- 1Université de Paris, Biologie Intégrée du Globule Rouge, UMR_S1134, BIGR, INSERM, Paris, France
- 2Institut National de la Transfusion Sanguine, Paris, France
- 3Laboratory of Excellence GR-Ex, Paris, France
Over 30 million women living in P. falciparum endemic areas are at risk of developing malaria during pregnancy every year. Placental malaria is characterized by massive accumulation of infected erythrocytes in the intervillous space of the placenta, accompanied by infiltration of immune cells, particularly monocytes. The consequent local inflammation and the obstruction of the maternofetal exchanges can lead to severe clinical outcomes for both mother and child. Even if protection against the disease can gradually be acquired following successive pregnancies, the malaria parasite has developed a large panel of evasion mechanisms to escape from host defense mechanisms and manipulate the immune system to its advantage. Infected erythrocytes isolated from placentas of women suffering from placental malaria present a unique phenotype and express the pregnancy-specific variant VAR2CSA of the Plasmodium falciparum Erythrocyte Membrane Protein (PfEMP1) family at their surface. The polymorphic VAR2CSA protein is able to mediate the interaction of infected erythrocytes with a variety of host cells including placental syncytiotrophoblasts and leukocytes but also with components of the immune system such as non-specific IgM. This review summarizes the described VAR2CSA-mediated host defense evasion mechanisms employed by the parasite during placental malaria to ensure its survival and persistence.
Introduction
Nearly half the world’s population, implicating 90 countries, lives in areas at risk of malaria transmission. In 2019, an estimated 11 million pregnant women were infected by Plasmodium in sub-Saharan Africa, where P. falciparum is the most prevalent parasite species, accounting for 99.7% of estimated malaria cases (1). P. falciparum infection contracted during pregnancy can lead to placental malaria (PM), a condition that could cause very serious clinical outcomes for both mother and child, including maternal anemia (2, 3), hypertension (4, 5), stillbirth (6, 7) as well as low birth-weight infants, which affected over 800,000 children in 2019 (1).
PM may result in significant morphological and immunological changes in the placenta. Focal syncytial necrosis, loss of syncytial microvilli, and proliferation of cytotrophoblastic cells are frequently observed as well as thickening of trophoblastic basement membranes together with the apparition of syncytial knots (8–10). Acute infection is also characterized by the substantial presence of infected erythrocytes (IEs) in the intervillous spaces of the placenta (Figure 1A).
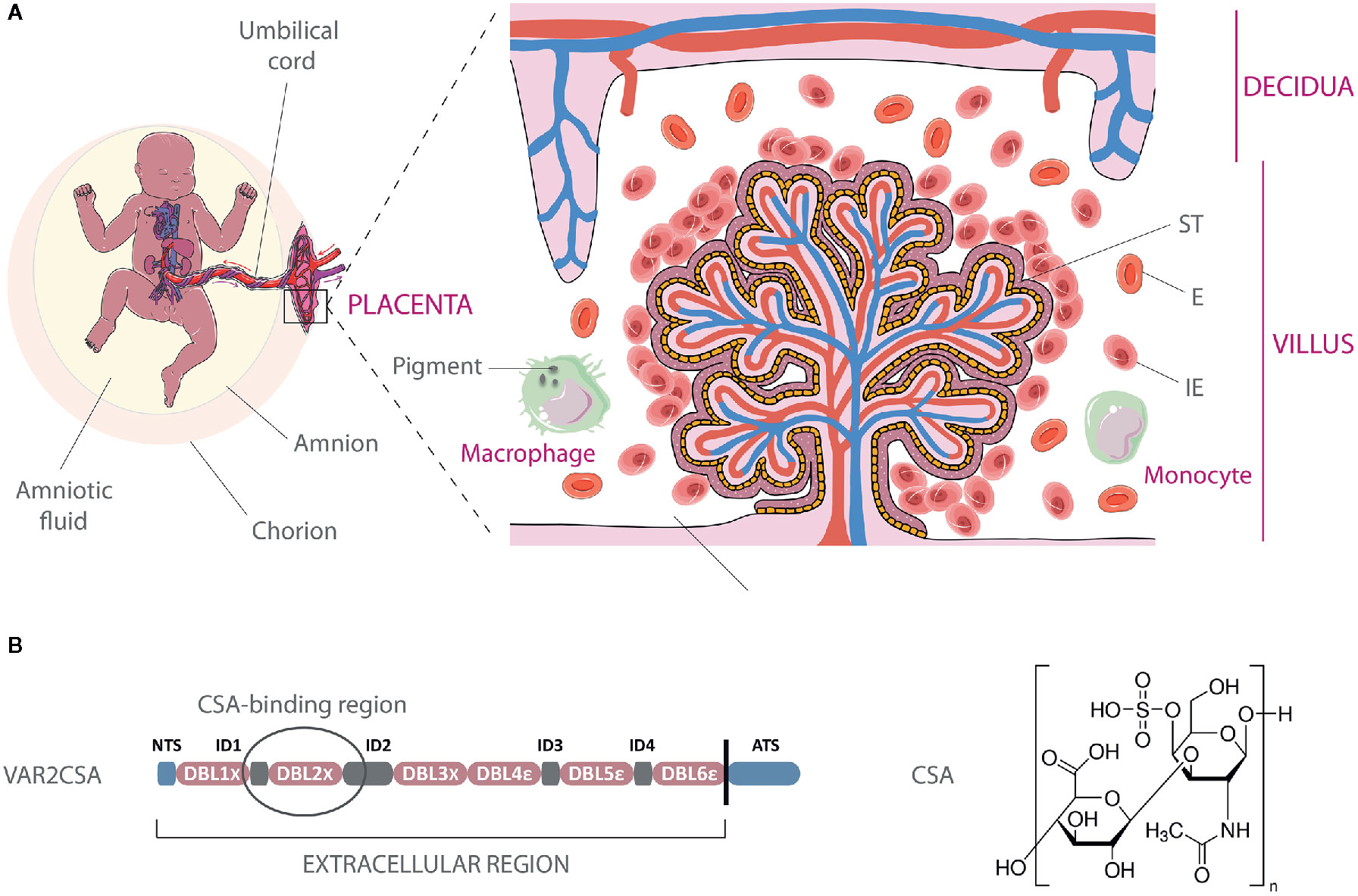
Figure 1 Infected erythrocyte sequestration within the intervillous space of the placenta. (A) Schematic representation of infected erythrocytes (IE) adhering to the syncytiotrophoblastic lining of the fetal villus, with increased presence of macrophages and monocytes in the maternal blood. Parasite pigments (hemozoin) remain visible in macrophages following IEs phagocytosis. The natural transfer of gases and nutrients between maternal blood in the intervillous space and fetal blood circulating in the villi is impaired by IEs sequestration. E, Erythrocyte; ST, syncytiotrophoblast. (B) Architecture of the VAR2CSA protein and chemical structure of chondroitin-4-sulfate A. The circled region within VAR2CSA (ID1-ID2a) represents the CSA-binding region. The art pieces used in this figure were modified from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com/).
Several transcriptomic and proteomic studies revealed that parasitized red blood cells isolated from P. falciparum-infected pregnant women display specific signatures, over-expressing a variety of different genes (11–13) and proteins (14–16) as compared to non-pregnancy-specific parasites. They also present a unique adhesive phenotype, interacting with chondroitin sulfate A (CSA), a low-sulfated glycosaminoglycan (GAG), which is the major host receptor involved in the adhesion of IEs to syncytiotrophoblastic cells (17–21) (Figure 1B). Chondroitin sulfate-proteoglycans (CSPGs) are present in the intervillous space of the placenta during the entire second and third trimesters and possibly during the latter part of the first trimester (22).
To date, the pregnancy-specific variant of the Plasmodium falciparum erythrocyte membrane protein 1 family (PfEMP1) VAR2CSA has been identified as the sole parasite-derived protein interacting with placental CSA (23–28).
This review focuses on the roles played by VAR2CSA in PM pathogenesis and introduces the latest information on its involvement in host defense evasion mechanisms ranging from cytoadhesion in the placenta, modulation of the placental microenvironment to escape of pregnancy-specific IEs from recognition by protective antibodies.
VAR2CSA Structure and Chondroitin Sulfate A (CSA)-Binding
VAR2CSA is a large protein of 350 kDa, with an extracellular region of approximately 300 kDa, displayed at the surface of IEs on membrane protrusions called knobs (29). PfEMP1 clustering on knob structures is thought to maximize cytoadhesion under flow conditions but also to act as an immune evasion mechanism, impairing antibody accessibility to key residues involved in CSA-binding (30, 31). Quantitative studies report an estimate of 3 to 80 VAR2CSA molecules per knob (32, 33). Knob density at the IEs surface has been shown to be linked to the PfEMP1 variant expressed by the parasite (34) and IEs stained by the monoclonal antibody PAM1.4 revealed that erythrocytes infected by the FCR3 parasite strains displayed more VAR2CSA clusters at the cell surface than erythrocytes infected by NF54 (35). Even if further studies are needed to precisely determine how these differences in PfEMP1 presentation impact antibody recognition, these observations highlight that P. falciparum is capable of complex variations at both intra- and inter-strain levels.
The cysteine-rich extracellular region of VAR2CSA has a complex architecture and is composed of six Duffy-Binding Like domains (DBLs), which are interspaced by four inter-domain regions (IDs) (Figure 1B). High-resolution structures have been obtained for the individual domains DBL3x, DBL6ϵ (36–40) as well as for the multidomain DBL3x-DBL4ϵ (41), providing a first step towards the definition of inter-domain interfaces and of the overall structure of the extracellular part of VAR2CSA. Low-resolution structures of the full-length extracellular part of VAR2CSA, obtained by small-angle X-ray scattering or single particle electron microscopy, reveal a compact organization of the protein maintained by specific inter-domain interactions (42–44). Nevertheless, the relative locations of the DBL domains within the overall structure of VAR2CSA significantly differ from one study to another (43, 44). In the recent work from Bewley et al., the VAR2CSA ectodomain low resolution structure appears as a duck-like shape with a packing of three tandem domains (DBL1x/DBL2x, DBL3x/DBL4ϵ, and DBL5ϵ/DBL6ϵ), which would form two pores, each theoretically susceptible to accommodate a 10-12-mer CSA. molecule (44). This model suggests that the higher-order structural organization of VAR2CSA is most likely allowing the formation of one, or maybe two, CSA-binding site(s), which comprise(s) several domains. The current definition of the boundaries of the core binding region, established using truncated fragments of recombinant VAR2CSA, localizes the high affinity CSA-binding site within the N-terminal part of the protein (45) between the ID1-ID2a section (46) even-though the accessory implication of other domains such as DBL4ϵ cannot be excluded (44). Additional VAR2CSA structural data at high resolution, ideally in complex with CSA, is still required to determine the precise determinants of CSA-binding, which might also include post-translational modifications (47).
VAR2CSA-Mediated Infected Erythrocytes Cytoadhesion in The Placenta and Evasion from Splenic Filtration
As parasites develop from ring stage to schizont stage within erythrocytes, the biomechanical properties of the host cells are subjected to considerable modifications, leading to decreased cellular deformability and loss of membrane elasticity [Reviewed in (48)]. Cytoadhesion of mature pregnancy-specific IEs to syncytiotrophoblasts leads to their sequestration in the intervillous spaces of the placenta. By sequestering in the placenta, biomechanically altered IEs avoid splenic retention at the level of the reticular mesh of the red pulp or during the challenging passage through the inter-endothelial slits of the organ (49–52). P. falciparum has therefore developed an efficient host defense evasion mechanism, which relies on a tight interaction between IEs and the syncytiotrophoblastic lining delimiting the intervillous spaces of the maternal portion of the placenta. As CSPGs are also present within the micro-vascular system, notably in the lungs and brain (53), the reason for exclusive placental sequestration of VAR2CSA-expressing IEs remains unclear. A body of work elucidated some comprehensive elements by demonstrating that the interaction of VAR2CSA with CSA is highly correlated with the degree of C-4 sulfation and the length of the CS chain (54–56), which may vary in different tissues. CSA density and wall shear stress also appear as two components influencing the IEs binding to CSA (57). CSA density on syncytiotrophoblasts and forces acting upon placental tissues could thus determine the selective cytoadhesion of IEs in the organ. If placental sequestration of IEs represents an effective immune evasion mechanism employed by P. falciparum to avoid its clearance by the spleen, this is not without harmful consequences for the women and the fetus. Sequestration is thought to be one of the prime mediators of biological alterations leading to placental insufficiency and subsequently to fetal growth restriction and poor birth outcomes [Reviewed in (58, 59)].
VAR2CSA-Mediated Modulation of The Placental Microenvironment
The placenta is a tightly controlled pro-inflammatory and anti-inflammatory environment, depending upon the stage of gestation. In healthy pregnancies, a pro-inflammatory milieu is required for fetal implantation, notably by promoting trophoblast invasion. A shift toward a type 2 cytokine/chemokine milieu gradually occurs during gestation favoring pregnancy maintenance and rapid fetal growth and development [reviewed in (60)]. P. falciparum infection during pregnancy can affect the placental environment, notably promoting inflammatory responses (61–65), some of which are associated with fetal growth retardation, low birth-weight babies, and in more extreme cases, poor pregnancy outcomes, such as preterm delivery and pregnancy loss (66–71). P. falciparum is thus able to upset the fine equilibrium between pro-inflammatory and anti-inflammatory responses, deregulating the immune system, with detrimental consequences for the human host.
Syncytiotrophoblast Activation
The syncytiotrophoblasts covering the placental villi are terminally differentiated cells, which result from the syncytialization of underlying villous cytotrophoblasts. They exhibit high metabolic activity and are involved in many physiological processes such as the active transport of molecules, the diffusion of gases, and the synthesis and secretion of large amounts of hormones, including steroids [Reviewed in (72)]. Experiments performed using primary placental cells, as well as the widely used choriocarcinoma cell line BeWo, revealed that VAR2CSA-dependent binding of IEs to syncytiotrophoblasts induces a broad range of cellular responses, notably activating MAPK pathways (73, 74). Activation of syncytiotrophoblasts leads to the secretion of pro-inflammatory cytokines/chemokines such as macrophage inflammatory protein (MIP), the neutrophil chemotactic factor interleukin (IL) 8 and IL-6 (74, 75), but also to the production of soluble ICAM-1 (75), which may act as a protection mechanism to regulate the inflammatory response (76). The interaction of syncytiotrophoblasts with VAR2CSA-expressing IEs might therefore participate in the immunological shaping of the local environment, establishing a complex network of factors which could promote the migration of immune cells to the intervillous space (74), as well as the in situ modulation of their activity.
Macrophage and Monocyte Immunomodulation
Sections taken from healthy placenta at different time-points throughout normal pregnancy showed that nearly half of the decidual cells are of bone marrow origin, comprising 18–20% macrophages (77, 78). Polarization of decidual macrophages varies with gestational age, shifting from an M1 polarization during fetal implantation, towards a mixed M1/M2 profile which remains until mid-pregnancy (79). After the placental development is completed, decidual macrophages are predominantly of the M2 phenotype, contributing to a tolerant immune environment and to fetal immunoprotection (80, 81).
PM is characterized by a significant increase in the number of monocytes and macrophages in the intervillous space (8–10, 82, 83), which is notably associated with elevated expression of the β chemokines IL-8 and MIP-1 (84). In vitro co-incubation experiments, performed in absence of human plasma/serum, i.e. in absence of opsonic antibodies, showed that VAR2CSA-expressing IEs are able to modulate specific transcription factor activation in RAW-macrophages, as compared to erythrocytes infected with genetically modified parasites presenting a deficiency in the export of PfEMP1 at the cell surface (PfEMP1-null) (85). The decreased activation of NF-κB-, CREB-, and GAS/ISRE-binding factors is accompanied by reduced production of TNF and IL-10. Similar experiments using human primary monocytes also revealed that VAR2CSA-expressing IEs are able to alter the production profiles of other cytokines/chemokines, limiting the release of IL-1β, IL-6, IL-10, MCP-1, MIP-1α, and MIP-1β, as compared to cells infected withfimmu.2020.624126 PfEMP1-null parasites (85). Although the precise nature of the monocyte receptor(s) involved still remains to be elucidated, these observations highlight how P. falciparum could exploit the host cellular pathways to modulate the immune response.
Interestingly, a study performed in an area of low prevalence of malaria, revealed gravidity-dependent differences in the capacity of peripheral blood mononuclear cells (PBMCs) to produce cytokines and chemokines in response to pregnancy-specific IEs (86). Despite no differences in opsonic antibody levels, cellular immune responses differed between women in their second to fourth pregnancy (G2-4) and grand multigravida (G5-G7). Indeed, more IL-10, IL-1β, IL-6, tumor necrosis factor (TNF) but less CXCL-8, CCL-8, IFNγ, and CXCL-10 were detected in G2-4 compared to G5-7, highlighting the modulation of immune cell function occurring during PM (86).
VAR2CSA Binding to Non-Specific IGM and Diversion of The Immune Response
PM induces VAR2CSA-specific immunoglobulin Gs (IgGs) belonging to the IgG1 subclass, and to a lower extent the IgG3 subclass (87, 88), both highly potent at interacting with Fcγ receptors present at the surface of phagocytic cells. Concordantly, women living in areas where malaria is endemic naturally acquire specific antibodies that promote the phagocytosis of VAR2CSA-expressing IEs (89–91), thus participating in parasite clearance. Binding of non-specific IgM on the surface of IEs was first demonstrated on rosetting parasites (92–94) and subsequently on VAR2CSA-expressing red blood cells (95). Following these observations, the function of IgM binding to VAR2CSA has been uncertain for several years. In 2011, a study performed by Barfod et al. showed that non-specific IgM binding participates in the masking of protective epitopes on VAR2CSA, leading to IE evasion of macrophage-mediated opsonic phagocytosis (96). The same study revealed that non-specific IgM binding to VAR2CSA-expressing IEs did not interfere with their capacity to adhere to CSA and did not increase their susceptibility to undergo complement-mediated lysis (96). The extensive analysis of non-specific IgM binding to large panels of PfEMP1 members demonstrated that IgM binding is a common functional phenotype found in multiple PfEMP1 variants across various parasite strains, thus providing a better understanding of the underlying molecular mechanisms (97–99). Although the CSA-binding site of VAR2CSA resides within the N-terminal region of the protein (100, 101), the IgM interacting residues appear to be mainly located within the C-terminal section, at the level of the DBL5ϵ or DBL6ϵ domains in VAR2CSA variants carried by the 3D7 and FCR3/IT parasite strains, respectively (102, 103) as well as in DBLϵ and DBLζ domains near the C-terminus of other PfEMP1 variants (98, 99, 104, 105).
The PfEMP1 binding sites on IgM have been located within the μ region of the fragment crystallizable (Fcμ) of polymeric immunoglobulins (97), and more precisely in the Cμ4 domain for the DBL4β domain of PfEMP1-VAR1 of the TM284 strain (106). These observations, together with the additional definition of the architecture of the IgM/PfEMP1 complex (107), provide critical molecular elements which could explain how PfEMP1s interfere with the binding of the complement component C1q to the adjacent Cμ3 domain, thus inhibiting complement-mediated lysis. Furthermore, these findings demonstrate how IgMs participate in PfEMP1 clustering on the cell surface, strengthening the interactions with host receptors (107–109). PfEMP1 binding to IgM has also been proposed as a non-exclusive molecular mechanism involved in the triggering of polyclonal B cell activation, a hall mark of malaria (110, 111). This activation would lead to hyper-gamma-immunoglobulinemia and the subsequent diversion of the specific humoral immune response towards antigens relevant for protection.
VAR2CSA Polymorphism
All the P. falciparum genomes sequenced to date reveal the presence of one or more var2csa gene copies (112–114). VAR2CSA is a highly polymorphic multidomain protein, usually consisting of six DBL domains; the first three DBL domains belong to the DBLx subtype and the three others to the DBLϵ subtype. The protein also contains a CIDRPAM domain (also referred to as ID2) between the DBL2x and DBL3x domains. A recent study has identified atypical extended or truncated VAR2CSA structures (115). Extended structures include one or two additional DBLε domains downstream of the conventional DBL1x-6ε domain structure (115). Within the conventional six DBL domain structure, DBL4ε is the most conserved DBL domain while DBL6ε is the most polymorphic DBL domain (112). Var2csa is present in all genomes of known Laverania sub-genus members (116). One of the closest P. falciparum relatives, the chimpanzee parasite Plasmodium reichenowi, possesses a var2csa-like gene which is annotated as a pseudogene and encodes a functional truncated protein (NTS-DBL1x-ID1-DBL2x-truncated ID2) (117).
Global sequence diversity and analysis of var2csa have been reported in different studies (118–121) and more recently for 1,249 sequences spanning 7 Kb of var2csa (NTS-DBL5ε) from various strains and field isolates (122). Although it was previously shown that the DBL6ε domain is the most polymorphic domain (112), this latest study, which does not include DBL6ε, demonstrates that the nucleotide diversity is higher towards the N-terminus of the protein and that the diversity is generally higher in African parasite populations than in South East Asian populations. While the DBL2x domain has the lowest nucleotide diversity (122), it possesses the highest density of insertions and deletions, with sequence length across samples ranging from 430 to 550 amino acids (122). In a population structure analysis performed on var2csa sequences from Benin and Malawi, five different clades of ID1-DBL2x (encoding for the CSA-binding region) were identified and the authors found an association between the 3D7-like clade and low birth-weight (120). Only four clades were identified, including a 3D7-like clade (clade 1) and an FCR3-like clade (clade 2) (120). Indeed, two of the previously identified clades could not be separated using this much larger dataset. Clades 1, 2, and 4 were present across all the P. falciparum malaria endemic areas and clade 1, which is associated with low birth-weight, is highly represented in the West African populations (41.7%), followed by East Africa (27.5%), South East Asia (23.5%), and South America (21.1%). However, clade 3 is exclusively found in African parasite populations but appears to represent less than 1% of the var2csa sequences.
A recent study, which used plasma obtained from Tanzanian and Malian women at the time of delivery, simultaneously examined the capability of antibodies to recognize native VAR2CSA expressed by either NF54 or FCR3, to inhibit the binding of IEs to CSA and to promote phagocytosis by THP1 cells. Plasma from Malian women reacted more strongly with VAR2CSA-expressing erythrocytes infected by the FCR3 parasite strain whereas Tanzanian plasma preferentially reacted with erythrocytes infected by NF54 (35). Analysis of antibody functionality showed that the balance between binding inhibition capability and opsonizing activity could be biased depending on the expressed VAR2CSA variant and on the geographical location (35), suggesting that epitopes involved in each functional process may differ among parasite strains and that parasite transmission in a given place could therefore shape antibody profiles. In addition, the multiplicity of var2csa genes within the parasite genome may also confer a greater capacity for antigenic variation and evasion of variant-specific immune responses (114).
Concluding Remarks
P. falciparum infection contracted during pregnancy elicits a broad range of immune responses, combining components of both the innate and the adaptive immunity, orchestrated by a complex network of pro- and anti-inflammatory cytokines (Figure 2). P. falciparum has developed the ability to manipulate the immune system to its advantage to ensure its survival and persistence within the human host.
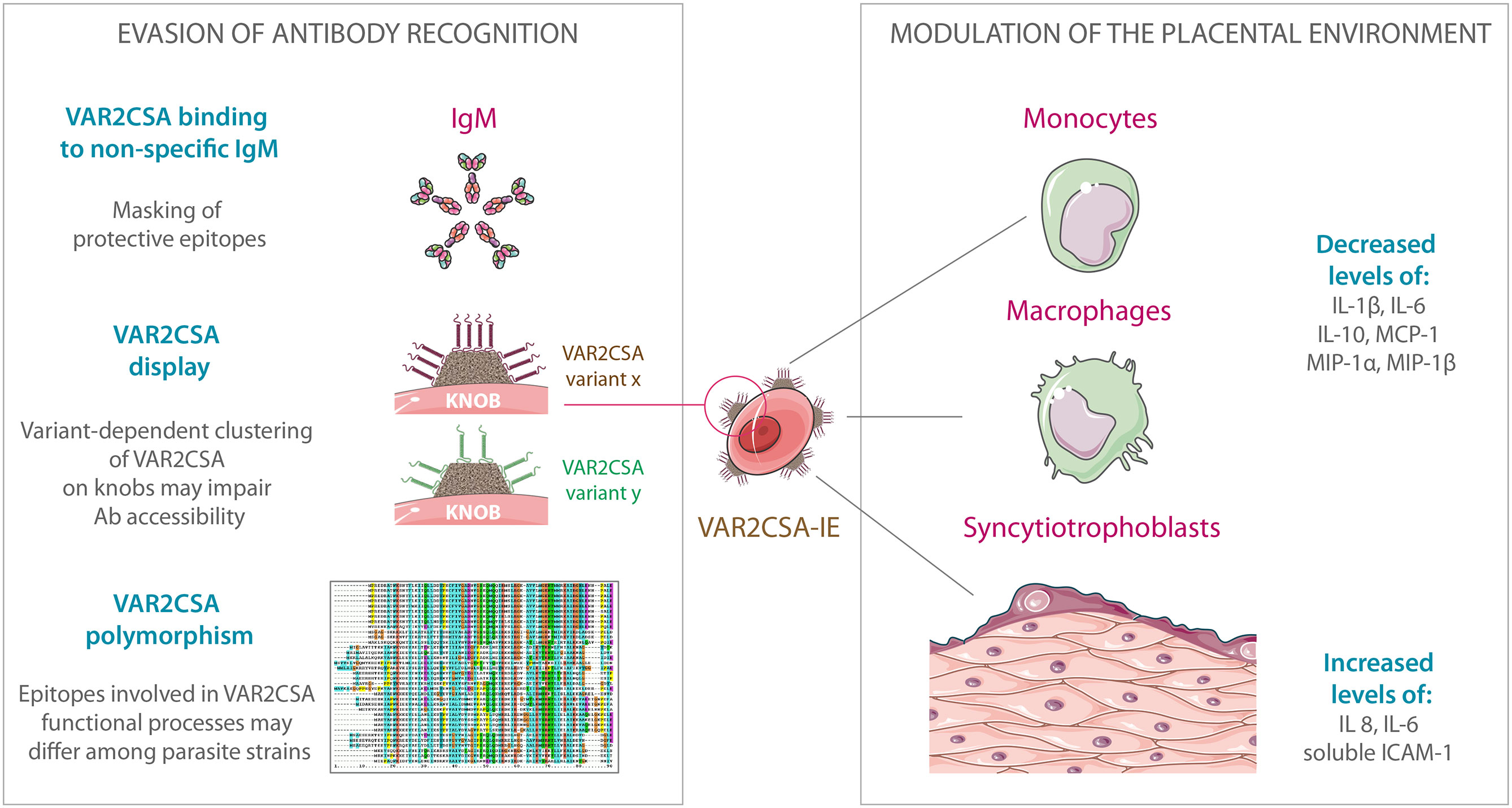
Figure 2 Evasion of antibody recognition and modulation of the placental environment by VAR2CSA-expressing infected erythrocytes. IgM binding to VAR2CSA could mask protein epitopes recognized by anti-VAR2CSA IgGs and consequently alter opsonic phagocytosis of IEs. PfEMP1 clustering on knob structures may act as an immune evasion mechanism, impairing antibody accessibility to key residues involved in CSA-binding. Due to extensive polymorphism, epitopes involved in each VAR2CSA functional process may differ among parasite strains. Furthermore, multiplicity of var2csa genes within the parasite genome may also confer a greater capacity for antigenic variation and evasion of variant-specific immune responses. The presence of VAR2CSA on the IEs surface could lead to decreased production of IL-1β, IL-6, IL-10, MCP-1, MIP-1α, and MIP-1β by monocytes and macrophages. VAR2CSA-dependent binding of IEs to syncytiotrophoblasts is able to activate MAPK pathways and lead to increased secretion of IL-8, IL-6, and soluble ICAM-1. The art pieces used in this figure were modified from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com/). The illustration of the protein sequence alignment is licensed under a Creative Commons Attribution, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/).
Although the parasite is able to escape host defense processes and manipulate the induced immune response using a variety of mechanisms described herein, women living in malaria endemic areas can gradually acquire protective clinical immunity against PM, depending on the intensity of parasite transmission (123). In moderate malaria transmission. PM adverse clinical outcomes can be seen in women of all parity status (124), whereas protection appears to develop in a more marked parity-dependent manner in high transmission settings (125). Importantly, PM protection has been linked to the presence of antibodies targeting PM-specific variant surface antigens (126) and more specifically VAR2CSA (127–129). These observations led to the belief that a VAR2CSA-based vaccine against PM could potentially be achieved. However, the high degree of sequence diversity within VAR2CSA represents a major hurdle for vaccine design.
Following extensive preclinical evaluation, two recombinant vaccine candidates PRIMVAC and PAMVAC, comprising the CSA-binding region of VAR2CSA from the 3D7 (clade 1) and FCR3 (clade 2) strains respectively, have been assessed in Phase I clinical trials in Europe and Africa (ClinicalTrials.gov identifiers NCT02658253 and NCT02647489, respectively) (130–134). The identification of immunological correlates of protection against PM being complex, there is to date no clear surrogate allowing an easy evaluation of the protective effects of vaccines in early clinical trials (135). Exploratory analyses performed for the PRIMVAC and PAMVAC trials nevertheless revealed that vaccine-induced antibodies had a limited capability to cross-react with VAR2CSA originating from heterologous parasite strains (133, 134), highlighting the difficulty to compose with the high degree of polymorphisms of the protein when designing vaccines. Alternative vaccine approaches, using VAR2CSA in combination with other P. falciparum antigens, such as the circumsporozoite protein (CSP) (136), or virus-like particles (VLPs) to display VAR2CSA-derived antigens are also currently under investigation (137–139).
Improving our understanding on how P. falciparum escapes host defenses, modulates the immune system and on how natural immunity develops during PM despite VAR2CSA polymorphisms is therefore crucial to design efficient and effective immuno-therapeutic approaches but also to appropriately evaluate them.
Author Contributions
AT, J-PS, BG, and AC wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the ANR-18-IDEX-0001, IdEx Université de Paris attributed to AT and BG.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Acknowledgments
We would like to thank Auria Godard for creating the illustration of the placental villus presented in Figure 1A. We also thank the peer-reviewers for critical review of the former version of the manuscript.
References
1. World Health Organization. World Malaria Report 2019. Geneva: World Health Organization (2019) Licence: CC BY-NC-SA 3.0 IGO.
2. Verhoeff FH, Brabin BJ, Chimsuku L, Kazembe P, Broadhead RL. An analysis of the determinants of anaemia in pregnant women in rural Malawi–a basis for action. Ann Trop Med Parasitol (1999) 93:119–33. doi: 10.1080/00034989958609
3. Shulman CE, Graham WJ, Jilo H, Lowe BS, New L, Obiero J, et al. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg (1996) 90:535–9. doi: 10.1016/s0035-9203(96)90312-0
4. Ndao CT, Dumont A, Fievet N, Doucoure S, Gaye A, Lehesran JY. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal, West Africa. Am J Epidemiol (2009) 170:847–53. doi: 10.1093/aje/kwp207
5. Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal-fetal conflict during placental malaria. PLoS Med (2006) 3:e446. doi: 10.1371/journal.pmed.0030446
6. Akuze J, Blencowe H, Waiswa P, Baschieri A, Gordeev VS, Kwesiga D, et al. Randomised comparison of two household survey modules for measuring stillbirths and neonatal deaths in five countries: the Every Newborn-INDEPTH study. Lancet Glob Health (2020) 8:e555–66. doi: 10.1016/S2214-109X(20)30044-9
7. Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI. Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. Lancet Glob Health (2017) 5:e1101–12. doi: 10.1016/S2214-109X(17)30340-6
8. Galbraith RM, Fox H, Hsi B, Galbraith GM, Bray RS, Faulk WP. The human materno-foetal relationship in malaria. II. Histological, ultrastructural and immunopathological studies of the placenta. Trans R Soc Trop Med Hyg (1980) 74:61–72. doi: 10.1016/0035-9203(80)90012-7
9. Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol (1982) 109:330–42.
10. Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol (2000) 31:85–93. doi: 10.1016/s0046-8177(00)80203-8
11. Tuikue Ndam N, Bischoff E, Proux C, Lavstsen T, Salanti A, Guitard J, et al. Plasmodium falciparum transcriptome analysis reveals pregnancy malaria associated gene expression. PLoS One (2008) 3:e1855. doi: 10.1371/journal.pone.0001855
12. Francis SE, Malkov VA, Oleinikov AV, Rossnagle E, Wendler JP, Mutabingwa TK, et al. Six genes are preferentially transcribed by the circulating and sequestered forms of Plasmodium falciparum parasites that infect pregnant women. Infect Immun (2007) 75:4838–50. doi: 10.1128/IAI.00635-07
13. Tuikue Ndam NG, Salanti A, Bertin G, Dahlbäck M, Fievet N, Turner L, et al. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J Infect Dis (2005) 192:331–5. doi: 10.1086/430933
14. Kamaliddin C, Salnot V, Leduc M, Ezinmegnon S, Broussard C, Fievet N, et al. PFI1785w: A highly conserved protein associated with pregnancy associated malaria. PLoS One (2017) 12:e0187817. doi: 10.1371/journal.pone.0187817
15. Bertin GI, Sabbagh A, Guillonneau F, Jafari-Guemouri S, Ezinmegnon S, Federici C, et al. Differential protein expression profiles between Plasmodium falciparum parasites isolated from subjects presenting with pregnancy-associated malaria and uncomplicated malaria in Benin. J Infect Dis (2013) 208:1987–97. doi: 10.1093/infdis/jit377
16. Fried M, Hixson KK, Anderson L, Ogata Y, Mutabingwa TK, Duffy PE. The distinct proteome of placental malaria parasites. Mol Biochem Parasitol (2007) 155:57–65. doi: 10.1016/j.molbiopara.2007.05.010
17. Fried M, Duffy PE. Adherence of Plasmodium falciparum to Chondroitin Sulfate A in the Human Placenta. Science (1996) 272:1502–4. doi: 10.1126/science.272.5267.1502
18. Achur RN, Valiyaveettil M, Alkhalil A, Ockenhouse CF, Gowda DC. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J Biol Chem (2000) 275:40344–56. doi: 10.1074/jbc.M006398200
19. Gysin J, Pouvelle B, Fievet N, Scherf A, Lépolard C. Ex vivo desequestration of Plasmodium falciparum-infected erythrocytes from human placenta by chondroitin sulfate A. Infect Immun (1999) 67:6596–602. doi: 10.1128/IAI.67.12.6596-6602
20. Maubert B, Guilbert LJ, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun (1997) 65:1251–7. doi: 10.1128/IAI.65.4.1251-1257.1997
21. Maubert B, Fievet N, Tami G, Boudin C, Deloron P. Cytoadherence of Plasmodium falciparum-infected erythrocytes in the human placenta. Parasite Immunol (2000) 22:191–9. doi: 10.1046/j.1365-3024.2000.00292.x
22. Agbor-Enoh ST, Achur RN, Valiyaveettil M, Leke R, Taylor DW, Gowda DC. Chondroitin Sulfate Proteoglycan Expression and Binding of Plasmodium falciparum-Infected Erythrocytes in the Human Placenta during Pregnancy. IAI (2003) 71:2455–61. doi: 10.1128/IAI.71.5.2455-2461.2003
23. Viebig NK, Gamain B, Scheidig C, Lépolard C, Przyborski J, Lanzer M, et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep (2005) 6:775–81. doi: 10.1038/sj.embor.7400466
24. Duffy MF, Byrne TJ, Elliott SR, Wilson DW, Rogerson SJ, Beeson JG, et al. Broad analysis reveals a consistent pattern of var gene transcription in Plasmodium falciparum repeatedly selected for a defined adhesion phenotype. Mol Microbiol (2005) 56:774–88. doi: 10.1111/j.1365-2958.2005.04577.x
25. Elliott SR, Duffy MF, Byrne TJ, Beeson JG, Mann EJ, Wilson DW, et al. Cross-reactive surface epitopes on chondroitin sulfate A-adherent Plasmodium falciparum-infected erythrocytes are associated with transcription of var2csa. Infect Immun (2005) 73:2848–56. doi: 10.1128/IAI.73.5.2848-2856.2005
26. Gamain B, Trimnell AR, Scheidig C, Scherf A, Miller LH, Smith JD. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2CSA gene transcribed in CSA-binding parasites. J Infect Dis (2005) 191:1010–3. doi: 10.1086/428137
27. Salanti A, Dahlbäck M, Turner L, Nielsen MA, Barfod L, Magistrado P, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med (2004) 200:1197–203. doi: 10.1084/jem.20041579
28. Salanti A, Staalsoe T, Lavstsen T, Jensen ATR, Sowa MPK, Arnot DE, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol (2003) 49:179–91. doi: 10.1046/j.1365-2958.2003.03570.x
29. Gruenberg J, Allred DR, Sherman IW. Scanning electron microscope-analysis of the protrusions (knobs) present on the surface of Plasmodium falciparum-infected erythrocytes. J Cell Biol (1983) 97:795–802. doi: 10.1083/jcb.97.3.795
30. Crabb BS, Cooke BM, Reeder JC, Waller RF, Caruana SR, Davern KM, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell (1997) 89:287–96. doi: 10.1016/s0092-8674(00)80207-x
31. Raventos-Suarez C, Kaul DK, Macaluso F, Nagel RL. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A (1985) 82:3829–33. doi: 10.1073/pnas.82.11.3829
32. Joergensen LM, Salanti A, Dobrilovic T, Barfod L, Hassenkam T, Theander TG, et al. The kinetics of antibody binding to Plasmodium falciparum VAR2CSA PfEMP1 antigen and modelling of PfEMP1 antigen packing on the membrane knobs. Malar J (2010) 9:100. doi: 10.1186/1475-2875-9-100
33. Sanchez CP, Karathanasis C, Sanchez R, Cyrklaff M, Jäger J, Buchholz B, et al. Single-molecule imaging and quantification of the immune-variant adhesin VAR2CSA on knobs of Plasmodium falciparum-infected erythrocytes. Commun Biol (2019) 2:172. doi: 10.1038/s42003-019-0429-z
34. Subramani R, Quadt K, Jeppesen AE, Hempel C, Petersen JEV, Hassenkam T, et al. Plasmodium falciparum-infected erythrocyte knob density is linked to the PfEMP1 variant expressed. mBio (2015) 6:e01456–01415. doi: 10.1128/mBio.01456-15
35. Doritchamou J, Teo A, Morrison R, Arora G, Kwan J, Manzella-Lapeira J, et al. Functional Antibodies against Placental Malaria Parasites Are Variant Dependent and Differ by Geographic Region. Infect Immun (2019) 87(7):e00865-18. doi: 10.1128/IAI.00865-18
36. Singh K, Gitti RK, Diouf A, Zhou H, Gowda DC, Miura K, et al. Subdomain 3 of Plasmodium falciparum VAR2CSA DBL3x is identified as a minimal chondroitin sulfate A-binding region. J Biol Chem (2010) 285:24855–62. doi: 10.1074/jbc.M110.118612
37. Vuchelen A, Pardon E, Steyaert J, Gamain B, Loris R, van Nuland NAJ, et al. Production, crystallization and X-ray diffraction analysis of two nanobodies against the Duffy binding-like (DBL) domain DBL6ϵ-FCR3 of the Plasmodium falciparum VAR2CSA protein. Acta Crystallogr Sect F Struct Biol Cryst Commun (2013) 69:270–4. doi: 10.1107/S1744309113001917
38. Gangnard S, Badaut C, Ramboarina S, Baron B, Ramdani T, Gamain B, et al. Structural and immunological correlations between the variable blocks of the VAR2CSA domain DBL6ϵ from two Plasmodium falciparum parasite lines. J Mol Biol (2013) 425:1697–711. doi: 10.1016/j.jmb.2013.02.014
39. Singh K, Gittis AG, Nguyen P, Gowda DC, Miller LH, Garboczi DN. Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nat Struct Mol Biol (2008) 15:932–8. doi: 10.1038/nsmb.1479
40. Khunrae P, Philip JMD, Bull DR, Higgins MK. Structural comparison of two CSPG-binding DBL domains from the VAR2CSA protein important in malaria during pregnancy. J Mol Biol (2009) 393:202–13. doi: 10.1016/j.jmb.2009.08.027
41. Gangnard S, Lewit-Bentley A, Dechavanne S, Srivastava A, Amirat F, Bentley GA, et al. Structure of the DBL3X-DBL4ϵ region of the VAR2CSA placental malaria vaccine candidate: insight into DBL domain interactions. Sci Rep (2015) 5:14868. doi: 10.1038/srep14868
42. Srivastava A, Gangnard S, Round A, Dechavanne S, Juillerat A, Raynal B, et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci U S A (2010) 107:4884–9. doi: 10.1073/pnas.1000951107
43. Clausen TM, Christoffersen S, Dahlbäck M, Langkilde AE, Jensen KE, Resende M, et al. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem (2012) 287:23332–45. doi: 10.1074/jbc.M112.348839
44. Bewley MC, Gautam L, Jagadeeshaprasad MG, Gowda DC, Flanagan JM. Molecular architecture and domain arrangement of the placental malaria protein VAR2CSA suggests a model for carbohydrate binding. J Biol Chem (2020) 295(52):18589–603. doi: 10.1074/jbc.RA120.014676
45. Srivastava A, Gangnard S, Dechavanne S, Amirat F, Lewit Bentley A, Bentley GA, et al. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS One (2011) 6:e20270. doi: 10.1371/journal.pone.0020270
46. Dahlbäck M, Jørgensen LM, Nielsen MA, Clausen TM, Ditlev SB, Resende M, et al. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J Biol Chem (2011) 286:15908–17. doi: 10.1074/jbc.M110.191510
47. Dorin-Semblat D, Tétard M, Claës A, Semblat J-P, Dechavanne S, Fourati Z, et al. Phosphorylation of the VAR2CSA extracellular region is associated with enhanced adhesive properties to the placental receptor CSA. PLoS Biol (2019) 17:e3000308. doi: 10.1371/journal.pbio.3000308
48. Lavazec C. Molecular mechanisms of deformability of Plasmodium-infected erythrocytes. Curr Opin Microbiol (2017) 40:138–44. doi: 10.1016/j.mib.2017.11.011
49. Nash GB, O’Brien E, Gordon-Smith EC, Dormandy JA. Abnormalities in the mechanical properties of red blood cells caused by Plasmodium falciparum. Blood (1989) 74:855–61. doi: 10.1182/blood.V74.2.855.855
50. Herricks T, Antia M, Rathod PK. Deformability limits of Plasmodium falciparum-infected red blood cells. Cell Microbiol (2009) 11:1340–53. doi: 10.1111/j.1462-5822.2009.01334.x
51. Herricks T, Seydel KB, Molyneux M, Taylor T, Rathod PK. Estimating physical splenic filtration of Plasmodium falciparum-infected red blood cells in malaria patients. Cell Microbiol (2012) 14:1880–91. doi: 10.1111/cmi.12007
52. Lavazec C, Deplaine G, Safeukui I, Perrot S, Milon G, Mercereau-Puijalon O, et al. Microsphiltration: a microsphere matrix to explore erythrocyte deformability. Methods Mol Biol (2013) 923:291–7. doi: 10.1007/978-1-62703-026-7_20
53. Robert C, Pouvelle B, Meyer P, Muanza K, Fujioka H, Aikawa M, et al. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res Immunol (1995) 146:383–93. doi: 10.1016/0923-2494(96)81042-x
54. Alkhalil A, Achur RN, Valiyaveettil M, Ockenhouse CF, Gowda DC. Structural requirements for the adherence of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. J Biol Chem (2000) 275:40357–64. doi: 10.1074/jbc.M006399200
55. Chai W, Beeson JG, Lawson AM. The structural motif in chondroitin sulfate for adhesion of Plasmodium falciparum-infected erythrocytes comprises disaccharide units of 4-O-sulfated and non-sulfated N-acetylgalactosamine linked to glucuronic acid. J Biol Chem (2002) 277:22438–46. doi: 10.1074/jbc.M111401200
56. Sugiura N, Clausen TM, Shioiri T, Gustavsson T, Watanabe H, Salanti A. Molecular dissection of placental malaria protein VAR2CSA interaction with a chemo-enzymatically synthesized chondroitin sulfate library. Glycoconj J (2016) 33:985–94. doi: 10.1007/s10719-016-9685-z
57. Rieger H, Yoshikawa HY, Quadt K, Nielsen MA, Sanchez CP, Salanti A, et al. Cytoadhesion of Plasmodium falciparum-infected erythrocytes to chondroitin-4-sulfate is cooperative and shear enhanced. Blood (2015) 125:383–91. doi: 10.1182/blood-2014-03-561019
58. Ngai M, Weckman AM, Erice C, McDonald CR, Cahill LS, Sled JG, et al. Malaria in Pregnancy and Adverse Birth Outcomes: New Mechanisms and Therapeutic Opportunities. Trends Parasitol (2020) 36:127–37. doi: 10.1016/j.pt.2019.12.005
59. Umbers AJ, Aitken EH, Rogerson SJ. Malaria in pregnancy: small babies, big problem. Trends Parasitol (2011) 27:168–75. doi: 10.1016/j.pt.2011.01.007
60. Yockey LJ, Iwasaki A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity (2018) 49:397–412. doi: 10.1016/j.immuni.2018.07.017
61. Fievet N, Moussa M, Tami G, Maubert B, Cot M, Deloron P, et al. Plasmodium falciparum induces a Th1/Th2 disequilibrium, favoring the Th1-type pathway, in the human placenta. J Infect Dis (2001) 183:1530–4. doi: 10.1086/320201
62. Suguitan AL, Leke RGF, Fouda G, Zhou A, Thuita L, Metenou S, et al. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis (2003) 188:1074–82. doi: 10.1086/378500
63. Diouf I, Fievet N, Doucouré S, Ngom M, Andrieu M, Mathieu J-F, et al. IL-12 producing monocytes and IFN-gamma and TNF-alpha producing T-lymphocytes are increased in placentas infected by Plasmodium falciparum. J Reprod Immunol (2007) 74:152–62. doi: 10.1016/j.jri.2006.10.001
64. Kabyemela ER, Muehlenbachs A, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Malar J (2008) 7:26. doi: 10.1186/1475-2875-7-26
65. Boström S, Ibitokou S, Oesterholt M, Schmiegelow C, Persson J-O, Minja D, et al. Biomarkers of Plasmodium falciparum infection during pregnancy in women living in northeastern Tanzania. PLoS One (2012) 7:e48763. doi: 10.1371/journal.pone.0048763
66. Chêne A, Briand V, Ibitokou S, Dechavanne S, Massougbodji A, Deloron P, et al. Placental cytokine and chemokine profiles reflect pregnancy outcomes in women exposed to Plasmodium falciparum infection. Infect Immun (2014) 82:3783–9. doi: 10.1128/IAI.01922-14
67. Umbers AJ, Boeuf P, Clapham C, Stanisic DI, Baiwog F, Mueller I, et al. Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. J Infect Dis (2011) 203:561–9. doi: 10.1093/infdis/jiq080
68. Silver KL, Conroy AL, Leke RGF, Leke RJI, Gwanmesia P, Molyneux ME, et al. Circulating soluble endoglin levels in pregnant women in Cameroon and Malawi–associations with placental malaria and fetal growth restriction. PLoS One (2011) 6:e24985. doi: 10.1371/journal.pone.0024985
69. Dong S, Kurtis JD, Pond-Tor S, Kabyemela E, Duffy PE, Fried M. CXC ligand 9 response to malaria during pregnancy is associated with low-birth-weight deliveries. Infect Immun (2012) 80:3034–8. doi: 10.1128/IAI.00220-12
70. Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol (1998) 160:2523–30.
71. Moormann AM, Sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, et al. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis (1999) 180:1987–93. doi: 10.1086/315135
72. Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci (2015) 370:20140066. doi: 10.1098/rstb.2014.0066
73. Lucchi NW, Koopman R, Peterson DS, Moore JM. Plasmodium falciparum-infected red blood cells selected for binding to cultured syncytiotrophoblast bind to chondroitin sulfate A and induce tyrosine phosphorylation in the syncytiotrophoblast. Placenta (2006) 27:384–94. doi: 10.1016/j.placenta.2005.04.009
74. Lucchi NW, Peterson DS, Moore JM. Immunologic activation of human syncytiotrophoblast by Plasmodium falciparum. Malar J (2008) 7:42. doi: 10.1186/1475-2875-7-42
75. Vásquez AM, Segura C, Blair S. Induction of pro-inflammatory response of the placental trophoblast by Plasmodium falciparum infected erythrocytes and TNF. Malar J (2013) 12:421. doi: 10.1186/1475-2875-12-421
76. Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw (2004) 15:91–8.
77. Mues B, Langer D, Zwadlo G, Sorg C. Phenotypic characterization of macrophages in human term placenta. Immunology (1989) 67:303–7.
78. Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods (1990) 132:181–9. doi: 10.1016/0022-1759(90)90028-t
79. Jaiswal MK, Mallers TM, Larsen B, Kwak-Kim J, Chaouat G, Gilman-Sachs A, et al. V-ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction (2012) 143:713–25. doi: 10.1530/REP-12-0036
80. Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol (2011) 187:3671–82. doi: 10.4049/jimmunol.1100130
81. Gustafsson C, Mjösberg J, Matussek A, Geffers R, Matthiesen L, Berg G, et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One (2008) 3:e2078. doi: 10.1371/journal.pone.0002078
82. Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol (1998) 22:1006–11. doi: 10.1097/00000478-199808000-00011
83. Ordi J, Menendez C, Ismail MR, Ventura PJ, Palacín A, Kahigwa E, et al. Placental malaria is associated with cell-mediated inflammatory responses with selective absence of natural killer cells. J Infect Dis (2001) 183:1100–7. doi: 10.1086/319295
84. Abrams ET, Brown H, Chensue SW, Turner GDH, Tadesse E, Lema VM, et al. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J Immunol (2003) 170:2759–64. doi: 10.4049/jimmunol.170.5.2759
85. Sampaio NG, Eriksson EM, Schofield L. Plasmodium falciparum PfEMP1 Modulates Monocyte/Macrophage Transcription Factor Activation and Cytokine and Chemokine Responses. Infect Immun (2018) 86(1):e00447-17. doi: 10.1128/IAI.00447-17
86. Ludlow LE, Hasang W, Umbers AJ, Forbes EK, Ome M, Unger HW, et al. Peripheral blood mononuclear cells derived from grand multigravidae display a distinct cytokine profile in response to P. falciparum infected erythrocytes. PLoS One (2014) 9:e86160. doi: 10.1371/journal.pone.0086160
87. Elliott SR, Brennan AK, Beeson JG, Tadesse E, Molyneux ME, Brown GV, et al. Placental malaria induces variant-specific antibodies of the cytophilic subtypes immunoglobulin G1 (IgG1) and IgG3 that correlate with adhesion inhibitory activity. Infect Immun (2005) 73:5903–7. doi: 10.1128/IAI.73.9.5903-5907.2005
88. Megnekou R, Staalsoe T, Taylor DW, Leke R, Hviid L. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infect Immun (2005) 73:4112–8. doi: 10.1128/IAI.73.7.4112-4118.2005
89. Keen J, Serghides L, Ayi K, Patel SN, Ayisi J, van Eijk A, et al. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Med (2007) 4:e181. doi: 10.1371/journal.pmed.0040181
90. Ataíde R, Mwapasa V, Molyneux ME, Meshnick SR, Rogerson SJ. Antibodies that induce phagocytosis of malaria infected erythrocytes: effect of HIV infection and correlation with clinical outcomes. PLoS One (2011) 6:e22491. doi: 10.1371/journal.pone.0022491
91. Jaworowski A, Fernandes LA, Yosaatmadja F, Feng G, Mwapasa V, Molyneux ME, et al. Relationship between human immunodeficiency virus type 1 coinfection, anemia, and levels and function of antibodies to variant surface antigens in pregnancy-associated malaria. Clin Vaccine Immunol (2009) 16:312–9. doi: 10.1128/CVI.00356-08
92. Clough B, Atilola FA, Black J, Pasvol G. Plasmodium falciparum: the importance of IgM in the rosetting of parasite-infected erythrocytes. Exp Parasitol (1998) 89:129–32. doi: 10.1006/expr.1998.4275
93. Scholander C, Treutiger CJ, Hultenby K, Wahlgren M. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat Med (1996) 2:204–8. doi: 10.1038/nm0296-204
94. Rowe JA, Shafi J, Kai OK, Marsh K, Raza A. Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am J Trop Med Hyg (2002) 66:692–9. doi: 10.4269/ajtmh.2002.66.692
95. Creasey AM, Staalsoe T, Raza A, Arnot DE, Rowe JA. Nonspecific immunoglobulin M binding and chondroitin sulfate A binding are linked phenotypes of Plasmodium falciparum isolates implicated in malaria during pregnancy. Infect Immun (2003) 71:4767–71. doi: 10.1128/iai.71.8.4767-4771.2003
96. Barfod L, Dalgaard MB, Pleman ST, Ofori MF, Pleass RJ, Hviid L. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc Natl Acad Sci U S A (2011) 108:12485–90. doi: 10.1073/pnas.1103708108
97. Jeppesen A, Ditlev SB, Soroka V, Stevenson L, Turner L, Dzikowski R, et al. Multiple Plasmodium falciparum Erythrocyte Membrane Protein 1 Variants per Genome Can Bind IgM via Its Fc Fragment Fcμ. Infect Immun (2015) 83:3972–81. doi: 10.1128/IAI.00337-15
98. Stevenson L, Huda P, Jeppesen A, Laursen E, Rowe JA, Craig A, et al. Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell Microbiol (2015) 17:819–31. doi: 10.1111/cmi.12403
99. Quintana MDP, Ecklu-Mensah G, Tcherniuk SO, Ditlev SB, Oleinikov AV, Hviid L, et al. Comprehensive analysis of Fc-mediated IgM binding to the Plasmodium falciparum erythrocyte membrane protein 1 family in three parasite clones. Sci Rep (2019) 9:6050. doi: 10.1038/s41598-019-42585-0
100. Srivastava A, Gangnard S, Dechavanne S, Amirat F, Lewit Bentley A, Bentley GA, et al. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS One (2011) 6:e20270. doi: 10.1371/journal.pone.0020270
101. Dahlbäck M, Jørgensen LM, Nielsen MA, Clausen TM, Ditlev SB, Resende M, et al. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J Biol Chem (2011) 286:15908–17. doi: 10.1074/jbc.M110.191510
102. Rasti N, Namusoke F, Chêne A, Chen Q, Staalsoe T, Klinkert M-Q, et al. Nonimmune immunoglobulin binding and multiple adhesion characterize Plasmodium falciparum-infected erythrocytes of placental origin. Proc Natl Acad Sci U S A (2006) 103:13795–800. doi: 10.1073/pnas.0601519103
103. Semblat J-P, Raza A, Kyes SA, Rowe JA. Identification of Plasmodium falciparum var1CSA and var2CSA domains that bind IgM natural antibodies. Mol Biochem Parasitol (2006) 146:192–7. doi: 10.1016/j.molbiopara.2005.12.007
104. Jeppesen A, Ditlev SB, Soroka V, Stevenson L, Turner L, Dzikowski R, et al. Multiple Plasmodium falciparum Erythrocyte Membrane Protein 1 Variants per Genome Can Bind IgM via Its Fc Fragment Fcμ. Infect Immun (2015) 83:3972–81. doi: 10.1128/IAI.00337-15
105. Akhouri RR, Goel S, Furusho H, Skoglund U, Wahlgren M. Architecture of Human IgM in Complex with P. falciparum Erythrocyte Membrane Protein 1. Cell Rep (2016) 14:723–36. doi: 10.1016/j.celrep.2015.12.067
106. Ghumra A, Semblat J-P, McIntosh RS, Raza A, Rasmussen IB, Braathen R, et al. Identification of residues in the Cmu4 domain of polymeric IgM essential for interaction with Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). J Immunol (2008) 181:1988–2000. doi: 10.4049/jimmunol.181.3.1988
107. Akhouri RR, Goel S, Furusho H, Skoglund U, Wahlgren M. Architecture of Human IgM in Complex with P. falciparum Erythrocyte Membrane Protein 1. Cell Rep (2016) 14:723–36. doi: 10.1016/j.celrep.2015.12.067
108. Stevenson L, Huda P, Jeppesen A, Laursen E, Rowe JA, Craig A, et al. Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cell Microbiol (2015) 17:819–31. doi: 10.1111/cmi.12403
109. Stevenson L, Laursen E, Cowan GJ, Bandoh B, Barfod L, Cavanagh DR, et al. α2-Macroglobulin Can Crosslink Multiple Plasmodium falciparum Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes. PLoS Pathog (2015) 11:e1005022. doi: 10.1371/journal.ppat.1005022
110. Donati D, Zhang LP, Chêne A, Chen Q, Flick K, Nyström M, et al. Identification of a polyclonal B-cell activator in Plasmodium falciparum. Infect Immun (2004) 72:5412–8. doi: 10.1128/IAI.72.9.5412-5418.2004
111. Donati D, Mok B, Chêne A, Xu H, Thangarajh M, Glas R, et al. Increased B cell survival and preferential activation of the memory compartment by a malaria polyclonal B cell activator. J Immunol (2006) 177:3035–44. doi: 10.4049/jimmunol.177.5.3035
112. Bockhorst J, Lu F, Janes JH, Keebler J, Gamain B, Awadalla P, et al. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol Biochem Parasitol (2007) 155:103–12. doi: 10.1016/j.molbiopara.2007.06.007
113. Sander AF, Salanti A, Lavstsen T, Nielsen MA, Magistrado P, Lusingu J, et al. Multiple var2csa-type PfEMP1 genes located at different chromosomal loci occur in many Plasmodium falciparum isolates. PLoS One (2009) 4:e6667. doi: 10.1371/journal.pone.0006667
114. Sander AF, Salanti A, Lavstsen T, Nielsen MA, Theander TG, Leke RGF, et al. Positive selection of Plasmodium falciparum parasites with multiple var2csa-type PfEMP1 genes during the course of infection in pregnant women. J Infect Dis (2011) 203:1679–85. doi: 10.1093/infdis/jir168
115. Doritchamou JYA, Morrison R, Renn JP, Ribeiro J, Duan J, Fried M, et al. Placental malaria vaccine candidate antigen VAR2CSA displays atypical domain architecture in some Plasmodium falciparum strains. Commun Biol (2019) 2:457. doi: 10.1038/s42003-019-0704-z
116. Otto TD, Gilabert A, Crellen T, Böhme U, Arnathau C, Sanders M, et al. Genomes of all known members of a Plasmodium subgenus reveal paths to virulent human malaria. Nat Microbiol (2018) 3:687–97. doi: 10.1038/s41564-018-0162-2
117. Larremore DB, Sundararaman SA, Liu W, Proto WR, Clauset A, Loy DE, et al. Ape parasite origins of human malaria virulence genes. Nat Commun (2015) 6:8368. doi: 10.1038/ncomms9368
118. Bordbar B, Tuikue Ndam N, Renard E, Jafari-Guemouri S, Tavul L, Jennison C, et al. Genetic diversity of VAR2CSA ID1-DBL2Xb in worldwide Plasmodium falciparum populations: impact on vaccine design for placental malaria. Infect Genet Evol (2014) 25:81–92. doi: 10.1016/j.meegid.2014.04.010
119. Doritchamou J, Sabbagh A, Jespersen JS, Renard E, Salanti A, Nielsen MA, et al. Identification of a Major Dimorphic Region in the Functionally Critical N-Terminal ID1 Domain of VAR2CSA. PLoS One (2015) 10:e0137695. doi: 10.1371/journal.pone.0137695
120. Patel JC, Hathaway NJ, Parobek CM, Thwai KL, Madanitsa M, Khairallah C, et al. Increased risk of low birth weight in women with placental malaria associated with P. falciparum VAR2CSA clade. Sci Rep (2017) 7:7768. doi: 10.1038/s41598-017-04737-y
121. Verity R, Hathaway NJ, Waltmann A, Doctor SM, Watson OJ, Patel JC, et al. Plasmodium falciparum genetic variation of var2csa in the Democratic Republic of the Congo. Malar J (2018) 17:46. doi: 10.1186/s12936-018-2193-9
122. Benavente ED, Oresegun DR, de Sessions PF, Walker EM, Roper C, Dombrowski JG, et al. Global genetic diversity of var2csa in Plasmodium falciparum with implications for malaria in pregnancy and vaccine development. Sci Rep (2018) 8:15429. doi: 10.1038/s41598-018-33767-3
123. Mayor A, Bardají A, Macete E, Nhampossa T, Fonseca AM, González R, et al. Changing Trends in P. falciparum Burden, Immunity, and Disease in Pregnancy. N Engl J Med (2015) 373:1607–17. doi: 10.1056/NEJMoa1406459
124. Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, et al. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health (2001) 6:770–8. doi: 10.1046/j.1365-3156.2001.00786.x
125. Walker PGT, ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health (2014) 2:e460–467. doi: 10.1016/S2214-109X(14)70256-6
126. Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet (2004) 363:283–9. doi: 10.1016/S0140-6736(03)15386-X
127. Gavina K, Gnidehou S, Arango E, Hamel-Martineau C, Mitran C, Agudelo O, et al. Clinical Outcomes of Submicroscopic Infections and Correlates of Protection of VAR2CSA Antibodies in a Longitudinal Study of Pregnant Women in Colombia. Infect Immun (2018) 86(4):e00797-17. doi: 10.1128/IAI.00797-17
128. Ndam NT, Denoeud-Ndam L, Doritchamou J, Viwami F, Salanti A, Nielsen MA, et al. Protective Antibodies against Placental Malaria and Poor Outcomes during Pregnancy, Benin. Emerg Infect Dis (2015) 21:813–23. doi: 10.3201/eid2105.141626
129. Tutterrow YL, Salanti A, Avril M, Smith JD, Pagano IS, Ako S, et al. High avidity antibodies to full-length VAR2CSA correlate with absence of placental malaria. PLoS One (2012) 7:e40049. doi: 10.1371/journal.pone.0040049
130. Chêne A, Gangnard S, Dechavanne C, Dechavanne S, Srivastava A, Tétard M, et al. Down-selection of the VAR2CSA DBL1-2 expressed in E. coli as a lead antigen for placental malaria vaccine development. NPJ Vaccines (2018) 3:28. doi: 10.1038/s41541-018-0064-6
131. Chêne A, Gangnard S, Guadall A, Ginisty H, Leroy O, Havelange N, et al. Preclinical immunogenicity and safety of the cGMP-grade placental malaria vaccine PRIMVAC. EBioMedicine (2019) 42:145–56. doi: 10.1016/j.ebiom.2019.03.010
132. Nielsen MA, Resende M, de Jongh WA, Ditlev SB, Mordmüller B, Houard S, et al. The Influence of Sub-Unit Composition and Expression System on the Functional Antibody Response in the Development of a VAR2CSA Based Plasmodium falciparum Placental Malaria Vaccine. PLoS One (2015) 10:e0135406. doi: 10.1371/journal.pone.0135406
133. Sirima SB, Richert L, Chêne A, Konate AT, Campion C, Dechavanne S, et al. PRIMVAC vaccine adjuvanted with Alhydrogel or GLA-SE to prevent placental malaria: a first-in-human, randomised, double-blind, placebo-controlled study. Lancet Infect Dis (2020) 20:585–97. doi: 10.1016/S1473-3099(19)30739-X
134. Mordmüller B, Sulyok M, Egger-Adam D, Resende M, de Jongh WA, Jensen MH, et al. First-in-human, Randomized, Double-blind Clinical Trial of Differentially Adjuvanted PAMVAC, A Vaccine Candidate to Prevent Pregnancy-associated Malaria. Clin Infect Dis (2019) 69:1509–16. doi: 10.1093/cid/ciy1140
135. McCall MBB, Kremsner PG, Mordmüller B. Correlating efficacy and immunogenicity in malaria vaccine trials. Semin Immunol (2018) 39:52–64. doi: 10.1016/j.smim.2018.08.002
136. Sungwa M, Susan T, Mikkel JC, Adolph KR, Boniface MS, Grundtvig TT, et al. A VAR2CSA:CSP conjugate capable of inducing dual specificity antibody responses. Afr Health Sci (2017) 17:373–81. doi: 10.4314/ahs.v17i2.11
137. Janitzek CM, Peabody J, Thrane S, Carlsen P HR, Theander T G, Salanti A, et al. A proof-of-concept study for the design of a VLP-based combinatorial HPV and placental malaria vaccine. Sci Rep (2019) 9:5260. doi: 10.1038/s41598-019-41522-5
138. Andersson A-MC, Resende M, Salanti A, Nielsen MA, Holst PJ. Novel adenovirus encoded virus-like particles displaying the placental malaria associated VAR2CSA antigen. Vaccine (2017) 35:1140–7. doi: 10.1016/j.vaccine.2017.01.016
Keywords: Plasmodium falciparum, placental malaria, VAR2CSA, PfEMP1, immune evasion, immuno-modulation, VAR2CSA polymorphism
Citation: Tomlinson A, Semblat J-P, Gamain B and Chêne A (2021) VAR2CSA-Mediated Host Defense Evasion of Plasmodium falciparum Infected Erythrocytes in Placental Malaria. Front. Immunol. 11:624126. doi: 10.3389/fimmu.2020.624126
Received: 30 October 2020; Accepted: 23 December 2020;
Published: 09 February 2021.
Edited by:
Justin Yai Alamou Doritchamou, National Institute of Allergy and Infectious Diseases (NIAID), United StatesReviewed by:
Mirja Hommel, Centre for Applied Medical Research (CIMA), SpainAndrew Teo, Nanyang Technological University, Singapore
Stephanie Yanow, University of Alberta, Canada
Copyright © 2021 Tomlinson, Semblat, Gamain and Chêne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arnaud Chêne, YXJuYXVkLmNoZW5lQGluc2VybS5mcg==
 Alice Tomlinson
Alice Tomlinson Jean-Philippe Semblat
Jean-Philippe Semblat Benoît Gamain
Benoît Gamain Arnaud Chêne
Arnaud Chêne