
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 21 May 2020
Sec. Immunological Tolerance and Regulation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.01000
 Daniel C. Julien1*
Daniel C. Julien1* Kara Woolgar2
Kara Woolgar2 Laura Pollard3
Laura Pollard3 Holly Miller1
Holly Miller1 Ankit Desai4
Ankit Desai4 Kristin Lindstrom2
Kristin Lindstrom2 Priya S. Kishnani4*
Priya S. Kishnani4*A 3.5 year old Hispanic female presented with signs and symptoms concerning for MPS II (Hunter Syndrome). The diagnosis of MPS II was confirmed by enzyme and molecular testing. Genetic evaluation revealed undetectable plasma iduronate-2-sulfatase enzyme activity and an inversion between intron 7 of the IDS gene and a region near exon 3 of IDS-2. This inversion is the molecular cause for ~8% of cases of MPS II and often results in a severe phenotype. X-inactivation studies revealed an inactivation ratio of 100:0. Given the patient's undetectable enzyme level, in combination with a severe IDS gene mutation, classic features at time of presentation, and the significantly skewed X inactivation, there was concern that she was at high risk of developing high and sustained antibody titers to idursulfase which would limit her benefit from enzyme replacement therapy (ERT). Anti-drug neutralizing antibodies to idursulfase have been associated with reduced systemic exposure to idursulfase and poorer clinical outcomes. Therefore, the decision was made to concurrently treat the patient with immune tolerance induction therapy during the first month of treatment with idursulfase in order to decrease the risk of developing high sustained antibody titers. The immune tolerance induction protocol consisted of rituximab weekly for 4 weeks, methotrexate three times a week for 3 weeks and monthly IVIG through B-cell and immunoglobulin recovery. Immune tolerance induction was initiated concurrently with the start of ERT. The patient had no significant adverse effects related to undergoing immune tolerance induction therapy and two and half years later is doing well with significantly reduced urine glycosaminoglycans and very low anti-drug antibody titers. This immune tolerance induction protocol could be considered for other patients with MPS II as well as patients with other lysosomal storage disorders who are starting on enzyme replacement therapy and are at high risk of developing neutralizing anti-drug antibodies.
Mucopolysaccharidosis type II (MPS II; OMIM # 309900), also known as Hunter syndrome, is a rare, X-linked lysosomal storage disease caused by a deficiency in the enzyme iduronate-2-sulfatase, encoded by the IDS gene (1–3). Deficiency of iduronate-2-sulfatase leads to accumulation of glycosaminoglycans dermatan and heparan sulfate in lysosomes, resulting in cellular and tissue damage, enlargement of the affected organs and ultimately disruption of normal cellular physiology and organ dysfunction (2). As MPS II is an X-linked disorder, it predominately affects males with an estimated occurrence of 1 in 130,000–170,000 live male births in those of Caucasian descent (4). Females may also rarely be affected due to certain X-chromosome deletions, chromosomal translocations, or unequal X-chromosome inactivation (5, 6). The clinical manifestations and phenotype of MPS II vary widely. Factors such as the age of presentation, severity of the disease, and rate of disease progression are correlated with the severity of a specific mutation. Indeed, over 450 unique IDS mutations have been documented in the literature (7). One of the most significant distinctions in the severity of MPS II is the presence or absence of cognitive impairment (8). Patients with the more severe form of the disease have a cognitive decline in the first few years of life, and often do not survive past 15 years of age, while those with the attenuated form of MPS II may have normal intelligence and live well into adulthood (8). Typically patients with absent or very little enzyme activity such as those with large gene deletions, gene rearrangements as well as nonsense, frameshift, and splice-site variants have a more severe phenotype compared to those with less significant mutations such as point mutations or deletions (9). The common clinical features of MPS II also include: joint stiffness and joint contractures leading to decreased range of motion, coarsening of the facies, macrocephaly, hepatomegaly, cardiomegaly with heart valve dysfunction, decreased growth velocity, reduced endurance, and decreased pulmonary function (10).
Historically, the management of MPS II had focused on relieving the symptoms of the disorder through surgical interventions and other supportive care measures (11). In the 1980's hematopoietic stem cell transplantation (HSCT), which had been successfully used in the treatment of mucopolysacharodosis type I, was first utilized for the treatment of MPS II, though with varying success (12–16). One limitation of HSCT in patients with MPS II is transplant-related morbidity or mortality. A review of SER data from patients that had undergone HSCT for MPS II between 1982 and 2007 revealed a 78% overall survival and 62% event free survival, but this data does not take into account the patients' age, phenotype, donor status or transplant protocols (17–19). However, newer data such as that from Japan, where HSCT for MPS II is routinely offered, shows a 5 year survival rate of 88.5% (20). Also, 11 of the 17 patients with MPS II that received HSCT in this study had stabilization of brain atrophy and were less likely to have speech deterioration compared to those who were untreated (20). However, HSCT prep regimens require the use of strong chemotherapeutics as well as radiation in some protocols. Long term complications from HSCT include graft vs. host disease, increased risk of malignancy, cataracts, as well as decreased fertility, to name a few.
In 2006 the treatment of MPS II was revolutionized with the Food and Drug Administration's approval of the intravenous (IV) infusion of idursulfase (Elaprase®, Shire HGT, Lexington, MA) for enzyme replacement therapy (ERT) in confirmed cases of MPS II. Though exogenous ERT is not able to efficiently cross the blood–brain barrier and therefore is not able to prevent the cognitive decline associated with the disease, clinical trials of intrathecal administration of idursulfase are underway (21, 22). ERT has been shown to be beneficial for various other aspects of the disease. For example patients receiving ERT have an improvement in their endurance on the 6-min walk test, reduction in liver and spleen size, improved pulmonary functional status, and reduction in urinary GAGs (23, 24). With the proven success of ERT, it has become the standard therapy for MPS II.
Though ERT has changed the landscape of management of MPS II, there are some significant challenges that can limit its clinical efficacy. ERT requires chronic (usually 0.5 mg/kg weekly) infusions of idursulfase, which may lead to the development of anti-drug antibodies to the exogenous enzyme. Although the presence of anti-idursulfase antibodies does not always translate into a confirmed decrease in the efficacy of ERT, 50% of treated patients go on to develop IgG antibodies within the first year of treatment (23–25). Of patients developing antibodies, 21% to 35% also have or go on to develop neutralizing IgG anti-drug antibodies to idursulfase (26, 27). Neutralizing anti-drug antibodies have been associated with reduced systemic exposure to idursulfase and subsequently less of a reduction of urinary GAGs, decreased improvements in pulmonary function, and diminished reduction in liver volume (7, 25, 27–29). With limited alternative therapeutic options, the development of strategies to eradicate or prevent the formation of neutralizing anti-drug antibodies is of vital importance. Though there is limited data in patients with MPS II, immune tolerance induction protocols using a combination of cytotoxic and immune suppressive agents have been successfully utilized in other types of lysosomal storage disorders (LSDs), particularly Pompe disease (30, 31). In this case study, we describe to our knowledge the first female patient with MPS II with zero IDS gene activity due to skewed X-inactivation to safely undergo concurrent immune tolerance induction therapy at the time of initiation of ERT with idursulfase.
A 3.5 year old ex 36 week female born via C-section after premature rupture of membranes presented to our hospital's Department of Genetics and Metabolism for evaluation of developmental delay. She was born to a 32 year old G3P3 mother and 32 year old father. Parents are non-consanguineous and both of Mexican descent. The patient was born via repeat cesarean section and spent ~2 weeks in the NICU for respiratory distress. Her past medical history was significant for global delay of milestones, snoring, and at time of presentation had recently been diagnosed with autism and attachment disorder. She had not had any prior surgical procedures. At the time of presentation, the patient was 3.5 years old and noted to have global developmental delay, coarse facies, macrocephaly, macroglossia, symmetric joint contractures, and hepatomegaly. Her head circumference was 53.8 cm (>99%), height was 108.8 cm (>99%), and weight was 26.9 kg (>99%). Brain MRI revealed delayed myelination, mildly low parenchymal volume, and mild brachycephaly. She was also noted to have palpable hepatosplenomegaly on exam. A comprehensive 4-generation family medical history revealed no other similarly affected individuals. Fragile X testing had been done and was normal. Based on the patient's features, exam, and imaging it was suspected that she may have a mucopolysaccharodosis disorder. Work up revealed elevated urine total glycosaminoglycans at 72.56 mg/mmol creatinine (4.5x ULN) with heparan sulfate 70.3 g/mol creatinine (12x ULN) and dermatan sulfate 58.07 g/mol creatinine (7x ULN). Functional enzyme testing results showed undetectable plasma iduronate-2-sulfatase enzyme activity and gene sequencing of the iduronate-2-sulfatase gene found an inversion between intron 7 and a region near exon 3, consistent with Hunter syndrome (MPS II). Of note, this specific mutation is responsible for ~8% of cases of MPS II. Interestingly, she was found to have a normal karyotype and chromosome microarray but was found to have X-chromosome inactivation at a ratio of 100:0. Echocardiogram revealed structurally normal cardiac anatomy as well as normal right and left ventricular size, but mildly thickened mitral and aortic valves. Sleep study revealed delayed sleep latency but no evidence of obstructive sleep apnea.
Due to the patient's IDS gene mutation, significantly skewed X-inactivation, as well as the severity of her phenotype with developmental delay, she was felt to likely have the severe/classic form of MPS II. Also, due to her age at the time of diagnosis, it was felt that she was not a good candidate for HSCT. Furthermore, there was a concern that she was at high risk of developing high and sustained anti-drug antibody titers upon initiation of idursulfase ERT, which would in turn limit the action or response to ERT. If the patient did go on to develop neutralizing anti-drug antibodies, there are limited alternative therapeutic options including eligibility for clinical trials, which would likely be further limited due to the patient being female.
With these factors in mind, the decision was made to start weekly idursulfase (0.5 mg/kg) with concurrent immune tolerance induction during the first month of ERT therapy with the goal of reducing the likelihood that the patient would go on to develop high sustained neutralizing antibody titers. The immune tolerance induction protocol utilized was first described by Kishnani et al. in cross-reactive immunologic material (CRIM)-negative infantile Pompe disease patients and as outlined in (Table 1) consisted of rituximab 375 mg/m2/dose IV weekly for 4 weeks (4 total doses), methotrexate 0.4 mg/kg orally three times weekly for 3 weeks (9 total doses), and IVIG 500 mg/kg IV weekly for 4 doses and then monthly through B-cell recovery (32, 33). The patient did not experience any infusion-related reactions with IV rituximab. Labs throughout treatment were within normal limits including her absolute neutrophil count, which ranged between 1,720 and 2,900 (NL > 1,500). Her immunoglobin levels, as well as a lymphocyte subset panel, were monitored monthly to determine B-cell recovery. While receiving methotrexate she had a very minimal elevation of liver function enzymes. Her maximum AST was 49 (1.3x ULN) and her maximum ALT was 115 (1.8x ULN). Total bilirubin was normal at all time points. The patient experienced no instances of illness necessitating emergency room evaluation or hospitalization while undergoing immune tolerance induction.
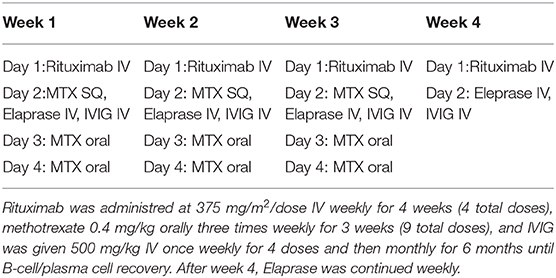
Table 1. Immune tolerance induction treatment protocol. ITI was administered shortly prior to Eleprase infusion.
Nearly 2.5 years after completion of the immune tolerance induction protocol she has been clinically stable. She has continued on weekly ERT and has had some notable improvement in her clinical status, including improved range of motion in her joints, a decrease in snoring and noisy breathing, and no evidence of worsening cardiomegaly, though has persistent valvular disease. MRI at time of initial presentation showed delayed myelination for age and global mildly low parenchymal volume, but no evidence of hydrocephalus and she has not had any seizures. In terms of cognitive ability, the patient remains substantially delayed (she is not toilet trained and is able to only count to the number 2), but she has made some improvements in language skills and is now able to form simple sentences, while before she communicated with single words or pointing. At this time she has an IgG titer of 1:160, these are very low titers, and which have no negative impact on clinical status.
At ~3–6 month intervals she has undergone monitoring of urine glycosaminoglycans, serum IgE, serum IgG, and anti-drug antibodies. Urine total glycosaminoglycans, dermatan sulfate, and heparan sulfate have significantly improved while receiving ERT as illustrated in (Figures 1–3), respectively. Serum IgE levels have been within normal limits at each time point monitored and clinically she has not had any signs or symptoms of allergic reaction or anaphylaxis during subsequent idursulfase infusions. Serum IgG levels have been within normal limits at each time point. The patient's anti-drug antibodies had been negative, however, her two most recent anti-drug antibody titers conducted by Convance Central Lab Services showed mild seroconversion (1:40 and 1:160, respectively) and have not had any negative impact on clinical status. This is in stark contrast to the high antibody titers in patients with MPS II, which typically range from 1:1000 to 1:100,000.
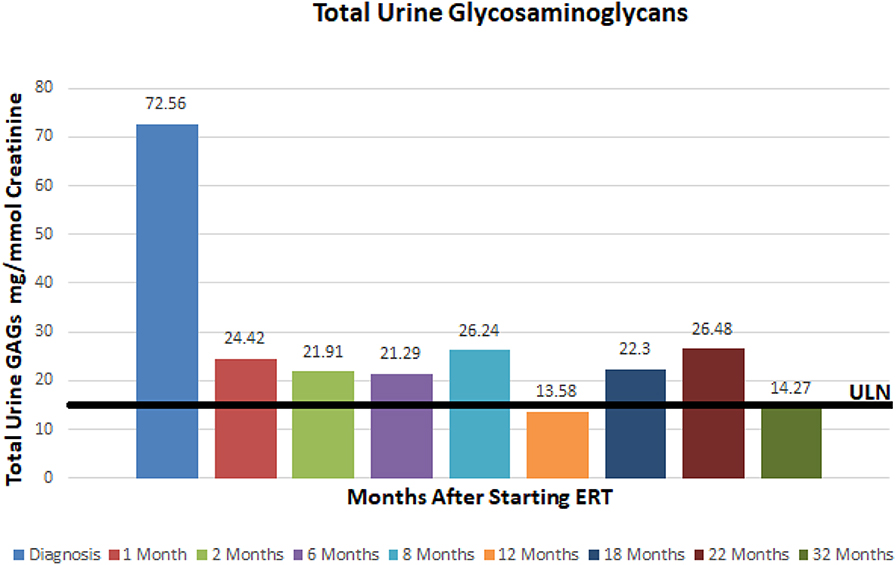
Figure 1. Total urine glycosaminoglycans at baseline and after starting enzyme replacement therapy. Normal range 0–16 mg/mmol creatinine.
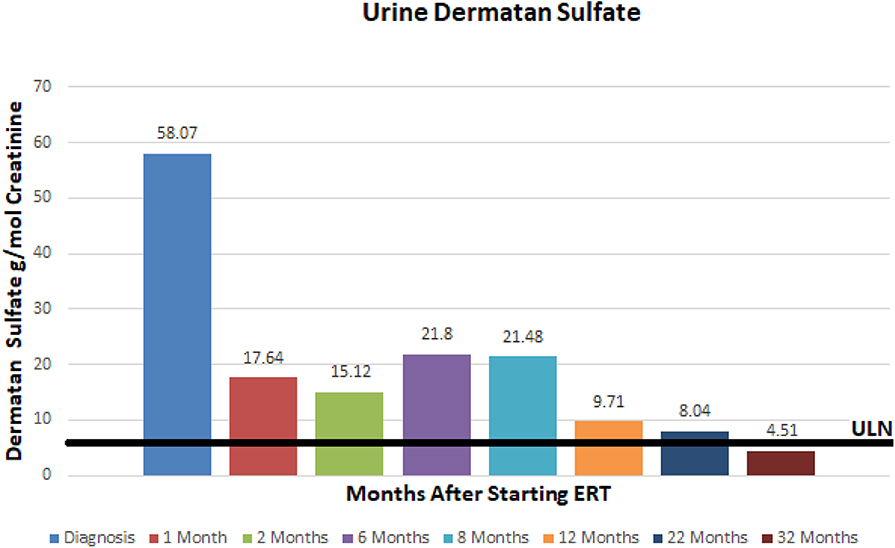
Figure 2. Urine dermatan sulfate at baseline and after initiation of enzyme replacement therapy. Normal range 0–7.93 g/mmol creatinine.
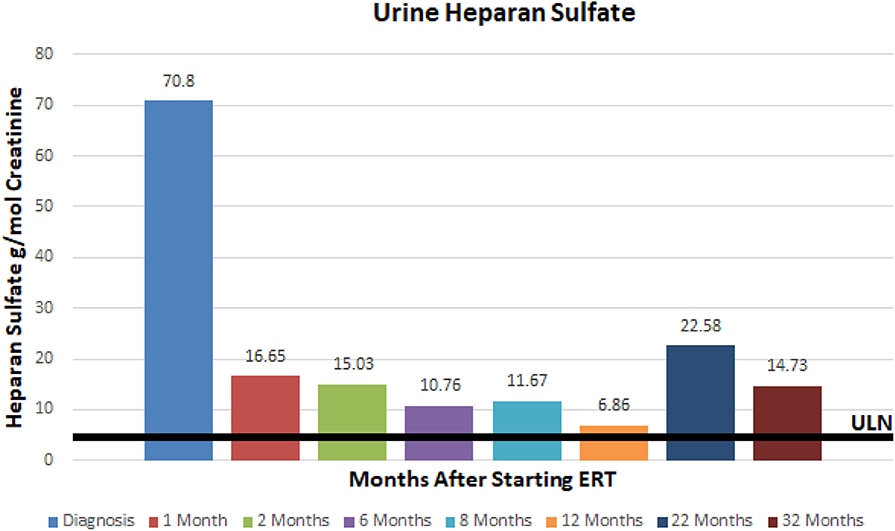
Figure 3. Urine heparan sulfate at baseline and after initiation of enzyme replacement therapy. Normal range 0–5.71 g/mmol creatinine.
MPS II is a rare, X-linked metabolic disorder caused by a deficiency of the lysosomal enzyme iduronate-2-sulfatase. Though it predominately affects males, there are rare cases in females such as the patient described in this case. The mainstay of treatment is ERT with idursulfase, which has been shown to improve lung function, liver and spleen size, increase 6 min walk time distance, and improve shoulder range of motion, ultimately resulting in an improved quality of life. However, the clinical trial for approval of idursulfase as well as several other studies have shown that patients with large gene deletions, gene rearrangements (such as our patient), and nonsense, frameshift, and splice site variants are at an increased risk of developing neutralizing anti-idursulfase antibodies compared to those with smaller gene alterations like missense mutations (21, 27, 34). Neutralizing anti-drug antibodies are associated with poor clinical outcomes such as decreased pulmonary function due to antibody-mediated inhibition of idursulfase therapeutic activity as well-decreased pulmonary expansion from hepatosplenomegaly and elevated urine glycosaminoglycans (27, 29, 34).
In this case presentation, we report on our experience with a short course of immune tolerance induction therapy in a female patient with severe MPS II as an approach to prevent the development of high sustained neutralizing antibodies. Due to the patient's IDS gene mutation, significantly skewed X-inactivation, as well as the severity of her phenotype with developmental delay, she was felt to likely have the severe/classic form of MPS II, as is illustrated in the photos provided by the patient's legal guardian in (Figure 4). Typically patients with absent or very little enzyme activity such as those with large gene deletions, gene rearrangements as well as nonsense, frameshift, and splice-site variants have a more severe phenotype compared to those with less significant mutations such as point mutations or deletions (9). Furthermore, those with the severe type of MPS II are more likely to develop anti-drug antibodies due to the lack of exposure to native enzyme. This placed her at high risk of developing high and sustained anti-drug antibody titers upon initiation of idursulfase ERT, which would in turn limit the action or response to ERT. If the patient did go on to develop neutralizing anti-drug antibodies, there are limited alternative therapeutic options including eligibility for clinical trials, which may have been limited due to patient's gender. HSCT was ruled out due to her age at the time of diagnosis and severity of symptoms.
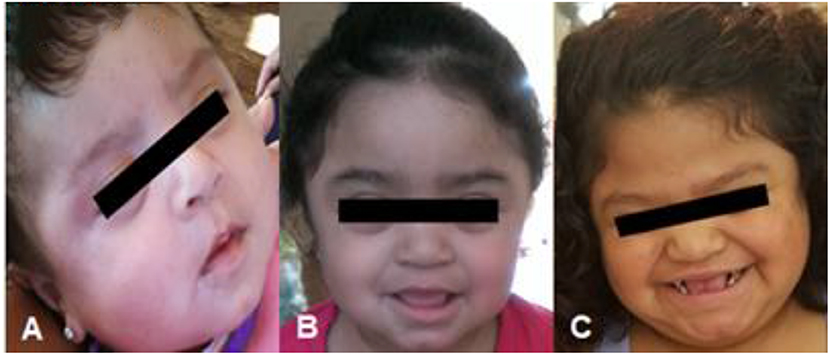
Figure 4. Patient as an (A) infant, (B) toddler, and (C) at time of presentation. Consent for use of patient photos was provided by the patient's parent/legal guardian.
Previous studies in patients with infantile Pompe disease who receive immune tolerance regimens at the initiation of ERT are less likely to develop neutralizing anti-drug antibodies (31). Unfortunately, once neutralizing anti-drug antibodies have developed, it is often extremely challenging to completely eliminate them so the focus has been on prevention of the development of antibodies. Utilizing a similar combination of immune suppressant medications that has been safe and used successfully for prevention of development of antibody titers in CRIM-negative infantile Pompe patients, the immune tolerance induction protocol used for this patient consisted of weekly infusion of the anti-CD20 monoclonal antibody rituximab, methotrexate, and IVIG. Some clinicians have used a different anti-CD20 monoclonal antibody called ofatumumab (29), which is a completely humanized antibody, in patients with MPS II that have already developed neutralizing anti-drug antibodies. Bortezomib, a proteasome inhibitor, has also been used due to its ability to eliminate long-lasting plasma cells. Prior studies of bortezomib have been conducted in patients with infantile Pompe disease patients who had high sustained antibody titers and a decline in clinical response to ERT. Bortezomib was felt to be safe and well-tolerated in these patients (35, 36). Additionally, corticosteroids such as dexamethasone have been used as part of the backbone of some immune tolerance induction protocols. The effects of corticosteroids on the immune system are many, but in general they are immune suppressive and have a synergistic effect in combination with medications like bortezomib in the rapid reduction of plasma cell populations (35).
Burton et al. have previously reported the effect of an immune tolerance induction protocol consisting of ofatumumab, methotrexate, intermittent corticosteroids, and IVIG in a patient with MPS II who already had high levels of neutralizing anti-drug antibodies to idursulfase (29). Though the patient did have a significant decline in neutralizing anti-drug antibodies over a 1.5 year period, full eradication was not achieved and urinary GAGs were only modestly reduced. As the patient continued to have decreased, but persistent neutralizing anti-drug antibodies, he continued on weekly IV methotrexate, IVIG, and IV rituximab every 3 months for a total of 1 year. Fortunately, the patient in this study had not been previously treated with ERT and she was able to undergo immune tolerance induction therapy concurrently with the induction of ERT. She experienced no obvious side effects from immune tolerance induction therapy including no illnesses or hospitalizations while receiving immune tolerance induction therapy. She was however noted to have a transient small rise in liver function enzymes, which may be related to the low doses of methotrexate she received, though could be from a variety of other causes such as a viral illness at the time of her lab draws as well.
This patient, as well as other patients with LSDs that have undergone immune tolerance induction therapy in the naïve setting, demonstrate the efficacy of immune tolerance induction strategies in preventing neutralizing anti-drug antibodies. The scientific community has made strides in recognizing the genetic alterations that put patients with LSDs and other metabolic disorders that are managed with ERT at risk of developing neutralizing anti-drug antibodies. However, several challenges and questions remain. For example, the testing for anti-drug antibodies is sometimes difficult to obtain and may have a slow turnaround time. The ideal timeframe of when to carryout immune tolerance induction therapy has not been identified, though it serves to consider that at initiation ERT could have the greatest chance of preventing the formation of neutralizing anti-drug antibodies, as this is when the patient's immune system is initially challenged with medication and before the development of memory B cells and long-lived plasma cells. Several combinations of immunosuppressive agents have been utilized in immune tolerance induction protocols, but it is unclear if one specific combination is superior. Also, though we understand that large genetic alterations lead to the development of neutralizing anti-drug antibodies, the immunologic factors that contribute to antibody development are complex and the precise interplay of factors remains unclear. As we gain further knowledge and experience in the use of immune tolerance induction therapy in LSDs these questions will be answered and will hopefully ultimately result in improved patient outcomes.
All datasets generated for this study are included in the article/supplementary material.
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
DJ was the primary author and wrote the manuscript and prepared tables and figures. KW, LP, HM, AD, and KL were secondary authors. PK was the final/supervising author.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Scarpa M. Mucopolysaccharidosis Type II. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, and Amemiya A, editors. GeneReviews is a Registered Trademark of the University of Washington, Seattle. All Rights Reserved. Seattle, WA: University of Washington (1993).
2. Coutinho MF, Lacerda L, Alves S. Glycosaminoglycan storage disorders: a review. Biochem Res Int. (2012) 2012:471325. doi: 10.1155/2012/471325
3. Glamuzina E, Fettes E, Bainbridge K, Crook V, Finnegan N, Abulhoul L, et al. Treatment of mucopolysaccharidosis type II (Hunter syndrome) with idursulfase: the relevance of clinical trial end points. J Inherit Metab Dis. (2011) 34:749–54. doi: 10.1007/s10545-011-9280-1
4. Kim S, Whitley CB, Jarnes Utz JR. Correlation between urinary GAG and anti-idursulfase ERT neutralizing antibodies during treatment with NICIT immune tolerance regimen: A case report. Mol Genet Metab. (2017) 122:92–9. doi: 10.1016/j.ymgme.2017.06.001
5. Lonardo F, Di Natale P, Lualdi S, Acquaviva F, Cuoco C, Scarano F, et al. Mucopolysaccharidosis type II in a female patient with a reciprocal X;9 translocation and skewed X chromosome inactivation. Am J Med Genet A. (2014) 164A:2627–32. doi: 10.1002/ajmg.a.36667
6. Pinto LL, Vieira TA, Giugliani R, Schwartz IV. Expression of the disease on female carriers of X-linked lysosomal disorders: a brief review. Orphanet J Rare Dis. (2010) 5:14. doi: 10.1186/1750-1172-5-14
7. Bradley LA, Haddow HRM, Palomaki GE. Treatment of mucopolysaccharidosis type II (Hunter syndrome): results from a systematic evidence review. Genet Med. (2017) 19:1187–201. doi: 10.1038/gim.2017.30
8. Guffon N, Heron B, Chabrol B, Feillet F, Montauban V, Valayannopoulos V. Diagnosis, quality of life, and treatment of patients with Hunter syndrome in the French healthcare system: a retrospective observational study. Orphanet J Rare Dis. (2015) 10:43. doi: 10.1186/s13023-015-0259-0
9. Vollebregt AAM, Hoogeveen-Westerveld M, Kroos MA, Oussoren E, Plug I, Ruijter GJA, et al. Genotype-phenotype relationship in mucopolysaccharidosis II: predictive power of IDS variants for the neuronopathic phenotype. Dev Med Child Neurol. (2017) 59:1063–70. doi: 10.1111/dmcn.13467
10. Martin R, Beck M, Eng C, Giugliani R, Harmatz P, Muñoz V, et al. Recognition and diagnosis of mucopolysaccharidosis II (hunter syndrome). Pediatrics. (2008) 121:e377–86. doi: 10.1542/peds.2007-1350
11. Muenzer J, Jones SA, Tylki-Szymanska A, Harmatz P, Mendelsohn NJ, Guffon N, et al. Ten years of the hunter outcome survey (HOS): insights, achievements, and lessons learned from a global patient registry. Orphanet J Rare Dis. (2017) 12:82. doi: 10.1186/s13023-017-0635-z
12. Kubaski F, Yabe H, Suzuki Y, Seto T, Hamazaki T, Mason R, et al. Hematopoietic Stem Cell Transplantation for Patients with Mucopolysaccharidosis II. Biol Blood Marrow Transplant. (2017) 23:1795–803. doi: 10.1016/j.bbmt.2017.06.020
13. Taylor M, Khan S, Stapleton M, Wang J, Chen J, Wynn R, et al. Hematopoietic stem cell transplantation for mucopolysaccharidoses: past, present, and future. Biol Blood Marrow Transplant. (2019) 25:e226–46. doi: 10.1016/j.bbmt.2019.02.012
14. Muenzer J, Giugliani R, Scarpa M, Tylki-Szymanska A, Jego V, Beck M. Clinical outcomes in idursulfase-treated patients with mucopolysaccharidosis type II: 3-year data from the hunter outcome survey (HOS). Orphanet J Rare Dis. (2017) 12:161. doi: 10.1186/s13023-017-0712-3
15. Prasad VK, Kurtzberg J. Umbilical cord blood transplantation for non-malignant diseases. Bone Marrow Transplant. (2009) 44:643–51. doi: 10.1038/bmt.2009.290
16. Boelens JJ, Prasad VK, Tolar J, Wynn RF, Peters C. Current international perspectives on hematopoietic stem cell transplantation for inherited metabolic disorders. Pediatr Clin North Am. (2010) 57:123-45. doi: 10.1016/j.pcl.2009.11.004
17. Annibali R, Caponi L, Morganti A, Manna M, Gabrielli O, Ficcadenti A. Hunter syndrome (mucopolysaccharidosis type II), severe phenotype: long term follow-up on patients undergone to hematopoietic stem cell transplantation. Minerva Pediatr. (2013) 65:487–96.
18. Guffon N, Bertrand Y, Forest I, Fouilhoux A, Froissart R. Bone marrow transplantation in children with hunter syndrome: outcome after 7 to 17 years. J Pediatr. (2009) 154:733–7. doi: 10.1016/j.jpeds.2008.11.041
19. Vellodi A, Young E, Cooper A, Lidchi V, Winchester B, Wraith JE. Long-term follow-up following bone marrow transplantation for Hunter disease. J Inherit Metab Dis. (1999) 22:638–48. doi: 10.1023/A:1005525931994
20. Tanaka A, Okuyama T, Suzuki Y, Sakai N, Takakura H, Sawada T, et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol Genet Metab. (2012) 107:513–20. doi: 10.1016/j.ymgme.2012.09.004
21. Whiteman DA, Kimura A. Development of idursulfase therapy for mucopolysaccharidosis type II (Hunter syndrome): the past, the present and the future. Drug Des Devel Ther. (2017) 11:2467–80. doi: 10.2147/DDDT.S139601
22. Chen HH, Sawamoto K, Mason RW, Kobayashi H, Yamaguchi S, Suzuki Y, et al. Enzyme replacement therapy for mucopolysaccharidoses; past, present, and future. J Hum Genet. (2019) 64:1153–71 doi: 10.1038/s10038-019-0662-9
23. Muenzer J, Gucsavas-Calikoglu M, McCandless SE, Schuetz TJ, Kimura A. A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (hunter syndrome). Mol Genet Metab. (2007) 90:329–37. doi: 10.1016/j.ymgme.2006.09.001
24. Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (hunter syndrome). Genet Med. (2006) 8:465–73. doi: 10.1097/01.gim.0000232477.37660.fb
25. Barbier AJ, Bielefeld B, Whiteman DA, Natarajan M, Pano A, Amato DA. The relationship between anti-idursulfase antibody status and safety and efficacy outcomes in attenuated mucopolysaccharidosis II patients aged 5 years and older treated with intravenous idursulfase. Mol Genet Metab. (2012) 110:303–10. doi: 10.1016/j.ymgme.2013.08.002
26. Muenzer J, Beck M, Eng CM, Giugliani R, Harmatz P, Martin R, et al. Long-term, open-labeled extension study of idursulfase in the treatment of hunter syndrome. Genet Med. (2011) 13:95–101. doi: 10.1097/GIM.0b013e3181fea459
27. Giugliani R, Harmatz P, Jones SA, Mendelsohn NJ, Vellodi A, Qiu Y, et al. Evaluation of impact of anti-idursulfase antibodies during long-term idursulfase enzyme replacement therapy in mucopolysaccharidosis II patients. Mol Genet Metab Rep. (2017). 12:2–7. doi: 10.1016/j.ymgmr.2017.01.014
28. Matzner U, Matthes F, Weigelt C, Andersson C, Eistrup C, Fogh J, et al. Non-inhibitory antibodies impede lysosomal storage reduction during enzyme replacement therapy of a lysosomal storage disease. J Mol Med. (2008) 86:433–42. doi: 10.1007/s00109-008-0309-3
29. Kim KH, Messinger YH, Burton BK. Successful reduction of high-sustained anti-idursulfase antibody titers by immune modulation therapy in a patient with severe mucopolysaccharidosis type II. Mol Genet Metab Rep. (2015) 2:20–4. doi: 10.1016/j.ymgmr.2014.11.007
30. Kishnani PS, Dickson PI, Muldowney L, Lee JJ, Rosenberg A, Abichandani R, et al. Immune response to enzyme replacement therapies in lysosomal storage diseases and the role of immune tolerance induction. Mol Genet Metab. (2016) 117:66–83. doi: 10.1016/j.ymgme.2015.11.001
31. Desai AK, Li C, Rosenberg AS, Kishnani PS. Immunological challenges and approaches to immunomodulation in Pompe disease: a literature review. Ann Transl Med. (2019) 7:285. doi: 10.21037/atm.2019.05.27
32. Banugaria SG, Prater SN, Patel TT, Dearmey SM, Milleson C, Sheets KB, et al. Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile pompe disease: a step towards improving the efficacy of ERT. PLoS ON0E. (2013) 8:e67052. doi: 10.1371/journal.pone.0067052
33. Messinger YH, Mendelsohn NJ, Rhead W, Dimmock D, Hershkovitz E, Champion M, et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med. (2012) 14:135–42. doi: 10.1038/gim.2011.4
34. Pano A, Barbier AJ, Bielefeld B, Whiteman DA, Amato DA. Immunogenicity of idursulfase and clinical outcomes in very young patients (16 months to 7.5 years) with mucopolysaccharidosis II (hunter syndrome). Orphanet J Rare Dis. (2015) 10:50. doi: 10.1186/s13023-015-0265-2
35. Banugaria SG, Prater SN, McGann JK, Feldman JD, Tannenbaum JA, Bailey C, et al. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from pompe disease. Genet Med. (2013) 15:123–31. doi: 10.1038/gim.2012.110
Keywords: MPS II, hunter syndrome, immune modulation, enzyme replacement therapy, neutralizing antibodies
Citation: Julien DC, Woolgar K, Pollard L, Miller H, Desai A, Lindstrom K and Kishnani PS (2020) Immune Modulation for Enzyme Replacement Therapy in A Female Patient With Hunter Syndrome. Front. Immunol. 11:1000. doi: 10.3389/fimmu.2020.01000
Received: 01 February 2020; Accepted: 27 April 2020;
Published: 21 May 2020.
Edited by:
Herman Waldmann, University of Oxford, United KingdomReviewed by:
Paul Richard Harmatz, University of California, San Francisco, United StatesCopyright © 2020 Julien, Woolgar, Pollard, Miller, Desai, Lindstrom and Kishnani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel C. Julien, ZGp1bGllbkBwaG9lbml4Y2hpbGRyZW5zLmNvbQ==; Priya S. Kishnani, cHJpeWEua2lzaG5hbmlAZHVrZS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.