- 1Radiation Oncology Unit, University Hospital of Siena, Siena, Italy
- 2Medical Oncology Unit, Grand Metropolitan Hospital “Bianchi Melacrino Morelli”, Reggio Calabria, Italy
- 3Sbarro Health Research Organization, Temple University, Philadelphia, PA, United States
- 4Department of Biology, College of Science and Technology, Temple University, Philadelphia, PA, United States
- 5Department of Medicine, Surgery and Neurosciences University of Siena, Siena, Italy
- 6Department of Experimental and Clinical Medicine, Magna Graecia University, Catanzaro, Italy
- 7Medical Oncology Unit, Azienda Ospedaliero – Universitaria “Mater Domini”, Catanzaro, Italy
An extraordinary large amount of strategies potentially able to elicit and empower an efficient anti-tumor immune-response in cancer patients, has already been described (1). However, a number of hurdles have delayed the translation of these results in efficacious treatments for many years leaving the immunological treatments confined to malignant melanoma and renal cell carcinoma(2, 3). In the latter few years, the discovery of priming (CTLA-4/B7.1) and effector (PD-1/PDL-1) immune-checkpoints and the availability of highly specific blocking mAbs has lead to a terrific clinical development of the immune-oncology approaches. Some of these mAbs, especially those directed to PD-1 (Nivolumab and Pembrolizumab) expressed on activated CTLs, or PDL-1 (Atezolizumab, Durvalumab, and Avelumab) expressed on inflammatory and cancer cells, have in fact, gained a stable role in the treatment of very common malignancies such as non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), and urological malignancies, where they are capable of producing significant benefit to many patients and prolonging their survival in about a quarter of the cases (4). Even though this kind of strategy is considered quite successful, it is however, hampered by the fact that its efficacy is unpredictable and is associated to immune-related adverse events (irAEs) and unsustainable costs. At the present, the identification of reliable biomarkers of response to immune-oncology treatments as well as the design of combined strategies to enhance their efficacy and field of action represent one of the mainstream immune-oncology research lines. PD-1/PDL-1 is a peripheral immune-checkpointaimed to attenuate the cytotoxic response of tumor-specific infiltrating lymphocytes. Thus, its blockade by anti PD-1 (Nivolumab and Pembrolizumab) or anti PDL-1 mAbs (Atezolizumab, Durvalumab, and Avelumab) rescues these CTLs and triggers a fast cytolytic effect in the tumor tissue (5). This effect may triggera rapid antitumor effect;neverthelessthis renewed CTL reaction is not sufficient alone to prolong patients' survival. In fact the antitumor activity of these reactivated cells, is more or less rapidly extinguished if a continuous and self-sustained supply of fresh tumor-specific immune-effectors does not occur (immunopriming) (6). Experimental evidence suggests in fact, the achievement of a prolonged patient survival requires a continuous immune-priming, in order to avoid CTL exhaustion in the tumor and to prevent an adaptive response by the tumor cells (7, 8). In this context, CTLA-4/B7.1 immune-check point, acts by attenuating the proliferative activity of antigen specific CTL clones, expressing CTLA-4 and by stimulating the immune-suppressive activity of immune-regulatory T cells (Tregs). Its blockade by Ipilimumab and Tremelimumab, two mAbs to CTLA-4, represents a valid therapeutic option for both metastatic malignant melanoma and renal cell carcinoma and is under clinical investigation in combination with effector PD1/PDL-1 immunocheckpoint blockade (9–12). An efficient Immune-priming however, requires the expression of multiple tumor associate (TAAs) and tumor specific antigens (TSAs) by cancer cells, released as consequence of cancer-associated inflammation, necrosis, previous use of cytotoxic drugs or radiation therapy (13). A number of studies have shown that the efficacy of both immune-effectors and antigen cross-priming may be hardened by cancer vaccines, specific anticancer treatments (radiotherapy, chemotherapy, steroid hormones, and immune-adjuvant agents), hypoxic response and/or tumor associated inflammation (14, 15) (Figure 1).
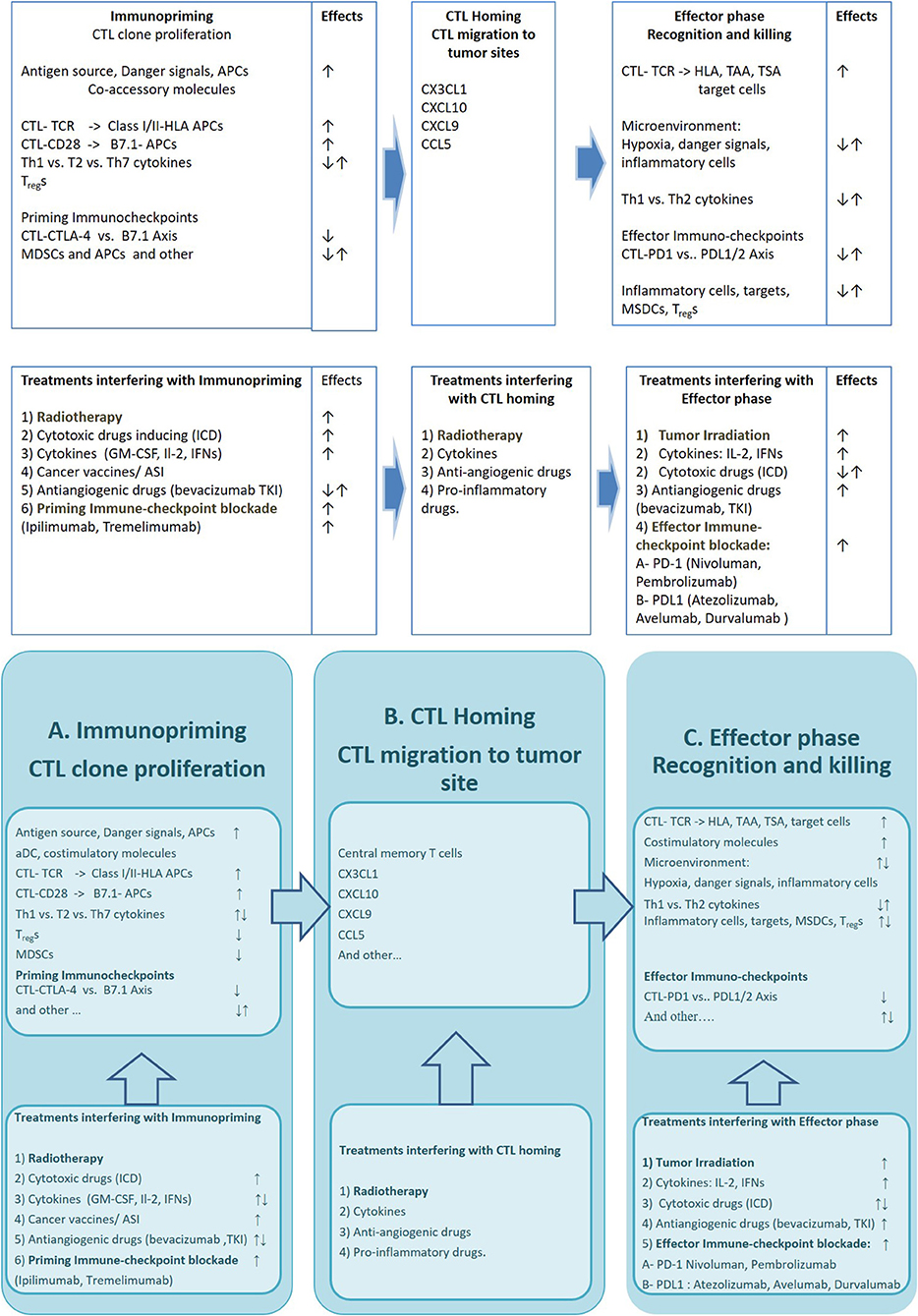
Figure 1. The figure describes the critical mechanisms involved in three phases of the immune-response against cancer and available drugs and strategies which may improve its efficacy Upper row: Specific cell lineages, molecular structures and immune-checkpoints involved in immunopriming process (A), T cell Homing (B), and modulation of CTL mediated Tumor cell killing (C). Bottom row: Strategies (AKA radiation therapy), cytotoxic Drugs, cytokines and Immunocheckpoint inhibitors interfering with the immunopriming (A), T cell Homing (B), and T cell mediated killing (C). APCs, antigen presenting cells; CTL-TCR, cytotoxic T lymphocites–T cell Receptor; HLA, Human Leucocyte Antigen; MDSCs, myeloid derived suppressor cells; TAA, Tumor Associated Antigen; TSA, Tumor Specific Antigen; ICD, Immunogenic Cell Death inducers; GM-CSF, Granulocyte-macrophage colony-stimulating factor; IFNs, interferons; ASI, active specific immunotherapy; TKI, tyrosine kinase inhibitor; CTLA-4, Cytotoxic T cell antigen−4; PD-1, Programmed cell death receptor-1; PDL, Programmed cell death ligand.
Radiation therapy in particular, together with its direct cyto-reductive activity on tumor burden is also capable of eliciting radio-induced DNA damage on target cells and triggering specific immunological effects (16) which are believed to be responsible for the “abscopal effects” observed in those rare cases, where tumor irradiation is paralleled by regression of non-irradiated tumor sites (17, 18). This hypothesis is in line with the results of a large number of studies showing that tumor irradiation may really influence all the phases of the immune-response. Tumor irradiation may in fact, trigger immunogenic cell death, and significant release of TAAs and TSAs in a context of immunological danger signal. The latter is consequent to DNA damage by radiation which is able to activate of Damage-Associated-Molecularbiochemical Patterns (DAMP) which in turn are able of enhancing tumor antigens presentation to CTL precursors and their proliferation in the draining lymph-nodes (19). Furthermore, the irradiated-tumor cells release inflammatory cytokines, chemokines (such as CXCL16) and tumor vessel associated adhesion molecules (VCAM-I and ICAM-I) able to reinforce the presence of activated CTLs in the tumor site (19–21). Finally, strong evidence does exists concerning the ability of radiation therapy to induce up-regulation of class I MHC, multiple death receptors (e.g., FAS, NKG2DL) in the target cells thus enhancing their susceptibility to recognition and killing by tumor specific CTLs (19). Clinical evidences in line with these preclinical results have also been reported.
IAn abscopal response to radiation was recorded in metastatic NSCLC patients who were receiving immunological treatment with ipilimumab (22). We recently carried out a retrospective analysis in advanced NSCLC patients enrolled in the BEVA2017, who had received an immune-modulating treatment with metronomic chemotherapy (mPE) +/– bevacizumab (mPEBev) reporting that that the use of radiotherapy given on palliative setting, was associated to a prolonged survival and that this effect was indeed correlated to a significant treatment-related increase in activated DCs and effector memory CTLs (23). Similarly, in a retrospective analysis of the KEYNOTE-001 phase I study aimed to investigate Pembrolizumab in a cohort of 495 patients advanced NSCLC patients, it has been detected a much longer PFS and OS in a group of 97 patients who had received radiation therapy prior immunotherapy (24). Finally, a perspective randomized phase III study in un-resectable lung stage III cancer patients aimed to receive chemoradiation followed by Durvalumab or placebo for 12 months (PACIFIC) reported a significant advantage in PFS in the experimental arm, which was unrelated to PDL-1 expression in the tumor (25).
In HNSCC, the immune system is known to have a pivotal role, as high density of tumor-infiltrating lymphocytes (TILs) is associated with improved outcome of patients (26, 27) while tumor tissues and draining lymph-nodes respectively, present a high density of CTLs expressing PD-1 and regulatory Tregs over-expressing CTLA-4; a finding that clearly suggests a high suppressive activity of either peripheral and central immune-checkpoints in these patients (28).
Based on this solid rationale, PD-1 blockade with Nivolumab, Pembrolizumab, and Durvalumab represented a concrete option for the treatment of recurrent or metastatic HNSCC to be investigated. At the present, the results of three large trials in HNSCC patients on or after frontline platinum-based chemotherapy, concur to show an median overall response rate of 11.3–18%, with a median time to progression of 9.7 months and a 32%reduced risk of death at 1 year of (29–33). These encouraging results led to the design of a number of clinical trials which are currently ongoing with the specific aim of combining tumor irradiation with immunological agents and/or immune-check point blockade in patients with advanced HNSCC (see Table 1).
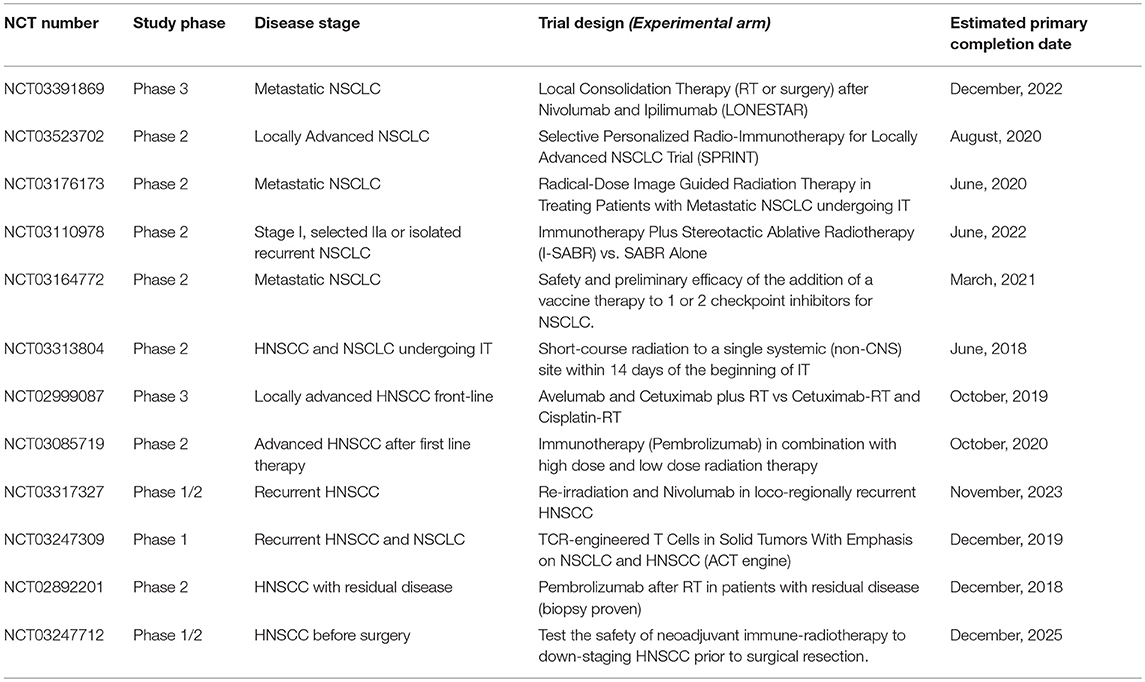
Table 1. Ongoing trials testing immunotherapy (IT) in combination with radiation therapy (RT) in patients with NSCLC or HNSCC.
On these premises, a rationale use of radiation therapy may be included among the various strategies that could potentially increase the efficacy of immunotherapy at different disease settings. We believe that more successful immune-oncological trials should take in consideration this knowledge to improve their benefit NSCLC and HNSCC patients.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Correale P, Cusi MG, Micheli L, Nencini C, Del Vecchio MT, Torino F, et al. Chemo-immunotherapy of colorectal carcinoma: preclinical rationale and clinical experience. Invest New Drugs (2006) 24:99–110. doi: 10.1007/s10637-006-5932-7
2. Bukowski RM. Natural history and therapy of metastatic renal cell carcinoma: the role of interleukin-2. Cancer (1997) 80:1198–220.
3. Sanlorenzo M, Vujic I, Carnevale-Schianca F, Quaglino P, Gammaitoni L, Fierro MT, et al. Role of interferon in melanoma: old hopes and new perspectives. Expert Opin Biol Ther. (2017) 17:475–83. doi: 10.1080/14712598.2017.1289169
4. Rosenberg SA. Decade in review-cancer immunotherapy: entering the mainstream of cancer treatment. Nat Rev Clin Oncol. (2014) 11:630–2. doi: 10.1038/nrclinonc.2014.174
5. Disis ML. Mechanism of action of immunotherapy. Semin Oncol. (2014) 41 (Suppl. 5):S3–13. doi: 10.1053/j.seminoncol.2014.09.004
6. Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. (2015) 33:445–74. doi: 10.1146/annurev-immunol-032414-112043
7. Shahabi V, Postow MA, Tuck D, Wolchok JD. Immune-priming of the tumor microenvironment by radiotherapy: rationale for combination with immunotherapy to improve anticancer efficacy. Am J Clin Oncol. (2015) 38:90–7. doi: 10.1097/COC.0b013e3182868ec8
8. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. (2015) 1:1325–32. doi: 10.1001/jamaoncol.2015.2756
9. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreatedmelanoma. N Engl J Med. (2015) 372:2006–17. doi: 10.1056/NEJMoa1414428
10. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-celllungcancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. (2016) 17:883–95. doi: 10.1016/S1470-2045(16)30098-5
11. Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol. (2017) 35:3851–8. doi: 10.1200/JCO.2016.72.1985
12. Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim YC, Ballas M, et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. (2016) 17:232–236.e1. doi: 10.1016/j.cllc.2016.03.003
13. Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. (2014) 88:986–97. doi: 10.1016/j.ijrobp.2013.08.035
14. Ladoire S1, Hannani D, Vetizou M, Locher C, Aymeric L, Apetoh L, et al. Cell-death-associated molecular patterns as determinants of cancer immunogenicity. Antioxid Redox Signal. (2014) 20:1098–116. doi: 10.1089/ars.2012.5133
15. Correale P, Botta C, Basile A, Pagliuchi M, Licchetta A, Martellucci I, et al. Phase II trial of bevacizumab and dose/dense chemotherapy with cisplatin and metronomicdailyoraletoposide in advanced non-small-cell-lungcancerpatients.Cancer Biol Ther. (2011) 12:112–8. doi: 10.4161/cbt.12.2.15722
16. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. (2007) 13:54–61. doi: 10.1038/nm1523
17. Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. (1953) 26:234–41. doi: 10.1259/0007-1285-26-305-234
18. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. (2004) 58:862–70. doi: 10.1016/j.ijrobp.2003.09.012
19. Levy A, Chargari C, Marabelle A, Perfettini JL, Magné N, Deutsch E. Can immunostimulatory agents enhance theabscopal effect of radiotherapy? Eur J Cancer (2016) 62:36–45. doi: 10.1016/j.ejca.2016.03.067
20. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer. (2016) 40:25–37. doi: 10.1016/j.currproblcancer.2015.10.001
21. Baird JR, Monjazeb AM, Shah O, McGee H, Murphy WJ, Crittenden MR, et al. stimulating innate immunity to enhance radiation therapy-induced tumor control. Int J Radiat Oncol Biol Phys. (2017) 99:362–73. doi: 10.1016/j.ijrobp.2017.04.014
22. Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. (2013) 1:365–72. doi: 10.1158/2326-6066.CIR-13-0115
23. Pastina P, Nardone V, Botta C, Croci S, Tini P, Battaglia G, et al. Radiotherapy prolongs the survival of advancednon-small-cell lung cancer patients undergone to an immune-modulating treatment with dosefractionedcisplatin and metronomic etoposide and bevacizumab (mPEBev). Oncotarget (2017) 8:75904–13. doi: 10.18632/oncotarget.20411
24. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinicalactivity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: asecondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. (2017) 18:895–903. doi: 10.1016/S1470-2045(17)30380-7
25. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
26. Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rödel F, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer (2014) 110:501–9. doi: 10.1038/bjc.2013.640
27. Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer (2014) 110:489–500. doi: 10.1038/bjc.2013.639
28. Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. (2014) 50:627–32. doi: 10.1016/j.oraloncology.2014.04.003
29. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
30. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. (2016) 17:956–65. doi: 10.1016/S1470-2045(16)30066-3
31. Chow LQM, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. (2016) 34:3838–45. doi: 10.1200/JCO.2016.68.1478
32. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. (2017) 35:1542–9. doi: 10.1200/JCO.2016.70.1524
33. Segal NH, Ou SI, Balmanoukian AS, Massarelli E, Brahmer JR, Weiss J, et al. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. Ann Oncol. (2016) 27(Suppl. 6):949. doi: 10.1093/annonc/mdw376.01
Keywords: NSCLC, radiation therapy, immunotherapy, HNSCC, chemotherapy
Citation: Nardone V, Pastina P, Giannicola R, Agostino R, Croci S, Tini P, Pirtoli L, Giordano A, Tagliaferri P and Correale P (2018) How to Increase the Efficacy of Immunotherapy in NSCLC and HNSCC: Role of Radiation Therapy, Chemotherapy, and Other Strategies. Front. Immunol. 9:2941. doi: 10.3389/fimmu.2018.02941
Received: 08 October 2018; Accepted: 30 November 2018;
Published: 12 December 2018.
Edited by:
Patrik Andersson, Massachusetts General Hospital, Harvard Medical School, United StatesReviewed by:
Charles A. Kunos, National Cancer Institute (NIH), United StatesAlessandro Isidori, Ospedali Riuniti Marche Nord, Italy
Copyright © 2018 Nardone, Pastina, Giannicola, Agostino, Croci, Tini, Pirtoli, Giordano, Tagliaferri and Correale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pierpaolo Correale, Y29ycmVhbGVwQHlhaG9vLml0
 Valerio Nardone
Valerio Nardone Pierpaolo Pastina1
Pierpaolo Pastina1