
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 14 February 2020
Sec. Comparative Immunology
Volume 10 - 2019 | https://doi.org/10.3389/fimmu.2019.03162
 Eamy Nursaliza Yaacob1,2†
Eamy Nursaliza Yaacob1,2† Parisa Norouzitallab1,2*†
Parisa Norouzitallab1,2*† Bruno G. De Geest3
Bruno G. De Geest3 Aline Bajek4
Aline Bajek4 Kristof Dierckens1
Kristof Dierckens1 Peter Bossier1‡
Peter Bossier1‡ Daisy Vanrompay2‡
Daisy Vanrompay2‡Vibrio anguillarum causes high mortality in European sea bass (Dicentrarchus labrax) larviculture and is a hindering factor for successful sustainable aquaculture of this commercially valuable species. Priming of the innate immune system through administration of immunostimulants has become an important approach to control disease outbreaks in marine fish larviculture. This study was conducted to evaluate immunostimulation by Escherichia coli HSP70 (DnaK) in axenic European sea bass larvae in order to protect the larvae against vibriosis. DnaK stimulates the immune response in crustaceans and juvenile fish against bacterial infections. The use of axenic fish larvae allows to study immunostimulation in the absence of an interfering microbial community. At 7 days post-hatching, larvae received a single dose of alginate encapsulated recombinant DnaK. Two non-treated control groups in which animals either received empty alginate microparticles (C1) or no alginante microparticles (C2 and C3) were included in the study. Eighteen hours later, all larvae, except the ones from group C3 (non-infected control) were challenged with V. anguillarum (105 CFU, bath infection). Mortality was daily recorded until 120 h post infection and at 18, 24, and 36 h post infection, larvae were sampled for expression of immune related genes. Results showed that V. anguillarum induced an immune response in axenic sea bass larvae but that the innate immune response was incapable to protect the larvae against deadly septicaemic disease. In addition, we showed that administration of alginate encapsulated DnaK to axenic European sea bass larvae at DAH7 resulted in a significant, DnaK dose dependent, upreglation of immune sensor, regulatory and effector genes. Significant upregulation of cxcr4, cas1 and especially of hep and dic was correlated with significant higher survival rates in V. anguillarum infected larvae. In the future recombinant DnaK might perhaps be used as a novel immunostimulant in sea bass larviculture.
The production of farmed fish has increases 12 times in the past three decades at an average annual growth of 8%, making making it the fastest growing food production sector (1). In Europe, the three major species reared are Atlantic salmon (Salmo salar), European sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata). In order to further support the growth of output, production of fish juveniles for stocking on growing farms has to be increased. However, the latter is hampered by high and unpredictable mortality of fish larvae after hatching (2). Compared to the other cultured fish species, highly sensitive to disease outbreaks. The increased sensitivity of sea bass is related to its strong and prolonged stress-induced cortisol increase and increased biosynthetic capacity of interrenal tissue involving a high turnover in the corticosteroid production and release (3). Sea bass larvae are highly susceptible to the opportunistic pathogen Vibrio anguillarum [reviewed in (4)]. V. anguillarum, a Gram-negative bacterium, causes a deadly septicaemic disease (5, 6). The skin, gills and intestinal epithelium are replication sites for Vibrio. In sea bass, V. anguillarum evades the innate immune response by inhibiting the respiratory burst reaction in macrophages and by downregulating the expression of apoptotic caspases (7). In addition, many Vibrio strains have siderophore-dependent and/or siderophore-independent iron-acquisition mechanisms [reviewed in (8, 9)], which enables them to use iron for growth even if iron-binding, fish proteins such as ferritin and transferrin are present.
Strategies for preventing vibriosis outbreaks in sea bass larviculture are urgently needed. Preventive use of antibiotics and chemotherapeutics is, for obvious reasons, no longer allowed. Stimulation of the innate immune system [reviewed by (10, 11)] of larvae or maternal transfer of immunity [maternal IgM, lectin, lysozyme, C3, factor B, phosvitin, lipovitellin; reviewed by (12–15)] is an alternative approach. Administration of immunostimulants in larvae is particularly interesting, as they might protect the larvae against vibriosis and at the same time create a broad spectrum innate immune memory (11). Thus, prophylaxis of infectious diseases in fish larviculture currently focuses on the stimulation of the innate immune system either by manipulating the environment for example with elevated temperature (16, 17) or supplying the diet with immunostimulants (18, 19).
Some of the immunostimulants, such as bacterial products (11), and plant constituents (20–24) are considered as the activators of endogenous heat shock proteins (HSPs). Heat shock proteins are a family of evolutionary conserved cellular proteins and they exist in all living organisms. Under normal physiological conditions, these proteins are produced constitutively and play a role in protein metabolism. However, following adverse conditions, the level of these proteins is increased in order to protect cells from lethal damage (12, 25). In the case of disease outbreaks, increased levels of HSPs can activate an innate immune response through a danger-associated molecular pattern (DAMP) cascade. HSP70 is a member of HSPs family with a size of 70KDa. The prokaryotic HSP70 is often referred to as DnaK. Eukaryotic HSP70 and DnaK have been reported to stimulate the immune response in brine shrimp (Artemia franciscana), Pacific white shrimp (Litopenaeus vannamei) and Southern platyfish (Xiphophorus maculatus) against bacterial infections (20, 26–29).
In this study, we examined the effectiveness of recombinant Escherichia coli DnaK as an immunostimulant for fish larvae and its potential for future oral mass application in sea bass larviculture for protecting the animals against vibriosis. To address this, a previously validated experimental infection model in gnotobiotic sea bass (D. labrax) larvae was used (30). Maintaining sea bass larvae under gnotobiotic conditions allows full control over the host-associated microbial communities (31). Therefore, it represents an exceptional experimental system for carrying out immunological studies related to host-pathogen interactions. The gnotobiotic system eliminates any possible microbial interference during the DnaK exposure period and hence facilitates the interpretation of the results in terms of a cause-effect relationship (32). DnaK was administered orally, encapsulated in alginate microparticles. The method for preparation and characterization of alginate microparticles for oral administration of immunostimulants to gnotobiotic sea bass larvae at day after hatching 7, has previously been established (33). The commercial alginate used is a polysaccharide, extracted from brown algae (kelp). Alginate possesses muco-adhesive properties and is considered as safe due to its biodegradability, its low toxicity and low immunogenicity (34). Alginate microparticles are stable in sea water and they are successfully ingested by sea bass larvae whereafter their content is released in the gut (33).
Recombinant DnaK (83 kDa), with additional amino acid residues being an N-terminal thioredoxin and C-terminal polyhistidine (6 × His) tag, was expressed by Enative, an E. coli strain (MG155; accession number: AIZ92815.1) over-expressing recombinant DnaK (28, 29) was cultured at 37°C in Luria Bertani (LB) broth containing 50 μg ml−1 kanamycin (Sigma-Aldrich, Diegem, Belgium). Over-expression of DnaK was induced by adding 0.5 mg/ml−1 of arabinose (Sigma-Aldrich) to the culture and incubation for 4 h at 37°C. For bacterial cell disruption and DnaK collection, cultured bacteria were transferred to sterile vials in which sterile glass beads (0.1 mm) were added. The vials were then placed in a bead beater (Lab Services, Breda, The Netherlands) and the cell wall of the bacteria was mechanically disrupted by agitation (twice for 30 s). The bacterial lysate was collected by centrifugation (2200 x g for 1 min at 4°C) and the subsequent purification of DnaK was performed using a Dynabeads® His-Tag Isolation & Pulldown kit (Life Technologies, Aalst, Belgium) following the manufacturer's protocol. The endotoxin content was determined using the Toxin Sensor Chromogenic LAL Endotoxin Assay Kit (GenScript, New Jersey, USA) following the manufacturer's protocol. Purified DnaK was stored at −80°C. The concentration of the purified DnaK was determined using a Quick Start™ Bradford protein assay (Bio-Rad Laboratories, Temse, Belgium) following the manufacturer's protocol. Samples were analyzed by SDS-PAGE using the procedure previously described (35). Precision Plus Protein™ (Bio-Rad Laboratories) was used as a pre-stained molecular weight marker (MW range 10–180 kDa). Proteins were stained with the Bio-Safe Coomassie stain (Bio-Rad Laboratories) or electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Immun-Blot® PVDF membrane, Bio-Rad Laboratories) using a semi-dry Western blotting system (250 mA, 1 h; Trans-Blot® Semi-Dry, Bio-Rad Laboratories). The PVDF membrane was blocked (PBS with 0.2% Tween-20 and 5% BSA) for 1 h at room temperature (RT) and subsequently incubated overnight (4°C) with a mouse monoclonal antibody against DnaK (8E2/2; diluted 1:5000) (Enzo Life Sciences, Antwerpen, Belgium). The membrane was washed and thereafter incubated with horseradish peroxidase-labeled donkey anti-mouse IgG (H + L) (Affinity BioReagents™, Colorado, USA) at a dilution of 1:2500 (1 h, RT). After washing, 0.7 mM 3,3′-diaminobenzidine (Sigma-Aldrich) and 0.01% H2O2 in 0.1 M Tris-HCl (pH 7.6) were added (10 min, RT). Membranes were washed and analyzed with Western blot imaging system ChemiDoc™ MP (Bio-Rad Laboratories) (Figure 1).
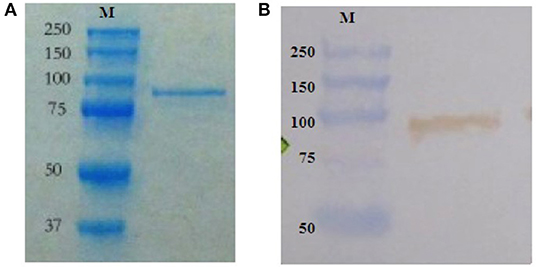
Figure 1. Arabinose-induced over-production of DnaK by the Enative E. coli strain. Purified recombinant DnaK (83 kDa), with additional amino acid residues being an N-terminal thioredoxin and C-terminal polyhistidine (6 × His) tag, by SDS-PAGE gel electrophoresis and Western blotting. (A) Coomassie staining of polyacrylamide gel loaded with purified recombinant DnaK. Molecular weight markers (M) in kDa are on the left. (B) PVDF membrane probed for purified recombinant DnaK using a mouse monoclonal antibody against DnaK followed by HRP-labeled donkey anti-mouse IgG (H + L), substrate and chromogen. Molecular weight markers (M) in kDa are on the left.
Recombinant E. coli DnaK was encapsulated in calcium alginate microparticles. Preparation of DnaK loaded microparticles (median size of 84 ± 4.7 μm) for oral administration to gnotobiotic sea bass larvae at day after hatching (DAH) 7, was done as previously described (33). Briefly, three ml of an aqueous sodium alginate solution prepared by using low viscosity alginate (A2158; Sigma-Aldrich, Diegem, Belgium), was dispersed in 12 ml iso-octane (Sigma-Aldrich) solution containing two lipophilic surfactants, 0.05% (v/v) Span-80 (Fagron, Weregem, Belgium) and 0.02% (v/v) Tween-80 (Fagron). The solution was stirred for 3 min using a magnetic stirrer. Subsequently, 1500 μg (500 μg ml−1 sodium alginate solution) recombinant DnaK was added to the aqueous sodium alginate solution. The emulsion was homogenized for 2 min using a Turrax homogenizer (Bodart & Co., Antwerp, Belgium). Next, 3 ml of a calcium chloride solution (700 mM; Sigma-Aldrich) was added to the mixture and was stirred for another 2 min. To further harden the formed microparticles, 15 ml of isopropyl alcohol (Fagron) was added. The resulting microparticles were separated and collected by centrifugation (300 x g) for 3 min after which the particles were washed three times with sterile deionized water. The microparticles were prepared fresh before every experiment and were used immediately. The efficiency of DnaK encapsulation was calculated as previously described (36). Thus, 200 mg (wet weight) of microparticles contained 300 μg of antigen or 1.5 μg antigen per mg microparticles.
European sea bass eggs that were produced by natural spawning and were pre-disinfected (20 ml l−1 0.5% active iodine for 10 min) were obtained from Écloserie Marine (Gravelines, France). Upon arrival, the eggs were transferred to cylindro-conical tank and were acclimatized in UV-treated sea water for 4 h. For obtaining axenic sea bass larvae, the disinfection of eggs was performed following the protocol developed by Dierckens et al. (37). All larvae used in this study belonged to the same full-sibling family batch. Once disinfected, 600 eggs were incubated in 400 ml of filtered (0.45 μm; Sartorius, Vilvoorde, Belgium) autoclaved artificial sea water (Instant Ocean, United Pet Group, Virginia, US) and in presence of gentle filtered aeration (0.1 μm). During the incubation, salinity of 36 g l−1 and temperature of 16 ± 1°C was maintained. To sustain axenity, solutions containing rifampicin and ampicillin were simultaneously added in the sea water at a final concentration of 10 mg l−1. Axenity testing was performed by inoculating 30 of eggs as well as 1 ml of culture medium (collected from individual bottles) in 9 ml of sterile marine broth (Carl Roth GmbH Co., Karlsruhe, Germany) followed by incubation at 28°C for 96 h. Incubation bottles that were positive for bacterial growth were excluded from the experiment. All the handlings and procedures including hatching, stocking, and experiments, were carried out under laminar flow hood and in a temperature-regulated room (16 ± 1°C) with dim light (100 lux).
For performing the experiment, axenic larvae on DAH6 were used. Larvae were stocked per 12 animals, in sterile screw cap vial containing 10 ml of filtered, autoclaved artificial sea water in which 10 mg l−1 rifampicin was added. The vials were placed on a rotor (4 rpm) with an axis tangential to the axis of the vials for providing aeration. Each group consisted out of 10 vials of 12 larvae (n = 120) (Table 1 and Figure 2).
At DAH7, larvae had a mean body weight of 0.5 mg. At this stage, the animals of groups Ahigh dose, Blow dose and C1 received a single dose of 1.0 mg alginate microparticles directly added into the sea water. Group Ahigh dose received 1.0 mg of loaded microparticles, representing 1.5 μg of DnaK. Group Blow dose received 0.5 mg empty microparticles and 0.5 mg loaded microparticles, representing 0.75 μg of DnaK. Group C1 received only 1 mg of empty microparticles. A control group (C2) in which animals received no microparticles was included in the study. Eighteen hours after administering the microparticles, groups Ahigh dose, Blow dose, C1 and C2 were experimentally infected (bath infection) with the virulent V. anguillarum strain HI-610 (serovar O2a, rifampicin resistant) using an infective dose of 105 CFU. The bath infection model was previously validated during a pathogenesis study of a V. anguillarum infection in gnotobiotic sea bass larvae (38, 39). An extra control group (C3) in which the animals were neither treated nor challenged was included. Mortality in all groups was monitored in all vials by counting the living larvae (transparent and swimming) under a dissecting microscope at 18, 24 and 36 h p.i. At each of these time points, 36 larvae (whole-body larva sampling) per group (3 biological replicates of 12 larvae = 3 vials) were euthanized by over-anesthetization using methylsulfonatetricaine (MS-222, Sigma) for analyzing the expression of innate immune-related genes. Euthanized larvae were immediately snap frozen in liquid nitrogen and were subsequently stored at −80°C until analysis. The remaining 12 larvae per group were monitored till 120 h p.i. and the mortality in each group was recorded daily. At the end of the trial, all remaining larvae were euthanized by over-anesthetization. The experiment was carried out in accordance with the recommendations in the European Union Ethical Guidelines for the care of animals used for experimental and other scientific purposes (2010/63/EU). The trial was approved by the UGhent Ethical Committee for Animal Experiments (EC2014/147).
At 18, 24, and 36 h p.i., the total RNA of the larvae was extracted following the protocol described by Reyes-López et al. (40). Briefly, the larvae of each vial were sacrificed by over- anesthetization using sea water containing methylsulfonatetricaine (MS-222) (Sigma). The animals were snap-frozen in liquid nitrogen and were stored at −80°C for further analysis. For immune gene expression analysis, total RNA was extracted of triplicates of 12 pooled homogenized larvae from each group using the Total RNA Isolation Reagent (TRI Reagent® Molecular Research Center, Inc., Cincinnati, USA) (41). The quantity and quality of the RNA was tested using a NanoDrop 2000 spectrophotometry (NanoDrop Technologies Inc.,). The extracted RNA was then treated with RNase-free amplification grade DNase I (Thermo Scientific, Aalst, Belgium) following the manufacturer's protocol and confirmed to be DNA free by PCR for the ribosomal protein S18 (rps18) gene. One μg of total RNA was reverse transcribed (RevertAid™ H Minus first-strand cDNA synthesis kit; Thermo Scientific) following the manufacturer's protocol. RNA and cDNA samples were stored at −80 and −20°C, respectively.
Selected immune genes and the primers that were used for mRNA expression analysis are presented in Table 2. Amplified cDNA samples were subjected to RT-qPCR using a Rotor-Gene Q (Qiagen®) instrument with each reaction containing 15 μl master mix containing iQ™ SYBR® Green Supermix (Bio-Rad Laboratories), the optimum concentration of primer pair (Table 2) and 5 μl of diluted cDNA to a final volume of 20 μl. The RT-qPCR reaction was performed with one cycle of 95°C for 3 min and 40 cycles of 95°C for 15s, 57–58°C for 1 min and 72°C for 15 s. Each of the three biological replicates was analyzed in duplicate. Plasmids (pGEM®-T) that contained gene-specific insert were used as positive controls. non-reverse transcribed total RNA of larvae and ddH2O were used as negative controls. The endogenous rps18 gene (housekeeping gene) was used as for normalization and the RT-qPCR efficiencies for each gene varied between 95 and 100%, allowing the use of the 2−ΔΔCT method for calculation of relative gene expression (fold-changes) (45).
For the effect of Vibrio infection on the larval immune system, C2 (no MPs and challenged with V. anguillarum) gene expression profile was studied by calculating the immune gene expression relative to group C3 (no MPs and no Vibrio) as the calibrator by calculating ΔΔCT. For studying the effect of DnaK on immune gene expression in European sea bass larvae, mRNA expression analysis was performed relative to C1 (fed with MPs and challenged with V. anguillarum) as the calibrator by calculating ΔΔCT.
To determine significant differences between various treatments, survival data were subjected to logistic regression analysis by using GenStat 16 (VSN International, Hemel Hempstead, UK). Statistical analysis of gene expression was conducted using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY). For comparing the sea bass relative gene expression (fold-changes) between different treatments at each time point t-test analysis (comparisons between C2 and C3) and one-way ANOVA (comparison between C1, Ahigh dose and Blow dose) were performed by setting the analyses at 95% confidence intervals (P < 0.05).
ANOVA test was followed by a post-hoc comparisons using Tukey HSD test and data for gene expression was log-transformed.
During the first 36 h p.i., the proportion of survived gnotobiotic European sea bass larvae was statistically the same for all groups (Figure 3). At 48 h p.i., significant (P < 0.05) mortality occurred in all V. anguillarum infected groups, except for the group receiving the high dose (1.5 μg) of DnaK (group Ahigh dose) (Figure 3). In the latter group, significant mortality, as compared to the non-infected control group C3, was only observed from 72 h p.i. onwards. Mortality in all infected groups, including group Ahigh dose, augmented further toward the end of the experiment (Figure 3). At the end of the experiment (120 h p.i.), only 8% of the larvae of group C2 (no MP, infected), 7% of the larvae of group Blow dose and 21% of the larvae of group C1 (empty MP, infected) had survived the infection, showing the ineffectiveness of DnaK administration at a low dose of 0.75 μg. However, at the same time point, 50% of the larvae of group Ahigh dose were still alive and the proportion of survived animals in group Ahigh dose was statistically higher than in all other infected groups (Figure 3) (C1, C2, and Blow dose), but lower than for group C3 (no MP, non-infected) which showed 73% survival. The survival results clearly demonstrated the beneficial effect of feeding European sea bass larvae with 1 mg of microparticles loaded with 1.5 μg DnaK (Figure 3). The administration of empty MPs (C1) also appeared to have some positive effect on survival (Figure 3) as at 120 h p.i., the survival rate in C1 was significantly higher than for group C2 (no MP, infected) and even group Blow dose. However, at 120 h p.i., a significant difference (P < 0.01) of 41% was recorded between the survival in group C1 and group Ahigh dose. Also, at h p.i. the proportion of survived animals in group Ahigh dose was significantly (P < 0.01) lower by 22%, as compared to the survival in the non-infected control group C3.

Figure 3. The proportion of surviving European sea bass larvae challenged with V. anguillarum. Animals of group C1 (1.0 mg of empty MPs), C2 (no MPs), Ahigh dose (1.0 mg of DnaK loaded MPs) or Blow dose (0.5 mg of empty MPs and 0.5 mg of DnaK loaded MPs) were challenged with 105 CFU of V. anguillarum. Group C3 received no MPs and was not infected. Survival of the animals was scored before challenge (0 h p.i.) and at several time points p.i. till the end of the trial at 120 h p.i. (A) The proportion of survived animals (mean ± SD) is represented at each time point. (B) Statistical differences in live animals for various groups at different time points after infection with V. anguillarum. For all groups, the proportion of live fish larvae was significantly the same before 48 h p.i. Statistical differences in the proportion of survived animals for various treatment groups, following infection are presented from 48 h p.i. till the end of the experiment at 120 h p.i. An asterisks represents a significant difference between the respective groups [not significant (NS) (P > 0.05), significant *(P < 0.05), significant ** (P < 0.01), significant *** (P < 0.001) and significant ****(P < 0.0001)]. Comparison between a group and itself is marked in black.
In order to examine the effect of a V. anguillarum infection on immune-related gene expression early upon infection, we compared the tlr3, tlr5m, cas1, il1β, tnfα, mif , il10, cc1, cxcl8, cxcr, ccr9, hpc, and dic gene transcript levels for groups C2 (no MP, infected) and C3 (no MP, non-infected) at 18, 24, and 36 h p.i. The mean gene transcript levels ± SD for group C2 relative to group C3 are presented in Figure 4. At 18 h p.i., only ccr9 (3.57-fold) was significantly upregulated. In addition, tlr3 (0.02-fold) was significantly, downregulated at this time point. At 24 h p.i., tnfα (20.25-fold), cc1 (12.11-fold), and cxcl8 (4.60-fold) were significantly upregulated. At, 36 h p.i., tlr3 (47.17-fold), tnfα (6.15-fold), and cc1 (13.73-fold) were significantly upregulated.
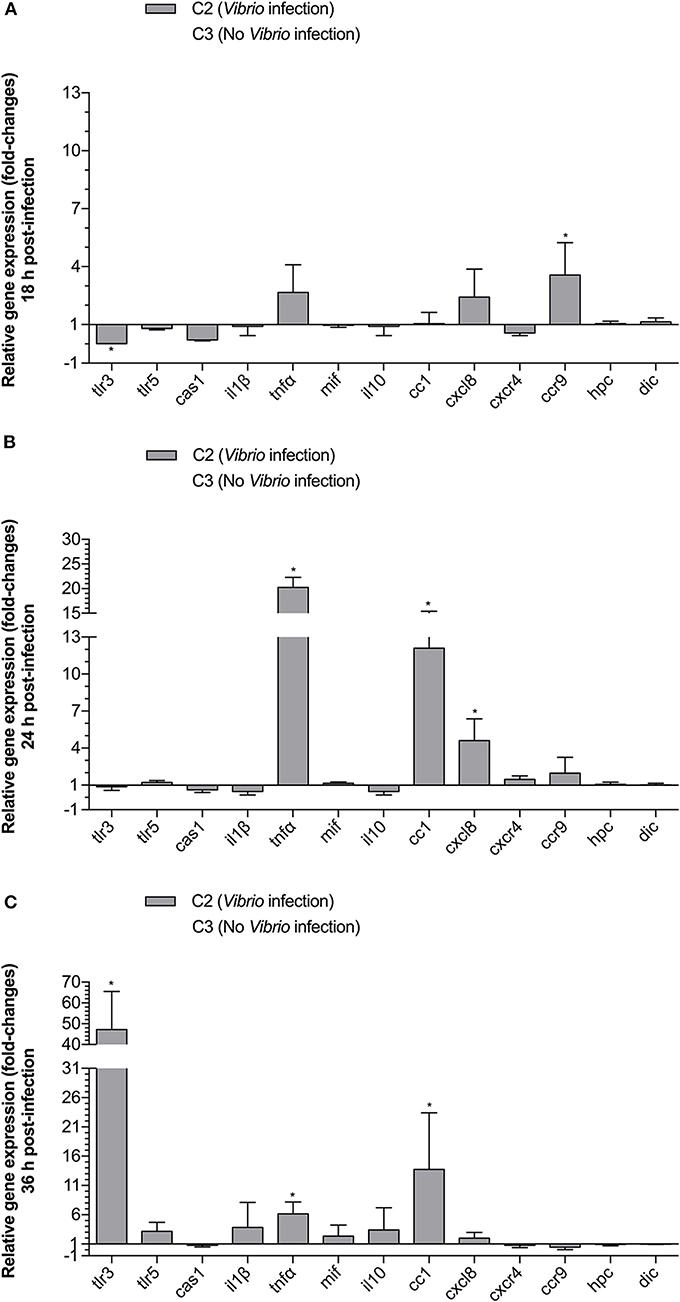
Figure 4. The effect of Vibrio challenge on relative expression of TLR, cytokine, chemokine and chemokine receptor genes in gnotobiotic sea bass larvae. Animals that were not fed with MPs were either cultured without challenge (C3) or were challenged with V. anguillarum at 105 CFU (C2). Animals were sampled at 18 (A), 24 (B), and 36 (C) h p.i. Gene expression results for C2 are presented relative to the C3 values. Bars represent the standard deviation (SD) of the mean. An asterisks (*) represents a significant (P < 0.05) change in gene expression for C2 compared to C3.
We examined the prophylactic effect of DnaK administration prior to a vibrio infection, by studying the survival of the larvae as well as the expression of tlr3, tlr5m, cas1, il1β, tnfα, mif , il10, cc1, cxcl8, cxcr, ccr9, hpc, and dic genes in live animals sampled at 18, 24, and 36 h p.i. The mean gene transcript levels ± SD for groups Ahigh dose and Blow dose relative to group C1 (empty MP, infected) are presented in Figure 5. At 18 h p.i., cas1 (6.2-fold), tnfα (17.24-fold) and cxcl8 (7.04-fold) were significantly upregulated in group Ahigh dose as compared to the control group C1. By 24 h p.i., more genes became upregulated in group Ahigh dose as transcript levels for cc1 (3.82-fold), cxcr4 (2.56-fold), hpc (4.27-fold), and dic (3.37-fold) had also significantly increased as compared to the control group C1. For group Ahigh dose, significant gene upregulation as compared to group C1 was no longer present at 36 h p.i. except for dic (1.43-fold). Significant downregulation of genes did not occur in group Ahigh dose, except for the mif gene, which was slightly downregulated at 18 and 24 h p.i. as compared to the control group C1.
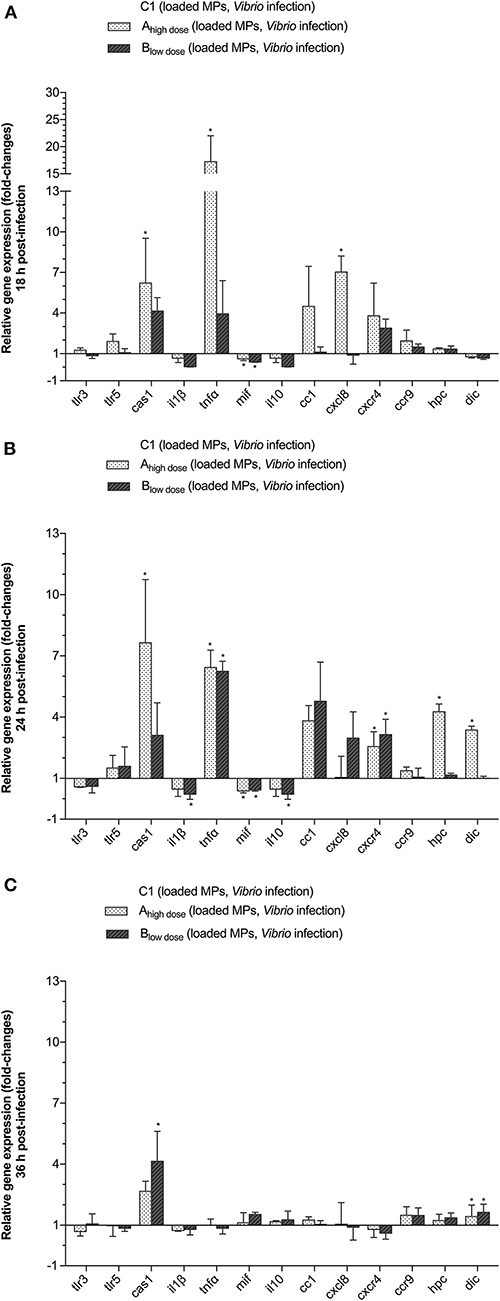
Figure 5. The effect of treating the animals with DnaK on the relative expression of TLR, cytokine, chemokine and chemokine receptor genes in gnotobiotic sea bass larvae. The larvae were divided into 3 different groups and were fed with either empty MPs (C1) or encapsulated DnaK at a high (Ahigh dose) or low dose (Blow dose) prior to infection with 105 CFU of V. anguillarum. Animals were sampled at 18 (A), 24 (B), and 36 (C) h p.i. Gene expression results for the Ahigh dose and Blow dose groups are presented as fold-changes relative to C1. Bars represent the standard deviation (SD) of the mean. An asterisks (*) indicates a significant difference (P < 0.05) for the high (Ahigh dose) or low dose (Blow dose) group compared to C1.
For group Blow dose, at 18 h p.i., only cas1 (4.14-fold) and cxcr4 (2.88-fold) transcript levels were significantly increased compared to the control group C1. At that time, il1β (25-fold), mif (2.04-fold), and il10 (25-fold) were significantly downregulated as compared to the control group C1. At 24 h p.i., only two genes mRNA were significantly upregulated in group Blow dose, being tnfα (6.24-fold) and cxcr4 (3.14-fold). Expression of mif (0.44-fold), il10 (0.22-fold), and il1B (0.22) was, albeit slightly, downregulated at that time. Later on, at 36 h p.i. only cas1 (4.14-fold) and dic (1.63-fold) were significantly upregulated as compared to the control group C1.
Regarding all genes under study, cas1, cc1, cxcl8, and cxcr4 were significantly upregulated, albeit not at the same time, in both group Ahigh dose and group Blow dose, as compared to the control group C1. In addition, for group Ahigh dose, significant gene upregulation, as compared to the control group C1, was also observed for tnfα (18 h p.i.), hpc (24 h p.i.), and dic (24 h p.i.).
Industrial production of sea bass is still hampered by low and unpredictable survival of larvae and juvenile fish due to disease outbreaks such as infections caused by Vibrio spp. (46, 47). Vibriosis is a bottleneck for the expansion of sustainable sea bass aquaculture and causes high mortality especially during the larviculture. Vibrio spp. are actually opportunistic bacteria and therefore, vibriosis outbreak in larviculture is the result of the combination of various factors. For example, high larval density, accumulation of dead larvae and the introduction of bacteria through live food that can promote selection and growth of opportunistic bacteria, including vibrio (48). The latter might lead to vibrio infections and in case of sea bass larvae to vibrio replication in the skin, gills and gastrointestinal tract resulting in deadly septiceamic disease (49).
Stimulation of the innate immune system of sea bass larvae could perhaps protect them against vibrio infections. Therefore, the current study focuses on oral administration of a candidate immunostimulant, being E. coli HSP70, also known as DnaK, to induce a protective innate immune response in sea bass larvae against mortality caused by V. anguillarum. For this purpose, a previously generated experimental infection model in axenic sea bass larvae (37) was used exposing the animals to vibrio through the sea water (49).
Immunostimulants are commercially available for fish farming. Seaweed-based (AQUAVAC® Ergosan™; MSD Animal Health, New Jersey, USA), yeast β-glucan-based (MacroGard® Biorigin, Antwerp, Belgium and Bio-Mos® Alltech Inc, USA) and Saccharomyces cerevisiae beta-glucans and mannanoligosaccharides-based (ExcelMOS®, Global Nutritech; VA, USA) immunostimulants have been successfully applied in juvenile rainbow trout (O. mykiss), juvenile/adult sea bass (D. labrax), juvenile carp (Cyprinus carpio) and seabream (S. aurata) broodstock (50–55). In fish farms, these commercial immunostimulants are being incorporated in the diet. The application of other immunostimulants such as vitamins (i.e., vitamin C and E), microorganisms (i.e., probiotic bacteria), prebiotics (Bio-Mos®), hormones (i.e., thyroxine), biologically active compounds (i.e., antimicrobial peptides, lectins, bovine lactoferrin), medicinal herbs [i.e., Astragalus membranaceus and Lonicera japonica (honeysuckle) extracts], amino acids (i.e., DL-arginine), poly-β-hydroxybutyrate and organic pigments (i.e., carotenoid and astaxanthin) has also been studied in various aquaculture species (44, 56–59). However, data from application of immunostimulants in fish larvae are limited [reviewed by (18, 60)]. Nevertheless, the existing data point in the direction of considerable benefit and little detrimental effect to the developing animal (44).
Microparticles (MPs) are delivery systems used for the administration of immunostimulants in a controlled manner (61). Delivery of immunostimulants in fish using different types of microparticles has been described. Microparticles out of a synthetic amino acid being poly(D,L-lactide-co-glycolide) or PLGA or out of natural polymers such as calcium phosphate particles, chitosan particles, liposomes, and alginate particles have been used for targeting the immune system of fish (62, 63). Inorganic materials such as carbon nanotubes have also been used as a delivery system. However, they are not biodegradable (64). Natural polymers are most widely used due to their bioactivity, biocompatibility, chemical stability, low toxicity, susceptibility to enzymatic degradation and low production costs (65). One of the commonly used natural polymers is alginate. Alginate MPs can be prepared by extruding a solution of sodium alginate containing proteins as a droplet into a divalent cross-linking solution such as a Ca2+ solution. Gelation and cross-linking of alginate polymers is than mainly achieved by the exchange of sodium ions from the guluronic acids of the alginate with the divalent cations of the cross linking solution. A characteristic “egg-box” structure is formed by the stacking of these guluronic groups (66). By selecting the alginate type and the formulation conditions it is possible to control the characteristics of the alginate MPs delivering the encapsulated immunostimulant in a controlled release manner.
In larviculture, encapsulated immunostimulants in alginate MPs can easily be introduced into the water. This action does not provoke stress to the fish larvae. Microparticles of the correct size can be designed to ensure the uptake of the particles by marine fish larvae. Formerly, we evaluated MP uptake in axenic European sea bass at day after hatching 7 (DAH7; the mouth opening begins between DAH4 and DAH5) using fluorescent MPs. We observed that smaller MPs (<20 μm) were taken up passively, thus unintentionally, through the natural drinking activity, which was actually rather low (4.1 ± 0.1 nl h−1 larva−1) in sea bass larvae. Larger MPs (45 to 80 μm) were taken up actively as they were more easily noticed by the larvae and probably regarded as prey (33). Alginate MPs with a median size of 83.19 ± 4.73 μm were optimal for administration to sea bass larvae at DAH7, as 2 h after feeding a mean of 81.9 ± 9.0% of the larvae had ingested the MPs and by 48 h after feeding 100% of the larvae showed an accumulation of MPs along the midgut. The MPs disintegrated in the hindgut releasing their content locally. So, in the correct study, we created an alginate carrier matrix for the recombinant DnaK for oral delivery to axenic sea bass larvae at DAH7. The encapsulation efficiency was 19.7% which was the same as described by Yaacob et al. (33). The carrier matrix protected the DnaK from the external environment as it was stable in sea water (33) and provided localized delivery to the gastrointestinal tract of fish larvae.
In the current study, we first examined the expression of innate immune-related genes during a V. anguillarum infection in axenic sea bass larvae, comparing group C2 (no MP, infected) to C3 (no MP, non-infected). The expression of sensor (tlr3, tlr5m, cxcr4, ccr9), regulatory (il10, cas1, il1β, tnfα, mif1, cxcl8) and effector (hpc, dic) genes was studied early upon infection as the first time points of infection are critical for fish immunity (67). Previously, we demonstrated the expression of all sensor and regulatory genes under study in axenic sea bass larvae (43). The expression of hpc and dic has been demonstrated by others in axenic and non-axenic sea bass larvae, respectively (44, 67). Possible Vibrio (Gram-negative bacterium with a flagellum) pathogen associated molecular patterns (PAMPs) that might stimulate the pathogen recognition receptors (PRRs) of fish are flagellin (TLR5), peptidoglycan (TLR1 and 2, and NOD1 and NOD2), lipoproteins (TLR1 and 2), lipo-arabinomannan (TLR1 and 2) and glycosyl-fosfatidylinositol (TLR1 and 2). Thus, TLR5M is expressed in European sea bass larvae (43), but we do not know if the other PRRs for Vibrio are present at these early live stages and if they are already expressed on innate immune cells and/or mucosal epithelial cells in the skin, the gills and the gut, which are the primary replication sites for Vibrio. At present, we noticed the expression of tlr5m (receptor for bacterial flagellin) in larvae but transcript levels were statistically the same for all groups. We noticed a significant upregulation for tlr3 in group C3, but we have no explanation for this observation. Actually, only few other immune-related genes were upregulated following infection. The latter is in contradiction with our previous results using the same experimental infection model, the same V. anguillarum strain and the same infective dose (43). During the latter study, V. anguillarum highly upregulated the expression of all genes under study (the same gene panel as currently with the exception of hpc and dic) except for cxcr4 and ccr9. Significant mortality occurred also later (from 72 hp.i. onwards) than in the current study. Perhaps, animals used in the present study, which came from another broodstock, were less robust and/or less immunocompetent, as at the end of the trial (120 h p.i.) only 73 ± 0.05% of the animals in group C3 (no MP, non-infected) were still alive, while in our former study 96.67 ± 3.33% were still alive at 120 h p.i. Indeed, we need to consider that all the individuals in the present and former study were full-sibling (limited genetic variability), but they originated from another broodstock. In Atlantic salmon (Salmo salar), it has been demonstrated that the same pathogen can differentially affect the survival of different full-sibling groups and that this is due to differences in their ability to mount an effective immune response (67). Differences in survival rates have also been observed by Dierckens et al. (37), performing two independent V anguillarum (strain HI-610 at 105 CFU) challenge trials in axenic European sea bass larvae.
In the current study, we showed that 1 mg alginate microparticles, containing in total 1.5 μg (group Ahigh dose) recombinant E. coli HSP70 (DnaK) could be added to the sea water, resulting in the uptake of microparticles and significantly augmented transcription levels for immune-related sensor (cxcr4), regulatory (cas1, tnfα, cc1, and cxcl8) and effector (hpc, dic) genes. Thus, significantly elevated transcription levels for sensor, regulatory and effector genes were correlated with increased resistance of the larvae to a lethal infection with V. anguillarum. Whether the augmented transcription of tnfα, cc1 and cxcl8 in group Ahigh dose was due to DnaK is not certain, as these genes were also upregulated by the Vibrio infection itself. However, in group Ahigh dose, significantly augmented expression levels for tnfα and cxcl8 were observed earlier (18 h p.i.) upon infection as compared to group C2 (no MP, infected). Group Blow dose was not significantly protected. This might be due to the fact that only sensor (cxcr4) and regulatory (cas1, cc1, and cxcl8) genes were significantly upregulated and not the effector genes hpc and dic, at least not at 18, 24, and 36 h p.i. Strangely, the administration of empty MPs also seemed to have some positive effect on survival following infection as at 120 h p.i., the survival rate in group C1 was significantly higher than in group C2 (no MP, infected). This is possibly due to the fact that animals received no larval fish food during this trial (to ensure axenic conditions), but alginate MPs can also be used as food, rendering the larvae of group C1 more robust than the ones from group C2. At 120 h p.i., the survival rate in group C1 was also significantly higher than in group Blow dose. Here, the metabolic cost of an albeit inefficient prophylactic strategy (too low dose of DnaK) might be the reason for the observed difference in survival.
Both cxcr4 and cas1 were significantly upregulated by DnaK administration, and in our study, not by Vibrio. CXCR4 is a master regulator of innate immune responses. CXCR4 signaling is important for cell migration, proliferation and differentiation (68). Cas1 mRNA levels were upregulated, but it was not accompanied or followed by an upregulation of il1β mRNA levels. Cas1 upreglation could have played a role in pyroptosis, a gasdermin (GSDM)-dependent inflammatory type of programmed cell death (69). Different from mammals, which have a panel of pyroptotic GSDM members (e.g., GSDMA-E), teleosts only possess GSDME. Jiang et al. (69) found that teleost GSDME exerted pyroptotic activity and bactericidal activities through its N-terminal domain. GSDME is specifically and highly efficiently cleaved by teleost caspase 1, releasing the N-terminal domain. The N-terminal domain exhibits lipid-binding and pore forming capacity, which, when recruited to the cell membrane, leads to cell death and massive release of proinflammatory cellular content. The N-terminal domain of GSDME was also lethal to E. coli. Thus, elevated cas1 expression could have contributed in this way to protection against V. anguillarum. However, this needs to be examined in more detail.
It is clear from our results that augmented expression levels for hpc and dic contributed to the observed partial protection in the infected groups. Recently, both hpc and dic mRNA levels and hepcidin and dicentracin peptides have been found in European sea bass eggs and throughout larval development (70). Also, upregulation of hpc was shown to be directly related to reduced mortality in European sea bass challenged with V. anguillarum HI-610 (bath 108 CFU) at DAH5 (40). In addition, it was shown that synthetic hepcidin of fish could induce significant protection in sea bass challenged with V. anguillarum (71). Meloni et al. (72) described the importance of dicentracin in protecting juvenile European sea bass against V. anguillarum and more recently, Franke et al. (44) suggested that poly-B-hydroxybutyrate (PHB)-induced dic expression might play a role in protection of European sea bass postlarvae (DAH 28–38) against vibriosis. Similar results were obtained in axenic Nile tilapia (Oreochromis niloticus) larvae (73).
In response to pathogens, fish cells secrete hepcidin and/or dicentracin amongst a number of other AMPs which possess different antimicrobial activities such as formation of transmembrane pores, hindering synthesis of cell-wall and inhibiting cytoplasmic membrane septum formation thus disrupting membrane structure. In addition, they can also deactivate enzymes and inhibit the synthesis of proteins as well as nucleic acids [reviewed by (74)]. Hepcidin has a dual function, it exerts antimicrobial activities but if also plays a role in iron regulation removing iron from the bloodstream and rendering it unavailable for bacterial growth. Hepcidin can bind and interfere with the outer membrane of bacteria leading to its destruction and eventual death of the bacteria. Furthermore, hepcidin can also destroy bacteria through interaction and hydrolysis of bacterial DNA (75). Dicentracin belongs to the family of piscidins. Fish piscidins are stored in granules of phagocytic granulocytes and are delivered to pathogen-containing phagosomes upon phagocytosis to kill the pathogens (76).
Our study shows that V. anguillarum induces an immune response in axenic sea bass larvae but that the innate immune response was incapable to protect the larvae against deadly septicemic disease. In addition, we showed that administration of alginate encapsulated recombinant E. coli HSP70, also known as DnaK, to axenic European sea bass larvae at DAH7 resulted in a significant, DnaK dose dependent, upreglation of immune sensor, regulatory and effector genes. Significant upregulation of cxcr4, cas1 and especially of hep and dic was correlated with significant higher survival rates in V. anguillarum infected larvae. In the future, recombinant DnaK might perhaps be used as a novel immunostimulant in sea bass larviculture. However, our study showed that there is still room for improvement as mortality still occurred in the DnaK treated larvae. A higher DnaK dose and/or repeated administrations instead of a single dose could perhaps further improve this immunostimulatory strategy.
All datasets generated for this study are included in the article/supplementary material.
The experiment was carried out in accordance with the recommendations in the European Union Ethical Guidelines for the care of animals used for experimental and other scientific purposes (2010/63/EU). The trial was approved by the UGhent Ethical Committee for Animal Experiments (EC2014/147).
EY and PN wrote the paper and PN assisted in reviewing the results. BD provided scientific advice for the preparation of the alginate microparticles. KD, PB, and DV designed and supervised the study, assisted in drafting the paper and critically reviewed the paper. AB provided the seabass larvae for the experiment.
EY received a doctoral scholarship from the Ministry of Higher Education Malaysia (MOHE) and the Universiti Malaysia Terengganu (UMT). The present study was financially supported by Ghent University (BOF-12-GOA-022). The authors also acknowledge the Research Foundation Flanders, FWO-Vlaanderen, Belgium [postdoc grant to PN, FWO 12P2519N).
AB is employed by the company Écloserie Marine de Gravelines.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Jorg De Smyter, Brigitte Van Moffaert and Tom Baelemans (Laboratory of Aquaculture & Artemia Reference Center; UGent) are acknowledged for their excellent technical support.
2. Galindo-Villegas J, Mulero V. Chapter 2.9: Current knowledge on the development and functionality of immune responses in the European sea bass (Dicentrarchus labrax). In: Vázquez FJS, Muñoz-Cueto JA, editors. Biology of European Sea Bass Edition. Boca Raton, FL: CRC Press, Taylor & Francis Group. p. 342–73. doi: 10.1201/b16043-14
3. Karakatsouli N, Katsakoulis P, Leondaritis G, Kalogiannis D, Papoutsoglou SE, Chadio S, et al. Acute stress response of European seabass Dicentrarchus labrax under blue and white light. Aquaculture. (2012) 364:48–52. doi: 10.1016/j.aquaculture.2012.07.033
4. Frans I, Michiels CW, Bossier P, Willems KA, Lievens B, Rediers H. Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J Fish Dis. (2011) 34:643–61. doi: 10.1111/j.1365-2761.2011.01279.x
5. Qiao G, Xu DH, Wang Z, Jang IK, Qi Z, Zhang M, et al. Comparison of immune response of Pacific white shrimp, Litopenaeus vannamei, after multiple and single infections with WSSV and Vibrio anguillarum. Fish Shellfish Immunol. (2015) 44:257–64. doi: 10.1016/j.fsi.2015.02.009
6. Balado M, Lages MA, Fuentes-Monteverde JC, Martínez-Matamoros D, Rodríguez J, Jiménez C, et al. The siderophore piscibactin is a relevant virulence factor for Vibrio anguillarum favored at low temperatures. Front Microbiol. (2018) 2:1766. doi: 10.3389/fmicb.2018.01766
7. Sepulcre MP, Sarropoulou E, Kotoulas G, Meseguer J, Mulero V. Vibrio anguillarum evades the immune response of the bony fish sea bass (Dicentrarchus labrax L.) through the inhibition of leukocyte respiratory burst and down-regulation of apoptotic caspases. Mol Immunol. (2007) 44:3751–57. doi: 10.1016/j.molimm.2007.03.021
8. Naka H, Dias GM, Thompson CC, Dubay C, Thompson FL, Crosa JH. Complete genome sequence of the marine fish pathogen Vibrio anguillarum harboring the pJM1 virulence plasmid and genomic comparison with other virulent strains of V. anguillarum and V. ordalii. Infect Immun. (2011) 79:2889–900. doi: 10.1128/IAI.05138-11
9. Li Y, MA Q. Iron acquisition strategies of Vibrio anguillarum. Front Cell Infect Microbiol. (2017) 7:342. doi: 10.3389/fcimb.2017.00342
10. Vadstein O, Bergh Ø, Gatesoupe FJ, Galindo-Villegas J, Mulero V, Picchietti S, et al. Microbiology and immunology of fish larvae. Rev Aquac. (2011) 5:S1–25. doi: 10.1111/j.1753-5131.2012.01082.x
11. Zhang Z, Chi H, Dalmo RA. Trained innate immunity of fish is a viable approach in larval aquaculture. Front Immunol. (2019) 10:42. doi: 10.3389/fimmu.2019.00042
12. Magnadóttir B. Innate immunity of fish (overview). Fish Shellfish Immunol. (2006) 20:137–51. doi: 10.1016/j.fsi.2004.09.006
13. Mulero I, García-Ayala A, Meseguer J, Mulero V. Maternal transfer of immunity and ontogeny of autologous immunocompetence of fish: a minireview. Aquaculture. (2007) 268:244–50. doi: 10.1016/j.aquaculture.2007.04.046
14. Swain P, Nayak SK. Role of maternally derived immunity in fish. Fish Shellfish Immunol. (2009) 27:89–99. doi: 10.1016/j.fsi.2009.04.008
15. Zhang S, Wang Z, Wang H. Maternal immunity in fish. Dev Comp Immunol. (2013) 39:72–8. doi: 10.1016/j.dci.2012.02.009
16. Sung YY, Pineda C, MacRae TH, Sorgeloos P, Bossier P. Exposure of gnotobiotic Artemia franciscana larvae to abiotic stress promotes heat shock protein 70 synthesis and enhances resistance to pathogenic Vibrio campbellii. Cell Stress Chaperones. (2008) 13:59–66. doi: 10.1007/s12192-008-0011-y
17. Norouzitallab P, Baruah K, Muthappa DM, Bossier P. Non-lethal heat shock induces HSP70 and HMGB1 protein production sequentially to protect Artemia franciscana against Vibrio campbellii. Fish Shellfish Immunol. (2015) 42:395–9. doi: 10.1016/j.fsi.2014.11.017
18. Bricknell I, Dalmo RA. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol. (2005) 19:457–72. doi: 10.1016/j.fsi.2005.03.008
19. Zhang A, Zhou X, Wang X, Zhou H. Characterization of two heat shock proteins (HSP70/Hsc70) from grass carp (Ctenopharyngodon idella): evidence for their differential gene expression, protein synthesis and secretion in LPS-challenged peripheral blood lymphocytes. Comp Biochem Physiol B Biochem Mol Bio. (2011) 159:109–14. doi: 10.1016/j.cbpb.2011.02.009
20. Baruah K, Norouzitallab P, Shihao L, Sorgeloos P, Bossier P. Feeding truncated heat shock protein 70s protects Artemia franciscana against virulent Vibrio campbellii challenge. Fish Shellfish Immunol. (2013) 34:183–91. doi: 10.1016/j.fsi.2012.10.025
21. Baruah K, Norouzitallab P, Linayati L, Sorgeloos P, Bossier P. Reactive oxygen species generated by a heat shock protein (Hsp) inducing product contributes to HSP70 production and HSP70-mediated protective immunity in Artemia franciscana against pathogenic vibrios. Dev Comp Immunol. (2014) 46:470–79. doi: 10.1016/j.dci.2014.06.004
22. Niu Y, Norouzitallab P, Baruah K, Don Sh, Bossier P. A plant-based heat shock protein inducing compound modulates host–pathogen interactions between Artemia franciscana and Vibrio campbellii. Aquaculture. (2014) 430:120–27. doi: 10.1016/j.aquaculture.2014.04.001
23. Baruah K, Phong HPPD, Norouzitallab P, Defoirdt T. The gnotobiotic brine shrimp (Artemia franciscana) model system reveals that the phenolic compound pyrogallol protects against infection through its prooxidant activity. Free Radic Biol Med. (2015) 89:593–601. doi: 10.1016/j.freeradbiomed.2015.10.397
24. Kumar V, Baruah K, Nguyen DV, Smagghe G, Vossen E, Bossier P. Phloroglucinol-mediated HSP70 production in crustaceans: protection against Vibrio parahaemolyticus in Artemia franciscana and Macrobrachium rosenbergii. Front Immunol. (2018) 9:1091. doi: 10.3389/fimmu.2018.01091
25. Vallejos-Vidal E, Reyes-López F, Teles M, MacKenzie S. The response of fish to immunostimulant diets. Fish Shellfish Immunol. (2016) 56:34–69. doi: 10.1016/j.fsi.2016.06.028
26. Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. (2002) 277:15028–34. doi: 10.1074/jbc.M200497200
27. Ryckaert J, Pasmans F, Tobback E, Duchateau L, Decostere A, Haesebrouck F, et al. Heat shock proteins protect platyfish (Xiphophorus maculatus) from Yersinia ruckeri induced mortality. Fish Shellfish Immunol. (2010) 28:228–31. doi: 10.1016/j.fsi.2009.09.005
28. Baruah K, Ranjan J, Sorgeloos P, Bossier P. Efficacy of heterologous and homologous heat shock protein 70s as protective agents to Artemia franciscana challenged with Vibrio campbellii. Fish Shellfish Immunol. (2010) 29:733–39. doi: 10.1016/j.fsi.2010.07.011
29. Hu B, Phuoc LH, Sorgeloos P, Bossier P. Bacterial HSP70 (DnaK) is an efficient immune stimulator in Litopenaeus vannamei. Aquaculture. (2014) 418–9:87–93. doi: 10.1016/j.aquaculture.2013.10.008
30. Barton BA. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol. (2002) 42:517–25. doi: 10.1093/icb/42.3.517
31. Marques A, Ollevier F, Verstraete W, Sorgeloos P, Bossier P. Gnotobiotically grown aquatic animals: opportunities to investigate host-microbe interactions. J Appl Microbiol. (2006) 100:903–18. doi: 10.1111/j.1365-2672.2006.02961.x
32. Baruah K, Norouzitallab P, Phong HPPD, Smagghe G, Bossier P. Enhanced resistance against Vibrio harveyi infection by carvacrol and its association with the induction of heat shock protein 72 in gnotobiotic Artemia franciscana. Cell Stress Chaperones. (2017) 22:377–87. doi: 10.1007/s12192-017-0775-z
33. Yaacob EN, Goethals J, Bajek A, Dierckens K, Bossier P, De Geest BG, et al. Preparation and characterization of alginate microparticles containing a model protein for oral administration in gnotobiotic European sea bass (Dicentrarchus labrax) larvae. Mar Biotechnol. (2017) 19:391–400. doi: 10.1007/s10126-017-9758-4
34. Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. (1998) 31:267–85. doi: 10.1016/S0169-409X(97)00124-5
35. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. (1970) 227:680–85. doi: 10.1038/227680a0
36. Jeffery H, Davis SS, O'Hagan DT. The preparation and characterization of poly(lactide-co-glycolide) microparticles. II. the entrapment of a model protein using a (water-in-oil)-in-water emulsion solvent evaporation technique. Pharm Res. (1993) 10:362–8.
37. Dierckens K, Rekecki A, Laureau S, Sorgeloos P, Boon N, Van den Broeck W, et al. Development of a bacterial challenge test for gnotobiotic sea bass (Dicentrarchus labrax) larvae. Environ Microbiol. (2009) 11:526–33 doi: 10.1111/j.1462-2920.2008.01794.x
38. Rekecki A, Gunasekara RAYSA, Dierckens K, Laureau S, Boon N, Favoreel H, et al. Bacterial host interaction of GFP-labelled Vibrio anguillarum HI-610 with gnotobiotic sea bass, Dicentrarchus labrax (L.) larvae. J Fish Dis. (2012) 35:265–73. doi: 10.1111/j.1365-2761.2011.01342.x
39. Li X, Defoirdt T, Yang Q, Laureau S, Bossier P, Dierckens K. Host-induced increase in larval sea bass mortality in a gnotobiotic challenge test with Vibrio anguillarum. Dis Aquat Organ. (2014) 108:211–16. doi: 10.3354/dao02722
40. Reyes-López FE, Aerts J, Vallejos-Vidal E, Ampe B, Dierckens K, Tort L, et al. Modulation of innate immune-related genes and glucocorticoid synthesis in gnotobiotic full-sibling European sea bass (Dicentrarchus labrax) larvae challenged with Vibrio anguillarum. Front Immunol. (2018) 9:914. doi: 10.3389/fimmu.2018.00914
41. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid gauanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. (1987) 162:156–59. doi: 10.1016/0003-2697(87)90021-2
42. Galindo-Villegas J, Mulero I, García-Alcazar A, Muñoz I, Peñalver-Mellado M, Streitenberger S, et al. Recombinant TNFα as oral vaccine adjuvant protects European sea bass against vibriosis: insights into the role of the CCL25/CCR9 axis. Fish Shellfish Immunol. (2013) 35:1260–71. doi: 10.1016/j.fsi.2013.07.046
43. Yaacob EN, De Geest BG, Goethals J, Bajek A, Dierckens K, Bossier P, et al. Recombinant ferritin-H induces immunosuppression in European sea bass larvae (Dicentrarchus labrax) rather than immunostimulation and protection against a Vibrio anguillarum infection. Vet Immunol Immunopathol. (2018) 204:19–27. doi: 10.1016/j.vetimm.2018.09.001
44. Franke A, Roth O, De Schryver P, Bayer T, Garcia-Gonzalez L, Kunzel S, et al. Poly-β-hydroxybutyrate administration during early life: effects on performance, immunity and microbial community of European sea bass yolk-sac larvae. Sci Rep. (2017) 7:15022. doi: 10.1594/PANGAEA.876665
45. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
46. Toranzo AE, Magariños B, Romalde JL. A review of the main bacterial fish diseases in mariculture systems. Aquaculture. (2005) 246:37–61. doi: 10.1016/j.aquaculture.2005.01.002
47. Touraki M, Niopas I, Karagiannis V. Treatment of vibriosis in European sea bass larvae, Dicentrarchus labrax L, with oxolinic acid administered by bath or through medicated nauplii of Artemia franciscana (Kellogg): efficacy and residual kinetics. J Fish Dis. (2012) 35:513–22. doi: 10.1111/j.1365-2761.2012.01387.x
48. Skjermo J, Vadstein O. Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture. (1999) 177:333–43 doi: 10.1016/S0044-8486(99)00096-4
49. Ina-Salwany MY, Al-Saari N, Mohamad A, Mursidi FA, Mohd-Aris A, Amal MNA, et al. Vibriosis in fish: a review on disease development and prevention. J Aquat Anim Health. (2019) 31:3–22. doi: 10.1002/aah.10045
50. Peddie S, Zou J, Secombes CJ. Immunostimulation in the rainbow trout (Oncorhynchus mykiss) following intraperitoneal administration of Ergosan. Vet Immunol Immunopathol. (2002) 86:101–13. doi: 10.1016/S0165-2427(02)00019-3
51. Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L, Real F, et al. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. (2007) 23:969–81. doi: 10.1016/j.fsi.2007.03.007
52. Heidarieh M, Mirvaghefi AR, Akbari M, Farahmand H, Sheikhzadeh N, Shahbazfar AA, et al. Effect of dietary Ergosan on growth performance, digestive enzymes, intestinal histology, hematological parameters and body composition of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem. (2012) 38:1169–74. doi: 10.1007/s10695-012-9602-8
53. Torrecillas S, Montero D, Izquierdo M. Improved health and growth of fish fed mannan oligosaccharides: potential mode of action. Fish Shellfish Immunol. (2014) 36:525–44. doi: 10.1016/j.fsi.2013.12.029
54. AbouShabana NM, Abdelkader R, Abdel-Rahman S, Abdel-Gawad HS, Abdel-Galil AM. Enhancement of broodstock health and maternal immunity in gilthead seabream (Sparus aurata L.) using ExcelMOS®. Fish Physiol Biochem. (2018) 44:1241–51. doi: 10.1007/s10695-018-0517-x
55. Petit J, Bailey EC, Wheeler RT, de Oliveira CAF, Forlenza M, Wiegertjes GF. Studies into β-glucan recognition in fish suggests a key role for the c-type lectin pathway. Front Immunol. (2019) 10:280. doi: 10.3389/fimmu.2019.00280
56. Galindo-Villegas J, Fukada H, Masumoto T, Hosokawa H. Effect of dietary immunostimulants on some innate immune responses and disease resistance against Edwardsiella tarda infection in japanese flounder (Paralichthys olivaceus). Aquacult Sci. (2006) 54:153–62. doi: 10.11233/aquaculturesci1953.54.153
57. Khuyen TD, Mandiki SNM, Cornet V, Douxfils J, Betoulle S, Bossier P, et al. Physiological and immune response of juvenile rainbow trout to dietary bovine lactoferrin. Fish Shellfish Immunol. (2017) 71:359–71. doi: 10.1016/j.fsi.2017.10.027
58. Wang W, Sun J, Liu C, Xue Z. Application of immunostimulants in aquaculture: current knowledge and future perspectives. Aquac Res. (2017) 48:1–23. doi: 10.1111/are.13161
59. Esmaeili A, Sotoudeh E, Morshedi V, Bagheri D, Dorafshan S. Effects of dietary supplementation of bovine lactoferrin on antioxidant status, immune response and disease resistance of yellowfin sea bream (Acanthopagrus latus) against Vibrio harveyi. Fish Shellfish Immunol. (2019) 93:917–23. doi: 10.1016/j.fsi.2019.08.045
60. Rojo-Cebreros AH, Ibarra-Castro L, Martínez-Brown JM. Immunostimulation and trained immunity in marine fish larvae. Fish Shellfish Immunol. (2018) 80:15–21. doi: 10.1016/j.fsi.2018.05.044
61. Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. (2010) 10:3223–30. doi: 10.1021/nl102184c
62. Ji J, Torrealba D, Ruyra A, Roher N. Nanodelivery systems as new tools for immunostimulant or vaccine administration: targeting the fish immune system. Biology. (2015) 4:664–96. doi: 10.3390/biology4040664
63. Rivas-Aravena A, Sandino AM, Spencer E. Nanoparticles and microparticles of polymers and polysaccharides to administer fish vaccines. Biol Res. (2013) 46:407–19. doi: 10.4067/S0716-97602013000400012
64. Yang M, Zhang M. Biodegradation of carbon nanotubes by macrophages. Front Mater. (2019) 6:225. doi: 10.3389/fmats.2019.00225
65. Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. (2010) 62:83–99. doi: 10.1016/j.addr.2009.07.019
66. George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—-a review. J Control Release. (2006) 114:1–14. doi: 10.1016/j.jconrel.2006.04.017
67. Reyes-López FE, Romeo JS, Vallejos-Vidal E, Reyes-Cerpa S, Sandino AM, Tort L, et al. Differential immune gene expression profiles in susceptible and resistant full-sibling families of Atlantic salmon (Salmo salar) challenged with infectious pancreatic necrosis virus (IPNV). Dev Comp Immunol. (2015) 53:210–21. doi: 10.1016/j.dci.2015.06.017
68. Smith N, Pietrancosta N, Herbeuval JP. CXCR4, master regulator of innate immune responses? Med Sci. (2017) 3:38–9. doi: 10.1051/medsci/20173308008
69. Jiang S, Gu H, Zhao Y, Sun Li. Teleost gasdermin E is cleaved by caspase 1, 3, and 7 and induces pyroptosis. J Immunol. (2019) 204:ji1900383. doi: 10.4049/jimmunol.1900383
70. Valero Y, Arizcun M, Cortés J, Ramírez-Cepeda F, Guzmán F, Mercado L, et al. NK-lysin, dicentracin and hepcidin antimicrobial peptides in European sea bass. ontogenetic development and modulation in juveniles by nodavirus. Dev Comp Immunol. (2019) 5:103516. doi: 10.1016/j.dci.2019.103516
71. Álvarez CA, Acosta F, Montero D, Guzmán F, Torres E, Vega B, et al. Synthetic hepcidin from fish: uptake and protection against Vibrio anguillarum in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol. (2016) 55:662–970. doi: 10.1016/j.fsi.2016.06.035
72. Meloni M, Candusso S, Galeotti M, Volpatti D. Preliminary study on expression of antimicrobial peptides in European sea bass (Dicentrarchus labrax) following in vivo infection with Vibrio anguillarum. a time course experiment. Fish Shellfish Immunol. (2015) 43:82–90. doi: 10.1016/j.fsi.2014.12.016
73. Situmorang ML, De Schryver P, Dierckens K, Bossier P. Effect of poly-β-hydroxybutyrate on growth and disease resistance of Nile tilapia Oreochromis niloticus juveniles. Vet Microbiol. (2016) 182:44–9. doi: 10.1016/j.vetmic.2015.10.024
74. Shabir U, Ali S, Magray AR, Ganai BA, Firdous P, Hassan T, et al. Fish antimicrobial peptides (AMP's) as essential and promising molecular therapeutic agents: a review. Microb Pathog. (2018) 114:50–6. doi: 10.1016/j.micpath.2017.11.039
75. Shirdel I, Kalbassi MR, Hosseinkhani S, Paknejad H, Wink M. Cloning, characterization and tissue-specific expression of the antimicrobial peptide hepcidin from caspian trout (Salmo caspius) and the antibacterial activity of the synthetic peptide. Fish Shellfish Immunol. (2019) 90:288–96. doi: 10.1016/j.fsi.2019.05.010
76. Mulero I, Noga E, Meseguer J, García-Ayala A, Mulero V. The antimicrobial peptides piscidins are stored in the granules of professional phagocytic granulocytes of fish and are delivered to the bacteria-containing phagosome upon phagocytosis. Dev Comp Immunol. (2008) 32:1531–8. doi: 10.1016/j.dci.2008.05.015
Keywords: gnotobiotic, European sea bass larvae, bacterial HSP70, DnaK, immunity, vibriosis, Vibrio anguillarum
Citation: Yaacob EN, Norouzitallab P, De Geest BG, Bajek A, Dierckens K, Bossier P and Vanrompay D (2020) Recombinant DnaK Orally Administered Protects Axenic European Sea Bass Against Vibriosis. Front. Immunol. 10:3162. doi: 10.3389/fimmu.2019.03162
Received: 19 August 2019; Accepted: 31 December 2019;
Published: 14 February 2020.
Edited by:
Brian Dixon, University of Waterloo, CanadaReviewed by:
Jorge Galindo-Villegas, Nord University, NorwayCopyright © 2020 Yaacob, Norouzitallab, De Geest, Bajek, Dierckens, Bossier and Vanrompay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parisa Norouzitallab, cGFyaXNhLm5vcm91eml0YWxsYWJAdWdlbnQuYmU=
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.