
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 10 February 2021
Sec. Microbial Immunology
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.620170
This article is part of the Research Topic Immunostimulatory Oral Microbiome in Health, Inflammation, and Autoimmune Diseases View all 20 articles
The human intestine contains thousands of bacterial species essential for optimal health. Aside from their pathogenic effects, these bacteria have been associated with the efficacy of various treatments of diseases. Due to their impact on many human diseases, intestinal bacteria are receiving increasing research attention, and recent studies on intestinal bacteria and their effects on treatments has yielded valuable results. Particularly, intestinal bacteria can affect responses to numerous forms of immunotherapy, especially cancer therapy. With the development of precision medicine, understanding the factors that influence intestinal bacteria and how they can be regulated to enhance immunotherapy effects will improve the application prospects of intestinal bacteria therapy. Further, biomaterials employed for the convenient and efficient delivery of intestinal bacteria to the body have also become a research hotspot. In this review, we discuss the recent findings on the regulatory role of intestinal bacteria in immunotherapy, focusing on immune cells they regulate. We also summarize biomaterials used for their delivery.
In the human intestine, there are more than 100 trillion bacterial cells that mainly exhibit commensalism with the host (1, 2) and play a role in the maintenance of host health (3). In this commensalistic relationship, intestinal bacteria not only participate in the regulation of the host immune system and the promotion of bone marrow hematopoiesis but also regulate the maturation and function of hematopoietic cells originating from the yolk sac (4, 5). Moreover, intestinal bacteria can regulate the barrier function via interaction with epithelial cells and stromal cells (6–8). The functions of intestinal bacteria widely range from local to systemic levels, including metabolism regulation, hematopoiesis, inflammation, immunity, and other physiological functions (8–11). However, changes in intestinal ecology can disrupt this commensalistic relationship. For instance, some symbiotic bacteria called pathobionts might cause, or even worsen, a number of diseases (10, 12, 13). Increasing evidence has demonstrated that dysbiosis has been connected to various diseases, including tumors, viral infection, inflammatory bowel disease (IBD), diabetes, and liver cirrhosis (10, 14, 15).
Owing to their impact on various human diseases, intestinal bacteria have been recently receiving increasing attention. Aside from their pathogenic effects, intestinal bacteria also exert beneficial effects in reducing gastrointestinal inflammation, preventing colorectal cancer, and treating some diseases (16–19). The mechanisms by which intestinal bacteria affect inflammation, immunity, and local therapeutic response have also been elucidated (8, 20, 21). Currently, intestinal bacteria, including Escherichia coli (22, 23), Bifidobacterium (24, 25), Filamentous fungus (10), Lactobacillus (26), Bacillus subtilis (27), and Bacteroides fragilis (28, 29), have been applied in the therapy of diseases such as diabetes (30), gastrointestinal diseases (31, 32), and allergic diseases (33, 34). Interestingly, intestinal bacteria can also potentiate antitumor therapies (35–37). With the advancements in precision medicine, intestinal bacteria have thus become increasingly important in the treatment of various diseases. Thus, studies aiming to understand the factors influencing intestinal bacteria and the strategies for their manipulation to enhance therapeutic efficacy are increasing (36).
Traditional tumor therapies, such as chemotherapy, surgery, radiation therapy, and molecular targeted therapy, are the primary methods used to treat tumors at different stages (31). However, they have some drawbacks, such as toxic side effects and recurrence after treatment. With the advancement in tumor research, immunotherapy has emerged as a promising therapeutic modality (38). Compared with chemotherapy, immunotherapy of tumors has fewer side effects. In cases wherein immunotherapy is effective in patients suffering from tumors, it could prolong their survival period and even cure tumors clinically. Although immunotherapy has shown promising potential in the treatment of hematological and solid tumors, its efficacy remains limited due to the variability in immune responses and susceptibilities to tumor types among patients (39). Therefore, only some patients benefit from immunotherapy. Recent studies have shown that the regulation of intestinal bacteria can influence the effects of immunotherapy (35, 40, 41). Moreover, intestinal bacteria have been applied to the immunotherapy of many diseases, such as type 1 diabetes and IBD, and yielded remarkable outcomes (42, 43). This treatment approach that uses intestinal bacteria is called bacterial therapy.
To delineate the factors that affect intestinal bacteria and improve their immunotherapeutic effects, many studies have attempted to regulate intestinal bacteria using antibiotic treatment or fecal microbiota transplantation (FMT) (14, 44, 45). However, both methods can cause large-scale and holistic changes in abundance and diversity of intestinal bacteria. Therefore, to regulate the population of a certain bacterium or transfer some type of probiotics into the host for bacterial therapy, an efficient way of delivering intestinal bacteria is necessary. Compared with intravenous (i.v.) injection, the oral administration of bacteria can improve patient compliance and avoid the risk of systemic infections that might be caused by the i.v. injection. However, because the oral administration route involves the passage of bacteria through the stomach and gut, various bacterial activities may be compromised due to the presence of gastric acid and bile salts in the gastrointestinal system. Therefore, the design and selection of biomaterials for the encapsulation and delivery of intestinal bacteria are essential. Many biomaterials, including Eudragit (46), chitosan (47), and alginate (48), have been widely used to encapsulate and protect intestinal bacteria against acid, bile, and other harsh components. Moreover, the properties of these biomaterials, including permeability, mechanical stability, and pH sensitivity, have been specially designed to improve the survival of intestinal bacteria in the acidic environment of the stomach and ensure their complete release in the intestine (49, 50).
In this review, we focused on the immunotherapeutic effects of intestinal bacteria mainly exerted by regulating various immune cells, as well as the biomaterials used to encapsulate and deliver the bacteria possessing these regulatory functions. We first discuss various immune cells, which are classified according to their types, regulated by intestinal bacteria. Then, we summarize the recent progress on biomaterial encapsulation methods in various intestinal bacterial species. Figure 1 schematically shows the oral administration of intestinal bacteria and their immunoregulatory function in various diseases.
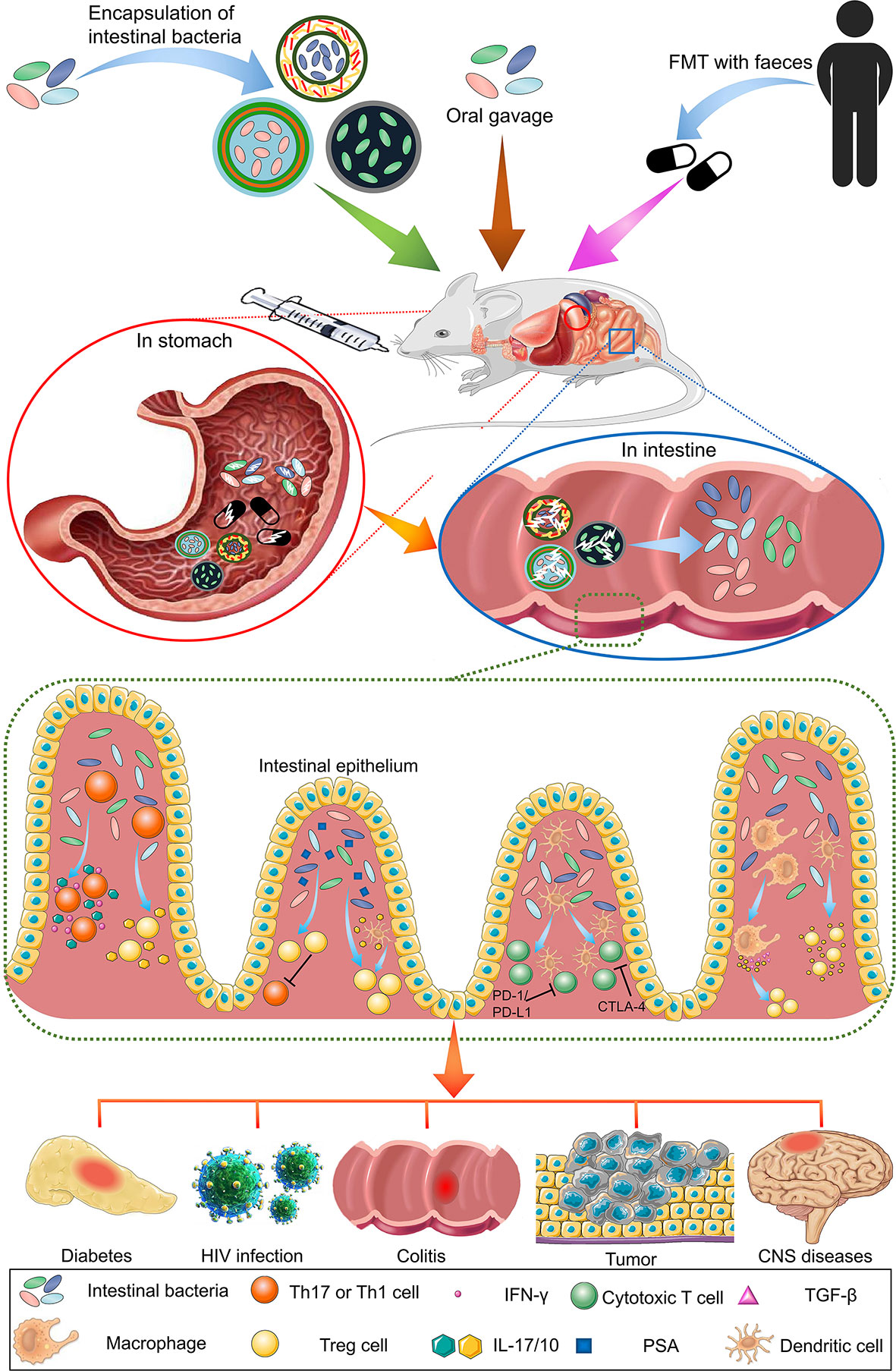
Figure 1 Overview of the oral administration of intestinal bacteria for immunotherapy in various diseases. Common intestinal bacterial delivery methods include oral delivery (gavage) and intravenous injection. Gavage is more widely used because of its safety profile. Compared with free bacteria and FMT, bacteria encapsulated by biomaterials can resist the acidic environment of the stomach, and their contents can be released in the intestine. The released bacteria exert immune regulation functions beneficial to the treatment of various diseases.
The immune system of the human body has a crucial impact on the development of various diseases. Diseases, such as inflammation and tumors, can alter the microenvironment of the body. For example, tumor cells in the tumor microenvironment (TME) have been reported to closely interact with the extracellular matrix (ECM) and stromal cells (51). A variety of immune and nonimmune cells that secrete cytokines and chemokines and express various surface receptors have been found in the TME. These cells have been shown to drive chronic inflammation and immunosuppression and promote the development of proangiogenic tumor environment (52). Immune cells, such as lymphocytes, dendritic cells (DCs), macrophages, and natural killer (NK) cells, are important in tumor development or suppression (53). A spatiotemporal dynamic analysis of 28 different kinds of immune cells that infiltrated tumors found that the composition of infiltrating immune cells changes at each tumor stage, with particular cells having a major impact on survival (54). Therefore, regulating these immune cells may improve the immunotherapy of tumors and other diseases.
Owing to some limitations in the application of immunotherapy (39, 55) and the regulation of the intestinal bacterial function (56, 57), many studies have combined the use of intestinal bacteria with immunotherapy, revealing their key role in regulating the response of immunotherapy, especially in tumors, by affecting immune cells (58–61). The findings of recent studies on bacteria used for immunotherapy and the immune cells they influenced are summarized in Table 1.
T-lymphocytes, also called T-cells, are derived from the bone marrow. Following their differentiation and development in the thymus, they are distributed to immune organs and tissues through the blood circulatory and lymphatic systems, thus exerting their immune function. T-cells are critical in many diseases, including tumors (75). According to their function and surface markers, T-cells can be classified as helper T-cells, regulatory T-cells, cytotoxic T-cells, suppressor T-cells, and memory T-cells. In this section, we summarized the recent findings on the regulation of these T-cells by intestinal bacteria.
Helper T-cells, whose major surface marker is CD4, play a role in intermediate immune response. They can proliferate and spread to activate other types of immune cells involved in direct immune response. Helper T-cells can be activated through an antigenic reaction with a polypeptide presented by the major histocompatibility complex II (MHC II). Once activated, they can secrete cytokines, regulate, or assist in the immune response.
Intestinal bacteria regulate immunotherapy for type 1 diabetes (T1D) via helper T-cells. A study analyzing the fecal samples of biobreeding diabetes-prone (BB-DP) and biobreeding diabetes-resistant (BB-DR) mice (56) found that the fecal matter of BB-DR mice is enriched in both Lactobacillus and Bifidobacterium species, whereas that of BB-DP mice is abundant in Bacteroides. The prevalence of Bacteroides in mice with T1D suggests that intestinal bacteria are involved in the occurrence and development of diseases. Furthermore, intestinal bacteria can regulate T-helper 17 (Th17) cells. Among non-obese diabetic (NOD) mice, female mice without segmented filamentous bacteria (SFB) showed a higher prevalence of diabetes, whereas those with SFB were resistant to diabetes (62). However, in male mice, there was no significant difference in the onset of diabetes between the two groups. To explore the relationship between SFB and diabetes, flow cytometry was performed on tissue derived from the small intestinal lamina and associated lymph nodes in SFB+ and SFB- female mice. The results showed an evident induction of Th17 cells in the small intestinal lamina propria of SFB+ females. Another study has demonstrated that the oral administration of the Lactobacillus johnsonii strain N6.2 (LjN6.2) from BB-DR rats conferred T1D resistance to BB-DP rats, but that of Lactobacillus reuteri strains did not (76). This resistance of LjN6.2-fed BB-DP mice was due to a change in Th17 cells within the mesenteric lymph nodes (63), which was not observed in non-gut–draining axillary lymph nodes, indicating that the change in Th17 cells was caused by LjN6.2 interactions within the mesenteric lymph node. Overall, these studies indicate that the induction of T1D could be circumvented by the intestinal bacterial-mediated differentiation of Th17 cells.
Most importantly, by affecting helper T cells, the intestinal bacteria can regulate immunotherapy for tumors. To investigate the potential mechanism underlying the inhibition of tumor progression and control of hepatocellular carcinoma (HCC) by intestinal bacteria feeding, Li and coworkers used Prohep to design their experiments (16). Prohep is a new mixture of intestinal bacteria comprising heat-inactivated VSL#3, viable Escherichia coli Nissle 1917 (EcN), and Lactobacillus rhamnosus GG (LGG) (1:1:1). Prohep not only significantly reduced tumor size and weight by 40% but also retarded tumor growth compared with the control (Figures 2A, B). In addition, after treatment with Prohep, the hypoxic region of the tumor was obviously increased, indicating that the reduction in tumor size might have been associated with cell death caused by hypoxia (Figure 2C). In Figure 2D, the confocal Z-stacks of 3D models show that compared with the control group, the number of vessel sprouts and the region of blood vessels in each tumor section were obviously decreased. The tumor group treated with intestinal bacteria had decreased number of Th17 cells. Moreover, metagenome sequencing revealed the association between intestinal bacteria and HCC development. Therefore, treatment with Prohep may promote the growth of beneficial bacterial colonies, including Oscillibacter and Prevotella, which are known producers of anti-inflammatory metabolites. Subsequently, these bacteria led to the reduction in the number of Th17 cells and promoted the differentiation of regulatory T-cells (Tregs; introduced in next section) in the intestine. In summary, tumor reduction induced by probiotic feeding relies on the downregulation of IL-17 and its major producer, Th17 cells.

Figure 2 The Prohep intestinal bacterial mixture improves hypoxia in tumor tissues and reduces tumor volume. (A) Changes in tumor size during 38 d. (B) Tumor weight of each group after the experiment. (C) Immunostaining of representative tumor sections using the CD31 angiogenesis (red) and GLUT-1 hypoxia (blue) markers. (D) Images of a 3D model acquired after superimposing multiple confocal planes by confocal Z-stack imaging (section thickness of 25 μm). *0.01 < P value < 0.05; **0.001 < P value < 0.01; ***P value < 0.001. Adapted from ref. (16).
Intestinal bacteria have also been found to prevent infections and suppress allergies. Chilba et al. demonstrated that a co-culture with Lactobacillus casei in vitro promoted the development of Th1 cells, resulting in an increased production of interferon γ (IFN-γ), and also stimulated CD11b+ cells to produce interleukin 12 (IL-12) in mouse spleen cells (77). Intestinal bacteria that increase the production of IL-12, such as L. casei, can effectively prevent infections. By controlling the balance of Th1/Th2 cells, L. casei has also been reported to suppress allergic diseases. This immunomodulation is partly due to the regulation of indoleamine 2,3-dioxygenase (IDO). IDO is widely distributed in the body and participates in tryptophan metabolism to produce 3-hydroxyanthranilic, quinolinic acid, and kynurenine (78). These three tryptophan degradation products have excellent blood–brain barrier penetration ability, which is a key obstacle to the application of many drugs to treat brain diseases (79). Moreover, 3-hydroxyanthranilic acid and quinolinic acid can selectively drive the apoptosis of Th1 cells, whereas kynurenines can promote the development of Tregs, suggesting that these metabolites may possess immunoregulatory effects (80). By expressing IDO and activating subsequent biological processes, the use of specific intestinal bacteria could also enhance the efficacy of immunotherapy for brain diseases.
Tregs exhibit immunosuppressive functions, and their deficiency contributes to the development of allergies and autoimmune diseases; thus, they are T-cells that regulate autoimmune activity in vivo. In addition, Tregs expressing specific tumor-associated antigens have been found in patients with tumor and aggregate in several kinds of solid tumors, where they may play a role in protecting tumors from cytotoxic immune responses (81). In fact, there is a negative correlation between the survival rate of patients with tumors and Treg infiltration. Therefore, the regulation of Tregs could be a promising treatment strategy in many diseases.
Bacterial metabolites affect the occurrence and progression of inflammation via mediating the communication between intestinal bacteria and the immune system. For example, butyrate, a short-chain fatty acid (SCFA) secreted by intestinal bacteria, promotes the extrathymic production of Tregs (21), indicating that intestinal bacteria exhibit a regulatory function in the immunotherapy of inflammatory diseases, such as IBD. In a prospective study of patients suffering from metastatic melanoma treated with ipilimumab, Dubin et al. compared the composition of preinflammation fecal microbiota before and after the development of colitis (65). Increased Bacteriodes were found in the stools of participants resistant to colitis, whereas enriched Faecalibacterium and other Firmicutes were detected in those with high incidence of checkpoint-block-induced colitis (57). Moreover, enrichment in the genus Bacteroides was observed in patients with resistant colitis. As one of the primary bacterial species in the human gut, Bacteroides can suppress inflammation via stimulating the differentiation of Tregs (82). This immunomodulatory effect was consistent with the results obtained by Dubin et al. and Chaput et al. (57, 65).
In addition, immunotherapy can also be regulated by specialized bacterial molecules, such as the polysaccharide A (PSA) of Bacteroides fragilis. Round et al. demonstrated that the Toll-like receptor (TLR) pathway could be activated in T-cells by the human commensal bacterium Bacteroides fragilis to establish an interaction between the host and intestinal bacteria (28). Deficiency in TLR2, which is located on CD4+ T-cells, can promote antimicrobial immune responses, resulting in reduced colonization of the mucosa by B. fragilis. However, this can be recovered by the PSA of B. fragilis that directly activates TLR2 on Foxp3+ regulatory T-cells, leading to the production of mucosal tolerance. Meanwhile, B. fragilis not expressing PSA could not suppress the host immune response. Another study confirmed the regulatory function of the PSA of B. fragilis and showed that B. fragilis induced the development of Foxp3+ Tregs with unique inducible hereditary characteristics (43). Unlike naturally produced Tregs, these inducible Tregs were found in peripheral tissues, such as the intestine, rather than in thymic tissues and can secrete cytokines, such as interleukin 10 (IL-10) (83). Round et al. found that germ-free animals colonized with B. fragilis had increased inhibitory capacity of Tregs and produced only anti-inflammatory cytokines from Foxp3+ T-cells in the intestine (43). In addition, they showed that as an immunomodulatory molecule, the PSA of B. fragilis induced CD4+ T-cells into inducible Foxp3+ Tregs. These processes required TLR2 signaling to induce Tregs and express IL-10. More importantly, the PSA of B. fragilis was demonstrated to not only prevent but also cure experimental colitis in animals, supporting that intestinal bacterial molecules not only mediate the balance between health and disease but also regulate the efficacy of immunotherapy for many diseases.
After recognizing that changes in the composition of intestinal bacteria can influence inflammation, the potential mechanisms of intestinal dysfunction following HIV-1 infection became clearer. Early in 1990, researchers found that most patients with acquired immunodeficiency syndrome (AIDS) exhibited gastrointestinal disorders of variable severity (84); thus, they assumed that maintaining the integrity of intestinal ecology could improve the clinical outcomes of patients suffering from AIDS. Consequently, increasing evidence showed that probiotic treatment can protect the intestinal surface, delay the progression of HIV-1 infection to AIDS, and provide specific benefits in patients with HIV-1 infection (64, 85). The balance between Th17 cells and Tregs is an important immunoregulatory mechanism that influences the production of functional host immune responses to infection (86, 87). This balance is mediated by tryptophan catabolites, tryptamine, indole-3-aldehyde (IAld), indoleacetic acid, and indolelactic acid (ILA), which act as aryl hydrocarbon receptor (AHR) ligands and promote the differentiation of naive CD4+ helper T-cells into Tregs (88). Intestinal bacteria, such as Lactobacilli (89) and Clostridium sporogenes, can metabolize and convert tryptophan into the above-mentioned AHR ligands, thereby affecting the Th17/Tregs balance and exerting a beneficial effect on autoimmune diseases and inflammation. More specifically, intestinal bacteria inducing the differentiation of Th17 cells or upregulating Tregs can suppress the progression of inflammation and provide benefits in patients with viral infection. In addition, by expressing IDO and producing tryptophan metabolites, intestinal bacteria can activate the AHR signaling pathway and induce IL-22 production (90). IL-22 mainly fulfills intestinal barrier function and maintains the intestinal homeostasis by mediating mucosal host defense, thereby helping the body resist the invasion of intestinal pathogenic bacteria (91, 92). Overall, intestinal bacteria can indirectly up-regulate IL-22 to counter pathogenic bacterial infections and diarrhea caused by gut dysbiosis.
Kwon et al. identified a mixture of probiotics, designated as IRT5, comprising L. casei, L. acidophilus, Bifidobacterium bifidium, L. reuteri, and Streptococcus thermophilus (67). After its oral administration for 20 days, IRT5 upregulated CD4+ Foxp3+ Tregs and induced the hypo-responsiveness of T-cells. In addition, the transformation of T-cells into Foxp3+ Tregs was shown to be directly mediated by DCs (discussed in Section of DCs) specialized to express IDO as well as suppressor cytokines such as TGF-β and IL-10.
By regulating Tregs, intestinal bacteria can also affect the immunotherapy of allergic diseases, such as allergic asthma. Russelle et al. showed that changes in the intestinal bacteria due to antibiotics increase the susceptibility of neonatal mice to allergic asthma (93). Compared with streptomycin, vancomycin reduced intestinal bacterial diversity, especially that of Bacteroides and Clostridiales, and the reduced Bacteroides were replaced by abundant Lactobacilli. As previously discussed, species under the genus Bacteroides, such as B. fragilis, are associated with T-cell differentiation. Additionally, treatment with vancomycin decreased the number of Foxp3+ Tregs in the colon, but not in the lungs, following the destruction of Clostridiales population. Atarashi et al. reached the same conclusion and identified the Clostridium species as potent inducers of Tregs (94). However, the role of Lactobacilli remains controversial as the said study showed its negative correlation with Tregs, whereas another study showed that it induces Tregs (95). Therefore, there might be other unknown factors participating in the regulation of immunotherapy by intestinal bacteria or an unknown interaction between other Lactobacilli species or strains. The specific mechanisms, however, remain unexplored. As there are thousands of intestinal bacteria in the body, maintaining commensalism with the host, all intestinal bacteria in each organ should be seen as a whole when we regulate intestinal bacteria in order to influence immunotherapy. Based on this view, FMT has been applied at the microbial level to alter immune responses in the body and has shown good results in the regulation of immunotherapy.
Cytotoxic T-cells are specific T-cells that secrete various cytokines and participate in immune function. They exert a killing effect on certain viruses, tumor cells, and other antigenic substances. In particular, cytotoxic T-cells and NK cells play an important role in defense against viruses and tumors. Recently, cytotoxic T-cells regulated by intestinal bacteria has been associated with the efficacy of the immune checkpoint inhibitors (ICIs).
ICI therapy leads to the activating of T-lymphocyte-mediated immune responses by inhibiting the interaction between T-cell inhibitory receptors and their homologous ligands on stromal cells or tumors (96). Currently, ICIs are mainly monoclonal antibodies targeting the programmed cell death protein 1 (PD-1)/PD ligand 1 (PD-L1) axis and cytotoxic T-lymphocyte antigen 4 (CTLA-4), and ICIs have achieved great success in the immunotherapy of tumors. However, ICIs cannot suppress tumor progression in most patients; thus, they often result in immune-related adverse events (irAEs) (97). The use of antibiotics could reduce the efficacy of ICIs in tumor immunotherapy, with ICIs showing excellent efficacy in the presence of specific intestinal bacterial species (98). For example, the immunostimulatory and antitumor effects of the CTLA-4 blockade was demonstrated to depend on the presence of various Bacteroides gut species (99). In patients suffering from renal cell carcinoma (RCC), advanced-stage non–small cell lung cancer (NSCLC), or bladder tumor treated with PD-1/PD-L1 blockade, the use of broad-spectrum antibiotics shortly before or after treatment was associated with adverse clinical outcomes (45).
Increasing evidences have demonstrated that intestinal bacteria can influence the effect of immune checkpoint blockade (ICB) treatment by regulating cytotoxic T-cells. Gopalakrishnan et al. observed notable differences in the composition and diversity of intestinal bacteria between responsive and non-responsive (NR) patients with melanoma receiving anti-PD-1 immunotherapy as shown in Figure 3A (68). Responsive patients had increased abundance of the Ruminococcaceae family (including Faecalibacterium spp.) compared to NR, and enrichment in Faecalibacterium spp. positively correlated with progression-free survival (PFS) and cytotoxic T-cell infiltration in the TME. To study the mechanism, FMT results confirmed the transferability of this phenotype. The detailed experimental design is presented in Figure 3B. Slower tumor growth and better immunotherapy efficacy were observed in mice subjected to FMT with stool samples from the responsive patients than in those from NR (Figures 3C, D). In a separate study, Matson and coworkers verified these findings using pretreated fecal samples from 42 patients with metastatic melanoma (24). Enterococcus faecium, Collinsella aerofaciens, and Bifidobacterium longum were found to be more enriched in responders. In addition, transferring fecal samples from the responders to germ-free mice has been shown to improve tumor control, enhance T-cell responses, and increase anti-PD-1 therapeutic effect. These effects are due to the regulation of cytotoxic T-cells by these intestinal bacteria.

Figure 3 Differences in the composition of intestinal bacteria related to the effects of anti-PD-1 immunotherapy and antitumor immunity. (A) Taxonomic cladogram from LEfSe showing differences in stool taxa. The size of dot is positively correlated with the abundance of the taxon. Letters a–r represent the following taxa respectively: (a) Gardnerella vaginalis, (b) Gardnerella, (c) Rothia, (d) Micrococcaceae, (e) Collinsella stercoris, (f) Bacteroides mediterraneensis, (g) Porphyromonas pasteri, (h) Prevotella histicola, (i) Faecalibacterium prausnitzii, (j) Faecalibacterium, (k) Clostridium hungatei, (l) Ruminococcus bromii, (m) Ruminococcaceae, (n) Phascolarctobacterium faecium, (o) Phascolarctobacterium, (p) Veilonellaceae, (q) Peptoniphilus, (r) Desulfovbrio alaskensis. (B) Experiment designed to study the GF mice. Relative to days (indicated as D) of tumor injection (2.5 - 8 × 105 tumor cells). (C) Tumor growth curves for each GF mouse from anti-PD-L1-treated R-FMT (blue, n = 2; median tumor volume = 403.7 mm3), NR-FMT (red, n = 3; median tumor volume = 2301 mm3), and Control (black, n = 2; median tumor volume = 771.35 mm3) mice. Statistics are as follows: p = 0.20 (R-FMT vs NR-FMT), p = 0.33 (NR-FMT vs Control) by the MW test. The black dotted line indicates the size limit of the tumor when treated with anti-PD-L1 (500 mm3). (D) Using the MW test, on the 14th day of implantation in NR-FMT mice (red) and R-FMT (blue), difference in tumor size expressed as fold change (FC) relative to the average tumor volume of Control GF mice. Data from 2 independent FMT experiments (R-FMT, n = 5, median FC = 0.18; NR-FMT, n = 6, median FC = 1.52). **P value < 0.01. Adapted from ref. (68).
Memory and effector T-cells are formed following the division and differentiation of T-cells, respectively. Memory T-cells are of vital importance in recurrent immune responses. When the host immune system is invaded by the same antigen, memory T-cells remobilize the mechanisms used to kill the antigen previously. The identity of memory T-cells can be determined via the expression of CD45RA, CD27, and CD62L (100). Currently, memory T-cells are used in vaccines for infectious diseases (101) and have recently been found to exert an anti-tumor function.
Routy et al. found a relationship among Akkermansia muciniphila, Enterococcus hirae 13144 (E. hirae), and memory T-cells (45). As shown in Figure 4A, patients with NSCLC and RCC responding to the PD-1/PD-L1 blockade had increased abundance of A. muciniphila in their feces than non-responders. They next investigated the responses of memory T-cells in the peripheral blood to the microbiota after initiating PD-1 blockade. Figure 4B presents the response of circulating memory CD4+ and CD8+ T-cells collected from 27 patients with NSCLC and 28 with RCC subjected to anti-PD-1 treatment. Due to the function of A. muciniphila and E. hirae, the responses of memory Th1 and Tc1 cells were enhanced in the responders, resulting in increased production of IFN-γ (Figure 4C). Figure 4D shows that based on 32 fecal samples, E. hirae was more abundant in responders with NSCLC than in non-responders. This further supports the relevance of probiotics, such as E. hirae and A. muciniphila, in predicting efficacious treatment.
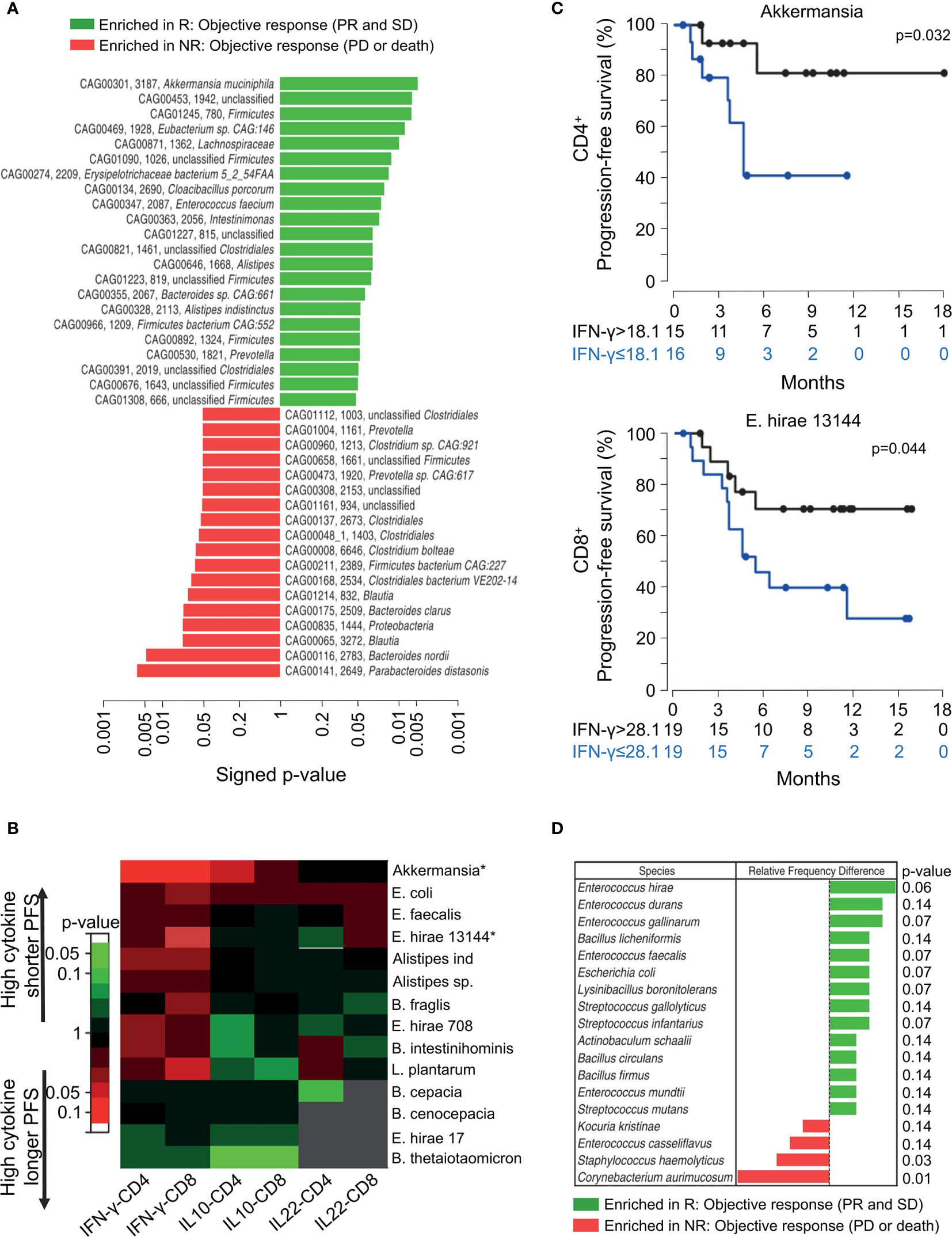
Figure 4 The composition of intestinal bacterial from stool samples determines the efficacy of PD-1 mAb therapy in cancer patients after 3 months. (A) Shotgun sequencing of stool samples at the time of diagnosis, using responders (R) (partial response or stable disease) determined according to the best clinical response relative to the non-responder (NR) (progress or death) of each MGS according to RECIST1.1 standard. P value of the entire cohort of n = 100 (60 patients with NSCLC and 40 with RCC). (B, C) Immune responses of circulating memory T-cells detected during PD-1 blockade and evaluation of the time to progression. (B) Heat map of the P values for each intestinal bacterium and each cytokine, classifying the PFS of patients with NSCLC RCC based on the median value of the production of cytokines in the entire cohort. Significant P values (<0.05, Student’s t test) are marked by asterisks as relevant intestinal bacteria. (C) Kaplan-Meier curves and Univariate analysis showing immune responses of PBS against peripheral blood memory Th1 and Tc1 directed against A. muciniphila and E. hirae 13144, respectively. (D) Stool samples of 16 R and 16 NR patients with NSCLC (defined as the best clinical outcome) analyzed based on culturomic before treatment; each intestinal bacterium having been identified by mass spectrometry. Colored bars show relative frequencies of each commensal in all stool cultures in R over NR patients, and the right graph shows P values with difference. *P value < 0.05. Adapted from ref. (45).
In addition, Vetizou and coworkers demonstrate the modulatory function of intestinal bacteria in responses to anti-CTLA-4 therapy by regulating of memory T-cells (44). In both mice and humans, T-cell specific response to B. fragilis or B. thetaiotaomicron was related to the effect of the CTLA-4 blockade. Tumors in germ-free (GF) or antibiotic-treated mice did not respond to CTLA-4 blockade. However, after the adoptive transfer of B. fragilis-specific T-cells or gavage with B. fragilis, the presence of Bacteroides species determined the antitumor effect of the CTLA-4 blockade. Subsequently, the dynamics of the response of memory T-cells in humans and mice during CTLA-4 blockade was analyzed and revealed that T-cell response to anti-CTLA-4 therapy was due to the production of Th1 cells induced by specific memory T-cells. Moreover, the adoptive transfer of this specific type of memory T-cells into GF or patients with antibiotic-treated tumors could partially restore the efficacy of the immune checkpoint blocker.
Antigen presenting cells (APCs) can absorb, process, and present antigens; hence, they constitute a key part of innate immunity. The major histocompatibility complex (MHC) class II molecule expressed on the surface of APCs can absorb pathogen proteins and help process them into short peptide segments, which are presented to T-cells. Thus, APCs are also known as the initiators of acquired immunity. The thymus-dependent antigen (TD-Ag) stimulated B-lymphocytes to produce antibodies, with the participation of not only T- and B-lymphocytes but also accessory cells. In general, APCs include macrophages, DCs, B–lymphocytes, and other cells that can express MHC class II molecules, the so-called full-time APCs. Other cells, such as the fibroblasts, endothelial cells, and various epithelial and mesothelial cells, also exhibit certain antigen presenting functions and are thus called non-full-time APCs. The expression of PD-L1 on DCs or macrophages might affect the efficacy of immunologic checkpoint inhibitors and therefore, in theory, could have the potential to predict the efficacy of drugs (102). We then speculate that intestinal bacteria could influence the efficacy of immunotherapy by regulating APCs.
Discovered by the Canadian scholar Steinman in 1973, DCs are the most powerful antigen-presenting cells at present. They were named for their dendritic or pseudopodal protrusions at their mature state. DCs can absorb, handle, and present antigens efficiently. Immature DCs show a strong ability to migrate, whereas mature DCs can effectively activate primary T-cells, which are essential for initiating, regulating, and maintaining immune responses. DCs have been closely linked with the occurrence and development of tumors. For instance, in the majority of solid tumors, higher DC infiltration correlates with better tumor prognosis. Effective antitumor immune responses mainly relies on the production of cellular immune responses based on CD8+ T-cells, which is also the basis of DCs in immunotherapy.
A previous study showed that C57BL/6 mice raised in Taconic Farms (TAC) or Jackson Laboratory (JAX) animal facilities showed differential colonization by segmented filamentous bacteria (SFB) (103). The difference in growth environment leads to the different composition of intestinal bacteria. Compared with JAX mice, an obvious enrichment of Th17 cells in TAC mice was observed. After 10 days of colonization of JAX-derived GF mice with SFB, the SFB-colonized lamina propria of both the small and large intestines were populated with increased Th17 cells. This suggests that intestinal bacteria, such as SFB, can stimulate immune response via direct contact with body tissues. However, further research found that SFB induced the production of serum amyloid A (SAA) by the terminal ileum, with SAA promoting the differentiation of Th17 cells under the action of DCs in vitro. The colonization of SFB led to the secretion of SAA, which further stimulated DCs in the intestine to induce the differentiation of Th17 cells, thus demonstrating that intestinal bacteria regulate host immunity through their secretory function.
In addition, substances with immunomodulatory functions, such as IDO, are not only regulated by intestinal bacteria (104, 105) but also expressed in immune cells, which makes the relationship between intestinal bacteria and immune cells more intricate. In fact, there are a series of human APCs that express IDO co-expressing cell surface markers CD123 and CCR6, by which they can be identified from other immune cells (106). In the family of DCs, IDO-expressing DCs can suppress inhibitory effector T cells and promote the differentiation of Tregs, proving to be beneficial in patients with autoimmune diseases like IgA nephropathy (IgAN) (107).
Sivan et al. studied the growth of melanoma in TAC and JAX mice with different commensal intestinal bacteria and observed differences in spontaneous antitumor immunity (15). Particularly, they found that as the most distinct intestinal bacterial species between the two groups, Bifidobacterium (Figure 5A) unexpectedly enhanced antitumor immunity in vivo. Bifidobacterium alone or combined with anti-PD-L1 treatment effectively inhibited tumor growth (Figure 5B). Additionally, after its oral administration, the antitumor activity of CD8+ T-cells was improved, and it was attributed to DC-induced accumulation of enhanced CD8+ T-cells in the TME. Figure 5C displays that the key genes related to antitumor immunity in DCs that are significantly enhanced by the administration of Bifidobacterium. Notably, only live Bifidobacterium produced this effect, suggesting that Bifidobacterium colonized intestinal niches that enabled them to interact with host cells that regulate DCs or to systemically release soluble factors that enhanced the function of DCs. In addition, Bifidobacterium was eliminated in CD8+ T-cell-depleted mice, indicating that the regulatory function of Bifidobacterium relied on the activity of cytotoxic T-cells. Therefore, the regulation between intestinal bacteria and immune cells is mutual. Furthermore, Bifidobacterium can activate other immune regulatory pathways. For instance, Bifidobacterium LMG 13195 or their membrane vesicles promoted the differentiation of immature T-cells into CD25+ Foxp3+ Treg cells by acting on DCs and inducing the production of IL-10 in vitro (69). Bifidobacterium has been extensively studied and can be detected in the intestines after the first meal. It is also a typical probiotic due to its health promoting functions. As a member of the Bifidobacterium family, Bifidobacterium LMG 13195 is safe for human consumption. Therefore, Bifidobacterium LMG 13195 has the potential as a safe and effective adjuvant for immunotherapy in clinical practice.
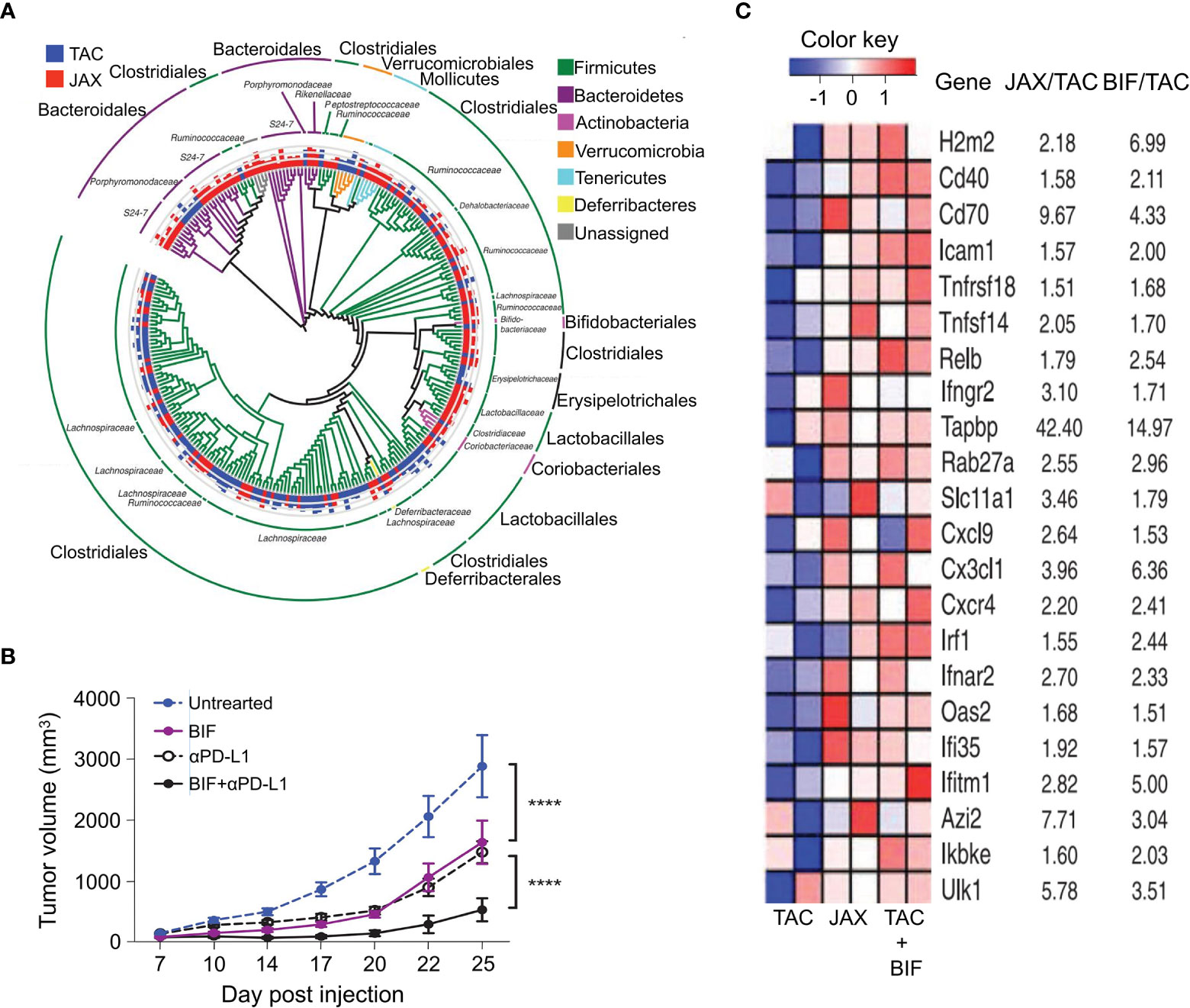
Figure 5 Tumor patients can benefit from direct administration of Bifidobacterium, which was shown to improve the DC cell-related tumor-specific immunity and effect of anti-PD-L1 monoclonal antibody treatment. (A) In newly obtained TAC and JAX mice, the phylogenetic analysis of taxa with obviously different abundance FDR < 0.05 (nonparametric t test); bars represent log-transformed fold changes, inner circle, log10(10); middle circle, log10(100); outer circle, log10(1000). (B) 7 and 14 d after the implantation of B16.SIY tumor, the tumor growth kinetics in TAC mice, untreated or treated with Bifidobacterium, anti-PD-L1 mAb 7, 10, 13, and 16 d after tumor implantation, or both regimens. (C) Heat map of key antitumor immunity genes in DCs isolated from untreated TAC, Bifidobacterium-treated TAC, and JAX mice. Mean fold change for each gene transcript is shown on the right. ****P value < 0.0001. Adapted from ref. (15).
Intestinal bacteria were also found to affect the immunotherapy of IBD by regulating DCs. As one of the most potent anti-inflammatory cytokines, IL-10 is required for protection during inflammation. In an experimental colitis model, researchers found that the PSA of B. fragilis protected subjects from inflammatory disease via promoting the proliferation of IL-10-producing CD4+ T-cells (70). Unlike other polysaccharides, PSA can be internalized by APCs and subsequently presented to T-cells, along with MHC class II molecules. Furthermore, the PSA of B. fragilis executed this process by regulating bone-marrow-derived DCs (BMDCs). Therefore, PSA may play a protective role during inflammation. The immunomodulatory capacity of symbiotic factors, such as PSA, may thus provide new treatment approaches for human inflammatory diseases.
In summary, the immunoregulatory role of DCs is multifaceted: DCs can inhibit immunity and exert a beneficial effect on autoimmune diseases such as IgAN, while they can also activate immune responses for immunotherapy of other diseases including tumors. Therefore, intestinal bacterial species that can regulate DCs may have diverse therapeutic applications. However, increasing attention must be paid to the changes in DCs mediated by pathogenic intestinal bacteria.
Macrophages have many functions and are important targets in the study of cell phagocytosis, cellular immunity, and molecular immunology. In vertebrate animals, macrophages are known to be involved in specific (cellular immunity) and nonspecific (innate immunity) defense. Their main function is to engulf pathogens and cellular debris. In addition, macrophages can activate multiple immune cells to release a variety of cytokines (108) and also regulate the differentiation and mobilization of neutrophils via secretion of granulocyte-colony stimulating factor (G-CSF) (109). Through these cytokines, macrophages can modulate the activity of other immune cells, such as neutrophils.
As we mentioned before, the PSA of B. fragilis provided protection against inflammatory disease via the production of the IL-10 cytokine. The relationship between macrophages and IL-10 was further demonstrated by Denning et al. In the lamina propria, some macrophages expressed several anti-inflammatory molecules, including IL-10; however, even after stimulation by Toll-like receptor ligands, the macrophages expressed only low levels of proinflammatory cytokines (110). The differentiation of Foxp3+ Tregs could be induced by these macrophages via a mechanism relying on the exogenous transforming growth factor-β, retinoic acid, and IL-10. Although this study did not use intestinal bacteria to regulate the immune process, the regulation of IL-10 by B. fragilis or the PSA they produce has been mentioned before. For example, Round and coworkers showed that the transformation of CD4+ T-cells into Foxp3+ Tregs that produce interleukin 10 (IL-10) is mediated by the PSA of B. fragilis (43). Therefore, we speculate that the PSA of B. fragilis synergizes with macrophage activity to enhance their immunomodulatory functions. In another study, the nucleoprotein fraction (NPF) of Bacillus subtilis 7025 cultural medium filtrate stimulated immune responses and exhibited antitumor effects (27). As NPF is mainly composed of histone and protamine, the NPF of Bacillus subtilis 7025 cultural medium filtrate has sufficient biological safety and can potentially be used in antitumor immunotherapy as it displays significant immunostimulatory effects through enhanced IFN-γ activity. Moreover, as one of the major cytokines involved in antitumor protection, IFN-γ can activate macrophages.
Remarkably, special macrophages called the microglia have also been reported to be regulated by intestinal bacteria. Microglia, which are found in the brain, are macrophages of the central nervous system (CNS) mainly involved in CNS diseases. Following the stimulation of inflammation, the antigenicity of microglia is enhanced. Host intestinal bacteria have been found to be vital in microglial homeostasis as microglia in GF mice exhibited obvious defects, changes in cell ratio, and immature phenotypes, resulting in impaired innate immune responses (71). In addition, less complex intestinal bacteria can lead to microglia defects, whereas the reconstitution of their complexity can partially restore the characteristics of microglia. Studies on SCFAs, which are bacterial fermentation products, found that SCFA influences microglial homeostasis and can be pathogenic to the CNS (111).
Progenitor cells of B lymphocytes (B-cells) are found in the island of hematopoietic cells in fetal liver. During development, B-cells are produced and differentiate in the bone marrow. B-cells can differentiate into plasma cells under antigenic stimulation, which can then synthesize and secrete antibodies, mainly involved in humoral immunity.
Intestinal bacterial DNA has been reported to have immunostimulatory effects on B-cells, thereby inducing the production of various cytokines critical in anti-tumor immunity (112). In 1991, researchers found that bacterial DNA could induce significant antibody responses in mice (72). When highly purified ssDNA collected from E. coli was used to stimulate lymphocytes, a dose-dependent response was observed, indicating lymphocyte proliferation. As the consumption of T-cells was not observed to reduce the proliferation of lymphocytes, bacterial DNA may have directly triggered the proliferation of B-cells. Another study showed that in bacterial DNA, unmethylated CpG dinucleotides rapidly activate B-cells to secrete IL-6 and IgM (73).
The DNA of intestinal bacteria can activate NK cells and thus enhance antitumor immunity (74, 113). The administration of mycobacterial DNA can induce the secretion of interferon and enhance the activity of NK cells, resulting in tumor regression (114). An in vitro experiment showed that the nucleic acid-rich component from Bacille Calmette-Guerinvaccine (BCG) enhanced the activity of NK cells in mouse spleen, induced antiviral activity, and induced macrophages to produce cytotoxic factors against tumor cells.
Group 3 innate lymphoid cells (ILC3) are tissue-resident lymphocytes abundant in mammalian intestine and play an important regulatory role in intestinal inflammation and homeostasis (115). ILC3 proliferation has been associated with L. paracasei abundance (116). This phenomenon was also observed in the lungs after viral infection, subsequently suppressing the inflammatory response to achieve a therapeutic effect via mediating the proliferation of Th2 cells (117).
In the previous section, we have introduced the effects of intestinal bacteria on the function of various types of immune cells, which ultimately enhanced immunotherapeutic effect in many diseases. For example, FMT is usually the preferred method to study of intestinal bacteria as a whole. When intestinal bacteria are regulated using FMT, their integrity can be maintained, and the influence of different bacteria on the regulation of the immune function does not need to be considered. In addition, the transfer of fecal samples from experimental mice to GF mice can not only reconstruct the composition of the intestinal bacteria but also facilitate the study of its effect on disease treatment.
However, FMT experiments cannot reveal the mechanisms and the role of certain intestinal bacteria in the regulation of immunotherapy. Therefore, to study the functions of several specific strains, single bacteria or combinations of several probiotics are delivered by oral administration (gavage) (26, 44) or intravenous injection (118). Intestinal bacteria can be delivered directly to the host through intravenous injection, gavage, and even anal perfusion (119, 120); however, these methods have certain disadvantages. Intravenous injection might lead to a variety of infectious diseases, such as bacteremia and septicemia. Especially in bacterial infectious diseases, there is a high risk of bacteria contaminating the blood. Thus, intravenous injection is rarely used in the studies we have discussed here. Anal infusion is more inconvenient than gavage and reduces patient compliance. Besides, intestinal bacteria are widely distributed in the colon. Therefore, the proper delivery of the target bacteria to various parts of the colon to simulate the situation of bacterial distribution in the human body is essential. However, anal perfusion can only deliver the target intestinal bacteria to areas near the descending colon and rectum and not the whole intestine. Thus, oral administration is the most widely used method for the delivery of intestinal bacteria.
Direct oral administration allows the passage of intestinal bacteria through the esophagus, stomach, small intestine, and colon in the proper order. However, this process involves the serious loss in bacterial activity due to the strong acidic environment in the stomach (pH = 2) and bile salts in the intestine (121, 122). This limitation might thus restrict the recognized roles of intestinal bacteria in promoting immunotherapy. Therefore, encapsulating intestinal bacteria with suitable materials conferring them protection and allowing their targeted release in the colon would be an effective method to address this issue.
Due to the strong acidic environment in the stomach, the materials used for bacterial encapsulation should be acid-resistant to maintain the integrity of the microcapsules during their travel to the colon. In addition, these materials should be biocompatible and automatically degraded in the colon to ensure host safety. For these purposes, various biomaterials, including alginate (123), enteric polymer (124), chitosan (125), and pectin (126), have been designed for the encapsulation and effective delivery of intestinal bacteria (122). Table 2 summarizes these biomaterials.
Bifidobacterium is the most abundant bacterial genus in the intestines of breastfed infants (148) and is one of the main components of the gut microbiome associated with the maintenance of human health. The abundance and diversity of Bifidobacterium species, including B. longum, B. breve, and B. adolescentis, change throughout life. In the human intestine, Bifidobacterium levels decrease with age. In the body of infants and children less than 3 years old, the primary Bifidobacterial species is B. breve; in breastfed infants and young adults, the proportion of B. adolescentis gradually increases and becomes the main species; in the elderly, B. longum becomes the representative species (149, 150). In addition, these Bifidobacterium subpopulations exhibit specific health-promoting functions. For example, both B. infantis and B. breve in the intestine of infants prolonged the immune memory of vaccines (151). B. longum in adult intestines promoted immunotherapy response to tumors (24). Because of their excellent health promoting effects and wide applicability, many studies on the microencapsulation of Bifidobacterium have been conducted.
Yeung et al. designed a core–shell microgel consisting of an alginate core and a chitosan shell to encapsulate B. longum (125). Under aerobic storage and simulated gastric fluid (SGF), the viability and resistance of encapsulated B. longum were significantly improved. In 2015, Varankovich et al. developed protein–polysaccharide capsules for B. adolescentis (145). Compared with free bacteria, the established capsules provided significant protection to bacterial cells at 37°C in SGF. Moreover, when alginate or iota-carrageenan was used as polysaccharide, the capsules were easily dissolved, releasing 70–79% of bacterial cells into the simulated intestinal fluid (SIF) within 3 h. Moreover, when the capsules were freeze-dried, the number of live bacterial cells released was increased. Another study employed an alginate matrix for B. breve followed by alternate encapsulation using alginate and chitosan to coat bacteria using layer-by-layer (LbL) method (146). These multilayer-coated alginate matrices enhanced the viability of Bifidobacterium breve in a low-pH environment and delivered bacterial cells into the intestine, wherein their load was gradually released. Moreover, D’Orazio et al. developed chitosan-coated alginate microcapsules for B. animalis subsp. lactis PBS075, L. rhamnosus PBS070, and L. plantarum PBS067 (147). They found that in SGF and other adverse conditions, the encapsulated probiotics showed significantly higher resistance.
L. rhamnosus has been extensively studied since the 1980s. This species has a certain tolerance to gastric acid and bile. By adhering to intestinal cells, it can colonize the human body and has been shown to exert a variety of effects, such as reducing cholesterol levels, inhibiting α-glucosidase activity, and antioxidant and anti-inflammatory effects (152). LGG, a strain of L. rhamnosus, can reduce the expression of some inflammation markers and increase the levels of tumor necrosis factor-α, IL-10, and IL-12 in the macrophages to promote a type 1 immune response (153).
Its encapsulation and delivery improved immune regulation. Huq and coworkers developed alginate-cellulose nanocrystals (CNC)-lecithin microbeads for Lactobacillus rhamnosus ATCC 9595 (135) and found that CNC-lecithin microbeads had higher compression strength than alginate microbeads alone. Additionally, lecithin reduced the damage to bacterial membranes caused by bile salts in the intestine, thereby protecting probiotics. Another study prepared core-shell alginate–silica microcapsules for LGG (154). After the ionogels were formed using LGG and alginate, a silica coat was applied via a mild reaction process. In addition to enhancing the viability of the encapsulated LGG, the mesopores in the silica shell prevented bacterial leakage and allowed the diffusion of nutrient metabolites, thus ensuring bacterial growth within the microcapsules.
Because of considerably low pH levels in the gastrointestinal tract, the development of pH-responsive carriers for the protection and controlled release of bacteria in the stomach and intestine is necessary. Based on this view, an ethylenediaminetetraacetic–calcium–alginate (EDTA–Ca–Alg) system for L. rhamnosus ATCC 53103 was prepared using emulsification (137). In the acidic environment of the stomach, the structure of hydrogels remained intact and provided protection for the encapsulated bacteria. However, in neutral pH, as the EDTA completely chelates Ca2+, Ca2+ was released from the hydrogel structure, leading to its gradual disintegration, which was kept in a soluble state, thus allowing the release of bacteria.
Escherichia coli is a gram-negative facultative anaerobe and is one of the most characterized model organisms (155). E. coli is not only an extensive intestinal symbiont of vertebrates, but also a multifunctional pathogen. Therefore, using the excellent symbiotic characteristics of E. coli and technology, such as genetic engineering or plasmid transfection, E. coli can serve as a vector to carry target gene for the stable and efficient expression of specific metabolites, such as monoclonal antibodies (156) and enzymes (118), in vivo.
Chowdhuri et al. encapsulated E. coli using the inverse-emulsion technique to generate unilamellar vesicles (GUV) and verified the protective effect of liposomes on bacterial viability and activity (138). They demonstrated that E. coli encapsulated in liposomes could be protected from degradation by proteases in the stomach by prolonging their dissolution under acidic conditions. In addition, alginate–chitosan–alginate (ACA) microcapsules, which showed strong resistance against enzymatic digestion, were developed for the oral delivery of live bacterial cells for therapy (139). In this study, E. coli DH5 were encapsulated in ACA microcapsules and exhibited normal survival and growth during its passage through the stomach and intestine. This is due to the stability of ACA microcapsules in SGF.
Lactobacillus plantarum is a lactic acid bacteria with numerous strains, such as L. plantarum 80, L. plantarum NCIMB 1193, and L. plantarum Hu (157). L. plantarum can secrete SCFAs, especially butyric acid and acetic acid, and strengthen the intestinal barrier by enhancing epithelial defense (158). In addition, due to its ability to produce various effective bacteriocins (antimicrobial peptides), L. plantarum has been extensively used as a food preservative and antibiotic supplement (159).
Li and co-workers used cellulose microgels (CMs) to encapsulate L. plantarum (140). The CMs with their porous structure were shown to have an improved ability of carrying bacteria. Conjugation with alginate contributed to better resistance to acidic conditions and enhancement of the survival of bacteria. In 2017, Dafe et al. synthesized pectin-starch hydrogels to encapsulate L. plantarum ATCC:13643 cells using the extrusion method (126). By mixing different concentrations of pectin (2, 1.5, 1, and 0.5% wt) and starch (0, 0.5, 1, and 1.5% wt), hydrogels were divided into 4 groups, named Pectin, Pectin/starch1, Pectin/starch2, and Pectin/starch3, respectively. Compared with unencapsulated bacterial cells, those encapsulated in all ratios of pectin–starch hydrogels were found to be highly resistant to SGF solutions and showed higher survival rate. Chen et al. prepared alginate–poly-L-lysine–alginate (APA) microcapsules for L. plantarum 80 (LP80) (160). However, APA microcapsules only provided short protection in SGF. Upon exposure to an acidic environment (pH = 2) for 5 min, 80.0% of the embedded bacterial cells remained viable. However, after 15 min and 1 h, bacterial viability significantly decreased to 8.3% and 0.2%, respectively. These results represent the limitations of APA microcapsules for the oral delivery of live bacteria. To address these, Nualkaekul et al. designed alginate beads coated with chitosan for L. plantarum. Their approach enhanced bacterial resistance to both SGF and highly acidic pomegranate juice and increased bacterial survival (142). Furthermore, the multilayer chitosan coating of alginate beads improved their protective function, which increased with the number of coatings.
To prepare sodium alginate/cellulose nanofiber gel macrospheres (ACMs), Zhang et al. extruded a mixture of TEMPO-oxidized cellulose nanofibers (CNF) and sodium alginate (SA) within a CaCl2 solution (Figure 6A) (161). To further study the influence of the ratio of SA and CNF on the macrospheres, microspheres were prepared with the ratios of SA and CNF as 1:0, 3:1, 1:1, 1:3, and 0:1, and named them ACM-1, ACM-2, ACM-3, ACM-4, and ACM-5, respectively. After encapsulating the probiotics, L. plantarum, ACMs were placed in SGF to simulate the acidic environment in the stomach. After 2 h in SGF, these macrospheres were found to shrink and provide protection for L. plantarum, which might be because of the decrease of electrostatic repulsion causing by the protonation of carboxylic chains in CNF and SA (Figure 6B). When placed in SIF, via deprotonation of carboxylic chains and eliminating intermolecular hydrogen bonds, the ACMs swelled and finally ruptured to release L. plantarum, which targeted delivery of probiotics in the intestine (Figure 6B). Moreover, among the five different proportions of ACMs, with the proportion of increased CNF, the shrinkage of ACMs decreased in SGF and their rupture delayed in SIF, which demonstrated that CNF improved the stability of ACMs and SA was used for supplying pH-responsive function. Figure 6C shows the SEM images of ACM1 to ACM5 after freeze-drying.
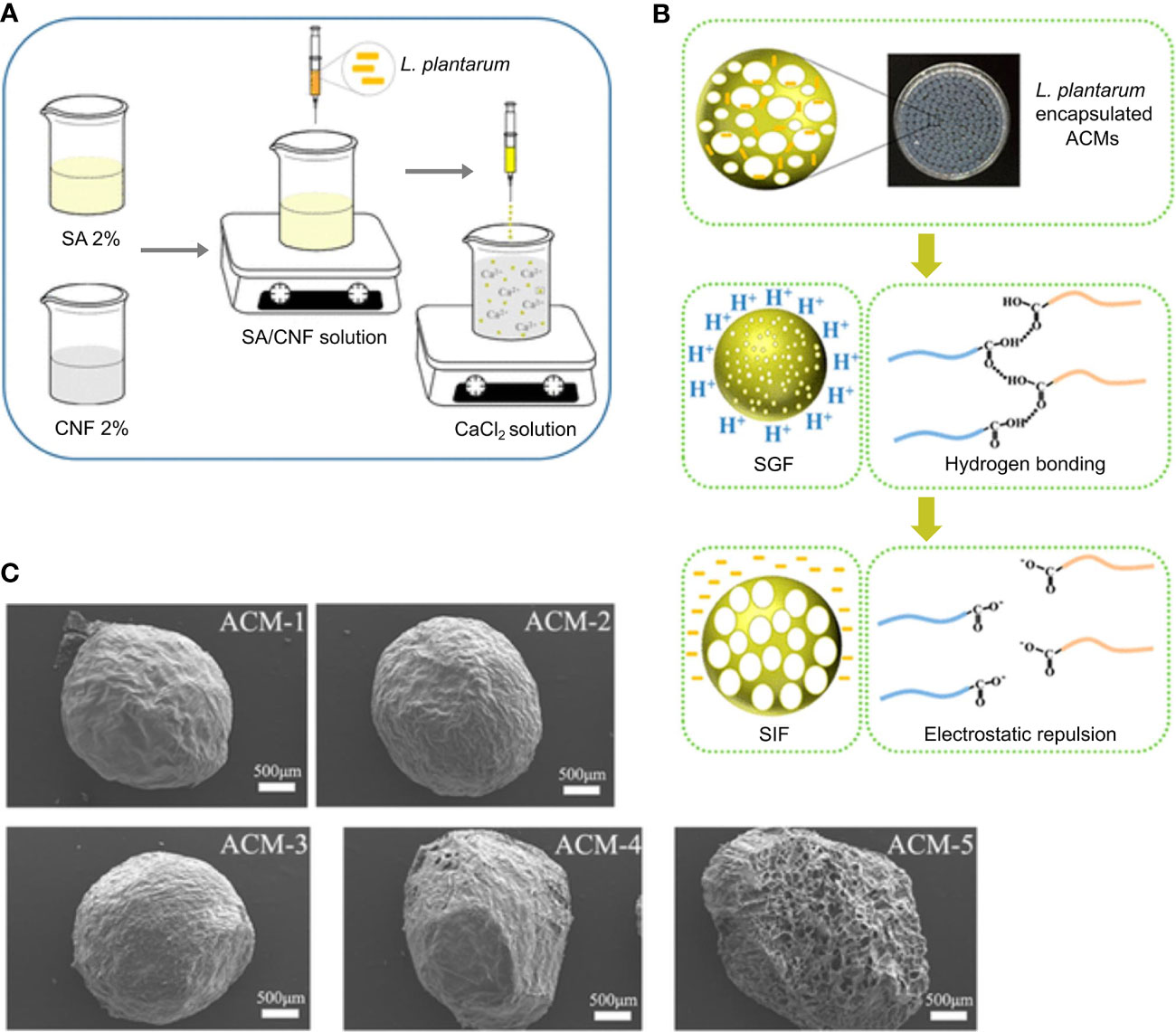
Figure 6 PH-responsive ACMs designed for delivery and release of L. plantarum. Overview of (A) the process of ACMs embeding L. plantarum and (B) ACMs designed for protecting L. plantarum against SGF and releasing in SIF. (C) SEM images of the freeze-dried ACMs. Adapted from ref. (161).
In addition to these synthetic materials, some natural substances have also exhibited good loading capacity of bacteria. For instance, naturally occurring cavities and channels can be found in corn starch. After being expanded by enzymatic hydrolysis, natural corn starch can accommodate probiotics. Based on this property, Li and co-workers used fungal α-amylase (FA), pancreatin (P), and pancreatic α-amylase (PA) to partially hydrolyze corn starch and encapsulate L. plantarum 299v (141). Furthermore in using different enzymes, corn starch was treated with each enzyme for different times (30 and 120 min), with the materials after treatment being referred to as FA30, FA120, P30, P120, PA30, and PA120, respectively. These differently treated bacteria-containing corn starch samples showed a better acidic resistance, bile salt resistance, and survival rate, as well as exhibited an elevated delivering efficiency compared with free bacteria.
Lactobacillus casei, a gram-positive stain, is one of the most widely studied strains applied as fermentation starter in cultures (162) and probiotics (163). Due to its health-promoting properties, L. casei has been extensively researched. It was reported to effectively lower blood pressure and cholesterol, enhance human immunity, and inhibit or even prevent tumor growth (164, 165).
To efficiently deliver L. casei, Li et al. designed a novel intestinal targeting carrier for the anti-acid protection of L. casei and its controlled releasing in the gastrointestinal tract (Figure 7A) (127). First, calcium alginate (CA) beads were manufactured using a coextrusion mini-fluidic method of combining pure Ca-alginate solution and Na-alginate solution containing L. casei. Then, by employing an adsorption method, the prepared CA beads were adsorbed by protamine molecules to form calcium alginate/protamine (CAP) beads. In addition, sodium caseinate (SC) and lately developed SGF-resistant fat SC (FSC) capsules were also reported to significantly increase the survival of L. casei when passing through the upper gastrointestinal tract (129). Although in vivo experiments revealed that both SC and FSC capsules were eventually digested in the stomach 3 or 24 h after oral delivery, a high buffer capacity and good emulsification properties were still showed, making them a very suitable encapsulating materials with good research potential. Moreover, de Barro and co-workers produced dried live probiotic spheres (DLPS) of L. casei and mixed them with microcrystalline cellulose (MCC), calcium cross-linked alginate, and lactose followed by granulation (128). Subsequently, a Eudragit coating was added using an extrusion method to protect bacteria from acid and maintain the integrity of the entire structure in the acidic environment of the stomach. When reaching the intestine, increase in the pH led to the change of the Eudragit coating from hydrophobic to hydrophilic and the concomitant release of the bacterial cells.
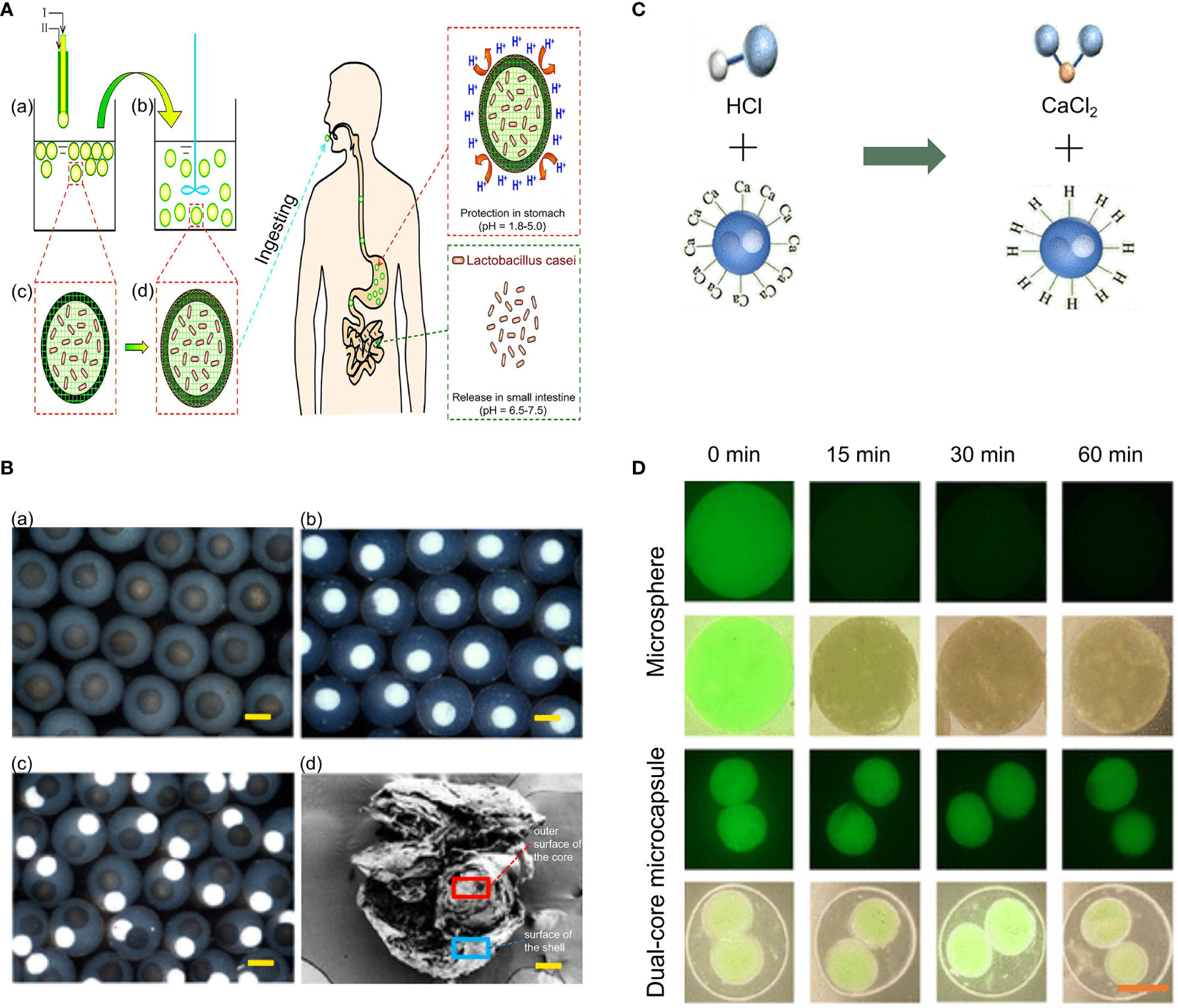
Figure 7 CAP beads and dual-core microcapsules prepared for encapsulating Lactobacillus casei, Bacillus subtilis, and Lactobacillus (NO. 21790). (A) Schematic illustration of the preparation process and the design concept of the proposed intestinal-targeted CAP carrier for the pH-responsive protection and release of L. casei. (a, c) CA beads prepared by a coextrusion method. “A” is Na-alginate solution containing L. casei, whereas “B” is pure Na-alginate solution. (b, d) CAP beads prepared by adsorption of protamine molecules. (e) Ingestion of CAP beads. (f) CAP beads offer improved protection to Lactobacillus in the stomach. (g) CAP beads rapidly release L. casei in the small intestine. (B) Characterization of microcapsules. optical microscope images of (a) the Lactobacillus microcapsules, (b) the Bacillus Subtilis microcapsules, and (c) dual-core microcapsules. (d) SEM images of dual-core microcapsules. Scale bars are 100 μm. (C) Alginate neutralizes HCL through metathesis reaction. (D) Detection of the activity of bacteria embeded in microspheres and dual-core microcapsules in SGF via fluorescent staining. The scale bar is 100 μm. Adapted from ref. (127, 166).
In addition to the intestinal bacteria mentioned above, there are also many other bacteria in the intestine that are known to have the function of promoting the health of the human body. To make the oral delivery of these intestinal bacteria more efficient, many suitable biomaterial schemes have been designed for their encapsulation.
Based on the yin–yang concept, microcapsules with independent internal compartments, which can encapsulate and deliver a variety of substances, such as drugs and microbes, to promote their functions without interference, have been designed (167, 168). Zhao and coworkers designed a dual-core microcapsule to encapsulate and deliver B. subtilis and Lactobacillus (NO. 21790) in separated microcompartments (166). First, each intestinal bacterium was mixed in a solution containing CNC, carboxymethyl cellulose (CMC), and sodium alginate. Then, it was electrosprayed to prepare the microspheres (containing only one kind of intestinal bacteria). In this technique, CMC had the function of neutralizing HCl to protect bacteria in an acidic environment, while CNC could restrict these bacteria to a certain range via depletion flocculation. Aluminum chloride (AlCl3) was the chosen crosslinking agent as it caused the microspheres that encapsulated the intestinal bacteria to form quickly. Subsequently, the outer phase fluid could be wrapped around the inner core by hydrodynamic focusing, and the alginate could be quickly solidified after the solution containing the dual cores was placed in the presence of electric field in a gel bath with 2% CaCl2, thereby forming the dual-core microcapsules. Figure 7B shows the optical microscope and SEM images of dual-core microcapsules. To further study the protective effect of dual-core microcapsules on bacteria in an acidic environment, dual-core microcapsules and microspheres without alginate shells were placed in simulated gastric juice (SGF) for 60 min to observe the bacterial activity. Theoretically, the dual-core microcapsules could provide protection to the encapsulated bacteria in an acidic environment because of the sodium alginate shell, which could neutralize HCL through a metathesis reaction (Figure 7C). As shown in Figure 7D, compared with dual-core microcapsules, intestinal bacteria encapsulated in microspheres without alginate shells lost most of their activity in the first 15 min. However even after 60 min, more than 70% of the probiotics encapsulated in dual-core microcapsules retained their activity in SGF.
Anselmo et al. combined chitosan (CHI) with alginate (ALG) as one CHI/ALG bilayer and then encapsulated intestinal bacteria using LbL method (Figure 8A) (143). In their study, Bacillus coagulans was used as a model strain. Several different LbL formulations and numbers of layer were designed to research their protecting function. An enteric polymer, L 100, was chosen to encapsulate probiotics in combination with chitosan. Compared with B. coagulans encapsulated using chitosan only, L100 combined with chitosan (denoted as CHI/L100) was able to protect the probiotic in SGF but failed against bile (Figure 8B). B. coagulans encapsulated by 2 CHI/ALG bilayers, expressed as (CHI/ALG)2, was reported to be well protected against both SGF and bile (Figure 8B). Figure 8C shows that bacteria encapsulated by (CHI/ALG)2 presented higher adhesion to the intestine in slices of freshly isolated small intestine. Additionally, the (CHI/ALG)2 coating was shown to improve the survival of probiotics in the intestine. Ouyang and co-workers improved the APA microcapsules and designed multilayer alginate/poly-L-lysine/pectin/poly-L-lysine/alginate (APPPA) microcapsules to encapsulate L. reuteri cells (144). Stability of APPPA microcapsules were tested as well as the activity of encapsulated bacterial cells at 37.2°C under various pH conditions. When microcapsules were placed into SGF and SIF, respectively, for a total of 24 h (12 h in SGF and 12 h in SIF) at 250 rpm mechanical shaking at 37.2°C, no obvious damage to bacterial cells was reported. After 24 h in GSF and SIF, respectively, more than 90% of APPPA microcapsules remained intact, exhibiting a good resistance to mechanical shocks. Compared with APA microcapsules, APPPA microcapsules showed an excellent stability. The reason behind this was the use of poly (amino acid), which is a material that has been widely used for encapsulating bacteria. It was therefore assumed that using more poly-L-lysine to encapsulate bacteria would have a better protection and delivery effect. Besides, when designing biomaterial for encapsulating bacteria, the number of layers is also considered to be an important factor affecting delivery efficiency. Accordingly, the effect of this protection was proportional to the number of layers in a certain range, rather than with the increasing in the number of layers (146).
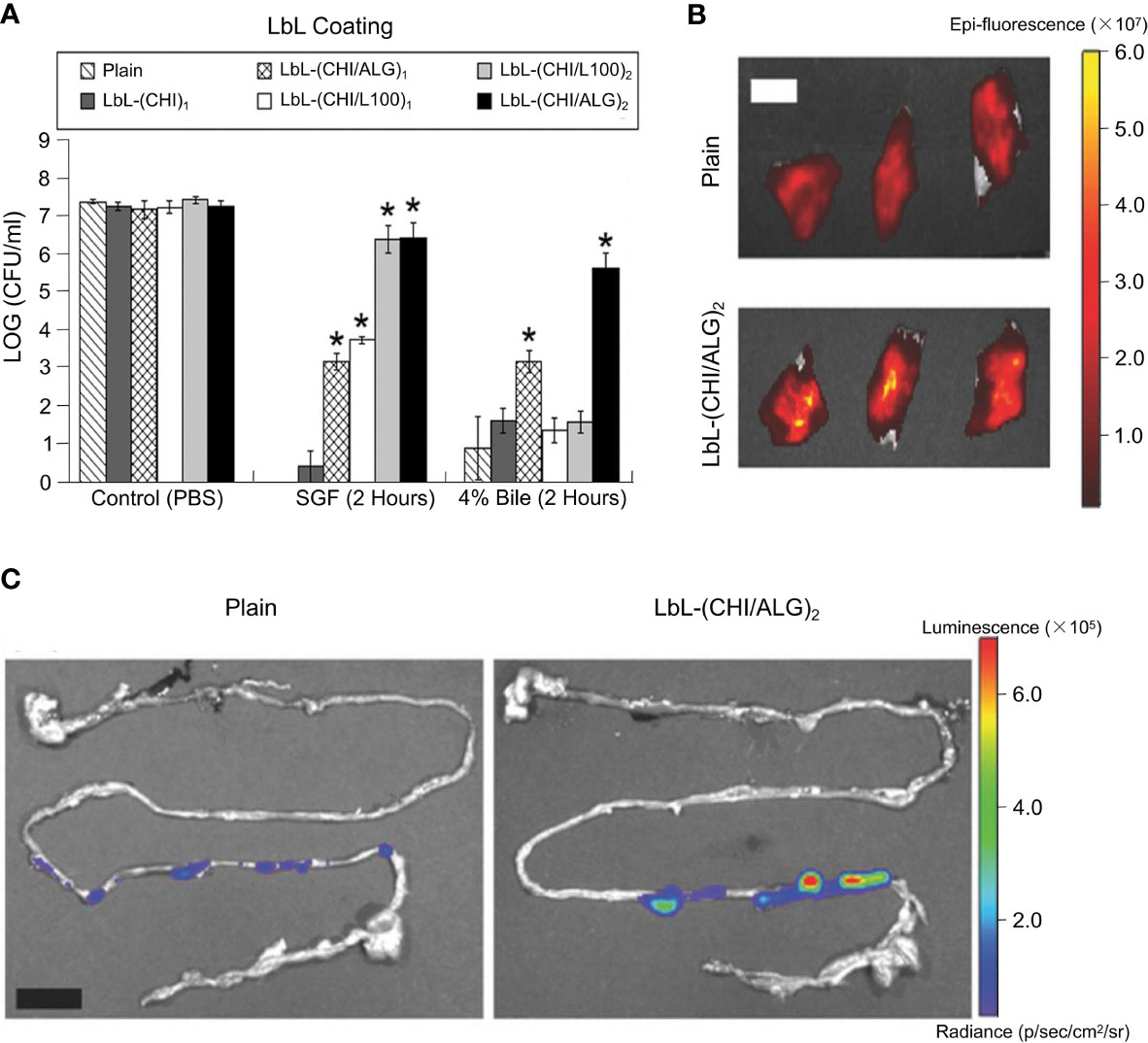
Figure 8 Layer-by-layer encapsulation of probiotics employed to enhance their survival rate against acidic and bile conditions, and their physical retention in the intestine. (A) Schematic LbL encapsulation of chitosan and alginate on probiotic. (B) LbL formulated (CHI/ALG)2 (black bars) BC were protected against both acidic and bile salt conditions at 37°C for up to 2 h. LbL coatings of chitosan (dark gray bars), (CHI/L100)1 (white bars), (CHI/L100)2 (light gray bars), and (CHI/ALG)1 (cross-hatched bars) were less effective at protecting BC against both acidic and bile conditions. Error bars represent standard deviation (n = 3). *denotes statistical difference (P < 0.05) using Student’s t-test between plain and LbL groups. **denotes statistical difference (P < 0.05) using individual Student’s t-test between the designated and any other group. (C) IVIS images of porcine intestine with plain- and (CHI/ALG)2-probiotics. Adapted from ref. (143).
Many intestinal bacteria can regulate the immune function and promote host health mainly via their DNA (169). Therefore, the encapsulation and delivery of bacterial DNA has great potential. As a positively charged natural polymer, chitosan can entrap nucleic acids (NA) (both RNA and DNA) and protect them from degradation by nuclease (170). However, chitosan is rarely used as NA carrier because of its poor water solubility and low transfection efficiency. To overcome these limitations, various modifications and formulations have been proposed (171, 172). For instance, Zhang et al. developed the PEGylation of chitosan nanoparticles for the delivery of DNA. These chitosan–DNA–PEG complexes increased the dose of DNA delivered and have been indicated to exhibit a liver tumor targeting (173). In another study, chitosan salts, such as chitosan glutamate, chitosan aspartate, chitosan acetate, chitosan lactate, and chitosan hydrochloride, were used to form chitosan–DNA complexes (174). Compared with standard chitosan, these complexes showed better transfection efficiency and lower cytotoxicity.
The regulation of intestinal bacteria to enrich target probiotics might be an effective strategy to improve the immunotherapeutic response. However, the best way to achieve this goal remains to be determined. When intestinal bacteria are transplanted as a whole (such as in FMT), thousands of bacterial species are simultaneously introduced. As such, this approach is not conducive to the in-depth study of the functions of each bacterial species. In addition, although the combination of intestinal bacteria in FMT has been shown to be of vital importance in this treatment, FMT can affect the abundance of main bacteria, while other bacteria do not exhibit any effect or even reduce the function of the main probiotic. Even if we could isolate the main bacteria, the immunomodulatory effects of the same strain would not appear to be completely consistent in different diseases and different studies. As we mentioned earlier, Lactobacilli populations showed diametrically opposite regulatory functions for Tregs in different studies. Therefore, we considered that the regulation of immunotherapy by intestinal bacteria could be affected by other factors.
Early in 2006, Hara et al. considered intestinal bacteria as a whole and proposed them as a potential organ of the human body (175). When artificially enriching or eliminating the number of intestinal bacterial species, this is generally accompanied by the reestablishment of a new multibacterial environment. This significant change in the composition of intestinal bacteria might explain the significant differences observed in the role of certain bacteria and might explain the positive effects of regulating intestinal bacteria on the efficacy of disease immunotherapy. In addition, intestinal bacteria are also known to be interdependent with the immune cells that they regulate. For example, Bifidobacterium was reported to improve the effect of PD-L1 inhibitor in antitumor treatment by regulating cytotoxic T-cells (15). However, Bifidobacterium could not exhibit this immunomodulatory effect in cytotoxic T-cell-depleted mice, suggesting that its effect was depended on the activity of cytotoxic T-cells. More specifically, it was assumed that intestinal bacteria serve more as aggregators or amplifiers of immune cells, which can in turn enhance the efficacy of immunotherapy. However, this regulatory function was demonstrated to rely on the adequacy of immune cells and the integrity of the immune system. Besides their interaction with immune cells, intestinal bacteria have also been shown to avoid or alleviate pathological conditions in various diseases, such as nonspecific inflammation. For example, Foxp3+ Tregs were found to be widely involved in the immunotherapy of various diseases. This was attributed to the role of Foxp3+ Tregs in affecting immune responses and suppressing the progress of inflammation. Based on this feature, several probiotics and the PSA of B. fragilis have been used to inhibit the progression of inflammation through the upregulation of Foxp3+ Tregs and to promote the immunotherapy of IBD (70) or the HIV-1 infection (67). Therefore, focusing on these common beneficial effects of intestinal bacteria and trying to combine them in treatment applications against various diseases or clinical problems might greatly expand the application prospects of intestinal bacteria.
The regulation of immune cells by intestinal bacteria is known to be accompanied by other substances in the microenvironment, cytokines, which can recruit and activate different kinds of immune cells. Many of the studies mentioned above have shown that the levels of interleukins change following the administration of intestinal bacteria. Independent of being upregulated or downregulated, the correlation between intestinal bacteria and interleukins has revealed the mechanism of the immune regulation by intestinal bacterial to a certain extent, and has also shown the feasibility of the combined use of these 2 factors for the immunotherapy of various diseases. Apart from interleukins, the levels of chemokines (75) and interferons (15) in the body have also been shown to be affected by intestinal bacteria. In particular, upregulation of IFN-γ by intestinal bacteria (such as Bifidobacterium) has also revealed to a certain extent the reason behind the ability of intestinal bacteria to promote the effect of ICB therapy in tumor immunotherapy. Recent studies have found that IFN-γ could upregulate the production of PD-L1 and establish the IFN-γ/PD-L1 axis based on the relationship between them (176–178). Furthermore, IFN-γ was found to exhibit the same effect on the expression of CTLA-4 (179, 180). Respectively, administration of intestinal bacteria to increase the expression of PD-L1 and CTLA-4, would undoubtedly greatly improve the effect of anti-CTLA-4 and anti-PD-1/PD-L1 therapies. Therefore, selecting the appropriate cytokines to synergize with intestinal bacteria could greatly enhance the effect of immunotherapy and the regulatory function of intestinal bacteria. Not only limited to cytokines, this synergy with intestinal bacteria established by immune cells could lead to more research ideas.
Regarding the intestinal bacteria used to regulate immunotherapy, there are also some problems that need in-depth study. One concern is the duration of their regulation of the immune system. As we know, intestinal bacteria are specific to people living in different areas or enjoying different diet habits, similar to our physical characteristics, and this specificity is known to determine the sensitivity of everybody to diseases and immunotherapy. As mentioned earlier, because of the differences in the composition of intestinal bacteria, patients who suffered from melanoma and were treated with PD-1 inhibitor were divided into responders (R) and non-responders (NR). This phenomenon implied that as long as the composition of intestinal bacteria remains stable in the body, their regulatory function might be long-term. Based on this view, intestinal bacteria could not only be used to regulate immunotherapy but might also be a major factor to prevent the diseases. Unlike conventional vaccines or vaccines based on genetically engineered bacteria, this kind of disease prevention via the direct use of intestinal bacteria might exhibit better compliance and longer-term preventive effects. Moreover, with deeper studies on their ability for immune regulation, it might be entirely possible to select the most suitable multi-bacterial environment and reconstruct it in unaffected or affected hosts by introducing various probiotics in order to achieve the prevention of many diseases and the maintenance of health status.
However, we would also need to consider how this multi-bacterial commensalism can be established and made stable in the body. First of all, in order to improve compliance and avoid infections caused by bacteria entering the blood, oral delivery of intestinal bacteria should be routinely selected. Second, in order to ensure the vitality of extraneous intestinal bacteria, these should be delivered in the form of microcapsules, that is, bacteria should be encapsulated into microspheres. Therefore, related encapsulation materials and technologies have to be developed to ensure the protection of bacteria from the acidic environment in the stomach and their complete release in the intestine. Furthermore, following the introduction of encapsulated bacteria in the body and the establishment of a new multi-bacterial environment, it would be necessary to maintain this multi-bacterial environment in the long term. As we all know, diet is an important factor in the composition of the intestinal bacteria. Therefore, providing an appropriate diet is an important method to this end. However, its precondition is the knowledge of the relationship between diet and intestinal bacteria. Hence, immunotherapy for many diseases, including tumors, is expected to open a new chapter through the dietary regulation of intestinal bacteria to achieve the prevention of diseases, promotion of immunotherapy during diseases, and maintenance of the curative effect after the resolution of diseases.
In this review, we have outlined the potential use of intestinal bacteria for the regulation of immunotherapy of diseases and the useful biomaterials employed for encapsulating these bacteria. Future studies should focus on developing efficient encapsulation and delivery methods and accurate targeting ability and establishing stable multi-bacterial environments to expand the clinical applicability of intestinal bacteria in disease treatment. In addition, dietary factors and genetic engineering can provide more methods and possibilities for their clinical application. We believe that with the continuous in-depth studies of intestinal bacteria and exploration of their synergistic strategies, better, safer, and more effective intestinal bacterial therapies will be used in clinical practice with more people benefiting from them.
All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XW declared a shared affiliation with several of the authors YL, ZL, YW, LL, XJ, XF, to the handling editor at time of review.
1. Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PloS Biol (2016) 14(8):e1002533. doi: 10.1371/journal.pbio.1002533
2. West NR, Powrie F. Immunotherapy Not Working? Check Your Microbiota. Cancer Cell (2015) 28(6):687–9. doi: 10.1016/j.ccell.2015.11.010
3. Hold GL. Gastrointestinal Microbiota and Colon Cancer. Dig Dis (2016) 34(3):244–50. doi: 10.1159/000443358
4. Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe (2014) 15(3):374–81. doi: 10.1016/j.chom.2014.02.006
5. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med (2014) 20(2):159–66. doi: 10.1038/nm.3444
6. De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M. Nutritional Keys for Intestinal Barrier Modulation. Front Immunol (2015) 6:612. doi: 10.3389/fimmu.2015.00612
7. Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA (2008) 105(52):20858–63. doi: 10.1073/pnas.0808723105
8. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol (2014) 14(3):141–53. doi: 10.1038/nri3608
9. Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer (2017) 17(5):271–85. doi: 10.1038/nrc.2017.13
10. Dzutsev A, Goldszmid RS, Viaud S, Zitvogel L, Trinchieri G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol (2015) 45(1):17–31. doi: 10.1002/eji.201444972
11. Pitt JM, Vetizou M, Waldschmitt N, Kroemer G, Chamaillard M, Boneca IG, et al. Fine-Tuning Cancer Immunotherapy: Optimizing the Gut Microbiome. Cancer Res (2016) 76(16):4602–7. doi: 10.1158/0008-5472.CAN-16-0448
12. Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol (2011) 23(4):473–80. doi: 10.1016/j.coi.2011.07.010
13. Vieira SM, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science (2018) 359(6380):1156–60. doi: 10.1126/science.aar7201
14. Jobin C. Precision medicine using microbiota. Science (2018) 359(6371):32–4. doi: 10.1126/science.aar2946
15. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (2015) 350(6264):1084–9. doi: 10.1126/science.aac4255
16. Li J, Sung CYJ, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci (2016) 113(9):E1306–15. doi: 10.1073/pnas.1518189113
17. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature (2011) 472(7341):57–U82. doi: 10.1038/nature09922
18. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA (2011) 108(38):16050–5. doi: 10.1073/pnas.1102999108
19. Zhan Y, Chen PJ, Sadler WD, Wang F, Poe S, Nunez G, et al. Gut microbiota protects against gastrointestinal tumorigenesis caused by epithelial injury. Cancer Res (2013) 73(24):7199–210. doi: 10.1158/0008-5472.CAN-13-0827
20. Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol (2013) 14(7):646–53. doi: 10.1038/ni.2604
21. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature (2013) 504(7480):451–5. doi: 10.1038/nature12726
22. Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol (2004) 22(3):313–20. doi: 10.1038/nbt937
23. Chua KJ, Kwok WC, Aggarwal N, Sun T, Chang MW. Designer probiotics for the prevention and treatment of human diseases. Curr Opin Chem Biol (2017) 40:8–16. doi: 10.1016/j.cbpa.2017.04.011
24. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science (2018) 359(6371):104–+. doi: 10.1126/science.aao3290
25. Routy B, Gopalakrishnan V, Daillere R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol (2018) 15(6):382–96. doi: 10.1038/s41571-018-0006-2
26. Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut (2012) 61(6):829–38. doi: 10.1136/gutjnl-2011-300367
27. Shlyakhovenko VA, Olishevsky SV, Kozak VV, Yanish YV, Rybalko SL. Anticancer and immunostimulatory effects of nucleoprotein fraction of Bacillus subtilis 7025 culture medium filtrate. Exp Oncol (2003) 25(2):119–23.
28. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science (2011) 332(6032):974–7. doi: 10.1126/science.1206095
29. Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO, dominant A. coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci USA (2009) 106(46):19256–61. doi: 10.1073/pnas.0812681106
30. Robert S, Gysemans C, Takiishi T, Korf H, Spagnuolo I, Sebastiani G, et al. Oral Delivery of Glutamic Acid Decarboxylase (GAD)-65 and IL10 by Lactococcus lactis Reverses Diabetes in Recent-Onset NOD Mice. Diabetes (2014) 63(8):2876–87. doi: 10.2337/db13-1236
31. Grierson P, Lim KH, Amin M. Immunotherapy in gastrointestinal cancers. J Gastrointest Oncol (2017) 8(3):474–84. doi: 10.21037/jgo.2017.05.01
32. Wei P, Yang Y, Liu Z, Huang J, Gong Y, Sun H. Oral Bifidobacterium longum expressing alpha-melanocyte-stimulating hormone to fight experimental colitis. Drug Delivery (2016) 23(6):2058–64. doi: 10.3109/10717544.2015.1122672
33. Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol (2011) 127(5):1087–94. doi: 10.1016/j.jaci.2011.02.015
34. Blumberg R, Powrie F. Microbiota, disease, and back to health: a metastable journey. Sci Transl Med (2012) 4(137):137rv7. doi: 10.1126/scitranslmed.3004184
35. Lucas C, Barnich N, Hang Thi Thu N. Microbiota, Inflammation and Colorectal Cancer. Int J Mol Sci (2017) 18(6):1310. doi: 10.3390/ijms18061310
36. Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell (2018) 33(4):570–80. doi: 10.1016/j.ccell.2018.03.015
37. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell (2014) 157(1):121–41. doi: 10.1016/j.cell.2014.03.011
38. Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and Anticancer Immunosurveillance. Cell (2016) 165(2):276–87. doi: 10.1016/j.cell.2016.03.001
39. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature (2011) 480(7378):480–9. doi: 10.1038/nature10673
40. Araya RE, Goldszmid RS. Two Bugs a NOD Away from Improving Cancer Therapy Efficacy. Immunity (2016) 45(4):714–6. doi: 10.1016/j.immuni.2016.10.007
41. York A. Microbiome: Gut microbiota sways response to cancer immunotherapy. Nat Rev Microbiol (2018) 16(3):121. doi: 10.1038/nrmicro.2018.12
42. Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PloS One (2011) 6(10):e25792. doi: 10.1371/journal.pone.0025792
43. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA (2010) 107(27):12204–9. doi: 10.1073/pnas.0909122107
44. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (2015) 350(6264):1079–+. doi: 10.1126/science.aad1329
45. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (2018) 359(6371):91–+. doi: 10.1126/science.aan3706
46. Ansari F, Pourjafar H, Jodat V, Sahebi J, Ataei A. Effect of Eudragit S100 nanoparticles and alginate chitosan encapsulation on the viability of Lactobacillus acidophilus and Lactobacillus rhamnosus. AMB Express (2017) 7(1):144. doi: 10.1186/s13568-017-0442-x
47. Singh P, Medronho B, Valente AJM, Miguel MG, Lindman B. Exploring the prebiotic effect of cyclodextrins on probiotic bacteria entrapped in carboxymetyl cellulose-chitosan particles. Colloids Surf B Biointerf (2018) 168:156–62. doi: 10.1016/j.colsurfb.2017.12.014
48. Alkotaini B, Tinucci SL, Robertson SJ, Hasan K, Minteer SD, Grattieri M. Alginate-Encapsulated Bacteria for the Treatment of Hypersaline Solutions in Microbial Fuel Cells. Chembiochem (2018) 19(11):1162–69. doi: 10.1002/cbic.201800142
49. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet (2012) 13(4):260–70. doi: 10.1038/nrg3182
50. Prakash S, Jones ML. Artificial Cell Therapy: New Strategies for the Therapeutic Delivery of Live Bacteria. J BioMed Biotechnol (2005) 2005(1):44–56. doi: 10.1155/JBB.2005.44
51. Hanahan D, Coussens LM. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell (2012) 21(3):309–22. doi: 10.1016/j.ccr.2012.02.022
52. Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol (2016) 27(8):1482–92. doi: 10.1093/annonc/mdw168
53. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol (2013) 14(10):1014–22. doi: 10.1038/ni.2703
54. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity (2013) 39(4):782–95. doi: 10.1016/j.immuni.2013.10.003
55. Kroemer G, Zitvogel L. Cancer immunotherapy in 2017: The breakthrough of the microbiota. Nat Rev Immunol (2018) 18(2):87–8. doi: 10.1038/nri.2018.4
56. Roesch LF, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM, et al. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J (2009) 3(5):536–48. doi: 10.1038/ismej.2009.5
57. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol (2017) 28(6):1368–79. doi: 10.1093/annonc/mdx108
58. Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PloS One (2012) 7(6):e39743. doi: 10.1371/journal.pone.0039743
59. Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. Isme J (2012) 6(2):320–9. doi: 10.1038/ismej.2011.109
60. Wei H, Dong L, Wang T, Zhang M, Hua W, Zhang C, et al. Structural shifts of gut microbiota as surrogate endpoints for monitoring host health changes induced by carcinogen exposure. FEMS Microbiol Ecol (2010) 73(3):577–86. doi: 10.1111/j.1574-6941.2010.00924.x
61. Marchesi JR, Dutilh BE, Hall N, Peters WHM, Roelofs R, Boleij A, et al. Towards the Human Colorectal Cancer Microbiome. PloS One (2011) 6(5):e20447. doi: 10.1371/journal.pone.0020447
62. Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice—-65. Proc Natl Acad Sci USA (2011) 108(28):11548–53. doi: 10.1073/pnas.1108924108
63. Lau K, Benitez P, Ardissone A, Wilson TD, Collins EL, Lorca G, et al. 3rd, Inhibition of type 1 diabetes correlated to a Lactobacillus johnsonii N6.2-mediated Th17 bias. J Immunol (2011) 186(6):3538–46. doi: 10.4049/jimmunol.1001864
64. Cunningham-Rundles S, Ahrne S, Johann-Liang R, Abuav R, Dunn-Navarra AM, Grassey C, et al. Effect of probiotic bacteria on microbial host defense, growth, and immune function in human immunodeficiency virus type-1 infection. Nutrients (2011) 3(12):1042–70. doi: 10.3390/nu3121042
65. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun (2016) 7:10391. doi: 10.1038/ncomms10391
66. Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PloS One (2010) 5(2):e9009. doi: 10.1371/journal.pone.0009009
67. Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA (2010) 107(5):2159–64. doi: 10.1073/pnas.0904055107
68. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (2018) 359(6371):97–103. doi: 10.1126/science.aan4236
69. Lopez P, Gonzalez-Rodriguez I, Sanchez B, Gueimonde M, Margolles A, Suarez A. Treg-inducing membrane vesicles from Bifidobacterium bifidum LMG13195 as potential adjuvants in immunotherapy. Vaccine (2012) 30(5):825–9. doi: 10.1016/j.vaccine.2011.11.115
70. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature (2008) 453(7195):620–5. doi: 10.1038/nature07008
71. Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci (2015) 18(7):965–77. doi: 10.1038/nn.4030
72. Messina JP, Gilkeson GS, Pisetsky DS. STIMULATION OF INVITRO MURINE LYMPHOCYTE-PROLIFERATION BY BACTERIAL-DNA. J Immunol (1991) 147(6):1759–64.
73. Yi AK, Klinman DM, Martin TL, Matson S, Krieg AM. Rapid immune activation by CpG motifs in bacterial DNA - Systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J Immunol (1996) 157(12):5394–402.
74. Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol (1996) 157(5):1840–5.
75. Cremonesi E, Governa V, Garzon JFG, Mele V, Amicarella F, Muraro MG, et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut (2018) 67(11):1984–94. doi: 10.1136/gutjnl-2016-313498
76. Valladares R, Sankar D, Li N, Williams E, Lai KK, Abdelgeliel AS, et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PloS One (2010) 5(5):e10507. doi: 10.1371/journal.pone.0010507
77. Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, et al. Well-controlled proinflammatory cytokine responses of Peyer’s patch cells to probiotic Lactobacillus casei. Immunology (2010) 130(3):352–62. doi: 10.1111/j.1365-2567.2009.03204.x
78. Gostner JM, Geisler S, Stonig M, Mair L, Sperner-Unterweger B, Fuchs D. Tryptophan Metabolism and Related Pathways in Psychoneuroimmunology: The Impact of Nutrition and Lifestyle. Neuropsychobiology (2020) 79(1):89–99. doi: 10.1159/000496293
79. Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci (2012) 13(7):465–77. doi: 10.1038/nrn3257
80. Fallarino I, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ (2002) 9(10):1069–77. doi: 10.1038/sj.cdd.4401073
81. Raghavan S, Quiding-Jarbrink M. Regulatory T cells in gastrointestinal tumors. Expert Rev Gastroenterol Hepatol (2011) 5(4):489–501. doi: 10.1586/egh.11.44
82. Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon J II. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med (2014) 6(220):220ra11. doi: 10.1126/scitranslmed.3008051
83. Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol (2009) 10(7):689–95. doi: 10.1038/ni.1760
84. Kotler DP, Francisco A, Clayton F, Scholes JV, Orenstein JM. SMALL INTESTINAL INJURY AND PARASITIC DISEASES IN AIDS. Ann Internal Med (1990) 113(6):444–9. doi: 10.7326/0003-4819-113-6-444
85. Bengmark S, Jeppsson B. GASTROINTESTINAL SURFACE PROTECTION AND MUCOSA RECONDITIONING. J Parenteral Enteral Nutr (1995) 19(5):410–5. doi: 10.1177/0148607195019005410
86. Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4(+) Regulatory T Cells Control T(H)17 Responses in a Stat3-Dependent Manner. Science (2009) 326(5955):986–91. doi: 10.1126/science.1172702
87. Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of T(H)17 cells and enhances function of FoxP3(+) natural regulatory T cells. Proc Natl Acad Sci USA (2009) 106(31):12885–90. doi: 10.1073/pnas.0812530106
88. Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun (2018) 9:3249. doi: 10.1038/s41467-018-05470-4
89. Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity (2013) 39(2):372–85. doi: 10.1016/j.immuni.2013.08.003
90. Hendrikx T, Schnabl B. Indoles: metabolites produced by intestinal bacteria capable of controlling liver disease manifestation. J Internal Med (2019) 286(1):32–40. doi: 10.1111/joim.12892
91. Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med (2008) 14(3):275–81. doi: 10.1038/nm1710
92. Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, et al. Th22 Cells Are an Important Source of IL-22 for Host Protection against Enteropathogenic Bacteria. Immunity (2012) 37(6):1061–75. doi: 10.1016/j.immuni.2012.08.024
93. Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep (2012) 13(5):440–7. doi: 10.1038/embor.2012.32
94. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science (2011) 331(6015):337–41. doi: 10.1126/science.1198469
95. Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced Regulatory T cells Protect against an Allergic Airway Response in Mice. Am J Respir Crit Care Med (2009) 179(3):186–93. doi: 10.1164/rccm.200806-951OC
96. Pardoll D. Cancer and the Immune System: Basic Concepts and Targets for Intervention. Semin Oncol (2015) 42(4):523–38. doi: 10.1053/j.seminoncol.2015.05.003
97. Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol (2006) 24(15):2283–9. doi: 10.1200/JCO.2005.04.5716
98. Zitvogel L, Ma Y, Raoult D, Kroemer G, Gajewski TF. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science (2018) 359(6382):1366–70. doi: 10.1126/science.aar6918
99. Pitt JM, Vétizou M, Gomperts Boneca I, Lepage P, Chamaillard M, Zitvogel L. Enhancing the clinical coverage and anticancer efficacy of immune checkpoint blockade through manipulation of the gut microbiota. OncoImmunology (2016) 6(1):e1132137. doi: 10.1080/2162402X.2015.1132137
100. Perfetto SP, Chattopadhyay PK, Roederer M. Innovation - Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol (2004) 4(8):648–U5. doi: 10.1038/nri1416
101. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol (2002) 2(4):251–62. doi: 10.1038/nri778
102. Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression (vol 128, pg 805, 2018). J Clin Invest (2018) 128(4):1708–8. doi: 10.1172/JCI120803
103. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell (2009) 139(3):485–98. doi: 10.1016/j.cell.2009.09.033
104. Xie R, Jiang P, Lin L, Jiang J, Yu B, Rao J, et al. Oral treatment with Lactobacillus reuteri attenuates depressive-like behaviors and serotonin metabolism alterations induced by chronic social defeat stress. J Psychiatr Res (2020) 122:70–8. doi: 10.1016/j.jpsychires.2019.12.013
105. Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med (2007) 175(6):561–9. doi: 10.1164/rccm.200606-821OC
106. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science (2002) 297(5588):1867–70. doi: 10.1126/science.1073514
107. Liu K, Yang Y, Chen Y, Li S, Gong Y, Liang Y. The therapeutic effect of dendritic cells expressing indoleamine 2,3-dioxygenase (IDO) on an IgA nephropathy mouse model. Int Urol Nephrol (2020) 52(2):399–407. doi: 10.1007/s11255-019-02365-1
108. Cavaillon JM. CYTOKINES AND MACROPHAGES. Biomed Pharmacother (1994) 48(10):445–53. doi: 10.1016/0753-3322(94)90005-1
109. Bleuze M, Auger J-P, Lavagna A, Gisch N, Gottschalk M, Segura M. In vitro characterization of granulocyte-colony stimulating factor (G-CSF) production by dendritic cells and macrophages during Streptococcus suis infection. Immunobiology (2020) 225(4):151979. doi: 10.1016/j.imbio.2020.151979
110. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol (2007) 8(10):1086–94. doi: 10.1038/ni1511
111. Wenzel TJ, Gates EJ, Ranger AL, Klegeris A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol Cell Neurosci (2020) 105:103493. doi: 10.1016/j.mcn.2020.103493
112. Hengge UR, Benninghoff B, Ruzicka T, Goos M. Topical immunomodulators–progress towards treating inflammation, infection, and cancer. Lancet Infect Dis (2001) 1(3):189–98. doi: 10.1016/S1473-3099(01)00095-0
113. Shimada S, Yano O, Tokunaga T. INVIVO AUGMENTATION OF NATURAL-KILLER CELL-ACTIVITY WITH A DEOXYRIBONUCLEIC-ACID FRACTION OF BCG. Japanese J Cancer Res (1986) 77(8):808–16.
114. Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, et al. DNA FROM BACTERIA, BUT NOT FROM VERTEBRATES, INDUCES INTERFERONS, ACTIVATES NATURAL-KILLER-CELLS AND INHIBITS TUMOR-GROWTH. Microbiol Immunol (1992) 36(9):983–97. doi: 10.1111/j.1348-0421.1992.tb02102.x
115. Zhou W, Sonnenberg GF. Activation and Suppression of Group 3 Innate Lymphoid Cells in the Gut. Trends Immunol (2020) 41(8):721–33. doi: 10.1016/j.it.2020.06.009
116. Qi H, Li Y, Yun H, Zhang T, Huang Y, Zhou J, et al. Lactobacillus maintains healthy gut mucosa by producing L-Ornithine. Commun Biol (2019) 2:171. doi: 10.1038/s42003-019-0424-4
117. Belkacem N, Serafini N, Wheeler R, Derrien M, Boucinha L, Couesnon A, et al. Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PloS One (2017) 12(9):e184976. doi: 10.1371/journal.pone.0184976
118. Zheng DW, Chen Y, Li ZH, Xu L, Li CX, Li B, et al. Optically-controlled bacterial metabolite for cancer therapy. Nat Commun (2018) 9(1):1680. doi: 10.1038/s41467-018-03233-9
119. Wu RY, Pasyk M, Wang B, Forsythe P, Bienenstock J, Mao YK, et al. Spatiotemporal maps reveal regional differences in the effects on gut motility for Lactobacillus reuteri and rhamnosus strains. Neurogastroenterol Motil (2013) 25(3):e205–14. doi: 10.1111/nmo.12072
120. West CL, Stanisz AM, Mao YK, Champagne-Jorgensen K, Bienenstock J, Kunze WA. Microvesicles from Lactobacillus reuteri (DSM-17938) completely reproduce modulation of gut motility by bacteria in mice. PloS One (2020) 15(1):e0225481. doi: 10.1371/journal.pone.0225481
121. Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. J Control Release (2012) 162(1):56–67. doi: 10.1016/j.jconrel.2012.06.003
122. Dong Q-Y, Chen M-Y, Xin Y, Qin X-Y, Cheng Z, Shi L-E, et al. Alginate-based and protein-based materials for probiotics encapsulation: a review. Int J Food Sci Technol (2013) 48(7):1339–51. doi: 10.1111/ijfs.12078
123. Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Delivery Rev (2012) 64:194–205. doi: 10.1016/j.addr.2012.09.007
124. de Barros JMS, Scherer T, Charalampopoulos D, Khutoryanskiy VV, Edwards AD. A laminated polymer film formulation for enteric delivery of live vaccine and probiotic bacteria. J Pharm Sci (2014) 103(7):2022–32. doi: 10.1002/jps.23997
125. Yeung TW, Ucok EF, Tiani KA, McClements DJ, Sela DA. Microencapsulation in Alginate and Chitosan Microgels to Enhance Viability of Bifidobacterium longum for Oral Delivery. Front Microbiol (2016) 7:494. doi: 10.3389/fmicb.2016.00494
126. Dafe A, Etemadi H, Dilmaghani A, Mandavinia GR. Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int J Biol Macromolecules (2017) 97:536–43. doi: 10.1016/j.ijbiomac.2017.01.060
127. Mei L, He F, Zhou R-Q, Wu C-D, Liang R, Xie R, et al. Novel Intestinal-Targeted Ca-Alginate-Based Carrier for pH-Responsive Protection and Release of Lactic Acid Bacteria. ACS Appl Mater Interf (2014) 6(8):5962–70. doi: 10.1021/am501011j
128. de Barros JM, Lechner T, Charalampopoulos D, Khutoryanskiy VV, Edwards AD. Enteric coated spheres produced by extrusion/spheronization provide effective gastric protection and efficient release of live therapeutic bacteria. Int J Pharm (2015) 493(1-2):483–94. doi: 10.1016/j.ijpharm.2015.06.051
129. Wuerth R, Hoermannsperger G, Wilke J, Foerst P, Haller D, Kulozik U. Protective effect of milk protein based microencapsulation on bacterial survival in simulated gastric juice versus the murine gastrointestinal system. J Funct Foods (2015) 15:116–25. doi: 10.1016/j.jff.2015.02.046
130. Damodharan K, Palaniyandi SA, Yang SH, Suh JW. Co-encapsulation of lactic acid bacteria and prebiotic with alginate-fenugreek gum-locust bean gum matrix: Viability of encapsulated bacteria under simulated gastrointestinal condition and during storage time. Biotechnol Bioprocess Eng (2017) 22(3):265–71. doi: 10.1007/s12257-017-0096-1
131. Wang J, Nickerson MT, Low NH, Van Kessel AG, Plaizier J. Efficacy of pea protein isolate–alginate encapsulation on viability of a probiotic bacterium in the porcine digestive tract. Can J Anim Sci (2016) 97(2):214–22. doi: 10.1139/cjas-2016-0090
132. Shi L-E, Li Z-H, Li D-T, Xu M, Chen H-Y, Zhang Z-L, et al. Encapsulation of probiotic Lactobacillus bulgaricus in alginate-milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J Food Eng (2013) 117(1):99–104. doi: 10.1016/j.jfoodeng.2013.02.012
133. de Barros JM, Costabile A, Charalampopoulos D, Khutoryanskiy VV, Edwards AD. Evaluating and optimizing oral formulations of live bacterial vaccines using a gastro-small intestine model. Eur J Pharm Biopharm (2016) 102:115–22. doi: 10.1016/j.ejpb.2016.03.010
134. Li W, Zhu Y, Ye F, Li B, Luo X, Liu S. Probiotics in cellulose houses: Enhanced viability and targeted delivery of Lactobacillus plantarum. Food Hydrocolloids (2017) 62:66–72. doi: 10.1016/j.foodhyd.2016.07.019
135. Huq T, Fraschini C, Khan A, Riedl B, Bouchard J, Lacroix M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr Polymers (2017) 168:61–9. doi: 10.1016/j.carbpol.2017.03.032
136. Diab R, Canilho N, Pavel IA, Haffner FB, Girardon M, Pasc A. Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv Colloid Interface Sci (2017) 249:346–62. doi: 10.1016/j.cis.2017.04.005
137. Zheng H, Gao M, Ren Y, Lou R, Xie H, Yu W, et al. An improved pH-responsive carrier based on EDTA-Ca-alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohydr Polym (2017) 155:329–35. doi: 10.1016/j.carbpol.2016.08.096
138. Chowdhuri S, Cole CM, Devaraj NK. Encapsulation of Living Cells within Giant Phospholipid Liposomes Formed by the Inverse-Emulsion Technique. Chembiochem (2016) 17(10):886–9. doi: 10.1002/cbic.201500643
139. Lin J, Yu W, Liu X, Xie H, Wang W, Ma X. In Vitro and in Vivo characterization of alginate-chitosan-alginate artificial microcapsules for therapeutic oral delivery of live bacterial cells. J Biosci Bioeng (2008) 105(6):660–5. doi: 10.1263/jbb.105.660
140. Li W, Luo X, Song R, Zhu Y, Li B, Liu S. Porous Cellulose Microgel Particle: A Fascinating Host for the Encapsulation, Protection, and Delivery of Lactobacillus plantarum. J Agric Food Chem (2016) 64(17):3430–6. doi: 10.1021/acs.jafc.6b00481
141. Li H, Van Thi Thuy H, Turner MS, Dhital S. Encapsulation of Lactobacillus plantarum in porous maize starch. Lwt Food Sci Technol (2016) 74:542–9. doi: 10.1016/j.lwt.2016.08.019
142. Nualkaekul S, Lenton D, Cook MT, Khutoryanskiy VV, Charalampopoulos D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr Polymers (2012) 90(3):1281–7. doi: 10.1016/j.carbpol.2012.06.073
143. Anselmo AC, McHugh KJ, Webster J, Langer R, Jaklenec A. Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv Mater (2016) 28(43):9486–90. doi: 10.1002/adma.201603270
144. Wei OY, Chen HM, Jones ML, Metz T, Haque T, Martoni C, et al. Artificial cell microcapsule for oral delivery of live bacterial cells for therapy: design, preparation, and in-vitro characterization. J Pharm Pharm Sci (2004) 7(3):315–24.
145. Varankovich NV, Khan NH, Nickerson MT, Kalmokoff M, Korber DR. Evaluation of pea protein-polysaccharide matrices for encapsulation of acid-sensitive bacteria—-104. Food Res Int (2015) 70:118–24. doi: 10.1016/j.foodres.2015.01.028
146. Cook MT, Tzortzis G, Khutoryanskiy VV, Charalampopoulos D. Layer-by-layer coating of alginate matrices with chitosan–alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J Mater Chem B (2013) 1(1):52–60. doi: 10.1039/C2TB00126H
147. D’Orazio G, Di Gennaro P, Boccarusso M, Presti I, Bizzaro G, Giardina S, et al. Microencapsulation of new probiotic formulations for gastrointestinal delivery: in vitro study to assess viability and biological properties. Appl Microbiol Biotechnol (2015) 99(22):9779–89. doi: 10.1007/s00253-015-6853-1
148. Turroni F, Duranti S, Milani C, Lugli GA, van Sinderen D, Ventura M. Bifidobacterium bifidum: A Key Member of the Early Human Gut Microbiota. Microorganisms (2019) 7(11):544. doi: 10.3390/microorganisms7110544
149. Kato K, Odamaki T, Mitsuyama E, Sugahara H, Xiao JZ, Osawa R. Age-Related Changes in the Composition of Gut Bifidobacterium Species. Curr Microbiol (2017) 74(8):987–95. doi: 10.1007/s00284-017-1272-4
150. Odamaki T, Bottacini F, Kato K, Mitsuyama E, Yoshida K, Horigome A, et al. Genomic diversity and distribution of Bifidobacterium longum subsp. longum across the human lifespan. Sci Rep (2018) 8(1):85. doi: 10.1038/s41598-017-18391-x
151. Huda MN, Ahmad SM, Alam MJ, Khanam A, Kalanetra KM, Taft DH, et al. Bifidobacterium Abundance in Early Infancy and Vaccine Response at 2 Years of Age. Pediatrics (2019) 143(2):e20181489. doi: 10.1542/peds.2018-1489
152. Oh NS, Joung JY, Lee JY, Kim Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PloS One (2018) 13(2):e0192021. doi: 10.1371/journal.pone.0192021
153. Capurso L. Thirty Years of Lactobacillus rhamnosus GG: A Review. J Clin Gastroenterol (2019) 53 Suppl 1:S1–S41. doi: 10.1097/MCG.0000000000001170
154. Haffner FB, Girardon M, Fontanay S, Canilho N, Duval RE, Mierzwa M, et al. Core–shell alginate@silica microparticles encapsulating probiotics—74. J Mater Chem B (2016) 4(48):7929–35. doi: 10.1039/C6TB02802K
155. Tenaillon O, Skurnik D, Picard B, Denamur E. The population genetics of commensal Escherichia coli. Nat Rev Microbiol (2010) 8(3):207–17. doi: 10.1038/nrmicro2298
156. Ahmadzadeh M, Farshdari F, Nematollahi L, Behdani M, Mohit E. Anti-HER2 scFv Expression in Escherichia coli SHuffle((R))T7 Express Cells: Effects on Solubility and Biological Activity. Mol Biotechnol (2020) 62(1):18–30. doi: 10.1007/s12033-019-00221-2
157. Cebeci A, Gürakan C. Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol (2003) 20(5):511–8. doi: 10.1016/S0740-0020(02)00174-0
158. Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, et al. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front Microbiol (2018) 9:1953. doi: 10.3389/fmicb.2018.01953
159. Seddik HA, Bendali F, Gancel F, Fliss I, Spano G, Drider D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob Proteins (2017) 9(2):111–22. doi: 10.1007/s12602-017-9264-z
160. Chen H, Ouyang W, Jones M, Haque T, Lawuyi B, Prakash S. In-vitro analysis of APA microcapsules for oral delivery of live bacterial cells. J Microencapsul (2005) 22(5):539–47. doi: 10.1080/02652040500162162
161. Zhang H, Yang C, Zhou W, Luan Q, Li W, Deng Q, et al. A pH-Responsive Gel Macrosphere Based on Sodium Alginate and Cellulose Nanofiber for Potential Intestinal Delivery of Probiotics. ACS Sustain Chem Eng (2018) 6(11):13924–31. doi: 10.1021/acssuschemeng.8b02237
162. Aryana KJ, Olson DW. A 100-Year Review: Yogurt and other cultured dairy products. J Dairy Sci (2017) 100(12):9987–10013. doi: 10.3168/jds.2017-12981
163. Ryan PM, Ross RP, Fitzgerald GF, Caplice NM, Stanton C. Sugar-coated: exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct (2015) 6(3):679–93. doi: 10.1039/C4FO00529E
164. Hill D, Sugrue I, Tobin C, Hill C, Stanton C, Ross RP. The Lactobacillus casei Group: History and Health Related Applications. Front Microbiol (2018) 9:2107. doi: 10.3389/fmicb.2018.02107
165. Jacouton E, Chain F, Sokol H, Langella P, Bermudez-Humaran LG. Probiotic Strain Lactobacillus casei BL23 Prevents Colitis-Associated Colorectal Cancer. Front Immunol (2017) 8:1553. doi: 10.3389/fimmu.2017.01553
166. Zhao C, Zhu Y, Kong B, Huang Y, Yan D, Tan H, et al. Dual-Core Prebiotic Microcapsule Encapsulating Probiotics for Metabolic Syndrome. ACS Appl Mater Interfaces (2020) 12(38):42586–94. doi: 10.1021/acsami.0c13518
167. Dilnawaz F, Singh A, Mohanty C, Sahoo SK. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials (2010) 31(13):3694–706. doi: 10.1016/j.biomaterials.2010.01.057
168. Li X, He Y, Hou J, Yang G, Zhou S. A Time-Programmed Release of Dual Drugs from an Implantable Trilayer Structured Fiber Device for Synergistic Treatment of Breast Cancer. Small (2020) 16(9):1902262. doi: 10.1002/smll.201902262
169. Lammers KM, Brigidi P, Vitali B, Gionchetti P, Rizzello F, Caramelli E, et al. Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol (2003) 38(2):165–72. doi: 10.1016/S0928-8244(03)00144-5
170. Lai W-F, Lin MC-M. Nucleic acid delivery with chitosan and its derivatives. J Controlled Release (2009) 134(3):158–68. doi: 10.1016/j.jconrel.2008.11.021
171. Zhao QQ, Chen JL, Han M, Liang WQ, Tabata Y, Gao JQ. Combination of poly(ethylenimine) and chitosan induces high gene transfection efficiency and low cytotoxicity. J Biosci Bioeng (2008) 105(1):65–8. doi: 10.1263/jbb.105.65
172. Kiang T, Bright C, Cheung CY, Stayton PS, Hoffman AS, Leong KW. Formulation of chitosan-DNA nanoparticles with poly(propyl acrylic acid) enhances gene expression. J Biomater Science Polymer Edition (2012) 15(11):1405–21. doi: 10.1163/1568562042368112
173. Zhang Y, Chen J, Zhang Y, Pan Y, Zhao J, Ren L, et al. A novel PEGylation of chitosan nanoparticles for gene delivery. Biotechnol Appl Biochem (2007) 46(Pt 4):197–204. doi: 10.1042/BA20060163
174. Weecharangsan W, Opanasopit P, Ngawhirunpat T, Apirakaramwong A, Rojanarata T, Ruktanonchai U, et al. Evaluation of chitosan salts as non-viral gene vectors in CHO-K1 cells. Int J Pharm (2008) 348(1-2):161–8. doi: 10.1016/j.ijpharm.2007.07.011
175. O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep (2006) 7(7):688–93. doi: 10.1038/sj.embor.7400731
176. Qian J, Wang C, Wang B, Yang J, Wang Y, Luo F, et al. The IFN-gamma/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. J Neuroinflamm (2018) 15(1):290. doi: 10.1186/s12974-018-1330-2
177. Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci (2018) 109(1):43–53. doi: 10.1111/cas.13424
178. Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep (2017) 19(6):1189–201. doi: 10.1016/j.celrep.2017.04.031
179. Mo X, Zhang H, Preston S, Martin K, Zhou B, Vadalia N, et al. Interferon-gamma Signaling in Melanocytes and Melanoma Cells Regulates Expression of CTLA-4. Cancer Res (2018) 78(2):436–50. doi: 10.1158/0008-5472.CAN-17-1615
Keywords: intestinal bacteria, probiotic, immunotherapy, immune cell, biomaterial, oral delivery
Citation: Liu Y, Li Z, Wu Y, Jing X, Li L and Fang X (2021) Intestinal Bacteria Encapsulated by Biomaterials Enhance Immunotherapy. Front. Immunol. 11:620170. doi: 10.3389/fimmu.2020.620170
Received: 22 October 2020; Accepted: 31 December 2020;
Published: 10 February 2021.
Edited by:
Gregg Joshua Silverman, New York University, United StatesReviewed by:
Xu Wang, First Affiliated Hospital of Jilin University, ChinaCopyright © 2021 Liu, Li, Wu, Jing, Li and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuedong Fang, ZmFuZ3hkQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.