- Department of Nuclear Medicine, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China
Inflammation is crucial to tumorigenesis and progression of many cancers. Inflammatory molecules in tumor microenvironment exert pro- or anti-tumor effects. Among them, interleukin, mainly produced by CD3+ and CD4+ T lymphocytes, is a class of small molecule proteins which play an important role in intercellular communication. Numerous studies have confirmed that interleukins are closely related to thyroid cancer. Interleukins regulate the proliferation and migration of thyroid cancer cells and they have prospects in discriminating benign and malignant thyroid diseases, predicting the risk of tumorigenesis, evaluating the prognosis and monitoring the recurrence of thyroid cancer. Besides, the effective application of interleukins in treatment of thyroid cancer has been confirmed by some cell and animal researches. The present review will introduce the potential mechanisms of interleukins in thyroid cancer and focus on the applications of interleukins in clinical practice of thyroid cancer, which will help update understanding of the progress of interleukins researches in thyroid cancer.
Introduction
Thyroid cancer is the most common endocrine malignancy with increasing incidence rate over the past decades (1). It happens as a result of hereditary susceptibility and environment factors such as iodine excess, radiation exposure, obesity (2). According to pathology types, thyroid cancer is divided into differentiated thyroid carcinoma (DTC), anaplastic thyroid carcinoma (ATC), and medullary thyroid carcinoma (MTC). DTC, including papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC), accounts for 90% of all thyroid cancers and has a relatively good prognosis. More than 80% of patients with DTC can achieve excellent response to current treatment model, such as surgery, radioiodine (RAI) therapy, and TSH suppressive therapy (3). Although ATC only accounts for 1–2% of thyroid cancer, it is responsible for 14–50% of all thyroid cancer-related deaths due to the lack of effective treatment (4). Molecular targeted therapy is the most promising emerging treatment for ATC and the involved drugs are multiple receptor tyrosine kinase inhibitors (5). MTC, derived from thyroid C cells, accounts for about 5–10% of thyroid cancer and the current treatment of MTC is limited to surgery (6).
Tumor microenvironment is closely related to the occurrence and development of cancer. It consists of immune cells, stroma cells, cytokines, and chemokines, which exert pro- or anti-tumor effects. Interleukins are small protein signaling molecules that belong to the superfamily of cytokines and are mainly produced by T lymphocytes, monocytes, macrophages, and endothelial cells. The main functions of interleukins include the facilitation of communication between cells of the immune system, regulation of transcription factors, and control of inflammation. The role of interleukins in cancer was first described by Vose (7), and in the following decades many studies have confirmed that interleukins, from IL-1 to IL-38, play significant roles in many cancers, such as breast cancer, hepatoma, thyroid cancer etc. (8, 9).
To date, numerous studies have confirmed that interleukins play significant roles in the diagnosis and treatment of thyroid cancer. This review will present the effects of interleukins in thyroid cancer and the clinical applications in the diagnosis and treatment of thyroid cancer in order to help update understanding of the progress of interleukins researches in thyroid cancer.
The Effects of Interleukins in Thyroid Cancer
Growing evidence suggests that imbalance of pro-inflammatory and anti-inflammatory cytokines is correlated to the pathogenesis of thyroid cancer. Inflammatory molecules in tumor microenvironment exert two main effects. One hand, they sustain features of the malignant phenotype of tumors, such as proliferation and invasiveness (10). Moreover, they recruit inflammatory and immune cells, and induce the remodeling of the tumor stroma and stimulate angiogenesis. Thus, inflammatory molecules could further promote tumor progression. In addition, the recruitment of immune cells into tumor sites could result in the immune escape of cancer cells, because cancer cells could induce the secretion of molecules that suppress immune responses and the recruitment of regulatory T cells (11). Interleukins are also crucial components of microenvironment of thyroid cancer and some studies confirmed that interleukins play significant roles in thyroid cancer through some potential mechanisms (Figure 1, Table 1).
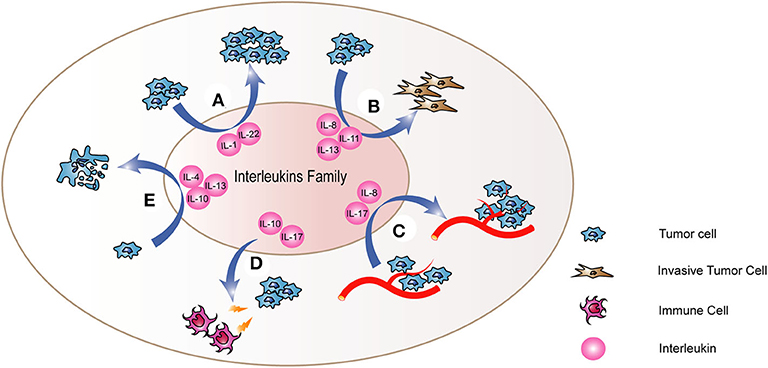
Figure 1. The mechanism of interleukins in thyroid cancer. Interleukins (A) regulate the proliferation of thyroid cancer cells and promote the process of (B) Epithelial-to-Mesenchymal Transition (EMT) and (C) angiogenesis. Besides, they also regulate the abilities of thyroid cancer cells to (D) resist to cell apoptosis and (E) escape the immune system. Through these mechanisms, interleukins cloud play important roles in the tumorigenesis and development of thyroid cancer.
Regulating Tumor Cell Proliferation
Tumor cell proliferation is an important step in tumor development. Several studies have demonstrated that interleukins could regulate the proliferation of thyroid cancer cells.
IL-1 includes two activator cytokines IL-1α and IL-1β, as well as an inhibitory cytokine, the IL-1 receptor antagonist (IL-1ra). IL-1α and IL-1β bind to the same receptor, the type 1 IL-1 receptor (IL-1R), and activate the downstream signaling cascades, ultimately promoting the immune and inflammatory responses (12). The role of IL-1 in cancer has been well-demonstrated (13) and it is well-demonstrated that IL-1 could regulate the proliferation of thyroid cancer through different mechanisms. Due to different thyroid cancer cell lines used in different studies, the results are contradictory. IL-1α could promote the proliferation of PTC cell line NIM1 via stimulation of Ca2+ channels (14). IL-1 could also suppress the proliferation of thyroid cancer cells. IL-1 inhibits the growth of the thyroid cancer cell line NPA, which was in part associated with the suppression of c-myc (15). IL-1β exerts strong antitumor effects on PTC (16, 17) and FTC cell lines (18) through suppressing proliferation and invasiveness. Furthermore, IL-1β did not have an anti-proliferative effect on ATC cell lines, which indicates that PTC cancer cells escaping from antitumor effect of IL-1β may be a step toward anaplasia change, resulting in more aggressiveness of thyroid cancer (17). However, the mechanisms of this process are not clear and further studies are needed.
IL-22, produced by Th17 and Th22 cells, exerts its biological effects through binding to IL-22 receptor and IL-10 receptor. IL-22 triggers a variety of downstream signaling pathways including JAK/STAT3 and MAPK, resulting in cancer progression (19). In thyroid cancer, IL-22 induces miR-595 expression, which in turn downregulates Sox17 expression, thereby enhancing the migration and invasion of thyroid cancer (20).
Promoting Epithelial-to-Mesenchymal Transition (EMT)
EMT is a process in which epithelial cells lose adhesion properties and turn into a mesenchymal phenotype, allowing non-invasive tumor cells to attain the ability of invasion and metastasis (21). It is an essential step in successful migration and metastasis of tumor cells. Some interleukins promote the EMT process of thyroid cancer and then enhance the aggressiveness of thyroid cancer.
IL-8, a pro-inflammatory chemokine, functions through binding to CXCR1 and CXCR2. Considering the characteristic expression of CXCR1 and CXCR2 on cancer cells, endothelial cells, and tumor-associated macrophages, the increased secretion of IL-8 from tumor cells has significance to the tumor microenvironment (22). IL-8 has been repeatedly reported to be a tumor-promoting cytokine in several cancers, but rarely reported in thyroid cancer. Mast cells, which correlate to malignant features and invasiveness of thyroid cancer, are the main source of IL-8 in thyroid cancer. IL-8 is required for mast cells mediated EMT in thyroid cancer through the IL-8-Akt-Slug pathway (23).
IL-11 interactives with its receptor IL-11Rα and activates signaling pathways of targeted cells such as JAK/STAT, MAPK, Src-family kinases, and PI3K pathway (24). The IL-11 gene is a hypoxia-inducible gene whose expression is induced by hypoxia via HIF-1α (25). IL-11 promotes the invasion, migration and EMT of ATC cell via the PI3K/Akt/GSK3β pathway (26). Higher expression of IL-11 in ATC tissues than in PTC could explain the higher metastasis rates of ATC (26). The promotion of EMT induced by IL-11 could take part in this process.
IL13Rα2 is a type II cytokine receptor with high binding affinity to IL-13 (27) and it has an oncogenic role in many cancers (28, 29). In PTC, IL13Rα2-induced cell migration is associated with the upregulation of EMT markers such as N-cadherin, Vimentin and Snail, indicating that IL13Rα2 enhances thyroid cancer aggressiveness through promoting EMT process (30). A recent study found that the number of invading cells in PTC declined significantly after IL13Rα2 knockdown, indicating that IL13Rα2 is involved in the invasion of PTC cells (31). However, the potential mechanism of how IL13Rα2 influence the EMT process of thyroid cancer is not clear.
Promoting Angiogenesis
To support the high proliferation of cancer cells, tumors need to rapidly develop a new vascular network. Angiogenesis, the formation of new blood vessels, is one of crucial steps in tumor progression. NF-κB is a key regulator of angiogenesis in thyroid cancer (32), and IL-8 may be a significant downstream effector of NF-κB signaling pathway in the progression of advanced thyroid cancer (33). IL-17 is involved in the pathogenesis of inflammatory responses and is known to induce the production of IL-1β and TNF-α in the tumor microenvironment (34). The expression of IL-17 is observed in various tumor tissues and considered as the most important pro-angiogenic mediator (35). The expression level of IL-17 is higher in DTC and MTC than that in benign thyroid neoplasms (36), which suggests that IL-17 is closely correlated to the aggressiveness of thyroid cancer, and the tumor pro-angiogenesis of IL-17 could have roles in this process. However, no study has ever reported the pro-angiogenesis of IL-17 in thyroid cancer.
Regulating Tumor Immune Escape
Human immune system is capable of recognizing and resisting cancer cells, however, by altering the host immune system, tumors can escape immune control and continue to progress (37). The tumor microenvironment provides conditions for tumors to escape the immune surveillance, and some interleukins play an important role in this process.
IL-10 is an anti-inflammatory and immunosuppressive cytokine that influence the course of cancer by promoting immune escape through inhibition of the antitumor activity of immune cells (38). IL-10 is expressed in thyroid cancer and influence the aggressiveness of it (39). The immunosuppressive effect of IL-10 may be involved in the immune escape of thyroid cancer cells and promote the aggressiveness of thyroid cancer. However, studies are needed to explain the mechanisms of this process.
Tumor cells achieve immune escape by downregulating the expression of major histocompatibility complex (MHC) class I and loss of MHC class I expression is a frequent mechanism of tumor immune escape in PTC (40). However, after IL-17 treatment, the membrane expression of MHC class I in K1 and PTC-1 increased significantly (41). Programmed cell death ligand 1 (PD-L1), expressed on the surface of tumor cells, binds to its receptor PD-1 on T cells membrane, inducing T cells anergy. The PD-1 expression of T cells reduced in the presence of IL-17 (41). It is suggested that IL-17 inhibit tumor immune escape by upregulating MHC class I expression on tumor cells and suppressing PD-L1/PD-1 pathway.
Some interleukins can inhibit the anti-tumor immune response, allowing tumor cells to escape recognition and attack by the immune system. Therefore, tumor cells can proliferate and metastasize to distant organs. Better understanding of the mechanisms of interleukins in immune escape will provide new targets for immunotherapy of thyroid cancer.
Inhibiting Cancer Cell Apoptosis
Apoptosis, also called programmed cell death, is finely regulated at the gene level to orderly remove damaged cells (42). Its alteration is not only responsible for tumor progression but also for tumor resistance to therapies.
Autocrine production of interleukins in thyroid cancer results in upregulation of anti-apoptotic proteins, which contributes to tumor progression. IL-4 is a pleiotropic cytokine produced by Th2 cells and exerts regulatory effect on the immune response. It can induce Th2 cell proliferation and differentiation, and inhibit apoptosis of B and T cells. A variety of malignancies, such as melanoma and breast cancer, express IL-4 receptor (IL-4Rα), and IL-4 has antiproliferative and/or proapoptotic effects in these cancer cells. On the contrary, IL-4 weakly stimulates the proliferation of thyroid cancer and protects it from apoptosis. The pro-tumor effect of IL-4 is associated with the up-regulation of anti-apoptotic molecule Bcl-2 and the weak down-regulation of the pro-apoptotic molecule Bax (43). Besides, autocrine production of IL-4 and IL-10 induces the over-expression of Bcl-xL and Bcl-2, two anti-apoptotic proteins, which subsequently protect thyroid cancer cells from the cytotoxic effects of antineoplastic drugs (44). Both PTC and FTC cells express CD95 and its ligand CD95L, which mediate cell apoptosis, however, expression of CD95 and CD95L does not affect tumorigenesis and progression in thyroid cancer (45). Therefore, there is a molecular mechanism that restrains the CD95-mediated apoptosis signaling pathway in thyroid cancer and autocrine production of IL-4 and IL-10 may have significant roles in this mechanism, because IL-4 and IL-10 promote resistance to CD95-mediated apoptosis via the activating the Jak/Stat pathway and up-regulating cFLIPL and PED (46). Besides, in PTC cell lines, transfection with siRNA targeting IL13Rα2 induces cell apoptosis by upregulation of caspase 3 and then results in inhibition of cell proliferation, which indicates that IL13Rα2 promotes the invasion and metastasis of tumors through inhibiting apoptosis (31).
Although the mechanisms of some interleukins in thyroid cancer have been described by some studies, how other interleukins confirmed to be correlated to thyroid cancer in clinical researches affect the progression of thyroid cancer is still not clear so far. Therefore, in order to provide more help for treatment of thyroid cancer, further studies focused on potential mechanisms in are still needed.
Clinical Utilities of Interleukins in Thyroid Cancer
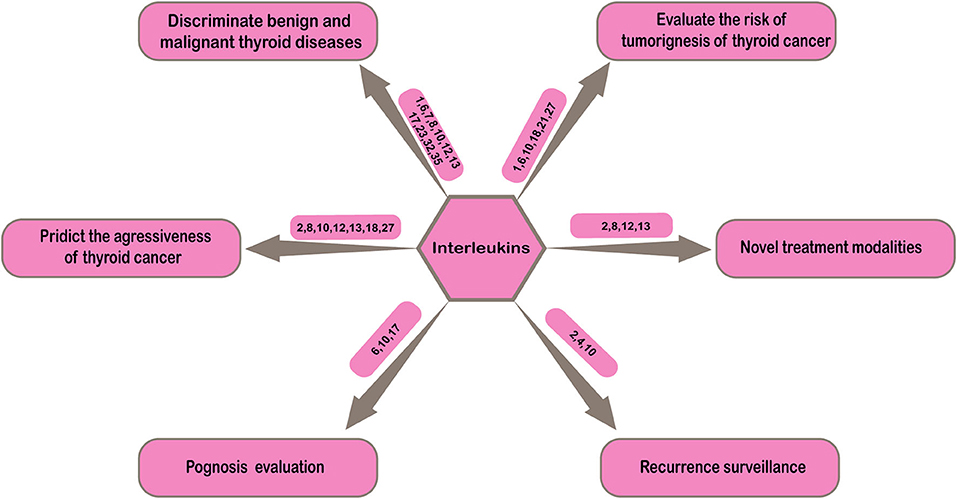
Thyroid cancer is the most common malignant tumor of endocrine system with increasing incidence over recent decades (1). How to improve the diagnosis and treatment quality of thyroid cancer has been paid more attention by clinicians. In clinical practice, the diagnosis, treatment, prognosis evaluation, and disease recurrence monitoring of thyroid cancer are challenging. Either in process of initial diagnosis or postoperative pathological classification, discriminating benign and malignant thyroid nodules is not easy. In order to achieve the purpose of early detection, early diagnosis and early treatment, determination of the high-risk population is significant in clinical practice. Evaluation of aggressiveness of thyroid cancer can provide more information for accurate risk stratification of patients. In addition, most thyroid cancer progress slowly and patients has long survival time after treatments, thus the prognosis evaluation and follow-up after treatment are particularly important. These problems in clinical practice suggest more effective biochemical indicators are needed to make up for the imperfections of current clinical diagnosis and treatment of thyroid cancer in order to improve the level of clinical diagnosis and treatment and make patients attain more benefits. This section will concentrate on the possible applications of interleukins in the clinical practice of thyroid cancer in order to provide more help for clinicians (Figure 2).
Discriminating Benign and Malignant Thyroid Diseases
Serum Interleukin Level
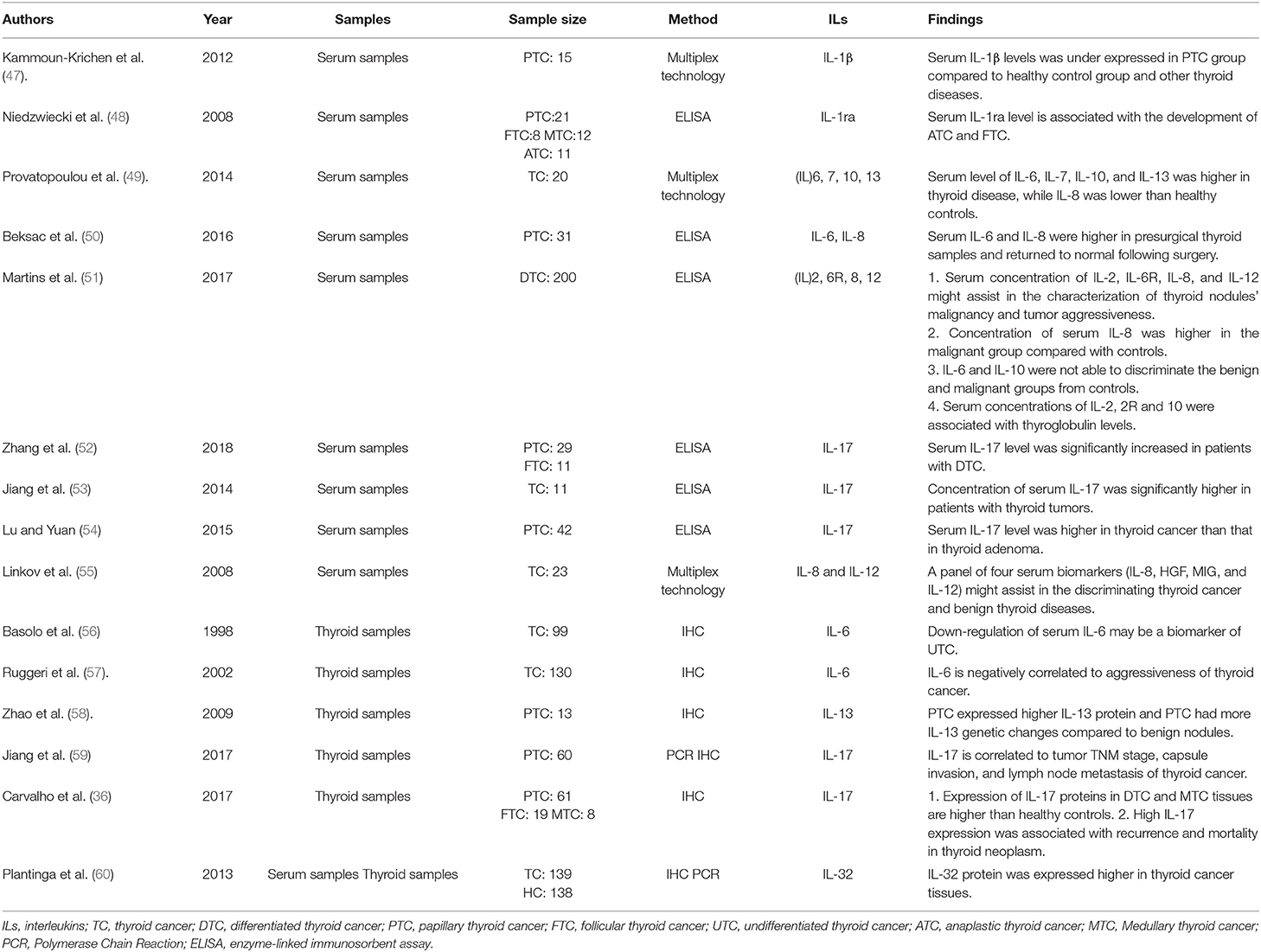
Serum biomarkers are generally used for tumor screening due to their effectiveness and convenience, and specific serum tumor biomarkers are important for tumor diagnosis. With the development of tumor immunity, some studies confirm the possibility of serum cytokine level for tumor diagnosis. It has been reported that the serum level of some interleukins could serve as potential biomarkers in the diagnosis of thyroid cancer, especially in discriminating benign and malignant thyroid diseases (Table 2). However, the results of different studies are contradictory.
IL-1 consists of IL-1α, IL-1β, and IL1 receptor antagonist (IL1ra). Compared with healthy controls, IL-1β was found to be underexpressed in the serum of patients with PTC, and it was considered to be a valuable factor in discriminating atrophic thyroiditis and thyroid cancer (47). IL1ra inhibits the pro-inflammatory effects of IL-1 through competitively binding to the IL-1 receptor (61). Compared with the control group, the serum level of IL1ra is higher in patients with FTC or ATC, but not in patients with PTC or MTC (48), which indicates that serum IL-1ra level could be used as a biomarker for FTC or ATC. However, this effect reaches significance only in women. It could due to the fact that production of IL-1ra in monocytes from female patients is increased (62).
IL-6 is a pleiotropic cytokine which could induce pro- and anti-inflammatory effects under specific conditions. In tumor microenvironment, IL-6 interacts with its receptor and associates with the target cell membrane glycoprotein 130, inducing pro-inflammation cytokines production to support the chronic inflammation. In addition, the IL-6 pathway could transmit positive signals to tumor cells, promoting the proliferation, invasion and anti-apoptosis of cancer. The published researches reported that IL-6 expression is higher in patients with benign and malignant thyroid neoplasms than healthy controls, and it is associated with tumor aggressiveness and poor survival (49). Besides, serum level of IL-6 is high in presurgical serum samples of PTC patients and returns to normal level following surgery (50). However, a recent study reported that IL-6 could not discriminate benign and malignant groups from healthy controls (51). This difference may due to different interleukins measurement methods and sample size.
The findings of serum IL-8 level in thyroid cancer are contradictory in different studies. Some studies found that the serum level of IL-8 is lower in thyroid disease than healthy controls (49), while others found that the level of IL-8 expression in serum samples of PTC patients is higher than healthy controls (50). Besides, a recent study (51) reported that compared with the benign thyroid disease groups, higher concentration of IL-8 in thyroid cancer was observed. The measurement methods and sample size in these studies are different, which indicates that a multicenter large sample study is needed to determine the serum expression level of IL-8 in thyroid cancer.
Serum level of IL-17 increases significantly in patients with thyroid tumors compared with healthy controls (52, 53). And its serum expression level of thyroid cancer is higher than that of thyroid adenoma, suggesting that it could be used as a potential biomarker to distinguish thyroid cancer from adenoma (54).
Serum level of other interleukins could also be used in discriminating benign and malignant thyroid diseases. Serum levels of IL-7, IL-10, and IL-13 are higher in thyroid diseases than healthy controls (49). However, a recent study (51) reported that serum concentrations of IL-10 could not discriminate benign and malignant groups from healthy controls. Besides, Serum concentration of IL-35 in thyroid cancer is lower than that in thyroid adenoma, which indicates IL-35 could be used in discriminating thyroid cancer and adenoma (54).
Interleukins are potential biomarkers in discriminating benign and malignant thyroid diseases, however, the clinical application value of single type of interleukin still have some limitations. In order to solve this problem, some researchers have begun to focus on the combined detection of different types of interleukins or the combined detection of interleukins and other biochemical indicators. It is reported that the combination of IL-13 and IL-8 is highly efficient in identify thyroid diseases (AUC 0.90) (49). Another study evidenced that a panel of four serum biomarkers (IL-8, HGF, MIG, and IL-12) might assist in discriminating thyroid cancer and benign thyroid diseases (AUC 0.81) (55).
Serum interleukin level is correlated to thyroid cancer and could be used to discriminate benign and malignant thyroid diseases. However, due to the different measurement methods and sample size, the results of different studies are not similar and even opposite. Besides, there are still some limitations in the effect of single type of interleukin. At present, few studies focused on the combined detection of different types of interleukins or the combined detection of interleukins and other biochemical indicators, which also limits the application of serum interleukins in clinical practice (49, 55). Therefore, in order to improve the clinical application value of interleukins, it is necessary to use the same detection methods for multi-center, large sample size research. In addition, studies on the combined detection are also needed.
Expression of Interleukins in Thyroid Tissues
Effective biomarkers are needed to improve the accuracy of pathological diagnosis of thyroid cancer. Even though serum interleukins could be used to discriminate benign and malignant thyroid diseases, they might not accurately reflect their actual expression level in thyroid tissues. Thus, studies on expression level of interleukins in thyroid tissues are still needed. Previous studies showed that IL-6 expression was significantly down-regulated in undifferentiated thyroid cancer tissues (56). Besides, PTC tissues had the highest level of IL-6 expression while FTC and ATC issues were consistently negative for IL-6 expression (57). Compared with benign or normal thyroid tissues, higher expressions of IL-13 (58) in PTC tissues, higher expressions of IL-17 (59) and IL-23 (36) in DTC and MTC tissues were observed. Besides, the expression of IL-32 (60) in thyroid cancer issues is also higher in benign thyroid tissues.
The expression of interleukins in thyroid cancer tissues is significantly different compared with benign or normal thyroid tissues. Therefore, interleukins could be potential biomarkers in the pathological diagnosis of thyroid cancer, and a simple immunohistochemical analysis in thyroid tissues could help pathologists discriminate benign and malignant thyroid disease accurately. However, similar to serum interleukins, further studies on the expression of different interleukins and other biomarkers in thyroid cancer are needed to improve their clinical value.
Evaluating the Risk of Tumorigenesis of Thyroid Cancer
Heritable factors are crucial to the occurrence and development of cancers. Several single nucleotide polymorphisms (SNPs) found in cytokine genes affect the expression or function of proteins which have been evaluated for their roles in cancer predisposition (63). Considering that genetically inherited predisposition is the initiating factor to thyroid cancer occurrence, some studies have focused on gene polymorphisms of interleukins and found some favorable results. The interleukins of whose SNPs are associated with the increased risk of PTC include IL-1α (64), IL6 (65, 66), IL10 (67, 68), IL-18 (69), and IL-27 (70). IL-1β (64, 71) and IL-21 (72) gene polymorphisms were related to the decreased risk of tumorigenesis in thyroid cancer. However, another study found that IL-27 gene polymorphism was not a risk factor of tumorigenesis but a risk factor lymph node metastasis in PTC (73). Patient selection bias may account for this difference. Moreover, the study focused on the serum interleukins has shown that high serum IL-10 level was positively associated with an increased risk of thyroid cancer, but it was significant only in women (74).
Multiple genetic studies have demonstrated the association between interleukins and the risk of thyroid cancer. Therefore, interleukin gene testing of high-risk populations, including patients with family history of thyroid cancer or radiation exposure, could help to assess the risk of thyroid cancer more accurately. Due to the differences of heritable factors among different populations, genetic studies are needed in different population to determine the clinical value of interleukins in the risk of thyroid cancer. In addition, interleukins in human body are affected by many other diseases, such as thyroiditis, immune diseases, or other malignancy. However, there are no reports on the tumor risk assessment of patients suffering from thyroid cancer combined with other diseases. Furthermore, it is unclear how these genetic polymorphisms affect the function and production of interleukins.
Predicting the Aggressiveness of Thyroid Cancer
Clinicopathological factors such as tumor size, extrathyroid extension, lymph node metastasis, and distant metastasis are common indicators of the aggressiveness of thyroid cancer. Aggressiveness is a significant factor for tumor risk stratification in clinical practice. Thyroid cancer patients with higher aggressiveness need more active surgical resection and radioiodine treatment. However, it is not always easy to distinguish these patients from others in initial diagnosis. At present, some studies have explored the possibility of interleukins in predicting the aggressiveness of thyroid cancer and have obtained promising results.
High levels of serum IL-2, IL-10, and IL-12 are correlated to the aggressive tumor characteristics in patients with DTC (51). Another study also reported that higher levels of positive expression of IL-10 in thyroid cancer tissues were significantly correlated to extrathyroidal invasion and larger tumor size (39). Recently, high expression of IL-13RA2 was also observed to be correlated with advanced tumor stage in PTC tissues (30).
Lymph node metastasis is the most common type of thyroid cancer metastasis. Most patients are detected with multiple lymph node metastases at the time of initial diagnosis. It is reported that interleukins are significantly associated with lymph node metastasis of thyroid cancer. The expression level of IL-8 is higher in thyroid cancer tissues with lymph node metastases than that without lymph node metastases (75). Gene polymorphism evidenced that IL-8 may contribute to DTC lymph node metastasis (76), and IL-1β may cause PTC lymph node metastasis (71). However, gene polymorphism IL-18 (69) and IL-27 (73) are negatively associated with lymph node metastasis in patients with PTC.
The correlation between interleukins and aggressiveness of thyroid cancer has been confirmed, indicating that interleukins might be used to predict the aggressiveness of thyroid cancer and provide some information for clinicians to make suitable treatment decisions. Distant metastasis is the main cause of disease-specific death in thyroid cancer patients (77). None of the above studies found the correlation between interleukins and distant metastasis of thyroid cancer. Considering the small proportion of patients with distant metastasis in these studies, the results may have some limitations. Therefore, further studies should focus on the patients with distant metastasis, and determine whether interleukins can be used to predict the risk of distant metastasis.
Prognosis Evaluation and Recurrence Surveillance
Considering the utility in predicting recurrence and mortality of thyroid cancer, postoperative risk estimation is recommended to guide radioiodine therapy and follow-up strategies (78). Molecule profile enriches the risk estimate system and is considered a prognostic factor of thyroid cancer. More and more studies reported that interleukins are associated with survival of thyroid cancer patients and are considered as potential predictors of prognosis. Elevated serum IL-6 level is significantly associated with poor overall survival in PTC patients (79). Besides, higher expression of IL-10 in cancer tissues (39) and IL-17 in serum (36) are related to shorter recurrence-free survival of thyroid cancer patients.
Although the prognosis of DTC patients is relatively favorable, the recurrence rate after initial treatment reaches up to 8–23% (80). Therefore, recurrence monitoring during follow-up is of great significance to provide timely and accurate information to patients for intensive treatment. Serum thyroglobulin (Tg) level after thyroidectomy and iodine ablation is the most effective indicator of disease recurrence during follow-up, with high sensitivity and accuracy. Interleukins such as IL-2 and IL-10 were correlated to tumor aggressiveness and to serum Tg level, which suggests the involvement of interleukins would improve the efficiency of Tg evaluation system (51). For DTC patients with autoimmune disease or post-trauma immune system response, the evaluation efficiency of Tg is greatly reduced due to interference of thyroglobulin antibody (TgAb). Thus, effective indicators are needed to monitor tumor recurrence in these patients. In PTC patients with or without Hashimoto's thyroiditis, serum IL-4 and IL-10 levels were reported to be higher in cases with persistent or recurrent disease than those without persistent or recurrent disease, suggesting that they could select patients who need close monitoring and intensive treatment (81).
Taken together, interleukins could be potential biomarkers used in prognosis evaluation and disease surveillance of thyroid cancer. The risk stratification information based on interleukin levels and clinicopathologic features could guide follow-up management decisions of patients with thyroid cancer. However, many other diseases also affect the production and function of interleukins in human body. Therefore, more studies are needed on thyroid cancer patients combined with other diseases to further confirm the potential utility of interleukins in prognosis evaluation and recurrence surveillance of thyroid cancer.
Current Situation of Interleukins in Treatment of Thyroid Cancer
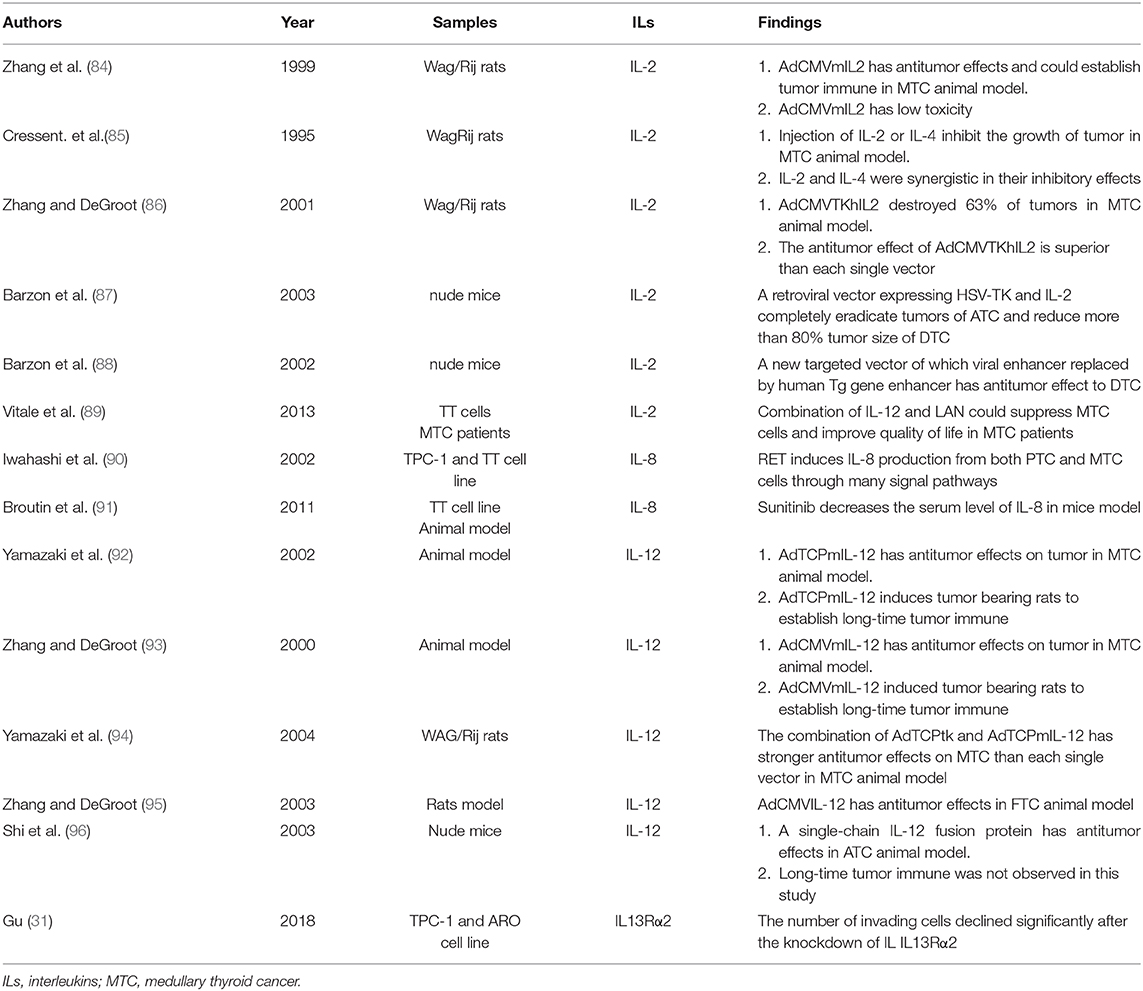
Eighty percentage of DTC patients respond well to the current combined treatment model of surgery, RAI therapy, and TSH suppression. However, ~10% of DTC patients have distant metastasis at the time of diagnosis or develop distant metastasis during follow-up, and these patients usually have poor response to treatments (3). Besides, some DTC patients gradually progress to be refractory to RAI (RR-DTC) during treatment. There is no effective treatment for aggressive pathological types of thyroid cancer, such as ATC. Therefore, new therapy model for these patients are needed. Over recent decades, it has been suggested that some interleukins have good prospects in the treatment of thyroid cancer (Table 3).
IL-2 exerts its antitumor effect by activating cytotoxic T lymphocytes, and natural killer (NK) cells. Genetic immunotherapy of IL-2 has a promising prospect in the application of the treatment of thyroid cancer, especially for ATC and MTC (82, 83). In animal models of MTC, about 42.9% of cases were cured by directly intratumoral injection of Replication-defective adenovirus expressing IL-2 (AdCMVmIL2) and most of the cured rats developed systemic immunity (84). Several studies have evaluated the possibility of IL-2 combined with other treatments to enhance the antitumor effects. The tumor growth can be inhibited and even eliminated after injection of IL-2 or IL-4. Moreover, IL-2 and IL-4 have a synergistic inhibitory effect (85). The combination of suicide gene transfer and immunotherapy provide promising results for gene therapy for thyroid cancer. An adenoviral vector expressing thymidine kinase of herpes simplex virus (HSV-TK) and IL-2 (AdCMVTKhIL2) was constructed, and about 63% of MTC tumors were destroyed after intratumoral injection of itAdCMVTKhIL2 and the antitumor effect of AdCMVTKhIL2 is superior than each single vector (86). Besides, a retroviral vector expressing HSV-TK and IL-2 could completely eradicate ATC tumors and reduce DTC tumor size by more than 80% (87). To further optimize this therapeutic approach, new vector was constructed by replacing the viral enhancer with the enhancer sequence of the human Tg gene. This new vector allows selective transgene expression and cell killing in DTC cells but not in ATC cells (88). In addition, an in vitro and vivo study suggested that combination of IL-12 and lanreotide (LAN), a somatostatin analog, could suppress and kill MTC cells and improve quality of life of MTC patients (89).
RET mutations are responsible for the course of human cancers (90). RET stimulation with its ligand GDNF induced IL-8 production in thyroid cancer (97), In MTC animal model, the decrease of serum IL-8 level is induced by RET inhibitor Sunitinib (91). IL-8 promotes the proliferation, survival, invasion, and angiogenesis of tumor. Therefore, inhibiting activation of RETof thyroid cancer, which results in the reduction of IL-8 secretion, may be an effective strategy for thyroid cancer treatment. And the decreased expression of IL-8 could be used to evaluate the curative effect of RET-inhibited treatment. It is gradually accepted that cancer stem cells (CSC) promote tumor growth, metastasis, recurrence and drug-resistance. CSC is more abundant in ATC sample than DTC (98). In human PTC specimens, a significant correlation between Mast cell (MC) density and stemness features was observed. It is reported that MC-dependent IL-8–Akt–Slug pathway that sustains EMT/stemness of thyroid cancer cells (23). Thus, the IL-8-CXCR1/2 axis might be used as a targeted therapy in advanced thyroid cancer.
Due to its most significant antitumor activity among all cytokines, IL-12 is one of the most promising interleukins in clinical application. Both IL-12 gene therapy and recombinant protein therapy inhibit thyroid cancer growth and prolonged survival (99). However, systemic administration of recombinant IL-12 caused dose-dependent toxicity in animals. In order to alleviate this situation, some studies have investigated the local expression of the IL-12 induced by transduction of IL-12 expressing vectors into the tumor tissue. Injection of AdTCPmIL-12 (92) and AdCMVmIL-12 (93) into MTC tumors has antitumor effect to both primary and distant lesions, and long-term antitumor immune was established. The combination of AdTCPtk and AdTCPmIL-12 has stronger antitumor effect to MTC than each single vector in vivo study (94). In addition, AdCMVIL-12 has the same antitumor effect in FTC animal model (95). Besides, a single-chain IL-12 fusion protein has antitumor effect in ATC animal model, however lone-time tumor immune was not observed in this study (96).
IL13Rα2 could serve as a target of therapeutic intervention of some malignant tumor and current trials mainly involve glioblastoma multiforme (100). In thyroid cancer, IL13Rα2 was observed to promote aggressive behavior of tumor through promoting EMT (30). The number of invading cells declined significantly after the knockdown of IL IL13Rα2, indicating the possibility of IL13Rα2 used as a novel target in the treatment of thyroid cancer (31). However, no study has reported IL13Rα2 could be used in treatment of thyroid cancer.
Interleukins have good prospects in the treatment of thyroid cancer. Studies mentioned above have reported that some interleukins could be used in the treatment of thyroid cancer and evaluation of curative effect. However, researches on the treatment of thyroid cancer by interleukin are limited to cell and animal researches. This may be because the mechanism of interleukins in thyroid cancer is not fully understood. Studies should further explore the mechanisms of interleukins in thyroid cancer in order to find more potential targets for the treatment of thyroid cancer. Besides, the interleukins, which has been proved to be effective in basic researches, should be studied in combination with other drugs.
Conclusion
Basic researches have confirmed that interleukins have significant roles in thyroid cancer through different potential mechanisms. Few interleukins exhibit anti-tumor roles in thyroid cancer, while most show pro-tumor effects. Interleukins have promising prospect in clinical practice of thyroid cancer. They enhance the accuracy of diagnosis of thyroid cancer and provide novel treatments approaches. Besides, interleukins may also be used as biomarkers of surveillance for recurrence and serve as prognostic factors in thyroid cancer. However, there still some limitations on the clinical applications of interleukins in thyroid cancer. Firstly, only a few interleukins in thyroid cancer have been described, and potential mechanisms of other interleukins are not very clear. Secondly, there are some differences in the results of the clinical study on the utility of interleukins in thyroid cancer, which may be due to the different methods and different sample size. In addition, interleukins level in human is affected by many diseases, and to date there is no studies focusing on the accuracy and clinical value of interleukins in discriminating thyroid tumors combined with other diseases. Finally, the use of interleukins in the treatment of thyroid cancer is limited to cell and animal researches. However, what certain is that with future researches, interleukins will provide more help for clinicians and patients.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This review was supported by the National Natural Science Foundation of China, under grant no. 81771865, and by the Shanghai Key Discipline of Medical Imaging, under grant no. 2017ZZ02005.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of this manuscript.
References
1. Harmer C. Foreword for special issue on thyroid cancer 2017. Clin Oncol. (2017) 29:275. doi: 10.1016/j.clon.2017.01.008
2. Nagataki S, Nystrom E. Epidemiology and primary prevention of thyroid cancer. Thyroid. (2002) 12:889–96. doi: 10.1089/105072502761016511
3. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. (1994) 97:418–28. doi: 10.1016/0002-9343(94)90321-2
4. Ito K, Hanamura T, Murayama K, Okada T, Watanabe T, Harada M, et al. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck. (2012) 34:230–7. doi: 10.1002/hed.21721
5. Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, et al. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. (2017) 13:644–60. doi: 10.1038/nrendo.2017.76
6. Vitale G, Caraglia M, Ciccarelli A, Lupoli G, Abbruzzese A, Tagliaferri P, et al. Current approaches and perspectives in the therapy of medullary thyroid carcinoma. Cancer. (2001) 91:1797–808. doi: 10.1002/1097-0142(20010501)91:9<1797::aid-cncr1199>3.0.co;2-p
7. Vose BM. Expansion of autorecognitive cytotoxic effectors in human cancer by T cell growth factor (Interleukin 2)1. Arch Geschwulstforsch. (1981) 51:317–26.
8. Fasoulakis Z, Kolios G, Papamanolis V, Kontomanolis EN. Interleukins associated with breast cancer. Cureus. (2018) 10:e3549. doi: 10.7759/cureus.3549
9. Yang H, Xuefeng Y, Jianhua X. Systematic review of the roles of interleukins in hepatocellular carcinoma. Clin Chim Acta. (2020) 506:33–43. doi: 10.1016/j.cca.2020.03.001
10. Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. (2005) 115:1068–81. doi: 10.1172/JCI22758
11. Ameri P, Ferone D. Diffuse endocrine system, neuroendocrine tumors and immunity: what's new? Neuroendocrinology. (2012) 95:267–76. doi: 10.1159/000334612
12. Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. (2010) 3:cm1. doi: 10.1126/scisignal.3105cm1
13. Baker KJ, Houston A, Brint E. IL-1 family members in cancer; two sides to every story. Front Immunol. (2019) 10:1197. doi: 10.3389/fimmu.2019.01197
14. Inokuchi N, Zeki K, Morimoto I, Nakano Y, Fujihira T, Yamashita U, et al. Stimulatory effect of interleukin-1 alpha on proliferation through a Ca2+/calmodulin-dependent pathway of a human thyroid carcinoma cell line, NIM 1. Jpn J Cancer Res. (1995) 86:670–6. doi: 10.1111/j.1349-7006.1995.tb02451.x
15. Kimura H, Yamashita S, Namba H, Tominaga T, Tsuruta M, Yokoyama N, et al. Interleukin-1 inhibits human thyroid carcinoma cell growth. J Clin Endocrinol Metab. (1992) 75:596–602. doi: 10.1210/jcem.75.2.1322431
16. Yip I, Pang XP, Berg L, Hershman JM. Antitumor actions of interferon-gamma and interleukin-1 beta on human papillary thyroid carcinoma cell lines. J Clin Endocrinol Metab. (1995) 80:1664–9. doi: 10.1210/jcem.80.5.7745015
17. Ohta K, Pang XP, Berg L, Hershman JM. Antitumor actions of cytokines on new human papillary thyroid carcinoma cell lines. J Clin Endocrinol Metab. (1996) 81:2607–12. doi: 10.1210/jcem.81.7.8675585
18. Lin JD, Chao TC, Weng HF, Lin KD. The roles of cytokines and retinoic acid in the regulation of human thyroid cancer cell growth. Cytokine. (1998) 10:536–9. doi: 10.1006/cyto.1997.0323
19. Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. (2001) 276:2725–32. doi: 10.1074/jbc.M007837200
20. Mei Z, Zhou L, Zhu Y, Jie K, Fan D, Chen J, et al. Interleukin-22 promotes papillary thyroid cancer cell migration and invasion through microRNA-595/Sox17 axis. Tumour Biol. (2016) 37:11753–62. doi: 10.1007/s13277-016-5030-1
21. Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. (2008) 14:818–29. doi: 10.1016/j.devcel.2008.05.009
22. Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. (2008) 14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843
23. Visciano C, Liotti F, Prevete N, Cali G, Franco R, Collina F, et al. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene. (2015) 34:5175–86. doi: 10.1038/onc.2014.441
24. Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. (1997) 15:797–819. doi: 10.1146/annurev.immunol.15.1.797
25. Onnis B, Fer N, Rapisarda A, Perez VS, Melillo G. Autocrine production of IL-11 mediates tumorigenicity in hypoxic cancer cells. J Clin Invest. (2013) 123:1615–29. doi: 10.1172/JCI59623
26. Zhong Z, Hu Z, Jiang Y, Sun R, Chen X, Chu H, et al. Interleukin-11 promotes epithelial-mesenchymal transition in anaplastic thyroid carcinoma cells through PI3K/Akt/GSK3beta signaling pathway activation. Oncotarget. (2016) 7:59652–63. doi: 10.18632/oncotarget.10831
27. Arima K, Sato K, Tanaka G, Kanaji S, Izuhara K. Characterization of the interaction between interleukin-13 and interleukin-13 receptors. J Biol Chem. (2005) 280:24915–22. doi: 10.1074/jbc.M502571200
28. Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. (2002) 4:388–99. doi: 10.1038/sj.neo.7900234
29. Fujisawa T, Joshi BH, Puri RK. IL-13 regulates cancer invasion and metastasis through IL-13Ralpha2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int J Cancer. (2012) 131:344–56. doi: 10.1002/ijc.26366
30. Chong ST, Tan KM, Kok CYL, Guan SP, Lai SH, Lim C, et al. IL13RA2 is differentially regulated in papillary thyroid carcinoma versus follicular thyroid carcinoma. J Clin Endocrinol Metab. (2019) 104:5573–84. doi: 10.1210/jc.2019-00040
31. Gu M. IL13Ralpha2 siRNA inhibited cell proliferation, induced cell apoptosis, and suppressed cell invasion in papillary thyroid carcinoma cells. Onco Targets Ther. (2018) 11:1345–52. doi: 10.2147/OTT.S153703
32. Bauerle KT, Schweppe RE, Haugen BR. Inhibition of nuclear factor-kappa B differentially affects thyroid cancer cell growth, apoptosis, and invasion. Mol Cancer. (2010) 9:117. doi: 10.1186/1476-4598-9-117
33. Bauerle KT, Schweppe RE, Lund G, Kotnis G, Deep G, Agarwal R, et al. Nuclear factor kappaB-dependent regulation of angiogenesis, and metastasis in an in vivo model of thyroid cancer is associated with secreted interleukin-8. J Clin Endocrinol Metab. (2014) 99:E1436–44. doi: 10.1210/jc.2013-3636
34. Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. (2008) 28:454–67. doi: 10.1016/j.immuni.2008.03.004
35. Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. (2004) 93:39–43. doi: 10.1016/j.imlet.2004.01.014
36. Carvalho DFG, Zanetti BR, Miranda L, Hassumi-Fukasawa MK, Miranda-Camargo F, Crispim JCO, et al. High IL-17 expression is associated with an unfavorable prognosis in thyroid cancer. Oncol Lett. (2017) 13:1925–31. doi: 10.3892/ol.2017.5638
37. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. (2011) 331:1565–70. doi: 10.1126/science.1203486
38. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. (2001) 19:683–765. doi: 10.1146/annurev.immunol.19.1.683
39. Cunha LL, Morari EC, Nonogaki S, Marcello MA, Soares FA, Vassallo J, et al. Interleukin 10 expression is related to aggressiveness and poor prognosis of patients with thyroid cancer. Cancer Immunol Immunother. (2017) 66:141–8. doi: 10.1007/s00262-016-1924-4
40. Angell TE, Lechner MG, Jang JK, LoPresti JS, Epstein AL. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin Cancer Res. (2014) 20:6034–44. doi: 10.1158/1078-0432.CCR-14-0879
41. Han LT, Hu JQ, Ma B, Wen D, Zhang TT, Lu ZW, et al. IL-17A increases MHC class I expression and promotes T cell activation in papillary thyroid cancer patients with coexistent Hashimoto's thyroiditis. Diagn Pathol. (2019) 14:52. doi: 10.1186/s13000-019-0832-2
42. Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. (2016) 8:603–19. doi: 10.18632/aging.100934
43. Vella V, Mineo R, Frasca F, Mazzon E, Pandini G, Vigneri R, et al. Interleukin-4 stimulates papillary thyroid cancer cell survival: implications in patients with thyroid cancer and concomitant Graves' disease. J Clin Endocrinol Metab. (2004) 89:2880–9. doi: 10.1210/jc.2003-031639
44. Stassi G, Todaro M, Zerilli M, Ricci-Vitiani L, Di Liberto D, Patti M, et al. Thyroid cancer resistance to chemotherapeutic drugs via autocrine production of interleukin-4 and interleukin-10. Cancer Res. (2003) 63:6784–90. doi: 10.1016/S0165-4608(02)00795-1
45. Mitsiades N, Poulaki V, Tseleni-Balafouta S, Koutras DA, Stamenkovic I. Thyroid carcinoma cells are resistant to FAS-mediated apoptosis but sensitive to tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. (2000) 60:4122–9. doi: 10.1097/00002820-200008000-00010
46. Todaro M, Zerilli M, Ricci-Vitiani L, Bini M, Perez Alea M, Maria Florena A, et al. Autocrine production of interleukin-4 and interleukin-10 is required for survival and growth of thyroid cancer cells. Cancer Res. (2006) 66:1491–9. doi: 10.1158/0008-5472.CAN-05-2514
47. Kammoun-Krichen M, Bougacha-Elleuch N, Mnif M, Bougacha F, Charffedine I, Rebuffat S, et al. IL-1beta a potential factor for discriminating between thyroid carcinoma and atrophic thyroiditis. Eur Cytokine Netw. (2012) 23:101–6. doi: 10.1684/ecn.2012.0312
48. Niedzwiecki S, Stepien T, Kuzdak K, Stepien H, Krupinski R, Seehofer D, et al. Serum levels of interleukin-1 receptor antagonist (IL-1ra) in thyroid cancer patients. Langenbecks Arch Surg. (2008) 393:275–80. doi: 10.1007/s00423-007-0251-9
49. Provatopoulou X, Georgiadou D, Sergentanis TN, Kalogera E, Spyridakis J, Gounaris A, et al. Interleukins as markers of inflammation in malignant and benign thyroid disease. Inflamm Res. (2014) 63:667–74. doi: 10.1007/s00011-014-0739-z
50. Beksac K, Sonmez C, Cetin B, Kismali G, Sel T, Tuncer Y, et al. Evaluation of proinflammatory cytokine and neopterin levels in women with papillary thyroid carcinoma. Int J Biol Markers. (2016) 31:e446–50. doi: 10.5301/jbm.5000214
51. Martins MB, Marcello MA, Batista FA, Peres KC, Meneghetti M, Ward MAL, et al. Serum interleukin measurement may help identify thyroid cancer patients with active disease. Clin Biochem. (2017) 52:1–7. doi: 10.1016/j.clinbiochem.2017.10.003
52. Jiang G, Ma S, Wei Y, Wu Y, Yu X, Liu H. The prevalence and distribution of Th17 and Tc17 cells in patients with thyroid tumor. Immunol Lett. (2014) 162(1 Pt A):68–73. doi: 10.1016/j.imlet.2014.07.005
53. Zhang L, Chen J, Xu C, Qi L, Ren Y. Effects of iodine-131 radiotherapy on Th17/Tc17 and Treg/Th17 cells of patients with differentiated thyroid carcinoma. Exp Ther Med. (2018) 15:2661–6. doi: 10.3892/etm.2017.5663
54. Lu Y, Yuan Y. Serum level of interleukin-17 and interleukin-35 as a biomarker for diagnosis of thyroid cancer. J Cancer Res Ther. (2015) 11(Suppl. 2):C209–11. doi: 10.4103/0973-1482.168187
55. Linkov F, Ferris RL, Yurkovetsky Z, Marrangoni A, Velikokhatnaya L, Gooding W, et al. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteomics Clin Appl. (2008) 2:1575–85. doi: 10.1002/prca.200780095
56. Basolo F, Fiore L, Pollina L, Fontanini G, Conaldi PG, Toniolo A. Reduced expression of interleukin 6 in undifferentiated thyroid carcinoma: in vitro and in vivo studies. Clin Cancer Res. (1998) 4:381–7.
57. Ruggeri RM, Villari D, Simone A, Scarfi R, Attard M, Orlandi F, et al. Co-expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) in thyroid nodules is associated with co-expression of CD30 ligand/CD30 receptor. J Endocrinol Invest. (2002) 25:959–66. doi: 10.1007/bf03344068
58. Zhao Z, Wei Q, Zhao Y, Sun F, Jin X, Cui B, et al. Genetic copy number alterations and IL-13 expression differences in papillary thyroid cancers and benign nodules. Endocrine. (2009) 36:155–60. doi: 10.1007/s12020-009-9206-y
59. Jiang XL, Zhang H, Chen YL, Peng L. [Expression of microRNA-221 and IL-17 in papillary thyroid carcinoma and correlation with clinicopathologic features]. Zhonghua Bing Li Xue Za Zhi. (2017) 46:160–5. doi: 10.3760/cma.j.issn.0529-5807.2017.03.004
60. Plantinga TS, Costantini I, Heinhuis B, Huijbers A, Semango G, Kusters B, et al. A promoter polymorphism in human interleukin-32 modulates its expression and influences the risk and the outcome of epithelial cell-derived thyroid carcinoma. Carcinogenesis. (2013) 34:1529–35. doi: 10.1093/carcin/bgt092
61. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. (2013) 39:1003–18. doi: 10.1016/j.immuni.2013.11.010
62. Witkin SS, Gerber S, Ledger WJ. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clin Infect Dis. (2002) 34:204–9. doi: 10.1086/338261
63. Balasubramanian SP, Azmy IA, Higham SE, Wilson AG, Cross SS, Cox A, et al. Interleukin gene polymorphisms and breast cancer: a case control study and systematic literature review. BMC Cancer. (2006) 6:188. doi: 10.1186/1471-2407-6-188
64. Li H, Duan N, Zhang Q, Shao Y. IL1A & IL1B genetic polymorphisms are risk factors for thyroid cancer in a Chinese Han population. Int Immunopharmacol. (2019) 76:105869. doi: 10.1016/j.intimp.2019.105869
65. Ozgen AG, Karadeniz M, Erdogan M, Berdeli A, Saygili F, Yilmaz C. The (-174) G/C polymorphism in the interleukin-6 gene is associated with risk of papillary thyroid carcinoma in Turkish patients. J Endocrinol Invest. (2009) 32:491–4. doi: 10.1007/BF03346494
66. Li H, Dai H, Li H, Li B, Shao Y. Polymorphisms of the highly expressed IL-6 gene in the papillary thyroid cancer susceptibility among Chinese. Curr Mol Med. (2019) 19:443–51. doi: 10.2174/1566524019666190426142432
67. Erdogan M, Karadeniz M, Ozbek M, Ozgen AG, Berdeli A. Interleukin-10 gene polymorphism in patients with papillary thyroid cancer in Turkish population. J Endocrinol Invest. (2008) 31:750–4. doi: 10.1007/bf03349252
68. Cil E, Kumral A, Kanmaz-Ozer M, Vural P, Dogru-Abbasoglu S, Altuntas Y, et al. Interleukin-10-1082 gene polymorphism is associated with papillary thyroid cancer. Mol Biol Rep. (2014) 41:3091–7. doi: 10.1007/s11033-014-3169-7
69. Chung JH, Lee YC, Eun YG, Chung JH, Kim SK, Chon S, et al. Single nucleotide polymorphism of interleukin-18 and interleukin-18 receptor and the risk of papillary thyroid cancer. Exp Clin Endocrinol Diabetes. (2015) 123:598–603. doi: 10.1055/s-0035-1559780
70. Nie X, Yuan F, Chen P, Pu Y, Zhu J, Wang Y, et al. Association between IL-27 gene polymorphisms and risk of papillary thyroid carcinoma. Biomark Med. (2016) 11:141–9. doi: 10.2217/bmm-2016-0283
71. Ban JY, Kim MK, Park SW, Kwon KH. Interleukin-1 beta polymorphisms are associated with lymph node metastasis in Korean patients with papillary thyroid carcinoma. Immunol Invest. (2012) 41:888–905. doi: 10.3109/08820139.2012.724751
72. Xiao M, Hu S, Tang J, Zhang L, Jiang H. Interleukin (IL)-21 promoter polymorphism increases the risk of thyroid cancer in Chinese population. Gene. (2014) 537:15–9. doi: 10.1016/j.gene.2013.12.050
73. Zhang S, Gao X, Wang Y, Jia J, Zhang Q, Ji Z. Interleukin 27−964A > G genetic polymorphism and serum IL-27p28 levels in Chinese patients with papillary thyroid cancer. Tumour Biol. (2015) 36:8207–11. doi: 10.1007/s13277-015-3570-4
74. Dossus L, Franceschi S, Biessy C, Navionis AS, Travis RC, Weiderpass E, et al. Adipokines and inflammation markers and risk of differentiated thyroid carcinoma: The EPIC study. Int J Cancer. (2018) 142:1332–42. doi: 10.1002/ijc.31172
75. Liotti F, Collina F, Pone E, La Sala L, Franco R, Prevete N, et al. Interleukin-8, but not the related chemokine CXCL1, sustains an autocrine circuit necessary for the properties and functions of thyroid cancer. Stem Cells. (2017) 35:135–46. doi: 10.1002/stem.2492
76. Kilic I, Guldiken S, Sipahi T, Palabiyik O, Akker M, Celik O, et al. Investigation of VEGF and IL-8 gene polymorphisms in patients with differentiated thyroid cancer. Clin Lab. (2016) 62:2319–25. doi: 10.7754/Clin.Lab.2016.160403
77. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. (2006) 91:2892–9. doi: 10.1210/jc.2005-2838
78. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
79. Kobawala TP, Trivedi TI, Gajjar KK, Patel DH, Patel GH, Ghosh NR. Significance of interleukin-6 in papillary thyroid carcinoma. J Thyroid Res. (2016) 2016:6178921. doi: 10.1155/2016/6178921
80. Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. (2013) 347:f4706. doi: 10.1136/bmj.f4706
81. Stanciu AE, Serdarevic N, Hurduc AE, Stanciu MM. IL-4, IL-10 and high sensitivity-CRP as potential serum biomarkers of persistent/recurrent disease in papillary thyroid carcinoma with/without Hashimoto's thyroiditis. Scand J Clin Lab Invest. (2015) 75:539–48. doi: 10.3109/00365513.2015.1057895
82. Drosten M, Putzer BM. Gene therapeutic approaches for medullary thyroid carcinoma treatment. J Mol Med. (2003) 81:411–9. doi: 10.1007/s00109-003-0455-6
83. Spitzweg C, Morris JC. Gene therapy for thyroid cancer: current status and future prospects. Thyroid. (2004) 14:424–34. doi: 10.1089/105072504323150732
84. Zhang R, Straus FH, DeGroot LJ. Effective genetic therapy of established medullary thyroid carcinomas with murine interleukin-2: dissemination and cytotoxicity studies in a rat tumor model. Endocrinology. (1999) 140:2152–8. doi: 10.1210/endo.140.5.6719
85. Cressent M, Pidoux E, Cohen R, Modigliani E, Roth C. Interleukin-2 and interleukin-4 display potent antitumour activity on rat medullary thyroid carcinoma cells. Eur J Cancer. (1995) 31A:2379–2384. doi: 10.1016/0959-8049(95)00445-9
86. Zhang R, DeGroot LJ. An adenoviral vector expressing functional heterogeneous proteins herpes simplex viral thymidine kinase and human interleukin-2 has enhanced in vivo antitumor activity against medullary thyroid carcinoma. Endocr Relat Cancer. (2001) 8:315–25. doi: 10.1677/erc.0.0080315
87. Barzon L, Bonaguro R, Castagliuolo I, Chilosi M, Franchin E, Del Vecchio C, et al. Gene therapy of thyroid cancer via retrovirally-driven combined expression of human interleukin-2 and herpes simplex virus thymidine kinase. Eur J Endocrinol. (2003) 148:73–80. doi: 10.1530/eje.0.1480073
88. Barzon L, Bonaguro R, Castagliuolo I, Chilosi M, Gnatta E, Parolin C, et al. Transcriptionally targeted retroviral vector for combined suicide and immunomodulating gene therapy of thyroid cancer. J Clin Endocrinol Metab. (2002) 87:5304–11. doi: 10.1210/jc.2002-020975
89. Vitale G, Lupoli G, Guarrasi R, Colao A, Dicitore A, Gaudenzi G, et al. Interleukin-2 and lanreotide in the treatment of medullary thyroid cancer: in vitro and in vivo studies. J Clin Endocrinol Metab. (2013) 98:E1567–74. doi: 10.1210/jc.2013-1443
90. Jhiang SM. The RET proto-oncogene in human cancers. Oncogene. (2000) 19:5590–7. doi: 10.1038/sj.onc.1203857
91. Broutin S, Ameur N, Lacroix L, Robert T, Petit B, Oumata N, et al. Identification of soluble candidate biomarkers of therapeutic response to sunitinib in medullary thyroid carcinoma in preclinical models. Clin Cancer. Res. (2011) 17:2044–54. doi: 10.1158/1078-0432.CCR-10-2041
92. Yamazaki M, Zhang R, Straus FH, Messina M, Robinson BG, Hashizume K, et al. Effective gene therapy for medullary thyroid carcinoma using recombinant adenovirus inducing tumor-specific expression of interleukin-12. Gene Ther. (2002) 9:64–74. doi: 10.1038/sj.gt.3301617
93. Zhang R, DeGroot LJ. Genetic immunotherapy of established tumours with adenoviral vectors transducing murine interleukin-12 (mIL12) subunits in a rat medullary thyroid carcinoma model. Clin Endocrinol. (2000) 52:687–94. doi: 10.1046/j.1365-2265.2000.01003.x
94. Yamazaki M, Straus FH, Messina M, Robinson BG, Takeda T, Hashizume K, et al. Adenovirus-mediated tumor-specific combined gene therapy using Herpes simplex virus thymidine/ganciclovir system and murine interleukin-12 induces effective antitumor activity against medullary thyroid carcinoma. Cancer Gene Ther. (2004) 11:8–15. doi: 10.1038/sj.cgt.7700636
95. Zhang R, DeGroot LJ. Gene therapy of a rat follicular thyroid carcinoma model with adenoviral vectors transducing murine interleukin-12. Endocrinology. (2003) 144:1393–8. doi: 10.1210/en.2002-221013
96. Shi Y, Parhar RS, Zou M, Baitei E, Kessie G, Farid NR, et al. Gene therapy of anaplastic thyroid carcinoma with a single-chain interleukin-12 fusion protein. Hum Gene Ther. (2003) 14:1741–51. doi: 10.1089/104303403322611755
97. Iwahashi N, Murakami H, Nimura Y, Takahashi M. Activation of RET tyrosine kinase regulates interleukin-8 production by multiple signaling pathways. Biochem Biophys Res Commun. (2002) 294:642–9. doi: 10.1016/S0006-291X(02)00528-4
98. Todaro M, Iovino F, Eterno V, Cammareri P, Gambara G, Espina V, et al. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. (2010) 70:8874–85. doi: 10.1158/0008-5472.CAN-10-1994
99. Parhar RS, Zou M, Al-Mohanna FA, Baitei EY, Assiri AM, Meyer BF, et al. IL-12 immunotherapy of Braf(V600E)-induced papillary thyroid cancer in a mouse model. Lab Invest. (2015) 96:89–97. doi: 10.1038/labinvest.2015.126
Keywords: interleukins, thyroid cancer, inflammation, tumor microenvironment, immunotherapy
Citation: Xi C, Zhang G-Q, Sun Z-K, Song H-J, Shen C-T, Chen X-Y, Sun J-W, Qiu Z-L and Luo Q-Y (2020) Interleukins in Thyroid Cancer: From Basic Researches to Applications in Clinical Practice. Front. Immunol. 11:1124. doi: 10.3389/fimmu.2020.01124
Received: 08 February 2020; Accepted: 07 May 2020;
Published: 12 June 2020.
Edited by:
Avery Dexter Posey Jr., University of Pennsylvania, United StatesReviewed by:
Evelien Smits, University of Antwerp, BelgiumConnie Jackaman, Curtin University, Australia
Copyright © 2020 Xi, Zhang, Sun, Song, Shen, Chen, Sun, Qiu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong-Ling Qiu, cWl1emhvbmdsaW5nMTIzQDE2My5jb20=; Quan-Yong Luo, bHF5bkBzaDE2My5uZXQ=
†These authors have contributed equally to this work
 Chuang Xi†
Chuang Xi† Quan-Yong Luo
Quan-Yong Luo