
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 16 December 2020
Sec. Viral Immunology
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.610300
 Rita Carsetti1,2*
Rita Carsetti1,2* Salvatore Zaffina3,4
Salvatore Zaffina3,4 Eva Piano Mortari1†
Eva Piano Mortari1† Sara Terreri1†
Sara Terreri1† Francesco Corrente2
Francesco Corrente2 Claudia Capponi2
Claudia Capponi2 Patrizia Palomba2
Patrizia Palomba2 Mattia Mirabella2
Mattia Mirabella2 Simona Cascioli5
Simona Cascioli5 Paolo Palange6
Paolo Palange6 Ilaria Cuccaro6
Ilaria Cuccaro6 Cinzia Milito7
Cinzia Milito7 Alimuddin Zumla8,9
Alimuddin Zumla8,9 Markus Maeurer10,11
Markus Maeurer10,11 Vincenzo Camisa3,4
Vincenzo Camisa3,4 Maria Rosaria Vinci3,4
Maria Rosaria Vinci3,4 Annapaola Santoro3,4
Annapaola Santoro3,4 Eleonora Cimini12
Eleonora Cimini12 Luisa Marchioni13
Luisa Marchioni13 Emanuele Nicastri13
Emanuele Nicastri13 Fabrizio Palmieri13
Fabrizio Palmieri13 Chiara Agrati12
Chiara Agrati12 Giuseppe Ippolito14
Giuseppe Ippolito14 Ottavia Porzio15,16
Ottavia Porzio15,16 Carlo Concato17
Carlo Concato17 Andrea Onetti Muda18
Andrea Onetti Muda18 Massimiliano Raponi4
Massimiliano Raponi4 Concetta Quintarelli19,20
Concetta Quintarelli19,20 Isabella Quinti7
Isabella Quinti7 Franco Locatelli19,21
Franco Locatelli19,21SARS-CoV-2 is a novel coronavirus, not encountered before by humans. The wide spectrum of clinical expression of SARS-CoV-2 illness suggests that individual immune responses to SARS-CoV-2 play a crucial role in determining the clinical course after first infection. Immunological studies have focused on patients with moderate to severe disease, demonstrating excessive inflammation in tissues and organ damage. In order to understand the basis of the protective immune response in COVID-19, we performed a longitudinal follow-up, flow-cytometric and serological analysis of innate and adaptive immunity in 64 adults with a spectrum of clinical presentations: 28 healthy SARS-CoV-2-negative contacts of COVID-19 cases; 20 asymptomatic SARS-CoV-2-infected cases; eight patients with Mild COVID-19 disease and eight cases of Severe COVID-19 disease. Our data show that high frequency of NK cells and early and transient increase of specific IgA, IgM and, to a lower extent, IgG are associated with asymptomatic SARS-CoV-2 infection. By contrast, monocyte expansion and high and persistent levels of IgA and IgG, produced relatively late in the course of the infection, characterize severe disease. Modest increase of monocytes and different kinetics of antibodies are detected in mild COVID-19. The importance of innate NK cells and the short-lived antibody response of asymptomatic individuals and patients with mild disease suggest that only severe COVID-19 may result in protective memory established by the adaptive immune response.
SARS-CoV-2 is a novel coronavirus, not encountered before by humans. Thus, everyone is susceptible to infection as the SARS-CoV-2 virus rapidly spreads in the current Coronavirus disease 2019 (COVID-19) pandemic. A wide spectrum of clinical expression of SARS-CoV-2 infection occurs, ranging from asymptomatic to mild upper respiratory tract illness, or moderate to severe disease with respiratory distress and multi-organ failure requiring intensive care and organ support (1). This variability of disease severity suggests that the individual immune responses to SARS-CoV-2 play a crucial role in determining the clinical course after first infection. Understanding the pathogenesis of COVID-19 disease requires in-depth study of underlying immune responses (2). This includes the cellular and molecular basis of the successful protective mechanisms and the role of dysregulated and excessive inflammation (3, 4). During the 2003 SARS outbreak, the efficacy of the innate immune responses to SARS-CoV-1 appeared to determine the extent of virus load (5) and adaptive immunity played a critical role during the later stages of infection (6).
In COVID-19, lymphopenia is common and correlates with severity of clinical disease similarly to severe influenza and other respiratory viral infections (7–9). Because of lymphopenia, neutrophil–lymphocyte ratio (NLR) and monocyte–lymphocyte ratio (MLR) increase in patients affected by severe COVID-19 (10, 11). Lymphopenia is caused by the reduction of both CD4+ and CD8+ T cells. Surviving T cells are functionally exhausted, and reduced T-cell count predicts an unfavorable clinical course (12, 13). T cells able to react to SARS-CoV-2 peptides can be demonstrated in healthy individuals, partly because of cross-reactivity with previous infections by other coronaviruses (14) and are expanded in individuals convalescent from COVID-19 (15).
Antibodies to SARS-CoV-2 are produced in large amounts in patients with severe disease, two–three weeks after the occurrence of first symptoms (16). The role of antibodies in viral elimination is supported by the successful use of convalescent plasma in patients with severe COVID-19 (17). Neutralizing antibodies are directed against the Receptor Binding Domain (RBD) or to other regions contained in the S1 subunit of the Spike protein (18–20). While immune responses to novel antigens encountered for the first time, are first dominated by antibodies of IgM isotype, followed by IgG (21, 22), the kinetics and protective or deleterious nature of the antibody responses to SARS-CoV-2 remains to be defined. Initial studies suggest that IgG may be produced earlier or at the same time with IgM (16, 23, 24). A recent study indicated that the IgA response to SARS-CoV-2 may be rapid, strong, and persistent (25, 26). The observation that the highest antibody levels are found in patients with severe COVID-19 disease led to the suggestion that antibodies to SARS-CoV-2 may be damaging or ineffective rather than protective (27–29), as was reported from very sick patients with Middle East respiratory syndrome (MERS) (30).
In order to identify the immunological features associated with the different clinical forms of SARS-CoV-2 infection, we performed a longitudinal study by standard 7–9 color flow-cytometry comparing innate and adaptive immune populations of adults with asymptomatic SARS-CoV-2 infection, mild and severe COVID-19 disease and healthy SARS-CoV-2 negative contacts. We also measured levels and kinetics of IgG, IgA, and IgM anti-SARS-CoV-2 antibodies in the serum.
Patients were enrolled in in-patient and out-patient settings if they agreed to participate and fulfilled the inclusion/exclusion criteria. Sixty-four adult patients were enrolled in the study (Supplementary Table S1A). At enrollment, after the protocol procedures (including medical history, physical examination, laboratory examination) participants were assigned to the study group: a) Contacts of SARS-CoV-2 confirmed cases who were negative by qPCR and were included as control group (28 patients); b) Asymptomatic cases (20 patients) tested positive for viral RNA and had no symptoms. Asymptomatic patients were quarantined and monitored for 14 days, and quarantine ended when two consecutive nasopharyngeal swabs showed negative results; c) Mild COVID-19 disease (eight patients), defined by positive SARS-CoV-2 nasopharyngeal swab qPCR test, with symptoms such as fever, myalgia, and fatigue without obvious chest HRCT findings for COVID-19, did not require hospitalization; d) Severe COVID-19 disease (eight patients) with clinical signs of pneumonia (fever, cough, dyspnoea, fast breathing) plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress; or SpO2 <90% on room air. Additional 77 patients with severe COVID-19 were recruited from the INMI, Lazzaro Spallanzani (34 ICU and 43 non-ICU, Supplementary Table S1B). Patients were admitted to ICU if they had pneumonia or new or worsening respiratory symptoms with chest imaging showing bilateral opacities, not fully explained by volume overload, lobar or lung collapse, or nodules with a oxygenation impairment:
● Mild ARDS: 200 mmHg < PaO2/FiO2a ≤300 mmHg (with PEEP or CPAP ≥5 cmH2O).
● Moderate ARDS: 100 mmHg <PaO2/FiO2 ≤200 mmHg (with PEEP ≥5 cmH2O).b
● Severe ARDS: PaO2/FiO2 ≤100 mmHg (with PEEP ≥5 cmH2O) (31).
Contacts, asymptomatic individuals and patients with mild disease were Health Care Workers (HCW) of the Bambino Gesù Children Hospital. We included in the study all HCWs that had a positive swab in the period between March 15 and May 31, 2020 and their contacts (who had a negative swab). Blood and serum samples were collected at weekly intervals since diagnosis (first positive nasopharyngeal swab). Severe cases were patients from the Pulmonary division of the Department of Public Health and infectious diseases, Policlinico Umberto I Hospital, Rome, Italy.
Ethical approval was obtained from the Medical Research and Ethics Committee at Sapienza, University of Rome and from the Ethics Committee at INMI, Lazzaro Spallanzani. According to the guidelines on Italian observational studies as established by the Italian legislation about the obligatory occupational surveillance and privacy management; HCWs’ confidentiality was safeguarded, and informed consent was obtained from all the participants. The study was performed in accordance with the Good Clinical Practice guidelines, the International Conference on Harmonization guidelines, and the most recent version of the Declaration of Helsinki.
Four leukocyte profiling panels computing seven- to nine-surface marker antigens for monitoring the major leukocyte subsets as well as characteristics of T cell, B cell, monocytes and NK cells subsets were designed (Supplementary Table S2). Results of immune-profile of analyzed patients are reported in Supplementary Tables S3–10. The graphs of the single time points refer to the sample obtained immediately after the first positive nasopharyngeal swab. When available, we also show the results obtained at different time points.
1 ml of total blood (EDTA) was incubated with the lysing solution Pharm Lyse (BD) to lyse red blood cells. Then, cells were divided in four equal aliquots and stained with the appropriate combination of fluorochrome-conjugated antibodies (Supplementary Table S2) to identify immune cell subsets according to standard techniques. For the staining of Supplementary Figure S7, heparinized blood of three healthy donors was isolated by Ficoll Paque™ Plus 206 (Amersham Pharmacia Biotech) density-gradient centrifugation. Peripheral blood mononuclear cells (PBMCs) were then stained with antibodies against CD19, CD24, CD27, CD38, IgM, IgG, IgA, and IgD (Supplementary Table S2). Cells were acquired on a BD FACSLyric™ (BD Biosciences). Data were analyzed with FlowJo ver. 10 (Treestar). Dead cells were excluded from analysis by side/forward scatter gating.
Included in this study were 160 serum samples obtained from subjects with available clinical records. In particular: fifty-one sera from SARS-CoV-2 negative contacts, sixty-three from SARS-CoV-2 asymptomatic patients, thirty-one from COVID-19 mild patients, and fifteen from COVID-19 severe patients. 86 samples from 28 patients were collected at different time points. All sera were kept on ice after collection and then stored at −80°C.
The Euroimmun Anti-SARS-CoV-2 ELISA IgG and IgA assays (Euroimmun), were performed on serum samples according to the manufacturer’s instructions. The recommended serum sample dilutions used were 1:100; in samples in which the IgA or IgG quantity was not detectable (overflow), we used 1:1,000, 1:3,000, 1:6,000, 1:25,000 dilutions. Values were then normalized for comparison. These ELISA assays provide a semi-quantitative in vitro determination of human antibodies of the immunoglobulin classes IgG and IgA against the SARS-CoV-2. The microplate wells are coated with recombinant S1 structural protein. The results were evaluated by calculation of the ratio between the extinction of samples and the extinction of the calibrator. The ratio interpretation was as follows: <0.8 = negative, ≥0.8 to <1.1 = borderline, ≥1.1 = positive.
For the detection of IgM anti RBD we developed an in-house ELISA. 96-well plates (Corning) were coated overnight with 1 μg/ml of purified SARS-CoV-2 RBD protein (Sino Biological). After washing with PBS/0.05% Tween and blocking with PBS/1% BSA, plates were incubated for 1 h at 37°C with diluted sera. Serum samples were measured at 1:100 dilutions. After washing, plates were incubated for 1 h at 37°C with peroxidase-conjugated goat anti-human IgM antibody (Jackons ImmunoResearch Laboratories). The assay was developed with o-phenylen-diamine tablets (Sigma-Aldrich) as a chromogen substrate. Absorbance at 450 nm was measured, and IgM concentrations were calculated by interpolation from the standard curve based on serial dilutions of monoclonal human IgM antibody against SARS-CoV-2 Spike-RBD (Invivogen). Due to the unavailability of a human IgM antibody against SARS-CoV-2 S1 to be used as standard we were unable to quantify the level of anti-S1 IgM. We could, however compare the OD measured in plates coated with either RBD or S1 (data not shown).
For the comparison of more than two independent groups, the non-parametric Kruskal–Wallis test was used, and if significant, pairwise comparisons were evaluated by the Mann–Whitney U-test. P values less than 0.05 were considered statistically significant.
SARS-CoV-2 asymptomatic patients (20 patients, M/F 4/16, mean age, 40.4 years, range 27–64) and SARS-CoV-2-negative contacts (28 patients, M/F 8/20, mean age, 40.8 years, range 27–68) had comparable demographic characteristics. Severe adult COVID-19 patients (eight patients, M/F 6/2, mean age, 65 years, range 30–90) and mild-symptoms adult COVID-19 patients (eight patients, M/F 5/3, mean age, 55.2 years, range 48–64) were older than asymptomatic patients and controls (Supplementary Table S1). During the study period, disease activity was regularly assessed, and COVID-19 patients continued their therapies according to the standard of care. Four out of eight severe cases were treated with anti-IL-6R monoclonal antibody (tocilizumab). All hospitalized COVID-19 patients were discharged and none died.
The PBMCs of patients with asymptomatic infection, mild and severe disease and their healthy contacts were compared. We correlated the immunological findings with the clinical course and studied the dynamic changes of cells of innate and adaptive immune response in time by analyzing blood samples obtained at weekly intervals beginning on the first or second week after diagnosis.
By flow-cytometry performed on the first sample obtained after diagnosis, we confirmed the increase of MLR in advanced COVID-19 cases, when T cells, normally representing the major lymphocyte population in the peripheral blood, are reduced (32) (Figures 1A, B). Previous studies indicated that neutrophils and macrophages infiltrate the lungs and are expanded in the peripheral blood of Intensive Care Unit (ICU)-admitted patients (33, 34). The increase of circulating neutrophils and monocytes, along with lymphopenia, explains why the NLR and MLR are significantly higher in patients with severe COVID-19 disease (10).
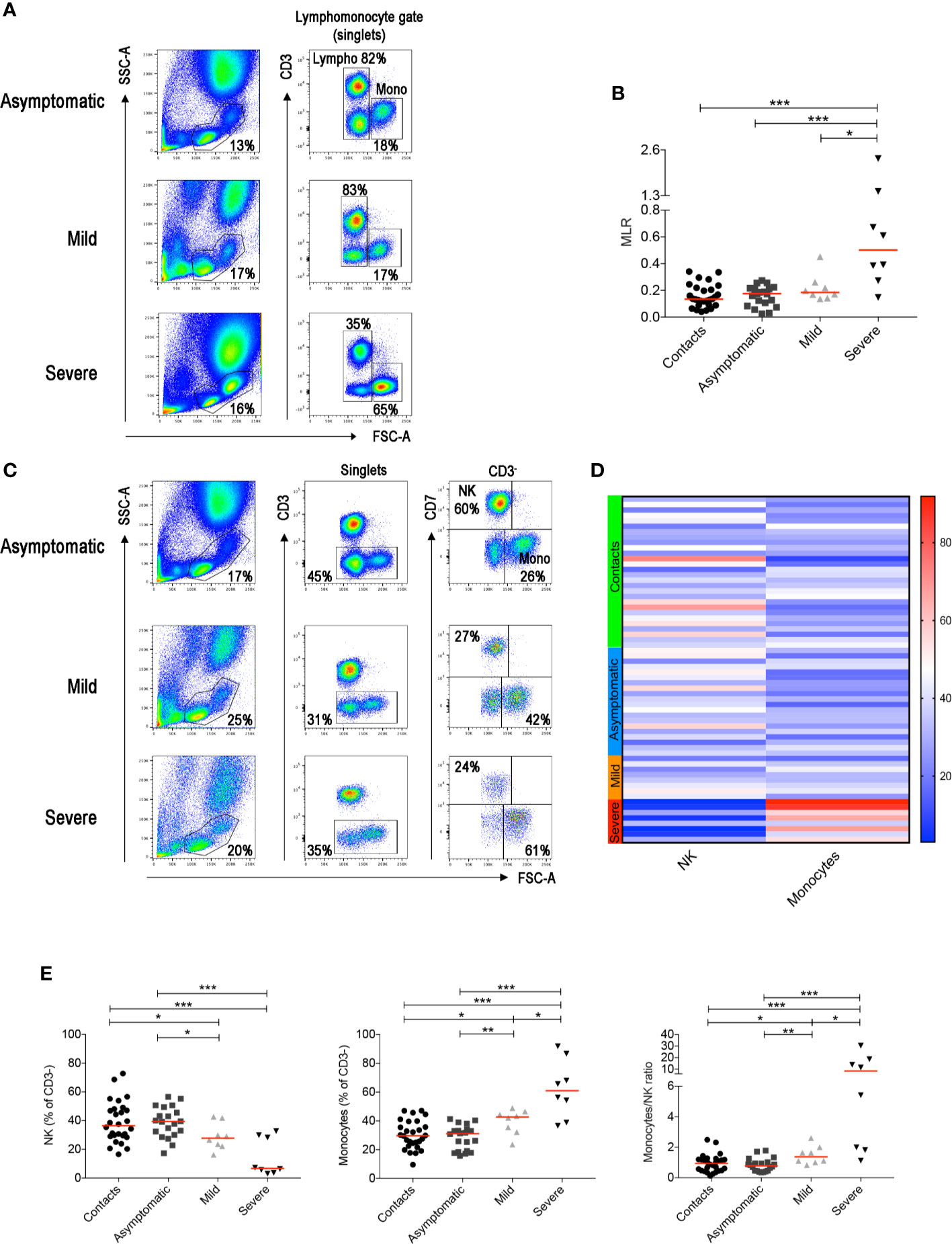
Figure 1 (A) Flow-cytometry analysis of the monocyte to lymphocyte ratio (MLR) in the blood of three representative patients with asymptomatic, mild and severe disease. Total blood (EDTA) was stained with antibodies against CD45, CD3, CD4, CD8, CD7, CD56, CD16. The lympho-monocyte gate was designed based on physical characteristics (FSC-A vs SSC-A). Lymphocytes were gated as FSC-Alow and CD3+ or CD3− and monocytes as CD3− FSC-Ahigh. (B) Scatter plot depicts the MLR in the sixty-four adult patients enrolled in the study (contacts n = 28; asymptomatic n = 20; mild n = 8; severe n = 8). (C) Gating strategy used to identify natural killer (NK) and monocytes inside the CD3- cells in three representative patients with asymptomatic, mild and severe disease. NK cells were defined as CD3−CD7+FSC-Alow and monocytes as CD3−CD7−FSC-Ahigh. (D) Heatmap shows percentages of NK and monocytes in contacts (indicated by the light green bar), asymptomatic (blue), mild (orange) and severe (red) patients. Percentages are represented by the different expression of red, blue and white as indicated in the color code. (E) Plots indicate the frequency of NK, monocytes and the monocytes/NK ratio (MNKR) in our patients. (B, E) Midlines indicate median. Statistical significances were determined using unpaired, two-tailed Mann–Whitney U-tests. *p ≤ 0.05, **p < 0.01, ***p < 0.001.
T-cell frequencies are preserved in asymptomatic individuals and in patients with mild disease. Thus, in order to investigate whether other lymphocyte populations than T cells change in asymptomatic and mild disease, we excluded T cells from the analysis (Figure 1C and Supplementary Figure S1). The CD3− gate, besides B cells discussed below, includes monocytes that can be distinguished by their larger size measured by the high FCS, and NK cells. NK cells express the markers CD7 (Figure 1C) and CD56 (Supplementary Figure S1).
We found that NK cells were reduced and monocytes increased in patients with severe COVID-19 (Figures 1C, D). Significant reduction of the frequency of NK cells and increase of monocytes were also observed in the group of patients with mild disease (Figures 1C–E). We calculated the Monocyte to NK ratio (MNKR), which was <1 in asymptomatic individuals, >1 in patients with mild disease and even higher in severe cases (Figure 1E).
These results were confirmed when we analyzed all the samples collected at different time points, as the MNKR remained stable throughout the follow up in asymptomatic and mild disease groups (Figure 2A), because each individual maintained his typical NK and monocyte frequency throughout the time of follow-up (Figure 2B). Thus, the different relative frequencies of monocytes and NK observed in asymptomatic, mild disease and severe cases were not incidental findings observed in a particular moment of the infection, but rather characteristics of the clinical course of the response of the individual immune system to SARS-CoV-2 (Figure 2B). We confirmed the importance of the frequency of NK cells by the analysis of another independent cohort of 77 patients hospitalized because of severe COVID-19. Cases who did not need ICU treatment had a significantly higher number of NK cells (CD56+ cells calculated in CD3− lympho-monocyte gate) than ICU patients (Figure 2C). In addition, the percentage of NK cells was low in patients with fatal COVID-19, whereas it increased in those individuals who recovered from severe disease (Figure 2C). These results are corroborated by the observation that ICU patients had lower perforin+ NK cells compared to non-ICU patients (35).
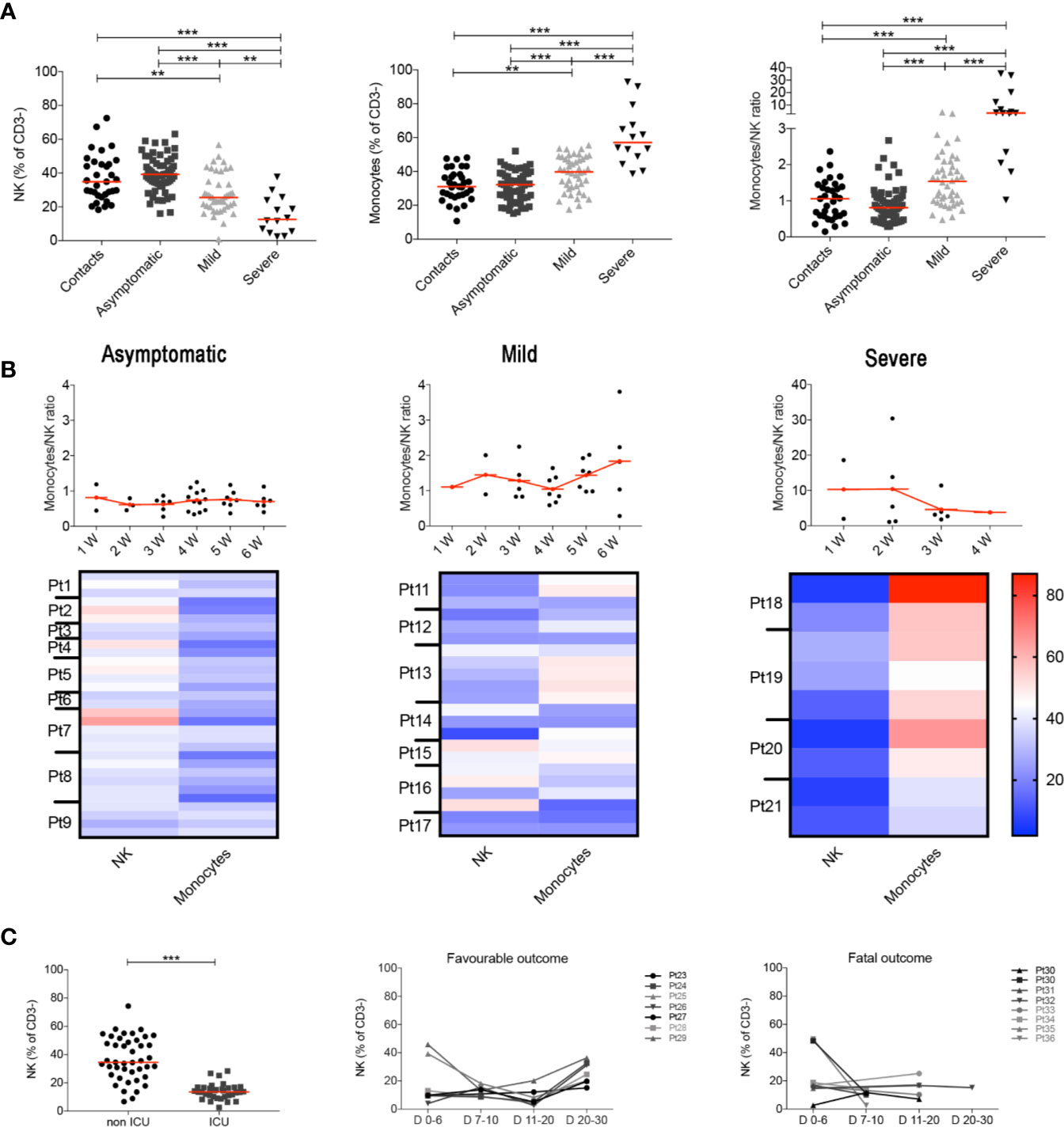
Figure 2 (A) Plots indicate the frequency of NK, monocytes and MNKR ratio in all analyzed samples collected at different time points. (B) Graphs depict the kinetics of the MNKR during the first 6 weeks of disease (midlines indicate mean) in all patients samples. Data referring to severe patients has a different scale due to the high value of MNKR. Heatmaps show the percentage of NK and monocytes in patients who had samples collected at different time points during the first 6 weeks. In the heatmap percentages are represented by the different expression of red, blue and white as indicated in the color code. (C) Scatter plot shows the percentage of NK cells in non-ICU (n = 43) and ICU (n = 34) patients. Graphs show the kinetics over time of NK cells percentage in patients with favorable and fatal outcome. (A, C) Midlines indicate median. Statistical significances were determined using unpaired, two-tailed Mann–Whitney U-tests. **p < 0.01, ***p < 0.001.
The increase of inflammatory cytokines mostly produced by monocytes plays an important role in determining systemic and local damage in COVID-19. Inflammatory cytokines are produced by intermediate monocytes that expand in the blood of patients with severe infections (36, 37). In order to measure the frequency of intermediate monocytes, we used CD16 and CD14 expression to differentiate the three types of CD14+ monocytes (Figure 3A) found in the peripheral blood, reflecting sequential stages of maturation and distinct functions (38, 39). CD14+CD16− classical monocytes are the precursors of the other types and play an important role in the response to pathogens (40). CD14−CD16+ non-classical monocytes contribute to the resolution of inflammation and maintain vascular homeostasis and endothelial integrity (41). Intermediate monocytes express CD14 with variable levels of CD16.
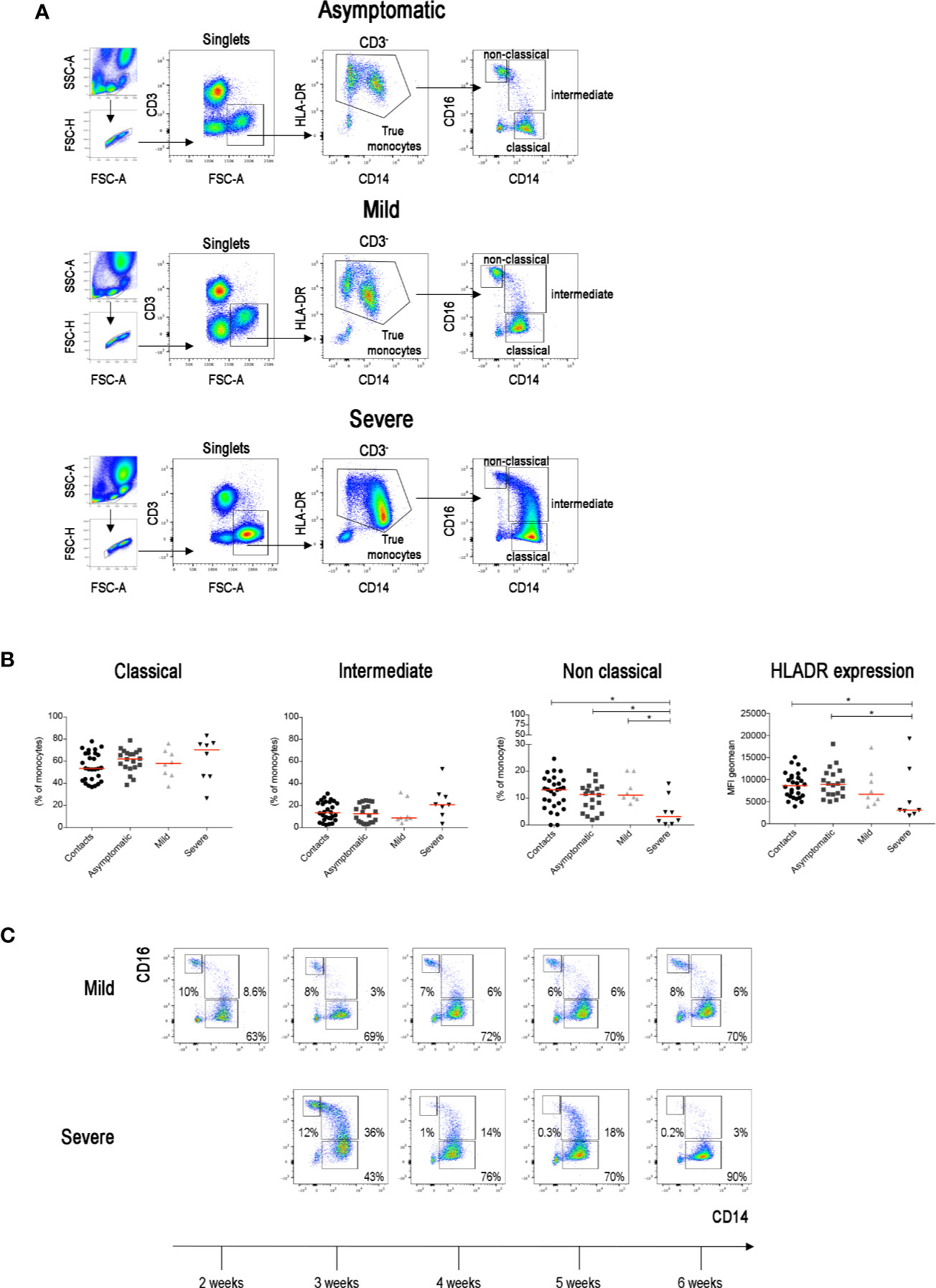
Figure 3 (A) Total blood was stained with antibodies against CD45, CD3, CD4, CD8, CD14, CD16, CD33, CD38 and HLADR. FACS plot show the gating strategy for the identification of monocytes (CD3−FSC-Ahigh) in three representative patients with asymptomatic, mild and severe disease. True monocytes are double positive for HLADR and CD14. In the true monocytes gate, we identified the classical (CD14++CD16−), intermediate (CD14+CD16+) and non-classical (CD14+CD16++) populations. (B) Scatter plots indicate the percentage of classical, intermediate and non-classical monocytes in each group of patients reported as single value. The Mean Fluorescence Intensity (MFI) of HLADR on total monocytes is shown by the last scatter plot. Midlines indicate median. Statistical significances were determined using unpaired, two-tailed Mann–Whitney U-tests. *p ≤ 0.05. (C) FACS plots show the different distribution of monocytes populations in two representative patients (one mild and one severe) during the course of the disease (2–6 weeks).
We confirm that, as reported in a recent paper (42), non-classical monocytes were significantly reduced in patients with severe COVID-19 when compared to SARS-CoV-2 negative contacts, SARS-CoV-2 positive asymptomatic and also mild COVID-19 disease patients (Figure 3B). We also confirm (43) that HLADR expression on CD14+ monocytes was significantly reduced in severe cases (Figure 3B) reflecting the complex immune-dysregulation triggered by the disease (43). Intermediate monocytes tended to increase in the severe cases. As the progression from the classical to intermediate stage is a dynamic step driven by infectious triggers (38), we compared the monocyte phenotype in the same patients at different time points during the course of the disease. Whereas intermediate monocytes were rare in the blood of contacts, asymptomatic and mild disease patients at all time points, transient increases were observed in patients with severe disease (Figure 3C).
In summary, we found that the MNKR reflects the clinical phenotype of the disease. Contact and asymptomatic patients had either higher representation of NK cells or a similar frequency of NK and monocytes (ratio around 1). The ratio was >1 in patients with mild disease and reached higher values in the severe cases (Figure 2B). Thus, the equilibrium between two cell types of the innate immune system may play a role in the control of SARS-CoV-2 infection. Prevalence of NK cells is associated to asymptomatic infection, while increased frequency of monocytes to severe disease.
T and B cells play key roles in response to viral infections. CD8+ T lymphocytes are crucial for the limitation of viral spread through their cytotoxic function. CD4+ T cells are indispensable for the expansion of CD8+ T cells (44) and the generation of CD8+ memory T cells (45, 46). In addition, CD4+ T cells are necessary for the germinal centre (GC) response and the production of memory B cells (MBCs) and plasma cells (47–49). As demonstrated before (35, 50), we found that CD3+ T cells were reduced only in severe COVID-19 patients (Figure 4A). In this group the frequency of activated HLADR+CD8+ T cells significantly increased in line with previous observations based on transcriptomic analysis (51), whereas activated HLADR+ CD4 T cells were measurable in patients with both mild and severe disease (Figure 4B). All these findings were confirmed when we included all serially collected samples in the analysis (Supplementary Figure S2A and B).
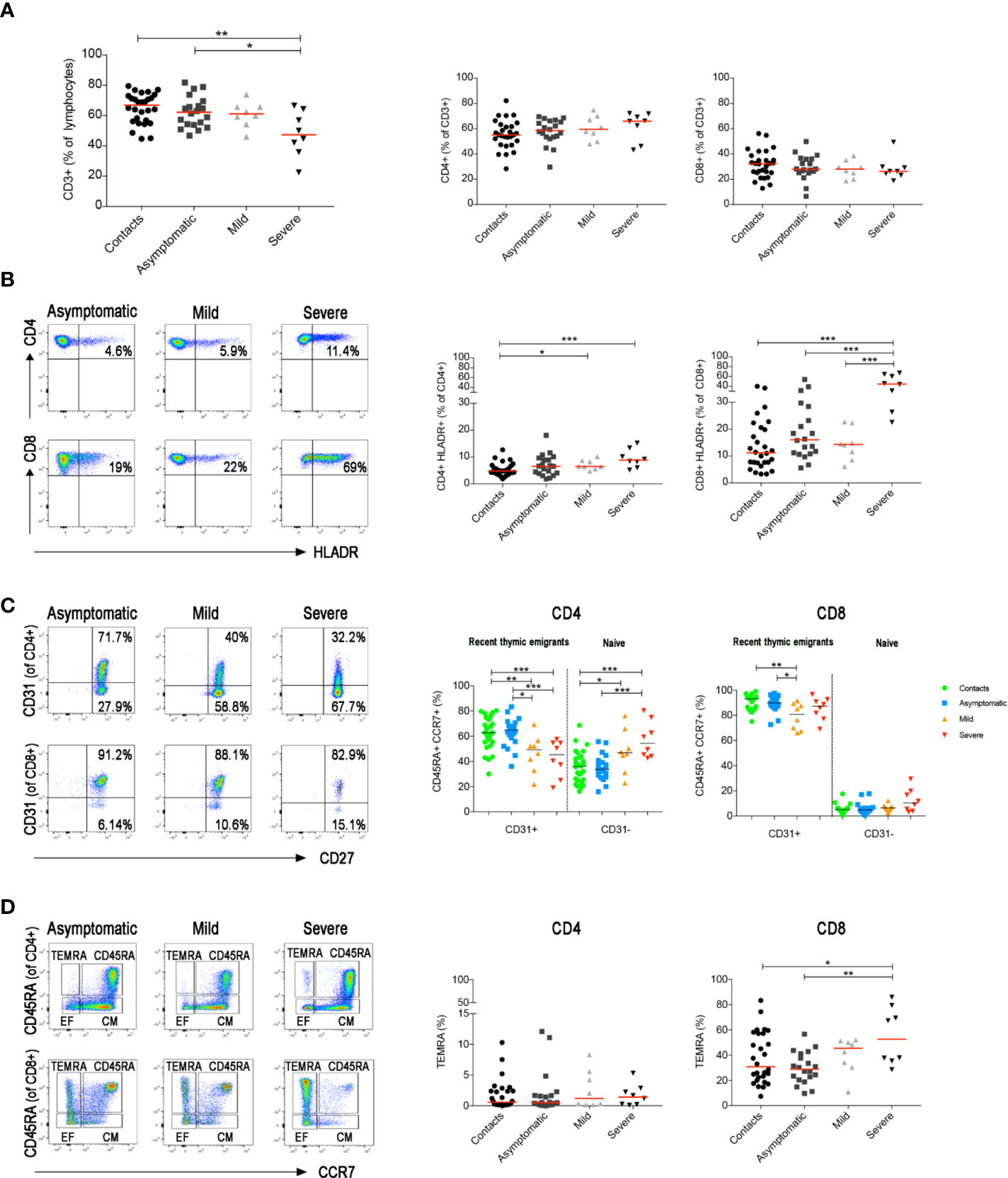
Figure 4 Total blood was stained with antibodies against CD45, CD3, CD4, CD8, CD14, CD16, CD33, CD38 and HLADR and in a second staining antibodies used were anti-CD3, CD4, CD8, CD45RA, CD27, CD28, CD31 and CCR7. (A) Graphs indicate the percentage of total CD3+, CD3+CD4+ and CD3+CD8+ T cells. (B) FACS plots depict the gating strategy to measure the frequency of CD4+ and CD8+ T cells expressing HLADR in three representative patients (asymptomatic, mild and severe). Percentage of CD4+HLADR+ or CD8+HLADR+ T cells is shown in the relative graphs (at the first sample collected after diagnosis). (C) Pseudocolor plots show CD31 expression in CD4+ and CD8+ naïve T cells (CD3+CCR7+CD45RA+). CD31+ are recent thymic emigrants and CD31− are naïve T cells. Graphs show the percentage of CD31+ and CD31− T cells in all patients (at the first sample collected after diagnosis). (D) FACS plots show CD4+ or CD8+ central memory (CD3+CCR7+CD45RA−), effector memory (CD3+CCR7−CD45RA−) T cells and TEMRA (CD3+CCR7−CD45RA+) T cells. Scatter plots depict the percentage of CD4+ and CD8+ TEMRA (at the first sample collected after diagnosis). Median is shown as midline. Statistical significances were determined using unpaired, two-tailed Mann–Whitney U-tests. *p ≤ 0.05, **p < 0.01, ***p < 0.001.
In a separate staining, we identified naïve and memory T cells, including central, effector and terminally differentiated (TEMRA) memory T cells (gating strategy in Supplementary Figure S3). We detected a reduction of recent thymic emigrants (CD45RA+CCR7+CD31+) and a relative increase of naïve (CD45RA+CCR7+CD31−) CD4+ T cells in both mild and severe disease patients (Figure 4C and Supplementary Figure S2C). CD8+ T cell distribution was only changed in patients with severe disease. CD8 naïve T cells were reduced and, as reported before (52, 53) exhausted CD8+ TEMRA were significantly increased (Figure 4D and Supplementary Figure S2D).
We identified the different B-cell populations in the peripheral blood by staining with a combination of antibodies able to distinguish transitional, naïve, memory, atypical MBCs and plasmablasts (PBs) (Figure 5A). In the CD27+ MBC population, we separately analyzed IgM+ and switched MBCs. The latter include IgG+ MBCs and IgG− MBCs. Most of the IgG− B cells correspond to IgA-expressing memory B and in minimal part to MBCs without detectable surface immunoglobulin (Supplementary Figure S4A). The most significant findings were the reduction of total B cells and the increase of PBs in the severe cases (Figure 5B), as reported in other studies (54–56). Among MBCs, we found an increase of IgM+ and a reduction of switched MBCs in asymptomatic and mild cases (Figure 5C). In patients with severe disease, in contrast, we observed an increase of switched MBCs negative for IgG and mostly expressing IgA (Figure 5D). All the findings were confirmed by the cumulative analysis of all samples (Supplementary Figures S4B–D).
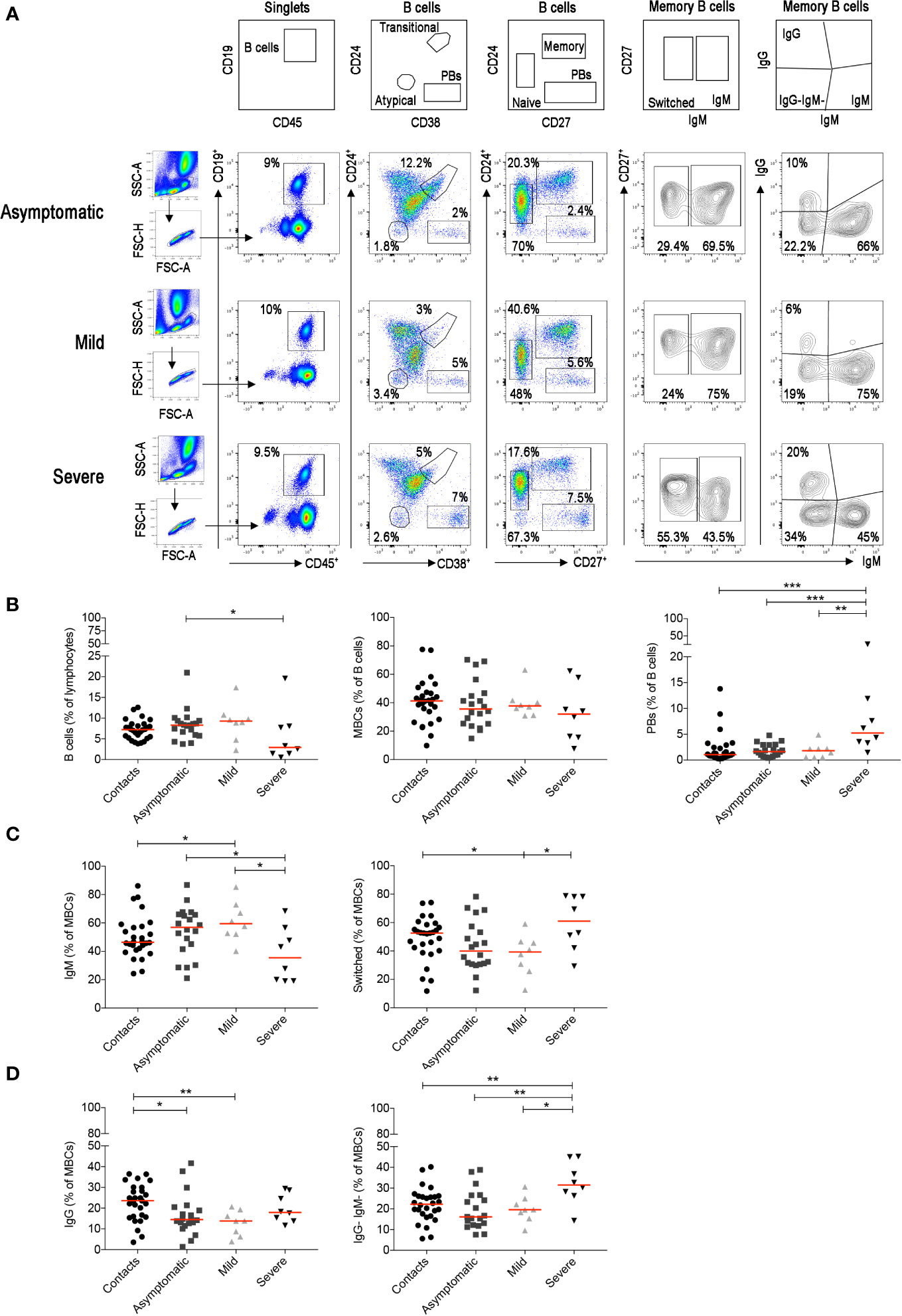
Figure 5 For the staining of the B cells we used the B-cell tube (BD biosciences) that includes: CD19, CD24, CD27, CD38, IgM, IgG, IgD, and CD21. (A) Viable lymphocytes were gated and then selected as CD19+ B cells in three representative patients with asymptomatic, mild and severe disease. The identification of the different B-cell populations is shown in the empty plots of the upper line. We identified transitional (CD24+CD38++), naïve (CD24+CD27−), memory (CD24+CD27+), atypical MBCs (CD24−CD38−) and plasmablasts (CD24−CD27++CD38++). In the CD27+ memory B-cell population based on IgM expression, we show IgM and switched (IgM−) MBCs. MBCs were also gated as IgM+, IgG+ and IgG-IgM− MBCs. (B) Plots indicate the percentage of B cells, MBCs and plasmablasts. In (C) the frequencies of IgM and switched MBCs are shown. In panel (D) we show the frequency of IgG+ and IgG−IgM−MBCs. Midlines indicate median. Statistical significances were determined using unpaired, two-tailed Mann–Whitney U-tests. *p ≤ 0.05, **p < 0.01, ***p < 0.001.
B cells fight viruses by producing antibodies when they differentiate into circulating PBs or tissue-resident plasma cells (57). The final stages of differentiation can be reached by B cells in the GCs, where either naïve or IgM+ MBCs (58, 59) acquire somatic mutations and are selected for their increased affinity to the stimulating antigen (48). T- and GC-independent antibody production is efficiently and rapidly triggered by TLR-mediated stimulation of MBCs (60, 61).
IgG and IgA antibodies directed against the S1 domain of the SARS-CoV-2 Spike protein were measured in the entire study cohort. We also measured the concentrations of IgM specific for the SARS-CoV-2 RBD.
When we analyzed all samples collected throughout the study we observed that both COVID-19 patients and SARS-CoV-2 positive asymptomatic individuals produced specific antibodies, with higher levels of IgG and IgA being detected in the serum of patients with severe disease (Figure 6A) as reported before (18, 26, 28, 52). IgM antibodies were instead equally increased in asymptomatic, mild and severe disease (Figure 6A).
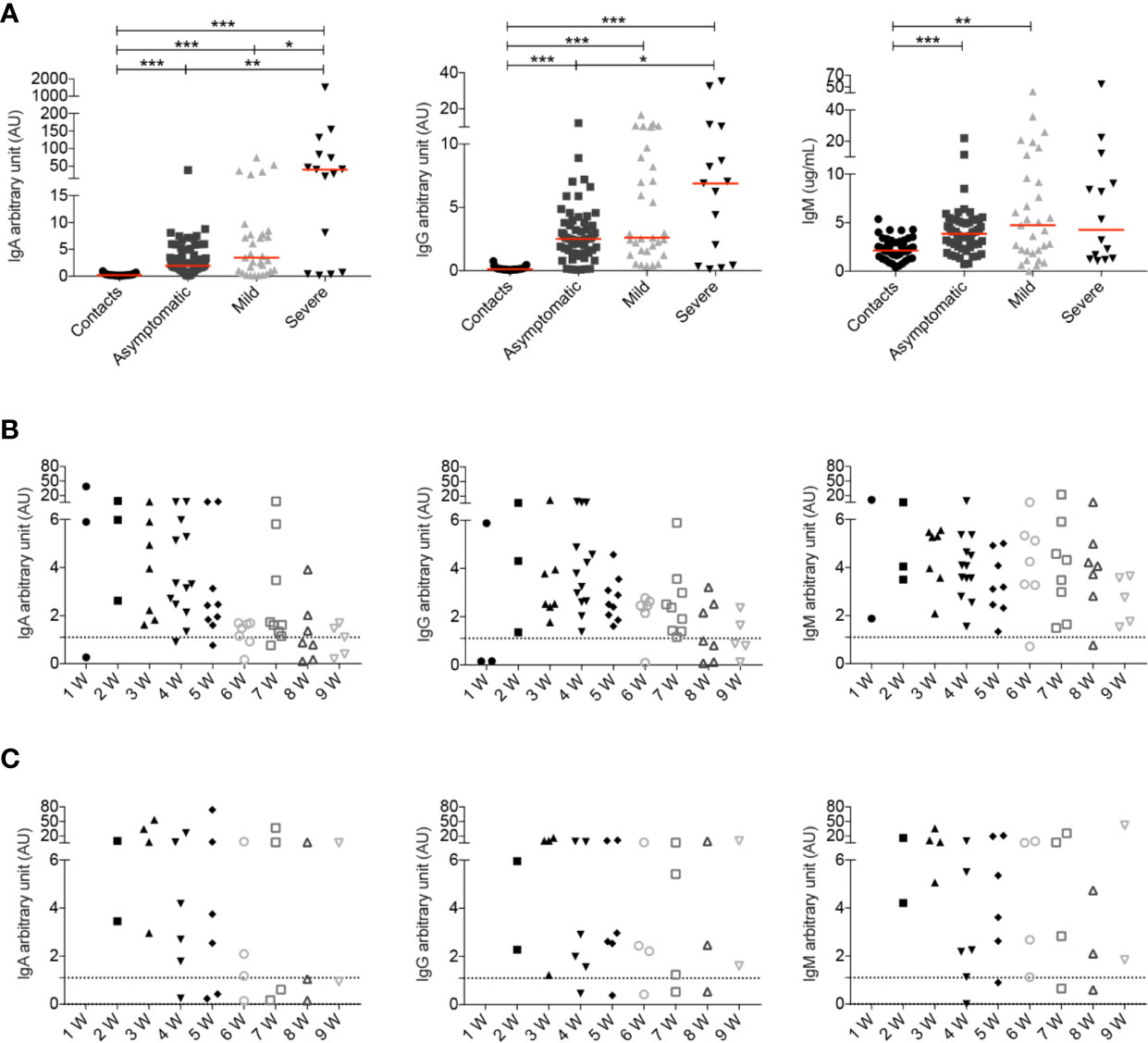
Figure 6 (A) Arbitrary units (AU) of IgG and IgA specific for the S1 domain of the SARS-CoV-2 Spike protein and concentration of RBD specific IgM were detected by ELISA at different time points. For some patients we had the opportunity to have serum samples at different time points of the disease. Data relative to all samples collected are shown (Contacts n = 51; Asymptomatic n = 63; Mild n = 31; Severe n = 15). Midlines indicate median. Statistical significances were determined using unpaired, two-tailed Mann–Whitney U-tests. *p ≤ 0.05, **p < 0.01, ***p < 0.001. (B, C) Graphs show the levels of IgA, IgG and IgM during the course of the disease in asymptomatic (B) and mild (C) patients. Time is indicated in weeks starting from the first positive nasopharyngeal swab. Dashed line indicates detection threshold (1.1).
We show in detail the kinetics of the antibody response of asymptomatic and mild disease individuals initiating from the earliest sample obtained after the first positive nasopharyngeal swab (Figures 6B, C).
We found that asymptomatic patients secrete specific IgA and IgM early after diagnosis. As for IgA and IgG there is an established threshold of positivity (dashed line), we could establish that individuals lacking IgG and IgA at week 1, later produced antibodies. Levels of IgA and IgG declined with time as reported by others (62–66). There is no established threshold for anti-RBD IgM, but we found that the median IgM concentration in 54 healthy contacts was 2.1 μg/ml (range 0.3–5.4). We found that IgM levels remained stable over the mean value of contacts in most asymptomatic individuals (Figure 6B).
In Figure 6C we show the antibody response of mild disease patients. In this group values below the threshold were found in two individuals, throughout the course of disease. Pt14, an otherwise healthy 48 year-old HCW, never produced IgG, IgA or IgM (five samples were evaluated). She had neurological symptoms and a positive nasopharyngeal swab PCR for 6 weeks. Pt12 (54-year-old), with respiratory symptoms and a PCR that remained positive for 8 weeks, had very low IgG, IgA and IgM levels fluctuating around the detection threshold. In the rest of the mild disease patients, IgA, IgG, and IgM abs increased later than in asymptomatic individuals but remained over the threshold for 9 weeks.
In summary, we observed that severe patients showed a strong antibody response in line with other observations (18, 26, 28, 52, 67). Levels of IgA and IgG in asymptomatic individuals returned to levels comparable to those measured in healthy contacts 6–8 weeks from the first positive swab. Antibodies did not decline rapidly in mild disease patients.
The first response to a novel virus is typically characterized by the cooperation between NK cells and natural antibodies, key components of innate immune system (68–72). Since the adaptive response requires time to build up after first encounter with an unknown pathogen, NK cells and natural antibodies contain the infection, whilst adaptive immune responses develop and generate highly-specific memory T and B cells that will clear the virus and prevent re-infection (22).
We still do not know whether the infection with SARS-CoV-2 triggers this typical response. We do know that individual immune responses play an important role in determining the clinical course of SARS-CoV-2 infection.
In order to understand the basis of the immune response in COVID-19, we performed a global analysis of innate and adaptive immunity in patients selected across the spectrum of disease severity, ranging from SARS-CoV-2 positive asymptomatic individuals to patients with mild and severe COVID-19. We used standard flow-cytometry and serology with the aim of finding an easy-to-use tool for the clinics.
Our data show that the balance between NK cells and monocytes is a sensitive indicator of the individual reaction to the virus and is related to the clinical course of the disease (Figures 1D, E). We calculated the ratio between the frequency of peripheral blood monocyte and NK cells (MNKR) and found that each individual included in our study maintained his typical MNKR throughout the time of follow-up (Figure 2B). The MNKR is below 1 in contacts and asymptomatic individuals and increases when monocytes expand, and NK cells are reduced. This phenomenon occurs in mild COVID-19, when the frequency of NK cell slightly declines and that of monocytes increases and is more and is more evident in patients with severe disease, where the loss of NK cells is associated to the expansion of monocytes (Figure 2B). Monocytes secrete inflammatory cytokines causing local and systemic damage (73–75). These alterations are reminiscent of Hemophagocytic Lymphohistiocytosis (HLH), a condition often related to mutations of genes governing the cytotoxic lymphocyte machinery indispensable for the function of NK cells. In HLH, chronic expansion and activation of monocytes cause the life-threatening condition known as cytokine storm (76–79). A similar “storm” is also responsible for the dramatic evolution of severe COVID-19. In these cases, therapeutic strategies aiming at controlling excessive pro-inflammatory cytokine levels have been successfully used (80, 81).
In most viral infection the production of type 1 Interferons (IFN-I) promotes NK cell expansion (82) and has a direct anti-viral effects (83). It has been demonstrated that antiviral IFN-I and III are not significantly induced by SARS-CoV-2 infection of respiratory epithelial cells, whereas a chemokines signature is established (84). As a consequence, NK cells survival and function are not supported, but monocytes are attracted to the site of infection. IFN beta-1 was administered as early treatment together with a triple combination of anti-viral drugs in a recently published multicenter, open randomized trial. The therapy was effective in suppressing the shedding of SARS-CoV-2 by acting on virus replication and innate immunity (85). In agreement with our hypothesis it has been demonstrated that inborn error of type I IFN immunity and neutralization of type I IFN function by autoantibodies are associated to the most severe forms of COVID-19 (86, 87).
It has been reported that antibodies are produced late in hospitalized patients with COVID-19: IgG increased after three weeks and IgM antibodies were transiently detected often later than IgG (16, 24). Earlier antibody production has been shown more recently (88, 89).
We measured the specific response to the S1 domain of the SARS-CoV-2 spike protein (IgG and IgA) and to the RBD (IgM) in the serum of all patients and controls in our study at different time points (Figure 6). We confirm that the highest levels of IgG and also IgA are produced by patients with severe disease. Our most interesting observation is the different kinetics of response in asymptomatic and mild disease forms of infection. The early and transient IgM, IgA, and IgG responses distinguish asymptomatic individuals from mild-disease patients, who have a slower, but more persistent antibody production (Figure 6).
In asymptomatic individuals, the early burst of IgA may rapidly and effectively eliminate the virus in the respiratory mucosa and prevent the development of a full adaptive immune reaction. The slightly slower IgG and IgA production that persist in time suggests that the adaptive immune response is triggered in mild disease and may be able to generate immunological memory. A long and severe disease fully activates the adaptive immune response and is associated with the production of anti-SARS-CoV-2 antibodies, PBs and memory B cells (90). Further studies are necessary to establish whether specific memory persist and for how long after asymptomatic and mild disease.
The particular antigen-specific IgA/IgG profile associated with clinical outcome may reflect TGFbeta production induced by coronavirus-species (91, 92). Augmented viral load may increase TGFbeta production, that, if locally secreted in the lung, facilitates neutrophil attraction and specifically induces the isotype switch to IgA (93), a situation that prompted the suggestion of anti-TGFbeta directed immunotherapies (94, 95).
Innate MBCs are increased in asymptomatic and mild disease. Innate MBCs produce natural antibodies in response to TLR stimulation (60, 96) but are also able to enter the GC where they remodel their antibodies to increase their affinity (58, 97). IgM+ MBCs are the precursors of most IgA+ and IgG+ switched MBCs (97) and give rise to IgA+ plasma cells at mucosal site (98). ‘Natural antibodies’, produced by innate MBCs, are antibodies that have a protective role in the early phases of the response independently of any previous encounter with antigen (96, 99, 100). These antibodies, have not yet been shaped by antigenic selection, carry few somatic mutations (58) and have broad reactivity (101). We recently suggested that natural antibodies might explain why most pediatric cases with laboratory-confirmed SARS-CoV-2 infection have either no or mild symptoms and recover within 1–2 weeks (102). Cross-reactive antibodies found in children and adult never exposed to SARS-CoV-2 (103) may correspond to natural antibodies. We speculate that the early IgA burst of asymptomatic individuals may derive from the rapid activation of pre-existing innate or cross-reactive IgM+ MBCs that switched to IgA in the respiratory mucosa (25), as suggested also by the demonstration that moderate levels of IgM and IgA cross-reactive to SARS-CoV-2 are present in the blood of healthy individuals never exposed to the infection (103). In addition, neutralizing IgG MBCs isolated from COVID-19 patients may have none or very few somatic mutations (104) thus suggesting that the pre-immune or innate MBC repertoire may contain SARS-CoV-2 specificities (89). In patients with severe COVID-19, IgM+ MBCs are reduced and switched MBCs are increased (Figure 5C). The increase of switched MBCs may reflect the immune reaction in the lymphoid tissue associated to the respiratory tree for local protection. Circulating PBs are also increased only in the severe cases in correlation with their higher antibody levels.
Our analysis of circulating T cells shows that SARS-COV-2 infection does not alter the T cell pool in asymptomatic individuals (Figure 4). In mild and severe COVID-19, instead, the increase of activated CD4+ T cells reflects the ongoing immune activation (Figure 4B). Activated CD4+ T cells are indispensable for the effector function during acute viral infections and for the expansion of CD8+ T cells (105). In severe cases, also CD8+ T cell are activated (Figure 4B), as also reported in a recent study analyzing the immune response in 76 COVID-19 patients from two independent cohorts (54), and TEMRA accumulate in the blood (Figure 4D). A persistent viral antigen stimulation and immune dysregulation may lead to T-cell exhaustion, a state of T-cell dysfunction demonstrated to occur during many chronic infections and cancer (106).
SARS-CoV-2 has evolved in bats, which control the infection through their innate immune system, enriched for NK receptors and different types of INF type I genes (107). Bats also produce antibodies that are highly diverse thanks to a repertoire of VH, DH and JH fragments that is much larger than that found in humans (108, 109). Antibodies do not undergo further improvement by introduction of somatic mutation. Thus, constitutive IFN type I secretion and ready-to-use antibodies may control viral infection in bats without the need of adaptive immune responses. For this reason, coronaviruses and other viruses remain endemic in bats, without damaging the host (107). Asymptomatic humans may behave like bats, controlling the infection thanks to NK cells and antibodies. The adaptive immune response is strongest in patients with severe disease, following the extensive tissue damage caused by the uncontrolled inflammatory reaction.
Our data may contribute to monitor the clinical disease. Although many large studies have described the inflammatory reaction in severe disease (76, 110), clinically it is indispensable to have prognostic markers early in the course of disease in order to promptly choose appropriate treatments (111). The increase of the ratio between Neutrophils (NLR) or Monocytes and lymphocytes (MLR), mainly caused by the loss of lymphocytes, is an indicator of severe disease (3), but does not change in less severe forms when lymphocytes numbers are still maintained (Figure 1B). We propose that the monocyte to NK ratio (MNKR) and the levels of specific IgG, IgA and IgM antibodies in the serum may be more sensible early markers of disease evolution. In particular, low level of antibodies in the first two weeks after diagnosis and increase of the MNKR may indicate patients at risk for increased severity of disease.
Our study has some limitations: 1) Patients with mild as well as severe disease were significantly older than the asymptomatic cases and contacts. The age range, however, reflects the epidemiology to COVID-19 at the study time. 2) All data on the kinetics of the COVID-19 are generated from the first blood sample collected at the time of the first positive PCR and not from to the date of first COVID-19 symptom. Due to variety of symptoms presentation and the inclusion of asymptomatic individuals, we preferred to use a uniform starting point for all subjects included in the study.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Bambino Gesù Children Hospital. The patients/participants provided their written informed consent to participate in this study.
RC, SZ, IQ, and FL designed the study and performed data analysis and manuscript preparation. EM and ST performed data experiments and data analysis and helped with manuscript preparation. FC, CCa, PatP, MMi, SC, EC, LM, EN, and FP performed data experiments. PaoP, IC, CM, VC, MV, AS, OP, CCo, AM, and MR helped with collection of study samples and clinical information. CA, GI, CQ, AZ, and MMa helped with manuscript preparation. All authors contributed to the article and approved the submitted version.
This work was funded by the RF2013-02358960 and COVID-2020-12371817 grants from the Italian Ministry of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This manuscript has been released as a pre-print at Medrxiv, doi:10.1101/2020.06.22.20137141.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.610300/full#supplementary-material
Supplementary Figure 1 | The lympho-monocyte gate was designed based on physical characteristics (FSC-A vs SSC-A). Singlets are identified by FSC-H vs FSC-A parameters. NK were identified as CD3-CD7+FSC-Alow and monocytes as CD3-CD7-FSC-Ahigh. NK cells identified as CD7+ in the CD3- gate also express CD56.
Supplementary Figure 2 | (A) Scatter plots show percentage of T cells (CD3+), CD4+ and CD8+. (B) Plots show the percentage of CD4+HLADR+and CD8+HLADR+ T cells in all sample serially collected and analyzed in the study. (C) Naïve T cells were divided based on CD31 expression (CD31+ and CD31-). (D) Plots show the percentage of TEMRA in CD4+ and CD8+ T cells in all sample serially collected and analyzed in the study. Midlines indicate median. Statistical significances were determined using unpaired, two-tailed Mann-Whitney U-tests. *p ≤ 0.05, **p < 0.01, ***p < 0.001.
Supplementary Figure 3 | Gating strategy to analyze T cells subset. The CD4+ and CD8+ T cells were subdivided into the main T cell subsets. HLADR was used to identify activated CD4+ and CD8+ T cells. Using CD45RA and CCR7: naïve (CD45RA+CCR7+), central memory (CM CD45RA-CCR7+), effector memory (EM CD45RA-CCR7−) and TEMRA (CD45RA+CCR7−) T cells were identified. Naïve T cells were further divided based on CD31 expression (CD31+ and CD31−). CM and EM were separated based on the expression of CD27 and CD28.
Supplementary Figure 4 | (A) FACS plots in three healthy donors indicates that most of IgG-IgM- MBCs correspond to IgA expressing MBCs and minimal part of these cells are IgA-IgG−IgM− MBCs. (B) Plots indicate the percentage of B cells, MBCs and plasmablasts. In (C) we show the frequencies of IgM and switched MBCs. In (D) we show the frequency of IgG+, IgG−IgM−MBCs. (B–D) Graphs refer to all samples analyzed in the study. Midlines indicate median. Statistical significances were determined using unpaired, two-tailed Mann–Whitney U-tests. *p ≤ 0.05, **p < 0.01, ***p < 0.001.
1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
2. Maggi E, Canonica GW, Moretta L. COVID-19: unanswered questions on immune response and pathogenesis. J Allergy Clin Immunol (2020) 146(1):18–22. doi: 10.1016/j.jaci.2020.05.001
3. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis (2020) 71(15):762–8. doi: 10.1093/cid/ciaa248
4. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (2020) 395(10229):1033–4. doi: 10.1016/S0140-6736(20)30628-0
5. Perlman S, Netland J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat Rev Microbiol (2009) 7(6):439–50. doi: 10.1038/nrmicro2147
6. Wong CK, Lam CWK, Wu AKL, Ip WK, Lee NLS, Chan IHS, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol (2004) 136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x
7. Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther (2020) 5(1):33. doi: 10.1038/s41392-020-0148-4
8. Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int J Infect Dis (2020) 96:131–5. doi: 10.1016/j.ijid.2020.04.086
9. Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: Implications for SARS. Nat Rev Immunol (2005) 5(12):917–27. doi: 10.1038/nri1732
10. Sun S, Cai X, Wang H, He G, Lin Y, Lu B, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chim Acta (2020) 507(8):174–80. doi: 10.1016/j.cca.2020.04.024
11. Peng J, Qi D, Yuan G, Deng X, Mei Y, Feng L, et al. Diagnostic value of peripheral hematologic markers for coronavirus disease 2019 (COVID-19): A multicenter, cross-sectional study. J Clin Lab Anal (2020) 34:e23475. doi: 10.1002/jcla.23475
12. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol (2020) 11:827. doi: 10.3389/fimmu.2020.00827
13. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol (2020) 17(5):533–5. doi: 10.1038/s41423-020-0402-2
14. Mateus J, Grifoni A, Tarke A, Sidney J, Ramirez SI, Dan JM, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Sci (80- ) (2020) 370(6512):89–94. doi: 10.1126/science.abd3871
15. Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol (2020) 5(48):eabd2071. doi: 10.1126/sciimmunol.abd2071
16. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med (2020) 71(16):2027–34. doi: 10.1038/s41591-020-0897-1
17. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci (2020) 117(17):9490–6. doi: 10.1073/pnas.2004168117
18. Okba N, Muller M, Li W, Wang C, GeurtsvanKessel C, Corman V, et al. SARS-CoV-2 specific antibody responses in COVID-19 patients. Emerg Infect Dis (2020) 26(7):1478–88. doi: 10.1101/2020.03.18.20038059
19. Liu L, Wang P, Nair MS, Yu J, Rapp M, Wang Q, et al. Potent neutralizing antibodies directed to multiple epitopes on SARS-CoV-2 spike. Nature (2020) 584:450–6. doi: 10.1038/s41586-020-2571-7
20. Wu F, Wang A, Liu M, Wang Q, Chen J, Xia S, et al. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. SSRN Electron J (2020) 2020.03.30.20047365. doi: 10.2139/ssrn.3566211
21. Phan TG, Tangye SG. Memory B cells: total recall. Curr Opin Immunol (2017) 45:132–40. doi: 10.1016/j.coi.2017.03.005
22. Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol (2015) 15:149–59. doi: 10.1038/nri3802
23. Lee YL, Liao CH, Liu PY, Cheng CY, Chung MY, Liu CE, et al. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect (2020) 81(2):e55–e8. doi: 10.1016/j.jinf.2020.04.019
24. To KK-W, Tsang OT-Y, Leung W-S, Tam AR, Wu T-C, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis (2020) 20(5):565–74. doi: 10.1016/s1473-3099(20)30196-1
25. Padoan A, Sciacovelli L, Basso D, Negrini D, Zuin S, Cosma C, et al. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin Chim Acta (2020) 507:164–7. doi: 10.1016/j.cca.2020.04.026
26. Yu H, Sun B, Fang Z, Zhao J, Liu X, Li Y, et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J (2020) 56(2):2001526. doi: 10.1183/13993003.01526-2020
27. Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol (2020) 20:269–70. doi: 10.1038/s41577-020-0308-3
28. Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis (2020) 71(16):2027–34. doi: 10.1093/cid/ciaa344
29. Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front Mol Biosci (2020) 7:157. doi: 10.3389/fmolb.2020.00157
30. Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J Virol (2019) 94(5):e02015–19. doi: 10.1128/jvi.02015-19
31. WHO Headquarters (HQ). Country & Technical Guidance - Coronavirus disease (COVID-19) (2020). Available at: https://www.who.int/publications/i/item/clinical-management-of-covid-19.
32. He R, Lu Z, Zhang L, Fan T, Xiong R, Shen X, et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol (2020) 127:104361. doi: 10.1016/j.jcv.2020.104361
33. Arabi YM, Balkhy HH, Hayden FG, Bouchama A, Luke T, Baillie JK, et al. Middle east respiratory syndrome. N Engl J Med (2017) 376:584–94. doi: 10.1056/NEJMsr1408795
34. Choi KW, Chau TN, Tsang O, Tso E, Chiu MC, Tong WL, et al. Outcomes and Prognostic Factors in 267 Patients with Severe Acute Respiratory Syndrome in Hong Kong. Ann Intern Med (2003) 139:715–23. doi: 10.7326/0003-4819-139-9-200311040-00005
35. Bordoni V, Sacchi A, Cimini E, Notari S, Grassi G, Tartaglia E, et al. An inflammatory profile correlates with decreased frequency of cytotoxic cells in COVID-19. Clin Infect Dis (2020) 71(16):2272–5. doi: 10.1093/cid/ciaa577
36. Fingerle G, Pforte A, Passlick B, Blumenstein M, Ströbel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood (1993) 82:3170–6. doi: 10.1182/blood.V82.10.3170.bloodjournal82103170
37. Nockher WA, Scherberich JE. Expanded CD14+ CD16+ Monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun (1998) 66(6):2782–90. doi: 10.1128/iai.66.6.2782-2790.1998
38. Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med (2017) 214(7):1913–23. doi: 10.1084/jem.20170355
39. Wildgruber M, Aschenbrenner T, Wendorff H, Czubba M, Glinzer A, Haller B, et al. The “intermediate” CD14++ CD16+ monocyte subset increases in severe peripheral artery disease in humans. Sci Rep (2016) 6:39483. doi: 10.1038/srep39483
40. Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol (2019) 10:2035. doi: 10.3389/fimmu.2019.02035
41. Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC. Nonclassical Monocytes in Health and Disease. Annu Rev Immunol (2019) 37:439–56. doi: 10.1146/annurev-immunol-042617-053119
42. Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell (2020) 182:1419–40. doi: 10.1016/j.cell.2020.08.001
43. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe (2020) 27(6):992–1000. doi: 10.1016/j.chom.2020.04.009
44. Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Sci (80- ) (2003) 300(5617):337–9. doi: 10.1126/science.1082305
45. Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4+ T cells in CD8+ T cell memory. Nat Rev Immunol (2016) 16(2):102–11. doi: 10.1038/nri.2015.10
46. Janssen EM, Lemmens EE, Wolfe T, Christen U, Von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature (2003) 421(6925):852–6. doi: 10.1038/nature01441
47. Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev (2013) 252(1):146–55. doi: 10.1111/imr.12031
48. Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell (2010) 143:592–605. doi: 10.1016/j.cell.2010.10.032
49. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol (2011) 29:621–63. doi: 10.1146/annurev-immunol-031210-101400
50. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (2020) 395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7
51. Zhang JY, Wang XM, Xing X, Xu Z, Zhang C, Song JW, et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat Immunol (2020) 21:1107–18. doi: 10.1038/s41590-020-0762-x
52. Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discovery (2020) 6:31. doi: 10.1038/s41421-020-0168-9
53. Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H, et al. Phenotype and kinetics of SARS-CoV-2{\textendash}specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol (2020) 5(48):eabd2071. doi: 10.1126/sciimmunol.abd2071
54. Arunachalam PS, Wimmers F, Mok CKP, Perera RAPM, Scott M, Hagan T, et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Sci (80- ) (2020) 369:1210–20. doi: 10.1126/science.abc6261
55. Zhu L, Yang P, Zhao Y, Zhuang Z, Wang Z, Song R, et al. Single-Cell Sequencing of Peripheral Mononuclear Cells Reveals Distinct Immune Response Landscapes of COVID-19 and Influenza Patients. Immunity (2020) 53(3):685–96. doi: 10.1016/j.immuni.2020.07.009
56. Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med (2020) 26:1070–6. doi: 10.1038/s41591-020-0944-y
57. Dörner T, Radbruch A. Antibodies and B Cell Memory in Viral Immunity. Immunity (2007) 27(3):384–92. doi: 10.1016/j.immuni.2007.09.002
58. Aranburu A, Piano Mortari E, Baban A, Giorda E, Cascioli S, Marcellini V, et al. Human B-cell memory is shaped by age- and tissue-specific T-independent and GC-dependent events. Eur J Immunol (2017) 47(2):327–44. doi: 10.1002/eji.201646642
59. Victora GD, Nussenzweig MC. Germinal Centers. Annu Rev Immunol (2011) 30:429–57. doi: 10.1146/annurev-immunol-020711-075032
60. Aranburu A, Ceccarelli S, Giorda E, Lasorella R, Ballatore G, Carsetti R. TLR ligation triggers somatic hypermutation in transitional B cells inducing the generation of IgM memory B cells. J Immunol (2010) 185:7293–301. doi: 10.4049/jimmunol.1002722
61. Capolunghi F, Rosado MM, Sinibaldi M, Aranburu A, Carsetti R. Why do we need IgM memory B cells? Immunol Lett (2013) 152:114–20. doi: 10.1016/j.imlet.2013.04.007
62. Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol (2020) 5:1598–607. doi: 10.1038/s41564-020-00813-8
63. Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med (2020). doi: 10.1056/nejmc2025179
64. Vabret N. Antibody responses to SARS-CoV-2 short-lived. Nat Rev Immunol (2020) 383:e74. doi: 10.1038/s41577-020-0405-3
65. Beaudoin-Bussières G, Laumaea A, Anand SP, Prévost J, Gasser R, Goyette G, et al. Decline of humoral responses against sars-cov-2 spike in convalescent individuals. MBio (2020) 11(5):e0250–20. doi: 10.1128/mBio.02590-20
66. Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med (2020) 26:1200–4. doi: 10.1038/s41591-020-0965-6
67. Rijkers G, Murk J-L, Wintermans B, van Looy B, van den Berge M, Veenemans J, et al. Differences in Antibody Kinetics and Functionality Between Severe and Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infections. J Infect Dis (2020) 222(8):1265–9. doi: 10.1093/infdis/jiaa463
68. Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol (2017) 44:43–51. doi: 10.1016/j.coi.2016.11.003
69. Vidal SM, Khakoo SI, Biron CA. Natural killer cell responses during viral infections: Flexibility and conditioning of innate immunity by experience. Curr Opin Virol (2011) 1(6):497–512. doi: 10.1016/j.coviro.2011.10.017
70. Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: Development, maturation, and clinical utilization. Front Immunol (2018) 9:1869. doi: 10.3389/fimmu.2018.01869
71. Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today (2000) 21(12):624–30. doi: 10.1016/S0167-5699(00)01754-0
72. Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Sci (80- ) (1999) 286(5447):2156–9. doi: 10.1126/science.286.5447.2156
73. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev (2020) 19(6):102537–30. doi: 10.1016/j.autrev.2020.102537
74. Sanchez-Cerrillo I, Landete P, Aldave B, Sanchez-Alonso S, Sanchez-Azofra A, Marcos-Jimenez A, et al. Differential Redistribution of Activated Monocyte and Dendritic Cell Subsets to the Lung Associates with Severity of COVID-19. medRxiv (2020). doi: 10.1101/2020.05.13.20100925
75. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol (2020) 20:355–62. doi: 10.1038/s41577-020-0331-4
76. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Sci (80- ) (2020) 55(5):105954. doi: 10.1126/science.abb8925
77. Hayden A, Park S, Giustini D, Lee AYY, Chen LYC. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: A systematic scoping review. Blood Rev (2016) 30(6):411–20. doi: 10.1016/j.blre.2016.05.001
78. Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao A, et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N Engl J Med (2020) 382:1811–22. doi: 10.1056/nejmoa1911326
79. Prilutskiy A, Kritselis M, Shevtsov A, Yambayev I, Vadlamudi C, Zhao Q, et al. SARS-CoV-2 Infection Associated Hemophagocytic Lymphohistiocytosis: An autopsy series with clinical and laboratory correlation. Am J Clin Pathol (2020) 154(4):466–74. doi: 10.1101/2020.05.07.20094888
80. Zhang S, Li L, Shen A, Chen Y, Qi Z. Rational Use of Tocilizumab in the Treatment of Novel Coronavirus Pneumonia. Clin Drug Invest (2020) 40(6):511–8. doi: 10.1007/s40261-020-00917-3
81. King A, Vail A, O’Leary C, Hannan C, Brough D, Patel H, et al. Anakinra in COVID-19: important considerations for clinical trials. Lancet Rheumatol (2020) 2(7):379–81. doi: 10.1016/S2665-9913(20)30160-0
82. Madera S, Rapp M, Firth MA, Beilke JN, Lanier LL, Sun JC. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J Exp Med (2016) 213(2):225–33. doi: 10.1084/jem.20150712
83. González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type i interferons. Nat Rev Immunol (2012) 12(2):125–35. doi: 10.1038/nri3133
84. Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell (2020) 181(5):1036–45. doi: 10.1016/j.cell.2020.04.026
85. Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet (2020) 395(10238):1695–704. doi: 10.1016/s0140-6736(20)31042-4
86. Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Sci (80- ) (2020) 370(6515):eabd4570. doi: 10.1126/science.abd4570
87. Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann H-H, Zhang Y, et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Sci (80- ) (2020) 370(6515):eabd4585. doi: 10.1126/science.abd4585
88. Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis (2020) 71(15):778–85. doi: 10.1093/cid/ciaa310
89. Nielsen SCA, Yang F, Jackson KJL, Hoh RA, Röltgen K, Jean GH, et al. Human B Cell Clonal Expansion and Convergent Antibody Responses to SARS-CoV-2. Cell Host Microbe (2020) 28(4):516–25. doi: 10.1016/j.chom.2020.09.002
90. Andreano E, Nicastri E, Paciello I, Pileri P, Manganaro N, Piccini G, et al. Identification of neutralizing human monoclonal antibodies from Italian Covid-19 convalescent patients. bioRxiv (2020). doi: 10.1101/2020.05.05.078154
91. Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, et al. An interferon-γ-related cytokine storm in SARS patients. J Med Virol (2005) 75(2):185–94. doi: 10.1002/jmv.20255
92. He L, Ding Y, Zhang Q, Che X, He Y, Shen H, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. J Pathol (2006) 210(3):288–97. doi: 10.1002/path.2067
93. Stavnezer J, Kang J. The Surprising Discovery That TGFβ Specifically Induces the IgA Class Switch. J Immunol (2009) 182(1):5–7. doi: 10.4049/jimmunol.182.1.5
94. Chen W. A potential treatment of COVID-19 with TGF-β blockade. Int J Biol Sci (2020) 16(11):1954–5. doi: 10.7150/ijbs.46891
96. Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol (2008) 180:800–8. doi: 10.4049/jimmunol.180.2.800
97. Grimsholm O, Piano Mortari E, Davydov AN, Shugay M, Obraztsova AS, Bocci C, et al. The Interplay between CD27dull and CD27bright B Cells Ensures the Flexibility, Stability, and Resilience of Human B Cell Memory. Cell Rep (2020) 30(9):2963–77. doi: 10.1016/j.celrep.2020.02.022
98. Carsetti R, Di Sabatino A, Rosado MM, Cascioli S, Piano Mortari E, Milito C, et al. Lack of Gut Secretory Immunoglobulin A in Memory B-Cell Dysfunction-Associated Disorders: A Possible Gut-Spleen Axis. Front Immunol (2020) 10:2937. doi: 10.3389/fimmu.2019.02937
99. Rosado MM, Aranburu A, Capolunghi F, Giorda E, Cascioli S, Cenci F, et al. From the fetal liver to spleen and gut: the highway to natural antibody. Mucosal Immunol (2009) 2:351–61. doi: 10.1038/mi.2009.15
100. Holodick NE, Rodríguez-Zhurbenko N, Hernández AM. Defining Natural Antibodies. Front Immunol (2017) 8:872. doi: 10.3389/fimmu.2017.00872
101. Throsby M, van den Brink E, Jongeneelen M, Poon LLM, Alard P, Cornelissen L, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PloS One (2008) 3(12):e3942. doi: 10.1371/journal.pone.0003942
102. Carsetti R, Quintarelli C, Quinti I, Piano Mortari E, Zumla A, Ippolito G, et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc Heal (2020) 4:414–6. doi: 10.1016/S2352-4642(20)30135-8
103. Selva KJ, van de Sandt CE, Lemke MM, Lee CY, Shoffner SK, Chua BY, et al. Distinct systems serology features in children, elderly and COVID patients. medRxiv (2020). doi: 10.1101/2020.05.11.20098459
104. Seydoux E, Homad LJ, MacCamy AJ, Parks KR, Hurlburt NK, Jennewein MF, et al. Analysis of a SARS-CoV-2 infected individual reveals development of potent neutralizing antibodies to distinct epitopes with limited somatic mutation. Immunity (2020) 53(1):98–105. doi: 10.1016/j.immuni.2020.06.001
105. Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev (1997) 159:105–17. doi: 10.1111/j.1600-065X.1997.tb01010.x
106. McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol (2019) 37:457–95. doi: 10.1146/annurev-immunol-041015-055318
107. Pavlovich SS, Lovett SP, Koroleva G, Guito JC, Arnold CE, Nagle ER, et al. The Egyptian Rousette Genome Reveals Unexpected Features of Bat Antiviral Immunity. Cell (2018) 173(5):1098–110. doi: 10.1016/j.cell.2018.03.070
108. Baker ML, Tachedjian M, Wang LF. Immunoglobulin heavy chain diversity in Pteropid bats: Evidence for a diverse and highly specific antigen binding repertoire. Immunogenetics (2010) 62:173–84. doi: 10.1007/s00251-010-0425-4
109. Banerjee A, Baker ML, Kulcsar K, Misra V, Plowright R, Mossman K. Novel Insights Into Immune Systems of Bats. Front Immunol (2020) 11:26. doi: 10.3389/fimmu.2020.00026
110. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine (2020) 55:102763. doi: 10.1016/j.ebiom.2020.102763
Keywords: SARS-CoV-2, COVID-19, innate and adaptiveimmune response, B cells, NK cell, monocytes, antibodies
Citation: Carsetti R, Zaffina S, Piano Mortari E, Terreri S, Corrente F, Capponi C, Palomba P, Mirabella M, Cascioli S, Palange P, Cuccaro I, Milito C, Zumla A, Maeurer M, Camisa V, Vinci MR, Santoro A, Cimini E, Marchioni L, Nicastri E, Palmieri F, Agrati C, Ippolito G, Porzio O, Concato C, Onetti Muda A, Raponi M, Quintarelli C, Quinti I and Locatelli F (2020) Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front. Immunol. 11:610300. doi: 10.3389/fimmu.2020.610300
Received: 28 September 2020; Accepted: 20 November 2020;
Published: 16 December 2020.
Edited by:
Francesca Chiodi, Karolinska Institutet (KI), SwedenReviewed by:
Anna Nilsson, Karolinska Institutet (KI), SwedenCopyright © 2020 Carsetti, Zaffina, Piano Mortari, Terreri, Corrente, Capponi, Palomba, Mirabella, Cascioli, Palange, Cuccaro, Milito, Zumla, Maeurer, Camisa, Vinci, Santoro, Cimini, Marchioni, Nicastri, Palmieri, Agrati, Ippolito, Porzio, Concato, Onetti Muda, Raponi, Quintarelli, Quinti and Locatelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita Carsetti, cml0YS5jYXJzZXR0aUBvcGJnLm5ldA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.