
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 09 October 2018
Sec. Viral Immunology
Volume 9 - 2018 | https://doi.org/10.3389/fimmu.2018.02312
This article is part of the Research Topic Understanding the Limitations of Current Influenza Vaccine Strategies and Future Development of More Efficacious Preventative and Therapeutic Interventions View all 7 articles
 Wei Dong1,2
Wei Dong1,2 Yoshita Bhide1
Yoshita Bhide1 Federica Sicca1
Federica Sicca1 Tjarko Meijerhof1
Tjarko Meijerhof1 Kate Guilfoyle3†
Kate Guilfoyle3† Othmar G. Engelhardt3
Othmar G. Engelhardt3 Louis Boon4
Louis Boon4 Cornelis A. M. de Haan5
Cornelis A. M. de Haan5 George Carnell6
George Carnell6 Nigel Temperton6
Nigel Temperton6 Jacqueline de Vries-Idema1
Jacqueline de Vries-Idema1 David Kelvin2,7
David Kelvin2,7 Anke Huckriede1*
Anke Huckriede1*Sequential infection with antigenically distinct influenza viruses induces cross-protective immune responses against heterologous virus strains in animal models. Here we investigated whether sequential immunization with antigenically distinct influenza vaccines can also provide cross-protection. To this end, we compared immune responses and protective potential against challenge with A(H1N1)pdm09 in mice infected sequentially with seasonal A(H1N1) virus followed by A(H3N2) virus or immunized sequentially with whole inactivated virus (WIV) or subunit (SU) vaccine derived from these viruses. Sequential infection provided solid cross-protection against A(H1N1)pdm09 infection while sequential vaccination with WIV, though not capable of preventing weight loss upon infection completely, protected the mice from reaching the humane endpoint. In contrast, sequential SU vaccination did not prevent rapid and extensive weight loss. Protection correlated with levels of cross-reactive but non-neutralizing antibodies of the IgG2a subclass, general increase of memory T cells and induction of influenza-specific CD4+ and CD8+ T cells. Adoptive serum transfer experiments revealed that despite lacking neutralizing activity, serum antibodies induced by sequential infection protected mice from weight loss and vigorous virus growth in the lungs upon A(H1N1)pdm09 virus challenge. Antibodies induced by WIV vaccination alleviated symptoms but could not control virus growth in the lung. Depletion of T cells prior to challenge revealed that CD8+ T cells, but not CD4+ T cells, contributed to cross-protection. These results imply that sequential immunization with WIV but not SU derived from antigenically distinct viruses could alleviate the severity of infection caused by a pandemic and may improve protection to unpredictable seasonal infection.
Influenza A virus (IAV) infections remain a worldwide public health threat. Influenza vaccination is the most reliable strategy to control annual epidemics and irregular pandemics (1). Current inactivated influenza vaccines (IIV) primarily induce strain-specific antibodies against the two major virus surface proteins, hemagglutinin (HA) and neuraminidase (NA). However, these strain-specific antibodies cannot provide protection against antigenically drifted and antigenically shifted strains. When a pandemic strain emerges, it takes around 6 months to develop and distribute a new vaccine (2), which is too late for a vaccine to provide effective protection during the first pandemic wave. Thus, a cross-protective vaccine that could provide immediate protection against unpredicted influenza virus strains is urgently needed.
Live virus infection has been shown to provide some degree of cross-protection against A(H1N1)pdm09 infection in animal models (3–8) and in humans (9, 10). However, the exact mechanisms involved in cross-protection remain elusive. Cross-reactive antibodies against conserved regions of viral proteins, such as the HA stalk, the M2 ectodomain (M2e) and nucleoprotein (NP), induced by (sequential) live virus infection, correlate with cross-protection (3, 11–13). Some anti-HA stalk antibodies can directly neutralize influenza virus particles in vitro (14). However, most of these antibodies target antigens that are expressed on the surface of infected cells and then provide cross-protection via a Fc receptor dependent mechanism (14–16).
Besides antibody responses, cross-reactive T cells induced by live virus infection have also been demonstrated to correlate with cross-protection (5, 6, 17). Cytotoxic CD8 T cells can recognize internal, conserved epitopes across different virus strains. In animal models, CD8 T cells induced by live virus infection have been shown to prevent A(H5N1) or A(H1N1)pdm09 virus infection (18). On the other hand, CD4 T cells specific for conserved epitopes have also been shown to provide protection against A(H1N1)pdm09 in mice (19, 20). These CD4 T cells could provide cross-protection through different mechanisms, including help for B cells, help for CD8 T cells and direct cytotoxic activity [reviewed in (21)]. Furthermore, it has been demonstrated in humans that the presence of memory cross-reactive CD4 or CD8 T cells is correlated with cross-protection against A(H1N1)pdm09 or A(H7N9) virus infection (9, 22, 23).
Vaccination with trivalent inactivated influenza vaccine (IIV) did not provide protection against A(H1N1)pdm09 virus infection and was even found to be associated with enhanced disease in observational studies from Canada in humans (24–28). In animal models, published studies indicate that vaccination with IIV could induce detectable levels of cross-reactive antibody against A(H1N1)pdm09 virus, yet, no cross-protection was observed (29–31). The exception is a recent study showing that non-neutralizing antibody induced by IIV could cause activation of influenza-specific CD8 T cells by promoting antigen presentation (32). If a broader immune response could be induced by the currently available influenza vaccines, it would benefit humans against novel virus infection.
Compared with a single virus infection, sequential infection with antigenically distinct live viruses was found to provide broader cross-protection (7, 8, 11). This is because the second infection can cause a quick recall immune response to epitopes shared between the two viruses. It has been shown that sequential influenza virus infection can boost antibody responses to the shared HA stalk region (11, 33).
Sequential immunization with antigenically distinct vaccines has also been used as a strategy to induce a broader immune response against influenza virus in animal models (34). However, most of these studies were focused on the cross-protective immune response induced by genetically modified vaccines (35–38). Little is known about the protective potential of sequential immunization with conventional inactivated vaccines derived from different seasonal influenza virus strains. In case of a pandemic, such a vaccination strategy could be a first means of intervention until a pandemic vaccine becomes available.
In this study, we assessed the cross-protective immune responses induced by sequential infection with A(H1N1) and A(H3N2) virus, or sequential immunization with whole inactivated virus (WIV) or subunit (SU) vaccine derived from these viruses in a mouse model. Sequential infection provided robust cross-protection which was mediated by non-neutralizing, cross-reactive antibody and CD8 effector memory T cells (TEM). Partial cross-protection was provided by sequential vaccination with WIV and was associated with CD8 central memory T cells (TCM), and to a minor extent, with cross-reactive antibodies. In contrast, sequential vaccination with SU vaccine induced low levels of cross-reactive serum antibodies and no T cell immunity against A(H1N1)pdm09, and did not provide cross-protection. These results imply that in case of a new pandemic, sequential immunization with WIV but not subunit vaccines derived from different seasonal virus strains could mitigate disease severity until a pandemic vaccine becomes available.
Influenza virus strains A/Puerto Rico/8/34 (H1N1)(PR8), X-31, a reassortant virus derived from A/Aichi/2/68 (H3N2), A/California/07/09 (H1N1)pdm09, and X-181 (a reassortant derived from (H1N1)pdm09 and PR8) were grown in embryonated chicken eggs, and the virus preparations were titrated on MDCK cells and in mice. Whole inactivated virus vaccines was produced from PR8, X31 and X-181 by treatment with β-propiolactone. PR8 SU and X-31 SU were prepared from PR8 and X-31 WIV, respectively, as described before (39).
Female 6–8 weeks old CB6F1 mice were purchased from Envigo, The Netherlands, and rested for at least 1 week. Mice were housed under SPF conditions in standard polycarbonate cages (5 animals per cage) with standard rodent bedding and cardboard cylinders as cage enrichment. Prior to the start of the experiment, animals were randomly allocated to the different treatment groups. All animal experiments were approved by the Central Committee for Animal Experiments CCD of the Netherlands (AVD105002016599). All experimental protocols were approved by the Animal Ethics Committee of the University Medical Center Groningen. Group sizes were determined using Piface software such that a power of at least 80% was reached.
Naive mice (n = 15) were immunized intramuscularly (i.m.) with 15 μg of PR8 WIV (containing around 5 μg of HA) or 5 μg of PR8 SU vaccine. Alternatively, mice were anesthetized and infected intranasally (i.n.) with a sublethal dose (103 TCID50) of PR8 virus (live virus = LV). Four weeks after immunization or infection, mice were i.m. immunized with 15 μg of X-31 WIV or 5 μg of X-31 SU or i.n. infected with a sublethal dose of (103 TCID50) X-31 virus. Mice injected twice with PBS i.m. with 28 days interval served as negative control (Table 1).
Four weeks after the second infection or immunization, 5 mice of each group were sacrificed for determination of infection- or vaccine-induced immune responses. The other 10 mice were anesthetized with isoflurane and challenged i.n. with 104.4 TCID50 of A/California/7/09 (H1N1)pdm09 in 40 μl PBS. Three days post infection, 5 mice were sacrificed for determination of immune responses and lung virus titers. The remaining 5 mice were monitored daily for body weight loss for 2 weeks. Body weight loss exceeding 20% was considered as humane endpoint.
On day 0 (before challenge) and day 3 post challenge, mice (n = 5 from each group) were sacrificed under isoflurane anesthesia. Serum, nose wash and bronchoalveolar lavage (BAL) were collected for further analysis. Lungs were perfused with 20 ml PBS containing 0.1% heparin through the right heart ventricle. Right lung lobes were collected, homogenized, snap-frozen and stored at −80°C for virus titration. The whole lung (day 0) or the left lung lobes (day 3) and the spleens were collected for lymphocyte isolation.
Lung tissue collected on day 3 post-challenge was weighed, homogenized in 1 ml of Episerf medium (Thermo Fisher Scientific) and then centrifuged at 1,200 rpm for 10 min. Supernatants were collected, aliquoted, snap-frozen and stored at −80°C until use. Lung virus titers were determined by infection of MDCK cells in 96-well plates with serial dilutions of the lung supernatants as described before (39). Viral titers, presented as log10 titer of 50% tissue culture infectious dose per gram lung (log10TCID50/g), were calculated based on the Reed-Muench method (40).
Spleens were homogenized in complete IMDM (with 10% FBS, 1% Penicillin-Streptomycin and 0.1% β-mercaptoethanol) using a GentleMACS dissociator (Miltenyi Biotec B, Leiden, The Netherlands). Cell suspensions were then forced through a cell strainer (BD Bioscience, Breda, The Netherlands) and treated with ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2) to remove erythrocytes.
PBS-perfused lungs for isolation of lymphocytes were homogenized using a GentleMACS dissociator (Miltenyi Biotec) and then digested by treatment with collagenase D (0.5 mg/lung) (Roche, Woerden, The Netherlands) in DMEM medium supplemented with 2% FBS at 37°C for 1.5 h. The cell suspension was passed through a cell strainer. Lung lymphocytes in the filtered suspensions were enriched using lymphocyte density gradients (Sanbio, Uden, The Netherlands) according to the manufacturer's protocol.
For the detection of IgG, IgG1, IgG2a, or IgA antibody against A(H1N1)pdm09 virus in serum and nasal wash, ELISA plates (Greiner, Alphen a/d Rijn, Netherlands) were coated with 0.3 μg/well of X-181 WIV, conserved M2e peptide (SLLTEVETPIRNEWGSRSNDSSD) or NP protein overnight at 37°C and ELISA assays were performed as described before (39). For NA-specific ELISA, recombinant NA protein of A(H1N1)pdm09 was expressed and purified as described previously (41). ELISA plates were coated with 0.1 μg/well of NA overnight at 4°C and assays were performed as described (39).
Pseudotyped viruses (PV) were produced by co-transfection of HEK293T/17 cells using the polyethylenimine transfection reagent (Sigma, cat: 408727). Lentiviral packaging plasmid p8.91 and vector pCSFLW bearing the luciferase reporter were transfected alongside the relevant HA glycoprotein genes in the plasmid pI.18 (42). Parental PV were produced bearing the HA of A/California/7/09 (H1), or A/duck/Memphis/546/1974 (H11). A chimeric HA (cHA) consisting of the stalk from A/California/7/09 (H1) and head from A/duck/Memphis/546/1974 (H11) was also produced (43). Pseudotype based microneutralisation assays (pMN) were performed as described previously (42). Briefly, serial dilutions of serum were incubated with 1 × 106 relative luminescence units (RLU) of HA bearing PV per well on a 96-well white plate for 1 h at 37°C 5% CO2 in a humidified incubator. 1.5 × 104 HEK293T/17 cells were then added per well and plates incubated at 37°C 5% CO2 for 48 h before addition of Bright-Glo™ reagent (Promega) and measurement of luciferase activity. Analysis was performed using Graph-Pad Prism. Stalk-directed antibody presence was measured via antibody titers recorded against the cHA and both of its parental strains (H11 and H1 PV). No (or negligible) antibodies should be present against the exotic H11 HA, restricting neutralization of the cHA PV to antibodies directed against the conserved H1 stalk of the cHA. Control antibodies used included mAb CR6261 (Crucell, Johnson, and Johnson) and polyclonal antiserum Anti H11N9 World Organization for Animal Health (OIE).
For IFNγ intracellular cytokine staining, lymphocytes (1.5–2 × 106) from lung or spleen in complete IMDM medium were stimulated with anti-CD28 (1 μg/ml, eBioscience), with or without X-181 WIV (10 μg/ml), overnight at 37°C in a 5% CO2 incubator. Protein transport inhibitor cocktail (eBioscience) was added for the last 4 h of stimulation. Stimulated cells were stained with fluorochrome conjugated antibodies, including Alexa Fluor 700-antiCD3 (clone 17A2), FITC-antiCD4 (GK1.5), PerCP-cy5.5-antiCD8α (53-6.7), eFlour 450-antiCD62L (MEL-14), APC-antiCD44 (IM7) for 45 min. After surface staining, cells were stained with Fixable Viability Dye eFluor 780 (eBioscience) to identify dead cells. Cells were then fixed with IC fixation buffer (eBioscience) and permeabilized with permeabilization buffer (eBioscience) before intracellular staining with PE-cy7-antiIFNγ (clone XMG1.2) (all monoclonal antibodies from eBioscience). Samples were acquired on a BD LSRII and data were analyzed by Kaluza® Flow Cytometry Analysis Software.
Influenza NP-specific IFNγ-producing T cells were enumerated using a commercial mouse IFNγ ELISpot kit (MABTEC, The Netherlands) according to the manufacturer's protocol. Briefly, splenocytes (2.5 × 105/well) collected on day 0 post-infection were incubated with or without 5 μg/ml of the PR8 NP366−374 epitope (ASNENMDAM) in a pre-coated 96-well plate. After overnight incubation, IFNγ-producing T cells were detected using alkaline phosphatase-conjugated anti-mouse IFNγ antibody. Spots were developed with BCIP/NBT substrate and counted with an AID Elispot reader (Autoimmune Diagnostika GmbH, Strassberg, Germany). The number of antigen-specific IFNγ-producing cells was calculated by subtracting the number of spots detected in the unstimulated samples from the number in stimulated samples.
Tetramer staining for lung samples was performed as follows: isolated lung lymphocytes were incubated with A(H1N1)pdm09 NP366−374-tetramer-PE (containing the A(H1N1)pdm09 epitope ASNENMETM) for 40 min and then stained with mouse anti-CD8α-PerCP-cy5.5 antibody for 40 min. Samples were acquired on a FACS Calibur™ BD II flow cytometer. Data were analyzed by Kaluza® Flow Cytometry Analysis Software.
Mice were sequentially infected or sequentially immunized with WIV as described above and serum was collected on day 28 post second infection or immunization. Serum collected from mice that were immunized twice i.m. with A(H1N1)pdm09 WIV served as positive control. Pooled serum was tested by ELISA for presence of anti-A(H1N1)pdm09 antibodies. Naive mice (n = 5/group) received 200 μl of pooled serum by intraperitoneal injection 1 day before challenge with A(H1N1)pdm09 virus. On day 6 post challenge, lungs were collected for virus titration.
For the T cell depletion study, mice were infected or vaccinated as described above and rested for 28 days. Groups of mice (n = 6/group) were injected with anti-CD4 T cell depletion antibody (200 μg/injection, GK1.5) or anti-CD8 T cell (200 μg/injection, YTS169). These antibodies were given i.p on day −1, 1, and 3 of A(H1N1)pdm09 virus (104.4 TCID50) challenge. On day 6 post challenge, lungs were collected for virus titration. Spleens were collected to confirm the depletion of T cells.
Mann-Whitney U-test was used to determine the differences between read-outs of two different groups. Statistical analyses were performed using GraphPad Prism version 6.01 for Windows. GraphPad Sofware, La Jolla, California, USA www.graphpad.com. P < 0.05, 0.01, 0.001 were considered as significantly different and were denoted by *, **, ***, individually.
To investigate the cross-protective immune response induced by sequential infection or vaccination with antigens from different influenza virus strains, we sequentially infected mice with PR8 and X-31 influenza virus or sequentially vaccinated mice with WIV or SU vaccines derived from these viruses. These viral strains were selected to reflect a heterosubtypic exposure history in humans. The cross-protective capacity of sequential infection or sequential immunization was determined by challenging the mice with virus A/California/7/09 (H1N1)pdm09.
After A(H1N1)pdm09 virus challenge, mice in the sequential SU vaccination group showed similar weight loss as mice in the PBS control group and developed severe symptoms, necessitating euthanasia on day 6 or 7 post challenge (Figures 1A,B). Mice that were sequentially vaccinated with WIV showed a similar trend of weight loss as mice in the PBS control group until day 6 post infection. Yet, from day 7 post infection onwards, WIV immunized mice recovered and none of the mice reached the humane endpoint. In the sequential infection group, mice showed no or only minor weight loss after challenge and none of them needed to be sacrificed.
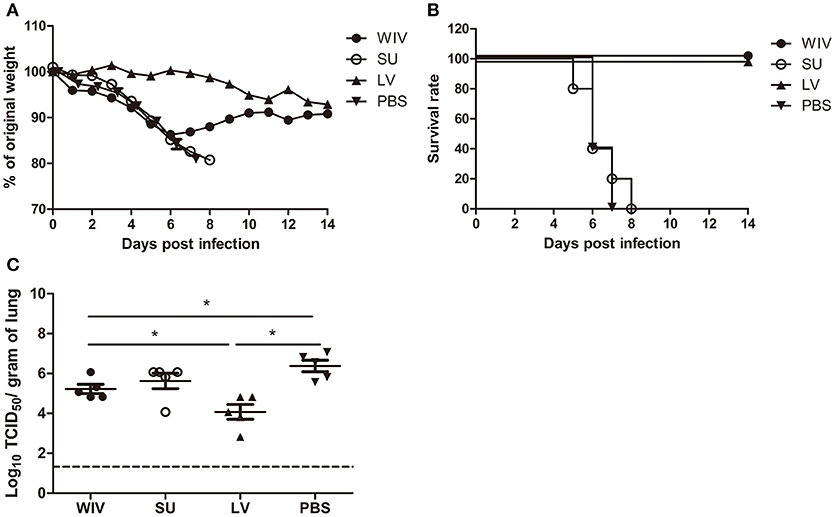
Figure 1. Weight loss, survival rate, and lung virus titer of immunized mice after A(H1N1)pdm09 virus challenge. Naïve mice (n = 10) were sequentially infected with sublethal doses of two different strains (PR8 and then X31) of live virus (LV) with 28 days interval or were sequentially immunized with vaccines (WIV, SU) derived from these virus strains and then challenged with virus A/California/7/2009 (H1N1)pdm09. After challenge, mice (n = 5) were monitored daily for weight loss (A) and survival (B) for a period of 14 days. On day 3 post-challenge, lung virus titers in 5 mice/group were determined by titration on MDCK cells (C). *p < 0.05, Mann-Whitney U-test. The dashed line represents the limit of detection.
On day 3 post-challenge, lung virus titers in the sequential SU vaccination group did not differ significantly from those in the PBS control group (Figure 1C). In the sequential WIV vaccination group, lung virus titers were decreased by 0.9 log10 as compared to the PBS group (p = 0.03). Sequential infection resulted in a significant decrease of the lung virus titer by 2 log10 relative to the control group (p = 0.015).
These data demonstrate that sequential immunization with WIV, although being less effective than sequential infection with live virus, provided a certain level of cross-protection against heterologous infection. In contrast, sequential SU vaccination did not provide cross-protection.
To explore the immune mechanisms involved in protection from weight loss and lung virus growth upon challenge, antibody responses induced by sequential infection with PR8 and X-31 or immunization with PR8 and X-31 derived vaccines were determined.
A single infection with PR8 or immunization with PR8-derived vaccines raised antibodies recognizing PR8 surface proteins in all animals. Although the dose of WIV and SU vaccines was chosen such that both contained the same amount of surface antigens, a single immunization with WIV induced around 30 times more PR8 HA/NA specific IgG than a single immunization with SU (p < 0.0001) (Figure 2A). Antibody titers raised by a sublethal infection were significantly higher than those raised by WIV (p < 0.01) but the difference was only 1.7-fold. Upon subsequent immunization/infection with X-31, on day 56, antibody titers to X-31 HA/NA in WIV-immunized mice were approximately 10-fold higher than in SU-immunized mice and around 3-fold higher than in sequentially infected mice (p < 0.0001) (Figure 2B).
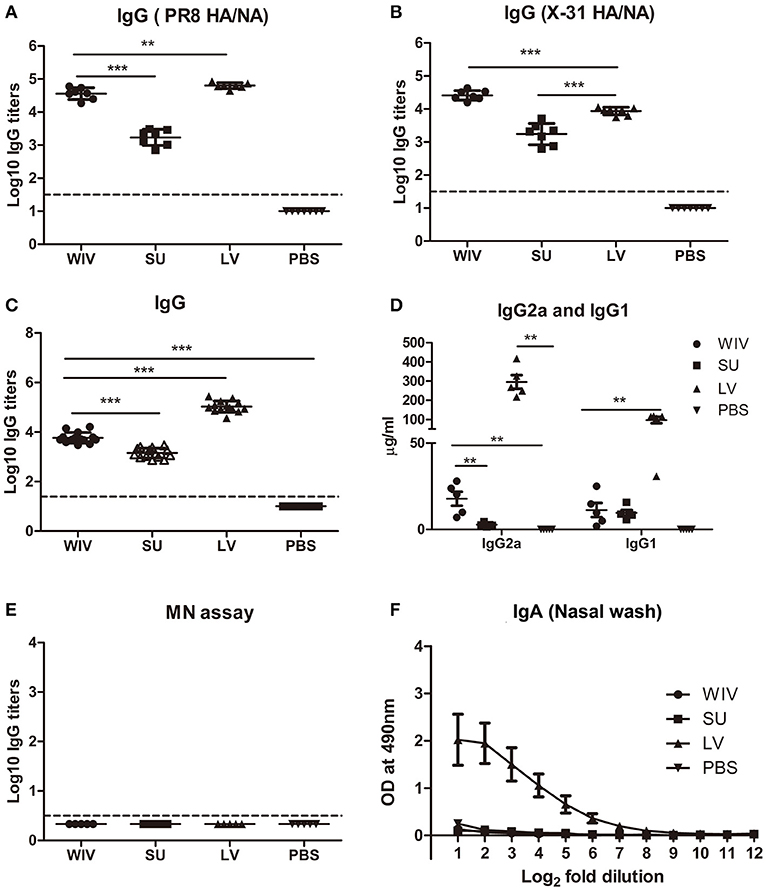
Figure 2. Cross-reactive antibody responses induced by sequential infection or immunization. On day 28 (the day of the second infection or immunization; A) and on day 56 (the day of sacrifice or challenge; B–F), serum samples and nasal washes were collected from the mice described in the legend to Figure 1. Anti-PR8 (HA/NA) IgG antibodies (A; n = 7) and Anti-X-31(HA/NA) IgG antibodies (B; n = 7) in serum samples were detected by ELISA using PR8 SU and X-31 SU for coating. Anti-H1N1pdm09-specific IgG (C; n = 15), IgG2a and IgG1 (D; n = 5) antibodies in serum samples were detected by ELISA. Microneutralization assay was used to determine the neutralizing ability of these antibodies toward A(H1N1)pdm09 virus (E; n = 5). Anti-H1N1pdm09 IgA antibody levels in nasal washes were determined by ELISA (F; n = 5). Data of individual animals (A–E) are depicted or mean values ± SEM (F) are given, **p < 0.01, ***p < 0.001. Mann-Whitney U-test. The dashed line represents the limit of detection.
On day 56, after sequential exposure to PR8 and X-31, we also determined antibody titers against heterologous A(H1N1)pdm09 virus. Sequential infection induced around 20 times more cross-reactive IgG antibody than sequential WIV vaccination and approximately 75 times more than sequential SU vaccination (p < 0.0001) (Figure 2C). With respect to the subtype profile of the IgG antibodies, sequential infection and WIV vaccination induced a Th1-type antibody response. The average ratio of serum IgG2a to IgG1 concentration was 3 for mice sequentially infected by live virus, compared with 1.5 induced by sequential WIV vaccination. In contrast, sequential SU vaccination induced a similar amount of IgG1 antibody as induced by sequential WIV vaccination but no IgG2a (Figure 2D). However, cross-reactive antibodies, irrespective of whether induced by sequential infection or immunization, did not neutralize A(H1N1)pdm09 virus (Figure 2E). With respect to mucosal antibodies, only sequential infection was found to induce cross-reactive IgA antibody against A(H1N1)pdm09 virus in the nose (Figure 2F).
In order to reveal the target protein(s) of the observed cross-reactive antibodies we first performed a pseudovirus-based assay to detect antibodies to the HA stalk domain. This assay uses a chimeric HA as antigen, with an H11 globular head, and an H1 stalk. The chimeric HA pseudovirus particles were effectively neutralized by the CR6261 mAb control which binds to the H1 stalk. However, no or very low amounts of antibodies reacting with the H1 stalk were observed in any of the experimental groups (Figure S1A). Next, we examined anti-NA antibodies against A(H1N1)pdm09 virus. The mice from the sequential infection group and 4 out of 5 mice from the WIV vaccination group developed anti-NA antibodies, while only 2 out of 5 mice from the sequential SU vaccination group did so and levels of anti-NA antibody were low (Figure S1B). Next, anti-M2e antibody titers were determined by coating conserved M2e peptide onto 96-well ELISA plate. Anti-M2e antibodies were only found in the sequential infection group (Figure S1C). We also analyzed the presence of cross-reactive antibodies against conserved internal proteins in serum using recombinant NP from HK68 (H3N2), which shows 90% of sequence homology with NP from A(H1N1)pdm09. Sequential infection and WIV vaccination induced similar though somewhat variable amounts of anti-NP antibodies (Figure S1D). As expected, no anti-NP antibody was found in the sequential SU vaccination group.
These data indicate that sequential infection induced broader and higher amounts of cross-reactive non-neutralizing antibodies than sequential WIV vaccination, while SU vaccination induced only antibodies against hemagglutinin and to a limited extent against neuraminidase. Moreover, responses to live virus and WIV were dominated by IgG2a while responses to SU consisted exclusively of IgG1 antibodies.
Apart from cross-reactive antibody response, cellular immune responses also play an important role in cross-protection. We first evaluated the overall memory T cell responses in spleen and lungs from mice after sequential infections or vaccinations. None of these immunization strategies could significantly enhance the number of memory CD4+CD44+ T cells in the spleen (Figure 3A). In contrast, numbers of CD8+CD44+ T cells were significantly enhanced in the spleens of sequentially infected mice compared with mice of the unvaccinated control group (p = 0.028). Also sequential WIV vaccination enhanced the numbers of memory CD8+CD44+ T cells in the spleen (p = 0.02) while SU did not have such an effect. In the lungs, only sequential infection resulted in a moderate but significant increase of memory T cells and this effect was restricted to CD8+CD44+ T cells (Figure S2A). Interestingly, while the CD8 memory T cell population in sequentially infected mice consisted of CD62L negative TEM as well as CD62L positive TCM, the majority of memory CD8 T cells from the sequential WIV vaccination group consisted of TCM (Figure 3B, Figure S2B). These data indicate that sequential infection and sequential immunization with WIV are capable of stimulating CD8 memory responses while immunization with SU is not.
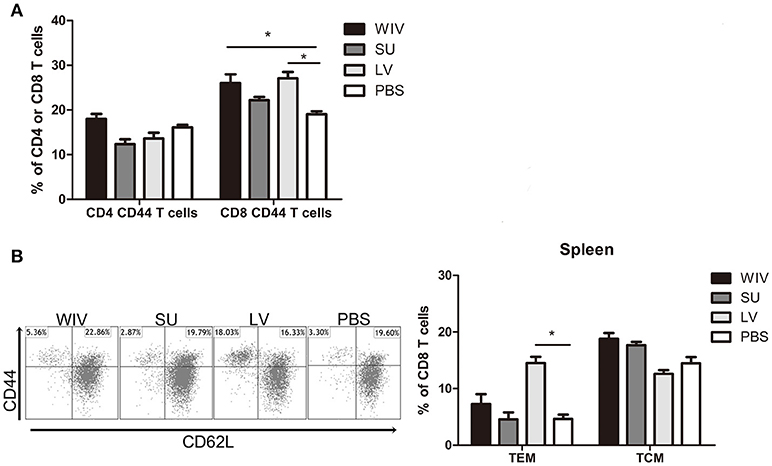
Figure 3. Memory T cell immune responses after sequential infection or immunization. Of the mice described in the legend to Figure 1, 5 animals/group were sacrificed 28 days after the second infection or immunization and spleen and lung were collected. (A) CD4+CD44+ and CD8+CD44+ memory T cells in spleen were determined by flow cytometry. (B) CD8+CD44+CD62L− effector memory T cells (TEM) and CD8+CD44+CD62L+ central memory T cells (TCM) in spleen. Left: representative dot plots depicting CD44 and CD62L expression on spleen CD8 T cells. Right: percentages of spleen CD8 TEM and TCM + SEM. (n = 4 or 5 per group, representative of two experiments, Mann-Whitney U-test, *p < 0.05).
For detection of CD4+ and CD8+ T cells responding to any influenza protein, splenocytes from sequentially infected or sequentially immunized mice were stimulated overnight with WIV, and IFNγ production was assessed by intracellular cytokine staining. In live virus infected mice, percentages of IFNγ-producing CD4+ and CD8+ memory T cells in spleen and lung were significantly higher than in mock immunized mice (Figure 4A, Figure S2C, p < 0.05). Moreover, around 90% of the IFNγ-producing CD8 T cells were effector memory cells (data not shown). Also in WIV immunized mice, enhanced percentages of IFNγ positive CD4+ and CD8+ memory T cells were found, yet lower than in the LV group. Significance as compared to PBS control animals was reached only for CD4+ T cells in spleen.
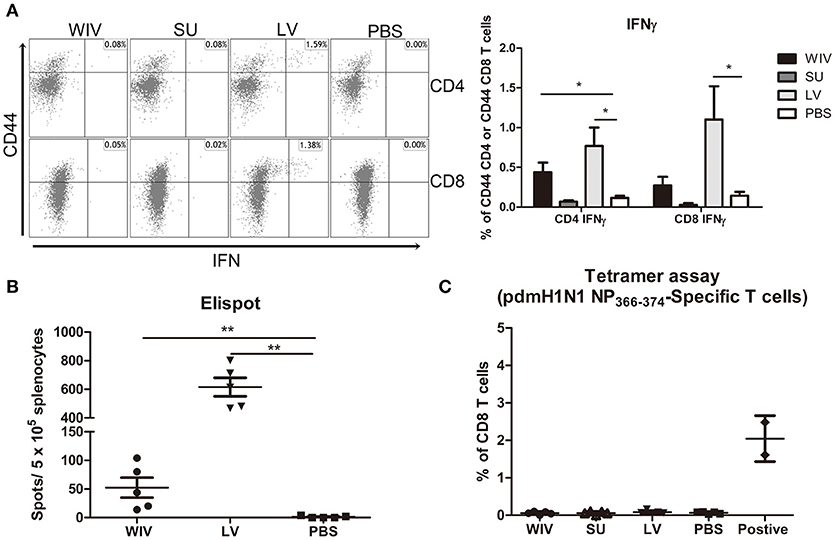
Figure 4. Influenza-specific T cell immune responses induced by sequential infection or immunization. (A) Splenocytes harvested on day 28 post the second infection/vaccination, were stimulated with A(H1N1)pdm09 WIV and anti-CD28 overnight in presence of protein transport inhibitor. Presence of intracellular IFNγ in CD4+CD44+ and CD8+CD44+ T cells was analyzed by flow cytometry. Left: representative dot plots of stimulated CD4 or CD8 T cells stained for CD44 and IFNγ. Right: percentages of IFNγ-producing cells among CD4+CD44+ and CD8+CD44+ T cells. (n = 4 or 5, representative of two experiments, Mann-Whitney U-test, *p < 0.05). (B) On day 28 post the second infection/immunization, NP366−374 of PR8 virus was used to stimulate mouse splenocytes and IFNγ-producing CD8 T cells were enumerated by ELISPOT. (n = 5, Mann-Whitney U test, ** p < 0.01). (C) A(H1N1)pdm09 NP366−374-specific CD8 T cells in spleens of infected/immunized mice (n = 5) were determined by tetramer assay. Lymphocytes from the blood of mice (n = 2) infected with A(H1N1)pdm09 virus served as positive control.
In order to determine whether infection of vaccination had induced CD8+ T cells to the dominant NP epitope an ELISPOT assay was performed for which the splenocytes were stimulated with NP366−374 peptide (ASNENMDAM) from PR8 virus (the epitope present in PR8 as well as X-31 virus). NP-specific CD8 T cells were detected in the WIV and the sequential infection group, but numbers were around 12 times higher in the latter (Figure 4B, p = 0.008). Next, we assessed the cross-reactivity of these NP-specific CD8 T cells to A(H1N1)pdm09 NP by staining with tetramers containing the ASENENMETM epitope (from A(H1N1)pdm09 virus). No tetramer positive CD8 T cells were observed in these groups of mice (Figure 4C) while tetramer positive cells were readily detected in blood of mice infected with A(H1N1)pdm09 virus.
Our data show that sequential infection and sequential immunization with WIV could provide protection against severe symptoms upon infection with an A(H1N1)pdm09 virus. To determine the contribution of cross-reactive antibodies against A(H1N1)pdm09 virus challenge, serum from sequentially virus infected, WIV vaccinated or PBS control mice was passively transferred to naive mice 1 day before A(H1N1)pdm09 virus challenge. Serum from mice vaccinated with WIV derived from A(H1N1)pdm09 virus served as positive control.
Mice receiving serum from mice immunized with A(H1N1)pdm09 WIV (positive control, neutralizing titer 330) via adoptive transfer did not show weight loss upon A(H1N1)pdm09 virus challenge (Figure 5A) and lung virus titers in these animals were decreased by more than 2 logs compared to the titers in the PBS control group (Figure 5B, p < 0.01). Similarly, mice receiving serum from the sequential infection group showed no or only mild weight loss. Interestingly, despite the fact that the transferred serum did not contain any neutralizing antibodies, lung virus titers in this group were decreased to the same low level as in mice which had received serum from A(H1N1)pdm09-immunized mice containing neutralizing antibodies. Also serum from the sequential WIV vaccination group provided partial protection; 4 out of 5 mice receiving this serum showed no or mild weight loss, while one mouse went down quickly. Yet, lung virus titers in the WIV vaccination group, though slightly lower, did not differ significantly from those in PBS-treated controls (p = 0.22) (Figure 5B).
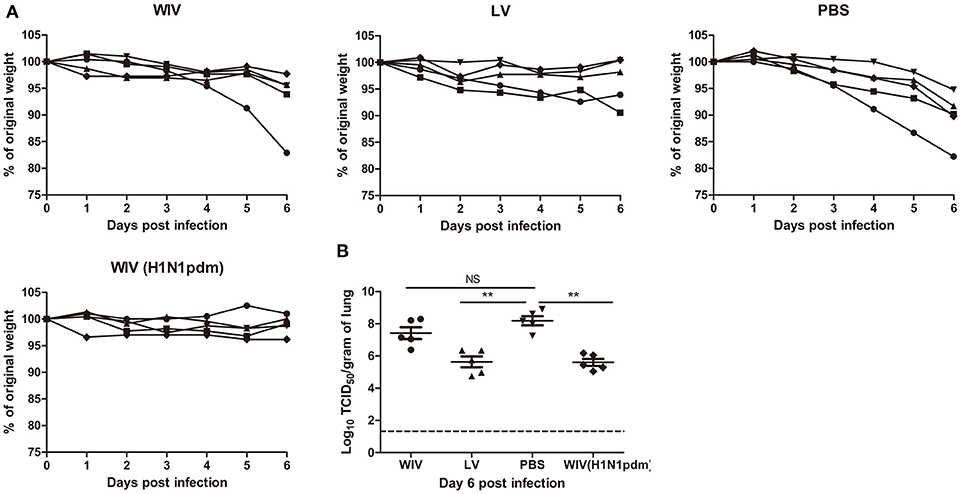
Figure 5. The cross-protective potential of antibodies induced by sequential infection or immunization. Mice (n = 5) were primed with PR8 virus (103TCID50) or PR8 WIV (15 μg) and boosted with X-31 virus (103TCID50) or X-31 WIV (15 μg). Mice primed and boosted with PBS served as negative control and mice primed and boosted with A(H1N1)pdm09 WIV (15 μg) served as positive control. Sera from these mice were collected 4 weeks after boost, pooled and injected into naïve mice 1 day before challenge with A/California/7/2009 (H1N1)pdm09 virus. Body weight loss (A) was monitored daily for 6 days. Virus titers in the lung tissue (B) on day 6 post-challenge were determined by titration on MDCK cells. **p < 0.01, Mann-Whitney U-test. The dashed line represents limit of detection. NS, not significant.
These data indicate that non-neutralizing antibodies induced by sequential infection were as effective as neutralizing antibodies induced by A(H1N1)pdm09 WIV vaccination in providing protection against A(H1N1)pdm09 virus challenge. However, non-neutralizing antibody induced by sequential WIV vaccination were not sufficient to provide full cross-protection.
To determine the contribution of T cell immune responses to cross-protection against A(H1N1)pdm09 virus infection, we used CD4 or CD8 specific antibodies to deplete T cells before and during A(H1N1)pdm09 challenge. On day 6 post-challenge, we confirmed that 95% of CD8 T cells or 96% of CD4 T cells in mice spleen were depleted by this treatment (data not shown).
Mice in the PBS mock vaccination group, no matter whether treated with PBS, CD4 depletion antibody or CD8 depletion antibody, showed continuous weight loss after A(H1N1)pdm09 challenge (Figure 6A, PBS) and displayed the same virus titers in lung tissue on day 6 post-infection (Figure 6B, PBS). In contrast, mice in the sequential infection group were protected from weight loss and showed low or undetectable lung virus titers (Figure 6A, LV). Depletion of CD4 T cells in these mice had no effect on protection. Depletion of CD8 T cells in the sequential infection group had some effect on protection from weight loss; on day 6 post A(H1N1)pdm09 virus challenge 3 out of 6 mice had lost >6.5% weight while in non-depleted mice the most severe weight loss was 2.1% and was observed in a single mouse only (Figure 6A, LV). In addition, lung virus titers were about 1.5 log10 higher in the CD8-depleted mice than in non-depleted control mice of the sequential infection group; yet, virus titers were still significantly lower than in non-immunized mice. In the WIV vaccination group, depletion of CD4 or CD8 T cell did not significantly alter the weight loss compared with mock depletion but a strong trend toward less weight loss was observed in mice depleted for CD4 T cells as compared to non-depleted mice of this group (P = 0.054, Figure 6A, WIV). Depletion of CD4 T cells decreased and depletion of CD8 T cells increased lung virus titers by about 1 log as compared to non-depleted animals on day 6 post challenge but these trends did not reach statistical significance (Figure 6B, WIV). Moreover, virus titers in WIV-immunized CD8 T cell-depleted mice were of the same magnitude as those in the PBS mock vaccination group.
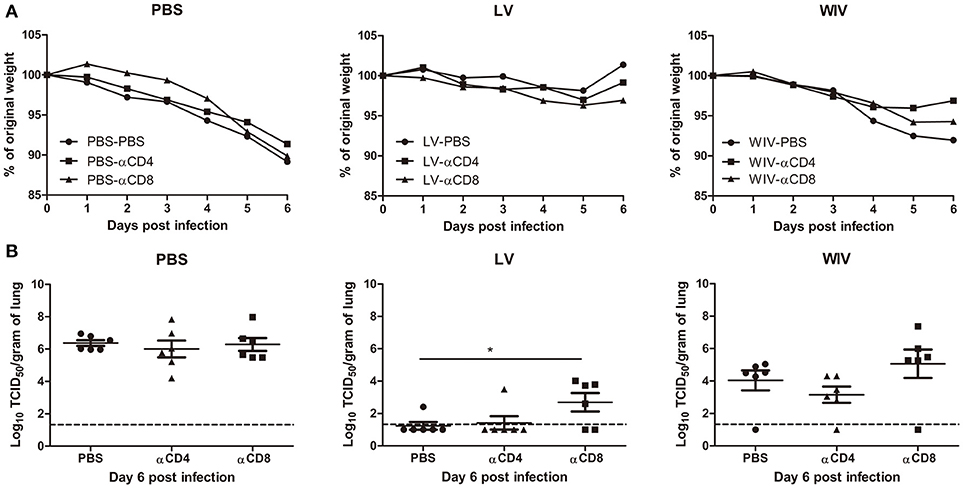
Figure 6. The cross-protective potential of CD4 T cells and CD8 T cells induced by sequential infection or immunization. Mice were primed with PR8 virus (103TCID50) or PR8 WIV (15 μg) and then boosted with X-31 virus (103TCID50) or X-31 WIV (15 μg). Mice primed and boosted with PBS served as control. Anti-CD4 or anti-CD8 T cell depletion antibody or PBS were injected intraperitoneally into mice on day −1, 1, and 3 of A(H1N1)pdm09challenge. Weight loss (A) was monitored for 6 days and lung virus titers (B) were determined on day 6 post-infection by titration on MDCK cells. *p < 0.05, Mann-Whitney U test. The dashed line represents limit of detection.
These data above suggests that CD4 memory T cells were most likely not involved in cross-protection while CD8 memory T cells induced by sequential infection or WIV immunization contributed decisively to cross-protection.
To determine whether sequential immunization with antigenically distinct traditional vaccines could provide cross-protection, mice were sequentially immunized with WIV or SU vaccines derived from PR8 and X-31 viruses and then challenged with an A(H1N1)pdm09 virus. Another group of mice was sequentially infected with sublethal doses of PR8 followed by X-31 prior to A(H1N1)pdm09 virus challenge. We demonstrate that sequential infection provided solid cross-protection which was correlated with cross-protective antibodies and CD8 TEM cells. Sequential vaccination with WIV provided partial cross-protection which also correlated with induction of cross-reactive antibodies and CD8 T cells. Yet, sequential SU vaccination did not provide cross-protection.
Neither sequential infection nor sequential immunization resulted in induction of antibodies capable of neutralizing A(H1N1)pdm09 virus. Yet, substantial amounts of cross-reactive non-neutralizing antibodies were induced. Previous publications have shown that non-neutralizing antibodies, for example anti-HA stem antibodies, can be induced by sequential infection with antigenically distinct viruses and may provide cross-protection against A(H1N1)pdm09 influenza virus infection (11, 12). In contrast to these findings, no anti-HA stem antibodies were found in this study. This may be due to the fact that the two virus strains (PR8 and X-31) used for infection/immunization belong to two different phylogenetic groups. The HA-stem regions from PR8 and X-31 virus show low similarity, which might have impaired boosting of HA-stem reactive B cells induced by PR8 through exposure to X-31. Nevertheless, we found cross-reactive antibodies against other conserved proteins in this study. Anti-M2e, anti-NP and anti-NA antibodies were induced by sequential infection and, although to a lesser extent, by sequential WIV immunization. In contrast, sequential SU immunization induced only very moderate amounts of anti-NA antibodies cross-reactive with A(H1N1)pdm09 virus.
Since no neutralizing antibodies were found, the cross-reactive but non-neutralizing antibodies likely are the reason for the cross-protection observed in the serum adoptive transfer experiment. Non-neutralizing antibodies can provide cross-protection via Fc receptor dependent mechanisms [reviewed in (44)]. Interestingly, control of lung virus growth by non-neutralizing antibodies evoked by sequential infection with PR8 and X-31 was as effective as by neutralizing antibodies evoked by A(H1N1)pdm09 WIV. Even in absence of antigen-specific T cells, neutralizing antibodies are thus not crucial for protection, suggesting that non-neutralizing antibodies maybe more important for cross-protection than generally thought. Our results are in line with studies in macaques which also demonstrate that non-neutralizing but cross-reactive antibodies can provide effective protection against heterosubtypic virus challenge, most likely through antibody dependent cellular cytotoxicity (45, 46). In this context, it is interesting that recent studies revealed that in humans antibodies cross-reacting with different influenza virus strains are common and are effectively enhanced by vaccination with seasonal influenza vaccines (47, 48). These antibodies might play a role in disease mitigation.
Hillaire et al. and Guo et al. have shown that one dose of serum from virus-infected animals could not provide cross-protection against A(H1N1)pdm09 virus infection in mice (5, 6), while Fang et al. have shown that four doses of serum could provide cross-protection(3). These studies imply that the amount of non-neutralizing cross-reactive antibodies may also play an important role in cross-protection. In the present study, cross-reactive antibody titers evoked by sequential WIV immunization were 20-fold lower than those evoked by sequential infection. We thus speculate that antibodies induced by WIV immunization, though in principle cross-protective as indicated by our data, were not present in sufficient amounts to confer complete protection.
Although sequential infection and sequential WIV immunization induced virus-specific IFNγ-producing CD4 T cells, depletion of CD4 T cells in this study did not influence the cross-protection, neither in the sequential infection group nor in the sequential WIV vaccination group. These results contrast with previous findings which indicate that CD4 T cells might play a role in cross-protection (5, 6, 21). Hillaire et al. reported that naïve mice that received T cells (a mixture of CD4 and CD8 T cells) induced by a single A(H3N2) (HK68) virus infection acquired better cross-protection against A(H1N1)pdm09 virus infection than naïve mice that received purified CD8 T cells only (6). Another study by Guo et al. reported that depletion of CD4 T cells induced by a single X-31 virus infection impaired the cross-protection against A(H1N1)pdm09 virus infection in mice (5). In this study, not only CD4 T cells, but also robust cross-reactive antibodies and CD8 T cell immune responses were induced by sequential infection. These antibodies or CD8 T cells alone could significantly reduce the virus titer in mice lung in the absence of CD4 T cells. We conclude that CD4 T cell are not essential for cross-protection against A(H1N1)pdm09 during infection in this mouse model.
CD8 T cells play an important role in cross-protection. In the present study, depletion of CD8 T cells induced by sequential WIV immunization resulted in lung virus titers similar to those in PBS mock vaccinated mice, implying that CD8 T cells are important for cross-protection induced by sequential WIV immunization. These results agree with those reported by Furuya et al. who showed that WIV (prepared by γ-irradiation) did not provide cross-protection against heterologous virus infection in mice defective in CD8 T cells (49). Another study by Budimir et al. also has shown that depletion of CD8 T cells induced by 2 doses of WIV abolished the cross-protection against heterologous virus challenge (50). Depletion of CD8 T cells in the sequential infection group prior to A(H1N1)pdm09 challenge had a significant though moderate effect on lung virus titers. This result implies that in the sequential infection group CD8 T cells do play a role in cross-protection, but team up with other mechanisms, eg antibodies (Figure 5), to provide full protection. Our findings are also in line with previous publications which demonstrate that CD4 T cells or antibody immune responses are required to cooperate with CD8 T cells for providing optimal cross-protection in live virus infected mice (5, 17, 51).
The tetramer experiment indicates that PR8 NP366−374 epitope-specific CD8 T cells elicited by PR8 and boosted by X-31 virus or WIV could not recognize the corresponding A(H1N1)pdm09 NP366−374 epitope. This result is in line with previous findings demonstrating that X-31 NP366−374 epitope cannot be recognized by A(H1N1)pdm09 NP-specific CD8 T cells (52). However, Guo et al. have reported that influenza NP and PA proteins from PR8 and A(H1N1)pdm09 virus share many conserved epitopes (52). It is possible that influenza-specific CD8 T cells against these shared conserved epitopes induced by sequential infection or WIV immunization contributed to cross-protection against A(H1N1)pdm09 influenza virus infection.
Different phenotypes of memory CD8 T cells show different capacities in cross-protection, for example Wu et al. have shown that CD8 TCM induced by influenza virus infection are not required for cross-protection (17). In the present study, we found that sequential infection mainly induced CD8 TEM. This result is in line with previous findings in mice and humans reporting that a single influenza infection predominantly induces influenza-specific CD8 TEM cells (53, 54). CD8 TEM have been shown to be associated with a fast recall immune response to the infection site, thus providing immediate cross-protection(53). Interestingly, we found that sequential WIV immunization was more likely to induce CD8 TCM. These cells have shown high proliferation ability in secondary lymphoid organs but to provide delayed cross-protection (55). Thus, we propose that CD8 TEM in lung and spleen induced by sequential infection provided immediate local antiviral effects, resulting in solid cross-protection. In contrast, CD8 TCM in spleen induced by sequential WIV immunization provided delayed antiviral effects in the lung, resulting in partial cross-protection.
A limitation of our study is the fact that we did not investigate whether sequential infection/immunization with the same virus could also provide protection from infection by a heterologous virus subtype. We therefore cannot formally conclude that antigenically distinct viruses are required to induce cross-protection. However, it has been well established that vaccination with distinct vaccines focuses the immune response on conserved antigens which would be a clear benefit when attempting to raise cross-protection.
In summary, sequential infection with antigenically distinct viruses provided robust cross-protection against A(H1N1)pdm09 virus infection. Yet, sequential immunization with antigenically distinct SU failed to provide cross-protection. Intriguingly, sequential immunization with antigenically distinct WIV provided partial cross-protection by a mechanism involving cross-reactive but non-neutralizing antibodies as well as CD8+ T cells. These results imply that sequential immunization with WIV prepared from antigenically distinct viruses could be used to alleviate the severity of virus infection if a new pandemic occurs.
WD, YB, FS, TM, and JdV-I performed animal experiments. KG, OE, LB, CdH, GC, and NT contributed materials and reagents. WD, CdH, GC, NT, and AH analyzed the data. WD and AH wrote the manuscript. WD, DK, and AH designed the study. All authors approved the manuscript.
LB is an employee of the company Bioceros, Utrecht, The Netherlands.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Meiling Dai, Utrecht University, Utrecht, The Netherlands, for technical assistance and David A. Price, Institute of Infection and Immunity, Cardiff University School of Medicine, Cardiff, United Kingdom, for a gift of tetramers. This research was funded by the European Union Seventh Framework Program 19 (FP7/2007-2013) Universal Influenza Vaccines Secured (UNISEC) consortium under grant agreement no. 602012 and by the EU Horizon 2020 Program under the Marie Skłodowska-Curie grant agreement 713660. DW received a PhD scholarship from the University Medical Center Groningen, Groningen, The Netherlands, and Shantou University Medical College, Shantou, China. This manuscript has been released as a preprint at https://www.biorxiv.org/content/early/2018/05/31/335281.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2018.02312/full#supplementary-material
1. Wiersma L, Rimmelzwaan G, de Vries R. Developing universal influenza vaccines: hitting the nail, not just on the head. Vaccines (2015) 3:239–62. doi: 10.3390/vaccines3020239
2. Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. (2015) 14:167–82. doi: 10.1038/nrd4529
3. Fang Y, Banner D, Kelvin AA, Huang SSH, Paige CJ, Corfe SA, et al. Seasonal H1N1 influenza virus infection induces cross-protective pandemic H1N1 virus immunity through a CD8-independent, B cell-dependent mechanism. J Virol. (2012) 86:2229–38. doi: 10.1128/JVI.05540-11
4. O'Donnell CD, Wright A, Vogel LN, Wei C-J, Nabel GJ, Subbarao K. Effect of priming with H1N1 influenza viruses of variable antigenic distances on challenge with 2009 pandemic H1N1 Virus. J Virol. (2012) 86:8625–33. doi: 10.1128/JVI.00147-12
5. Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J Virol. (2011) 85:448–55. doi: 10.1128/JVI.01812-10
6. Hillaire MLB, van Trierum SE, Kreijtz JHCM, Bodewes R, Geelhoed-Mieras MM, Nieuwkoop NJ, et al. Cross-protective immunity against influenza pH1N1 2009 viruses induced by seasonal influenza A (H3N2) virus is mediated by virus-specific T-cells. J General Virol. (2011) 92:2339–49. doi: 10.1099/vir.0.033076-0
7. Carter DM, Bloom CE, Nascimento EJM, Marques ETA, Craigo JK, Cherry JL, et al. Sequential seasonal H1N1 influenza virus infections protect ferrets against novel 2009 H1N1 influenza virus. J Virol. (2013) 87:1400–10. doi: 10.1128/JVI.02257-12
8. Laurie KL, Carolan LA, Middleton D, Lowther S, Kelso A, Barr IG. Multiple infections with seasonal influenza A virus induce cross-protective immunity against A(H1N1) pandemic influenza virus in a ferret model. J Infect Dis. (2010) 202:1011–20. doi: 10.1086/656188
9. Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. (2013) 19:1305–12. doi: 10.1038/nm.3350
10. Hayward AC, Wang L, Goonetilleke N, Fragaszy EB, Bermingham A, Copas A, et al. Natural T cell-mediated protection against seasonal and pandemic influenza: results of the flu watch cohort study. Am J Resp Crit Care Med. (2015) 191:1422–31. doi: 10.1164/rccm.201411-1988OC
11. Kirchenbaum GA, Carter DM, Ross TM. Sequential Infection in Ferrets with Antigenically Distinct Seasonal H1N1 Influenza Boosts Hemagglutinin Stalk-Specific Antibodies. J Virol. (2015) 90:1116–28. doi: 10.1128/JVI.02372-15
12. Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, et al. H3N2 Influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol. (2013) 87:4728–37. doi: 10.1128/JVI.03509-12
13. Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis. (2013) 207:974–81. doi: 10.1093/infdis/jis935
14. DiLillo DJ, Tan GS, Palese P, Ravetch J V. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med. (2014) 20:143–51. doi: 10.1038/nm.3443
15. El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. (2011) 186:1022–31. doi: 10.4049/jimmunol.0902147
16. LaMere MW, Lam H-T, Moquin A, Haynes L, Lund FE, Randall TD, et al. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol. (2011) 186:4331–9. doi: 10.4049/jimmunol.1003057
17. Wu T, Hu Y, Lee Y-T, Bouchard KR, Benechet A, Khanna K, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukocyte Biol. (2014) 95:215–24. doi: 10.1189/jlb.0313180
18. Kreijtz JHCM, Bodewes R, van den Brand JM, de Mutsert G, Baas C, van Amerongen G, et al. Infection of mice with a human influenza A/H3N2 virus induces protective immunity against lethal infection with influenza A/H5N1 virus. Vaccine (2009) 27:4983–9. doi: 10.1016/j.vaccine.2009.05.079
19. Alam S, Sant AJ. Infection with seasonal influenza virus elicits CD4 T cells specific for genetically conserved epitopes that can be rapidly mobilized for protective immunity to pandemic H1N1 influenza virus. J Virol. (2011) 85:13310–21. doi: 10.1128/JVI.05728-11
20. Eliasson DG, Omokanye A, Schön K, Wenzel UA, Bernasconi V, Bemark M, et al. M2e-tetramer-specific memory CD4 T cells are broadly protective against nfluenza infection. Mucosal Immunol. (2018) 11:273–89. doi: 10.1038/mi.2017.14
21. Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. (2012) 12:136–48. doi: 10.1038/nri3152
22. Wang Z, Wan Y, Qiu C, Quiñones-Parra S, Zhu Z, Loh L, et al. Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8+ T cells. Nat Commun. (2015) 6:6833. doi: 10.1038/ncomms7833
23. Wilkinson TM, Li CKF, Chui CSC, Huang AKY, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. (2012) 18:274–80. doi: 10.1038/nm.2612
24. Jefferies S, Earl D, Berry N, Blackmore T, Rooker S, Raymond N, et al. Effectiveness of the 2009 seasonal influenza vaccine against pandemic influenza A(H1N1)2009 in healthcare workers in New Zealand, June-August 2009. Euro Surveill (2011) 16:19761. doi: 10.2807/ese.16.02.19761-en
25. Hardelid P, Fleming DM, Mcmenamin J, Andrews N, Robertson C, SebastianPillai P, et al. Effectiveness of pandemic and seasonal influenza vaccine in preventing pandemic influenza A(H1N1)2009 infection in England and Scotland 2009-2010. Eurosurveillance (2011) 16:1–7. doi: 10.2807/ese.16.02.19763-en
26. Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med. (2009) 361:1945–52. doi: 10.1056/NEJMoa0906453
27. Skowronski DM, de Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, et al. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: Four observational studies from Canada. PLoS Med. (2010) 7:e1000258. doi: 10.1371/journal.pmed.1000258
28. Janjua NZ, Skowronski DM, Hottes TS, Osei W, Adams E, Petric M, et al. Seasonal influenza vaccine and increased risk of pandemic A/H1N1-related illness: first detection of the association in British Columbia, Canada. Clin Infect Dis. (2010) 51:1017–27. doi: 10.1086/656586
29. Chen GL, Min J-Y, Lamirande EW, Santos C, Jin H, Kemble G, et al. Comparison of a live attenuated 2009 H1N1 vaccine with seasonal influenza vaccines against 2009 pandemic H1N1 virus infection in mice and ferrets. J Infect Dis. (2011) 203:930–6. doi: 10.1093/infdis/jiq144
30. Ellebedy AH, Ducatez MF, Duan S, Stigger-Rosser E, Rubrum AM, Govorkova EA, et al. Impact of prior seasonal influenza vaccination and infection on pandemic A (H1N1) influenza virus replication in ferrets. Vaccine (2011) 29:3335–39. doi: 10.1016/j.vaccine.2010.08.067
31. Pascua PNQ, Song M-S, Lee JH, Park KJ, Kwon H-I, Baek YH, et al. Evaluation of the efficacy and cross-protectivity of recent human and swine vaccines against the pandemic (H1N1) 2009 virus infection. PLoS ONE (2009) 4:e8431. doi: 10.1371/journal.pone.0008431
32. Kim JH, Reber AJ, Kumar A, Ramos P, Sica G, Music N, et al. Non-neutralizing antibodies induced by seasonal influenza vaccine prevent, not exacerbate A(H1N1)pdm09 disease. Sci Rep. (2016) 6:1–13. doi: 10.1038/srep37341
33. Krammer F, Pica N, Hai R, Tan GS, Palese P. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J Virol. (2012) 86:10302–7. doi: 10.1128/JVI.01336-12
34. Krammer F. Strategies to induce broadly protective antibody responses to viral glycoproteins. Exp Rev Vaccines (2017) 16:503–13. doi: 10.1080/14760584.2017.1299576
35. Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol. (2013) 87:6542–50. doi: 10.1128/JVI.00641-13
36. Wei C-J, Boyington JC, McTamney PM, Kong W-P, Pearce MB, Xu L, et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science (2010) 329:1060–4. doi: 10.1126/science.1192517
37. Ryder AB, Nachbagauer R, Buonocore L, Palese P, Krammer F, Rose JK. Vaccination with vesicular stomatitis virus-vectored chimeric hemagglutinins protects mice against divergent influenza virus challenge strains. J Virol. (2016) 90:2544–50. doi: 10.1128/JVI.02598-15
38. Zhou H, Huang Y, Yuan S, Li Y, Wu S, Xu J, et al. Sequential immunization with consensus influenza hemagglutinins raises cross-reactive neutralizing antibodies against various heterologous HA strains. Vaccine. (2017) 35:305–12. doi: 10.1016/j.vaccine.2016.11.051
39. Liu H, Patil HP, de Vries-Idema J, Wilschut J, Huckriede A. Enhancement of the immunogenicity and protective efficacy of a mucosal influenza subunit vaccine by the saponin adjuvant GPI-0100. PLoS ONE (2012) 7:e52135. doi: 10.1371/journal.pone.0052135
40. Reed LJ, Muench H A simple method of estimating fifty percent endpoints. Am J Hyg. (1938) 27:493–497.
41. Dai M, Guo H, Dortmans JCFM, Dekkers J, Nordholm J, Daniels R. Identification of residues that affect oligomerization and / or enzymatic activity of influenza virus H5N1 Neuraminidase Proteins. J Virol. (2016) 90:9457–70. doi: 10.1128/JVI.01346-16
42. Temperton NJ, Hoschler K, Major D, Nicolson C, Manvell R, Hien M. A sensitive retroviral pseudotype assay for influenza H5N1-neutralizing antibodies. Influenza Other Respir Viruses (2007) 1:105–12. doi: 10.1111/j.1750-2659.2007.00016.x
43. Ferrara F, Temperton N. MethodsX Chimeric in fl uenza haemagglutinins : generation and use in pseudotype neutralization assays. MethodsX (2017) 4:11–24. doi: 10.1016/j.mex.2016.12.001
44. Jegaskanda S, Vanderven HA, Wheatley AK, Stephen J. Fc or not Fc; that is the question : antibody Fc-receptor interactions are key to universal influenza vaccine design. Hum Vaccin Immunother. (2017) 13:1–9. doi: 10.1080/21645515.2017.1290018
45. Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol. (2013) 87:5512–22. doi: 10.1128/JVI.03030-12
46. Carroll TD, Jegaskanda S, Matzinger SR, Fritts L, McChesney MB, Kent SJ, et al. A Lipid/DNA adjuvant-inactivated influenza virus vaccine protects rhesus macaques From uncontrolled virus replication after heterosubtypic influenza a virus challenge. J Infect Dis. (2018) 218:856–67. doi: 10.1093/infdis/jiy238
47. McCarthy KR, Watanabe A, Kuraoka M, Do KT, McGee CE, Sempowski GD, et al. Memory B cells that cross-react with group 1 and group 2 influenza a viruses are abundant in adult human repertoires article memory b cells that cross-react with group 1 and group 2 influenza a viruses are abundant in adult human repertoires. Immunity (2018) 48:174–83.e9. doi: 10.1016/j.immuni.2017.12.009
48. Lee J, Boutz DR, Chromikova V, Joyce MG, Vollmers C, Leung K, et al. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat Med. (2016) 22:1456–64. doi: 10.1038/nm.4224
49. Furuya Y, Chan J, Regner M, Lobigs M, Koskinen A, Kok T, et al. Cytotoxic T cells are the predominant players providing cross-protective immunity induced by {gamma}-irradiated influenza A viruses. J Virol. (2010) 84:4212–21. doi: 10.1128/JVI.02508-09
50. Budimir N, Huckriede A, Meijerhof T, Boon L, Gostick E, Price DA, et al. Induction of heterosubtypic cross-protection against influenza by a whole inactivated virus vaccine: the role of viral membrane fusion activity. PLoS ONE (2012) 7:e30898. doi: 10.1371/journal.pone.0030898
51. Laidlaw BJ, Decman V, Ali M-A a, Abt MC, Wolf AI, Monticelli L, et al. Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity. PLoS Pathog. (2013) 9:e1003207. doi: 10.1371/journal.ppat.1003207
52. Guo H, Topham DJ. Multiple distinct forms of CD8+ T cell cross-reactivity and specificities revealed after 2009 H1N1 influenza a virus infection in mice. PLoS ONE (2012) 7:e46166. doi: 10.1371/journal.pone.0046166
53. Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T Cells Play a prominent role in recall responses to secondary viral infection in the lung. The J Immunol. (2004) 172:6533–7. doi: 10.4049/jimmunol.172.11.6533
54. Sridhar S, Begom S, Bermingham A, Ziegler T, Roberts KL, Barclay WS, et al. Predominance of heterosubtypic IFN-γ-only-secreting effector memory T cells in pandemic H1N1 naive adults. Eur J Immunol. (2012) 42:2913–24. doi: 10.1002/eji.201242504
Keywords: sequential vaccination, cross-protection, antigenically distinct influenza virus strains, immune mechanism, non-neutralizing antibody
Citation: Dong W, Bhide Y, Sicca F, Meijerhof T, Guilfoyle K, Engelhardt OG, Boon L, de Haan CAM, Carnell G, Temperton N, de Vries-Idema J, Kelvin D and Huckriede A (2018) Cross-Protective Immune Responses Induced by Sequential Influenza Virus Infection and by Sequential Vaccination With Inactivated Influenza Vaccines. Front. Immunol. 9:2312. doi: 10.3389/fimmu.2018.02312
Received: 13 July 2018; Accepted: 17 September 2018;
Published: 09 October 2018.
Edited by:
John Stambas, Deakin University, AustraliaReviewed by:
Stephen Kent, The University of Melbourne, AustraliaCopyright © 2018 Dong, Bhide, Sicca, Meijerhof, Guilfoyle, Engelhardt, Boon, de Haan, Carnell, Temperton, de Vries-Idema, Kelvin and Huckriede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anke Huckriede, YS5sLncuaHVja3JpZWRlQHVtY2cubmw=
†Present Address: Kate Guilfoyle, Viroclinics Biosciences B.V., Rotterdam, Netherlands
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.