- 1Department of Urology, The Third Affiliated Hospital of Soochow University, Changzhou, China
- 2Department of Anesthesiology, University of Connecticut, Mansfield, CT, United States
- 3Shanghai Key Laboratory of Acupuncture Mechanism and Acupoint Function, Fudan University, Shanghai, China
Hypoxia and ischemia are the main underlying pathogenesis of stroke and other neurological disorders. Cerebral hypoxia and/or ischemia (e.g., stroke) can lead to neuronal injury/death and eventually cause serious neurological disorders or even death in the patients. Despite knowing these serious consequences, there are limited neuroprotective strategies against hypoxic and ischemic insults in clinical settings. Recent studies indicate that microRNAs (miRNAs) are of great importance in regulating cerebral responses to hypoxic/ischemic stress in addition to the neuroprotective effect of the δ-opioid receptor (DOR). Moreover, new discovery shows that DOR can regulate miRNA expression and inhibit inflammatory responses to hypoxia/ischemia. We, therefore, summarize available data in current literature regarding the role of DOR and miRNAs in regulating the neuroinflammatory responses in this article. In particular, we focus on microglia activation, cytokine production, and the relevant signaling pathways triggered by cerebral hypoxia/ischemia. The intent of this review article is to provide a novel clue for developing new strategies against neuroinflammatory injury resulting from cerebral hypoxia/ischemia.
Introduction
Neurons in the mammalian central nervous system are extremely vulnerable to deprivation of oxygen and blood supply. Once the neurons are deprived of oxygen or blood supply, many pathological events are triggered including inflammatory changes in the brain. In addition, local accumulation of metabolic wastes also leads to local/regional tissue dysfunction and/or damage (1). Moreover, the reestablishment of blood flow and/or oxygen supply further enlarges the area of cell death and/or tissue damage secondary due to the activation of immune responses and cell death programs (2). Although the past studies have identified numerous events/molecules that are critical determinants of neural survival/death under hypoxic/ischemic conditions, there is still a paucity of neuroprotective strategies against hypoxic/ischemic insults in the clinical settings. Therefore, there is an urgent need to advance our understanding of hypoxic/ischemic process and explore various novel therapies against the cerebral injury caused by hypoxia/ischemia.
Thus far, microRNAs (miRNAs) and DOR have been identified as key factors in regulating neuroinflammatory and other biological processes under cerebral hypoxia and ischemia. MicroRNAs are short RNA molecules (a class of ~21- to 25-ribonucleotide single-strand non-coding RNA molecules) and can be found in all eukaryote cells (3). They bind to target messenger RNAs (mRNAs) through base pairing with the 3′ untranslated regions (3′-UTRs), modulating direct cleavage and/or translational repression of target mRNAs (4). Up to now, more than 2,000 human miRNAs have been identified (5). These miRNAs are found to play important roles in a wide spectrum of processes under both physiological and pathological conditions, for example, hypoxia and ischemia. The spatiotemporal properties of miRNA expression provide complex regulatory networks in the mammalian cells. Thus, a better understanding of miRNAs and their roles in repression of target mRNA and gene silencing is warranted to unveil the potential therapeutics against hypoxic/ischemic injury by targeting specific miRNA molecules in the brain.
Opioid receptors belong to the large family of seven transmembrane G protein–coupled receptors. There are three major types of opioid receptors, the μ-, κ-, and δ-opioid receptors (known as MOR, KOR, and DOR) (6). Although there exists only one DOR gene, two DOR subtypes, namely, DOR1 and DOR2, are identified according to their pharmacological attributes (7). High levels of endogenous DOR mRNA are expressed in the brain (especially in cortex and striatum) and dorsal root ganglion (6, 8–10). Our previous studies have shown that DOR activation and/or overexpression induce a protective effect against neuronal injury in hypoxic/ischemic conditions (11, 12). Such observations were made through serial studies on primary cultured neurons under hypoxia (13–15), brain slices in oxygen–glucose deprivation (OGD) (16–20), and in vivo brain exposed to cerebral hypoxia/ischemia (21–24). Our observations on DOR neuroprotective effect have been confirmed by other independent laboratories (25–32). δ-opioid receptor neuroprotection is mediated by several important pathways, including an increase in cellular antioxidant activity and inhibition of cell death signaling. Moreover, there is growing evidence suggesting that the DOR neuroprotection against hypoxic/ischemic injury may be achieved by modulating miRNAs because DOR regulates miRNA expression in different organs such as brain, kidney, heart, and liver in hypoxia (33–36). Therefore, it is possible to protect organs against hypoxic/ischemic injury by targeting specific miRNA molecules directly or indirectly through DOR signaling.
In this article, we reviewed the effects of DOR activation on miRNAs and neuroinflammatory responses to hypoxic/ischemic insults. First, we discussed the effect of hypoxia/ischemia on the expression of cerebral miRNAs. Second, we summarized the miRNA-mediated neuroinflammatory events under hypoxic/ischemic conditions. Third, we indicated various miRNAs involved in microglia activation, cytokine production, and cell signaling under hypoxia and ischemia. Fourth, we discussed the effect of DOR activation on the miRNA expression. Finally, we briefly commented on the potential use of circulating miRNAs as biomarkers and possible targets for clinical treatment against hypoxic/ischemic injury.
Effects of Hypoxia/Ischemia on Cerebral miRNA Expression
The biogenesis of miRNA in mammalian cells required multistep process that begins with transcription of the primary miRNAs (pri-miRNAs) by RNA polymerase II in the nucleus. MicroRNA genes are transcribed either from introns of protein-coding genes or by intergenic miRNAs under the control of their own promoters (37). Primary miRNAs are cleaved by microprocessors including DROSHA and DiGeorge syndrome critical region 8 (DGCR8), to produce the ~60- to 70-nucleotide stem-loop precursor miRNAs (pre-miRNAs). The pre-miRNAs are then exported to the cytoplasm via exportin-5 and further processed by Dicer. One strand of the mature miRNA is loaded into the RNA-induced silencing complex (RISC), whereas the remaining strand is released and degraded. Mature miRNA guides RISC to target transcripts by sequence complementary binding and mediates gene suppression (38). Current literature suggests that hypoxia/ischemia can regulate miRNA expression at various steps throughout its biogenesis pathway. For instance, transcriptional activities of miRNA genes can be affected by epigenetic modifications (e.g., DNA methylation and histone modification) and/or binding of different transcriptional factors [e.g., HIF, nuclear factor (NF-κB), and p53] that are involved in various biological and inflammatory responses. Hypoxia/ischemic condition also affects the expression of some enzymes, e.g., Drosha, Dicer, and AGO2, which participate in the regulation of pri-miRNA processing and the maturation of various miRNAs. Finally, miRNA–RISC complex configurations are modulated under hypoxic/ischemic conditions (39, 40).
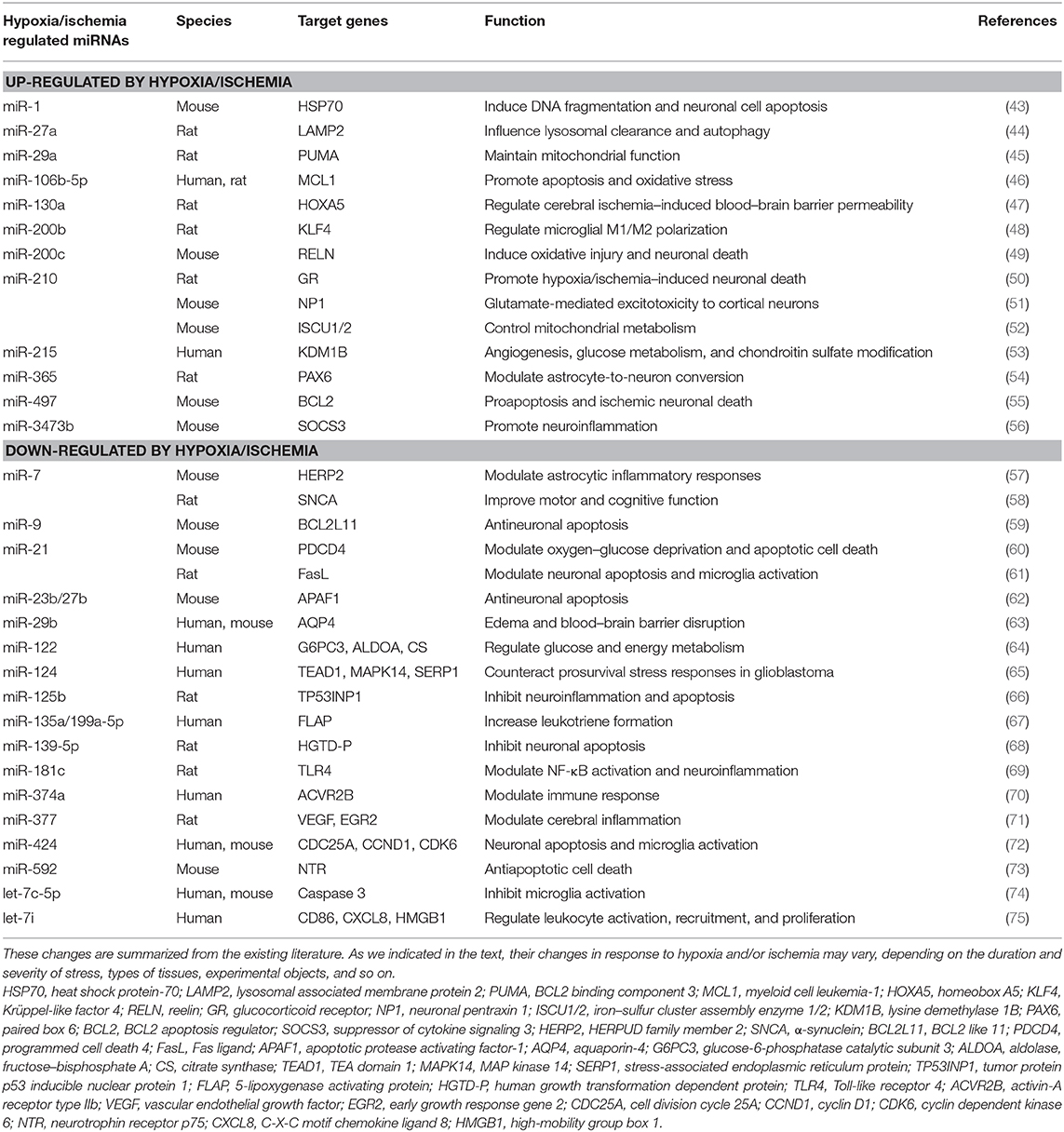
Current research has shown that miRNAs play an important role in response to the hypoxic/ischemic insults to the brain, a hypoxia/ischemia–sensitive organ (34, 39–42). Indeed, cerebral hypoxic/ischemic stress regulates miRNA expression and significantly affects neuronal functions and survival (Table 1). In addition, clinical studies also showed that stroke patients had a dysregulation in global miRNA profiles several months after the original hypoxic/ischemic insults (76, 77). It is reported that mild/moderate hypoxic/ischemic stress may induce cell proliferation, migration, and angiogenesis (78, 79), whereas a severe/prolonged hypoxia/ischemic stress causes apoptosis and necrosis. The differential cellular signaling is partially mediated by the miRNA-induced repression of gene expression (62, 80).
Hypoxia-inducible factor 1 (HIF-1) lies at the epicenter of the complex regulatory network that involves hundreds of protein-coding genes and directly regulates the expression of certain miRNAs via its transcriptional factor activity in the brain. It is a key transcriptional factor for cerebral hypoxic/ischemic responses (81, 82). MiR-210 was one of the first hypoxia-sensitive miRNAs discovered as a direct transcriptional target of HIF (83). Upon hypoxic exposure, miR-210 transcription is dynamically induced through HIF-1α interaction with the hypoxia-response element (HRE) located within its promoter region (40, 52). Evidence indicates that HIF-1 may also contribute to the transcriptional activation of other hypoxia-sensitive miRNAs (e.g., miR-26, miR-181, and miR-26) through direct binding to HREs in their respective promoter regions (83, 84). The regulation has been confirmed by experiments including luciferase-based promoter-reporter assays and chromatin immunoprecipitation (ChIP) assays (83).
Several studies have attempted to summarize the complex hypoxia/ischemia–induced miRNA alternations in the brain and found that the hypoxic/ischemic miRNA changes are highly variable following the different durations of hypoxia/ischemia, the level of oxygen concentration, and the types of cell/animal models. Different miRNAs exhibit diverse responses to different durations of hypoxic/ischemic insults in the same brain region. Our previous study examined the effects of hypoxia on miRNA expression in the rat cortex. The expression of miR-29b, miR-101b, miR-298, miR-324-3p, miR-347, and miR-466b were significantly down-regulated following 1-day exposure to hypoxia. However, some other miRNAs were more tolerant to hypoxic insult. For instance, a down-regulated expression of miR-31 and miR-186 was observed only following a continuous 5-day hypoxia exposure. The expression of miR-29a, let-7f, and miR-511 remain unchanged until a continuous 10-day exposure of hypoxia (34). These findings suggest that cortical miRNAs respond differently to hypoxic stress, depending on the durations of exposure.
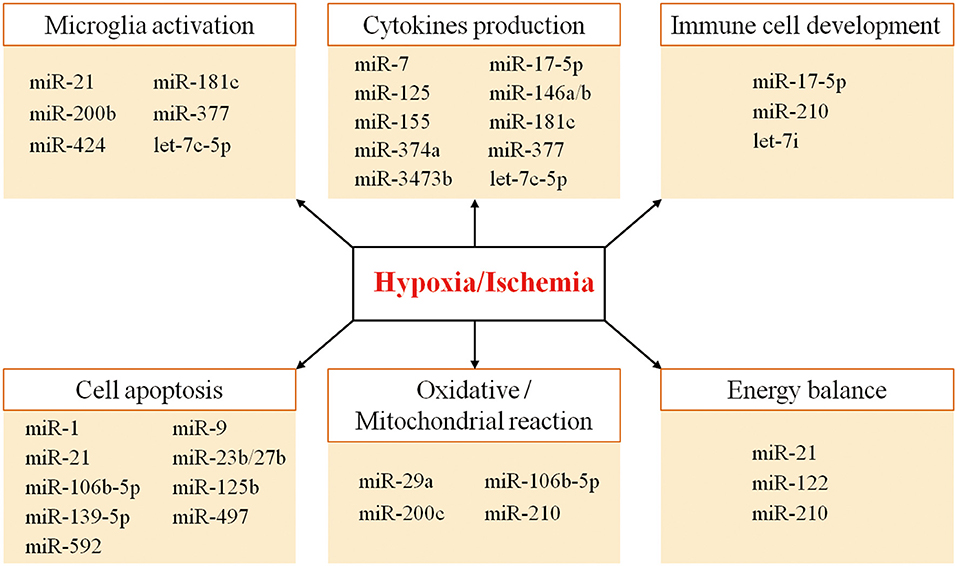
Thus, the combinatorial and coordinated regulation of miRNAs forms a complex network to regulate target genes in response to hypoxic/ischemic insult. As illustrated in Figure 1, many miRNAs regulate neuroinflammation events such as microglia activation, cytokine production, and immune cell development, as well as other biological processes such as apoptosis, oxidative/mitochondrial reaction, and energy balance in responding to cerebral hypoxia/ischemia (Figure 1). For instance, the hypoxia-sensitive miR-210 is potently induced by hypoxia via an HIF-1–dependent manner and in turn stabilizes HIF-1 by targeting glycerol-3-dehydrogenase-like 1, forming a positive feedback regulatory loop (85). Table 1 summarizes miRNA changes in cerebral hypoxia/ischemia with defined target genes based on both animal and clinical studies.
The miRNA-Mediated Neuroinflammatory Events Under Hypoxia/Ischemia
Hypoxic and/or ischemic injuries are well-documented entities in the pathogenesis of cerebrovascular diseases such as stroke, and neurodegenerative diseases, including Alzheimer's disease (AD) and Parkinson's disease (PD). The effects of hypoxia/ischemia on miRNA expression in the brain have been widely investigated in different animal models and clinical settings. Khoshnam et al. (86) reviewed the interplay of miRNAs in the inflammatory processes following ischemic stroke.
Cerebral hypoxia/ischemia greatly affects key inflammatory transcription factors including NF-κB (87). It also induces the synthesis and release of inflammatory mediators, enzymes, and cytokines (87, 88). On the other side, hypoxia/ischemia can greatly alter miRNA expression. A brief summary of hypoxia/ischemia–sensitive miRNAs and their targeting genes involved in neuronal inflammatory and immune responses is shown in Table 1.
MicroRNAs regulate inflammatory response by affecting microglia activation in cerebral hypoxia/ischemia. A significant reduction of miR-424 expression was discovered in circulating lymphocytes of patients with ischemic stroke (72). Similar findings are also observed in mouse plasma and brain tissues after ischemia. In contrast, miR-424 overexpression caused G1 phase cell-cycle arrest by translational suppression of key activators of G1/S transition (e.g., CDC25A, cyclin D1, and CDK6) in microglia cells, thus attenuating ischemic brain injury by inhibiting neuronal apoptosis and microglia activation (72). In an animal model of middle cerebral artery occlusion (MCAO), there is an elevation of miR-200b expression after brain ischemia. MiR-200b up-regulation was able to induce proneuroinflammatory cytokine production and microglia M1 polarization via directly targeting KLF4 (48). Similarly, let-7c-5p was significantly altered in the plasma of patients with ischemic stroke and in MCAO mice (74). The ischemic induction of proinflammatory mediators [e.g., inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6)] in the ischemic cortex were attenuated by the overexpression of let-7c-5p (74). The neuroprotective effect is likely achieved by the direct regulation of let-7c-5p on caspase 3 levels, which is an important regulator of microglia M1/M2 polarization (89–91). MiR-377 plays a proinflammatory role following ischemic stroke by modulating the anti-inflammation effect of EGR2 and the proangiogenesis effect of vascular endothelial growth factor (VEGF) (71). Fan et al. (71) found that the level of miR-377 decreased in the rat brain after MCAO and cultured microglia cells exposed to OGD. Furthermore, they found that miR-377 knockdown could attenuate microglia activation and the release of proinflammatory cytokines after OGD. In addition, miR-21 can induce neuronal protection via suppression of FasL and inhibition of microglia activation (61), whereas OGD down-regulates miR-21 expression in rat primary microglial cells, suggesting an up-regulation of miR-21 may induce a protective effect against ischemic injury.
Hypoxia/ischemia–sensitive miRNAs can alter the production of inflammatory cytokines through direct or indirect pathways. For instance, miRNA-181c suppresses the expression of TNF-α, a key inflammatory cytokine, by binding to the 3′-UTR (92). Toll-like receptor 4 (TLR4) was confirmed to be another direct target of miR-181c. Down-regulation of miR-181c expression in primary microglia under hypoxia promoted TLR4 expression and NF-κB activation, increasing the downstream production of proinflammatory mediators (69). MiR-125b was also found to activate the NF-κB signals by targeting a negative NF-κB regulator, for example, the TNF-α-induced protein 3. On the other hand, miR-125b is a direct NF-κB transcriptional target. Thus, there is a positive self-regulatory loop of miR-125–NF-κB for strengthening and prolonging NF-κB activity (93).
Ischemia–reperfusion injury resulted in a reduction of miR-125b in rat brain and increase in protein levels of TP53INP1, p53, cytokines IL-1β, and TNF-α (66). Two hypoxia-related miRNAs, miR-146a and miR-155, are found to play an important role in NF-κB–mediated inflammatory responses following hypoxia (40, 42). Tumor necrosis factor receptor–associated factor 6, IL-1 receptor–associated kinase 1, and TLR4 are direct target genes of miR-146a/b (94–96). Hypoxia can promote miR-146a expression, thus down-regulating expression of proinflammation targets (97). In rat ischemic model, the brain and circulation miR-155 was down-regulated (98). Reducing miR-155 facilitates the downstream proinflammatory signaling via multiple targets including inositol phosphatase SHIP1, MyD88, and SOCS1 required for TLR/IL-1R signaling (99–102). In the endotoxin mouse model, Alexander et al. (103) further confirmed the importance of exosome-delivered miR-155 and miR-146a in regulating the immune responses; for example, miR-146a reduced, whereas miR-155 enhanced the inflammatory responses. There are several examples for the miRNA-modulated production of inflammatory cytokines, including the miR-3473b-SOCS3-iNOS/COX-2/TNF-α/IL-6 axis in stroke pathology (56), miR-374a-ACVR2B-IL-6/IL-8 axis in the infants with hypoxic/ischemic encephalopathy (70), and endoplasmic reticulum stress-related miR-7-HERP2-TNF-α/IL-1β axis in mouse astrocytes exposed to OGD insult (57).
Various studies have demonstrated that miR-210 serves as a key mediator of hypoxia/ischemia–dependent responses. Thus far, a complex spectrum of target genes for miR-210 has been identified. These genes are involved in angiogenesis, cell proliferation, mitochondrial metabolism, inflammatory response, and so on (52, 104–107). MiR-210, through HIF-1α-dependent pathway, can modulate T-cell differentiation in hypoxia, limit inflammatory cytokine production, and decrease the severity of disease (108). MiR-210 also mediates immunosuppression and immune escape under hypoxic conditions in cancer cells (109, 110). Elevated miR-210 in oxygen-deprived regions of tumors decreases cell susceptibility to lysis by antigen-specific cytotoxic T lymphocytes (109). In addition, miR-210 was shown to enhance myeloid-derived suppressor cell–mediated T-cell suppression by targeting Arg1, CXCL12, and IL-16 expression, thus facilitating tumor growth by increasing arginase activity and nitric oxide production (110). Interestingly, germline deletion of miR-210 in mice resulted in the development of autoantibodies, whereas overexpressing miR-210 exhibited impaired class-switched antibody responses, expanding the immune regulatory function of miR-210 to B cell activation and autoantibody production (111). Taken together, these findings underscored a key regulatory role for hypoxia-induced miR-210 in immune cell development. Apart from well-studied miR-210, decreased expression of let-7i and miR-17-5p were discovered in patients with acute ischemic stroke and in animal post–hypoxia/ischemia models. Let-7i regulates several signals involved in leukocyte activation, recruitment, and proliferation involving of CD86 signaling in T helper cells, high-mobility group box 1 (HMGB1) signaling, and CXCL8 signaling (75). MiR-17-5p regulates NLRP3 inflammasome activation, caspase 1 cleavage, and IL-1β production in the rat model of brain damage (112).
The NF-κB signaling is a key factor in inflammatory responses and functions to modulate several hypoxic/ischemic–sensitive miRNAs. This phenomenon has been observed in both acute and chronic hypoxic conditions (113, 114). Systematic screening approach was applied in identifying several NF-κB–regulated miRNAs, including miR-146a, miR-155, miR-21, miR-130, miR-210, and so on (94, 115–117). Researchers have discovered the NF-κB binding sites located at the promoter regions of miR-21, miR-130, and miR-210. Using ChIP and luciferase reporter assays, direct interactions between NF-κB and promoters of miR-21, miR-130, and miR-210 have been confirmed (116, 117).
In conclusion, multiple miRNAs and transcriptional factors form positive or negative feedback regulatory loops that actively participate in hypoxia/ischemia–induced neuroinflammatory responses.
δ-Opioid Receptor–Induced Alterations in miRNA Expression in the Brain Under Hypoxic Condition
Our recent studies and those of others have provided a strong evidence supporting DOR-mediated neuroprotection in the brain, especially in the cortex (7). An important aspect of the DOR-mediated neuroprotection is its ability in maintaining cellular ionic homeostasis. Specially, DOR activation attenuates Na+ influx through the membrane, reduces the increase in intracellular Ca2+, and decreases the excessive leakage of intracellular K+ (12, 16–20). δ-opioid receptor activation reduces hypoxic/ischemic disruption of ionic homeostasis by inhibiting the Na+ channels and N-methyl-D-aspartate receptors (19) and activating a PKC-dependent signaling pathway (16, 20). Another aspect of DOR-mediated neuroprotection is the reduction of glutamate-induced excitotoxic neuronal injury by enhancing antioxidant ability and inhibiting caspase signaling (15, 21, 118, 119). δ-opioid receptor activation can also inhibit the production of inflammatory cytokines in hypoxic/ischemic conditions. In a rat model, DOR activation improved rat survival, which is associated with a significant decrease in release of proinflammatory molecules (e.g., TNF-α, IL-1β, and HMGB1) (120). δ-opioid receptor activation suppressed TNF-α expression and retinal ischemia-induced cell death (121). Our studies discovered that DOR activation has an inhibitory effect on hypoxia-induced increase in TNF-α in cultured rat astrocytes (122). Moreover, DOR plays an important role in neuroprotection against hypoxic/ischemic stress by regulating the expression of inflammatory and anti-inflammatory cytokines in the neonatal brain (123). The underlying mechanism is partially mediated by Nrf-2/HO-1/NQO-1 signaling (123) and, at least partially, involved in DOR-mediated miRNA regulation.
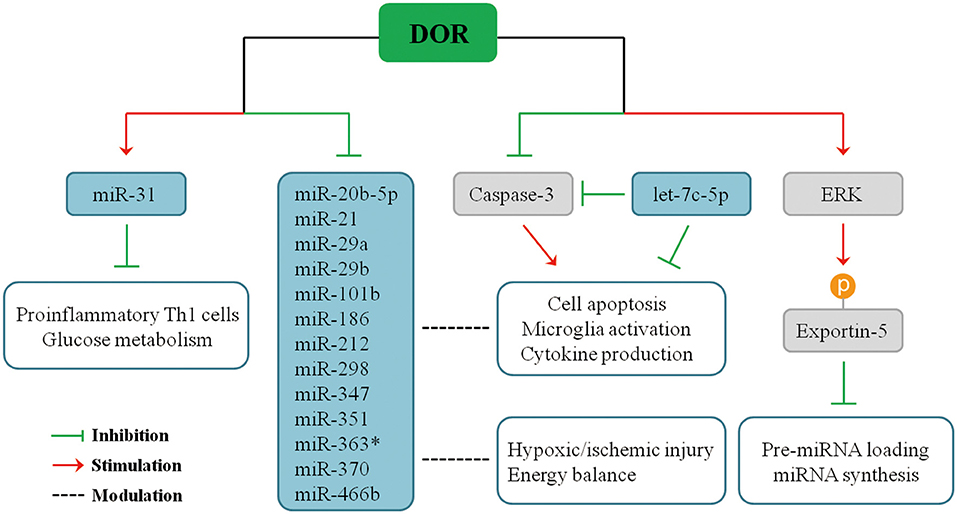
Recent studies demonstrated that DOR activation modulates the expression of miRNAs in multiple organs in response to hypoxic stress. Our serial studies demonstrated that DOR activation has a positive or negative impact on different miRNAs in the brain, kidney, heart, and liver in hypoxia. Using microarray and quantitative real-time polymerase chain reaction (PCR) analysis to measure miRNA expression and applying UFP-512, a potent and specific DOR agonist, to activate DOR in Sprague–Dawley rats (33–36), we found that some miRNAs significantly change their expression upon DOR activation in the brain under normoxic condition, and such modulation becomes more profound in hypoxic/ischemic conditions, especially after a prolonged period. For instance, DOR activation did not alter the brain miR-31 expression under normoxic condition, but it led to a 50% increase in miR-31 levels after 1-day hypoxic exposure, suggesting that DOR activation up-regulates miR-31 in hypoxia (34), thus inhibiting proinflammatory TH1 cells and cell glucose metabolism (124, 125). In the same animal model, DOR activation reduced the levels of miR-347 and miR-466b in the cortex after prolonged hypoxia as compared to DOR activation in normoxic animals (34). Moreover, 5-day hypoxia had no significant effect on cortical miR-21 and miR-370 expression. However, the combination of hypoxia and DOR activation produced a dramatic 70% suppression in both miR-21 and miR-370 levels. Moreover, cortical miR-21 and miR-370 expression remained unchanged when DOR agonist was applied under normoxic conditions, and the regulatory effect of DOR was shown when the animals were exposed to prolonged hypoxia (34). Similarly, many other cortical miRNAs had a sensitive response to DOR activation and changed their expression in hypoxic condition, including miR-20-5p, miR-29a, miR-29b, miR-101b, miR-186, miR-212, miR-298, miR-351, and miR-363* (34). The effects of DOR activation on miRNA expression after pronged hypoxia are summarized in Figure 2.
Therefore, DOR activation can affect miRNA-mediated neuroinflammatory responses under hypoxic/ischemic conditions through direct or indirect pathways. The expression of miR-21, miR-29a, and miR-29b are directly regulated upon DOR activation and thus affecting cellular events mediated by target genes. On the other side, DOR activation modulates some key molecules and transcriptional factors and affects miRNA biogenesis or cellular inflammatory responses, eventually affecting neuronal survival/death. We have found that DOR activation up-regulates extracellular-signal-regulated kinase (ERK) activity, and ERK signaling mediates DOR neuroprotection (14). ERK is found to suppress pre-miRNA export from the nucleus to cytoplasm through phosphorylation of exportin-5, resulting in a global reduction of pre-miRNA loading and miRNA synthesis in cancer cells (126, 127). The similar regulatory mechanism may exist in the brain tissue. Besides ERK signaling, DOR activation also reduces caspase 3 expression (21). Mounting evidence suggests that caspase 3 is an important regulator of microglia activation and inflammation-mediated neurotoxicity independently from its apoptotic activity. Indeed, activated caspase 3 in microglia was found in several neurodegenerative disease models including PD and AD (91), partially accounting for neuroinflammation in the neurodegenerative brains. Caspase inhibitors can hinder microglia activation and consequently reduce neuroinflammation (91, 128, 129). Interestingly, caspase 3 is a direct target of let-7c-5p (74), suggesting a potential regulatory network among DOR, let-7c-5p, caspase signaling, and microglia-mediated neuroinflammation.
Potential Clinical Applications and Therapeutic Targets
MicroRNAs have been implicated as an important player in response to brain hypoxia/ischemia. Emerging evidence shows that multiple hypoxia/ischemia–sensitive miRNAs may serve as potential clinical biomarkers for brain injury and the progression of neurological disorders (130). In addition, certain circulatory miRNAs are proved to correlate with their changes in the brain with pathological alterations (131). These specific circulatory miRNA(s) may serve as a biomarker(s) for neuropathological changes and the prognosis. For example, a positive correlation between blood and brain levels of miR-210, a master and pleiotropic miRNA sensitive to hypoxia, was reported in patients with ischemic stroke and ischemic mouse models. Moreover, the levels of miR-210 in stroke patients with good outcomes were significantly higher than those in patients with poor outcomes (132). Tiedt et al. (133) identified circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers for acute ischemic stroke by RNA sequencing followed by real-time PCR validation. Liu et al. (98) also performed brain and whole-blood miRNA expression profiles in rat models with different neurological disorders and identified a set of blood miRNAs (e.g., miR-298, miR-155, and miR-362-3p) that are correlated with different disease statuses. All these studies strongly suggest that certain blood miRNAs can serve as biomarkers for hypoxia/ischemia–relevant neurological diseases. Further studies on miRNAs that respond to hypoxia/ischemia and their regulatory mechanisms may lead to major improvements in diagnostic medicine and disease prognosis.
A large number of mediators and intracellular signaling are actively participating in neuroinflammatory responses to hypoxia/ischemia. Targeting selected miRNAs that have the capacity to modulate these inflammatory mediators and signaling may be potentially used to develop new therapies. For instance, IL-10 is an anti-inflammatory molecule participating in tissue repair and cytoprotective effects in ischemic brain tissue (134). Interleukin 10 can be directly regulated by several miRNAs, for example, miR-106a, miR-4661, miR-98, miR-27, let-7, and miR-1423 post-transcriptionally (135). Moreover, miR-21 can indirectly regulate IL-10 via down-regulation of the IL-10 inhibitor PDCD4 [Table 1; (136)]. An interaction between miRNAs and IL-10 has been implicated to play a vital role in inflammatory and autoimmune diseases (137, 138). Moreover, inhibition of miR-155 can trigger IL-10–mediated anti-inflammatory responses, resulting in improved post-stroke recovery (139). Furthermore, modulating miR-210 and miR-107 can result in VEGF-associated post-stroke angiogenesis and neurogenesis, suggesting that miR-210 and miR-107 may serve as a potential strategy for stroke treatment (140, 141).
Because DOR is neuroprotective and has a broad influence on miRNA expression in hypoxia, it is likely to develop new protective strategies against hypoxic/ischemic brain injury by directly or indirectly targeting certain miRNAs. There is a high level of endogenous DOR expression in the cortex, striatum, temporal lobe, putamen, and caudate nucleus (6, 8–10, 142). All these brain regions are commonly attacked by stroke and other neurodegenerative diseases. A short-term attack, for example, acute hypoxia, may up-regulate DOR expression (7), but prolonged hypoxia largely reduced the level of DOR in the brain (7, 11, 14). An increase in the DOR expression and/or activity may greatly render these brain regions more tolerant to hypoxic/ischemic insults. Unfortunately, this is a difficult, if not impossible, strategy for the patients at present. Alternatively, applying specific DOR agonist, through miRNA- and non–miRNA-mediated neuroprotection, may be a more reliable clinical approach. However, there are still some limitations in the clinical application of DOR agonists because of opioid addiction/tolerance and other issues such as low specificity of opioid ligands (6, 143). Also, chronic opioid administration has an inhibitory effect on immune ability, for example, immunosuppression, including antibody production, natural killer cell activity, cytokine expression, and phagocytic activity (144). More in-depth research on the molecular mechanisms of DOR-mediated miRNA regulation, including the effects of DOR on miRNA splicing and maturation process, may lead to an alternative and reliable way for the DOR-miRNA–mediated neuroprotection against hypoxic/ischemic brain injury.
Author Contributions
Y-MC searched the literature and prepared the manuscript. X-ZH and S-MW participated in the preparation of the manuscript. YX initiated the project, made the outline and revised the manuscript.
Funding
YX was supported by the National Natural Science Foundation of China (81873361) and Science and Technology Commission of Shanghai Municipality (18401970100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
3′-UTRs, 3′ untranslated regions; AD, Alzheimer disease; BBB, Blood–brain barrier; ChIP, chromatin immunoprecipitation; DGCR8, DiGeorge syndrome critical region 8; DOR, δ-opioid receptors; GPD1L, glycerol-3-dehydrogenase-like 1; HIF-1, hypoxia inducible-factor 1; HRE, hypoxia-response-element; IR, Ischemia–reperfusion; IRAK1, interleukin-1 receptor-associated kinase 1; KOR, kappa-opioid receptor; MCAO, middle cerebral artery occlusion; miRNAs, microRNAs; MOR, mu-opioid receptor; mRNA, messenger RNA; OGD, oxygen–glucose deprivation; PD, Parkinson disease; pre-miRNAs, precursor miRNAs; pri-miRNAs, primary miRNAs; RISC, RNA-induced silencing complex; TLR4, Toll-like receptor 4; TRAF6, TNF receptor associated factor 6.
References
1. Zhao H, Alam A, Soo AP, George AJT, Ma D. Ischemia-reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine. (2018) 28 31–42. doi: 10.1016/j.ebiom.2018.01.025
2. Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. (2011) 17:1391–401. doi: 10.1038/nm.2507
3. Guo L, Zhao Y, Yang S, Zhang H, Chen F. Integrative analysis of miRNA-mRNA and miRNA-miRNA interactions. BioMed Res Int. (2014) 2014:907420. doi: 10.1155/2014/345605
4. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. (2009) 136:215–33. doi: 10.1016/j.cell.2009.01.002
5. Friedlander MR, Lizano E, Houben AJ, Bezdan D, Banez-Coronel M, Kudla G, et al. Evidence for the biogenesis of more than 1,000 novel human microRNAs. Genome Biol. (2014) 15:R57. doi: 10.1186/gb-2014-15-4-r57
6. Xia Y. Neural Functions of the Delta-Opioid Receptor. New York, NY; Heidelberg; Dordrecht; London: Springer (2015). p. 1–684. doi: 10.1007/978-3-319-25495-1
7. Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. (2012) 13:230–46. doi: 10.2174/138945012799201612
8. Xia Y, Haddad GG. Ontogeny and distribution of opioid receptors in the rat brainstem. Brain Res. (1991) 549:181–93. doi: 10.1016/0006-8993(91)90457-7
9. Xia Y, Haddad GG. Major difference in the expression of δ-and μ-opioid receptors between turtle and rat brain. J Comp Neurol. (2001) 436:202–10. doi: 10.1002/cne.1061
10. Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. (2012) 124:223–8. doi: 10.1016/j.drugalcdep.2012.01.013
11. He X, Sandhu HK, Yang Y, Hua F, Belser N, Kim DH, et al. Neuroprotection against hypoxia/ischemia: delta-opioid receptor-mediated cellular/molecular events. Cell Mol Life Sci. (2013) 70:2291–303. doi: 10.1007/s00018-012-1167-2
12. Chao D, Xia Y. Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Progr Neurobiol. (2010) 90:439–70. doi: 10.1016/j.pneurobio.2009.12.007
13. Zhang J, Gibney GT, Zhao P, Xia Y. Neuroprotective role of delta-opioid receptors in cortical neurons. Am J Physiol Cell Physiol. (2002) 282:C1225–34. doi: 10.1152/ajpcell.00226.2001
14. Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen-sensitive {delta}-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem. (2005) 280:16208–18. doi: 10.1074/jbc.M408055200
15. Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke. (2006) 37:1094–9. doi: 10.1161/01.STR.0000206444.29930.18
16. Chao D, Donnelly DF, Feng Y, Bazzy-Asaad A, Xia Y. Cortical delta-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J Cereb Blood Flow Metab. (2007) 27:356–68. doi: 10.1038/sj.jcbfm.9600352
17. Chao D, Bazzy-Asaad A, Balboni G, Xia Y. delta-, but not mu-, opioid receptor stabilizes K(+) homeostasis by reducing Ca(2+) influx in the cortex during acute hypoxia. J Cell Physiol. (2007) 212:60–7. doi: 10.1002/jcp.21000
18. Chao D, Bazzy-Asaad A, Balboni G, Salvadori S, Xia Y. Activation of DOR attenuates anoxic K+ derangement via inhibition of Na+ entry in mouse cortex. Cereb Cortex. (2008) 18:2217–27. doi: 10.1093/cercor/bhm247
19. Chao D, Balboni G, Lazarus LH, Salvadori S, Xia Y. Na+ mechanism of delta-opioid receptor induced protection from anoxic K+ leakage in the cortex. Cell Mol Life Sci. (2009) 66:1105–15. doi: 10.1007/s00018-009-8759-5
20. Chao D, He X, Yang Y, Bazzy-Asaad A, Lazarus LH, Balboni G, et al. DOR activation inhibits anoxic/ischemic Na+ influx through Na+ channels via PKC mechanisms in the cortex. Exp Neurol. (2012) 236:228–39. doi: 10.1016/j.expneurol.2012.05.006
21. Yang Y, Xia X, Zhang Y, Wang Q, Li L, Luo G, et al. delta-Opioid receptor activation attenuates oxidative injury in the ischemic rat brain. BMC Biol. (2009) 7:55. doi: 10.1186/1741-7007-7-55
22. Feng Y, He X, Yang Y, Chen J, Yin K, Xia Y. Effect of delta-opioid receptor over-expression on cortical expression of GABAA receptor alpha1-subunit in hypoxia. Chinese J Phys. (2011) 54:118–23. doi: 10.4077/CJP.2011.AMM047
23. Tian X, Hua F, Sandhu HK, Chao D, Balboni G, Salvadori S, et al. Effect of delta-opioid receptor activation on BDNF-TrkB vs. TNF-alpha in the mouse cortex exposed to prolonged hypoxia. Int J Mol Sci. (2013) 14:15959–76. doi: 10.3390/ijms140815959
24. Tian X, Guo J, Zhu M, Li M, Wu G, Xia Y. delta-Opioid receptor activation rescues the functional TrkB receptor and protects the brain from ischemia-reperfusion injury in the rat. PLoS ONE. (2013) 8:e69252. doi: 10.1371/journal.pone.0069252
25. Borlongan CV, Hayashi T, Oeltgen PR, Su TP, Wang Y. Hibernation-like state induced by an opioid peptide protects against experimental stroke. BMC Biol. (2009) 7:31. doi: 10.1186/1741-7007-7-31
26. Lim YJ, Zheng S, Zuo Z. Morphine preconditions Purkinje cells against cell death under in vitro simulated ischemia-reperfusion conditions. Anesthesiology. (2004) 100:562–8. doi: 10.1097/00000542-200403000-00015
27. Charron C, Messier C, Plamondon H. Neuroprotection and functional recovery conferred by administration of kappa- and delta 1-opioid agonists in a rat model of global ischemia. Physiol Behav. (2008) 93:502–11. doi: 10.1016/j.physbeh.2007.10.015
28. Pamenter ME, Buck LT. delta-Opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex. J Exp Biol. (2008) 211(Pt 21):3512–7. doi: 10.1242/jeb.021949
29. Horiuchi T, Kawaguchi M, Kurita N, Inoue S, Sakamoto T, Nakamura M, et al. Effects of delta-opioid agonist SNC80 on white matter injury following spinal cord ischemia in normothermic and mildly hypothermic rats. J Anesthesia. (2008) 22:32–7. doi: 10.1007/s00540-007-0576-0
30. Zhu M, Li MW, Tian XS, Ou XM, Zhu CQ, Guo JC. Neuroprotective role of delta-opioid receptors against mitochondrial respiratory chain injury. Brain Res. (2009) 1252 183–91. doi: 10.1016/j.brainres.2008.11.030
31. Wang S, Duan Y, Su D, Li W, Tan J, Yang D, et al. Delta opioid peptide [D-Ala2, D-Leu5] enkephalin (DADLE) triggers postconditioning against transient forebrain ischemia. Eur J Pharmacol. (2011) 658:140–4. doi: 10.1016/j.ejphar.2011.02.006
32. Yang L, Wang H, Shah K, Karamyan VT, Abbruscato TJ. Opioid receptor agonists reduce brain edema in stroke. Brain Res. (2011) 1383 307–16. doi: 10.1016/j.brainres.2011.01.083
33. He X, Yang Y, Zhi F, Moore ML, Kang X, Chao D, et al. δ-Opioid receptor activation modified microRNA expression in the rat kidney under prolonged hypoxia. PLoS ONE. (2013) 8:e61080. doi: 10.1371/journal.pone.0061080
34. Yang Y, Zhi F, He X, Moore ML, Kang X, Chao D, et al. δ-opioid receptor activation and microRNA expression of the rat cortex in hypoxia. PLoS ONE. (2012) 7:e51524. doi: 10.1371/journal.pone.0051524
35. Zhi F, Xue L, Shao N, Deng D, Kang X, Chao D, et al. delta-opioid receptor activation and MicroRNA expression in the rat heart under prolonged hypoxia. Cell Physiol Biochem. (2016) 39:1118–28. doi: 10.1159/000447815
36. Zhi F, Shao N, Xue L, Xu Y, Kang X, Yang Y, et al. Characteristic MicroRNA expression induced by delta-opioid receptor activation in the rat liver under prolonged hypoxia. Cell Physiol Biochem. (2017) 44:2296–309. doi: 10.1159/000486067
37. Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. (2009) 10:126–39. doi: 10.1038/nrm2632
38. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. (2015) 15:321–33. doi: 10.1038/nrc3932
39. Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Rad Biol Med. (2013) 64:20–30. doi: 10.1016/j.freeradbiomed.2013.05.022
40. Bertero T, Rezzonico R, Pottier N, Mari B. Impact of MicroRNAs in the cellular response to hypoxia. Int Rev Cell Mol Biol. (2017) 333:91–158. doi: 10.1016/bs.ircmb.2017.03.006
41. Minhas G, Mathur D, Ragavendrasamy B, Sharma NK, Paanu V, Anand A. Hypoxia in CNS pathologies: emerging role of miRNA-based neurotherapeutics and yoga based alternative therapies. Front Neurosci. (2017) 11:386. doi: 10.3389/fnins.2017.00386
42. Yang Y, Sandhu HK, Zhi F, Hua F, Wu M, Xia Y. Effects of hypoxia and ischemia on microRNAs in the brain. Curr Med Chem. (2015) 22:1292–301. doi: 10.2174/0929867322666150209154755
43. Chang CY, Lui TN, Lin JW, Lin YL, Hsing CH, Wang JJ, et al. Roles of microRNA-1 in hypoxia-induced apoptotic insults to neuronal cells. Archiv Toxicol. (2016) 90:191–202. doi: 10.1007/s00204-014-1364-x
44. Che H, Yan Y, Kang XH, Guo F, Yan ML, Liu HL, et al. MicroRNA-27a promotes inefficient lysosomal clearance in the hippocampi of rats following chronic brain hypoperfusion. Mol Neurobiol. (2017) 54:2595–610. doi: 10.1007/s12035-016-9856-8
45. Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, et al. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia. (2013) 61:1784–94. doi: 10.1002/glia.22556
46. Li P, Shen M, Gao F, Wu J, Zhang J, Teng F, et al. An antagomir to MicroRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. (2017) 54:2901–21. doi: 10.1007/s12035-016-9842-1
47. Wang Y, Wang MD, Xia YP, Gao Y, Zhu YY, Chen SC, et al. MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J. (2018) 32:935–44. doi: 10.1096/fj.201700139RRR
48. Wen M, Ye J, Han Y, Huang L, Yang H, Jiang W, et al. Hypertonic saline regulates microglial M2 polarization via miR-200b/KLF4 in cerebral edema treatment. Biochem Biophys Res Commun. (2018) 499:345–53. doi: 10.1016/j.bbrc.2018.03.161
49. Stary CM, Xu L, Sun X, Ouyang YB, White RE, Leong J, et al. MicroRNA-200c contributes to injury from transient focal cerebral ischemia by targeting Reelin. Stroke. (2015) 46:551–6. doi: 10.1161/STROKEAHA.114.007041
50. Ma Q, Dasgupta C, Li Y, Bajwa NM, Xiong F, Harding B, et al. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis. (2016) 89:202–12. doi: 10.1016/j.nbd.2016.02.011
51. Pulkkinen K, Malm T, Turunen M, Koistinaho J, Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. (2008) 582:2397–401. doi: 10.1016/j.febslet.2008.05.048
52. Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. (2009) 10:273–84. doi: 10.1016/j.cmet.2009.08.015
53. Hu J, Sun T, Wang H, Chen Z, Wang S, Yuan L, et al. MiR-215 Is Induced Post-transcriptionally via HIF-drosha complex and mediates glioma-initiating cell adaptation to hypoxia by targeting KDM1B. Cancer Cell. (2016) 29:49–60. doi: 10.1016/j.ccell.2015.12.005
54. Mo JL, Liu Q, Kou ZW, Wu KW, Yang P, Chen XH, et al. MicroRNA-365 modulates astrocyte conversion into neuron in adult rat brain after stroke by targeting Pax6. Glia. (2018) 66:1346–62. doi: 10.1002/glia.23308
55. Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, et al. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. (2010) 38:17–26. doi: 10.1016/j.nbd.2009.12.021
56. Wang X, Chen S, Ni J, Cheng J, Jia J, Zhen X. miRNA-3473b contributes to neuroinflammation following cerebral ischemia. Cell Death Dis. (2018) 9:11. doi: 10.1038/s41419-017-0014-7
57. Dong YF, Chen ZZ, Zhao Z, Yang DD, Yan H, Ji J, et al. Potential role of microRNA-7 in the anti-neuroinflammation effects of nicorandil in astrocytes induced by oxygen-glucose deprivation. J Neuroinflam. (2016) 13:60. doi: 10.1186/s12974-016-0527-5
58. Kim T, Mehta SL, Morris-Blanco KC, Chokkalla AK, Chelluboina B, Lopez M, et al. The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing alpha-synuclein. Sci Signal. (2018) 11:eaat4285. doi: 10.1126/scisignal.aat4285
59. Wei N, Xiao L, Xue R, Zhang D, Zhou J, Ren H, et al. MicroRNA-9 mediates the cell apoptosis by targeting Bcl2l11 in ischemic stroke. Mol Neurobiol. (2016) 53:6809–17. doi: 10.1007/s12035-015-9605-4
60. Yan H, Rao J, Yuan J, Gao L, Huang W, Zhao L, et al. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate ischemic neuronal death by targeting miR-21/PDCD4 signaling pathway. Cell Death Dis. (2017) 8:3211. doi: 10.1038/s41419-017-0047-y
61. Zhang L, Dong LY, Li YJ, Hong Z, Wei WS. miR-21 represses FasL in microglia and protects against microglia-mediated neuronal cell death following hypoxia/ischemia. Glia. (2012) 60:1888–95. doi: 10.1002/glia.22404
62. Chen Q, Xu J, Li L, Li H, Mao S, Zhang F, et al. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. (2014) 5 e1132. doi: 10.1038/cddis.2014.92
63. Wang Y, Huang J, Ma Y, Tang G, Liu Y, Chen X, et al. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J Cereb Blood Flow Metab. (2015) 35:1977–84. doi: 10.1038/jcbfm.2015.156
64. Zeng Y, Lv Y, Tao L, Ma J, Zhang H, Xu H, et al. G6PC3, ALDOA and CS induction accompanies mir-122 down-regulation in the mechanical asphyxia and can serve as hypoxia biomarkers. Oncotarget. (2016) 7:74526–36. doi: 10.18632/oncotarget.12931
65. Mucaj V, Lee SS, Skuli N, Giannoukos DN, Qiu B, Eisinger-Mathason TS, et al. MicroRNA-124 expression counteracts pro-survival stress responses in glioblastoma. Oncogene. (2015) 34:2204–14. doi: 10.1038/onc.2014.168
66. Li XQ, Yu Q, Tan WF, Zhang ZL, Ma H. MicroRNA-125b mimic inhibits ischemia reperfusion-induced neuroinflammation and aberrant p53 apoptotic signalling activation through targeting TP53INP1. Brain Behav Immun. (2018) 74 154–65. doi: 10.1016/j.bbi.2018.09.002
67. Gonsalves CS, Kalra VK. Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a-5p. J Immunol. (2010) 184:3878–88. doi: 10.4049/jimmunol.0902594
68. Qu Y, Wu J, Chen D, Zhao F, Liu J, Yang C, et al. MiR-139-5p inhibits HGTD-P and regulates neuronal apoptosis induced by hypoxia-ischemia in neonatal rats. Neurobiol Dis. (2014) 63:184–93. doi: 10.1016/j.nbd.2013.11.023
69. Zhang L, Li YJ, Wu XY, Hong Z, Wei WS. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J Neurochem. (2015) 132:713–23. doi: 10.1111/jnc.13021
70. Looney AM, Ahearne CE, Hallberg B, Boylan GB, Murray DM. Downstream mRNA target analysis in neonatal hypoxic-ischaemic encephalopathy identifies novel marker of severe injury: a proof of concept paper. Mol Neurobiol. (2017) 54:8420–8. doi: 10.1007/s12035-016-0330-4
71. Fan Y, Ding S, Sun Y, Zhao B, Pan Y, Wan J. MiR-377 regulates inflammation and angiogenesis in rats after cerebral ischemic injury. J Cell Biochem. (2018) 119:327–37. doi: 10.1002/jcb.26181
72. Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, et al. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke. (2013) 44:1706–13. doi: 10.1161/STROKEAHA.111.000504
73. Irmady K, Jackman KA, Padow VA, Shahani N, Martin LA, Cerchietti L, et al. Mir-592 regulates the induction and cell death-promoting activity of p75NTR in neuronal ischemic injury. J Neurosci. (2014) 34:3419–28. doi: 10.1523/JNEUROSCI.1982-13.2014
74. Ni J, Wang X, Chen S, Liu H, Wang Y, Xu X, et al. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behave Immun. (2015) 49:75–85. doi: 10.1016/j.bbi.2015.04.014
75. Jickling GC, Ander BP, Shroff N, Orantia M, Stamova B, Dykstra-Aiello C, et al. Leukocyte response is regulated by microRNA let7i in patients with acute ischemic stroke. Neurology. (2016) 87:2198–205. doi: 10.1212/WNL.0000000000003354
76. Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, et al. Expression profile of MicroRNAs in young stroke patients. PLoS ONE. (2009) 4:e7689. doi: 10.1371/journal.pone.0007689
77. Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. (2011) 43:521–8. doi: 10.1152/physiolgenomics.00158.2010
78. Hadjipanayi E, Schilling AF. Hypoxia-based strategies for angiogenic induction: the dawn of a new era for ischemia therapy and tissue regeneration. Organogenesis. (2013) 9:261–72. doi: 10.4161/org.25970
79. Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. (2011) 2:1117–33. doi: 10.1177/1947601911423654
80. Chio CC, Lin JW, Cheng HA, Chiu WT, Wang YH, Wang JJ, et al. MicroRNA-210 targets antiapoptotic Bcl-2 expression and mediates hypoxia-induced apoptosis of neuroblastoma cells. Archiv Toxicol. (2013) 87:459–68. doi: 10.1007/s00204-012-0965-5
81. Koh HS, Chang CY, Jeon SB, Yoon HJ, Ahn YH, Kim HS, et al. The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat Commun. (2015) 6:6340. doi: 10.1038/ncomms7340
82. Guo M, Ma X, Feng Y, Han S, Dong Q, Cui M, et al. In chronic hypoxia, glucose availability and hypoxic severity dictate the balance between HIF-1 and HIF-2 in astrocytes. FASEB J. (2019) 33:11123–36. doi: 10.1096/fj.201900402RR
83. Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. Mol Cell Biol. (2007) 27:1859–67. doi: 10.1128/MCB.01395-06
84. Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Different. (2008) 15:667–71. doi: 10.1038/sj.cdd.4402310
85. Kelly TJ, Souza AL, Clish CB, Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol. (2011) 31:2696–706. doi: 10.1128/MCB.01242-10
86. Khoshnam SE, Winlow W, Farzaneh M. The interplay of MicroRNAs in the inflammatory mechanisms following ischemic stroke. J Neuropathol Exp Neurol. (2017) 76:548–61. doi: 10.1093/jnen/nlx036
87. Taylor CT, Doherty G, Fallon PG, Cummins EP. Hypoxia-dependent regulation of inflammatory pathways in immune cells. J Clin Investig. (2016) 126:3716–24. doi: 10.1172/JCI84433
88. Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. (2010) 345:105–20. doi: 10.1007/82_2010_74
89. Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, et al. MicroRNA let-7c regulates macrophage polarization. J Immunol. (2013) 190:6542–9. doi: 10.4049/jimmunol.1202496
90. Zhang W, Liu H, Liu W, Liu Y, Xu J. Polycomb-mediated loss of microRNA let-7c determines inflammatory macrophage polarization via PAK1-dependent NF-kappaB pathway. Cell Death Different. (2015) 22:287–97. doi: 10.1038/cdd.2014.142
91. Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, et al. Caspase signalling controls microglia activation and neurotoxicity. Nature. (2011) 472:319–24. doi: 10.1038/nature09788
92. Zhang L, Dong LY, Li YJ, Hong Z, Wei WS. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J Neuroinflam. (2012) 9:211. doi: 10.1186/1742-2094-9-211
93. Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC. MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci USA. (2012) 109:7865–70. doi: 10.1073/pnas.1200081109
94. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. (2006) 103:12481–6. doi: 10.1073/pnas.0605298103
95. Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. (2011) 208:1189–201. doi: 10.1084/jem.20101823
96. Nahid MA, Satoh M, Chan EK. Mechanistic role of microRNA-146a in endotoxin-induced differential cross-regulation of TLR signaling. J Immunol. (2011) 186:1723–34. doi: 10.4049/jimmunol.1002311
97. Chen G, Gao X, Wang J, Yang C, Wang Y, Liu Y, et al. Hypoxia-induced microRNA-146a represses Bcl-2 through Traf6/IRAK1 but not Smad4 to promote chondrocyte autophagy. Biol Chem. (2017) 398:499–507. doi: 10.1515/hsz-2016-0211
98. Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. (2010) 30:92–101. doi: 10.1038/jcbfm.2009.186
99. O'connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. (2009) 106:7113–8. doi: 10.1073/pnas.0902636106
100. Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, et al. Requirement of bic/microRNA-155 for normal immune function. Science. (2007) 316:608–11. doi: 10.1126/science.1139253
101. Virtue A, Wang H, Yang XF. MicroRNAs and toll-like receptor/interleukin-1 receptor signaling. J Hematol Oncol. (2012) 5:66. doi: 10.1186/1756-8722-5-66
102. Tang B, Xiao B, Liu Z, Li N, Zhu ED, Li BS, et al. Identification of MyD88 as a novel target of miR-155, involved in negative regulation of Helicobacter pylori-induced inflammation. FEBS Lett. (2010) 584:1481–6. doi: 10.1016/j.febslet.2010.02.063
103. Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. (2015) 6 7321. doi: 10.1038/ncomms8321
104. Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. (2010) 122(Suppl. 11):S124–31. doi: 10.1161/CIRCULATIONAHA.109.928424
105. Qiu J, Zhou XY, Zhou XG, Cheng R, Liu HY, Li Y. Neuroprotective effects of microRNA-210 on hypoxic-ischemic encephalopathy. Bio Med Res Int. (2013) 2013:350419. doi: 10.1155/2013/350419
106. Huang X, Le QT, Giaccia AJ. MiR-210–micromanager of the hypoxia pathway. Trends Mol Med. (2010) 16:230–7. doi: 10.1016/j.molmed.2010.03.004
107. Tili E, Michaille JJ, Croce CM. MicroRNAs play a central role in molecular dysfunctions linking inflammation with cancer. Immunol Rev. (2013) 253:167–84. doi: 10.1111/imr.12050
108. Wang H, Flach H, Onizawa M, Wei L, Mcmanus MT, Weiss A. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol. (2014) 15:393–401. doi: 10.1038/ni.2846
109. Noman MZ, Buart S, Romero P, Ketari S, Janji B, Mari B, et al. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. (2012) 72:4629–41. doi: 10.1158/0008-5472.CAN-12-1383
110. Noman MZ, Janji B, Hu S, Wu JC, Martelli F, Bronte V, et al. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res. (2015) 75:3771–87. doi: 10.1158/0008-5472.CAN-15-0405
111. Mok Y, Schwierzeck V, Thomas DC, Vigorito E, Rayner TF, Jarvis LB, et al. MiR-210 is induced by Oct-2, regulates B cells, and inhibits autoantibody production. J Immunol. (2013) 191:3037–48. doi: 10.4049/jimmunol.1301289
112. Chen D, Dixon BJ, Doycheva DM, Li B, Zhang Y, Hu Q, et al. IRE1alpha inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic-ischemic brain injury in rats. J Neuroinflam. (2018) 15:32. doi: 10.1186/s12974-018-1077-9
113. Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. (2011) 3:159–66. doi: 10.1093/jmcb/mjr007
114. Yang Y, Wang JK. The functional analysis of MicroRNAs involved in NF-kappaB signaling. Eur Rev Med Pharmacol Sci. (2016) 20:1764–74. Available online at: https://www.europeanreview.org/article/10746
115. Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. (2010) 11:141–7. doi: 10.1038/ni.1828
116. Zhou R, Hu G, Gong AY, Chen XM. Binding of NF-kappaB p65 subunit to the promoter elements is involved in LPS-induced transactivation of miRNA genes in human biliary epithelial cells. Nucleic Acids Res. (2010) 38:3222–32. doi: 10.1093/nar/gkq056
117. Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li L, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med. (2012) 16:249–59. doi: 10.1111/j.1582-4934.2011.01291.x
118. Zhang J, Haddad GG, Xia Y. δ-, but not mu- and kappa-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res. (2000) 885:143–53. doi: 10.1016/S0006-8993(00)02906-1
119. Xu Y, Zhi F, Shao N, Wang R, Yang Y, Xia Y. Cytoprotection against hypoxic and/or MPP(+) injury: effect of delta-opioid receptor activation on caspase 3. Int J Mol Sci. (2016) 17:1179. doi: 10.3390/ijms17081179
120. Tang CW, Feng WM, Du HM, Bao Y, Zhu M. Delayed administration of D-Ala2-D-Leu5-enkephalin, a delta-opioid receptor agonist, improves survival in a rat model of sepsis. Tohoku J Exp Med. (2011) 224:69–76. doi: 10.1620/tjem.224.69
121. Husain S, Abdul Y, Potter DE. Non-analgesic effects of opioids: neuroprotection in the retina. Curr Pharm Design. (2012) 18:6101–8. doi: 10.2174/138161212803582441
122. Wang Q, Chao D, Chen T, Sandhu H, Xia Y. delta-Opioid receptors and inflammatory cytokines in hypoxia: differential regulation between glial and neuron-like cells. Transl Stroke Res. (2014) 5:476–83. doi: 10.1007/s12975-014-0342-1
123. Qiu J, Chao D, Sheng S, Khiati D, Zhou X, Xia Y. delta-opioid receptor-Nrf-2-mediated inhibition of inflammatory cytokines in neonatal hypoxic-ischemic encephalopathy. Mol Neurobiol. (2019) 56:5229–40. doi: 10.1007/s12035-018-1452-7
124. Johansson A, Nyberg WA, Sjostrand M, Moruzzi N, Bergman P, Khademi M, et al. miR-31 regulates energy metabolism and is suppressed in T cells from patients with Sjogren's syndrome. Eur J Immunol. (2019) 49:313–22. doi: 10.1002/eji.201747416
125. Bardua M, Haftmann C, Durek P, Westendorf K, Buttgereit A, Tran CL, et al. MicroRNA-31 reduces the motility of proinflammatory T helper 1 lymphocytes. Front Immunol. (2018) 9:2813. doi: 10.3389/fimmu.2018.02813
126. Sun HL, Cui R, Zhou J, Teng KY, Hsiao YH, Nakanishi K, et al. ERK activation globally downregulates miRNAs through phosphorylating exportin-5. Cancer Cell. (2016) 30:723–36. doi: 10.1016/j.ccell.2016.10.001
127. Qu Y, Shi B, Hou P. Activated ERK: an emerging player in miRNA downregulation. Trends Cancer. (2017) 3:163–5. doi: 10.1016/j.trecan.2017.01.002
128. Fricker M, Vilalta A, Tolkovsky AM, Brown GC. Caspase inhibitors protect neurons by enabling selective necroptosis of inflamed microglia. J Biol Chem. (2013) 288:9145–52. doi: 10.1074/jbc.M112.427880
129. Kim SJ, Li J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis. (2013) 4:e716. doi: 10.1038/cddis.2013.238
130. Vijayan M, Reddy PH. Peripheral biomarkers of stroke: focus on circulatory microRNAs. Biochim Biophys Acta. (2016) 1862:1984–93. doi: 10.1016/j.bbadis.2016.08.003
131. Martinez B, Peplow PV. Blood microRNAs as potential diagnostic and prognostic markers in cerebral ischemic injury. Neural Regen Res. (2016) 11:1375–8. doi: 10.4103/1673-5374.191196
132. Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, et al. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci. (2011) 3:1265–72. doi: 10.2741/e330
133. Tiedt S, Prestel M, Malik R, Schieferdecker N, Duering M, Kautzky V, et al. RNA-Seq Identifies Circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers for acute ischemic stroke. Circ Res. (2017) 121:970–80. doi: 10.1161/CIRCRESAHA.117.311572
134. Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. (2006) 117:433–42. doi: 10.1111/j.1365-2567.2006.02321.x
135. Quinn SR, O'neill LA. The role of microRNAs in the control and mechanism of action of IL-10. Curr Top Microbiol Immunol. (2014) 380:145-55. doi: 10.1007/978-3-662-43492-5_7
136. Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, et al. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. (2010) 87:431–9. doi: 10.1093/cvr/cvq082
137. Sharma A, Kumar M, Ahmad T, Mabalirajan U, Aich J, Agrawal A, et al. Antagonism of mmu-mir-106a attenuates asthma features in allergic murine model. J Appl Physiol. (2012) 113:459–64. doi: 10.1152/japplphysiol.00001.2012
138. Takuse Y, Watanabe M, Inoue N, Ozaki R, Ohtsu H, Saeki M, et al. Association of IL-10-regulating MicroRNAs in peripheral blood mononuclear cells with the pathogenesis of autoimmune thyroid disease. Immunol Invest. (2017) 46:590–602. doi: 10.1080/08820139.2017.1322975
139. Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci. (2015) 35:12446–64. doi: 10.1523/JNEUROSCI.1641-15.2015
140. Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai H, et al. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther. (2014) 21:37–43. doi: 10.1038/gt.2013.55
141. Li Y, Mao L, Gao Y, Baral S, Zhou Y, Hu B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci Rep. (2015) 5:13316. doi: 10.1038/srep13316
142. Nielsen CK, Simms JA, Li R, Mill D, Yi H, Feduccia AA, et al. δ-opioid receptor function in the dorsal striatum plays a role in high levels of ethanol consumption in rats. J Neurosci. (2012) 32:4540–52. doi: 10.1523/JNEUROSCI.5345-11.2012
143. Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol. (2013) 23:473–9. doi: 10.1016/j.conb.2013.02.005
Keywords: brain injury, hypoxia, ischemia, microRNAs, δ-opioid receptor (DOR), neuroinflammatory response
Citation: Chen Y-M, He X-Z, Wang S-M and Xia Y (2020) δ-Opioid Receptors, microRNAs, and Neuroinflammation in Cerebral Ischemia/Hypoxia. Front. Immunol. 11:421. doi: 10.3389/fimmu.2020.00421
Received: 30 September 2019; Accepted: 24 February 2020;
Published: 25 March 2020.
Edited by:
Heng Zhao, Stanford University, United StatesReviewed by:
Ana Lloret, University of Valencia, SpainQiang Liu, Barrow Neurological Institute (BNI), United States
Fudong Liu, University of Texas Health Science Center at Houston, United States
Awadhesh K. Arya, University of Maryland, Baltimore, United States
Copyright © 2020 Chen, He, Wang and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xia, eTU1NzM4MDg4QGdtYWlsLmNvbQ==; Xiao-Zhou He, Y3p5eWh4ekBzaW5hLmNvbQ==
 Yi-Meng Chen
Yi-Meng Chen Xiao-Zhou He1*
Xiao-Zhou He1* Ying Xia
Ying Xia