- 1Department of Medicine, Center for Global Health, Weill Cornell Medicine, New York, NY, United States
- 2Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, Netherlands
- 3National Institute for Medical Research, Mwanza, Tanzania
- 4Department of Medicine, Bugando Medical Centre, Mwanza, Tanzania
- 5Department of Parasitology, Leiden University Medical Center, Leiden, Netherlands
Studies of the role of Schistosoma co-infections on plasma HIV-1 RNA (HIV-1 viral load) have yielded incongruent results. The role of duration of HIV-1 infection on the link between Schistosoma and HIV-1 viral load has not been previously investigated. We aimed to assess the impact of HIV-1/Schistosoma co-infections on viral load in Antiretroviral Treatment (ART)-naïve HIV-1 infected people taking into account the duration of HIV-1 infection. We describe 79 HIV-infected outpatients greater than 18 years of age who had never used ART in Mwanza, Tanzania. Schistosomiasis testing was done by urine and stool microscopy and by serum Schistosoma circulating anodic antigen (CAA) testing. Schistosoma positivity was defined as having either test positive. We conducted univariable and multivariable linear regressions to assess the relationship between Schistosoma infection and the log10 of viral load. Duration of HIV infection was calculated using the first measured CD4+ T-cell (CD4) count as a function of normal CD4 count decay per calendar year in drug naïve individuals. An active Schistosoma infection was demonstrated in 46.8% of the patients. The median log10 viral load was 4.5[3.4–4.9] log10 copies/mL in Schistosoma uninfected patients and 4.3[3.7–4.6] log10 copies/mL in Schistosoma infected patients. Schistosoma co-infection was negatively associated with the log10 of viral load after adjustment for Schistosoma intensity as measured by CAA, CD4 counts at time of testing, and duration of HIV-1 infection (β = −0.7[−1.3;−0.1], p = 0.022). Schistosoma co-infection was not associated with viral load in univariable analysis. There was also no interaction between Schistosoma positivity and duration of HIV-1 infection. Our study is the first, to our knowledge, to report adjustment for duration of HIV-1 infection when analyzing the relationship between HIV-1 viral load and Schistosoma spp. We found that time infected with HIV-1 has a major effect on the relationship between HIV-1 viral load and Schistosoma infection and may be a critical explanatory factor in the disparate findings of studies on HIV-1 viral load and schistosomiasis. The log10 viral load difference found indicates that Schistosoma co-infection does not make HIV progression worse, and could possibly lead to slower HIV disease progression.
Introduction
Although Africa makes up just 15% of the worldwide population, it is burdened by 70% of the world's 36.7 million HIV infections and 91% of the world's 240 million Schistosoma infections (1, 2). The 2013 Global Burden of Disease Study estimated that schistosomiasis alone causes 2.6 million disability-adjusted life years (DALYs) lost annually, while HIV infection alone causes 66.7 million DALYs (3). Of note, DALY calculations for HIV and schistosomiasis account for each infection separately and do not consider impacts that they may have on one another.
A growing body of animal and human studies supports a complex relationship between HIV and schistosomiasis. Animal studies suggest that schistosomiasis may alter immune control of viral co-infections, facilitating viral reactivation and replication (4–6). However the role of Schistosoma spp. co-infections on plasma HIV-1 RNA (HIV-1 viral load) is still unclear, with various studies reporting higher, lower, or equivalent HIV-1 viral loads in those with Schistosoma co-infection (7–15). Within this body of evidence, the longest time that people with HIV and Schistosoma co-infection have been followed was approximately 24 months. Our group has recently documented an unexpected improved long-term HIV disease-free survival in those with HIV/Schistosoma co-infections at time of HIV-1 seroconversion (16). This suggests that chronic Schistosoma infection may downregulate HIV-1 viral replication even though the opposite has been observed during acute infection (6, 15), or that time infected with HIV may have been a confounder in studies that examine the link between Schistosoma spp. and HIV-1 viral load.
We thus aimed to assess the impact of HIV-1/Schistosoma spp. co-infections on viral load in Antiretroviral Treatment (ART)-naïve HIV-1 infected people taking into account the duration of HIV-1 infection. To investigate this question, we designed a study situated within an outpatient HIV clinic at which approximately 30% of individuals are Schistosoma -infected (17, 18), and enrolled patients who would shortly be starting ART.
Methods
Study Participants and Enrollment
This study was conducted in April and May 2015 in an HIV outpatient clinic at Bugando Medical Centre (BMC) in Mwanza. The participants were HIV-infected adults greater than 18 years of age who had never used ART according to clinic records and patient report.
Eligible patients provided a single urine and stool sample for schistosomiasis testing by microscopy in order to determine which species of schistosomes were present, as well as serum for quantitation of Schistosoma Circulating Anodic Antigen (CAA), which measures the intensity of infection (19). Plasma was also collected for viral load measurement. Additional information was extracted from the HIV clinic database and the patient's chart.
Laboratory Methods
Microscopic testing was performed on 10 mL of urine by the filtration technique and on feces following the Kato Katz method. Testing was performed by parasitologists at the National Institute of Medical Research (NIMR) in Mwanza, Tanzania. Five Kato Katz slides using 41.7 mg of stool per slide were used, which has been shown to have a sensitivity comparable to collecting three stool samples on different days (20). CAA testing was performed at NIMR in Mwanza as previously described, using a positivity threshold of 30 pg/mL (dry reagent SCAA20 assay format) (19). In order to maximize sensitivity of testing for Schistosoma infections in this HIV-infected population, we used a composite score in which we defined Schistosoma infection as having either a microscopy or CAA positive test. Plasma viral load was quantified using the COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test (Roche Molecular Systems Inc., Pleasanton, California, USA) at the BMC clinical laboratory, with a lower limit of detection of 20 copies/mL.
Statistical Analysis
Data was double entered, verified and cleaned using Microsoft Excel 2013 and analysis was performed using STATA version 13. Categorical data were described with proportions and continuous data were described with median and interquartile range. Chi-square tests and t-tests were used to compare presence of demographic and clinical factors between those co-infected with Schistosoma/HIV-1 and those infected with HIV-1 only. Both viral loads and CAA values had extreme outliers and were skewed to the right. We thus used the log10 of viral load and natural log of CAA, by convention. Univariable and multivariable linear regressions were used to assess the relationship between Schistosoma infection and the log10 of viral load. We also assessed the association between Schistosoma infection and CD4 counts. All variables significantly associated with the outcome in the univariable analysis were included in the multivariable analysis. A stepwise analysis was conducted for the multivariable analysis. A quantile regression was used to assess the interaction between Schistosoma infection and duration of HIV-1 infection and its impact on the difference in median of the log10 of viral load.
Time infected with HIV was defined using the first CD4+ T-cell counts (CD4 counts) reported at the clinic. This method has been previously used with some variation (21–25). Due to similarity in the available data and study setting, we used the method of Forbi et al. (23). The CD4 counts at time of enrollment at the clinic were used to approximate the time delay between HIV infection and enrollment as a function of normal CD4 decay per calendar year in drug naïve individuals. The normal reference values of CD4 counts in healthy Tanzanians have been estimated at a median of 596.5 [291.2–1278.9] cells/μL for men and 764.5 [288.5–1406.8] cells/μL for women (26). In addition, the most prevalent HIV infecting clade in the Lake Zone in Tanzania is clade A (27) and in Mwanza, Tanzania, Clade A and D viruses make up the majority (34 and 28% respectively) of HIV infections (28). This is similar to proportions found on the Ugandan side of the lake, in Rakai (29) and Entebbe (30) and the overall normal CD4 decay per calendar year, across all clades, has been shown to be approximately 34.5 cells/μL per year in Rakai (29).
Starting from the upper range of the normal reference values for CD4 counts, we modeled decay by the square-root function as suggested by Kiwanuka et al. (29), which meant we subtracted 5.87 cells1/2/μL1/2 from 35.76 cells1/2/μL1/2 for men and 37.51 cells1/2/μL1/2 for women per calendar year period until the square root of the first CD4 count reported at the clinic was reached. The time period for this to happen was considered to be the estimated period between HIV-1 acquisition and enrollment at the clinic. The time between the first CD4 count reported at the clinic and the date of viral load testing was then added to this variable to obtain the duration of HIV-1 infection. This led to an estimated median time from seroconversion to enrollment of 2.5[1.7–3.0] years, which is similar to the median time from seroconversion to enrollment estimated by our group within the Kisesa Lake region cohort using mid-dates between two serosurveys as date of seroconversion (manuscript submitted). Finally, to look at the interaction between Schistosoma infection and time infected with HIV, we categorized the latter using tertiles.
Ethical Considerations
All participants were recruited after providing written informed consent in accordance with the declaration of Helsinki. Clearance was obtained from the joint CUHAS/BMC Research Ethics Committee, the National Institute for Medical Research in Dar es Salaam, Tanzania, and Weill Cornell Medical College, New York. All clinical data were made available immediately to clinicians and recorded in the patient's medical record. All patients with Schistosoma infection received praziquantel 40 mg/kg free of charge.
Results
We enrolled 83 HIV-infected patients who presented at the clinic and had never initiated ART. Thirty-seven out of seventy-nine (46.8%) were positive for Schistosoma spp. either by CAA or microscopy test. 33/81 (40.7%) were positive by CAA and the median CAA was 18.3[5.6–517.2] pg/mL. The distribution of CAA values was skewed to the right. Therefore we log-transformed it and the median ln CAA was 3.0[1.9–6.3] ln pg/mL. None had a positive urine microcopy and among those with positive stool microscopy (20/81–24.7%), the median of the mean egg count was 21.6[4.8–52.8] eggs/gram. Seventeen patients were CAA positive but urine and stool negative, while 4 patients were stool positive but CAA negative. Patients had a median age of 36[29-41] years, and 67/83 (80.7%) were female. Median CD4 counts at enrollment was 504[395-749] cells/μL and median CD4 counts at time of viral load testing was 455[328-614] cells/μL.
Patients had enrolled in the HIV clinic a median of 2.5[1.7–3.0] years after acquiring HIV, and provided viral loads for this study a median of 3.7[3.0–5.7] (minimum = 1.7, maximum = 12.0) years after acquiring HIV. The median viral load was 21,670.5[2,852.0–56,160.0] copies/mL, or 4.3[3.5–4.7] log10 copies/mL. The median log10 viral load was 4.5[3.4–4.9] log10 copies/mL in Schistosoma uninfected patients and 4.3[3.7–4.6] log10 copies/mL in Schistosoma infected patients. The main variables are presented in Table 1 by Schistosoma infection status.

Table 1. Characteristics of the 79 HIV-1 infected patients tested for Schistosoma infection who had never initiated ART.
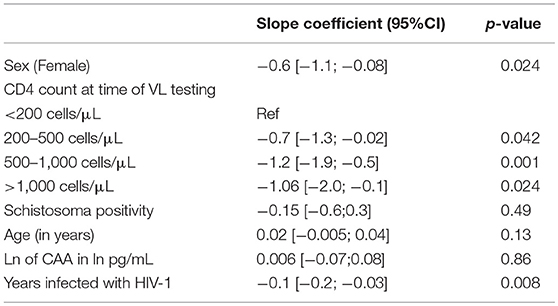
After univariable linear regression, female sex, higher CD4 counts and longer time infected with HIV-1, were all significantly associated with lower log10 of the viral load. Schistosoma positivity and ln of CAA were not associated with log10 of the viral load (Table 2).
Our unadjusted data shows a typical relationship between viral load and time infected with HIV-1, as described by other studies (Figure 1A) (31).
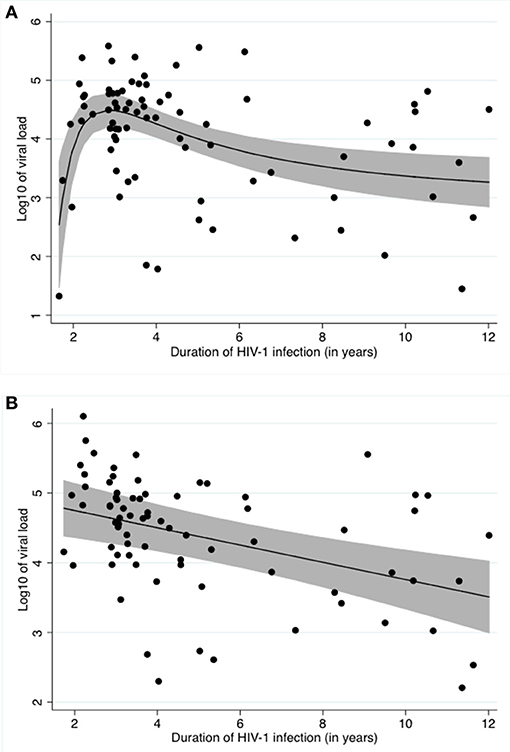
Figure 1. Relationship between log10 of the viral load and time infected with HIV (A) Unadjusted, (B) Adjusted for Schistosoma status, ln of CAA and CD4 counts. (A) shows the crude relationship between log10 of viral load and duration of HIV-1 infection. A fractional polynomial was fitted to the data. The black line represents the predicted log10 of viral load after applying the resulting function to the data. The grey area represents the 95% confidence limits around the fitted values. The black dots represent the residuals. (B) shows the relationship between log10 of viral load and duration of HIV-1 infection after adjustment for Schistosoma infection status, ln of CAA and CD4 counts using a fractional polynomial. The black line represents the predicted log10 of viral load after applying the resulting function to the data. The grey area represents the 95% confidence limits around the fitted values. The black dots represent the residuals.
After stepwise multivariable analysis, the best-fit model for log10 viral load included Schistosoma positivity, ln of CAA, CD4 counts at time of study enrollment and time infected with HIV-1. Schistosoma positivity was negatively associated with the log10 of viral load after adjustment (−0.7[−1.3;−0.1], p = 0.022). Sex and age were not part of the best-fit model. The best-fit model is presented in Table 3. Of note, this model includes both the Schistosoma infection status as a binary variable and also the natural log of the CAA value to assess whether the intensity of the Schistosoma infection impacted the viral load. The relationship between duration of HIV-1 infection and log10 of viral load adjusted for Schistosoma status, ln of CAA, and CD4 counts is shown in Figure 1B.
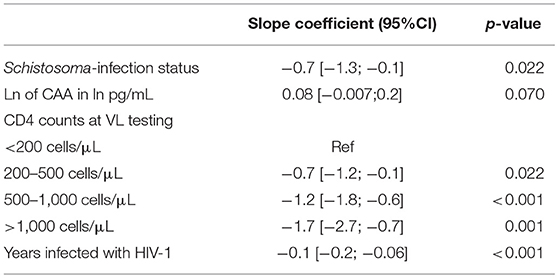
Table 3. Results of the multivariable linear regression with log10 of viral load as a continuous outcome.
When looking at whether the difference in median log10 of viral load between Schistosoma infected and uninfected patients changed over time, we found no statistical significance. Figure 2 assesses the difference in median of log10 viral load between Schistosoma infected and uninfected patients within each HIV-1 infected time category.
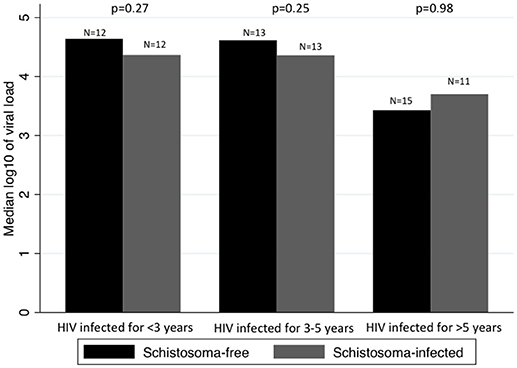
Figure 2. Comparison between median viral loads by time infected with HIV-1 and by Schistosoma-infection status. Panel shows the median log10 of viral load in Schistosoma-free and Schistosoma-infected patients, by category of duration of HIV-1 infection. The difference in median log10 of viral load was assessed by rank-sum test. There was no difference in median log10 of viral load between Schistosoma-free and Schistosoma-infected, regardless of the duration of HIV-1 infection.
We assessed the impact of time on the difference in median of log10 viral load between Schistosoma infected and uninfected patients using a quantile regression with an interaction term between Schistosoma status and duration of HIV-1 infection. In other words, we assessed whether the difference in viral loads between Schistosoma infected and uninfected patients seen in people HIV infected for 3–5 years is significantly different from the difference seen in people HIV infected for <3 years, and again for >5 vs. <3 years. The quantile regression showed no significant interactions between Schistosoma status and time infected with HIV-1.
In our cohort, Schistosoma status was not associated with CD4 counts (slope coefficient = −48.2 [−184.8; 88.4], p = 0.48).
Discussion
Our study showed that current infection with Schistosoma spp. was associated with significantly lower HIV-1 viral loads after adjusting for CD4 counts and time infected with HIV-1. The viral load difference of 0.7 log10 copies/mL would be expected to lead to over a 60% decrease in risk of HIV transmission and of reaching AIDS-related death for Schistosoma -co-infected patients compared to Schistosoma -free patients (32). This is in close alignment with the 82% decrease in risk of reaching lower CD4 counts and/or death found by our group (16) using a different analysis technique and in a different population. Taken together, these findings suggest the possibility that long-term HIV outcomes may be positively affected by Schistosoma infection.
Our study is the first, to our knowledge, to report adjusting for duration of HIV-1 infection when studying the relationship between HIV-1 viral load and Schistosoma spp. Time infected with HIV-1 was a main confounder of the relationship between HIV-1 viral load and Schistosoma infection. It is well known that CD4 counts and viral load in HIV-1 infected individuals, and the rates at which they change, differ over time (33–35), which makes it difficult to compare changes in these parameters between two individuals at different periods of their HIV-1 infection (36). Duration of HIV-1 infection may thus be a critical explanatory factor in the disparate findings of studies on HIV-1 viral load and Schistosoma infections (7–15), as suggested by Walson et al. (36). Other studies' lack of control for duration of HIV-1 infection may have hindered accurate analysis of the relationship between HIV-1 and Schistosoma infections. This may also be true for most studies looking at the relationship between other helminths and HIV-1 infections.
In addition, the control for ART initiation has been inconsistent in most studies. Many investigators have either assumed that all participants were ART naïve due to past limited availability of ART, or have not mentioned ART intake at all in their studies (7, 8, 10, 11, 15). Importantly, given the drastic effect of ART on CD4 counts and HIV-1 viral load (31), failure to account for ART intake in even a few individuals could have biased the results. By choosing patients who had been followed in the HIV outpatient clinic and would be beginning ART in the near future, and by measuring their viral load before initiation of ART, we avoided having the relationship between Schistosoma spp. and HIV-1 viral load distorted by the effect of ART on viral loads.
The association between sex and viral load on univariable analysis is unsurprising. Male sex has previously been shown to be associated with higher viral loads (37–39). The decrease in viral load with increasing CD4 counts has also been previously documented in sub-Saharan Africa (40, 41). The overall decrease in viral load over time infected with HIV-1 is logical, as too few of our patients have been infected with HIV-1 for over 10 years to see the late-stage re-increase in viral loads.
Our results are to be interpreted in light of some limitations. The sample size was small due to the expense and relative unavailability of viral load testing at a time when viral load testing was just becoming available at our clinic. In addition, despite not being significant in our study or in a study in South Africa (42), others have shown an impact of Schistosoma spp on CD4 counts (8, 10, 11, 16, 43–45), potentially biasing our calculation of the duration of HIV infection. Assessing this relationship using other methods to determine the length of HIV infection would be useful. Nonetheless, the facts that significance is still attained and that variables expected to impact viral load do impact it strengthen confidence in the quality and accuracy of our analysis. Larger, longitudinal studies would be useful in order to investigate the potential interactions between duration of HIV-1 infection and Schistosoma infection and its effect on HIV-1 viral load. Investigating the effect of praziquantel treatment would also be of interest given studies that suggest that tissue lesions may not regress after treatment (46).
In conclusion, our work demonstrates that individuals with HIV-1 and Schistosoma co-infections had lower viral loads than those with HIV-1 alone, when accounting for time infected with HIV-1. The difference in viral load suggests that Schistosoma infection may not lead to worse HIV-1 outcomes nor higher HIV-1 transmission. Future studies of interactions between HIV-1 and Schistosoma spp. should account for the duration of HIV-1 infection in their analyses.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
SC participated in data collection, analyzed the data and wrote the original manuscript. CdD, PC, and GvD assisted with laboratory and data analysis and reviewed the final manuscript. DM, RM, and JM conducted laboratory testing and review the final manuscript. LvL assisted with data analysis and reviewed the final manuscript. SK designed the study and reviewed the final manuscript. JD designed the study, enrolled the patients, analyzed the data and wrote the original manuscript. All authors have reviewed and approved the current submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We want to thank Jim Todd (London School of Tropical Hygiene and Medicine) for his input on the calculation of the duration of HIV-1 infection. We also want to thank BMC HIV clinic staff for their help in retrieving the data and the study participants for their enthusiastic involvement in this project. This study was funded by K23 AI 110238 (to JD).
References
1. WHO. HIV/AIDS Fact Sheet. (2018) Available Online at: http://www.who.int/en/news-room/fact-sheets/detail/hiv-aids
2. WHO. Schistosomiasis Fact Sheet. (2018) Available Online at: http://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis
3. Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (2016) 388:1603–58. doi: 10.1016/S0140-6736(16)31460-X
4. Chenine AL, Buckley KA, Li PL, Rasmussen RA, Ong H, Jiang S, et al. Schistosoma mansoni infection promotes SHIV clade C replication in rhesus macaques. AIDS (2005) 19:1793–7. doi: 10.1097/01.aids.0000189857.51935.0b
5. Ayash-Rashkovsky M1, Chenine AL, Steele LN, Lee SJ, Song R, Ong H, et al. Coinfection with Schistosoma mansoni reactivates viremia in rhesus macaques with chronic simian-human immunodeficiency virus clade C infection. Infect Immun. (2007) 75:1751–6. doi: 10.1128/IAI.01703-06
6. Chenine AL, Shai-Kobiler E, Steele LN, Ong H, Augostini P, Song R, et al. Acute Schistosoma mansoni infection increases susceptibility to systemic SHIV clade C infection in rhesus macaques after mucosal virus exposure. PLoS Negl Trop Dis. (2008) 2:e265. doi: 10.1371/journal.pntd.0000265
7. Lawn SD, Karanja DM, Mwinzia P, Andove J, Colley DG, Folks TM, et al. The effect of treatment of Schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS (2000) 14:2437–43. doi: 10.1097/00002030-200011100-00004
8. Elliott AM, Mawa PA, Joseph S, Namujju PB, Kizza M, Nakiyingi JS, et al. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans R Soc Trop Med Hyg. (2003) 97:103–8. doi: 10.1016/S0035-9203(03)90040-X
9. Brown M, Kizza M, Watera C, Quigley MA, Rowland S, Hughes P, et al. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. J Infect Dis. (2004) 190:1869–79. doi: 10.1086/425042
10. Brown M, Mawa PA, Joseph S, Bukusuba J, Watera C, Whitworth JA, et al. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J Infect Dis. (2005) 191:1648–57. doi: 10.1086/429668
11. Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, van Dam GJ, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of Schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis. (2005) 192:1956–61. doi: 10.1086/497696
12. Sangaré LR, Herrin BR, John-Stewart G, Walson JL. Species-specific treatment effects of helminth/HIV-1 co-infection: a systematic review and meta-analysis. Parasitology (2011) 138:1546–58. doi: 10.1017/S0031182011000357
13. Walson J, Singa B, Sangaré L, Naulikha J, Piper B, Richardson B, et al. Empiric deworming to delay HIV disease progression in adults with HIV who are ineligible for initiation of antiretroviral treatment (the HEAT study): a multi-site, randomised trial. Lancet Infect Dis. (2012) 12:925–32. doi: 10.1016/S1473-3099(12)70207-4
14. Obuku AE, Asiki G, Abaasa A, Ssonko I, Harari A, van Dam GJ, et al. Effect of Schistosoma mansoni infection on innate and HIV-1-specific T-cell immune responses in HIV-1-infected ugandan fisher folk. AIDS Res Hum Retroviruses (2016) 32:668–75. doi: 10.1089/aid.2015.0274
15. Downs JA, Dupnik KM, van Dam GJ, Urassa M, Lutonja P, Kornelis D, et al. Effects of Schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: a nested case-control study. PLoS Negl Trop Dis. (2017) 11:e0005968. doi: 10.1371/journal.pntd.0005968
16. Colombe S, Machemba R, Mtenga B, Lutonja P, Kalluvya SE, de Dood CJ, et al. Impact of Schistosome infection on long-term HIV/AIDS outcomes. PLoS Negl Trop Dis. (2018) 12:e0006613. doi: 10.1371/journal.pntd.0006613
17. Efraim L, Peck RN, Kalluvya SE, Kabangila R, Mazigo HD, Mpondo B, et al. Schistosomiasis and impaired response to antiretroviral therapy among HIV-infected patients in Tanzania. J Acquir Immune Defic Syndr. (2013) 62:e153–6. doi: 10.1097/QAI.0b013e318282a1a4
18. Marti AI, Colombe S, Masikini PJ, Kalluvya SE, Smart LR, Wajanga BM, et al. Increased hepatotoxicity among HIV-infected adults co-infected with Schistosoma mansoni in Tanzania: a cross-sectional study. PLoS Negl Trop Dis. (2017) 11:e0005867. doi: 10.1371/journal.pntd.0005867
19. Corstjens PLAM, De Dood CJ, Kornelis D, Fat EM, Wilson RA, Kariuki TM, et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology (2014) 141:1841–55. doi: 10.1017/S0031182014000626
20. Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, et al. Variations in helminth faecal egg counts in Kato-Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Trop. (2004) 92:205–12. doi: 10.1016/j.actatropica.2004.06.011
21. Vanhems P, Lambert J, Guerra M, Hirschel B, Allard R. Association between the rate of CD4+ T cell decrease and the year of human immunodeficiency virus (HIV) type 1 seroconversion among persons enrolled in the Swiss HIV cohort study. J Infect Dis. (1999) 180:1803–8. doi: 10.1086/315110
22. Taffé P, May M, Study SHC. A joint back calculation model for the imputation of the date of HIV infection in a prevalent cohort. Stat Med. (2008) 27:4835–53. doi: 10.1002/sim.3294
23. Forbi JC, Forbi TD, Agwale SM. Estimating the time period between infection and diagnosis based on CD4+ counts at first diagnosis among HIV-1 antiretroviral naïve patients in Nigeria. J Infect Dev Ctries. (2010) 4:662–7. doi: 10.3855/jidc.1015
24. Van Sighem A, Nakagawa F, De Angelis D, Quinten C, Bezemer D, de Coul EO, et al. Estimating HIV incidence, time to diagnosis, and the undiagnosed HIV epidemic using routine surveillance data. Epidemiology (2015) 26:653–60. doi: 10.1097/EDE.0000000000000324
25. Wong NS, Wong KH, Lee MP, Tsang OTY, Chan DPC, Lee SS. Estimation of the undiagnosed intervals of HIV-infected individuals by a modified back-calculation method for reconstructing the epidemic curves. PLoS ONE (2016) 11:e0159021. doi: 10.1371/journal.pone.0159021
26. Ngowi BJ, Mfinanga SG, Bruun JN, Morkve O. Immunohaematological reference values in human immunodeficiency virus-negative adolescent and adults in rural northern Tanzania. BMC Infect Dis. (2009) 9:1. doi: 10.1186/1471-2334-9-1
27. Shao ER, Kifaro EG, Kimaro J, Mrema JG, Mwasamwaja AO, Kayandabila J, et al. HIV-1 diversity in Tanzania and its implication toward development of effective vaccines: a review article. J Vaccines Vaccin. (2014) 5:249. doi: 10.4172/2157-7560.1000249
28. Kasang C, Kalluvya S, Majinge C, Stich A, Bodem J, Kongola G, et al. HIV drug resistance (HIVDR) in antiretroviral therapy-naive patients in Tanzania not eligible for WHO threshold HIVDR survey is dramatically high. PLoS ONE (2011) 6:e23091. doi: 10.1371/journal.pone.0023091
29. Kiwanuka N, Robb M, Laeyendecker O, Kigozi G, Wabwire-Mangen F, Makumbi FE, et al. HIV-1 viral subtype differences in the rate of CD4+ T-cell decline among HIV Seroincident antiretroviral naive persons in Rakai district, Uganda. J Acquir Immune Defic Syndr. (2010) 54:180–4. doi: 10.1097/QAI.0b013e3181c98fc0
30. Ndembi N, Lyagoba F, Nanteza B, Kushemererwa G, Serwanga J, Katongole-Mbidde E. Transmitted antiretroviral drug resistance surveillance among newly HIV type 1-diagnosed women attending an antenatal clinic in Entebbe, Uganda. AIDS Res Hum Retroviruses (2008) 24:889–95. doi: 10.1089/aid.2007.0317
31. Landi A, Mazzoldi A, Andreoni C, Bianchi M, Cavallini A, Laurino M, et al. Modelling and control of HIV dynamics. Comp Methods Prog Biomed. (2008) 89:162–8. doi: 10.1016/j.cmpb.2007.08.003
32. Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS (2008) 22:2179–85. doi: 10.1097/QAD.0b013e328312c756
33. Patrikar S, Basannar DR, Bhatti VK, Kotwal A, Gupta RM, Grewal RS. Rate of decline in CD4 count in HIV patients not on antiretroviral therapy. Med J Armed Forces India. (2014) 70:134–8. doi: 10.1016/j.mjafi.2013.08.005
34. Arnaout RA, Lloyd AL, O'Brien TR, Goedert JJ, Leonard JM, Nowak MA. A simple relationship between viral load and survival time in HIV-1 infection. Proc Natl Acad Sci USA. (1999) 96:11549–53.
35. Martinson NA, Gupte N, Msandiwa R, Moulton LH, Barnes GL, Ram M, et al. (2014). CD4 and viral load dynamics in antiretroviral-naïve HIV-infected adults from Soweto, South Africa: a prospective cohort. PLoS ONE (2014) 9:e96369. doi: 10.1371/journal.pone.0096369
36. Walson JL, John-Stewart G. Treatment of helminth co-infection in individuals with HIV-1: a systematic review of the literature. PLoS Negl Trop Dis. (2007) 1:e102. doi: 10.1371/journal.pntd.0000102
37. Sterling TR, Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, et al. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. (2001) 344:720–5. doi: 10.1056/NEJM200103083441003
38. Donnelly CA, Bartley LM, Ghani AC, Le Fevre AM, Kwong GP, Cowling BJ, et al. Gender difference in HIV-1 RNA viral loads. HIV Med. (2005) 6:170–8. doi: 10.1111/j.1468-1293.2005.00285.x
39. Ballesteros-Zebadúa P, Villarreal C, Cocho G, Huerta L, Estrada JL. Differences in HIV-1 viral loads between male and female antiretroviral-untreated Mexican patients. Arch Med Res. (2013) 44:296–301. doi: 10.1016/j.arcmed.2013.04.003
40. Govender S, Otwombe K, Essien T, Panchia R, De Bruyn G, Mohapi L, et al. CD4 counts and viral loads of newly diagnosed HIV-infected individuals: implications for treatment as prevention. PLoS ONE (2014) 9:e90754. doi: 10.1371/journal.pone.0090754
41. Ochieng W, Ogoyi D, Mulaa FJ, Ogola S, Musoke R, Otsyula MG. Viral load, CD4+ T-lymphocyte counts and antibody titres in HIV-1 infected untreated children in Kenya; implication for immunodeficiency and AIDS progression. Afr Health Sci. (2006) 6:3–13. doi: 10.5555/afhs.2006.6.1.3
42. Kleppa E, Klinge KF, Galaphaththi-Arachchige HN, Holmen SD, Lillebø K, Onsrud M, et al. Schistosoma haematobium infection and CD4+ T-cell levels: a cross-sectional study of young South African women. PLoS ONE (2015) 10:e0119326. doi: 10.1371/journal.pone.0119326
43. Li Y, Zeng Q, Ellis MK, Xiong T, Balen J, McManus DP. CD4+ T-cell counts, CD4+/CD8+ T-cell count ratios, and antibody levels in migrant fishermen infected with Schistosoma japonicum in the Dongting Lake, China. Am J Trop Med Hyg. (2006) 75:910–3. doi: 10.4269/ajtmh.2006.75.910
44. Oliveira-Prado R, Caldas IR, Teixeira-Carvalho A, Andrade MV, Gazzinelli A, Correa-Oliveira R, et al. CD4 and CD8 distribution profile in individuals infected Schistosoma mansoni. Scand J Immunol. (2009) 69:521–8. doi: 10.1111/j.1365-3083.2009.02247.x
45. Nausch N, Bourke CD, Appleby LJ, Rujeni N, Lantz O, Trottein F, et al. Proportions of CD4+ memory T cells are altered in individuals chronically infected with Schistosoma haematobium. Sci Rep. (2012) 2:472. doi: 10.1038/srep00472
Keywords: Schistosoma spp., HIV-1, Viral load, Plasma HIV-1 RNA, Tanzania
Citation: Colombe S, Corstjens PLAM, de Dood CJ, Miyaye D, Magawa RG, Mngara J, Kalluvya SE, van Lieshout L, van Dam GJ and Downs JA (2018) HIV-1 Viral Loads Are Not Elevated in Individuals Co-infected With Schistosoma spp. After Adjustment for Duration of HIV-1 Infection. Front. Immunol. 9:2005. doi: 10.3389/fimmu.2018.02005
Received: 13 June 2018; Accepted: 14 August 2018;
Published: 06 September 2018.
Edited by:
Michael Harrison Hsieh, Children's National Health System, United StatesReviewed by:
William Evan Secor, Centers for Disease Control and Prevention (CDC), United StatesKeke Celeste Fairfax, Purdue University, United States
Copyright © 2018 Colombe, Corstjens, de Dood, Miyaye, Magawa, Mngara, Kalluvya, van Lieshout, van Dam and Downs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soledad Colombe, c29sZWRhZC5jb2xvbWJlQGdtYWlsLmNvbQ==
 Soledad Colombe
Soledad Colombe Paul L. A. M. Corstjens2
Paul L. A. M. Corstjens2 Lisette van Lieshout
Lisette van Lieshout Govert J. van Dam
Govert J. van Dam Jennifer A. Downs
Jennifer A. Downs