- 1Department of Surgical and Biomedical Sciences, Paediatric Clinic, Università degli Studi di Perugia, Perugia, Italy
- 2Department of Infectious Diseases, Istituto Superiore di Sanità, Rome, Italy
- 3Immunisation and Countermeasures Division, Public Health England–National Infection Service, London, United Kingdom
- 4Institute of Biomedicine, University of Turku, Turku, Finland
- 5Department of Medical Microbiology, Capital Medical University, Beijing, China
- 6Department of Infectious Disease Epidemiology, The Vaccine Confidence Project TM, London School of Hygiene & Tropical Medicine, London, United Kingdom
- 7Division of Pediatric Infectious Diseases, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States
- 8Children's Hospital, Helios HSk, Wiesbaden, Germany
- 9Department of Pediatrics, University Medicine, Mainz, Germany
- 10Department of Pediatrics, Vall d'Hebron University Hospital, Barcelona, Spain
- 11School of Medicine-Germans Trias i Pujol University Hospita, Universidad Autónoma de Barcelona, Barcelona, Spain
- 12Retired, Neuilly-sur-Seine, France
- 13School of Medicine, College of Health and Medicine, University of Tasmania, Hobart, TAS, Australia
- 14School of Health and Biomedical Science, RMIT University, Melbourne, VIC, Australia
- 15Department of Immunology and Pathology, Monash University, Melbourne, VIC, Australia
- 16Department of Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong, China
- 17Department of Microbiology, Institute of Biomedicine and Translational Medicine, University of Tartu, Tartu, Estonia
- 18Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, United States
- 19Microbiology and Mycology Program, Faculty of Medicine, Institute of Immunology and Immunotherapy, University of Chile, Santiago, Chile
- 20Retired, Milan, Italy
Pertussis is an acute respiratory disease caused by Bordetella pertussis. Due to its frequency and severity, prevention of pertussis has been considered an important public health issue for many years. The development of the whole-cell pertussis vaccine (wPV) and its introduction into the pediatric immunization schedule was associated with a marked reduction in pertussis cases in the vaccinated cohort. However, due to the frequency of local and systemic adverse events after immunization with wPV, work on a less reactive vaccine was undertaken based on isolated B. pertussis components that induced protective immune responses with fewer local and systemic reactions. These component vaccines were termed acellular vaccines and contained one or more pertussis antigens, including pertussis toxin (PT), filamentous haemagglutinin (FHA), pertactin (PRN), and fimbrial proteins 2 (FIM2) and 3 (FIM3). Preparations containing up to five components were developed, and several efficacy trials clearly demonstrated that the aPVs were able to confer comparable short-term protection than the most effective wPVs with fewer local and systemic reactions. There has been a resurgence of pertussis observed in recent years. This paper reports the results of a Consensus Conference organized by the World Association for Infectious Disease and Immunological Disorders (WAidid) on June 22, 2018, in Perugia, Italy, with the goal of evaluating the most important reasons for the pertussis resurgence and the role of different aPVs in this resurgence.
Introduction
Pertussis is an acute respiratory disease caused by Bordetella pertussis, a Gram-negative bacterial pathogen (1). Before the availability of vaccines, pertussis was a frequent cause of morbidity and mortality, particularly in infants and young children. The introduction of pertussis-containing vaccines into the immunization schedule of infants and children has reduced pertussis incidence, although sporadic outbreaks remain relatively common. In a recent publication (2), it was projected that in 2014, 24. One million cases of pertussis occurred around the world in children aged <5 years with ~160,000 deaths and many hospitalization admissions, some to pediatric intensive care units. Due to its frequency and severity, pertussis prevention has been considered an important public health issue for many years. The development of the whole-cell pertussis vaccine (wPV) and its introduction into the pediatric immunization schedule was associated with a marked reduction in pertussis cases in the vaccinated cohort (3). With widespread use of wPV, reporting of pertussis declined significantly worldwide, and both hospitalization rates and deaths due to pertussis were greatly reduced. However, due to the frequency of local and systemic adverse events after immunization with wPV, many parents were refusing vaccination for their children and lawsuits against the vaccine manufacturers were forcing many of them to stop producing the vaccine (4–6). Currently, wPV is no longer being used in most developed countries, but remains in use in most low and middle income countries (LMIC) (4, 7–9).
Work on a less reactive vaccine was undertaken based on the isolation of B. pertussis components that induced protective immune responses with fewer local and systemic reactions. These component vaccines were termed acellular vaccines and were composed of one or more pertussis antigens, including pertussis toxin (PT), filamentous haemagglutinin (FHA), pertactin (PRN), and fimbrial proteins 2 (FIM2) and 3 (FIM3). Preparations containing up to five components were developed, and several efficacy trials clearly demonstrated that the aPVs were able to confer comparable short-term protection to the most effective wPVs with fewer local and systemic reactions (10–15) (Figure 1). With this enhanced safety profile and despite being more expensive than wPVs, aPVs were included in the pediatric immunization schedules of many countries, particularly in the industrialized world (16, 17). After over a decade of use, a rise in pertussis incidence was demonstrated in several industrialized countries, including Australia and the United States (18–22). Some of this apparent increase may also be due to improved diagnostic methods, such as the use of molecular techniques to diagnose pertussis, but most experts think that it is also a result of more rapid waning of immunity after immunization with the acellular vaccines. Data from the United States document that 4,000 cases were reported annually in the 1980s, but increases to 25,827, 25,616, 27,500, and 48,277 cases were reported in 2004, 2005, 2010, and 2012, respectively (19–21). In 2016, 17,972 cases were reported, with an incidence rate of 70.9 per 100,000 in children <6 months, 31.9 per 100 000 in 6 to 11 month-olds, and 13.7, 14.8, and 16.3 for those aged 1–6, 7–10, and 11–19 years, respectively (23). However, the under-reporting of mild cases would be lower these rates.
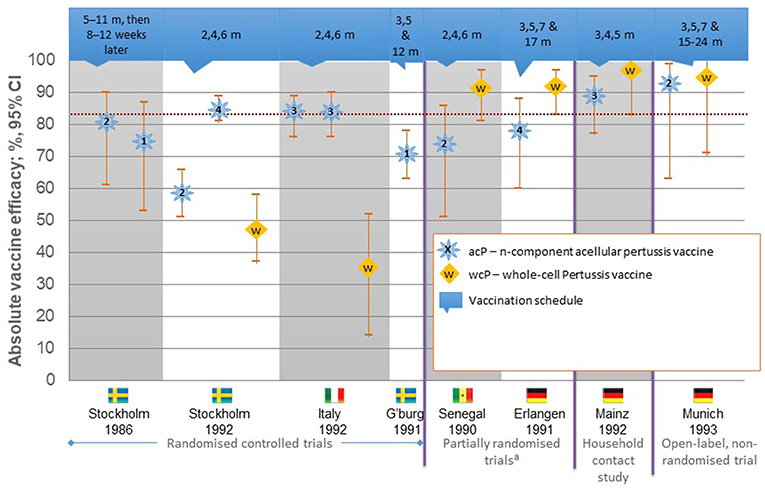
Figure 1. Efficacy of pertussis vaccines against World Health Organization-defined typical pertussis.
Trends that are not substantially different have been registered in other areas. In Europe, 48,446 pertussis cases were reported to the European Surveillance System by 30 EU/EEA countries in 2016. This number was slightly higher than that reported in 2012 (42,572), the year of peak pertussis incidence in Europe. As in the USA, rates were higher among children <1 year. In this younger group, the notification rate was 73.6 cases per 100,000 population, a value significantly higher than that reported in 2014 (51.6 per 100,000 population). Moreover, children between 10 and 14 years of age were reported to have the second highest incidence rates of pertussis, ~30 cases per 100,000 population (24).
The reasons for pertussis resurgence have been investigated, and several possibilities have been considered. This paper reports the results of a Consensus Conference organized by the World Association for Infectious Disease and Immunological Disorders (WAidid) on June 22, 2018, in Perugia, Italy, with the goal of evaluating the most important reasons for the pertussis resurgence and the role of different aPVs in this resurgence.
Laboratory Methods for Pertussis Diagnosis
Several reports indicate that resurgence of pertussis might be due, at least in part, to an artifact, resulting from an incomplete identification of pertussis cases in the past (Figure 2). The sole use of cultures to confirm B. pertussis infection has contributed to this phenomenon. Although the culture method has 100% specificity, its sensitivity is very low (25). Moreover, nasopharyngeal samples for culture have to be collected within the first 15 days of the disease, when symptoms are frequently non-specific, and such a diagnosis is rarely suspected. Furthermore, isolation can become difficult if the patient has recently been treated with antibiotics that are active against B. pertussis (26). In addition, pertussis cases occurring outside the first year of age (including in adulthood) frequently present as only prolonged cough and are undiagnosed, resulting in an underestimation of the true disease incidence (25, 26).
In recent years, more attention has been given to the epidemiology of pertussis disease in all age groups. New criteria for the clinical definition of pertussis according to age have been suggested (27). The enhanced identification of pertussis cases using molecular methods, particularly in adolescents and adults, has increased the total number of reported cases significantly (28). The molecular techniques, particularly real-time-polymerase chain reaction (RT-PCR), are widely available in clinical practice and can be completed in a few hours, allowing rapid diagnosis of pertussis. Moreover, since the techniques do not require viable bacteria, they are more sensitive (70–99% vs. 12–60%). However, similar to culture, they are maximally sensitive during the first 2–3 weeks of disease (29). Finally, to confirm B. pertussis infection beyond this period in previously undiagnosed patients, serologic methods detecting anti-pertussis toxin IgG in serum and saliva have been developed and validated (30).
The availability of reliable tests for pertussis diagnosis to physicians was rapidly associated with a relevant increase in their use and in the number of pertussis cases being reported. In Australia, the use of both both PCR and serology in patients with pertussis-like symptoms increased from 0.5% in the period from April 2000 to March 2004 to 1.7% in the period between March 2010 and March 2011. At the same time in Australia, the proportion of pertussis notifications with a PCR-confirmed diagnosis increased from 16.3% in the period from April 2000 to March 2004 to 65.3% in the period between April 2010 and March 2011 (31). In Canada, the availability of a more sensitive PCR assay was associated with a concomitant 6-fold increase in specimen submissions and a 5-fold increase in pertussis incidence (32).
Assessing the impact of the enhanced diagnostic methods to the resurgence of disease is difficult, given the periodicity of naturally occurring pertussis, the different control measures and reporting standards for different countries, and the different vaccines and schedules recommended in each country. However, a study carried out by a working group of the WHO seems to indicate that although improved diagnosis may play a role, a real increase in pertussis incidence has occurred in recent years (33). The group prepared a questionnaire to obtain information on pertussis incidence, vaccination coverage and schedules, surveillance methods, case definitions, and the type of vaccine used. The questionnaire was widely distributed to countries thought to have achieved long-standing high vaccine coverage and effective disease control. Resurgence was defined as evidence of a burden of disease higher than that expected when compared to previous reporting cycles in the same setting. A total of 19 countries participated. Although the increased pertussis incidence could be ascribed to cyclic patterns amplified by detection bias in most of these countries, in five of them (Australia, Chile, Portugal, USA, and UK), the presence of a true resurgence of pertussis in recent years was clearly demonstrated. Only one country using a wPV, Chile, reported a resurgence. However, this resurgence was mainly ascribed to decreased of vaccine coverage, variable coverage within the various districts, changes in the surveillance practices, and problems with the specificity of diagnostic tests. The WHO group concluded that pertussis resurgence was not observed in any country using wPVs, suggesting that a link between aPV use and increased pertussis reporting existed (33).
Reasons for Pertussis Resurgence After Acellular Pertussis Vaccine Introduction
Waning of Immunity
As is the case with immunization, natural B. pertussis infection does not assure permanent protection against pertussis. Several studies have documented that a second episode of pertussis can occur some years after the first one. Current estimates of the duration of protection due to natural infection range from 7 to 10 years (34, 35) to 20 years (36), but there is evidence that it can be as short as 3.5 years (37). Differences may be due to methods used to evaluate protection and pertussis epidemiology in different countries. However, duration of protection due to natural B. pertussis infection can vary from subject to subject as evidenced by Wearing and Rohani (38) who, exploring the inter-epidemic period and fade-out-frequency with a mathematical model, concluded that more than 10% of individuals lose protection within 10 years from infection whereas others are protected for more than 30 years.
A similar or only slightly reduced duration of protection has been calculated after immunization with wPV. Lambert (39) studied a pertussis outbreak that occurred in 1962 in Kent County, Michigan, USA. He found that the incidence of pertussis in vaccinated persons was directly related to the interval since the last immunization with wPV. Among the 210 vaccinated individuals with pertussis, attack rates were 21, 47, and 65% in groups of people who had received the vaccine within the last 4, 4 to 7 years earlier and 8–11 years earlier, respectively. Jenkinson (40) conducted a 10 year study of pertussis in a discrete general practice community in the UK and reported that protection due to immunization with wPV was still effective in 85% of children 4 years after immunization, but was reduced to 50% in the following 3 years.
In contrast, the duration of immunity after immunization with aPVs appears to be shorter, independent of the schedule used, the numbers, and concentrations of antigens included in each vaccine and the methods used to prepare the vaccines. Reports also suggested that pertussis occurred significantly earlier in subjects fully vaccinated with aPV than in those given wPV (41–43). Clark et al. (41) in 2012 reported that children who were fully immunized during infancy with an aPV had pertussis more often in the first years of school, while those given a wPV were at higher risk later, mainly during adolescence. Similar differences were demonstrated by Vickers et al. (43), who found that children who had received an aPV during infancy were already at risk of pertussis within the first 4 years of life, whereas those vaccinated with wPV did not contract pertussis until 5–9 years. Finally, Klein et al. (44) demonstrated that most of the pertussis cases diagnosed in adolescents during an outbreak were seen in individuals fully immunized during infancy with an aPV, rather than in those who had received wPV. Individuals receiving only aPV had five times higher odds of pertussis disease than those receiving wPV (OR 5.63, 95% CI 2.55–12.46). When aPV effectiveness (VE) was measured over time, its effectiveness was lower than that expected after wPV or natural infection (44). Early waning of immunity was reported regardless of the schedule used for immunization (44).
The addition of booster doses of aPV to prolong protection was also assessed. Although there was transient protection afforded by additional booster doses, the protection waned rapidly. A meta-analysis of 11 studies (45) that measured long-term immunity to pertussis after three or five doses of diphtheria-tetanus-aP (DTaP), according to the schedules used in many European countries and in the USA, respectively, did not reveal a significant difference between the annual odds of pertussis for the three or five dose regimens. It was calculated that for every additional year after the last dose of DTaP, the risk of pertussis increased 1.33 times (95% CI 1.23–1.43), leading to the conclusion that 8.5 years after the last aPV dose, only 10% of children were still protected against disease. Similar results were reported in another meta-analysis including studies of aPVs administered according to the USA schedule (46). VE was compared after the childhood series (five doses) and after an adolescent booster dose (sixth dose). Relative VE was defined as VE in the population given prior doses of an aPV and absolute VE was defined as VE in an aPV-naïve population. Absolute VE after the childhood series was 91% (95% CI 87–95%) but declined annually by 9.6% (46). Initial relative VE after adolescent boosting was 70% (95% CI: 54 to 86%) and declined by 45.3% annually. The absolute VE of the full six-dose aPV series was estimated to be 85% (95% CI: 84–86%) in the first year after series completion. However, it declined by 11.7% (95% CI: 11.1 to 12.3%) per year, and at 18 years of age, protection was limited to 28.2% of immunized patients (95% CI: 27 to 29%) (46).
Wang et al. (47) studied 279 children aged 5 to 15 years who presented to primary care with a persistent cough of 2 to 8 weeks duration. Evidence of recent B. pertussis infection based on a high oral fluid anti-pertussis toxin IgG titer was demonstrated in 215 children who had been fully vaccinated. Risk was higher in those who had been immunized ≥7 years earlier, but in 12% of these cases, chronic cough was demonstrated in patients given an aPV <7 years before. Further evidence of waning immunity after recent aPV immunization was reported by Principi et al. (48) who documented B. pertussis infection in 18.7% (95% CI 11.5–28.0) of children and adolescents with chronic cough who had been immunized with an aPV a few years previously (<2 years in some cases).
Immune Responses to Pertussis Vaccines and Natural Infection
Studies that have compared immune responses after natural B. pertussis infection and the administration of both wPVs and aPVs have clearly shown that the immune stimulation evoked by aPVs is different from that due to natural infection and wPVs (49–51). Natural infection evokes both mucosal and systemic immune responses, while aPVs induce only a systemic immune response. As B. pertussis is a mucosal pathogen and only exceptionally causes infection outside the respiratory tract, this difference is of particular importance in pertussis control. Mucosal immunity is essential to prevent colonization and transmission of B. pertussis organisms. Consequently, preventive measures such as aPVs that do not induce a valid mucosal response can prevent disease but cannot avoid infection and transmission. Animal studies have shown that natural infection is associated with a strong secretory IgA response in both the upper and lower airways and induction of resident memory T cells (TRM) (52, 53). Moreover, it has been recently reported (54) that IL-17 and IFN-γ-secreting CD69+CD4+ TRM cells were expanded in the respiratory tract after B. pertussis challenge of mice immunized with wP, but not aP vaccines. However, natural infection was associated with the most persistent protection against nasal colonization and this correlated with potent induction of nasal tissue TRM cells. These animal data suggest that the lack of mucosal immune response after aPV administration might explain its lower efficacy when compared to wPVs and the shorter duration of protection compared to both wPV vaccination and natural infection.
Clear differences between systemic immune response after natural infection and aP and wP vaccines. Natural infection and wPvs induce antibodies of the IgG1, IgG2, and IgG3 subclasses, with marginal production of IgG4 (55), suggesting a strong Th1 response. In contrast, the immune response after aPVs evoke a mixed Th2 and Th17 response (56). APVs evoke the production of IgG1 and IgG4 antibodies, which is consistent with a Th2 response. Furthermore, aPVs evoke CD4+ T-cells that produce high concentrations of IL-4 and IL-5 and low amounts of IFNγ, again consistent with a Th2 response (57).
Since Th1 cytokines play an important role in protection against pertussis (58, 59), this finding can further explain the better protection offered by wPVs and natural infection. Studies carried out in children who have received infant series of either wPV or aPVs have shown children given aPVs exhibited higher pertussis-specific antibody levels and higher memory B- and T-cell responses (5, 60–63). Although no correlates of antibody protection for pertussis have been established (64), the higher IgG levels in aPV-immunized children could lead to the conclusion that better humoral protection was afforded by the aP rather than wP vaccines. However, the antigens measured were only those included in the aPVs and not the additional antigens included in the wPs.
These differences in immune responses persist over time, even after booster aPVs (65, 66). The administration of aPV booster doses at 4 and 9 years of age was associated with an increase in the production of IgG4, regardless of the type of vaccine used for priming, but was significantly higher in aPV-primed children (66). IgG4 antibodies are unable to activate the complement system and lead to a suboptimal inflammatory response with impaired phagocytosis and antimicrobial defense, another potential mechanism for the lower efficacy of aPVs compared to wPVs (67). Moreover, the evidence that production of IgG4 after immunization with aPV increases with each dose seems to indicate that the protection offered by aPVs tends to be as shorter with each subsequent boosters (68, 69). Preadolescent booster vaccination with an aPV was found to induce lower B-cell and Th1 cell responses in aPV-primed compared with wPV-primed children, resulting in significantly lower Th1/Th2 ratios. Confirming this, it has been shown that wPv or aPV primary immunizations in infancy determines adolescent cellular immune profiles, showing a beneficial Th1-dominated response after wP-priming (69). These findings of a preferential Th1 response were also shown in the baboon model, with aPV vaccines preventing disease after natural pertussis challenge, but not preventing transmission of pertussis organisms (70). All these findings indicate that although aPVs are as individually protective as wPVs in the first years after priming, they induce shorter long-term protection than wPVs and a different profile of pertussis-specific immunity.
Finally, aPV pertussis vaccines do not prevent colonization. Consequently, they do not reduce the circulation of B. pertussis and do not exert any herd immunity effect. These findings at least partly explain the resurgence of pertussis.
Genetic Modifications of Bordetella pertussis
Circulation of B. pertussis strains with modified or absent antigens included in the aPV have been reported in both the pre-vaccine era and the aPV era (71–73). Moreover, strains with polymorphisms of the PT gene resulting in the production of greater amounts of this protein have been detected (74–79). Although it cannot be excluded that this phenomenon might simply be derived from the natural evolutionary course of B. pertussis, it has been proposed that it might be a consequence of B. pertussis adaptation to aPV use (80).
Genes encoding antigens included in the aPV vaccines have evolved at higher rates than other non-vaccine surface protein-encoding genes soon after the introduction of aPVs into the pediatric immunization schedule (81). The most compelling data have been the evolution of PRN-negative B. pertussis strains according to the use of vaccines PRN-containing vaccines. With some exceptions (82–84), studies have demonstrated that the emergence of PRN-deficient strains has resulted as a consequence of aPV-induced selection pressure. The rate of PRN-negative isolates is significantly correlated with aPV use in the USA (85). In Denmark, where an aPV without PRN is used, no PRN-deficient isolates have been detected (86). In Japan where aPVs with PRN were administered for many years (87), consistent rates of PRN-negative strains have been demonstrated over time (2005–2007, 41%; 2008–2010, 35%; and 2011–2013, 25%) (88, 89). However, when these aPV vaccines were replaced with a preparation without PRN in November 2012, a marked reduction of PRN-deleted strains was observed (2014–2016, 8%) (90). The clinical relevance of PRN-deleted strains has not been precisely defined (80), but children infected with these strains do not have more severe pertussis (91, 92), In contrast, B. pertussis strains with the enhanced PT promoter allele PTP3, instead of the common PTP2 allele, were found to produce greater amounts of PT (74) and cause more severe disease in younger infants (92).
Interesting, B. pertussis strains lacking the PRN gene show increased fitness and/or prolonged infection times in animals immunized with ACVs (74, 93, 94). This finding suggests that loss of PRN could lead to a reduced immune response to aPVs and favor pertussis resurgence. However, clinical studies that have evaluated the effectiveness of aPVs containing PRN in the setting of PRN-deficient pertussis have produced conflicting results. One study in the US (80) assessed the VEs of a five-dose DTaP series among 4–10 year-olds and a Tdap booster among 11–19 year-olds in an area where >90% of B. pertussis strains were PRN deficient. It was found that overall DTaP VE was 84% (95% CI 58–94%) while that of TdaP was 70% (95% CI 54–81%), which do not substantially differ than rates reported during the circulation of PRN-positive strains. In contrast, a second US study revealed that in vaccinated persons, the likelihood of suffering from pertussis disease was greater if the infecting strain was PRN-negative than if it is PRN-positive (85).
In conclusion, aPV use seems to favor adaptation of B. pertussis strains with emergence of mutated strains. However, the role of genetic modification in reducing aPV protection remains unclear with future studies needed.
Role of Antigens Included in Presently Available Vaccines in Conditioning Protection
Although pertussis resurgence has been demonstrated to be independent of the type of aPV used, it is theoretically possible that the composition of vaccines and the immunization strategies may have played a role in modifying the pertussis incidence. However, estimates of aPV efficacy and comparisons between different aPVs are very problematic for several reasons. First, the criteria for the diagnosis of pertussis used in the various aPV effectiveness trials have not been uniform. In some cases, significant underestimations of the real pertussis incidence may have limited the reliability of final results. When the WHO's clinical case definition of pertussis as prolonged paroxysmal cough is used, it is highly likely that most of the mild cases are not included. Second, study designs, administration schedules, and duration of follow-up have not been consistent in the effectiveness trials. In many European countries, the primary series includes only two doses of an aPV with a booster dose at ~1 year. In contrast, in other countries, including the US, the primary series is based on three doses within the first 6 months of life, with a booster dose given after the first birthday. Third, most, but not all, national immunization schedules include a booster before entering school and during adolescence. Fourth, the composition of the administered aPV can vary. Most of these studies have been carried out with vaccines containing three or five antigens, but in earlier studies vaccines with only PT have been included. In addition, the quantity of antigen can differ among the preparations. For example, GSK DTaP vaccines contain 25 μg PT, 25 μg FHA, and 8 μg PRN, while the Sanofi preparation also includes FIM2 and three different amounts of PT, FHA, and PRN for the primary and booster doses. Tdap contains 10 μg PT, 5 μg FHA, and 3 μg PRN when administered alone, but when Tdap is combined with polio, hepatitis B, and Haemophilus influenzae type b, the PT and FHA content is increased to 20 μg. In addition, the type of aluminum salt used as an adjuvant and its content vary slightly among between vaccines. Finally, there has been no single study that directly compares all aPV vaccines with different numbers and quantities of included antigens.
Role of the Number of Pertussis Antigens
In those studies that directly compared vaccines using similar vaccine schedules, similar definitions of pertussis disease, and comparable durations of follow-up, it can be concluded that the 3-component aPV (3aPV) and the 5-component aPV (5aPV) have comparable efficacy. Greco et al. evaluated two 3aPVs produced by different pharmaceutical companies (12), and Gustafsson et al. (13) studied a 5aPV, with both studies being conducted in children that had received three doses at ~2, 4, and 6 months of age and were followed for 2 years. For both 3aPVs, the overall efficacy was 84% (95% CI 75.8–89.4 for the first and 76.2–89.7 for the second 3aPV), while that of the 5aPV was 85.2% (95% CI 80.6–88.8) 1 year after first dose (13).
All these vaccines contain PT, which might explain these relatively similar results. PT seems to be essential for conferring protection (95, 96). In Sweden (10) and Denmark (97) a vaccine containing only PT demonstrated effectiveness that did not differ from that of multivalent aPVs concomitantly used in other countries. However, lower levels of anti-PT antibodies have been associated with increased susceptibility to B. pertussis infection (95, 96). It has not been determined which type and quantity of PT is able to confer the greatest short- and long-term protection. Because of its various noxious effects, PT must be detoxified before inclusion in aPVs. Detoxification can be achieved genetically or through chemical treatment. The results of these methods are quite different, as genetic detoxification does not modify the antigenic characteristics of PT and leads to a protein with superior immunogenicity to chemically detoxified PT, as clearly demonstrated by an efficacy trial showing that the PT-9K/129G-based vaccine induces earlier and longer-lasting protection (98). Additionally, lower doses of genetically detoxified PT seem to stimulate comparable protection as higher doses of the chemically detoxified product. In the study by Greco et al. (12) in which two 3aPVs were evaluated, the protection offered by the two vaccines was comparable but was achieved with 5 μg of genetically detoxified PT and with 25 μg of chemically detoxified PT.
Finally, it cannot be forgotten that not all chemical treatments have the same impact on PT. Detoxification with formaldehyde, glutaraldehyde, or with combined procedures are more destructive of epitopes than detoxification with hydrogen peroxide, evoking a lower immune response (50). Confirming this notion, important differences have been evidenced when comparing two aPVs detoxified by different chemical procedures in terms of long-term B and T-cell immune responses (99, 100).
The relevance of FHA for inducing protection is less clear. Several studies in mice suggest that pre-existing serum anti-FHA antibodies do not protect against pertussis in mice (101). FHA antibody induced by passive or active immunization did not protect mice against intracerebral or pulmonary challenge with B. pertussis (102). Finally, human studies conducted in Japan suggested that comparable protection was seen after either an aPV containing PT and FHA or only an aPV including only PT, although the results of this study remain debatable, since the PT and FHA combination vaccine contained only approximately 50% of the PT in PT only product. In contrast, another study in humans found that the relative efficacy of a monovalent PT vaccine was significantly less than that seen after combined PT-FHA vaccine during 3 years of passive surveillance (103). However, as FHA is an adhesin that is essential for the adherence of B. pertussis to the respiratory epithelium, it has been suggested that mucosal antibodies against this antigen could block adherence, thereby protecting against colonization, and, consequently, disease (104). This hypothesis is supported by the evidence that FHA delivered to experimental animals intranasally can provide protection against aerosol challenge with B. pertussis (105, 106).
The importance of PRN has already been discussed, and whether the lack of PRN in the infecting strain can reduce aPV efficacy remains controversial. Although protection against pertussis after aPV administration has been correlated with high serum anti-PRN antibody concentrations, pre-existing PRN antibodies do not protect against B. pertussis infection (97). Further studies are in progress to evaluate the impact of circulating B. pertussis strains lacking pertactin in a systematic project among EU countries.
Fimbriae are adhesins, and the hypothesis of a potential role reported previously for FHA can also be suggested for Fim2 and Fim3. However, data on the potential ability of these antigens to induce protective mucosal immunity are lacking. Serum levels of antibodies against Fim2 and Fim3 have been correlated with protection after household exposure to B. pertussis (107). Moreover, in a clinical trial, when mild cases of pertussis were included in the case definition, the efficacy of a 5aPV was found to be significantly higher than that of a two or three component aPV. Comparing the relative risk of 3aPV recipients acquiring pertussis to those receiving 5aPV was 1.82 (95% CI 1.14–2.90), suggesting a protective role of fimbriae (10, 107). In the presently available vaccines, FIM2 seems to be more important than FIM 3, as demonstrated by data collected during long-term evaluation of children included in a study by Olin et al. (108). A slight but significant reduction in 5aPV efficacy was seen over time when compared to 3aPV. During that time period, the expression of fimbriae in circulating B. pertussis changed from predominantly Fim2 to Fim3; suggesting that the stronger immune responses to the Fim2 antigen than the Fim3 antigen in the vaccinated subjects was the cause of the reduced long-term efficacy of the 5aPV, suggesting that FIM 2 evokes more relevant protection after 5aPV administration (109).
Vaccination Coverage and Vaccine Hesitancy
Although coverage with one dose of diphtheria- tetanus-pertussis vaccine (DTP1) is extremely high globally with overall levels at 90% in 2017, the coverage varies between widely 49 and 99%, depending on the country (110, 111). In 2017, the greatest number of cases of pertussis were reported in in India (23,766), Germany (16,183), Australia (12,114), and China (10,390), countries with national vaccination coverage rates of DTP1 all above 90% and coverage rates for three doses of DTP (DTP3) above 88% (112). However, coverage may be high nationally, there can be regional variation leading to sporadic pertussis cases and disease outbreaks (111).
Pertussis is an important cause of childhood morbidity and mortality, especially in infants under 6 months of age. Maternal pertussis immunization is an effective strategy to protect infants during this vulnerable period, prior to them being protected during their childhood vaccination. Maternal pertussis immunization has been included in national recommendations across several high-income countries, including the United States in 2011, the United Kingdom in 2012, and in Australia in 2015 (113, 114). Reasons for non-vaccination in pregnant women are context specific, and include a lack of awareness, a lack of access, a lack of perceived need for the vaccine, and concerns about its safety and effectiveness (115).
Conclusions
The resurgence of pertussis observed in recent years seems to be a complex but real phenomenon resulting from a number of cases, including the use of aPV in many locales. Lack of mucosal immune responses after aPV administration favor infection, persistent colonization, and transmission of the pathogen. Moreover, earlier waning of protective immunity and the circulation of B. pertussis variants depleted of vaccine-included antigens further favor the increase in pertussis disease. Several different aPVs are available, but it has yet to be determined which of them confers the highest and the most-prolonged protection. Further studies are needed to evaluate the importance of individual antigens included in aPVs in conferring protection against disease, colonization, and transmission. However, present knowledge seems to indicate that PT, particularly if genetically detoxified, represents the main antigen that ensures protection from disease even if not from infection. The contribution of FHA, PRN, and FIM2 and FIM3 in vaccine efficacy and long-lasting protection is still under discussion and needs further study.
The optimal pertussis vaccine would be one that induced both a mucosal and systemic responses similar to those occurring under natural infection, leading to a long-term protection against both disease and infection. Such a vaccine might increase public confidence and result in better vaccine uptake. Meanwhile, the identification of more efficacious vaccination strategies with currently available vaccines reaching high vaccination coverage rates is required, including the vaccination of pregnant women (50).
Author Contributions
SE proposed the project, coordinated the Study Group, and wrote the first draft of the study. PS, NF, and GF coordinated the sections of the project on Vaccines, Microbiology, and Immunology, respectively, giving a substantial scientific contribution. QH, PP, TT, MK, CR, CW, KF, IH, IL, KE, and MO gave a substantial scientific contribution. NP co-wrote the first draft of the manuscript and supervised the project. All authors approved the final submitted version of the manuscript.
Funding
This study was supported by WAidid 2018_11 grant.
Conflict of Interest Statement
SE has received consultancy fees and independent research grants from GlaxoSmithKline group of companies, Sanofi-Pasteur, Merck, Vifor, and DMG. MK was member of advisory boards for GSK, Pfizer, Baxter, Novartis, Astra Zeneca, MedImmune, SPMSD, Sanofi, MSD, Jansen, and performed for these companies presentations during industry symposia. These activities were done as a service task for his employer. Personally, he did not receive any fees from companies. There was also no target agreement with his employer in this respect. MO has received an investigator initiative research grant from Merck to evaluate population perception on hexavalent vs. heptavalent vaccines in Chile. KF is on the vaccine advisory boards for Sanofi Pasteur and Seqiris and have received honoraria from AstraZeneca and Pfizer for giving talks. PP has received research funding from the National Institute for Health Research and from GlaxoSmithKline, and has received honorariums from Sanofi Pasteur and Pfizer. CR has received consultancy fees and independent research grants from GlaxoSmithKline group of companies, Sanofi-Pasteur MSD, Wyeth, Pfizer, Astra-Zeneca, and Astellas.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Centers for Disease Control and Prevention. Pertussis (whooping cough). Causes and Transmission. (2017). Available online at: https://www.cdc.gov/pertussis/about/causes-transmission.html (accessed August 15, 2018).
2. Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. (2017) 17:974–80. doi: 10.1016/S1473-3099(17)30390-0
3. Cherry JD, Brunnel P, Golden G. Report of the task force on pertussis and pertussis immunization. Pediatrics. (1988) 81(Suppl.) 933–84.
4. Plotkin SA, Orenstein W, Offit PA. (editors). Vaccines. Philadelphia, PA: Elsevier Saunders (2013).
5. Berkovic SF, Harkin L, McMahon JM, Pelekanos JT, Zuberi SM, Wirrell EC, et al. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. (2006) 5:488–92. doi: 10.1016/S1474-4422(06)70446-X
6. Reyes IS, Hsieh DT, Laux LC, Wilfong AA. Alleged cases of vaccine encephalopathy rediagnosed years later as Dravet syndrome. Pediatrics. (2011) 128:e699–702. doi: 10.1542/peds.2010-0887
7. Sato Y, Kimura M, Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. (1984) 1:122–6. doi: 10.1016/S0140-6736(84)90061-8
8. Romanus V, Jonsell R, Bergquist SO. Pertussis in Sweden after the cessation of general immunization in 1979. Pediatr Infect Dis J. (1987) 6:364–71. doi: 10.1097/00006454-198704000-00005
9. Gangarosa EJ, Galazka AM, Wolfe CR, Phillips LM, Gangarosa RE, Miller E, et al. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet. (1998) 351:356–61. doi: 10.1016/S0140-6736(97)04334-1
10. Trollfors B, Taranger J, Lagergard T, Lind L, Sundh V, Zackrisson G, et al. A placebo-controlled trial of a pertussis-toxoid vaccine. N Engl J Med. (1995) 333:1045–50. doi: 10.1056/NEJM199510193331604
11. Storsaeter J, Hallander H, Farrington CP, Olin P, M€ollby R, Miller E. Secondary analyses of the efficacy of two acellular pertussis vaccines evaluated in a Swedish phase III trial. Vaccine. (1990) 8:457–61. doi: 10.1016/0264-410X(90)90246-I
12. Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med (1996) 334:341–8. doi: 10.1056/NEJM199602083340601
13. Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. (1996) 334:349–55. doi: 10.1056/NEJM199602083340602
14. Edwards KM, Decker MD. Acellular pertussis vaccines for infants. N Engl J Med. (1996) 334:391. doi: 10.1056/NEJM199602083340609
15. Hallander HO, Gustafsson L. Efficacy and effectiveness of acellular pertussis vaccines: a 20-year Swedish experience. Expert Rev Vacc. (2009) 8:1303–7. doi: 10.1586/erv.09.88
16. European Centre for Disease Prevention and Control. Vaccine Scheduler. Pertussis: Recommended Vaccinations. Available online at: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=3&SelectedCountryIdByDisease=-1 (accessed August 15, 2018).
17. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Available online at: https://www.cdc.gov/vaccines/pubs/pinkbook/pert.html (accessed August 15, 2018).
18. McIntyre P, Gidding H, Gilmour R, Lawrence G, Hull B, Horby P, et al. Vaccine preventable diseases and vaccination coverage in Australia, 1993–1998. Commun Dis Intell. (2000) 24(Suppl):22–26.
19. Jajosky RA, Hall PA, Adams DA, Dawkins FJ, Sharp P, Anderson WJ, et al. Summary of notifiable diseases—United States, 2004. MMWR Morb Mortal Wkly Rep. (2006) 53:1–79.
20. McNabb SJ, Jajosky RA, Hall-Baker PA, Adams DA, Sharp P, Anderson WJ, et al. Summary of notifiable diseases—United States, 2005. MMWR Morb Mortal Wkly Rep. (2007) 54:1–92.
21. Adams DA, Gallagher KA, Jajosky RA, Ward J, Sharp P, Anderon WJ, et al. Summary of notifiable diseases—United States, 2010. MMWR Morb Mortal Wkly Rep. (2012) 59:1–111.
22. Adams DA, Jajosky RA, Ajani U, Kriseman J, Sharp P, Onwen DH, et al. Summary of notifiable diseases—United States, 2012. MMWR Morb Mortal Wkly Rep. (2014) 61:1–121.
23. Centers for Disease Control and Prevention. Final Pertussis Surveillance Report. (2016) Available online at: https://www.cdc.gov/mmwr/volumes/66/wr/mm6652md.htm?s_cid=mm6652md_w (accessed August 15, 2018).
24. European Centre for Disease Prevention and Control. Surveillance Report. Annual Epidemiological Report for 2016 Pertussis. Available online at: https://ecdc.europa.eu/sites/portal/files/documents/AER-for-2016-pertussis.pdf (accessed on August 15, 2018).
25. Wendelboe AM, Van Rie A. Diagnosis of pertussis: a historical review and recent developments. Expert Rev Mol Diagn. (2006) 6:857–64. doi: 10.1586/14737159.6.6.857
26. Faulkner AE, Skoff T, Martin S, Cassiday P, Tondella ML, Liang J, et al. Pertussis. In: Roush SW, McIntyre L, Baldy LM, editors. VPD Surveillance Manual. 5th ed. Atlanta, GA: CDC (2011). p. 1–12.
27. Cherry JD, Tan T, Wirsing von König CH, Forsyth KD, Thisyakorn U, Greenberg D. Clinical definitions of pertussis: summary of a global pertussis initiative roundtable meeting, February 2011. Clin Infect Dis. (2012) 54:1756–64. doi: 10.1093/cid/cis302
28. Marchant CD, Loughlin AM, Lett SM, Todd CW, Wetterlow LH, Bicchieri R, et al. Pertussis in Massachusetts, 1981–1991: incidence, serologic diagnosis, and vaccine effectiveness. J Infect Dis. (1994) 169:1297–305. doi: 10.1093/infdis/169.6.1297
29. Centers for Disease Control and Prevention. Pertussis (Whooping cough) Best Practices for Healthcare Professionals on the Use of Polymerase Chain Reaction (PCR) for Diagnosing Pertussis. (2017). Available online at: https://www.cdc.gov/pertussis/clinical/diagnostic-testing/diagnosis-pcr-bestpractices.html (accessed August 15, 2018).
30. Campbell H, Amirthalingam G, Fry NK, Litt D, Harrison TG, Wagner K, et al. Oral fluid testing for pertussis, England and Wales, June 2007-August 2009. Emerg Infect Dis. (2014) 20:968–75. doi: 10.3201/eid2006.131069
31. Kaczmarek MC, Valenti L, Kelly HA, Ware RS, Britt HC, Lambert SB. Sevenfold rise in likelihood of pertussis test requests in a stable set of Australian general practice encounters, 2000–2011. Med J Aust. (2013) 198:624–8. doi: 10.5694/mja13.10044
32. Fisman DN, Tang P, Hauck T, Richardson S, Drews SJ, Low DE. Pertussis resurgence in Toronto, Canada: a population-based study including test-incidence feedback modeling. BMC Public Health. (2011) 11:694. doi: 10.1186/1471-2458-11-694
33. World Health Organization. WHO SAGE pertussis working group Background paper SAGE April 2014. (2014). Available online at: http://www.who.int/immunization/sage/meetings/2014/april/1_Pertussis_background_FINAL4_web.pdf (accessed August 15, 2018).
34. Miller E, Gay NJ. Epidemiological determinants of pertussis. Dev Biol Stand. (1997) 89:15–23 doi: 10.1016/S0099-1767(97)90076-3
35. Broutin H, Simondon F, Rohani P, Guéan JF, Grenfell BT. Loss of immunity to pertussis in a rural community in Senegal. Vaccine. (2004) 22:594–6. doi: 10.1016/j.vaccine.2003.07.018
36. Wirsing von König CH, Postels-Multani S, Bock HL, Schmitt HJ. Pertussis in adults: frequency of transmission after household exposure. Lancet. (1995) 346:1326–9. doi: 10.1016/S0140-6736(95)92343-8
37. Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. (2005) 24(5 Suppl):S58–61. doi: 10.1097/01.inf.0000160914.59160.41
38. Wearing HJ, Rohani P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog. (2009) 5:e1000647. doi: 10.1371/journal.ppat.1000647
39. Lambert HJ. Epidemiology of a small pertussis outbreak in Kent county, Michigan. Public Health Rep. (1965) 80:365–9. doi: 10.2307/4592424
40. Jenkinson D. Duration of effectiveness of pertussis vaccine: evidence from a 10 year community study. Br Med J. (1988) 296:612–4. doi: 10.1136/bmj.296.6622.612
41. Clark TA, Messonnier NE, Hadler SC. Pertussis control: time for something new? Trends Microbiol. (2012) 20:211–3. doi: 10.1016/j.tim.2012.03.003
42. Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. (2012) 308:454–6. Erratum in: JAMA (2012) 308:1432. doi: 10.1001/jama.2012.6364
43. Vickers D, Ross AG, Mainar-Jaime RC, Neudorf C, Shah S. Whole-cell and acellular pertussis vaccination programs and rates of pertussis among infants and young children. CMAJ. (2006) 175:1213–7. doi: 10.1503/cmaj.051637
44. Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics. (2013) 131:e1716–22. doi: 10.1542/peds.2012-3836
45. McGirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics. (2015) 135:331–43. doi: 10.1542/peds.2014-1729
46. Chit A, Zivaripiran H, Shin T, Lee JKH, Tomovici A, Macina D, et al. Acellular pertussis vaccines effectiveness over time: a systematic review, meta-analysis and modeling study. PLoS ONE. (2018) 13:e0197970. doi: 10.1371/journal.pone.0197970
47. Wang K, Fry NK, Campbell H, Amirthalingam G, Harrison TG, Mant D, et al. Whooping cough in school age children presenting with persistent cough in UK primary care after introduction of the preschool pertussis booster vaccination: prospective cohort study. BMJ. (2014) 348:g3668. doi: 10.1136/bmj.g3668
48. Principi N, Litt D, Terranova L, Picca M, Malvaso C, Vitale C, et al. Pertussis-associated persistent cough in previously vaccinated children. J Med Microbiol. (2017) 66:1699–1702.
49. Esposito S, Agliardi T, Giammanco A, Faldella G, Cascio A, Bosis S, et al. Long-term pertussis-specific immunity after primary vaccination with a combined diphtheria, tetanus, tricomponent acellular pertussis, and hepatitis B vaccine in comparison with that after natural infection. Infect Immun. (2001) 69:4516–20. doi: 10.1099/jmm.0.000607
50. Esposito S, Principi N. Prevention of pertussis: an unresolved problem. Hum Vaccin Immunother. (2018) 14:2452–9. doi: 10.1080/21645515.2018.1480298
51. Periscope Consortium. Periscope: road towards effective control of pertussis. Lancet Infect Dis. (2019) 19:e179–86. doi: 10.1016/S1473-3099(18)30646-7
52. Solans L, Locht C. The Role of mucosal immunity in pertussis. Front Immunol. (2019) 9:3068. doi: 10.3389/fimmu.2018.03068
53. Allen AC, Wilk MM, Misiak A, Borkner L, Murphy D, Mills KHG. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol. (2018) 11:1763–76. doi: 10.1038/s41385-018-0080-x
54. Wilk MM, Borkner L, Misiak A, Curham L, Allen AC, Mills KHG. Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg Microbes Infect. (2019) 8:169–185. doi: 10.1080/22221751.2018.1564630
55. Raeven RH, van der Maas L, Tilstra W, Uittenbogaard JP, Bindels TH, Kuipers B, et al. Immunoproteomic profiling of Bordetella pertussis outer membrane vesicle vaccine reveals broad and balanced humoral immunogenicity. J Proteome Res. (2015) 14:2929–42. doi: 10.1021/acs.jproteome.5b00258
56. Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, et al. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. (2013) 9:e1003264. doi: 10.1371/journal.ppat.1003264
57. Palazzo R, Carollo M, Bianco M, Fedele G, Schiavoni I, Pandolfi E, et al. Persistence of T-cell immune response induced by two acellular pertussis vaccines in children five years after primary vaccination. New Microbiol. (2016) 39:35–47.
58. Higgs R, Higgins S C, Ross PJ, Mills KH. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol. (2012) 5:485–500. doi: 10.1038/mi.2012.54
59. Ryan M, Gothefors L, Storsaeter J, Mills KH. Bordetella pertussis-specific Th1/Th2 cells generated following respiratory infection or immunization with an acellular vaccine: comparison of the T cell cytokine profiles in infants and mice. Dev Biol Stand. (1997) 89:297–305.
60. Hendrikx LH, Berbers GA, Veenhoven RH, Sanders EA, Buisman AM. IgG responses after booster vaccination with different pertussis vaccines in Dutch children 4 years of age: effect of vaccine antigen content. Vaccine. (2009) 27:6530–6. doi: 10.1016/j.vaccine.2009.08.052
61. Schure RM, Hendrikx LH, de Rond LG, Oztürk K, Sanders EA, Berbers GA, et al. T-cell responses before and after the fifth consecutive acellular pertussis vaccination in 4-year-old Dutch children. Clin Vaccine Immunol. (2012) 19:1879–86. doi: 10.1128/CVI.00277-12
62. Hendrikx LH, de Rond LG, Oztürk K, Veenhoven RH, Sanders EA, Berbers GA, et al. Impact of infant and preschool pertussis vaccinations on memory B-cell responses in children at 4 years of age. Vaccine. (2011) 29:5725–30. doi: 10.1016/j.vaccine.2011.05.094
63. Smits K, Pottier G, Smet J, Dirix V, Vermeulen F, De Schutter I, et al. Different T cell memory in preadolescents after whole-cell or acellular pertussis vaccination. Vaccine. (2013) 32:111–8. doi: 10.1016/j.vaccine.2013.10.056
64. Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine. (1998) 16:1907–16. doi: 10.1016/S0264-410X(98)00227-8
65. Brummelman J, Helm K, Hamstra HJ, van der Ley P, Boog CJ, Han WG, et al. Modulation of the CD4(+) T cell response after acellular pertussis vaccination in the presence of TLR4 ligation. Vaccine. (2015) 33:1483–91. doi: 10.1016/j.vaccine.2015.01.063
66. van der Lee S, Sanders EAM, Berbers GAM, Buisman AM. Whole-cell or acellular pertussis vaccination in infancy determines IgG subclass profiles to DTaP booster vaccination. Vaccine. (2018) 36:220–6. doi: 10.1016/j.vaccine.2017.11.066
67. Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. (2009) 39:469–77. doi: 10.1111/j.1365-2222.2009.03207.x
68. da Silva Antunes R, Babor M, Carpenter C, Khalil N, Cortese M, Mentzer AJ, et al. Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters. J Clin Invest. (2018) 128:3853–65. doi: 10.1172/JCI121309
69. van der Lee S, Hendrikx LH, Sanders EAM, Berbers GAM, Buisman AM. Whole-cell or acellular pertussis primary immunizations in infancy determines adolescent cellular immune profiles. Front Immunol. (2018) 9:51. doi: 10.3389/fimmu.2018.00051
70. Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA. (2014) 111:787–92. doi: 10.1073/pnas.1314688110
71. Bart MJ, Harris SR, Advani A, Arakawa Y, Bottero D, Bouchez V, et al. Global population structure and evolution of Bordetella pertussis and their relationship with vaccination. MBio. (2014) 5:e01074. doi: 10.1128/mBio.01074-14
72. Belcher T, Preston A. Bordetella pertussis evolution in the (functional) genomics era. Pathog Dis. (2015) 73:ftv064. doi: 10.1093/femspd/ftv064
73. van Gent M, Heuvelman CJ, van der Heide HG, Hallander HO, Advani A, Guiso N, et al. Analysis of Bordetella pertussis clinical isolates circulating in European countries during the period 1998–2012. Eur J Clin Microbiol Infect Dis. (2015) 34:821–30. doi: 10.1007/s10096-014-2297-2
74. Mooi FR, van Loo IH, van Gent M, He Q, Bart MJ, Heuvelman KJ, et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg Infect Dis. (2009) 15:1206–13. doi: 10.3201/eid1508.081511
75. Advani A, Gustafsson L, Ahrén C, Mooi FR, Hallander HO. Appearance of Fim3 and ptxP3-Bordetella pertussis strains, in two regions of Sweden with different vaccination programs. Vaccine. (2011) 29:3438–42. doi: 10.1016/j.vaccine.2011.02.070
76. Hegerle N, Paris AS, Brun D, Dore G, Njamkepo E, Guillot S, et al. Evolution of French Bordetella pertussis and Bordetella parapertussis isolates: increase of Bordetellae not expressing pertactin. Clin Microbiol Infect. (2012) 18:E340–6. doi: 10.1111/j.1469-0691.2012.03925.x
77. Kallonen T, Mertsola J, Mooi FR, He Q. Rapid detection of the recently emerged Bordetella pertussis strains with the ptxP3 pertussis toxin promoter allele by real-time PCR. Clin Microbiol Infect. (2012) 18:E377–9. doi: 10.1111/j.1469-0691.2012.04000.x
78. Lam C, Octavia S, Bahrame Z, Sintchenko V, Gilbert GL, et al. Selection and emergence of pertussis toxin promoter ptxP3 allele in the evolution of Bordetella pertussis. Infect Genet Evol. (2012) 12:492–5. doi: 10.1016/j.meegid.2012.01.001
79. Octavia S, Sintchenko V, Gilbert GL, Lawrence A, Keil AD, Hogg G, et al. Newly emerging clones of Bordetella pertussis carrying prn2 and ptxP3 alleles implicated in Australian pertussis epidemic in 2008–2010. J Infect Dis. (2012) 205:1220–4. doi: 10.1093/infdis/jis178
80. Breakwell L, Kelso P, Finley C, Schoenfeld S, Goode B, Misegades LK, et al. Pertussis vaccine effectiveness in the setting of pertactin-deficient pertussis. Pediatrics. (2016) 137:e20153973. doi: 10.1542/peds.2015-3973
81. Sealey KL, Harris SR, Fry NK, Hurst LD, Gorringe AR, Parkhill J, et al. Genomic analysis of isolates from the United Kingdom 2012 pertussis outbreak reveals that vaccine antigen genes are unusually fast evolving. J Infect Dis. (2015) 212:294–301. doi: 10.1093/infdis/jiu665
82. Barkoff AM, Mertsola J, Guillot S, Guiso N, Berbers G, He Q. Appearance of Bordetella pertussis strains not expressing the vaccine antigen pertactin in Finland. Clin Vaccine Immunol. (2012) 19:1703–4. doi: 10.1128/CVI.00367-12
83. Bouchez V, Brun D, Cantinelli T, Dore G, Njamkepo E, Guiso N. First report and detailed characterization of B. pertussis isolates not expressing pertussis toxin or pertactin. Vaccine. (2009) 27:6034–49. doi: 10.1016/j.vaccine.2009.07.074
84. Mastrantonio P, Spigaglia P, van Oirschot H, van der Heide HG, Heuvelman K, Stefanelli P, et al. Antigenic variants in Bordetella pertussis strains isolated from vaccinated and unvaccinated children. Microbiology. (1999) 145:2069–75. doi: 10.1099/13500872-145-8-2069
85. Martin SW, Pawloski L, Williams M, Weening K, Debolt C, et al. Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis. (2015) 60:223–7. doi: 10.1093/cid/ciu788
86. Zeddeman A, van Gent M, Heuvelman CJ, van der Heide HG, Bart MJ, Advani A, et al. Investigations into the emergence of pertactin-deficient Bordetella pertussis isolates in six European countries, 1996 to 2012. Euro Surveill. (2014) 19:20881. doi: 10.2807/1560-7917.ES2014.19.33.20881
87. Okada K, Komiya T, Yamamoto A, Takahashi M, Kamachi K, Nakano T, et al. Safe and effective booster immunization using DTaP in teenagers. Vaccine. (2010) 28:7626–33. doi: 10.1016/j.vaccine.2010.09.050
88. Otsuka N, Han HJ, Toyoizumi-Ajisaka H, Nakamura Y, Arakawa Y, Shibayama K, et al. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS ONE. (2012) 7:e31985. doi: 10.1371/journal.pone.0031985
89. Miyaji Y, Otsuka N, Toyoizumi-Ajisaka H, Shibayama K, Kamachi K. Genetic analysis of Bordetella pertussis isolates from the 2008–2010 pertussis epidemic in Japan. PLoS ONE. (2013) 8:e77165. doi: 10.1371/journal.pone.0077165
90. Hiramatsu Y, Miyaji Y, Otsuka N, Arakawa Y, Shibayama K, Kamachi K. Significant decrease in pertactin-deficient Bordetella pertussis isolates, Japan. Emerg Infect Dis. (2017) 23:699–701. doi: 10.3201/eid2304.161575
91. Bodilis H, Guiso N. Virulence of pertactin-negative Bordetella pertussis isolates from infants, France. Emerg Infect Dis. (2013) 19:471–4. doi: 10.3201/eid1903.121475
92. Clarke M, Mcintyre PB, Blyth CC, Wood N, Octavia S, Sintchenko V, et al. The relationship between Bordetella pertussis genotype and clinical severity in Australian children with pertussis. J Infect. (2016) 72:171–8. doi: 10.1016/j.jinf.2015.11.004
93. Hegerle N, Dore G, Guiso N. Pertactin deficient Bordetella pertussis present a better fitness in mice immunized with an acellular pertussis vaccine. Vaccine. (2014) 32:6597–600. doi: 10.1016/j.vaccine.2014.09.068
94. Safarchi A, Octavia S, Luu LDW, Tay CY, Sintchenko V, Wood N, et al. Pertactin negative Bordetella pertussis demonstrates higher fitness under vaccine selection pressure in a mixed infection model. Vaccine. (2015) 33:6277–81. doi: 10.1016/j.vaccine.2015.09.064
95. Robbins JB, Schneerson R, Keith JM, Miller MA, Kubler-Kielb J, Trollfors B. Pertussis vaccine: a critique. Pediatr Infect Dis J. (2009) 28:237–41. doi: 10.1097/INF.0b013e31818a8958
96. Storsaeter J, Hallander HO, Gustafsson L, Olin P. Low levels of anti-pertussis antibodies plus lack of history of pertussis correlate with susceptibility after household exposure to Bordetella pertussis. Vaccine. (2003) 21:3542–9. doi: 10.1016/S0264-410X(03)00407-9
97. Thierry-Carstensen B, Dalby T, Stevner MA, Robbins JB, Schneerson R, Trollfors B. Experience with monocomponent acellular pertussis combination vaccines for infants, children, adolescents and adults–a review of safety, immunogenicity, efficacy and effectiveness studies and 15 years of field experience. Vaccine. (2013) 31:5178–91. doi: 10.1016/j.vaccine.2013.08.034
98. Seubert A, D'Oro U, Scarselli M, Pizza M. Genetically detoxified pertussis toxin (PT-9K/129G): implications for immunization and vaccines. Expert Rev Vaccines. (2014) 13:1191–204. doi: 10.1586/14760584.2014.942641
99. Carollo M, Pandolfi E, Tozzi AE, Buisman AM, Mascart F, Ausiello CM. Humoral and B-cell memory responses in children five years after pertussis acellular vaccine priming. Vaccine. (2014) 32:2093–9. doi: 10.1016/j.vaccine.2014.02.005
100. Palazzo R, Carollo M, Bianco M, Fedele G, Schiavoni I, Pandolfi E, et al. Persistence of T-cell immune response induced by two acellular pertussis vaccines in children five years after primary vaccination. New Microbiol. (2016) 39:35–47.
101. Taranger J, Trollfors B, Lagergård T, Zackrisson G. Parapertussis infection followed by pertussis infection. Lancet. (1994) 344:1703. doi: 10.1016/S0140-6736(94)90485-5
102. Sato H, Sato Y. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in an intracerebral or aerosol challenge system. Infect Immun. (1984) 46:415–21.
103. Sorsaeter J, Olin P. Relative efficacy of two acellular pertussis vaccines during three years of passive surveillance. Vaccine. (1992) 10:142–4. doi: 10.1016/0264-410X(92)90002-2
104. Scheller EV, Cotter PA. Bordetella filamentous hemagglutinin and fimbriae: critical adhesins with unrealized vaccine potential. Pathog Dis. (2015) 73:ftv079. doi: 10.1093/femspd/ftv079
105. Alonso S, Reveneau N, Pethe K, Locht C. Eighty-kilodalton N-terminal moiety of Bordetella pertussis filamentous hemagglutinin: adherence, immunogenicity, and protective role. Infect Immun. (2002) 70: 4142–7. doi: 10.1128/IAI.70.8.4142-4147.2002
106. Knight JB, Huang YY, Halperin SA, Anderson R, Morris A, Macmillan A, et al. Immunogenicity and protective efficacy of a recombinant filamentous haemagglutinin from Bordetella pertussis. Clin Exp Immunol. (2006) 144:543–51. doi: 10.1111/j.1365-2249.2006.03097.x
107. Cherry JD, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. (1998) 16:1901–6. doi: 10.1016/S0264-410X(98)00226-6
108. Olin P, Rasmussen F, Gustafsson L, Hallander HO, Heijbel H. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Lancet. (1997) 350:1569–77. doi: 10.1016/S0140-6736(97)06508-2
109. Hallander H, Advani A, Alexander F, Gustafsson L, Ljungman M, Pratt C, et al. Antibody responses to Bordetella pertussis Fim2 or Fim3 following immunization with a whole-cell, two-component, or five-component acellular pertussis vaccine and following pertussis disease in children in Sweden in 1997 and 2007. Clin Vaccine Immunol. (2014) 21:165–73. doi: 10.1128/CVI.00641-13
110. WHO. Global and regional immunization profile. Data received as of 21 September 2018. (2018). Available online at: http://www.who.int/immunization/monitoring_surveillance/data/gs_gloprofile.pdf?ua=1 (accessed October 4, 2018).
111. WHO. Official country reported coverage estimates time series. Immunization, Vaccines and Biologicals. Data, statistics and graphics. (2018). Available online at: http://www.who.int/immunization/monitoring_surveillance/data/en/ (accessed October 4, 2018).
112. WHO. Disease incidence time series. Immunization, Vaccines and Biologicals. Data, statistics and graphics. (2018). Available online at: http://www.who.int/immunization/monitoring_surveillance/data/en/ (accessed October 4, 2018).
113. Advisory Committee on Immunization Practices (ACIP). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women–Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. (2013) 62:131–5.
114. Australian Technical Advisory Group on Immunisation (ATAGI). The Australian Immunisation Handbook. 10th ed. Canberra, ACT: Australian Government Department of Health (2017).
Keywords: acellular pertussis vaccine, Bordetella pertussis, pertussis, whole-cell pertussis vaccine, pertussis prevention
Citation: Esposito S, Stefanelli P, Fry NK, Fedele G, He Q, Paterson P, Tan T, Knuf M, Rodrigo C, Weil Olivier C, Flanagan KL, Hung I, Lutsar I, Edwards K, O'Ryan M and Principi N (2019) Pertussis Prevention: Reasons for Resurgence, and Differences in the Current Acellular Pertussis Vaccines. Front. Immunol. 10:1344. doi: 10.3389/fimmu.2019.01344
Received: 24 February 2019; Accepted: 28 May 2019;
Published: 03 July 2019.
Edited by:
Luciana Leite, Instituto Butantan, BrazilReviewed by:
Camille Locht, Institut National de La Santé et de la Recherche Médicale (INSERM), FranceCarmen Alvarez-Dominguez, Instituto de Investigación Marques de valdecilla (IDIVAL), Spain
Kingston H. Mills, Trinity College Dublin, Ireland
Copyright © 2019 Esposito, Stefanelli, Fry, Fedele, He, Paterson, Tan, Knuf, Rodrigo, Weil Olivier, Flanagan, Hung, Lutsar, Edwards, O'Ryan and Principi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanna Esposito, c3VzYW5uYS5lc3Bvc2l0b0B1bmltaS5pdA==
 Susanna Esposito
Susanna Esposito Paola Stefanelli
Paola Stefanelli Norman K. Fry
Norman K. Fry Giorgio Fedele
Giorgio Fedele Qiushui He4,5
Qiushui He4,5 Catherine Weil Olivier
Catherine Weil Olivier Katie L. Flanagan
Katie L. Flanagan Nicola Principi
Nicola Principi