/s41590-020-00814-z
30. Wlodarczyk M, Nowicka G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int J Mol Sci (2019) 20(5):1146–63. doi: 10.3390/ijms20051146
31. Sankhla M, Sharma TK, Mathur K, Rathor JS, Butolia V, Gadhok AK, et al. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin Lab (2012) 58(5-6):385–92. doi: 10.1172/JCI21625
32. Yesilbursa D, Serdar Z, Serdar A, Sarac M, Coskun S, Jale C. Lipid peroxides in obese patients and effects of weight loss with orlistat on lipid peroxides levels. Int J Obes (Lond) (2005) 29(1):142–5. doi: 10.1038/sj.ijo.0802794
33. Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Secretion of autoimmune antibodies in the human subcutaneous adipose tissue. PloS One (2018) 13(5):e0197472. doi: 10.1371/journal.pone.0197472
34. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem (1976) 72:248–54. doi: 10.1006/abio.1976.9999
35. Cherukuri A, Cheng PC, Pierce SK. The role of the CD19/CD21 complex in B cell processing and presentation of complement-tagged antigens. J Immunol (2001) 167(1):163–72. doi: 10.4049/jimmunol.167.1.163
36. Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, et al. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum (2008) 58(6):1762–73. doi: 10.1002/art.23498
37. Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J Immunol (2015) 195(1):71–9. doi: 10.4049/jimmunol.1500055
38. June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today (1994) 15(7):321–31. doi: 10.1016/0167-5699(94)90080-9
39. Schwartz BD. HLA molecules: sentinels of the immune response. Am J Respir Cell Mol Biol (1991) 5(3):211–2. doi: 10.1165/ajrcmb/5.3.211
40. Thibult ML, Mamessier E, Gertner-Dardenne J, Pastor S, Just-Landi S, Xerri L, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol (2013) 25(2):129–37. doi: 10.1093/intimm/dxs098
41. Frasca D, Diaz A, Romero M, Garcia D, Jayram D, Thaller S, et al. Identification and Characterization of Adipose Tissue-Derived Human Antibodies With “Anti-self” Specificity. Front Immunol (2020) 11:392. doi: 10.3389/fimmu.2020.00392
42. Ruffatti A, Calligaro A, Del Ross T, Bertoli MT, Doria A, Rossi L, et al. Anti-double-stranded DNA antibodies in the healthy elderly: prevalence and characteristics. J Clin Immunol (1990) 10(6):300–3. doi: 10.1007/BF00917474
43. Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell (2010) 9(5):667–84. doi: 10.1111/j.1474-9726.2010.00608.x
44. Zamboni M, Rossi AP, Fantin F, Zamboni G, Chirumbolo S, Zoico E, et al. Adipose tissue, diet and aging. Mech Ageing Dev (2014) 136-137:129–37. doi: 10.1016/j.mad.2013.11.008
45. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature (2017) 542(7640):177–85. doi: 10.1038/nature21363
46. Muller DC, Elahi D, Tobin JD, Andres R. The effect of age on insulin resistance and secretion: a review. Semin Nephrol (1996) 16(4):289–98.
47. Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension (2011) 58(5):784–90. doi: 10.1161/HYPERTENSIONAHA.111.175315
48. Machann J, Thamer C, Schnoedt B, Stefan N, Stumvoll M, Haring HU, et al. Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: a whole body MRI/MRS study. MAGMA (2005) 18(3):128–37. doi: 10.1007/s10334-005-0104-x
49. Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord (1999) 23(2):126–32. doi: 10.1038/sj.ijo.0800777
50. Saisho Y, Butler AE, Meier JJ, Monchamp T, Allen-Auerbach M, Rizza RA, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat (2007) 20(8):933–42. doi: 10.1002/ca.20543
51. Silaghi A, Piercecchi-Marti MD, Grino M, Leonetti G, Alessi MC, Clement K, et al. Epicardial adipose tissue extent: relationship with age, body fat distribution, and coronaropathy. Obesity (Silver Spring) (2008) 16(11):2424–30. doi: 10.1038/oby.2008.379
52. Robert L. Aging of the vascular-wall and atherosclerosis. Exp Gerontol (1999) 34(4):491–501. doi: 10.1016/s0531-5565(99)00030-3
53. Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev (2005) 204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x
54. Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood (2011) 118(5):1294–304. doi: 10.1182/blood-2011-01-330530
55. Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood (2011) 118(5):1305–15. doi: 10.1182/blood-2011-01-331462
56. Frasca D, Diaz A, Romero M, Vazquez T, Blomberg BB. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mech Ageing Dev (2017) 162:91–9. doi: 10.1016/j.mad.2017.01.004
57. Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A (2002) 99(8):5545–50. doi: 10.1073/pnas.082114899
58. Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, et al. Cutting Edge: IL-4, IL-21, and IFN-gamma Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells. J Immunol (2016) 197(4):1023–8. doi: 10.4049/jimmunol.1600522
59. Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, et al. Age-Associated B Cells Express a Diverse Repertoire of VH and Vkappa Genes with Somatic Hypermutation. J Immunol (2017) 198(5):1921–7. doi: 10.4049/jimmunol.1601106
60. Johnson JL, Rosenthal RL, Knox JJ, Myles A, Naradikian MS, Madej J, et al. The Transcription Factor T-bet Resolves Memory B Cell Subsets with Distinct Tissue Distributions and Antibody Specificities in Mice and Humans. Immunity (2020) 52(5):842–55 e6. doi: 10.1016/j.immuni.2020.03.020
61. Swain SL, Kugler-Umana O, Kuang Y, Zhang W. The properties of the unique age-associated B cell subset reveal a shift in strategy of immune response with age. Cell Immunol (2017) 321:52–60. doi: 10.1016/j.cellimm.2017.05.009
Keywords: aging, B cells, obesity, inflammation, antibody responses
Citation: Frasca D, Diaz A, Romero M and Blomberg BB (2021) Phenotypic and Functional Characterization of Double Negative B Cells in the Blood of Individuals With Obesity. Front. Immunol. 12:616650. doi: 10.3389/fimmu.2021.616650
Received: 12 October 2020; Accepted: 11 January 2021;
Published: 23 February 2021.
Edited by:
Marcella Visentini, Sapienza Università di Roma, ItalyReviewed by:
Massimo Fiorilli, Sapienza University of Rome, ItalyCopyright © 2021 Frasca, Diaz, Romero and Blomberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela Frasca, ZGZyYXNjYUBtZWQubWlhbWkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.