- Willem-Alexander Children's Hospital, Department of Pediatrics and Laboratory for Pediatric Immunology, Leiden University Medical Center, Leiden, Netherlands
B cell reconstitution after hematopoietic stem cell transplantation (HSCT) is variable and influenced by different patient, donor, and treatment related factors. In this review we describe B cell reconstitution after pediatric allogeneic HST, including the kinetics of reconstitution of the different B cell subsets and the development of the B cell repertoire, and discuss the influencing factors. Observational studies show important roles for stem cell source, conditioning regimen, and graft vs. host disease in B cell reconstitution. In addition, B cell recovery can play an important role in post-transplant infections and vaccine responses to encapsulated bacteria, such as pneumococcus. A substantial number of patients experience impaired B cell function and/or dependency on Ig substitution after allogeneic HSCT. The underlying mechanisms are largely unresolved. The integrated aspects of B cell recovery after HSCT, especially BCR repertoire reconstitution, are awaiting further investigation using modern techniques in order to gain more insight into B cell reconstitution and to develop strategies to improve humoral immunity after allogeneic HSCT.
Introduction
Hematopoietic stem cell transplantation (HSCT) is a treatment modality in which hematopoietic stem cells are used as curative therapy for congenital and acquired disorders of the hematopoietic system and metabolic diseases (1). During HSCT, the hematopoietic system is replaced using donor-derived hematopoietic stem cells as allograft. Stem cells can give progeny to functioning erythrocytes, thrombocytes, myeloid lineages and/or lymphocytes, achieving recovery of normal hematopoiesis and immunity.
Restoration of the individual components of the immune system occurs with different dynamics in which innate immunity (neutrophils, monocytes and natural killer cells) typically precedes adaptive immunity (T- and B-lymphocytes). Complete immune reconstitution can take several months up to 2 years after HSCT. Immune reconstitution after allogeneic HSCT has been studied extensively with a main focus on T cell reconstitution. Only limited information is available about B cell reconstitution. In this review we summarize the existing knowledge on B cell reconstitution after pediatric allogeneic HSCT and point out the need and challenges for further investigations. We included studies via systematic literature search in Embase, Medline Epub (Ovid), Cochrane Central and Web of Science including the terms “hematopoietic stem cell transplantation,” “B lymphocyte,” “immune reconstitution,” “child” and synonyms between 01-01-1980 and 31-12-2018. Additional relevant studies were included through references within the identified studies. Lastly, top results of Google Scholar were screened.
B Cell Reconstitution After HSCT
Compared to other hematopoietic cell types, B cell reconstitution occurs relatively late after HSCT. The first emerging B cells can be detected in peripheral blood after 1.5–2 months. These are mainly CD24high CD38high transitional B cells (2) (See Figure 1A for all peripheral B-cell subsets). A subdivision of these transitional B cells can be made in the early appearing CD21low B cells, called the T1 cells, and the CD21high B (or T2) cells which develop later (3). Additionally, expansion of CD5+ B cells is reported early after HSCT (4, 5). CD5 is a known marker of human immature B cells, but its expression covers a broader range of B cell developmental/differentiation stages. More mature CD5+ B cell subpopulations are characterized by their regulatory properties such as IL-10 production upon activation by bacterial or parasitic antigens, and their constitutive death-inducing ligand expression (6–10). The CD5+ B cell expansion early after HSCT is likely to be a reflection of B cell immaturity, but may also point to a role for regulatory B cells in controlling immune reactions and autoimmunity after HSCT. Several months after HSCT, the transitional B cells decrease in number and gradually naive mature (CD24int CD38int) B cells emerge to become the predominant population. In the course of the first year following HSCT, naive mature B cells represent more than 80% of the peripheral blood B lymphocytes. (2). To obtain more insight in the mechanism and dynamics of B cell regeneration, κ-deleting recombination excision circles (KRECs) might serve as a useful biomarker for replication history and to evaluate the onset of de novo B-cells, as KRECs have been reported to be positively correlated with B cell numbers after transplantation (11–15). At one year after HSCT, the B cell reconstitution stabilizes reaching age-corrected normal total B cell counts in peripheral blood in most patients (Figure 1B) (16–20). Looking further into the B cell populations, non-switched (CD27+IgM+IgD+/-) and switched (CD27+IgD-IgM-) memory B-cells appear slowly, taking up to two years or longer after HSCT to reach normal age matched levels (16, 17, 20, 21). Especially non-switched B cells seem to remain below normal values, suggesting defects in this maturation stage. During the process of B cell maturation in general, mature B lymphocytes further differentiate into memory B cells, and may undergo isotype switching and affinity maturation in a T cell dependent germinal center reaction (Figure 1A). In this process, cognate interaction between T follicular helper (Tfh) cells and specialized follicular dendritic cells is pivotal. As a consequence, the quality and dynamics of CD4 T cell, and thus also Tfh, reconstitution after HSCT will also impact on B cell differentiation and may thus contribute to an impaired or arrested maturation of B cells. (22–24). However, even in the presence of donor CD4+ T cells that are capable of supporting the process of somatic hyper mutation, the incidence of somatic hypermutation is decreased in recipient B cells in cell culture (25). It could be that treatment given prior to transplantation disrupts secondary lymphoid organs, which are necessary for the introduction of somatic hypermutations in the variable domains of the immunoglobulin molecules and affinity maturation in the germinal centers (26). Immune responses against polysaccharides seem frequently impaired in HSCT patients (27, 28). Polysaccharide antibody responses are important for the T cell independent defense to encapsulated bacteria, in which marginal zone B cells play an important role (29, 30). The impaired reconstitution of this subset might indicate why certain patients encounter specific problems with susceptibility to encapsulated bacteria such as pneumococcus. The counterpart of marginal zone B cells, IgM memory B cells, seems also to be reduced in long term transplanted patients (16, 17, 20, 21).
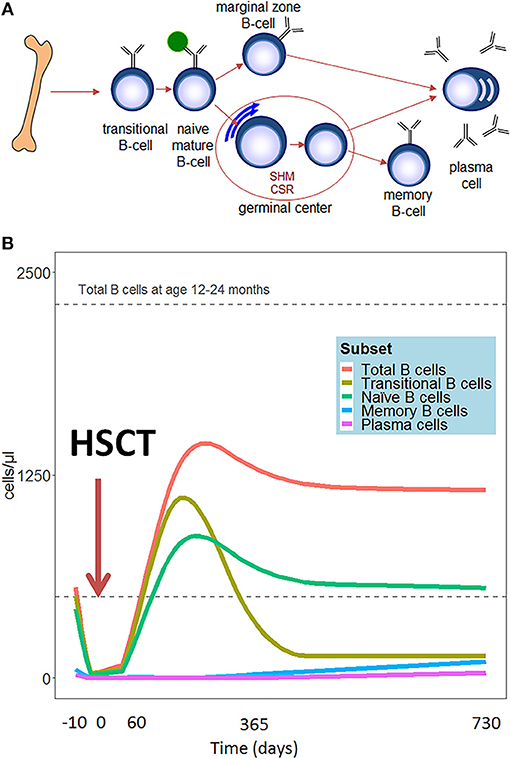
Figure 1. (A) Schematic representation of peripheral B-cell development. (B) Hypothetical scheme of B cell subset reconstitution after HSCT based on literature. The first cells emerging in the peripheral blood are the transitional B cells. In the course of the first year, the transitional B cells decrease in number and are replaced by mature naïve B cells. These mature B lymphocytes further differentiate into memory B cells and plasma cells.
Immunoglobulin (Ig) levels seem to recover in parallel to B cell reconstitution, in which recovery of Ig subclasses usually occurs in a distinctive order (16, 31–33). After HSCT, Ig levels drop, reflecting the absence of Ig producing B cells. Some Ig production may persist, probably due to surviving long-lived plasma cells of host origin (34). As a reflection of normal ontogeny, IgM production will reconstitute relatively early and, on average, reaches normal levels within the first 6 months after HSCT. Similar to IgM, IgG generally reaches normal levels in the second half of the first year, whereas normalization of IgA levels may take up to 5 years after HSCT. IgG subclasses, on average, reach normal serum levels within 5 months (IgG1), 9 months (IgG3), or up to 2 years (IgG2 and IgG4). However, the time frame is highly variable and can be influenced by several factors such as the underlying disease, stem cell source, and type of donor (16, 31–33).
For complete humoral immune reconstitution after HSCT, generation of a diverse BCR repertoire is necessary. Literature on the diversification of the BCR after HSCT is limited. In the last 10 years, investigation of BCR diversification after HSCT has stagnated and studies performed are limited by older techniques or are difficult to generalize because of the small sample size. Analysis of the pattern of VH3- and VH4-gene usage based on 700 rearrangements in four patients suggested that the B-cell receptor repertoire shows the same (limited) repertoire of VH genes at 90 days and 1 year after HSCT (35). Other studies showed evidence that generation of the new repertoire occurs gradually and suggest that the CDR3 regions post HSCT are similar to CDR3 regions in adults and do not follow fetal ontogeny (36–38). The CDR3 length in the memory B cell compartment has a specific restriction compared to healthy controls, resulting in an oligoclonal repertoire early after HSCT (39). These methods only provide rough information about the BCR repertoire post HSCT. In adults, one study investigated the IGH repertoire before and after HSCT in acute myeloid leukemia patients using next generation sequencing. In general, they observed lower repertoire diversity after HSCT than before (40). Furthermore, each individual appeared to have highly unique and characteristic IGH repertoire of switched memory B-cells, which allowed the investigators to separate donor and recipient derived B cell clones. Interestingly, this showed in some cases persistence of recipient B cells which indicates that recipient B cells may still contribute to protective immunity after HSCT. The study analyzed the VH1 Ig repertoire, which represents only about 10% of all Ig sequences in humans. All of these studies exclusively investigated Ig heavy chain diversity. To the best of our knowledge, there are no studies investigating Ig light chain.
Based on available literature, HSCT-treated patients are frequently affected by an unbalanced, incomplete and, therefore, abnormal BCR repertoire, leading to impaired humoral immunity with associated risks of infectious and/or auto-immune complications. So far, studies have been limited by small cohorts and low-throughput or low resolution techniques. More in-depth analyses are needed to understand the integrated evolution of cellular and repertoire reconstitution and the influence of different HSCT-related factors on immune repertoire formation and B cell function require after transplantation.
Factors Influencing B Cell Reconstitution
Stem Cell Source
Although all different stem cell sources have curative potential, they differ in qualitative and quantitative characteristics of immune recovery and risk of specific HSCT-related complications such as graft vs. host disease (GVHD) (Table 1). Several studies suggest that umbilical cord blood (CB) is superior over bone marrow (BM) or peripheral blood stem cells (PBSC) with regard to time to B cell recovery and B cell differentiation (16, 41, 42). Total B cell counts, non-switched memory and switched memory B cells are higher in CB compared to BM and PBSC. In a mismatched donor setting, CB recipients show no significant better outcome for B cell reconstitution (62). The rapid reconstitution and better differentiation of B cells when using CB could be explained by the higher number of B lymphocyte progenitors in CB compared to BM (63). However, as precursor composition in matched and unmatched CB are the same, differences in CD19+ counts cannot be explained by higher progenitor numbers alone.
Between BM and PBSCs, differences in B cell reconstitution mainly exist in the early stage after HSCT. B cell numbers seem to recover faster when using PBSCs compared to BM (33, 43, 44). Still, with both stem cell sources, naïve, non-switched memory and switched memory B cells will remain below the normal values on the long term (16, 43). After 6 months, no differences are shown in B cell recovery using BM compared to PBSC (33, 43, 44).
Stromal cells and T cells are important for optimal B cell development and functionality in CB, BM and PBSC grafts (64–67). In CB more primitive and higher numbers of the stromal progenitors and primitive HSCs are reported compared to BM and PBSC (67). Cotransfer of stromal cells and T cells in the graft, as well as ex vivo modification of the grafts, such as CD34 selection, CD3/CD19 depletion and TCRα/β depletion could indirectly cause differences in functional and quantitative B cell reconstitution after HSCT.
Serotherapy and Chemotherapeutic Conditioning
Serotherapy makes use of antilymphocyte antibodies which target T cells and other leukocyte populations, with the aim to reduce the risk of graft rejection and acute graft vs. host disease (aGVHD) (68, 69). The most prominent agents used are anti-thymocyte globulin (ATG) and alemtuzumab. Whereas ATG is a polyclonal immunoglobulin, alemtuzumab is a monoclonal antibody against CD52 (70). Both agents induce elimination of B cell populations (71, 72). A number of studies have reported on the impact of ATG on B cell reconstitution. Although some studies have reported a delay on immune reconstitution others have indicated the opposite (19, 31, 45–47). Whether these differences are explained by disease-specific characteristics, donor type, ATG exposure or the type of ATG is currently unresolved and requires further study. Similar to ATG, alemtuzumab has been reported to result in delayed B cell reconstitution. The kinetics of B cell reconstitution after alemtuzumab are variable and may as well be dependent on the same parameters as in case of ATG (16, 48).
Both in malignant and non-malignant diseases, achievement of full donor chimerism is often preferred to cure the primary disease and obtain stable graft function. In those cases myeloablative conditioning (MAC) is usually required. MAC often results in donor chimerism of all lineages and thereby in robust B cell reconstitution and function (20, 39, 49–51). However, in an increasing proportion of children a reduced intensity regimen is preferred either because the underlying disease or the pre-existing co-morbidities preclude a MAC approach. In these patients reduced intensity conditioning (RIC) is used, causing incomplete and reversible myelosuppression. Conditioning with RIC is associated with better survival, due to favorable toxicity profile and thus lower transplant-related mortality (52). However, RIC is associated with an increased incidence of partial/mixed chimerism and graft rejection which may result in suboptimal B cell function and the need for immunoglobulin supplementation (52, 53). Furthermore, the use of total body irradiation (TBI) is associated with delayed B cell immune reconstitution (16, 19). The mechanism behind TBI and the impaired reconstitution is not fully understood, but lower naïve B cells and switched memory B cells have been observed for up to 2 years.
Graft vs. Host Disease
GVHD is a frequent complication of allogeneic HSCT which is responsible for significant transplant-related morbidity and mortality. aGVHD is mediated primarily by alloreactive donor T cells. The donor T cells are activated by host antigen presenting cells, which could be B cells. In general, GVHD is associated with significantly poorer B cell reconstitution, in both function and numbers (16, 54, 55). Higher grades of aGHVD seem to be associated with more extensively impaired humoral immunity, to which probably both GVHD itself as well as the associated immunosuppressive therapies contribute (56, 57). In chronic GVHD (cGVHD), poor B cell reconstitution seems mainly due to reduced numbers of B cell progenitors and unswitched memory B cells (16, 21, 33, 55, 58). In adults, regulatory B cells (Bregs) are found to be reduced in patients with cGVHD compared to no cGVHD and healthy controls. Severity of cGVHD seems to correlate with the number of Bregs, indicating a role for these B cell subset in cGVHD (59, 60). Using mass cytometry, it appeared that specific B cell subpopulations can be distinguished in patients suffering from different grades of cGVHD (61). Patients with severe cGVHD had an increased frequency of activated B cells, defined as CD38+ CD39+ CXCR5+ HLA-DR+ B-cells, compared to patients with moderate cGVHD. Furthermore, activated B cells were found at a reduced frequency in patients with mild cGVHD compared to patients without cGVHD (61). Regarding pathophysiology, whereas aGVHD is considered to be primarily mediated by T cells, an important role for donor B cells is assumed in the complex immune pathology of cGVHD (73–77). However, through antigen presentation, cytokine production and other immunoregulatory functions, it is hypothesized that B cells take part in the pathophysiology of all types of GHVD (78–82). Therefore, B cells have been targeted with several therapies such as Rituximab, Bortezomib, and Ibrutinib in both murine models and patients with GVHD, with promising clinical results (83–85).
Infections and Vaccination
In a significant proportion of HSCT recipients, antibody titers of vaccine-preventable diseases decline over the years, if these recipients are not revaccinated (86–89). Vaccination responses after HSCT are dependent on both T- and B cell reconstitution. An exception is the polysaccharide antibody response, which is completely B cell dependent (90). The polysaccharide antibody response plays a role in the immunization against encapsulated bacteria, such as pneumococcus. HSCT recipients are more susceptible to infections during the post transplantation period (91–93). The risk of pneumococcal invasive disease is increased both early and late after HSCT, reaching 30-fold higher risks compared to the general population after 10 years, suggesting long lasting defects in B cell reconstitution even after revaccination (94–97). The risk of pneumococcal disease correlates with presence/occurrence of GVHD, suggesting a link between the functional dysregulation of B cells and GVHD (21, 94, 96). Whereas, an association with hypogammaglobulinemia has been reported, increased susceptibility to pneumococcal disease is most often due to a more selectively impaired immune response against polysaccharides (27, 28, 95). A poor response to polysaccharide vaccines, is hypothesized to be caused by the lack of unswitched memory B cells, which are reduced after transplantation (98). A poor response to polysaccharide vaccines is hypothesized to be caused by reduced MZ B cells or IgM memory B cells, which are reduced after transplantation (16, 17, 20, 21, 98). Reduced numbers of IgM memory B cells and increased risk of encapsulated bacterial infection has also been observed in young children, patients with common variable immune deficiency (CVID) and splenectomized patients (29, 99–101). Revaccination usually occurs with inactivated vaccines, as live attenuated vaccines have the potential to induce active disease in immunosuppressed patients. However below normal values, class switched memory B cells are observed as early as 3 months after HSCT (17). It is largely unknown if these cells are already capable of an immune response, taking into account the slow reconstitution of CD4+ T cells. In current guidelines, revaccination starts 3-6 months after HSCT, but looking at thresholds of the CD4+ T cells and ability for class switch recombination might be a useful biomarker to guide the timing of vaccination compared to fixed time point after HSCT. Live attenuated vaccines could be considered two years after HSCT, in patients without cGVHD or immunosuppression (102).
Future Prospects
B cell reconstitution after HSCT including BCR repertoire formation and diversification, is awaiting new insights in order to develop better treatment strategies for prevention of clinical complications due to defects in B cell mediated immunity. For example, clinical outcome is still suboptimal in a proportion of HSCT-treated severe combined immunodeficiency (SCID) patients, due to humoral immune dysfunction. Some of these patients still suffer from persistent (humoral) immunodeficiency, auto-immunity and/or immune dysregulation leading to impaired quality of life (103). Although SCID represents the prototype of inherited immune disorders, an increasing spectrum of patients with inherited immune disorders is being genetically identified (>250 monogenetic diseases) (104). This steadily growing group of transplanted patients with non-SCID inherited immune disorders faces similar challenges regarding the long term quality of their (sometimes partially) corrected immune system. To evaluate the long term quality of immune reconstitution, B cell immunity recovery can serve as an indicator of immune fitness after HSCT.
We have limited information on the diversity of the BCR repertoire in the various B cell subsets in both peripheral blood and BM of HSCT-treated patients. Important insights have been obtained through flow cytometric analysis of peripheral B-cells after HSCT. However, to gain better understanding of numerical and functional B-cell reconstitution more in depth-analysis of cellular dynamics (using flow cytometry and KREC analysis), molecular aspects (BCR repertoire analysis) and antigen specificity is needed. Extensive analysis of B cell reconstitution can be done through modern flow cytometry and sequence techniques or even simultaneously with mass cytometry (105, 106). With modern sequencing techniques, it is possible to look at both the BCR heavy and light chain. Combined with single cell RNA sequencing, the integrated gene expression per cell within individual patients can be investigated (107). These modern techniques/methodology will make it possible to investigate the influence of aforementioned parameters on B cell repertoire development after HSCT in an innovative and more accurate way. This could revolutionize the knowledge about the B cell reconstitution and pinpoint the individual B cell maturation problems of transplanted patients.
Author Contributions
NvdM and MvdB contributed conception of the paper; NvdM did literature search and wrote the first draft of the manuscript; DB, MvdB, and AL wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. (2017) 52:811–7. doi: 10.1038/bmt.2017.34
2. Marie-Cardine A, Divay F, Dutot I, Green A, Perdrix A, Boyer O, et al. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol. (2008) 127:14–25. doi: 10.1016/j.clim.2007.11.013
3. Suryani S, Fulcher DA, Santner-Nanan B, Nanan R, Wong M, Shaw PJ, et al. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. (2010) 115:519–29. doi: 10.1182/blood-2009-07-234799
4. Antin JH, Ault KA, Rappeport JM, Smith BR. B lymphocyte reconstitution after human bone marrow transplantation. Leu-1 antigen defines a distinct population of B lymphocytes. J Clin Invest. (1987) 80:325–32. doi: 10.1172/JCI113076
5. Antin JH, Emerson SG, Martin P, Gadol N, Ault KA. Leu-1+ (CD5+) B cells. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. (1986) 136:505–10.
6. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. (2002) 3:944–50. doi: 10.1038/ni833
7. Lundy SK, Boros DL. Fas ligand-expressing B-1a lymphocytes mediate CD4+-T-cell apoptosis during schistosomal infection: induction by interleukin 4 (IL-4) and IL-10. Infect Immun. (2002) 70:812–9. doi: 10.1128/IAI.70.2.812-819.2002
8. Palanivel V, Posey C, Horauf AM, Solbach W, Piessens WF, Harn DA. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp Parasitol. (1996) 84:168–77. doi: 10.1006/expr.1996.0102
9. Spencer NF, Daynes RA. IL-12 directly stimulates expression of IL-10 by CD5+ B cells and IL-6 by both CD5+ and CD5- B cells: possible involvement in age-associated cytokine dysregulation. Int Immunol. (1997) 9:745–54. doi: 10.1093/intimm/9.5.745
10. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. (2008) 28:639–50. doi: 10.1016/j.immuni.2008.03.017
11. Mensen A, Johrens K, Anagnostopoulos I, Demski S, Oey M, Stroux A, et al. Bone marrow T-cell infiltration during acute GVHD is associated with delayed B-cell recovery and function after HSCT. Blood. (2014) 124:963–72. doi: 10.1182/blood-2013-11-539031
12. Serana F, Sottini A, Chiarini M, Zanotti C, Ghidini C, Lanfranchi A, et al. The different extent of B and T cell immune reconstitution after hematopoietic stem cell transplantation and enzyme replacement therapies in SCID patients with adenosine deaminase deficiency. J Immunol. (2010) 185:7713–22. doi: 10.4049/jimmunol.1001770
13. Mensen A, Ochs C, Stroux A, Wittenbecher F, Szyska M, Imberti L, et al. Utilization of TREC and KREC quantification for the monitoring of early T- and B-cell neogenesis in adult patients after allogeneic hematopoietic stem cell transplantation. J Transl Med. (2013) 11:188. doi: 10.1186/1479-5876-11-188
14. Nakatani K, Imai K, Shigeno M, Sato H, Tezuka M, Okawa T, et al. Cord blood transplantation is associated with rapid B-cell neogenesis compared with BM transplantation. Bone Marrow Transplant. (2014) 49:1155–61. doi: 10.1038/bmt.2014.123
15. van Zelm MC, Szczepanski T, van der Burg M, van Dongen JJ. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J Exp Med. (2007) 204:645–55. doi: 10.1084/jem.20060964
16. Abdel-Azim H, Elshoury A, Mahadeo KM, Parkman R, Kapoor N. Humoral immune reconstitution kinetics after allogeneic hematopoietic stem cell transplantation in children: a maturation block of IgM memory B cells may lead to impaired antibody immune reconstitution. Biol Blood Marrow Transplant. (2017) 23:1437–46. doi: 10.1016/j.bbmt.2017.05.005
17. Avanzini MA, Locatelli F, Dos Santos C, Maccario R, Lenta E, Oliveri M, et al. B lymphocyte reconstitution after hematopoietic stem cell transplantation: functional immaturity and slow recovery of memory CD27+ B cells. Exp Hematol. (2005) 33:480–6. doi: 10.1016/j.exphem.2005.01.005
18. Park BG, Park CJ, Jang S, Chi HS, Kim DY, Lee JH, et al. Reconstitution of lymphocyte subpopulations after hematopoietic stem cell transplantation: comparison of hematologic malignancies and donor types in event-free patients. Leuk Res. (2015) 39:1334–41. doi: 10.1016/j.leukres.2015.09.010
19. Bae KW, Kim BE, Koh KN, Im HJ, Seo JJ. Factors influencing lymphocyte reconstitution after allogeneic hematopoietic stem cell transplantation in children. Korean J Hematol. (2012) 47:44–52. doi: 10.5045/kjh.2012.47.1.44
20. Scarselli A, Di Cesare S, Capponi C, Cascioli S, Romiti ML, Di Matteo G, et al. Longitudinal evaluation of immune reconstitution and B-cell function after hematopoietic cell transplantation for primary immunodeficiency. J Clin Immunol. (2015) 35:373–83. doi: 10.1007/s10875-015-0154-4
21. D'Orsogna LJ, Wright MP, Krueger RG, McKinnon EJ, Buffery SI, Witt CS, et al. Allogeneic hematopoietic stem cell transplantation recipients have defects of both switched and igm memory B cells. Biol Blood Marrow Transplant. (2009) 15:795–803. doi: 10.1016/j.bbmt.2008.11.024
22. de Vries E, van Tol MJ, van den Bergh RL, Waaijer JL, ten Dam MM, Hermans J, et al. Reconstitution of lymphocyte subpopulations after paediatric bone marrow transplantation. Bone Marrow Transplant. (2000) 25:267–75. doi: 10.1038/sj.bmt.1702141
23. Olkinuora H, Talvensaari K, Kaartinen T, Siitonen S, Saarinen-Pihkala U, Partanen J, et al. T cell regeneration in pediatric allogeneic stem cell transplantation. Bone Marrow Transplant. (2007) 39:149–56. doi: 10.1038/sj.bmt.1705557
24. Kalwak K, Gorczynska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz M, et al. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br J Haematol. (2002) 118:74–89. doi: 10.1046/j.1365-2141.2002.03560.x
25. Glas AM, van Montfort EH, Storek J, Green EG, Drissen RP, Bechtold VJ, et al. B-cell-autonomous somatic mutation deficit following bone marrow transplant. Blood. (2000) 96:1064–9.
26. Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol. (2014) 14:495–504. doi: 10.1038/nri3689
27. Slatter MA, Bhattacharya A, Flood TJ, Spickett GP, Cant AJ, Abinun M, et al. Polysaccharide antibody responses are impaired post bone marrow transplantation for severe combined immunodeficiency, but not other primary immunodeficiencies. Bone Marrow Transplant. (2003) 32:225–9. doi: 10.1038/sj.bmt.1704109
28. Giebink GS, Warkentin PI, Ramsay NK, Kersey JH. Titers of antibody to pneumococci in allogeneic bone marrow transplant recipients before and after vaccination with pneumococcal vaccine. J Infect Dis. (1986) 154:590–6. doi: 10.1093/infdis/154.4.590
29. Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. (2003) 197:939–45. doi: 10.1084/jem.20022020
30. Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. (2004) 104:3647–54. doi: 10.1182/blood-2004-01-0346
31. Bosch M, Dhadda M, Hoegh-Petersen M, Liu Y, Hagel LM, Podgorny P, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. (2012) 14:1258–75. doi: 10.3109/14653249.2012.715243
32. Gerritsen EJ, van Tol MJ, Lankester AC, van der Weijden-Ragas CP, Jol-van der Zijde CM, Oudeman-Gruber NJ, et al. Immunoglobulin levels and monoclonal gammopathies in children after bone marrow transplantation. Blood. (1993) 82:3493–502.
33. Abrahamsen IW, Somme S, Heldal D, Egeland T, Kvale D, Tjonnfjord GE. Immune reconstitution after allogeneic stem cell transplantation: The impact of stem cell source and graft-versus-host disease. Haematologica. (2005) 90:86–93.
34. Kroger N, Zagrivnaja M, Schwartz S, Badbaran A, Zabelina T, Lioznov M, et al. Kinetics of plasma-cell chimerism after allogeneic stem cell transplantation by highly sensitive real-time PCR based on sequence polymorphism and its value to quantify minimal residual disease in patients with multiple myeloma. Exp Hematol. (2006) 34:688–94. doi: 10.1016/j.exphem.2006.01.011
35. Suzuki I, Milner EC, Glas AM, Hufnagle WO, Rao SP, Pfister L, et al. Immunoglobulin heavy chain variable region gene usage in bone marrow transplant recipients: lack of somatic mutation indicates a maturational arrest. Blood. (1996) 87:1873–80.
36. Di Martino D, Terranova MP, Scuderi F, Di Michele P, Iacovone S, Scarso L, et al. VH3 and VH6 immunoglobulin M repertoire reconstitution after hematopoietic stem-cell transplantation in children. Transplantation. (2005) 79:98–107. doi: 10.1097/01.TP.0000147461.71610.66
37. Di Martina D, Dallarsa S, Terranava P, Scarsa L, Morreale G, Valetta A. B-cell repertoire reconstitution after hematopoietic stem cell transplantation in children evaluated by immunoglobulin heavy chain third complementarity determining region fingerprinting. Haematologica. (2004) 89:506–8.
38. Gokmen E, Raaphorst FM, Boldt DH, Teale JM. Ig heavy chain third complementarity determining regions (H CDR3s) after stem cell transplantation do not resemble the developing human fetal H CDR3s in size distribution and Ig gene utilization. Blood. (1998) 92:2802–14.
39. Omazic B, Hentschke P, Näsman-Björk I, Mattsson J, Oxelius VA, Ringdén O, et al. Reconstitution of the Ig heavy chain CDR3 repertoire after allogeneic haematopoietic stem cell transplantation with myeloablative or reduced-intensity conditioning regimens. Scand J Immunol. (2005) 61:72–81. doi: 10.1111/j.0300-9475.2005.01528.x
40. Sethi MK, Thol F, Stadler M, Heuser M, Ganser A, Koenecke C, et al. VH1 family immunoglobulin repertoire sequencing after allogeneic hematopoietic stem cell transplantation. PLoS ONE. (2017) 12:e0168096. doi: 10.1371/journal.pone.0168096
41. Bartelink IH, Belitser SV, Knibbe CAJ, Danhof M, de Pagter AJ, Egberts TCG, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant. (2013) 19:305–13. doi: 10.1016/j.bbmt.2012.10.010
42. Renard C, Barlogis V, Mialou V, Galambrun C, Bernoux D, Goutagny MP, et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. Br J Haematol. (2011) 152:322–30. doi: 10.1111/j.1365-2141.2010.08409.x
43. Storek J, Dawson MA, Storer B, Stevens-Ayers T, Maloney DG, Marr KA, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. (2001) 97:3380–9. doi: 10.1182/blood.V97.11.3380
44. Baron F, Storer B, Maris MB, Storek J, Piette F, Metcalf M, et al. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. (2006) 12:1176–87. doi: 10.1016/j.bbmt.2006.07.004
45. Liu J, Xu LP, Bian Z, Chang YJ, Wang Y, Zhang XH, et al. Differential impact of two doses of antithymocyte globulin conditioning on lymphocyte recovery upon haploidentical hematopoietic stem cell transplantation. J Transl Med. (2015) 13:391. doi: 10.1186/s12967-015-0748-x
46. Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant. (2014) 49:426–33. doi: 10.1038/bmt.2013.191
47. Roll P, Muhammad K, Stuhler G, Grigoleit U, Einsele H, Tony HP. Effect of ATG-F on B-cell reconstitution after hematopoietic stem cell transplantation. Eur J Haematol. (2015) 95:514–23. doi: 10.1111/ejh.12524
48. Willemsen L, Jol-van der Zijde CM, Admiraal R, Putter H, Jansen-Hoogendijk AM, Ostaijen-Ten Dam MM, et al. Impact of serotherapy on immune reconstitution and survival outcomes after stem cell transplantations in children: thymoglobulin versus alemtuzumab. Biol Blood Marrow Transplant. (2015) 21:473–82. doi: 10.1016/j.bbmt.2014.11.674
49. Recher M, Berglund LJ, Avery DT, Cowan MJ, Gennery AR, Smart J, et al. IL-21 is the primary common gamma chain-binding cytokine required for human B-cell differentiation in vivo. Blood. (2011) 118:6824–35. doi: 10.1182/blood-2011-06-362533
50. Abd Hamid IJ, Slatter MA, McKendrick F, Pearce MS, Gennery AR. Long-term outcome of hematopoietic stem cell transplantation for IL2RG/JAK3 SCID: a cohort report. Blood. (2017) 129:2198–201. doi: 10.1182/blood-2016-11-748616
51. Miggelbrink AM, Logan BR, Buckley RH, Parrott RE, Dvorak CC, Kapoor N, et al. B cell differentiation and IL-21 response in IL2RG/JAK3 SCID patients after hematopoietic stem cell transplantation. Blood. (2018). 131:2967–77. doi: 10.1182/blood-2017-10-809822
52. Chiesa R, Veys P. Reduced-intensity conditioning for allogeneic stem cell transplant in primary immune deficiencies. Expert Rev Clin Immunol. (2012) 8:255–66; quiz 67. doi: 10.1586/eci.12.9
53. Rao K, Amrolia PJ, Jones A, Cale CM, Naik P, King D. Improved survival after unrelated donor bone marrow transplantation in children with primary immunodeficiency using a reduced-intensity conditioning regimen. Blood. (2005). 105:879–85. doi: 10.1182/blood-2004-03-0960
54. Bohmann EM, Fehn U, Holler B, Weber D, Holler E, Herr W, et al. Altered immune reconstitution of B and T cells precedes the onset of clinical symptoms of chronic graft-versus-host disease and is influenced by the type of onset. Ann Hematol. (2017) 96:299–310. doi: 10.1007/s00277-016-2881-x
55. Hilgendorf I, Mueller-Hilke B, Kundt G, Holler E, Hoffmann P, Edinger M, et al. The lack of memory B cells including T cell independent IgM+ IgD+ memory B cells in chronic graft-versus host disease is associated with susceptibility to infection. Transplant International. (2012) 25:87–96. doi: 10.1111/j.1432-2277.2011.01388.x
56. Xie M, Fu HX, Chang YJ, Xu LP, Liu DH, Zhang XH, et al. Characteristics and influencing factors of CD19+ B cell reconstitution in patients following haploidentical/mismatched hematopoietic stem cell transplantation. Int J Hematol. (2012) 96:109–21. doi: 10.1007/s12185-012-1099-5
57. Storek J, Wells D, Dawson MA, Storer B, Maloney DG. Factors influencing B lymphopoiesis after allogeneic hematopoietic cell transplantation. Blood. (2001) 98:489–91. doi: 10.1182/blood.V98.2.489
58. Fedoriw Y, Samulski TD, Deal AM, Dunphy CH, Sharf A, Shea TC, et al. Bone marrow B cell precursor number after allogeneic stem cell transplantation and GVHD development. Biol Blood Marrow Transplant. (2012) 18:968–73. doi: 10.1016/j.bbmt.2012.03.005
59. de Masson A, Bouaziz JD, Le Buanec H, Robin M, O'Meara A, Parquet N, et al. CD24hiCD27+ and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood. (2015) 125:1830–9. doi: 10.1182/blood-2014-09-599159
60. Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. (2014) 124:2034–45. doi: 10.1182/blood-2014-04-571125
61. Stikvoort A, Chen Y, Radestad E, Torlen J, Lakshmikanth T, Bjorklund A, et al. Combining flow and mass cytometry in the search for biomarkers in chronic graft-versus-host disease. Front Immunol. (2017) 8:717. doi: 10.3389/fimmu.2017.00717
62. Servais S, Lengline E, Porcher R, Carmagnat M, Peffault de Latour R, Robin M, et al. Long-term immune reconstitution and infection burden after mismatched hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2014) 20:507–17. doi: 10.1016/j.bbmt.2014.01.001
63. Arakawa-Hoyt J, Dao MA, Thiemann F, Hao QL, Ertl DC, Weinberg KI, et al. The number and generative capacity of human B lymphocyte progenitors, measured in vitro and in vivo, is higher in CB than in adult or pediatric bone marrow. Bone Marrow Transplant. (1999) 24:1167–76. doi: 10.1038/sj.bmt.1702048
64. Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. (2007) 110:4543–51. doi: 10.1182/blood-2007-05-092130
65. Funk PE, Witte PL. Enrichment of primary lymphocyte-supporting stromal cells and characterization of associated B lymphocyte progenitors. Eur J Immunol. (1992) 22:1305–13. doi: 10.1002/eji.1830220528
66. Kurosaka D, LeBien TW, Pribyl JA. Comparative studies of different stromal cell microenvironments in support of human B-cell development. Exp Hematol. (1999) 27:1271–81. doi: 10.1016/S0301-472X(99)00067-3
67. Hordyjewska A, Popiolek L, Horecka A. Characteristics of hematopoietic stem cells of CB. Cytotechnology. (2015) 67:387–96. doi: 10.1007/s10616-014-9796-y
68. Theurich S, Fischmann H, Shimabukuro-Vornhagen A, Chemnitz JM, Holtick U, Scheid C, et al. Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochr Database Syst Rev. (2012) 2012:CD009159. doi: 10.1002/14651858.CD009159.pub2
69. van Besien K, Kunavakkam R, Rondon G, De Lima M, Artz A, Oran B, et al. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. (2009) 15:610–7. doi: 10.1016/j.bbmt.2009.01.021
70. Ali R, Ramdial J, Algaze S, Beitinjaneh A. The role of anti-thymocyte globulin or alemtuzumab-based serotherapy in the prophylaxis and management of graft-versus-host disease. Biomedicines. (2017) 5:E67. doi: 10.3390/biomedicines5040067
71. Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. (2007) 21:1387–94. doi: 10.1038/sj.leu.2404683
72. Nuckel H, Frey UH, Roth A, Duhrsen U, Siffert W. Alemtuzumab induces enhanced apoptosis in vitro in B-cells from patients with chronic lymphocytic leukemia by antibody-dependent cellular cytotoxicity. Eur J Pharmacol. (2005) 514:217–24. doi: 10.1016/j.ejphar.2005.03.024
73. Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. (2005) 105:2973–8. doi: 10.1182/blood-2004-09-3660
74. Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. (2009) 113:3865–74. doi: 10.1182/blood-2008-09-177840
75. Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. (2012) 119:1570–80. doi: 10.1182/blood-2011-07-364414
76. Sarantopoulos S, Blazar BR, Cutler C, Ritz J. B cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2015) 21:16–23. doi: 10.1016/j.bbmt.2014.10.029
77. Zeiser R, Sarantopoulos S, Blazar BR. B-cell targeting in chronic graft-versus-host disease. Blood. (2018) 131:1399–405. doi: 10.1182/blood-2017-11-784017
78. Do RK, Chen-Kiang S. Mechanism of BLyS action in B cell immunity. Cytokine Growth Factor Rev. (2002) 13:19–25. doi: 10.1016/S1359-6101(01)00025-9
79. Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. (2000) 1:475–82. doi: 10.1038/82717
80. Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. (2002) 17:515–24. doi: 10.1016/S1074-7613(02)00425-9
81. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. (2000) 192:1553–62. doi: 10.1084/jem.192.11.1553
82. Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. (1997) 100:2757–65. doi: 10.1172/JCI119822
83. Kharfan-Dabaja MA, Cutler CS. Rituximab for prevention and treatment of graft-versus-host disease. Int J Hematol. (2011) 93:578–85. doi: 10.1007/s12185-011-0855-2
84. Pai CC, Chen M, Mirsoian A, Grossenbacher SK, Tellez J, Ames E, et al. Treatment of chronic graft-versus-host disease with bortezomib. Blood. (2014) 124:1677–88. doi: 10.1182/blood-2014-02-554279
85. Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. (2017) 130:2243–50. doi: 10.1182/blood-2017-07-793786
86. Parkkali T, Ruutu T, Stenvik M, Kuronen T, Kayhty H, Hovi T, et al. Loss of protective immunity to polio, diphtheria and Haemophilus influenzae type b after allogeneic bone marrow transplantation. APMIS. (1996) 104:383–8. doi: 10.1111/j.1699-0463.1996.tb00731.x
87. Ljungman P, Lewensohn-Fuchs I, Hammarstrom V, Aschan J, Brandt L, Bolme P, et al. Long-term immunity to measles, mumps, and rubella after allogeneic bone marrow transplantation. Blood. (1994) 84:657–63.
88. Ljungman P, Aschan J, Barkholt L, Broliden PA, Gustafsson B, Lewensohn-Fuchs I, et al. Measles immunity after allogeneic stem cell transplantation; influence of donor type, graft type, intensity of conditioning, and graft-versus host disease. Bone Marrow Transplant. (2004) 34:589–93. doi: 10.1038/sj.bmt.1704634
89. Guinan EC, Molrine DC, Antin JH, Lee MC, Weinstein HJ, Sallan SE, et al. Polysaccharide conjugate vaccine responses in bone marrow transplant patients. Transplantation. (1994) 57:677–84. doi: 10.1097/00007890-199403150-00009
90. Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. (1992) 165(Suppl. 1):S49–52.
91. Leather HL, Wingard JR. Infections following hematopoietic stem cell transplantation. Infect Dis Clin. (2001) 15:483–520.
92. Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Hematol Oncol Clin North Am. (2011) 25:101–16. doi: 10.1016/j.hoc.2010.11.008
93. Corre E, Carmagnat M, Busson M, de Latour RP, Robin M, Ribaud P, et al. Long-term immune deficiency after allogeneic stem cell transplantation: B-cell deficiency is associated with late infections. Haematologica. (2010) 95:1025–9. doi: 10.3324/haematol.2009.018853
94. Witherspoon RP, Storb R, Ochs HD, Fluornoy N, Kopecky KJ, Sullivan KM, et al. Recovery of antibody production in human allogeneic marrow graft recipients: influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood. (1981) 58:360–8.
95. Hammarstrom V, Pauksen K, Svensson H, Lonnqvist B, Simonsson B, Ringden O, et al. Serum immunoglobulin levels in relation to levels of specific antibodies in allogeneic and autologous bone marrow transplant recipients. Transplantation. (2000) 69:1582–6. doi: 10.1097/00007890-200004270-00011
96. Engelhard D, Cordonnier C, Shaw PJ, Parkalli T, Guenther C, Martino R, et al. Early and late invasive pneumococcal infection following stem cell transplantation: a European bone marrow transplantation survey. Br J Haematol. (2002) 117:444–50. doi: 10.1046/j.1365-2141.2002.03457.x
97. Kumar D, Humar A, Plevneshi A, Siegal D, Franke N, Green K, et al. Invasive pneumococcal disease in adult hematopoietic stem cell transplant recipients: a decade of prospective population-based surveillance. Bone Marrow Transplant. (2008) 41:743–7. doi: 10.1038/sj.bmt.1705964
98. Moens L, Wuyts M, Meyts I, De Boeck K, Bossuyt X. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J Immunol. (2008) 181:5306–12. doi: 10.4049/jimmunol.181.8.5306
99. Borrow R, Heath PT, Siegrist CA. Use of pneumococcal polysaccharide vaccine in children: what is the evidence? Curr Opin Infect Dis. (2012) 25:292–303. doi: 10.1097/QCO.0b013e3283531b0f
100. Carsetti R, Rosado MM, Donnanno S, Guazzi V, Soresina A, Meini A, et al. The loss of IgM memory B cells correlates with clinical disease in common variable immunodeficiency. J Allergy Clin Immunol. (2005) 115:412–7. doi: 10.1016/j.jaci.2004.10.048
101. Cameron PU, Jones P, Gorniak M, Dunster K, Paul E, Lewin S, et al. Splenectomy associated changes in IgM memory B cells in an adult spleen registry cohort. PLoS ONE. (2011) 6:e23164. doi: 10.1371/journal.pone.0023164
102. Conrad A, Alcazer V, Valour F, Ader F, Lyon HSG. Vaccination post-allogeneic hematopoietic stem cell transplantation: what is feasible? Expert Rev Vaccines. (2018) 17:299–309. doi: 10.1080/14760584.2018.1449649
103. Abd Hamid IJ, Slatter MA, McKendrick F, Pearce MS, Gennery AR. Long-Term Health outcome and quality of life post-HSCT for IL7Ralpha-, Artemis-, RAG1- and RAG2-deficient severe combined immunodeficiency: a single center report. J Clin Immunol. (2018) 38:727–32. doi: 10.1007/s10875-018-0540-9
104. Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol. (2018) 38:96–128. doi: 10.1007/s10875-017-0464-9
105. Stern L, McGuire H, Avdic S, Rizzetto S, Fazekas de St Groth B, Luciani F, et al. Mass cytometry for the assessment of immune reconstitution after hematopoietic stem cell transplantation. Front Immunol. (2018) 9:1672. doi: 10.3389/fimmu.2018.01672
106. H IJ, van Schouwenburg PA, van Zessen D, Pico-Knijnenburg I, Stubbs AP, van der Burg M. Antigen receptor galaxy: a user-friendly, web-based tool for analysis and visualization of T and B cell receptor repertoire data. J Immunol. (2017) 198:4156–65. doi: 10.4049/jimmunol.1601921
Keywords: hematopoietic stem cell transplantation, allogeneic, immune reconstitution, B lymphocyte, subsets, pediatric
Citation: van der Maas NG, Berghuis D, van der Burg M and Lankester AC (2019) B Cell Reconstitution and Influencing Factors After Hematopoietic Stem Cell Transplantation in Children. Front. Immunol. 10:782. doi: 10.3389/fimmu.2019.00782
Received: 03 January 2019; Accepted: 25 March 2019;
Published: 12 April 2019.
Edited by:
Geraldo Aleixo Passos, University of São Paulo, BrazilReviewed by:
Dário Ligeiro, Instituto Português do Sangue e Transplantação (IPST), PortugalMichael Uhlin, Karolinska Institute (KI), Sweden
Copyright © 2019 van der Maas, Berghuis, van der Burg and Lankester. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arjan C. Lankester, YS5sYW5rZXN0ZXJAbHVtYy5ubA==
 Nicolaas G. van der Maas
Nicolaas G. van der Maas Dagmar Berghuis
Dagmar Berghuis Mirjam van der Burg
Mirjam van der Burg Arjan C. Lankester
Arjan C. Lankester