- Department of Dermatology, Ghent University Hospital, Ghent, Belgium
The impressive potential of biologics has been demonstrated in psoriasis, hidradenitis suppurativa, and urticaria. Numerous biologicals are entering the field for a restricted number of skin disorders. Off-label use of biologics in other recalcitrant skin diseases has increased. Mounting data point to the potential of already existing biologics acting on the IL-17/IL-23 pathway in skin disorders with epidermal hyperkeratosis (e.g., pityriasis rubra pilaris), acneiform inflammation (e.g., hidradenitis suppurativa), and loss of mucosal integrity (e.g., aphthosis). TNF-α blockers are also effective in the latter conditions but seem of particular value in granulomatous (e.g., granuloma annulare) and neutrophilic disorders (e.g., pyoderma gangrenosum). Failure of IL-17 blockade in skin diseases resulting from immune-mediated cell destruction (e.g., alopecia areata and vitiligo) illustrates its limited involvement in Th1-dependent skin immunology. Overall, disappointing results of TNF-α blockers in alopecia areata and vitiligo point to the same conclusion although promising results in toxic epidermal necrolysis suggest TNF-α exerts at least some in vivo Th1-related activities. Acting on both the Th1 and Th17 pathway, ustekinumab has a rather broad potential with interesting results in lupus and alopecia areata. The efficacy of omalizumab in bullous pemphigoid has revealed an IgE-mediated recruitment of eosinophils leading to bullae formation. Reconsidering reimbursement criteria for less common but severe diseases seems appropriate if substantial evidence is available (e.g., pityriasis rubra pilaris). For other disorders, investigator- and industry-initiated randomized clinical trials should be stimulated. They are likely to improve patient outcome and advance our understanding of challenging skin disorders.
Introduction
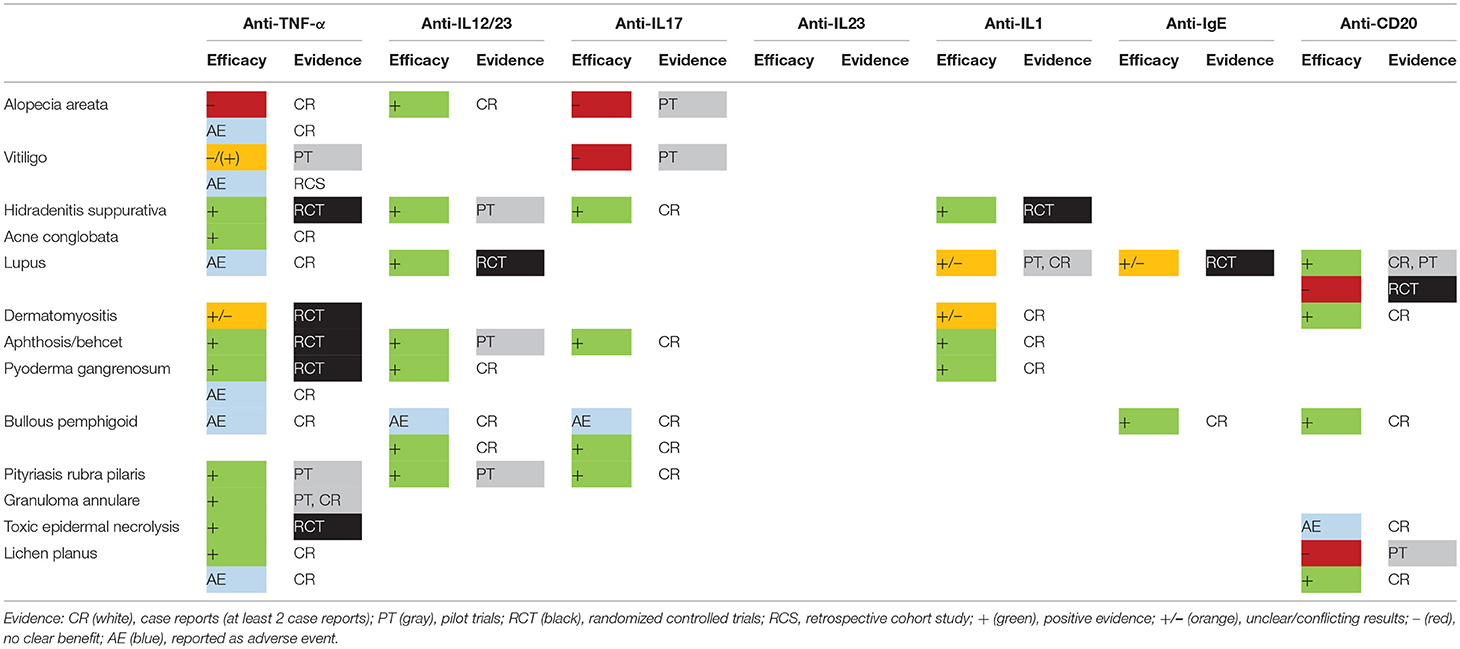
A large number of dermatological disorders is mediated by a deregulated skin immune response. In the last 2 decades, biologics have revolutionized the traditional approach by achieving unprecedented results with limited adverse events. Unfortunately, their use is restricted to a very small number of diseases (psoriasis, urticaria, and hidradenitis suppurativa). The promise of a targeted approach triggers clinicians to test these drugs in recalcitrant disorders despite their off-label status. More than 100 articles have been published on the off-label use of biologics. This is in obvious contrast with the limited number of prospective randomized trials that have been carried out. In this review we summarize the evidence of the currently available biologics targeting IL-1, IL-12/IL-23, IL-17, IL-23, TNF-α, CD20, and IgE in different “off-label” skin conditions (Supplementary Table S1).
Disorders Based on Targeted Immune-Based Cell Destruction: Alopecia Areata and Vitiligo
Given the role of TNF-α in the Th1 response, it made sense to test TNF-α inhibitors in alopecia areata and vitiligo. Nonetheless, adalimumab fails to induce hair regrowth in most alopecia areata patients (1). Several cases have been published mentioning de novo development of alopecia areata due to TNF-α blockers (2, 3). However, some successful cases have also been reported (4). The risk of vitiligo is significantly increased in patients receiving this class of biologics [hazard ratio: 1.99 (95% confidence interval: 1.06–3.75)] (5). Overall, repigmentation using TNF-α blockers is limited in vitiligo and this approach was considered to be ineffective (6). Nonetheless, TNF-α inhibitors can be useful to halt disease progression in active vitiligo (7). No data on the combination of TNF-α inhibitors and phototherapy are available.
Increased IL-17 levels and Th17 lymphocytes have been observed in the skin and blood of patients with alopecia areata and vitiligo (8, 9). Unfortunately, most alopecia areata patients did not show any response to secukinumab, an anti-IL17 monoclonal antibody (10). The same result was observed in our trial using secukinumab in progressive vitiligo which showed that 7/8 patients developed new skin depigmentations leading to an early halt of further recruitment. In subsequent experiments, we showed that Th17.1 cells rather than Th17 cells are increased in active vitiligo (11). Th17.1 lymphocytes are a subgroup of Th17 cells gradually differentiating into non-classical Th1 cells. IL-12 and IL-23 (in combination with low TGF-β) are the main cytokines driving Th17 > Th1 polarization. In that regard, ustekinumab, a combined anti-IL12/23 monoclonal antibody, might represent an attractive treatment option. Despite some reports mentioning new onset vitiligo and alopecia areata during ustekinumab, cases showing repigmentation have been published (12). In alopecia areata, some impressive cases of hair regrowth following the initiation of ustekinumab illustrate the interesting potential of this biologic (13, 14). Increased serum levels of IL-23 have been found in vitiligo although no clinical data are available for IL-23 blockers (15).
Acneiform Disorders With Neutrophils: Hidradenitis Suppurativa and Acne Conglobata
The introduction of TNF-α blockers in the therapeutic arsenal of hidradenitis suppurativa has increased the scientific interest for this disorder. The improvement of abscesses and pustules with beneficial effects on the quality of life are clear although treatment failure is more common compared to psoriasis (16).
IL-17 is considered to be an important factor in the detrimental inflammatory responses in HS. Case reports and a small pilot trial mention clear improvement in HS patients receiving secukinumab (17, 18). In contrast, an ex vivo study on lesional skin samples showed a more pronounced decrease in pro-inflammatory cytokine production and antimicrobial peptides with TNF-α inhibitors compared to IL-17 inhibition (19). Treatment with ustekinumab leads to moderate-marked responses in 82% of HS patients (20). In a retrospective analysis, guselkumab showed improvement in 8/11 (73%) of HS patients (21, 22).
In a small randomized controlled trial (RCT) (10:10), anakinra—a recombinant IL-1 receptor antagonist—displayed promising results with a 67% decreased activity score compared to 20% in the placebo arm (23). Nonetheless, some HS patients fail to improve with both anakinra and canakinumab (= monoclonal antibody against IL-1β) (24, 25). Up till now, large RCTs are missing.
Acne conglobata can be a disfiguring disease with limited treatment options in case of failure of systemic retinoids. Sand and Thomsen reported benefit from TNF-α inhibitors in 64% (7/11) of patients with severe refractory acne conglobata (26). Propionibacterium acnes stimulates keratinocytes to produce IL-1α and TNF-α. Additionally, peripheral blood mononuclear cells (PBMCs) of acne patients stimulated with P. acnes produce increased amounts of TNF-α and IL-8 pointing to the central role of TNF-α in this condition (27). P. acnes also promotes Th17 and Th17.1 responses (28) although no data have been published on the efficacy of IL-17 blockers or ustekinumab.
Systemic Disorders: Lupus and Dermatomyositis
A phase II RCT of patients with active systemic lupus erythematosus (SLE) demonstrated that the addition of ustekinumab to standard care resulted in an improved efficacy (29). After 6 months, a Systemic Lupus Erythematosus Responder Index (SRI)-4 response was achieved in 62% of ustekinumab treated patients compared to 33% in the placebo group. Although some patients have been reported to exhibit lupus-like cutaneous disorders following ustekinumab, several successfully treated cases of subacute and discoid cutaneous lupus can be found in literature with ustekinumab (30–32). Regarding TNF-α blockers, lupus is a well-known adverse event which can display diverse presentation patterns (33).
Rituximab showed promising results in open-label studies but failed to reach the primary endpoints in 2 RCTs (34, 35). However, some confounding factors such as concomitant immunosuppressive medication and problems in study design (e.g., outcome measures with limited sensitivity) might have played a role. Nonetheless, cutaneous lesions of SLE showed beneficial results in a retrospective study. At 6 months, 76% of patients (n = 50) showed improvement in mucocutaneous lesions with 40% complete responses. The response to rituximab in subacute cutaneous lupus erythematosus and chronic cutaneous lupus erythematosus seems more variable (36).
Omalizumab was tested in a small RCT (n = 15) which enrolled SLE patients with increased levels of IgE anti-dsDNA, anti-Sm or anti-SSA autoantibodies. A significant improvement in Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2k) scores was found at 16 weeks although the difference was lower than the clinically minimal important change (37).
A similar pattern emerges with anti-TNF-α antibodies in dermatomyositis which can develop during therapy. Nonetheless, an RCT demonstrated that infliximab can be of value in a subset of patients despite failure to reach the endpoints after 16 weeks (38).
In a retrospective series of patients with refractory idiopathic inflammatory myopathies (including antisynthetase syndrome, dermatomyositis and polymyositis) rituximab resulted in a significantly decreased dependency on glucocorticoids and favorable clinical responses (39).
Abnormal IL-1 receptor antagonist production has been observed in dermatomyositis and polymyositis. 4/15 (36.4%) patients with dermatomyositis or polymyositis improved with anakinra (40).
Aphthosis (and Behcet's Syndrome)
Anti-TNF-α inhibitors were useful in resistant cases of recurrent aphthous stomatitis. Adalimumab, etanercept and infliximab have all shown good efficacy. Complete responses have been documented in approximately two-thirds of patients treated with adalimumab (41). Adalimumab is also effective to reduce other manifestations of Behcet's syndrome including the resolution of venous thrombosis (42).
Secukinumab showed benefit in five patients with Behcet's syndrome (and concomitant ankylosing spondylitis or psoriatic arthritis) refractory to conventional treatment and anti-TNF-α therapy. Improvement of active mucocutaneous manifestations was evident. Especially the rapid resolution of oral ulcerations was remarkable (43). Different studies have confirmed the involvement of IL-17 in the pathogenesis. Ustekinumab was also effective in a small prospective study of 14 patients. After 12 weeks, complete response was seen in 64%, partial response in 21% with 14% non-responders (44).
IL-1 antagonism (Anakinra) is mainly tested on uveitis in Behcet's syndrome although a small pilot study has investigated the effect on the mucocutaneous complaints. In five of six patients the severity and number of ulcers improved with two cases of complete response (45).
Neutrophilic Disorders: Pyoderma Gangrenosum (PG)
Infliximab is the only biologic with an RCT for classic PG showing improvement in 20/29 patients (46). Etanercept and adalimumab have variable efficacy in small studies. Paradoxical new onset PG has also been linked to TNF-α inhibitors. Beneficial results have been ascribed to ustekinumab in case reports (47). This is in line with the role of the IL-17/23 pathway in neutrophil migration (48). A retrospective study found complete responses for infliximab in 63.6% (n = 33), for adalimumab in 57.1% (n = 28), for etanercept in 71.4% (n = 7) and for ustekinumab in 66.6% (n = 9) of patients. These results were all superior to corticosteroids (48.8%; n = 78) (49). Anakinra and canakinumab lead to variable outcomes (50, 51).
Occurrence of Sweet syndrome is possible during anti-TNF-α treatment although it can also be effective in pre-existing Sweet syndrome (52, 53). Some isolated cases responded to IL-1 inhibition (anakinra) (54, 55). Neutrophilic dermatoses associated with auto-inflammatory disorders (e.g., Schnitzler syndrome, Muckle-Wells syndrome) respond well to IL-1 inhibitors (56).
Bullous Disorders: Bullous Pemphigoid (BP) and Other Bullous Diseases
Remarkable improvements have been seen for omalizumab in BP. A systematic review found 22 reported cases with 84% complete responses. Anti-BP180 IgE antibodies correlate with disease activity in BP supporting their pathogenic relevance (57). IgE autoantibodies against BP180 and BP230 were found in, respectively 21/36 (58.3%) and 18/36 (50%) BP sera (58). The favorable safety profile of omalizumab makes it an attractive treatment option for BP (59).
Rituximab (anti-CD20) also led to 85% complete responses in BP (in 62 patients). Rituximab was associated with lower recurrence rates and a longer disease-free period compared to omalizumab (60). Patients that first did not respond to omalizumab but improved using rituximab (61), but also cases with improvement on omalizumab after failure to rituximab have been published.
Development of BP using anti-TNF-α treatment can occur sporadically although this link remains controversial. Both for ustekinumab and secukinumab cases of successful treatment of BP and new onset BP during treatment have been described (62–65). IL-17 production by innate immune cells, especially neutrophils, is characteristic in lesional BP skin. IL-17 upregulates matrix-metalloproteinase-9 and neutrophil elastase expression which are involved in blister formation (66). Elevated serum concentrations of IL-23 were confirmed in BP patients and rising levels were associated with disease relapse. This pathway could therefore be potentially interesting in BP (66).
Besides pemphigus, rituximab has been administered successfully in other autoimmune bullous diseases. In a retrospective cohort, mucous membrane pemphigoid (n = 14) exhibited disease control in 85.7% (partial response: 64.3%, complete response: 28.6%) (67). Similarly, some encouraging data have been obtained in ocular cicatricial pemphigoid (68). A meta-analysis showed that rituximab is also a promising option in epidermolysis bullosa acquisita (69).
Psoriasiform Disorders: Pityriasis Rubra Pilaris
Off-label use of biologics has been extensively performed in PRP given its high clinical and pathogenic similarity with psoriasis. Anti-TNF-α, anti-IL-17 and anti-IL-12/23 treatments are all effective with marked to complete responses ranging around 50–78%. Partial responses were seen in 11–25% of patients and lack of response in 11–25% (70). Infliximab exhibited superior results compared to adalimumab while ustekinumab is currently most widely used for this disorder. Recent case series on IL-17 inhibition (secukinumab and ixekizumab) show a fast clearance and limited risk of recurrence after therapy withdrawal (71, 72). Nonetheless, treatment response is difficult to predict due to the heterogeneity of the disease (73). Both cases with progression using IL-17 inhibition that subsequently improved using ustekinumab (74) as a patient with limited response to ustekinumab who benefited from secukinumab, have been published (73).
Granulomatous Disorders: Granuloma Annulare
The role of TNF-α producing macrophages in granuloma formation points to a key role of this cytokine in granuloma annulare. A summary of published cases showed that 20/26 patients with granuloma annulare responded to anti-TNF-α treatment (75). Overall, these data support a high efficacy of TNF-α inhibitors in granuloma annulare although well-designed RCTs are missing. Nonetheless, granuloma annulare has also been reported as a side effect of biologics including adalimumab, infliximab and etanercept but also during treatment with secukinumab (76–78).
Mastocytosis
Given its excellent efficacy in urticaria, the use of omalizumab in other mast cell mediated diseases such as mastocytosis looks promising. Most published cases report excellent efficacy on systemic symptoms (e.g., gastrointestinal problems, pruritus). Some interesting patients with resolution of cutaneous lesions have been observed (79, 80). A multicenter RCT with 7 patients receiving omalizumab and 9 placebo was conducted. After 6 months, improvement in French Association for the Initiatives of Research on Mastocyte and Mastocytosis (AFIRMM) score was higher in the omalizumab group (52–26 vs. 104–102) although significance was not reached (81).
Toxic Epidermal Necrolysis
Being one of the most life-threatening skin disorders, treatment of toxic epidermal necrolysis is extremely important. TNF-α has been identified in the blister fluid and serum of TEN patients (82). Most evidence is gathered from case series and case reports. Nonetheless, the majority of cases show excellent responses with improved outcomes compared to the expected mortality (83). An interesting case series of 10 patients with excellent outcome using etanercept was published (84). Adequate screening for infections is necessary given the high prevalence of soft tissue infections (S. aureus, P. aeruginosa, K. pneumoniae) (83). An RCT comparing etanercept (n = 48) with corticosteroids (n = 43) showed a lower mortality rate after etanercept (8.3%) compared to predicted outcome (17.7%). The difference with the corticosteroid group (16.3%) was not significant although the time for complete skin healing was lower (85). Nonetheless, more than 50 cases have been described developing TEN despite concurrent treatment with TNF-α blockers.
Lichen Planus
Lichenoid drug eruptions are a well-known side effect of TNF-α blockade (86). Cutaneous and oral lichen planus, lichen planopilaris and lichen striatus have all developed in patients receiving anti-TNF- α treatment. Nonetheless, in some cases of extensive lichen planus adalimumab showed improvement and isolated cases of oral lichen planus and nail lichen planus improved using etanercept (87–89). Despite some promising reports, rituximab failed to show efficacy in a small trial with five consecutive patients with erosive lichen planus (90). Data on other biologics are very limited.
Discussion
Several promising results have been obtained by the off-label use of biologics and by pilot trials (Table 1, Figure 1) although disappointing results can be equally important to clarify the underlying pathogenic pathways. Failure of both TNF-α inhibitors and IL-17A-blockers in alopecia areata and vitiligo limits the involvement of these cytokines in the immune-mediated destruction of skin cells (10, 11). However, the apparent efficacy of TNF-α inhibitors in toxic epidermal necrolysis and occasional cases of alopecia areata and vitiligo suggest that the role of TNF-α is more complex (4, 7, 91). Alopecia areata and vitiligo remain biologic orphan diseases although some exciting cases have been published showing hair regrowth after initiation of ustekinumab (13, 14).
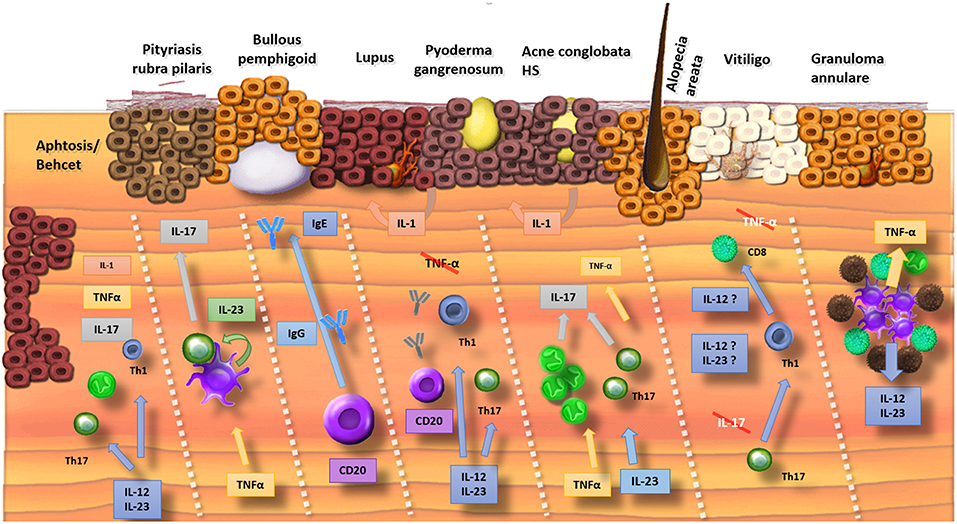
Figure 1. Summary of the involved cytokines in cutaneous disorders (Crossed interleukins: trials were conducted but failed). Mucosal ulceration involves a wide range of cytokines and therefore biologics inhibiting different pathways (IL-1, TNF-α, and IL-17/23) result in improvement. Pityriasis rubra pilaris has a psoriasis-like pathophysiology with a TNF-α stimulated production of IL-23 by dendritic cells. This results in the release of IL-17. Bullous pemphigoid is an antibody-mediated disorder where both inhibition of B lymphocytes (e.g., rituximab) as binding of IgE-antibodies with omalizumab is efficacious. The pathophysiology of lupus is complex with a possible driving role for IL-12 and IL-23. Mixed results for inhibition of B-lymphocytes with rituximab were obtained. TNF-α inhibitors are associated with drug-induced lupus. In neutrophilic pustular disorders (pyoderma gangrenosum, acne, hidradenitis), the IL-17 pathway seems crucial with beneficial results if cytokines of this pathway are targeted (IL-17, IL-23, and TNF-α). In alopecia areata and vitiligo, antibodies against TNF-α and IL-17 were disappointing. Some evidence exists for a role of ustekinumab (IL-12/23) in alopecia areata. TNF-α and IL-12/23 can be targeted in granuloma annulare.
Promising early data concerning the blockage of the IL-23/IL17 pathway in HS confirm that this pathway is an important initiator of a suppurative neutrophilic inflammation (19, 20, 22, 92). The benefit of blocking IL-17 in aphthosis is in agreement with its defensive capacities against mucosal invasion of pathogens and its protective actions to maintain epithelial barrier integrity.
The results of the phase II trial of ustekinumab in lupus could be a major breakthrough although phase III trials have to be awaited. Increased IL-12 and IL-23 levels have been reported in SLE patients and genetic research links the IL-12 pathway with an increased susceptibility for SLE (29). In general, interferon is considered the main driving factor in lupus. In contrast, the efficacy of ustekinumab was independent of interferon levels (29, 31, 32).
One of the most remarkable findings was undoubtedly the impressive resolution of bullae in BP following the administration of anti-IgE antibodies (59, 59, 60, 93, 94). This confirms the pathogenic role of anti-IgE antibodies which seem actively involved in the development of bullae. Mice injected with IgE autoantibodies from BP patients in grafted human skin developed erythema and infiltration of eosinophils in the skin and ultimately histological dermal-epidermal separation (95). Further studies on omalizumab in bullous pemphigoid seem of particular value given its efficacy and improved safety profile compared to the alternative options.
While current biologics cover mainly the Th17 pathway, strong inhibition of important Th1 cytokines such as IFN-γ is not (yet) available leading to disappointing results in vitiligo and alopecia areata. Dupilumab, a monoclonal antibody directed against IL-4 and IL-13, developed for atopic dermatitis interferes with key cytokines in the Th2 pathway and offers new possibilities for off-label use. Case reports of good response to different types of chronic itch including prurigo nodularis, uremic pruritus and genital pruritus illustrate the interesting potential of dupilumab (96–99). New development of alopecia areata has been observed during treatment with dupilumab (100–102). Nonetheless, patients with both atopic dermatitis and alopecia areata treated with dupilumab have repeatedly demonstrated hair regrowth (103–105). Furthermore, some isolated cases were published with beneficial results in bullous pemphigoid (106), eosinophilic annular erythema (107), and papuloerythroderma of Ofuji (108).
Biologics have been linked to the occasional development of alopecia areata, vitiligo, lupus, dermatomyositis and bullous diseases. However, as we have learned from TNF-α antagonists in psoriasis, this finding does not exclude their efficacy. Increased serum or lesional levels of specific interleukins do not ensure the efficacy of the corresponding biologic. As seen in alopecia areata and vitiligo, numerous studies documented increased TNF-α and IL-17 levels while the inhibition of both cytokines failed in these disorders (10, 11). Unfortunately, the lack of convincing mouse models for most dermatologic disorders limits the evidence that can be obtained before conducting a clinical trial.
Getting biologics approved and reimbursed for these new indications is another challenge. Weighing economic implications vs. health improvement will remain a difficult balance in the next decades. The rise of biosimilars may improve access to off-label use in disorders which are unlikely to be of economic interest due to their rare incidence or limited patients with severe disease requiring biologics (109). An increased appreciation and funding of investigator-initiated clinical trials seem of particular relevance to explore the full capacity of biologics.
Author Contributions
RS reviewed the literature, drafted the manuscript, and made the figures. JL and NvG critically revised the manuscript.
Funding
The research activities of RS and NvG are supported by the Scientific Research Foundation-Flanders (Krediet aan Navorsers: 1504718N and FWO Senior Clinical Investigator: 1831512N, respectively).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01918/full#supplementary-material
References
1. Bolduc C, Bissonnette R. Safety and efficacy of adalimumab for the treatment of severe alopecia areata: case series of three patients. J Cutan Med Surg. (2012) 16:257–60. doi: 10.1177/120347541201600407
2. Melé-Ninot G, Expósito-Serrano V, Quintana Codina M, Iglesias Sancho M, Sánchez-Regaña M, Umbert Millet P, et al. Adalimumab-related alopecia in a patient affected by psoriasis. Dermatol Online J. (2017) 23.
3. Ostojic P, Pavlov-Dolijanovic S. Alopecia universalis in a patient with rheumatoid arthritis developed during treatment with adalimumab. Z Rheumatol. (2018) 77:412–5. doi: 10.1007/s00393-018-0464-z
4. Gorcey L, Gordon Spratt EA, Leger MC. Alopecia universalis successfully treated with adalimumab. JAMA Dermatol. (2014) 150:1341–4. doi: 10.1001/jamadermatol.2014.1544
5. Bae JM, Kim M, Lee HH, Kim K-J, Shin H, Ju HJ, et al. Increased risk of vitiligo following anti-tumor necrosis factor therapy: a 10-year population-based cohort study. J Invest Dermatol. (2018) 138:768–74. doi: 10.1016/j.jid.2017.11.012
6. Alghamdi KM, Khurrum H, Taieb A, Ezzedine K. Treatment of generalized vitiligo with anti-TNF-α Agents. J Drugs Dermatol. (2012) 11:534–9.
7. Webb KC, Tung R, Winterfield LS, Gottlieb AB, Eby JM, Henning SW, et al. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol. (2015) 173:641–50. doi: 10.1111/bjd.14016
8. Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol. (2011) 36:292–7. doi: 10.1111/j.1365-2230.2010.03972.x
9. Atwa MA, Youssef N, Bayoumy NM. T-helper 17 cytokines (interleukins 17, 21, 22, and 6, and tumor necrosis factor-α) in patients with alopecia areata: association with clinical type and severity. Int J Dermatol. (2016) 55:666–72. doi: 10.1111/ijd.12808
10. Guttman-Yassky E, Nia JK, Hashim PW, Mansouri Y, Alia E, Taliercio M, et al. Efficacy and safety of secukinumab treatment in adults with extensive alopecia areata. Arch Dermatol Res. (2018) 310:607–14. doi: 10.1007/s00403-018-1853-5
11. Speeckaert R, Mylle S, van Geel N. IL-17A is not a treatment target in progressive vitiligo. Pigm Cell Mel Res. (2019). doi: 10.1111/pcmr.12789. [Epub ahead of print].
12. Elkady A, Bonomo L, Amir Y, Vekaria AS, Guttman-Yassky E. Effective use of ustekinumab in a patient with concomitant psoriasis, vitiligo, and alopecia areata. JAAD Case Rep. (2017) 3:477–9. doi: 10.1016/j.jdcr.2017.07.009
13. Guttman-Yassky E, Ungar B, Noda S, Suprun M, Shroff A, Dutt R, et al. Extensive alopecia areata is reversed by IL-12/IL-23p40 cytokine antagonism. J Allergy Clin Immunol. (2016) 137:301–4. doi: 10.1016/j.jaci.2015.11.001
14. Aleisa A, Lim Y, Gordon S, Her MJ, Zancanaro P, Abudu M, et al. Response to ustekinumab in three pediatric patients with alopecia areata. Pediatr Dermatol. (2018) 36:e44–5. doi: 10.1111/pde.13699
15. Vaccaro M, Cannavò SP, Imbesi S, Cristani M, Barbuzza O, Tigano V, et al. Increased serum levels of interleukin-23 circulating in patients with non-segmental generalized vitiligo. Int J Dermatol. (2015) 54:672–4. doi: 10.1111/ijd.12392
16. Kyriakou A, Trigoni A, Galanis N, Sotiriadis D, Patsatsi A. Efficacy of adalimumab in moderate to severe hidradenitis suppurativa: real life data. Dermatol Rep. (2018) 10:7859. doi: 10.4081/dr.2018.7859
17. Prussick L, Rothstein B, Joshipura D, Saraiya A, Turkowski Y, Abdat R, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. (2019). doi: 10.1111/bjd.17822. [Epub ahead of print].
18. Schuch A, Fischer T, Boehner A, Biedermann T, Volz T. Successful treatment of severe recalcitrant hidradenitis suppurativa with the interleukin-17A antibody secukinumab. Acta Derm Venereol. (2018) 98:151–2. doi: 10.2340/00015555-2794
19. Vossen ARJV, Ardon CB, van der Zee HH, Lubberts E, Prens EP. The anti-inflammatory potency of biologics targeting TNF-α, IL-17A, IL-12/23 and CD20 in hidradenitis suppurativa: an ex vivo study. Br J Dermatol. (2019) 181. doi: 10.1111/bjd.17641
20. Blok JL, Li K, Brodmerkel C, Horvátovich P, Jonkman MF, Horváth B. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol. (2016) 174:839–46. doi: 10.1111/bjd.14338
21. Casseres RG, Kahn JS, Her MJ, Rosmarin D. Guselkumab in the treatment of hidradenitis suppurativa: a retrospective chart review. J Am Acad Dermatol. (2018) 81:265–7. doi: 10.1016/j.jaad.2018.12.017
22. Kovacs M, Podda M. Guselkumab in the treatment of severe Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. (2018) 33:e140–1. doi: 10.1111/jdv.15368
23. Tzanetakou V, Kanni T, Giatrakou S, Katoulis A, Papadavid E, Netea MG, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. (2016) 152:52–9. doi: 10.1001/jamadermatol.2015.3903
24. Russo V, Alikhan A. Failure of anakinra in a case of severe hidradenitis suppurativa. J Drugs Dermatol. (2016) 15:772–4.
25. Tekin B, Salman A, Ergun T. Hidradenitis suppurativa unresponsive to canakinumab treatment: a case report. Indian J Dermatol Venereol Leprol. (2017) 83:615–7. doi: 10.4103/ijdvl.IJDVL_147_16
26. Sand FL, Thomsen SF. Off-label use of TNF-alpha inhibitors in a dermatological university department: retrospective evaluation of 118 patients. Dermatol Ther. (2015) 28:158–65. doi: 10.1111/dth.12222
27. Yiu ZZN, Madan V, Griffiths CEM. Acne conglobata and adalimumab: use of tumour necrosis factor-α antagonists in treatment-resistant acne conglobata, and review of the literature. Clin Exp Dermatol. (2015) 40:383–6. doi: 10.1111/ced.12540
28. Kistowska M, Meier B, Proust T, Feldmeyer L, Cozzio A, Kuendig T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. (2015) 135:110–8. doi: 10.1038/jid.2014.290
29. van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. (2018) 392:1330–9. doi: 10.1016/S0140-6736(18)32167-6
30. Winchester D, Duffin KC, Hansen C. Response to ustekinumab in a patient with both severe psoriasis and hypertrophic cutaneous lupus. Lupus. (2012) 21:1007–10. doi: 10.1177/0961203312441982
31. De Souza A, Ali-Shaw T, Strober BE, Franks AG. Successful treatment of subacute lupus erythematosus with ustekinumab. Arch Dermatol. (2011) 147:896–8. doi: 10.1001/archdermatol.2011.185
32. Dahl C, Johansen C, Kragballe K, Olesen AB. Ustekinumab in the treatment of refractory chronic cutaneous lupus erythematosus: a case report. Acta Derm Venereol. (2013) 93:368–9. doi: 10.2340/00015555-1467
33. Shovman O, Tamar S, Amital H, Watad A, Shoenfeld Y. Diverse patterns of anti-TNF-α-induced lupus: case series and review of the literature. Clin Rheumatol. (2018) 37:563–8. doi: 10.1007/s10067-017-3884-2
34. Scherlinger M, Carcaud C, Truchetet M-E, Barnetche T, Duffau P, Couzi L, et al. Rituximab in moderate to severe non-renal systemic lupus erythematosus: a reanalysis of the EXPLORER study. Ann Rheum Dis. (2019) 78:1007–10. doi: 10.1136/annrheumdis-2018-214833
35. Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. (2010) 62:222–33. doi: 10.1002/art.27233
36. Quelhas da Costa R, Aguirre-Alastuey ME, Isenberg DA, Saracino AM. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol. (2018) 154:1432–40. doi: 10.1001/jamadermatol.2018.3793
37. Hasni S, Gupta S, Davis M, Poncio E, Temesgen-Oyelakin Y, Joyal E, et al. Safety and tolerability of omalizumab, a randomized clinical trial of humanized anti-IgE monoclonal antibody in systemic lupus erythematosus? Arthritis Rheumatol. (2019) 71: 1135–40. doi: 10.1002/art.40828
38. Schiffenbauer A, Garg M, Castro C, Pokrovnichka A, Joe G, Shrader J, et al. A randomized, double-blind, placebo-controlled trial of infliximab in refractory polymyositis and dermatomyositis. Semin Arthritis Rheum. (2018) 47:858–64. doi: 10.1016/j.semarthrit.2017.10.010
39. de Souza FHC, Miossi R, de Moraes JCB, Bonfá E, Shinjo SK. Favorable rituximab response in patients with refractory idiopathic inflammatory myopathies. Adv Rheumatol. (2018) 58:31. doi: 10.1186/s42358-018-0030-z
40. Svensson J, Tjärnlund A, Askling J, Dastmalchi M, Hanna B, Bucher SM, et al. FRI0197 use of BIOLOGICS in PM and DM in Sweden - a national register study. Ann Rheum Dis. (2014) 73:453–4. doi: 10.1136/annrheumdis-2014-eular.4966
41. Ranganath SP, Pai A. Is optimal management of recurrent aphthous stomatitis possible? A reality check. J Clin Diagn Res. (2016) 10:ZE08–13. doi: 10.7860/JCDR/2016/19519.8643
42. Emmi G, Bettiol A. Adalimumab-based treatment versus disease-modifying antirheumatic drugs for venous thrombosis in Behçet's syndrome: a retrospective study of seventy patients with vascular involvement. Arthritis Rheumatol. (2018) 70:1500–7. doi: 10.1002/art.40531
43. Di Scala G, Bettiol A, Cojan RD, Finocchi M, Silvestri E, Emmi G. Efficacy of the anti-IL 17 secukinumab in refractory Behçet's syndrome: a preliminary study. J Autoimmun. (2019) 97:108–13. doi: 10.1016/j.jaut.2018.09.002
44. Mirouse A, Barete S, Monfort J-B, Resche-Rigon M, Bouyer A-S, Comarmond C, et al. Ustekinumab for Behçet's disease. J Autoimmun. (2017) 82:41–6. doi: 10.1016/j.jaut.2017.05.002
45. Grayson PC, Yazici Y, Merideth M, Sen HN, Davis M, Novakovich E, et al. Treatment of mucocutaneous manifestations in Behçet's disease with anakinra: a pilot open-label study. Arthritis Res Ther. (2017) 19:69. doi: 10.1186/s13075-017-1222-3
46. Brooklyn TN, Dunnill MGS, Shetty A, Bowden JJ, Williams JDL, Griffiths CEM, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. (2006) 55:505–9. doi: 10.1136/gut.2005.074815
47. de Risi-Pugliese T, Seksik P, Bouaziz J-D, Chasset F, Moguelet P, Gornet J-M, et al. Ustekinumab treatment for neutrophilic dermatoses associated with Crohn's disease: a multicenter retrospective study. J Am Acad Dermatol. (2019) 80:781–4. doi: 10.1016/j.jaad.2018.06.065
48. Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. (2018) 14:225–33. doi: 10.1080/1744666X.2018.1438269
49. Herberger K, Dissemond J, Brüggestrat S, Sorbe C, Augustin M. Biologics and immunoglobulins in the treatment of pyoderma gangrenosum - analysis of 52 patients. J Dtsch Dermatol Ges. (2019) 17:32–41. doi: 10.1111/ddg.13741
50. Sun NZ, Ro T, Jolly P, Sayed CJ. Non-response to Interleukin-1 Antagonist canakinumab in two patients with refractory pyoderma gangrenosum and hidradenitis suppurativa. J Clin Aesthet Dermatol. (2017) 10:36–8.
51. Beynon C, Chin MF, Hunasehally P, Bhagwandas K, Bevan M, Taylor M, et al. Successful treatment of autoimmune disease-associated pyoderma gangrenosum with the IL-1 receptor antagonist anakinra: a case series of 3 patients. J Clin Rheumatol. (2017) 23:181–3. doi: 10.1097/RHU.0000000000000511
52. Banse C, Sobocinski V, Savoye G, Avenel G, Vittecoq O. Occurrence of sweet syndrome under anti-TNF. Clin Rheumatol. (2015) 34:1993–4. doi: 10.1007/s10067-015-3054-3
53. Agarwal A, Barrow W, Selim MA, Nicholas MW. Refractory subcutaneous sweet syndrome treated with adalimumab. JAMA Dermatol. (2016) 152:842–4. doi: 10.1001/jamadermatol.2016.0503
54. Kluger N, Gil-Bistes D, Guillot B, Bessis D. Efficacy of anti-interleukin-1 receptor antagonist anakinra (Kineret®) in a case of refractory Sweet's syndrome. Dermatology (Basel). (2011) 222:123–7. doi: 10.1159/000326112
55. Delluc A, Limal N, Puéchal X, Francès C, Piette JC, Cacoub P. Efficacy of anakinra, an IL1 receptor antagonist, in refractory Sweet syndrome. Ann Rheum Dis. (2008) 67:278–9. doi: 10.1136/ard.2006.068254
56. Lipsker D, Lenormand C. Indications and modes of use for interleukin (IL)-1 antagonists in inflammatory dermatosis: a new therapeutic approach to immune-mediated inflammatory diseases. Ann Dermatol Venereol. (2012) 139:459–67. doi: 10.1016/j.annder.2012.03.012
57. van Beek N, Lüttmann N, Huebner F, Recke A, Karl I, Schulze FS, et al. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatol. (2017) 153:30–8. doi: 10.1001/jamadermatol.2016.3357
58. Hashimoto T, Ohzono A, Teye K, Numata S, Hiroyasu S, Tsuruta D, et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. Br J Dermatol. (2017) 177:141–51. doi: 10.1111/bjd.15114
59. James T, Salman S, Stevenson B, Bundell C, Kelly G, Nolan D, et al. IgE blockade in autoimmunity: omalizumab induced remission of bullous pemphigoid. Clin Immunol. (2018) 198:54–6. doi: 10.1016/j.clim.2018.12.015
60. Kremer N, Snast I, Cohen ES, Hodak E, Mimouni D, Lapidoth M, et al. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. Am J Clin Dermatol. (2018) 20:209–16. doi: 10.1007/s40257-018-0401-6
61. Bilgiç Temel A, Bassorgun CI, Akman-Karakaş A, Alpsoy E, Uzun S. Successful treatment of a bullous pemphigoid patient with rituximab who was refractory to corticosteroid and omalizumab treatments. Case Rep Dermatol. (2017) 9:38–44. doi: 10.1159/000452828
62. Loget J, Plée J, Antonicelli F, Bernard P. A successful treatment with ustekinumab in a case of relapsing bullous pemphigoid associated with psoriasis. J Eur Acad Dermatol Venereol. (2017) 31:e228–30. doi: 10.1111/jdv.14002
63. Le Guern A, Alkeraye S, Vermersch-Langlin A, Coupe P, Vonarx M. Bullous pemphigoid during ustekinumab therapy. JAAD Case Rep. (2015) 1:359–60. doi: 10.1016/j.jdcr.2015.07.014
64. Kamata M, Asano Y, Shida R, Maeda N, Yoshizaki A, Miyagaki T, et al. Secukinumab decreased circulating anti-BP180-NC16a autoantibodies in a patient with coexisting psoriasis vulgaris and bullous pemphigoid. J Dermatol. (2019) 46:e216–7. doi: 10.1111/1346-8138.14760
65. Ho P-H, Tsai T-F. Development of bullous pemphigoid during secukinumab treatment for psoriasis. J Dermatol. (2017) 44:e2201. doi: 10.1111/1346-8138.13909
66. Le Jan S, Plée J, Vallerand D, Dupont A, Delanez E, Durlach A, et al. Innate immune cell-produced IL-17 sustains inflammation in bullous pemphigoid. J Invest Dermatol. (2014) 134:2908–17. doi: 10.1038/jid.2014.263
67. Lamberts A, Euverman HI, Terra JB, Jonkman MF, Horváth B. Effectiveness and safety of rituximab in recalcitrant pemphigoid diseases. Front Immunol. (2018) 9:248. doi: 10.3389/fimmu.2018.00248
68. You C, Lamba N, Lasave AF, Ma L, Diaz MH, Foster CS. Rituximab in the treatment of ocular cicatricial pemphigoid: a retrospective cohort study. Graefes Arch Clin Exp Ophthalmol. (2017) 255:1221–8. doi: 10.1007/s00417-017-3603-3
69. Iwata H, Vorobyev A, Koga H, Recke A, Zillikens D, Prost-Squarcioni C, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. (2018) 13:153. doi: 10.1186/s13023-018-0896-1
70. Napolitano M, Abeni D, Didona B. Biologics for pityriasis rubra pilaris treatment: a review of the literature. J Am Acad Dermatol. (2018) 79:353–9 e11. doi: 10.1016/j.jaad.2018.03.036
71. Bonomo L, Levitt JO. Secukinumab emerges as a rapidly effective therapy for pityriasis rubra pilaris. Cutis. (2018) 101:367–9.
72. Heibel MD, Heibel HD. Successful treatment of type I pityriasis rubra pilaris with ixekizumab. JAAD Case Rep. (2018) 4:774–6. doi: 10.1016/j.jdcr.2018.05.006
73. Wain T, Choy B, Satchell AC, Woods JA, Frew JW. Secukinumab in pityriasis rubra pilaris: a case series demonstrating variable response and the need for minimal clinical datasets. JAAD Case Rep. (2018) 4:500–5. doi: 10.1016/j.jdcr.2018.02.007
74. Matsuda T, Yamazaki F, Ueda-Hayakawa I, Kambe N, Okamoto H. Case of pityriasis rubra pilaris progressed to generalized erythroderma following blockade of interleukin-17A, but improved after blockade of interleukin-12/23 p40. J Dermatol. (2019) 46:70–2. doi: 10.1111/1346-8138.14709
75. Chen A, Truong AK, Worswick S. The role of biologics in the treatment of chronic granuloma annulare. Int J Dermatol. (2019) 58:622–6. doi: 10.1111/ijd.14350
76. Ratnarathorn M, Raychaudhuri SP, Naguwa S. Disseminated granuloma annulare: a cutaneous adverse effect of anti-tnf agents. Indian J Dermatol. (2011) 56:752–4. doi: 10.4103/0019-5154.91847
77. Voulgari PV, Markatseli TE, Exarchou SA, Zioga A, Drosos AA. Granuloma annulare induced by anti-tumour necrosis factor therapy. Ann Rheum Dis. (2008) 67:567–70. doi: 10.1136/ard.2007.075663
78. Bonomo L, Ghoneim S, Levitt J. A case of granuloma annulare associated with secukinumab use. Case Rep Dermatol Med. (2017) 2017:5918708. doi: 10.1155/2017/5918708
79. Lieberoth S, Thomsen SF. Cutaneous and gastrointestinal symptoms in two patients with systemic mastocytosis successfully treated with omalizumab. Case Rep Med. (2015) 2015:903541. doi: 10.1155/2015/903541
80. Hinojosa T, Lewis DJ, Vangipuram R, Laraib Safeer BA, Mui UN, Haley C, et al. The efficacy of omalizumab in cutaneous mastocytosis: a case series. Dermatol Ther. (2019) 32:e12848. doi: 10.1111/dth.12848
81. Distler M, Maul T, Steiner U, Jandus P, Murer C, Graf N, et al. The effect of omalizumab in mastocytosis patients. Prospective double-blind, placebo-controlled multicentre study. J Allergy Clin Immunol. (2019) 143:Supplement (AB134). doi: 10.1016/j.jaci.2018.12.407
82. Wang F, Ye Y, Luo Z-Y, Gao Q, Luo D-Q, Zhang X. Diverse expression of TNF-α and CCL27 in serum and blister of Stevens–Johnson syndrome/toxic epidermal necrolysis. Clin Transl Allergy. (2018) 8:12. doi: 10.1186/s13601-018-0199-6
83. Woolridge KF, Boler PL, Lee BD. Tumor necrosis factor alpha inhibitors in the treatment of toxic epidermal necrolysis. Cutis. (2018) 101:E15–21.
84. Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. (2014) 71:278–83. doi: 10.1016/j.jaad.2014.04.044
85. Wang C-W, Yang L-Y, Chen C-B, Ho H-C, Hung S-I, Yang C-H, et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. (2018) 128:985–96. doi: 10.1172/JCI93349
86. Garcovich S, De Simone C, Genovese G, Berti E, Cugno M, Marzano AV. Paradoxical skin reactions to biologics in patients with rheumatologic disorders. Front Pharmacol. (2019) 10:282. doi: 10.3389/fphar.2019.00282
87. Holló P, Szakonyi J, Kiss D, Jokai H, Horváth A, Kárpáti S. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. (2012) 92:385–6. doi: 10.2340/00015555-1249
88. Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. (2007) 8:121. doi: 10.2165/00128071-200708020-00010
89. Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. (2009) 84:325–8.
90. Tétu P, Monfort J-B, Barbaud A, Francès C, Chasset F. Failure of rituximab in refractory erosive lichen planus. Br J Dermatol. (2018) 179:980–1. doi: 10.1111/bjd.16704
91. Woolridge KF, Boler PL, Lee BD. Tumor necrosis factor alpha inhibitors in the treatment of toxic epidermal necrolysis. Cutis. (2018) 101:E15–21.
92. Thorlacius L, Theut Riis P, Jemec GBE. Severe hidradenitis suppurativa responding to treatment with secukinumab: a case report. Br J Dermatol. (2018) 179:182–5. doi: 10.1111/bjd.15769
93. Balakirski G, Alkhateeb A, Merk HF, Leverkus M, Megahed M. Successful treatment of bullous pemphigoid with omalizumab as corticosteroid-sparing agent: report of two cases and review of literature. J Eur Acad Dermatol Venereol. (2016) 30:1778–82. doi: 10.1111/jdv.13758
94. Menzinger S, Kaya G, Schmidt E, Fontao L, Laffitte E. Biological and clinical response to omalizumab in a patient with bullous pemphigoid. Acta Derm Venereol. (2018) 98:284–6. doi: 10.2340/00015555-2845
95. Fairley JA, Burnett CT, Fu C-L, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol. (2007) 127:2605–11. doi: 10.1038/sj.jid.5700958
96. Rambhia PH, Levitt JO. Recalcitrant prurigo nodularis treated successfully with dupilumab. JAAD Case Rep. (2019) 5:471–3. doi: 10.1016/j.jdcr.2019.03.016
97. Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. (2019) 5:339–41. doi: 10.1016/j.jdcr.2019.01.024
98. Yang EJ, Murase JE. Recalcitrant anal and genital pruritus treated with dupilumab. Int J Womens Dermatol. (2018) 4:223–6. doi: 10.1016/j.ijwd.2018.08.010
99. Mollanazar NK, Elgash M, Weaver L, Valdes-Rodriguez R, Hsu S. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. (2019) 155:121–2. doi: 10.1001/jamadermatol.2018.3906
100. Kanda N, Koto M, Hoashi T, Saeki H. Case of alopecia areata during dupilumab treatment for atopic dermatitis. J Dermatol. (2019). doi: 10.1111/1346-8138.14880. [Epub ahead of print].
101. Maloney NJ, Worswick S, Cheng K. Development of alopecia in patients treated with dupilumab. Dermatol Ther. (2019) 32:e12869. doi: 10.1111/dth.12869
102. Yazdanyar S, Jemec GBE. Alopecia areata after treatment with dupilumab. Dermatitis. (2019) 30:175–6. doi: 10.1097/DER.0000000000000458
103. Darrigade A-S, Legrand A, Andreu N, Jacquemin C, Boniface K, Taïeb A, et al. Dual efficacy of dupilumab in a patient with concomitant atopic dermatitis and alopecia areata. Br J Dermatol. (2018) 179:534–6. doi: 10.1111/bjd.16711
104. Dobkin H, Mullen R, Zirwas M. Alopecia universalis and atopic dermatitis improvement with dupilumab: demonstration of a shared pathophysiology and clinical efficacy. Skinmed. (2019) 17:139–40.
105. Ludriksone L, Elsner P, Schliemann S. Zwei patienten mit abheilung einer alopecia areata unter dupilumab. J Dtsch Dermatol Ges. (2019) 17(Suppl. 2):1–3. doi: 10.1111/ddg.13778
106. Kaye A, Gordon SC, Deverapalli SC, Her MJ, Rosmarin D. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. (2018) 154:1225–6. doi: 10.1001/jamadermatol.2018.2526
107. Gordon SC, Robinson SN, Abudu M, Her M, Deverapalli S, Levin A, et al. Eosinophilic annular erythema treated with dupilumab. Pediatr Dermatol. (2018) 35:e255–6. doi: 10.1111/pde.13533
108. Teraki Y, Taguchi R, Takamura S, Fukuda T. Use of dupilumab in the treatment of papuloerythroderma of ofuji. JAMA Dermatol. (2019). doi: 10.1001/jamadermatol.2019.0946
Keywords: biologics, off-label, TNF-α, IL-1, IL-12, IL-23, IgE, CD20
Citation: Speeckaert R, Lambert J and van Geel N (2019) Learning From Success and Failure: Biologics for Non-approved Skin Diseases. Front. Immunol. 10:1918. doi: 10.3389/fimmu.2019.01918
Received: 31 May 2019; Accepted: 29 July 2019;
Published: 08 August 2019.
Edited by:
Katja Bieber, Universität zu Lübeck, GermanyReviewed by:
Takashi Hashimoto, Osaka City University, JapanMassimo Cugno, University of Milan, Italy
Angelo Valerio Marzano, University of Milan, Italy
Copyright © 2019 Speeckaert, Lambert and van Geel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reinhart Speeckaert, cmVpbmhhcnQuc3BlZWNrYWVydEB1Z2VudC5iZQ==
 Reinhart Speeckaert
Reinhart Speeckaert Jo Lambert
Jo Lambert