
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 03 April 2020
Sec. Alloimmunity and Transplantation
Volume 11 - 2020 | https://doi.org/10.3389/fimmu.2020.00489
This article is part of the Research TopicNovel Immunological Biomarkers for Allogeneic HSCT OutcomeView all 21 articles
 Francesca Bonifazi1*
Francesca Bonifazi1* Francesco Barbato1
Francesco Barbato1 Federico Ravaioli2
Federico Ravaioli2 Mariarosaria Sessa3
Mariarosaria Sessa3 Irene Defrancesco1,4
Irene Defrancesco1,4 Mario Arpinati1
Mario Arpinati1 Michele Cavo1,3
Michele Cavo1,3 Antonio Colecchia2,5
Antonio Colecchia2,5Hepatic veno-occlusive disease (VOD) or sinusoidal obstruction syndrome (SOS) is a rare complication characterized by hepatomegaly, right-upper quadrant pain, jaundice, and ascites, occurring after high-dose chemotherapy, hematopoietic stem cell transplantation (HSCT) and, less commonly, other conditions. We review pathogenesis, clinical appearance and diagnostic criteria, risk factors, prophylaxis, and treatment of the VOD occurring post-HSCT. The injury of the sinusoidal endothelial cells with loss of wall integrity and sinusoidal obstruction is the basis of development of postsinusoidal portal hypertension responsible for clinical syndrome. Risk factors associated with the onset of VOD and diagnostic tools have been recently updated both in the pediatric and adult settings and here are reported. Treatment includes supportive care, intensive management, and specific drug therapy with defibrotide. Because of its severity, particularly in VOD with associated multiorgan disease, prophylaxis approaches are under investigation. During the last years, decreased mortality associated to VOD/SOS has been reported being it attributable to a better intensive and multidisciplinary approach.
Hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), is a clinical syndrome occurring after high-dose chemotherapy, hematopoietic stem cell transplantation (HSCT) (1, 2), and, less commonly, after ingestion of toxic alkaloids (toxic injury) (3), after high doses of radiotherapy (4) or liver transplantation (5). Clinical diagnosis criteria include hepatomegaly, right-upper quadrant pain, ascites, and jaundice (6), although anicteric forms may occur, particularly, but not exclusively among pediatric population (7). The onset or the progression can be complicated by a multiorgan disease (MOD), characterized by functional disorders affecting lungs (pleural effusion, pulmonary infiltrates, hypoxia), kidneys (renal insufficiency/failure), and central nervous system (confusion, encephalopathy). Multiorgan disease is associated with high mortality rate (exceeding 80%), and it has been identified as the best predictive marker of severe VOD/SOS (8–10).
In HSCT patients, endothelial cell injury leads to loss of sinusoidal wall integrity, endothelial cell detachment, sinusoidal obstruction, and development of postsinusoidal portal hypertension (PH) (11). The incidence of posttransplant VOD/SOS is highly variable, ranging from 5.3% (12) to 13.7% (9) to higher percentages, according to transplant settings and different studies; particularly in pediatric high-risk populations, the incidence could be 20 to 30% up to 60% (7, 13–15).
Transplant outcome is significantly affected by VOD/SOS occurrence, where the mortality rates can reach up to 80% in the severe forms, in older series (9), whereas more recent studies report lower mortality rates (16, 17), in patients treated with defibrotide. Early diagnosis and treatment are positively correlated to a survival benefit (16). Treatment includes supportive care, intensive treatment, and specific drug therapy.
The initial step of VOD/SOS pathogenesis is the injury of sinusoidal endothelium of the liver (Figure 1) leading to loss of endothelial cell cohesions: gaps appear in the endothelial barrier, and red blood cells pass through these gaps and accumulates in the Disse space, causing the detachment of the endothelial cells with downstream embolization of the centrilobular vein and subsequent postsinusoidal obstruction (18).
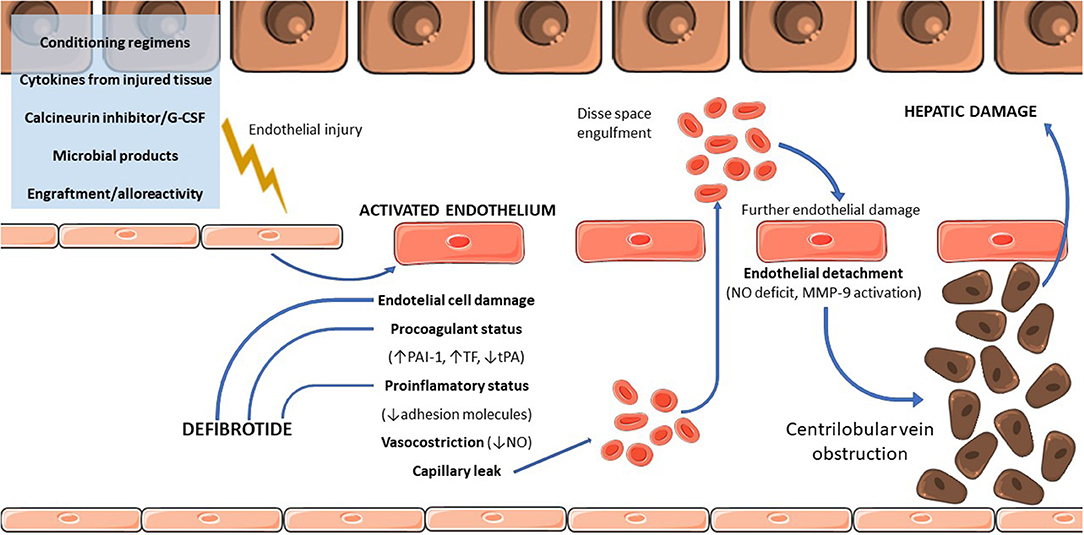
Figure 1. Physiopathology of VOD/SOS. G-CSF, granulocyte colony-stimulating factor; PAI, plasminogen activator inhibitor-1; TF, tissue factor; tPA, tissue plasminogen activator; MMP-9, matrix metallopeptidase 9.
Several causes (Figure 1) are incriminated into initial endothelial damage, including conditioning regimens (19), cytokines produced by injured tissues (20), endogenous microbial products migrating through damaged mucosal barriers (21), drugs used during the transplant [such as granulocyte colony-stimulating factor (G-CSF) or calcineurin inhibitors] (22–24), and the engraftment process itself (25). Conditioning regimens have a crucial role in the pathogenesis as highlighted by the increased risk of VOD/SOS associated with higher plasma levels of cytotoxic drugs, such as busulfan or metabolites of cyclophosphamide (26). Chemotherapy drugs are metabolized by the cytochrome P450 complex, producing toxic metabolites subsequently converted to non-toxic ones by the glutathione (GSH) enzymatic system and then eliminated (27). Centrilobular regions of the liver are poor in GSH, making them more sensitive to toxic agents and explaining the predominant damage of centrilobular regions (28, 29). Moreover, a GSH S-transferase M1 null genotype reducing the detoxifying capacity of the liver parenchyma predisposes to SOS/VOD (30), and the reduced detoxifying ability due to immature enzymatic system could, at least partially, explain the higher incidence of VOD/SOS in children (13).
Some clinical observations led to the hypothesis that alloreactivity plays a role in VOD/SOS. Incidence of VOD/SOS is higher after allogenic compared to autologous HSCT and is higher in patients receiving a transplant from a mismatched unrelated donor (31). These observations are supported by findings in experimental models where endothelial cells are targets of alloreactive T cells (32).
Endothelial cells after HSCT show signs of injury characterized by procoagulant and proinflammatory status (Figure 1). This status is confirmed by the presence of increased levels of circulating markers of endothelial activation after HSCT, such as endothelial procoagulant factors and adhesion molecules (20), circulating endothelial cells (33), endothelial progenitor cells (34), and microparticles (35).
Endothelial cell detachment seems to be correlated with nitric oxide deficiency caused by postconditioning toxicity (36). Nitric oxide deficiency promotes increased endothelial cell production of matrix metalloproteinase 9 (MMP-9) that seems to be strongly involved in VOD/SOS development, probably promoting the endothelial cell detachment. The role of MMP-9 in the VOD/SOS pathogenesis is supported by the evidence that the in vivo inhibition of MMPs completely prevents its occurrence (37).
Along with the embolization by detached endothelial cells, blood flow obstruction is promoted by the proliferation of perisinusoidal stellate cells and subendothelial fibroblasts in the terminal hepatic vein followed by the deposition of the extracellular matrix (38). Then perivenular fibrosis spreads into the liver parenchyma (39). All these events lead to a block in liver blood outflow, with progressive obliteration of the venules and centrilobular sinusoidal, causing hepatic congestion and the development of postsinusoidal PH (40).
Because of the central role of endothelial injury in its pathogenesis, VOD/SOS is now classified as a transplant-related endothelial dysfunction, as well as posttransplant microangiopathy, idiopathic pneumonia, diffuse alveolar hemorrhage, and engraftment syndrome (11).
The clinical presentation of VOD/SOS is the consequence of the PH, being characterized by rapid weight gain, tendentially unresponsive to diuretics, hyperbilirubinemia, painful hepatomegaly, and ascites. It generally occurs within 21 days after transplant, late-onset VOD/SOS is nowadays recognized as distinct VOD/SOS feature by recent diagnostic criteria elaborated by the European Society for Blood and Marrow Transplantation (EBMT) (41) (Table 1). It has been already reported that late VOD/SOS occurs at least in 39.3% and 16.7%, respectively, in the adult and pediatric setting (16).
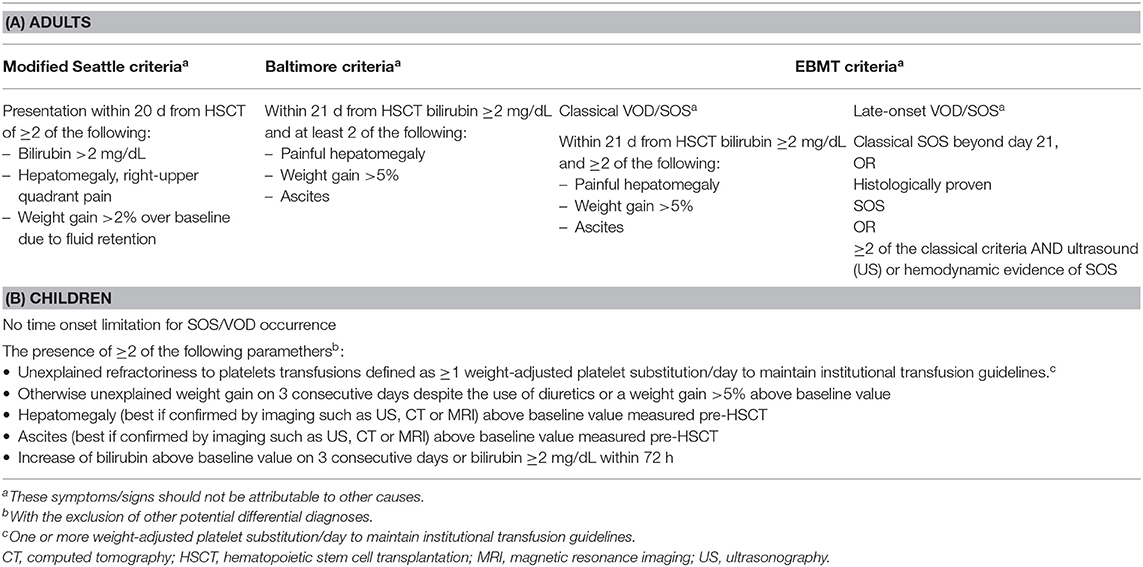
Table 1. Modified seattle, Baltimore, and EBMT diagnostic criteria in adults (A) and in children (B).
The onset of VOD/SOS can be either smoldering or disruptive, ranging from mild forms spontaneously resolving within few weeks to severe forms with organ damage and MOD. Multiorgan disease, involving generally pulmonary and renal functions, can rapidly occur, significantly worsening the outcome (17, 41, 42). Because of the high mortality rate of severe VOD/SOS, daily monitoring for prompt detection of symptoms, such as jaundice, hepatomegaly, fluid overload with weight gain and ascites (42), is required. Although it remains a life-threatening condition, progresses in the management of severe VOD/SOS improved the outcome compared to the past (43).
The “traditional” diagnosis of VOD/SOS is based on fulfillment of either Baltimore (44) or modified Seattle criteria (6) (Table 1) and the exclusion of differential diagnosis.
Several conditions, such as fluid overload, constrictive pericarditis, ascites of different origin (pancreatic, chylous), drug-induced cholestasis and more generally drug-induced liver injury (DILI), cholangitis lenta, sepsis, infectious hepatitis, parenteral nutrition, cholestasis, and hepatic graft-versus-host disease (GvHD), can mimic VOD/SOS and still make real-life differential diagnosis a true challenge or pitfall.
The main difference between the two diagnostic systems is hyperbilirubinemia being mandatory in the Baltimore criteria, which implies longer time waiting for its development or intrinsically more aggressive forms. Up to 30% of children with VOD/SOS was anicteric (7, 45, 46) compared to 12% of adults. The clinical scenario can be variable, in particular in children where anicteric forms are not rare (13, 47) and dynamically changing.
For these reasons, the EBMT proposed, both in adult (Table 1A) and in pediatric (Table 1B) setting, new different diagnostic criteria and a scale for severity grading of suspected VOD/SOS (13, 41).
The EBMT criteria for adult patients (41) foresee two clinical entities: the classical VOD/SOS appearing within 21 days after HSCT with bilirubin ≥2 mg/mL and two of the following criteria: painful hepatomegaly, weight gain, and ascites. The late-onset VOD/SOS occurs beyond 21 days after transplantation and potentially presents as follows:
1. Same feature as the classical one,
2. It should be histologically proven, and
3. Two out of four criteria for the classical VOD/SOS (bilirubin ≥2 mg/mL, weight gain >5%, painful hepatomegaly, and ascites) plus hemodynamic or ultrasound (US) evidence of VOD/SOS.
In the pediatric setting (13), there are no distinctions related to the time of onset, and no time limitations are given. The fulfillment of at least two of the following criteria is required for diagnosis: the unexplained consumptive and transfusion-refractory thrombocytopenia, an otherwise unexplained weight gain on 3 consecutive days despite the use of diuretics or a weight gain 5% above the baseline value, hepatomegaly (best if confirmed by imaging) above the baseline value, ascites (best if confirmed by imaging) above the baseline value, and rising bilirubin from the baseline on 3 consecutive days or bilirubin ≥2 mg/dL within 72h.
The main differences between the diagnostic criteria of adult and children are the bilirubin increase, which can be missing mainly in the pediatric setting, in a significant proportion of cases and the presence of refractory thrombocytopenia. It should be reminded that the criteria have been established from different panels of experts, following a consensus-based approach; the refractoriness of thrombocytopenia to transfusion has been called in to discussion also for the adult criteria system but not finally adopted as a criterion because of lack of panel consensus. These criteria need to be further validated by forthcoming prospective studies (48).
Both adult and pediatric criteria have been associated to severity grading scales that are related to the dynamic changes, mainly the evolution of hepatic and renal function tests (Table 2). The speed of changes is considered a warning sign belonging to higher severity grading scale (for suspected VOD/SOS) and hence supporting early treatment initiation with potential clinical outcome improvement. This score system can be also used in case of suspected VOD/SOS, before patients meet the diagnostic criteria, especially before day 21 (41).
The EBMT diagnostic criteria for adults include a late-onset VOD/SOS where both histology and US attain key roles for the diagnosis itself. In pediatric setting, the role of imaging has been significantly upgraded, as suggested by the EBMT diagnostic criteria, which recommend hepatomegaly and ascites to be confirmed by imaging during the clinical course and immediately before HSCT (13).
Among imaging techniques, US is certainly one of the most commonly studied as it allows assessment of both parenchymal and vascular changes; it is cheap and can be used bedside. However, even though US has been recognized as an EBMT diagnostic criterion, its role is restricted to diagnosis confirmation, when clinical signs are already noticeable. Ultrasound and Doppler US can easily detect the typical signs of PH such as ascites, hepatomegaly, splenomegaly, and dilatation of portal vein, which are commonly present in symptomatic VOD/SOS. The first article describing systematically these typical US and US Doppler diagnostic criteria was published by Lassau et al. (49). The prospective study included 100 patients having undergone HSCT; 25 of 100 patients developed VOD/SOS. The authors used seven morphologic and seven Doppler criteria to define the value of US in the prediction, diagnosis, and prognostic assessment of VOD/SOS. Based on these 14 criteria, a diagnostic score was then produced; a score of 6 had a sensitivity of 83% and a specificity of 87%. However, at the best of US performance, ~20% of the VOD/SOS could be misdiagnosed according to the Lassau score. A recent article by Park et al. (50) confirmed that some morphological parameters such as ascites and gallbladder wall thickening were significantly associated (odds ratio, respectively, 56.3 and 36.3) to VOD/SOS diagnosis. Nishida et al. (51) proposed a novel scoring system (HokUS-10) based on 10 US variables, which was able to predict VOD/SOS diagnosis with sensitivity of 100% and specificity of 95.8% in 10 patients. Although HokUS-10 score is easier to apply than the Lassau score, it still has to be adequately validated. There is much evidence on the utility of US imaging as a diagnostic tool; nevertheless, its role is still controversial because of lack of reproducibility and the requirement need of an expert sonographer, especially for US Doppler. Furthermore, some US Doppler signs (e.g., patency of paraumbilical vein) appear when an advanced stage of VOD/SOS has already been developed; thus, its application may be very limited to early diagnose or to anticipate the clinical VOD/SOS diagnosis. The use of ultrasonographic contrast agent, which is able to assess the hepatic vascularization, has been used to facilitate the diagnosis and to evaluate treatment response (52, 53) in VOD/SOS setting.
Because magnetic resonance and computed tomography represent the gold standard techniques for focal liver lesions identification, particularly in cancer staging and surveillance, their use is still pivotal in post-HSCT VOD/SOS (42, 54, 55). However, the potential role of these imaging techniques can be further increased in all types of VOD/SOS (56). Major limitation for a broader use is related to logistic issues, mainly in critical patients.
Because of the potential complications of hepatic biopsy in thrombocytopenic patients (i.e., hemorrhage, hemobilia, shock), the possibility of a histologic diagnosis of VOD/SOS is quite limited to well-trained centers with dedicated multidisciplinary team and cannot be considered a routine practice. Transjugular biopsy can limit the risk of bleeding and allow the measurement of the hepatic venous pressure gradient (HVPG), although the risk of unreadable specimens can be accounted (57). Hepatic venous pressure gradient is the hallmark of PH: its measurement is a very specific tool for VOD/SOS diagnosis, and values >10 mm Hg predict VOD/SOS with good level of accuracy and specificity (58). The main limitation consists in being an invasive procedure.
In patients with advanced chronic hepatic disease, the measurement of PH via HVPG has been replaced by hepatic stiffness measurement performed by elastography, which is a non-invasive method. Liver stiffness measurement (LSM) by transient elastography has been introduced several years ago to stage liver diseases (59); since then, numerous experiences have demonstrated a good correlation between liver stiffness and liver disease grading (60). Thus, LSM progressively allowed reducing the number of liver biopsies performed in patients with advanced liver disease. Moreover, it was observed that LSM could also be useful to measure PH, because it closely correlates with HVPG (61). Elastography was used to predict VOD/SOS in HSCT patients. Recent studies (62–64) investigated the predictive role of LSM changes, assessed by transient elastography (TE) or shear wave elastography, in post-HSCT VD/SOS in pediatric and adult patients. Liver stiffness measurement values assessed by TE in healthy subjects without liver pathology range between 4.3 and 5.3 kPa (65, 66), whereas a threshold of 21 kPa holds a high specificity (>90%) and can be used to confirm the presence of clinically significant portal hypertension (67, 68). In HSCT patients, LSMs were carried out at baseline and once a week after HSCT. Only in patients who developed VOD/SOS, LSM values markedly increased compared to previous measurement (from 10.3–59.3 vs. 3.5–7.5 kPa) (62, 63). Liver stiffness measurement increases from 1 to 15 days before clinical VOD/SOS diagnosis and most intriguingly LSM decreased after the start of defibrotide treatment parallel to clinical signs of VOD/SOS (e.g., bilirubin, weight) (63–69). Based on these results, it was speculated that LSM, a non-invasive method, executable bedside, can be useful to perform both a preclinical diagnosis of VOD/SOS and to monitor treatment response. Main limitations for a wide application of this method are the need of a specific training of the operator, the presence of significant amount of ascites, and a body mass index >30 kgm2. Based on preliminary results, an Italian national multicenter prospective trial (“ElastoVOD/SOS Study,” ClinicalTrial.gov NCT03426358) is actually running, aimed to confirm the prognostic role of LSM in a prospective multicenter context.
Several biomarkers (70) have been proposed for VOD/SOS diagnosis and/or prevention; they are markers of hemostasis and coagulation such as plasminogen activator inhibitor 1 (PAI-1) or other markers of endothelial injury, such as elevated levels of von Willebrand factor, thrombomodulin, soluble intercellular adhesion molecule 1, suppressor of tumorigenicity 2, angiopoietin 2, hyaluronic acid (HA), or markers of inflammation [interleukin 6 (IL-6), IL-10, CD97].
The increased level of PAI-1 antigen is the most studied marker for its role as a predictor of VOD/SOS (71–74), whereas a decrease of its level has been correlated with better treatment outcome (75). Anyway, the proteomic-based approach published by Akil et al. (76) failed to include PAI-1 in the final predictive model. In this model only L-ficolin, HA, and vascular cell adhesion molecule 1 showed a prognostic value for diagnosis. Available data on single or combined panel of biomarkers for VOD/SOS are still inconclusive, and a wide application in the real world is so far marginal.
The incidence of VOD/SOS after transplantation varies substantially from 2 to 60% (6, 7, 12, 13, 16, 47) because of both different setting of patients and transplant procedures and of application of different diagnostic criteria.
The incidence of VOD/SOS is higher in children than in adults (7, 9, 13–16, 47), although a retrospective analysis of a large Italian pediatric cohort (47) found a surprisingly very low incidence of VOD/SOS [2% (95% confidence interval, 1.7–2.5)].
Risk factors are generally classified as either patient related or transplantation related (77). Among the former ones, age, Karnofsky index, any preexisting liver disease, altered liver function tests, advanced hematological disease, second transplant thalassemia and ferritin level, and abdominal radiation are risk factors reported in literature since the last two decades.
The use of new immunotherapies for the therapy of acute leukemias, such as gemtuzumab ozogamicin for acute myeloid leukemia and inotuzumab ozogamicin for acute lymphoblastic leukemia, is associated with a significant increase of VOD/SOS risk (77–79), mainly related to the subsequent HSCT. In this respect, avoidance of more than two inotuzumab ozogamicin cycles and double alkylators in the preparing regimen and the use of ursodeoxycholic acid are recommended in patients suffering from relapsed/refractory acute lymphoblastic leukemia undergoing allogeneic HSCT after inotuzumab ozogamicin treatment (80).
The following transplantation-related risk factors should be mentioned (77): allogeneic vs. autologous transplant, mismatched/haploidentical transplant, T-replete transplants, and myeloablative-preparing regimen containing either busulfan or total body irradiation.
The odds ratios of each risk factor reported by the review from Dalle and Giralt (77) are those reported from each reference, sic et simpliciter, without a risk score–building purpose.
Recently, the Center for International Blood and Marrow Transplant Research developed a risk score built on a large population series of more than 13,000 patients (81). Younger age, positive hepatitis B/C serology, lower Karnofsky index, use of sirolimus, disease at transplant, and myeloablative-conditioning regimen were associated to higher risk of VOD/SOS. The authors did not include pretransplant therapies impacting on VOD/SOS, so the applicability of this model to patient receiving either gemtuzumab ozogamicin or inotuzumab ozogamicin is still unknown. Prospective validation of risk factors is yet to be completed and needs further assessment to provide a more precise estimation of the magnitude of each risk factor (70).
The treatment of VOD/SOS includes supportive and intensive care in addition to the specific therapy with defibrotide.
Supportive care and clinical monitoring are primary issues in the management of VOD/SOS throughout the whole HSCT course, in order to promptly capture clinical diagnostic criteria, to timely record all dynamic changes and to follow both the response to treatment and disease progression. Daily reports of several parameters, such as abdominal circumference, weight, and diuresis, are recommended (13, 41). The nurse group of the Italian Society of Bone Marrow Transplantation elaborated an operational flowchart for a dynamic nursing monitoring of patients with suspected or proven VOD/SOS (82). Supportive care includes a careful evaluation of fluid balance with diuretics, as well as all therapeutic measures aimed to reduce the discomfort of massive ascites, pleural effusion, hypoxia, pain, and renal dysfunction such as paracentesis, thoracentesis, oxygen therapy according to the respiratory parameters, analgesic therapy, hemodialysis, or hemofiltration. A transfer to the intensive care unit can be required. The therapy at the intensive care unit is symptomatic and may differ among centers.
Defibrotide is the only registered drug for the treatment of moderate/severe VOD/SOS; it is a mixture of polydeoxyribonucleotide, mainly single-stranded, derived from the porcine intestinal mucosa. Its mechanism of action is not yet fully understood (83, 84). Oligonucleotides interact with heparin-binding proteins such as fibroblast growth factors, which exert fibrogenetic as well as angiogenetic effects with endothelial stabilization. Moreover, defibrotide acts as an antithrombotic and profibrinolytic drug; it reduces platelet adhesion and activation, without systemic anticoagulant effects, by means of inhibition of PAI-1, thrombin, and leukocyte adhesion process (via inhibition of P-selectin expression), and also decreases vascular permeability and apoptosis due to calcineurin inhibitors and chemotherapy, without interfering with antitumor effect of cytotoxic drugs (85). Because of the capacity of defibrotide to protect endothelium from toxic, inflammatory, and ischemic damage, its potential therapeutic use has been tested, some decades ago, in several vascular disorders such as thrombophlebitis (86, 87), in postsurgery deep vein thrombosis prophylaxis (88, 89), and peripheral arterial diseases (90) with significant benefits. It has been used, even in a pivotal way, in acute myocardial infarction (91), in postthrombolysis reocclusion of coronary (92), ischemic damage of the liver (93), diabetic microangiopathy, and Reynaud phenomenon (94).
The efficacy and safety of defibrotide in the setting of VOD/SOS, especially after HSCT, have been extensively evaluated by different authors. The first study is a historically controlled multicenter open-label phase III study (95) recruiting patients from 1995 to 2008; participating centers prospectively enrolled patients with established hepatic VOD/SOS to receive defibrotide 25 mg/kg per day, whereas the placebo cohort (32 patients) was retrospectively identified from 6,867 medical charts of HSCT patients by blinded independent reviewers in order to minimize the selection bias. The unusual study design (retrospective vs. prospective comparison) is due to the refusal of participating centers to accept a prospective randomization with placebo resulting unethical (orphan disease with high mortality). This study adopted the VOD/SOS diagnosis criteria, and severe VOD/SOS was defined as a VOD/SOS complicated by MOD. The primary endpoint was 100-day mortality; secondary endpoints were 100-day complete response (CR) rate and 6-month overall survival. The study demonstrated both 100-day survival and CR benefit favoring the defibrotide arm (38.2 vs. 25.0% and 25.5 vs. 12.5%, respectively). Median duration of therapy was 21.5 days, and 10.7% of patients discontinued defibrotide for treatment-related adverse event (AE). Adverse events were similar in the two arms, particularly hemorrhagic events (64% in the experimental arm vs. 75% in the historical control arm). Pulmonary alveolar hemorrhage occurred in 11.8 and 15.6% of the patients, gastrointestinal bleeding in 7.8 vs. 9.4%, and cerebral hemorrhage in 2.9 vs. 3.1%, respectively, in the experimental and control arms.
Concurrently the aforementioned phase III study, an international compassionate use program (CUP) (17), has been implemented, aimed to ensure drug supply to a wider range of transplant centers across the world. Transplant centers adhering to the CUP program enrolled patients developing severe VOD/SOS either after HSCT or after radiotherapy/chemotherapy. Both the Baltimore- and Seattle-modified (6, 44) diagnosis criteria were used; when the Seattle criteria were not met, the presence of US changes or histological diagnosis could be sufficient for patient recruitment and drug supply. Severe VOD/SOS was defined by the presence of MOD or by >30% of predicted risk retrospectively evaluated according to the Bearman model (96). Defibrotide doses ranged from 10 to 80 mg/kg, because no specific treatment protocol has been adopted. Participating centers voluntarily provided demographic and clinical data for the analysis. Overall 1,169 patients received at least one dose of defibrotide, whereas data were finally retrieved on 710 patients. Six hundred eighty-nine of 710 patients developed VOD/SOS after HSCT: 499 after an allogeneic HSCT, and 112 after autologous HSCT; 60% were transplanted for acute lymphoblastic leukemia, and 57% of the study population was adults (≥18 years old). Two hundred ninety-two of 710 patients were treated for a severe VOD/SOS. One hundred-day survival was 54% in the overall population (58% of those patients receiving defibrotide at the dose of 25 mg/kg) and was higher in the pediatric cohort (65.4 vs. 46.1%), in the group without MOD (64.7 vs. 39.7%), and in patients developing VOD/SOS after a non-HSCT therapy (74.2 vs. 67.5%). Adverse events occurred in 51% of patients, whereas overall discontinuation of the drug occurred in 28%; 9% of patients discontinued defibrotide because of AEs, mainly hemorrhages (gastrointestinal). No clinically meaningful trends in AE occurrence were identified by gender, age, or dose group.
The third study was a prospective open-label, single-arm study in an expanded access program (16) enrolling, from 2007 to 2016, patients with hepatic VOD/SOS, both post-HSCT and non-HSCT treatments, with the aim to evaluate 100-day overall survival (primary endpoint) and safety of defibrotide given at the dose of 25 mg/kg for at least 21 days. The inclusion criteria changed over time: initially, VOD/SOS should be diagnosed according to the Baltimore criteria by day +35 post-HSCT or by biopsy as well as MOD (by day +45 post-HSCT); then, VOD/SOS was diagnosed based on Seattle criteria, with onset after day +35, secondary to non-transplant treatment, also including VOD/SOS without MOD. A total number of 1,137 patients were enrolled, 1,000 with VOD/SOS after HSCT (85% allogeneic HSCT and 15% autologous HSCT). The pediatric group represented 82% of postautologous HSCT VOD/SOS and 52.3% of postallogeneic HSCT VOD/SOS. One hundred-day overall survival was 58.9% in the whole population, 68.5% in patients who developed VOD/SOS without MOD, and 49.5% in patients with MOD; VOD/SOS was significantly associated with MOD occurrence in all transplant types and all age groups. Late-onset VOD/SOS was more frequent in adults than in children (39.3% of adult patients and 16.7% of children) and was associated with lower survival only in the pediatric group. Earlier initiation of defibrotide treatment was significantly associated with higher day +100 survival (P < 0.001). Treatment-emergent AEs (in patients who received at least one dose of defibrotide) were more frequent in adults than in children (77.9 and 65.5%, respectively) and in patients with MOD (75.2% overall, 81% in adults, and 70.5% in children). Twenty-one percent of patients had at least one treatment-related AE (TRAE), which represented the reason for treatment discontinuation in 12.4% of patients. Treatment-related AEs were not different according in relation to VOD/SOS time of onset. The most important TRAEs were pulmonary hemorrhage (4.6%), gastrointestinal hemorrhage (3.0%), epistaxis (2.3%), and hypotension (2.0%).
A postmarketing phase IV study on defibrotide has been required by French regulatory authorities as a source of real-world data (48). Patients treated with defibrotide as prophylaxis were included, although there is no registration of defibrotide for this indication. In this study, VOD/SOS diagnosis was performed according to the EBMT criteria and the primary endpoints were both 100-day survival and 100-day complete response of severe VOD/SOS. Three hundred twenty-four French patients received defibrotide from July 2014 to October 2018; 40 developed severe VOD/SOS, and 120 after HSCT; overall, 105 patients developed a severe/very severe VOD/SOS (89 after HSCT). More than 30% of patients with VOD/SOS showed a concomitant MOD. One hundred-day survival in the overall population (140 patients), in severe VOD/SOS, and in very severe VOD/SOS were 58%, 79%, and 34%, respectively. The proportion of patients experiencing any AEs was 54% in the overall population. The study is still active, and definitive data are forthcoming.
Data from these important studies are quite superimposable and further confirmed by a systematic review of the literature, which found out 100-day survival of 41% in patients with MOD and 71% in those without MOD (97).
Corticosteroids, which have been used both in adult (98) and pediatric (99) setting, achieved the 2C level of recommendation in British guidelines (100); their use should be cautiously considered because of the increased risk of infections. Tissue plasminogen activator and N-acetylcysteine are not recommended for increased bleeding risk and lack of efficacy, respectively (100).
In case of no response and progression of VOD/SOS, the prognosis is dismal, and few further treatments are available, with limited efficacy. Transjugular intrahepatic portosystemic shunting placement has been reported in few anecdotal cases in literature for the treatment of VOD/SOS, but currently its use is not recommended because of poor outcomes (101). It has been considered sometimes when a severe VOD/SOS refractory to medical treatment occurred in a liver transplant recipient (102). Similarly, the role of orthotopic liver transplantation is controversial; its use has been described in few case reports in patients with severe VOD/SOS associated with life-threatening liver failure (103).
Several pharmacological approaches have been tested with the purpose of preventing VOD/SOS, including heparin, antithrombin, prostaglandin E1, pentoxifylline, and ursodeoxycholic acid (9, 96). All these agents showed little or no efficacy or caused intolerable rates of adverse effects for a prophylactic strategy apart from ursodeoxycholic acid, which is recommended by British guidelines (100). Unfractionated heparin and low-molecular-weight heparin have been extensively studied, including some randomized trials, but with inconclusive results (12, 104–108). No efficacy was demonstrated for antithrombin and pentoxifylline (109–111). Also, the use of prostaglandin E1 was abandoned because of inconclusive results and excess of toxicity (112–114). The use of ursodeoxycholic acid (UDCA) in VOD/SOS prophylaxis has been investigated, in comparison to placebo, in three different randomized trials. Two of them (115, 116) demonstrated a significant reduction of VOD/SOS incidence in the UDCA arm; one revealed no differences between the two arms (117). A meta-analysis of the three trials comparing UDCA with placebo supported the use of UDCA as a possible effective prevention strategy, also because of its safety profile (118). Another randomized study compared prophylactic use of UDCA in association with heparin against heparin alone and revealed no differences in VOD/SOS incidence between the two groups (119).
The use of defibrotide as prophylactic agent has been tested in several retrospective studies (120–122) and in one prospective randomized trial in the pediatric setting. This phase III, randomized, open-label, multicenter trial compared defibrotide to placebo as VOD/SOS prophylaxis in pediatric patients undergoing allogeneic or autologous HSCT (7). In this study, each patient had one or more VOD/SOS risk factor including preexisting hepatic disease, second myeloablative transplant, allogeneic transplant for leukemia beyond second relapse, conditioning with busulfan and melphalan, prior treatment with gemtuzumab ozogamicin or a diagnosis of primary hemophagocytic lymph histiocytosis, adrenoleukodystrophy, or osteopetrosis. The trial included 365 patients younger than 18 years equally allocated in two arms. Patients in the treatment group received defibrotide (DF) 25 mg/kg per day in four divided intravenous infusions, starting with the initiation of conditioning regimen and continuing for 30 days after transplantation or, if discharged from hospital before 30 days after HSCT, for at least 14 days. The primary endpoint was the incidence of VOD by 30 days after HSCT. Twenty-two patients (12%) in the DF group developed VOD/SOS compared with 35 patients (20%) in the control group (Z-test for competing risk analysis P = 0.0488; log-rank test P = 0.0507).
Based on these results, the British Committee for Standards in Hematology and the British Society for Blood and Marrow Transplantation guidelines recommend the use of defibrotide for VOD/SOS prophylaxis in children undergoing HSCT with at least one risk factor for VOD/SOS (evidence IA) (100).
In adults, evidences are far less conclusive, and consistent results from randomized trials are still lacking. Even if some retrospective studies suggest a possible role of defibrotide for prophylaxis of VOD/SOS, particularly in high-risk patients, there is no clear evidence of its efficacy (123–125). There is no physiological reason why defibrotide should not work for VOD/SOS prophylaxis in adult, but it is yet to be proved if a prophylactic strategy would grant a better outcome than an early treatment strategy. A prospective randomized trial is ongoing, aimed to clarify these issues (ClinicalTrials.gov NCT02851407).
Diagnosis of VOD/SOS is currently based mainly on clinical criteria; biomarkers for VOD/SOS diagnosis are not yet validated. The most reliable imaging method supporting VOD/SOS diagnosis is US, which is now recognized as an essential diagnosis criterion of late-onset VOD/SOS and highly recommended to assess hepatomegaly and ascites in children. Nevertheless, VOD/SOS diagnosis remains difficult in real-life setting, despite the availability of different diagnostic criteria systems; differential diagnosis is quite challenging because several other conditions could meet the VOD/SOS criteria, such as sepsis, cholangitis lenta, constrictive pericarditis, hepatic GvHD, hepatitis, or DILI. Moreover, more than one complication can occur simultaneously in the same patient leading to a substantial delay of final diagnosis. When clinical criteria are not fully met, invasive diagnostic methods are still hard to be widely used because they need well-trained multidisciplinary teams to perform and read biopsy or HVPG measurement. Even in the presence of these facilities, however, patients with suspicious or proven VOD/SOS can be critically instable, and the risk of further severe procedure-related complications cannot be prevented.
For these reasons additional tools for the diagnosis are most welcome. Elastometry is a non-invasive method to perform LSM, which is a validated surrogate of HVPG, in advanced chronic liver disease. If ongoing studies confirm the role of elastometry in HSCT patients, this non-invasive imaging technique will allow an earlier VOD/SOS diagnosis and an accurate monitoring of treatment response. The use of elastometry underpinned the importance of a multidisciplinary approach to VOD/SOS with specialists in radiology, hepatology, intensive care, and nephrology supporting and helping physicians performing HSCT in the management of VOD/SOS. Anyway, the use of elastometry for VOD/SOS diagnosis and for treatment response evaluation deserves further validation by prospective studies.
In the past, the mortality risk for patients who develop posttransplant VOD/SOS with MOD, typically characterized by pulmonary and/or renal dysfunction, has been estimated to be >80% (8–10). In more recent reports, mortality rates are significantly lower: 22 and 35% at 100 days and 5 years, respectively, in the retrospective large Italian pediatric cohort (47), and 49.5% estimated survival at 100 days in the T-IND study (16).
Decreased mortality can be attributed to a better intensive care, to increasing proportion of centers with multidisciplinary teams, to a wider use of risk stratification, to earlier treatment. Prevention of MOD occurrence and progression of severity grading significantly increased survival in all HSCT transplants settings (16, 49). Finally, a greater knowledge on risk factors will lead to a more tailored approach to both prevention and treatment of VOD/SOS.
FBon and AC conceived and designed the study. FBon wrote the first draft. AC, MS, FBar, ID, FR, and MC wrote sections of the manuscript. All the authors contributed to manuscript revision, read and approved the submitted version.
FBon and AC participated on advisory boards and received speaker fess from JAZZ Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
The authors acknowledged BolognAIL and REUSE WITH LOVE for supporting the Transplant Program at the Hematology Department Seràgnoli in Bologna; the authors thank Dr. Rita Bertoni and Dr. Enrica Tomassini for the technical support.
1. Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. (1995) 85:3005–20. doi: 10.1182/blood.V85.11.3005.bloodjournal85113005
2. Kumar S, DeLeve LD, Kamath PS, Tefferi A. Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clin Proc. (2003) 78:589–98. doi: 10.4065/78.5.589
3. Valla DC, Cazals-Hatem D. Sinusoidal obstruction syndrome. Clin Res Hepatol Gastroenterol. (2016) 40:378–85. doi: 10.1016/j.clinre.2016.01.006
4. Fajardo LF, Colby TV. Pathogenesis of veno-occlusive liver disease after radiation. Arch Pathol Lab Med. (1980) 104:584–8.
5. Takamura H, Nakanuma S, Hayashi H, Tajima H, Kakinoki K, Kitahara M, et al. Severe veno-occlusive disease/sinusoidal obstruction syndrome after deceased-donor and living-donor liver transplantation. Transplant Proc. (2014) 46:3523–35. doi: 10.1016/j.transproceed.2014.09.110
6. McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Veno-occlusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. (1984) 4:116–22. doi: 10.1002/hep.1840040121
7. Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. (2012) 379:1301–9. doi: 10.1016/S0140-6736(11)61938-7
8. Sakai M, Strasser SI, Shulman HM, McDonald SJ, Schoch HG, McDonald GB. Severe hepatocellular injury after hematopoietic cell transplant: incidence, etiology and outcome. Bone Marrow Transplant. (2009) 44:441–7. doi: 10.1038/bmt.2009.56
9. Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. (2010) 16:157–68. doi: 10.1016/j.bbmt.2009.08.024
10. Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic transplantation. Br J Haematol. (2015) 168:481–91. doi: 10.1111/bjh.13215
11. Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. (2011) 46:1495–502. doi: 10.1038/bmt.2011.65
12. Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. (1998) 92:3599–604.
13. Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. (2018) 53:138–45. doi: 10.1038/bmt.2017.161
14. Barker CC, Butzner JD, Anderson RA, Brant R, Sauve RS. Incidence, survival and risk factors for the development of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Transplant. (2003) 32:79–87. doi: 10.1038/sj.bmt.1704069
15. Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica. (2005) 90:1396–404
16. Kernan NA, Grupp S, Smith AR, Arai S, Triplett B, Antin JH, et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br J Haematol. (2018) 181:816–27. doi: 10.1111/bjh.15267
17. Corbacioglu S, Carreras E, Mohty M, Pagliuca A, Boelens JJ, Damaj G, et al. Defibrotide for the Treatment of Hepatic Veno-Occlusive Disease: Final Results from the International Compassionate-Use Program. Biol Blood Marrow Transplant. (2016) 22:1874–82. doi: 10.1016/j.bbmt.2016.07.001
18. DeLeve LD, Wang X, Kuhlenkamp JF, Kaplowitz N. Toxicity of azathioprine and monocrotaline in murine sinusoidal endothelial cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology. (1996) 23:589–99. doi: 10.1002/hep.510230326
19. Zeng L, Jia L, Xu S, Yan Z, Ding S, Xu K. (2010) Vascular endothelium changes after conditioning in hematopoietic stem cell transplantation: role of cyclophosphamide and busulfan. Transplant Proc. 42:2720–4. doi: 10.1016/j.transproceed.2010.04.024
20. Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Escolar G, et al. The release of soluble factors contributing to endothelial activation and damage after hematopoietic stem cell transplantation is not limited to the allogeneic setting and involves several pathogenic mechanisms. Biol Blood Marrow Transplant. (2009) 15:537–46. doi: 10.1016/j.bbmt.2009.01.013
21. Eissner G, Multhoff G, Holler E. Influence of bacterial endotoxin on the allogenicity of human endothelial cells. Bone Marrow Transplant. (1998) 21:1286–8. doi: 10.1038/sj.bmt.1701264
22. Fuste B, Escolar G, Marin P, Mazzara R, Ordinas A, Diaz-Ricart M. (2004) G-CSF increases the expression of VCAM-1 on stromal cells promoting the adhesion of CD34+ hematopoietic cells: studies under flow conditions. Exp Hematol. 32:765–72 doi: 10.1016/j.exphem.2004.05.023
23. Mercanoglu F, Turkmen A, Kocaman O, Pinarbasi B, Dursun M, Selcukbiricik F, et al. Endothelial dysfunction in renal transplant patients is closely related to serum cyclosporine levels. Transplant Proc. (2004) 36:1357–60. doi: 10.1016/j.transproceed.2004.05.073
24. Zoja C, Furci L, Ghilardi F, Zilio P, Benigni A, Remuzzi G. Cyclosporin-induced endothelial cell injury. Lab Invest. (1986) 55:455–62. doi: 10.1055/s-0038-1644123
25. Biedermann BC, Sahner S, Gregor M, Tsakiris DA, Jeanneret C, Pober JS, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. (2002) 359:2078–83. doi: 10.1016/S0140-6736(02)08907-9
26. Hassan M, Ljungman P, Ringdén O, Hassan Z, Oberg G, Nilsson C, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant. (2000) 25:915–24. doi: 10.1038/sj.bmt.1702377
27. Wolf CR, Moll E, Friedberg T, Oesch F, Buchmann A, Kuhlmann WD, et al. Characterization, localization and regulation of a novel phenobarbital-inducible form of cytochrome P450, compared with three further P450-isoenzymes, NADPH P450-reductase, glutathione transferases and microsomal epoxide hydrolase. Carcinogenesis. (1984) 5:993–1001. doi: 10.1093/carcin/5.8.993
28. DeLeve LD. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. (1996) 24:830–7. doi: 10.1002/hep.510240414
29. DeLeve LD, Wang X. Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology. (2000) 60:143–54. doi: 10.1159/000028359
30. Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M, et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. (2004) 104:1574–7. doi: 10.1182/blood-2003-11-3778
31. Carreras E, Dufour C, Mohty M, Kröger. The EBMT Handbook. Cham: Springer (2019). doi: 10.1007/978-3-030-02278-5
32. Biedermann BC. Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol. (2008) 21:129–38. doi: 10.1016/j.beha.2008.02.003
33. Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. (2009) 13:454–71. doi: 10.1111/j.1582-4934.2008.00639.x
34. Pytlík R, Kideryová L, Benesová K, Cechová H, Veselá R, Rychtrmocová H, et al. Circulating endothelial precursor cells (EPC) in patients undergoing allogeneic haematopoietic progenitor cell transplantation. Folia Biol. (2010) 56:32–5
35. Pihusch V, Rank A, Steber R, Pihusch M, Pihusch R, Toth B, et al. Endothelial cell-derived microparticles in allogeneic hematopoietic stem cell recipients. Transplantation. (2006) 81:1405–9. doi: 10.1097/01.tp.0000209218.24916.ba
36. DeLeve LD, Wang X, Kanel GC, Ito Y, Bethea NW, McCuskey MK, et al. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. (2003) 38:900–8. doi: 10.1002/hep.1840380416
37. Deleve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. (2003) Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 125:882–90. doi: 10.1016/S0016-5085(03)01056-4
38. Ueno T, Bioulac-Sage P, Balabaud C, Rosenbaum J. Innervation of the sinusoidal wall: regulation of the sinusoidal diameter. Anat Rec A Discov Mol Cell Evol Biol. (2004) 280:868–73. doi: 10.1002/ar.a.20092
39. Valla DC. Budd-Chiari syndrome and veno-occlusive disease/sinusoidal obstruction syndrome. Gut. (2008) 57:1469–78. doi: 10.1136/gut.2007.133637
40. European Association for the Study of the Liver. Electronic address:ZWFzbG9mZmljZUBlYXNsb2ZmaWNlLmV1. (2016). EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 64:179–202. doi: 10.1016/j.jhep.2015.07.040
41. Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. (2016) 51:906–12. doi: 10.1038/bmt.2016.130
42. Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. (2015) Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 50:781–9. doi: 10.1038/bmt.2015.52
43. Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. (2011) 17:1713–20. doi: 10.1016/j.bbmt.2011.06.006
44. Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. (1987) 44:778–83. doi: 10.1097/00007890-198712000-00011
45. Myers KC, Dandoy C, El-Bietar J, Davies SM, Jodele S. Veno-occlusive disease of the liver in the absence of elevation in bilirubin in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2015) 21:379–81. doi: 10.1016/j.bbmt.2014.09.026
46. Naples JC, Skeens MA, Auletta J, Rangarajan H, Abu-Arja R, Horwitz E, et al. Anicteric veno-occlusive disease after hematopoietic stem cell transplantation in children. Bone Marrow Transplant. (2015) 51:135–7. doi: 10.1038/bmt.2015.208
47. Faraci M, Bertaina A, Luksch R, Calore E, Lanino E, Saglio F, et al. Sinusoidal Obstruction Syndrome/Veno-Occlusive Disease after Autologous or Allogeneic Hematopoietic Stem Cell Transplantation in Children: a retrospective study of the Italian Hematology-Oncology Association-Hematopoietic Stem Cell Transplantation Group. Biol Blood Marrow Transplant. (2019) 25:313–20. doi: 10.1016/j.bbmt.2018.09.027
48. Mohty M, Labopin M, Lebon D, Berceanu A, Jubert C, Yakoub-Agha I, et al. Efficacy and safety of defibrotide in the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome following hematopoietic stem cell transplantation: interim results from the defifrance study. Bone Marrow Transplant. (2019) 54(Suppl.):231–2. doi: 10.1038/s41409-019-0559-4
49. Lassau N, Leclère J, Auperin A, Bourhis JH, Hartmann O, Valteau-Couanet D, et al. Hepatic veno-occlusive disease after myeloablative treatment and bone marrow transplantation: value of grayscale and Doppler US in 100 patients. Radiology. (1997) 204:545–52. doi: 10.1148/radiology.204.2.9240551
50. Park JE, Choi YH, Cheon JE, Kim WS, Kim IO, Ryu YJ, et al. Gallbladder wall oedema and ascites are independent predictors of progression to hepatic veno-occlusive disease for children with hematopoietic stem celltransplantation. Eur Radiol. (2018) 28:2291–8. doi: 10.1007/s00330-017-5137-9
51. Nishida M, Kahata K, Hayase E, Shigematsu A, Sato M, Kudo Y, et al. Novel ultrasonographic scoring system of sinusoidal obstruction syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2018) 24:1896–900. doi: 10.1016/j.bbmt.2018.05.025
52. Fontanilla T, Hernando CG, Claros JC, Bautista G, Minaya J, Del Carmen Vega M, et al. Acoustic radiation force impulse elastography and contrast-enhanced sonography of sinusoidal obstructive syndrome (Veno-occlusive Disease): preliminary results. J Ultrasound Med. (2011) 30:1593–8. doi: 10.7863/jum.2011.30.11.1593
53. Trenker C, Sohlbach K, Dietrich CF, Görg C. Clinical diagnosis ofveno-occlusive disease using contrast enhanced ultrasound. Bone MarrowTransplant. (2018) 53:1369–371. doi: 10.1038/s41409-018-0198-1
54. Bandali MF, Mirakhur A, Lee EW, Ferris MC, Sadler DJ, Gray RR, et al. Portal hypertension: Imaging of portosystemic collateral pathways and associated image-guided therapy. World J Gastroenterol. (2017) 23:1735–46. doi: 10.3748/wjg.v23.i10.1735
55. Atilla E, Toprak SK, Demirer T. Current review of iron overload and related complications in hematopoietic stem cell transplantation. Turk J Haematol. (2017) 34:1–9. doi: 10.4274/tjh.2016.0450
56. Ravaioli F, Colecchia A, Alemanni LV, Vestito A, Dajti E, Marasco G, et al. Role of imaging techniques in liver veno-occlusive disease diagnosis: recent advances and literature review. Expert Rev Gastroenterol Hepatol. (2019) 13:463–84. doi: 10.1080/17474124.2019.1588111
57. Kis B, Pamarthi V, Fan CM, Rabkin D, Baum RA. Safety and utility of transjugular liver biopsy in hematopoietic stem cell transplant recipients. J Vasc Interv Radiol. (2013) 24:85–9. doi: 10.1016/j.jvir.2012.09.011
58. Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. (2009) 6:573–82. doi: 10.1038/nrgastro.2009.149
59. Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. (2003) 29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001
60. Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. (2012) 142:1293–302.e4. doi: 10.1053/j.gastro.2012.02.017
61. Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. (2012) 143:646–54. doi: 10.1053/j.gastro.2012.05.035
62. Colecchia A, Marasco G, Ravaioli F, Kleinschmidt K, Masetti R, Prete A, et al. Usefulness of liver stiffness measurement in predicting hepatic veno-occlusive disease development in patients who undergo HSCT. Bone Marrow Transplant. (2017) 52:494–7. doi: 10.1038/bmt.2016.320
63. Colecchia A, Ravaioli F, Sessa M, Alemanni VL, Dajti E, Marasco G, et al. Liver stiffness measurement allows early diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome in adult patients who undergo hematopoietic stem cell transplantation: results from a monocentric prospective study. Biol Blood Marrow Transplant. (2019) 25:995–1003. doi: 10.1016/j.bbmt.2019.01.019
64. Reddivalla N, Robinson AL, Reid KJ, Radhi MA, Dalal J, Opfer EK, et al. Using liver elastography to diagnose sinusoidal obstruction syndrome in pediatric patients undergoing hematopoietic stem cell transplant. Bone Marrow Transplant. (2018) 15:523–30. doi: 10.1038/s41409-017-0064-6
65. Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. (2008) 48:606–13. doi: 10.1016/j.jhep.2007.11.020
66. Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, et al. (2017). EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (Long Version). Ultraschall Med. 38:e48. doi: 10.1055/a-0641-0076
67. De Franchis R, Baveno VI Faculty. (2015). Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 63:743–52. doi: 10.1007/978-3-319-23018-4_1
68. Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. (2017) 67:399–411. doi: 10.1016/j.jhep.2017.02.003
69. Zama D, Bossù G, Ravaioli F, Masetti R, Prete A, Festi D, et al. Longitudinal evaluation of liver stiffness in three pediatric patients with veno-occlusive disease. Pediatr Transplant. (2019) 23:e13456. doi: 10.1111/petr.13456
70. Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transplant. (2019) 25:1271–80. doi: 10.1016/j.bbmt.2019.02.018
71. Lee JH, Lee KH, Lee JH, Kim S, Seol M, Park CJ, et al. Plasminogen activator inhibitor-1 is an independent diagnostic marker as well as severity predictor of hepatic veno-occlusive disease after allogeneic bone marrow transplantation in adults conditioned with busulphan and cyclophosphamide. Br J Haematol. (2002) 118:1087–94. doi: 10.1046/j.1365-2141.2002.03748.x
72. Pihusch M, Wegner H, Goehring P, Salat C, Pihusch V, Hiller E, et al. Diagnosis of hepatic veno-occlusive disease by plasminogen activator inhibitor-1 plasma antigen levels: a prospective analysis in 350 allogeneic hematopoietic stem cell recipients. Transplantation. (2005) 80:1376–82. doi: 10.1097/01.tp.0000183288.67746.44
73. Sartori MT, Cesaro S, Peruzzo M, Messina C, Saggiorato G, Calore E, et al. Contribution of fibrinolytic tests to the differential diagnosis of veno-occlusive disease complicating pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. (2012) 58:791–7. doi: 10.1002/pbc.23213
74. Piccin A, Sartori MT, Bisogno G, Van Schilfgaarde M, Saggiorato G, Pierro AMD, et al. New insights into sinusoidal obstruction syndrome. Intern Med J. (2017) 47:1173–83. doi: 10.1111/imj.13550
75. Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. (2010) 16:1005–17. doi: 10.1016/j.bbmt.2010.02.009
76. Akil A, Zhang Q, Mumaw CL, Raiker N, Yu J, Velez de Mendizabal N, et al. Biomarkers for diagnosis and prognosis of sinusoidal obstruction syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2015) 21:1739–45. doi: 10.1016/j.bbmt.2015.07.004
77. Dalle JH, Giralt SA. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: risk factors and stratification, prophylaxis, and treatment. Biol Blood Marrow Transplant. (2016) 22:400–9. doi: 10.1016/j.bbmt.2015.09.024
78. Kantarjian HM, DeAngelo DJ, Advani AS, Stelljes M, Kebriaei P, Cassaday RD, et al. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. (2017) 4:e387–98. doi: 10.1016/S2352-3026(17)30103-5
79. Kantarjian HM, Vandendries E, Advani AS. Inotuzumab ozogamicin for acute lymphoblastic leukemia. N Engl J Med. (2016) 375:2100–1. doi: 10.1056/NEJMc1612040
80. Kebriaei P, Cutler C, de Lima M, Giralt S, Lee SJ, Marks D, et al. Management of important adverse events associated with inotuzumab ozogamicin: expert panel review. Bone Marrow Transplant. (2018) 53:449–56. doi: 10.1038/s41409-017-0019-y
81. Strouse C, Zhang Y, Zhang MJ, DiGilio A, Pasquini M, Horowitz MM, et al. Risk score for the development of veno-occlusive disease after allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant. (2018) 24:2072–80. doi: 10.1016/j.bbmt.2018.06.013
82. Botti S, Orlando L, Gargiulo G, Cecco VD, Banfi M, Duranti L, et al. Veno-occlusive disease nurse management: development of a dynamic monitoring tool by the GITMO nursing group. Ecancermedicalscience. (2016) 10:661. doi: 10.3332/ecancer.2016.661
83. Richardson PG, Carreras E, Iacobelli M, Nejadnik B. The use of defibrotide in blood and marrow transplantation. Blood Adv. (2018) 2:1495–509. doi: 10.1182/bloodadvances.2017008375
84. Pescador R, Capuzzi L, Mantovani M, Fulgenzi A, Ferrero ME. Defibrotide: properties and clinical use of an old/new drug. Vascul Pharmacol. (2013) 59:1–10. doi: 10.1016/j.vph.2013.05.001
85. Eissner G, Multhoff G, Gerbitz A, Kirchner S, Bauer S, Haffner S, et al. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide. Blood. (2002) 100:334–40. doi: 10.1182/blood.V100.1.334
86. Coccheri S, Andreozzi GM, D'Addato M, Gensini GF, PROVEDIS Study Group. Effects of defibrotide in patients with chronic deep insufficiency. The PROVEDIS study. Int Angiol. (2004) 23:100–7.
87. Di Perri T, Vittoria A, Messa GL, Cappelli R. Defibrotide therapy for thrombophlebitis–controlled clinical trial. Haemostasis Suppl. (1986) 16:42–7. doi: 10.1159/000215340
88. Mozzi E, Chiurazzi D, Germiniani R, Pacini F. Effectiveness of defibrotide for prophylaxis of deep venous thrombosis after general surgery: a double-blind, placebo-controlled clinical trial. Haemostasis. Suppl. (1986) 1:36–8. doi: 10.1159/000215338
89. Ciavarella N, Ettorre C, Schiavoni M, Schonauer S, Cicinelli E, Cagnazzo G. Effectiveness of defibrotide for prophylaxis of deep venous thrombosis in gynecological surgery: a double-blind, placebo-controlled clinical trial. Haemostasis. Suppl. (1986) 1:39–41. doi: 10.1159/000215339
90. Craveri A, Tornaghi G, Ranieri R, Stanzani M, Landi G, Paganardi L, et al. Effects of defibrotide after oral and parenteral administration in patients with peripheral obliterative arterial disease (POAD). Int Angiol. (1990) 9:274–7. doi: 10.1016/0268-9499(90)90075-U
91. Milazzotto F, Carelli M, Citone C, Di Marcotullio G, Giampaolo P, Malinconico U, et al. Use of defibrotide in the treatment of acute myocardial infarction. Semin Thromb Hemost. (1989) 15:464–9. doi: 10.1055/s-2007-1002745
92. Tubaro M, Mattioli G, Matta F, Cappello C, Natale E, Ricci R, et al. Defibrotide versus heparin in the prevention of coronary reocclusion after thrombolysis in acute myocardial infarction. Cardiovasc Drugs Ther. (1993) 7:809–16. doi: 10.1007/BF00878935
93. Ferrero ME, Marni A, Rovati M, Aimini R, Salari PC, Gaja G. Defibrotide, a stimulator of prostacyclin (PGI2) production, prevents the effects of ischaemic damage. Int J Tissue React. (1989) 11:179–84.
94. Cimminiello C. Clinical trials with defibrotide in vascular disorders. Semin Thromb Hemost. Suppl. (1996) 1:29–34.
95. Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. (2016) 127:1656–65. doi: 10.1182/blood-2015-10-676924
96. Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Veno-occlusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. (1993) 11:1729–36. doi: 10.1200/JCO.1993.11.9.1729
97. Richardson P, Aggarwal S, Topaloglu O, Villa KF, Corbacioglu S. Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). Bone Marrow Transplant. (2019) 54:1951–62. doi: 10.1038/s41409-019-0474-8
98. Al Beihany A, Al Omar H, Sahovic E, Chaudhri N, Al Mohareb F, et al. Successful treatment of hepatic veno-occlusive disease after myeloablative allogeneic hematopoietic stem cell transplantation by early administration of a short course of methylprednisolone. Bone Marrow Transplant. (2008) 41:287–91. doi: 10.1038/sj.bmt.1705896
99. Myers KC, Lawrence J, Marsh RA, Davies SM, Jodele S. High-dose methylprednisolone for veno-occlusive disease of the liver in pediatric hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. (2013) 19:500–3. doi: 10.1016/j.bbmt.2012.11.011
100. Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following hematopoietic stem cell transplantation. Br J Haematol. (2013) 163:444–57. doi: 10.1111/bjh.12558
101. Smith FO, Johnson MS, Scherer LR, Faught P, Breitfeld PP, Albright E, et al. Transjugular intrahepatic portosystemic shunting (TIPS) for treatment of severe hepatic veno-occlusive disease. Bone Marrow Transplant. (1996) 18:643–6.
102. Campos-Varela I, Castells L, Dopazo C, Pérez-Lafuente M, Allende H, Len O, et al. (2012) Transjugular intrahepatic portosystemic shunt for the treatment of sinusoidal obstruction syndrome in a liver transplant recipient and review of the literature. Liver Transpl. 18:201–5. doi: 10.1002/lt.22351
103. Rapoport AP1, Doyle HR, Starzl T, Rowe JM, Doeblin T, DiPersio JF. Orthotopic liver transplantation for life-threatening veno-occlusive disease of the liver after allogeneic bone marrow transplant. Bone Marrow Transplant. (1991) 8:421–4.
104. Marsa-Vila L, Gorin NC, Laporte JP, Labopin M, Dupuy-Montbrun MC, Fouillard L, et al. Prophylactic heparin does not prevent liver veno-occlusive disease following autologous bone marrow transplantation. Eur J Haematol. (1991) 47:346–54. doi: 10.1111/j.1600-0609.1991.tb01859.x
105. Bearman SI, Hinds MS, Wolford JL, Petersen FB, Nugent DL, Slichter SJ, et al. A pilot study of continuous infusion heparin for the prevention of hepatic veno-occlusive disease after bone marrow transplantation. Bone Marrow Transplant. (1990) 5:407–11.
106. Imran H, Tleyjeh IM, Zirakzadeh A, Rodriguez V, Khan SP. Use of prophylactic anticoagulation and the risk of hepatic veno-occlusive disease in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. (2006) 37:677–86. doi: 10.1038/sj.bmt.1705297
107. Simon M, Hahn T, Ford LA, Anderson B, Swinnich D, Baer MR, et al. Retrospective multivariate analysis of hepatic veno-occlusive disease after blood or marrow transplantation: possible beneficial use of low molecular weight heparin. Bone Marrow Transplant. (2001) 27:627–33. doi: 10.1038/sj.bmt.1702854
108. Forrest DL, Thompson K, Dorcas VG, Couban SH, Pierce R. Low molecular weight heparin for the prevention of hepatic veno-occlusive disease (VOD/SOS) after hematopoietic stem cell transplantation: a prospective phase II study. Bone Marrow Transplant. (2003) 31:1143–9. doi: 10.1038/sj.bmt.1704087
109. Haussmann U, Fischer J, Eber S, Scherer F, Seger R, Gungor T. Hepatic veno-occlusive disease in pediatric stem cell transplantation: impact of pre-emptive antithrombin III replacement and combined antithrombin III/defibrotide therapy. Haematologica. (2006) 91:795–800.
110. Ferrà C, de Sanjosé S, Lastra CF, Martí F, Mariño EL, Sureda A, et al. Pentoxifylline, ciprofloxacin and prednisone failed to prevent transplant-related toxicities in bone marrow transplant recipients and were associated with an increased incidence of infectious complications. Bone Marrow Transplant. (1997) 20:1075–80. doi: 10.1038/sj.bmt.1701023
111. Attal M, Huguet F, Rubie H, Charlet JP, Schlaifer D, Huynh A, et al. Prevention of regimen-related toxicities after bone marrow transplantation by pentoxifylline: a prospective, randomized trial. Blood. (1993) 82:732–6. doi: 10.1182/blood.V82.3.732.732
112. Song JS, Seo JJ, Moon HN, Ghim T, Im HJ. Prophylactic low-dose heparin or prostaglandin E1 may prevent severe veno-occlusive disease of the liver after allogeneic hematopoietic stem cell transplantation in Korean children. J Korean Med Sci. (2006) 21:897–903. doi: 10.3346/jkms.2006.21.5.897
113. Bearman SI, Shen DD, Hinds MS, Hill HA, McDonald GB. A phase I/II study of prostaglandin E1 for the prevention of hepatic venocclusive disease after bone marrow transplantation. Br J Haematol. (1993) 84:724–30. doi: 10.1111/j.1365-2141.1993.tb03152.x
114. Gluckman E, Jolivet I, Scrobohaci ML, Devergie A, Traineau R, Bourdeau-Esperou H, et al. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br J Haematol. (1990) 74:277–81. doi: 10.1111/j.1365-2141.1990.tb02583.x
115. Essell JH, Schroeder MT, Harman GS, Halvorson R, Lew V, Callander N, et al. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. (1998) 128(12 Pt 1):975–81. doi: 10.7326/0003-4819-128-12_Part_1-199806150-00002
116. Ohashi K, Tanabe J, Watanabe R, Tanaka T, Sakamaki H, Maruta A, et al. The Japanese multicenter open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol. (2000) 64:32–8. doi: 10.1002/(SICI)1096-8652(200005)64:1<32::AID-AJH6>3.0.CO;2-N
117. Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. (2002) 100:1977–83. doi: 10.1182/blood-2001-12-0159
118. Tay J, Tinmouth A, Fergusson D, Huebsch L, Allan DS. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2007) 13:206–17. doi: 10.1016/j.bbmt.2006.09.012
119. Park SH, Lee MH, Lee H, Kim HS, Kim K, Kim WS, et al. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. (2002) 29:137–43. doi: 10.1038/sj.bmt.1703342
120. Corbacioglu S, Hönig M, Lahr G, Stöhr S, Berry G, Friedrich W, et al. Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD/SOS, which could be prevented with defibrotide. Bone Marrow Transplant. (2006) 38:547–53. doi: 10.1038/sj.bmt.1705485
121. Qureshi A, Marshall L, Lancaster D. Defibrotide in the prevention and treatment of veno-occlusive disease in autologous and allogeneic stem cell transplantation in children. Pediatr Blood Cancer. (2008) 50:831–2. doi: 10.1002/pbc.21425
122. Cappelli B, Chiesa R, Evangelio C, Biffi A, Roccia T, Frugnoli I, et al. Absence of VOD/SOS in paediatric thalassaemic HSCT recipients using defibrotide prophylaxis and intravenous Busulphan. Br J Haematol. (2009) 147:554–60. doi: 10.1111/j.1365-2141.2009.07871.x
123. Chalandon Y, Roosnek E, Mermillod B, Newton A, Ozsahin H, Wacker P, et al. Prevention of veno-occlusive disease with defibrotide after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. (2004) 10:347–54. doi: 10.1016/j.bbmt.2004.01.002
124. Dignan F, Gujral D, Ethell M, Evans S, Treleaven J, Morgan G, et al. Prophylactic defibrotide in allogeneic stem cell transplantation: minimal morbidity and zero mortality from veno-occlusive disease. Bone Marrow Transplant. (2007) 40:79–82. doi: 10.1038/sj.bmt.1705696
Keywords: VOD/SOS, HSCT, defibrotide, elastometry, liver stiffness measurement
Citation: Bonifazi F, Barbato F, Ravaioli F, Sessa M, Defrancesco I, Arpinati M, Cavo M and Colecchia A (2020) Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 11:489. doi: 10.3389/fimmu.2020.00489
Received: 01 September 2019; Accepted: 03 March 2020;
Published: 03 April 2020.
Edited by:
Hildegard Theresia Greinix, Medical University of Graz, AustriaReviewed by:
Tapani Ruutu, Helsinki University Central Hospital, FinlandCopyright © 2020 Bonifazi, Barbato, Ravaioli, Sessa, Defrancesco, Arpinati, Cavo and Colecchia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Bonifazi, ZnJhbmNlc2NhLmJvbmlmYXppQHVuaWJvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.