
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 30 October 2020
Sec. Cancer Immunity and Immunotherapy
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.592389
This article is part of the Research Topic The State-of-Art in Immuno-oncology, what to do with Glioblastoma? View all 27 articles
Gliomas, particularly high-grade gliomas including glioblastoma (GBM), represent the most common and malignant types of primary brain cancer in adults, and carry a poor prognosis. GBM has been classified into distinct subgroups over the years based on cellular morphology, clinical characteristics, biomarkers, and neuroimaging findings. Based on these classifications, differences in therapeutic response and patient outcomes have been established. Recently, the identification of complex molecular signatures of GBM has led to the development of diverse targeted therapeutic regimens and translation into multiple clinical trials. Chemical-, peptide-, antibody-, and nanoparticle-based probes have been designed to target specific molecules in gliomas and then be visualized with multimodality molecular imaging (MI) techniques including positron emission tomography (PET), single-photon emission computed tomography (SPECT), near-infrared fluorescence (NIRF), bioluminescence imaging (BLI), and magnetic resonance imaging (MRI). Thus, multiple molecules of interest can now be noninvasively imaged to guide targeted therapies with a potential survival benefit. Here, we review developments in molecular-targeted diagnosis and therapy in glioma, MI of these targets, and MI monitoring of treatment response, with a focus on the biological mechanisms of these advanced molecular probes. MI probes have the potential to noninvasively demonstrate the pathophysiologic features of glioma for diagnostic, treatment, and response assessment considerations for various targeted therapies, including immunotherapy. However, most MI tracers are in preclinical development, with only integrin αVβ3 and isocitrate dehydrogenase (IDH)-mutant MI tracers having been translated to patients. Expanded international collaborations would accelerate translational research in the field of glioma MI.
Gliomas, especially glioblastoma (GBM), are the most malignant primary brain tumors in adults (1). Numerous in vitro, in vivo, and ex vivo studies have revealed multiple molecular fingerprints of gliomas, such as methylation of the O(6)-methylguanine-DNA methyltransferase (MGMT) promoter, mutant isocitrate dehydrogenase (IDH), platelet-derived growth factor receptor (PDGFR), vascular endothelial growth factor receptor (VEGFR), integrin αvβ3 receptor, epidermal growth factor receptor (EGFR), c-Met, etc. These tumor-specific molecules can be used not only as targets for diagnosis and therapeutic response assessment, but also as potential targets for glioma treatment. Recently, advances in techniques for identifying new molecules of interest and the rapid development of novel molecular targeted inhibitors have given rise to new molecular imaging (MI) agents that have been developed using this highly selective approach.
Developments in MI techniques enable the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in living systems (2). MI probes are introduced noninvasively to determine the expression of molecular targets of interest in tumors and, when evaluated repeatedly over time in the same subject, enable the evaluation of tumor response to a given therapy. Considering the spatial and temporal heterogeneity are inherent in gliomas, MI can serve as a useful tool for overcoming some of the limitations of routine diagnostics. For example, although pathological diagnosis is considered the gold standard, it provides molecular characterization of the glioma at a single snapshot in time (e.g., prior to chemoradiation, or in the case of recurrent disease, after multiple treatments including chemoradiation) and is limited in scope to the tumor region sampled by neurosurgeon. In addition, multiple reports have demonstrated inter-rater variability for glioma pathology diagnosis among trained experts, and the superiority of molecular and genetic profiles compared to histological analyses for prediction of overall survival (OS) in patients with glioma (3, 4). Instead, by implementing an advanced MI-based approach, the molecular marker status of tumors could be interrogated repeatedly in vivo over the course of the patient’s treatment regimens. Accordingly, translational research involving these methods is currently underway at different stages including subcutaneous glioma animal models, orthotopic glioma animal models, and patients with glioma (e.g., NCT03539731).
Here, we searched PubMed (2000 to 2020) using the search terms “glioma” or “glioblastoma” in combination with “molecular imaging”, “positron emission tomography (PET)”, “fluorescence”, “magnetic resonance spectroscopy (MRS)”, and “single-photon emission computed tomography (SPECT)”. We included only articles published in English. The articles relevant to this topic were included for analysis. Next, we address the MI tracers developed for glioma and review their current stage of clinical translation. We also discuss nonspecific tracers (e.g., 18F-fluoro-2-deoxyglucose [18F-FDG] and radiolabeled amino acids) that are used to monitor for treatment response to anti-glioma therapies. Additional details about the tracers routinely utilized in glioma diagnosis and therapy have been reviewed previously (5–8). The goal of this review is to narrow the gap between multidisciplinary researchers in the fields of glioma molecular diagnosis, therapy, and imaging techniques, in order to ultimately help improve targeted diagnosis and therapy in glioma.
In Table 1, we summarize distinct MI modalities, and their corresponding tracers, in the context of targeted therapies against glioma. Other advanced MR imaging (MRI) techniques such as MR perfusion imaging, dynamic susceptibility contrast (DSC) MRI, and diffusion-weighted MRI are summarized elsewhere (18, 19).
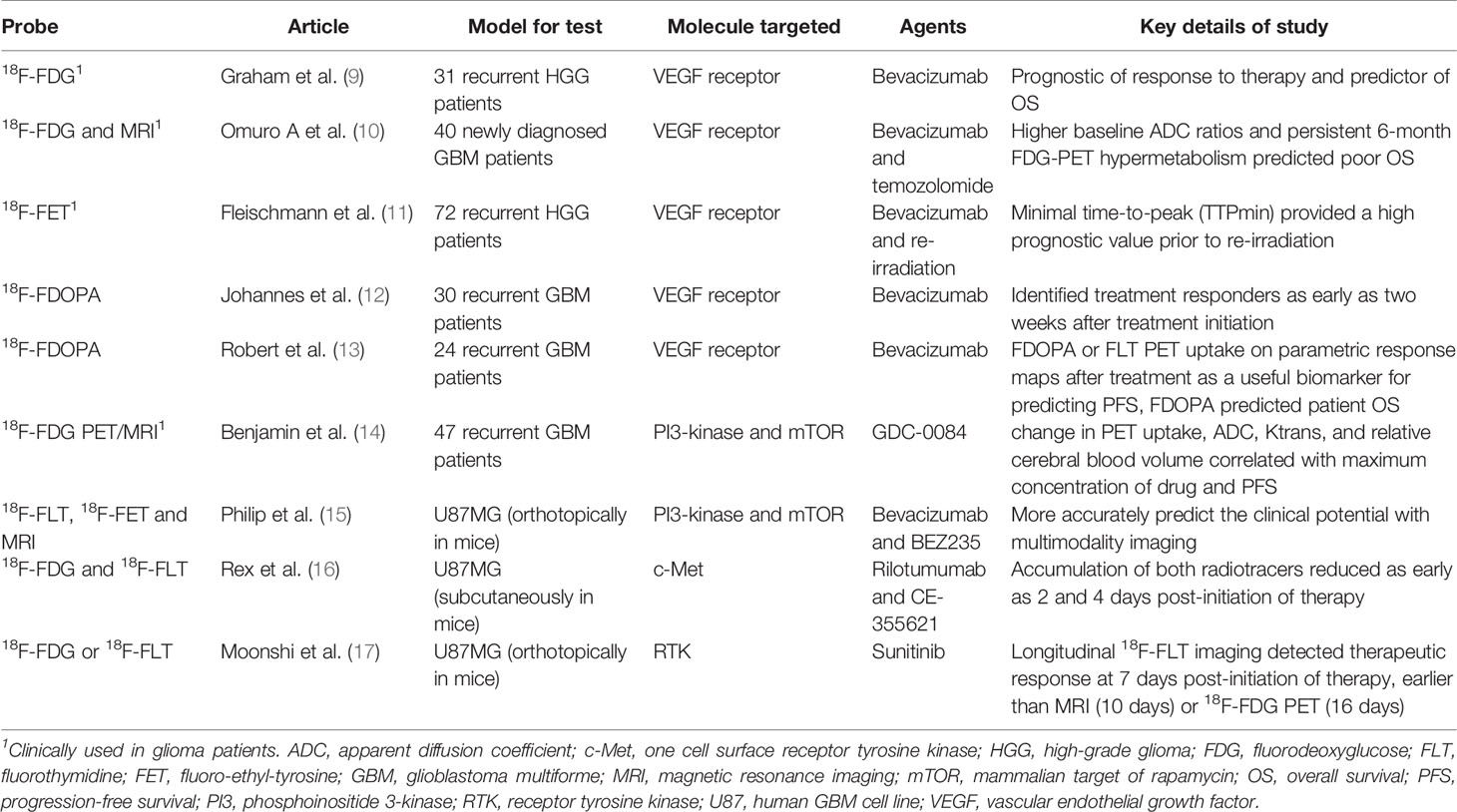
Table 1 Widely used nonspecific molecular imaging tracers to assess glioma response to targeted inhibitor therapies.
The widely used oncologic and neurologic radiotracer, 18F-FDG, has been employed not only for evaluating the efficacy of bevacizumab [the only U.S. Food and Drug Administration (FDA)–approved targeted inhibitor for recurrent GBM (20)] for newly diagnosed and recurrent GBM (9, 10), but also for monitoring efficacy of novel inhibitors against molecular targets of interest in glioma, such as c-Met [a receptor tyrosine kinase (RTK) whose ligand is hepatocyte growth factor] (16), phosphoinositide 3 (PI3)-kinase (21), mammalian target of rapamycin (mTOR) (22), and other RTKs (17). These studies demonstrate that 18F-FDG PET/computed tomography (PET/CT) can potentially detect early metabolic changes that occur before alterations discernable on traditional anatomic MRI (e.g., tumor volume) and can thus help predict OS in these patients.
To evaluate the efficacy of novel targeted medications in glioma, other MI tracers besides 18F-FDG have been used. Goggi et al. compared various PET imaging radiotracers, including 18F-FDG, 3’-deoxy-3’-18F-fluorothymidine (18F-FLT), and 2-18F-fluoroethyl-triazolyl-conjugated c(RGDyK) peptide (18F-FtRGD), for early determination of tumor response to the antiangiogenic agent axitinib in mice bearing U87MG subcutaneous tumors (23). The results showed that the retention of 18F-FtRGD exhibited a much earlier attenuation in the tumor by Day 7 (Day 3 for 18F-FLT), compared to Day 10 for 18F-FDG. Moreover, a prospective study of 16 patients with recurrent high-grade glioma (HGG) treated with bevacizumab and irinotecan concluded that both 18F-FLT-avid and 18F-fluoro-ethyl-tyrosine (18F-FET)-avid volume reduction after two months of therapy predicted progression-free survival (PFS) and OS, and the volume-based analysis of 18F-FET uptake was superior to that of 18F-FLT in predicting patient survival (24).
18F-FLT PET has gained traction in neuro-oncology imaging in Europe to help guide targeted therapy for gliomas. The use of this probe allows for direct and correlated quantification of proliferation rates through expression of the enzyme thymidine kinase-1 during DNA synthesis at an early stage (25, 26). Other studies have evaluated the 11C-methyl-L-methionine (11C-Met) radiotracer, which has been demonstrated to be an early indicator, at 3 weeks, of tumor proliferation and vessel remodeling. By comparison, 18F-FLT uptake correlated with positive Ki-67 staining only at 6 weeks in an analysis of the dynamic growth of angiogenesis-dependent/independent experimental GBM (27). Compared to the 110-min half-life of 18F, the 20-min half-life of 11C makes the latter radioisotope less amenable to practical clinical translation.
In the United States, the more commonly used amino acid-based PET radiotracer is 18F-FDOPA and its uptake has been prospectively shown to be correlated with glioma grade and cellularity (28). A prospective study of 30 patients with recurrent HGG on bevacizumab therapy demonstrated that 18F-FDOPA PET identified treatment responders as early as two weeks after starting treatment (12). In an earlier study of 18F-FDOPA and 18F-FLT PET in recurrent HGG patients treated with bevacizumab, a post-treatment increase in uptake of both radiotracers on parametric response maps (PRMs) predicted PFS, but only the 18F-FDOPA PET PRMs predicted OS (13). One advantage of the amino acid-based tracers, including 11C-Met, 18F-FET, 18F-FLT and 18F-FDOPA, etc., is the fact that their uptake does not depend on blood-brain barrier (BBB) permeability.
In another study, patients treated with the indoleamine 2,3 dioxygenase 1 (IDO1) pathway inhibitor indoximod (D1-MT) and temozolomide underwent pre-treatment and on-treatment α-11C-methyl-L-tryptophan (AMT) PET, and post-treatment imaging showed decreased regional uptake of the radiotracer (29). Because IDO1 metabolizes tryptophan into kynurenine, this strategy of using AMT PET to monitor therapeutic response with an IDO1 inhibitor serves as an example of a PET radiotracer “companion diagnostic” to targeted molecular therapy in GBM.
Noninvasive imaging of the molecular events that occur in glioma has attracted increased research interest. Several promising molecular targets have been identified, including mutant IDH, PDGFR, VEGFR, integrin αvβ3 receptor, EGFR, c-Met, etc., These molecules and their specific inhibitors have been studied in multiple trials, and we summarize the MI modalities that are being used to visualize them in the context of glioma therapy. With a focus on translation from pre-clinical models to human trials, relevant studies are summarized in Table 2.
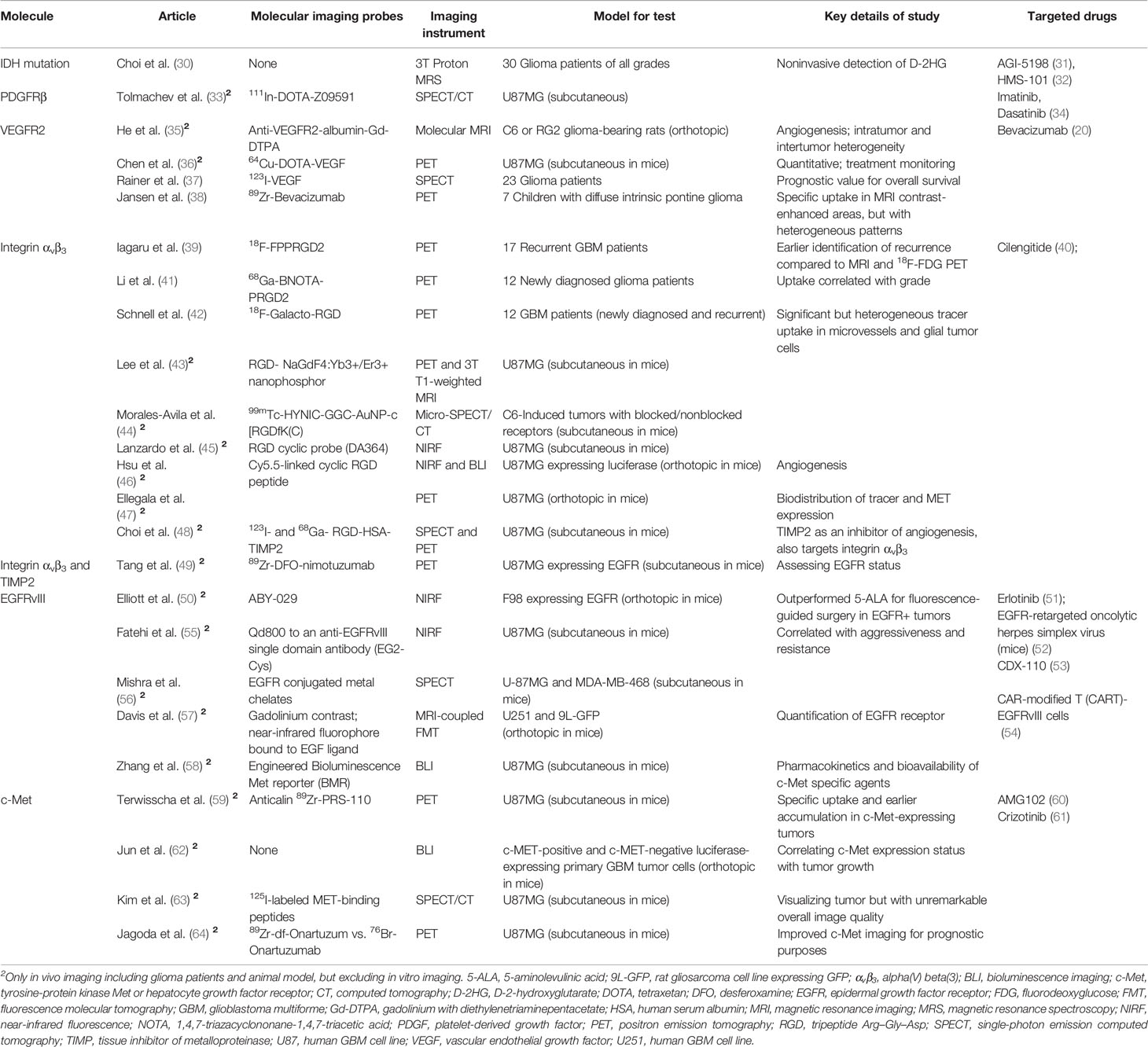
Table 2 List of in vivo visualization of specific molecules whose targeted inhibitors are under evaluation in clinical trials.
IDH mutation was identified in most astrocytomas and secondary GBM as an early and inducing event in gliomagenesis (65, 66). IDH mutation status is a predictive marker of the therapeutic efficacy of alkylating chemotherapy in HGG patients (67, 68) and has also been associated with improved prognostic (i.e., OS) value in HGG and low-grade glioma (LGG) (65, 69). Therefore, IDH mutational status was introduced into the 2016 World Health Organization (WHO) classification of cancers of the central nervous system as a crucial molecular genetic feature (70). In addition, the presence of IDH mutation itself represents a therapeutic target in glioma, and several IDH1 mutation inhibitors have been evaluated in IDH-mutant glioma patients (71).
IDH mutation can be detected using various ex vivo methods, including direct sequencing (65, 72), allele-specific PCR (73), and immunohistochemistry (IHC) (74). Several studies have also focused on D-2-hydroxyglutarate (D-2HG). Santagata et al. used desorption electrospray-ionization mass spectrometry to detect D-2HG ex vivo and found that its signal overlaps with areas of tumor and correlates with the tumor contents. They further suggested that mapping the D-2HG signal onto anatomic 3D reconstructed MR images of tumors can be integrated with advanced multimodality image-guided neurosurgical procedures to enable rapid molecular analysis of surgical tissue intraoperatively (75).
In vivo imaging of IDH mutation has attracted considerable attention. However, because of the technical challenges associated with imaging the gene mutation itself, the MI approaches are currently based on D-2HG. Choi et al. estimated the concentration of D-2HG by performing spectral fitting in the case of tumors from 30 patients. Numerical and phantom analyses of MRS pulse sequences were performed, and the results were validated with mass spectrometry of ex vivo tissues and then successfully translated to clinic with a larger prospective trial (30, 76). Such in vivo MRS methods have also been shown to detect IDH mutations (Figures 1A, B) that were missed in IHC analyses, and the reduction in D-2HG levels has been used to monitor treatment response in patients with IDH-mutant gliomas and correlated with clinical status (82, 83). A recent clinical trial and pooled analysis demonstrated the high sensitivity and specificity of MRS compared to other imaging modalities for the detection of IDH mutational status (84, 85). MRS was used to serially monitor for a decrement of D-2HG levels in gliomas in a Phase I clinical trial of a new mutant IDH1 inhibitor (86). To date, no specific IDH-mutant-specific targeted MI probe has been developed for PET or SPECT. Nonspecific probes such as 18F-FDOPA were shown to accumulate in LGG with IDH mutation (87). A more recent study suggests that dynamic 18F-FDOPA uptake parameters (e.g., time to peak SUV) rather than static uptake parameters (e.g., SUVmax) may be able to discriminate between IDH mutant and IDH wild-type gliomas (88).
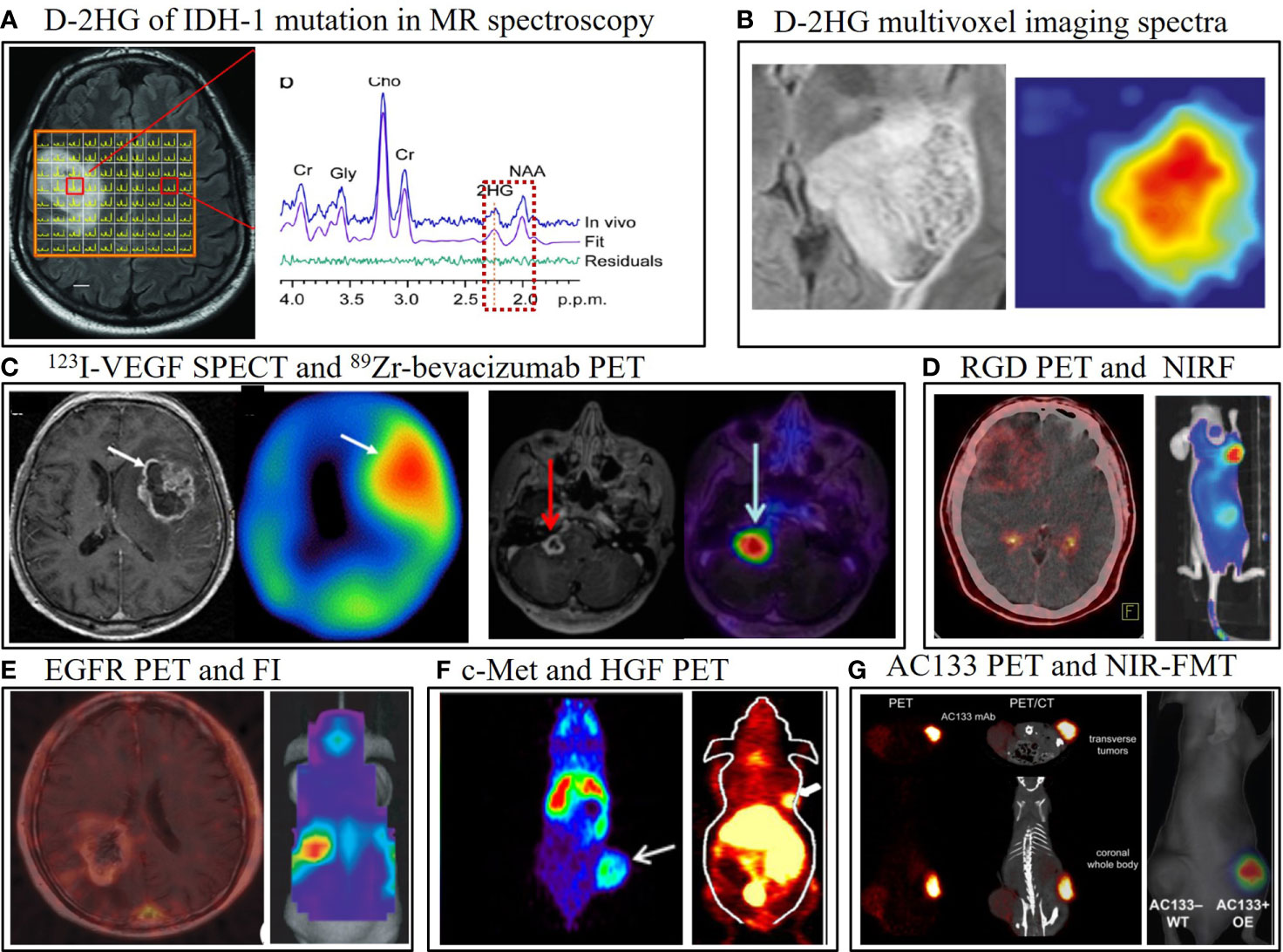
Figure 1 Representative multimodality molecular imaging in glioma, including positron emission tomography (PET), single-photon emission computed tomography (SPECT), optical, and MR spectroscopy (MRS). (A) The major catabolite of IDH-1 mutation in gliomas, D-2-hydroxyglutarate (D-2HG), can be visualized by MRS, and this technique has been translated to clinical trials (30). (B) T2/FLAIR abnormal signal area in MRI is overlaid with the D-2HG multivoxel imaging spectra in MRS (76). (C) Glioblastoma lesion uptake with the 123I-VEGF SPECT tracer (left) (37) and the 89Zr-bevacizumab PET radiotracer (144 h post-injection) fused with gadolinium-enhanced T1-weighted MRI in a child with diffuse intrinsic pontine glioma (right) (38). (D) Integrin αvβ3 visualized in a patient with glioblastoma using 68Ga-PRGD2 PET/CT by our team; RGD-Cy5.5 conjugate near-infrared fluorescence (NIRF) image showing integrin αvβ3 in a mouse bearing a subcutaneous U87MG tumor (77). (E) 11C-PD153035 PET/CT for visualization of EGFR in human glioblastoma (78); in vivo optical imaging of epidermal growth factor receptor variant III (EGFRvIII)-expressing U87MG cells orthotopically implanted in a mouse identifies the tumor after intravenous injection of a EGFRvIII single-domain antibody bioconjugated to near-infrared quantum dots, with an extra cysteine for site-specific conjugation (55). (F) 89Zr-PRS-110 PET noninvasively shows c-Met positivity in a U87MG subcutaneous tumor model (59). 64Cu-labeled recombinant human hepatocyte growth factor PET also detects c-Met expression in nude mice bearing U87MG xenografted tumors (79). (G) Mouse bearing AC133/CD133-overexpressing U251 gliomas in a subcutaneous tumor model can be imaged with 64Cu-NOTA-AC133 mAb PET/CT (80); IR700-conjugated AC133 can also identify the tumor using near-infrared fluorescence (NIRF) molecular tomography (FMT) (81). All images have been reprinted with permission; (D) is previously unpublished data.
MI of D-2HG as a marker of IDH mutant status by MRS has achieved successful clinical translation in glioma patients and can be used to serially and noninvasively monitor for this important pathophysiologic molecular marker. Further research should be conducted to integrate this imaging modality as a neuroimaging “companion diagnostic” in clinical trials of therapies targeting the IDH1 mutation, to determine whether it can stratify patients into the responder and non-responder subsets. More novel MI techniques with higher sensitivity, higher specificity, and lower dependence on BBB permeability should be developed, in light of the low sensitivity of MRS for detecting IDH mutant status in smaller tumors due to partial-volume effects (89).
PDGFR plays a critical role in HGG and synergizes with SFKs, which are nonreceptor membrane-associated tyrosine kinases. PDGFR and SFKs are both associated with the invasiveness (90), self-renewal of glioma-initiating cells, and growth of tumor vasculature in HGG (91). PDGFRβ is expressed not only in vasculature, but also in GBM-associated stromal cells, which exert tumor-promoting effects on glioma cells in vitro and in vivo (92).
Specific targeted inhibitors of PDGFRβ include first-generation single-kinase inhibitors (e.g., imatinib) and second-generation inhibitors of multiple protein tyrosine kinases (e.g., dasatinib, which targets both PDGFR and SFKs). Dasatinib has been shown to inhibit bevacizumab-induced glioma cell invasion in an orthotopic xenograft model, supporting the human translation of combining dasatinib with bevacizumab in HGG (93). However, recent clinical trials showed that dasatinib in conjunction with bevacizumab did not appear to benefit patients with newly diagnosed and recurrent GBM (94, 95). MEDI-575, an immunoglobulin G2κ monoclonal antibody that selectively binds to platelet-derived growth factor receptor α (PDGFRα), also showed limited clinical efficacy in recurrent GBM in a Phase II clinical trial (96).
Developments in visualizing PDGFR expression in glioma via MI are relatively insufficient. Tolmachev et al. designed a PDGFRβ-binding affibody molecule, Z09591, which was labeled with 111In to specifically visualize PDGFRβ expression; the affibody was used for imaging in an U87MG xenograft model by applying small-animal SPECT/CT (33). Future studies of novel PET radiotracers are warranted because they may provide increased sensitivity, specificity, and quantification accuracy. In conclusion, PDGFR can be used as a pathophysiologic marker of glioma but much work still remains for further PDGFR-based targeted therapy and imaging.
VEGF is the key pro-angiogenic protein that is overexpressed in and released by gliomas into their microenvironment (97). Glioma treatment with bevacizumab, an inhibitor of VEGF receptor (VEGFR) expressed on vascular endothelium, has led to increased PFS but no OS benefit in the patients with recurrent GBM and was approved for GBM therapy in 2009 (98). However, bevacizumab failed to show a survival advantage in two large studies of patients with newly diagnosed GBM: AVaglio in Europe and RTOG-0825 in North America (99, 100).
Selecting appropriate candidates for optimal antiangiogenic therapy is critical, and this has recently attracted considerable research attention. EGFR gene amplification are associated with shorter time to progression in patients with recurrent GBM while treated with bevacizumab (101). Other tissue-based and advanced neuroimaging parameters that are used as potential biomarkers in the setting of anti-VEGFR therapy are reviewed elsewhere (102). The 18F-radiolabeled FET, FLT, and FDG PET tracers mentioned earlier are based on cell proliferation and metabolism and can be used to indirectly assess anti-VEGFR treatment response (103). Here, we focus on VEGFR-specific MI, which may help in identifying suitable candidates for antiangiogenic treatment, as well as in evaluating treatment response and disease progression. An anti-VEGFR probe (anti-VEGFR-albumin-gadolinium) was designed to image VEGFR in C6 and RG2 glioma-bearing rats with MRI, and the findings were further confirmed through fluorescence staining and quantification of the fluorescence intensity of the anti-VEGFR probe (35). Moreover, a PET tracer, 64Cu-DOTA-VEGF, was developed for use in small-animal PET to quantify VEGFR expression levels in animal models in vivo (36).A clinical research demonstrated the SPECT using recombinant human VEGF labeled with 123I can visualize GBM rather than LGG and stratify patients’ OS based on specific T/N ratio threshold (37) (Figure 1C, left). In HGG, VEGF-based radiotracer approaches used to assess response to therapy may be confounded by endogenous VEGF levels in the tumor microenvironment that compete to bind for the same VEGFR’s on the vascular endothelium. Therefore, another approach would be to develop an anti-VEGFR-based radiolabeled antibody. An immunoPET tracer, 89Zr-bevacizumab, was designed using a diagnostic radioisotope with the commercial antibody drug (Avastin®) to visualize the heterogeneity of binding of this drug on the vascular endothelium in diffuse intrinsic pontine glioma (DIPG) (38) (Figure 1C, right).
In conclusion, VEGFR has been successfully targeted with bevacizumab as an approved therapy for recurrent GBM, and its effects could be monitored with several MI techniques. Further investigation is required to correlate these VEGF- and VEGFR-targeted MI techniques with treatment efficacy in clinical trials of bevacizumab therapy for GBM, which has potential to identify the patient subset that is most likely to respond to therapy. Taking the relatively large molecular weights of VEGF or antibody into consideration, the BBB influence of these tracers should be investigated further. The newer anti-angiogenic agents in GBM, e.g., anti-VEGF therapies like TTAC-0001 (NCT03856099), could similarly be evaluated with this MI-based approach.
Integrin alpha(V)beta(3) (αvβ3) was shown to be overexpressed in neogenic vessels and glioma cells in vitro (104) and ex vivo (105); the expression of this integrin generally correlates with malignancy grade and is a negative prognostic factor (105). Several inhibitors targeting integrin αvβ3 are under development. Cilengitide, a selective αVβ3 and αVβ5 integrin inhibitor, has been shown to inhibit GBM growth in preclinical tumor models, as well as in patients with newly diagnosed and recurrent GBM in Phase I and II clinical trials (106–110). However, in the Phase III CENTRIC EORTC 26071-22072 trial, Stupp et al. reported no OS benefit when this inhibitor was combined with standard chemotherapy in newly diagnosed GBM patients with methylation of the MGMT promoter (111).
Chinot noted several possible reasons for the failure of that trial, including screening based on MGMT promoter methylation status when this biomarker may not necessarily be associated with integrin biology (112). Another reason for failure of that trial may be the heterogeneity of integrin αvβ3 expression in GBM, which was clearly demonstrated by ex vivo IHC (105) and in vivo MI studies (42). Targeted therapy is likely to be effective only when the defined target molecule is expressed at high levels. Thus, for GBM treatment, a rational MI-based approach for future clinical trials would be to (1) confirm the existence of the target as a screening inclusion criterion before initiating integrin-inhibitor treatment and (2) serially track expression of the molecular target as a physiologic surrogate for monitoring tumor response alongside traditional anatomic MRI.
Noninvasive visualization of integrins in the setting of cancer has been developed over the past decades. Sipkins et al. visualized integrin αvβ3 by using Gd-containing liposomes coated with a monoclonal antibody (mAb) in animal models of breast cancer and malignant melanoma (113). Integrin imaging for several tumor types via multimodality imaging including MRI, ultrasound, near-infrared fluorescence (NIRF) imaging, SPECT, and PET has been reviewed elsewhere (114).
NIRF dyes conjugated with a cyclic arginine-glycine-aspartic acid (RGD) peptide were applied to visualize subcutaneously inoculated integrin-positive gliomas (46, 77, 115). Chen et al. confirmed that the specific RGD peptide−integrin interaction which was detected using the NIRF technique could be employed to noninvasively image integrin expression in almost real-time in U87MG GBM xenografts (Figure 1D, right) (77). A study using 64Cu-cyclam-RAFT-c(-RGDfK-)4 in a mouse model of glioma demonstrated its therapeutic efficacy and suitability for integrin imaging in the tumor (116).
The RGD-based MI tracers and techniques have been successfully translated to patients in clinical trials. 18F-FPPRGD2, an RGD-dimer PET tracer, was evaluated for imaging the expression of integrin αvβ3 in healthy volunteers and in patients with GBM and other cancers requiring antiangiogenic treatment (117). 18F-galacto-RGD was found to have marked yet heterogeneous uptake in microvessels and glial tumor cells (42). In another study, a relatively more specific dimer, 68Ga-BNOTA-PRGD2, was utilized (Figure 1D, left) and a semiquantitative feature of uptake was correlated with tumor grade (41). A clinical study of 18F-AlF-NOTA-PRGD2 PET/CT in newly diagnosed GBM patients showed that this integrin-targeting PET approach predicted response to chemoradiation (84.6% sensitivity, 90.0% specificity, and 87.0% accuracy) as early as 3 weeks post-initiation of treatment when using a SUVmax threshold of 1.35 (118). How much these typical peptide-based imaging tracers depend on BBB breakdown for imaging have not thoroughly assessed in suitable models.
Although integrin αvβ3-targeted inhibitors were effective in preclinical studies and small cohorts of GBM patients in phase I and II clinical trials, they failed to demonstrate a survival benefit in a Phase III trial. However, integrin receptor imaging has been successfully translated to small pilot clinical studies of GBM patients and can be used to noninvasively demonstrate the integrin receptor distribution and expression density, which supports its use as a predictive neuroimaging biomarker during screening for prospective trial participants. Before this imaging can become a reliable predictive indicator for a specific subgroup of glioma patients, the imaging probes and techniques should be further validated for improved sensitivity and specificity in human patients.
EGFR gene amplification and overexpression are striking features of GBM, particularly primary GBM. In approximately 50% of tumors showing EGFR amplification, a specific EGFR mutant, EGFR variant III (EGFRvIII), can be detected. EGFRvIII is specifically expressed in 31% of primary GBM patients, and compared to patients with wild-type EGFR GBM, those with EGFR-mutant GBM tend to have an older age at diagnosis, worse prognosis, and resistance to chemoradiotherapy (119, 120).
In addition to EGFR inhibitors (e.g., erlotinib), oncolytic HSV retargeted to GBM EGFR (52) and EGFRvIII vaccines have been evaluated in clinical trials. Rindopepimut (CDX-110) was designed to generate a specific immune response against EGFRvIII-expressing tumors, and the drug was demonstrated to benefit EGFRvIII-positive GBM patients in a Phase II trial, although it failed in a Phase III trial (ACT IV) of newly diagnosed, EGFRvIII-positive GBM patients (121, 122). Binder and colleagues reviewed possible reasons for failure of that trial, including loss of GBM EGFRvIII expression in ~60% of cases regardless of whether rindopepimut or control treatment was administered, and the lack of control arms in the previous promising Phase II trials (123). The incorporation of MI in such clinical trials to non-invasively detect the loss of expression of the target protein could prompt an earlier determination of lack of treatment efficacy, so a new therapy could be initiated that may lead to improved patient outcomes.
The first-in-human study of the chimeric antigen receptor modified T cell (CART)-EGFRvIII, as a cellular immunotherapy, in 10 recurrent GBM patients demonstrated on-target activity in brain. One patient had stable disease for over 18 months. However, the investigators found that the antigen expression decreased in the biopsied tissue in most patients (54). We believe that MI of antigen heterogeneity and reductions in antigen expression may provide earlier detection that the current therapy may no longer be efficacious, so that a different therapeutic strategy can be pursued earlier on.
EGFR-specific tracers were developed for multiple imaging modalities including SPECT, optical imaging, and MRI. Mishra et al. used anti-EGFR antibody-conjugated metal chelates in SPECT to image EGFR expression in mice bearing glioma cell lines (56). In another study, near-infrared imaging was performed on mice bearing orthotopic GBM by using a method in which a near-infrared quantum dot (Qd800) was conjugated to an anti-EGFRvIII single-domain (sd) antibody containing an extra cysteine to enable site-specific conjugation (EG2-Cys) (Figure 1E, right); this quantum dot-modified probe showed increased accumulation in tumors relative to the unconjugated quantum dot or the quantum dot conjugated to the Fc region of the antibody (EG2-hFc) (55). Another specific NIRF tracer, ABY-029, outperformed 5-ALA in detecting the tumor margin of EGFR-positive tumors and has the potential to enhance fluorescence-guided surgery (50). Lastly, 11C-PD153035 PET/CT was demonstrated to be positively correlated with ex vivo EGFR immunostaining and Western blot analysis in the case of glioma patients (Figure 1E, left) (78).
Davis et al. designed a MRI-coupled fluorescence molecular tomography (FMT) system in which gadolinium (Gd)–based contrast was used and a near-infrared fluorophore was bound to EGF, the ligand of EGFR. By using this system, the EGFR expression status in animal models of U251 and 9L-GFP tumors was quantified with 100% sensitivity and specificity (57). The FMT system was particularly effective when used in combination with the anatomy-based information provided by the Gd-enhanced MRI scan data.
Therefore, specific types of EGFR mutations should be screened with MI probes to investigate their utilization as imaging biomarkers for selecting patients for oncologic vaccine-based approaches. Future studies should also examine whether targeted EGFR-mutant MI tracers can be used to direct EGFR-targeted therapy in vivo.
Hepatocyte growth factor/scatter factor (HGF/SF) and its cell-surface receptor, the tyrosine kinase c-Met, were found to be closely linked with glioma cell invasion and tumor progression (124), and c-Met has been widely confirmed as a crucial predictor of GBM patient outcomes (125).
Nearly two decades ago, c-Met expression was not only demonstrated in glioma cells and tumor microvasculature, but was also shown to be associated with astrocytic tumors through immunohistochemical staining of ex vivo glioma samples. Elevated c-Met expression levels paralleled higher tumor grades: 21.4% positive in astrocytoma (WHO grade II) and 53.8% positive in anaplastic astrocytoma as compared with 87.5% in GBM (126). Moreover, recent research has demonstrated increased efficacy of a prognosis model that includes c-Met protein expression (127). Jun et al. found c-Met was preferentially localized in the perivascular regions of human GBM tissues that are highly clonogenic, tumorigenic, and resistant to radiation. Bioluminescence imaging (BLI) was used to monitor tumor growth in nude mouse brains implanted with c-Met-positive and c-Met-negative luciferase-expressing GBM tumor cells, and this confirmed the relationship between c-Met expression tumor growth in vivo (62).
Both c-Met pathway-targeting small molecules and mAbs have been investigated in GBM, yielding promising results. AMG 102 (rilotumumab) enhanced the efficacy of temozolomide or docetaxel in U87MG cells and xenografts (60). However, in a Phase II clinical trial of rilotumumab in heavily pretreated patients with recurrent GBM, monotherapy was not associated with significant antitumor activity (128). Cabozantinib (XL184), an oral inhibitor of multiple RTKs such as c-Met and VEGFR2, yielded favorable results in the case of advanced prostate cancer (129), thyroid cancer (130), and was approved by the U.S. FDA in 2012. Interestingly, the Phase II trial of XL184 in recurrent GBM demonstrated antitumor activity, particularly in the antiangiogenic treatment-naive cohort, with a median PFS of 3.7 months in both the 140 mg/day and 100 mg/day groups (131). In the subset of patients who had received prior antiangiogenic therapy, the objective response rate was only 4.3% with a median duration of response of 4.2 months (132).
Knockdown of the c-Met protein can make tumor necrosis factor related apoptosis-inducing ligand (TRAIL)-resistant brain tumor cells sensitive to TRAIL treatment in vitro; moreover, in nude mice intracerebrally implanted with a c-Met-knockdown tumor cell line, the effect of stem cell-delivered S-TRAIL in vivo was confirmed using BLI (133). Zhang et al. monitored gene expression quantitatively and dynamically in cultured cells and in a U87MG tumor xenograft model by using a genetically engineered bioluminescent c-Met reporter gene (58). This novel MI of the reporter gene has been gradually used to visualize the crosstalk among different relevant molecular targets in glioma animal models.
Several groups have developed new radionuclide tracers to image c-Met expression in gliomas in vivo. With SPECT imaging, the tumor can be visualized using 125I-labeled c-Met-binding peptides in human U87MG tumor-bearing mice (63). Onartuzumab, an experimental therapeutic anti-c-Met mAb, was radiolabeled with 76Br or 89Zr, and the resulting probes showed minimal background in normal brain (64). Terwisscha van Scheltinga et al. visualized c-Met expression by using an anticalin 89Zr-PRS-110 PET radiotracer in U87MG xenografts (Figure 1F, left); however, nearly 40% nonspecific uptake of this probe was confirmed in the blocking experiment, and thus further investigation is necessary (59). In another study, recombinant human HGF was labeled with 64Cu, and this probe had strong and specific binding to c-Met in a U87MG tumor model (Figure 1F, right) (79).
In summary, all the MI techniques for visualizing c-Met expression are in the preclinical phase, and they will be clinically translated after the development of targeted drugs evaluated in clinical trials.
In addition to the molecular targets for diagnosis, treatment, and imaging, other molecules exist that better characterize glioma pathophysiology including glioma stem-like cells, newly formed tumor blood vessels, etc. However, specific inhibitors against these emerging molecular biomarkers have not yet been evaluated in clinical trials. The relevant studies are summarized in Table 3.
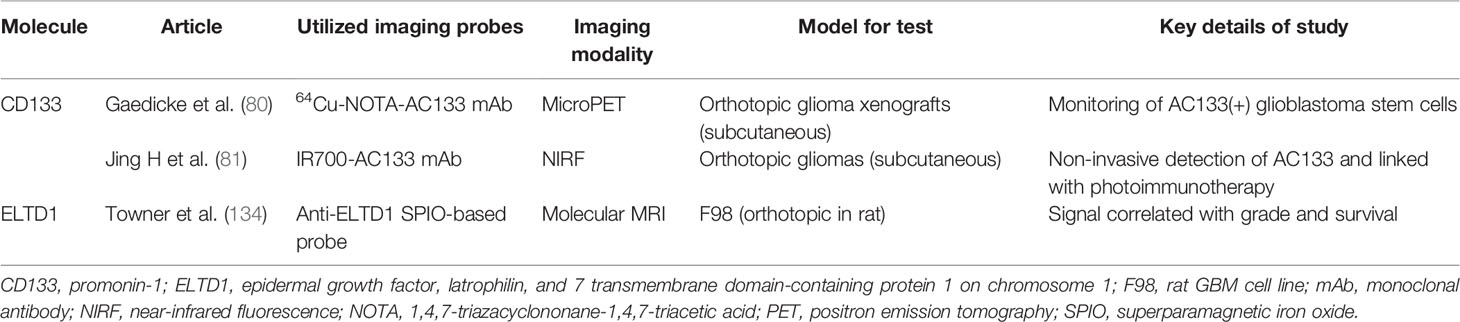
Table 3 List of in vivo visualization of specific molecules that do not yet have inhibitors under evaluation in clinical trials.
Glioma cancer stem cells (CSCs) are resistant to chemoradiotherapy and have attracted the attention of multidisciplinary researchers. Gaedicke et al. developed a new imaging tracer targeting the AC133 epitope of CD133, which is a well-investigated CSC marker. An AC133-specific mAb was radiolabeled with 64Cu to generate 64Cu-NOTA-AC133 mAb, which was used to monitor AC133-positive GBM CSCs. High-sensitivity and high-resolution images were obtained in animal models using both PET and NIRF imaging (Figure 1G) (80). A novel small peptide, CBP4, was linked to gold nanoparticles and the resultant probe was shown to be suitable as an imaging agent for CD133-expressing GBM CSCs (135). Jing et al. conjugated the AC133 antibody with an IR700 dye and showed that the resulting probe can be used noninvasively to assess AC133-positive gliomas via near-infrared FMT; the probe was employed in near-infrared photoimmunotherapy to effectively induce cell death and tumor shrinkage in an animal model (81).
EGF, latrophilin, and 7-transmembrane domain-containing protein 1 on chromosome 1 (ELTD1) was identified as a putative glioma-associated marker using a bioinformatics method and reported to be associated with glioma grade and patient survival by Towner et al. (134). An anti-ELTD1 superparamagnetic iron oxide (SPIO)-based probe was designed by coating SPIO nanoparticles with dextran and conjugating an anti-ELTD1 antibody. This probe was used to assess the in vivo levels of ELTD1, and further investigation revealed that the anti-ELTD1 antibody inhibited glioma growth in mouse glioma models, an effect that could be attributed to diminished vascularization (136).
We divided the translation process (from bench to bedside) into three stages of development: (1) Preclinical stage that includes subcutaneous animal models with glioma cell lines; (2) Preclinical stage that includes orthotopic animal models with glioma cell lines; and (3) Clinical stage that involves glioma patients. In Figure 2, we summarize the progress from pre-clinical to clinical translation of the abovementioned targeted MI tracers. Most of the targeted tracers have only been studied in animal models. The MI studies evaluated in human glioma patients target integrin αVβ3, IDH-mutation and VEGFR, pyruvate kinase M2 and have been imaged using PET/CT, SPECT and MRI modalities. The superior molecular sensitivity of PET, the lack of radiation, and high spatial resolution of MRI render these techniques much easier to translate, along with the fact that they are routinely used in the medical field. Optical imaging (e.g., NIRF and BLI), have also been utilized to image molecular expression in glioma xenografts in subcutaneous and orthotopic animal models. Although penetration depth remains a challenge in optical imaging, intraoperative imaging could represent a promising area of research following further development in both imaging technique and tracer design. Multimodality imaging can provide a possible solution to overcome certain limitations of current methods (e.g., PET and MRI for imaging integrin αvβ3, or optical imaging and MRI for imaging EGFR and IGFBP7). This strategy could enable imaging to be performed, using a single probe, on multiple imaging platforms with diverse disease models, ranging from small animal models to large animal models and even humans.
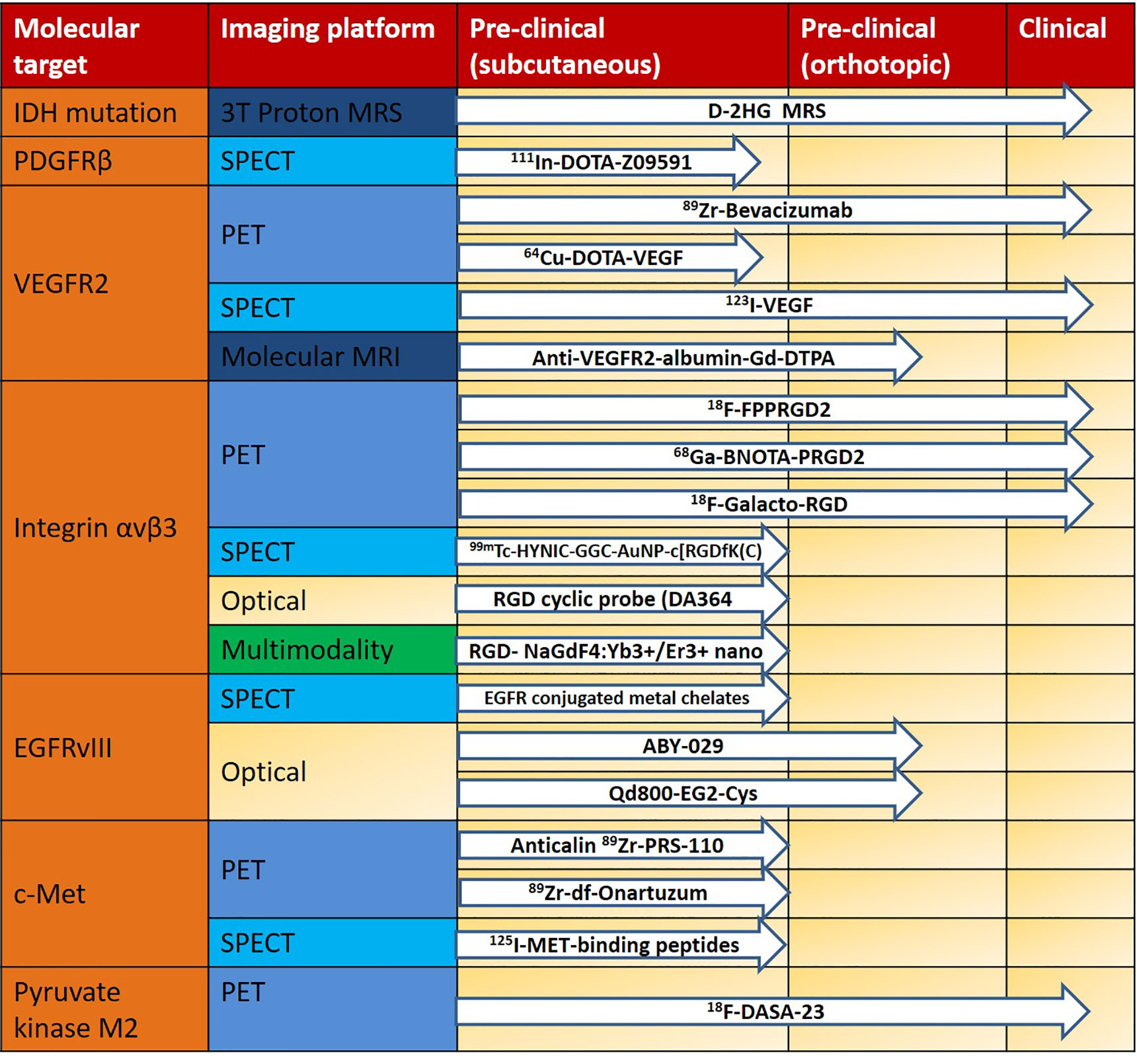
Figure 2 Translational pipeline of molecular imaging probes in glioma using different imaging platforms. IDH, isocitrate dehydrogenase; MRS, magnetic resonance spectroscopy; PDGFRβ, platelet-derived growth factor receptor beta; SPECT, single-photon emission computed tomography; VEGFR2, vascular endothelial growth factor receptor 2; PET, positron emission tomography; MRI, magnetic resonance imaging; Integrin αvβ3, integrin alpha(V)beta(3); EGFRvIII, epidermal growth factor receptor variant III.
With the discovery of multiple new molecular targets in glioma, the design and clinical translation of novel targeted diagnostics, treatments, and MI techniques have rapidly developed. MI offers several promising advantages over conventional anatomic imaging in glioma. Firstly, specific molecular expression patterns and therapeutic responses can be serially imaged in vivo, particularly for HGG patients, who typically undergo surgical treatment once at the time of initial diagnosis. Because of the minimal risk to patients, MI can be performed repeatedly if necessary, and can be used to evaluate tumor heterogeneity across the entire tumor, including its resected and residual components. Secondly, MI can potentially visualize prognostic and predictive biomarkers of interest to aid in selecting appropriate patients for molecular-targeted therapy. This approach would promote the evidence-based selection of patients for molecular-targeted therapeutic clinical trials and thereby possibly increase the success of improving survival in the appropriate patient cohort. Thirdly, MI can be applied routinely for the development and assessment of novel anti-glioma drugs or immunotherapy agents, because it can accurately monitor the pharmacodynamic and bioavailability of therapeutics in tumors.
Multimodality imaging probes can be designed to detect multiple biomarkers concurrently in glioma patients, and thus noninvasively map crucial molecules in this heterogenous and challenging disease. Given the advantages mentioned above, MI can represent an optimal method for achieving personalized medical care for glioma patients (137). To the previously identified “3 Rs” (right patient, right time, and right drug), MI enables us to add a fourth “R”: right dosing.
Although MI offers several advantages, the use of this method in clinical research and practice currently remains at an early stage. Most MI probes are in the preclinical stage, while MI tracers targeting integrin αVβ3, VEGF receptor, and IDH-mutation have been successfully translated to pilot studies in glioma patients. Another potential limitation is that most of these studies are based on the use of peptides, proteins, and even nanoparticles. Demand exists for designing small-molecule tracers that can cross the BBB, which generally hinders the use of MI in the case of LGG with relatively more intact BBB functionality compared to HGG.
Accelerating the clinical translation of MI to benefit patients with glioma will only be achieved with deft navigation of regulatory requirements and multi-center, international cooperation. Firstly, after the potential toxicity of MI probes has been tested in small-animal models, we recommend taking advantage of early exploratory Investigational New Drug studies (138). Due to the very low concentrations of injected tracers visualized on exquisitely sensitive MI platforms, this regulatory compliance strategy is more apt for MI research in an incurable disease such as GBM. Secondly, accrual of a sufficient number of patients into MI studies to make meaningful conclusions will require international multi-center clinical trials that are guided by uniform research protocols with built-in continual quality assessment and quality control.
DL and CP wrote the manuscript, under the supervision of LZ and ZC. Other authors participated in some discussions. All authors contributed to the article and approved the submitted version.
This paper was partially sponsored by the Beijing Medical Research (2018-7), the National Natural Science Foundation of China Projects (81971668), Beijing Nova Program (xx2017017), Beijing Talents foundation, Clinical Scientist Supporting grant of Beijing Tiantan Hospital (YSP201902), as well as the funding from the Radiology Department at Stanford University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Tracy Burk for helping with communication among co-authors with different backgrounds. We also thank William Ding for proofreading this article and language revision. Finally, we dedicate this manuscript to the memory of Sanjiv S. Gambhir, our dear colleague, advisor, and friend.
1. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol (2019) 21(Suppl 5):v1–v100. doi: 10.1093/neuonc/noz150
3. Gravendeel LA, Kouwenhoven MC, Gevaert O, de Rooi JJ, Stubbs AP, Duijm JE, et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res (2009) 69(23):9065–72. doi: 10.1158/0008-5472.CAN-09-2307
4. Holdhoff M, Ye X, Piotrowski AF, Strowd RE, Seopaul S, Lu Y, et al. The consistency of neuropathological diagnoses in patients undergoing surgery for suspected recurrence of glioblastoma. J Neurooncol (2019) 141(2):347–54. doi: 10.1007/s11060-018-03037-3
5. Ahmed R, Oborski MJ, Hwang M, Lieberman FS, Mountz JM. Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag Res (2014) 6:149–70. doi: 10.2147/CMAR.S54726
6. Sharma A, McConathy J. Overview of PET tracers for brain tumor imaging. PET Clin (2013) 8:129–46. doi: 10.1016/j.cpet.2013.02.001
7. Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol (2016) 18(9):1199–208. doi: 10.1093/neuonc/now058
8. Muoio B, Giovanella L, Treglia G. Recent Developments of 18F-FET PET in Neuro-oncology. Curr Med Chem (2018) 25(26):3061–73. doi: 10.2174/0929867325666171123202644
9. Graham MS, Krebs S, Bale T, Domfe K, Lobaugh SM, Zhang Z, et al. Value of [(18)F]-FDG positron emission tomography in patients with recurrent glioblastoma receiving bevacizumab. Neurooncol Adv (2020) 2(1):vdaa050. doi: 10.1093/noajnl/vdaa050
10. Omuro A, Beal K, Gutin P, Karimi S, Correa DD, Kaley TJ, et al. Phase II Study of Bevacizumab, Temozolomide, and Hypofractionated Stereotactic Radiotherapy for Newly Diagnosed Glioblastoma. Clin Cancer Res (2014) 20(19):5023–31. doi: 10.1158/1078-0432.CCR-14-0822
11. Fleischmann DF, Unterrainer M, Bartenstein P, Belka C, Albert NL, Niyazi M. (18)F-FET PET prior to recurrent high-grade glioma re-irradiation-additional prognostic value of dynamic time-to-peak analysis and early static summation images? J Neurooncol (2017) 132(2):277–86. doi: 10.1007/s11060-016-2366-8
12. Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Grogan T, et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res (2014) 20(13):3550–9. doi: 10.1158/1078-0432.CCR-13-1440
13. Harris RJ, Cloughesy TF, Pope WB, Nghiemphu PL, Lai A, Zaw T, et al. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol (2012) 14(8):1079–89. doi: 10.1093/neuonc/nos141
14. Ellingson BM, Yao J, Raymond C, Nathanson DA, Chakhoyan A, Simpson J, et al. Multiparametric MR-PET Imaging Predicts Pharmacokinetics and Clinical Response to GDC-0084 in Patients with Recurrent High-Grade Glioma. Clin Cancer Res (2020) 26(13):3135–44. doi: 10.1158/1078-0432.CCR-19-3817
15. O’Halloran PJ, Viel T, Murray DW, Wachsmuth L, Schwegmann K, Wagner S, et al. Mechanistic interrogation of combination bevacizumab/dual PI3K/mTOR inhibitor response in glioblastoma implementing novel MR and PET imaging biomarkers. Eur J Nucl Med Mol Imaging (2016) 43(9):1673–83. doi: 10.1007/s00259-016-3343-3
16. Rex K, Lewis XZ, Gobalakrishnan S, Glaus C, Silva MD, Radinsky R, et al. Evaluation of the antitumor effects of rilotumumab by PET imaging in a U-87 MG mouse xenograft model. Nucl Med Biol (2013) 40(4):458–63. doi: 10.1016/j.nucmedbio.2013.01.004
17. Moonshi SS, Bejot R, Atcha Z, Vijayaragavan V, Bhakoo KK, Goggi JL. A comparison of PET imaging agents for the assessment of therapy efficacy in a rodent model of glioma. Am J Nucl Med Mol Imaging (2013) 3(5):397–407.
18. Hutterer M, Hattingen E, Palm C, Proescholdt MA, Hau P. Current standards and new concepts in MRI and PET response assessment of antiangiogenic therapies in high-grade glioma patients. Neuro Oncol (2015) 17(6):784–800. doi: 10.1093/neuonc/nou322
19. Shukla G, Alexander GS, Bakas S, Nikam R, Talekar K, Palmer JD, et al. Advanced magnetic resonance imaging in glioblastoma: a review. Chin Clin Oncol (2017) 6(4):40. doi: 10.21037/cco.2017.06.28
20. Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist (2009) 14(11):1131–8. doi: 10.1634/theoncologist.2009-0121
21. Cawthorne C, Burrows N, Gieling RG, Morrow CJ, Forster D, Gregory J, et al. [18F]-FLT positron emission tomography can be used to image the response of sensitive tumors to PI3-kinase inhibition with the novel agent GDC-0941. Mol Cancer Ther (2013) 12(5):819–28. doi: 10.1158/1535-7163.MCT-12-0905
22. Keen HG, Ricketts SA, Maynard J, Logie A, Odedra R, Shannon AM, et al. Examining changes in [18 F]FDG and [18 F]FLT uptake in U87-MG glioma xenografts as early response biomarkers to treatment with the dual mTOR1/2 inhibitor AZD8055. Mol Imaging Biol (2014) 16(3):421–30. doi: 10.1007/s11307-013-0705-0
23. Goggi JL, Bejot R, Moonshi SS, Bhakoo KK. Stratification of 18F-labeled PET imaging agents for the assessment of antiangiogenic therapy responses in tumors. J Nucl Med (2013) 54(9):1630–6. doi: 10.2967/jnumed.112.115824
24. Martha Nowosielski MH, Putzer D, Iglseder S, Seiz M, Jacobs AH, Gobel G, et al. FET- and FLT-PET response assessment of anti-angiogenic therapy in recurrent high-grade glioma comparing tracer uptake and volume reduction. Neuro Oncol (2012) 14(vi):122–3. doi: 10.1093/neuonc/nos236
25. Ullrich R, Backes H, Li H, Kracht L, Miletic H, Kesper K, et al. Glioma proliferation as assessed by 3’-fluoro-3’-deoxy-L-thymidine positron emission tomography in patients with newly diagnosed high-grade glioma. Clin Cancer Res (2008) 14(7):2049–55. doi: 10.1158/1078-0432.CCR-07-1553
26. Backes H, Ullrich R, Neumaier B, Kracht L, Wienhard K, Jacobs AH. Noninvasive quantification of 18F-FLT human brain PET for the assessment of tumour proliferation in patients with high-grade glioma. Eur J Nucl Med Mol Imaging (2009) 36(12):1960–7. doi: 10.1007/s00259-009-1244-4
27. Viel T, Talasila KM, Monfared P, Wang J, Jikeli JF, Waerzeggers Y, et al. Analysis of the growth dynamics of angiogenesis-dependent and -independent experimental glioblastomas by multimodal small-animal PET and MRI. J Nucl Med (2012) 53(7):1135–45. doi: 10.2967/jnumed.111.101659
28. Pafundi DH, Laack NN, Youland RS, Parney IF, Lowe VJ, Giannini C, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol (2013) 15(8):1058–67. doi: 10.1093/neuonc/not002
29. Lukas RV, Juhasz C, Wainwright DA, James CD, Kennedy E, Stupp R, et al. Imaging tryptophan uptake with positron emission tomography in glioblastoma patients treated with indoximod. J Neurooncol (2019) 141(1):111–20. doi: 10.1007/s11060-018-03013-x
30. Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med (2012) 18(4):624–9. doi: 10.1038/nm.2682
31. Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science (2013) 340(6132):626–30. doi: 10.1126/science.1236062
32. Chaturvedi A, Araujo Cruz MM, Jyotsana N, Sharma A, Yun H, Gorlich K, et al. Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood (2013) 122(16):2877–87. doi: 10.1182/blood-2013-03-491571
33. Tolmachev V, Varasteh Z, Honarvar H, Hosseinimehr SJ, Eriksson O, Jonasson P, et al. Imaging of platelet-derived growth factor receptor beta expression in glioblastoma xenografts using affibody molecule 111In-DOTA-Z09591. J Nucl Med (2014) 55(2):294–300. doi: 10.2967/jnumed.113.121814
34. Ahluwalia MS, de Groot J, Liu WM, Gladson CL. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett (2010) 298(2):139–49. doi: 10.1016/j.canlet.2010.08.014
35. He T, Smith N, Saunders D, Pittman BP, Lerner M, Lightfoot S, et al. Molecular MRI differentiation of VEGF receptor-2 levels in C6 and RG2 glioma models. Am J Nucl Med Mol Imaging (2013) 3(4):300–11.
36. Chen K, Cai W, Li ZB, Wang H, Chen X. Quantitative PET imaging of VEGF receptor expression. Mol Imaging Biol (2009) 11(1):15–22. doi: 10.1007/s11307-008-0172-1
37. Rainer E, Wang H, Traub-Weidinger T, Widhalm G, Fueger B, Chang J, et al. The prognostic value of [(123)I]-vascular endothelial growth factor ([(123)I]-VEGF) in glioma. Eur J Nucl Med Mol Imaging (2018) 45(13):2396–403. doi: 10.1007/s00259-018-4088-y
38. Jansen MH, Veldhuijzen van Zanten SEM, van Vuurden DG, Huisman MC, Vugts DJ, Hoekstra OS, et al. Molecular Drug Imaging: (89)Zr-Bevacizumab PET in Children with Diffuse Intrinsic Pontine Glioma. J Nucl Med (2017) 58(5):711–6. doi: 10.2967/jnumed.116.180216
39. Iagaru A, Mosci C, Mittra E, Zaharchuk G, Fischbein N, Harsh G, et al. Glioblastoma Multiforme Recurrence: An Exploratory Study of (18)F FPPRGD2 PET/CT. Radiology (2015) 277(2):497–506. doi: 10.1148/radiol.2015141550
40. Scaringi C, Minniti G, Caporello P, Enrici RM. Integrin inhibitor cilengitide for the treatment of glioblastoma: a brief overview of current clinical results. Anticancer Res (2012) 32(10):4213–23.
41. Li D, Zhao X, Zhang L, Li F, Ji N, Gao Z, et al. (68)Ga-PRGD2 PET/CT in the evaluation of Glioma: a prospective study. Mol Pharm (2014) 11(11):3923–9. doi: 10.1021/mp5003224
42. Schnell O, Krebs B, Carlsen J, Miederer I, Goetz C, Goldbrunner RH, et al. Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol (2009) 11(6):861–70. doi: 10.1215/15228517-2009-024
43. Lee J, Lee TS, Ryu J, Hong S, Kang M, Im K, et al. RGD peptide-conjugated multimodal NaGdF4:Yb3+/Er3+ nanophosphors for upconversion luminescence, MR, and PET imaging of tumor angiogenesis. J Nucl Med (2013) 54(1):96–103. doi: 10.2967/jnumed.112.108043
44. Morales-Avila E, Ferro-Flores G, Ocampo-Garcia BE, De Leon-Rodriguez LM, Santos-Cuevas CL, Garcia-Becerra R, et al. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c[RGDfK(C)] for molecular imaging of tumor alpha(v)beta(3) expression. Bioconjug Chem (2011) 22(5):913–22. doi: 10.1021/bc100551s
45. Lanzardo S, Conti L, Brioschi C, Bartolomeo MP, Arosio D, Belvisi L, et al. A new optical imaging probe targeting alphaVbeta3 integrin in glioblastoma xenografts. Contrast Media Mol Imaging (2011) 6(6):449–58. doi: 10.1002/cmmi.444
46. Hsu AR, Hou LC, Veeravagu A, Greve JM, Vogel H, Tse V, et al. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in an orthotopic glioblastoma model. Mol Imaging Biol (2006) 8(6):315–23. doi: 10.1007/s11307-006-0059-y
47. Ellegala DB, Leong-Poi H, Carpenter JE, Klibanov AL, Kaul S, Shaffrey ME, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation (2003) 108(3):336–41. doi: 10.1161/01.CIR.0000080326.15367.0C
48. Choi N, Kim SM, Hong KS, Cho G, Cho JH, Lee C, et al. The use of the fusion protein RGD-HSA-TIMP2 as a tumor targeting imaging probe for SPECT and PET. Biomaterials (2011) 32(29):7151–8. doi: 10.1016/j.biomaterials.2011.06.007
49. Tang Y, Hu Y, Liu W, Chen L, Zhao Y, Ma H, et al. A radiopharmaceutical [(89)Zr]Zr-DFO-nimotuzumab for immunoPET with epidermal growth factor receptor expression in vivo. Nucl Med Biol (2019) 70:23–31. doi: 10.1016/j.nucmedbio.2019.01.007
50. Elliott JT, Marra K, Evans LT, Davis SC, Samkoe KS, Feldwisch J, et al. Simultaneous In Vivo Fluorescent Markers for Perfusion, Protoporphyrin Metabolism, and EGFR Expression for Optically Guided Identification of Orthotopic Glioma. Clin Cancer Res (2017) 23(9):2203–12. doi: 10.1158/1078-0432.CCR-16-1400
51. Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia (2010) 12(9):675–84. doi: 10.1593/neo.10688
52. Uchida H, Marzulli M, Nakano K, Goins WF, Chan J, Hong CS, et al. Effective treatment of an orthotopic xenograft model of human glioblastoma using an EGFR-retargeted oncolytic herpes simplex virus. Mol Ther (2013) 21(3):561–9. doi: 10.1038/mt.2012.211
53. Swartz AM, Li QJ, Sampson JH. Rindopepimut: a promising immunotherapeutic for the treatment of glioblastoma multiforme. Immunotherapy (2014) 6(6):679–90. doi: 10.2217/imt.14.21
54. O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med (2017) 9(399):1–30. doi: 10.1126/scitranslmed.aaa0984
55. Fatehi D, Baral TN, Abulrob A. In vivo imaging of brain cancer using epidermal growth factor single domain antibody bioconjugated to near-infrared quantum dots. J Nanosci Nanotechnol (2014) 14(7):5355–62. doi: 10.1166/jnn.2014.9076
56. Mishra G, Panwar P, Mishra AK. Tumor targeting using anti-epidermal growth factor receptor (ior egf/r3) immunoconjugate with a tetraaza macrocyclic agent (DO3A-EA). Mol Imaging (2012) 11(5):408–16.
57. Davis SC, Samkoe KS, O’Hara JA, Gibbs-Strauss SL, Payne HL, Hoopes PJ, et al. MRI-coupled fluorescence tomography quantifies EGFR activity in brain tumors. Acad Radiol (2010) 17(3):271–6. doi: 10.1016/j.acra.2009.11.001
58. Zhang L, Virani S, Zhang Y, Bhojani MS, Burgess TL, Coxon A, et al. Molecular imaging of c-Met tyrosine kinase activity. Anal Biochem (2011) 412(1):1–8. doi: 10.1016/j.ab.2011.01.028
59. Terwisscha van Scheltinga AG, Lub-de Hooge MN, Hinner MJ, Verheijen RB, Allersdorfer A, Hulsmeyer M, et al. In vivo visualization of MET tumor expression and anticalin biodistribution with the MET-specific anticalin 89Zr-PRS-110 PET tracer. J Nucl Med (2014) 55(4):665–71. doi: 10.2967/jnumed.113.124941
60. Jun HT, Sun J, Rex K, Radinsky R, Kendall R, Coxon A, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res (2007) 13(22 Pt 1):6735–42. doi: 10.1158/1078-0432.CCR-06-2969
61. Goodwin CR, Rath P, Oyinlade O, Lopez H, Mughal S, Xia S, et al. Crizotinib and erlotinib inhibits growth of c-Met(+)/EGFRvIII(+) primary human glioblastoma xenografts. Clin Neurol Neurosurg (2018) 171:26–33. doi: 10.1016/j.clineuro.2018.02.041
62. Jun HJ, Bronson RT, Charest A. Inhibition of EGFR induces a c-MET-driven stem cell population in glioblastoma. Stem Cells (2014) 32(2):338–48. doi: 10.1002/stem.1554
63. Kim EM, Park EH, Cheong SJ, Lee CM, Kim DW, Jeong HJ, et al. Characterization, biodistribution and small-animal SPECT of I-125-labeled c-Met binding peptide in mice bearing c-Met receptor tyrosine kinase-positive tumor xenografts. Nucl Med Biol (2009) 36(4):371–8. doi: 10.1016/j.nucmedbio.2009.01.005
64. Jagoda EM, Lang L, Bhadrasetty V, Histed S, Williams M, Kramer-Marek G, et al. Immuno-PET of the hepatocyte growth factor receptor Met using the 1-armed antibody onartuzumab. J Nucl Med (2012) 53(10):1592–600. doi: 10.2967/jnumed.111.102293
65. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med (2009) 360(8):765–73. doi: 10.1056/NEJMoa0808710
66. Kloosterhof NK, Bralten LB, Dubbink HJ, French PJ, van den Bent MJ. Isocitrate dehydrogenase-1 mutations: a fundamentally new understanding of diffuse glioma? Lancet Oncol (2011) 12(1):83–91. doi: 10.1016/S1470-2045(10)70053-X
67. Leibetseder A, Ackerl M, Flechl B, Wohrer A, Widhalm G, Dieckmann K, et al. Outcome and molecular characteristics of adolescent and young adult patients with newly diagnosed primary glioblastoma: a study of the Society of Austrian Neurooncology (SANO). Neuro Oncol (2013) 15(1):112–21. doi: 10.1093/neuonc/nos283
68. SongTao Q, Lei Y, Si G, YanQing D, HuiXia H, XueLin Z, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci (2012) 103(2):269–73. doi: 10.1111/j.1349-7006.2011.02134.x
69. Labussiere M, Boisselier B, Mokhtari K, Di Stefano AL, Rahimian A, Rossetto M, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology (2014) 83(13):1200–6. doi: 10.1212/WNL.0000000000000814
70. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
71. Schiff D, Van den Bent M, Vogelbaum MA, Wick W, Miller CR, Taphoorn M, et al. Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-Oncology (SNO) and European Association of Neuro-Oncology (EANO) consensus. Neuro Oncol (2019) 21(7):837–53. doi: 10.1093/neuonc/noz033
72. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science (2008) 321(5897):1807–12. doi: 10.1126/science.1164382
73. Perizzolo M, Winkfein B, Hui S, Krulicki W, Chan JA, Demetrick DJ. IDH mutation detection in formalin-fixed paraffin-embedded gliomas using multiplex PCR and single-base extension. Brain Pathol (2012) 22(5):619–24. doi: 10.1111/j.1750-3639.2012.00579.x
74. Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol (2009) 118(5):599–601. doi: 10.1007/s00401-009-0595-z
75. Santagata S, Eberlin LS, Norton I, Calligaris D, Feldman DR, Ide JL, et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci U S A (2014) 111(30):11121–6. doi: 10.1073/pnas.1404724111
76. Choi C, Raisanen JM, Ganji SK, Zhang S, McNeil SS, An Z, et al. Prospective Longitudinal Analysis of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy Identifies Broad Clinical Utility for the Management of Patients With IDH-Mutant Glioma. J Clin Oncol (2016) 34(33):4030–9. doi: 10.1200/JCO.2016.67.1222
77. Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenografts. Cancer Res (2004) 64(21):8009–14. doi: 10.1158/0008-5472.CAN-04-1956
78. Sun J, Cai L, Zhang K, Zhang A, Pu P, Yang W, et al. A pilot study on EGFR-targeted molecular imaging of PET/CT With 11C-PD153035 in human gliomas. Clin Nucl Med (2014) 39(1):e20–6. doi: 10.1097/RLU.0b013e3182a23b73
79. Luo H, Hong H, Slater MR, Graves SA, Shi S, Yang Y, et al. PET of c-Met in Cancer with (6)(4)Cu-Labeled Hepatocyte Growth Factor. J Nucl Med (2015) 56(5):758–63. doi: 10.2967/jnumed.115.154690
80. Gaedicke S, Braun F, Prasad S, Machein M, Firat E, Hettich M, et al. Noninvasive positron emission tomography and fluorescence imaging of CD133+ tumor stem cells. Proc Natl Acad Sci U S A (2014) 111(6):E692–701. doi: 10.1073/pnas.1314189111
81. Jing H, Weidensteiner C, Reichardt W, Gaedicke S, Zhu X, Grosu AL, et al. Imaging and Selective Elimination of Glioblastoma Stem Cells with Theranostic Near-Infrared-Labeled CD133-Specific Antibodies. Theranostics (2016) 6(6):862–74. doi: 10.7150/thno.12890
82. Andronesi OC, Loebel F, Bogner W, Marjanska M, Vander Heiden MG, Iafrate AJ, et al. Treatment Response Assessment in IDH-Mutant Glioma Patients by Noninvasive 3D Functional Spectroscopic Mapping of 2-Hydroxyglutarate. Clin Cancer Res (2016) 22(7):1632–41. doi: 10.1158/1078-0432.CCR-15-0656
83. de la Fuente M II, Young RJ, Rubel J, Rosenblum M, Tisnado J, Briggs S, et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol (2016) 18(2):283–90. doi: 10.1093/neuonc/nov307
84. Branzoli F, Di Stefano AL, Capelle L, Ottolenghi C, Valabregue R, Deelchand DK, et al. Highly specific determination of IDH status using edited in vivo magnetic resonance spectroscopy. Neuro Oncol (2018) 20(7):907–16. doi: 10.1093/neuonc/nox214
85. Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: a systemic review and meta-analysis. Eur Radiol (2019) 29(2):745–58. doi: 10.1007/s00330-018-5608-7
86. Andronesi OC, Arrillaga-Romany IC, Ly K II, Bogner W, Ratai EM, Reitz K, et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun (2018) 9(1):1474. doi: 10.1038/s41467-018-03905-6
87. Verger A, Metellus P, Sala Q, Colin C, Bialecki E, Taieb D, et al. IDH mutation is paradoxically associated with higher 18F-FDOPA PET uptake in diffuse grade II and grade III gliomas. Eur J Nucl Med Mol Imaging (2017) 44(8):1306–11. doi: 10.1007/s00259-017-3668-6
88. Ginet M, Zaragori T, Marie PY, Roch V, Gauchotte G, Rech F, et al. Integration of dynamic parameters in the analysis of (18)F-FDopa PET imaging improves the prediction of molecular features of gliomas. Eur J Nucl Med Mol Imaging (2020) 47(6):1381–90. doi: 10.1007/s00259-019-04509-y
89. Wenger KJ, Hattingen E, Harter PN, Richter C, Franz K, Steinbach JP, et al. Fitting algorithms and baseline correction influence the results of non-invasive in vivo quantitation of 2-hydroxyglutarate with (1) H-MRS. NMR BioMed (2019) 32(1):e4027. doi: 10.1002/nbm.4027
90. Lu KV, Zhu S, Cvrljevic A, Huang TT, Sarkaria S, Ahkavan D, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res (2009) 69(17):6889–98. doi: 10.1158/0008-5472.CAN-09-0347
91. Jeon HM, Kim SH, Jin X, Park JB, Joshi K, Nakano I, et al. Crosstalk between Glioma-Initiating Cells and Endothelial Cells Drives Tumor Progression. Cancer Res (2014) 74(16):4482–92. doi: 10.1158/0008-5472.CAN-13-1597
92. Clavreul A, Etcheverry A, Chassevent A, Quillien V, Avril T, Jourdan ML, et al. Isolation of a new cell population in the glioblastoma microenvironment. J Neurooncol (2012) 106(3):493–504. doi: 10.1007/s11060-011-0701-7
93. Huveldt D, Lewis-Tuffin LJ, Carlson BL, Schroeder MA, Rodriguez F, Giannini C, et al. Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS One (2013) 8(2):e56505. doi: 10.1371/journal.pone.0056505
94. Lassman AB, Pugh SL, Gilbert MR, Aldape KD, Geinoz S, Beumer JH, et al. Phase 2 trial of dasatinib in target-selected patients with recurrent glioblastoma (RTOG 0627). Neuro Oncol (2015) 17(7):992–8. doi: 10.1093/neuonc/nov011
95. Galanis E, Anderson SK, Twohy EL, Carrero XW, Dixon JG, Tran DD, et al. A phase 1 and randomized, placebo-controlled phase 2 trial of bevacizumab plus dasatinib in patients with recurrent glioblastoma: Alliance/North Central Cancer Treatment Group N0872. Cancer (2019) 125(21):3790–800. doi: 10.1002/cncr.32340
96. Phuphanich S, Raizer J, Chamberlain M, Canelos P, Narwal R, Hong S, et al. Phase II study of MEDI-575, an anti-platelet-derived growth factor-alpha antibody, in patients with recurrent glioblastoma. J Neurooncol (2017) 131(1):185–91. doi: 10.1007/s11060-016-2287-6
97. Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer (1994) 59(4):520–9. doi: 10.1002/ijc.2910590415
98. Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol (2009) 27(5):740–5. doi: 10.1200/JCO.2008.16.3055
99. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med (2014) 370(8):699–708. doi: 10.1056/NEJMoa1308573
100. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med (2014) 370(8):709–22. doi: 10.1056/NEJMoa1308345
101. Hovinga KE, McCrea HJ, Brennan C, Huse J, Zheng J, Esquenazi Y, et al. EGFR amplification and classical subtype are associated with a poor response to bevacizumab in recurrent glioblastoma. J Neurooncol (2019) 142(2):337–45. doi: 10.1007/s11060-019-03102-5
102. Thomas AA, Omuro A. Current role of anti-angiogenic strategies for glioblastoma. Curr Treat Options Oncol (2014) 15(4):551–66. doi: 10.1007/s11864-014-0308-2
103. Nedergaard MK, Michaelsen SR, Perryman L, Erler J, Poulsen HS, Stockhausen MT, et al. Comparison of (18)F-FET and (18)F-FLT small animal PET for the assessment of anti-VEGF treatment response in an orthotopic model of glioblastoma. Nucl Med Biol (2016) 43(3):198–205. doi: 10.1016/j.nucmedbio.2015.12.002
104. Mattern RH, Read SB, Pierschbacher MD, Sze C II, Eliceiri BP, Kruse CA. Glioma cell integrin expression and their interactions with integrin antagonists: Research Article. Cancer Ther (2005) 3A:325–40.
105. Schittenhelm J, Schwab E II, Sperveslage J, Tatagiba M, Meyermann R, Fend F, et al. Longitudinal expression analysis of alphav integrins in human gliomas reveals upregulation of integrin alphavbeta3 as a negative prognostic factor. J Neuropathol Exp Neurol (2013) 72(3):194–210. doi: 10.1097/NEN.0b013e3182851019
106. MacDonald TJ, Stewart CF, Kocak M, Goldman S, Ellenbogen RG, Phillips P, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium Study PBTC-012. J Clin Oncol (2008) 26(6):919–24. doi: 10.1200/JCO.2007.14.1812
107. Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol (2010) 28(16):2712–8. doi: 10.1200/JCO.2009.26.6650
108. Gilbert MR, Kuhn J, Lamborn KR, Lieberman F, Wen PY, Mehta M, et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery. J Neurooncol (2012) 106(1):147–53. doi: 10.1007/s11060-011-0650-1
109. Ishida J, Onishi M, Kurozumi K, Ichikawa T, Fujii K, Shimazu Y, et al. Integrin inhibitor suppresses bevacizumab-induced glioma invasion. Transl Oncol (2014) 7(2):292–302 e1. doi: 10.1016/j.tranon.2014.02.016
110. Nabors LB, Fink KL, Mikkelsen T, Grujicic D, Tarnawski R, Nam DH, et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol (2015) 17(5):708–17. doi: 10.1093/neuonc/nou356
111. Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2014) 15(10):1100–8. doi: 10.1016/S1470-2045(14)70379-1
112. Chinot OL. Cilengitide in glioblastoma: when did it fail? Lancet Oncol (2014) 15(10):1044–5. doi: 10.1016/S1470-2045(14)70403-6
113. Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med (1998) 4(5):623–6. doi: 10.1038/nm0598-623
114. Cai W, Sam Gambhir S, Chen X. Multimodality tumor imaging targeting integrin alphavbeta3. Biotechniques (2005) 39(6 Suppl):S14–25. doi: 10.2144/000112091
115. Handgraaf HJM, Boonstra MC, Prevoo H, Kuil J, Bordo MW, Boogerd LSF, et al. Real-time near-infrared fluorescence imaging using cRGD-ZW800-1 for intraoperative visualization of multiple cancer types. Oncotarget (2017) 8(13):21054–66. doi: 10.18632/oncotarget.15486
116. Jin ZH, Furukawa T, Degardin M, Sugyo A, Tsuji AB, Yamasaki T, et al. alphaVbeta3 Integrin-Targeted Radionuclide Therapy with 64Cu-cyclam-RAFT-c(-RGDfK-)4. Mol Cancer Ther (2016) 15(9):2076–85. doi: 10.1158/1535-7163.MCT-16-0040
117. Iagaru A, Mosci C, Shen B, Chin FT, Mittra E, Telli ML, et al. (18)F-FPPRGD2 PET/CT: Pilot Phase Evaluation of Breast Cancer Patients. Radiology (2014) 273(2):549–59. doi: 10.1148/radiol.14140028
118. Zhang H, Liu N, Gao S, Hu X, Zhao W, Tao R, et al. Can an (1)(8)F-ALF-NOTA-PRGD2 PET/CT Scan Predict Treatment Sensitivity to Concurrent Chemoradiotherapy in Patients with Newly Diagnosed Glioblastoma? J Nucl Med (2016) 57(4):524–9. doi: 10.2967/jnumed.115.165514
119. Heimberger AB, Crotty LE, Archer GE, Hess KR, Wikstrand CJ, Friedman AH, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res (2003) 9(11):4247–54.
120. Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res (2005) 11(4):1462–6. doi: 10.1158/1078-0432.CCR-04-1737
121. Schuster J, Lai RK, Recht LD, Reardon DA, Paleologos NA, Groves MD, et al. multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol (2015) 17(6):854–61. doi: 10.1093/neuonc/nou348
122. Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol (2017) 18(10):1373–85. doi: 10.1016/S1470-2045(17)30517-X
123. Binder DC, Ladomersky E, Lenzen A, Zhai L, Lauing KL, Otto-Meyer SD, et al. Lessons learned from rindopepimut treatment in patients with EGFRvIII-expressing glioblastoma. Transl Cancer Res (2018) 7(Suppl 4):S510–3.
124. Moriyama T, Kataoka H, Seguchi K, Tsubouchi H, Koono M. Effects of hepatocyte growth factor (HGF) on human glioma cells in vitro: HGF acts as a motility factor in glioma cells. Int J Cancer (1996) 66(5):678–85. doi: 10.1002/(SICI)1097-0215(19960529)66:5<678::AID-IJC16>3.0.CO;2-0
125. Li MY, Yang P, Liu YW, Zhang CB, Wang KY, Wang YY, et al. Low c-Met expression levels are prognostic for and predict the benefits of temozolomide chemotherapy in malignant gliomas. Sci Rep (2016) 6:21141. doi: 10.1038/srep21141
126. Nabeshima K, Shimao Y, Sato S, Kataoka H, Moriyama T, Kawano H, et al. Expression of c-Met correlates with grade of malignancy in human astrocytic tumours: an immunohistochemical study. Histopathology (1997) 31(5):436–43. doi: 10.1046/j.1365-2559.1997.3010889.x
127. Bell EH, Pugh SL, McElroy JP, Gilbert MR, Mehta M, Klimowicz AC, et al. Molecular-Based Recursive Partitioning Analysis Model for Glioblastoma in the Temozolomide Era: A Correlative Analysis Based on NRG Oncology RTOG 0525. JAMA Oncol (2017) 3(6):784–92. doi: 10.1001/jamaoncol.2016.6020
128. Wen PY, Schiff D, Cloughesy TF, Raizer JJ, Laterra J, Smitt M, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol (2011) 13(4):437–46. doi: 10.1093/neuonc/noq198
129. Smith MR, Sweeney CJ, Corn PG, Rathkopf DE, Smith DC, Hussain M, et al. Cabozantinib in Chemotherapy-Pretreated Metastatic Castration-Resistant Prostate Cancer: Results of a Phase II Nonrandomized Expansion Study. J Clin Oncol (2014) 32(30):3391–9. doi: 10.1200/JCO.2013.54.5954
130. Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol (2013) 31(29):3639–46. doi: 10.1200/JCO.2012.48.4659
131. Wen PY, Drappatz J, de Groot J, Prados MD, Reardon DA, Schiff D, et al. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients naive to antiangiogenic therapy. Neuro Oncol (2018) 20(2):249–58. doi: 10.1093/neuonc/nox154
132. Cloughesy TF, Drappatz J, de Groot J, Prados MD, Reardon DA, Schiff D, et al. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients with prior antiangiogenic therapy. Neuro Oncol (2018) 20(2):259–67. doi: 10.1093/neuonc/nox151
133. Du W, Uslar L, Sevala S, Shah K. Targeting c-Met receptor overcomes TRAIL-resistance in brain tumors. PLoS One (2014) 9(4):e95490. doi: 10.1371/journal.pone.0095490
134. Towner RA, Jensen RL, Colman H, Vaillant B, Smith N, Casteel R, et al. ELTD1, a potential new biomarker for gliomas. Neurosurgery (2013) 72(1):77–90. doi: 10.1227/NEU.0b013e318276b29d. discussion 91.
135. Cho JH, Kim AR, Kim SH, Lee SJ, Chung H, Yoon MY. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater (2017) 47:182–92. doi: 10.1016/j.actbio.2016.10.009
136. Ziegler J, Pody R, Coutinho de Souza P, Evans B, Saunders D, Smith N, et al. ELTD1, an effective anti-angiogenic target for gliomas: preclinical assessment in mouse GL261 and human G55 xenograft glioma models. Neuro Oncol (2017) 19(2):175–85. doi: 10.1093/neuonc/now147
137. Weller M, Stupp R, Hegi M, Wick W. Individualized targeted therapy for glioblastoma: fact or fiction? Cancer J (2012) 18(1):40–4. doi: 10.1097/PPO.0b013e318243f6c9
138. Guidance for Industry, Investigators, and Reviewers. Exploratory IND Studies. Biotechnol Law Rep (2005) 24(5):603–11. doi: 10.1089/blr.2006.25.167
139. Phase III Study of Rindopepimut/GM-CSF in Patients With Newly Diagnosed Glioblastoma (ACT IV). NIH. U.S. National Library of Medicine, ClinicalTrials. gov (2011). Available at: http://clinicaltrials.gov/ct2/show/record/ NCT01480479.
Keywords: glioma, molecular imaging, probes, targeted therapy, precision medicine
Citation: Li D, Patel CB, Xu G, Iagaru A, Zhu Z, Zhang L and Cheng Z (2020) Visualization of Diagnostic and Therapeutic Targets in Glioma With Molecular Imaging. Front. Immunol. 11:592389. doi: 10.3389/fimmu.2020.592389
Received: 07 August 2020; Accepted: 08 October 2020;
Published: 30 October 2020.
Edited by:
Xiaoxing Xiong, Renmin Hospital of Wuhan University, ChinaReviewed by:
Yoshua Esquenazi, University of Texas Health Science Center at Houston, United StatesCopyright © 2020 Li, Patel, Xu, Iagaru, Zhu, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liwei Zhang, neurochina106@126.com; Zhen Cheng, zcheng@stanford.edu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.