- 1Duke University Human Vaccine Institute, Duke University School of Medicine, Durham, NC, United States
- 2Department of Immunology, Duke University School of Medicine, Durham, NC, United States
- 3Department of Pediatrics, Duke University School of Medicine, Durham, NC, United States
Despite the availability of yearly vaccinations, influenza continues to cause seasonal, and pandemic rises in illness and death. An error prone replication mechanism results in antigenic drift and viral escape from immune pressure, and recombination results in antigenic shift that can rapidly move through populations that lack immunity to newly emergent strains. The development of a “universal” vaccine is a high priority and many strategies have been proposed, but our current understanding of influenza immunity is incomplete making the development of better influenza vaccines challenging. Influenza immunity has traditionally been measured by neutralization of virions and hemagglutination inhibition, but in recent years there has been a growing appreciation of other responses that can contribute to protection such as antibody-dependent cellular cytotoxicity (ADCC) that can kill influenza-infected cells. ADCC has been shown to provide cross-strain protection and to assist in viral clearance, making it an attractive target for “universal” vaccine designs. Here we provide a brief overview of the current state of influenza research that leverages “the other end of the antibody.”
Introduction
Influenza causes 3–5 million cases of severe illness and 290,000–650,000 deaths annually (1). Influenza is caused by orthomyxoviruses that have a segmented, negative-strand RNA genome that encodes its own RNA-dependent RNA polymerase that results in ~1 error per replicated genome (2–4). Accumulating errors cause small changes over time that allow viral escape (5, 6), a process called antigenic drift. Reassorting of the segmented influenza genome can produce novel influenza strains to which there is no preexisting immunity in the human population, a process called antigenic shift, and it is thought this mechanism produced the 1918, 1957, and 1968 pandemics (7).
The primary means of combatting both seasonal and pandemic influenza are quarantine and isolation (8), strict hygiene (9), and vaccination (10–12). Influenza vaccines were developed starting in the 1940s (13–16), and seasonal vaccines now include multiple antigens either as inactivated or live-attenuated products (17–20). Selection of representative influenza strains requires the ongoing worldwide analysis of circulating influenza (21), leading to potential mismatch with low vaccine efficacy (22). Public health leaders have called for the development of “universal” influenza vaccines (23), but at this time it is not clear what kinds of immunity such a vaccine should elicit.
Vaccines can prevent symptomatic disease, reduce disease duration, and reduce viral shedding, and infectivity to other persons. To accomplish these, a vaccine can elicit responses that inhibit influenza virions and/or enhance the clearance of influenza-infected cells. The influenza virion has three virally-encoded surface antigens (Figure 1A): a trimeric glycoprotein hemagglutinin (HA) that binds to sialic acid on cell surface receptors promoting virion endocytosis followed by fusion of viral and host cell membranes, a tetrameric neuraminidase (NA) that cleaves sialic acid to release virions from infected cells, and enhance passage through respiratory mucins, and a proton channel (M2 matrix) that helps the release of the viral genome after acidification in endocytic vacuoles. These proteins are present in most split-virus vaccine products (24), but the exact contribution of each of these, or of other influenza proteins that are not surface expressed, to vaccine efficacy is unclear.
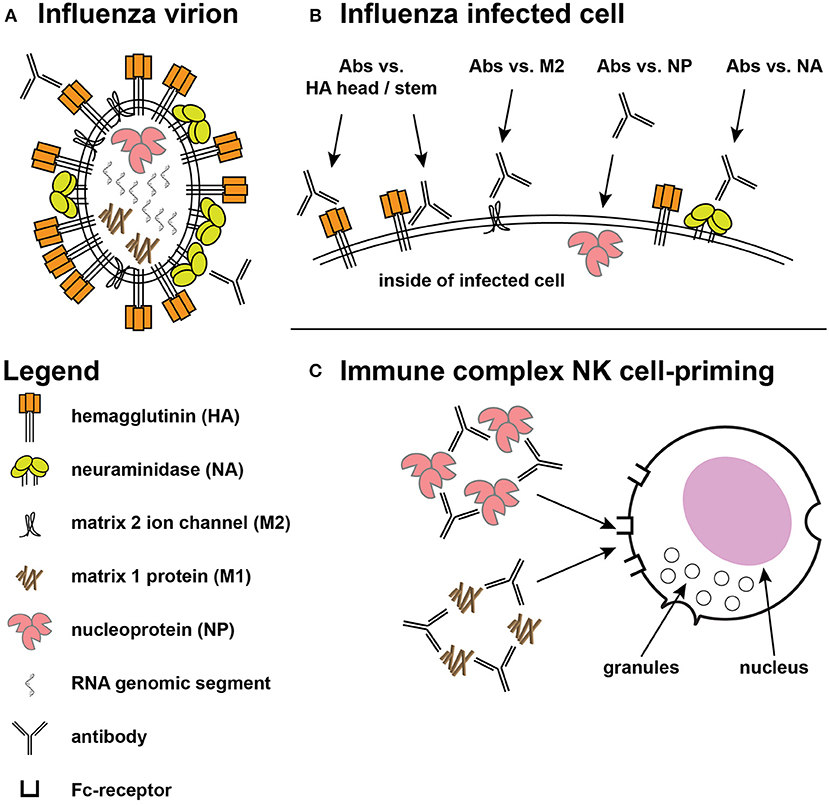
Figure 1. Targets for protective influenza responses. (A) Influenza virion showing the tight packing of viral hemagglutinin (HA), neuraminidase (NA), and matrix 2 (M2) ion channels on the surface. Inside the virion are the 8 genomic segments, nucleoprotein (NP), and the structural matrix 1 (M1) protein. (B) The surface of an infected cell shows the same antigens spread out on the surface, providing access for ADCC-mediating antibodies directed at the HA head and stem, along with greater access to the M2 ion channel. Antibodies against NP have also shown ADCC activity. (C) Immune complexes of NP and M1 have been shown to prime NK cells to secrete cytokines.
ADCC Activity Against Influenza
Antibody specificity is mediated by binding of Fab (fragment antigen binding) domains to their antigenic target, while the other end of the antibody is a constant region, the Fc (fragment crystallizable) domain, that provides a link between antibody recognition of infected cells and effector cells. NK cells, monocytes/macrophages, and neutrophils all have Fc-receptors (FcRs) on their surface, and the combination of both activating and inhibitory signals direct the immune response (25). In humans, NK cells are generally thought of as the primary effector for ADCC, but ADCC of influenza-infected cells has been demonstrated using neutrophils, monocytes, lymphocytes, and cord blood cells (26–28).
An increasing body of literature has demonstrated the possible protective role of non-neutralizing antibodies for pathogens as diverse as HIV-1 (29, 30), herpes simplex virus (31–33), Ebola (34, 35), and influenza (36–38). Influenza-infected cells can be recognized by antibodies that bind to proteins that play important roles within the viral life cycle. As noted above, HA initiates the viral life cycle by binding sialic acid and mediating viral entry into cells via fusion after receptor-mediated endocytosis (2). During this process, M2 matrix channels promote pH equilibration, a critical step in the release of the viral genome from the endosome (39). After influenza genome replication and protein synthesis, viral components are transferred to the plasma membrane where viral budding occurs. NA removes sialic acid from glycoproteins allowing the release of newly assembled virions from the surface of infected cells and preventing aggregation (2). HA has been the focus of most vaccine designs because HA evolves more rapidly than other antigens (40), but more conserved antigens like NA, M2, and nucleoprotein (NP) have also been considered attractive targets for vaccine designs. Antibodies against these other antigens do not appear to directly prevent infection but can target infected cells (19), and antibodies against all of these proteins have mediated killing of infected cells in vitro (Figure 1B).
Antibody-dependent cellular cytotoxicity (ADCC) against influenza was described by Greenberg et al. (26) and ADCC activity can be mediated by multiple cell types (27, 28, 41) and arises after infection or vaccination (41–43). Most early work suggested that ADCC was primarily directed against HA (44), although the contribution of other antigens to ADCC could not be ruled out (43). Study of influenza-specific monoclonal antibodies (mAbs) have shown that antibodies directed against the globular head often lack breadth while those directed against the more conserved HA stem can recognize multiple strains and subtypes of HA (4). Both specificities of HA antibodies have mediated ADCC (Figure 1B) but antibodies with neutralization and hemagglutination inhibition (HAI) activity tend to be directed against the HA head domain (Figure 1A).
ADCC-mediating antibodies also target other proteins on the virus surface (Figure 1B). Murine mAbs against influenza B NA mediated ADCC and provided protection in mouse challenge (45), but whether this activity contributes significantly to human protection is not known. NA antibody titers have correlated with protection against influenza infection in humans (46–50), but NA responses have not been studied at the same level of molecular detail as HA responses (51). NA antibodies are thought to act by decorating infected cells thus making them targets for complement fixation and ADCC, and by inhibiting enzymatic activity and preventing virions from escaping respiratory mucins (19). NA is present in most vaccines derived from influenza virions (24), but few vaccine candidates focus on NA antigens and their relative contribution to protection is not well-quantified. There is growing interest in NA as a component vaccine-mediated protection, and the recent formation of the NAction! working group (51) is focused on filling key knowledge gaps.
M2 is a highly conserved antigen necessary for the virus life cycle (39) and a number of studies showed antibodies to M2 mediated ADCC and protected in mouse studies (36, 52, 53). M2 is present at low copy number on virions and may be occluded by the larger HA and NA proteins (19) (Figure 1A), and while vaccine candidates based on M2 have advanced to human clinical trials (54, 55), it is not yet known whether these approaches will provide broad cross-protection when tested in human efficacy trials.
Curiously, a protein that might not be expected on the surface of either virions or virus-infected cells has been shown to mediate ADCC in vitro. Influenza nucleoprotein (NP) is involved in replication and packaging of virus RNA segments into virions (56), but studies have shown that NP is surface expressed on infected cells (57, 58). Humans given seasonal vaccine demonstrated H7N9 cross-reactive ADCC activity that correlated with binding to NP (59). Antibodies against both NP and the internal RNA-binding and structural matrix 1 (M1) protein did not mediate ADCC against target cells expressing those specific antigens, but immune complexes of those proteins primed natural killer (NK) cells to secrete cytokines (60) (Figure 1C); the authors suggested such activity could contribute to an anti-viral environment. Whether these kinds of activities play an important role in cross-protection against human infection is not yet known.
ADCC in Mouse Models
The receptors and cell types important for human immune response may not have the same effect or use the same pathways in animal models. In fact, there are numerous differences among antibody isotypes and subclasses (61) in animal models typically used for immune studies, as well as in FcR sequences and cellular distributions (62), and such differences may result in species-specific mechanisms of ADCC activity against influenza.
With that caveat, mouse models have provided suggestive evidence for the kinds of immunity that might be protective in humans. DiLillo et al. administered human mAbs to transgenic mice that expressed human FcRs, and demonstrated that for both HA stem-directed antibodies (37) and HA head-directed antibodies (63) that Fc-FcR interactions were required for protection against lethal challenge. Interestingly, transgenic mice receiving neutralizing anti-HA and anti-NA antibodies were only protected against challenge if they were matched for Fc-Fc?R binding while strain specific anti-HA and anti-NA antibodies protected regardless of Fc-FcR matching (63). Similar studies of antibodies against M2 have also shown Fc-FcR dependence, with IgG1 M2-directed antibodies requiring matching FcRs to protect against lethal challenge (52, 53).
Wild-type mice have also demonstrated ADCC-mediating antibody protection. For example, murine antibodies generated against H7N9 influenza were protective, but ADCC-mediating antibodies required efficient Fc-FcR interaction to protect (64). Vaccination against H5N1 that elicited ADCC activity protected against lethal H5N8 challenge in mice (65), and protection against H7 influenza challenge also correlated with ADCC activity (66). Passive transfer of HA stem-specific human sera demonstrated protection against lethal challenge in a manner highly correlated with ADCC activity (67). Protection is not limited to HA-directed antibodies, as antibodies against influenza B NA also mediate ADCC and protect against lethal virus challenge (45).
However, not all mouse studies have suggested a protective effect of ADCC. Evaluation of candidate vaccines that targeted specific epitopes on the HA head domain were capable of eliciting potent ADCC responses in vitro, but mice given these immunogens were more sensitive to lethal challenge (68, 69), and examination of lung tissue suggested damage caused by the immune response. Whether this kind of damage could occur in humans is not known.
Other Non-Primate Models
Swine are susceptible to influenza and they are thought to be a key species in development of new strains by antigenic shift (70). Because of the major economic impact of swine influenza disease, vaccination is common and new vaccine candidates are tested in swine (71). Experimental infection models have been used to test swine for protection against challenge following vaccination (72) and as a model of enhanced influenza disease (73). Studies in this latter model have suggested that a lack of neutralizing activity against the challenge strain combined with ADCC-mediating antibodies can produce enhanced disease in swine (73, 74), suggesting that caution may be appropriate in the development of vaccines that do not elicit traditional correlates of influenza protection (75). Furthermore, differences between swine and human FcRs make direct measures of ADCC in swine challenging, and have confounded passive infusion studies of human antibodies (76). The swine model is important for improving our understanding of influenza pathogenesis, but it remains to be seen whether success or failure in the swine model will directly translate to human trials.
The ferret model may also provide insights into influenza immunity, although direct measures of ADCC activity in ferrets is also challenging. For example, immunization of ferrets to elicit HA stem-directed antibodies showed that ADCC in a reporter assay correlated with protection, though the assay used human FcRs due to the lack of ferret-specific reagents (77). Passive transfer of immune sera directed against the HA stem (78), infusion of ADCC-mediating mAbs (79), and immunization to induce ADCC-mediating antibodies (65) have all protected ferrets against heterologous influenza challenges. Unfortunately, the lack of ferret-specific reagents limits the depth of investigation possible at this time, and as with swine, it is not clear whether these studies will translate directly to human trials.
Non-human Primate Studies
Non-human primate models have not traditionally been used for influenza research due to high cost and the low level of symptoms following experimental infection (80), but recently a number of studies have evaluated the protective capacity of ADCC in non-human primates. For example, protection was observed in rhesus macaques infected with a pre-pandemic H1N1 A/Kawasaki/173/2001 who were subsequently challenged with H1N1 A/California/04/2009; in this study ADCC activity correlated with control of the second H1N1 infection (81). Protection was also observed in a study of H5 immunization of rhesus macaques challenged with pandemic H1N1, and ADCC activity correlated with reduced viral shedding after infection (82). In cynomolgus macaques, vaccines that elicit ADCC-mediating antibodies have been shown to decrease shedding of influenza (83), and passive infusion of a human ADCC-mediating mAb protected against infection (84). However, given the differences between human and non-human primate Fc-FcR biology (85), it is not clear if non-human primate studies will be predictive of human study outcomes.
Human Studies
Human studies have focused on examining people after natural infection, vaccination, and/or the isolation of human monoclonal antibodies. Natural infection studies have shown that influenza can imprint the immune system in a manner that provides protection against heterologous influenza exposure. For example, healthy adults and children in the US were found to have ADCC-mediating antibodies against H5N1 and H7N9 strains (86), despite the fact that those strains have not circulated in the US. Intravenous immune globulin, a product derived from plasma donation, contained ADCC-mediating antibodies that cross-reacted with multiple influenza strains; e.g., immune globulin collected from donors prior to the emergence of the 2009 pandemic strain was active against the newly emergent strain (87). During the last influenza pandemic a number of studies suggested that older adults were less likely to be infected by the newly emergent strain, and older persons were more likely to have ADCC-mediating antibodies against the 2009 H1N1 pandemic strain (88), suggesting that these responses may have contributed to the relative protection observed. However, such antibodies were not well correlated with protection in children (89, 90), indicating that in vitro activity alone may not provide an accurate correlate of efficacy.
ADCC-mediating antibodies that arise during severe infection may also correlate with outcomes. A study of seasonal and H7N9 human infections demonstrated that persons who did not survive infection were more likely to have low ADCC activity (91). It is not known whether ADCC-mediating antibodies being present prior to infection would have been protective.
Vaccine studies have examined the ability of different constructs to elicit cross-reactive ADCC-mediating antibodies. For example, seasonal vaccination of older adults indicated that they had strong boosting of ADCC activity, including activity against H5 and H7 strains (92). Similarly, a study of H7 vaccination found ADCC activity against many group 2 influenza strains (93), and H5 vaccination induced ADCC responses to multiple strains (94).
Testing of many of human-derived antibodies showed protection in mouse studies as described above and have helped define vaccine design targets. For example, antibodies against the vestigial esterase domain of H3 HA (95) or influenza B (96) mediate ADCC and block other viral activities, but whether such antibodies can be easily elicited by vaccination and protect is not known. Others have examined why HA head-directed antibodies are less efficient at mediating ADCC (97) or why antibodies that bind at or near the sialic acid receptor binding site of HA lack ADCC activity (98, 99). Data suggested that ADCC activity against influenza-infected cells requires that sialic acid on NK cells must also bind HA. Several studies have identified possible vaccine targets on HA, including a pH-sensitive epitope on H7 HA (100), receptor binding site antibodies for influenza B (79), and the influenza B HA stem (101). Furthermore, recently isolated human antibodies against epitopes in the HA trimer interface provided protection in an Fc-dependent manner (38, 102), and such antibodies were elicited in animal models by vaccination (103). These studies suggest that probing the human immune system could identify additional targets for vaccine design.
Most human studies measure responses to influenza infection or vaccination, and some correlate those responses with epidemiologic data, but far fewer perform experimental infectious challenge to correlate a response with protection. In 2016, Jegaskanda et al. reported that high levels of pre-existing ADCC-mediating antibodies protected against experimental infection (104), suggesting that if ADCC activity is present prior to infection it can protect. However, it is not clear whether ADCC activity alone is protective, and as of this writing, there do not appear to be any reports of human challenge studies investigating the protective capacity of passively administered ADCC-mediating influenza antibodies. Essentially all humans over the age of 6 years have evidence of prior influenza infection (105), meaning that vaccination or passive infusion studies of ADCC-based protection in humans have to be interpreted in the light of prior immunity. Despite this, the studies described in this review and other work are leading to new vaccine designs, and it is hoped that one or more of these new designs will provide long-lasting protection to all people worldwide.
Conclusions
Influenza remains an important cause of human disease that often resists effective control by current vaccination strategies and there is a current push for the development of “universal vaccine” candidates. Antibody binding to Fc receptors with effector cell activation has been shown to protect in numerous animal models and harnessing this activity could be an important component of universal vaccine designs. ADCC and other activities based on the “other end of the antibody,” in combination with traditional activities like neutralization, might be harnessed to contribute to protection from this ever-changing pathogen.
Author Contributions
TV wrote the first draft and participated in editing. MM edited the draft and prepared the final version.
Funding
This work was supported by NIH grant P01 AI120756.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Health Organization. Influenza Update – 331. (2018). Available online at: https://www.who.int/influenza/surveillance_monitoring/updates/latest_update_GIP_surveillance/en/ (accessed Jan 01, 2019).
2. Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. (2008) 26 (Suppl. 4):D49–53. doi: 10.1016/j.vaccine.2008.07.039
3. Boivin S, Cusack S, Ruigrok RW, Hart DJ. Influenza A virus polymerase: structural insights into replication and host adaptation mechanisms. J Biol Chem. (2010) 285:28411–7. doi: 10.1074/jbc.R110.117531
4. Air GM. Influenza virus antigenicity and broadly neutralizing epitopes. Curr Opin Virol. (2015) 11:113–21. doi: 10.1016/j.coviro.2015.03.006
5. Huang KY, Rijal P, Schimanski L, Powell TJ, Lin TY, McCauley JW, et al. Focused antibody response to influenza linked to antigenic drift. J Clin Invest. (2015) 125:2631–45. doi: 10.1172/JCI81104
6. Schmidt AG, Do KT, McCarthy KR, Kepler TB, Liao HX, Moody MA, et al. Immunogenic stimulus for germline precursors of antibodies that engage the influenza hemagglutinin receptor-binding site. Cell Rep. (2015) 13:2842–50. doi: 10.1016/j.celrep.2015.11.063
7. Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. (2006) 12:9–14. doi: 10.3201/eid1201.051254
8. World Health Organization Writing G, Bell D, Nicoll A, Fukuda K, Horby P, Monto A, et al. Non-pharmaceutical interventions for pandemic influenza, national and community measures. Emerg Infect Dis. (2006) 12:88–94. doi: 10.3201/eid1201.051371
9. Blanco N, Eisenberg MC, Stillwell T, Foxman B. What transmission precautions best control influenza spread in a hospital? Am J Epidemiol. (2016) 183:1045–54. doi: 10.1093/aje/kwv293
10. Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2017-18 influenza season. MMWR Recomm Rep. (2017) 66:1–20. doi: 10.15585/mmwr.rr6602a1
11. Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) - United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. (2018) 67:643–5. doi: 10.15585/mmwr.mm6722a5
12. Robinson CL, Romero JR, Kempe A, Pellegrini C, Szilagyi P. Advisory committee on immunization practices recommended immunization schedule for children and adolescents aged 18 years or younger - United States, 2018. MMWR Morb Mortal Wkly Rep. (2018) 67:156–7. doi: 10.15585/mmwr.mm6705e2
13. Francis T, Salk JE, Pearson HE, Brown PN. Protective effect of vaccination against induced influenza A. J Clin Invest. (1945) 24:536–46. doi: 10.1172/JCI101633
14. Salk JE, Pearson HE, Brown PN, Francis T. Protective effect of vaccination against induced influenza B. J Clin Invest. (1945) 24:547–53. doi: 10.1172/JCI101634
15. Francis T, Salk JE, Quilligan JJ. Experience with vaccination against influenza in the spring of 1947: a preliminary report. Am J Public Health Nations Health. (1947) 37:1013–6. doi: 10.2105/AJPH.37.8.1013
16. Salk JE, Suriano PC. Importance of antigenic composition of influenza virus vaccine in protecting against the natural disease; observations during the winter of 1947-1948. Am J Public Health Nations Health. (1949) 39:345–55. doi: 10.2105/AJPH.39.3.345
17. Allison JE, Glezen WP, Taber LH, Paredes A, Webster RG. Reactogenicity and immunogenicity of bivalent influenza A and monovalent influenza B virus vaccines in high-risk children. J Infect Dis. (1977) 136 (Suppl):S672–6. doi: 10.1093/infdis/136.Supplement_3.S672
18. Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. (2000) 181:1133–7. doi: 10.1086/315323
19. Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. (2015) 14:167–82. doi: 10.1038/nrd4529
20. Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: formulation and production strategies. Eur J Pharm Biopharm. (2015) 94:251–63. doi: 10.1016/j.ejpb.2015.05.023
21. Barr IG, McCauley J, Cox N, Daniels R, Engelhardt OG, Fukuda K, et al. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 northern hemisphere season. Vaccine. (2010) 28:1156–67. doi: 10.1016/j.vaccine.2009.11.043
22. Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. (2013) 11:153. doi: 10.1186/1741-7015-11-153
23. Erbelding EJ, Post D, Stemmy E, Roberts PC, Augustine AD, Ferguson S, et al. A universal influenza vaccine: the strategic plan for the national institute of allergy and infectious diseases. J Infect Dis. (2018) 218:347–54. doi: 10.1093/infdis/jiy103
24. Renfrey S, Watts A. Morphological and biochemical characterization of influenza vaccines commercially available in the United Kingdom. Vaccine. (1994) 12:747–52. doi: 10.1016/0264-410X(94)90227-5
25. Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. (2008) 8:34–47. doi: 10.1038/nri2206
26. Greenberg SB, Criswell BS, Six HR, Couch RB. Lymphocyte cytotoxicity to influenza virus-infected cells. II requirement for antibody and non-T lymphocytes. J Immunol. (1977) 119:2100–6.
27. Hashimoto G, Wright PF, Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis. (1983) 148:785–94. doi: 10.1093/infdis/148.5.785
28. Hashimoto G, Wright PF, Karzon DT. Ability of human cord blood lymphocytes to mediate antibody-dependent cellular cytotoxicity against influenza virus-infected cells. Infect Immun. (1983) 42:214–8.
29. Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. (2001) 75:6953–61. doi: 10.1128/JVI.75.15.6953-6961.2001
30. Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. (2012) 366:1275–86. doi: 10.1056/NEJMoa1113425
31. Kohl S, Loo LS, Pickering LK. Protection of neonatal mice against herpes simplex viral infection by human antibody and leukocytes from adult, but not neonatal humans. J Immunol. (1981) 127:1273–5.
32. Kohl S, Loo LS. Protection of neonatal mice against herpes simplex virus infection: probable in vivo antibody-dependent cellular cytotoxicity. J Immunol. (1982) 129:370–6.
33. Wang K, Tomaras GD, Jegaskanda S, Moody MA, Liao HX, Goodman KN, et al. Monoclonal antibodies, derived from humans vaccinated with the RV144 HIV vaccine containing the HVEM binding domain of herpes simplex virus (HSV) glycoprotein D, neutralize HSV infection, mediate antibody-dependent cellular cytotoxicity, and protect mice from ocular challenge with HSV-1. J Virol. (2017) 91. doi: 10.1128/JVI.00411-17
34. Gunn BM, Yu WH, Karim MM, Brannan JM, Herbert AS, Wec AZ, et al. A role for Fc function in therapeutic monoclonal antibody-mediated protection against ebola virus. Cell Host Microbe. (2018) 24:221–33 e225. doi: 10.1016/j.chom.2018.07.009
35. Saphire EO, Schendel SL, Fusco ML, Gangavarapu K, Gunn BM, Wec AZ, et al. Systematic analysis of monoclonal antibodies against ebola virus GP defines features that contribute to protection. Cell. (2018) 174:938–52.e913. doi: 10.1016/j.cell.2018.07.033
36. Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. (2004) 172:5598–605. doi: 10.4049/jimmunol.172.9.5598
37. DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med. (2014) 20:143–51. doi: 10.1038/nm.3443
38. Watanabe A, McCarthy KR, Kuraoka M, Schmidt AG, Adachi Y, Onodera T, et al. Antibodies to a conserved influenza head interface epitope protect by an IgG subtype-dependent mechanism. Cell. (2019) 177:1124–35 e1116. doi: 10.1016/j.cell.2019.03.048
39. Pielak RM, Chou JJ. Influenza M2 proton channels. Biochim Biophys Acta. (2011) 1808:522–9. doi: 10.1016/j.bbamem.2010.04.015
40. Kilbourne ED, Johansson BE, Grajower B. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc Natl Acad Sci USA. (1990) 87:786–90. doi: 10.1073/pnas.87.2.786
41. Greenberg SB, Criswell BS, Six HR, Couch RB. Lymphocyte cytotoxicity to influenza virus-infected cells: response to vaccination and virus infection. Infect Immun. (1978) 20:640–5.
42. Greenberg SB, Six HR, Drake S, Couch RB. Cell cytotoxicity due to specific influenza antibody production in vitro after recent influenza antigen stimulation. Proc Natl Acad Sci USA. (1979) 76:4622–6. doi: 10.1073/pnas.76.9.4622
43. Vella S, Rocchi G, Resta S, Marcelli M, De Felici A. Antibody reactive in antibody-dependent cell-mediated cytotoxicity following influenza virus vaccination. J Med Virol. (1980) 6:203–11. doi: 10.1002/jmv.1890060303
44. Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol. (2014) 193:469–75. doi: 10.4049/jimmunol.1400432
45. Wohlbold TJ, Podolsky KA, Chromikova V, Kirkpatrick E, Falconieri V, Meade P, et al. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat Microbiol. (2017) 2:1415–24. doi: 10.1038/s41564-017-0011-8
46. Murphy BR, Kasel JA, Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med. (1972) 286:1329–32. doi: 10.1056/NEJM197206222862502
47. Couch RB, Kasel JA, Gerin JL, Schulman JL, Kilbourne ED. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis. (1974) 129:411–20. doi: 10.1093/infdis/129.4.411
48. Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. (2013) 207:974–81. doi: 10.1093/infdis/jis935
49. Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, et al. Antibody to influenza virus neuraminidase: an independent correlate of protection. J Infect Dis. (2015) 212:1191–9. doi: 10.1093/infdis/jiv195
50. Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, et al. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. MBio. (2016) 7:e00417–6. doi: 10.1128/mBio.00417-16
51. Krammer F, Fouchier RAM, Eichelberger MC, Webby RJ, Shaw-Saliba K, Wan H, et al. NAction! How can neuraminidase-based immunity contribute to better influenza virus vaccines? MBio. (2018) 9:e02332–17. doi: 10.1128/mBio.02332-17
52. El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. (2011) 186:1022–31. doi: 10.4049/jimmunol.0902147
53. Van den Hoecke S, Ehrhardt K, Kolpe A, El Bakkouri K, Deng L, Grootaert H, et al. Hierarchical and redundant roles of activating fcgammars in protection against influenza disease by M2e-specific IgG1 and IgG2a antibodies. J Virol. (2017) 91:e02500–16. doi: 10.1128/JVI.02500-16
54. Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. (2011) 29:5145–52. doi: 10.1016/j.vaccine.2011.05.041
55. Ibanez LI, Roose K, De Filette M, Schotsaert M, De Sloovere J, Roels S, et al. M2e-displaying virus-like particles with associated RNA promote T helper 1 type adaptive immunity against influenza A. PLoS ONE. (2013) 8:e59081. doi: 10.1371/journal.pone.0059081
56. Portela A, Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J Gen Virol. (2002) 83(Pt 4):723–34. doi: 10.1099/0022-1317-83-4-723
57. Yewdell JW, Frank E, Gerhard W. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J Immunol. (1981) 126:1814–9.
58. Fujimoto Y, Tomioka Y, Takakuwa H, Uechi G, Yabuta T, Ozaki K, et al. Cross-protective potential of anti-nucleoprotein human monoclonal antibodies against lethal influenza A virus infection. J Gen Virol. (2016) 97:2104–16. doi: 10.1099/jgv.0.000518
59. Jegaskanda S, Co MDT, Cruz J, Subbarao K, Ennis FA, Terajima M. Induction of H7N9-cross-reactive antibody-dependent cellular cytotoxicity antibodies by human seasonal influenza A viruses that are directed toward the nucleoprotein. J Infect Dis. (2017) 215:818–23. doi: 10.1093/infdis/jiw629
60. Vanderven HA, Ana-Sosa-Batiz F, Jegaskanda S, Rockman S, Laurie K, Barr I, et al. What lies beneath: antibody dependent natural killer cell activation by antibodies to internal influenza virus proteins. EBioMed. (2016) 8:277–90. doi: 10.1016/j.ebiom.2016.04.029
61. Stavnezer J, Amemiya CT. Evolution of isotype switching. Semin Immunol. (2004) 16:257–75. doi: 10.1016/j.smim.2004.08.005
62. Kacskovics I. Fc receptors in livestock species. Vet Immunol Immunopathol. (2004) 102:351–62. doi: 10.1016/j.vetimm.2004.06.008
63. DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest. (2016) 126:605–10. doi: 10.1172/JCI84428
64. Henry Dunand CJ, Leon PE, Huang M, Choi A, Chromikova V, Ho IY, et al. Both neutralizing and non-neutralizing human H7N9 influenza vaccine-induced monoclonal antibodies confer protection. Cell Host Microbe. (2016) 19:800–13. doi: 10.1016/j.chom.2016.05.014
65. Park SJ, Si YJ, Kim J, Song MS, Kim SM, Kim EH, et al. Cross-protective efficacies of highly-pathogenic avian influenza H5N1 vaccines against a recent H5N8 virus. Virology. (2016) 498:36–43. doi: 10.1016/j.virol.2016.08.010
66. Tan GS, Leon PE, Albrecht RA, Margine I, Hirsh A, Bahl J, et al. Broadly-reactive neutralizing and non-neutralizing antibodies directed against the H7 influenza virus hemagglutinin reveal divergent mechanisms of protection. PLoS Pathog. (2016) 12:e1005578. doi: 10.1371/journal.ppat.1005578
67. Jacobsen H, Rajendran M, Choi A, Sjursen H, Brokstad KA, Cox RJ, et al. Influenza virus hemagglutinin stalk-specific antibodies in human serum are a surrogate marker for in vivo protection in a serum transfer mouse challenge model. MBio. (2017) 8:e01463–17. doi: 10.1128/mBio.01463-17
68. Ye ZW, Yuan S, Poon KM, Wen L, Yang D, Sun Z, et al. Antibody-dependent cell-mediated cytotoxicity epitopes on the hemagglutinin head region of pandemic H1N1 influenza virus play detrimental roles in H1N1-infected mice. Front Immunol. (2017) 8:317. doi: 10.3389/fimmu.2017.00317
69. Wang J, Liu M, Ding N, Li Y, Shao J, Zhu M, et al. Vaccine based on antibody-dependent cell-mediated cytotoxicity epitope on the H1N1 influenza virus increases mortality in vaccinated mice. Biochem Biophys Res Commun. (2018) 503:1874–9. doi: 10.1016/j.bbrc.2018.07.129
70. Shao W, Li X, Goraya MU, Wang S, Chen JL. Evolution of influenza A virus by mutation and Re-assortment. Int J Mol Sci. (2017) 18:1650. doi: 10.3390/ijms18081650
71. Rajao DS, Anderson TK, Gauger PC, Vincent AL. Pathogenesis and vaccination of influenza A virus in swine. Curr Top Microbiol Immunol. (2014) 385:307–26. doi: 10.1007/82_2014_391
72. Kitikoon P, Vincent AL, Janke BH, Erickson B, Strait EL, Yu S, et al. Swine influenza matrix 2 (M2) protein contributes to protection against infection with different H1 swine influenza virus (SIV) isolates. Vaccine. (2009) 28:523–31. doi: 10.1016/j.vaccine.2009.09.130
73. Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, et al. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med. (2013) 5:200ra114. doi: 10.1126/scitranslmed.3006366
74. Gauger PC, Vincent AL, Loving CL, Lager KM, Janke BH, Kehrli ME Jr, et al. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (delta-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine. (2011) 29:2712–9. doi: 10.1016/j.vaccine.2011.01.082
75. Crowe JE Jr. Universal flu vaccines: primum non nocere. Sci Transl Med. (2013) 5:200fs234. doi: 10.1126/scitranslmed.3007118
76. Morgan SB, Holzer B, Hemmink JD, Salguero FJ, Schwartz JC, Agatic G, et al. Therapeutic Administration of broadly neutralizing FI6 antibody reveals lack of interaction between human IgG1 and Pig Fc receptors. Front Immunol. (2018) 9:865. doi: 10.3389/fimmu.2018.00865
77. Nachbagauer R, Miller MS, Hai R, Ryder AB, Rose JK, Palese P, et al. Hemagglutinin stalk immunity reduces influenza virus replication and transmission in ferrets. J Virol. (2015) 90:3268–73. doi: 10.1128/JVI.02481-15
78. Yassine HM, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med. (2015) 21:1065–70. doi: 10.1038/nm.3927
79. Shen C, Chen J, Li R, Zhang M, Wang G, Stegalkina S, et al. A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci Transl Med. (2017) 9:eaam5752. doi: 10.1126/scitranslmed.aam5752
80. Bouvier NM, Lowen AC. Animal models for influenza virus pathogenesis and transmission. Viruses. (2010) 2:1530–63. doi: 10.3390/v20801530
81. Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol. (2013) 87:5512–22. doi: 10.1128/JVI.03030-12
82. Florek NW, Kamlangdee A, Mutschler JP, Kingstad-Bakke B, Schultz-Darken N, Broman KW, et al. A modified vaccinia Ankara vaccine vector expressing a mosaic H5 hemagglutinin reduces viral shedding in rhesus macaques. PLoS ONE. (2017) 12:e0181738. doi: 10.1371/journal.pone.0181738
83. Florek NW, Weinfurter JT, Jegaskanda S, Brewoo JN, Powell TD, Young GR, et al. Modified vaccinia virus Ankara encoding influenza virus hemagglutinin induces heterosubtypic immunity in macaques. J Virol. (2014) 88:13418–28. doi: 10.1128/JVI.01219-14
84. Song A, Myojo K, Laudenslager J, Harada D, Miura T, Suzuki K, et al. Evaluation of a fully human monoclonal antibody against multiple influenza A viral strains in mice and a pandemic H1N1 strain in nonhuman primates. Antiviral Res. (2014) 111:60–8. doi: 10.1016/j.antiviral.2014.08.016
85. Cocklin SL, Schmitz JE. The role of Fc receptors in HIV infection and vaccine efficacy. Curr Opin HIV AIDS. (2014) 9:257–62. doi: 10.1097/COH.0000000000000051
86. Terajima M, Co MD, Cruz J, Ennis FA. High antibody-dependent cellular cytotoxicity antibody titers to H5N1 and H7N9 avian influenza A viruses in healthy US adults and older children. J Infect Dis. (2015) 212:1052–60. doi: 10.1093/infdis/jiv181
87. Jegaskanda S, Vandenberg K, Laurie KL, Loh L, Kramski M, Winnall WR, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity in intravenous immunoglobulin as a potential therapeutic against emerging influenza viruses. J Infect Dis. (2014) 210:1811–22. doi: 10.1093/infdis/jiu334
88. Jegaskanda S, Laurie KL, Amarasena TH, Winnall WR, Kramski M, De Rose R, et al. Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. J Infect Dis. (2013) 208:1051–61. doi: 10.1093/infdis/jit294
89. Co MD, Terajima M, Thomas SJ, Jarman RG, Rungrojcharoenkit K, Fernandez S, et al. Relationship of preexisting influenza hemagglutination inhibition, complement-dependent lytic, and antibody-dependent cellular cytotoxicity antibodies to the development of clinical illness in a prospective study of A(H1N1)pdm09 Influenza in children. Viral Immunol. (2014) 27:375–82. doi: 10.1089/vim.2014.0061
90. Mesman AW, Westerhuis BM, Ten Hulscher HI, Jacobi RH, de Bruin E, van Beek J, et al. Influenza virus A(H1N1)2009 antibody-dependent cellular cytotoxicity in young children prior to the H1N1 pandemic. J Gen Virol. (2016) 97:2157–65. doi: 10.1099/jgv.0.000552
91. Vanderven HA, Liu L, Ana-Sosa-Batiz F, Nguyen TH, Wan Y, Wines B, et al. Fc functional antibodies in humans with severe H7N9 and seasonal influenza. JCI Insight. (2017) 2:e92750. doi: 10.1172/jci.insight.92750
92. Vanderven HA, Jegaskanda S, Wines BD, Hogarth PM, Carmuglia S, Rockman S, et al. Antibody-dependent cellular cytotoxicity responses to seasonal influenza vaccination in older adults. J Infect Dis. (2017) 217:12–23. doi: 10.1093/infdis/jix554
93. Stadlbauer D, Rajabhathor A, Amanat F, Kaplan D, Masud A, Treanor JJ, et al. Vaccination with a recombinant H7 hemagglutinin-based influenza virus vaccine induces broadly reactive antibodies in humans. mSphere. (2017) 2:e00502–17. doi: 10.1128/mSphere.00502-17
94. de Vries RD, Altenburg AF, Nieuwkoop NJ, de Bruin E, van Trierum SE, Pronk MR, et al. Induction of cross-clade antibody and T-cell responses by a modified vaccinia virus ankara-based influenza A(H5N1) vaccine in a randomized phase 1/2a clinical trial. J Infect Dis. (2018) 218:614–23. doi: 10.1093/infdis/jiy214
95. Bangaru S, Zhang H, Gilchuk IM, Voss TG, Irving RP, Gilchuk P, et al. A multifunctional human monoclonal neutralizing antibody that targets a unique conserved epitope on influenza HA. Nat Commun. (2018) 9:2669. doi: 10.1038/s41467-018-04704-9
96. Chai N, Swem LR, Park S, Nakamura G, Chiang N, Estevez A, et al. A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action. Nat Commun. (2017) 8:14234. doi: 10.1038/ncomms14234
97. He W, Tan GS, Mullarkey CE, Lee AJ, Lam MM, Krammer F, et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci USA. (2016) 113:11931–6. doi: 10.1073/pnas.1609316113
98. Cox F, Kwaks T, Brandenburg B, Koldijk MH, Klaren V, Smal B, et al. HA antibody-mediated FcgammaRIIIa activity is both dependent on fcr engagement and interactions between HA and sialic acids. Front Immunol. (2016) 7:399. doi: 10.3389/fimmu.2016.00399
99. Leon PE, He W, Mullarkey CE, Bailey MJ, Miller MS, Krammer F, et al. Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact. Proc Natl Acad Sci USA. (2016) 113:E5944–51. doi: 10.1073/pnas.1613225113
100. Yu F, Song H, Wu Y, Chang SY, Wang L, Li W, et al. A potent germline-like human monoclonal antibody targets a pH-sensitive epitope on H7N9 influenza hemagglutinin. Cell Host Microbe. (2017) 22:471–83.e475. doi: 10.1016/j.chom.2017.08.011
101. de Vries RD, Nieuwkoop NJ, van der Klis FRM, Koopmans MPG, Krammer F, Rimmelzwaan GF. Primary human influenza B virus infection induces cross-lineage hemagglutinin stalk-specific antibodies mediating antibody-dependent cellular cytoxicity. J Infect Dis. (2017) 217:3–11. doi: 10.1093/infdis/jix546
102. Bangaru S, Lang S, Schotsaert M, Vanderven HA, Zhu X, Kose N, et al. A site of vulnerability on the influenza virus hemagglutinin head domain trimer interface. Cell. (2019) 177:1136–52.e1118. doi: 10.1016/j.cell.2019.04.011
103. Bajic G, Maron MJ, Adachi Y, Onodera T, McCarthy KR, McGee CE, et al. Influenza antigen engineering focuses immune responses to a subdominant but broadly protective viral epitope. Cell Host Microbe. (2019) 25:P827-835.e6. doi: 10.1016/j.chom.2019.04.003
104. Jegaskanda S, Luke C, Hickman HD, Sangster MY, Wieland-Alter WF, McBride JM, et al. Generation and protective ability of influenza virus-specific antibody-dependent cellular cytotoxicity in humans elicited by vaccination, natural infection, and experimental challenge. J Infect Dis. (2016) 214:945–52. doi: 10.1093/infdis/jiw262
Keywords: ADCC—antibody dependent cellular cytotoxicity, influenza, antibodies, animal models, vaccine targets, mAb
Citation: Von Holle TA and Moody MA (2019) Influenza and Antibody-Dependent Cellular Cytotoxicity. Front. Immunol. 10:1457. doi: 10.3389/fimmu.2019.01457
Received: 25 March 2019; Accepted: 10 June 2019;
Published: 25 June 2019.
Edited by:
Gabriella Scarlatti, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Mirella Salvatore, Weill Cornell Medicine, United StatesDavid Pejoski, Université de Genève, Switzerland
Copyright © 2019 Von Holle and Moody. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Anthony Moody, bW9vZHkwMDdAbWMuZHVrZS5lZHU=
 Tarra A. Von Holle
Tarra A. Von Holle M. Anthony Moody
M. Anthony Moody