- 1Department of Laboratory Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Department of Oral Biology, University of Florida, Gainesville, FL, United States
- 3Department of Laboratory Medicine, Huashan Hospital, Fudan University, Shanghai, China
- 4Department of Laboratory Medicine, Jinhua People’s Hospital, Jinhua, China
- 5Department of Clinical Laboratory, Linyi People’s Hospital, Linyi, China
- 6Department of Laboratory Medicine, People’s Hospital of Rongcheng, Rongcheng, China
- 7Department of Rheumatology Laboratory, Futian District Hospital of Rheumatism, Shenzhen, China
- 8Department of Laboratory Medicine, Ruijing Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 9Department of Laboratory Medicine, Zhongshan Hospital, Fudan University, Shanghai, China
- 10Department of Laboratory Medicine, General Hospital of Northern Theater Command, Shengyang, China
- 11Department of Laboratory Medicine, Zhejiang Provincial Hospital of TCM, The First Affiliated Hospital of Zhejiang Chinese Medicine University, Hangzhou, China
- 12Department of Rheumatology Laboratory, People’s Hospital of Xinjiang Ugyur Autonomous Region, Urumqi, China
- 13Department of Laboratory Medicine, Weihai Central Hospital, Weihai, China
- 14Department of Clinical Laboratory, Ninbo No.6 Hospital, Ninbo, China
- 15Department of Laboratory Medicine, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, China
- 16Department of Laboratory Medicine, The Third People’s Hospital of Changzhou, Changzhou, China
- 17Department of Laboratory Medicine, Jiujiang First People’s Hospital, Jiujiang, China
- 18Department of Laboratory Medicine, Obstetrics & Gynecology Hospital Affiliated to Fudan University, Shanghai, China
- 19Department of Laboratory Medicine, The People’s Hospital of Guangxi Zhuang Autonomous Regain, Nanning, China
- 20Department of Laboratory Medicine, People’s Hospital of Sanmen, Taizhou, China
- 21Department of Laboratory Medicine, The Fifth Affiliated Hospital Sun Yat-Sen University, Zhuhai, China
- 22Department of Laboratory Medicine, Zhenggu Hospital, School of Medicine, Fujian University of Traditional Chinese Medicine, Quanzhou, China
- 23Department of Laboratory Medicine, Zibo Maternal and Child Health Hospital, Zibo, China
- 24Department of Laboratory Medicine, The 940th Hospital of PLA Joint Logistics Support Force, Lanzhou, China
- 25Department of Laboratory Medicine, Daqing Oilfield General Hospital, Daqing, China
- 26Departments of Microbiological and Immunology, 3201 Hospital, Hanzhong, China
- 27Department of Laboratory Medicine, The Red Cross Hospital, Xining, China
- 28Department of Laboratory Medicine, Weihai Municipal Hospital, Shandong University, Weihai, China
- 29Department of Laboratory Medicine, Yili Kazak Autonomous Prefecture Hospital of Traditional Chinese Medicine, Yili, China
- 30Department of Laboratory Medicine, Yinzhou People’s Hospital, Ningbo, China
- 31Center of Pathology and Clinical Laboratory, Sir Run Run Hospital, Nanjing Medical University, Nanjing, China
Objective: Anti-DFS70 antibodies correlating with the nuclear dense fine speckled (DFS) pattern in the HEp-2 indirect immunofluorescence assay (IFA) are less common in patients with systemic autoimmune rheumatic disease (SARD) than in healthy subjects and their clinical associations remain elusive. We hosted a multi-center HEp-2 IFA training program to improve the ability of clinical laboratories to recognize the DFS pattern and to investigate the prevalence and relevance of anti-DFS70 antibodies.
Methods: DFS pattern sera identified by HEp-2 IFA in 29 centers in China were redirected to a central laboratory for anti-DFS70 testing by line immunoblot assay (LIA), enzyme-linked immunosorbent assay (ELISA), and IFA with HEp-2 ELITE/DFS70-KO substrate. Anti-extractable nuclear antigen antibodies were measured by LIA and the clinical relevance was examined in adult and pediatric patients.
Results: HEp-2 IFA positive rate and DFS pattern in positive sera were 36.2% (34,417/95,131) and 1.7% (582/34,417) in the patient cohort, and 10.0% (423/4,234) and 7.8% (33/423) in a healthy population, respectively. Anti-DFS70 prevalence among sera presenting the DFS pattern was 96.0, 93.7, and 49.6% by ELISA, LIA, and HEp-2 ELITE, respectively. 15.5% (52/336) of adult and 50.0% (20/40) of pediatric anti-DFS70 positive patients were diagnosed with SARD. Diseases most common in anti-DFS70 positive patients were spontaneous abortion (28.0%) in adults and juvenile idiopathic arthritis (22.5%) in pediatric patients.
Conclusion: Accurate DFS pattern identification increased the detection rate of anti-DFS70 antibodies by ELISA and LIA. Anti-DFS70 antibodies are remarkably high in cases of spontaneous abortion and in pediatric SARD patients, but not prevalent in adult SARD patients.
Introduction
Antinuclear antibodies (ANA) are commonly regarded as serological hallmarks of systemic autoimmune rheumatic disease (SARD) (1). However, antibodies against the 70 kDa dense fine speckled protein (DFS70), also known as transcription coactivator p75 (2) and lens epithelium-derived growth factor (3), are purported to be an interesting immunological paradox as they are commonly detected in apparently healthy individuals (4–9) but are rare in patients with SARD (5, 10). In the last decade, many studies have focused on the clinical relevance of anti-DFS70 antibodies [reviewed in (5)] and their prevalence has been reported in some chronic inflammatory diseases (2, 11, 12) and cancers [e.g. prostate cancer (13, 14)], but still no clear disease association has been found. In fact, the presence of isolated anti-DFS70 antibodies has been proposed to serve as a diagnostic biomarker to help rule out SARD (4, 15, 16), which highlights the importance of correctly identifying these antibodies in clinical laboratories.
The HEp-2 cell indirect immunofluorescence assay (HEp-2 IFA) is considered the “gold standard” method for ANA screening by the American College of Rheumatology (17). Typical images of the DFS pattern show dense fine speckled staining of interphase nuclei and strong coarse speckled staining of the metaphase plate. DFS pattern is defined as the anti-cell-2 (AC-2) pattern by the International Consensus on ANA Patterns (ICAP) (18). Due to its unique features, only trained and experienced technicians may recognize DFS pattern. Correlations between the anti-DFS70 antibodies detected by specific assays and the DFS pattern have been reported higher than 90% (19). Considering HEp-2 IFA pattern interpretation is largely dependent on the experience of the technologist, reading of the DFS pattern continues to challenge researchers and clinicians alike. Bentow et al. reported that the reading accuracy of DFS unmixed and mixed patterns were ~50% and <10%, respectively, based on an international internet-based survey (20). Recently, the Autoantibody Standardization Committee has made available a reference material for anti-DFS70 antibodies (21), which may assist clinical laboratories in the recognition of DFS pattern to some extent. However, further efforts still need to be placed on training to recognize the DFS pattern.
Our research focused on exploring cost-effective training models to improve the consistency of DFS pattern recognition in laboratories from various regions with unevenly distributed medical resources. Thus, we organized a multi-center DFS pattern identification mutual aid program. The prevalence and clinical associations of anti-DFS70 in both Han Chinese and minority populations were investigated in routine HEp-2 IFA screening cohorts from 30 centers and in healthy individuals from a physical examination cohort from one center in China.
Materials and Methods
Study Design
From July to September 2019, a total of 645 serum specimens were sent from 29 research centers across China to the organizing laboratory at the Renji Hospital, which is affiliated with Jiao Tong University (Shanghai, China). Figure 1 and Table 1 detail the distribution and specific information (e.g. type of hospital, location, etc.) of the 30 participating laboratories.
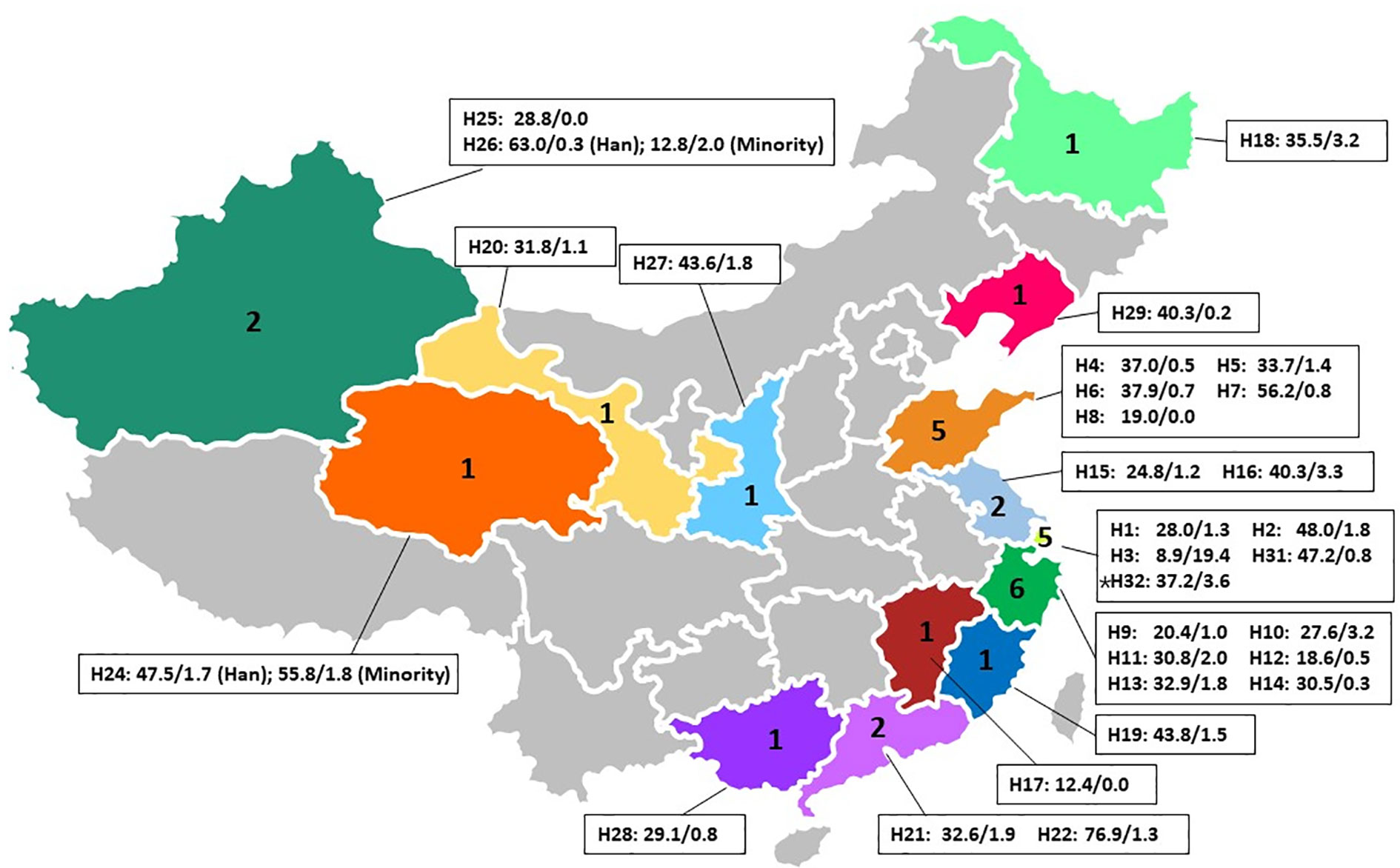
Figure 1 Distribution of one central laboratory ‘(*H32)’ and 29 participating laboratories across China and the positive rates of the HEp-2 IFA screening test and DFS pattern in IFA positive sera. H(number) is the ID for different centers as listed in ‘Table 1’. Following the code name is the HEp-2 IFA positive rate (%)/DFS pattern rate in IFA positive sera (%). Han: Han Chinese. Minorities in H24 included Tibetans, Huis, and Mongolians. Minorities in H26 included Uygurs and Kazakhs.
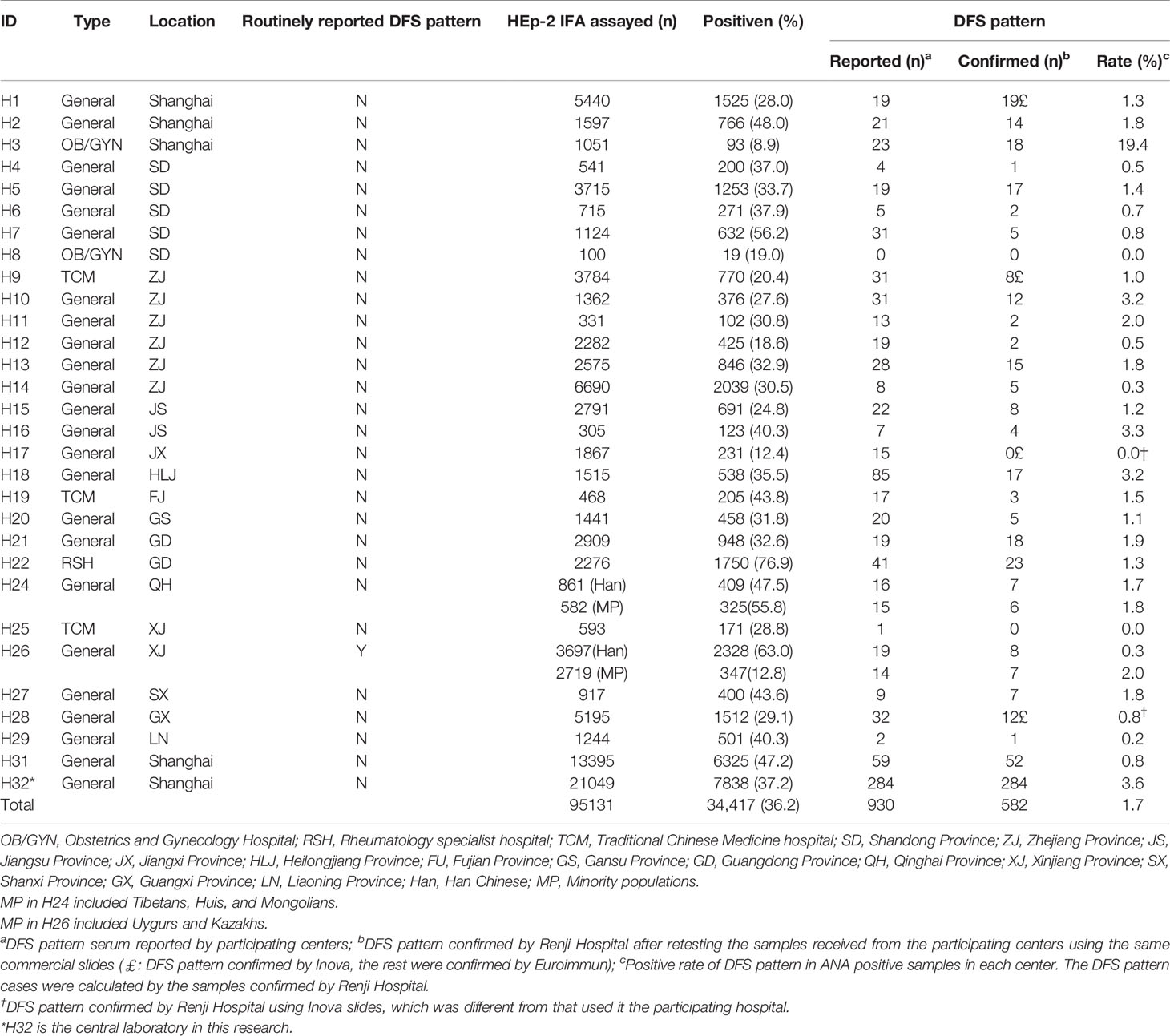
Table 1 Detailed information of HEp-2 IFA positive and DFS pattern rates of one central laboratory and 29 participating centers during a 3-month study period.
At the initiation of the study, corresponding author BZ at the Renji Hospital organizing center directed an online training course to all participating laboratories detailing how to identify the DFS pattern based on the ICAP classification criteria (22). In brief, interphase nuclei show characteristic speckles with heterogeneity in brightness and distribution, while the metaphase plate depicts a strong speckled pattern with some coarse speckles (22). At the end of each month, participating laboratories shipped sera they interpreted as DFS pattern positive during routine HEp-2 IFA tests to Renji Hospital. Upon arrival, two experienced research technicians re-tested the samples by HEp-2 IFA and reported patterns according to ICAP classification (22). Inconsistent interpretations were discussed between two independent evaluators. If they could not reach a consensus, a third evaluator adjudicated. A video conference was offered monthly to review all inconsistent ANA pattern interpretations as part of the ongoing training. Figure 2A shows a flowchart of the study design.
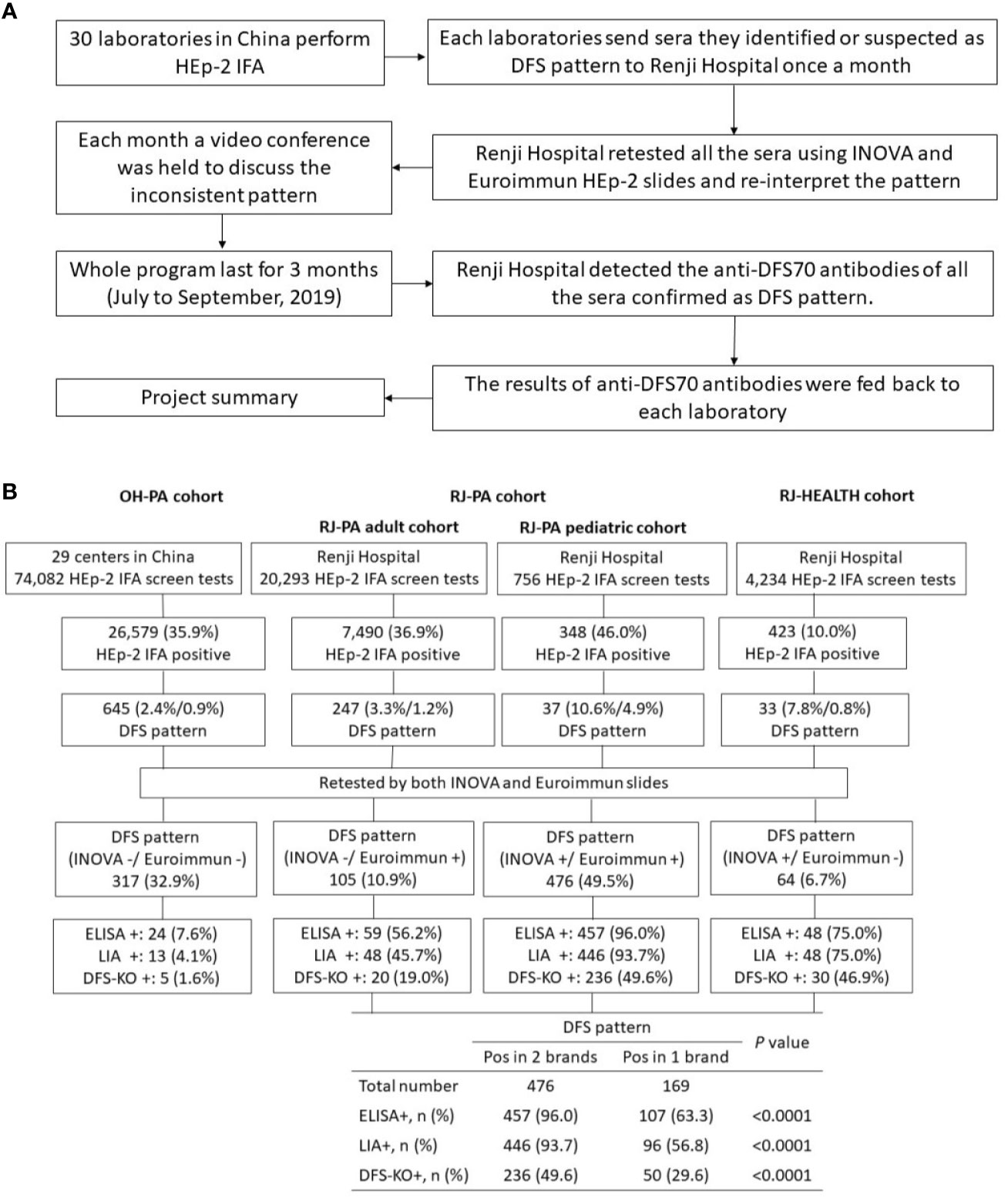
Figure 2 Summary of DFS pattern recognition and training project. (A) Project workflow for organizing sample referrals sent to Renji Hospital from participating training laboratories for validation of DFS pattern and detection of anti-DFS70 antibody. (B) Summary of results of the HEp-2 cell indirect immunofluorescence assay (HEp-2 IFA) and anti-DFS70 antibody assays in three Chinese cohorts collected between July and September 2019. The patient cohort from other hospitals (OH-PA) included samples from 29 participating laboratories in China, excluding the organizing laboratory at Renji Hospital. The RJ-PA cohort included both adult and pediatric patients subjected to routine HEp-2 IFA screening in Renji Hospital. The RJ-HEALTH cohort represents the generally healthy population in the Physical Examination Center of Renji Hospital during the same period of this project. DFS pattern positive rates were shown by the percentage of DFS pattern in HEp-2 IFA positive sera/DFS pattern in HEp-2 IFA screen tests. All samples were re-tested by two commercial HEp-2 IFA kits and further analyzed by ELISA, LIA, and IFA on ELITE/DFS KO substrate. The positive rates of anti-DFS70 were compared between sera showing DFS pattern in both or either one of the commercial HEp-2 IFA kits. Pos in 2 brands: DFS pattern was observed in both Inova and Euroimmun slides. Pos in 1 brand: DFS patterns were observed only in Inova or Euroimmun. ELISA: enzyme-linked immunosorbent assay; LIA: line immunoblot assay; DFS-KO: IFA performed on HEp-2 ELITE/DFS70-KO substrate.
Additionally, the prevalence of ANA and DFS patterns were analyzed in a healthy population (age≥18) from the Renji Hospital Physical Examination Center for routine physical examination (RJ-HEALTH cohort, n=4,234) from July to September 2019. This cohort according to a clinical questionnaire had no history of SARD, malignancies or chronic infections, and were considered healthy by judgment of the chief physician based on physical examination reports. This healthy cohort was compared to the adults in RJ-PA cohort, which consisted of adult (≥18 years, n=20,293) and pediatric (<18 years, n=756) patients undergoing routine HEp-2 IFA screening in the same hospital during the same period (RJ-PA cohort, n=21,049). The percentage of SARD patients in RJ-PA cohort were also investigated according to medical record. All serum specimens collected in this study were approved by the Institutional Review Board of Renji Hospital (No. KY[2019]121). No consent was required for this study.
ANA Screening
Of the 30 participating laboratories, including Renji Hospital, 25 of the laboratories performed ANA screening on HEp-2 slides from Euroimmun AG (Lübeck, Germany), 3 used slides from Inova Diagnostics (San Diego, CA, USA), and 2 used HEp-2 IFA kits from two companies in mainland China (H&J NovoMed, Beijing and HOB, Suzhou).
All samples received by the organizing center at Renji Hospital were re-tested using HEp-2 slides from both Euroimmun and Inova Diagnostics. Euroimmun HEp-2 slides were prepared using the Sprinter XL automated IFT/ELISA analyzer (Euroimmun). Images were acquired with EUROPattern Suite version 3.4.24.0 with a cutoff of 80 AU per the manufacturer’s instructions. NOVA Lite HEp-2 IgG ANA with DAPI slides were processed using the IFA-ELISA processor platform of the QUANTA-Lyser instrument (Inova Diagnostics) and scanned via the NOVA View automated microscope with software version 1.0.4.3, using a cutoff of 49 nuclear intensities (NI) per the manufacturer’s instructions. Samples positive for any nuclear/cytoplasmic autoantibody by IFA were considered HEp-2 IFA positive. Anti-DFS70 reference material IS2726 ANA #24 (21) for ICAP pattern AC-2 was applied as the quality control for HEp-2 IFA.
Anti-DFS70 Antibodies Detection
Anti-DFS70 antibodies were detected by both line immunoblot assay (LIA) and enzyme-linked immunosorbent assay (ELISA). For LIA, the IMTEC-ANA-LIA XL assay (Human Worldwide, Weisbaden, Germany) was used. HumaScan was used to analyze and interpret the results according to the manufacturer’s instructions. For ELISA, 96-well plates were coated with 0.5 µg/mL purified recombinant DFS70 antigen (DIARECT AG, Freiburg, Germany) overnight at 4°C. After blocking with 10 mg/mL gelatin, patient sera were diluted 1:200 in serum diluent (0.75 mg/mL bovine gamma globulin, 0.15 mg/mL bovine serum albumin, 10 mg/mL gelatin, 0.05% Tween 20, 0.5M phosphate buffer, pH 7.4) and added to each well for 2 h incubation at room temperature. For the secondary antibody, horseradish peroxidase-conjugated AffiniPure rabbit anti-human IgG (Jackson ImmunoResearch, West Grove, PA) was diluted 1:1,000 in serum diluent. Samples were developed by TMB (3,3′,5,5′-tetramethylbenzidine, Euroimmun) and the optical density (O.D.) value was read at 450 nm by microplate reader (Multiskan FC, Thermo Fisher, Waltham, MA, USA). Sixty serum samples showing negative reaction to DFS70 by IMTEC-ANA-LIA XL assay (Human Worldwide) were used as negative controls. These included twenty ANA negative from healthy population and forty homogenous and speckled patterns from SARD patients with various titers by HEp-2 IFA. Cutoff was determined by mean O.D. value +3 standard deviations (SD). Anti-DFS70 reference material (21) was used as the positive control for both assays.
Immunofluorescence Assay Using HEp-2 ELITE/DFS70-KO Substrate
IFA was also performed using HEp-2 ELITE/DFS70-KO slides (Immco Diagnostics, Trinity Biotech, Buffalo, NY, USA), which consist of a mixture of 10% conventional HEp-2 cells and 90% engineered HEp-2 cells that do not express DFS70 antigen (23). Results were interpreted using a manual fluorescence microscope (Nikon Eclipse Ni, Tokyo, Japan). Anti-DFS70 antibodies were confirmed by bright staining of interphase nuclei with the corresponding DFS pattern in ~10% of cells.
Line Immunoblot Assay (LIA)
To examine potential association with other autoantibodies, sera positive for anti-DFS70 by ELISA and LIA were further screened for fifteen autoantibodies (nRNP/Sm, Sm, SSA/Ro60, Ro52/TRIM21, SSB/La, Scl-70, PM-Scl, Jo-1, CENP-B, PCNA, dsDNA, nucleosomes, histones, ribosomal P protein (Rib-P), AMA-M2) using the Euroline ANA Profile 3 (Euroimmun) according to the manufacturer’s instructions.
Questionnaire for HEp-2 IFA Screening in Participating Centers
The following were determined by questionnaire for each participating center: which commercial kits they used for routine HEp-2 IFA screening; whether the laboratory routinely reports the DFS pattern; the working experience of their research technicians; whether they apply third-party quality control for HEp-2 IFA. Moreover, the clinical diagnoses of sera with the DFS pattern, which were re-tested and confirmed by Renji Hospital, were retrospectively analyzed by reviewing the medical records including the age, gender, ethnicity and clinical diagnosis from each center.
Statistical Analysis
Statistical Package for Social Sciences (SPSS) (IBM-SPSS, Inc., Armonk, New York) was used to perform statistical analysis. Cohen’s kappa was applied to analyze the interrater agreement between two commercial slides. Differences in the distribution of anti-extractable nuclear antigen (ENA) between SARD versus non-SARD were evaluated by the two-tailed Chi-squared (χ2) test or Fisher’s exact test. The correlations between anti-DFS70 ELISA O.D. values and HEp-2 IFA nuclei fluorescence intensity read by NOVA View or titer by HEp-2 IFA were calculated by Spearman’s rank correlation test. In our study, a two-sided t-test with a P-value <0.05 was considered significant.
Results
Prevalence of ANA and DFS Pattern in China
The 30 participating laboratories consisted of one rheumatology specialist hospital in southern China, three traditional Chinese medicine hospitals in eastern and western China, two obstetrics and gynecology specialist hospitals in eastern China, and twenty-four general hospitals across mainland China. Each laboratory was assigned a unique hospital identification number for the study as listed in Table 1. Overall, the HEp-2 IFA positive rate was 36.2% (34,417/95,131) with some significant differences among the centers. For example, rheumatology specialist hospital H22 had the highest HEp-2 IFA positive rate (76.9%), while obstetrics and gynecology specialist hospital H3 had the lowest rate (8.9%, Table 1).
The prevalence of the DFS pattern was 0.6% (582/95,131) of total patients and 1.7% (582/34,417) in the HEp-2 IFA positive samples, respectively. Interestingly, an obstetrics and gynecology specialist hospital (H3) had the lowest overall HEp-2 IFA positive rate (8.9%), but the highest positive rate of DFS pattern (19.4%) among the participating centers. Note that this high percent of positive DFS pattern could not be accounted for by unusual local environmental exposure when compared to four neighboring centers in the same region (H1, H2, H31, and H32, Figure 1). Three centers, including another obstetrics and gynecology specialist hospital (H8), a Traditional Chinese Medicine hospital (H25), and a general hospital (H17), did not have any confirmed samples with DFS pattern.
To determine the effects of geographical location and potential differences in dietary and lifestyle choices, HEp-2 IFA results were compared between local Han Chinese and minority populations within the H24 and H26 cohorts (Table 1). In H24, the HEp-2 IFA positive rate was higher among the minority population (325/582, 55.8%), which included Tibetans, Huis, and Mongolians than in Han Chinese (409/861, 47.5%, P=0.002). However, the prevalence of the DFS pattern in HEp-2 IFA positive samples was similar in both groups (Han: 7/409, 1.7%; minorities: 6/325, 1.8%, P=0.891). In H26, the HEp-2 IFA positive rate was significantly lower among the minority population (347/2,719, 12.8%) than in Han Chinese (2,328/3,697, 63.0%, P<0.001), yet the prevalence of the DFS pattern was substantially higher among the minority population (7/347, 2.0%), which included Uygurs and Kazakhs than in Han Chinese (8/2,328, 0.3%, P<0.0001).
Comparison of the Prevalence of the DFS Pattern in Adult and Pediatric Patients Versus Healthy Population Cohort
Quantitative comparisons between the RJ-PA and RJ-HEALTH cohorts, along with patient distributions in different departments and antibody titers, are shown in Table 2 and Supplementary Table 1. It should be noted that the prevalence of the DFS pattern in HEp-2 IFA positive sera was significantly higher in pediatric (10.6%) compared to adult patients (3.3%, P<0.001) in the RJ-PA cohort, but no statistical difference was observed in the RJ-HEALTH cohort (7.8%, P=0.173). The same trend was observed when investigating association with gender in various populations in Table 2. DFS pattern was more common in females in the HEp-2 IFA screening cohort (females: 3.6%, 231/6,461, males: 1.6%, 16/1,029, P<0.0001), while no gender differences were observed in pediatric patients (females: 9.2%, 26/284, males: 17.2%, 11/64, P=0.060) or the healthy population (females: 6.3%, 17/268, males: 10.3%, 16/155, P=0.141). Moreover, the same distributions of DFS pattern titers were detected among adult and pediatric RJ-PA and RJ-HEALTH cohorts, as over 40% of titers were ≥1/640 (Supplementary Table 1).
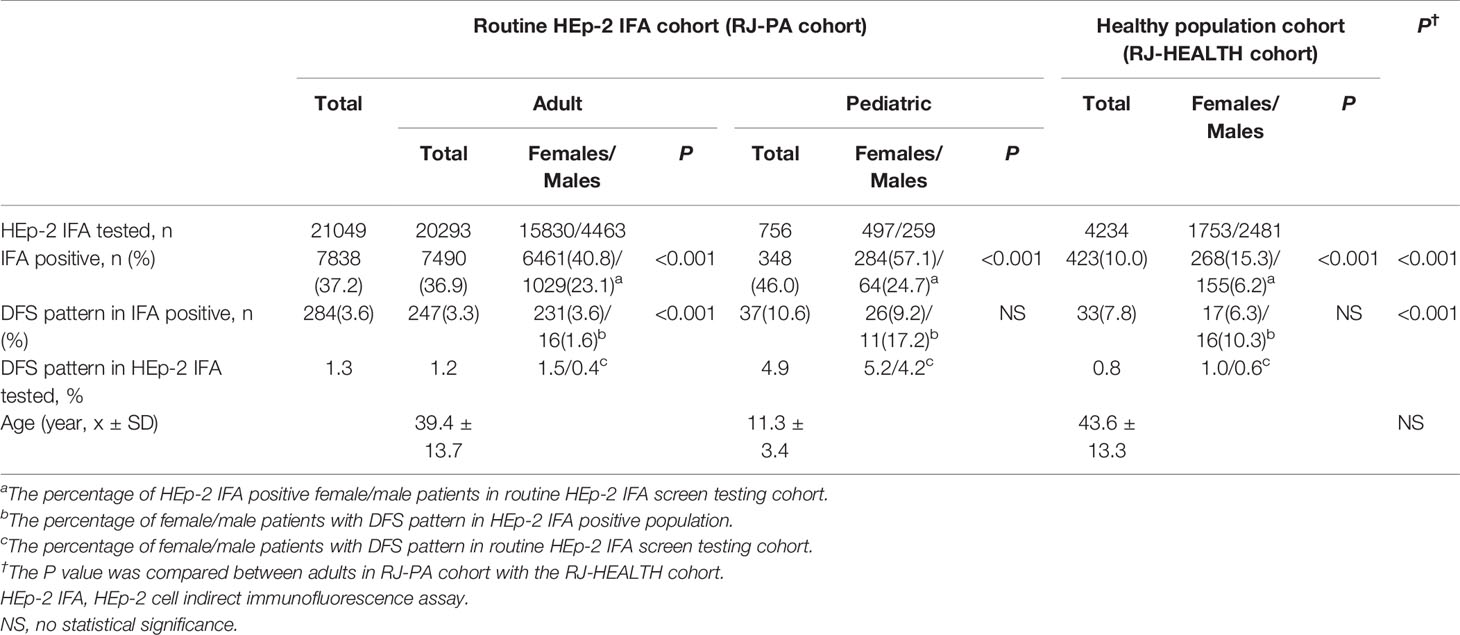
Table 2 Comparison of the DFS pattern between adult and pediatric patient and healthy population cohorts by routine HEp-2 IFA in Renji Hospital during a 3-month study period.
In the RJ-PA cohort, the percentages of SARD patients with DFS pattern were compared respectively with HEp-2 IFA negative (AC-0) and three other common patterns including nuclear homogeneous (AC-1), fine speckled (AC-4), and large/coarse speckled (AC-5), and remaining patterns (Table 3). In the RJ-PA adult cohort, the percentage of SARD in each AC pattern was much lower in patients with DFS pattern compared to those with AC-1, AC-4, AC-5 or other remaining patterns, but higher than the HEp-2 IFA negatives. In contrast, the percentage of SARD in pediatric patients with DFS pattern was comparable to those with HEp-2 IFA negative or AC-1, lower than AC-4 or AC-5, and higher than other remaining patterns.
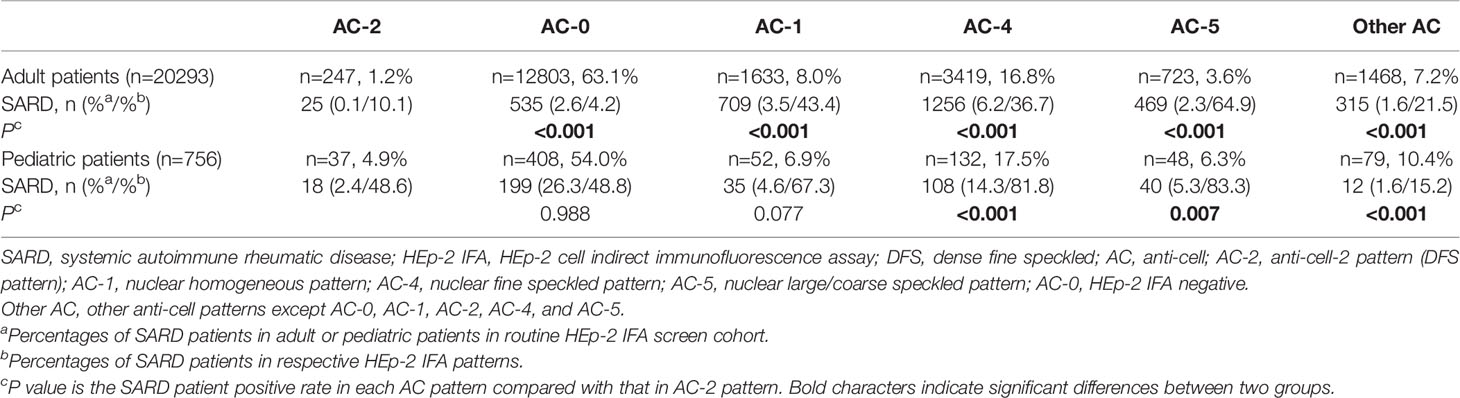
Table 3 Comparison of the percentages of SARD patients with DFS pattern (AC-2) versus with ANA-negative (AC-0) and other AC patterns in the RJ-PA adult and pediatric cohorts.
Consistency of Interpretation Rates of the DFS Pattern Between Renji Hospital and Participating Centers
At the onset of this study, only 14 of the 29 participating laboratories routinely reported the DFS pattern (Table 1). Among the participating centers, the mean years of experience with reading ANA patterns was 4.4 ± 2.9 years. The interpretation consistency rates between Renji Hospital and other centers were low, as only 46.2% (298/645) of delivered sera were confirmed positive for the DFS pattern. Heterogeneity in medical resources might be a reason for the lack of expertise in the recognition of the DFS pattern in some centers. Figure 3A shows a relatively small change in interpretation consistency from 80.8% to 89.5% (P=0.535) in Shanghai centers from July to September, compared to a more obvious increase from 29.7% to 60.6% (P<0.0001) in other regions. An apparent improvement in DFS pattern interpretation was observed in many participating centers throughout the program (Figure 3B).

Figure 3 The apparent improvement (%) in correctly recognizing DFS pattern in many participating centers during the training program from July to September 2019. Putative DFS sera sent to the organizing center at Renji Hospital were re-analyzed and the accuracy of DFS pattern reporting was calculated as interpretation consistency rate. (A) Comparison of the interpretation consistency rate of DFS pattern by Renji Hospital versus 4 centers in Shanghai area and 25 centers in more remote provinces in China (Other Regions). (B) Apparent improvement in DFS pattern interpretation in representative hospitals with codes as indicated in Circles mark months when serum samples were not delivered to Renji Hospital. Centers with statistical differences showing improvement of their interpretation consistency rate between July and September were marked with asterisk. (C) Comparison of results in the re-testing of serum samples sent by the 29 centers to Renji Hospital using Inova and Euroimmun ANA HEp-2 cell slides. AC-2: dense fine speckled (DFS) pattern, AC-2 + Other pattern: DFS pattern mixed with other ANA pattern, AC-1: nuclear homogeneous pattern, AC-4: nuclear speckled pattern, Neg: ANA negative results. a: AC-1 mixed with another pattern (AC-1 + AC-4 not included); b: AC-4 mixed with another pattern (AC-1 + AC-4 not included); c: Other single patterns including AC-5 - nuclear large/coarse speckled, AC-8 - homogeneous nucleolar and AC-28 - mitotic chromosomal.
Comparison of DFS Pattern Interpretation by Two Commercial HEp-2 IFA Kits
All 645 serum samples delivered from the participating centers were tested on two different commercial HEp-2 IFA slides to evaluate the agreement in identifying the DFS pattern. There was good agreement between the two brands (kappa=0.598) as 31.2% (201/645) of sera showed single or mixed DFS pattern on both slides, while 4.3% (28/645) and 15.3% (99/645) exhibited the DFS pattern only in Inova Diagnostics or Euroimmun slides, respectively. Some of the typical inconsistent cases are shown in Supplementary Figure 1. Moreover, 49.1% (317/645) of sera did not show the DFS pattern on either type of slide. All the misinterpreted patterns are listed in Figure 3C, which shows that the nuclear homogeneous pattern (AC-1) was the most difficult pattern to distinguish from DFS. 18.1% and 12.4% of sera were misinterpreted as nuclear homogeneous on Inova and Euroimmun slides, respectively.
Anti-DFS70 Antibodies Detected by ELISA, LIA, and HEp-2 ELITE
All 645 sera interpreted as single or mixed DFS pattern on Inova or Euroimmun HEp-2 slides were further analyzed by ELISA, LIA, and IFA on HEp-2 ELITE/DFS KO substrate. Sera with DFS pattern on both Inova and Euroimmun slides were significantly more likely to be positive for anti-DFS70 antibody in ELISA, LIA, or HEp-2 ELITE assays than sera showing positive in only one of the slides. Anti-DFS70 positive rates among the samples positive on both versus only one commercial slide were 96.0% (457/476) and 63.3% (107/169) (P<0.0001) by ELISA, 93.7% (446/476) and 56.8% (96/169) (P<0.0001) by LIA, and 49.6% (236/476) and 29.6% (50/169) (P<0.0001) by HEp-2 ELITE, respectively (Figure 2B). Anti-DFS70 reference material was tested by HEp-2 ELITE/DFS KO substrate as a positive control (Supplementary Figure 2). In our in-house anti-DFS70 ELISA, 60 samples negative for anti-DFS70, confirmed by LIA, were used to establish a cutoff of 0.6 (mean O.D. value of 0.214 + 3 × 0.130 SD). The O.D. value for anti-DFS70 reference material was 2.240. The correlations measured between ELISA O.D. value with HEp-2 IFA titer (Spearman r=0.501, P<0.0001) and NI read by NOVA VIEW 1.0 (Spearman r=0.506, P<0.0001) were considered significant (Supplementary Figure 3).
Clinical Phenotypes and Additional ENA Specificities in Anti-DFS70 Positive Sera
In total, 84.7% (546/645) of serum samples with the DFS pattern, including 517 samples from the HEp-2 IFA screening cohort and 29 sera from the RJ-HEALTH cohort, showed positive reactivity to DFS70 by both ELISA and LIA. Of these positive patients, 336 adult (89.4%) and 40 pediatric (10.6%) patients had traceable clinical phenotypes. The spectrum of disease and clinical conditions of adult and pediatric patients with positive reactivity to DFS70 by both ELISA and LIA were analyzed respectively in Table 4 and the disease groups were detailed in Supplementary Table 2. For SARD group, the most prevalent diseases among DFS70-positive adult and pediatric patients were spontaneous abortion (28.0%, 94/336) and juvenile idiopathic arthritis (JIA, 22.5%, 9/40), respectively (Supplementary Table 2). In investigating anti-DFS70 positive patients with SARD, half of the anti-DFS70 positive pediatric patients were diagnosed with SARD including 9 JIA (22.5%, 9/40), 8 undifferentiated connective tissue disease (UCTD) (20.0%, 8/40) and 3 SLE (7.5%, 3/40) while anti-DFS70 positive. In contrast, adult patients included 28 UCTD (8.3%, 28/336), 13 rheumatoid arthritis (3.9%, 13/336), 5 SLE (1.5%, 5/336), 5 Sjogren’s syndrome (1.5%, 5/336) and 1 antiphospholipid syndrome (0.3%, 1/336).
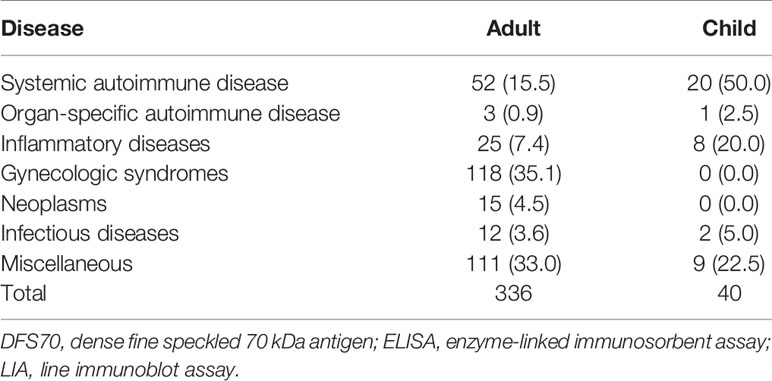
Table 4 Clinical diagnoses of adult and pediatric patients with positive reactivity to DFS70 by both ELISA and LIA.
All 376 patients were further tested for additional anti-ENA antibodies by LIA. The prevalence of “monospecific” anti-DFS70 was higher in non-SARD patients (76.8%, 289/376) than in SARD patients (15.2%, 57/376; P<0.0001) as shown in Supplementary Table 3. The additional anti-ENA positive rates were as follows: anti-Ro52/TRIM21 4.8% (SARD: 9.7%, non-SARD: 3.7%, P=0.059), anti-SSA/Ro60 2.7% (SARD: 6.9%, non-SARD: 1.7%, P=0.027), anti-Rib-P 1.1% (SARD: 4.2%, non-SARD: 0.3%, P=0.024), anti-histone 1.1% (SARD: 2.8%, non-SARD: 0.7%, P=0.170), anti-nRNP/Sm 0.8% (SARD: 4.2%, non-SARD: 0.0%, P=0.007), and anti-SSB/La 0.3% (SARD: 1.4%, non-SARD: 0.0%, P=0.194). The details of additional ENAs in adult and pediatric patients with anti-DFS70 in different clinical diagnoses are listed in Supplementary Table 4. Moreover, additional anti-ENAs were not detected in DFS sera from the RJ-HEALTH cohort.
Discussion
A large-scale, short-term multi-center study was conducted with 30 laboratories from various regions in China, of which 16 started with no experience of reporting DFS pattern and 14 had begun reporting DFS pattern only one or two years prior to the study. After the DFS pattern training/re-orientation program, we found that: 1) the short-term training greatly improved DFS pattern recognition in many of the participating laboratories as the interpretation consistency increased from 29.7 to 60.6% in the areas other than Shanghai, 2) the pattern identification consistency was higher in areas with well-developed health facilities (i.e. the consistency rate in the four laboratories in Shanghai was 80.8 versus 29.7% for the other participating laboratories at the start of the program), 3) sera with DFS pattern confirmed by two commercial HEp-2 slides showed higher positive rates for anti-DFS70 antibodies than by only one slide brand (96.0 versus 56.2, or 75.0%).
To better understand the prevalence of DFS pattern in the routine HEp-2 IFA screening cohort of this study, we have summarized HEp-2 IFA positive rates from different reports worldwide in Supplementary Table 5. The reported HEp-2 IFA positive rates in Supplementary Table 5 based on routine screen test cohorts range from 11.6–82.0% with a median of 28.5%, which is close to our present study of 36.2%. However, our prevalence of the DFS pattern was 0.6% in the routine HEp-2 IFA screening cohort, which is relatively low compared with reports from other countries (Supplementary Table 5). There are a few possible explanations for this low frequency. First, ethnicity may have played a role in the prevalence of the DFS pattern/anti-DFS70. To put this into perspective, the highest prevalence of 27% was only reported in the United States (16) compared to 0.3–8.4% reported in other continents (Supplementary Table 5). In the present study, the HEp-2 IFA positive rate was significantly higher for Han Chinese than for Uygurs and Kazakhs, yet DFS pattern positive rates were higher among these minority populations. In contrast, the HEp-2 IFA positive rate was higher in Tibetans, Huis, and Mongolians than Han Chinese, while the DFS pattern positive rate in HEp-2 IFA positive sera showed no statistical difference. To our knowledge, this is the first report to compare HEp-2 IFA positive rates between national minority groups and Han Chinese in China. Second, reported DFS pattern positive rates can also be affected by the experience of IFA evaluators (16, 23). In Supplementary Table 5, the reported prevalence of the DFS pattern in Turkey varied widely from 0.3 to 8.1% (24). In North America, the prevalence of DFS has been reported as 27.0% in the United States (16) but only 1.6% in Canada (6). As the present study shows, improving the accuracy of DFS pattern interpretation in some of the participating centers, especially those in areas with relatively poor medical resources, will affect the overall reported anti-DFS70 prevalence. Finally, application of different commercial HEp-2 IFA kits may contribute to the discrepancy in prevalence of the DFS pattern (25, 26). Due to the inevitable heterogeneity in performance of various slides, the consistency of the two kits used in our study only exhibited moderate agreement (kappa=0.598) in DFS pattern identification.
We further investigated the prevalence of the DFS pattern in pediatric patients. Schmeling et al. (27) reported a 4.5% positive rate (titer ≥1:80) of anti-DFS70 in pediatric patients referred for ANA testing, which is comparable to the 4.9% reported in the present study. Anti-DFS70 antibodies have been reported to be more prevalent in younger age groups (4, 28). This may partly explain why the positive rate of DFS pattern was higher in pediatric versus adult patients in this study. However, no statistical difference between pediatric patients and adult healthy individuals was found. Notably, the DFS pattern was more prevalent in adult female patients (females 3.6%, males 1.6%), while no gender differences were observed among the pediatric patient and healthy populations. Therefore, the prevalence features of anti-DFS70 in pediatric patients were similar to those in the healthy adult population. To date, few studies have focused on anti-DFS70 in pediatric patients. The prevalence in healthy children should be further investigated and compared to pediatric patients in the same age category (29).
As accurate DFS pattern interpretation by IFA alone is challenging, additional objective tests such as ELISA, LIA, or chemiluminescence immunoassay (CLIA) are necessary to identify anti-DFS70 antibodies (25, 30–32). ELITE/DFS KO substrate also offers a unique possibility of evaluating anti-DFS70 antibodies at the IFA stage (23, 33). Reports have shown that ELITE/DFS KO substrate can improve the sensitivity of confirming anti-DFS70 antibodies to 65% compared to 61% by CLIA (23). In our study, the sensitivity of ELITE/DFS KO substrate was far below both ELISA and LIA. Thus, we recommend clinical laboratories apply anti-DFS70 antibody methodologies like ELISA and LIA instead of ELITE/DFS KO substrate.
Carter et al. reported 73.1% monospecific DFS70 in anti-DFS70 positive sera from a HEp-2 IFA screening cohort which was lower than our findings of 92.0%. Monospecific anti-DFS70 is considered rare in SARD and may serve as an exclusion biomarker for SARD (5, 7, 34). In a multi-center study, Choi et al. reported 1.1% monospecific anti-DFS70 in a large cohort of SLE patients (34). In our study, the percentage of monospecific anti-DFS70 patients was much higher in non-SARD (76.8%) than in SARD (15.2%) patients in HEp-2 IFA screening cohort. This to some extent supports that monospecific anti-DFS70 is a reliable biomarker to rule out diagnosis of SARD (35). Moreover, anti-Ro52/TRIM21 and anti-SSA/Ro60 were the most commonly detected autoantibodies accompanying anti-DFS70 antibodies in our LIA ENA profiles, while SLE-specific antibodies including Sm and dsDNA were not observed. Choi et al. (34) also reported anti-SSA/Ro60 (34.6%) and anti-Ro52/TRIM21 (27.2%) detected by addressable laser bead immunoassay array, as the most common autoantibodies found with anti-DFS70 antibodies in SLE patients, which is consistent with our results.
Regarding the clinical relevance of anti-DFS70 antibodies, there were some unexpected findings. First, the effect of DFS pattern in helping to rule out SARD in the routine HEp-2 IFA screening cohort was not in fact stronger than negative ANA in adults, while no statistical difference was observed between DFS pattern and negative ANA in pediatric patients. The latter can be explained partly to the high percentage of JIA patients (26.6%, 201/756) in the pediatric ANA screen cohort and the HEp-2 IFA positive rate of JIA was 21.9% (44/201) (data not shown). Moreover, it is still worth noting that the frequency of SARD in anti-DFS70 positive pediatric patients was unexpectedly as high as 50.0%. We consider this data significant because in this RJ-PA pediatric cohort, the percentage of SARD patients with DFS pattern was similar to those with AC-1 and much higher than those with other ANA patterns except AC-4 and AC-5. However, in the RJ-PA adult cohort, the percentage of SARD patients with DFS pattern was much lower than those with AC-1, AC-4, AC-5 or even those with other patterns. Together, this data strongly suggests that anti-DFS70 prevalence was different in pediatric and adults patients with SARD. Sperotto et al. tested four monospecific anti-DFS70 positive cases out of a population of 261 school-age children and found that three of the cases (75%) had a family history of autoimmune disease, but no disease symptoms (29). Moreover, none of the ANA-positive (anti-DFS70 positive/negative) subjects developed SARD in a three-year follow up (29). Therefore, future multi-center studies should focus on the underlying role of anti-DFS70 in pediatric cohorts. Second, the proportion of anti-DFS70 positive adult patients who had experienced spontaneous abortion from the ANA screening cohort was remarkably high at 28.0%. The highest rate of DFS pattern among the HEp-2 IFA positive population was observed in an obstetrics and gynecology hospital in Shanghai, China. For the first time, these data suggest that anti-DFS70 antibodies may be associated with female reproductive diseases. Furthermore, in an ongoing study in the Renji Hospital, the prevalence of DFS pattern in females with spontaneous abortion (3.1%, 94/2990) was much higher than age-matched healthy females (1.5%, 45/2990; P<0.0001, data not shown), which also support the association between them. Isolated studies have also reported anti-DFS70 in eye diseases, like sympathetic ophthalmia (9), Vogt-Koyanagi-Harada syndrome (11), and atopic dermatitis with cataracts (36), and as tumor-associated antibodies present in prostate cancer patients (8, 13, 37). Marlet et al. (38) was the first to report that anti-DFS70 antibodies may be correlated with thrombosis and obstetric complications. DFS70 has been shown to be upregulated by human papilloma virus (HPV) infection and its associated oncogenes E6/E7 (39) and has been implicated in autoimmune thyroiditis (40). Both diseases are also associated with several reproductive pathology (41). However, anti-DFS70 have previously been shown to be associated with young age and female sex (40). Therefore, to better investigate the association between spontaneous abortions and anti-DFS70, the prevalence of anti-DFS70 needs to be compared between the pregnant women who completed the pregnancy versus those with spontaneous abortions. Further studies are necessary to explore the clinical association between anti-DFS70 and reproductive diseases.
In addition to those mentioned above, UCTD and RA were the most common SARD in adults associated with anti-DFS70, while JIA, UCTD, and pediatric SLE were seen in anti-DFS70 positive pediatric patients. This is consistent with some previous reports that anti-DFS70 antibodies may be restricted to SARD patients without typical ANA-associated antibodies, and only rarely found in patients with ANA-associated rheumatic disease (6, 7, 42). Infantino et al. also reported high prevalence of anti-DFS70 antibodies in UCTD cases (13.3%) and suggested that anti-DFS70 antibodies could serve as an appropriate biomarker for the development of UCTD to CTD (28).
One limitation of this study was that we were unable to obtain some additional laboratory test results, including anti-thyroid peroxidase antibodies, serum free triiodothyronine, thyroxine, thyroid-stimulating hormone, and activated partial thromboplastin time for the anti-DFS70 positive cohort from the participating laboratories to further explore their relationship to anti-DFS70 positive patients.
In conclusion, DFS pattern interpretation can be a challenging task for many clinical laboratories. However, a short-term training course and inter-laboratory comparison of HEp-2 IFA results can improve IFA pattern reading accuracy. The prevalence of ANA and the DFS pattern may vary between different Chinese ethnic groups. The clinical usefulness of anti-DFS70 may help to exclude SARD in adult patients. The increased prevalence of spontaneous abortion and pediatric SARD in anti-DFS70 positive patients will require further follow-up studies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics Statement
All serum specimens collected in this study were approved by the Institutional Review Board of Renji Hospital (No. KY[2019]121). No consent was required for this study.
Author Contributions
BZ, ZW, AL, CL, DL, FZ, HL, HG, JZ, JL, LC, LW, LY, LNY, LJ, MFH, MH, PX, QL, SH, SSC, SMC, SZ, WS, XG, YC, YW, YQ, ZL, ZN, and ZH assisted HEp-2 IFA pattern interpretation. ZW performed the experiments. BZ analyzed the experiments and wrote the manuscript. EC supervised the study and revised the manuscript. BZ and RM read and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Shanghai Medical and Health Development Foundation grant 2019 and National Natural Science Foundation of China (grants 81772139). EC is supported by NIH grant R01DE028536.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors EC.
Acknowledgments
The authors would like to thank Mr. Enling Li, Ms. Chongzhao Yu (Renji Hospital, School of Medicine, Shanghai Jiao Tong University), Ms. Zhijuan Gong and Mr. Mingxin Shi (Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University) for their excellent technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.562138/full#supplementary-material
Abbreviations
ANA, antinuclear antibodies; AC-2, anti-cell-2; DFS70, dense fine speckled 70 kDa antigen; CLIA, chemiluminescence immunoassay; CTD, connective tissue disease; ELISA, enzyme-linked immunosorbent assay; ENA, extractable nuclear antigen; HEp-2 IFA, HEp-2 cell indirect immunofluorescence assay; ICAP, International Consensus on Antinuclear Antibody Patterns; LIA, line immunoblot assay; NI, nuclear intensities; O.D., optical density; Rib-P, ribosomal P proteins; RA, rheumatoid arthritis; SARD, systemic autoimmune rheumatic disease; SD, standard deviations; UCTD, undifferentiated connective tissue disease.
References
1. Mahler M, Fritzler MJ. Epitope specificity and significance in systemic autoimmune diseases. Ann N Y Acad Sci (2010) 1183:267–87. doi: 10.1111/j.1749-6632.2009.05127.x
2. Ochs RL, Muro Y, Si Y, Ge H, Chan EK, Tan EM, et al. Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allergy Clin Immunol (2000) 105:1211–20. doi: 10.1067/mai.2000.107039
3. Singh DP, Ohguro N, Kikuchi T, Sueno T, Reddy VN, Yuge K, et al. Lens epithelium-derived growth factor: effects on growth and survival of lens epithelial cells, keratinocytes, and fibroblasts. Biochem Biophys Res Commun (2000) 267:373–81. doi: 10.1006/bbrc.1999.1979
4. Watanabe A, Kodera M, Sugiura K, Usuda T, Tan EM, Takasaki Y, et al. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheumatol (2004) 50:892–900. doi: 10.1002/art.20096
5. Conrad K, Röber N, Andrade LE, Mahler M. The clinical relevance of anti-DFS70 autoantibodies. Clin Rev Allergy Immunol (2017) 52:202–16. doi: 10.1007/s12016-016-8564-5
6. Mahler M, Parker T, Peebles CL, Andrade LE, Swart A, Carbone Y, et al. Anti-DFS70/LEDGF antibodies are more prevalent in healthy individuals compared to patients with systemic autoimmune rheumatic diseases. J Rheumatol (2012) 39:2104–10. doi: 10.3899/jrheum.120598
7. Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE, et al. Pattern on the antinuclear antibody–HEp-2 test is a critical parameter for discriminating antinuclear antibody–positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheumatol (2011) 63:191–200. doi: 10.1002/art.30084
8. O’Rourke DJ, DiJohnson DA, Caiazzo RJ Jr., Nelson JC, Ure D, O’Leary MP, et al. Autoantibody signatures as biomarkers to distinguish prostate cancer from benign prostatic hyperplasia in patients with increased serum prostate specific antigen. Clin Chim Acta (2012) 413:561–7. doi: 10.1016/j.cca.2011.11.027
9. Sugiura K, Muro Y, Nishizawa Y, Okamoto M, Shinohara T, Tomita Y, et al. LEDGF/DFS70, a major autoantigen of atopic dermatitis, is a component of keratohyalin granules. J Invest Dermatol (2007) 127:75–80. doi: 10.1038/sj.jid.5700487
10. Mahler M, Andrade LE, Casiano CA, Malyavantham K, Fritzler MJ. Anti-DFS70 antibodies: an update on our current understanding and their clinical usefulness. Expert Rev Clin Immunol (2019) 15:241–50. doi: 10.1080/1744666X.2019.1562903
11. Yamada K, Senju S, Shinohara T, Nakatsura T, Murata Y, Ishihara M, et al. Humoral immune response directed against LEDGF in patients with VKH. Immunol Lett (2001) 78:161–8. doi: 10.1016/s0165-2478(01)00243-7
12. Chin MS, Caruso RC, Detrick B, Hooks JJ. Autoantibodies to p75/LEDGF, a cell survival factor, found in patients with atypical retinal degeneration. J Autoimmun (2006) 27:17–27. doi: 10.1016/j.jaut.2006.04.002
13. Daniels T, Zhang J, Gutierrez I, Elliot ML, Yamada B, Heeb MJ, et al. Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate (2005) 62:14–26. doi: 10.1002/pros.20112
14. Dai L, Li J, Ortega R, Qian W, Casiano CA, Zhang JY. Preferential autoimmune response in prostate cancer to cyclin B1 in a panel of tumor-associated antigens. J Immunol Res (2014) 2014:827827. doi: 10.1155/2014/827827
15. Shovman O, Gilburd B, Chayat C, Amital H, Langevitz P, Watad A, et al. Prevalence of anti-DFS70 antibodies in patients with and without systemic autoimmune rheumatic diseases. Clin Exp Rheumatol (2018) 36:121–6.
16. Carter JB, Carter S, Saschenbrecker S, Goeckeritz BE. Recognition and relevance of anti-DFS70 autoantibodies in routine antinuclear autoantibodies testing at a community hospital. Front Med (2018) 5:88. doi: 10.3389/fmed.2018.00088
17. Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis (2010) 69:1420–2. doi: 10.1136/ard.2009.127100
18. Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis (2019) 2019) 78:879–89. doi: 10.1136/annrheumdis-2018-214436
19. Miyara M, Albesa R, Charuel J-L, El Amri M, Fritzler MJ, Ghillani-Dalbin P, et al. Clinical phenotypes of patients with anti-DFS70/LEDGF antibodies in a routine ANA referral cohort. Clin Dev Immunol (2013) 2013:703759. doi: 10.1155/2013/703759
20. Bentow C, Fritzler MJ, Mummert E, Mahler M. Recognition of the dense fine speckled (DFS) pattern remains challenging: results from an international internet-based survey. Auto Immun Highlights (2016) 7:8. doi: 10.1007/s13317-016-0081-2
21. Dellavance A, Baldo DC, Zheng B, Mora RA, Fritzler MJ, Hiepe F, et al. Establishment of an international autoantibody reference standard for human anti-DFS70 antibodies: proof-of-concept study for a novel Megapool strategy by pooling individual specific sera. Clin Chem Lab Med (2019) 57:1754–63. doi: 10.1515/cclm-2019-0087
22. Chan EKL, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PL, et al. Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014-2015. Front Immunol (2015) 6:412. doi: 10.3389/fimmu.2015.00412
23. Malyavantham K, Suresh L. Analysis of DFS70 pattern and impact on ANA screening using a novel HEp-2 ELITE/DFS70 knockout substrate. Auto Immun Highlights (2017) 8:3. doi: 10.1007/s13317-017-0091-8
24. Deng C, Qu X, Cheng S, Zeng X, Li Y, Fei Y. Decision-making value of nuclear dense fine speckled pattern in systemic autoimmune rheumatic disease: trick or treat? Ann Rheum Dis (2020) 79:e92. doi: 10.1136/annrheumdis-2019-215587
25. Bizzaro N, Tonutti E, Villalta D. Recognizing the dense fine speckled/lens epithelium–derived growth factor/p75 pattern on HEp-2 cells: Not an easy task! Comment on the article by Mariz et al. Arthritis Rheumatol (2011) 63:4036–7. doi: 10.1002/art.30621
26. Pisetsky DS, Spencer DM, Lipsky PE, Rovin BH. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann Rheum Dis (2018) 77:911–3. doi: 10.1136/annrheumdis-2017-212599
27. Schmeling H, Mahler M, Levy DM, Moore K, Stevens AM, Wick J, et al. Autoantibodies to dense fine speckles in pediatric diseases and controls. J Rheumatol (2015) 42:2419–26. doi: 10.3899/jrheum.150567
28. Infantino M, Shovman O, Pérez D, Manfredi M, Grossi V, Benucci M, et al. Anti-DFS70 autoantibodies in undifferentiated connective tissue diseases subjects: what’s on the horizon? Rheumatol (Oxford) (2018) 57:1293–8. doi: 10.1093/rheumatology/key012
29. Sperotto F, Seguso M, Gallo N, Plebani M, Zulian F. Anti-DFS70 antibodies in healthy schoolchildren: a follow-up analysis. Autoimmun Rev (2017) 16:210–1. doi: 10.1016/j.autrev.2017.01.001
30. Fritzler MJ. The antinuclear antibody test: last or lasting gasp? Arthritis Rheumatol (2011) 63:19–22. doi: 10.1002/art.30078
31. Mahler M, Hanly JG, Fritzler MJ. Importance of the dense fine speckled pattern on HEp-2 cells and anti-DFS70 antibodies for the diagnosis of systemic autoimmune diseases. Autoimmun Rev (2012) 11:642–5. doi: 10.1016/j.autrev.2011.11.005
32. Basu A, Woods-Burnham L, Ortiz G, Rios-Colon L, Figueroa J, Albesa R, et al. Specificity of antinuclear autoantibodies recognizing the dense fine speckled nuclear pattern: preferential targeting of DFS70/LEDGFp75 over its interacting partner MeCP2. Clin Immunol (2015) 161:241–50. doi: 10.1016/j.clim.2015.07.014
33. Bizzaro N, Fabris M. New genetically engineered DFS70 knock-out HEp-2 cells enable rapid and specific recognition of anti-DFS70 antibodies. Autoimmunity (2018) 51:152–6. doi: 10.1080/08916934.2018.1469013
34. Choi M, Clarke A, St Pierre Y, Hanly J, Urowitz M, Romero-Diaz J, et al. The prevalence and determinants of anti-DFS70 autoantibodies in an international inception cohort of systemic lupus erythematosus patients. Lupus (2017) 26:1051–9. doi: 10.1177/0961203317692437
35. Ortiz-Hernandez GL, Sanchez-Hernandez ES, Casiano CA. Twenty years of research on the DFS70/LEDGF autoantibody-autoantigen system: many lessons learned but still many questions. Auto Immun Highlights (2020) 11:3. doi: 10.1186/s13317-020-0126-4
36. Ayaki M, Ohoguro N, Azuma N, Majima Y, Yata K, Ibaraki N, et al. Detection of cytotoxic anti-LEDGF autoantibodies in atopic dermatitis. Autoimmunity (2002) 35:319–27. doi: 10.1080/0891693021000003198
37. Xie C, Kim HJ, Haw JG, Kalbasi A, Gardner BK, Li G, et al. A novel multiplex assay combining autoantibodies plus PSA has potential implications for classification of prostate cancer from non-malignant cases. J Transl Med (2011) 9:43. doi: 10.1186/1479-5876-9-43
38. Marlet J, Ankri A, Charuel J-L, Ghillani-Dalbin P, Perret A, Martin-Toutain I, et al. Thrombophilia associated with anti-DFS70 autoantibodies. PLoS One (2015) 10(9):e0138671. doi: 10.1371/journal.pone.0138671
39. Leitz J, Reuschenbach M, Lohrey C, Honegger A, Accardi R, Tommasino M, et al. Oncogenic human papillomaviruses activate the tumor-associated lens epithelial-derived growth factor (LEDGF) gene. PLoS Pathog (2014) 10:e1003957. doi: 10.1371/journal.ppat.1003957
40. Mahler M, Meroni PL, Andrade LE, Khamashta M, Bizzaro N, Casiano CA, et al. Towards a better understanding of the clinical association of anti-DFS70 autoantibodies. Autoimmun Rev (2016) 15:198–201. doi: 10.1016/j.autrev.2015.11.006
41. Jahdi F, Khademi K, Khoei EM, Haghani H, Yarandi F. Reproductive factors associated to human papillomavirus infection in Iranian woman. J Family Reprod Health (2013) 7:145–9.
Keywords: antinuclear antibodies, DFS70, pediatric rheumatic disease, spontaneous abortion, systemic autoimmune rheumatic disease
Citation: Zheng B, Wang Z, Mora RA, Liu A, Li C, Liu D, Zhai F, Liu H, Gong H, Zhou J, Liu J, Chen L, Wu L, Yuan L, Ying L, Jie L, He M, Hao M, Xu P, Lu Q, Han S, Chen S, Chen S, Zhu S, Sun W, Guo X, Chen Y, Wang Y, Qu Y, Li Z, Niu Z, Han Z and Chan EKL (2020) Anti-DFS70 Antibodies Among Patient and Healthy Population Cohorts in China: Results From a Multicenter Training Program Showing Spontaneous Abortion and Pediatric Systemic Autoimmune Rheumatic Diseases Are Common in Anti-DFS70 Positive Patients. Front. Immunol. 11:562138. doi: 10.3389/fimmu.2020.562138
Received: 14 May 2020; Accepted: 14 September 2020;
Published: 02 October 2020.
Edited by:
Pier Luigi Meroni, Istituto Auxologico Italiano (IRCCS), ItalyReviewed by:
Tadej Avcin, University Medical Centre Ljubljana, SloveniaJohan Rönnelid, Uppsala University, Sweden
Copyright © 2020 Zheng, Wang, Mora, Liu, Li, Liu, Zhai, Liu, Gong, Zhou, Liu, Chen, Wu, Yuan, Ying, Jie, He, Hao, Xu, Lu, Han, Chen, Chen, Zhu, Sun, Guo, Chen, Wang, Qu, Li, Niu, Han and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Zheng, zhengbing_renji@shsmu.edu.cn; Edward K. L. Chan, echan@ufl.edu
 Bing Zheng
Bing Zheng Zhiqing Wang1
Zhiqing Wang1 Rodrigo A. Mora
Rodrigo A. Mora Loujian Jie
Loujian Jie Edward K. L. Chan
Edward K. L. Chan