- 1Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, Netherlands
- 2Department of Parasitology, Leiden University Medical Center, Leiden, Netherlands
- 3National Institute for Medical Research, Mwanza, Tanzania
- 4Department of Medicine, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
- 5Center for Global Health, Weill Cornell Medicine, New York, NY, United States
- 6Department of Medicine, Bugando Medical Centre, Mwanza, Tanzania
Background: Traditional microscopic examination of urine or stool for schistosome eggs lacks sensitivity compared to measurement of schistosome worm-derived circulating antigens in serum or urine. The ease and non-invasiveness of urine collection makes urine an ideal sample for schistosome antigen detection. In this study several user-friendly, lateral-flow (LF) based urine assays were evaluated against a composite reference that defined infection as detection of either eggs in urine or anodic antigen in serum.
Method: In a Tanzanian population with a S. haematobium prevalence of 40–50% (S. mansoni prevalence <2%), clinical samples from 44 women aged 18 to 35 years were analyzed for Schistosoma infection. Urine and stool samples were examined microscopically for eggs, and serum samples were analyzed for the presence of the anodic antigen. Urines were further subjected to a set of LF assays detecting (circulating) anodic (CAA) and cathodic antigen (CCA) as well as antibodies against soluble egg antigens (SEA) and crude cercarial antigen preparation (SCAP).
Results: The urine LF anodic antigen assay utilizing luminescent upconverting reporter particles (UCP) confirmed its increased sensitivity when performed with larger sample volume. Qualitatively, the anodic antigen assay performed on 250 μL urine matched the performance of the standard anodic antigen assay performed on 20 μL serum. However, the ratio of anodic antigen levels in urine vs. serum of individual patients varied with absolute levels always higher in serum. The 10 μL urine UCP-LF cathodic antigen assay correlated with the commercially available urine POC-CCA (40 μL) test, while conferring better sensitivity with a quantitative result. Urinary antibodies against SEA and SCAP overlap and correlate with the presence of urinary egg and serum anodic antigen levels.
Conclusions: The UCP-LF anodic antigen assay using 250 μL of urine is an expedient user-friendly assay and a suitable non-invasive alternative to serum-based antigen testing and urinary egg detection. Individual biological differences in the clearance process of the circulating antigens are thought to explain the observed high variation in the type and level of antigen (anodic or cathodic) measured in urine or serum. Simultaneous detection of anodic and cathodic antigen may be considered to further increase accuracy.
Introduction
Schistosomiasis is a neglected parasitic infection that may affect over 700 million people worldwide (1), and optimal diagnostic strategies have still not been established. Accurate diagnosis of an active Schistosoma infection requires elaborate parasitology on multiple samples as eggs cluster together and shedding is irregular (2); these factors are known to limit the sensitivity of the egg detection methods in urine (i.e., S. haematobium) as well as stool (i.e., S. mansoni). This traditional diagnostic method of microscopic examination of urine (3) or stool (4) for schistosome eggs in medium to low endemic areas lacks sensitivity compared to measurement of schistosome worm antigen in serum or urine (5, 6). Maximizing sensitivity becomes particularly pressing in the setting of low-intensity infections (more common among adults), and also as efforts to interrupt transmission accelerate (7). Schistosome circulating anodic and cathodic antigens (CAA and CCA, respectively) are glycosaminoglycan-like carbohydrates regurgitated from the gut of live schistosome worms into the host bloodstream (8). Both antigens are cleared from the human circulation via the kidneys and can be detected in serum as well as urine, allowing non-invasive diagnosis. Antigen levels diminish rapidly following anti-schistosome praziquantel treatment (9, 10). Detection of these antigens has been shown to be more accurate to identify active and low grade infections and does not require the analyses of different sample types in case of unknown species or mixed infection. A urine cathodic antigen assay is available as a low cost point-of-care test (POC-CCA) with excellent diagnostic capability for moderate to high-grade S. mansoni infection (5, 11, 12), but to a lesser extent for S. haematobium detection (13). This assay is mostly used for mapping and monitoring of endemic areas (14–16), but also showed its use in non-endemic countries for individual diagnosis of e.g. immigrants (17–19).
A more sensitive genus-specific anodic antigen LF assay, applicable for all Schistosoma species including veterinary infections, was developed several years ago (20). Multiple studies have utilized this up-converting phosphor LF assay (UCP-LF CAA) to investigate prevalence in S. mansoni, S. haematobium, S. mekongi, and S. japonicum settings using serum, urine, or both (21–29). The test platform includes a sample preparation step that allows analysis of increased sample volume providing ultimate sensitivity down to detection of single worm (single sex) infections. A study in the People's Republic of China showed a sensitivity of >90% for diagnosis of S. japonicum using the assay on concentrated urine samples, as compared to a composite reference that defined infection as detection of either eggs or anodic antigen in serum or urine (30). Anodic antigen concentrations were approximately 10-fold lower in urine than in serum similar to what has been described previously (31).
We sought to determine the performance of several user-friendly LF based urine assays in a population in which S. haematobium is endemic. Urine LF assays were evaluated against a composite reference of either detecting eggs in urine and/or schistosome anodic antigen in serum. Eggs were detected by standard urine filtration microscopy and the level of schistosome anodic antigen in serum was determined using the UCP-LF CAA assay. We also compared these findings to the commercially-available POC-CCA urine test, as well as an experimental UCP-LF urine assay, both detecting the cathodic antigen. In addition, two rapid UCP-LF antibody assays detecting antibodies against crude cercarial and soluble egg preparations were evaluated for use with urine samples.
Methods
Study Site and Population
This study was conducted in four rural inland villages in northwest Tanzania in which S. haematobium is highly endemic (32). The prevalence of S. haematobium infection among adults in these villages is ~40–50%, and the prevalence of S. mansoni infection is ≤2% (25). All women between the ages of 18 and 35 in each village were invited to receive free screening for schistosomiasis. Women provided urine, stool, and blood samples. Urine and stool were examined microscopically for Schistosoma eggs, and serum was used to determine schistosome anodic antigen concentration. Urine samples were further analyzed for anodic and cathodic antigens and anti-schistosome antibodies. Women were also screened for HIV and those enrolled in this study were all HIV-uninfected. Individuals found to be HIV-infected were given referral letters to the nearest HIV Care and Treatment Centre, where they could access HIV care free of charge.
Sample Collection and Field Analysis
All samples were collected between 10:00 a.m. and 2:00 p.m. on study enrollment days. Women provided at least 25 mL of urine. Ten mL of urine was filtered in the field and immediately examined microscopically for S. haematobium eggs. Fifteen mL of urine was placed into Falcon tubes and stored at −20°C at the reference laboratory in Mwanza until transport to Leiden University Medical Center for schistosome antigen and antibody testing. To test for S. mansoni eggs, five Kato-Katz slides were prepared from each stool sample, a strategy with a sensitivity comparable to testing three stool samples obtained on separate days (33). Serum was centrifuged and separated each day upon return to Mwanza and subsequently stored at −20°C.
Serum Anodic Antigen Testing in Local Laboratory
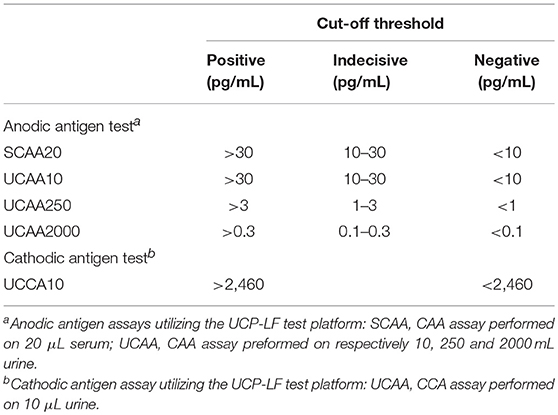
Serum anodic antigen testing using the (UCP-LF) technology was performed at the National Institute for Medical Research in Mwanza as previously described (20, 34). In this dry-reagent format of the assay, 20 μL of serum was used (SCAA20) (12). Serum was pretreated with an equal volume of 4% (v/v) trichloroacetic acid (TCA); applied cut-off thresholds are indicated in Table 1. Cut-offs are derived from Corstjens et al. (12) and are determined from testing series of negative samples.
Urine Anodic Antigen Lateral Flow Assays
UCP-LF anodic antigen assays using increasing volumes of urine were performed at Leiden University Medical Center as previously described (35). All urine samples were tested using 10 μL of urine (UCAA10) and 250 μL of urine (UCAA250) (12). Samples with negative or indecisive results in the UCAA250 assay were retested using 2,000 μL of urine (UCAA2000). All samples were extracted with an equal volume of 4% (v/v) TCA. Concentration of the clear supernatant was performed using 0.5 and 4 mL single-use 10 kD concentration devices (Amicon Ultra Centrifugal Filters, Millipore Corp) for the UCAA250 and UCAA2000, respectively. The resulting 20 μL concentrate was used in the assay. Applied cut-off thresholds are indicated in Table 1.
Additional Urine Tests
Besides the UCP-LF anodic antigen assay, urine samples were also examined with four additional tests. The commercially-available urine point-of-care (POC)-CCA test (Rapid Medical Diagnostics, Pretoria, South Africa; format 2013, utilizing chase buffer) was used to evaluate its performance in an S. haematobium-endemic setting. Furthermore, a prototype UCP-LF cathodic antigen assay, quantitating cathodic antigen in urine, was performed with 10 μL urine (UCCA10), following the same protocol as described for the anodic antigen assay (UCAA10), but with a cathodic antigen specific UCP reporter conjugate and LF strips, as described earlier (12). Cut-off thresholds for the UCCA10 assay are indicated in Table 1. The UCP-LF cathodic antigen assay has not been explored with concentrated urines and therefore does not include an indecisive (potential positive) range; the applied cutoff is according to the threshold as determined previously with ELISA (36). The UCCA10 utilizes the same antibody on the UCP reporter as on the LF strip (mouse monoclonal 54-4C2), whereas the POC-CCA uses a second antibody on the LF strip (mouse monoclonal 54-5C10). Sample input differs as the POC-CCA requires 40 μL of untreated urine whereas the UCCA10 assay utilizes 10 μL urine which is extracted with an equal volume of TCA (4% w/v). In addition, two UCP-LF-based assays for antibody detection against soluble egg antigens (SEA) and crude cercarial antigen preparation (SCAP) in urine were applied, as previously described (12). The cut-off threshold was calculated from 11 samples that were negative in all antigen assays as well as with microscopy.
Data Analysis
Sensitivity (Sn) and specificity (Sp) were calculated for each diagnostic test, considering the combined results from microscopy (egg count) and SCAA20 (serum anodic antigen) as the “composite reference.” Hence, any positive test result by urinary microscopy or SCAA20 (indecisive results considered as negative) was considered a true-positive result. Consequently, the specificity was set to be 100% for these two assays. To indicate the weight of indecisive (potentially positive) and trace results, Sn and Sp were calculated for each test separately including indecisive or trace results either as positive or negative. Additionally, the performance of each test was compared to UCAA2000 and a combination of UCAA2000 and UCCA10 (indecisive results of the UCAA2000 were considered as negative). The correlation between the two antibody assays was calculated using the Spearman rho. All statistical analysis were performed using IBM SPSS Statistics v 23.0 (IBM Corp, Armonk, NY).
Ethics
This project was approved by the Catholic University for Health and Allied Sciences/Bugando Medical Centre (Mwanza, Tanzania), the National Institute for Medical Research (Dar es Salaam, Tanzania), and Weill Cornell Medical College (New York, New York). All study participants provided written informed consent and were given praziquantel on the day of study enrollment in accordance with World Health Organization recommendations for treatment of adults in areas in which schistosome infections are highly endemic (37).
Results
Serum Anodic Antigen and Urine Microscopy Findings—A Composite Reference
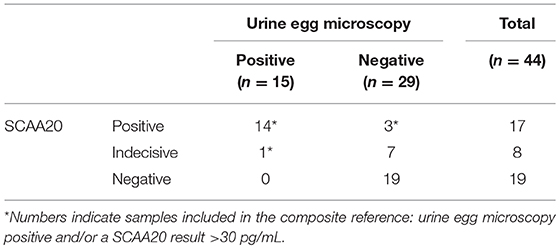
Between June and August 2013, we obtained 39 samples from women who presented for schistosomiasis screening, and 5 additional post-treatment samples from women with a confirmed S. haematobium infection and who received praziquantel treatment 2 months previously. The distribution of locally determined serum anodic antigen levels (SCAA20) and urine microscopy egg-count results are shown in Table 2. Of the 44 samples, 14 were egg and serum anodic antigen positive. The serum anodic antigen assay detected 3 additional positives with concentrations ≥30 pg/mL; 8 other samples were ranked indecisive of which one had 2 eggs per 10 mL. In total, 18 women met the criteria for the composite reference of S. haematobium infection, defined as either a positive test result by urine egg microscopy or a serum anodic antigen level >30 pg/mL. S. mansoni eggs in stool or urine were not identified in any study participant.
Urine Anodic Antigen (UCAA) Assay Results—Performance Utilizing Large Sample Volume
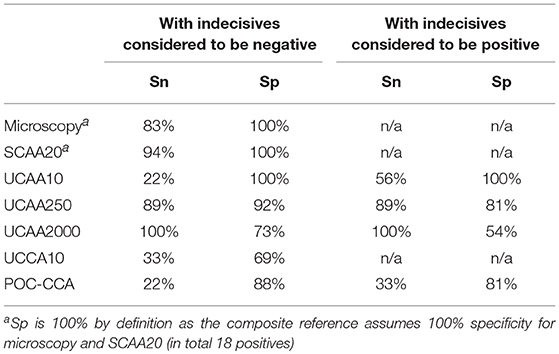
Urine samples transported to LUMC (the Netherlands) were analyzed utilizing increased sample volume for enhanced sensitivity as well as to resolve the status of samples with indecisive results. Applying the dry reagent cutoff threshold of 30 pg/mL (12), the UCAA10 assay was positive for 4 out of the 44 samples, and indecisive for 6. These 10 positive/indecisive results were all part of the 18 composite reference positives (Table 3). The 25-fold larger volume UCAA250 assay identified 16 out of 18 composite reference positives, 2 additional positives not detected by the UCAA10 assay, and another 3 as indecisive. The 200-fold-larger volume UCAA2000 assay correctly identified all 18 composite reference positives, as well as 7 additional positives that were negative by both urine microscopy and serum anodic antigen. Additionally, 5 indecisive samples were obtained with the UCAA2000, one also scoring a SCAA20 indecisive result (13 pg/mL). All samples positive with a lower volume UCAA assay remained positive when retested at a higher volume. Results are summarized in Figure 1 showing the percentage of positive and indecisive samples for all applied diagnostics.
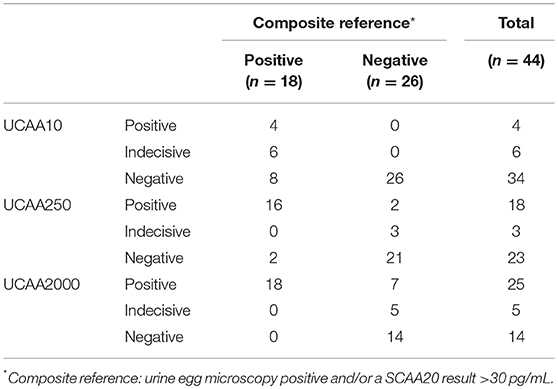
Table 3. Performance of the increased volume UCP-LF anodic antigen assays vs. the composite reference.
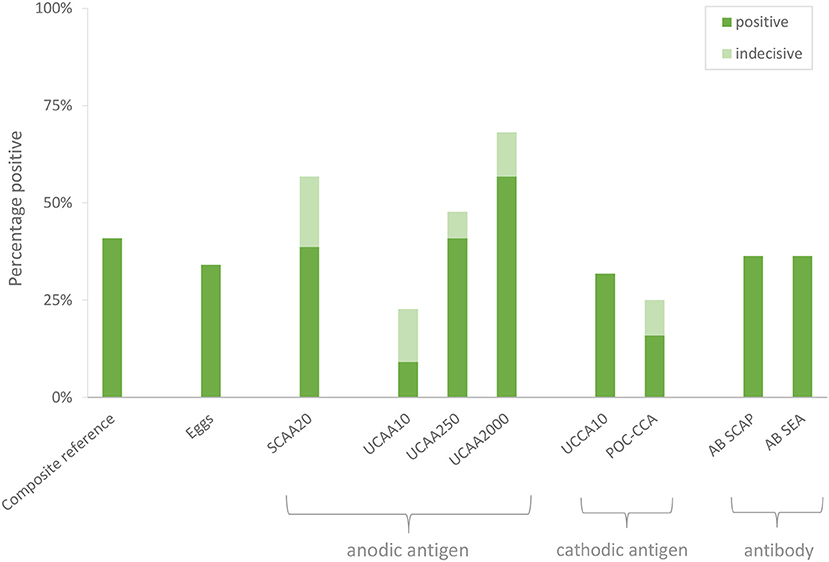
Figure 1. Performance of individual diagnostic tests to identify S. haematobium infection. A composite reference was defined, based on assays performed locally, ranking samples positive based on either a positive test result by urine egg microscopy and/or a serum anodic antigen level >30 pg/mL.
Urine Cathodic Antigen Results
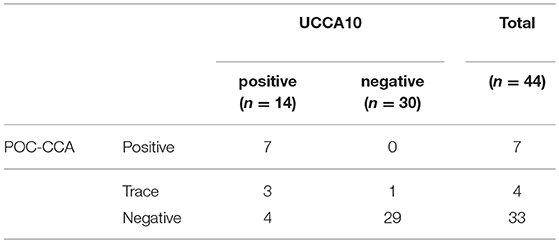
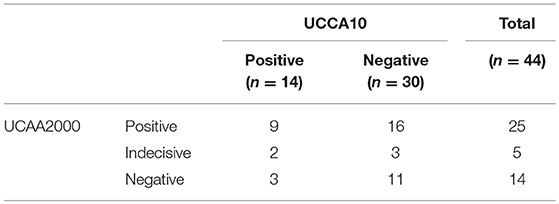
In this S. haematobium endemic setting the POC-CCA test identified 7 positives and 4 traces. These results are in agreement with the results of the prototype UCP-LF cathodic antigen assay (UCCA10). All 7 POC-CCA positives and the 3 out of 4 traces tested positive with the UCCA10 assay, which overall indicated 14 positive samples (Table 4). One UCCA10 negative sample with a POC-CCA trace result tested positive for anodic antigen in serum as well as in urine. The POC-CCA identified only 4 out of 18 composite reference positives, and of the 4 traces, only 1 was also positive by the composite reference. Table 5 shows the cathodic antigen (UCCA10) and the anodic antigen (UCAA2000) assay results. From the 25 UCAA2000 positives, 9 were also identified with the UCCA10, while the latter identified 3 additional positives that were not identified by the UCAA2000, and which were also negative according to the composite reference. For the few samples with a quantified level of both antigens, there was no obvious correlation observed between the absolute levels of the anodic and cathodic antigen in urine.
Performance of the Urine Anodic and Cathodic Antigen Tests
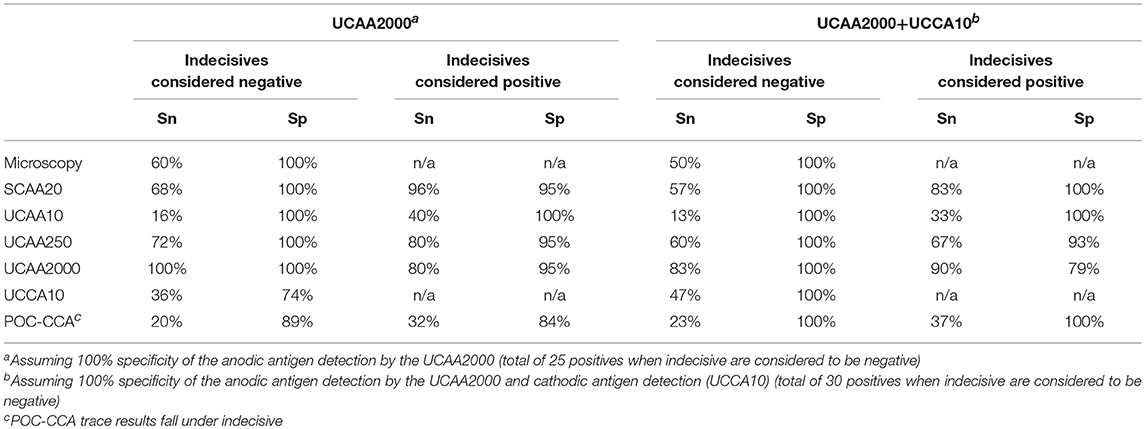
Table 6 shows the clinical performance of each test compared to the composite reference. By definition, the clinical specificity (Sp) of both microscopy and SCAA20 is 100%. Clinical sensitivity (Sn) of anodic antigen detection in urine increases when using larger sample volume. Sp decreases with the increased urine volume as a consequence of detecting antigen positives that were not identified with the assays used to define the local composite reference. Sn and Sp values when using the assumed most sensitive urine antigen diagnostics as a gold standard are indicated in Table 7. Values are calculated for either the anodic antigen only, or for the combined anodic and cathodic antigen detection results. To indicate the weight of indecisive and trace results, Sn and Sp were calculated either including indecisive or trace results as positive or negative. Table 7 indicates Sn and Sp against the UCAA2000 and the UCAA2000 combined with the UCCA10. In this table indecisive results obtained with the UCAA2000 are categorized as negative as larger volume testing was not applied to resolve their status.
Serology Status Determined Through Antibody Detection in Urine
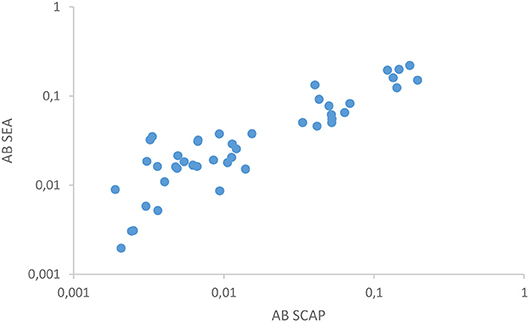
The two antibody detection assays in urine correlated well (Figure 2) as was similarly demonstrated in serum in a previous study (12). Out of the 18 composite reference positives 14 showed clear reactivity with at least one of the antibody assays. Serology turned out to be negative in two egg positive cases. One of these samples (7 eggs per 10 mL urine) had a remarkably high level of the anodic antigen in serum and cathodic antigen in urine, respectively >10,000 and 5,900 pg/mL as determined with the SCAA20 (serum) and UCCA10 (urine) assays. In contrast, the urine anodic antigen level was 27.8 pg/mL (UCAA250), more than 100-fold less than the concentration in serum. This sample was also easily identified with the POC-CCA test. The second sample (5 eggs per 10 mL urine) was negative for urine cathodic antigen, but positive for anodic antigen with levels in serum and urine showing a 50-fold difference (50 and 1 pg/mL, respectively). Tentatively, urine antibody assays generally seem reactive at anodic antigen levels above 100 pg/mL in serum as well as with the presence of urinary eggs.
Status of Previously Infected, Praziquantel-Treated Participants
The serum anodic antigen assay (SCAA10) indicated that 3 of the 5 previously infected and treated individuals were still carrying an active infection, with levels ranging between 10 and 100 pg/mL. This was confirmed by urine anodic antigen, indicating levels between 1 and 15 pg/mL. A fourth sample was only positive for urine cathodic antigen, with a level of 4,500 pg/mL, while the POC-CCA test was negative. Eggs were not detected in any of the 5 individuals, but based on the outcome of these tests, 3 individuals remained positive by the composite reference and the individual with the positive UCCA10 is likely still infected as well. Thus, 4 of the 5 recently-treated women would require an additional drug treatment.
Discussion
Although the number of individuals included in this study was relatively small (a study limitation), it clearly demonstrates that the use of larger volumes of urine enhances the sensitivity of diagnosis with the anodic antigen assay in individuals with S. haematobium infection. Diagnostic tests performed in Tanzania identified 18 who met the criteria for the composite reference, defined here as being urine egg microscopy positive and/or serum anodic antigen (SCAA20) positive. As a non-invasive and thus patient-friendly alternative, the UCAA250 assay utilizing 250 μL could be considered. This assay identified 18 positives, 16 of whom were identified by the composite reference. Allowing for a less than optimal specificity, indecisive results could be interpreted as positive, which would yield an additional 3 positive scores. The higher volume format UCAA2000 confirmed that this would be correct for 2 out of the 3 indecisive samples. The UCAA250 assay would then have successfully detected 20 positives out of the 25 samples that tested positive with the UCAA2000 anodic antigen assay. The UCAA2000 assay identified all 18 composite reference positive samples as well as 7 additional positives. However, this assay is more time-intensive and costly as it requires larger tubes and concentration devices that are no longer compliant with micro-centrifuges and tubes. Therefore, we would recommend accepting a slightly lower sensitivity by using 250 μL urine rather than 2000 μL. Alternative lower-cost methods to concentrate the CAA from large urine volume are being explored to improve field applicability of the UCAA2000 (38).
Utilization of a single, non-invasive urine sample, rather than performing both urine microscopy and serum anodic antigen testing, is more user-friendly, less prone to error and likely more economical. Moreover, because the anodic antigen is genus-specific, collection and testing of urine only is sufficient for diagnosis of all Schistosoma species infections. The same may hold for the cathodic antigen if concentrated, although this antigen has some additional biological activity due to homology to Lewis X structures (39).
The commercially available POC-CCA test for urine samples is recommended for diagnosis of Schistosoma mansoni infections. Use of this assay for diagnosis of S. haematobium infections shows variable results with generally lower performances than for S. mansoni (13, 40–43). Our study also documented a lower sensitivity of this test for the diagnosis of urinary schistosomiasis. This might be due to lower levels of cathodic antigen produced by S. haematobium worms as compared to S. mansoni (unpublished observations). Use of the more sensitive UCP-LF cathodic antigen assay (UCCA10) indeed increased the number of positive samples to 14 compared to 7 for the POC-CCA. Importantly, of 4 samples generating a POC-CCA trace result, 3 samples tested positive with the UCCA10 assay. Clearly, weak signals (traces) in visually interpreted tests as the POC-CCA may be more difficult to interpret. However, considering traces as negative resulted in a higher specificity, but subsequently lower sensitivity, which may reduce the usefulness of the test for mapping purposes (44, 45). Overall, the overlap between POC-CCA and UCCA10 results confirm the specificity of the POC-CCA test. The UCP-LF cathodic antigen test was developed to increase analytical sensitivity through the application of a luminescent reporter, and to explore the effect of TCA treatment on the biological background. Further development of this assay into a high sensitivity format, identical to the UCP-LF anodic antigen assay and potentially utilizing larger volumes of urine, may provide better insight on the ratio of the levels of both antigens in urine samples. When the cathodic antigen assay is amenable to larger volume testing, a duplex LF strip detecting both antigens in a single sample will further increase sensitivity. Moreover, the relation between the two antigens might provide some information regarding the infecting species.
When using (rapid) LF based assays, indecisive test results remain an important issue. For visual assays, weak signals may be scored as trace by some observers whereas others would score it as negative. Whether a trace is false positive or true positive may be difficult to determine without alternative diagnostics. The intensity of the signal might also vary per production batch of test strips, as well as whether the manufacturer is targeting highest sensitivity or specificity of the test. Further, the choice to rank trace results as a positive or negative will depend on the goal of the survey (prevalence studies vs. elimination studies) as well as potential complications of drug treatment. A strength of the UCP-LF test format is that it utilizes a luminescent reporter label that requires a reader (scanner) to analyze the LF strip and generate the test result. Besides providing extra sensitivity as compared to a visual test, this reader removes operator interpretation flaws. Moreover, the reader can be set either to include or exclude indecisive results as positive. This allows manufacturers to optimize the test for sensitivity and adjust for specificity by programming the reader.
In contrast to the detection of eggs and circulating worm antigens, the presence of host antibodies against Schistosoma derived biomolecules is not necessarily a measure for ongoing active infection, except in those who would not previously have been exposed to Schistosoma parasites [i.e., individuals from non-endemic settings or travelers; (46)]. In the current small endemic cohort, antibody detection in urine could not contribute to resolving disputes regarding anodic antigen negatives and cathodic antigen positives or indecisive/trace results as the analytical sensitivity of the assay was not sufficient to identify all infected individuals. This may have been a consequence of setting relatively high cutoff thresholds. Studies with larger cohorts are needed to determine accurate values; appropriate thresholds may also provide insight in potential differences of reactivity against SCAP and SEA. Although the assay is not yet fully optimized, this study has demonstrated that the detection of specific host antibodies in non-invasive urine samples is feasible. This would constitute a rapid and low cost assay, but it remains to be determined whether urine would be a good enough alternative to the more invasive fingerstick blood.
Conclusion
The non-invasive UCP-LF anodic antigen (CAA) assay utilizing 250 μL of urine has been demonstrated to be a valid alternative for the current locally used diagnostic strategy, which is based on egg microscopy combined with anodic antigen serum analysis. Furthermore, testing for the presence of the worm derived anodic antigen in large volume urine samples is a sensitive tool to monitor treatment efficiency. An important feature of the UCP-LF antigen urine assay is the potential confirmation of indecisive test results by using larger sample volumes. Testing for the presence of the cathodic antigen (CCA) would have added value also in S. haematobium settings, although more studies are needed to validate the status of anodic antigen negative and cathodic antigen positive samples. Anticipated development of a UCP-LF duplex test combining the anodic and cathodic antigen may improve sensitivity.
Author Contributions
JD and PC designed the study. CdD was responsible for the production of all UCP-LF test materials and performed UCP-LF urine assay. JD, JM, and SK were responsible for the collection of samples and Schistosoma diagnostics performed in Tanzania. CdD, PH, GvD, JD, and PC performed the data analysis and interpretation of the results. All authors were involved in drafting the manuscript and approved the final version.
Funding
This work was supported in part by a grant from the National Institutes of Health (K23 AI 110238) and the University of Georgia Research Foundation, Inc., through the SCORE award (#50186) from the Bill & Melinda Gates Foundation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the National Institute for Medical Research—Mwanza Research Centre for their invaluable assistance with this research project, as well as the study participants for their enthusiastic participation.
References
1. World Health Organization. Schistosomiasis. (2018). Available online at: http://www.who.int/schistosomiasis/disease/en/ (Accessed August 29, 2018).
2. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet (2014) 383:2253–64. doi: 10.1016/S0140-6736(13)61949-2
3. Peters PA, Mahmoud AA, Warren KS, Ouma JH, Siongok TK. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Heal Organ (1976) 54:159–62.
4. Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. (1972) 14:397–400.
5. Colley DG, Binder S, Campbell C, King CH, Tchuem Tchuenté L-A, N'Goran EK, et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am J Trop Med Hyg. (2013) 88:426–32. doi: 10.4269/ajtmh.12-0639
6. van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. (2000) 77:69–80. doi: 10.1016/S0001-706X(00)00115-7
7. World Health Organization Sixty-Fifth World Health Assembly. Elimination of Schistosomiasis. in Provisional Agenda Item 13-11 Geneva: World Health Organization. Available online at: http://apps.who.int/gb/ebwha/pdf_files/WHA65/A65_21-en.pdf (Accessed August 30, 2018).
8. de Water R, Fransen JA, Deelder AM. Ultrastructural localization of the circulating anodic antigen in the digestive tract of Schistosoma mansoni using monoclonal antibodies in an immunogold labeling procedure. Am J Trop Med Hyg (1986) 35:549–58.
9. van Lieshout L, de Jonge N, Bassily S, Mansour MM, Deelder AM. Assessment of cure in schistosomiasis patients after chemotherapy with praziquantel by quantitation of circulating anodic antigen (CAA) in urine. Am J Trop Med Hyg. (1991) 44:323–8.
10. van Lieshout L, De Jonge N, Mansour MM, Bassily S, Krijger FW, Deelder AM. Circulating cathodic antigen levels in serum and urine of schistosomiasis patients before and after chemotherapy with praziquantel. Trans R Soc Trop Med Hyg. (1993) 87:311–2.
11. Ochodo EA, Gopalakrishna G, Spek B, Reitsma JB, van Lieshout L, Polman K, et al. Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Cochrane Database Sys Rev. (2015) 3:CD009579. doi: 10.1002/14651858.CD009579.pub2
12. Corstjens PLAM, De Dood CJ, Kornelis D, Fat EMTK, Wilson RA, Kariuki TM, et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology (2014) 141:1841–55. doi: 10.1017/S0031182014000626
13. Midzi N, Butterworth AE, Mduluza T, Munyati S, Deelder AM, van Dam GJ. Use of circulating cathodic antigen strips for the diagnosis of urinary schistosomiasis. Trans R Soc Trop Med Hyg. (2009) 103:45–51. doi: 10.1016/j.trstmh.2008.08.018
14. Sousa-Figueiredo JC, Stanton MC, Katokele S, Arinaitwe M, Adriko M, Balfour L, et al. Mapping of schistosomiasis and soil-transmitted helminths in namibia: the first large-scale protocol to formally include rapid diagnostic tests. PLoS Negl Trop Dis. (2015) 9:e0003831. doi: 10.1371/journal.pntd.0003831
15. Danso-Appiah A, Minton J, Boamah D, Otchere J, Asmah R, Rodgers M, et al. Accuracy of point-of-care testing for circulatory cathodic antigen in the detection of schistosome infection: systematic review and meta-analysis. Bull World Heal Organ. (2016) 94:522–33A. doi: 10.2471/BLT.15.158741
16. Colley DG, Andros TS, Campbell CH. Schistosomiasis is more prevalent than previously thought: what does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect Dis Poverty (2017) 6:63. doi: 10.1186/s40249-017-0275-5
17. Chernet A, Kling K, Sydow V, Kuenzli E, Hatz C, Utzinger J, et al. Accuracy of diagnostic tests for schistosoma mansoni infection in asymptomatic eritrean refugees: serology and point-of-care circulating cathodic antigen against stool microscopy. Clin Infect Dis. (2017) 65:568–74. doi: 10.1093/cid/cix366
18. Becker SL, Marti H, Zimmermann S, Vidacek D, Herrmann M, Utzinger J, et al. Application in Europe of a urine-based rapid diagnostic test for confirmation of Schistosoma mansoni infection in migrants from endemic areas. Euro Surveill. (2015) 20:23.
19. Beltrame A, Guerriero M, Angheben A, Gobbi F, Requena-Mendez A, Zammarchi L, et al. Accuracy of parasitological and immunological tests for the screening of human schistosomiasis in immigrants and refugees from African countries: an approach with Latent Class Analysis. PLoS Negl Trop Dis. (2017) 11:e0005593. doi: 10.1371/journal.pntd.0005593
20. Corstjens PL, van Lieshout L, Zuiderwijk M, Kornelis D, Tanke HJ, Deelder AM, et al. Up-converting phosphor technology-based lateral flow assay for detection of Schistosoma circulating anodic antigen in serum. J Clin Microbiol. (2008) 46:171–6. doi: 10.1128/JCM.00877-07
21. Downs JA, van Dam GJ, Changalucha JM, Corstjens PLAM, Peck RN, de Dood CJ, et al. Association of schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg. (2012) 87:868–73. doi: 10.4269/ajtmh.2012.12-0395
22. Wilson S, Jones FM, van Dam GJ, Corstjens PLAM, Riveau G, Fitzsimmons CM, et al. Human Schistosoma haematobium antifecundity immunity is dependent on transmission intensity and associated with immunoglobulin G1 to worm-derived antigens. J Infect Dis. (2014) 210:2009–16. doi: 10.1093/infdis/jiu374
23. Knopp S, Corstjens PLAM, Koukounari A, Cercamondi CI, Ame SM, Ali SM, et al. Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of schistosoma haematobium in low endemic settings. PLoS Negl Trop Dis. (2015) 9:e0003752. doi: 10.1371/journal.pntd.0003752
24. Ssetaala A, Nakiyingi-Miiro J, Asiki G, Kyakuwa N, Mpendo J, Van Dam GJ, et al. Schistosoma mansoni and HIV acquisition in fishing communities of Lake Victoria, Uganda: a nested case-control study. Trop Med Int Heal. (2015) 20:1190–5. doi: 10.1111/tmi.12531
25. Downs JA, De Dood CJ, Dee HE, McGeehan M, Khan H, Marenga A, et al. Schistosomiasis and human immunodeficiency virus in men in Tanzania. Am J Trop Med Hyg. (2017) 96:856–62. doi: 10.4269/ajtmh.16-0897
26. Clements MN, Corstjens PLAM, Binder S, Campbell CH, de Dood CJ, Fenwick A, et al. Latent class analysis to evaluate performance of point-of-care CCA for low-intensity Schistosoma mansoni infections in Burundi. Parasit Vectors (2018) 11:111. doi: 10.1186/s13071-018-2700-4
27. Vonghachack Y, Sayasone S, Khieu V, Bergquist R, van Dam GJ, Hoekstra PT, et al. Comparison of novel and standard diagnostic tools for the detection of Schistosoma mekongi infection in Lao People's Democratic Republic and Cambodia. Infect Dis Poverty (2017) 6:127. doi: 10.1186/s40249-017-0335-x
28. Balahbib A, Amarir F, Corstjens PLAM, de Dood CJ, van Dam GJ, Hajli A, et al. Selecting accurate post-elimination monitoring tools to prevent reemergence of urogenital schistosomiasis in Morocco: a pilot study. Infect Dis Poverty (2017) 6:75. doi: 10.1186/s40249-017-0289-z
29. van Dam GJ, Odermatt P, Acosta L, Bergquist R, de Dood CJ, Kornelis D, et al. Evaluation of banked urine samples for the detection of circulating anodic and cathodic antigens in Schistosoma mekongi and S. japonicum infections: a proof-of-concept study. Acta Trop. (2015) 141:198–203. doi: 10.1016/j.actatropica.2014.09.003
30. van Dam GJ, Xu J, Bergquist R, de Dood CJ, Utzinger J, Qin Z-Q, et al. An ultra-sensitive assay targeting the circulating anodic antigen for the diagnosis of Schistosoma japonicum in a low-endemic area, People's Republic of China. Acta Trop. (2015) 141:190–7. doi: 10.1016/j.actatropica.2014.08.004
31. van Dam GJ, Bogitsh BJ, van Zeyl RJ, Rotmans JP, Deelder AM. Schistosoma mansoni: in vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J Parasitol. (1996) 82:557–64.
32. Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, Simplice H, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania's Lake Victoria region. Am J Trop Med Hyg. (2011) 84:364–9. doi: 10.4269/ajtmh.2011.10-0585
33. Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, et al. Variations in helminth faecal egg counts in Kato-Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop. (2004) 92:205–12. doi: 10.1016/j.actatropica.2004.06.011
34. Downs JA, Corstjens PLAM, Mngara J, Lutonja P, Isingo R, Urassa M, et al. Correlation of serum and dried blood spot results for quantitation of Schistosoma circulating anodic antigen: a proof of principle. Acta Trop. (2015) 150:59–63. doi: 10.1016/j.actatropica.2015.06.026
35. Corstjens PLAM, Nyakundi RK, de Dood CJ, Kariuki TM, Ochola EA, Karanja DMS, et al. Improved sensitivity of the urine CAA lateral-flow assay for diagnosing active Schistosoma infections by using larger sample volumes. Parasit Vectors (2015) 8:241. doi: 10.1186/s13071-015-0857-7
36. Polman K, Diakhate MM, Engels D, Nahimana S, van Dam GJ, Falcão Ferreira ST, et al. Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Trop Med Int Heal. (2000) 5:534–7. doi: 10.1046/j.1365-3156.2000.00600.x
37. World Health Organization. Preventive Chemotherapy in Human Helminthiasis. (2006) Available online at: http://www.who.int/neglected_diseases/preventive_chemotherapy/pct_manual/en/ (Accessed August 30, 2018).
38. Markwalter C, Corstjens P, Mammoser C, Camps G, van Dam G, Wright D. Poly(amidoamine)-coated magnetic particles for enhanced detection of Schistosoma circulating anodic antigen in endemic urine samples. Analyst (2018). doi: 10.1039/c8an00941d. [Epub ahead of print].
39. Van Dam GJ, Bergwerff AA, Thomas-Oates JE, Rotmans JP, Kamerling JP, Vliegenthart JF, et al. The immunologically reactive O-linked polysaccharide chains derived from circulating cathodic antigen isolated from the human blood fluke Schistosoma mansoni have Lewis x as repeating unit. Eur J Biochem. (1994) 225:467–82.
40. Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. (2008) 102:625–33. doi: 10.1179/136485908X337490
41. Stothard JR, Sousa-Figueiredo JC, Standley C, van Dam GJ, Knopp S, Utzinger J, et al. An evaluation of urine-CCA strip test and fingerprick blood SEA-ELISA for detection of urinary schistosomiasis in schoolchildren in Zanzibar. Acta Trop. (2009) 111:64–70. doi: 10.1016/j.actatropica.2009.02.009
42. Sanneh B, Joof E, Sanyang AM, Renneker K, Camara Y, Sey AP, et al. Field evaluation of a schistosome circulating cathodic antigen rapid test kit at point-of-care for mapping of schistosomiasis endemic districts in The Gambia. PLoS ONE (2017) 12:e0182003. doi: 10.1371/journal.pone.0182003
43. Rubaba O, Chimbari MJ, Soko W, Manyangadze T, Mukaratirwa S. Validation of a urine circulating cathodic antigen cassette test for detection of Schistosoma haematobiumin uMkhanyakude district of South Africa. Acta Trop. (2018) 182:161–5. doi: 10.1016/j.actatropica.2018.02.029
44. Coulibaly JT, Knopp S, N'Guessan NA, Silué KD, Fürst T, Lohourignon LK, et al. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d'Ivoire. PLoS Negl Trop Dis. (2011) 5:e1384. doi: 10.1371/journal.pntd.0001384
45. Tchuem Tchuenté L-A, Kueté Fouodo CJ, Kamwa Ngassam RI, Sumo L, Dongmo Noumedem C, Kenfack CM, et al. Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in cameroon. PLoS Negl Trop Dis. (2012) 6:e1758. doi: 10.1371/journal.pntd.0001758
46. van Grootveld R, van Dam GJ, de Dood C, de Vries JJC, Visser LG, Corstjens PLAM, et al. Improved diagnosis of active Schistosoma infection in travellers and migrants using the ultra-sensitive in-house lateral flow test for detection of circulating anodic antigen (CAA) in serum. Eur J Clin Microbiol Infect Dis. (2018) 37:1709–16. doi: 10.1007/s10096-018-3303-x
Keywords: Schistosoma haematobium, CAA anodic antigen, CCA cathodic antigen, antibody, urine, lateral flow assay, UCP upconverting reporter particle
Citation: de Dood CJ, Hoekstra PT, Mngara J, Kalluvya SE, van Dam GJ, Downs JA and Corstjens PLAM (2018) Refining Diagnosis of Schistosoma haematobium Infections: Antigen and Antibody Detection in Urine. Front. Immunol. 9:2635. doi: 10.3389/fimmu.2018.02635
Received: 02 September 2018; Accepted: 26 October 2018;
Published: 14 November 2018.
Edited by:
Thiago Almeida Pereira, Stanford University, United StatesReviewed by:
Paulo Marcos Zech Coelho, Fundação Oswaldo Cruz (Fiocruz), BrazilWilliam Evan Secor, Centers for Disease Control and Prevention (CDC), United States
Copyright © 2018 de Dood, Hoekstra, Mngara, Kalluvya, van Dam, Downs and Corstjens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul L. A. M. Corstjens, cC5sLmEubS5jb3JzdGplbnNAbHVtYy5ubA==
†These authors have contributed equally to this work
 Claudia J. de Dood1
Claudia J. de Dood1 Pytsje T. Hoekstra
Pytsje T. Hoekstra Samuel E. Kalluvya
Samuel E. Kalluvya Jennifer A. Downs
Jennifer A. Downs Paul L. A. M. Corstjens
Paul L. A. M. Corstjens