- 1Department of Oncology, The First Medical Center of Chinese PLA General Hospital, Beijing, China
- 2Medical School of Chinese PLA, Beijing, China
- 3The 78th Group Army Hospital of Chinese PLA, Mudanjiang, China
- 4Departments of Thoracic/Head and Neck Medical Oncology and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 5Department of Radiotherapy, The First Medical Center of Chinese PLA General Hospital, Beijing, China
- 6BeiGene (Shanghai) Co., Ltd., Shanghai, China
Background: Serum tumor markers carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), cytokeratin 19 fragment (CYFRA21-1) and squamous-cell carcinoma-related antigen (SCC-Ag) are routinely used for monitoring the response to chemotherapy or targeted therapy in advanced-stage non-small cell lung cancer (NSCLC), however their role in immunotherapy remains unclear. The aim of this study was to investigate whether dynamics of these serum markers were associated with the efficacy and prognosis of Chinese late-stage NSCLC patients treated with programmed cell death-1/programmed cell death ligand-1 (PD-1/PD-L1) inhibitors.
Methods: We initiated a longitudinal prospective study on advanced NSCLC patients treated with PD-1/PD-L1 inhibitors in Chinese PLA general hospital (Beijing, China). Blood samples of baseline and after 6 weeks' treatment were collected. CT scan were used by all patients to evaluate treatment efficacy according to RECIST 1.1. Serum tumor markers levels were measured with an electrochemical luminescence for SCC-Ag and with a chemiluminescent microparticle immunoassay for serum CEA, CA125, and CYFRA21-1. At least 20% decreases of the biomarkers from baseline were considered as meaningful improvements after 6 weeks of treatment with immune checkpoint inhibitors (ICIs). Optimization-based method was used to balance baseline covariates between different groups. Associations between serum tumor biomarker improvements and objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) were analyzed.
Results: A total of 308 Chinese patients with advanced NSCLC were enrolled in the study. After balancing baseline covariates, patients with meaningful improvements in <2 out of 4 biomarkers (CEA, CA125, CYFRA21-1, and SCC-Ag) was ended up with lower ORR (0.08 vs. 0.35, p < 0.001), shorten PFS (median: 5.4 vs. 12.5 months, p < 0.001), and OS (median: 11.7 vs. 25.6 months, p < 0.001) in the total population. Subgroup analysis of patients with adenocarcinoma revealed that patients with meaningful improvements in <2 out of 4 biomarkers had significant lower ORR (0.06 vs. 0.36, p < 0.001), shorten PFS (median: 4.1 vs. 11.9 months, p < 0.001), and OS (median: 11.9 vs. 24.2 months, p < 0.001). So as in patients with squamous cell carcinoma, meaningful improvements in at least 2 out of 4 biomarkers were linked to better ORR (0.42 vs. 0.08, p = 0.014), longer PFS (median: 13.1 vs. 5.6 months, p = 0.001), and OS (median: 25.6 vs. 10.9 months, p = 0.06).
Conclusions: The dynamic change of CEA, CA125, CYFRA21-1, and SCC-Ag from baseline have prognostic value for late-stage NSCLC patients treated with PD-1/PD-L1 inhibitors. Decrease of associated biomarkers serum levels were associated with favorable clinical outcomes.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide (1, 2). As the most common subtype of lung cancer, non-small cell lung cancer (NSCLC) accounts for 80–85% of the total cases. Over 60% of the NSCLC patients present with locally advanced or metastatic diseases at the time of diagnosis, and surgical resection may not be a treatment option (3). For these patients, although chemotherapy or targeted therapy has improved clinical outcomes in certain subtypes of lung cancer, up to 90% of patients inevitably relapse with the 5-year survival rate below 20% (4–6).
The emergence of immune checkpoint blockade (ICB) therapy targeting programmed cell death-1/programmed cell death ligand-1 (PD-1/PD-L1) have revolutionized the treatment of NSCLC, with large number of clinical trials demonstrating their increased effectiveness (7–10). Unfortunately, response rate is only ~20% for advanced NSCLC in unselected populations, thus biomarker development remains critical to avoid ineffective treatments (11). PD-L1 expression and tumor mutation burden (TMB) are the most studied and validated predictors of clinical benefit in NSCLC patients with ICB therapy (12–15), while their roles are still controversial (7, 16–19). Moreover, detecting these biomarkers usually requires and invasive procedures followed by pathological assessment or even complicated and expensive methodologies such as the next generation sequencing (NGS). Therefore, non-invasive method and convenient biomarkers with relatively low cost are urgently needed.
Serum carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), cytokeratin 19 fragment (CYFRA21-1), and squamous-cell carcinoma-related antigen (SCC-Ag) might be relevant for the prognosis of patients and have been widely used as biomarkers predicting the efficacy of chemotherapy or targeted therapy in NSCLC patients (20–27). However, their roles and post-treatment changes from baseline in advanced NSCLC treated by immune checkpoint inhibitors (ICIs) remains unclear. The aim of this study was to investigate whether dynamics of serum tumor markers were associated with the efficacy and prognosis of Chinese late-stage NSCLC patients treated with ICIs.
Methods
Study Design
This observational study was performed in a real-life clinical practice setting. A total of 308 consecutive NSCLC patients from stage IIIB to IV receiving PD-1/PD-L1 checkpoint inhibitors were prospectively enrolled in Chinese PLA general hospital (Beijing, China) from January 2015 to January 2019. ICIs were treated for at least 6 weeks, and serum biomarkers (CEA, CA125 CYFRA21-1, and SCC-Ag) were measured at ICIs treatment initiation and after 6 weeks. During treatment, response was evaluated at least once.
The efficacy of immunotherapy was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (28), including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). ORR was defined as the percentage of patients who have ever achieved a CR or PR since the first ICIs treatment. The time interval between date of commencement of PD-1/PD-L1 inhibitors treatment and date of disease progression or death (PFS) or death alone (OS) was calculated for each patient. The data cut-off date was Oct 6, 2019.
The baseline covariates including age, gender, histological type, clinical stage, smoking history, Eastern Cooperative Oncology Group Performance Status (ECOG PS), metastatic sites (lung, liver, and brain), radiotherapy, treatment (monotherapy or combination therapy), and prior lines of therapy (one line, two lines, and at least three lines) were collected. Lab test results including hemoglobin, white blood count, neutrophil, lymphocyte, monocyte, lactate dehydrogenase, platelet, and albumin were also routinely recorded.
Specimen Collection and Tumor Markers Assay
Blood samples were collected before the first ICIs treatment and after 6 weeks. Serum levels of CEA, CA125, and CYFRA21-1 were detected with electrochemical luminescence (CEA assay kit, CA125 quantitative determination kit and Non-small cell lung cancer associated antigen 21-1 detection kit; Roche), whereas SCC-Ag was measured with chemiluminescent microparticle immunoassay (Architect SCC reagent kit; Abbott). According to instructions of manufacturers, the reference range was 0–5.0 ng/ml for CEA, 0.1–35.0 ng/ml for CA125, 0.1–4.0 ng/ml for CYFRA21-1, and 0–1.8 ng/ml for SCC-Ag. Lab test results and levels of serum tumor markers were categorized by low, normal, and high based on the reference range, respectively (Supplementary Table 1). PD-L1 expression was evaluated by immunohistochemistry and tumor proportion score using PD-L1 antibody (Dako 22C3) before ICIs treatment.
The study protocol was approved by the Ethics Committee of Chinese PLA General Hospital. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines defined by the International Conference on Harmonization. Written informed consent was collected from all patients before enrollment.
Statistical Analysis
A post-treatment decline in serum marker level ≥20% from baseline was considered as meaningful improvement. Two groups were subsequently divided based on whether meaningful improvements of at least two serum biomarkers or not. Optimization-based methods were utilized to balance the baseline covariates between different groups (29). A weight under the following criteria was assigned to each patient: (1) Absolute value of standardized mean difference no more than 0.15; (2) Variance ratio between 0.67 (1/1.5) and 1.5. The effective sample sizes in the weighted sample were calculated by Kish's approximate formula. Group difference in ORR was calculated by Chi-square test. Median PFS and OS were estimated by Kaplan-Meier method and their 95% confidence intervals (CIs) were constructed by Brookmeyer and Crowley method, group difference was assessed by Log-rank test. Hazard ratio (HR) with its 95% CI were calculated using Cox proportional hazards models. All statistical tests were bilateral with significance level 0.05. All analyses were performed in R, with the R packages WeightIt version 0.5.1 (https://cran.r-project.org/web/packages/WeightIt/index.html) for optimization-based methods and survey version 3.36 (https://cran.r-project.org/web/packages/survey/index.html) in the weighted sample.
Results
Baseline Patient Characteristics
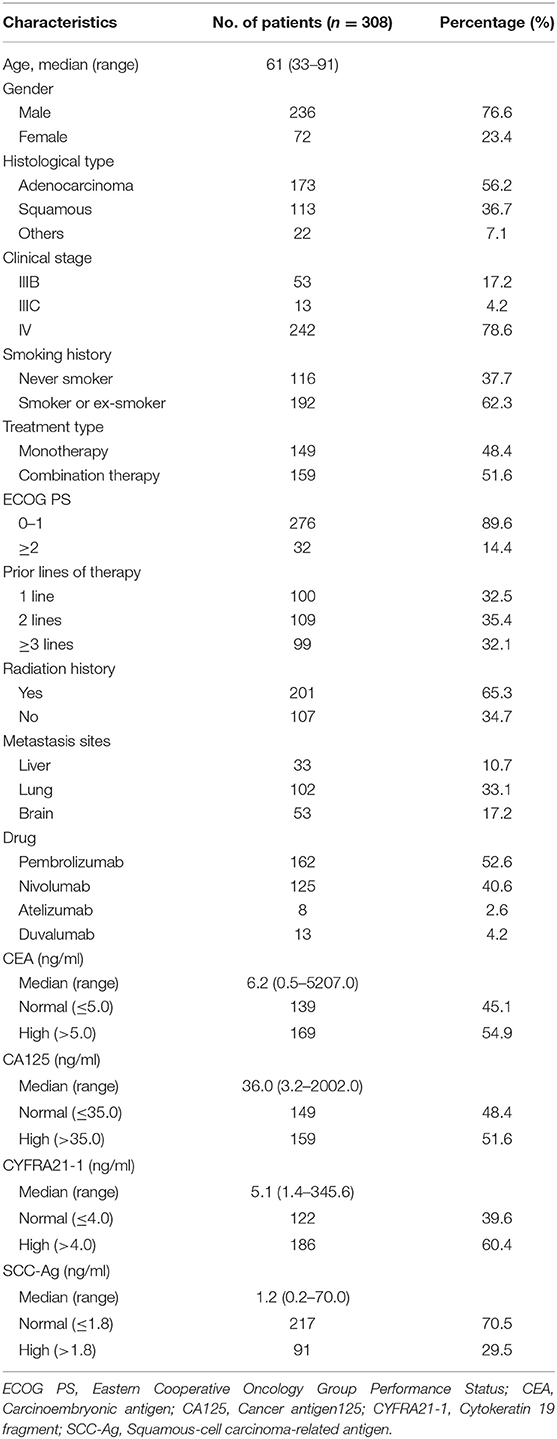
The main clinical characteristics of all the participants at baseline were presented in Table 1. Among 308 included patients, 56.2% were adenocarcinoma (ADC), 36.7% were squamous cell carcinoma (SCC) and the rest 7.1% belong to other subtypes. According to the eighth edition TNM staging of International Lung Cancer Research Association (30), 17.2% were stage IIIB, 4.2% were stage IIIC, and 78.6% were stage IV. 52.6% of patients used the drug of Pembrolizumab, 40.6% used Nivolumab, and the remaining patients used Atelizumab or Duvalumab. The median level of serum markers at baseline was 6.2 ng/ml for CEA (range 0.5–5207.0), 36.0 ng/ml for CA125 (range 3.2–2002.0), 5.1 ng/ml for CYFRA21-1 (range 1.4–345.6), and 1.2 ng/ml for SCC-Ag (range 0.2–70.0). Proportion of patients with elevated levels of CEA, CA125, CYFRA21-1, and SCC-Ag were 54.9, 51.6, 60.4, and 29.5%, respectively.
Association Between Dynamics of Tumor Markers and Clinical Outcomes
The Total Population
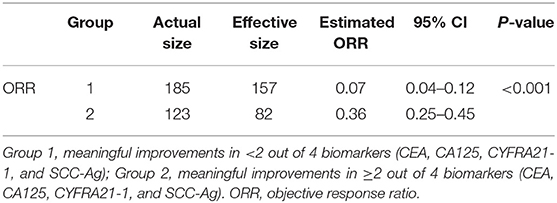
The total population was divided into two groups by meaningful improvements in <2 out of 4 biomarkers (CEA, CA125, CYFRA21-1, and SCC-Ag) (“<2/4 biomarkers improvement group”) and at least 2 out of 4 biomarkers (“≥2/4 biomarkers improvement group”). Standardized mean difference values of treatment type (combination therapy) and prior lines of therapy (one line, two lines) before balancing was 0.25, 0.24, and 0.18, respectively, followed by optimization-based weighting procedure to balance all baseline covariates between the two groups (Supplementary Table 2).
In the weighted samples, the ORR in the “<2/4 biomarker improvement group” was significantly lower than the “≥2/4 biomarkers improvement group” (0.08 vs. 0.35, p < 0.001) (Table 2). The patients in the “<2/4 biomarker improvement group” also had significantly shorten PFS (median: 5.4 vs. 12.5 months, p < 0.001) and OS (median: 11.7 vs. 25.6 months, p < 0.001) compared with the “≥2/4 biomarkers improvement group.” The Kaplan-Meier curves of PFS and OS in both original and weighted sample were presented in Figure 1.
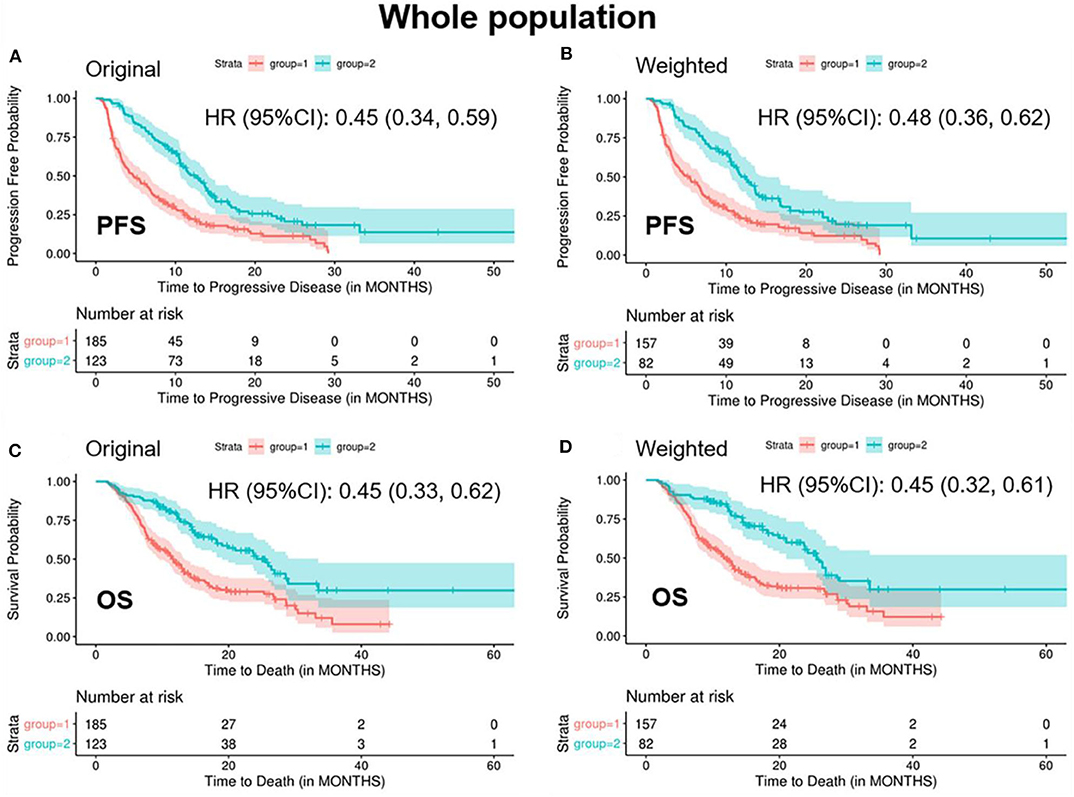
Figure 1. Kaplan-Meier curves of PFS/OS in the original and weighted sample of whole population. group 1: meaningful improvements in <2 out of 4 biomarkers (CEA, CA125, CYFRA21-1, and SCC-Ag); group 2: meaningful improvements in ≥2 out of 4 biomarkers (CEA, CA125, CYFRA21-1, and SCC-Ag). Kaplan-Meier curves of (A,C) were based on the original sample; Kaplan-Meier curves of (B,D) were based on the weighted sample.
Subgroup Analysis of ADC
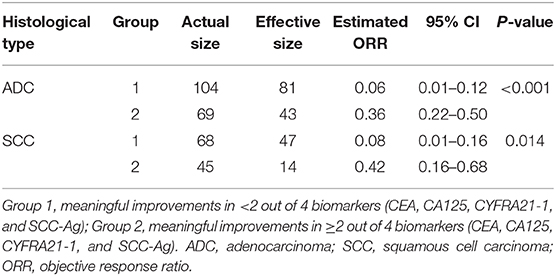
In patients with ADC, standardized mean difference of treatment type (combination therapy), prior lines of therapy (one line), and platelets (high level) was 0.25, 0.21, and 0.16, respectively, between the two groups before balancing (Supplementary Table 3). After balancing by the optimization-based method, patients in the “<2/4 biomarkers improvement group” were less likely to respond to treatment (ORR: 0.06 vs. 0.36, p < 0.001), more likely to progress (median PFS: 4.1 vs. 11.9 months, p < 0.001) and decease (median OS: 11.9 vs. 24.2 months, p < 0.001) (Table 3 and Figure 2).
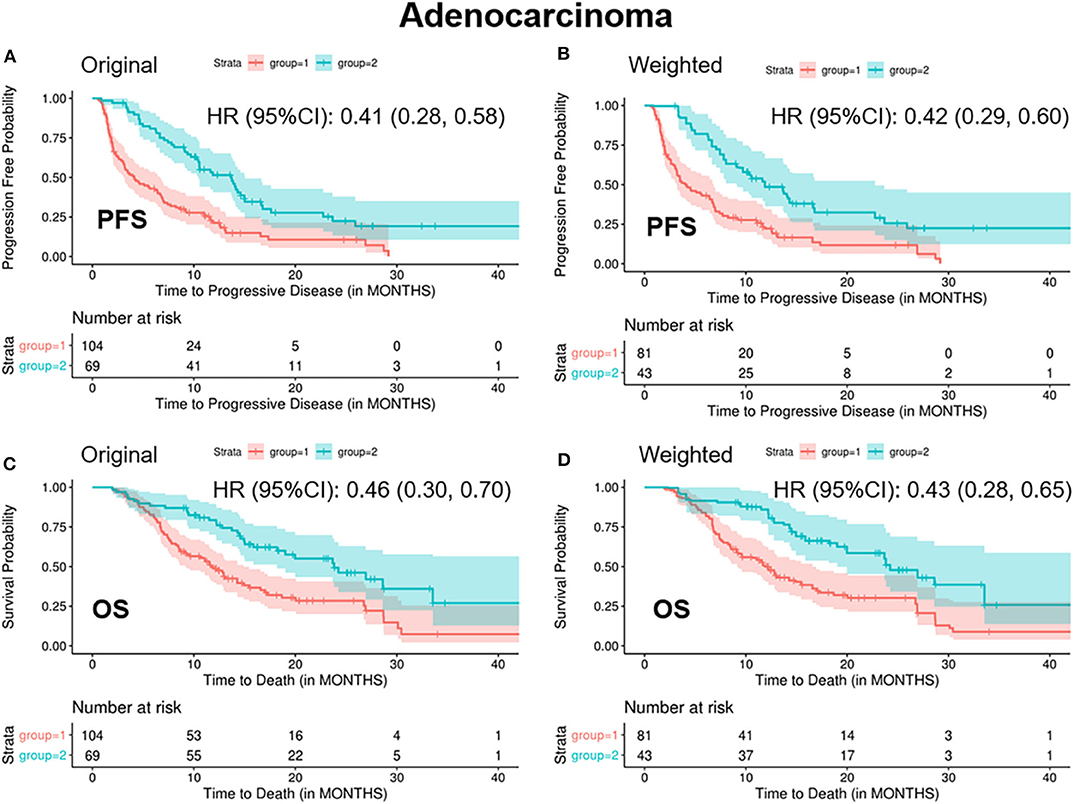
Figure 2. Kaplan-Meier curves of PFS/OS in the original and weighted sample of adenocarcinoma. group 1: meaningful improvements in <2 out of 4 biomarkers (CEA, CA125, CYFRA21-1, and SCC-Ag); group 2: meaningful improvements in ≥2 out of 4 biomarkers (CEA, CA125, CYFRA21-1, and SCC-Ag). Kaplan-Meier curves of (A,C) were based on the original sample; Kaplan-Meier curves of (B,D) were based on the weighted sample.
Subgroup Analysis of SCC
In patients with SCC, standardized mean difference of the baseline covariates stage (IV), treatment type (combination therapy), prior lines of therapy (one line, two lines), and radiation history (yes) before balancing was 0.16, 0.26, 0.29, 0.34, and 0.19, respectively (Supplementary Table 4). After balancing by the optimization-based method, patients in the “<2/4 biomarkers improvement group” were less likely to respond to treatment (ORR: 0.08 vs. 0.42, p = 0.014), more likely to progress (median PFS: 5.6 vs. 13.1 months, p = 0.001) and decease (median OS: 10.2 vs. 25.6 months, p = 0.06) (Table 3 and Figure 3).
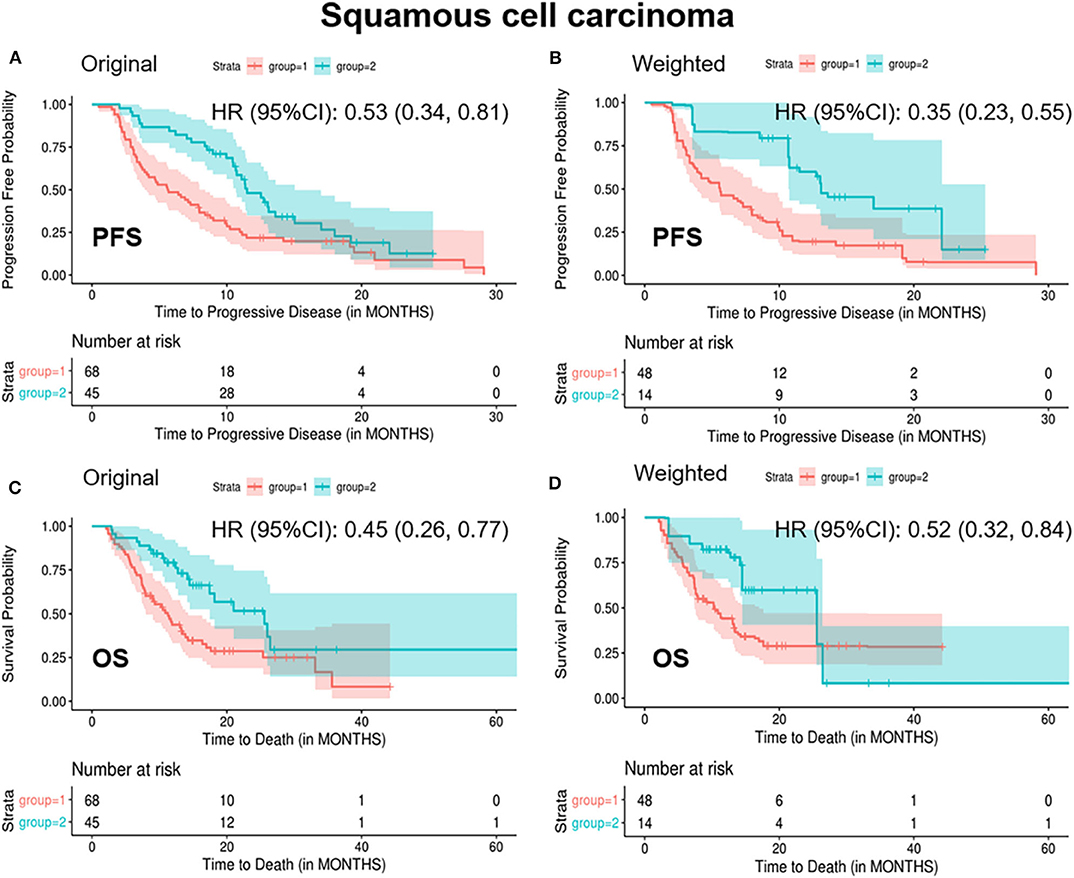
Figure 3. Kaplan-Meier curves of PFS/OS in the original and weighted sample of squamous cell carcinoma. group 1: meaningful improvements in <2 out of 4 biomarkers (CEA, CA125, CYFRA21-1, and SCC-Ag); group 2: meaningful improvements in ≥2 out of 4 biomarkers (CEA, CA125, CYFRA21-1, and SCC-Ag). Kaplan-Meier curves of (A,C) were based on the original sample; Kaplan-Meier curves of (B,D) were based on the weighted sample.
Association Between Dynamics of Tumor Markers and PD-L1 Expression
PD-L1 expression was measured before ICIs treatment in 70 patients, of which 44 (62.8%) were diagnosed with ADC and 26 (37.2%) were SCC. Overall, there were 12 (17.1%) patients with PD-L1 expression negative, 25 (35.7%) patients with PD-L1 expression 1–50%, and 33 (47.1%) patients with PD-L1 expression >50%. However surprisingly, our analysis showed no correlations of PD-L1 expression with dynamics of tumor markers, either in the whole group or any subgroups.
Discussion
Recently, immune checkpoint inhibitors such as PD-1/PD-L1 inhibitors, have been widely used for advanced-stage cancer treatment. Despite of enormous success in treatment of NSCLC (31), not all patients could get long-term benefit from the treatment of ICIs (11). PD-L1 expression and TMB have been widely used as predictive markers, but their roles are still controversial (32). Reliable markers remain to be detected to identify patients who would get benefit from ICIs treatment.
In this study, we evaluated the baseline levels as well as post-treatment changes of routinely measured serum tumor markers in clinical practice to explore their associations with response to ICB therapy in patients with late-stage NSCLC. We demonstrated that dynamic changes of CEA, CA125, CYFRA21-1, and SCC-Ag were associated with the efficacy and prognosis of late-stage NSCLC patients treated with PD-1/PD-L1 inhibitors. Similar results were also observed in the subsequent subgroup analysis on ADC and SCC. Therefore, monitoring the changes in levels of serum tumor markers could be a promising prognostic factor for advanced NSCLC patients with ICIs treatment.
The approach of monitoring dynamic changes of serum tumor markers is more convenient and affordable compared to the most adopted PD-L1 expression or TMB. In contrast to other non-invasive biomarkers like lactate dehydrogenase (LDH) and neutrophil-to-lymphocyte ratio (NLR) (33–36), dynamics of serum tumor markers were also found to be more remarkably associated with response and survival according to our results, and this could also be supported by two recent studies (37, 38). Overall, as far as we know, this is the first and largest cohort study evaluating the relationship of routinely measured serum tumors markers with the efficacy and prognosis of patients receiving ICB therapy.
Optimization-based methods were used in our study. It considered the balance of baseline covariates between two groups compared to inverse propensity score weighting methods, in which only the balance of propensity score was considered in the algorithm. After balancing baseline covariates, possible confounding effects from clinical characteristics could be avoided and the collinearity in baseline covariates could also be controlled. Of noted, this is the first application of this novel statistical method in the clinical observational study.
Although we balanced all measurable baseline variates to avoid bias, there were still some limitations in our study. Firstly, the results may be influenced by the method used for choosing the cut-off point. Twenty percent was selected as a threshold to identify meaningful change in biomarkers according to previous reports, and meaningful improvement in at least two biomarkers was considered as a prognostic factor which was not data-driven. Secondly, only patients receiving more than 6 weeks of ICB treatment were enrolled in this study with baseline and post-treatment serum markers been measured, which may increase selective bias. Thirdly, dynamic change of baseline and after 6 weeks' tumor levels were used for our analysis, whether a shorter interval time is better need further investigation. Fourthly, this observational study was based on the single institution which may cause selection bias. Fifthly, we used the methods of electrochemical luminescence and chemiluminescent microparticle immunoassay for testing tumor markers, some new methods with high sensitivity and specificity may be more helpful for early detection of tumor markers (39, 40). Last but not least, though weighting method were used to balance all measurable baseline covariates, some unrecorded baseline covariates such as TMB could be potential confounders.
Conclusions
In summary, we proposed a new strategy of monitoring dynamics of serum tumor markers and highlight their importance as a potential prognostic biomarker of advanced NSCLC treated with ICIs. Decrease of associated biomarkers serum levels were associated with favorable clinical outcomes. Further investigations will be required to evaluate the roles of these serum markers with different cut-off values as well as earlier dynamic changes from baseline in larger multi-center patient populations.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by People's Liberation Army General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZZ, FY, RC, YL, JM, XY, LW, FZ, HT, DG, ZH, SZ, XL, XZ, XG, YH, and JW contributed to the study design. YH and JW were responsible for interpretation of the results. DG and ZH contributed to statistical analysis. ZZ, FY, and RC were prepared for the manuscript. All authors contributed to data analysis.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YM declared a shared affiliation, with no collaboration, with one of the authors, RC, to the handling editor at the time of review.
Acknowledgments
We thank all the participants who made the study possible.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.01173/full#supplementary-material
Abbreviations
NSCLC, non-small cell lung cancer; ADC, adenocarcinoma; SCC, squamous cell carcinoma; CA125, cancer antigen 125; CEA, carcinoembryonic antigen; CYFRA21-1, cytokeratin 19 fragment; SCC-Ag, squamous-cell carcinoma-related antigen; ICB, immune checkpoint blockade; ICIs, immune checkpoint inhibitors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; PD-1/PD-L1, programmed cell death-1/programmed cell death ligand-1; RECIST, Response Evaluation Criteria in Solid Tumors; TMB, tumor mutation burden.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics (2019). CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Tan WL, Jain A, Takano A, Newell EW, Iyer NG, Lim WT, et al. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol. (2016) 17:e347–e62. doi: 10.1016/S1470-2045(16)30123-1
3. Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. (2018) 52:103–9. doi: 10.1016/j.semcancer.2017.11.019
4. Siegel RL, Miller KD, Jemal A. Cancer statistics (2017). CA Cancer J Clin. (2017) 67:7–30. doi: 10.3322/caac.21387
5. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015. World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. (2015) 10:1240–42. doi: 10.1097/JTO.0000000000000663
6. Ke B, Wei T, Huang Y, Gong Y, Shi L. Interleukin-7 resensitizes non-small-cell lung cancer to cisplatin via inhibition of ABCG2. Mediat Inflamm. (2019) 2019:7241418. doi: 10.1155/2019/7241418
7. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
8. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
9. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
10. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
11. Barbee MS, Ogunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. (2015) 49:907–37. doi: 10.1177/1060028015586218
12. Aguiar PN Jr, Santoro IL, Tadokoro H, de Lima Lopes G, Filardi BA, Oliveira P, et al. A pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarker. Immunotherapy. (2016) 8:1011–9. doi: 10.2217/imt-2016-0032
13. Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. (2019) 5:696–702. doi: 10.1001/jamaoncol.2018.7098
14. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First-Line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (checkmate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. (2019) 37:992–1000. doi: 10.1200/JCO.18.01042
15. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. (2018) 24:1441–8. doi: 10.1038/s41591-018-0134-3
16. Bhaijee F, Anders RA. PD-L1 expression as a predictive biomarker: is absence of proof the same as proof of absence? JAMA Oncol. (2016) 2:54–5. doi: 10.1001/jamaoncol.2015.3782
17. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. (2018) 362:eaar3593. doi: 10.1126/science.aar3593
18. Buttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. (2017) 35:3867–76. doi: 10.1200/JCO.2017.74.7642
19. Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S, Bouhlel L, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. (2016) 27:147–53. doi: 10.1093/annonc/mdv489
20. Zhang ZH, Han YW, Liang H, Wang LM. Prognostic value of serum CYFRA21-1 and CEA for non-small-cell lung cancer. Cancer Med. (2015) 4:1633–8. doi: 10.1002/cam4.493
21. Yang Q, Zhang P, Wu R, Lu K, Zhou H. Identifying the best marker combination in CEA, CA125, CY211, NSE, and SCC for lung cancer screening by combining roc curve and logistic regression analyses: is it feasible? Dis Markers. (2018) 2018:2082840. doi: 10.1155/2018/2082840
22. Masaki T, Takanori A, Eiichi C, Kunihide N. Postoperative serum CEA level is a more significant prognostic factor than post/preoperative serum CEA ratio in non-small cell cancer patients. Asian Pac J Cancer Prev. (2015) 16:7809–12. doi: 10.7314/APJCP.2015.16.17.7809
23. Susana C, Isaac NE, Marina L, Pablo M, Eva C, Davis T, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer. (2011) 12:172–9. doi: 10.1016/j.cllc.2011.03.019
24. Jiang ZF, Wang M, Xu JL. Thymidine kinase 1 combined with CEA, CYFRA21-1 and NSE improved its diagnostic value for lung cancer. Life Sci. (2018) 194:1–6. doi: 10.1016/j.lfs.2017.12.020
25. Yoshimura A, Uchino J, Hasegawa K, Tsuji T, Shiotsu S, Yuba T, et al. Carcinoembryonic antigen and CYFRA 21-1 responses as prognostic factors in advanced non-small cell lung cancer. Transl Lung Cancer Res. (2019) 8:227–34. doi: 10.21037/tlcr.2019.06.08
26. Holdenrieder S, Wehnl B, Hettwer K, Simon K, Uhlig S, Dayyani F. Carcinoembryonic antigen and cytokeratin-19 fragments for assessment of therapy response in non-small cell lung cancer: a systematic review and meta-analysis. Br J Cancer. (2017) 116:1037–45. doi: 10.1038/bjc.2017.45
27. Ma R, Xu H, Wu J, Sharma A, Bai S, Dun B, et al. Identification of serum proteins and multivariate models for diagnosis and therapeutic monitoring of lung cancer. Oncotarget. (2017) 8:18901–13. doi: 10.18632/oncotarget.14782
28. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
29. Zubizarreta JR. Stable weights that balance covariates for estimation with incomplete outcome data. J Am Stat Assoc. (2015) 110:910–22. doi: 10.1080/01621459.2015.1023805
30. Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, et al. The international association for the study of lung cancer lung cancer staging project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. (2016) 11:300–11. doi: 10.1016/j.jtho.2015.10.008
31. Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. (2017) 14:655–68. doi: 10.1038/nrclinonc.2017.88
32. Dal Bello MG, Alama A, Coco S, Vanni I, Grossi F. Understanding the checkpoint blockade in lung cancer immunotherapy. Drug Discov Today. (2017) 22:1266–73. doi: 10.1016/j.drudis.2017.05.016
33. Zhang Z, Li Y, Yan X, Song Q, Wang G, Hu Y, et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Cancer Med. (2019) 8:1467–73. doi: 10.1002/cam4.2024
34. Jiang T, Qiao M, Zhao C, Li X, Gao G, Su C, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother. (2018) 67:713–27. doi: 10.1007/s00262-018-2126-z
35. Jiang T, Bai Y, Zhou F, Li W, Gao G, Su C, et al. Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer. (2019) 130:76–83. doi: 10.1016/j.lungcan.2019.02.009
36. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Oncol Targets Ther. (2018) 11:955–65. doi: 10.2147/OTT.S153290
37. Dal Bello MG, Filiberti RA, Alama A, Orengo AM, Mussap M, Coco S, et al. The role of CEA, CYFRA21-1 and NSE in monitoring tumor response to nivolumab in advanced non-small cell lung cancer (NSCLC) patients. J Transl Med. (2019) 17:74. doi: 10.1186/s12967-019-1828-0
38. Lang D, Horner A, Brehm E, Akbari K, Hergan B, Langer K, et al. Early serum tumor marker dynamics predict progression-free and overall survival in single PD-1/PD-L1 inhibitor treated advanced NSCLC-A retrospective cohort study. Lung Cancer. (2019) 134:59–65. doi: 10.1016/j.lungcan.2019.05.033
39. Wang H, Zhai X, Liu T, Liang J, Bian L, Lin L, et al. Development of a novel immunoassay for the simple and fast quantitation of neutrophil gelatinase-associated lipocalin using europium(III) chelate microparticles and magnetic beads. J Immunol Methods. (2019) 470:15–9. doi: 10.1016/j.jim.2019.04.004
Keywords: non-small cell lung cancer, serum tumor markers, Chinese patients, immune checkpoint inhibitors, prognostic biomarker
Citation: Zhang Z, Yuan F, Chen R, Li Y, Ma J, Yan X, Wang L, Zhang F, Tao H, Guo D, Huang Z, Zhang S, Li X, Zhi X, Ge X, Hu Y and Wang J (2020) Dynamics of Serum Tumor Markers Can Serve as a Prognostic Biomarker for Chinese Advanced Non-small Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors. Front. Immunol. 11:1173. doi: 10.3389/fimmu.2020.01173
Received: 12 March 2020; Accepted: 12 May 2020;
Published: 10 June 2020.
Edited by:
Maysaloun Merhi, Hamad Medical Corporation, QatarReviewed by:
Ying Ma, University of Texas MD Anderson Cancer Center, United StatesPierre Busson, Centre National de la Recherche Scientifique (CNRS), France
Tiancai Liu, Southern Medical University, China
Copyright © 2020 Zhang, Yuan, Chen, Li, Ma, Yan, Wang, Zhang, Tao, Guo, Huang, Zhang, Li, Zhi, Ge, Hu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Hu, aHV5aTA0MDEmI3gwMDA0MDthbGl5dW4uY29t; Jinliang Wang, d2FuZ2ppbmxpYW5nMzAxJiN4MDAwNDA7MTYzLmNvbQ==
†These authors have contributed equally to this work
 Zhibo Zhang
Zhibo Zhang Fang Yuan1†
Fang Yuan1† Runzhe Chen
Runzhe Chen Ye Li
Ye Li Zhiyue Huang
Zhiyue Huang Yi Hu
Yi Hu Jinliang Wang
Jinliang Wang