- Department of Immunology, Duke University Medical Center, Durham, NC, United States
γδ T cells are the first T cell lineage to develop in the thymus and take up residence in a wide variety of tissues where they can provide fast, innate-like sources of effector cytokines for barrier defense. In contrast to conventional αβ T cells that egress the thymus as naïve cells, γδ T cells can be programmed for effector function during development in the thymus. Understanding the molecular mechanisms that determine γδ T cell effector fate is of great interest due to the wide-spread tissue distribution of γδ T cells and their roles in pathogen clearance, immunosurveillance, cancer, and autoimmune diseases. In this review, we will integrate the current understanding of the role of the T cell receptor, environmental signals, and transcription factor networks in controlling mouse innate-like γδ T cell effector commitment.
Introduction
γδ T cells are part of the three evolutionary conserved lymphocyte lineages (with αβ T cells and B cells) that undergo somatic gene rearrangement for the generation of antigen receptors (1). While immune cells can broadly be divided by adaptive vs. innate, γδ T cells straddle this classification by having properties of both. Although γδ T cells are capable of generating unique T cell receptors (TCRs), many γδ T cells express TCRs with limited diversity (2). Innate-like γδ T cells, also referred to as “natural” γδ T cells, are endowed with their effector functions early during development in the thymus and consequently do not require clonal expansion or differentiation from a naïve cell for their effector responses (3, 4). Importantly, innate-like γδ T cells exhibit the four hallmark characteristics of tissue-resident lymphocytes; (1) self-renewal and long-term maintenance, (2) enrichment at barrier tissues, (3) tissue sensing capabilities, and (4) rapid effector responses (5). These tissue-resident properties combined with early seeding during fetal life enable innate-like γδ T cells to act as a first line of defense in the skin, gut, and reproductive tract while other lymphocytes are still being developed.
γδ T cells play innumerable roles in pathogen clearance, wound healing, autoimmunity, and cancer, largely through the production of soluble mediators (6). The two major effector subsets of γδ T cells can be distinguished based on cytokine production: IFNγ producers (Tγδ1) and IL-17A producers (Tγδ17), although γδ T cells are capable of producing many other cytokines (6). IFNγ production by γδ T cells is associated with clearance of intracellular pathogens and anti-tumor responses, while IL-17A production is linked to clearance of extracellular bacteria and fungi (7, 8). Although protective against infectious diseases, cytokine production by γδ T cells is involved in many immune pathologies and autoimmune diseases when dysregulated (9). Remarkably, the presence of γδ T cells within tumors was found to be the most significant favorable cancer-wide prognostic population in humans (10). While enriched at mucosal and barrier tissues, γδ T cells are also present in many other non-lymphoid tissues where they support steady-state tissue homeostasis (6, 11). Recent studies have shown that IL-17A production by γδ T cells regulates adipose tissue immune cell homeostasis and thermogenesis (12), bone regeneration (13), and the promotion of short-term memory in the brain meninges (14). As innate-like lymphocytes, γδ T cells sense their local environment and are regulated through a combination of the TCR, cytokine receptors, co-stimulatory receptors, inhibitory receptors, and natural killer receptors (15). These receptors recognize various environmental ligands or stimuli that induce signaling cascades that lead to expression of key transcription factors (TFs) that can then dictate the identity and effector function of γδ T cells. This review will focus on the integration of TCR and environmental cues with downstream TF modules that govern the effector fate of mouse innate-like γδ T cells.
γδ Lineage Commitment in the Thymus
In the thymus, double-negative CD4− CD8− (DN) thymocytes give rise to two distinct T cell lineages defined by the expression of either an αβTCR or a γδTCR (16). DN thymocytes are a heterogeneous group of developmentally linked progenitor cells distinguished by the expression of CD44, CD117 (also known as c-kit), and CD25 that encompass the transition of early thymocyte progenitor cells (ETP/DN1) through the DN2, DN3, and DN4 cell stages (16). Rearrangement of the TCRβ, TCRγ, and TCRδ gene loci begin in DN2 cells and are completed in DN3 cells (17), a time frame that coincides with the divergence of the αβ and γδ lineages (18, 19). Indeed, the DN3 stage represents an obligatory checkpoint at which productive rearrangement and expression of either a pre-TCR (TCRβ + invariant pTα) or γδTCR complex signals the rescue of cells from apoptosis, proliferation, and αβ or γδ lineage differentiation (17). β-selected cells undergo further development to the CD4+CD8+ double positive (DP) stage, where TCRα rearrangement and additional selection events yield mature CD4+ or CD8+ single positive αβ T cells (16, 20). Unlike αβ T cells, γδ T cells develop following a single γδ-selection step mediated by the γδTCR, do not progress through to a DP stage, and rather most γδ T cells remain DN instead (16).
Developing DN thymocytes integrate signals from the TCR complex expressed on their cell surface along with myriad environmental cues. As such, two models were proposed to explain αβ vs. γδ lineage choice: the signal strength model and the stochastic-selective (pre-commitment) model (16). The major difference between these models is the importance placed on TCR signaling and the timing of its influence. The pre-commitment model is founded on the idea that lineage fate is determined prior to rearrangement of TCR loci. The expression of γδTCR on γδ T cell precursors or pre-TCR on αβ precursors simply confirms their fate and cells pre-committed to one fate with a mismatched TCR were hypothesized to die. Initial studies supporting this model showed that DN thymocytes lacking TCR expression but expressing high levels of IL-7Rα (21) or the high mobility group (HMG) box TF Sox13 (22) were predisposed to becoming γδ T cells. However, more recent evidence that Sox13 is not required for the generation of all γδ T cells, but rather only for a select subset of IL-17-producing γδ T cells marked by Vγ4 usage (23) [Tonegawa nomenclature (24)], is at odds with the pre-commitment model.
In contrast, the signal strength model of αβ vs. γδ lineage commitment has garnered widespread support. It posits that the strength of TCR signal that DN thymocytes receive dictates the lineage decision; weak signals promote αβ fate, while strong signals promote the γδ fate. The extensive evidence in favor of this model has been previously reviewed in detail (16, 25). Most notably, key support was provided by elegant experiments demonstrating that a single γδTCR transgene can mediate both γδ and αβ lineage fates, dependent on the signal strength of the TCR (26, 27). In particular, lineage fate toggled between αβ and γδ outcomes when TCR signal strength was tuned by genetic alterations in TCR ligand availability, TCR surface expression levels, or in expression of TCR signaling factors (26, 27). Enhanced or prolonged activation of the extracellular signal-regulated kinase (ERK) pathway and downstream Egr, and Id3 targets are important mediators of strong γδTCR signals that promote γδ lineage commitment (25, 26, 28). More recent work has begun to shed light on the mechanism by which DN cells translate differences in signal strength and ERK signaling into alternative lineage fates. γδ T cell development is dependent on a non-canonical mode of ERK action mediated by its DEF-binding pocket (29). This domain is favored by strong and more prolonged signals and enables ERK to bind a distinct set of proteins required for γδ lineage adoption. Thus, strong signals mediated primarily by γδTCR complexes are required for DN cell commitment to the γδ T cell lineage.
Effector Programming of γδ T Cells
Waves of γδ T Cell Development
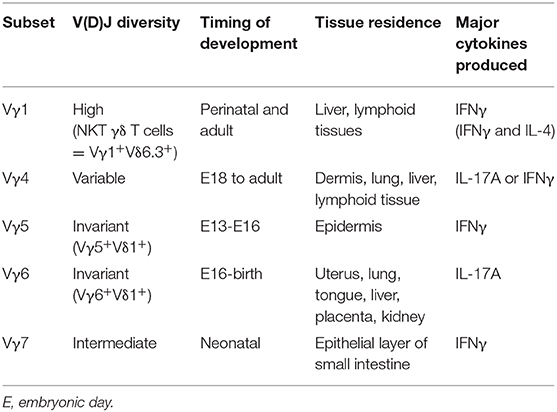
A distinctive and poorly understood feature of γδ T cell ontogeny is the development of γδ thymocytes in a series of “waves” that are defined by γ-chain variable regions (Vγ) usage (Table 1). Interestingly, the waves of Vγ subsets are highly correlated with homing abilities to specific tissues early in life, where they become long-lived tissue-resident cells. This process begins when the fetal thymus is seeded as early as embryonic day 13.5 (E13.5) by fetal liver progenitors to generate the first wave of γδ T cells, known as Vγ5+Vδ1+ dendritic epidermal T cells (DETCs) that exclusively home to the epidermis of the skin (30). The second wave of γδ T cells, expressing an invariant Vγ6Vδ1 TCR, develop around E16 and primarily seed epithelial layers of the female reproductive tract, lung, and tongue (31). Next, the late fetal stages give rise to Vγ4+ and Vγ1+ γδ T cells that express more varied TCRs due to pairing with several Vδ chains and can be found in many tissues such as peripheral lymphoid organs, blood, lung, liver, and dermis (2, 31). Unlike Vγ5+ and Vγ6+ γδ T cells, these subsets are not restricted to the fetal window and can also develop during neonatal and adult life (2, 31). Of note, the Vγ7+ γδ T cells that reside in the intraepithelial layer of the small intestine are thought to mature extrathymically (2, 32). While the link between Vγ usage and tissue homing can be explained in DETCs with upregulation of CCR10 in the thymus before trafficking to the epidermis (33, 34), this association is not yet understood for other Vγ subsets. Moreover, the molecular mechanisms governing the unique sequential development of Vγ subsets are unknown, however features of both the fetal progenitors and environment have been implicated (35–38).
Effector Diversification of γδ Thymocytes
In contrast to αβ T cells that leave the thymus as naïve cells and acquire their effector function in the periphery, γδ T cells can commit to an effector fate during development in the thymus. The pre-programming in the thymus allows γδ T cells to be early innate-like responders to infection and tissue-damage, without the delay that is required for αβ T cell responses. While this review focuses on “pre-programmed” innate-like or “natural” γδ T cells, some γδ T cells exit the thymus as naïve cells and acquire effector function following activation in the periphery; these are referred to as “inducible” γδ T cells (4, 39). Similar to αβ T cells, innate lymphoid cells (ILCs), and other lymphocyte lineages, γδ T cells can be divided into effector subsets based on the expression of either T-bet/IFNγ (Tγδ1) or RORγt/IL-17A (Tγδ17). During ontogeny, effector γδ T cell subsets differentiate in functional waves encompassing DETCs, IL-17A producers, and NKT γδ T cells, which are also partially associated with Vγ usage (40). Specifically, Vγ5+ DETCs preferentially produce IFNγ, while Vγ6+ γδ T cells mainly produce IL-17A (41). Later waves, such as Vγ4 and Vγ1, are more heterogenous in their capacity to produce various effector cytokines. While IL-17A production is not limited to a specific Vγ subset, innate-like Tγδ17 cell generation is restricted to a window of time during fetal life, approximately E16 to birth, that enriches for Vγ6+ and Vγ4+ γδ T cell subsets (42). Within the third functional wave, Vγ1+Vδ6.3+ NKT γδ T cells express PLZF and are capable of producing both IL-4 and IFNγ (43, 44). Therefore, the fate decisions of developing thymocytes during fetal life impacts the adult reservoir of innate-like γδ T cell effectors.
γδ T cell effectors can be defined by various cell surface markers: IFNγ producing γδ T cells typically express CD27, CD122, NK1.1, and high levels of CD45RB, while IL-17A producing γδ T cells lack expression of CD27, CD122, and NK1.1 but usually express CCR6 and low levels of CD45RB (41, 45, 46) (Figure 1). Nevertheless, the study of γδ effector diversification has been hampered by the lack of definitive markers that distinguish Tγδ1 and Tγδ17 precursors. Before effector commitment, CD25 is expressed by the earliest γδ T cells in the thymus (47), as γδ-selected thymocytes are derived from CD25+ DN2 and DN3 T cell precursors (18, 48). Post-selection γδ thymocytes are also distinguished by CD27 upregulation (48), and these CD25+CD27+ are the earliest progenitors of IL-17A and IFNγ γδ effectors (46). Emerging γδ thymocytes with low levels of γδTCR also express intermediate levels of CD45RB, and have molecular signatures and developmental potential consistent with being precursors to both Tγδ17 and Tγδ1 cells (41, 49). Indicative of their immature status, these pioneer γδ T cells are marked by high levels of CD24 expression, which is later downregulated upon maturation (50).
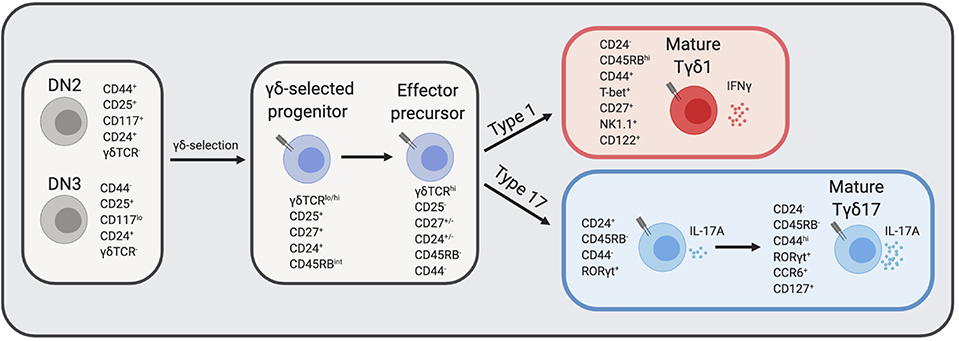
Figure 1. γδ T cell development in the thymus. DN thymocytes undergo γδ-selection and become immature γδ thymocytes that eventually diverge into either IFNγ producers or IL-17A producers. The expression of cell surface markers and transcription factors that define transitional precursors and mature effector γδ T cells are listed next to each cell type. CD24 and CD27 expression at the “effector precursor” stage is heterogenous and is marked by +/–, however, cells transition from CD24+ to CD24−. DN, double negative; TCR, T cell receptor. Figure made with biorender.com.
Several recent studies have provided clarity regarding the developmental trajectories of innate-like γδ T cell effector subsets beyond the precursor stage (49, 51). Recent work by Sumaria and colleagues identified CD45RB−CD44− γδ thymocytes as precursors of both type 1 and type 17 effectors, suggesting that all γδ T cells downregulate CD45RB prior to effector diversification (Figure 1) (52). Consistent with this view, the absolute block in Tγδ17 development in the absence of c-Maf revealed an effector specialization checkpoint at the immature CD45RB−CD24+ γδ thymocyte stage (49). This block also provides genetic support for a model in which effector programming is molecularly distinct from γδ-selection (3). Among mature CD24− γδ thymocytes, CD45RB and CD44 distinguish effector lineages: CD44hiCD45RBlo γδ T express high levels of RORγt and IL-7Rα and are committed to IL-17A production, whereas CD44+CD45RB+ γδ T cells express T-bet, but lack RORγt or IL-7Rα expression and are committed to IFNγ production (Figure 1) (51). Additionally, CD73 expression, which is linked to strong ligand-dependent γδTCR signaling (53), is significantly more expressed on IFNγ-committed than IL-17A-committed γδ thymocytes (51), and CD73− γδ thymocytes are enriched for those undergoing type 17 differentiation in the perinatal thymus (54). Interestingly, although CD24+ γδ thymocytes are considered “immature,” they nonetheless express key TFs necessary for their effector acquisition, such as RORγt for Tγδ17 cells (49, 54, 55), and are surprisingly also functionally competent to produce IL-17A (51). The application of global single cell transcriptomic analysis to fetal γδ thymocytes is likely to add significant granularity to the developmental trajectories of effector programming [preprint (56)].
Role of γδTCR
Similar to the role of TCR in αβ vs. γδ lineage choice, the γδTCR is important for determining the effector fate of γδ T cells. The current understanding supports a model with two sequential steps in commitment; first, the decision of αβ vs. γδ, and second, the decision to become an IFNγ- or IL-17A-secreting γδ T cell (3). Both steps in development are dependent on TCR signal strength integrated with numerous environmental signals. The idea that thymic selection determines the effector fate of γδ T cells was first supported by the finding that γδ T cells exposed to a TCR ligand leading to a strong TCR signal become IFNγ producers, whereas the absence of ligand or weak γδTCR signal result in the IL-17A effector fate (57). Further supporting the notion that ligand-dependent strong γδTCR signals promote the type 1 fate, DETCs, known to produce IFNγ, adopt an IL-17A producing γδ T cell fate in the absence of their selecting ligand, Skint-1 (discussed further below) (41). Conversely, enhancing γδTCR signal strength through the addition of crosslinking γδTCR antibody GL3 to fetal thymic organ cultures (FTOC) significantly reduced the number of CD44hiCD45RB− IL-17A-committed cells while increasing type 1-associated CD44+CD45RBhi cells (51). A similar outcome was achieved when strong TCR signals were mimicked by transduction of T cell progenitors with a constitutively active form of the kinase Lck (LckF505) (49). Together, these studies suggest that the type 17 program is the default effector pathway that is otherwise repressed by strong or ligand-dependent TCR signals. Whether Tγδ17 development supported by weak TCR signaling is truly or universally ligand-independent remains to be determined.
γδ T cell effector fate choice is also influenced by specific TCR signal transduction pathways. For example, ERK signals support the type 1 program as ERK-deficient TCRβ−/− mice have an increased frequency of CD27− γδ T cells, and ERK-deficient KN6 γδ TCR transgenic thymocytes are skewed toward IL-17A production compared to the controls that predominately produce IFNγ (29). More recently, it was revealed that the tyrosine kinase Syk is selectively required for Tγδ17 development, through activation of the PI3K/Akt pathway downstream of γδTCR signaling (58). Studies show that impairment of TCR signal strength with SKG [Zap70 mutant (59)] and CD3DH (CD3γ and CD3δ double heterozygous) mice both have reduced frequencies of IL-17A-producing Vγ6+ γδT cells (60, 61). Notably, the defect in Zap70 signaling impacts Vγ4+ Tγδ17s as well, just to a lesser extent, while the Vγ4+ γδT cells in the CD3DH mice are not impaired (60, 61). These findings imply that while we group Tγδ17s into one effector class, the Vγ subsets may require specific signal strengths and downstream signaling molecules for their effector programs. Taken together, these findings also support the model that IFNγ producing γδ T cells require strong TCR signals, while IL-17A producing γδ T cells generally require weaker TCR signal strength (41, 46, 51).
Environmental Cues
Environmental cues in the thymus are derived from both thymic epithelial cells (TECs), developing thymocytes, and other hematopoietic cells. Timing is also a critical factor, as the developmental windows in which progenitors seed the thymus influence their exposure to signals integrated from both the stromal microenvironment and resident developing thymocytes. Therefore, γδ T cell effector specialization can be influenced by various environmental cues during ontogeny.
Lymphotoxin Signaling
One of the best-studied examples of such signals is a process called “trans-conditioning.” This phenomenon was initially discovered in TCRβ−/− mice that have an altered γδ T cell gene profile and significantly reduced secretion of IFNγ by splenic γδ T cells (62). The authors concluded that αβ T cells are required for the normal development of γδ T cells (62). Subsequent work identified lymphotoxin production by DP thymocytes as the mechanism, in part, responsible for the regulation of γδ T cell maturation and differentiation toward an IFNγ-producing fate (63). Mechanistically, this was extended with the finding that CD27, a tumor necrosis factor (TNF) receptor superfamily member, engages CD70 and positively upregulates lymphotoxin beta receptor (LTBR) expression on γδ T cells (46). Accordingly, the function of CD27 in supporting IFNγ production coincides with its selective expression by mature Tγδ1 as compared to Tγδ17 cells (Figures 1, 2) (46). The role of lymphotoxin signaling in γδ T cell effector commitment is complex as the thymic differentiation of IL-17A-producing γδ T cells is also dependent on this pathway (64). Indeed, by way of the lymphotoxin signaling pathway, the NF- κb family members, RelA and RelB, play distinct roles in the thymic preprogramming of Tγδ17 cells. RelA regulates lymphotoxin ligand expression in accessory thymocytes, thereby indirectly controlling IL-17A production by γδ T cells. On the other hand, γδ T cell precursors require RelB downstream of LTBR to maintain Rorc expression for differentiation into mature Tγδ17 cells (Figure 2) (64). Taken together, lymphotoxin signaling regulates the effector fate acquisition of γδ T cells through integration of γδ T cell-intrinsic and extrinsic pathways.
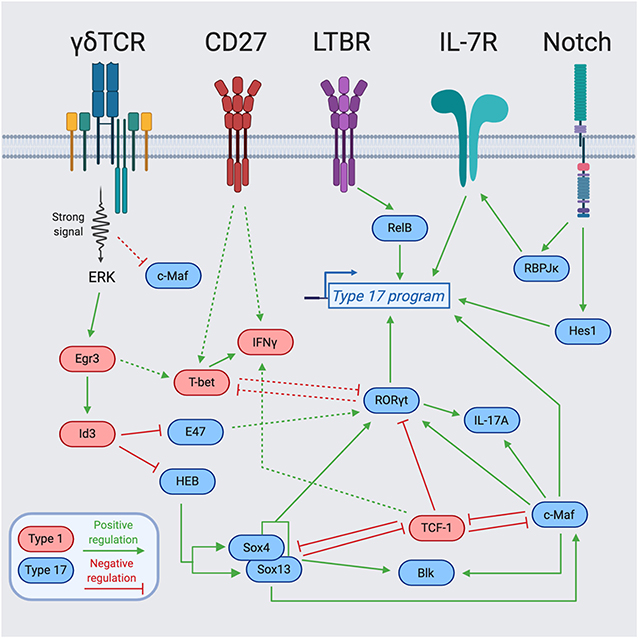
Figure 2. Transcription factor network regulating γδ T cell effector programming. Integration of cell surface receptors [TCR, Lymphotoxin Beta Receptor (LTBR), CD27, and Notch] with downstream transcription factors for the programming of γδ T cell effector function. Blue-colored TFs support the type 17 program, while red-colored TFs support the type 1 program. The dotted lines represent indirect regulation or that the supporting data was described in another cell type. The solid lines represent more direct regulation. Figure made with biorender.com.
Cytokines and Notch Signaling
IL-7 is known for being a non-redundant, key regulator of lymphocyte homeostasis through promotion of survival and proliferation (65–68). The IL-7/IL-7R pathway plays essential roles at distinct stages in the development of multiple lymphocyte lineages (69). In particular, γδ T cells require IL-7Rα for their development, as IL-7R-deficient mice lack all γδ T cells (70). Follow-up work by several groups demonstrated that IL-7Rα-deficient mice have a block in V-J recombination of the TCRγ genes (71), and that IL-7R controls the accessibility of the TCRγ locus (72–74). While IL-7 signaling is required for all γδ T cell development, high levels of IL-7Rα expression and IL-7 signaling preferentially favor the differentiation of IL-17A-producing γδ T cells (75, 76). In line with this notion, Aire-deficient mice have increased production of IL-7 by medullary thymic epithelial cells (mTECs) that results in expanded populations of IL-17A-producing Vγ6+Vδ1+ T cells in the thymus and the periphery (77). The IL-7 signaling pathway also integrates with additional environmental signals and transcriptional regulators, most notably, the Notch signaling pathway. The Notch target and transcriptional repressor, Hes1, is specifically expressed in IL-17A-producing γδ T cells and Hes1 ablation significantly decreases IL-17A production with no effect on IFNγ secretion in peripheral γδ T cells (Figure 2) (78). Notch also regulates Tγδ17 differentiation in a Hes1-independent, but RBPJκ-dependent manner (79). Mechanistically, Notch signaling and RBPJκ are required for IL-7Rα expression, and IL-7Rα-mediated signaling is indispensable for the homeostasis of IL-17+ γδ T cells (Figure 2) (79). Future studies further exploring the transcriptional activators and repressors of Il7r will help elucidate how IL-7 signaling integrates with other environmental cues to control γδ T cell fate.
IL-17 is another interesting example of a soluble mediator produced in the thymus that regulates the development of γδ T cells. The development of innate-like Tγδ17 cells is restricted to a functional embryonic wave during fetal life from E16 to birth, resulting in long-lived, self-renewing cells that are found in adult mice (42). Surprisingly, it was found that IL-17 production in the thymus influences the development of Tγδ17 cells through a negative feedback loop such that CCR6+CD27− Tγδ17 cell numbers are increased in Il17af−/− mice (mice with deletion of the entire Il17a and Il17f locus) compared to wild-type controls (42). Interestingly, IL-17-producing Thy1+ cells resembling group 3 innate lymphoid cells (ILC3s) were found in the thymus of Rag1−/− mice (42). Therefore, the restriction of Tγδ17 cell development may be attributed to IL-17 production from both innate lymphoid cells and IL-17+ αβ and γδ T cells (42).
TGF-β signaling has pleiotropic effects on immune cells. Among type 17 lineages, a specific role for TGF-β was first defined for the differentiation of naïve CD4+ T cells into Th17 cells. Specifically, TGF-β1−/− mice have severely diminished Th17 cells in peripheral lymphoid organs (80). Despite major distinctions between Th17 cells and Tγδ17 cells, IL-17A-producing γδ T cells are also significantly reduced in mice deficient for either TGF-β1 or Smad3, the TGF-β signaling adaptor molecule, suggesting a similar dependence of TGF-β signaling for IL-17 production in the γδ lineage (81). However, this study was performed in neonates at a time point when innate-like Tγδ17 cells have left the thymus, therefore, the precise role of TGF-β signaling in Tγδ17 cell development is still unclear. In this regard, TGF-β may support Tγδ17 cells as a driver of Ras signaling (82), a signaling cascade that strongly promotes the type 17 program in γδ T cells (49).
Butyrophilins
Whether γδ T cells undergo thymic selection analogous to αβ T cells has been a major question in the field. In order to explain the domination of tissue-specific γδ T cell compartments by particular Vγ subsets, it was hypothesized that the same γδTCR-specific ligands expressed in both the fetal thymus and target tissues could mediate positive selection during ontogeny and thereafter, tissue localization and maintenance cues for long-term residence (83). FVB-Tac mice harboring a spontaneous mutation that selectively disrupts the DETC compartment was reported to map back to a single gene expressed by TECs and keratinocytes, representing the first support for the hypothesis that DETCs undergo positive selection in the thymus (84). A few years later, the phenotype of FVB-Tac mice was attributed to a mutation in the Skint1 gene (85). Skint1 is a member of the butyrophilin-like (Btnl) family that structurally resembles the B7 superfamily molecules CD80 and PD-L1 (86–88). Skint gene expression is restricted to the thymus and skin, therefore, the broader applicability of this mechanism of selection for other intraepithelial γδ T cells was questioned (85). Recently, expression of Btnl1 by villus epithelial cells in the small intestine was shown to mediate the extrathymic selection of Vγ7+ intraepithelial lymphocytes (IELs), driving their expansion and maturation (89). In particular, joint expression of Btnl1 and Btnl6 by intestinal epithelial cells regulates the TCR-dependent responses of Vγ7+ IELs (89). Importantly, human intestinal epithelium co-expressing BTNL3 and BTNL8 selectively regulated Vγ4+ γδ T cells, indicating an evolutionary conserved mechanism of γδ T cell regulation across mouse and human (89). While extensive progress has been made, much remains unknown regarding the identity of γδTCR ligands that drive specific γδ T cell subset selection for tissue homeostasis (90).
γδ T Cell Crosstalk With mTECs
Aire-expressing mTECs are necessary for central tolerance through expression of tissue-restricted antigens (91). Previous work identified the importance of RANKL-RANK signaling for induction of mTEC Aire expression by lymphoid tissue inducer (LTi) cells (92, 93). Notably, the timing of Aire expression on mTECs coincides with the first wave of Vγ5+ DETC precursors seeding the thymus (94). Interestingly, RANKL-RANK interactions between RANKL+ Vγ5+ DETC thymocytes and RANK+ mTECs also induce Aire expression and mTEC maturation. Such RANKL-RANK signaling is additionally required for Skint-1 expression by mTECs, and thus is reciprocally necessary for Vγ5+ DETC development. Taken together, this study elegantly demonstrates the crosstalk between developing DETC progenitors and immature mTECs that each rely on shared RANKL-RANK signals for maturation. While DETCs are the first γδ thymocytes to emerge in ontogeny, similar crosstalk between resident immune cells and TECs may account for the discrete developmental windows of other innate-like γδ T cell subsets.
Transcriptional Networks Regulating γδT Cell Identity
γδ T cell effector acquisition is regulated by a highly-integrated network of transcriptional regulators. The lineage-defining transcription factors (LDTFs), RORγt and T-bet, promote the effector fates of IL-17A vs. IFNγ producers in various lymphocyte lineages, respectively (95–97). Although these LDTFs are integral to programming γδ T cell effector function, many other signal-dependent and collaborating TFs play essential roles in establishing and maintaining γδ T cell identity downstream of TCR signaling and various environmental signaling cascades (Figure 2).
In order to better understand the effector diversification of γδ T cells from a global perspective, the Immgen consortium performed gene-expression profiling of isolated ex vivo γδ T cells subsets (55). Among these, distinct clusters of immature γδ T cells could be distinguished based on their transcriptomes, reflecting three unique effector programs: IL-17A producers (Vγ6+ and Vγ4+), IFNγ producers (Vγ1+, Vγ1+Vδ6.3+, Vγ7+), and DETCs (Vγ5+) (55). Importantly, key TFs are enriched in specific γδ effector subsets, such as Rorc, Maf, Sox13, and Sox4 for the IL-17A producers and Tcf7 (TCF-1), Lef1, Tbx21 (T-bet), and Eomes for the IFNγ producers (55). The dual action of many of these TFs in both promoting one effector fate, while repressing the alternative fate leads to a complex TF network in γδ T cells (Figure 2). Interestingly, TFs associated with type 17 programming in adaptive Th17 cells—namely, IRF4, BATF, and STAT3—are dispensable for Tγδ17 cells (64, 98–100).
TCR-Independent Transcriptional Regulators
Independent of conventional TCR signaling, innate-like γδ T cell effector programming is regulated by a quartet of HMG box TFs including Sox4, Sox13, TCF-1, and Lef1 (101). Among these, Sox13 and Sox4 are essential for the differentiation of Vγ4+ IL-17A-producing cells (101). This Vγ-specific requirement is intriguing as it implies that discrete regulators drive the specification of distinct subsets of Tγδ17 cells, although it remains possible that redundancy between Sox13 and Sox4 masks a global role for Sox TFs in γδ T cell type 17 programming. Within the Vγ4+ subset, Sox13 and Sox4 regulate key Tγδ17 program genes such as Rorc and Blk (23, 101), a tyrosine protein kinase that is selectively required for the development of Tγδ17 cells (102). While Sox proteins positively regulate type 17 fate, TCF-1 and Lef1 function to restrain Tγδ17 cell generation and gene expression (101). TCF-1 is targeted by multiple environmental signals; it is a Notch-induced TF that plays critical stage-specific roles in T cell differentiation (103, 104), and is also influenced by the Wnt signaling pathway through its β-catenin interaction domain, which is required to ensure DP thymocyte survival (104). In γδ T cells, TCF-1 promotes the expression of Lef1 and the IFNγ producing fate (101). Sox13 may also counteract the type 1 program through direct antagonism of TCF-1 via its β-catenin interaction domain (22), and indirectly via TCF-1 targets, as evidenced by Sox13 Tg mice expressing greatly diminished levels of Lef1 (101). The mutually opposing functions of Sox proteins and TCF-1/Lef1 in Tγδ1 and Tγδ17 differentiation likely reinforces and stabilizes effector fate. Together, TCR-independent HMG box TFs represent key interconnected nodes in the transcriptional network of γδ T cells.
TCR-Dependent Transcriptional Regulators
A crucial question in γδ T cell biology is how distinct functional potentials arise from differential TCR signal strengths? (41). Broadly, effector commitment to an IFNγ-producing fate through strong TCR signaling requires both promotion of drivers of the type 1 program, and simultaneous neutralization of drivers of the type 17 program. TCR signaling can be linked to γδ T cell lineage and effector commitment through the Egr-Id3 pathway. Downstream of strong TCR signaling, Erk induced Egr1 promotes the development of γδ T cells through activation of the E protein inhibitor Id3 (26, 28). Induction of Id3 is also required for functional IFNγ production, providing a mechanism by which signal strength is translated into downstream effectors (28). This signal is key in suppression of E proteins that otherwise support Tγδ17 features (Figure 2). Indeed, it has been demonstrated in DP thymocytes that E proteins enhance RORγt expression, while Egr3 negatively regulates RORγt expression by inducing Id3 (105). Similarly, Id3 can antagonize the type 17 program by forming an inactive heterodimer with HEB, an E protein TF that is required for direct promotion of Sox13 and Sox4 expression and CD73− Tγδ17 cell development (54). Along these lines, Egr3 is highly expressed in Vγ5+Vδ1+ thymocytes and upregulation of Egr3 after Skint-1-mediated selection or strong TCR signal represses Rorc and Sox13 but supports Tbx21 expression and commitment toward an IFNγ producing fate (41). Therefore, Egr3 downstream of Skint-1-mediated selection directs the TF balance necessary for proper DETC development through restraint of the “default” type 17 program. These findings highlight that TCR-dependent and TCR-independent TFs both antagonize and promote each other to regulate the effector fate of γδ T cells.
Regulation of Type 17 Commitment
In contrast to Tγδ17 specification factors important for type 17 differentiation of distinct Vγ subsets [e.g., Sox13, Sox4, and HEB (54, 101)], the AP-1 factor c-Maf was recently identified as universally required for the generation and maintenance of all IL-17A-producing γδ T cells (49). As a canonical commitment factor, c-Maf directly activates Rorc and key Tγδ17 effector genes (Il17a and Blk), while also antagonizing the expression or function of negative regulators of the type 17 program (TCF-1 and Lef1) that promote the alternative Tγδ1 fate (Figure 2) (49). c-Maf globally supports a Tγδ17 chromatin accessibility landscape, with a particularly important role in the establishment of an active regulatory status at Rorc involving the recruitment of the histone acetyltransferase p300, and H3K27 acetylation (49). The signals that directly activate c-Maf in γδ thymocytes remain to be defined, but may involve known Tγδ17-promoting factors such as Notch, TGF-β, and IL-7 that have been described as c-Maf activators in CD4+ T cells or ILCs (75, 78, 79, 81, 106–108). There is some evidence that Sox TFs function upstream of c-Maf and can regulate its protein expression (49). Interestingly, unlike Sox13 expression that is independent of TCR signaling (101, 109), c-Maf expression is tuned by TCR signal strength in fetal γδ thymocytes; strong TCR signals lead to low c-Maf and weak signals result in high c-Maf protein levels, providing a mechanism by which weak γδTCR signals can be translated into Tγδ17 regulatory programming (49).
Integration of Type 17 Regulators
A highly-integrated network of regulators control type 17 programming (Figure 2). Sox13 and Sox4 collaborate with c-Maf in the direct activation of Rorc and other key Tγδ17 genes such as Blk and Il17a (49, 101). The close proximity of Maf recognition element (MARE) and HMG box consensus sites in the c-Maf-dependent Rorc enhancer (CNS+10) suggests that c-Maf and Sox TFs may bind and function cooperatively in γδ T cells (49), as has been described in multiple other cell types (110–112). Of particular relevance, Sox5 and c-Maf can cooperatively bind the Rorc promoter and drive its expression in Th17 cells (112). Additionally, c-Maf and RORγt collaborate in the activation of Il17a and potentially other type 17 signature genes, however, c-Maf also functions independently of its direct target RORγt in regulating key Tγδ17 lineage-modulating factors (e.g., Blk, Lef1, and Syk) (49). Aside from activation of the type 17 program, both Sox13 and c-Maf repress the alternative type 1 fate by targeting TCF-1/Lef1 (49, 101). TCF-1 negatively regulates the Rorc locus (101), and its occupancy at Rorc CNS+10 is antagonized by c-Maf in γδ thymocytes (49). As TCF-1 harbors intrinsic HDAC activity (113), this antagonism may represent another mechanism by which c-Maf promotes H3K27 acetylation at the Rorc locus (49). Intriguingly, c-Maf also restrains the expression and function of TCF-1 in ILC3s (106), while TCF-1 represses the c-Maf/RORγt axis to limit the formation of Tc17 cells in CD8+ T cells (114). This suggests that c-Maf/TCF-1 antagonism is conserved across multiple lymphocyte lineages to regulate the balance of the type 1 vs. type 17 specialization.
The integration of various signals in the effector programming of γδ thymocytes suggests several tiers of regulators in specialization. In building a model, this includes: (1) specification factors (e.g., RelB, Notch, HEB, Sox13, and TCF-1) that perceive environmental signals to support type 1 or type 17 programming either universally or in the establishment of discrete Tγδ17 subsets; (2) commitment factors (e.g., c-Maf, Egr-Id3) that impart or reinforce effector identity programs, and (3) LDTFs (e.g., RORγt, T-bet) that control genes for key canonical effector functions (Figure 2). As γδ T cell selection and effector diversification occur across various DN and γδ thymocyte developmental intermediates, with numerous thymus and TCR-derived signals likely occurring over a protracted period, the temporal contributions of such inputs with respect to effector commitment remains unclear. In this regard, a recent intriguing study employing a Sox13 reporter mouse, identified DN1-like (CD117−CD24+CD25+) precursors in the perinatal to day 10 thymus that are prewired for the expression of the Tγδ17 gene network (e.g., Rorc, Sox4, Tcf7, Tcf12, Maf, Il7r, Scart2, and Blk) and are generated in a TCR-independent manner (109). Remarkably, such Sox13+ DN1d cells are predisposed to become CCR6+ IL-17A-producing cells, suggesting they are pre-committed to the Tγδ17 fate (109). Future work focused on how such effector-committed precursors intersect with the rearrangement of particular Vγ TCRs and signal strengths will broaden our understanding of the integration of environmental and TCR inputs in the effector programming of γδ thymocytes during ontogeny.
Concluding Remarks
The last decade of research has led to enormous leaps in the understanding of tissue-resident lymphocytes, with newfound appreciation for the diversity of innate lymphocytes. Although dependent on the same LDTFs, innate-like γδ T cells and ILCs have unique transcriptional networks that control their effector fates. Such underlying distinctions in regulatory programming may translate into functional differences or non-redundant roles for innate-like γδ T cells vs. ILCs. Indeed, γδ T cells possess a TCR complex that endow them with additional environmental sensing capacities. Thus, uniquely, innate-like γδ T cell effector commitment can be controlled, in part, by the fine-tuning of key transcriptional regulators downstream of TCR signaling to both promote one fate while repressing the other. However, there is still much to be learned with respect to the establishment of transcriptional programs independent of TCR signaling and the elements that predispose γδ thymocytes to an effector fate prior to TCR expression. In the future, taking advantage of advances in single-cell sequencing and genomics techniques will lead to a higher resolution picture of γδ T cell trajectories and lineage decisions.
Author Contributions
MP prepared and wrote the manuscript. MC edited the manuscript.
Funding
MP and MC were supported by National Institutes of Health grant RO1 GM115474. MC was additionally supported by NIH grant UM1 HG009428 and a Whitehead Charitable Foundation Scholar Award.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hirano M, Guo P, McCurley N, Schorpp M, Das S, Boehm T, et al. Evolutionary implications of a third lymphocyte lineage in lampreys. Nature. (2013) 501:435–8. doi: 10.1038/nature12467
2. Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. (2002) 2:336–45. doi: 10.1038/nri797
3. Munoz-Ruiz M, Sumaria N, Pennington DJ, Silva-Santos B. Thymic determinants of γδ T cell differentiation. Trends Immunol. (2017) 38:336–44. doi: 10.1016/j.it.2017.01.007
4. Chien YH, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)-17-producing γδ T cells. Trends Immunol. (2013) 34:151–4. doi: 10.1016/j.it.2012.11.004
5. Fan X, Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell. (2016) 164:1198–211. doi: 10.1016/j.cell.2016.02.048
6. Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. (2013) 13:88–100. doi: 10.1038/nri3384
7. Amatya N, Childs EE, Cruz JA, Aggor FEY, Garg AV, Berman AJ, et al. IL-17 integrates multiple self-reinforcing, feed-forward mechanisms through the RNA binding protein Arid5a. Sci Signal. (2018) 11:eaat4617. doi: 10.1126/scisignal.aat4617
8. Castro F, Cardoso AP, Goncalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol. (2018) 9:847. doi: 10.3389/fimmu.2018.00847
9. Papotto PH, Reinhardt A, Prinz I, Silva-Santos B. Innately versatile: γδ17 T cells in inflammatory and autoimmune diseases. J Autoimmun. (2018) 87:26–37. doi: 10.1016/j.jaut.2017.11.006
10. Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. (2015) 21:938–45. doi: 10.1038/nm.3909
11. Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. (2017) 17:733–45. doi: 10.1038/nri.2017.101
12. Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Koay HF, et al. γδ T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol. (2018) 19:464–74. doi: 10.1038/s41590-018-0094-2
13. Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, et al. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun. (2016) 7:10928. doi: 10.1038/ncomms10928
14. Ribeiro M, Brigas HC, Temido-Ferreira M, Pousinha PA, Regen T, Santa C, et al. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol. (2019) 4:eaay5199. doi: 10.1126/sciimmunol.aay5199
15. Ribeiro ST, Ribot JC, Silva-Santos B. Five layers of receptor signaling in γδ t-cell differentiation and activation. Front Immunol. (2015) 6:15. doi: 10.3389/fimmu.2015.00015
16. Ciofani M, Zuniga-Pflucker JC. Determining gammadelta versus alphass T cell development. Nat Rev Immunol. (2010) 10:657–63. doi: 10.1038/nri2820
17. Livak F, Tourigny M, Schatz DG, Petrie HT. Characterization of TCR gene rearrangements during adult murine T cell development. J Immunol. (1999) 162:2575–80.
18. Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zuniga-Pflucker JC. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. (2006) 25:105–16. doi: 10.1016/j.immuni.2006.05.010
19. Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis. J Exp Med. (2008) 205:1173–86. doi: 10.1084/jem.20072425
20. Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. (1995) 375:795–8. doi: 10.1038/375795a0
21. Kang J, Volkmann A, Raulet DH. Evidence that gammadelta versus alphabeta T cell fate determination is initiated independently of T cell receptor signaling. J Exp Med. (2001) 193:689–98. doi: 10.1084/jem.193.6.689
22. Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, et al. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. (2007) 315:230–3. doi: 10.1126/science.1135344
23. Gray EE, Ramirez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, et al. Deficiency in IL-17-committed Vgamma4(+) gammadelta T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat Immunol. (2013) 14:584–92. doi: 10.1038/ni.2585
24. Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. (1986) 322:836–40. doi: 10.1038/322836a0
25. Fahl SP, Kappes DJ, Wiest DL. TCR Signaling circuits in alphabeta/gammadelta T lineage choice. In: Soboloff J, Kappes DJ, editors. Signaling Mechanisms Regulating T Cell Diversity and Function. Boca Raton, FL: Taylor & Francis Group, LLC (2018). p. 85–104.
26. Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, et al. Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity. (2005) 22:595–606. doi: 10.1016/j.immuni.2005.04.003
27. Hayes SM, Li L, Love PE. TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity. (2005) 22:583–93. doi: 10.1016/j.immuni.2005.03.014
28. Lauritsen JP, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, et al. Marked induction of the helix-loop-helix protein Id3 promotes the gammadelta T cell fate and renders their functional maturation Notch independent. Immunity. (2009) 31:565–75. doi: 10.1016/j.immuni.2009.07.010
29. Lee SY, Coffey F, Fahl SP, Peri S, Rhodes M, Cai KQ, et al. Noncanonical mode of ERK action controls alternative alphabeta and gammadelta T cell lineage fates. Immunity. (2014) 41:934–46. doi: 10.1016/j.immuni.2014.10.021
30. Havran WL, Chien YH, Allison JP. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science. (1991) 252:1430–2. doi: 10.1126/science.1828619
31. Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, et al. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. (1990) 343:754–7. doi: 10.1038/343754a0
32. Carding SR, Kyes S, Jenkinson EJ, Kingston R, Bottomly K, Owen JJ, et al. Developmentally regulated fetal thymic and extrathymic T-cell receptor gamma delta gene expression. Genes Dev. (1990) 4:1304–15. doi: 10.1101/gad.4.8.1304
33. Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal gammadelta T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. (2004) 21:121–31. doi: 10.1016/j.immuni.2004.06.008
34. Jin Y, Xia M, Saylor CM, Narayan K, Kang J, Wiest DL, et al. Cutting edge: intrinsic programming of thymic gammadeltaT cells for specific peripheral tissue localization. J Immunol. (2010) 185:7156–60. doi: 10.4049/jimmunol.1002781
35. Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien YH, et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. (1990) 62:863–74. doi: 10.1016/0092-8674(90)90262-D
36. Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. (1990) 344:68–70. doi: 10.1038/344068a0
37. Ramond C, Berthault C, Burlen-Defranoux O, de Sousa AP, Guy-Grand D, Vieira P, et al. Two waves of distinct hematopoietic progenitor cells colonize the fetal thymus. Nat Immunol. (2014) 15:27–35. doi: 10.1038/ni.2782
38. Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. (2012) 335:1195–200. doi: 10.1126/science.1216557
39. Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA, et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. (2012) 37:524–34. doi: 10.1016/j.immuni.2012.06.011
40. Prinz I, Silva-Santos B, Pennington DJ. Functional development of gammadelta T cells. Eur J Immunol. (2013) 43:1988–94. doi: 10.1002/eji.201343759
41. Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity. (2011) 35:59–68. doi: 10.1016/j.immuni.2011.04.018
42. Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, et al. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity. (2012) 37:48–59. doi: 10.1016/j.immuni.2012.06.003
43. Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, et al. Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3. J Immunol. (2010) 184:1268–79. doi: 10.4049/jimmunol.0903218
44. Pereira P, Berthault C, Burlen-Defranoux O, Boucontet L. Critical role of TCR specificity in the development of Vgamma1Vdelta6.3+ innate NKTgammadelta cells. J Immunol. (2013) 191:1716–23. doi: 10.4049/jimmunol.1203168
45. Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur J Immunol. (2009) 39:3488–97. doi: 10.1002/eji.200939922
46. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. (2009) 10:427–36. doi: 10.1038/ni.1717
47. Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat Immunol. (2006) 7:995–1003. doi: 10.1038/ni1371
48. Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging beta- and gammadelta-selected pre-T cells in the adult mouse thymus. Immunity. (2006) 24:53–64. doi: 10.1016/j.immuni.2005.11.012
49. Zuberbuehler MK, Parker ME, Wheaton JD, Espinosa JR, Salzler HR, Park E, et al. The transcription factor c-Maf is essential for the commitment of IL-17-producing gammadelta T cells. Nat Immunol. (2019) 20:73–85. doi: 10.1038/s41590-018-0274-0
50. Pereira P, Zijlstra M, McMaster J, Loring JM, Jaenisch R, Tonegawa S. Blockade of transgenic gamma delta T cell development in beta 2-microglobulin deficient mice. EMBO J. (1992) 11:25–31. doi: 10.1002/j.1460-2075.1992.tb05023.x
51. Sumaria N, Grandjean CL, Silva-Santos B, Pennington DJ. Strong TCRgammadelta signaling prohibits thymic development of IL-17A-secreting gammadelta T Cells. Cell Rep. (2017) 19:2469–76. doi: 10.1016/j.celrep.2017.05.071
52. Sumaria N, Martin S, Pennington DJ. Developmental origins of murine gammadelta T-cell subsets. Immunology. (2019) 156:299–304. doi: 10.1111/imm.13032
53. Coffey F, Lee SY, Buus TB, Lauritsen JP, Wong GW, Joachims ML, et al. The TCR ligand-inducible expression of CD73 marks gammadelta lineage commitment and a metastable intermediate in effector specification. J Exp Med. (2014) 211:329–43. doi: 10.1084/jem.20131540
54. In TSH, Trotman-Grant A, Fahl S, Chen ELY, Zarin P, Moore AJ, et al. HEB is required for the specification of fetal IL-17-producing gammadelta T cells. Nat Commun. (2017) 8:2004. doi: 10.1038/s41467-017-02225-5
55. Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, et al. Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nat Immunol. (2012) 13:511–8. doi: 10.1038/ni.2247
56. Sagar, Pokrovskii M, Herman JS, Naik S, Sock E, Lausch U, et al. Deciphering the regulatory landscape of γδ T cell development by single-cell RNA-sequencing. bioRxiv. (2018). doi: 10.1101/478529
57. Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. (2008) 29:90–100. doi: 10.1016/j.immuni.2008.04.022
58. Muro R, Nitta T, Nakano K, Okamura T, Takayanagi H, Suzuki H. gammadeltaTCR recruits the Syk/PI3K axis to drive proinflammatory differentiation program. J Clin Invest. (2018) 128:415–26. doi: 10.1172/JCI95837
59. Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. (2003) 426:454–60. doi: 10.1038/nature02119
60. Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A, et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat Immunol. (2014) 15:80–7. doi: 10.1038/ni.2773
61. Munoz-Ruiz M, Ribot JC, Grosso AR, Goncalves-Sousa N, Pamplona A, Pennington DJ, et al. TCR signal strength controls thymic differentiation of discrete proinflammatory gammadelta T cell subsets. Nat Immunol. (2016) 17:721–7. doi: 10.1038/ni.3424
62. Pennington DJ, Silva-Santos B, Shires J, Theodoridis E, Pollitt C, Wise EL, et al. The inter-relatedness and interdependence of mouse T cell receptor gammadelta+ and alphabeta+ cells. Nat Immunol. (2003) 4:991–8. doi: 10.1038/ni979
63. Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of gammadelta cell differentiation by alphabeta T cell progenitors. Science. (2005) 307:925–8. doi: 10.1126/science.1103978
64. Powolny-Budnicka I, Riemann M, Tanzer S, Schmid RM, Hehlgans T, Weih F. RelA and RelB transcription factors in distinct thymocyte populations control lymphotoxin-dependent interleukin-17 production in gammadelta T cells. Immunity. (2011) 34:364–74. doi: 10.1016/j.immuni.2011.02.019
65. Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. (1994) 180:1955–60. doi: 10.1084/jem.180.5.1955
66. von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. (1995) 181:1519–26. doi: 10.1084/jem.181.4.1519
67. Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. (1997) 89:1033–41. doi: 10.1016/S0092-8674(00)80291-3
68. Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. (2008) 111:2101–11. doi: 10.1182/blood-2007-06-096297
69. Kang J, Coles M. IL-7: the global builder of the innate lymphoid network and beyond, one niche at a time. Semin Immunol. (2012) 24:190–7. doi: 10.1016/j.smim.2012.02.003
70. He YW, Malek TR. Interleukin-7 receptor alpha is essential for the development of gamma delta + T cells, but not natural killer cells. J Exp Med. (1996) 184:289–93. doi: 10.1084/jem.184.1.289
71. Maki K, Sunaga S, Ikuta K. The V-J recombination of T cell receptor-gamma genes is blocked in interleukin-7 receptor-deficient mice. J Exp Med. (1996) 184:2423–7. doi: 10.1084/jem.184.6.2423
72. Ye SK, Agata Y, Lee HC, Kurooka H, Kitamura T, Shimizu A, et al. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity. (2001) 15:813–23. doi: 10.1016/S1074-7613(01)00230-8
73. Schlissel MS, Durum SD, Muegge K. The interleukin 7 receptor is required for T cell receptor gamma locus accessibility to the V(D)J recombinase. J Exp Med. (2000) 191:1045–50. doi: 10.1084/jem.191.6.1045
74. Agata Y, Katakai T, Ye SK, Sugai M, Gonda H, Honjo T, et al. Histone acetylation determines the developmentally regulated accessibility for T cell receptor gamma gene recombination. J Exp Med. (2001) 193:873–80. doi: 10.1084/jem.193.7.873
75. Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing gammadelta cells. Proc Natl Acad Sci USA. (2012) 109:17549–54. doi: 10.1073/pnas.1204327109
76. Baccala R, Witherden D, Gonzalez-Quintial R, Dummer W, Surh CD, Havran WL, et al. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. (2005) 174:4606–12. doi: 10.4049/jimmunol.174.8.4606
77. Fujikado N, Mann AO, Bansal K, Romito KR, Ferre EMN, Rosenzweig SD, et al. Aire inhibits the generation of a perinatal population of interleukin-17A-producing gammadelta T cells to promote immunologic tolerance. Immunity. (2016) 45:999–1012. doi: 10.1016/j.immuni.2016.10.023
78. Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, et al. Notch-Hes1 pathway is required for the development of IL-17-producing gammadelta T cells. Blood. (2011) 118:586–93. doi: 10.1182/blood-2011-02-334995
79. Nakamura M, Shibata K, Hatano S, Sato T, Ohkawa Y, Yamada H, et al. A genome-wide analysis identifies a notch-RBP-Jkappa-IL-7Ralpha axis that controls IL-17-producing gammadelta T cell homeostasis in mice. J Immunol. (2015) 194:243–51. doi: 10.4049/jimmunol.1401619
80. Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. (2006) 441:231–4. doi: 10.1038/nature04754
81. Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, et al. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol. (2010) 184:1675–9. doi: 10.4049/jimmunol.0903539
82. Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. (2009) 19:128–39. doi: 10.1038/cr.2008.328
83. Allison JP, Havran WL. The immunobiology of T cells with invariant gamma delta antigen receptors. Annu Rev Immunol. (1991) 9:679–705. doi: 10.1146/annurev.iy.09.040191.003335
84. Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nat Immunol. (2006) 7:843–50. doi: 10.1038/ni1363
85. Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, et al. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. (2008) 40:656–62. doi: 10.1038/ng.108
86. Barbee SD, Woodward MJ, Turchinovich G, Mention JJ, Lewis JM, Boyden LM, et al. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci USA. (2011) 108:3330–5. doi: 10.1073/pnas.1010890108
87. Salim M, Knowles TJ, Hart R, Mohammed F, Woodward MJ, Willcox CR, et al. Characterization of a putative receptor binding surface on Skint-1, a critical determinant of dendritic epidermal T cell selection. J Biol Chem. (2016) 291:9310–21. doi: 10.1074/jbc.M116.722066
88. Rhodes DA, Reith W, Trowsdale J. Regulation of immunity by butyrophilins. Annu Rev Immunol. (2016) 34:151–72. doi: 10.1146/annurev-immunol-041015-055435
89. Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, et al. Epithelia use butyrophilin-like molecules to shape organ-specific gammadelta T cell compartments. Cell. (2016) 167:203–18 e17. doi: 10.1016/j.cell.2016.08.030
90. Willcox BE, Willcox CR. gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol. (2019) 20:121–8. doi: 10.1038/s41590-018-0304-y
91. Mathis D, Benoist C. Aire. Annu Rev Immunol. (2009) 27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532
92. Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, et al. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. (2007) 204:1267–72. doi: 10.1084/jem.20062497
93. White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJ, et al. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol. (2008) 38:942–7. doi: 10.1002/eji.200738052
94. Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, et al. Rank signaling links the development of invariant gammadelta T cell progenitors and Aire(+) medullary epithelium. Immunity. (2012) 36:427–37. doi: 10.1016/j.immuni.2012.01.016
95. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. (2006) 126:1121–33. doi: 10.1016/j.cell.2006.07.035
96. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. (2000) 100:655–69. doi: 10.1016/S0092-8674(00)80702-3
97. De Obaldia ME, Bhandoola A. Transcriptional regulation of innate and adaptive lymphocyte lineages. Annu Rev Immunol. (2015) 33:607–42. doi: 10.1146/annurev-immunol-032414-112032
98. Barros-Martins J, Schmolka N, Fontinha D, Pires de Miranda M, Simas JP, Brok I, et al. Effector gammadelta T cell differentiation relies on master but not auxiliary Th cell transcription factors. J Immunol. (2016) 196:3642–52. doi: 10.4049/jimmunol.1501921
99. Raifer H, Mahiny AJ, Bollig N, Petermann F, Hellhund A, Kellner K, et al. Unlike alphabeta T cells, gammadelta T cells, LTi cells and NKT cells do not require IRF4 for the production of IL-17A and IL-22. Eur J Immunol. (2012) 42:3189–201. doi: 10.1002/eji.201142155
100. Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, et al. A validated regulatory network for Th17 cell specification. Cell. (2012) 151:289–303. doi: 10.1016/j.cell.2012.09.016
101. Malhotra N, Narayan K, Cho OH, Sylvia KE, Yin C, Melichar H, et al. A network of high-mobility group box transcription factors programs innate interleukin-17 production. Immunity. (2013) 38:681–93. doi: 10.1016/j.immuni.2013.01.010
102. Laird RM, Laky K, Hayes SM. Unexpected role for the B cell-specific Src family kinase B lymphoid kinase in the development of IL-17-producing gammadelta T cells. J Immunol. (2010) 185:6518–27. doi: 10.4049/jimmunol.1002766
103. Weber BN, Chi AW, Chavez A, Yashiro-Ohtani Y, Yang Q, Shestova O, et al. A critical role for TCF-1 in T-lineage specification and differentiation. Nature. (2011) 476:63–8. doi: 10.1038/nature10279
104. Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin–TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat Immunol. (2001) 2:691–7. doi: 10.1038/90623
105. Xi H, Schwartz R, Engel I, Murre C, Kersh GJ. Interplay between RORgammat, Egr3, and E proteins controls proliferation in response to pre-TCR signals. Immunity. (2006) 24:813–26. doi: 10.1016/j.immuni.2006.03.023
106. Parker ME, Barrera A, Wheaton JD, Zuberbuehler MK, Allan DSJ, Carlyle JR, et al. c-Maf regulates the plasticity of group 3 innate lymphoid cells by restraining the type 1 program. J Exp Med. (2019) 217:e20191030. doi: 10.1084/jem.20191030
107. Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, et al. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nat Immunol. (2011) 12:1238–45. doi: 10.1038/ni.2134
108. Auderset F, Schuster S, Fasnacht N, Coutaz M, Charmoy M, Koch U, et al. Notch signaling regulates follicular helper T cell differentiation. J Immunol. (2013) 191:2344–50. doi: 10.4049/jimmunol.1300643
109. Spidale NA, Sylvia K, Narayan K, Miu B, Frascoli M, Melichar HJ, et al. Interleukin-17-producing gammadelta T cells originate from SOX13(+) progenitors that are independent of gammadeltaTCR signaling. Immunity. (2018) 49:857–72 e5. doi: 10.1016/j.immuni.2018.09.010
110. Huang W, Lu N, Eberspaecher H, De Crombrugghe B. A new long form of c-Maf cooperates with Sox9 to activate the type II collagen gene. J Biol Chem. (2002) 277:50668–75. doi: 10.1074/jbc.M206544200
111. Rajaram N, Kerppola TK. Synergistic transcription activation by Maf and Sox and their subnuclear localization are disrupted by a mutation in Maf that causes cataract. Mol Cell Biol. (2004) 24:5694–709. doi: 10.1128/MCB.24.13.5694-5709.2004
112. Tanaka S, Suto A, Iwamoto T, Kashiwakuma D, Kagami S, Suzuki K, et al. Sox5 and c-Maf cooperatively induce Th17 cell differentiation via RORgammat induction as downstream targets of Stat3. J Exp Med. (2014) 211:1857–74. doi: 10.1084/jem.20130791
113. Xing S, Li F, Zeng Z, Zhao Y, Yu S, Shan Q, et al. Tcf1 and Lef1 transcription factors establish CD8(+) T cell identity through intrinsic HDAC activity. Nat Immunol. (2016) 17:695–703. doi: 10.1038/ni.3456
Keywords: γδ T cells, thymus, TCR signal strength, transcriptional regulation, innate-like lymphocyte, IL-17A, IFNγ
Citation: Parker ME and Ciofani M (2020) Regulation of γδ T Cell Effector Diversification in the Thymus. Front. Immunol. 11:42. doi: 10.3389/fimmu.2020.00042
Received: 22 November 2019; Accepted: 08 January 2020;
Published: 24 January 2020.
Edited by:
Arthur Mortha, University of Toronto, CanadaReviewed by:
Immo Prinz, Hannover Medical School, GermanyMaria L. Toribio, Severo Ochoa Molecular Biology Center (CSIC-UAM), Spain
Copyright © 2020 Parker and Ciofani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Ciofani, bWFyaWEuY2lvZmFuaUBkdWtlLmVkdQ==
 Morgan E. Parker
Morgan E. Parker Maria Ciofani
Maria Ciofani