- 1Department of Kidney Transplantation, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Clinical Research Center for Organ Transplantation, Changsha, China
- 3Clinical Immunology Center, Central South University, Changsha, China
Since December 2019, the ongoing coronavirus disease 2019 (COVID-19) pandemic has significantly affected solid organ transplantation (SOT) worldwide and has become a threat to the lives of SOT recipients. Here, we have reviewed, condensed, and organized the available information on COVID-19 to provide recommendations to transplant healthcare workers. Our review of reported cases shows that the symptoms of SOT patients with COVID-19 are similar to those of the normal population, but their severity and outcomes are worse. Thus far, there is no evidence that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly causes permanent damage to kidney, liver, or heart allografts.
Introduction
In December 2019, a cluster of patients with pneumonia of unknown cause were reported (1). The pathogen was promptly isolated on January 7, 2020 and its whole genome was shared later on January 12, which identified it as a novel member of the β coronavirus family (2, 3). On February 11, 2020 this virus was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV) (4); on the same day the disease caused by SARS-CoV-2 was named as coronavirus disease (COVID-19) by the WHO (5). On March 11, 2020, the WHO made the assessment that COVID-19 can be characterized as a pandemic (6), and as of April 17, 2020, nearly 2,074,529 cases and 139,378 deaths have been confirmed worldwide (7).
Droplet and contact transmission are the main routes of viral transmission; airborne transmission is only possible in specific circumstances such as endotracheal intubation, bronchoscopy, and open suctioning (8, 9). Although SARS-CoV-2 has been cultured from the stool (10), fecal–oral transmission has not been reported. The general population lacks immunity and is susceptible to SARS-CoV-2 due to the novelty of the virus. The average incubation period for COVID-19 is 5.2 days (95% confidence interval [CI], 4.1–7.0) with the 95th percentile at 12.5 days. The basic reproduction number (R0) value has been estimated to be 2.2 (95% CI, 1.4–3.9) (11). It should be noted that asymptomatic patients with SARS-CoV-2 account for 1.2% of the infected cases according to a retrospective study of 72,314 confirmed cases in China (12). Other studies have concluded that the percentage of asymptomatic patients range from 8.6 to 15.7% (13). Further, evidence indicates that asymptomatic patients can also transmit the disease (14, 15).
The spread of COVID-19 has significantly restricted the transplantation program in epidemic areas due to the high risk of infection in immunosuppressed patients, risk of transmission in health workers, impact of travel bans on organ procurement organizations' (OPO) activities, and lack of medical resources in certain circumstances. In this paper, we explore ways to safely continue transplantation under the threat of COVID-19 and review the current reported cases of organ transplant in COVID-19 patients.
Organ Transplantation Proceeds With Extra Precautions in Low-Risk Areas
Transplant Activity Report During the COVID-19 Pandemic
In March 2020, organ transplant centers in Wuhan completely halted all transplant surgeries. A suspension on living donor transplants, pancreas transplants, and renal transplants was first considered in other less affected regions; however, lifesaving urgent transplant surgery still proceeded in most centers (16, 17). Statistics showed a drastic 90.6% and 51.1% reduction in deceased donor transplant in France and USA, respectively, which is mostly driven by kidney transplant (18). Total amount of all organ transplants reduced from 100~150 to 40 transplants per month in Netherlands (19). Kumar et al. have suggested a phase down approach to new transplant activity during this unprecedented crisis. The percentage of transplant activity reduction should be based on risk tolerance, the degree of local COVID-19 activity, and medical resource capacity (20). Generally, according to the transmission patterns defined by the World Health Organization (21), when sporadic cases and clusters of cases with clear traceable transmission chains are present, transplantation can be performed with precautions. However, in regions where community transmission (large number of untraceable cases) is on the rise, a temporary tiered suspension on transplantation is recommended due to increased amount of infection risk among patients and healthcare workers (22).
Donor and Recipient Screening
No donor-derived COVID-19 case has been reported thus far. Although 10.4–15% of COVID-19 patients have been detected with serum SARS-CoV-2 nucleic acid (RNAaemia), no infectious live virus has been found in the blood (23, 24). Angiotensin-converting enzyme 2 (ACE2), which has been identified as a receptor for SARS-CoV-2 (25), is abundant in virtually all organs including the lung, kidney, liver, heart, and intestine (26); this implies that SARS-CoV-2 viremia could possibly infect any transplant organ and conceal itself until the immunosuppressed status exists. Postmortem autopsy has shown that SARS-CoV-2 mainly exists in the lungs (27, 28), although involvement of the kidney, liver, and heart has also been reported (29, 30). Considering the real risk of donor-derived infection under immunosuppression and the risk of viral transmission to health workers during coordination, procurement, surgery, and nursing, both donor and recipient screening should be mandated in the affected areas.
Due to the existence of asymptomatic patients, clinical screening methods such as inquiry into potential exposure and suspected symptoms, laboratory blood examination, or a chest computed tomography (CT) (31) are not sufficient to completely rule out the possibility of infection. A nucleic acid test (NAT) remains the cornerstone of COVID-19 screening. The positive rate using nasopharyngeal swabs (63%) is lower than that using the bronchoalveolar lavage fluid (93%) and the sputum (72%), but is significantly higher than using oropharyngeal swabs (32%); nasopharyngeal swabs have the highest viral load among all types of clinical specimens (32). Because of its accessibility and sensitivity, NAT nasopharyngeal swabs are preferred for screening purposes (33). Universal NAT testing should be recommended in community transmission areas (20, 22). However, due to high false negative rate of one-time NAT testing, if feasible, two consecutive NAT tests conducted ≥24 h apart should be considered (34, 35).
However, when life-saving urgent transplantation is required and NAT kits are limited, testing should be done in the following order of priority: suspected donor/recipient, donor, and recipient, because the donors have a higher exposure risk (ICU experience for instance) compared with low-risk recipients who follow social distancing rules. Suspected donor/recipient cases (different from the suspected cases for epidemiological surveillance purposes) are determined based on the following (36–38):
• Possible exposure to COVID-19 confirmed or suspected patients.
• Suspected symptoms such as fever (reported symptom prevalence: 83–99%), cough (59–82%), fatigue (44–70%), anorexia (40–84%), shortness of breath (31–40%), sputum production (28–33%), and myalgias (11–35%).
• Conditions such as lymphocytopenia (83.2%), thrombocytopenia (36.2%), and leukopenia (33.7%).
• Abnormal CT results (86.2%) with ground-glass opacity (56.4%), and bilateral patchy shadows (51.8%).
Any suspected donor/recipient (who fulfills any of the above conditions) should be screened by NAT for SARS COV-2. Any COVID-19 confirmed donor/recipient, suspected donor/recipient unable to rule out COVID-19, and donor/recipient with fever of unknown cause should be contraindicated for organ transplant.
Safety of OPO Coordinators
In regions where organ transplant programs are still ongoing, OPO coordinators generally have to travel more compared with transplant doctors and nurses and social contact is unavoidable. It is thus essential to impart training on epidemiology, prevention, risk assessment of COVID-19, and psychological tutoring on stress coping (39). Coordinators should perform risk assessment depending on the epidemic situation of the area where the donor originates from, the hospital/ICU where the donor currently stays, wear personal protective equipment, and practice hand hygiene during the whole donation coordination. Further, they should reduce unnecessary in-person contact by utilizing online communication tools.
COVID-19 in Transplant Recipients
According to a survey conducted on 88 major transplant centers in the United States between March 24 and March 31, 2020, 31 (35.2%) respondents reported 148 COVID-19 SOT patients altogether. Of these patients, 80 (54.1%) were mildly ill (no pneumonia), 31 (20.9%) were moderately ill (pneumonia), and 37 (25.0%) were critically ill (17). Although the classification is subjective, the results indicate a greater disease severity compared with normal COVID-19 patients. Herein, we reviewed all reported COVID-19 SOT cases prior to April 17, 2020 to show the manifestation, treatment, and prognosis.
COVID-19 in Kidney Transplantation Recipients
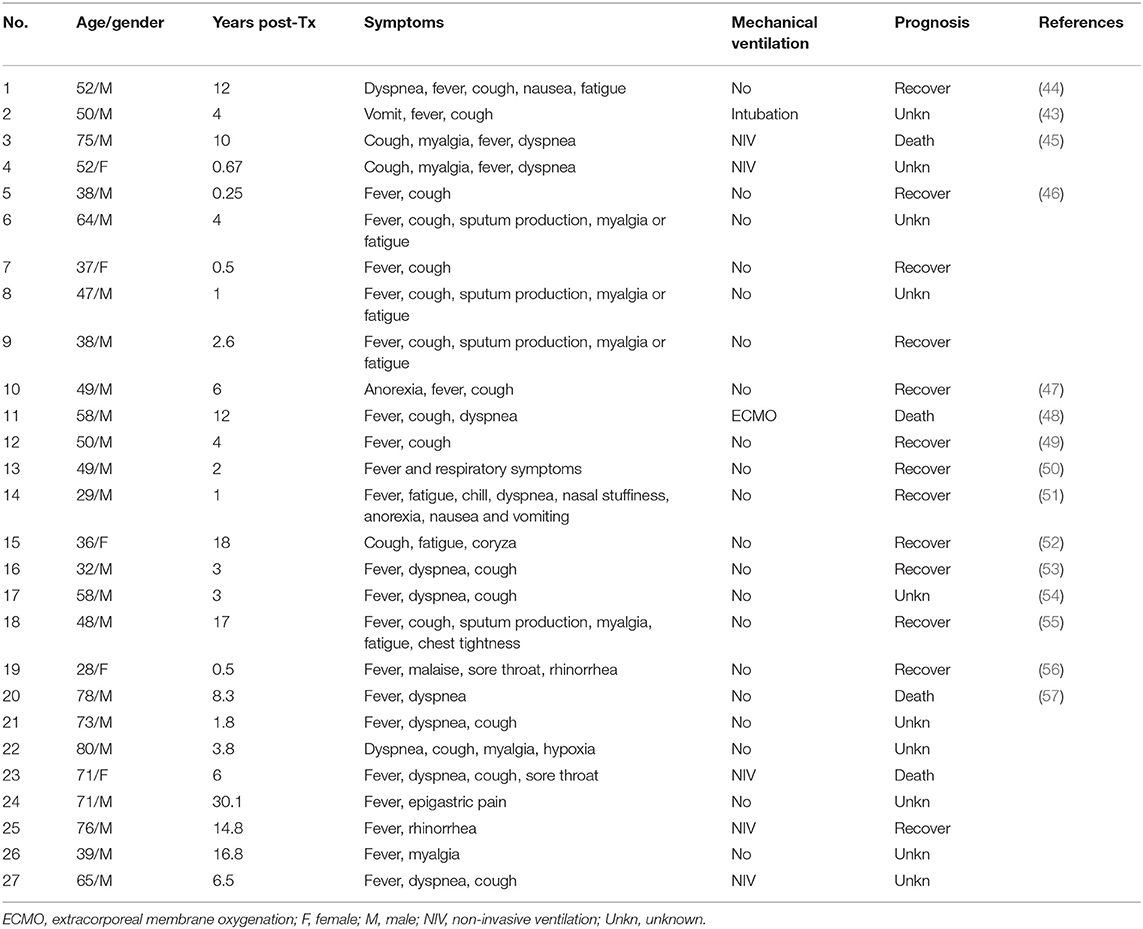
Kidney transplantation (KT) recipients account for the largest proportion of SOT recipients (40), which explains why they form the majority of COVID-19 SOT patients (103/148, 69.6%) (17). As of April 17, 2020, 27 COVID-19 KT patients have been reported on PubMed. The mean age was 53.4 ± 16 years, and the median post-transplantation (post-TX) interval was 4 years [interquartile range (IQR): 1.9–9.6]. No pattern was observed between the KT recipients' susceptibility and post-TX interval. It has been suggested that the atypical symptoms and absence of fever seen in COVID-19 SOT patients might be due to immunosuppression (41–43). Our review (Table 1) shows that COVID-19 KT patients presented with fever (25/27, 92.6%), cough (20/27, 74.0%), dyspnea (12/27, 44.4%), myalgia (8/27, 29.6%), fatigue (7/27, 25.9%), and sputum production (4/27, 14.8%), which is similar to COVID-19 symptoms in the general population (37). The gastrointestinal symptoms seen in general COVID-19 patients (58) such as anorexia (2/27, 7%), nausea (2/27, 7%), and vomiting (2/27, 7%) are also present in COVID-19 KT patients. It should be noted that (as shown in case No. 2) the initial symptoms are fever and vomiting, which leads to a presumption of viral gastroenteritis. In this case, cough did not develop until 5 days later (43).
Of the 27 COVID-19 KT patients, 7 (25.9%) required mechanical ventilation (Table 1). Based on the severity, this indicates that at least 25.9% of the COVID-19 KT patients were critical (defined as COVID-19 positive along with mechanical ventilation or evidence of multi-organ failure or shock) (59). In comparison, the condition of about 5% of COVID-19 patients in the general population are critical (60), which implies a worse prognosis among infected SOT recipients. The mortality rate was 30.8% (4/13) for all known outcomes (Table 1). This is consistent with a recent single-center study with a mortality rate of 28% (10/36) (61).
Antiviral treatment remains controversial since sufficient evidence is not available to prove its efficacy against SARS-CoV-2 (62, 63). The current proposed antiviral drugs with small volume randomized controlled trials include antimalarial drugs (chloroquine and hydroxychloroquine), HIV protease inhibitors (lopinavir/ritonavir, darunavir/cobicistat, darunavir/ritonavir), and remdesivir (64). On May 1, 2020, US FDA (Food and Drug Administration) issued an emergency use authorization for remdesivir for treatment of severe COVID-19 patients (65, 66). Of the 27 COVID-19 KT patients, 12 (44.4%) were given hydroxychloroquine, while 10 were prescribed lopinavir/ritonavir (Table 2). Patient No. 12 recovered with unaltered immunosuppressive therapy and no antiviral drug. Antiviral drugs must be handled with caution due to potential drug–drug interactions and adverse effects. The elimination of calcineurin inhibitors (CNI) and rapamycin inhibitors (mTORi) is mainly controlled by cytochrome P450 3A4 (CYP3A4), CYP3A5, and the efflux pump P-glycoprotein (67). However, both chloroquine and hydroxychloroquine are P-glycoprotein inhibitors and lopinavir/ritonavir are potent CYP3A4 inhibitors (68). In patient No. 15, tacrolimus trough level reached 90.5 ng/mL with a combination of lopinavir/ritonavir, hydroxychloroquine, and tacrolimus. The high tacrolimus level induced renal failure in patient No. 27. Transitional discontinuation and dose reduction of CNI are necessary before the initiation of the aforementioned antiviral drugs.
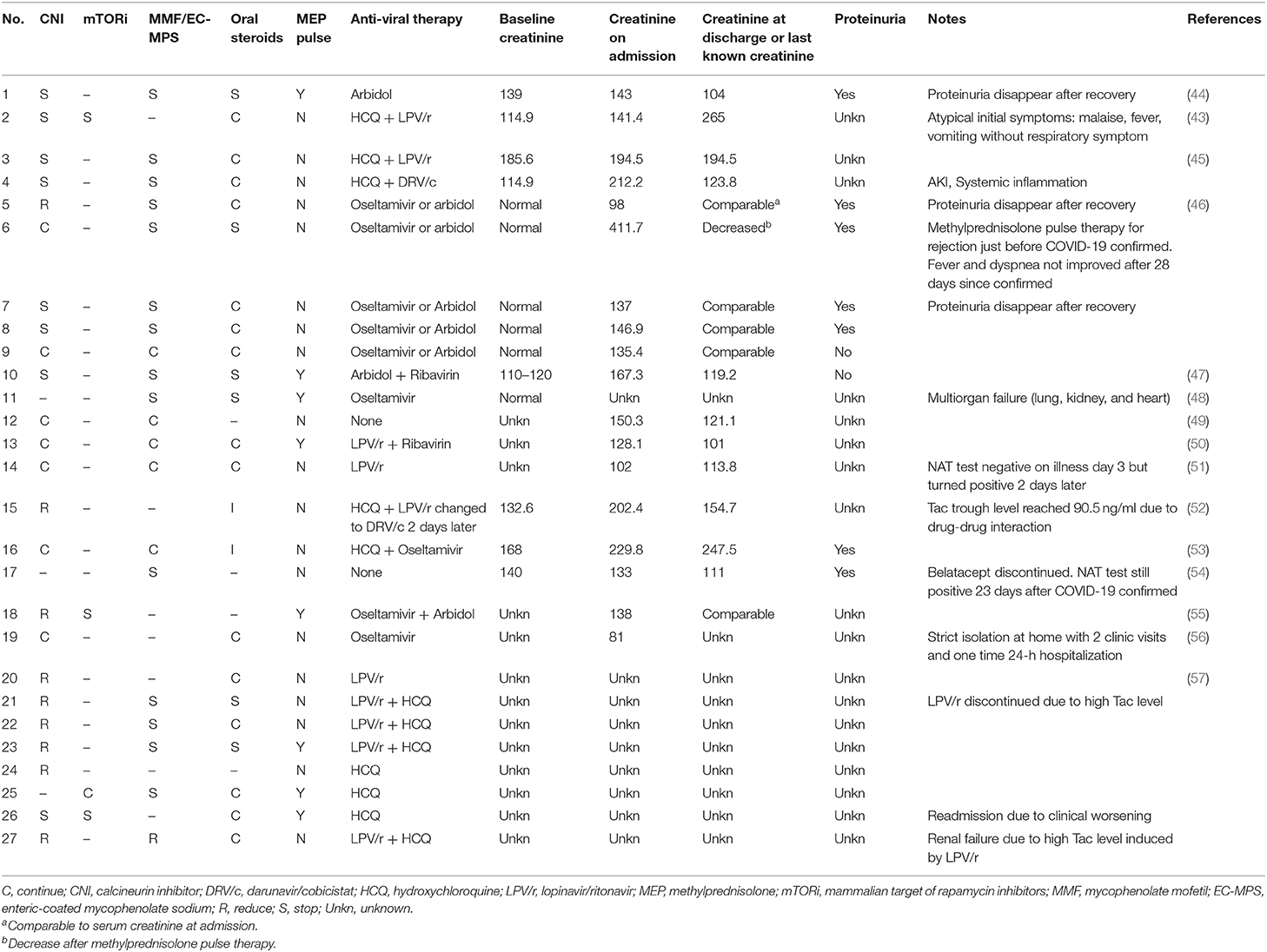
Table 2. Immunosuppressive agents changes, treatment, and allograft function of 27 COVID-19 KT patients.
In vitro study shows that cyclosporin A inhibits diverse coronavirus replication including severe acute respiratory syndrome coronavirus (SARS-CoV) and middle east respiratory syndrome coronavirus (MERS-CoV) (69, 70). However, further in vivo and clinical studies are needed to support the conversion from tacrolimus to cyclosporine in COVID-19 SOT patients. Since mTOR inhibitors can cause interstitial pneumonitis and lead to ground glass opacity abnormality in chest CT scan (71), switching mTOR inhibitor to CNI should be considered among COVID-19 SOT patients with typical ground opacity chest CT scan.
The lack of an effective treatment strategy against COVID-19 implies that the recovery depends primarily on our immune system itself. Regular immunosuppression in SOT patients leads to a longer viral shedding time as observed from past experience with RNA respiratory viral infections (42). Although further evidence is needed, reduction of immunosuppression might contribute to a better prognosis and a shorter course of the disease. Of the 27 COVID-19 KT patients, 21 (77.8%) reduced or stopped their immunosuppressive agents (Table 2). CNI was reduced in 9/24 (37.5%) and stopped in 8/24 (33.3%) patients. Mycophenolate mofetil (MMF) or enteric-coated mycophenolate sodium (EC-MPS) was stopped in 14/20 (70%) patients and reduced in 1/20 (5%) patients. However, it is riskier to reduce immunosuppression in COVID-19 SOT cases than in SOT patients with opportunistic infections because immunocompetent people are generally susceptible to COVID-19 and it does not imply deficient immune status. It should be noted that 6 of the 7 (85.7%) COVID-19 KT patients with proteinuria were treated with reduced immunosuppression. Certainly, proteinuria could be caused directly by COVID-19 (72, 73), but it could also be an early sign of renal rejection (74). Further, the disappearance of proteinuria after COVID-19 recovery in patient Nos. 1, 5, and 7 might be related to the resumption of immunosuppression or remission from potential COVID-19 kidney impairment.
KT recipients are deeply concerned with changes in renal function. However, a recent postmortem renal histopathology study in COVID-19 patients showed the direct renal impairment caused by SARS-CoV-2, such as diffuse brush border damage of proximal tubule, vacuolar degeneration, and necrosis (75). Acute kidney injury (AKI) occurrence in COVID-19 patients varies drastically from 0 to 28% [0/116, 0% (76); 55/287, 19.2% (77); 523/2,634, 19.9% (78); 55/193, 28% (72)]. This variability may be related to the differences in therapy and treatment availability. Proteinuria is found in 28.57–59% of COVID-19 patients and hematuria is seen in 41.17–44% of COVID-19 patients (72, 73). This impairment of the kidney may be caused by hypoxemia, hemodynamic changes, and most importantly by circulating inflammatory mediators (79). During our review, we paid particular attention to kidney allograft function alteration. Only 1 of the 27 (3.7%) COVID-19 KT patients were mentioned with AKI. Besides proteinuria (7/9, 77.8%), no abnormal serum creatinine change was observed between creatinine level at admission and at discharge (or last follow-up).
Eight of the 27 (29.6%) COVID-19 KT patients were treated with methylprednisolone pulse therapy. Altogether, 81.5% (22/27) of the patients were prescribed with steroids including oral steroids. Theoretically, corticosteroid administration in COVID-19 KT patients could be beneficial in two ways: (1) immunosuppression to prevent allograft rejection and (2) alleviation of immune-related injury to the lung (80) or kidney (79). However, a retrospective cohort study showed that high-dose corticosteroids had a limited beneficial effect on SARS because it increased the risks of virus dissemination, opportunistic infection, and osteopathy (81). In patient No. 6, methylprednisolone pulse therapy (1,000 mg) was administrated to treat acute rejection just 1 day before the confirmation of COVID-19. A large dose of steroids could be an important cause of constant fever and dyspnea during hospitalization (28 days since the confirmation of COVID-19). Corticosteroid therapy has been a long-lasting controversy in viral pneumonias caused by coronaviruses. Its use in the critically ill subgroup may help improve the outcome (80); however, further studies are essential to establish guidelines for steroid therapy in COVID-19 SOT patients.
In short, compared with general population current reports on COVID-19 KT patients showed similar symptoms and implied worse outcomes. Further solid evidences are needed to prove the efficacy of immunosuppression reduction strategy, current antiviral treatment, and methylprednisolone pulse therapy. Despite of involvement of kidney in COVID-19 KT patients, no permanent renal function impairment was observed.
COVID-19 in Liver Transplant Patients
Regarding COVID-19 infection in liver transplantation, a 37-year-old man developed fever with abdominal discomfort at day 9 (D9) after liver transplant and was subsequently confirmed as COVID-19 positive by RT-PCR and CT examination. The patients' immunosuppression was discontinued and he was administered low-dose methylprednisolone for 2 weeks. His temperature recovered normally at D18 post-transplant; however, he developed rejection at D27 post-transplant, which was eventually controlled using tacrolimus and an infusion of methylprednisolone for 3 days. His RT-PCR test for COVID-19 was negative at D45 post-transplant and he was discharged after achieving clinical cure standards at D51 post-transplant (55). Another 37-year-old man presented with fever (with a temperature of up to 39°C) 4 days prior to liver transplant, which persisted for 30 days even after antimicrobial agents and caspofungin were prescribed. Thoracic CT and RT-PCT test confirmed COVID-19 infection at D15 post-transplant that was eventually cleared at D49 post-transplant. However, acute cellular rejection was suspected due to low-dose immunosuppression at D36 post-transplant and the patient did not recover during hospitalization (82). Unfortunately, this patient did not undergo COVID-19 associated examinations before liver transplant; therefore, it is not known whether COVID-19 infection occurred before or after the transplant. A 50-year-old man who had a cadaveric liver transplant in July 2017 contracted COVID-19 and presented with a representative clinical course of the disease, including persistent fever, significant lymphocytopenia, and mixed diffuse ground-glass opacities on CT. This patient had no rejection phase and successfully recovered after 1 month of comprehensive treatment including immunosuppression adjustment, oxygen therapy, antivirus, systemic low-dose methylprednisolone, intravenous immunoglobulin, prophylactic antibiotic, alpha interferon, and nutritional support (83).
Unfortunately, several cases with fatal outcomes have also been reported. A 59-year old patient who had undergone a liver transplant 3 years earlier was infected by his wife on February 1, 2020. His symptoms progressed rapidly from mild to critical illness with several nosocomial infections, and although several standard rescue efforts were attempted, he eventually died 45 days after the diagnosis. The authors believe that an earlier withdrawal of immunosuppression along with a more aggressive approach that includes antiviral and antibacterial therapy might have averted the fatal outcome (84). A transplant center from Italy reported that three long-term (more than 10 years) liver transplant survivors died between 3 and 12 days after the onset of COVID-19 pneumonia. All three patients were men and older than 65 years with diabetes. The authors declare that long-term liver transplant patients with metabolic comorbidities are associated with a poor prognosis (85).
Similar with the difficulty we face in COVID-19 KT patients, aforementioned COVID-19 liver transplant cases show that it is tricky to regulate immune system and balance between infection and rejection. The liver tissue damage might be due to an immune-mediated inflammatory response to the virus or a direct virus-induced cytopathogenic effect that has been reviewed elsewhere (86).
COVID-19 in Heart and Lung Transplant Patients
Compared with the COVID-19 infection in kidney and liver transplant patients, there are fewer reported cases for heart transplant patients. The first two cases of COVID-19 infected heart transplant recipients from China were cured after treatments that were similar to those in non-transplant recipients (87). Two of the three heart transplant recipients reported from Spain were discharged after 20 days of treatment, which included hydroxychloroquine (HCQ) therapy and discontinuation of immunosuppression; however, the third one died at day 10 after confirmed COVID-19 infection although lopinavir/ritonavir, HCQ, and interferon–β (IFN-β) were administered (57).
Lung transplantation is the only effective treatment modality for end-stage pulmonary chronic diseases (88). A 59-year-old woman 13 months post-bilateral lung transplant was reported with confirmed COVID-19. Only mild exercise dyspnea and dry cough were present without any fever or diarrhea. Without any administration of antiviral drugs or change of immunosuppression therapy or mechanical ventilation, the viral load decreased overtime and turned negative after 21 days hospitalization (89). Currently, no guidelines are available to treat lung transplant patients with COVID-19 pneumonia.
Lung transplant as a treatment of acute infectious pneumonia was relatively rare (90). During this COVID-19 pandemic, three chronic pulmonary disease patients infected with COVID-19 (for more than a month) received lung transplants after full ethical approval. Two of them survived during the short-term follow-up; however, the third patient died post-transplant (91). Soon afterwards, the first description of lung transplantation for COVID-19 confirmed elderly patients with end-stage pulmonary disease was reported by Han et al. (90). It is recommended that the RT-PCR test for COVID-19 should be negative at least twice (24-h interval) for transplant candidates; else, the virus may damage the transplanted lung and medical staff will be at a high risk of transmission. These five reported cases suggest that lung transplant is a potential option for patients when internal medicine treatments including mechanical ventilation and extracorporeal membrane oxygenation (ECMO) cannot enhance lung function.
Conclusion
The COVID-19 pandemic will continue to threaten global public health and transplantation programs until effective antiviral drugs and vaccines are developed. In order to ensure transplantation safety, donor and recipient screening is necessary in COVID-19 spreading areas. The SOT recipients are an immunologically vulnerable group and it is crucial to prevent viral infection in such patients. Both online education to spread awareness about COVID-19 and online follow-up could minimize the risk of infection in SOT patients. Compared with the general population, our review of current COVID-19 KT cases show a similar clinical manifestation and inferior outcomes. The long-lasting controversy over immunoregulation to balance infection and rejection continues during this battle with COVID-19. Antiviral drugs and methylprednisolone pulse therapy should be administered with great caution. Current antiviral therapy needs more evidence to prove that its efficacy can outweigh its potential adverse effects and drug-drug interactions. Lung transplant could be a possible way out for end stage COVID-19 patients, but great caution should be taken before COVID-19 lung injury is proven irreversible. Generally, much more studies and specific clinical guidelines are needed to protect transplantation from the adverse effect of COVID-19.
Author Contributions
HZ and HD wrote the manuscript. HZ generated the tables. HZ, HD, and XX revised the manuscript. All authors have contributed to the editing of the manuscript.
Funding
This authors' work was supported by the National Science Foundation of China (NSFC; Nos. 81800664 and 81900370) to HD and HZ, Natural Science Foundation of Hunan Province of China (2019JJ50842), and Huxiang Young Talents of Hunan Province (2019RS2013) to HD.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. World Health Organization. Situation Report - 1 Novel Coronavirus (2019-nCoV) 21 January 2020. (2020). Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf (accessed April 4, 2020).
3. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
4. Gorbalenya AE, Baker SC, Baric RS, Groot RJ de, Drosten C, Gulyaeva AA, et al. Severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the Coronavirus Study Group. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.02.07.937862
5. World Health Organization. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes It. (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 (accessed April 4, 2020).
6. World Health Organization. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020. (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 (accessed April 4, 2020).
7. COVID-19 Situation Reports. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed April 17, 2020).
8. World Health Organization. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. (2020). Available online at: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed April 4, 2020).
9. Ye Q, Wang B, Mao J, Fu J, Shang S, Shu Q, et al. Epidemiological analysis of COVID-19 and practical experience from China. J Med Virol. (2020). doi: 10.1002/jmv.25813. [Epub ahead of print].
10. Zhang Y, Chen C, Zhu S, Shu C, Wang D, Song J. Isolation of 2019-nCoV from a stool specimen of a laboratory- confirmed case of the coronavirus disease 2019 (COVID-19). China CDC Wkly. (2020) 2:123–4. doi: 10.46234/ccdcw2020.033
11. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
12. Team TNCPERE. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol. (2020) 41:145–51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003
13. Wu ZY. [Asymptomatic and pre-symptomatic cases of COVID-19 contribution to spreading the epidemic and need for targeted control strategies]. Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:E036. doi: 10.3760/cma.j.cn112338-20200406-00517
14. Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. (2020) 323:1406–7. doi: 10.1001/jama.2020.2565
15. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N Engl J Med. (2020) 382:970–1. doi: 10.1056/NEJMc2001468
16. Global Transplantation Covid Report March 2020. (2020). Available online at: https://tts.org/index.php?option=com_content&view=article&id=696&Itemid=115 (accessed April 9, 2020).
17. Boyarsky BJ, Chiang TP-Y, Werbel WA, Durand CM, Avery RK, Getsin SN, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. (2020). doi: 10.1111/ajt.15915. [Epub ahead of print].
18. Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. (2020) 395:e95–6. doi: 10.1016/S0140-6736(20)31040-0
19. de Vries APJ, Alwayn IPJ, Hoek RAS, van den Berg AP, Ultee FCW, Vogelaar SM, et al. Immediate impact of COVID-19 on transplant activity in the Netherlands. Transpl Immunol. (2020) 61:101304. doi: 10.1016/j.trim.2020.101304
20. Kumar D, Manuel O, Natori Y, Egawa H, Grossi P, Han S-H, et al. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant. (2020). doi: 10.1111/ajt.15876. [Epub ahead of print].
21. Global Surveillance for COVID-19 Caused by Human Infection with COVID-19 Virus: Interim Guidance 20 March 2020. Available online at: https://apps.who.int/iris/handle/10665/331506 (accessed April 17, 2020).
22. The Transplantation Society. Guidance on Coronavirus Disease 2019 (COVID-19) for Transplant Clinicians. (2020). Available online at: https://tts.org/tid-about/tid-presidents-message/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians (accessed May 26, 2020).
23. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
24. Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv [Preprint]. (2020). doi: 10.1101/2020.02.29.20029520
25. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Crystal structure of the 2019-nCoV spike receptor-binding domain bound with the ACE2 receptor. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.02.19.956235
26. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. (2004) 203:631–7. doi: 10.1002/path.1570
27. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
28. Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. [A pathological report of three COVID-19 cases by minimally invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. (2020) 49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193
29. Diao B, Feng Z, Wang C, Wang H, Liu L, Wang C, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.04.20031120
30. Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through post-mortem core biopsies. Modern Pathol. (2020). doi: 10.20944/preprints202003.0311.v1. [Epub ahead of print].
31. ACR Recommendations for the Use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. (2020). Available online at: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection (accessed April 15, 2020).
32. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. (2020) 323:1843–4. doi: 10.1001/jama.2020.3786
33. Tang Y-W, Schmitz JE, Persing DH, Stratton CW. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol. (2020) 58:e00512-20. doi: 10.1128/JCM.00512-20
34. Wang Y, Kang H, Liu X, Tong Z. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J Med Virol. (2020) 92:538–9. doi: 10.1002/jmv.25721
35. Xiao AT, Tong YX, Zhang S. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol. (2020). doi: 10.1002/jmv.25855. [Epub ahead of print].
36. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
37. CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control Prevention (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (accessed April 15, 2020).
38. Chunhua F, Liping W, Manhua NIE, Yajie LIU, Jin H, Xubiao XIE. Emergency management for kidney transplantation in the epidemic period of coronavirus disease 2019. J Cent South Univ Med Sci. (2020) 45:0–2.
39. CDC. Healthcare Personnel and First Responders: How to Cope with Stress and Build Resilience during the COVID-19 Pandemic. Centers for Disease Control Prevention (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/community/mental-health-healthcare.html (accessed May 23, 2020).
40. Organ Procurement and Transplantation Network (OPTN) Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2018 annual data report: introduction. Am J Transplant. (2020) 20:11–19. doi: 10.1111/ajt.15671
41. Romanelli A, Mascolo S. Immunosuppression drug-related and clinical manifestation of coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. (2020). doi: 10.1111/ajt.15905. [Epub ahead of print].
42. Manuel O, Estabrook M, the American Society of Transplantation Infectious Diseases Community of Practice. RNA respiratory viral infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. (2019) 33:e13511. doi: 10.1111/ctr.13511
43. Guillen E, Pineiro GJ, Revuelta I, Rodriguez D, Bodro M, Moreno A, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. (2020). doi: 10.1111/ajt.15874. [Epub ahead of print].
44. Zhu L, Xu X, Ma K, Yang J, Guan H, Chen S, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. (2020). doi: 10.1111/ajt.15869. [Epub ahead of print].
45. Gandolfini I, Delsante M, Fiaccadori E, Zaza G, Manenti L, Degli Antoni A, et al. COVID-19 in kidney transplant recipients. Am J Transplant. (2020). doi: 10.1111/ajt.15891. [Epub ahead of print].
46. Zhang H, Chen Y, Yuan Q, Xia Q-X, Zeng X-P, Peng J-T, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. (2020) 77:742–7. doi: 10.1016/j.eururo.2020.03.030
47. Chen S, Yin Q, Shi H, Du D, Chang S, Ni L, et al. A familial cluster, including a kidney transplant recipient, of Coronavirus Disease 2019 (COVID-19) in Wuhan, China. Am J Transplant. (2020). doi: 10.1111/ajt.15903. [Epub ahead of print].
48. Huang J, Lin H, Wu Y, Fang Y, Kumar R, Chen G, et al. COVID-19 in post-transplantation patients- report of two cases. Am J Transplant. (2020). doi: 10.1111/ajt.15896. [Epub ahead of print].
49. Seminari E, Colaneri M, Sambo M, Gallazzi I, Di Matteo A, Silvia R, et al. SARS Cov2 infection in a renal transplanted patients. A case report. Am J Transplant. (2020). doi: 10.1111/ajt.15902. [Epub ahead of print].
50. Wang J, Li X, Cao G, Wu X, Wang Z, Yan T. COVID-19 in a kidney transplant patient. Eur Urol. (2020) 77:761–70. doi: 10.1016/j.eururo.2020.03.036
51. Ning L, Liu L, Li W, Liu H, Wang J, Yao Z, et al. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: case report. Am J Transplant. (2020). doi: 10.1111/ajt.15897. [Epub ahead of print].
52. Bartiromo M, Borchi B, Botta A, Bagala A, Lugli G, Tilli M, et al. Threatening drug-drug interaction in a kidney transplant patient with Coronavirus Disease 2019 (COVID-19). Transpl Infect Dis. (2020). doi: 10.1111/tid.13286. [Epub ahead of print].
53. Bussalino E, De Maria A, Russo R, Paoletti E. Immunosuppressive therapy maintenance in a kidney transplant recipient SARS-CoV-2 pneumonia: a case report. Am J Transplant. (2020). doi: 10.1111/ajt.15920. [Epub ahead of print].
54. Marx D, Moulin B, Fafi-Kremer S, Benotmane I, Gautier G, Perrin P, et al. First case of COVID-19 in a kidney transplant recipient treated with belatacept. Am J Transplant. (2020). doi: 10.1111/ajt.15919. [Epub ahead of print].
55. Zhong Z, Zhang Q, Xia H, Wang A, Liang W, Zhou W, et al. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. (2020) e13296. doi: 10.1111/ajt.15928. [Epub ahead of print].
56. Arpali E, Akyollu B, Yelken B, Tekin S, Turkmen A, Kocak B. Case report: a kidney transplant patient with mild COVID-19. Transpl Infect Dis. (2020). doi: 10.1111/tid.13296. [Epub ahead of print].
57. Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. (2020). doi: 10.1111/ajt.15929. [Epub ahead of print].
58. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. (2020) 51:843–51. doi: 10.1111/apt.15731
59. Siordia JA. Epidemiology and clinical features of COVID-19: a review of current literature. J Clin Virol. (2020) 127:104357. doi: 10.1016/j.jcv.2020.104357
60. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
61. Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al. Covid-19 and kidney transplantation. N Engl J Med. (2020). doi: 10.1056/NEJMc2011117. [Epub ahead of print].
62. Antiviral Therapy | Coronavirus Disease COVID-19. COVID-19 Treatment Guidelines. (2020). Available online at: https://www.covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/antiviral-therapy/ (accessed April 30, 2020).
63. Yousefifard M, Zali A, Mohamed Ali K, Madani Neishaboori A, Zarghi A, Hosseini M, et al. Antiviral therapy in management of COVID-19: a systematic review on current evidence. Arch Acad Emerg Med. (2020) 8:e45. doi: 10.1111/ijcp.13557
64. Table 2a Potential Antiviral Agents Clinical Data | Coronavirus Disease COVID-19. COVID-19 19 Treatment Guideline. (2020). Available online at: https://www.covid19treatmentguidelines.nih.gov/tables/table-2a/ (accessed April 30, 2020).
65. Remdesivir EUA Letter of Authorization. (2020). Available online at: https://www.fda.gov/media/137564/download (accessed May 21, 2020).
66. Remdesivir | Coronavirus Disease COVID-19. COVID-19 Treatment Guideline. (2020). Available online at: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/remdesivir/ (accessed May 21, 2020).
67. Vanhove T, Remijsen Q, Kuypers D, Gillard P. Drug–drug interactions between immunosuppressants and antidiabetic drugs in the treatment of post-transplant diabetes mellitus. Transplant Rev. (2017) 31:69–77. doi: 10.1016/j.trre.2016.09.001
68. Table 2b Characteristics of Potential Antiviral Agents | Coronavirus Disease COVID-19. COVID-19 Treatment Guideline. (2020). Available online at: https://www.covid19treatmentguidelines.nih.gov/tables/table-2b/ (accessed April 30, 2020).
69. de Wilde AH, Raj VS, Oudshoorn D, Bestebroer TM, van Nieuwkoop S, Limpens RWAL, et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J Gen Virol. (2013) 94:1749–60. doi: 10.1099/vir.0.052910-0
70. Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses. (2013) 5:1250–60. doi: 10.3390/v5051250
71. Molas-Ferrer G, Soy-Muner D, Anglada-Martínez H, Riu-Viladoms G, Estefanell-Tejero A, Ribas-Sala J. Interstitial pneumonitis as an adverse reaction to mTOR inhibitors. Nefrol Publicacion Of Soc Espanola Nefrol. (2013) 33:297–300. doi: 10.3265/Nefrologia.pre2013.Jan.11439
72. Anti-2019-nCoV Volunteers, Li Z, Wu M, Yao J, Guo J, Liao X, et al. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. (2020). doi: 10.1101/2020.02.08.20021212. [Epub ahead of print].
73. Liu R, Ma Q, Han H, Su H, Liu F, Wu K, et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin Chem Lab Med. (2020). doi: 10.1515/cclm-2020-0220. [Epub ahead of print].
74. Shamseddin MK, Knoll GA. Posttransplantation proteinuria: an approach to diagnosis and management. Clin J Am Soc Nephrol. (2011) 6:1786–93. doi: 10.2215/CJN.01310211
75. Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. (2020). doi: 10.1016/j.kint.2020.04.003. [Epub ahead of print].
76. Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. (2020) 51:343–8. doi: 10.1159/000507471
77. Xiao G, Hu H, Wu F, Sha T, Huang Q, Li H, et al. Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.06.20055194
78. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
79. Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, Ronco C, et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care. (2020) 24:155. doi: 10.1186/s13054-020-02872-z
80. Zhou W, Liu Y, Tian D, Wang C, Wang S, Cheng J, et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. (2020) 5:18. doi: 10.1038/s41392-020-0127-9
81. Auyeung T, Lee J, Lai W, Choi C, Lee H, Lee J, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. (2005) 51:98–102. doi: 10.1016/j.jinf.2004.09.008
82. Qin J, Wang H, Qin X, Zhang P, Zhu L, Cai J, et al. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatol Baltim Md. (2020). doi: 10.1002/hep.31257. [Epub ahead of print].
83. Bin L, Yangzhong W, Yuanyuan Z, Huibo S, Fanjun Z, Zhishui C. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. (2020). doi: 10.1111/ajt.15901. [Epub ahead of print].
84. Huang J-F, Zheng KI, George J, Gao H-N, Wei R-N, Yan H-D, et al. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. (2020). doi: 10.1111/ajt.15909. [Epub ahead of print].
85. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. (2020) 5:532–3. doi: 10.1016/S2468-1253(20)30116-3
86. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. (2020) 40:998–1004. doi: 10.1111/liv.14435
87. Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. (2020) 39:496–7. doi: 10.1016/j.healun.2020.03.006
88. Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report−2014; focus theme: retransplantation. J Heart Lung Transplant. (2014) 33:996–1008. doi: 10.1016/j.healun.2014.08.003
89. Aigner C, Dittmer U, Kamler M, Collaud S, Taube C. COVID-19 in a lung transplant recipient. J Heart Lung Transplant. (2020)39:610–1. doi: 10.1016/j.healun.2020.04.004
90. Han W, Zhu M, Chen J, Zhang J, Zhu S, Li T, et al. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann Surg. (2020). doi: 10.1097/SLA.0000000000003955. [Epub ahead of print].
Keywords: COVID-19, solid organ transplantation, immunosuppressant, transplant safety, clinical characteristics
Citation: Zhang H, Dai H and Xie X (2020) Solid Organ Transplantation During the COVID-19 Pandemic. Front. Immunol. 11:1392. doi: 10.3389/fimmu.2020.01392
Received: 10 May 2020; Accepted: 01 June 2020;
Published: 16 June 2020.
Edited by:
Zhenhua Dai, Guangdong Provincial Academy of Chinese Medical Sciences, ChinaReviewed by:
Cheng Yang, Zhongshan Hospital, Fudan University, ChinaDali Sun, North Dakota State University, United States
Copyright © 2020 Zhang, Dai and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helong Dai, aGVsb25nNjg4ODhAY3N1LmVkdS5jbg==; Xubiao Xie, eGlleHViaWFvQGNzdS5lZHUuY24=
 Hedong Zhang
Hedong Zhang Helong Dai
Helong Dai Xubiao Xie1,2,3*
Xubiao Xie1,2,3*