
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 21 March 2019
Sec. Multiple Sclerosis and Neuroimmunology
Volume 10 - 2019 | https://doi.org/10.3389/fimmu.2019.00412
A commentary has been posted on this article:
Commentary: New Variant of MELAS Syndrome With Executive Dysfunction, Heteroplasmic Point Mutation in the MT-ND4 Gene (m.12015T>C; p.Leu419Pro) and Comorbid Polyglandular Autoimmune Syndrome Type 2
 Dominique Endres1,2*
Dominique Endres1,2* Patrick Süß1,2
Patrick Süß1,2 Simon J. Maier1,2
Simon J. Maier1,2 Evelyn Friedel1,2
Evelyn Friedel1,2 Kathrin Nickel1,2
Kathrin Nickel1,2 Christiane Ziegler2
Christiane Ziegler2 Bernd L. Fiebich2
Bernd L. Fiebich2 Franz X. Glocker3
Franz X. Glocker3 Friedrich Stock4
Friedrich Stock4 Karl Egger5
Karl Egger5 Thomas Lange6
Thomas Lange6 Michael Dacko6
Michael Dacko6 Nils Venhoff7
Nils Venhoff7 Daniel Erny8,9
Daniel Erny8,9 Soroush Doostkam8
Soroush Doostkam8 Katalin Komlosi10
Katalin Komlosi10 Katharina Domschke2
Katharina Domschke2 Ludger Tebartz van Elst1,2
Ludger Tebartz van Elst1,2Background: Mitochondrial diseases are caused by dysfunctions in mitochondrial metabolic pathways. MELAS syndrome is one of the most frequent mitochondrial disorders; it is characterized by encephalopathy, myopathy, lactic acidosis, and stroke-like episodes. Typically, it is associated with a point mutation with an adenine-to-guanine transition at position 3243 of the mitochondrial DNA (mtDNA; m.3243A>G) in the mitochondrially encoded tRNA leucine 1 (MT-TL1) gene. Other point mutations are possible and the association with polyglandular autoimmune syndrome type 2 has not yet been described.
Case presentation: We present the case of a 25-year-old female patient with dysexecutive syndrome, muscular fatigue, and continuous headache. Half a year ago, she fought an infection-triggered Addison crisis. As the disease progressed, she had two epileptic seizures and stroke-like episodes with hemiparesis on the right side. Cerebral magnetic resonance imaging showed a substance defect of the parieto-occipital left side exceeding the vascular territories with a lactate peak. The lactate ischemia test was clearly positive, and a muscle biopsy showed single cytochrome c oxidase-negative muscle fibers. Genetic testing of blood mtDNA revealed a heteroplasmic base exchange mutation in the mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 4 (MT-ND4) gene (m.12015T>C; p.Leu419Pro; heteroplasmy level in blood 12%, in muscle tissue: 15%). The patient suffered from comorbid autoimmune polyglandular syndrome type 2 with Hashimoto's thyroiditis, Addison's disease, and autoimmune gastritis. In addition, we found increased anti-glutamic acid decarboxylase 65, anti-partial cell, anti-intrinsic factor, and anti-nuclear antibodies.
Conclusion: We present an atypical case of MELAS syndrome with predominant symptoms of a dysexecutive syndrome, two stroke-like episodes, and fast-onset fatigue. The symptoms were associated with a not yet described base and aminoacid exchange mutation in the MT-ND4 gene (m.12015T>C to p.Leu419Pro). The resulting changed protein complex in our patient is part of the respiratory chain multicomplex I and might be the reason for the mitochondriopathy. However, different simulations for pathogenetic relevance are contradictory and rather speak for a benign variant. To our knowledge this case report is the first reporting MELAS syndrome with comorbid polyglandular autoimmune syndrome type 2. Screening for autoimmune alterations in those patients is important to prevent damage to end organs.
Mitochondrial diseases are caused by malfunctions surrounding the mitochondrial metabolic pathways. They are clinically, genetically, and biochemically heterogeneous, can occur at any age, often involve the central nervous system (CNS), and are usually associated with multiorgan involvement (1, 2). Mitochondria produce energy for the cell in form of adenosine triphosphate (ATP) generated by oxidative phosphorylation within the respiratory chain. The respiratory chain is composed of five enzymatic multiprotein complexes (I, II, III, IV, and V). Four of these complexes contain both proteins encoded by nuclear DNA (nDNA) and proteins encoded by mitochondrial DNA (mtDNA). Therefore, mutations in nuclear and mitochondrial DNA may affect the respiratory chain function resulting in impaired ATP synthesis and cellular dysfunction or death (3). The mtDNA mutations can be divided into structural rearrangements (e.g., deletions), quantitative disturbances of mtDNA also being called mtDNA depletion (reduction of the mtDNA copy number), and point mutations (base exchange mutations) (1). Some known inherited mitochondrial disorders are MELAS (Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes), LHON (Leber hereditary optic neuropathy), or MERRF (myoclonic epilepsy and ragged red fibers) (1, 2). Because of the mixed genetics and the broad spectrum of biochemical functions of mitochondria the clinical manifestations of mitochondrial disorders are very heterogeneous. Typically, post-mitotic long-living cells like neurons, muscle cells or retinal cells are predominantly affected by these diseases. MELAS, one of the most common mitochondrial disorders, is characterized mainly by symptoms of myopathy, encephalopathy, lactic acidosis, stroke-like episodes, and other organ-related complications such dementia, epilepsy, headache, hearing impairment, short stature, or type 1 diabetes mellitus (3, 4).
Autoimmune polyendocrine syndromes (APS) are a heterogeneous family of autoimmune diseases. The two major types, APS-1 and APS-2 are both associated with adrenal cortex insufficiency (Addison's disease). While APS type 2 is most frequently associated with diabetes mellitus and/or autoimmune thyroiditis, APS type 1 is often characterized by the coexistence of hypoparathyroidism and mucocutaneous candidiasis, a condition which is also called autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED). Both types can be associated with other autoimmune diseases (5, 6).
The most prevalent immunological alteration in mitochondrial disease is type 1 diabetes mellitus, which is associated with the point mutation m.3243A > G and is frequently found in patients with mitochondrial disease (7, 8). Autoimmune polyglandular syndrome type 2 (with Addison's disease, autoimmune thyroiditis, diabetes mellitus, and primary ovarian failure) was reported once in a patient with mitochondrial disease, more specifically in a female patient with KSS and a consistent 2,532-bp deletion of her mtDNA, and—beyond that—with a heteroplasmic m.3243A > G mutation (9)—but not in patients with MELAS syndrome.
We present the case of a 25-year-old female German patient with dysexecutive syndrome (affective instability, sensory overload, concentration, and impulse control problems), rapid-onset physical fatigue, and continuous headache.
The patient's history was negative for in-utero or birth complications, inflammatory brain diseases, or febrile convulsions. She reported cognitive deficits (memory difficulties and needing more time to learn than her siblings) and impulsive behaviors (especially when things change) since her first decade of life. Both symptoms were mild and did not meet the diagnostic criteria for attention-deficit/hyperactivity disorder or autism. The subject did not encounter tics, or extraordinary personality traits, though she had known a physical lack of energy for as long as she could remember. She was easily exhausted in sports at school and could not keep up with her family on hikes. She had to go to bed early, upon which she fell asleep swiftly. Since she was 6 years old, she experienced repeated syncopations which based on history probably represented vasovagal pathomechanisms. There was no history of increased frequency of infections, especially no signs of mucocutaneous candidiasis. Dental history was unremarkable.
When she was seventeen, she fell off her bike in an unclear way and suffered brain trauma with cerebral hemorrhage. The cerebral magnetic resonance imaging (MRI) showed left hemispheric subdural hemorrhage and “contre-coup” right fronto-opercular as well as multiple right temporal cerebral contusions. Neither a subsequent change of personality nor other neurocognitive or psychomotor symptoms were observed by the patient or her family. Five years ago, she noticed hyperpigmentation (Figure 1). Four years ago, she developed early morning nausea that occurred almost on a daily base. She had no appetite and could eat very little. At age 25 (half a year before presentation in our clinic), she developed an infection-triggered Addison crisis with hypotension, diarrhea/vomiting, hypoglycemia, vigilance reduction, and metabolic acidosis. The diagnosis of Addison's disease was suspected due to the Addison crisis, anti-adrenal cortex autoantibodies (titer of 1:100; reference < 1:10) and indicative laboratory values (ACTH increased, 71.4 pg/ml; reference: 5–50 pg/ml). During her inpatient stay, the patient already noticed a self-limiting, discrete hemiparesis on the right side. Treatment was started with a glucocorticoid (hydrocortisone) and a mineralocorticoid (fludrocortisone). Later, the patient developed two grand mal seizures, for which she was admitted to an intensive care unit. An anti-epileptic treatment with levetiracetam was initiated. While being on intensive care treatment, she developed progressive disorientation, reduced vigilance, headache, coordination disturbances and, again, hemiparesis on the right side. The MRI showed a subacute left parieto-occipital cortical/subcortical lesion involving the precentral gyrus with T2-weighted FLAIR signal increase and diffusion restriction without respecting vascular territories and the multiple right-hemispheric chronic posttraumatic lesions. There was a clear lactate peak in the lesion (Figure 2). The EEG showed an intermittent slowing temporoparietal on both sides (stronger on the left) and singular spike-wave complexes temporal (once temporoparietal) on the left. During her subsequent stay in a neurological rehabilitation clinic, her neurological deficits receded almost completely. On occasion her right hand may feel clumsier than her left, but this occurs rarely. Following the grand mal seizures symptoms of sensory overload and affective instability evolved and persisted ever since. She has not experienced any further early morning nausea following treatment with hydro- and fludrocortisone. Several attempts to reduce levetiracetam led to an exacerbation of cognitive deficits.
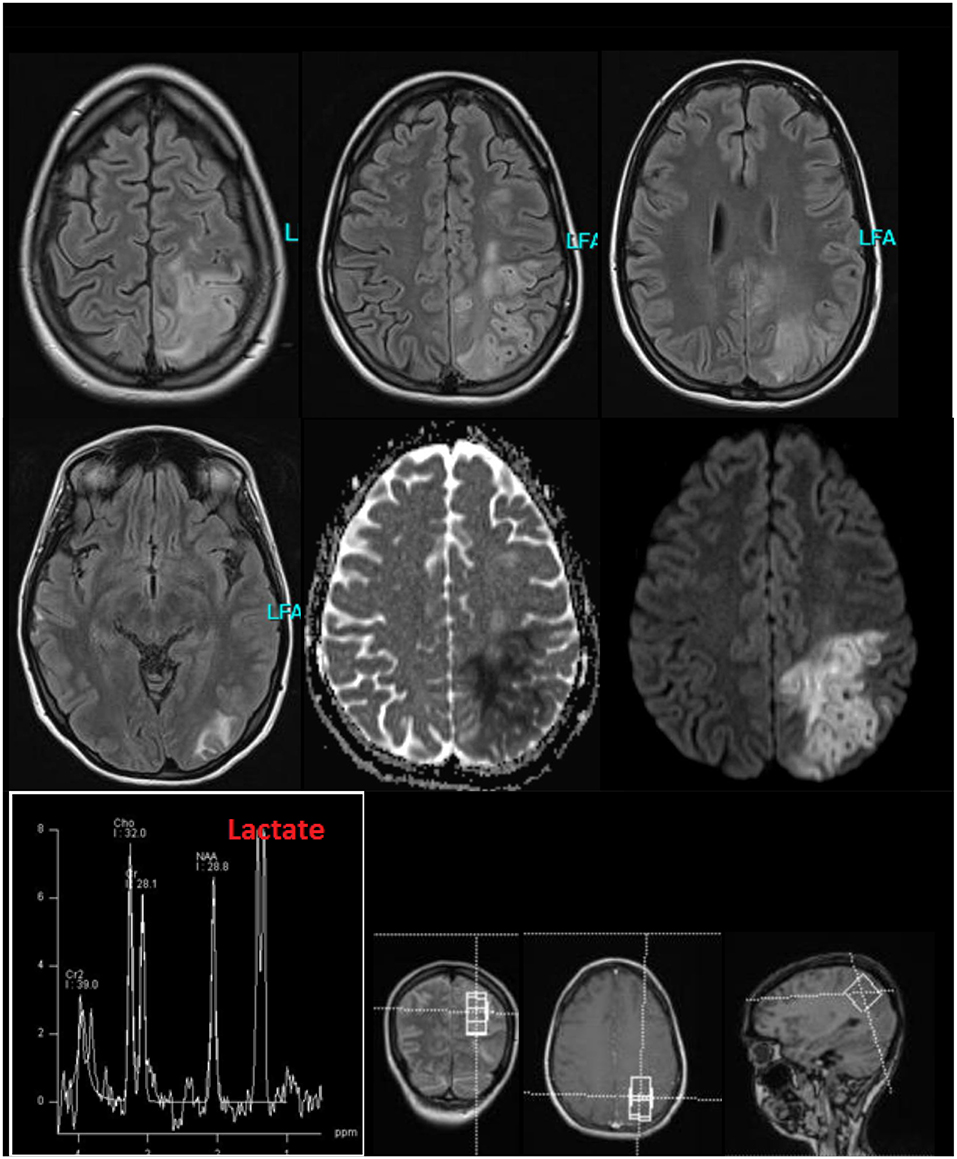
Figure 2. Cerebral magnetic resonance imaging (MRI) findings during the stay in the intensive care unit because of stroke like episodes. The MRI showed a subacute left parieto-occipital cortical/subcortical lesion involving the precentral gyrus with T2-weighted FLAIR signal increase and diffusion restriction without respecting vascular territories and the multiple right-hemispheric chronic posttraumatic lesions. The former subdural hemorrhage showed complete resolution. In the lesion, an increased lactate peak was registered (uncorrected data).
The patient suffered from Hashimoto's thyroiditis for years (substituted with L-thyroxine) and, as already mentioned, Addison's disease. When being diagnosed for the Addison crisis normochromic, normocytic anemia was recognized (hemoglobin: 10.4 g/dl; reference 12–16 g/dl). Two years prior to present admission a gastroscopy was done revealing mild to moderate antrum gastritis suspicious of chronic toxic (type C) gastritis. Her mother, sister, and maternal grandfather all suffered from type 1 diabetes mellitus, while another sister is healthy.
Analysis of the complete mitochondrial genome from peripheral blood leukocytes showed a heteroplasmic base exchange mutation in the mitochondrially encoded NADH:ubiquinone oxidoreductase core subunit 4 (MT-ND4) gene (m.12015T>C; p.Leu419Pro). Heteroplasmy level for the mutation in blood showed 12%, in muscle tissue 15%. This point mutation was not detected in the blood of the patient's mother, no other tissues were analyzed in the mother. No other pathogenic or likely pathogenic alteration was detected in the mtDNA of the patient.
In MITOMAP, the human mitochondrial genome database (www.mitomap.org/MITOMAP) the alteration is only reported as a mtDNA coding region sequence variant and has not been reported in association with disease yet. Several simulations (prediction tools) assume a benign change (fathmm, MutationTaster, PROVEAN), according to SIFT it is a pathogenic variant (Table 1). Besides the mtDNA analysis, only the nuclear DNA polymerase subunit gamma (POLG) gene was analyzed in the patient. In the POLG gene (part of mitochondria-related nDNA) no pathogenic variant (mutation), but the well-known polymorphism c.156_158delGCA; p.Q55del (rs727504102) was detected in heterozygous state. For this deletion, an association with diabetes mellitus has been reported in the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php). It is classified as a clearly benign polymorphism in several databases (https://www.ncbi.nlm.nih.gov/clinvar/variation/167521/).
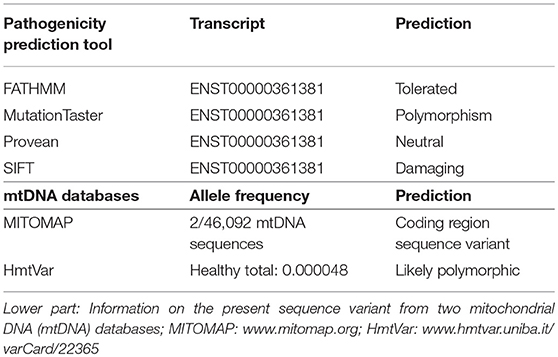
Table 1. Upper part: Different in silico prediction scores for the pathogenic relevance of the novel base exchange variant in the MT-ND4 gene of our patient.
The psychiatric investigation showed attention and concentration deficits, memory difficulties, affective instability, impulsive behaviors and sensory overload. Cognitive performance showed partially below-average results (Figure 3). The lactate ischemia test revealed a more than three-fold increase in lactate levels compared to resting state values. The lactate concentration in the cerebrospinal fluid (CSF) was not increased. In the muscle biopsy, single cytochrome c oxidase (COX)-negative muscle fibers were identified. In the immunological screening, increased anti-thyroglobulin, increased anti-thyroid peroxidase, anti-adrenal cortex, anti-glutamic acid decarboxylase (GAD-65), anti-partial cell, anti-intrinsic factor, and anti-nuclear antibodies (ANAs) were discerned. Serum concentration for immunoglobulin (Ig)G, IgM and especially IgA were within normal range. The B-cell panel showed clearly increased number of IgD-CD27+B-cells, IgA+CD27+memory B-cells, and IgM+/–CD38++plasma blasts of B-cells. The urine analyses revealed only slightly increased albumin-creatinine quotient and therefore no typical signs of focal segmental glomerulosclerosis (10). A current gastroscopy showed signs of autoimmune gastritis. The examination's findings are summarized in Table 2.
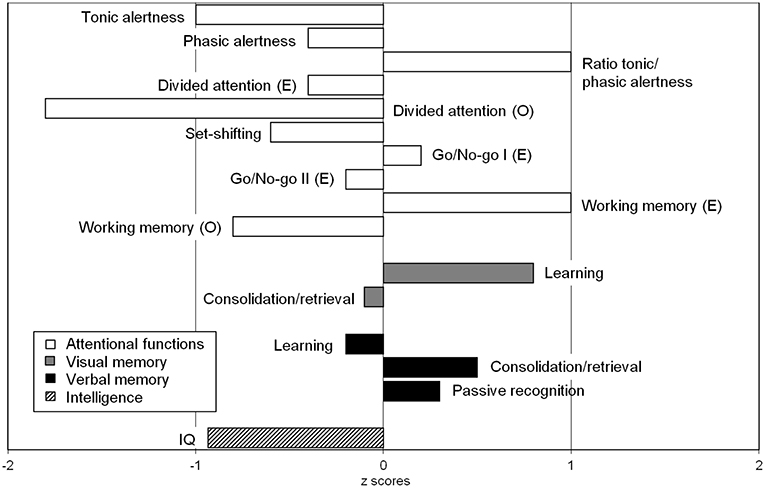
Figure 3. Neuropsychological findings showed significant impairments in divided attention for visual and auditory stimuli combined. The recorded intelligence level was in the lower normal range (IQ = 86), which appeared discrepant with the patient's educational background (this may also be attributed to fluctuations in attention). A detailed description of all test results can be found in Table 2 in the bottom column on neuropsychological test results.
The follow-up cMRI 6 months after the stroke like events showed a chronic cortical to subcortical parieto-occipital brain lesion on the left side involving the posterior margin of the precentral gyrus with signs of chronic hemorrhagic transformation and the known multiple post-traumatic right hemispheric lesions (Figure 4). Single-voxel magnetic resonance spectroscopy results show slightly increased lactate (Lac)+/creatine ratios in the dorsal anterior cingulate cortex (dACC), dorsolateral prefrontal cortex (DLPFC), and orbitofrontal cortex (OFC; Figure 5).
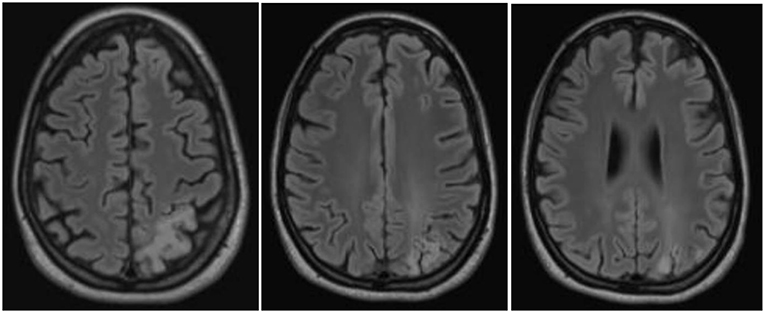
Figure 4. Magnetic resonance imaging findings a half year after the stroke-like episodes showed a chronic cortical to subcortical parieto-occipital brain lesion on the left side involving the posterior margin of the precentral gyrus with signs of chronic hemorrhagic transformation.
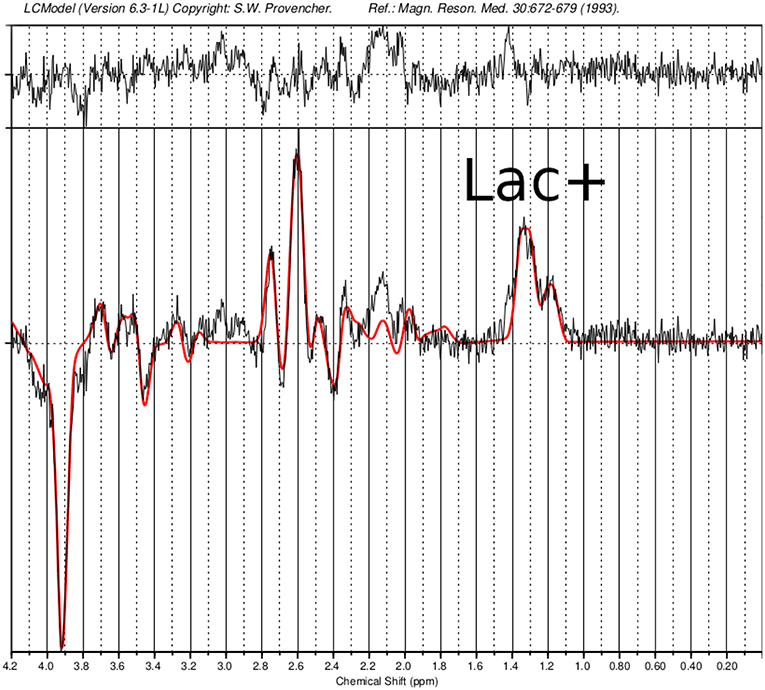
Figure 5. Single-voxel magnetic resonance spectroscopy imaging. Presented is a MEGA-sLASER difference spectrum from the orbitofrontal cortex (OFC) of the patient, with indicated lactate (Lac)+ resonance. In the OFC, a Lac+/Creatine concentration ratio of 0.182 was found compared to a control group (N=6; 5 males and 1 female; 28 ± 2 years) with 0.159 ± 0.01.
In this paper, we present the case of a female patient with a new variant of MELAS with the clinical phenomenology of a dysexecutive syndrome and a history of two stroke-like episodes, “novel” heteroplasmic base exchange mutation in the MT-ND4 gene (m.12015T>C; p.Leu419Pro), and comorbid polyglandular autoimmune syndrome type 2.
The patient suffered from myopathy with reduced physical capacity, a positive lactate ischemia test [following the criteria of Finsterer et al. (16)], discrete myopathic changes, and slightly pathological muscle biopsy results with single COX-negative muscle fibers and a discrete unspecific type 2 fiber atrophy. Myopathy clearly existed before steroid treatment began. Moreover, she suffered from encephalopathy with chronical dysexecutive syndrome, headache, EEG pathologies, and epileptic seizures (successfully treated with high doses of levetiracetam). She had two earlier stroke-like episodes with a typical parieto-occipital lesion and a lactate peak. Lactate levels outside the lesion (in the different prefrontal areas: ACC, OFC, and DLPFC) were slightly higher compared with controls, however the lactate concentration in the CSF was normal, which might indicate to a milder form of mitochondriopathy. Therefore, the patient fulfilled three main criteria of MELAS: Myopathy, encephalopathy and stroke-like episodes (3). The patient may have previously suffered from the fourth main symptom, lactate acidosis. Currently, the subject's serum and CSF lactate levels were normal. However, the result of the lactate ischemia test makes earlier episodes of lactate acidosis likely. With a body height of 172 cm, the patient does not have a short stature or growth hormone deficiency. However, she is the smallest person in her family. Her testosterone concentrations were extremely low (not measurable) and secondary hair was reduced. In a cohort of 99 MELAS patients abnormal growth was described in 50% of the individuals (8). No involvement of eyes, inner ears, the heart, or kidneys was found.
A diagnosis of Hashimoto's thyroiditis was made based on the clinical findings of hypothyroidism combined with increased antibodies against thyroglobulin and thyroid peroxidase, and with typical alterations in thyroid sonography. The findings to justify the diagnosis of Addison's disease were hyperpigmentation, loss of weight, gastrointestinal symptoms, and a history of Addison crisis with hypotension, diarrhea/vomiting, hypoglycemia, and vigilance reduction, alongside with metabolic acidosis and compatible laboratory findings (anti-adrenal cortex antibodies and increased ACTH-levels) and the normalization of these symptoms following treatment with gluco- and mineralocorticoids. There might be an overlap between Addison's disease and MELAS syndrome leading to the symptom of vomiting (could also be due to lactate acidosis in the context of MELAS). IgA serum concentration was within normal range and there was no evidence for mucocutaneous candidiasis, enamel hypoplasia, or hypoparathyroidism in the patients' medical history. Therefore, the diagnosis of APECED or autoimmune polyglandular syndrome type I (APS-I) was very unlikely. The combination of Hashimoto's thyroiditis, Addison's disease, and pernicious anemia led to the diagnosis of the more frequent autoimmune polyglandular syndrome type II. The course of MELAS syndrome was complicated by comorbid Addison's disease. Moreover, the present patient suffered from an autoimmune gastritis with anti-partial gastric cells, and further immunological activation with antibodies against GAD-65 (without clear signs for APECED), intrinsic factor, and nuclear antigens with negative DFS70 testing. Currently, the patient was not experiencing manifest type 1 diabetes. The finding of an ANA with homogeneous nuclear staining pattern in indirect immunofluorescence was suspicious for connective tissue disease like systemic lupus erythematosus. Because of the low ANA titer (1:200), no specificity in ENA-testing, unremarkable complement diagnostics, and no clinical symptoms indicative of a connective tissue disease (such as joint pain) this diagnosis was excluded.
MELAS syndrome is classically (in over 80% of all cases) associated with a point mutation with adenine-to-guanine transition at position 3243 of mtDNA (m.3243A>G) in the mitochondrially encoded tRNA leucine 1 (MT-TL1) gene encoding tRNALeu(UUR) (1, 3). Further underlying mutations were found in the mitochondrial genes MT-TL1 (in particular, mutation 3271T>C) (17), MTTQ, MTTH, MTTK, MTTC, MTTS1, MTND1, MTND5, MTND6, and MTTS2. One case report describes a case of MELAS syndrome due to mutation in the nuclear POLG1 gene (18). The present patient displayed a heteroplasmic base exchange mutation in the MT-ND4 gene (m.12015T>C; p.Leu419Pro). Changes in the MT-ND4 gene were earlier published in one paper regarding two patients with MELAS syndrome (PubMed search for “MELAS” and “ND4” performed on December 23, 2018). An A-to-G base substitution at 11084, which leads to a Thr to Ala amino acid substitution was suggested to be disease-related (19). That association was later proven wrong as this very mutation was found in 14% of Asians (thus classifying it as a polymorphism). The point mutation affecting the patient described here has thus far only been detected in two of 46,092 examined sequences and has not yet been associated with mitochondriopathy (https://www.mitomap.org/MITOMAP and HmtVar: www.hmtvar.uniba.it/varCard/22365. It is possible that the two subjects with the same variant, who were documented earlier, had less-severe heteroplasmia, so that penetrance was low, i.e., the critical threshold value, which leads to the appearance of symptoms, was not exceeded. A very low and therefore undetectable heteroplasmia level in blood or a de novo mutation might explain the inconspicuous findings in the mtDNA of the present patients' mother. An examination of the asymptomatic mother from a second tissue was not desired. The resulting protein complex with the changed protein in the present patient (see Figure 6; marked in blue) is part of the respiratory complex I (https://www.ebi.ac.uk/pdbe/entry/pdb/5xtc/protein/25; Figure 6), which is comparable with genetic alterations in earlier published cases with MELAS syndrome (1). Different simulations resulted in contradictory results (from mostly benign to pathophysiologically relevant). From a clinical perspective, the association seems logical (the phenotype, especially including the results of the muscle biopsy and the MRI underline a clinical picture of MELAS), however, currently there is not enough evidence available to prove the association. Further data on patients with a similar phenotype harboring the same MT-ND4 alteration are needed to evaluate the association of this novel mtDNA point mutation and MELAS syndrome. Moreover, there might be further mutations or variants in the nDNA regarding Addison's disease or polyglandular autoimmune syndrome, which could contribute to this complex disorder.
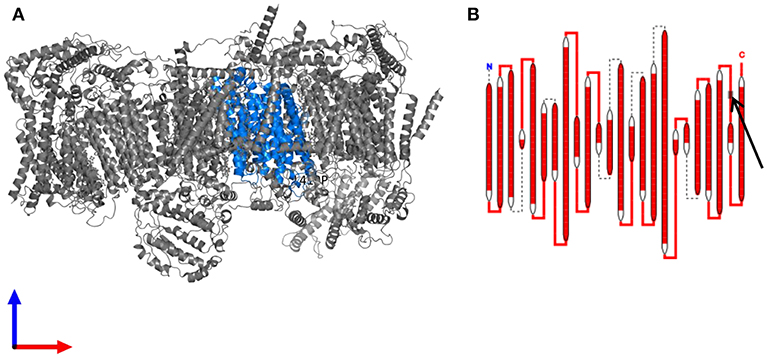
Figure 6. The resulting protein complex with the changed protein (A) Structure of the human respiratory chain complex I. The protein subunit carrying the mutation is highlighted in blue. (B) Schematic drawing of the complex I subunit altered in the patient. The site of the mutation in an extramembranous domain is indicated by an arrow. Data are derived from https://www.ebi.ac.uk/pdbe/entry/pdb/5xtc/protein/25 (20).
The most common immunological comorbidity in MELAS syndrome is type 1 diabetes mellitus (4), especially due to the frequent m.3243A > G mutation in MELAS syndrome, which is associated with diabetes mellitus (7). The present patient had increased diabetes-associated anti-GAD65 antibodies and a carried a polymorphism in the POLG gene, which is known to be associated with diabetes mellitus; however, clinically, she did not suffer from diabetes mellitus. The prevalence of growth and sexual hormone alterations was also high in earlier studies (8). In line with that observation, our patient suffered from low testosterone levels and is the shortest in her family. Hashimoto's thyroiditis and Addison's disease were earlier described as comorbid disorders in patients with mitochondriopathies; however, generally they do not seem to occur more often in this condition (7, 8). The association between MELAS syndrome and Addison's disease was explored in earlier papers (21, 22). O'Grady and colleagues presented an overview of earlier cases with a combination of mitochondriopathy and primary adrenal insufficiency. All the cases had manifested in childhood and lacked predominant psychiatric symptoms (i.e., dysexecutive symptoms), as was the case with our patient (22).
To our knowledge, this is the first case report of a patient with MELAS syndrome with comorbid autoimmune polyglandular syndrome type 2. This aspect is clinically important, as shown in the patient's history. Addison's disease and MELAS syndrome (lactate acidosis/myopathy) share clinical features with respect to weakness, fatigue, and abdominal complaints such as nausea or diarrhea. Hence, it is important to think of both diseases when considering the differential diagnosis. Additionally, our patient was tested positive for further antibodies. It is important to recognize these immunological changes to prevent end organ damage. In our patient, gastritis or anemia could be avoided by administering stomach protection and a vitamin B12 substitute, Addison's disease was successfully treated with steroids; L-thyroxin was substituted, and regular controls for early detection of diabetes mellitus were recommended.
Autoimmune polyendocrine syndromes are frequently associated with a broad spectrum of antibodies. In contrast, mitochondrial disorders like MELAS are generally not characterized by the presence of antibodies or autoimmune phenomena. Therefore, it is most likely that the broad spectrum of antibodies described here is part of the APS in our patient. On the other hand, it cannot be excluded for sure that mitochondrial disorders themselves may have impact on components of the immune system. Like neurons, cardiac myocytes, and skeletal muscle fibers, which are frequently affected in mitochondrial disorders because of their postmitotic state and longevity, antibody-producing plasma cells are postmitotic, long-living cells and highly dependent on sufficient energy supply by their mitochondria. A decline in ATP production could impair the quantity of ab production and result in hypogammaglobulinemia. An increased formation of reactive oxygen species might result in increased mutation rates and activation of apoptotic pathways within immune cells. Since mitochondria also play an important role in intracellular calcium homeostasis mitochondrial disorders might affect cellular mobility and signaling processes (8).
In summary, to our knowledge this is the first case presentation of a patient with atypical MELAS syndrome presenting with a neuropsychiatric syndrome of predominantly executive dysfunction, together with a polyglandular autoimmune syndrome type 2, and a heteroplasmic point mutation in the MT-ND4 gene. The recognition of such constellations is important for further research and clinical differential diagnosis in complex neuropsychiatric cases.
All datasets generated for this study are included in the manuscript and/or the supplementary files.
The patient has given her signed written informed consent for this case report, including the presented images, to be published.
DoE treated the patient, performed the data research, and wrote the paper. PS, KK, KD, and LT supported the interpretation. SD, DaE, and PS performed the neuropathological analyses and interpretation. KE performed and interpreted the MRIs. SM, KN, MD, and TL performed and interpreted the magnetic resonance spectroscopy. FS, CZ, BF, and KK interpreted the genetic testing. FG performed the neurological investigation and interpretation. NV performed the rheumatological measurements and interpreted the results. EF performed the opthalmoscopic examinations. All authors were critically involved in the theoretical discussion and composition of the manuscript. All authors read and approved the final version of the manuscript.
The article processing charge was funded by the German Research Foundation (DFG) and the University of Freiburg in the funding program Open Access Publishing.
LT has received fees for lectures from Medice, Sevier, Shire, and Actelion within the last three years.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Kornblum C. Mitochondriale Erkrankungen. S1-Leitlinie der DGN für Diagnostik und Therapie in der Neurologie. (2012). Available online at: https://www.dgn.org/leitlinien/2385-ll-20-2012-mitochondriale-erkrankungen
2. Lightowlers RN, Taylor RW, Turnbull DM. Mutations causing mitochondrial disease: what is new and what challenges remain? Science. (2015) 349:1494–9. doi: 10.1126/science.aac7516
3. El-Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. (2015) 116:4–12. doi: 10.1016/j.ymgme.2015.06.004
4. Yatsuga S, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T, et al. Taro matsuoka for MELAS study group in Japan. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta. (2012) 1820:619–24. doi: 10.1016/j.bbagen.2011.03.015
5. Brabant G, Manns MP, Vogel A, Strassburg CP. Autoimmun polyglanduläre syndrome: aspekte zu pathogenese, prognose und therapie. Dtsch Arztebl. (2002) 99:A1428–34.
6. Cutolo M. Autoimmune polyendocrine syndromes. Autoimmun Rev. (2014) 13:85–9. doi: 10.1016/j.autrev.2013.07.006
7. Schaefer AM, Walker M, Turnbull DM, Taylor RW. Endocrine disorders in mitochondrial disease. Mol Cell Endocrinol. (2013) 379:2–11. doi: 10.1016/j.mce.2013.06.004
8. Al-Gadi IS, Haas RH Falk MJ, Goldstein A, McCormack SE. Endocrine disorders in primary mitochondrial disease. J Endocr Soc. (2018) 2:361–73. doi: 10.1210/js.2017-00434
9. Ohno K, Yamamoto M, Engel AG, Harper CM, Roberts LR, Tan GH, et al. MELAS- and kearns-sayre-type co-mutation [corrected] with myopathy and autoimmune polyendocrinopathy. Ann Neurol. (1996) 39:761–6.
10. Seidowsky A, Hoffmann M, Glowacki F, Dhaenens CM, Devaux JP, de Sainte Foy CL, et al. Renal involvement in MELAS syndrome - a series of 5 cases and review of the literature. Clin Nephrol. (2013) 80:456–63. doi: 10.5414/CN107063
11. Nieves-Moreno M, Martínez-de-la-Casa JM, Cifuentes-Canorea P, Sastre-Ibáñez M, Santos-Bueso E. (2017) Normative database for separate inner retinal layers thickness using spectral domain optical coherence tomography in caucasian population. PLoS ONE 12:e0180450. doi: 10.1371/journal.pone.0180450
12. Arteaga de Castro CS, Boer VO, Andreychenko A, Wijnen JP, van der Heide UA, Luijten PR, et al. Improved efficiency on editing MRS of lactate and γ-aminobutyric acid by inclusion of frequency offset corrected inversion pulses at high fields. NMR Biomed. (2013) 26:1213–9. doi: 10.1002/nbm.2937
13. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. (1998) 11:266–72. doi: 10.1002/(SICI)1099-1492(199810)11:6<266::AID-NBM530>3.0.CO;2-J
14. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. (1993) 30:672–9. doi: 10.1002/mrm.1910300604
15. Behar KL, Rothman DL, Spencer DD, Petroff OAC. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. (1994) 32:294–302. doi: 10.1002/mrm.1910320304
16. Finsterer J, Shorny S, Capek J, Cerny-Zacharias C, Pelzl B, Messner R, et al. Lactate stress test in the diagnosis of mitochondrial myopathy. J Neurol Sci. (1998) 159:176–80. doi: 10.1016/S0022-510X(98)00170-1
17. Tarnopolsky MA, Maguire J, Myint T, Applegarth D, Robinson BH. Clinical, physiological, and histological features in a kindred with the T3271C melas mutation. Muscle Nerve. (1998) 21:25–33.
18. Deschauer M, Tennant S, Rokicka A, He L, Kraya T, Turnbull DM, et al. MELAS associated with mutations in the POLG1 gene. Neurology. (2007) 68:1741–2. doi: 10.1212/01.wnl.0000261929.92478.3e
19. Lertrit P, Noer AS, Jean-Francois MJ, Kapsa R, Dennett X, Thyagarajan D, et al. A new disease-related mutation for mitochondrial encephalopathy lactic acidosis and strokelike episodes (MELAS) syndrome affects the ND4 subunit of the respiratory complex I. Am J Hum Genet. (1992) 51:457–68.
20. Velankar S, van Ginkel G, Alhroub Y, Battle GM, Berrisford JM, Conroy MJ, et al. PDBe: improved accessibility of macromolecular structure data from PDB and EMDB. Nucleic Acids Res. (2016) 44:D385–95. doi: 10.1093/nar/gkv1047
21. Afroze B, Amjad N, Ibrahim SH, Humayun KN, Yakob Y. Adrenal insufficiency in a child with MELAS syndrome. Brain Dev. (2014) 36:924–7. doi: 10.1016/j.braindev.2013.12.009
Keywords: MELAS, mitochondrial disease, polyglandular autoimmune syndrome, ND4 gene, dysexecutive syndrome
Citation: Endres D, Süß P, Maier SJ, Friedel E, Nickel K, Ziegler C, Fiebich BL, Glocker FX, Stock F, Egger K, Lange T, Dacko M, Venhoff N, Erny D, Doostkam S, Komlosi K, Domschke K and Tebartz van Elst L (2019) New Variant of MELAS Syndrome With Executive Dysfunction, Heteroplasmic Point Mutation in the MT-ND4 Gene (m.12015T>C; p.Leu419Pro) and Comorbid Polyglandular Autoimmune Syndrome Type 2. Front. Immunol. 10:412. doi: 10.3389/fimmu.2019.00412
Received: 24 December 2018; Accepted: 15 February 2019;
Published: 21 March 2019.
Edited by:
Tjalf Ziemssen, Zentrum für Klinische Neurowissenschaften (ZKN), GermanyReviewed by:
Silvia Corrochano, Medical Research Council, United KingdomCopyright © 2019 Endres, Süß, Maier, Friedel, Nickel, Ziegler, Fiebich, Glocker, Stock, Egger, Lange, Dacko, Venhoff, Erny, Doostkam, Komlosi, Domschke and Tebartz van Elst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominique Endres, ZG9taW5pcXVlLmVuZHJlc0B1bmlrbGluaWstZnJlaWJ1cmcuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.