- Division of Immunodermatology and Allergy Research, Department of Dermatology and Allergy, Hannover Medical School, Hanover, Germany
Atopic dermatitis (AD), one of the most frequent inflammatory skin diseases worldwide, is believed to result from a disturbed skin barrier as well as aberrant immune reactions against per se harmless allergens. Starting mostly during childhood with a chronic, remitting relapsing course, the disease can persist into adulthood in about one fifth of patients. Immune reactions to self-proteins have been observed in AD patients already in the beginning of the Twentieth century, when human cellular extracts were shown to provoke skin lesions. However, the term “autoimmunity” has never been claimed, since AD is first and foremost an atopic disease. In contrast, this IgE-hallmarked autoreactivity was termed “autoallergy” and is ongoing discussed regarding its impact on the disease. Since severely affected patients tend to develop IgE-hypersensitivity reactions to numerous environmental allergens, the impact of immune responses to self-proteins is difficult to determine. On the other hand: any autoreactivity, irrespective of the magnitude, implicates the potential of driving the chronification of the disease while shaping the immune response. This review article revisits the observations made on autoallergy from an actual point of view and tries to approach the question whether these still point to a contribution to the disease.
Autoreactive IgE—an AD-Specific Phenomenon?
First of all, autoreactivity accompanying atopy is a historical observation. The first studies date back to the 1920s, where human skin extract, injected into the skin led to visible skin inflammation (1, 2). These observations have been made long before IgE was discovered (3). In these days, disease criteria and names were not as clearly defined as today, making it somewhat speculative from today's perspective to draw precise conclusions. From what we know today, we can only speculate that in these experiments antigens of the skin dander extracts were captured by recipient's IgE, which was bound to Fc-receptors on the cell surface of mast cells or basophils. This would have led to IgE crosslinking, the release of pro-inflammatory mediators, and finally type I hypersensitivity reactions of the skin.
Serum total IgE levels are often drastically increased in atopic patients and are applied as diagnostic tools and therapeutical targets (4, 5). However, the specificity of the majority often remains unclear. By now, also several modern approaches of controlled experiments focused on the question of autoreactive IgE. Summarizing 14 studies involving 2,644 patients in total, Tang et al. finally conclude that AD is indeed associated with IgE-autoreactivity (6). In this meta-analysis the authors summarize the frequency of affected AD patients to be between 23 and 91% without finding a correlation to age, sex, or disease duration. Interestingly, two of 14 studies detected a significant correlation between IgE-autoreactivity and AD disease severity (7, 8); and in further three studies this became apparent by trend (9–11). As mentioned above, severely affected patients do indeed show strongly elevated total IgE levels, with a bouquet of allergen-sensitizations including e.g., aero-, food-, and microbial allergens. Therefore, it might seem not surprising to find IgE also against self. Nevertheless, autoreactive IgE may play a role in the pathogenicity of the disease, since compared to pollen or food allergens, self-antigens are by nature perennial and inescapable.
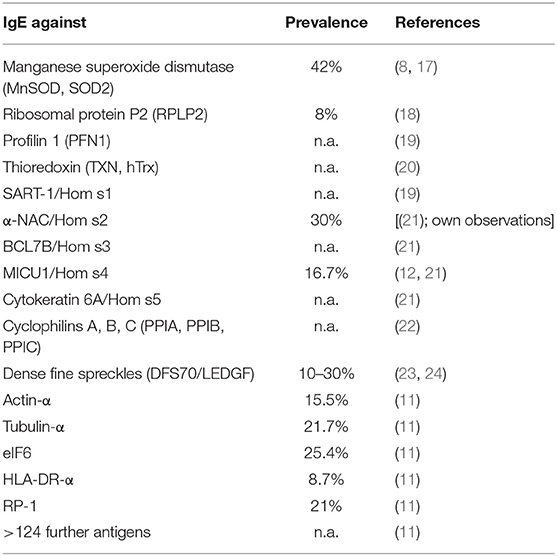
The 14 studies mentioned above contained different control entities including other skin autoimmune diseases as psoriasis (8, 10–12) or systemic lupus erythematosus (13), of which none had detectable autoreactive IgE toward the respective autoallergens tested. Nevertheless, autoreactive IgE can be found also in other inflammatory skin diseases [for a review see (14)]: For example, patients suffering from bullous pemphigoid are IgE-(as well as IgG- and IgA-)sensitized against the hemidesmosomal proteins BP180 (BP antigen 2) and BP230 (BP antigen 1). These and other antigens of autoimmune blistering diseases lead to destruction of skin integrity, and the mechanisms are meanwhile quite well understood (15). In systemic lupus erythematosus, patients often display autoreactive IgE to double-stranded DNA or P2 proteins. Recently, an extensive study revealed that autoreactive IgE can be found in the majority of patients with chronic spontaneous urticaria: Reporting over 30 autoreactivities, most prominently the cytokine IL-24 appears as a target for specific IgE in these patients (16). Maurer et al. summarized different studies and come to the point that autoreactive IgE is not exclusively found in AD (14), however, the large number of >140 autoallergens described in this disease up to now appears indeed to be unique (see Table 1).
The Origin of Autoreactive IgE—a Remnant of the Childhood, Crossreactivity, or de novo Sensitization?
The frequent occurrence of IgE sensitization to autoallergens in patients with AD was considered as a result of tissue damage and thereby release of auto-antigens that are commonly invisible to T cells (25). Since AD starts in most cases during infancy, several studies investigated autoreactive IgE in children: In a study from 2005, Mothes et al. investigated retrospectively a cohort of 174 adult AD patients regarding the presence of auto-IgE and found 23% to be positive (10). These displayed generally stronger disease symptoms, including clinical signs and scores, increased pruritus, more often a positive history of food allergy, higher levels of total as well as aero-allergen-, food-allergen-, and microbial allergen-specific serum IgE. These patients also reported more frequently to suffer from recurrent bacterial and viral infections of the skin such as impetigo contagiosa or eczema herpeticatum. But most interestingly, an early onset of AD and manifestation of clinically symptomatic AD between the 2nd and 6th years of life was associated with auto-IgE (10). In that work, also sera from 102 children aged 1–12 suffering from AD were analyzed and the authors detected auto-IgE in a substantial subgroup. Children aged 2–13 were affected more often than 1-year-olds. Longitudinal sampling suggested a development of auto-IgE in younger years. However, this study lacks a control cohort of healthy children (10).
In adult patients, auto-IgE in healthy children aged 10–15 was measured by Kistler et al. (26). Samples were generated within the birth cohorts GINIplus and LISAplus and therefore are population-representative. The authors agree with the finding by Mothes et al. that auto-IgE is quite frequently detectable in children of that age, however, the occurrence of auto-IgE was unexpectedly decreased in children suffering from AD and allergic asthma compared to healthies. Therefore, the occurrence of auto-IgE in children appears to be a general phenomenon with so far unknown meaning, but is not a predictor regarding AD. The authors speculate that a general type-2 immune prevalence in early life may be an opposing mechanism to more harmful type-1 (auto)inflammation (26).
Autoreactive IgE antibodies have been identified by detecting interactions between self-antigens and IgE in the serum of patients. In order to define single Aspergillus allergens, Crameri et al. established an Aspergillus fumigatus phage display library and applied sera of patients with known respective sensitization (17, 18). The discovery of two autoallergens occurred subsequently by investigating sequence homology of the newly identified allergens manganese superoxide dismutase (MnSOD, later termed Asp f6) and ribosomal protein P2 (termed Asp f8) to human proteins. Both of the human homologs, MnSOD and P2 shared strong sequence similarities and subsequent IgE-immunoblotting confirmed a cross-reactivity of the IgE between human and Aspergillus proteins. While P2-specific IgE was found in around 8% of 75 AD patients investigated (18), MnSOD sensitization was observed in more than 40% of 69 AD patients tested (8). By comparing results from cDNA libraries that displayed putative allergens from the fungi Aspergillus and Malassezia, respectively, Limacher et al. came across the thioredoxins that were later termed Asp f28, Asp f29, and Mala s13 (20). Sequence homology led to the identification of the highly homologous human variant, hTrx, and those from further organisms, defining a pan-allergen family. Competetive ELISAs confirmed IgE-crossreactivity, especially between the microorganisms, but also between Malassezia and the autoallergen hTrx. Malassezia has been known for decades as a trigger factor in AD, colonizing the skin as a facultative pathogen (27). Therefore, a sensitization to Malassezia was suggested to be underlying the cross-reactivity to hTrx, although these hypotheses are difficult to prove.
In direct approaches to identify autoallergens, cDNA phage libraries were generated from human proteins. Therefore, again a crude extract from the human epithelial cell line A431 was applied (21, 28). Binding to full length recombinant and native proteins was validated after recombinant protein expression and (competitive) IgE-blotting experiments. In total, five autoallergens were identified in these fundamental studies that were termed according to the IUIS nomenclature “Homo sapiens allergen 1 to 5” (Hom s1-s5). MICU1/Hom s4-specific IgE was found in a subsequent study to cross-react to homologous proteins of different species, all bearing calcium-binding abilities, namely Phl p7 (timothy grass) and Cyp c1 (common carp) (12). Finally, 10 years ago, a comprehensive phage display approach mapped in total 140 bona fide autoallergens, while confirming 16 that had already been described (11).
In order to approach the question of clinical relevance, recombinantly produced versions of several autoallergens were successfully tested toward IgE-reactivity in patient's skin by means of prick testing [Hom s2, s3, s4, s5 (21) and the ribosomal protein P2 (18)]. While this approach underlines the clinical relevance of auto-IgE, the sensitization to autoallergens and the mechanism of cross-reactivity was further addressed in vivo. Upon sensitization with α-NAC/Hom s2, mice developed skin symptoms as well as specific crossreactive IgE and IgG antibody responses. Intradermal administration of the autoallergen led to skin symptoms in sensitized mice as well as in non-sensitized mice after passive transfer of serum of sensitized ones (29).
Regarding the specificity of allergen extract-based IgE-binding assays, one has to consider that also in healthy individuals certain irrelevant cross-reactive carbohydrate antigens bind IgE without mounting a pro-inflammatory response (25). Further, some studies assume that IgE antibodies show a strong potential of crossreactivity, binding to a wide range of epitopes (30). This implicates that identified autoreactive IgE may not be a result of an interaction between B and autoreactive T helper cells. Therefore, the T cell response to putative autoallergens has to be investigated, too, in order to draw a comprehensive picture.
Autoreactive T Cells as a Result of Crossreactivity or de novo Sensitization?
T helper cells of the Th2 subtype are capable of initiating the class switch in B cells to induce the production of antigen-specific IgE. In AD, the T cell response is together with skin barrier disturbance regarded as the central disease mechanism. T cells home to the skin in AD patients (31), and those isolated from the inflamed, lesional AD skin have been shown to react to environmental allergens (32). It has been observed that during an ongoing acute or chronic AD inflammation, skin-infiltrating T cells are mostly T helper cells. Nevertheless, CD8+ T cells are also present and furthermore, these have been described to be crucial in initiating the skin inflammation (33, 34). Regarding T cell polarizations, first of all Th2, but also Th1, Th17, and Th22 T cells have been described to contribute to the pathogenesis of AD (35). These appear to be a result of (a) the allergen, (b) the inflammatory milieu, and/or (c) the disease progression. To explain this heterogeneity, it has been proposed that the Th1-predominance in chronic AD lesions might be a result of T cell responses to non-classical allergens like autoallergens. However, analyzes of autoallergen-specific T cell responses confirm this theory only partially.
Again, the primary observations regarding autoreactive T cells in AD have been made astonishingly long ago. Hashem et al. reported 1,963 proliferation of lymphocytes after stimulation with autologous skin extracts, detected by analyzing cell shape and division (36). In these tests, two patients with severe eczema reacted stronger compared to two healthy controls and one asthmatic. The first observations by Crameri et al. on T cell reactions toward the self-antigen MnSOD were made on T cells from AD donors sensitized to fungal and human MnSOD. However, that time no control donors were compared (17). A second trial showed in a proof-of-concept approach that non-sensitized AD patients do not show T cell proliferative responses to human-, Aspergillus- or Malassezia-MnSOD (8), while in a third approach also healthy controls next to sensitized and non-sensitized AD patients were enrolled in T cell proliferation studies (37). The human ribosomal P2 protein led to T cell responses in six out of six senzitized AD individuals, but not in four non-sensitized AD or three healthy donors (18). These experiments show altogether a clear-cut cross-reactivity between human and fungal allergen homologs and further a tight correlation between IgE and T cell responses, which appears to have clinical implications in allergic diseases (38, 39).
In a laborious approach T cell clones were generated from Malassezia-sensitized AD donors, from blood as well as from APT-lesions induced by Mala s13 or Malassezia extract. These clones did cross-react to both proteins and were assigned regarding the respective cytokine production to Th1, Th2, Th17, as well as Th22 T helper cell subsets (40). Later, our group described that hTrx upregulates the Th2 cytokine IL-13 in an IgE-dependent manner and showed further an impaired upregulation of IL-10 by hTrx in specifically sensitized AD patients (41). hTrx is known to act also as an alarmin, being secreted upon cellular stress (42). This suggests that the protein is often recognized by the immune system within danger situations, which may favor sensitization. Recent studies show that hTrx as well as Mala s13 are effectively bound by pattern recognition receptors on myeloid cells, and that hTrx directly induces pro-inflammatory responses that promote the survival of Th17 cells (Roesner et al., unpublished). Both crossreactivity as well as intrinsic properties of hTrx may therefore underlie the observed cellular and humoral responses.
Regarding the autoallergens that were identified by the human protein-directed approach by Natter et al. (21), α-NAC/Hom s2 as well as MICU1/Hom s4 have been shown to evoke IFN-γ responses from peripheral blood cells of sensitized donors. These reactions were found to be stronger compared to those by the classical pollen allergen Phl p1 (12, 43). MICU1/Hom s4, however, also promoted IFN-γ in healthy individuals, suggesting that the protein might activate parts of the innate immune system. The Th1-response by α-NAC/Hom s2 was later shown to be dependent on IL-12 and mediated through TLR-2 on monocytes, also describing an effect aside from the adaptive T cell response (44). Proliferation testing of T cells from the blood of sensitized donors, however, revealed that α-NAC/Hom s2 specifically triggered skin-homing T cells in AD patients. Generating subsequently α-NAC/Hom s2-specific T cell clones, CD4+ but interestingly also CD8+ T cells were could be established. While from lesional AD skin only 10% of T cell clones were CD8+, surprisingly 61% clones generated from the circulation of sensitized donors were found to be cytotoxic T cells (45). These did secrete IFN-γ and/or IL-4 as well as occasionally IL-17 upon autologous, specific re-stimulation.
The identification of autoreactive T cells homing to / infiltrating in the skin clearly indicates that these promote the pro-inflammatory milieu in the inflammatory response in affected patients. However, relatively low numbers of participants narrow the impact of these experiments.
Deeper insights come from approaches evoking skin lesions in patients' skin. Recombinantly produced human MnSOD has been applied to patient's skin in the context of an atopy patch test (APT) (8). This test aims for the late type (type IV-like) hypersensitivity skin reaction to protein allergens in a controlled fashion (46). This positive skin reaction can therefore be seen as a proof-of-concept-observation of the clinical relevance of a given sensitization. Within the EU, only those test substances are allowed nowadays to be applied on the skin, which have been produced under GMP conditions. This production process however is often financially not profitable which explains that the APT is generally not available as a routine diagnostic tool (47, 48).
Further deciphering of the T cell reactivity to autoallergens is achieved by identifying immunodominant epitopes within the amino acid sequence. T cells recognize linear epitopes that are presented by MHC complexes of antigen presenting cells. Breaking-down the amino acid sequence into immunodominant epitopes can therefore be achieved by applying single synthetic peptides in stimulation or binding assays. Therefore, candidate peptides are often produced in an overlapping fashion, covering the complete sequence of the protein of interest. A possibility to downsize these laborious approaches is to apply prediction algorithms upfront that identify MHC-binding motifs within the primary sequence [like SYFPEITHI (49) and consensus (50)]. For hTrx and α-NAC/Hom s2 candidate T cell epitopes were presented to PBMC of sensitized donors, which led to modest but measureable T cell proliferation in sensitized donors and finally to the identification of MHC-I and MHC-II epitopes (51, 52).
Are Autoallergens Presented via MHC?—a Deeper Look Into the “Peptidome”
Cross-reactivity of T cells is nowadays an accepted immunological phenomenon. The specificity of the T cell is given by the highly diverse TCR, which is generated by the process of V(D)J recombination and can theoretically lead to 1017 different receptors (53, 54). However, the naïve T cell pool of a human being consists “only” of 107 to 1013 different TCR (55–57), what raises the question how these are able to cover a virtually unlimited set of pathogenic molecules (>1015 possibilities from 20 proteogenic amino acids) (58). Recent studies showed that each TCR is able to get activated by millions of different peptide/MHC complexes (58). While at least two amino acids within an epitope were considered as nearly irreplaceable since serving as anchor positions for the MHC, also these have been shown to be variable to a certain extent (59). It is believed that the high variability is not leading to a complete autoimmune-chaos, since a) not every protein is present in every tissue, and b) not every possible combination is generated by the antigen-presenting cells. The question whether a putative immunodominant epitope is presented via MHC is therefore of central interest.
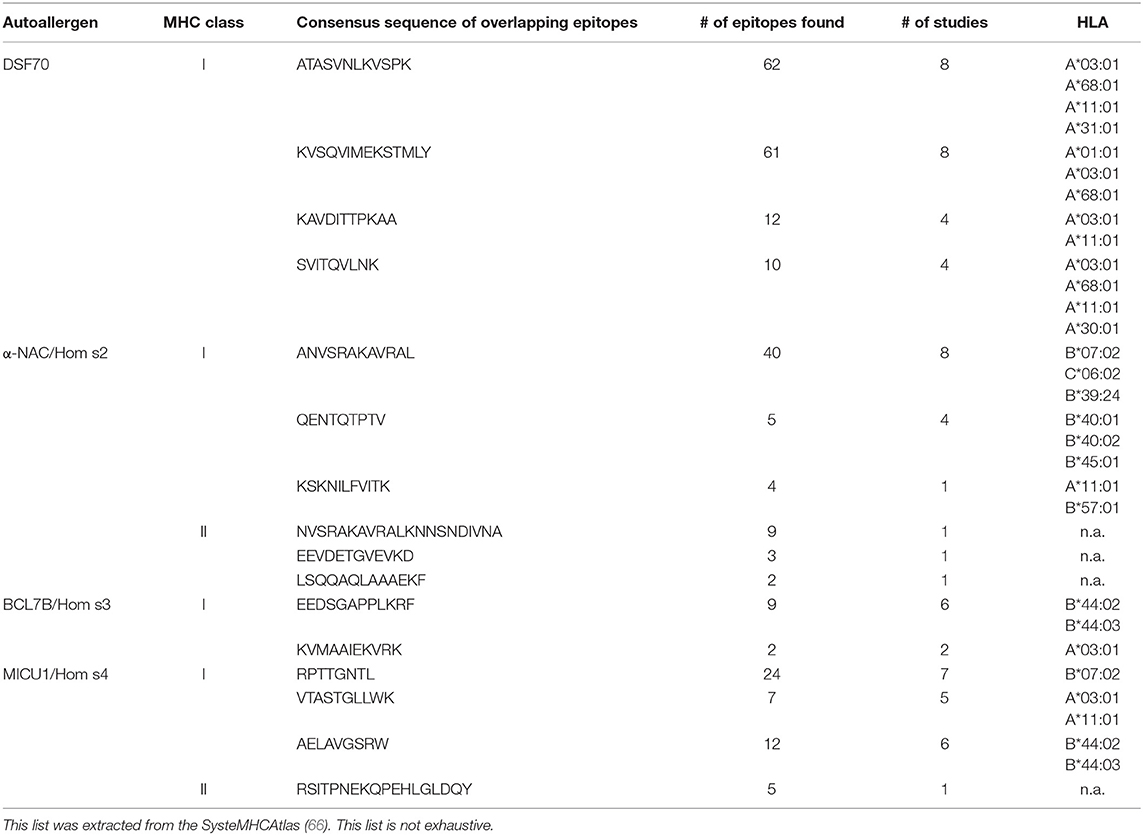
Meanwhile, harnessing the power of high-throughput proteomics, T cell epitopes presented by MHC-molecules on the surface of antigen-presenting cells can be identified by mass spectrometry. These immuno assays represent beautiful but laborious as well as money-consuming approaches enabling an objective view into what T cells might react to. However, caution has to be taken since donors differ regarding the MHC molecules and every MHC has different binding abilities, leading to a patient-specific set of presented epitopes for each antigen. This is usually taken into account by enlargement of the test cohort. Epitopes discovered to be presented in different donors are of special interest, since these may represent targets for vaccination or allergen immunotherapy. To date, most of these assays have been performed on cells derived from healthy donors to get a picture of the status quo of antigen presentation (60–63); but also data on specific diseases are available (64, 65). These studies are listed within the PRoteomics IDEntifications (PRIDE) database. The consortium Human Immuno-Peptidome Project (HUPO-HIPP) intends to define experimental standards, to connect labs generating these data, and to gather available information in order to display the sequences to scientists around the world on a single platform, the SysteMHCAtlas (66). This database harbors today data from 23 experimental approaches. Data mining in this archive reveals that autoallergens are indeed frequently presented. For example regarding the autoallergen α-NAC/Hom s2, 66 presented epitopes have been identified by mass spectroscopy. Interestingly, 49 of 66 are mapped within the sequence stretch α-NAC/Hom s2181−209 VKLVMSQANVSRAKAVRALKNNSNDIVNA, which has also been found to be immunogenic in our studies on autoallergic AD patients (51). Further, these data match our own findings, that MHC-I and MHC-II epitopes overlap in this region (52). Also epitopes of DSF70, Hom s3, and Hom s4 could be identified: Table 2 lists the most commonly identified epitopes and their appearances in the assays. Epitopes of hTrx were found in at least two studies (60, 67).
These data support the probability of autoallergens to play a role in disease, since their epitopes are commonly presented. It appears even more compelling, that presented natural epitopes of α-NAC/Hom s2 have also been described to evoke pro-inflammatory responses in sensitized subjects.
Autoreactive T Cell Receptors, a Matter of Specificity
MHC-multimers are further tools to investigate the question whether a certain epitope is recognized by T cells. These multimerized, labeled MHC/peptide-complexes have been shown to bind to matching T cells with strong specificity, allowing their enumeration and characterization (68). With MHC class I-multimers harboring α-NAC/Hom s2 epitopes, we observed specific staining of a subgroup of CD8+ T cells in patients that displayed detectable levels of specific IgE (51). This T cell fraction showed specific characteristics of effector/memory (TEM) of terminally differentiated effector T (TEMRA) cells, arguing for a contribution in an ongoing inflammatory process. Measuring cytokines secreted by these cells, we detected first of all IL-4, and further (but less) IFN-γ. While this phenotype, also termed Tc2, is relatively uncommon in healthy donors, it reflects the cytokine milieu in AD. Again, quality and quantity of the immune response suggest a contribution to the disease pathogenesis.
Interestingly, the binding affinity of identified peptides to the MHC-I molecules, as well as the binding avidity of the tetramer to the TCR were both observed at a rather low level (51). Based on this, it could be hypothesized that autoallergen-specific T cells do not harbor perfectly matching T cell receptors (TCR). This might indeed be the case, since those would have been eradicated during negative selection in the thymus. The only possible explanation of autoreactivity in AD, where a fully functional thymus can be assumed, is that T cells with minimal autoreactive potential escape the negative selection. Upon encounter of the autoallergen within a highly inflammatory milieu, however, the suboptimal recognition may be sufficient to mount a pro-inflammatory response against self. The expression of a low avidity TCR can be seen as a major mechanism by which autoreactive T cells escape tolerance. Different studies have demonstrated that that autoreactive T cells that are not completely eliminated by negative selection due to low avidity are quiescent under steady-state conditions in the presence of their target, but during an infection they are able to respond to the respective antigen and differentiate into effector T cells and form memory T cells (69, 70). Interestingly, these low avidity T cells in contrast to high avidity T cells appear to persist without losing their self-destructive potential (70). Szomolay described that also other TCR that recognize self-antigens may do so with low avidity: The MART-1/melan-A antigen, which is specific for the melanocyte lineage and also found in normal skin, evokes T cell reactivity, but the respective TCR could be shown to react much better to other (hypothetical) peptides (71). Contrary to that, TCR that recognize pathogen epitopes (which are not displayed during negative selection) are often perfectly suitable to the respective epitope (71). These findings match to the idea of Wooldridge et al., who describe that TCR do not recognize one single peptide epitope, but broader signatures and many different epitopes (58, 59).
Conclusion
Historical observations in the patients' skin argue for a pathogenic role of autoallergy in AD. A high number of IgE-reactive auto-antigens have been identified by now, what appears to be specific for AD. These bear the potential to mount an inflammatory response. For several autoantigens immune-stimulatory functions have been described, and different receptors have been shown to bind self-antigens. The limited success of anti-IgE treatment may underlie the fact that AD is strongly driven by specific T cell responses. However, a subgroup of patients may indeed benefit from this therapeutic (4, 72, 73). T cells recognizing autoallergens have been shown to be of effector subtype, to home to and infiltrate the skin. These have been observed to respond with different cytokines involving IFN-γ, IL-4, and IL-13, but also IL-17 and IL-22. Further in vitro data were generated on T cell epitopes. Although this is known to bear certain risks, recent data corroborate these findings. Difficulties in detecting the immune responses may most probably result from the fact that autoallergens do not harbor perfectly matching T cell epitopes, since such T cells are eliminated efficiently in the thymus during maturation. Nevertheless, reviewing data that are available today, there is no plausible reason to deny an impact of autoreactivity in AD.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Keller P. Beitrag zu den beziehungen zwischen asthma und ekzem. Arch Dermatol Syph. (1924) 148. 82–97. doi: 10.1007/BF01827500
2. Storm Van Leuwen W, Bien Z, Varekamp H. Über die hautreaktion mit extrakten menschlicher hautschuppen bei allergischen krankheiten. Klin Wochenschr. (1926) 5:1023–5. doi: 10.1007/BF01717944
3. Prausnitz C, Küstner H. Studien über die ueberempfindlichkeit. Zentralbl Bakteriol. (1921) 86:160–9.
4. Kasperkiewicz M, Mook SC, Knuth-Rehr D, Vorobyev A, Ludwig RJ, Zillikens D, et al. IgE-selective immunoadsorption for severe atopic dermatitis. Front Med. (2018) 5:27. doi: 10.3389/fmed.2018.00027
5. Gomez G. Current strategies to inhibit high affinity FcepsilonRI-mediated signaling for the treatment of allergic disease. Front Immunol. (2019) 10:175. doi: 10.3389/fimmu.2019.00175
6. Tang TS, Bieber T, Williams HC. Does “autoreactivity” play a role in atopic dermatitis? J Allergy Clin Immunol. (2012) 129:1209–15.e2. doi: 10.1016/j.jaci.2012.02.002
7. Szakos E, Lakos G, Aleksza M, Gyimesi E, Pall G, Fodor B, et al. Association between the occurrence of the anticardiolipin IgM and mite allergen-specific IgE antibodies in children with extrinsic type of atopic eczema/dermatitis syndrome. Allergy. (2004) 59:164–7. doi: 10.1046/j.1398-9995.2003.00367.x
8. Schmid-Grendelmeier P, Fluckiger S, Disch R, Trautmann A, Wuthrich B, Blaser K, et al. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol. (2005) 115:1068–75. doi: 10.1016/j.jaci.2005.01.065
9. Ohkouchi K, Mizutani H, Tanaka M, Takahashi M, Nakashima K, Shimizu M. Anti-elongation factor-1alpha autoantibody in adult atopic dermatitis patients. Int Immunol. (1999) 11:1635–40. doi: 10.1093/intimm/11.10.1635
10. Mothes N, Niggemann B, Jenneck C, Hagemann T, Weidinger S, Bieber T, et al. The cradle of IgE autoreactivity in atopic eczema lies in early infancy. J Allergy Clin Immunol. (2005) 116:706–9. doi: 10.1016/j.jaci.2005.06.025
11. Zeller S, Rhyner C, Meyer N, Schmid-Grendelmeier P, Akdis CA, Crameri R. Exploring the repertoire of IgE-binding self-antigens associated with atopic eczema. J Allergy Clin Immunol. (2009) 124:278–85, 285.e1–7. doi: 10.1016/j.jaci.2009.05.015
12. Aichberger KJ, Mittermann I, Reininger R, Seiberler S, Swoboda I, Spitzauer S, et al. Hom s 4, an IgE-reactive autoantigen belonging to a new subfamily of calcium-binding proteins, can induce Th cell type 1-mediated autoreactivity. J Immunol. (2005) 175:1286–94. doi: 10.4049/jimmunol.175.2.1286
13. Valenta R, Maurer D, Steiner R, Seiberler S, Sperr WR, Valent P, et al. Immunoglobulin E response to human proteins in atopic patients. J Invest Dermatol. (1996) 107:203–8. doi: 10.1111/1523-1747.ep12329617
14. Maurer M, Altrichter S, Schmetzer O, Scheffel J, Church MK, Metz M. Immunoglobulin E-mediated autoimmunity. Front Immunol. (2018) 9:689. doi: 10.3389/fimmu.2018.00689
15. Stevens NE, Cowin AJ, Kopecki Z. Skin barrier and autoimmunity-mechanisms and novel therapeutic approaches for autoimmune blistering diseases of the skin. Front Immunol. (2019) 10:1089. doi: 10.3389/fimmu.2019.01089
16. Schmetzer O, Lakin E, Topal FA, Preusse P, Freier D, Church MK, et al. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. (2018) 142:876–82. doi: 10.1016/j.jaci.2017.10.035
17. Crameri R, Faith A, Hemmann S, Jaussi R, Ismail C, Menz G, et al. Humoral and cell-mediated autoimmunity in allergy to Aspergillus fumigatus. J Exp Med. (1996) 184:265–70. doi: 10.1084/jem.184.1.265
18. Mayer C, Appenzeller U, Seelbach H, Achatz G, Oberkofler H, Breitenbach M, et al. Humoral and cell-mediated autoimmune reactions to human acidic ribosomal P2 protein in individuals sensitized to Aspergillus fumigatus P2 protein. J Exp Med. (1999) 189:1507–12. doi: 10.1084/jem.189.9.1507
19. Valenta R, Duchene M, Pettenburger K, Sillaber C, Valent P, Bettelheim P, et al. Identification of profilin as a novel pollen allergen; IgE autoreactivity in sensitized individuals. Science. (1991) 253:557–60. doi: 10.1126/science.1857985
20. Limacher A, Glaser AG, Meier C, Schmid-Grendelmeier P, Zeller S, Scapozza L, et al. Cross-reactivity and 1.4-A crystal structure of Malassezia sympodialis thioredoxin (Mala s 13), a member of a new pan-allergen family. J Immunol. (2007) 178:389–96. doi: 10.4049/jimmunol.178.1.389
21. Natter S, Seiberler S, Hufnagl P, Binder BR, Hirschl AM, Ring J, et al. Isolation of cDNA clones coding for IgE autoantigens with serum IgE from atopic dermatitis patients. FASEB J. (1998) 12:389–96. doi: 10.1096/fasebj.12.14.1559
22. Fluckiger S, Fijten H, Whitley P, Blaser K, Crameri R. Cyclophilins, a new family of cross-reactive allergens. Eur J Immunol. (2002) 32:10–7. doi: 10.1002/1521-4141(200201)32:1<10::AID-IMMU10>3.0.CO;2-I
23. Ochs RL, Muro Y, Si Y, Ge H, Chan EK, Tan EM. Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allergy Clin Immunol. (2000) 105:1211–20. doi: 10.1067/mai.2000.107039
24. Watanabe K, Muro Y, Sugiura K, Tomita Y. IgE and IgG(4) autoantibodies against DFS70/LEDGF in atopic dermatitis. Autoimmunity. (2011) 44:511–9. doi: 10.3109/08916934.2010.549157
25. Campana R, Dzoro S, Mittermann I, Fedenko E, Elisyutina O, Khaitov M, et al. Molecular aspects of allergens in atopic dermatitis. Curr Opin Allergy Clin Immunol. (2017) 17:269–77. doi: 10.1097/ACI.0000000000000378
26. Kistler T, Thiering E, Janmohamed SR, Haak S, Heinrich J, Ollert M, et al. Autoreactive IgE occurs naturally during childhood and is negatively correlated with allergic sensitization at age 10. Allergy. (2018) 73:829. doi: 10.1111/all.13540
27. Hradetzky S, Heratizadeh A. Malassezia sympodialis und atopische Dermatitis. Allergologie. (2014) 38:30–7. doi: 10.5414/ALX01702
28. Valenta R, Natter S, Seiberler S, Wichlas S, Maurer D, Hess M, et al. Molecular characterization of an autoallergen, Hom s 1, identified by serum IgE from atopic dermatitis patients. J Invest Dermatol. (1998) 111:1178–83. doi: 10.1046/j.1523-1747.1998.00413.x
29. Mossabeb R, Seiberler S, Mittermann I, Reininger R, Spitzauer S, Natter S, et al. Characterization of a novel isoform of alpha-nascent polypeptide-associated complex as IgE-defined autoantigen. J Invest Dermatol. (2002) 119:820–9. doi: 10.1046/j.1523-1747.2002.00518.x
30. Gueant JL, Mata E, Masson C, Gerard P, Moneret-Vautrin DA, Mouton-Faivre C, et al. Non-specific cross-reactivity of hydrophobic serum IgE to hydrophobic drugs. Mol Immunol. (1995) 32:259–66. doi: 10.1016/0161-5890(94)00152-Q
31. Santamaria Babi LF, Picker LJ, Perez Soler MT, Drzimalla K, Flohr P, Blaser K, et al. Circulating allergen-reactive T cells from patients with atopic dermatitis and allergic contact dermatitis express the skin-selective homing receptor, the cutaneous lymphocyte-associated antigen. J Exp Med. (1995) 181:1935–40. doi: 10.1084/jem.181.5.1935
32. Werfel T, Morita A, Grewe M, Renz H, Wahn U, Krutmann J, et al. Allergen specificity of skin-infiltrating T cells is not restricted to a type-2 cytokine pattern in chronic skin lesions of atopic dermatitis. J Invest Dermatol. (1996) 107:871–6. doi: 10.1111/1523-1747.ep12331164
33. Hennino A, Vocanson M, Toussaint Y, Rodet K, Benetiere J, Schmitt AM, et al. Skin-infiltrating CD8+ T cells initiate atopic dermatitis lesions. J Immunol. (2007) 178:5571–7. doi: 10.4049/jimmunol.178.9.5571
34. Hennino A, Jean-Decoster C, Giordano-Labadie F, Debeer S, Vanbervliet B, Rozieres A, et al. CD8+ T cells are recruited early to allergen exposure sites in atopy patch test reactions in human atopic dermatitis. J Allergy Clin Immunol. (2011) 127:1064–7. doi: 10.1016/j.jaci.2010.11.022
35. Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. (2016) 138:336–49. doi: 10.1016/j.jaci.2016.06.010
36. Hashem N, Hirschhorn K, Sedlis E, Holt LE Jr. Infantile eczema. Evidence of autoimmunity to human skin. Lancet. (1963) 282. 269–70. doi: 10.1016/S0140-6736(63)90171-5
37. Fluckiger S, Scapozza L, Mayer C, Blaser K, Folkers G, Crameri R. Immunological and structural analysis of IgE-mediated cross-reactivity between manganese superoxide dismutases. Int Arch Allergy Immunol. (2002) 128:292–303. doi: 10.1159/000063862
38. Schwienbacher M, Israel L, Heesemann J, Ebel F. Asp f6, an Aspergillus allergen specifically recognized by IgE from patients with allergic bronchopulmonary aspergillosis, is differentially expressed during germination. Allergy. (2005) 60:1430–5. doi: 10.1111/j.1398-9995.2005.00904.x
39. Gabriel MF, González-Delgado P, Postigo I, Fernández J, Soriano V, Cueva B, et al. From respiratory sensitization to food allergy: anaphylactic reaction after ingestion of mushrooms (Agaricus bisporus). Med Mycol Case Rep. (2015) 8:14–16. doi: 10.1016/j.mmcr.2015.02.003
40. Balaji H, Heratizadeh A, Wichmann K, Niebuhr M, Crameri R, Scheynius A, et al. Malassezia sympodialis thioredoxin-specific T cells are highly cross-reactive to human thioredoxin in atopic dermatitis. J Allergy Clin Immunol. (2011) 128:92–9.e4. doi: 10.1016/j.jaci.2011.02.043
41. Hradetzky S, Roesner LM, Heratizadeh A, Crameri R, Garbani M, Scheynius A, et al. Differential cytokine induction by the human skin-associated autoallergen thioredoxin in sensitized patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. (2015) 135:1378–80.e1–5. doi: 10.1016/j.jaci.2014.10.038
42. Sahaf B, Rosen A. Secretion of 10-kDa and 12-kDa thioredoxin species from blood monocytes and transformed leukocytes. Antioxid Redox Signal. (2000) 2:717–26. doi: 10.1089/ars.2000.2.4-717
43. Mittermann I, Reininger R, Zimmermann M, Gangl K, Reisinger J, Aichberger KJ, et al. The IgE-reactive autoantigen Hom s 2 induces damage of respiratory epithelial cells and keratinocytes via induction of IFN-gamma. J Invest Dermatol. (2008) 128:1451–9. doi: 10.1038/sj.jid.5701195
44. Hradetzky S, Balaji H, Roesner LM, Heratizadeh A, Mittermann I, Valenta R, et al. The Human skin-associated autoantigen alpha-NAC activates monocytes and dendritic cells via TLR-2 and primes an IL-12-dependent Th1 response. J Invest Dermatol. (2013) 133:2289–92. doi: 10.1038/jid.2013.161
45. Heratizadeh A, Mittermann I, Balaji H, Wichmann K, Niebuhr M, Valenta R, et al. The role of T-cell reactivity towards the autoantigen alpha-NAC in atopic dermatitis. Br J Dermatol. (2011) 164:316–24. doi: 10.1111/j.1365-2133.2010.10090.x
46. Kerschenlohr K, Darsow U, Burgdorf WH, Ring J, Wollenberg A. Lessons from atopy patch testing in atopic dermatitis. Curr Allergy Asthma Rep. (2004) 4:285–9. doi: 10.1007/s11882-004-0072-7
47. Schnuch A, Mahler V, Elsner P, John SM, Reusch M, Merk H, et al. Allergiediagnostik mit dem Epikutantest droht das Aus. Hautarzt. (2012) 63:250–2. doi: 10.1007/s00105-012-2345-8
48. Bayerl C. Totgeglaubte leben länger – der Epikutantest. Akt Dermatol. (2017) 43:6. doi: 10.1055/s-0043-101322
49. Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. (1999) 50:213–9. doi: 10.1007/s002510050595
50. Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. (2006) 24:817–9. doi: 10.1038/nbt1215
51. Roesner LM, Heratizadeh A, Wieschowski S, Mittermann I, Valenta R, Eiz-Vesper B, et al. alpha-NAC-specific autoreactive CD8+ T cells in atopic dermatitis are of an effector memory type and secrete IL-4 and IFN-gamma. J Immunol. (2016) 196:3245–52. doi: 10.4049/jimmunol.1500351
52. Roesner LM, Wieschowski S, Vauth M, Valenta R, Crameri R, Werfel T. Antigene bei atopischer dermatitis weisen auf Ebene der immundominanten epitope homologien zu mikrobiellen Antigenen auf. Allergo J Int. (2016) 25:69–70. doi: 10.1007/s40629-016-0130-4
53. Dornmair K, Goebels N, Weltzien HU, Wekerle H, Hohlfeld R. T-cell-mediated autoimmunity: novel techniques to characterize autoreactive T-cell receptors. Am J Pathol. (2003) 163:1215–2611. doi: 10.1016/S0002-9440(10)63481-5
54. Sewell AK. Why must T cells be cross-reactive? Nat Rev Immunol. (2012) 12:669–77. doi: 10.1038/nri3279
55. Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. (1999) 286:958–61. doi: 10.1126/science.286.5441.958
56. Laydon DJ, Bangham CR, Asquith B. Estimating T-cell repertoire diversity: limitations of classical estimators and a new approach. Philos Trans R Soc Lond B Biol Sci. (2015) 370:20140291. doi: 10.1098/rstb.2014.0291
57. Lythe G, Callard RE, Hoare RL, Molina-Paris C. How many TCR clonotypes does a body maintain? J Theor Biol. (2016) 389:214–24. doi: 10.1016/j.jtbi.2015.10.016
58. Wooldridge L, Ekeruche-Makinde J, Van Den Berg HA, Skowera A, Miles JJ, Tan MP, et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem. (2012) 287:1168–77. doi: 10.1074/jbc.M111.289488
59. Ekeruche-Makinde J, Miles JJ, Van Den Berg HA, Skowera A, Cole DK, Dolton G, et al. Peptide length determines the outcome of TCR/peptide-MHCI engagement. Blood. (2013) 121:1112–23. doi: 10.1182/blood-2012-06-437202
60. Bassani-Sternberg M, Pletscher-Frankild S, Jensen LJ, Mann M. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol Cell Proteomics. (2015) 14:658–73. doi: 10.1074/mcp.M114.042812
61. Caron E, Espona L, Kowalewski DJ, Schuster H, Ternette N, Alpizar A, et al. An open-source computational and data resource to analyze digital maps of immunopeptidomes. Elife. (2015) 4. doi: 10.7554/eLife.07661
62. Mommen GP, Marino F, Meiring HD, Poelen MC, Van Gaans-Van Den Brink JA, Mohammed S, et al. Sampling from the proteome to the human leukocyte antigen-DR (HLA-DR) ligandome proceeds via high specificity. Mol Cell Proteomics. (2016) 15:1412–23. doi: 10.1074/mcp.M115.055780
63. Pearson H, Daouda T, Granados DP, Durette C, Bonneil E, Courcelles M, et al. MHC class I-associated peptides derive from selective regions of the human genome. J Clin Invest. (2016) 126:4690–701. doi: 10.1172/JCI88590
64. Bassani-Sternberg M, Braunlein E, Klar R, Engleitner T, Sinitcyn P, Audehm S, et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun. (2016) 7:13404. doi: 10.1038/ncomms13404
65. Wang Q, Drouin EE, Yao C, Zhang J, Huang Y, Leon DR, et al. Immunogenic HLA-DR-presented self-peptides identified directly from clinical samples of synovial tissue, synovial fluid, or peripheral blood in patients with rheumatoid arthritis or lyme arthritis. J Proteome Res. (2017) 16:122–36. doi: 10.1021/acs.jproteome.6b00386
66. Shao W, Pedrioli PGA, Wolski W, Scurtescu C, Schmid E, Vizcaino JA, et al. The SysteMHC Atlas project. Nucleic Acids Res. (2018) 46:D1237–47. doi: 10.1093/nar/gkx664
67. Schellens IM, Hoof I, Meiring HD, Spijkers SN, Poelen MC, Van Gaans-Van Den Brink JA, et al. Comprehensive analysis of the naturally processed peptide repertoire: differences between HLA-A and B in the immunopeptidome. PLoS ONE. (2015) 10:e0136417. doi: 10.1371/journal.pone.0136417
68. Wooldridge L, Lissina A, Cole DK, Van Den Berg HA, Price DA, Sewell AK. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. (2009) 126:147–64. doi: 10.1111/j.1365-2567.2008.02848.x
69. De Visser KE, Cordaro TA, Kioussis D, Haanen JB, Schumacher TN, Kruisbeek AM. Tracing and characterization of the low-avidity self-specific T cell repertoire. Eur J Immunol. (2000) 30:1458–68. doi: 10.1002/(SICI)1521-4141(200005)30:5<1458::AID-IMMU1458>3.0.CO;2-2
70. Enouz S, Carrie L, Merkler D, Bevan MJ, Zehn D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J Exp Med. (2012) 209:1769–79. doi: 10.1084/jem.20120905
71. Szomolay B, Liu J, Brown PE, Miles JJ, Clement M, Llewellyn-Lacey S, et al. Identification of human viral protein-derived ligands recognized by individual MHCI-restricted T-cell receptors. Immunol Cell Biol. (2016) 94:573–82. doi: 10.1038/icb.2016.12
72. Wang HH, Li YC, Huang YC. Efficacy of omalizumab in patients with atopic dermatitis: a systematic review and meta-analysis. J Allergy Clin Immunol. (2016) 138:1719–22.e1. doi: 10.1016/j.jaci.2016.05.038
Keywords: autoimmunity, autoallergy, atopic dermatitis (AD), skin, allergy, T cell, IgE, autoreactivity
Citation: Roesner LM and Werfel T (2019) Autoimmunity (or Not) in Atopic Dermatitis. Front. Immunol. 10:2128. doi: 10.3389/fimmu.2019.02128
Received: 17 April 2019; Accepted: 23 August 2019;
Published: 10 September 2019.
Edited by:
Ralf J. Ludwig, Universität zu Lübeck, GermanyReviewed by:
Jarmila Celakovska, Charles University, CzechiaCharlotte Menné Bonefeld, University of Copenhagen, Denmark
Copyright © 2019 Roesner and Werfel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lennart M. Roesner, cm9lc25lci5sZW5uYXJ0QG1oLWhhbm5vdmVyLmRl
 Lennart M. Roesner
Lennart M. Roesner Thomas Werfel
Thomas Werfel