
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 30 July 2020
Sec. Microbial Immunology
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.01489
This article is part of the Research Topic Recent Advances in the Immunology of Helminth Infection – Protection, Pathogenesis and Panaceas View all 21 articles
Human intestinal helminth infection affects more than 1 billion people often in the world's most deprived communities. These parasites are one of the most prevalent neglected tropical diseases worldwide bringing huge morbidities to the host population. Effective treatments and vaccines for helminths are currently limited, and therefore, it is essential to understand the molecular sensors that the intestinal epithelium utilizes in detecting helminths and how the responding factors produced act as modulators of immunity. Defining the cellular and molecular mechanisms that enable helminth detection and expulsion will be critical in identifying potential therapeutic targets to alleviate disease. However, despite decades of research, we have only recently been able to identify the tuft cell as a key helminth sensor at the epithelial barrier. In this review, we will highlight the key intestinal epithelial chemosensory roles associated with the detection of intestinal helminths, summarizing the recent advances in tuft cell initiation of protective type 2 immunity. We will discuss other potential sensory roles of epithelial subsets and introduce enteroendocrine cells as potential key sensors of the microbial alterations that a helminth infection produces, which, given their direct communication to the nervous system via the recently described neuropod, have the potential to transfer the epithelial immune interface systemically.
Soil-transmitted helminths (STHs) affect >1 billion people in the world's most deprived communities (1). These parasites are one of the most prevalent neglected tropical diseases worldwide bringing huge morbidities to the host population. Sub-Saharan Africa alone is estimated to lose 2.3 million disability-adjusted life-years annually (2). Constant advances have been made in identifying type 2 immune responses as key to helminth control and expulsion (3–8), with the cytokine interleukin (IL)-13 being crucial in driving the characteristic “allergic” immune response (3). CD4+ T-cells and more recently type 2 innate lymphoid cells (ILC2s) are key producers of these cytokines, with ILC2 believed to be the major initiators of type 2 immunity (9, 10) driven by the release of the epithelial alarmins IL-33, thymic stromal lymphopoietin (TSLP), and IL-25 (11).
Following the identification that tuft cells of the rat epithelium (12) possessed alpha-gustducin, the G-protein subunit of numerous taste receptors, it had been postulated that these cells could act as solitary chemosensory apparatus within tissues. The identification of the tuft cell-specific marker doublecortin like kinase 1 (DCLK1) (13–17) and the discovery of the downstream master transcription factors, Pou domain, class 2, transcription factor 3 (Pou2f3) and growth factor-independent 1b (GFI1b) (15, 18–20), allowed further elucidation of this tuft cell chemosensory hypothesis. In 2016, three key papers cemented the importance of tuft cells in sensing parasites and brought tuft cell biology to the forefront of helminth immunity (21). Through examination of Pou2f3 null mice during a small intestinal helminth infection, Gerbe et al. (19) defined that IL-13 acted downstream of the tuft cell lineage, suggesting a tuft cell initiated IL-25-driven positive feed-forward loop resulting in ILC2 expansion and IL-13-driven tuft and goblet cell hyperplasia (Figure 1), essential for helminth expulsion (22, 23). In parallel to these studies, von Moltke et al. (24) confirmed tuft cells as IL-25 expressers that, following small intestinal helminthiasis, underwent hyperplasia via an ILC2 derived IL-13 interaction with the IL-4Rα in a feed-forward loop, presumably via stem cell niche signaling (25, 26). Finally, mice null for the G-protein subunit gustducin or the transient receptor potential cation channel, subfamily M, member 5 (TRMP5), a cation channel known to be important in the signaling cascade of chemosensory cells in the gut, mirrored the delayed tuft and goblet cell hyperplasia following a small intestinal helminth infection, giving the first indication of the chemosensory mechanisms of initial parasite detection (27). This minireview will focus on recent advancements in tuft cell biology as well as examining the potential for other epithelial chemosensory responses to helminths themselves and the microbial dysbiosis infection induces.
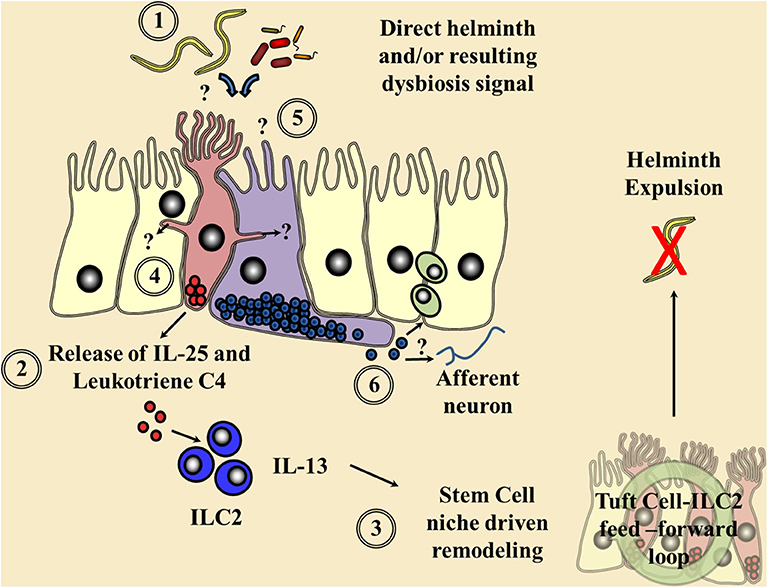
Figure 1. Current understanding of chemosensory detection of helminths at the epithelial barrier and a flavor of possible future perspectives. (1) Helminths are detected by tuft cells (red) through an as yet undefined receptor and ligand, although microbial dysbiosis produced via helminth colonization may be a potential candidate. (2) Gustducin-α and the transient receptor potential cation channel, subfamily M, member 5 (TRMP5) are required for the signaling cascade and Ca2+ flux, allowing the secretion of the alarmin interleukin (IL)-25 and leukotriene C4 in an arachidonate 5-lipoxygenase (ALOX5)-dependent mechanism signaling to resident type 2 innate lymphoid cells (ILC2s). (3) These factors in turn increase ILC2 numbers and their secretion of the cytokine IL-13, driving a feed-forward loop via the stem cell niche resulting in helminth expulsion. Tuft cell-derived acetylcholine could also possibly alter this epithelial stem cell niche and local immune responses. (4) Potential cross communication of tuft cells via cytospinules and the relay of helminth-derived signals to coordinate surrounding epithelial response. (5) The potential of enteroendocrine cells (purple), which host an array of chemosensory apparatus, to directly sense a helminth infection or infection-induced microbial dysbiosis. (6) The release of enteroendocrine peptide hormones signaling to the surrounding immune system either directly or via neuronal communication is proposed.
Single-cell analysis of the intestinal epithelium now suggests that tuft cells are a heterogenous population, with two proposed distinct subsets—tuft-1 and tuft-2—with differing cytokine profiles (28, 29). In response to undefined helminth antigens, small intestinal tuft cells produce TSLP as well as IL-25, which are crucial for the initiation of the anthelmintic mucosal response (28, 30). Only tuft-2 cells produce TSLP, although a functional role for TSLP from tuft cells has yet to be demonstrated (28). Schneider et al. (31) showed that genetic deletion of tumor necrosis factor alpha-induced protein 3 (Tnfaip3), a negative regulator of IL-25 signaling in ILC2, caused tuft cell and goblet cell hyperplasia, as well as small intestinal lengthening. Tissue remodeling that mimics the histological features of a helminth infection (31), reinforcing the importance of IL-25 signaling in type 2 immunity.
The initial identification of the potential taste receptor signaling pathways involved in parasite recognition at the epithelial barrier by Howitt et al. (27) has since been expanded upon. Luo et al. (32) further elucidated the Trpm5-dependent sensory pathway by showing that stimulation of intestinal organoids using larvae and antigens of the small intestinal helminth Trichinella spiralis stimulates increased intracellular calcium levels, resulting in tuft cell depolarization. They further observed that IL-13 administration promotes tuft cell hyperplasia as well as upregulation of genes including the Tas2r family of bitter taste G-protein coupled receptors (GPCRs) and the succinate receptor Sucnr1, indicating an adaptive ability of these chemosensory cells during T. spiralis infection. The importance of Tas2r in tuft cell recognition of helminths was demonstrated when pretreatment of small intestinal villi with allyl isothiocyanate, an inhibitor of bitter taste receptors, abolished T. spiralis-induced tuft cell-derived IL-25. Conversely, increased tuft cell production of IL-25 was seen after the administration of salicin, a Tas2r agonist (32). Two groups have also demonstrated the importance of Sucnr1 with Lei et al. (33) demonstrating that tuft cells are the sole epithelial expressers of this receptor in the small intestine. They, in parallel with Schneider et al. (31) demonstrated that dietary succinate increases small intestinal tuft cell secretion of IL-25 and promotes hyperplasia. Interestingly, examination of Sucnr1 null mice demonstrated a prevention of succinate-induced tuft and goblet cell hyperplasia (33). Nadjsombati et al. (34) demonstrated that succinate metabolites are produced by the small intestinal helminth Nippostrongylus brasiliensis in vitro; yet, immunity against N. brasiliensis is not abrogated in Sucnr1−/− mice, suggesting no requirement or at least redundancy in this potential helminth recognition pathway.
Schneider et al. (31) also reported that small intestinal tuft cells on Tritrichomonas-colonized mice highly express not only Sucnr1 but also GPCRs for short-chain fatty acids Ffar3. This result is also corroborated by other groups, who reported that Ffar3 is highly expressed by intestinal tuft-2 cells, but not intestinal tuft-1 cells or tracheal tuft cells (28, 34). Although the discovery of Ffar3 expression on small intestinal tuft cells is an interesting find, little is known at the moment on how the receptor impacts anthelmintic immunity. Interestingly, in a murine model of allergic airway inflammation, Ffar3 knockout abrogates Heligmosomoides polygyrus-induced alleviation of airway inflammation but did not affect worm burden in the small intestinal niche of this helminth (35). Murine small and large intestinal tuft cells also express choline acetyltransferase, which catalyzes the production of acetylcholine (36). Although the close interaction between airway tuft cells and cholinergic neurons has been previously demonstrated (37), with recent demonstrations of tuft cell acetylcholine driving ciliary beating in a Trpm5-dependent fashion (38), their role in cholinergic neuron signaling in the intestine is less clear. There is evidence that during genetic and antagonist muscarinic receptor blockade, small intestinal tuft cells arise with an enteroendocrine-like phenotype to sustain the murine intestinal epithelial cholinergic niche (39). Moreover, as acetylcholine receptors are also expressed on diverse cell types, including goblet cells (where acetylcholine promotes mucus secretion), dendritic cells, macrophages, as well as B and T cells (40, 41), there is the unexplored possibility that tuft cells may also play a larger role in anthelmintic immunity via their production of acetylcholine.
Recent findings have also begun to elucidate the initial on switch of the tuft cell/ILC2 feed-forward loop, which, given the production of IL-25 in the naive state (24), was likely to be another messenger or danger signal. Tuft cells had previously been shown to produce leukotriene C4 (13), but McGinty et al. (42) have demonstrated that following small intestinal helminth infection, tuft cells secrete leukotriene C4, in an Alox5-dependent manner, that could signal to surrounding ILC2s via their expression of leukotriene receptors CYSLTR1 and 2 (Figure 1). Given that several immune cells can produce leukotrienes and the long-lived nature of tuft cells (43), bone marrow transfer experiments were superseded by targeted cell-specific null models demonstrating that it was indeed tuft cell-derived leukotriene that was key in driving ILC2 expansion early during small intestinal N. brasiliensis infection (42). However, the precise chemoreceptor or the helminth products they detect remain unknown. Parallel studies by Ualiyeva et al. (44) have also demonstrated that tuft cells located in the lung can release leukotrienes in response to aeroallergens via the P2Y2 receptor, indicating systemic potential for helminth detection. Interestingly, although tuft cells also produce IL-25 in response to protist-derived succinate via SUCNR1, McGinty et al. (42) demonstrated that stimulation of tuft cells with succinate, although driving IL-25, resulted in no leukotriene production but importantly no defect in ILC2-driven responses. Furthermore, TAS1R3 also expressed on tuft cells responds to Tritrichomonas muris and succinate, but not to a helminth infection (45), indicating an ability of tuft cells to selectively respond to different parasites.
This variety and flexibility of the cellular secretome of tuft cells further mirror the responses of enteroendocrine cells (EECs), key chemosensory cells of nutrient detection, in being able to orchestrate an array of digestive requirements to the numerous nutrients detected. Therefore, it is likely that these pathways have been utilized by the innate immune system in evolution to allow the “tasting” of parasites and allow an equally diverse response in immunity as digestion. Furthermore, EECs demonstrate heterogeneity spatially to respond to the nutrients in the likely locations they would appear (46–49); so it is likely that chemosensory cells detecting and responding to parasites would also differentiate in a spatiotemporal fashion to specific parasite niches along the intestinal tract and beyond, as indicated in spatial studies of tuft cells (28, 29, 36, 50). Tuft cells also possess cytospinules which project into the nuclei of neighboring cells, providing them with a unique ability to communicate cellular cargo to the surrounding epithelium (51). Moreover, tuft cells are often associated with EECs (52), while both cell types can act as reserve stem cell niche in the small intestine upon Paneth cell ablation (53), indicating potential overlap and collective function (Figure 1).
EECs are specialized trans-epithelial signal transduction conduits which respond to luminal nutrients by secreting peptide hormones to control gastrointestinal enzyme secretion, motility, and appetite regulation (54, 55). Despite constituting only 1% of the total epithelium, these cells span from the entire length of the gastrointestinal tract and collectively form the largest endocrine system of the body (56). Peptide-secreting intestinal epithelial cells described as having a high degree of amine precursor uptake were reported as early as the 1960s (57). Initially thought to arise from neural crest cells due to their production of neuropeptides such as serotonin, lineage tracing on avian embryos proved that these cells do not arise from the ectoderm (58, 59). Like other intestinal epithelial cells, EECs originate from Lgr5+ intestinal stem cells within the intestinal crypt, integrating Wnt, Notch, and mitogen-activated protein kinase-dependent signaling (60), and require the expression of the secretory cell lineage transcription factor atonal bHLH transcription factor 1 (61–65), finally forming EECs via the expression of the transcription factors neurogenin3 and neurogenic differentiation 1 (NeuroD1) (64, 66, 67). Neurogenin3+ EEC progenitor cells will further differentiate to give rise to multiple mature EEC types, traditionally identified with a one-cell one-peptide dogma. This historic classification included glucagon-like-peptide-1 (GLP-1)-producing L-cells, cholecystokinin (CCK)-producing I-cells, gastrin-producing G-cells, gastric inhibitory peptide-producing K-cells, somatostatin-secreting D-cells, secretin-producing S-cells, and serotonin-producing enterochromaffin cells. However, it is now known that there is considerable secretome overlap and plasticity between the different EEC lineages. Using transgenic reporter mice, multiple groups have shown that CCK, GLP-1, and secretin are coexpressed by a large subset of EECs (68, 69). A recent single-cell transcriptional analysis using Neurog3 reporter mice showed that hormonal co-secretion differs by cell lineage, with a large proportion of EECs secreting multiple hormones (70). Furthermore, EECs also show hormonal plasticity in response to various extracellular cues, such as the upregulation of secretin production in response to bone morphogenic protein as well as their physical location within the crypt/villi dictating their secretome (70, 71).
Although still incompletely understood, recent evidence has shown that EECs have a huge potential to interact with the immune system, with a strong potential for playing a role in the chemosensory sensing of helminths and orchestrating immunity (56). Indeed, helminth infections in particular can drive hyperplasia of EECs in a variety of animal species, often thought to be the driving force to alterations in feeding that accompany a helminth infection in the upper small intestine (72–77). These alterations in EEC hyperplasia, like tuft cells, are also driven by type 2 cytokines in both small intestinal (T. spiralis) and large intestinal (Trichuris muris) helminth infections (78–81), with the EEC peptide CCK shown to influence the resulting immune response via driving weight loss in a feed-forward loop (81). Moreover, EECs can secrete peptide hormones as well as cytokines in response to pathogen-associated molecules (82), and given that intestinal immune cells potentially express peptide hormone receptors (83–85), there is the intriguing possibility that EECs are critical and novel modulators of barrier immunity to helminths (Figure 1).
Interestingly, EECs are the chief epithelial expressers of the receptors that sense bacterial metabolites, such as Ffar3/2 (86, 87), and therefore have the unique ability to relay dysbiosis into physiological adaptation (88). It is now well-established that microbial dysbiosis occurs during an intestinal helminth infection (89, 90), and these changes are transient following helminth expulsion (91, 92), meaning microbial alterations may provide a clear signal to the epithelium of a helminth infection. Indeed, the microbiota is a well-established essential signal for repair during intestinal inflammation (93) and antibiotic-induced microbial dysbiosis alters succinate levels altering tuft cell numbers in the absence of a helminth infection (33). Microbial load increases greatly in the cecum and large intestine, but small intestinal dysbiosis does occur during a large intestinal helminth infection (94). Although these microbial changes are not as instantaneous as detecting the helminths themselves, they can occur within days of infection (95). Moreover, helminth-driven dysbiosis may actually strengthen existing innate barrier responses, as during large intestinal Trichuris suis infection, the addition of the dietary supplement inulin heightens the microbial changes T. suis initiates (96), resulting in tuft cell hyperplasia (97).
EECs have a heightened ability to potentially sense helminths and/or the microbial dysbiosis they produce via the huge array of chemosensory apparatus they possess. Classically, the peptide hormones secreted by EECs signal to the brain in a paracrine fashion via local vagal afferents to mediate digestion and satiety. Recently, Bohórquez et al. (98) demonstrated that CCK-expressing EECs possess basal axon-like cytoplasmic processes, termed neuropods, which transpose nutritional and microbial intestinal signals directly to the brain (99). Neuropods are rich in mitochondria, dense secretory vesicles, presynaptic proteins, and neurofilaments and lie in close contact to enteric glia (100). Neuropods are present in both ileal and colonic EECs (98) and have the capacity to respond to and transmit glucose stimuli to vagal neurons in milliseconds (99). The EEC neuropod therefore has the exciting potential to communicate intestinal chemosensory information directly to the brain and, given the novel neurological control of ILCs (101), presents an exciting immunological addition to the gut–brain axis.
Given that RNA-seq analysis of bitter taste receptor-expressing cells in multiple barrier tissues is strongly linked to innate immune transcripts (102), it is clear that we are only at the beginning of fully elucidating the complex interactions of chemosensory, immune, and neuronal cellular interactions during infection. It still remains imperative to define the helminth products that initiate these epithelial cascades which drive immunity. Although tuft cells are reported to respond almost instantly to a helminth infection (42), it remains a possibility that helminth-derived microbial alterations could be a potential slower innate trigger, particularly in the large intestine where reports of helminth-induced tuft cell alterations have so far been absent. Alternatively, chemosensing may fall to EECs in the large intestinal niche and tuft cells may even act in concert with EECs utilizing microspinule communication to harness neighboring EECs neuropod signaling to help drive the systemic immunity often seen during a helminth infection. In summary, the initial fascinating epithelial chemosensory discoveries discussed above could simply be a taste of things to come.
JW contributed to the conceptualization. AF, KW, JT, and JW contributed to writing the original draft. JW contributed to the writing, review, and editing. All authors contributed to the article and approved the submitted version.
The Worthington lab was supported by a Wellcome Trust Seed Corn Award-209087/Z/17/Z.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Eugene Jarvis for proofreading.
1. McCarty TR, Turkeltaub JA, Hotez PJ. Global progress towards eliminating gastrointestinal helminth infections. Curr Opin Gastroenterol. (2014) 30:18–24. doi: 10.1097/MOG.0000000000000025
2. Lo NC, Addiss DG, Hotez PJ, King CH, Stothard JR, Evans DS, et al. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect Dis. (2017) 17:e64–9. doi: 10.1016/S1473-3099(16)30535-7
3. Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. (1998) 160:3453–61.
4. Urban JFJr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. (1998) 8:255–64. doi: 10.1016/S1074-7613(00)80477-X
5. McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie ANJ. Simultaneous disruption of interleukin. (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. (1999) 189:1565–72. doi: 10.1084/jem.189.10.1565
6. Bancroft AJ, Artis D, Donaldson DD, Sypek JP, Grencis RK. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. Eur J Immunol. (2020) 30:2083–91. doi: 10.1002/1521-4141(200007)30:7andlt;2083::AID-IMMU2083andgt;3.0.CO;2-3
7. Urban JF, Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ, et al. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. (2020) 164:2046–52. doi: 10.4049/jimmunol.164.4.2046
8. Jackson JA, Turner JD, Rentoul L, Faulkner H, Behnke JM, Hoyle M, et al. T helper cell type 2 responsiveness predicts future susceptibility to gastrointestinal nematodes in humans. J Infect Dis. (2004) 190:1804–11. doi: 10.1086/425014
9. Kim BS, Artis D. Group 2 innate lymphoid cells in health and disease. Cold Spring Harb Perspect Biol. (2015) 7:a016337. doi: 10.1101/cshperspect.a016337
10. Bouchery T, Le Gros G, Harris N. ILC2s-trailblazers in the host response against intestinal helminths. Front Immunol. (2019) 10:623. doi: 10.3389/fimmu.2019.00623
11. Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. (2019) 129:1441–51. doi: 10.1172/JCI124606
12. Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA. (1996) 93:6631–4. doi: 10.1073/pnas.93.13.6631
13. Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, et al. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. (2008) 509:514–25. doi: 10.1002/cne.21768
14. Gerbe F, Brulin B, Makrini L, Legraverend C, Jay P. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology. (2009) 137:2179–80; author reply 2180-2171. doi: 10.1053/j.gastro.2009.06.072
15. Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. (2011) 192:767–80. doi: 10.1083/jcb.201010127
16. Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol. (2011) 14:106–14. doi: 10.1038/ncb2384
17. Saqui-Salces M, Keeley TM, Grosse AS, Qiao XT, El-Zaatari M, Gumucio DL, et al. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol. (2011) 136:191–204. doi: 10.1007/s00418-011-0831-1
18. Bjerknes M, Khandanpour C, Möröy T, Fujiyama T, Hoshino M, Klisch TJ, et al. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev Biol. (2012) 362:194–218. doi: 10.1016/j.ydbio.2011.12.009
19. Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. (2016) 529:226–30. doi: 10.1038/nature16527
20. Yamashita J, Ohmoto M, Yamaguchi T, Matsumoto I, Hirota J. Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS ONE. (2017) 12:e0189340. doi: 10.1371/journal.pone.0189340
21. Grencis RK, Worthington JJ. Tuft cells: a new flavor in innate epithelial immunity. Trends Parasitol. (2016) 32:583–5. doi: 10.1016/j.pt.2016.04.016
22. Hasnain SZ, Wang H, Ghia JE, Haq N, Deng Y, Velcich A, et al. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. (2010) 138:1763–71. doi: 10.1053/j.gastro.2010.01.045
23. Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med. (2011) 208:893–900. doi: 10.1084/jem.20102057
24. von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. (2016) 529:221–5. doi: 10.1038/nature16161
25. Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A, et al. T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell. (2018) 175:1307–20.e1322. doi: 10.1016/j.cell.2018.10.008
26. Zhu P, Zhu X, Wu J, He L, Lu T, Wang Y, et al. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat Immunol. (2019) 20:183–94. doi: 10.1038/s41590-018-0297-6
27. Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. (2016) 351:1329–33. doi: 10.1126/science.aaf1648
28. Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, et al. A single-cell survey of the small intestinal epithelium. Nature. (2017) 551:333–9. doi: 10.1038/nature24489
29. Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. (2018) 560:319–24. doi: 10.1038/s41586-018-0393-7
30. Schneider C, O'Leary CE, Locksley RM. Regulation of immune responses by tuft cells. Nat Rev Immunol. (2019) 19:584–93. doi: 10.1038/s41577-019-0176-x
31. Schneider C, O'Leary CE, von Moltke J, Liang HE, Ang QY, Turnbaugh PJ, et al. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell. (2018) 174:271–84.e214. doi: 10.1016/j.cell.2018.05.014
32. Luo XC, Chen ZH, Xue JB, Zhao DX, Lu C, Li YH, et al. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc Natl Acad Sci USA. (2019) 116:5564–9. doi: 10.1073/pnas.1812901116
33. Lei W, Ren W, Ohmoto M, Urban JFJr, Matsumoto I, Margolskee RF, et al. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc Natl Acad Sci USA. (2018) 115:5552–7. doi: 10.1073/pnas.1720758115
34. Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, et al. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity. (2018) 49:33–41.e37. doi: 10.1016/j.immuni.2018.06.016
35. Zaiss MM, Rapin A, Lebon L, Dubey LK, Mosconi I, Sarter K, et al. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity. (2015) 43:998–1010. doi: 10.1016/j.immuni.2015.09.012
36. Schütz B, Ruppert A-L, Strobel O, Lazarus M, Urade Y, Büchler MW, et al. Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci Rep. (2019) 9:17466. doi: 10.1038/s41598-019-53997-3
37. Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA. (2011) 108:9478–83. doi: 10.1073/pnas.1019418108
38. Perniss A, Liu S, Boonen B, Keshavarz M, Ruppert AL, Timm T, et al. Chemosensory cell-derived acetylcholine drives tracheal mucociliary clearance in response to virulence-associated formyl peptides. Immunity. (2000) 52:683–99.e611. doi: 10.1016/j.immuni.2020.03.005
39. Middelhoff M, Nienhuser H, Valenti G, Maurer HC, Hayakawa Y, Takahashi R, et al. Prox1-positive cells monitor and sustain the murine intestinal epithelial cholinergic niche. Nat Commun. (2020) 11:111. doi: 10.1038/s41467-019-13850-7
40. Birchenough GMH, Johansson MEV, Gustafsson JK, Bergström JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol. (2015) 8:712–9. doi: 10.1038/mi.2015.32
41. Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, et al. Expression and function of the cholinergic system in immune cells. Front Immunol. (2017) 8:1085. doi: 10.3389/fimmu.2017.01085
42. McGinty JW, Ting HA, Billipp TE, Nadjsombati MS, Khan DM, Barrett NA, et al. Tuft-cell-derived leukotrienes drive rapid anti-helminth immunity in the small intestine but are dispensable for anti-protist immunity. Immunity. (2000) 52:528–41.e527. doi: 10.1016/j.immuni.2020.02.005
43. Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. (2014) 124:1283–95. doi: 10.1172/JCI73434
44. Ualiyeva S, Hallen N, Kanaoka Y, Ledderose C, Matsumoto I, Junger WG, et al. Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Sci Immunol. (2020) 5:eaax7224. doi: 10.1126/sciimmunol.aax7224
45. Howitt MR, Cao YG, Gologorsky MB, Li JA, Haber AL, Biton M, et al. The taste receptor TAS1R3 regulates small intestinal tuft cell Homeostasis. Immunohorizons. (2020) 4:23–32. doi: 10.4049/immunohorizons.1900099
46. Svendsen B, Pedersen J, Albrechtsen NJ, Hartmann B, Torang S, Rehfeld JF, et al. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. (2015) 156:847–57. doi: 10.1210/en.2014-1710
47. Svendsen B, Pais R, Engelstoft MS, Milev NB, Richards P, Christiansen CB, et al. GLP1- and GIP-producing cells rarely overlap and differ by bombesin receptor-2 expression and responsiveness. J Endocrinol. (2016) 228:39–48. doi: 10.1530/JOE-15-0247
48. Adriaenssens AE, Reimann F, Gribble FM. Distribution and stimulus secretion coupling of enteroendocrine cells along the intestinal tract. Compr Physiol. (2018) 8:1603–38. doi: 10.1002/cphy.c170047
49. Fazio Coles TE, Fothergill LJ, Hunne B, Nikfarjam M, Testro A, Callaghan B, et al. Quantitation and chemical coding of enteroendocrine cell populations in the human jejunum. Cell Tissue. (2020) 379:109–20. doi: 10.1007/s00441-019-03099-3
50. McKinley ET, Sui Y, Al-Kofahi Y, Millis BA, Tyska MJ, Roland JT, et al. Optimized multiplex immunofluorescence single-cell analysis reveals tuft cell heterogeneity. JCI Insight. (2017) 2:e93487. doi: 10.1172/jci.insight.93487
51. Hoover B, Baena V, Kaelberer MM, Getaneh F, Chinchilla S, Bohórquez DV. The intestinal tuft cell nanostructure in 3D. Sci Rep. (2017) 7:1652. doi: 10.1038/s41598-017-01520-x
52. Cheng X, Voss U, Ekblad E. Tuft cells: distribution and connections with nerves and endocrine cells in mouse intestine. Exp Cell Res. (2018) 369:105–11. doi: 10.1016/j.yexcr.2018.05.011
53. van Es JH, Wiebrands K, López-Iglesias C, van de Wetering M, Zeinstra L, van den Born M, et al. Enteroendocrine and tuft cells support Lgr5 stem cells on Paneth cell depletion. Proc Natl Acad Sci USA. (2019) 116:26599–05. doi: 10.1073/pnas.1801888117
54. Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. (2013) 9:584–97. doi: 10.1038/nrendo.2013.136
55. Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. (2019) 15:226–37. doi: 10.1038/s41574-019-0168-8
56. Worthington JJ, Reimann F, Gribble FM. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol. (2017) 11:3–20. doi: 10.1038/mi.2017.73
57. Pearse AG. The calcitonin secreting C cells and their relationship to the APUD cell series. J Endocrinol. (1969) 45:Suppl:13–14.
58. Andrew A, Kramer B. An experimental investigation into the possible origin of pancreatic islet cells from rhombencephalic neurectoderm. J Embryol Exp Morphol. (1979) 52:23.
59. Andrew A, Kramer B, Rawdon BB. Gut and pancreatic amine precursor uptake and decarboxylation cells are not neural crest derivatives. Gastroenterology. (1983) 84:429–31. doi: 10.1016/S0016-5085(83)80148-6
60. Basak O, Beumer J, Wiebrands K, Seno H, van Oudenaarden A, Clevers H. Induced quiescence of Lgr5+ stem cells in intestinal organoids enables differentiation of hormone-producing enteroendocrine cells. Cell Stem Cell. (2017) 20:177–190.e174. doi: 10.1016/j.stem.2016.11.001
61. Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. (2001) 294:2155–8. doi: 10.1126/science.1065718
62. Shroyer NF, Helmrath MA, Wang VYC, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1. (Math1) reveals a role in cellular homeostasis. Gastroenterology. (2007) 132:2478–88. doi: 10.1053/j.gastro.2007.03.047
63. May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. (2010) 323:70–5. doi: 10.1016/j.mce.2009.12.009
64. Li HJ, Ray SK, Singh NK, Johnston B, Leiter AB. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes Metab. (2011b) 13 Suppl 1:5–12. doi: 10.1111/j.1463-1326.2011.01438.x
65. Gerbe F, Legraverend C, Jay P. The intestinal epithelium tuft cells: specification and function. CMLS. (2012) 69:2907–17. doi: 10.1007/s00018-012-0984-7
66. Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine V. Unitarian theory of the origin of the four epithelial cell types. Am J Anatomy. (1974) 141:537–61. doi: 10.1002/aja.1001410407
67. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. (2007) 449:1003–7. doi: 10.1038/nature06196
68. Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. (2012) 153:5782–95. doi: 10.1210/en.2012-1595
69. Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. (2012) 153:3054–65. doi: 10.1210/en.2011-2170
70. Gehart H, van Es JH, Hamer K, Beumer J, Kretzschmar K, Dekkers JF, et al. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell. (2019) 176:1158–73.e1116. doi: 10.1016/j.cell.2018.12.029
71. Beumer J, Artegiani B, Post Y, Reimann F, Gribble F, Nguyen TN, et al. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol. (2018) 20:909–16. doi: 10.1038/s41556-018-0143-y
72. Yang S, Gaafar SM, Bottoms GD. Effects of multiple dose infections with ascaris-suum on blood gastrointestinal hormone levels in pigs. Vet Parasitol. (1990) 37:31–44. doi: 10.1016/0304-4017(90)90023-5
73. Dynes RA, Poppi DP, Barrell GK, Sykes AR. Elevation of feed intake in parasite-infected lambs by central administration of a cholecystokinin receptor antagonist. Br J Nutr. (1998) 79:47–54. doi: 10.1079/BJN19980008
74. Scott I, Hodgkinson SM, Lawton DEB, Khalaf S, Reynolds GW, Pomroy WE, et al. Infection of sheep with adult and larval Ostertagia circumcincta: gastrin. Int J Parasitol. (1998) 28:1393–401. doi: 10.1016/S0020-7519(98)00112-X
75. Bosi G, Shinn AP, Giari L, Simoni E, Pironi F, Dezfuli BS. Changes in the neuromodulators of the diffuse endocrine system of the alimentary canal of farmed rainbow trout, Oncorhynchus mykiss. (Walbaum), naturally infected with Eubothrium crassum. (Cestoda). J Fish Dis. (2005) 28:703–11. doi: 10.1111/j.1365-2761.2005.00674.x
76. Dezfuli BS, Pironi F, Shinn AP, Manera M, Giari L. Histopathology and ultrastructure of Platichthys flesus naturally infected with Anisakis simplex s.l. larvae. (Nematoda: anisakidae). J Parasitol. (2007) 93:1416–23. doi: 10.1645/GE-1214.1
77. Forbes AB, Warren M, Upjohn M, Jackson B, Jones J, Charlier J, et al. Associations between blood gastrin, ghrelin, leptin, pepsinogen and Ostertagia ostertagi antibody concentrations and voluntary feed intake in calves exposed to a trickle infection with O. ostertagi. Vet Parasitol. (2009) 162:295–305. doi: 10.1016/j.vetpar.2009.03.010
78. McDermott JR, Leslie FC, D'Amato M, Thompson DG, Grencis RK, McLaughlin JT. Immune control of food intake: enteroendocrine cells are regulated by CD4(+) T lymphocytes during small intestinal inflammation. Gut. (2006) 55:492–7. doi: 10.1136/gut.2005.081752
79. Wang HQ, Steeds J, Motomura Y, Deng YK, Verma-Gandhu M, El-Sharkawy RT, et al. CD4(+) T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. (2007) 56:949–57. doi: 10.1136/gut.2006.103226
80. Motomura Y, Ghia JE, Wang H, Akiho H, El-Sharkawy RT, Collins M, et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. (2008) 57:475–81. doi: 10.1136/gut.2007.129296
81. Worthington JJ, Samuelson LC, Grencis RK, McLaughlin JT. Adaptive immunity alters distinct host feeding pathways during nematode induced inflammation, a novel mechanism in parasite expulsion. PLoS Pathog. (2013) 9:e1003122. doi: 10.1371/journal.ppat.1003122
82. Palazzo M, Balsari A, Rossini A, Selleri S, Calcaterra C, Gariboldi S, et al. Activation of enteroendocrine cells via TLRs induces hormone, chemokine, and defensin secretion. J Immunol. (2007) 178:4296–303. doi: 10.4049/jimmunol.178.7.4296
83. Genton L, Kudsk KA. Interactions between the enteric nervous system and the immune system: role of neuropeptides and nutrition. Am J Surgery. (2003) 186:253–8. doi: 10.1016/S0002-9610(03)00210-1
84. Worthington JJ. The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem Soc Transact. (2015) 43:727–33. doi: 10.1042/BST20150090
85. Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, et al. GLP-1 receptor. (GLP-1R) agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte. (IEL) GLP-1R. Diabetes. (2015) 64:2537–49. doi: 10.2337/db14-1577
86. Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. (2006) 324:353–60. doi: 10.1007/s00441-005-0140-x
87. Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. (2013) 154:3552–64. doi: 10.1210/en.2013-1142
88. Cani PD, Knauf C. How gut microbes talk to organs: the role of endocrine and nervous routes. Mol Metab. (2016) 5:743–52. doi: 10.1016/j.molmet.2016.05.011
89. Cortes A, Peachey L, Scotti R, Jenkins TP, Cantacessi C. Helminth-microbiota cross-talk - A journey through the vertebrate digestive system. Mol Biochem Parasitol. (2019) 233:111222. doi: 10.1016/j.molbiopara.2019.111222
90. Jenkins TP, Brindley PJ, Gasser RB, Cantacessi C. Helminth microbiomes - a hidden treasure trove? Trends Parasitol. (2019) 35:13–22. doi: 10.1016/j.pt.2018.10.007
91. Holm JB, Sorobetea D, Kiilerich P, Ramayo-Caldas Y, Estelle J, Ma T, et al. Chronic trichuris muris infection decreases diversity of the intestinal microbiota and concomitantly increases the abundance of lactobacilli. PLoS ONE. (2015) 10:e0125495. doi: 10.1371/journal.pone.0125495
92. Houlden A, Hayes KS, Bancroft AJ, Worthington JJ, Wang P, Grencis RK, et al. Chronic trichuris muris infection in C57BL/6 mice causes significant changes in host microbiota and metabolome: effects reversed by pathogen clearance. PLoS ONE. (2015) 10:e0125945. doi: 10.1371/journal.pone.0125945
93. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. (2004) 118:229–41. doi: 10.1016/j.cell.2004.07.002
94. Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, et al. Helminth infection promotes colonization resistance via type 2 immunity. Science. (2016) 352:608–12. doi: 10.1126/science.aaf3229
95. Fredensborg BL, Fossdal Í Kálvalíð* I, Johannesen TB, Stensvold CR, Nielsen HV, Kapel CMO. Parasites modulate the gut-microbiome in insects: a proof-of-concept study. PLoS ONE. (2020) 15:e0227561. doi: 10.1371/journal.pone.0227561
96. Stolzenbach S, Myhill LJ, Andersen LO, Krych L, Mejer H, Williams AR, et al. Dietary inulin and trichuris suis infection promote beneficial bacteria throughout the porcine gut. Front Microbiol. (2020) 11:312. doi: 10.3389/fmicb.2020.00312
97. Myhill LJ, Stolzenbach S, Hansen TVA, Skovgaard K, Stensvold CR, Andersen LO, et al. Mucosal barrier and Th2 immune responses are enhanced by dietary inulin in pigs infected with Trichuris suis. Front Immunol. (2018) 9:2557. doi: 10.3389/fimmu.2018.02557
98. Bohórquez DV, Chandra R, Samsa LA, Vigna SR, Liddle RA. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol. (2011) 42:3–13. doi: 10.1007/s10735-010-9302-6
99. Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, et al. A gut-brain neural circuit for nutrient sensory transduction. Science. (2018) 361:eaat5236. doi: 10.1126/science.aat5236
100. Kaelberer MM, Rupprecht LE, Liu WW, Weng P, Bohórquez DV. Neuropod cells: emerging biology of the gut-brain sensory transduction. Annu Rev Neurosci. (2020) 43:337–53. doi: 10.1146/annurev-neuro-091619-022657
101. Jowett GM, Neves JF. Commentary: Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Front Pharmacol. (2018) 9:230. doi: 10.3389/fphar.2018.00230
Keywords: tuft cells, enteroendocrine cell (EEC), microbiome, epithelium, helminth, G protein-coupled receptor (GPCR)
Citation: Faniyi AA, Wijanarko KJ, Tollitt J and Worthington JJ (2020) Helminth Sensing at the Intestinal Epithelial Barrier—A Taste of Things to Come. Front. Immunol. 11:1489. doi: 10.3389/fimmu.2020.01489
Received: 24 April 2020; Accepted: 08 June 2020;
Published: 30 July 2020.
Edited by:
Kara Filbey, University of Manchester, United KingdomReviewed by:
Jakob Von Moltke, University of Washington, United StatesCopyright © 2020 Faniyi, Wijanarko, Tollitt and Worthington. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John J. Worthington, ai5qLndvcnRoaW5ndG9uQGxhbmNhc3Rlci5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.