
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 17 November 2020
Sec. Autoimmune and Autoinflammatory Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.581673
Autoimmune uveitis (AU), being one of the sight-threatening ocular inflammatory disorders, has been widely regarded by ophthalmologists and immunologists as a great challenge. Apremilast, a phosphodiesterase-4 inhibitor (PDE4i), which was approved by the U.S. Food and Drug Administration (FDA) for the treatment of active psoriatic arthritis in 2014, has been attracting researchers, who are exploring its efficiency and mechanism on uveitis. In this study, we used an experimental autoimmune uveitis (EAU), a representative model for human AU, to investigate the effect of apremilast on regulating anti-inflammatory mediators. Our study demonstrated that apremilast treatment resulted in a decrease in vascular leakage, macular edema, and inflammatory cell infiltration in the retina, corresponding to decreased clinical and pathological scores. Specifically, apremilast decreased the proportion and population of Th17 cells and increased the proportion and population of T regulatory (Treg) cells. Mechanistically, apremilast may regulate Th17 and Treg cells by inhibiting the phosphorylation of the phosphoinositide 3-kinase (PI3K)/protein kinase B(AKT)/Forkhead box O1 (FoxO1) signaling pathway. These findings suggested that apremilast alleviated EAU by regulating Th17 and Treg through the PI3K/AKT/FoxO1 pathway.
Autoimmune uveitis (AU) has been widely considered as one of the ocular inflammatory disorders that cause significant damage to vision (1). Glucocorticoids, immune-suppressants, or biological agents, which are used for uveitis, are still faced with huge challenges, including restricted efficiency and long-term adverse events (2, 3). Therefore, it is necessary to search for new medications that could treat this refractory sight-threatening disease.
Apremilast, a small molecule-inhibiting phosphodiesterase-4(PDE4), has been confirmed to be able to regulate downstream inflammatory mediators by preventing cyclic adenosine monophosphate (cAMP) degradation into adenosine monophosphate (AMP) (4). cAMP plays an essential role as a second messenger in diverse intracellular pathways, involving many metabolic reactions and inflammatory activities (5). Convincingly, apremilast is supposed to act as a therapeutic agent for various autoimmune disorders by increasing intracellular cAMP (6). The effect of apremilast on several chronic inflammatory disorders, such as psoriasis (7, 8), dermatitis (9), and rheumatoid arthritis (10, 11, 12) has been studied and verified in animal models. Inspiringly, apremilast was approved by the U.S. Food and Drug Administration (FDA) in 2014 to treat adult active psoriatic arthritis (13, 14). To search for new drugs for AU, we determined to explore the effects of apremilast on uveitis.
PI3Ks can be classified into three subtypes and are involved in various biochemical reactions, including cell growth, survival, differentiation, material transport, and metabolism (15). AKT, belonging to the AGC family of serine/threonine protein kinase, acts as the main downstream molecule of PI3K signaling (16). The PI3K/AKT pathway plays a core role in the process and release of pro-inflammatory factors (17, 18). Few studies confirmed the relationship between PED4 inhibitors (including apremilast) and PI3K/AKT. Peter G. Smith et al. confirmed PDE4B is one risk-related factor in an independent series of primary DLBCLs, and cAMP-mediated apoptosis in DLBCL is related to inhibition of the PI3K/AKT pathway (19). A single-center, exploratory phase Ib open-label, nonrandomized study was further performed, and it showed that the PDE4 inhibitor roflumilast is a safe therapy for treatment of B-cell malignancies by suppressing the activity of the oncogenic PI3K/AKT kinases (20). Torbafylline, another PDE inhibitor, was illustrated to attenuate burn-induced rat skeletal muscle proteolysis through the PDE4/cAMP/EPAC/PI3K/Akt pathway. Phosphodiesterase (PDE) inhibitor torbafylline (HWA 448) attenuates burn-induced rat skeletal muscle proteolysis through the PDE4/cAMP/EPAC/PI3K/Akt pathway (21). It is interesting to explore whether, in experimental AU (EAU), apremilast could influence the PI3K/AKT pathway. In addition, transcription factor forkhead-box O1 (FoxO1), among the FoxO family in mammals, is modulated mainly by the PI3K/AKT signal via phosphorylation (22), and FoxO1 has been reported to regulate several downstream gene targets including pro-inflammatory molecules, adhesion molecules, B-cell regulators, and T-regulatory modulators (23). It has been recently shown to increase Foxp3 expression of CD4+ T-cells and strengthen the population and capability of Treg cells (24). The imbalance between regulatory CD4+ T (Treg) and effector CD4+ T-cells (Teffs, such as Th17 and Th1) has been widely accepted to be the core mechanism of autoimmune diseases including AU (2). The key to controlling the inflammation of AU is to rebuild the balance by strengthening the population and capability of Treg cells. We sought to understand whether apremilast could modulate Treg/Teff cells to ameliorate EAU via the PI3K/AKT/FoxO1 pathway.
Adult female C57BL/6J mice, age 6–8 weeks and weighing 20–25 g, were purchased from the Guangzhou Animal Experiment Center. Each animal used for the study was bred in a specific pathogen-free condition with a 12-h light–dark cycle as well as steady temperature and humidity. During the whole breeding and experimental period, clean food and water were provided to the mice. Mouse experiments were strictly conducted according to the guidelines of the Institutional Animal Care Committee affiliated to Zhongshan Ophthalmic Center, Sun Yat-sen University.
Complete Freund’s adjuvant (CFA, BD Difco, San Jose, CA, USA) containing 5 mg Mycobacterium tuberculosis H37Ra (BD Difco, San Jose, CA, USA) was mixed with 200 μg hIRBP1-20 (GPTHLFQPSLVLDMAKVLLD, GL Biochem, Shanghai, China) in a 1:1 volume ratio (v/v). The emulsion was injected into the back spot of each mouse, near the tail and two flanks subcutaneously. In addition, 250 ng Bordetella pertussis toxin (PTX, List Biological Laboratories, Campbell, California, USA) dissolved in phosphate-buffered saline (PBS, Gibco, Grand Island, New York, USA) was intraperitoneally injected to the immunized mice on days 0 and 2 after immunization (25–27).
Apremilast, at a purity of more than 95% purchased from Selleck Chemicals (Houston, TX, USA), was dissolved into dimethyl sulfoxide (DMSO, Sigma, 0.1%) for storage at -80°C. It was further dissolved by PBS containing carboxymethyl cellulose (CMC, Sigma, 0.5%) and Tween 80 (Sigma, 0.25%) for in vivo and in vitro experiments (11).
All the immunized mice were administrated with vehicle (0.1% DMSO, 0.5% CMC, 0.25% Tween 80) or apremilast at different dosages (5, 15, or 25 mg/kg) by oral gavage from days 7 to 21 after immunization (11).
On the 21st day after immunization, fundus photos were taken to observe the retinal hemorrhage, vascular leakage, macular edema, retinal folds, retinal detachment, etc. Clinical scores were graded from 0 to 4 according to the fundus images (Phoenix Co., Campbell, California, USA) (27, 28).
On the 21st day after immunization, the eyeballs gathered from the experimental mice were fixed in 4% PFA at room temperature for 48 h and paraffin embedded. The fixed eyeballs were cut into 5-μm-thick slides at the margin of the optic nerve for hematoxylin and eosin (H&E) staining. Histopathologic manifestations, including inflammatory cell infiltration and the retinal folds of each retina level, were photographed and evaluated with a score from 0 to 4 (27, 28).
To isolate retinal cells, the eyeballs were removed from the orbital cavity of the mice and were temporarily stored in an RPMI-1640 culture medium (Gibco, USA) with 10% fetal bovine serum (FBS, Gibco) on ice. Retinas were dissected from the eyes under the microscope and were then polished and cut into small pieces. To deeply digest the retinas into single cell suspensions, they were incubated at 37° for 60 min in an RPMI-1640 culture medium containing collagenase D (Roche, Basel, Switzerland) and 10% FBS. Finally, after washing twice with PBS, the retinal infiltrated cells were obtained for flow cytometry analysis (29).
To isolate the cells of draining lymph nodes (DLNs), inguinal, axillary, and cervical lymph nodes were harvested from the mice on day 21 after immunization and ground. Cells were filtered through a cell strainer to make single-cell suspensions. All the cells were plated in a 96-well plate (approximately 5*105 cell/well) and incubated with IRBP1-20 (20 μg/ml) at 37°C for 72 h in a humidified incubator with 5% CO2. After treatment with different doses of apremilast (0, 50, 100, 200, 400, and 800 nM), the cells were analyzed by flow cytometry.
To test the CD4+ lymphocytes, LIVE/DEAD (Thermo Fisher Scientific, Waltham, MA, USA) was first labeled to distinguish the living cells. Surface marker antimouse CD4 and CD45 were labeled to all the cultured cells. After further fixation and permeabilization were performed for intracellular staining, intracellcular inflammatory cytokines were labeled and evaluated using an LSRFortessa (BD Biosciences). The following antimouse antibodies used for the cytometry analysis were purchased from BioLegend (San Diego, CA, USA) or Abcam (Cambridge, MA, USA) including antimouse CD4 Percp-cy5.5 (Biolegend), antimouse CD45 BV510 (Biolegend), antimouse IL17A APC (Biolegend), antimouse IFN-γ PE (Biolegend), antimouse TNFα BV421 (Biolegend), antimouse Foxp3 FITC (Biolegend), antimouse pPI3K PE (Biolegend), antimouse pAKT APC (Biolegend), antimouse FoxO1 (Abcam), and antimouse pFoxO1 (Abcam). SC79 (Selleck Chemicals, Houston, TX), as an AKT activator, was also used to stimulate the cells isolated from the DLNs and spleens of the EAU group to further determine whether the PI3K/AKT/FoxO1 pathway was involved in the mechanism of apremilast treatment on EAU. All the figures and data were collected and analyzed with FlowJo software (Tree Star, Ashland, OR).
To further investigate the effect of apremilast on pathological IRBP-specific CD4+ T-lymphocytes, we designed a T-lymphocyte adoptive transfer experiment. Healthy C57BL/6J mice were chosen as recipients and were divided into three groups: healthy control group (CT), apre- adoptive transfer group (apre- AT), and apre+ adoptive transfer group (apre+ AT). Cells were separated from the inguinal, axillary, and cervical LNs of EAU on the 14th day after immunization. After being co-cultured with/without apremilast and stimulated with IRBP1-20 (20 μg/ml) for 72 h in a 96-well plate, CD4+ T-cells were sorted, purified, and collected. Recipient mice of the apre+ AT or apre-AT groups, respectively, received CD4+ T-cells co-cultured with or without apremilast through tail vein injection (2×107 living cells/mouse). Mice of the CT received an equal volume of PBS. Different groups of mice were raised under the same condition (as in 1.1 animals).
Student’s t test and Mann-Whitney test, as well as one-way ANOVA were all performed for statistical analysis using the Graph Pad Prism software (Version 8.0, La Jolla, CA, USA). All the data were presented as mean±SD and Plt;0.05 was considered to be statistically significant.
To determine the therapeutic effect of apremilast on EAU, different doses of apremilast (5, 15, or 25 mg/kg) or vehicle were orally administered to the mice from the 7th day after immunization. On day 21, fundus photos were taken to evaluate the clinical scores of their fundus. Eyeballs were taken to make sections for pathological scores. Compared with the EAU mice of the vehicle (apre- group), apremilast treatment decreased the ocular inflammation features of the chorioretinal lesions, vascular leakage, and vasculitis dose-dependently. Representative fundus images are shown in Figure 1A. We found that apremilast at 5 mg/kg had little effect to alleviate EAU. However, apremilast at 15 and 25 mg/kg showed obvious better efficacy on EAU although no significant different effect between the two groups (Figure 1B). Apremilast treatment also decreased the retina folds and inflammatory cell infiltrations, which were summarized and calculated as pathological scores (Figure 1C). The pathological scores revealed consistent dosage effects of apremilast with the clinical scores (Figure 1D). Based on drug safety, we selected the dose of 15 mg/kg for the subsequent experiments. These results demonstrated that apremilast (15 mg/kg) reduced both the clinical and histological scores and then ameliorated the EAU manifestations strikingly and safely.
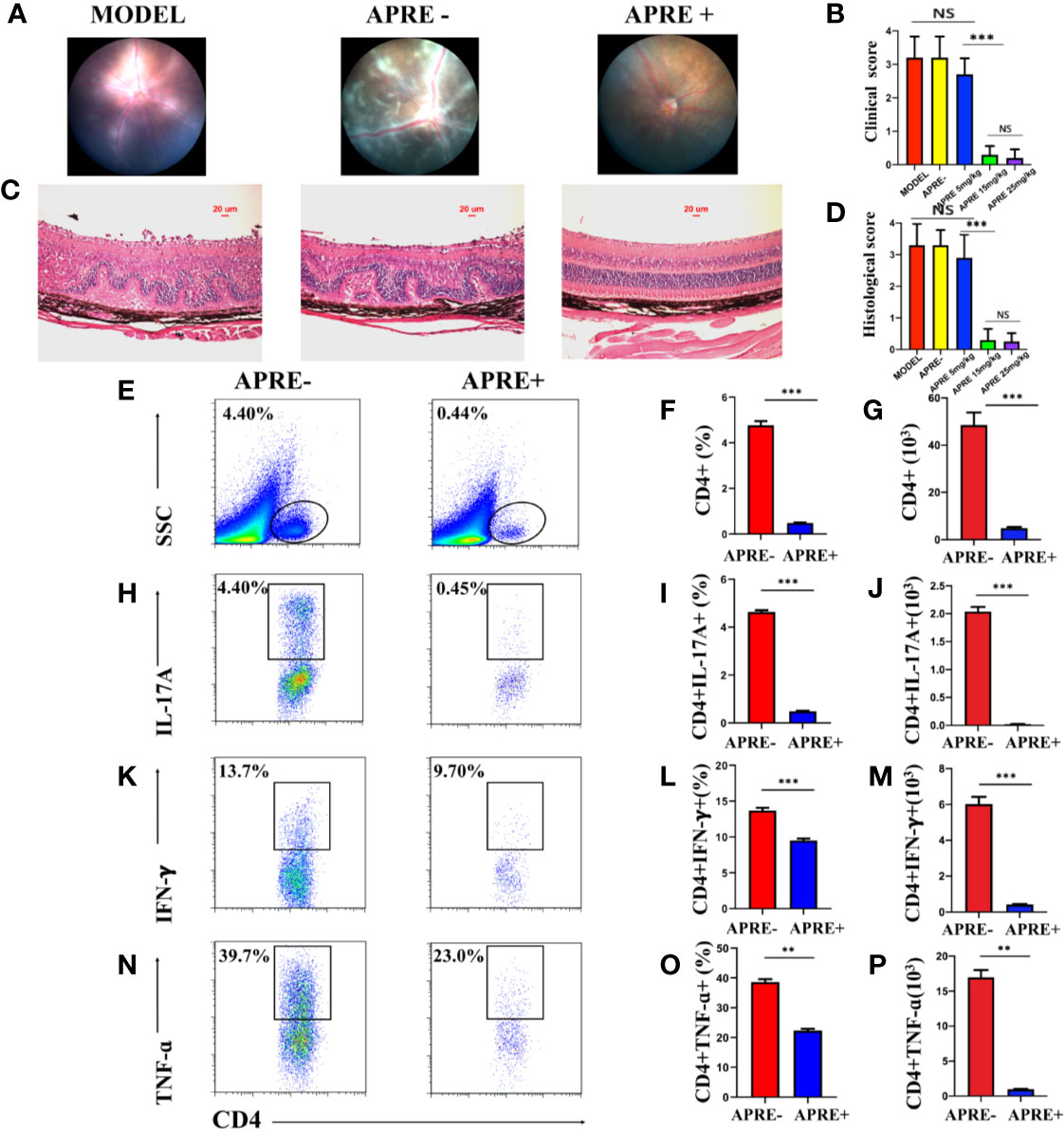
Figure 1 Apremilast ameliorated the inflammation of EAU. (A) The typical fundus images of the different administration on the 21st day after immunization. (B) Clinical scores chart of the five groups. (C) The representative histological images of H&E stain on the 21st day after immunization. (D) Histological scores of five groups. (E–P) The frequency and number of CD4+ T-cells, IL-17A-, and IFN-γ-producing cells that infiltrated the retina were measured by flow cytometry on day 21 after immunization. Apremilast blocked the CD4+ T-cells from entering the retina and reduced the frequency and number the of IL-17A-and IFN-γ-producing cells. Data expressed the mean ± SD, **P < 0.01, ***P < 0.001. N = 6.
It is widely well known that the infiltration of Teff cells, especially Th17 and Th1, into the retina is crucial in the pathologic retinal damage of EAU. To explore whether apremilast could prevent Teff cells from getting into the eyes, we isolated retinal infiltrated cells for further flow cytometry analysis. The population and proportion of CD4+ T-cells as well as inflammatory mediators, including IL17A, IFNγ, and TNFα in the retina were compared between the EAU mice of the apre+ and apre- groups. We found that CD4+ T-cells in the retina of the apre- group EAU mice appeared to be much more abundant than those in the retina of the apre+ group EAU mice (Figures 1E–G). Furthermore, Th17, Th1, and TNFα-producing cells isolated from the retina of the apre+ group EAU mice were fewer than those in the retina of the apre- group EAU mice (Figures 1K–P). Apremilast significantly decreased the migration of CD4+ T-cells, especially Th17, and IFNγ-producing cells into the retina. These results indicate that apremilast could prevent CD4+ T-cells, especially Teff cells, from infiltrating the retina and then alleviate uveitis.
Imbalance of Treg and Teff cells is regarded as one of the dominant mechanisms of uveitis. To evaluate whether apremilast could regulate Treg and Teff in vivo, we isolated T-cells from the DLNs of EAU mice from the apre+ and apre- groups and analyzed them by flow cytometry. We found that the frequency and number of IL17A-, IFNγ-, and TNFα-producing cells of the apre+ group were significantly lower than those of the apre- group, suggesting that apremilast suppressed Th17 and Th1 (Figures 2A–I). Meanwhile, higher frequency and number of Tregs (CD4+CD25+Foxp3+) were measured in the apre+ group (Figures 2J–L). These results show that apremilast can increase the population and proportion of Tregs and decrease the number and frequency of Teffs in EAU.
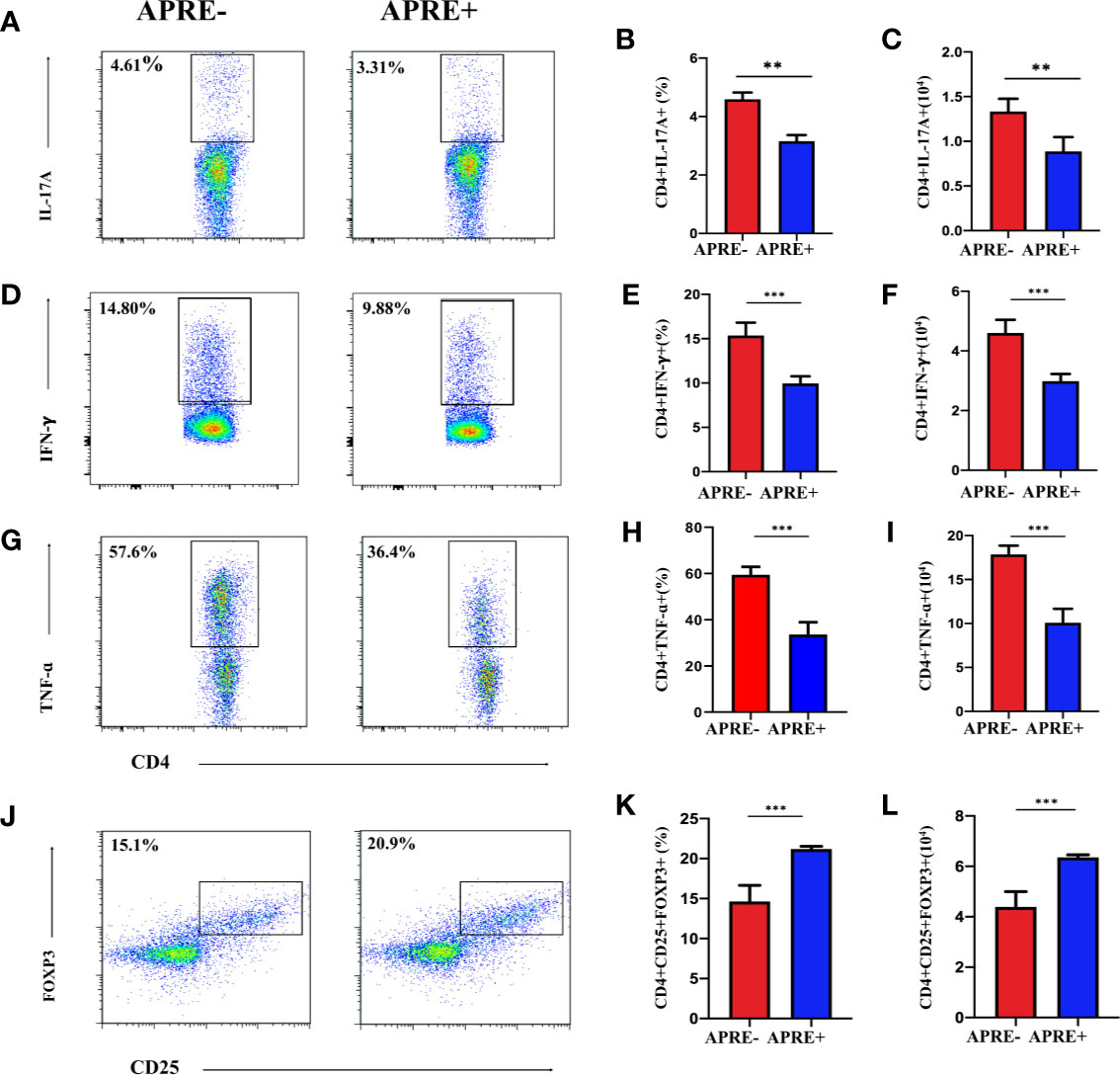
Figure 2 Apremilast inhibited Teff and strengthened Treg in the DLNs. CD4+ T-lymphocyte subtype on day 21 after immunization were compared between the apremilast treatment (15 mg/kg) and vehicle groups by flow cytometry quantitation. (A–I) The frequency and number of IL17A-, IFNγ-, and TNFα-producing cells. (J–L) The proportion and population of CD4+CD25+Foxp3+ T-cells. Data expressed as mean±SD, **P < 0.01, ***P < 0.001, N = 6.
To further assess the ability of apremilast to regulate Treg and Teff in vitro, lymphocytes separated from the DLNs of EAU were cultured with or without apremilast. A gradient concentration of apremilast was applied in the DLNs, and 200 nM apremilast showed the greatest effects. As shown in Figure 3, apremilast decreased the proportion and population of Th17 and Th1 (Figures 3A–I) and increased Treg cells (Figures 3J–L). Overall, apremilast could regulate CD4+ T cells in vitro.
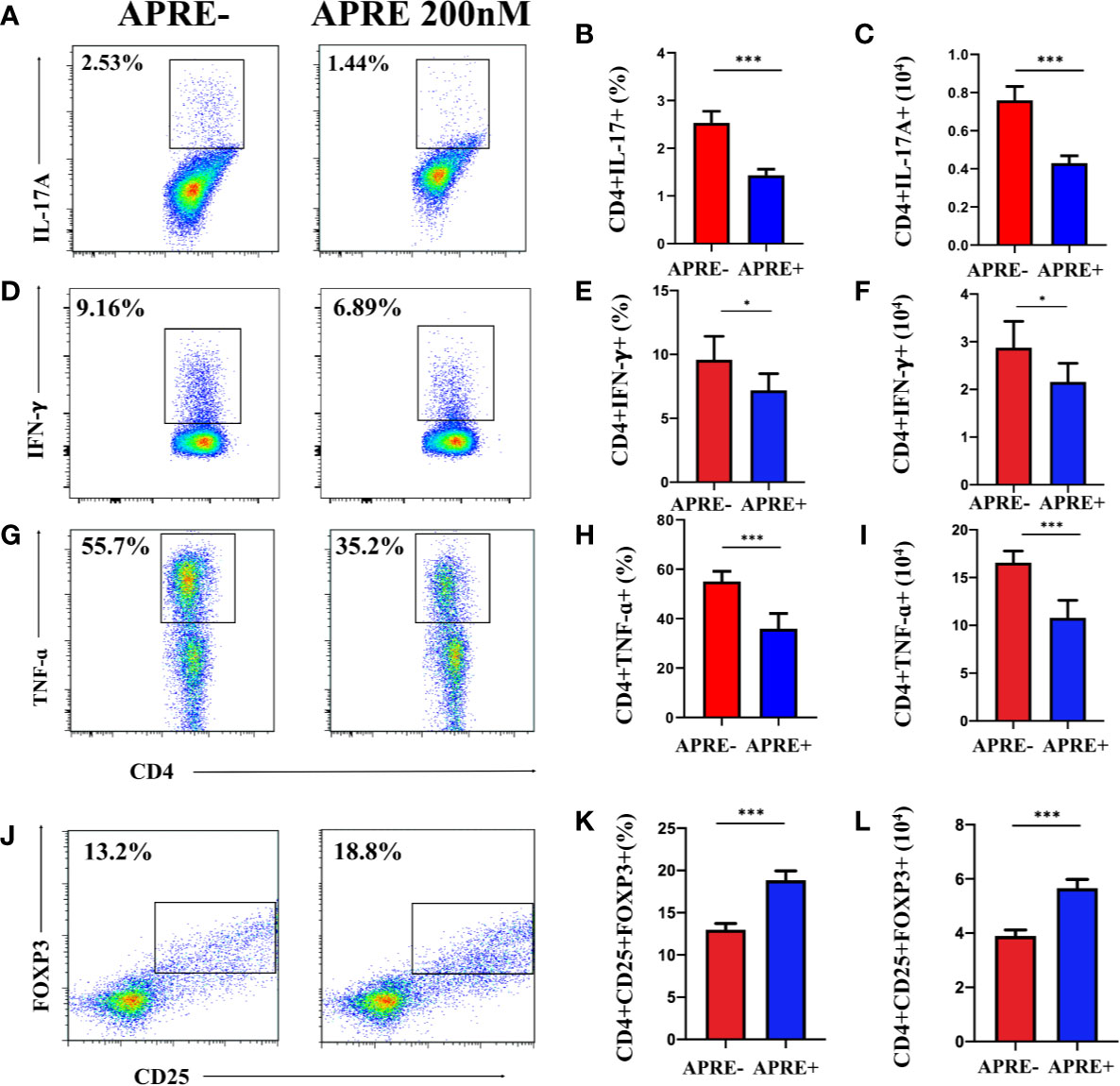
Figure 3 Apremilast modulated naïve CD4+ T-cells in vitro. Cells isolated from the DLNs stimulated with IRBP1-20 for 3 days with or without apremilast were analyzed by flow cytometry. (A–I) Apremilast suppressed the frequency and number of IL17A-, IFNγ-, and TNFα-producing cells. (J–L) Apremilast upregulated the proportion and population of CD4+CD25+Foxp3+ T-cells. Data expressed as mean±SD, **P < 0.01, ***P < 0.001, N = 6.
We conducted the adoptive transfer experiment to confirm whether apremilast could ameliorate EAU by regulating CD4+ T-cells. Mice of apre- AT, apre+ AT, and CT were injected with CD4+ T-cells co-cultured with or without apremilast or PBS through the tail vein. During the feeding period, fundus photos were taken to assess the clinical scores every 7 days, and eyeballs were taken to make sections for the pathological scores. Representative fundus photos of apre- AT and apre+ AT on day 21 are exhibited in Figure 4A (photos of the CT aere not shown). Mice of the apre+ AT group showed fewer ocular inflammation features, including chorioretinal lesions, vascular leakage, and vasculitis, than the mice of the apre- AT group (Figure 4A). There were fewer retina folds and inflammatory cell infiltrations in apre+ AT than those in apre- AT, which was identical results to the fundus images (Figure 4C). To sum up, apremilast treatment significantly decreased the inflammation of EAU induced by the adoptive transfer experiment clinically and pathologically (Figures 4B, D). This adoptive transfer experiment declared that apremilast ameliorated EAU by modulating IRBP-specific CD4+ T-lymphocytes.
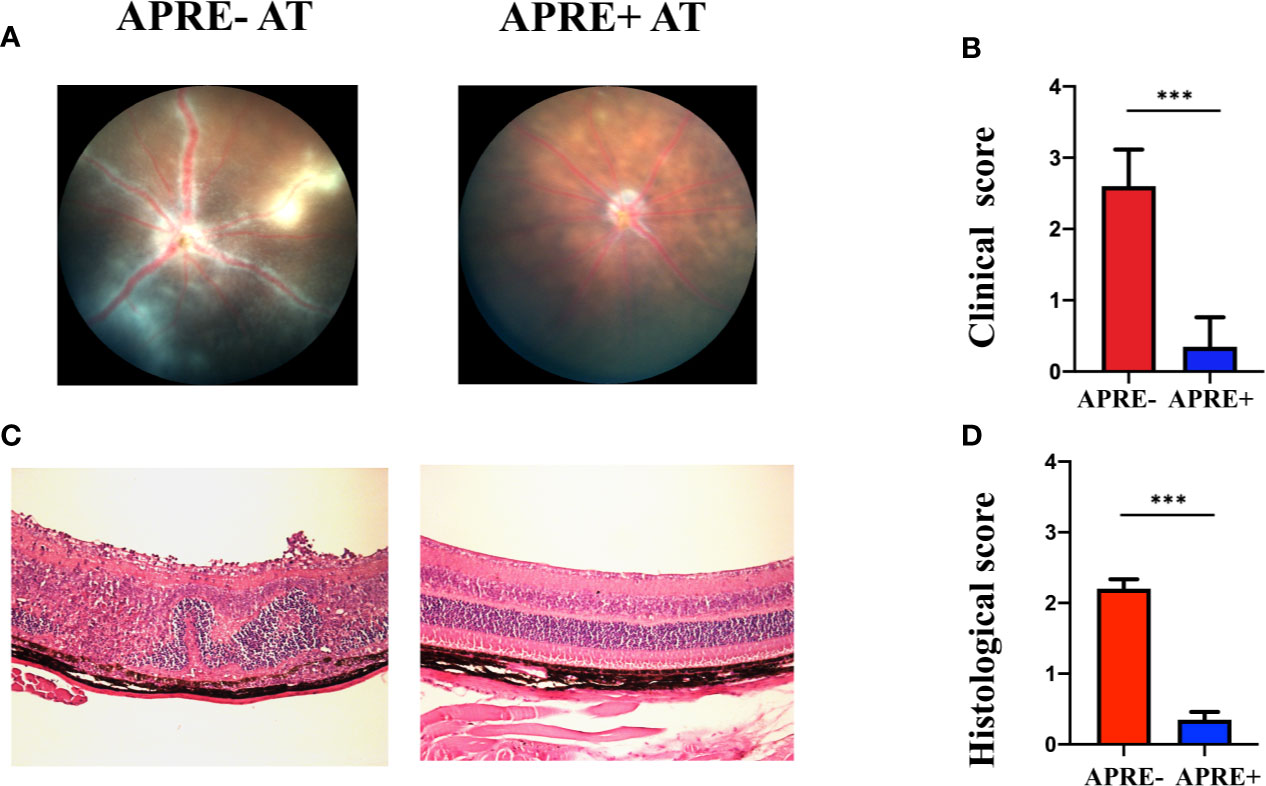
Figure 4 Apremilast prevented IRBP1-20 induced T-cells from entering the EAU. (A, B) On the 21st day after injection of the cultured IRBP1-20-specific T-cells treated with or without apremilast, typical fundus photographs were taken, and the two groups’ clinical scores were calculated. (C, D) The histological images and scores. Data expressed as mean±SD, ***P < 0.001, N = 6.
The PI3K/AKT pathway is reported to be important for its signal regulation function in several autoimmune diseases. PI3K/AKT are often dysregulated in autoimmune diseases, including AU. To investigate whether PI3K/AKT/FoxO1 is implicated in the therapeutic effect of apremilast on EAU, we evaluated the levels of pPI3K, pAKT, and pFoxO1 of the CD4+ T-lymphocytes of EAU with apremilast (200 nM). Meanwhile, we checked the level of FoxO1. We found that apremilast upregulated PI3K/AKT/FoxO1 phosphorylation of the CD4+ T-lymphocytes isolated from the EAU and suppressed the phosphorylation of the intracellular protein significantly (Figures 5A–I). In addition, FoxO1 was consistently upregulated by apremilast (Figures 5J–L).
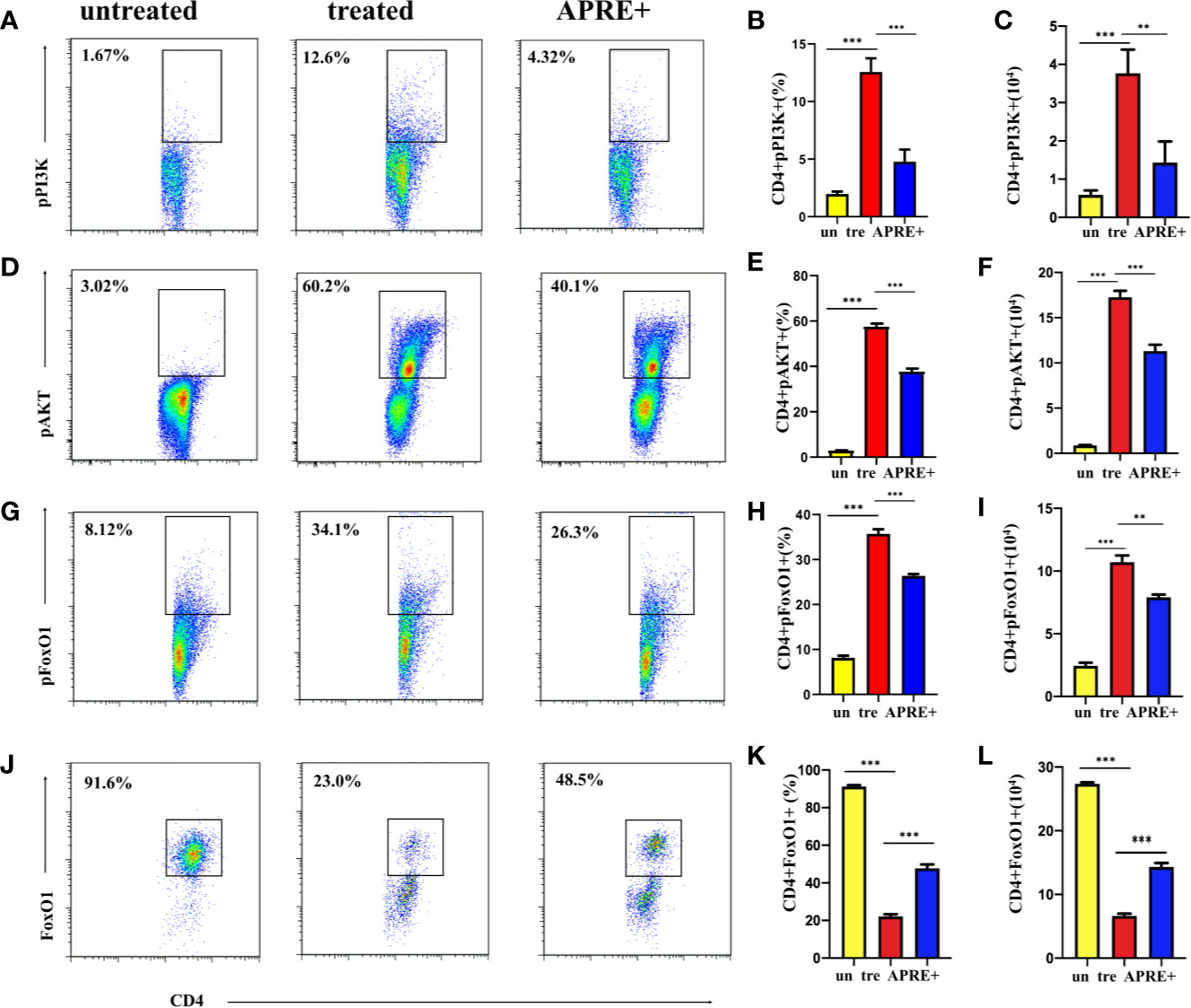
Figure 5 Apremilast attenuated the EAU via the PI3K/AKT/FoxO1 signaling pathway. (A–I) CD4+ T-lymphocytes from the DLNs of the EAU group induced by IRBP1-20 for 3 days with or without apremilast treatment were analyzed; the proportion and population of pPI3K+, pAKT+, and pFoxO1+. (J–L) The frequency and number of FoxO1+ CD4+ T-cells with or without apremilast treatment. Data expressed as mean±SD, **P < 0.01, ***P < 0.001, N = 6.
SC79 is a specific AKT activator that can upregulate the phosphorylation of intracellular AKT and is often used to promote the phosphorylation of the PI3K/AKT pathway. We used SC79 to treat CD4+ T-cells from the DLNs of EAU with apremilast. We wanted to determine whether SC79 could reverse the effect of apremilast on downregulating inflammatory mediators. This experiment would also help us to confirm that the PI3K/AKT/FoxO1 pathway is involved in the apremilast’s therapeutic effect on EAU. Flow cytometry showed that SC79 promoted the expression of inflammatory mediators, including IL17A, IFNγ, and TNFα (Figures 6A–I), while it inhibited Treg (Figures 6J–L). These results indicate that the differentiation and function of CD4+ T-cells might be regulated by the phosphorylation of intracellular AKT. It is reasonable to presume that apremilast alleviated EAU by reducing the phosphorylation of the PI3K/AKT/FoxO1 pathway.
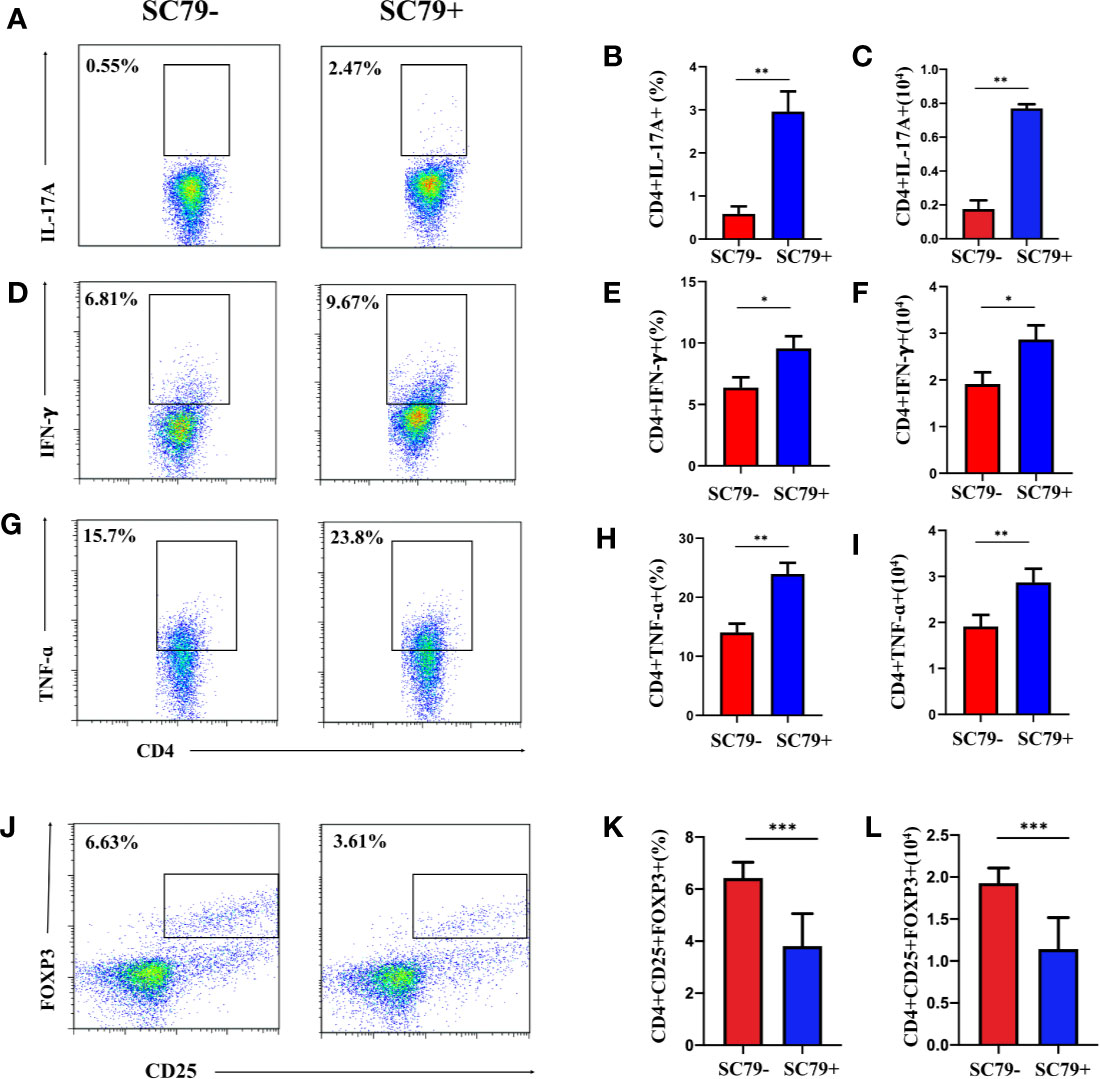
Figure 6 Apremilast failed to rebuild the imbalance between Treg/Teff of EAU co-stimulated with SC79. Cells from the DLNs of the EAU group were cultured in IRBP1-20 and apremilast with or without SC79 (16 mg/ml). (A–I) The proportion and population of IL17A-, IFNγ-, and TNFα-producing CD4+ T-cells in the two groups. (J–L) The proportion and population of CD4+CD25+Foxp3+ T-cells of the two groups. Data expressed as mean±SD, *P < 0.05, **P < 0.01, ***P < 0.001, N = 6.
For the first time, this study shows that apremilast could treat AU effectively in an animal model. First, we demonstrate that apremilast could prevent CD4+ T-cells, especially Teff cells, from infiltrating the eyes of EAU mice. Next, we demonstrated that apremilast significantly suppressed Th17 and Th1 cells and enhanced Treg in the DLNs of the EAU group in vivo and in vitro, meaning that it could harmonize the balance between Teff/Treg induced by the autoimmune inflammation. We also demonstrate that phosphorylation and activation of the PI3K/AKT/FoxO1 pathway could act as a new pathogenic mechanism involved in EAU. Finally, we find that apremilast could reduce the phosphorylation of PI3K/AKT/FoxO1 to alleviate EAU.
In the past few years, the efficacy of PDE4 inhibitors on autoimmune diseases has been reported. R. Caspi et al. found that rolipram, the first selective PDE4 inhibitor, had a protective effect in an EAU model (30), and Zai-Long Chi et al. found that rolipram could inhibit the pathogenesis of LPS-induced uveitis (31). Roflumilast, a second-generation PDE4 inhibitor, was approved for chronic obstructive pulmonary disease by the FDA in 2011 (32). However, severe unacceptable adverse events, including nausea and vomiting, blocked the clinical application of the abovementioned PDE4 inhibitors (33). Researchers subsequently invented apremilast, a newer third-generation PDE4 inhibitor, by using a recognized functional pharmacophore of the earlier PDE4 inhibitor and adding a series of active groups in order to reduce the side effects and optimize the pharmaceutical effects (34). Apremilast has been studied on some chronic inflammatory disorders, such as psoriasis (35), dermatitis (36), Behcet’s disease (37, 38), and rheumatoid arthritis (39), appearing to have fewer side effects compared with those previous inhibitors (33). It was approved to treat adult patients with PsA and moderate-to-severe psoriasis (PsO) by the FDA in 2014 (40). Apremilast has been shown to be efficacious with an acceptable safety profile in several PsA clinical trials (40). However, no results of apremilast studies on uveitis have been reported until now (37).
In this study, using the EAU model, we first found that apremilast treatment not only significantly decreased the clinical inflammation score, including chorioretinal lesions, vascular leakage, exudate, vasculitis, and even retinal detachment of the EAU mice, but it also dwindled the degree of retinal folds and inflammatory cell infiltrations dose-dependently. These results show that apremilast could be an effective drug for AU.
The imbalance between Treg and Teff cells is the core mechanism of AU. The increase of Teff cells, especially Th17, and the decrease of Treg contributes to AU (41). In addition, the infiltration of pathological CD4+ T cells, especially Th17 and Th1, into the retina is involved in the pathogenesis of uveitis (42). Few studies have elucidated the association between PDE4 inhibitors and Treg/Th17. Zheng et al. reported that apremilast ameliorated the experimental arthritis via regulating the imbalance between Treg and Th17 cells (11).
In this study, we demonstrated that more CD4+ T-cells, especially Th17 and IFNγ-producing cells, were present in the retina of EAU mice than in the retina of the blank control mice. When apremilast was administered, the number of pathogenic cells in the retina appeared to fall. This implies that apremilast could block CD4+ T-cells, especially Teff cells, from attacking the retina in EAU. Additionally, we found that apremilast could rebuild the balance of Treg and Teff both in vivo and in vitro. Compared with the group without treatment, the apremilast group obviously upregulated the population and frequency of Treg and downregulated Th17 and Th1. For the first time, we determined that, in the EAU model, apremilast suppresses Th1 and Th17 cells and enhances Treg cells. These results were consistent with the discovery in the study by Zheng et al., who reported that apremilast ameliorated experimental arthritis via regulating the imbalance between Treg and Th17 cells (11).
PI3Ks are a family of heterodimeric lipid kinases and can be divided into three classes based on different stimuli (43, 44). AKT is one kind of serine protein kinase from the protein kinase AGC subfamily and acts as one of essential downstream factors of PI3K (44, 45). PI3K/AKT is widely regarded as an important pathway to trigger several biochemical reactions that are closely related to metabolism and disease occurrence (46). Activation of this pathway starts by different and initial upstream cell-surface receptors, including growth factor, antigen, costimulatory cytokine, chemokine, and Toll-like receptors (TLRs) (44). Once stimulated, PI3K is phosphorylated and activated, which then catalyzes the formation of the second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3) (43). Then, AKT is phosphorylated to be activated by PIP3 binding together with PDK1 and AKT. Once activated, AKT phosphorylates many downstream targets, including FoxO1 (47). FoxO1 transcriptionally mediates pathways responsible for many metabolic diseases that are translated by the FoxO1 gene, a member of FoxO genes belonging to the transcription factor (TF) family (48). The PI3K/AKT pathway has been reported to be important for Treg development both in vitro and in vivo. Factors downstream of AKT activation, particularly the FoxO1 transcription factors, have also been reported to play important roles in Treg development. The involvement of PI3K/AKT/FoxO1 in CD4+ T-cell differentiation by maintaining Foxp3 expression has been proven (24, 49). FoxO1 resides in the nucleus of Tregs, where it helps maintain Foxp3 expression. When phosphorylated by AKT, they are excluded from the nucleus and, thus, not able to regulate their transcriptional targets (24). PI3K/AKT are often dysregulated in autoimmune disease, and the expression of a constitutively active form of AKT leads to autoimmunity. Some autoimmune diseases, such as rheumatoid arthritis (50) and multiple sclerosis (51) show increased activity of the PI3K/AKT pathway (31). However, few studies have determined the function of the PI3K/AKT/FoxO1 pathway in the pathogenesis of uveitis. Yang et al. found that, compared with the peripheral blood mononuclear cells (PBMCs) of healthy controls, those from AAU patients with AS revealed a higher FoxO1 by a PCR-restricted fragment length polymorphism (RFLP) assay. However, further research concerning the role of FoxO1 and the biochemical pathways that control T-cell homeostasis is urgently needed to elucidate their role in the development of AAU with AS (52).
In this study, we first found that cells isolated from EAU showed higher PI3K/AKT/FoxO1 phosphorylation compared with cells from the blank control mice. In addition, apremilast prevented PI3K, AKT, and FoxO1 from phosphorylating. Furthermore, the in vitro study showed that SC79, an AKT activator, promoted the expression of inflammatory mediators, IL17A, IFNγ, and TNFα while inhibiting Foxp3, which indicated that the function of CD4+ T-cells could be regulated by the phosphorylation of intracellular AKT. Thus, we could presume that apremilast alleviates EAU by suppressing the phosphorylation of PI3K/AKT/FoxO1.
On all these counts, apremilast could alleviate EAU significantly by rebuilding the balance of Treg/Teff CD4+ T-cells via phosphorylating and activating the PI3K/AKT/FoxO1 pathway. Obviously, these results enriched our understanding of the mechanism of EAU and provided a new convincing option for the treatment of AU.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by the Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-Sen University.
YC was responsible for the conception and design of the study, experiments, data collection, and manuscript writing. ZL and HL provided guide of experiments. WS helped in the experiment design. YX and YP were helpful in manuscript writing. XC and DL were responsible for conception and design, revision of the manuscript, and final manuscript approval. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Science Foundation of China (8187040615, Guangzhou, Guangdong, China).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bertrand PJ, Jamilloux Y, Ecochard R, Richard-Colmant G, Gerfaud-Valentin M, Guillaud M, et al. Uveitis: Autoimmunity and beyond. Autoimmun Rev (2019) 18(9):102351. doi: 10.1016/j.autrev.2019.102351
2. MS Patrizia Fasching, Winfried Graninger. Therapeutic Potential of Targeting the Th17/Treg Axis in Autoimmune Disorders. Molecules (2017) 22(1):E134. doi: 10.3390/molecules22010134
3. Wildner G, Diedrichs-Mohring M. Resolution of uveitis. Semin Immunopathol (2019) 41(6):727–36. doi: 10.1007/s00281-019-00758-z
4. Zebda R, Paller AS. Phosphodiesterase 4 inhibitors. J Am Acad Dermatol (2018) 78(3):S43–52. doi: 10.1016/j.jaad.2017.11.056
5. Ghigo A, Mika D. cAMP/PKA signaling compartmentalization in cardiomyocytes: Lessons from FRET-based biosensors. J Mol Cell Cardiol (2019) 131:112–21. doi: 10.1016/j.yjmcc.2019.04.020
6. Montesinos C, Miguel Perez-Aso M, Mediero A. Apremilast, a novel phosphodiesterase 4 (PDE4) inhibitor, regulates inflammation through multiple cAMP downstream effectors. Arthritis Res Ther (2015) 17(249):1–13. doi: 10.1186/s13075-015-0771-6
7. Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol (2010) 159(4):842–55. doi: 10.1111/j.1476-5381.2009.00559.x
8. Gooderham M, Papp K. Selective Phosphodiesterase Inhibitors for Psoriasis: Focus on Apremilast. BioDrugs (2015) 29(5):327–39. doi: 10.1007/s40259-015-0144-3
9. Adams M, Schafer PH, Horan G. Apremilast Normalizes Gene Expression of In ammatory Mediators in Human Keratinocytes and Reduces Antigen-Induced Atopic Dermatitis in Mice. Drugs R&D (2019) 19:329–38. doi: 10.1007/s40268-019-00284-1
10. McCann FE, Palfreeman AC, Andrews M, Perocheau DP, Inglis JJ, Schafer P, et al. Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor-alpha from human rheumatoid synovial cells and ameliorates experimental arthritis. Arthritis Res Ther (2010) 12(3):R107. doi: 10.1186/ar3041
11. Weiqian Chen JW, Xu Z. apremilast ameliorates experimental arthritis via suppression of Th1 and Th17 cells and enhancement of cD4+Foxp3+ regulatory T cells Differentiation. Front Immunol (2018) 9(1662):1–10. doi: 10.3389/fimmu.2018.01662
12. Kragstrup TW, Adams M, Lomholt S, Nielsen MA, Heftdal LD, Schafer P, et al. IL-12/IL-23p40 identified as a downstream target of apremilast in ex vivo models of arthritis. Ther Adv Musculoskelet Dis (2019) 11:1759720X19828669. doi: 10.1177/1759720X19828669
13. Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheumatic Dis (2014) 73(6):1020–6. doi: 10.1136/annrheumdis-2013-205056
14. Felquer MLA, Soriano ER. New treatment paradigms in psoriatic arthritis. Curr Opin Rheumatol (2015) 27(2):99–106. doi: 10.1097/BOR.0000000000000151
15. Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Bioscience (2020) 10(54):1–12. doi: 10.1186/s13578-020-00416-0
16. Yudushkin I. Getting the Akt Together: Guiding Intracellular Akt Activity by PI3K. Biomolecules (2019) 9(2):67. doi: 10.3390/biom9020067
17. Villegas SN, Gombos R, Garcia-Lopez L, Gutierrez-Perez I, Garcia-Castillo J, Vallejo DM, et al. PI3K/Akt Cooperates with Oncogenic Notch by Inducing Nitric Oxide-Dependent Inflammation. Cell Rep (2018) 22(10):2541–9. doi: 10.1016/j.celrep.2018.02.049
18. Khan H, Sureda A, Belwal T, Cetinkaya S, Suntar I, Tejada S, et al. Polyphenols in the treatment of autoimmune diseases. Autoimmun Rev (2019) 18(7):647–57. doi: 10.1016/j.autrev.2019.05.001
19. Menon M BP, Isenberg DA, Mauri C. A Regulatory Feedback between Plasmacytoid Dendritic Cells and Regulatory B Cells Is Aberrant in Systemic Lupus Erythematosus. Immunity (2016) 44(3):683–97. doi: 10.1016/j.immuni.2016.02.012
20. Kelly K, Mejia A, Suhasini AN, Lin PA, Kuhn J, Karnad AB, et al. Safety and Pharmacodynamics of the PDE4 Inhibitor Roflumilast in Advanced B-cell Malignancies. Clin Cancer Res (2017) 23(5):1186–92. doi: 10.1158/1078-0432.CCR-16-1207
21. Joshi R, Kadeer N, Sheriff S, Friend LA, James JH, Balasubramaniam A, et al. Phosphodiesterase (PDE) inhibitor torbafylline (HWA 448) attenuates burn-induced rat skeletal muscle proteolysis through the PDE4/cAMP/EPAC/PI3K/Akt pathway. Mol Cell Endocrinol (2014) 393(1-2):152–63. doi: 10.1016/j.mce.2014.06.012
22. Lainé A, Martin B, Luka M, Mir L, Auffray C, Lucas B, et al. Foxo1 Is a T Cell–Intrinsic Inhibitor of the RORγt-Th17 Program. J Immunol (2015) 195(4):1791–803. doi: 10.4049/jimmunol.1500849
23. Graves DT, Milovanova TN. Mucosal Immunity and the FOXO1 Transcription Factors. Front Immunol (2019) 10(2530):1–12. doi: 10.3389/fimmu.2019.02530
24. Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature (2016) 529(7587):532–6. doi: 10.1038/nature16486
25. Walker JM. Autoimmunity methods and protocols. (2012) 900:443–69. doi: 10.1007/978-1-60761-720-4
26. Zhu W, Chen X, Yu J, Xiao Y, Li Y, Wan S, et al. Baicalin modulates the Treg:Teff balance to alleviate uveitis by activating the aryl hydrocarbon receptor. Bbiochem Pharmacol (2018) 154(2018):18–27. doi: 10.1016/j.bcp.2018.04.006
27. Caspi RR, Chen J. Clinical and Functional Evaluation of Ocular Inflammatory Disease Using the Model of Experimental Autoimmune Uveitis. Methods Mmol Biol (2019) 1899:211–27. doi: 10.1007/978-1-4939-8938-6_15
28. Li Z, Chen X, Chen Y, Li H, Yu J, Li Y, et al. Teriflunomide suppresses T helper cells and dendritic cells to alleviate experimental autoimmune uveitis. Biochem Pharmacol (2019) 170:113645. doi: 10.1016/j.bcp.2019.113645
29. Dhanesh Amarnani AIM-P, Wong LL. Effect of Methotrexate on an In Vitro Patient-Derived Model of Proliferative Vitreoretinopathy. Invest Ophthalmol Visual Sci (2017) 58(10):3940–9. doi: 10.1167/iovs.16-20912
30. Xu H, Strassmann G, Chan C-C, Rizzo LV, Silver PB, Wiggert B, et al. Protective Effect of the Type IV Phosphodiesterase Inhibitor Rolipram in EAU: Protection Is Independent of IL-10-Inducing Activity. Invest Ophthalmol Visual Sci (1999) 40(5):942–50.
31. Chi ZL, Hayasaka S, Zhang XY, Hayasaka Y, Cui HS. Effects of rolipram, a selective inhibitor of type 4 phosphodiesterase, on lipopolysaccharide-induced uveitis in rats. Invest Ophthalmol Vis Sci (2004) 45(8):2497–502. doi: 10.1167/iovs.03-1373
32. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev (2017) 9:CD002309. doi: 10.1002/14651858.CD002309.pub5
33. Goldminz AM, Kumar N, Kim N, Gottlieb AB. Phosphodiesterase 4-targeted treatments for autoimmune diseases. BMC Med (2013) 11(96):1–8. doi: 10.1186/1741-7015-11-96
34. Hon-Wah Man PS, Wong LM, Patterson RT. Discovery of (S)-N-{2-[1-(3-Ethoxy-4-methoxyphenyl)-2-methanesulfonylethyl]-1,3-dioxo2,3-dihydro-1H-isoindol-4-yl}acetamide (Apremilast), a Potent and Orally Active Phosphodiesterase 4 and Tumor Necrosis Factor-r Inhibitor. J Med Chem (2009) 52(6):1522–4. doi: 10.1021/jm900210d
35. Cather JC, Papp K, Rosoph L. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet (2012) 380:738–46. doi: 10.1016/S0140-6736(12)60642-4
36. Abrouk M, Farahnik B, Zhu TH, Nakamura M, Singh R, Lee K, et al. Apremilast treatment of atopic dermatitis and other chronic eczematous dermatoses. J Am Acad Dermatol (2017) 77(1):177–80. doi: 10.1016/j.jaad.2017.03.020
37. Hatemi G, Mahr A, Ishigatsubo Y, Song YK, Takeno M, Kim D, et al. Trial of Apremilast for Oral Ulcers in Behcet’s Syndrome. N Engl J Med (2019) 381(20):1918–28. doi: 10.1056/NEJMoa1816594
38. Lopalco G, Venerito V, Leccese P, Emmi G, Cantarini L, Lascaro N, et al. Real-world effectiveness of apremilast in multirefractory mucosal involvement of Behcet’s disease. Ann Rheum Dis (2019) 78(12):1736–7. doi: 10.1136/annrheumdis-2019-215437
39. Genovese MC, Jarosova K, Cieślak D, Alper J, Kivitz A, Hough DR, et al. Apremilast in Patients With Active Rheumatoid Arthritis: A Phase II, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study. Arthritis Rheumatol (2015) 67(7):1703–10. doi: 10.1002/art.39120
40. Fala L. Otezla (Apremilast), an Oral PDE-4 Inhibitor, Receives FDA Approval for the Treatment of Patients with Active Psoriatic Arthritis and Plaque Psoriasis. Am Health Drug Benefits (2015) 8:105–10.
41. Zhang L, Wan F, Song J, Tang K, Zheng F, Guo J, et al. Imbalance Between Th17 Cells and Regulatory T Cells During Monophasic Experimental Autoimmune Uveitis. Inflammation (2015) 39(1):113–22. doi: 10.1007/s10753-015-0229-7
42. Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukocyte Biol (2003) 74:179–85. doi: 10.1189/jlb.1102574
43. Moncayo G, Fayard E, Hemmings BA. Phosphati- dylinositol 3-kinase signaling in thymocytes- the need for stringent control. Sci Signaling (2010) 3(135):re5. doi: 10.1126/scisignal.3135re5
44. Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J, et al. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep (2020) 47(6):4587–629. doi: 10.1007/s11033-020-05435-1
45. Vidotto T, Melo CM, Castelli E, Koti M, Reis RB, Squire JA, et al. Emerging role of PTEN loss in evasion of the immune response to tumours. Br J Cancer (2020) 122(12):1732–43. doi: 10.1038/s41416-020-0834-6
46. Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev (2012) 64(4):972–1003. doi: 10.1124/pr.111.004846
47. Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang RY, et al. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflamm (2019) 16(1):95. doi: 10.1186/s12974-019-1474-8
48. Peng S, Li W, Hou N, Huang N. A Review of FoxO1-Regulated Metabolic Diseases and Related Drug Discoveries. Cells (2020) 9(1):184. doi: 10.3390/cells9010184
49. Kitz A, de Marcken M, Gautron AS, Mitrovic M, Hafler DA, Dominguez-Villar M, et al. AKT isoforms modulate Th1-like Treg generation and function in human autoimmune disease. EMBO Rep (2016) 17(8):1169–83. doi: 10.15252/embr.201541905
50. Ma Z, Yu R, Zhu Q, Sun L, Jian L, Wang X, et al. CXCL16/CXCR6 axis promotes bleomycin-induced fibrotic process in MRC-5 cells via the PI3K/AKT/FOXO3a pathway. Int Immunopharmacol (2019) 81:106035. doi: 10.1016/j.intimp.2019.106035
51. Zheng Q, Liu L, Liu H, Zheng H, Sun H, Ji J, et al. The Bu Shen Yi Sui Formula Promotes Axonal Regeneration via Regulating the Neurotrophic Factor BDNF/TrkB and the Downstream PI3K/Akt Signaling Pathway. Front Pharmacol (2019) 10:796. doi: 10.3389/fphar.2019.00796
Keywords: apremilast, uveitis, PI3K/AKT/FoxO1 signal pathway, Teff/Treg, PDE4, experimental autoimmune uveitis
Citation: Chen Y, Li Z, Li H, Su W, Xie Y, Pan Y, Chen X and Liang D (2020) Apremilast Regulates the Teff/Treg Balance to Ameliorate Uveitis via PI3K/AKT/FoxO1 Signaling Pathway. Front. Immunol. 11:581673. doi: 10.3389/fimmu.2020.581673
Received: 09 July 2020; Accepted: 12 October 2020;
Published: 17 November 2020.
Edited by:
Lazaros Ignatios Sakkas, University of Thessaly, GreeceReviewed by:
Peter H. Schafer, Bristol Myers Squibb, United StatesCopyright © 2020 Chen, Li, Li, Su, Xie, Pan, Chen and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqing Chen, aHVpY2hlbjExOTExMEAxNjMuY29t; Dan Liang, bGlhbmdkYW5AZ3p6b2MuY29t
†First author
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.