- 1Department for Congenital Disorders, Statens Serum Institut, Copenhagen, Denmark
- 2Centre for Medical Parasitology at Department of International Health, Immunology and Microbiology, University of Copenhagen and Department of Infectious Diseases, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark
- 3Department of Medical Microbiology, Radboud University Medical Center, Nijmegen, Netherlands
The Plasmodium falciparum Pfs230 and Pfs48/45 proteins are expressed during transmission from man to mosquito and are leading candidates for a malaria transmission blocking vaccine. Individually they generate transmission blocking (TB) antibodies in rodent models. Whether the single protein vaccines are suitable to use in field settings will primarily depend on their potency to elicit functional antibodies. We hypothesized that a combination of both proteins will be more potent than each protein individually. Therefore we designed chimeric proteins composed of fragments of both Pfs230 and Pfs48/45 as well as single protein fragments, and expressed these in Lactoccus lactis. Both the individual Pfs230 and Pfs48/45 fragments and chimeras elicited high levels of functional antibodies in mice. Importantly, one of the chimeric proteins elicited over threefold higher transmission blocking antibody responses than the single antigens alone. Furthermore the immunogenicity of one of the chimeras could be enhanced through coupling to a virus-like particle (VLP). Altogether these data support further clinical development of these novel constructs.
Introduction
The transmission of Plasmodium falciparum from one person to another relies on the generation of male and female gametocytes in the human host that can be picked up and spread by a mosquito. The aim of a malaria transmission blocking vaccine (MTBV) is to effectively block malaria transmission at the population level thereby contributing to malaria elimination.
Several MTBV candidates have been identified by screening monoclonal antibodies generated against P. falciparum mosquito stages for TB activity (1–4). Three proteins, Pf s48/45, Pf s230, and Pf s25 are currently targeted as lead candidates for an MTBV. Of these, Pf s48/45 and Pf s230 are expressed in the gametocyte as it develops from stage III through V in the human host. Shortly, after being taken up by a blood-feeding mosquito, the parasite emerges from the RBC as a gamete and after a few rounds of replication motile males fertilize female gametes to form zygotes. Pf s48/45 is expressed on the surface of both male and female gametes where it is bound to the plasma membrane through a GPI-anchor (5) and forms a stable complex with Pf s230 (6). Both Pf s48/45 and Pf s230 are important for male fertility (7).
Humans develop naturally acquired immunity against P. falciparum gametocytes (8–10) and antibodies against Pf s230 and Pf s48/45 have been associated with TB activity in some but not all immune epidemiological studies (11, 12). Recently, we demonstrated that Pf s48/45- and Pf s230-specific antibodies exhibit strong TB activity in the standard membrane feeding assay (SMFA) (13), the gold standard for assessing transmission blockade ex vivo (2, 14–16). Whether such antibodies act synergistically, as observed for combinations of mAbs targeting different epitopes on Pf s48/45 (2), is not yet known.
Pf s48/45 and Pf s230 are members of the six-cysteine (6-Cys) s48/45 protein family and contain three and fourteen 6-Cys domains respectively (17). Each 6-Cys domain contains up to six cysteine residues that are involved in intra-domain disulfide bond formation which results in conformational antibody epitopes. The C-terminal 6-Cys domain of Pfs48/45 contains the conformational epitope I, which is targeted by the most potent TB monoclonal antibody described to date, mAb45.1 (18). We have recently used the Lactococcus lactis expression system for the production of the C-terminal 6-Cys domain of Pf s48/45 (6C) as a fusion protein (R0.6C) with the N-terminal GLURP-R0 region (19, 20). The resulting fusion protein can be produced in high yields of properly folded monomeric protein which elicited high levels of TB antibodies in small rodents (19, 20). In the case of Pf s230, the C fragment spanning the N-terminal pro-domain and first three 6-Cys domains has been shown to elicit the most potent TB antibodies (21). The presence of three 6-Cys domains suggests that disulfide bonds may be critical for proper folding of each of these domains. Accordingly, a series of Pf s230-specific transmission-blocking monoclonal antibodies did not recognize reduced Pf s230 (22). In an attempt to identify the minimal Pf s230-domain involved in the generation of TB antibodies, Pf s230 constructs containing the Pro, Pro+I, Pro+I,II, and Pro+I,II,III domains were produced individually in the wheat germ cell-free system (23). Interestingly, the N-terminal Pro domain, which lacks cysteines, was sufficient to elicit complement-dependent TB activity in the SMFA, suggesting that TB antibodies may also be directed against non-conformational epitopes (23). With respect to Pf s230, the C-fragment was the first construct to elicit TB antibodies; however, oocyst reduction was incomplete suggesting that folding was compromised by an incorrect cysteine connectivity (21). Another construct, Pf s230D1, corresponding to amino acid residues 444 to 736 was produced in Pichia pastoris as a properly folded protein and elicited TB antibodies in rodents (24).
While clinical trials with Pf s230D1 are ongoing (ClinicalTrials.gov Identifier: NCT02334462) and R0.6C is in early clinical development phase, we sought to identify more potent Pf s48/45- and Pf s230-based immunogens. We hypothesized that a combination of antibodies against both proteins would be more potent than against each antigen individually. Therefore we constructed chimeric proteins composed of fragments of both Pf s230 and Pf s48/45, expressed these in L. lactis and evaluated antibody responses in rodents. A multicomponent hybrid protein containing both Pf s48/45 and Pf s230 holds the potential to lower the required threshold of functional antibodies and to reduce the risk of escape mutations.
Methods
Preparation of Constructs
Three different truncated forms of Pf s230 from N-terminus, i.e. Pro (pro domain AA 443 to 590), Pro+I (pro domain and domain I, AA 443 to 736) and Pro+I,II,III (pro domain through domain III, AA 443 to 1132) were amplified by PCR from P. falciparum 3D7 DNA (GenBank accession number L08135) and cloned into the BglII restriction site of pSS5 plasmid containing N-terminus Spycatcher (25). Pf s48/45291−428 (6C) was amplified from an expression vector encoding R0.6C (19, 20) using the forward primer 5′-CCATGGATCCGAAAAAAAAGTCATACACGGATGTAACTTC-3′ and the reverse primer 5′-CCATAGATCTTGCTGAATCTATAGTAACTGTCATATAAGC-3′. The amplified PCR product was digested with BamHI and BglII (underlined) and cloned in frame into plasmids containing the Pro or Pro+I inserts to generate Pro-6C and Pro+I-6C fusion constructs, respectively. All the constructs were verified by DNA sequencing and transformed into L. lactis MG1363 by electroporation for expression of recombinant proteins with 6xHis tags.
Fermentation and Protein Purification
Fermentation of L. lactis MG1363, containing Pf s230 or Pf s230-Pf s48/45 fusion constructs were carried out as described previously (19, 26). Briefly, cell-free culture-filtrates were concentrated five-fold and buffer exchanged into Tris buffer (50 mM Tris, 50mM NaCl pH 8.0 supplemented with 10mM Imidazole) using a Quix Stand Benchtop system (Hollow fiber cartridge with cutoff at 10,000 or 30,000 Da, surface area 650cm2, GE Healthcare, Sweden) followed by filtration through a Durapore filter (PVDF, 0.22 μm, Millipore) and applied to a 5 ml HisTrap HP column (GE Healthcare, Sweden). Bound protein was eluted with 500mM Imidazole in Tris buffer pH 8.0 (50 mM Tris, 50mM NaCl) at a flow rate of 4 ml/min. Fractions containing the desired protein were further applied to a 5 ml HiTrap Q HP column (GE Healthcare, Sweden) for purification of monomeric proteins. Bound protein was eluted through step gradient elution in Tris buffer pH 8.0 (50 mM Tris, 1mM EDTA, 1 M NaCl) and fractions containing monomers were concentrated by a VIVA spin column with a 10 or 30 kDa cutoff (Vivascience, Germany), and kept in 50 mM Tris, 250 mM NaCl and 1 mM EDTA, pH 8.0 at −80°C until use. Immune purification for Pro-6C and Pro+I-6C was done as previously described (26). Fractions containing the desired protein were pooled and then concentrated and buffer exchanged against 50 mM Tris, 100 mM NaCl, and 1 mM EDTA, pH 8.0 and kept at −80°C until use. Fractions were analyzed by SDS-PAGE and immune blotting with mAb45.1 against Pf s48/45 conformational epitope I. Protein concentrations were measured using a BCA kit (Thermo Fisher Scientific, USA).
Protein Characterization
Analytical size exclusion high-performance liquid chromatography (SE-HPLC) of purified fusion proteins was performed as described previously (19, 20). Briefly, 5 μl of protein was loaded on an Agilent advance Bio SEC 300 Å, 2.7 μm, 4.6 × 300 mm SEC column (Agilent Technologies, GB) and eluted with a 0.1 ml/min flow of elution buffer (phosphate buffer) at room temperature. Protein standards (Sigma Aldrich) were also run using the same conditions mentioned above for sizing of the purified recombinant proteins. The amount of free cysteine residues was measured using Ellman's Reagent (Thermo Fisher Scientific, USA) following the manufacturer's instructions. A standard curve was constructed using known concentrations of free cysteine (Sigma-Aldrich, USA). Folding was determined in the mAb45.1 sandwich ELISA as described (19, 26).
Production of VLPs
SpyTag was genetically fused to the N-terminus of AP205, as previously described (27). In brief, the SpyTag peptide sequence (AHIVMVDAYKPTK) was fused to the gene sequence encoding the major AP205 coat protein (Gene ID: 956335) using a flexible linker (GSGTAGGGSGS) between the two sequences. The SpyTag-AP205 VLPs were expressed in Escherichia coli One Shot® BL21 Star™ (DE3) cells (Thermo Fisher Scientific, USA) and purified by ultracentrifugation using an Optiprep™ (Sigma-Aldrich, USA) gradient. For conjugation to VLPs, purified soluble Pro-6C or Pro+I-6C proteins were incubated at a molar ratio of 1:1 (VLP/antigen) in a 1xPBS buffer for 2 h at room temperature. Unbound protein was removed by dialysis against PBS using 1,000 MWCO dialysis tubing (Spectrum Labs, USA). Densitometric analysis of SDS-PAGE gels was used to estimate protein concentrations.
Dynamic Light Scattering
Uncoupled VLP, soluble proteins and proteins conjugated to VLP were adjusted to 0.5–1 mg/ml in PBS and spun at 15,000 g for 10 min. Seventy Microliter sample was loaded into a disposable Eppendorf Uvette cuvette (Sigma-Aldrich, USA) and measured at 25°C on a DynoPro NanoStar (WYATT Technology, USA) equipped with a 658 nm laser. Each sample was measured 20 times and intensity-average size and percentage polydispersity (PD) was estimated using Dynamic software (Version 7.5.0).
Electron Microscopy
Pro-6C or Pro+I-6C coupled to VLP (with concentrations between 0.4 and 0.5 mg/ml based on antigen content) were incubated on carbon-coated and glow-discharged grids and negatively stained with 2% phosphotungstic acid (pH 7.4). The particles were analyzed on a CM 100 BioTWIN electron microscope with an accelerating voltage of 80 kV. Images were acquired using an Olympus Veleta camera and particle size was estimated using iTEM software.
Animals and Immunogenicity Studies
In the first experiment, groups (n = 5) of CD-1 mice 5–7 weeks of age (Janvier Labs, Denmark) were immunized 3 times at 3-week interval by the intramuscular injection of equimolar amounts of immune-purified Pro-6C and the individual Pf s230 and Pfs48/45 recombinant protein constructs formulated with Alhydrogel® (Brenntag, Denmark) to a final concentration of 2 mg/ml Aluminum. Please note that six mice received R0.6C. Each dose contained 128 pmoles of soluble protein (equivalent to 2 μg 6C). Serum was collected on days 14, 35, and 56. In the second experiment, groups (n = 8) of CD-1 mice were immunized with 64 pmoles (equivalent to 1 μg 6C) Pro-6C or Pro+I-6C (soluble or conjugated to VLP) as described above for the first experiment. One mouse receiving Pro+I-6C was terminated due to behavioral abnormalities not related to vaccination. All animals were treated in accordance with the regulations and guidelines of the European and National authorities.
Enzyme-Linked Immunosorbent Assay (ELISA) for Antibody Response Measurement
Gametocyte extract ELISA was performed with cultured sexual stage of Pf NF54 parasites as previously described (26). Serum immunoglobulin (IgG) subclass levels were measured using ELISA as previously described (28). For antigen-specific ELISA, 96-well plates (Nunc MaxiSorp) were coated with 0.5 μg/well of Pf s48/45-6C (25), Pro+I, or Pro+I,II,II as appropriate. Antigen-specific antibodies were detected using HRP-conjugated polyclonal goat anti-mouse IgG (Novex A16072, diluted 1:3000). Antibody midpoint titer (EC50) was calculated using sigmoidal curve fitting. One-sided analysis of variance on the log-transformed values was used to confirm that ELISA data contain essential differences. Mann-Whitney test was then used to investigate whether the chimera elicited higher levels of specific antibodies compared to individual components. p-Values are two-sided and quoted without adjustment for multiple testing since the significance of the chimera was the primary problem under investigation. p-values ≤ 0.05 were considered significant. Statistical analysis was conducted using GraphPad Prism 7 (GraphPad Software, USA).
Standard Membrane Feeding Assay (SMFA)
The biological activity of specific antisera was assessed in the SMFA as previously described (26, 29). Depending on availability, wild type P. falciparum NF54 gametocytes or transgenic P. falciparum NF54 (NF54-HGL) gametocytes expressing luciferase (29) were fed to Anopheles stephensi mosquitoes that were reared and maintained at Radboudumc, The Netherlands. Non-heat inactivated mice sera, and active or heat-inactivated human complement was added to the cultured material prior to feeding to mosquitoes. After 6–8 days, oocysts in 20 fed mosquitoes were counted by microscopy, or quantified in four pools of five mosquitoes (NF54-HGL) by measuring luciferase levels (29). Samples were tested in two independent SMFA experiments. Luminescence-based TRA estimates from two independent feeds with each of four pools of five mosquitoes were made using generalized linear mixed models (GLMMs) without zero-inflated negative binomial error structure (30, 31). Microscopy-based estimates from two feeds with 20 mosquitoes each were made using GLMMs with zero-inflated negative binomial error structure (30, 31). Statistical differences between test samples were determined using General Linear Mixed Regression analysis. Statistical analyses were performed using R studio (v. 3.2.4, The R Foundation, Boston, USA). Pre-immune pooled mice serum samples were also tested in a single independent SMFA experiment (Figure S2).
Depletion of Antigen-Specific IgGs
Pf s230- and Pf s48/45-specific antibodies were depleted from serum using Pf s230-CMB (32) and R0.10C-containing columns respectively, as previously described (13). The Pf s230-CMB fragment contains AA 444-730 and thus covers the Pro+I fragment expressed in L. lactis. To test if all antigen-specific IgGs were depleted, the flow through serum (depleted serum) was tested in ELISA using plates coated with Pf s230-CMB or SpyC-6C as appropriate (25, 26).
Results
Expression of a Multivalent Pfs230-Pfs48/45 Chimera in L. Lactis
To test whether a multivalent vaccine targeting Pf s48/45 and Pf s230 is immunogenic, we generated a chimeric construct containing the Pro domain of Pf s230 fused to the 6C fragment of Pf s48/45 (Figure 1A). We anticipated that this Pro-6C fusion protein would express well in L. lactis since the Pf s230-Pro domain is glutamate-rich, does not contain cysteines, and is similar to the R0 domain which previously enhanced expression of properly-folded 6C in L. lactis (25, 26). In addition to Pro-6C, we made constructs that either contained Pf s48/45 or Pf s230 fragments (Figure 1A). L. lactis MG1363 harboring these constructs were grown in a 1L bioreactor and the respective recombinant proteins were purified from the clarified supernatant through the C-terminal His-tag by immobilized metal affinity chromatography and ion exchange chromatography (Figure 1B). As expected, protein yields decreased with increasing number of Pf s230 domains (Table S1). Pro-6C was further immune-purified on a mAb 45.1-column to enrich for properly folded protein species (Figure 1B). The yield of immune-purified Pro-6C was 15 mg/L, similar to that of R0.6C. Conformational mAb 45.1 against the Pf s48/45 epitope I reacted with Pro-6C and this binding was equivalent to that of immune-purified R0.6C, suggesting that they exhibit similar cysteine-connectivity (Figure 1C).

Figure 1. Production of recombinant Pfs230 and Pfs48/45. (A) Schematic representation of Pfs230 constructs and Pfs230-Pfs48/45 chimera. Each construct contains the SpyCatcher sequence at the N-terminus and a His-tag at the C-terminus. 6-Cys domains are shown as boxes and numbers indicate the number of cysteines. The Pro-domain does not contain cysteines. (B) Coomassie blue stained 4–12.5% polyacrylamide gel of conventionally purified Pfs230 constructs and immune-purified Pro-6C chimera. Protein was loaded in each lane with (+) or without (–) DTT (10 mM). The sizes (kDa) of the molecular mass markers are indicated. (C) Sandwich ELISA of purified Pro-6C chimera. The antigens were captured with mAb45.1 and detected with anti-His-HRP. Immune purified R0.6C were used as a reference. X-axis is shown on a logarithmic scale.
Immunogenicity of Soluble Pfs48/45 and Pfs230 Protein Constructs
Groups of mice were immunized 3 times at 3-week interval with equimolar amounts of Pro-6C and individual Pf s230 and Pf s48/45 recombinant protein constructs formulated on Alhydrogel®. We used a suboptimal antigen dose to detect differences in immunogenic properties between protein constructs. Chimeric Pro-6C elicited significantly higher levels of gametocyte-specific antibodies than those obtained with the individual Pro domain (Mann-Whitney test, p = 0.0079) and levels comparable to those obtained with Pro+I, and R0.6C (Figure 2A). We found that levels of specific antibodies against the Pro and 6C domains were similar in mice immunized with Pro-6C compared to mice immunized with the individual Pro and 6C (R0.6C) antigens, suggesting that these domains do not exhibit antigenic competition (Figures 2B,C). As expected, levels of Pf s230-specific antibodies increased with Pf s230 fragment length (Figure 2C). The functional activity of vaccine-induced antibodies was determined by testing pooled antisera from each group in serial dilutions in the SMFA. All proteins except the Pro domain, elicited a TB response of >80% at a 1/9 dilution. Interestingly, Pro-6C induced higher levels of functional antibodies than the other recombinant proteins including R0.6C (p < 0.001) (Figure 2D), supporting further investigation of multi-component Pf s230-Pf s48/45 vaccines.
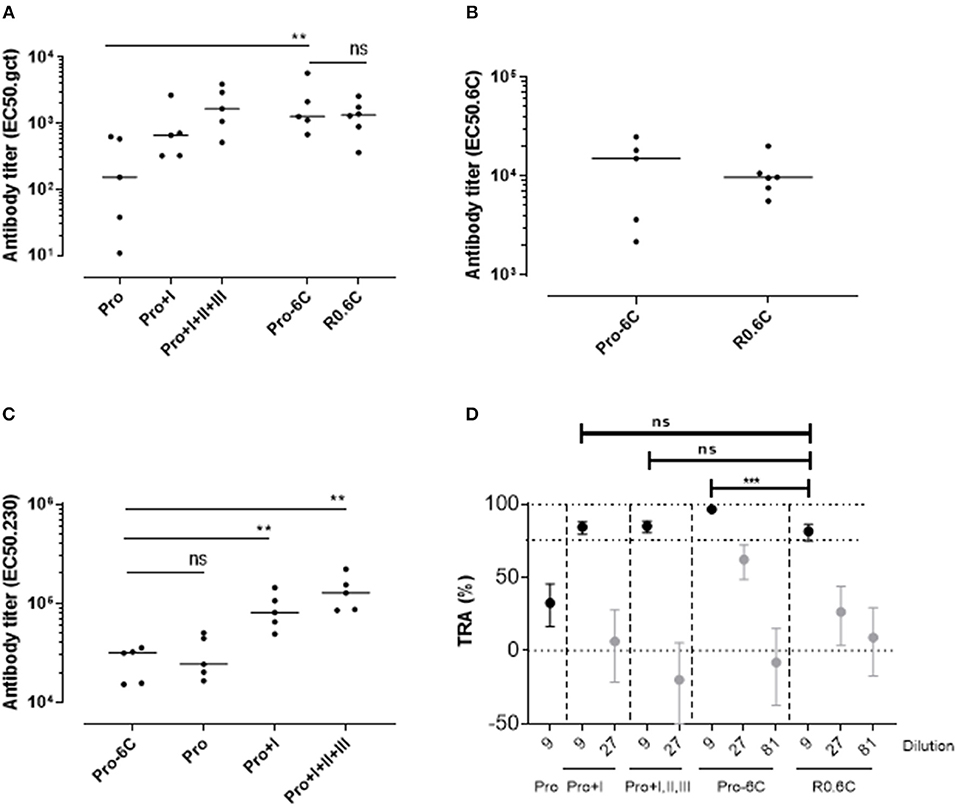
Figure 2. Immunogenicity of Pro-6C and individual fragments. Groups of mice (n = 5) were immunized with immune purified Pro-6C in a comparative study with individual Pro, Pro+I, Pro+I,II,III and R0.6C constructs. Day 56 serum was tested for antibody reactivity on ELISA plates coated with (A) gametocyte extract, (B) Pfs48/45-6C or (C) Pfs230 Pro+I,II,III. Antibody titers (dots represent individual mice) are expressed as EC50 values. Horizontal lines represent median values. The asterisks represent statistical significance determined by Mann-Whitney test (**p < 0.01, ns not significant). (D) Functional activity of serial dilutions of pooled sera in the SMFA. Transmission reducing activity (TRA) is the reduction of oocyst numbers compared to a serum control. All samples were tested in the presence of active complement. Data points are best estimates of two independent SMFA experiments and error bars represent 95% confidence intervals. Comparison between test samples was done using General Linear Mixed Regression analysis (***p < 0.005, ns, not significant).
Generation of Soluble and VLP-Based Chimeric Constructs
Next, we tested whether the potency of Pro-6C could be further increased by including the first 6-Cys domain of Pf s230 (Pro+I-6C) (Figure 3A). The Pro+I-6C chimera was purified following the same workflow developed for Pro-6C. The yield of immune-purified Pro+I-6C was 5 mg/L, which was 3-fold lower than that of Pro-6C most likely due to the additional cysteine residues (Table S1). The folding of both chimera was similar as determined in the mAb45.1 sandwich ELISA (Figure 3B). Disulfide-bonding was confirmed by demonstrating very low levels of free thiol groups (<1%) under native conditions (data not shown). Immune purified Pro-6C and Pro+I-6C eluted as single peaks by analytical size exclusion chromatography demonstrating that they form homogeneous solutions of monomeric protein species (Figures 3C,D). Before starting in vivo immunogenicity studies with both soluble constructs, we also coupled Pro-6C and Pro+I-6C to virus-like particles (VLPs) to see if this would further increase the immunogenicity of the chimeras. Both Pro-6C and Pro+I-6C contained a SpyCatcher domain allowing covalent coupling to SpyTag-decorated AP205 VLPs (25, 33). Spy-Catcher Pro-6C and Pro+I-6C coupled to SpyTag VLPs efficiently (Figure 4A) and properly folded Pf s48/45 epitope I was retained during conjugation, as shown by western blot and mAb45.1 sandwich ELISA (Figures 4A,B). Both VLPs formed homogenous populations of non-aggregated antigen-VLP complexes as demonstrated by transmission electron microscopy (Figure 4C). Furthermore, the VLP-particles displaying Pro-6C and Pro+I-6C demonstrated a low percentage of polydispersity (<16%) measured by dynamic light scattering (DLS) experiments and an average size of 71.8 and 73.7 nm, respectively (Figure 4D).
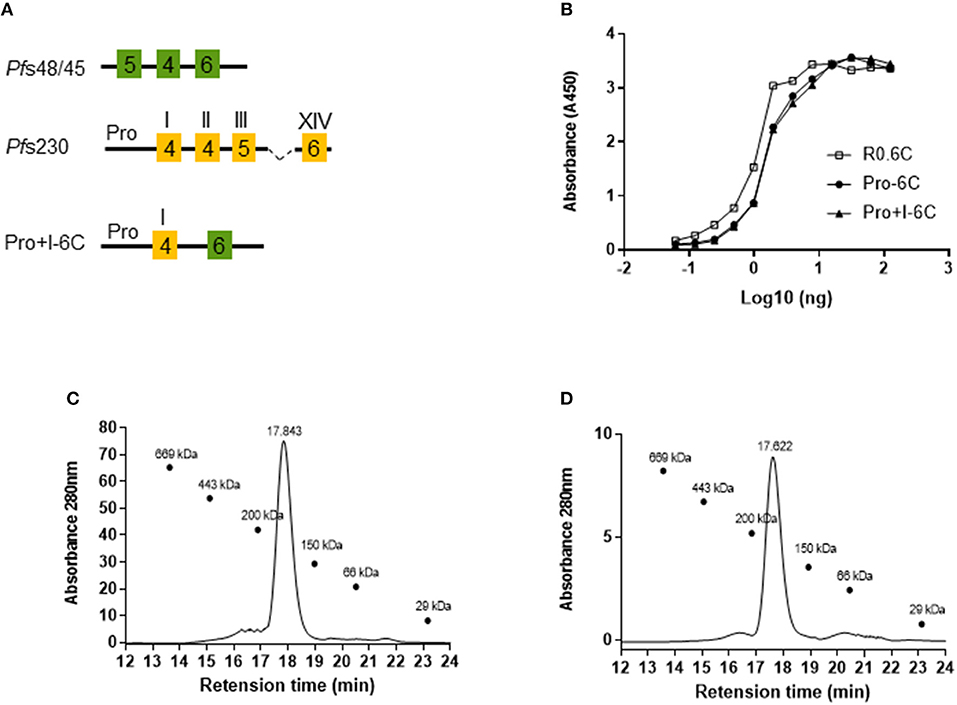
Figure 3. Design and characterization of a multi-domain Pfs230-Pfs48/45 chimera. (A) Schematic representation of the Pro+I-6C chimera. This protein contains the SpyCatcher sequence at the N-terminus and a His-tag at the C-terminus. 6-Cys domains are shown as boxes and numbers indicate the number of cysteines. The Pro-domain does not contain cysteines. (B) Sandwich ELISA of purified chimera. Immune purified R0.6C was used as a reference. Size exclusion chromatography analysis of (C) Pro-6C and (D) Pro+I-6C. SE-HPLC was performed under native conditions in a phosphate buffer of pH 7.2 to determine the amount of monomer in the sample. The sizes (kDa) of the molecular mass markers are indicated.

Figure 4. Characterization of virus-like particle-based vaccines. SpyCatcher tagged Pro-6C and Pro+I-6C were mixed with SpyTag-AP205 resulting in a unidirectional display of the chimeras on the VLP surface. (A) Reduced and non-reduced SDS-PAGE gel and western blot. The gels (left) are stained with coomassie blue, while the western blots (right) are developed with mAb45.1 as primary antibody. The following was loaded corresponding to the numbers; Lane 1: AP205 (VLP); lane 2: Pro-6C; lane 3: Pro-6C-VLP; lane 4: Pro+I-6C; Lane 5: Pro+I-6C-VLP. (B) Sandwich ELISA, using mAb 45.1 as the solid phase capture antibody. (C) Transmission electron microscopy images (negative stain) of the VLP-based vaccines after assembly. Both Pro-6C-VLPs and Pro+I-6C-VLPs appear non-aggregated, uniformly dispersed and have an estimated size of 30 nm. Scale bar 100 nm. (D) Dynamic light scattering (DLS) profile of the vaccine components Pro-6C [10.5 nm, polydispersity (PD) 10.7%], Pro+I-6C (10.8 nm, PD 20.7%), VLP (25.6 nm, PD 16.8%) and the purified vaccine products; Pro-6C-VLP (71.8 nm, PD 11.5%), and Pro+I-6C-VLP (73.7 nm, PD 15.8%).
Immunogenicity of Soluble and VLP-Based Chimeric Constructs
The immunogenicity of soluble Pro-6C and Pro+I-6C, and of Pro-6C and Pro+I-6C coupled to VLPs was assessed in vivo. Groups of CD-1 mice (n = 8) were immunized 3 times at 3-week intervals with equimolar amounts of antigen adjuvanted on Alhydrogel®. Soluble Pro+I-6C elicited significantly (Mann-Whitney test, p = 0.0079) higher levels of Pf s230-specific responses than soluble Pro-6C (Figure 5A). However, this increase was not associated with higher levels of gametocyte-specific antibodies (Figure 5C), possibly due to the difference in Pfs230-specific antibody levels being masked by higher 6C-specific signals in the gametocyte ELISA, as observed for single antigen constructs (Figure 2A). The functional activity of pooled anti-sera from each group was then tested at serial dilutions in the SMFA. Antibodies against soluble Pro+I-6C promoted higher SMFA activity than antibodies against Pro-6C at a 1/27 dilution (p < 0.001) (Figure 5D), in line with the SMFA results obtained with the single domain constructs (Figure 2D). VLP display did not provide an increase in gametocyte-, Pf s48/45-, or Pf s230-specific antibodies for both Pro-6C and Pro+I-6C (Figures 5A–C). VLP-display of Pro-6C enhanced (Mann-Whitney test, p < 0.001) the production of functional antibodies as demonstrated in the SMFA while there was no such effect for Pro+I-6C (Figure 5D).
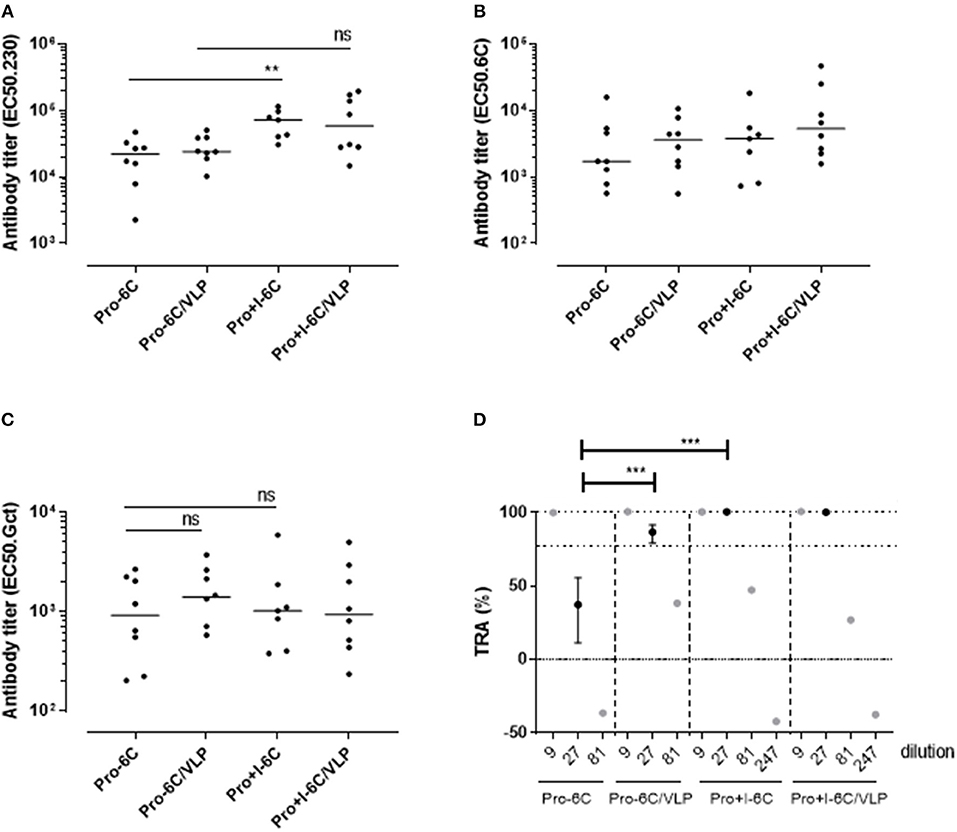
Figure 5. VLP-delivery of Pro-6C and Pro+I-6C. Groups of mice (n = 8) were immunized with soluble Pro-6C and Pro+I-6C or bound to AP205. Day 56 serum was tested for antibody reactivity on ELISA plates coated with (A) Pfs230 Pro+I,II,III (B), Pfs48/45-6C or (C) gametocyte extract. Antibody titers (dots represents individual mice) are expressed as EC50 values. Horizontal lines represent median values. The asterisks represent statistical significance determined by Mann-Whitney test (**p < 0.01, ns not significant). (D) Functional activities of serial diluted sera were assessed in the SMFA. Transmission reducing activity (TRA) is the reduction of oocyst load compared to a serum control. All samples are tested in the presence of active complement. Data points are best estimates of two independent SMFA experiments and error bars represent 95% confidence intervals. Note that 1/81 and 1/247 samples were tested in SMFA only once and therefore no confidence intervals are given. Comparison between samples was done using General Linear Mixed Regression analysis (***p < 0.005, ns, not significant).
Functional Activity of Domain Specific Antibodies in the SMFA
To investigate the functional activity of domain-specific antibodies, pooled sera from mice immunized with soluble Pro+I-6C (no VLP) were depleted for Pf s230- and Pf s48/45-specific antibodies using affinity columns with immobilized Pf s230 and Pf s48/45 respectively, as previously described for human antibodies (13). Antibody depletion was confirmed by ELISA (data not shown). The functional activity of antibody-depleted sera was then tested in the SMFA in 2-fold serial dilutions in the presence of complement. Sera depleted of Pf s230-specific or Pf s48/45-specific antibodies retained transmission blocking activity at 9- and 18-fold dilutions, respectively, demonstrating that functional antibodies against both proteins are induced by the Pro+I-6C construct (Figure 6A). Since the TB activity of Pf s230-specific antibodies depends on complement (22), depleted anti-sera were re-tested in SMFA with and without active complement. As expected, Pf s230-specific antibodies (Δ6C) lost their functional activity in the absence of active complement (Figure 6B). Since complement fixation is dependent on specific antibody subclasses (34), we determined the IgG subclass profile elicited by the Pro+I-6C formulation. The chimera elicited predominantly IgG1 antibodies, and to a lower extent IgG2a and IgG2b antibodies (Figure S1). Altogether, these data show that functional antibodies are generated against both domains of the chimeric construct and that Pf s230-specific antibodies are complement dependent.
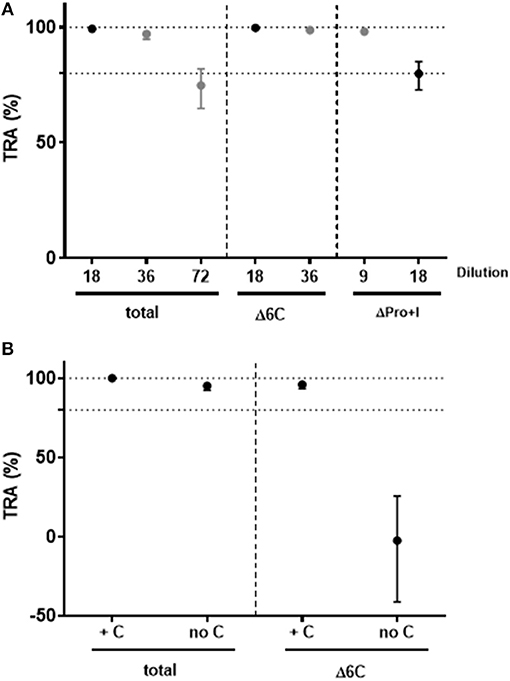
Figure 6. Functional activity of domain-specific antibodies generated by Pro+I-6C. Domain-specific antibodies were depleted from pooled sera of mice immunized with Pro+I-6C, using columns containing Pfs230-Pro+I and Pfs48/45-10C (13), to generate sera that recognize either 6C or Pro+I respectively. (A) Serial dilutions of sera containing Pro+I antibodies only (Δ6C) and sera containing 6C antibodies only (ΔPro+I) were tested in the SMFA, in the presence of active complement. (B) Total sera and sera containing Pro+I antibodies only were re-tested with (+C) and without (no C) active complement at a dilution of 1/9. Transmission reducing activity (TRA) is the reduction of oocyst numbers compared to a serum control. Data points are best estimates of two independent SMFA experiments and error bars represent 95% confidence intervals.
Discussion
Pf s230 and Pf s48/45 are expressed during the P. falciparum sexual stages in humans and elicit antibodies which effectively prevent parasite multiplication in the infected mosquito (13). Promising Pf s230- and Pf s48/45-based MTBV candidates are currently entering clinical development individually. Here we set out to produce constructs with higher potency and to this end produced and tested chimeric proteins based on both vaccine candidates. To the best of our knowledge, this is the first study that explores a multivalent MTBV based on Pf s230 and Pf s48/45. Our chimeric proteins induce antibodies that have >80% transmission reducing activity in the SMFA, which is high enough to meet the no/go decision criterion for selection and further vaccine development (35). Overall, our data show that these chimeric proteins elicited antibodies with higher TB activity in the SMFA than the single proteins alone (Figures 2D, 5D) and are therefore attractive next generation vaccine candidates.
One concern when generating multivalent vaccines is that one of the components is immunodominant and that responses against the other component are therefore compromised (36, 37). To investigate this we tested specific antibody responses against the individual domains by ELISA and demonstrated that these are not affected when the domains are presented as part of chimeric proteins. Moreover, the depletion experiments showed that antibodies against the Pro+I- and 6C-domains are functional in the SMFA. Interestingly, the Pro+I-6C chimera elicited higher levels of functional Pf s230-specific antibodies than the Pro+I domain alone; sera from Pro+I immunized mice showed no TRA in the SMFA at 1/27 dilution, whereas sera depleted of 6C-specific antibodies from mice immunized with Pro+I-6C still retained 99% TRA at 1/36 dilution. This apparent difference was not reflected in levels of specific antibodies detected in the Pf s230-ELISA indicating that functional activity in the SMFA is not only dependent on quantity but also the quality of antibodies. Importantly, there was no difference in levels of functional antibodies against the Pf s48/45-6C-domain when comparing Pro+I-6C and R0.6C immunized mice suggesting that the increase in functional activity of Pf s230-specific antibodies is, at least in part, related to a better presentation of antibody epitopes in the Pf s230 domain I of the Pro+I-6C chimera.
Adjuvants with the ability to enhance antigen immunogenicity are critical components of an efficacious subunit vaccine. In the case of chimeras that include Pf s230, it is particularly important that vaccination elicit antibodies with complement-fixing activity. Here we show that Pro+I-6C formulated on Alhydrogel® elicited high levels of IgG1 antibodies, but also Pro+I specific IgG2a and IgG2b antibodies in mice. Murine IgG2a and IgG2b are subclasses of IgG that bind with high affinity to human complement (34). The activation of complement may subsequently trigger lysis of gametes in the infected mosquito (22). It remains to be determined whether the Pro+I-6C chimera may also elicit complement-fixing antibodies in humans.
Both Pf s230- and Pf s48/45-based vaccine constructs are rich in cysteine and proper disulfide bond formation is critical for functional antibody responses (22, 38). Therefore, successful construction and production of chimeric proteins depend on the maintenance of conformational integrity of immunologically relevant regions of the individual domains. The mAb45.1 is a conformational mAb (2) that reacts with properly folded Pf s48/45 but not disulphide reduced Pfs48/45 (38). Its reactivity with the fusion proteins indicate proper cysteine connectivity of the Pf s48/45 domain. Accordingly, our results from ELISA and immunoblotting analysis showed that the purified chimeras expressed in L. lactis were strongly recognized by mAb45.1 and had thus retained conformational epitope I in the Pf s48/45-6C domain. It was particularly encouraging that recombinant Pro+I-6C reacted with mAb45.1 since Pf s230 domain I contains four cysteine residues which may potentially interfere with disulfide bonding of the Pf s48/45-6C domain. While disulphide bond studies have not been completed on the two chimera, the positive reactivity with mAb45.1, indicate that the Pf s230 sequence does not disrupt the proper disulphide formation of the Pf s48/45 6C domain contained within. Correct folding was further supported by antibody depletion experiments showing that both domains of the chimeras elicited functional antibodies. The data thus demonstrate that L. lactis is not only suitable for expression of Pf s48/45 fragments (19), but also for Pf s230 fragments and fusions thereof. The immune purification of the two chimeras used here is not compliant with cGMP manufacturing since a monoclonal antibody of rat origin was used. However, a purification process based on conventional chromatographic procedures is currently being developed. Although yields and purity remain to be determined, it is likely that high product yields can be obtained through upscaling the fermentation process which is straightforward since there is no requirement for oxygen and vigorous stirring during fermentation (39). Additionally, yields of properly folded protein species may also be increased through protein refolding processes as those developed for R0.6C (20).
In conclusion, we have produced two chimeras composed of leading vaccine candidates against the transmission stages of P. falciparum. Both chimera elicited high levels of functional antibodies in rodents and outperformed the corresponding individual protein fragments. Previously, rodents have been immunized with Pf s25 administered together with either Pf s28 or Pf s230C (40). In contrast to our findings for Pfs48/45-Pfs230 chimeras, the Pf s25-based dual-antigen vaccines did not elicit higher levels of functional antibodies than the corresponding single antigen vaccines (40). Together these data demonstrate that additive effects can only be achieved for certain antigen combinations. Our results do not only support the use of chimeric proteins for MTBV development but also for malaria vaccine development in general (26, 28, 41, 42). Another advantage of multi-component vaccines is that the production of antibodies against multiple antigens might help reduce the spread of potential escape mutants in the population. Such escape mutants may compromise overall vaccine efficacy as exemplified with the RTS,S malaria vaccine (43). This multivalent strategy is thus conceptually attractive and will constitute a novel means toward control and eventually eradication of malaria once clinical efficacy has been demonstrated.
Ethics Statement
The animal studies were approved by the Danish Animal Experiments Inspectorate. Approval number: 2013-15-2934-00902/BES.
Author Contributions
SS, ST, BC, KT, RS, WG, G-JvG, MvdV-B, and MJ: performed the experiments. ST, MN, AS, and AFS: designed the VLP constructs. SS, MT, and MJ: designed the experiments. MT, MJ, and RWS: wrote the manuscript. All authors reviewed the manuscript.
Funding
This work was supported by the Danish Ministry of Foreign Affairs (DFC file no.14-P01-GHA), the Danish Council for Strategic research (grant 13127) and by the European Union's Horizon 2020 research and innovation program under grant agreement No. 733273. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
A patent application covering the VLP spy-technology has been filed by the University of Copenhagen.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Tenna Jensen and Magnus Friis Søndergaard for technical assistance with protein purification and characterization and Laura Pelser, Jolanda Klaassen, Astrid Pouwelsen, and Jacqueline Kuhnen for mosquito infection and dissection work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01256/full#supplementary-material
Figure S1. Subclass-specific antibody responses in mice immunized with Pro+I-6C. Day 56 sera were tested for antibody reactivity on ELISA plates coated (A) Pro+I or (B) 6C. Results for individual mice are shown, and a horizontal bar represents each median value.
Figure S2. Pre-immune mouse serum contains low intrinsic TRA. Pooled mouse serum from each group was tested at 1/9 dilution in a single SMFA experiment. TRA is the reduction of oocyst numbers compared to a serum control. Error bars represent 95% confidence intervals. TRA values below 50% have large confidence intervals and this can explain the observed variation between different groups. The mean TRA of all groups is 21% (CIs: 5.7–34.1%) demonstrating that mouse serum has low intrinsic TRA.
Table S1. Production and quantification of Pfs230 and Pfs230/Pfs48/45 chimeric protein in L. lactis.
References
1. Carter R Transmission blocking malaria vaccines. Vaccine. (2001) 19:2309–14. doi: 10.1016/S0264-410X(00)00521-1
2. Vermeulen AN, Ponnudurai T, Beckers PJ, Verhave JP, Smits MA, Meuwissen JH. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. (1985) 162:1460–76. doi: 10.1084/jem.162.5.1460
3. Vermeulen AN, Roeffen WF, Henderik JB, Ponnudurai T, Beckers PJ, Meuwissen JH. Plasmodium falciparum transmission blocking monoclonal antibodies recognize monovalently expressed epitopes. Dev Biol Stand. (1985) 62:91–7.
4. Nikolaeva D, Draper SJ, Biswas S. Toward the development of effective transmission-blocking vaccines for malaria. Expert Rev Vaccines. (2015) 14:653–80. doi: 10.1586/14760584.2015.993383
5. Kocken CH, Jansen J, Kaan AM, Beckers PJ, Ponnudurai T, Kaslow DC, et al. Cloning and expression of the gene coding for the transmission blocking target antigen Pf s48/45 of Plasmodium falciparum. Mol Biochem Parasitol. (1993) 61:59–68. doi: 10.1016/0166-6851(93)90158-T
6. Kumar N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. (1987) 9:321–35. doi: 10.1111/j.1365-3024.1987.tb00511.x
7. van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell. (2001) 104:153–64. doi: 10.1016/S0092-8674(01)00199-4
8. Drakeley CJ, Bousema JT, Akim NI, Teelen K, Roeffen W, Lensen AH, et al. Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol. (2006) 28:185–90. doi: 10.1111/j.1365-3024.2005.00818.x
9. Bousema JT, Drakeley CJ, Sauerwein RW. Sexual-stage antibody responses to P. falciparum in endemic populations. Curr Mol Med. (2006) 6:223–9. doi: 10.2174/156652406776055140
10. Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci USA. (2010) 107:6958–63. doi: 10.1073/pnas.1001323107
11. Theisen M, Jore MM, Sauerwein R. Towards clinical development of a Pf s48/45-based transmission blocking malaria vaccine. Expert Rev Vaccines. (2017) 16:329–36. doi: 10.1080/14760584.2017.1276833
12. Stone WJ, Dantzler KW, Nilsson SK, Drakeley CJ, Marti M, Bousema T, et al. Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology. (2016) 143:187–98. doi: 10.1017/S0031182015001341
13. Stone WJR, Campo JJ, Ouedraogo AL, Meerstein-Kessel L, Morlais I, Da D, et al. Unravelling the immune signature of Plasmodium falciparum transmission-reducing immunity. Nat Commun. (2018) 9:558. doi: 10.1038/s41467-018-03769-w
14. Carter R, Graves PM, Keister DB, Quakyi IA. Properties of epitopes of Pf s 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol. (1990) 12:587–603. doi: 10.1111/j.1365-3024.1990.tb00990.x
15. Rener J, Graves PM, Carter R, Williams JL, Burkot TR. Target antigens of transmission-blocking immunity on gametes of Plasmodium falciparum. J Exp Med. (1983) 158:976–81. doi: 10.1084/jem.158.3.976
16. Ponnudurai T, Lensen AH, Van Gemert GJ, Bensink MP, Bolmer M, Meuwissen JH. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology. (1989) 98(Pt 2):165–73. doi: 10.1017/S0031182000062065
17. Gerloff DL, Creasey A, Maslau S, Carter R. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc Natl Acad Sci USA. (2005) 102:13598–603. doi: 10.1073/pnas.0502378102
18. Roeffen W, Teelen K, van As J, vd Vegte-Bolmer M, Eling W, Sauerwein R. Plasmodium falciparum: production and characterization of rat monoclonal antibodies specific for the sexual-stage Pf s48/45 antigen. Exp Parasitol. (2001) 97:45–9. doi: 10.1006/expr.2000.4586
19. Singh SK, Roeffen W, Andersen G, Bousema T, Christiansen M, Sauerwein R, et al. A Plasmodium falciparum 48/45 single epitope R0.6C subunit protein elicits high levels of transmission blocking antibodies. Vaccine. (2015) 33:1981–6. doi: 10.1016/j.vaccine.2015.02.040
20. Singh SK, Roeffen W, Mistarz UH, Chourasia BK, Yang F, Rand KD, et al. Construct design, production, and characterization of Plasmodium falciparum 48/45 R0.6C subunit protein produced in Lactococcus lactis as candidate vaccine. Microbial Cell Factories. (2017) 16:97. doi: 10.1186/s12934-017-0710-0
21. Williamson KC, Keister DB, Muratova O, Kaslow DC. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol Biochem Parasitol. (1995) 75:33–42. doi: 10.1016/0166-6851(95)02507-3
22. Read D, Lensen AH, Begarnie S, Haley S, Raza A, Carter R. Transmission-blocking antibodies against multiple, non-variant target epitopes of the Plasmodium falciparum gamete surface antigen Pfs230 are all complement-fixing. Parasite Immunol. (1994) 16:511–9. doi: 10.1111/j.1365-3024.1994.tb00305.x
23. Tachibana M, Wu Y, Iriko H, Muratova O, MacDonald NJ, Sattabongkot J, et al. N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin Vaccine Immunol. (2011) 18:1343–50. doi: 10.1128/CVI.05104-11
24. MacDonald NJ, Nguyen V, Shimp R, Reiter K, Herrera R, Burkhardt M, et al. Structural and immunological characterization of recombinant 6-cysteine domains of the Plasmodium falciparum sexual stage protein Pfs230. J Biol Chem. (2016) 291:19913–22. doi: 10.1074/jbc.M116.732305
25. Singh SK, Thrane S, Janitzek CM, Nielsen MA, Theander TG, Theisen M, et al. Improving the malaria transmission-blocking activity of a Plasmodium falciparum 48/45 based vaccine antigen by SpyTag/SpyCatcher mediated virus-like display. Vaccine. (2017) 35:3726–32. doi: 10.1016/j.vaccine.2017.05.054
26. Theisen M, Roeffen W, Singh SK, Andersen G, Amoah L, van de Vegte-Bolmer M, et al. A multi-stage malaria vaccine candidate targeting both transmission and asexual parasite life-cycle stages. Vaccine. (2014) 32:2623–30. doi: 10.1016/j.vaccine.2014.03.020
27. Thrane S, Janitzek CM, Matondo S, Resende M, Gustavsson T, de Jongh WA, et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J Nanobiotechnol. (2016) 14:30. doi: 10.1186/s12951-016-0181-1
28. Lousada-Dietrich S, Jogdand PS, Jepsen S, Pinto VV, Ditlev SB, Christiansen M, et al. A synthetic TLR4 agonist formulated in an emulsion enhances humoral and Type 1 cellular immune responses against GMZ2–a GLURP-MSP3 fusion protein malaria vaccine candidate. Vaccine. (2011) 29:3284–92. doi: 10.1016/j.vaccine.2011.02.022
29. Vos MW, Stone WJ, Koolen KM, van Gemert GJ, van Schaijk B, Leroy D, et al. A semi-automated luminescence based standard membrane feeding assay identifies novel small molecules that inhibit transmission of malaria parasites by mosquitoes. Sci Rep. (2015) 5:18704. doi: 10.1038/srep18704
30. Churcher TS, Blagborough AM, Delves M, Ramakrishnan C, Kapulu MC, Williams AR, et al. Measuring the blockade of malaria transmission–an analysis of the standard membrane feeding assay. Int J Parasitol. (2012) 42:1037–44. doi: 10.1016/j.ijpara.2012.09.002
31. Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MH, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. (2009) 24:127–35. doi: 10.1016/j.tree.2008.10.008
32. Farrance CE, Rhee A, Jones RM, Musiychuk K, Shamloul M, Sharma S, et al. A plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin Vaccine Immunol. (2011) 18:1351–7. doi: 10.1128/CVI.05105-11
33. Veggiani G, Zakeri B, Howarth M. Superglue from bacteria: unbreakable bridges for protein nanotechnology. Trends Biotechnol. (2014) 32:506–12. doi: 10.1016/j.tibtech.2014.08.001
34. Stewart WW, Johnson A, Steward MW, Whaley K, Kerr MA. The activation of C3 and C4 in human serum by immune complexes containing mouse monoclonal antibodies of different isotype and affinity: effects on solubilisation. Mol Immunol. (1988) 25:1355–61.
35. Sauerwein RW, Bousema T. Transmission blocking malaria vaccines: assays and candidates in clinical development. Vaccine. (2015) 33:7476–82. doi: 10.1016/j.vaccine.2015.08.073
36. Theisen M, Cox G, Hogh B, Jepsen S, Vuust J. Immunogenicity of the Plasmodium falciparum glutamate-rich protein expressed by vaccinia virus. Infect Immun. (1994) 62:3270–5.
37. Johansson BE, Kilbourne ED. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J Virol. (1993) 67:5721–3.
38. Outchkourov N, Vermunt A, Jansen J, Kaan A, Roeffen W, Teelen K, et al. Epitope analysis of the malaria surface antigen pf s48/45 identifies a subdomain that elicits transmission blocking antibodies. J Biol Chem. (2007) 282:17148–56. doi: 10.1074/jbc.M700948200
39. Singh SK, Tiendrebeogo RW, Chourasia BK, Kana IH, Singh S, Theisen M. Lactococcus lactis provides an efficient platform for production of disulfide-rich recombinant proteins from Plasmodium falciparum. Microbial Cell Factories. (2018) 17:55. doi: 10.1186/s12934-018-0902-2
40. Menon V, Kapulu MC, Taylor I, Jewell K, Li Y, Hill F, et al. Assessment of antibodies induced by multivalent transmission-blocking malaria vaccines. Front Immunol. (2017) 8:1998. doi: 10.3389/fimmu.2017.01998
41. Pan W, Huang D, Zhang Q, Qu L, Zhang D, Zhang X, et al. Fusion of two malaria vaccine candidate antigens enhances product yield, immunogenicity, and antibody-mediated inhibition of parasite growth in vitro. J Immunol. (2004) 172:6167–74. doi: 10.4049/jimmunol.172.10.6167
42. Kalra A, Edula JR, Gupta PK, Pandey AK, Chauhan VS. Antigenicity of a bacterially expressed triple chimeric antigen of Plasmodium falciparum AARP, MSP-311 and MSP-119: PfAMSP-Fu35. PLoS ONE. (2016) 11:e0165720. doi: 10.1371/journal.pone.0165720
Keywords: malaria, vaccines, multivalent, transmission blocking, Lactococcus lactis
Citation: Singh SK, Thrane S, Chourasia BK, Teelen K, Graumans W, Stoter R, van Gemert G-J, van de Vegte-Bolmer MG, Nielsen MA, Salanti A, Sander AF, Sauerwein RW, Jore MM and Theisen M (2019) Pfs230 and Pfs48/45 Fusion Proteins Elicit Strong Transmission-Blocking Antibody Responses Against Plasmodium falciparum. Front. Immunol. 10:1256. doi: 10.3389/fimmu.2019.01256
Received: 13 December 2018; Accepted: 17 May 2019;
Published: 05 June 2019.
Edited by:
Julius Clemence Hafalla, London School of Hygiene and Tropical Medicine (LSHTM), United KingdomReviewed by:
Rhoel Dinglasan, University of Florida, United StatesTakafumi Tsuboi, Ehime University, Japan
Copyright © 2019 Singh, Thrane, Chourasia, Teelen, Graumans, Stoter, van Gemert, van de Vegte-Bolmer, Nielsen, Salanti, Sander, Sauerwein, Jore and Theisen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthijs M. Jore, TWF0dGhpanMuSm9yZUByYWRib3VkdW1jLm5s; Michael Theisen, bXRoQHNzaS5kaw==
†These authors have contributed equally to this work
 Susheel K. Singh
Susheel K. Singh Susan Thrane2†
Susan Thrane2† Karina Teelen
Karina Teelen Wouter Graumans
Wouter Graumans Marga G. van de Vegte-Bolmer
Marga G. van de Vegte-Bolmer Matthijs M. Jore
Matthijs M. Jore Michael Theisen
Michael Theisen